Abstract
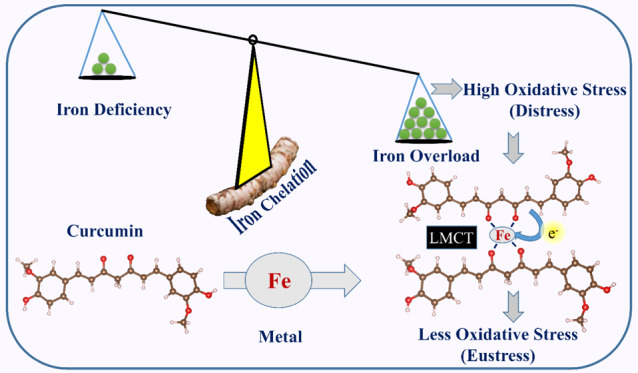
Chelation therapy is one of the most effective and widely accepted methods of treatment to reduce metal toxicity caused by an excess amount of essential metals. Essential minerals play an important role in maintaining healthy human physiology. However, the presence of an excess amount of such essential metals can cause cell injury, which finally leads to severe life-threatening diseases. Chelating complexes can efficiently capture the targeted metal and can easily be excreted from the body. Commonly utilized metal chelators have major side effects including long-term damage to some organs, which has pointed out the need of less harmful biocompatible chelating agents. In this work, we have investigated the iron chelating property of curcumin through various spectroscopic tools by synthesizing and characterizing the iron–curcumin (Fe–Cur) complex. We have also investigated whether the synthesized materials are able to retain their antioxidant activity after the chelation of a substantial amount of metal ion. Our study unravels the improved antioxidant activity of the synthesized chelate complex. We further demonstrate that the proposed complex generates no significant reactive oxygen species (ROS) under dark conditions, which makes it a promising candidate for chelation therapy of iron toxicity. Femtosecond-resolved fluorescence studies further provide insight into the mechanism of activity of the new complex where electron transfer from ligand to metal has been observed prominently. Thus, the Fe–Cur complex has a potential to act as a dual activity medicine for excretion of toxic metal ions via chelation and as a therapeutic agent of oxidative stress caused by the metal ion as well.
Introduction
Essential metals such as cobalt (Co), iron (Fe), chromium (Cr), copper (Cu), nickel (Ni), manganese (Mn), molybdenum (Mo), zinc (Zn), and selenium (Se) are fundamental supplements that are essential for several physiological and biochemical purposes.1−3 Nevertheless, a portion of these substantial metals in high dosages can be dangerous to the body, while others (e.g., cadmium, mercury, lead, chromium, silver, and arsenic) in minute amounts have incoherent impacts in the body, causing critical and chronic toxicities in humans.4,5 Excess amounts of essential metals are a source of neurotoxicity. Moreover, excess metal in the human body can generate free radicals, which can increase the oxidative stress and can destroy lipids, proteins, and DNA molecules. These free radicals also promote carcinogenesis. Excess amount of iron has largely been reported to be one of the reasons for death in children6,7 and has a long history of research in therapeutics.8,9 After the discovery of numerous oral medicines for iron toxicity, child mortalities are under control, but rigorous poisonings due to excess iron still take place.10
So far, chelation therapy is the most powerful and commonly utilized strategy for the treatment of substantial metal overburden and mitigation of a number of diseases such as Parkinsonʼs disease, Alzheimerʼs disease, Wilsonʼs ailment, and Friedreichʼs ataxia.11 The most commonly utilized chelating agents such as CaNa2EDTA—1985,9 BAL—1949,9 DMSA—1978,12 and DMPS—195813 were utilized to treat metal overburden issues for a long time. However, these chelators have harmful impacts that may damage a few fundamental organs.14,15 The accumulation of a substantial amount of metals in cerebrum by some chelators can affect intellectual capacity of teenagers.16 Thus, nanoscience-related innovation can open a new approach for the treatment of metal toxicity-related issues.17,18 Various in vitro investigations have portrayed the capacity of metallic nanoparticles to tie or detect the excess amount of metals.7,19 For example, nanosized silver and gold particles can agglomerate with bivalent metal ions such as mercury (Hg2+), copper (Cu2+), iron (Fe2+), and lead (Pb2+), which can shift the surface plasmon band, useful for detection purposes.20−22 However, low biocompatibility limits their in vivo applications.20 In the present study, we have developed a new technique that could be a potential option for the treatment of substantial cationic metal poisoning.
Among all of the cationic metals, iron is an essential mineral in the human body as it constitutes the important biological molecule hemoglobin, and thus maintaining the proper iron balance in body is important to regulate different physiological activities.23 Nevertheless, iron is one of the heavy metals commonly known to produce hydroxyl radicals (•OH)1. Excess amount of iron leads to greater formation of free radicals that can cause carcinogenesis.24 Iron-instigated free radicals can produce malignancy by oxidation of DNA, prompting to DNA damage.25 In this work, through various spectroscopy tools, we have investigated the different properties of the synthesized iron–curcumin (Fe–Cur) complex, which provide us the details of the Fe-chelating action by the promising chelator curcumin. We have also investigated whether the synthesized complex provides antioxidant activity. The role of metal particles in the antioxidant action of the complex is assessed in detail using a well-known radical scavenger 2,2-diphenyl-1-picrylhydrazyl (DPPH) in aqueous media under light irradiation as well as under dark conditions. This analysis reveals that the antioxidant activity of curcumin increases after the chelation of Fe, and a consequent decrease is also observed in free radical generation under dark conditions. We have extended our studies on reactive oxygen species (ROS) in aqueous solution, which show that this complex does not generate any significant ROS in the dark. The results validate its acceptability as a therapeutic agent. Femtosecond-resolved fluorescence studies further highlight the mechanistic approach of the therapeutic activity of the metal–ligand complex. Through the photophysical study, it has been found that the ligand-to-metal charge transfer plays the key role to control the entire ROS generation and radical scavenging activity of the proposed complex. For a comparison of the activity and probable side effects of the Fe–Cur complex, we have introduced and investigated another transition metal complex with the ligand curcumin (Zn–Cur). Although these two complexes are structurally similar, the difference in electronic distribution causes a decreased efficiency in the activities. The trend in radical scavenging activity is observed to be similar for both the metal curcumin complexes, which validates the role of electron transfer in controlling the activities of the complex. Thus, the entire study provides deep insight into the photophysical behavior of a potential chelating therapeutic agent curcumin in mitigation of iron toxicity with validation of in vitro applications that may uncover a dual activity nanotherapeutic approach in future.
Results and Discussion
The schematic representation of Fe–Cur complexes is depicted in Figure 1a, and the photographs of curcumin (yellow) and Fe–Cur (brown) in the dimethyl sulfoxide (DMSO) solvent under visible light are shown in Figure 1b. The formation of metallo-organic complexes causes significant alterations in the electronic configuration of the free ligands. UV–visible absorption spectroscopy is a suitable method to determine complexation or metalation. Figure 1c represents the absorption spectra of FeCl3 (blue) with an absorption peak at 340 nm and of curcumin (black) with a peak at 435 nm in DMSO solvent. The inset shows the absorption maximum of FeCl3 (blue) with an absorption peak at 390 nm and of curcumin (black) appears at 440 nm in an aqueous solvent. The absorption peaks of the ligand curcumin can be assigned to a π–π* transition from (HOMO-1) to (LUMO) and (HOMO) to (LUMO) separately.26 The blue shift in the absorption spectra of curcumin after attachment to the metal particle provides a signature of the covalent attachment. For the Fe–curcumin complex, a clear peak can be observed at 440 nm, with a wide absorption peak at around 520 nm in DMSO as well as in water as shown in Figure 1c (red). Some earlier reports27,28 have depicted the comparative Fourier transform infrared (FTIR) and nuclear magnetic resonance (NMR) spectra of Fe–Cur and curcumin and concluded effective complex formation.
Figure 1.
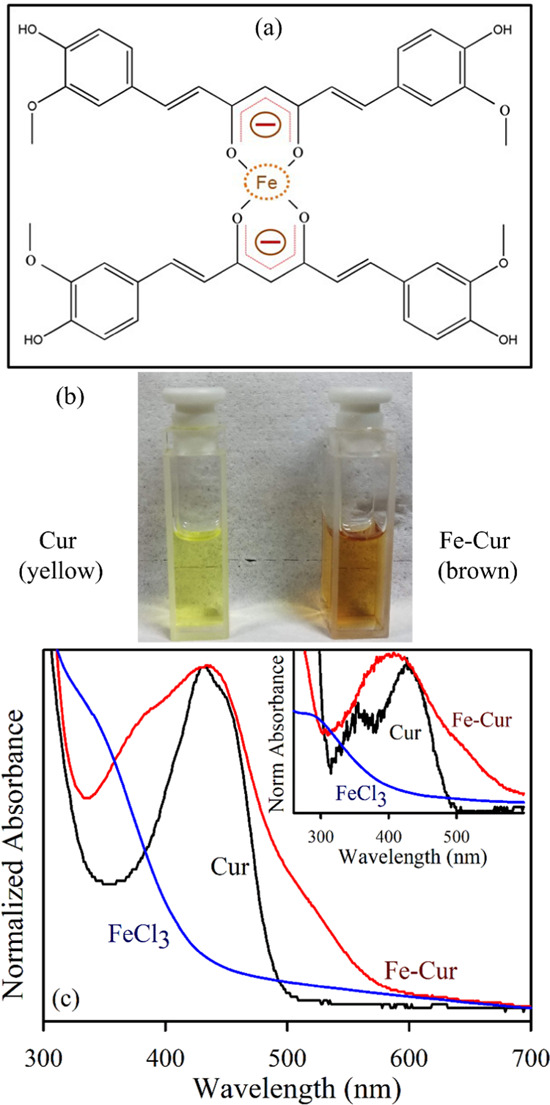
(a) Schematic representation of Fe–Cur complexes. (b) Photographs of curcumin (yellow) and Fe–Cur (brown) in DMSO under visible light. (c) Absorption spectra of FeCl3 (blue), Cur (black), and Fe–Cur (red) using DMSO as the solvent. The inset shows the absorption spectra of FeCl3 (blue), Cur (black), and Fe–Cur (red) using water as the solvent.
The room-temperature steady-state photoluminescence (PL) spectrum (Figure 2a) of curcumin (black) shows a peak at 520 nm upon excitation at 400 nm in DMSO solvent, and the inset shows the same measurement in an aqueous solvent. However, after metalation with iron, the steady-state emission intensity is significantly quenched (in both the solvents) in spite of the fact that the absorption peaks of the complex in both the solutions are comparable. The observation leads to the possibility of nonradiative charge transfer.29Figure 2b shows the excitation spectra of all of the samples in DMSO solvent, and the inset shows the excitation of all of the samples in an aqueous solvent. A significant decrease in intensity for both emission and excitation spectra after metalation can be ascribed to the signature of complexation as well as nonradiative charge transfer from the ligand (Cur) to the chelated metal ion. There is also a possibility of the development of a new energetically low lying charge-transfer state due to electronic transition from curcumin to the chelated metal. The curcumin moiety having delocalized π-electron density behaves as a donor to the positively charged metal center (which has empty d-orbitals) behaving as an acceptor and exhibiting a ligand-to-metal charge-transfer (LMCT) band.30,31
Figure 2.
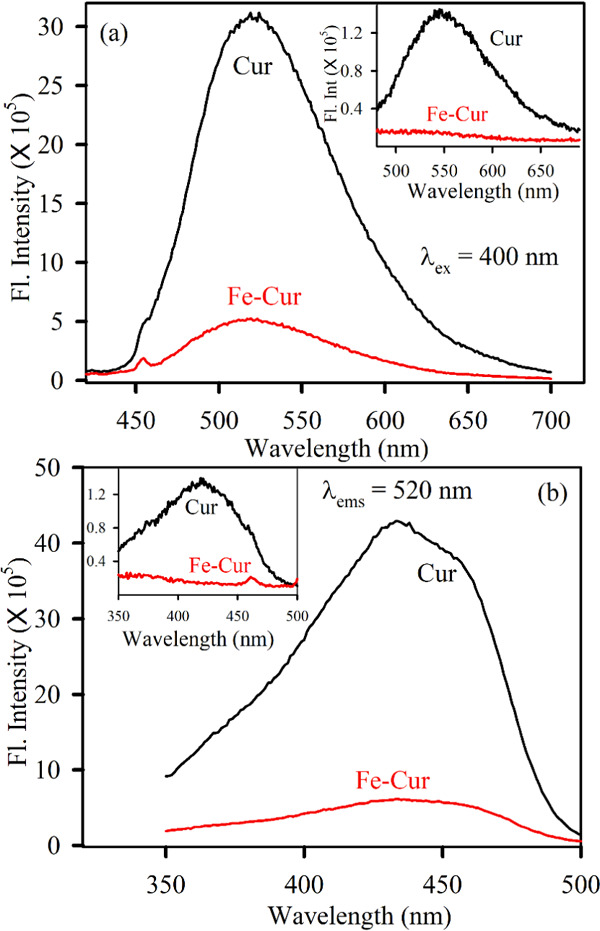
(a) Room-temperature emission spectra of Cur (black) and Fe–Cur (red) in DMSO are shown. The excitation wavelength was 400 nm. The inset shows the emission spectra of the same samples using water as a solvent. (b) Room-temperature excitation spectra of Cur (black) and Fe–Cur (red) in DMSO are shown. The emission wavelength was set at 530 nm. The inset shows the excitation spectra of the same samples using water as a solvent.
Figure 3a demonstrates the antioxidant activities of curcumin and the Fe–Cur complex in the dark as well as under green light irradiation and constant stirring conditions. The antioxidant activity of samples is observed by the decolorization kinetics of the stable free radical DPPH in an ethanol–water mixture.32 DPPH, a stable free radical having violet color, is reduced to DPPH2, which is yellow in color, due to donation of a H-atom from the polyphenolic antioxidant to the radical.33 As the DPPH assay is performed under stirring conditions, there is no possibility of precipitation. Figure 3a shows an increase in radical scavenging activity for Fe–Cur in the dark, whereas under green light conditions, no effect has been observed in this assay. The electronic configuration of Fe (at the ground state) allows it to capture electron density from the peripheral O–H bond and initiate the breaking of the peripheral O–H bond.26 Thus, it can show more antioxidant property. However, in the case of the light-irradiated system, the electronic configuration alters significantly in the case of Fe–Cur complex. This may possibly induce stability in the complex that could lead to less electron affinity of the metal center, which results in a lower antioxidant activity. Thus, the improved antioxidant property of Fe–Cur in the dark results in formation of a more vulnerable ArO–H bond in curcumin and a resulting simpler H-atom loss process.34 The dose-dependent antioxidant activities of Fe–Cur have been performed at three different concentrations (OD 0.07, 0.10, and 0.15) as shown in Figure 3b. It has been clearly observed that the antioxidant capability of Fe–Cur increases more for the concentration of OD 0.10 compared to other two concentrations in the dark (Figure 3b). The results demonstrate that Fe–Cur serves as an extremely effective free-radical scavenger compared to free curcumin in water in the absence of light.
Figure 3.
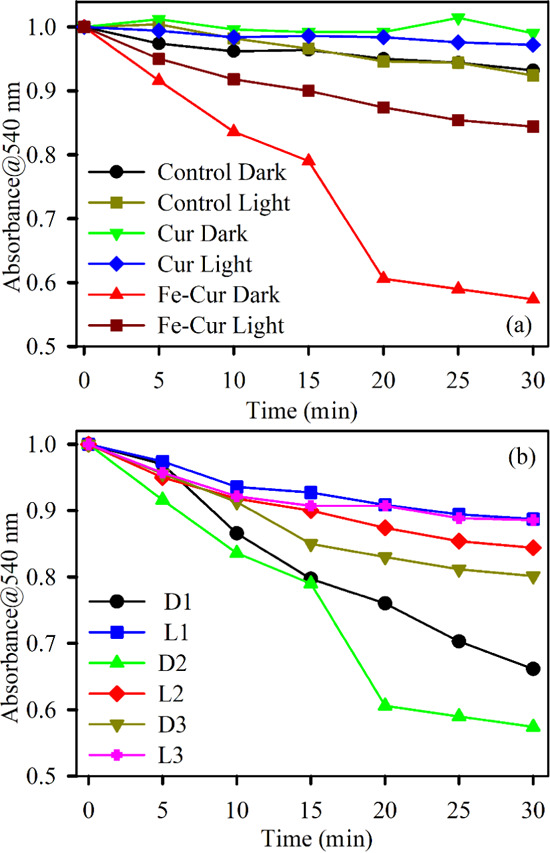
Absorption kinetics of DPPH degradation (monitored at 540 nm) (a) in the absence of light using samples Cur (green) and Fe–Cur (red) and in the presence of green light with Cur (blue) and Fe–Cur (brown) and (b) in the absence (D1: 0.07 OD, D2: 0.10, and D3: 0.15 OD) and presence (L1: 0.07 OD, L2: 0.10 OD, and L3: 0.15 OD) of light using samples Fe–Cur with variable concentrations.
We have assessed the ROS generation capability by Fe–Cur with respect to curcumin by performing the dichlorofluorescein (DCFH) assay in the presence of light and in the dark as well. The rise in the emission intensity of DCF, which is the oxidized form of DCFH, has been monitored to quantify the amount of ROS in the system. The Fe–Cur complex shows 8.5 times additional ROS generation compared to curcumin under green light irradiation, as represented in Figure 4a. The dose-dependent capability of Fe–Cur has been performed at three different concentrations (OD 0.07, 0.10, and 0.15) as shown in Figure 4b. It has been clearly observed that the ROS generation capability of Fe–Cur increases with increasing concentration (Figure 4b) in the presence of green light. Fe–Cur almost does not generate any ROS in the absence of light.
Figure 4.
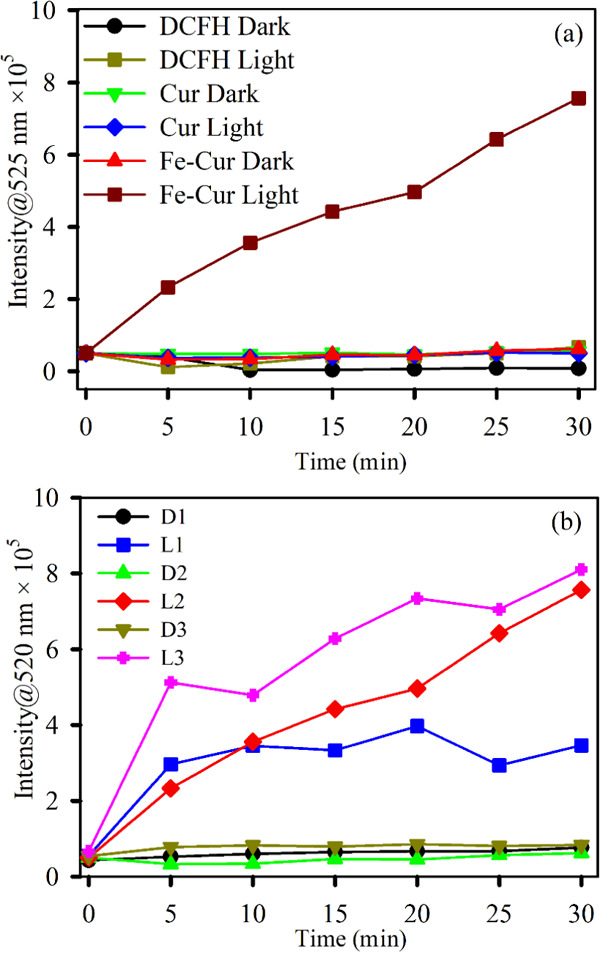
DCFH oxidation (monitored at 525 nm) with time (a) in the absence of light with samples Cur (green) and Fe–Cur (red) and in the presence of light with Cur (blue) and Fe–Cur (brown) and (b) in the absence (D1: 0.07 OD, D2: 0.10 OD, and D3: 0.15 OD) and presence (L1: 0.07 OD, L2: 0.10 OD, and L3: 0.15 OD) of light using samples Fe–Cur with variable concentrations. The excitation wavelength was 488 nm.
Femtosecond-resolved fluorescence transients of Fe–Cur samples have been measured to find out the excited-state dynamics of the complex, which provides further evidence of the metal–ligand chelation. The femtosecond-resolved decay profiles of the free ligand curcumin and the Fe–Cur complex are compared in Figure 5. The fluorescence decay of curcumin is fitted by a double exponential decay with a lifetime of 3.2 ps (faster part: corresponds to solvation dynamics) and another 73.5 ps (longer part: corresponds to excited-state intramolecular H-atom transfer, ESIHT).35 The average lifetime of the system is 50.5 ps. The femtosecond-resolved decay profile of Fe–Cur shows a faster time component of 0.09 ps, and the corresponding average lifetime is 25.6 ps. The lifetime components of the fluorescence transients are summarized in Table 1. The faster time scale in the decay pattern of the Fe complex could be ascribed to the electron transfer from the ligand to the chelated metal center.36 Therefore, this electron transfer process can be correlated to the enhanced antioxidant activity and photoinduced ROS generation by Fe–Cur.
Figure 5.
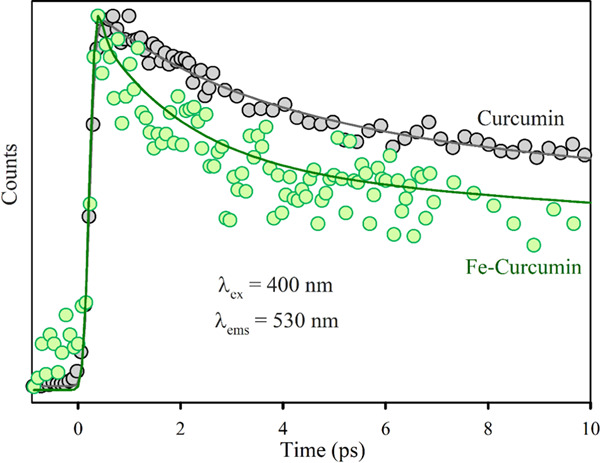
Femtosecond-resolved fluorescence transients of Cur (gray) and Fe–Cur (green) in DMSO. The excitation wavelength was 400 nm, and the detection wavelength was 530 nm. The circles are experimental data, and the solid lines are the best multiexponential fit.
Table 1. Lifetime of Femtosecond-Resolved Fluorescence Transients of Cur and Fe–Cur Complexes, Detected at 530 nm (PL Maxima) upon Excitation at 400 nm Wavelengtha.
| system | τ1 (ps) | τ2 (ps) | τ3 (ps) | τavg (ps) |
|---|---|---|---|---|
| Cur | 3.19 (32%) | 73.50 (68%) | 50.50 | |
| Fe–Cur | 0.09 (32%) | 1.82 (28%) | 62 (40%) | 25.60 |
Numbers in parentheses indicate relative contributions.
Additionally, we have performed a study on the antioxidant activity and photoinduced ROS generation by the Zn–Cur complex to compare its activity with the Fe–Cur one. It has been found that the antioxidant activity of Zn–Cur increases under dark conditions, but the increment is much less as compared to Fe–Cur. The trend is due to the presence of stronger O–H bonds in the case of the Zn complex as Zn(II) has a filled d orbital showing lower interactions compared to Fe(II).37Figure 6b shows the ROS generation capability of Zn–Cur in the presence of green light (blue) and in the absence of light (red). The ROS generation capability of Zn–Cur is increasing, but the ability is much less than that of Fe–Cur. Therefore, antioxidant activity and photoinduced ROS generation by Zn–Cur are consistent with the Fe–Cur results. However, the activity of Fe–Cur is far better than that of Zn–Cur.
Figure 6.
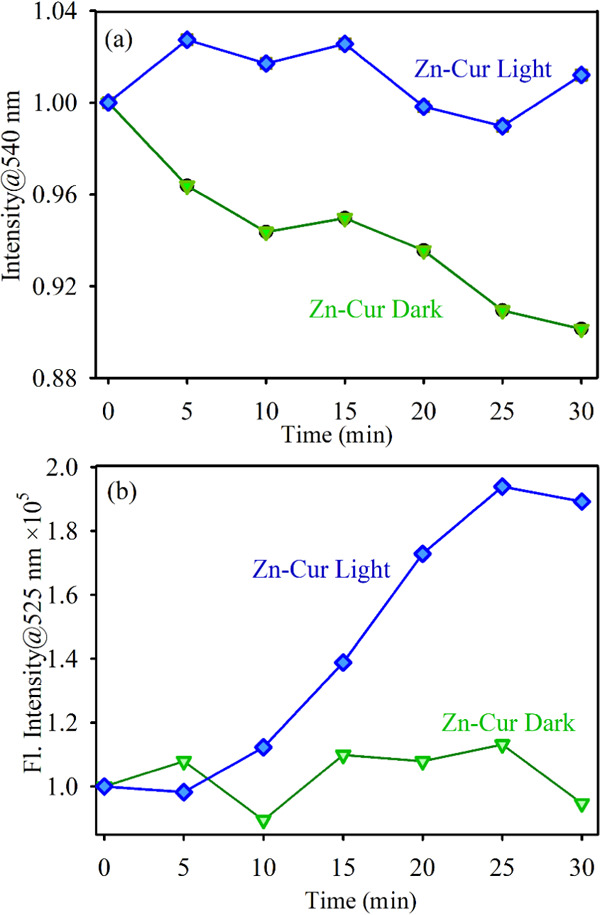
(a) Absorption kinetics of DPPH degradation (monitored at 540 nm) in the absence and presence of light using Zn–Cur. (b) DCFH emission (monitored at 525 nm) in the absence and presence of light using Zn–Cur samples. The excitation wavelength was 488 nm.
Conclusions
In the present study, the biocompatible ligand curcumin shows a high affinity to chelate with metallic Fe. Curcumin is known to have less or minimal side effects. Thus, it can be used as an alternative to conventional chelating agents. The Fe–Cur complex has enhanced the antioxidant activity and negligible ROS generation capability in the dark. The prominent ligand-to-metal charge transfer in the case of the Fe–Cur complex is found to be the mechanistic pathway to control the radical scavenging and the ROS generation trends. The proposed mechanism has further been validated by a comparison of activity with the structural analogue Zn–Cur. Thus, the electron rearrangement process in the Fe–Cur complex leading to efficient radical scavenging activity may pave a new way for the effective treatment of iron overload diseases and the Fe–Cur complex can be considered as an alternative medicine.
Experimental Section
Materials
All of the chemicals used in this study were of analytical grade and were used without any further refinement. Curcumin, anhydrous iron(III) chloride (FeCl3), and Zn(CH3CO2)2·2H2O were bought from Sigma-Aldrich. The appropriate solvent for the samples, methanol was purchased from Merck. For various spectroscopic studies, Millipore water and dimethyl sulfoxide (DMSO) from Spectrochem were used as solvents.
Preparation of Metallo-Curcumin Complexes
Fe–Cur Composite
This compound was prepared by mixing curcumin with iron(III) chloride at a molar ratio of 1:1 in methanolic solution.26,34 First, 50 mL of 2 mM curcumin in methanol was heated at 60 °C for dissolution. Iron(III) chloride (2 mM) was dissolved in 100 mL of methanol by heating and added into the curcumin solution. A brown solid precipitate was formed immediately, and it was refluxed for 2.5 h.38 The brown product was filtered and washed first with cold methanol and then with water to eliminate the remaining reactants. The purified product was dried in vacuum overnight at 60 °C, and the final product was brown.
Zn(II)–Cur Compound
The compound Zn(II)–Cur was synthesized at a molar ratio of 1:1.26,34 First, 50 mL of 2 mM solution of Cur in methanol was heated at 60 °C for dissolution. Then, 2 mM zinc acetate dihydrate was dissolved in 100 mL of methanol by heating. It was added into the Cur solution, and the solution was refluxed for 2.5 h.38 The red solid sample was filtered and washed to eliminate the remaining reactants. The purified sample was dried in vacuum overnight at 60 °C, and the final product was red.34
Characterization Methods
Optical Studies
Absorption spectra of the samples were measured using a Shimadzu UV-2600 spectrophotometer in quartz cell of path length 1 cm. The steady-state excitation and emission spectra were recorded in a Jobin-Yvon Fluorolog fluorimeter. The femtosecond time-resolved fluorescence transients were collected using the femtosecond upconversion technique (FOG 100, CDP) with a full width at half-maximum (FWHM) of 195 fs. The fluorescence decay patterns were measured at the emission wavelength of 530 nm upon excitation at 400 nm using a femtosecond pulsed laser source. The fluorescence transients were fitted in Scientist software. The details of the upconversion setup and the fitting procedures were explained in the earlier publications of our group.26,39
DPPH Assay for In Vitro Antioxidant Activity Study
The UV–vis-assisted DPPH assay was performed to study the antioxidant activity of the samples using reported methodology.26 The degradation kinetics of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was observed and monitored by measuring the decrease in the characteristic absorption peak of DPPH at 520 nm as a function of time. The assay was performed in the dark as well as in the presence of green light (wavelength ∼540 nm) irradiation for a 30 min time window, and the time interval between two consecutive data was 5 min.
Preparation of Dichlorofluorescein for In Vitro Measurement of ROS
DCFH was synthesized using the procedure reported in the literature.40,41 First, 0.5 mL of 1.0 mM DCFH-DA (dichlorofluorescein diacetate purchased from Calbiochem) in methanol was mixed with 2.0 mL of 0.01 N aqueous NaOH for 30 min.40 The solution was then neutralized to pH 7.4 by NaH2PO4. This solution was preserved under dark conditions in ice.
Acknowledgments
The authors are extremely indebted to the Deanship of the Scientific Research (DSR), Umm Al-Qura University, for full budgetary help of this work through project number 18-SCI-1-01-0024. M.N.H. and J.P. would like to thank CSIR (India) for fellowship. The authors are thankful to Anindita Bhattacharya for careful reading of our manuscript and critical comments.
The authors declare no competing financial interest.
References
- Engwa G. A.; Ferdinand P. U.; Nwalo F. N.; Unachukwu M. N.. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog?; IntechOpen, 2019. [Google Scholar]
- Nagajyoti P. C.; Lee K. D.; Sreekanth T. Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 2010, 8, 199–216. 10.1007/s10311-010-0297-8. [DOI] [Google Scholar]
- Soetan K. O.; Olaiya C. O.; Oyewole O. E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Mahurpawar M. Effects of heavy metals on human health. Int. J. Res. 2015, 530, 1–7. [Google Scholar]
- Mudgal V.; Madaan N.; Mudgal A.; Singh R.; Mishra S. Effect of toxic metals on human health. Open Nutraceuticals J. 2010, 3, 94–99. 10.2174/18763960010030100094. [DOI] [Google Scholar]
- Tchounwou P. B.; Yedjou C. G.; Patlolla A. K.; Sutton D. J.. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer, 2012; pp 133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink D. N.; Tenenbein M.; Koren G.; Redelmeier D. A. Iron poisoning in young children: association with the birth of a sibling. Can. Med. Assoc. J. 2003, 168, 1539–1542. [PMC free article] [PubMed] [Google Scholar]
- Wuana R. A.; Okieimen F. E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647 10.5402/2011/402647. [DOI] [Google Scholar]
- Adhikari A.; Biswas P.; Mondal S.; Das M.; Darbar S.; Hameed A. M.; Alharbi A.; Ahmed S. A.; Bhattacharya S. S.; Pal D.; Pal S. K. A Smart Nanotherapeutic Agent for in vitro and in vivo Reversal of Heavy-Metal-Induced Causality: Key Information from Optical Spectroscopy. ChemMedChem 2020, 15, 420–429. 10.1002/cmdc.201900543. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Prevention, Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003–2005, Morb. Mortal. Wkly. Rep. 2006, 55, 204, 207. [PubMed]
- Zahir F.; Rizwi S. J.; Haq S. K.; Khan R. H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. 10.1016/j.etap.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Friedheim E.; Graziano J.; Popovac D.; Dragovic D.; Kaul B. Treatment of lead poisoning by 2,3-dimercaptosuccinic acid. Lancet 1978, 312, 1234–1236. 10.1016/S0140-6736(78)92103-7. [DOI] [PubMed] [Google Scholar]
- Aposhian H. V.; Bruce D. C.; Alter W.; Dart R. C.; Hurlbut K. M.; Aposhian M. M. Urinary mercury after administration of 2,3-dimercaptopropane-1-sulfonic acid: correlation with dental amalgam score. FASEB J. 1992, 6, 2472–2476. 10.1096/fasebj.6.7.1563599. [DOI] [PubMed] [Google Scholar]
- Aposhian H. V.; Maiorino R. M.; Gonzalez-Ramirez D.; Zuniga-Charles M.; Xu Z.; Hurlbut K. M.; Junco-Munoz P.; Dart R. C.; Aposhian M. M. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology 1995, 97, 23–38. 10.1016/0300-483X(95)02965-B. [DOI] [PubMed] [Google Scholar]
- Liu G.; Garrett M. R.; Men P.; Zhu X.; Perry G.; Smith M. A. Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochim. Biophys. Acta, Mol. Basis Dis. 2005, 1741, 246–252. 10.1016/j.bbadis.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Houghton D. Strain relaxation kinetics in Si1–xGex/Si heterostructures. J. Appl. Phys. 1991, 70, 2136–2151. 10.1063/1.349451. [DOI] [Google Scholar]
- Pasha S. S.; Fageria L.; Climent C.; Rath N. P.; Alemany P.; Chowdhury R.; Roy A.; Laskar I. R. Evaluation of novel platinum (II) based AIE compound-encapsulated mesoporous silica nanoparticles for cancer theranostic application. Dalton Trans. 2018, 47, 4613–4624. 10.1039/C7DT04232A. [DOI] [PubMed] [Google Scholar]
- Hutchison J. E. Greener Nanoscience: A Proactive Approach to Advancing Applications and Reducing Implications of Nanotechnology. ACS Nano 2008, 2, 395–402. 10.1021/nn800131j. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Yang F.; Yang X. Colorimetric detection of mercury (II) ion using unmodified silver nanoparticles and mercury-specific oligonucleotides. ACS Appl. Mater. Interfaces 2010, 2, 339–342. 10.1021/am9007243. [DOI] [PubMed] [Google Scholar]
- Lin C.-Y.; Yu C.-J.; Lin Y.-H.; Tseng W.-L. Colorimetric sensing of silver (I) and mercury (II) ions based on an assembly of Tween 20-stabilized gold nanoparticles. Anal. Chem. 2010, 82, 6830–6837. 10.1021/ac1007909. [DOI] [PubMed] [Google Scholar]
- Xue X.; Wang F.; Liu X. One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J. Am. Chem. Soc. 2008, 130, 3244–3245. 10.1021/ja076716c. [DOI] [PubMed] [Google Scholar]
- Li F.; Wang J.; Lai Y.; Wu C.; Sun S.; He Y.; Ma H. Ultrasensitive and selective detection of copper (II) and mercury (II) ions by dye-coded silver nanoparticle-based SERS probes. Biosens. Bioelectron. 2013, 39, 82–87. 10.1016/j.bios.2012.06.050. [DOI] [PubMed] [Google Scholar]
- Nies D. H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- Lobo V.; Patil A.; Phatak A.; Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin G.; Kauser H.; Athar M. Iron augments stage-I and stage-II tumor promotion in murine skin. Cancer Lett. 2002, 183, 113–122. 10.1016/S0304-3835(02)00116-7. [DOI] [PubMed] [Google Scholar]
- Bagchi D.; Chaudhuri S.; Sardar S.; Choudhury S.; Polley N.; Lemmens P.; Pal S. K. Modulation of stability and functionality of a phyto-antioxidant by weakly interacting metal ions: curcumin in aqueous solution. RSC Adv. 2015, 5, 102516–102524. 10.1039/C5RA21593E. [DOI] [Google Scholar]
- Bicer N.; Yildiz E.; Yegani A. A.; Aksu F. Synthesis of curcumin complexes with iron (iii) and manganese (ii), and effects of curcumin–iron (iii) on Alzheimerʼs disease. New J. Chem. 2018, 42, 8098–8104. 10.1039/C7NJ04223J. [DOI] [Google Scholar]
- Khalil M. I.; Al-Zahem A. M.; Al-Qunaibit M. H. Synthesis, characterization, Mössbauer parameters, and antitumor activity of Fe (III) curcumin complex. Bioinorg. Chem. Appl. 2013, 2013, 982423 10.1155/2013/982423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera A.; Bagchi D.; Pal S. K. Improvement of Photostability and NIR Activity of Cyanine Dye through Nanohybrid Formation: Key Information from Ultrafast Dynamical Studies. J. Phys. Chem. A 2019, 123, 7550–7557. 10.1021/acs.jpca.9b04100. [DOI] [PubMed] [Google Scholar]
- Giri A.; Goswami N.; Sasmal C.; Polley N.; Majumdar D.; Sarkar S.; Bandyopadhyay S. N.; Singha A.; Pal S. K. Unprecedented catalytic activity of Mn3O4 nanoparticles: potential lead of a sustainable therapeutic agent for hyperbilirubinemia. RSC Adv. 2014, 4, 5075–5079. 10.1039/c3ra45545a. [DOI] [Google Scholar]
- Patwari J.; Chatterjee A.; Sardar S.; Lemmens P.; Pal S. K. Ultrafast dynamics in co-sensitized photocatalysts under visible and NIR light irradiation. Phys. Chem. Chem. Phys. 2018, 20, 10418–10429. 10.1039/C7CP08431E. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S.; Batabyal S.; Polley N.; Pal S. K. Vitamin B2 in nanoscopic environments under visible light: photosensitized antioxidant or phototoxic drug?. J. Phys. Chem. A 2014, 118, 3934–3943. 10.1021/jp502904r. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S.; Sardar S.; Bagchi D.; Singha S. S.; Lemmens P.; Pal S. K. Sensitization of an endogenous photosensitizer: electronic spectroscopy of riboflavin in the proximity of semiconductor, insulator, and metal nanoparticles. J. Phys. Chem. A 2015, 119, 4162–4169. 10.1021/acs.jpca.5b03021. [DOI] [PubMed] [Google Scholar]
- Zhao X.-Z.; Jiang T.; Wang L.; Yang H.; Zhang S.; Zhou P. Interaction of curcumin with Zn (II) and Cu (II) ions based on experiment and theoretical calculation. J. Mol. Struct. 2010, 984, 316–325. 10.1016/j.molstruc.2010.09.049. [DOI] [Google Scholar]
- Adhikary R.; Mukherjee P.; Kee T. W.; Petrich J. W. Excited-state intramolecular hydrogen atom transfer and solvation dynamics of the medicinal pigment curcumin. J. Phys. Chem. B 2009, 113, 5255–5261. 10.1021/jp901234z. [DOI] [PubMed] [Google Scholar]
- Maji T. K.; Hasan M. N.; Ghosh S.; Wulferding D.; Bhattacharya C.; Lemmens P.; Karmakar D.; Pal S. K. Development of a magnetic nanohybrid for multifunctional application: From immobile photocatalysis to efficient photoelectrochemical water splitting: A combined experimental and computational study. J. Photochem. Photobiol., A 2020, 397, 112575 10.1016/j.jphotochem.2020.112575. [DOI] [Google Scholar]
- Addicoat M. A.; Metha G. F.; Kee T. W. Density functional theory investigation of Cu (I)- and Cu (II)-curcumin complexes. J. Comput. Chem. 2011, 32, 429–438. 10.1002/jcc.21631. [DOI] [PubMed] [Google Scholar]
- Hariharan R.; Senthilkumar S.; Suganthi A.; Rajarajan M. Photodynamic action of curcumin derived polymer modified ZnO nanocomposites. Mater. Res. Bull. 2012, 47, 3090–3099. 10.1016/j.materresbull.2012.08.028. [DOI] [Google Scholar]
- Choudhury S.; Mondal P. K.; Sharma V.; Mitra S.; Sakai V. G.; Mukhopadhyay R.; Pal S. K. Direct observation of coupling between structural fluctuation and ultrafast hydration dynamics of fluorescent probes in anionic micelles. J. Phys. Chem. B 2015, 119, 10849–10857. 10.1021/jp511899q. [DOI] [PubMed] [Google Scholar]
- Ahmed S. A.; Bagchi D.; Katouah H. A.; Hasan M. N.; Altass H. M.; Pal S. K. Enhanced Water Stability and photoresponsivity in Metal-organic framework (MOF): A potential tool to combat Drug-resistant Bacteria. Sci. Rep. 2019, 9, 19372 10.1038/s41598-019-55542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwari J.; Joshi H.; Mandal H.; Roy L.; Bhattacharya C.; Lemmens P.; Pal S. K. Exciton dissociation in an NIR-active triohybrid nanocrystal leading to efficient generation of reactive oxygen species. Phys. Chem. Chem. Phys. 2019, 21, 10667–10676. 10.1039/C9CP01923E. [DOI] [PubMed] [Google Scholar]


