Abstract
Background
Despite successful clinical outcomes of biologic medications in patients with chronic rheumatic diseases, some considerable adverse effects such as infections remain a major concern. Possibility of tuberculosis (TB) reactivation over treatment with anti-tumor necrotizing factor (TNF) alpha agents has necessitated a screening test before initiation of treatment. However, screening over the course of treatment is not recommended in those patients with negative baseline screening tests. This study aimed to evaluate the efficacy of tuberculin skin test (TST) before treatment in patients with chronic rheumatologic diseases who were indicated to receive anti-TNF-alpha therapy and the necessity of repeating this test over the course of treatment.
Methods
In this prospective study, patients with chronic rheumatologic diseases receiving anti-TNF-alpha agents were studied in a two-year period. TST was performed before treatment and those with positive results were excluded from the study. Thereafter, treatment with anti-TNF-alpha agents was initiated with the indicated dose. TST was repeated before administration of biologic treatment until TST became positive or 16 weeks after the initiation of treatment with anti-TNF-alpha.
Results
A total of 51 cases were studied, of whom one patient (1.9%) was excluded due to positive TST before treatment. All participants received infliximab and the TST test became positive in one patient (2%) 2 weeks after receiving the first dose. Also, the results of further tests at weeks 6, 10, and 14 were all negative for the remaining patients.
Conclusion
Due to the possibility of TST conversion after administration of anti-TNF-alpha therapy, it is important to consider TB monitoring in patients under treatment with these agents using available methods such as TST.
Keywords: Tuberculin skin test, Anti-TNF-alpha agents, Tuberculosis
Background
The Community Oriented Program for Control of Rheumatic Diseases (COPCORD) and the International League of Associations for Rheumatology (ILAR) by the collaboration of the World Health Organization (WHO) revealed that rheumatic complains were the commonest complaint in the community, and soft tissue rheumatism, ill-defined musculoskeletal symptoms, and osteoarthritis were the most prevalent disorders [1]. The urban COPCORD study in developing countries such as Iran demonstrated that in the population over the age of 15 years rheumatic complains were seen in 41.9% of people. Degenerative joint disease and inflammatory disorders were also reported in a considerable proportion of patients [2]. Different therapeutic options have been recommended for rheumatologic diseases, such as non-steroidal anti-inflammatory drugs, traditional disease-modifying anti-rheumatic drugs (DMARDs), and glucocorticoids [3, 4]. Moreover, numerous biologic therapies have emerged in the recent decades with significantly successful outcomes, including tumor necrosis factor-alpha (TNF-alpha) blockers, CTLA4-Ig, anti-interleukin I (IL-1) and anti-IL 6 receptors, and rituximab (an anti CD20 antibody) [5–7]. However, some complications, particularly infections, are not uncommon by using these medications, both as a direct consequence of the treatment or due to the underlying disease process [8–10]. Reactivation of tuberculosis (TB) has also been widely reported in patients receiving biologic therapies, in particular anti-TNF-alpha agents [11–13]. Therefore, tuberculin skin test (TST) or interferon-gamma release assay (IGRA) is strictly recommended before the initiation of therapy [13]. Most current guidelines and expert reviews recommend that in case of the absence of risk factors and clinical suspicion for TB, there is no need for repeating TB screening tests [13, 14]. However, there are some reports of TB infection in patients under treatment with biologic therapies and negative TST at initiation [15–17]. These reports raise the concern about the inadequacy of a single TST test before initiation of treatment. However, no prospective study has been conducted in this regard. Therefore, we aimed to evaluate the efficacy of TST before treatment in patients with chronic rheumatologic diseases who were indicated to receive anti-TNF-alpha therapy and necessity of repeating this test over the course of treatment.
Methods
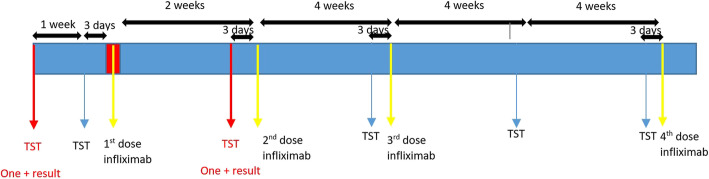
This prospective observational study was conducted on patients (in any age or sex) with a chronic rheumatologic disease referred to Imam Reza Teaching Hospital of Tabriz University of Medical Sciences for receiving anti-TNF-alpha agents in a two-year period (March 2017 to March 2019). Patients were excluded if they had a medically confirmed history of active or latent TB infection, household TB contact, or unevaluated symptoms that could possibly be due to TB infection, such as chronic cough. Informed consent was obtained from all participants. TST was performed 10 days before treatment with the standard method by an internal medicine specialist and was confirmed by another internal medicine specialist. Patients with positive TST tests were excluded from the study and referred to the TB control centers for further diagnostic and/or therapeutic procedures. The study was continued with TST negative patients. One week later, TST test was repeated and the positive tests were considered as booster effect; these cases were also excluded from the study. Thereafter, treatment with anti-TNF-alpha agents was initiated with the indicated dose. Infliximab was administered at weeks 0, 2, and 6 and then every 8 weeks. The timing of the TST test and infliximab administrations are illustrated in Fig. 1. TST was repeated by the same person with the same procedure before administration of treatment until TST became positive or 16 weeks after the initiation of treatment with anti-TNF-alpha.
Fig. 1.
Timing of the administration of infliximab and tuberculin skin test (TST)
TST was done by an internal specialist on the volar side of the left forearm with the Mantoux method. Ten units of tuberculin purified protein derivative (PPD) was injected intradermally and the injected site was marked (PPD RT23; Staten Serum Institute, Copenhagen, Denmark). The appearance of any induration was evaluated 72 h after injection using the ballpoint method [18]. The same procedure was repeated each time the TST was performed. An induration of more than 5 mm was considered in patients receiving immunosuppressive drugs, such as methotrexate or cyclosporine; otherwise, 10 mm or higher was considered positive. Also, any increase in diameter of induration of TST was defined as positive TST [19, 20].
Statistical analysis
Continuous data were reported as mean and standard deviation. The frequency and percentage of qualitative variables were also reported. SPSS version 24 was used for all analyses.
Results
A total of 51 patients participated in this study, out of whom one patient with ankylosing spondylitis (AS) and a positive TST before initiation of treatment was excluded from the study. The study was conducted on 50 patients, including 28 males (56%) and 22 females (44%). The mean age was 31.2 ± 6.55 years (range: 20–50 years). Also, 33 patients (66%) had AS and 17 patients (34%) had rheumatoid arthritis (RA). Concurrent use of methylprednisolone was reported in 17 (34%) patients (Table 1). All patients had received Bacillus Calmette–Guérin (BCG) vaccination in their childhood.
Table 1.
Characteristics of included patients
| Total number | 50 |
|---|---|
| Age (mean ± SD years) | 31.2 ± 6.6 |
| ≤ 18 (n, %) | 0 |
| 18–39 (n, %) | 42 (84%) |
| 40–49 (n, %) | 7 (14%) |
| ≥ 50 (n, %) | 1 (2%) |
| BCG vaccination at birth (n, %) | 50 (100%) |
| Type of disease | |
| Ankylosing spondylitis | 33 (66%) |
| Rheumatoid arthritis | 17 (34%) |
| Biologic treatment | |
| Infliximab (n, %) | 50 (100%) |
| Each dose (mg) | 200 |
| Other treatments | |
| Indomethacin (n, %) | 13 (26%) |
| Daily dose (mg) | 150 |
| Naproxen (n, %) | 4 (8%) |
| Daily dose (mg) | 1500 |
| Diclofenac (n, %) | 16 (32%) |
| Daily dose (mg) | 200 |
| Hydroxychloroquine (n, %) | 17 (34%) |
| Daily dose mean ± SD dose (mg) | 270.5 ± 98.5 |
| Methotrexate (n, %) | 17 (34%) |
| Weekly dose mean ± SD dose (mg) | 13.8 ± 3.3 |
| Prednisolone | |
| Daily dose mean ± SD (mg) | 5.29 ± 1.21 |
| ≤ 2.5 mg (n, %) | 1 (2%) |
| = 5 mg (n, %) | 13 (26%) |
| ≥ 7.5 mg (n, %) | 3 (6%) |
| No prednisolone (n, %) | 33 (66%) |
All patients with negative TST included in our study received infliximab with the standard dose. Before administration of the second dose of infliximab (2 weeks after the first dose of infliximab), a male 37-year-old patient with AS developed a positive TST (induration, 8 mm; Table 2). The TST induration of this patient prior to biologic treatment was 3 mm; he received indomethacin concomitantly but did not receive prednisolone or other non-steroidal anti-inflammatory drugs. Moreover, he had no household TB contact. The patient was referred to TB control center for further evaluation and the study continued with the remaining patients. However, no other positive TST cases were seen when TST was repeated in the following weeks. In addition, none of the patients had symptoms of TB.
Table 2.
TST results
| # | Time of test | Number of tested patients | Result | |
|---|---|---|---|---|
| 1# | 10 days before treatment | 51 | Positive * | 1 (1.9%) |
| Negative | 50 (98.1%) | |||
| 2# | 3 days before treatment | 50 | Positive | 0 |
| Negative | 50 (100) | |||
| 3# | 3 days before the second dose | 50 | Positive * | 1 (2%) |
| Negative | 49 (98%) | |||
| 4# | 3 days before the third dose | 49 | Positive | 0 |
| Negative | 49 (100) | |||
| 5# | Four weeks after the third dose | 49 | Positive | 0 |
| Negative | 49 (100) | |||
| 6# | 3 days before fifth dose | 49 | Positive | 0 |
| Negative | 49 (100) | |||
TST positive patients were referred to TB control centers and TB infection was confirmed
Discussion
The possibility of TB reactivation by anti-TNF-alpha treatment has been well-established by several studies, and guidelines recommend performing screening tests before initiation of these drugs. However, the majority of current guidelines suggest that there is no need for re-screening TB infection after initiation of biologic treatments [14]. We evaluated the sufficiency of TST in patients with chronic rheumatic diseases indicated to receive biologic therapy. Our results demonstrated that there is a possibility of positive TB infection after administration of biologic drugs despite a negative prior screening test (conversion of TST) that can be detected by repeating TST over the course of treatment. Although this was seen only in one over 50 patients included in the study, neglecting this finding and poor detection and management of this highly communicable disease can lead to terrible consequences.
Several studies have revealed the risk of TB infection in patients who receive TNF-alpha inhibitors [21–23]. Askling et al. investigated the Swedish Inpatient Register RA cohort (62.321 patients) and reported that 230 individuals in this cohort were diagnosed with TB during the 14 years follow-up period, of whom, 15 patients had received TNF-alpha inhibitors (11 patients were treated with infliximab) [24]. A conversion from negative TST to positive after treatment with anti-TNF-alpha has also been reported by some studies. Park et al. reported a considerable ratio of 32.6% of patients having a conversion from negative to positive TST by using biologic medications [17]. Also, Slouma et al. reported that there were two cases of active pulmonary TB among patients receiving anti-TNF-alpha therapy with initially negative TST and QuantiFERON-TB Gold test [16]. Another study by Mobini et al. also reported a case of seropositive RA under treatment with infliximab that got an active TB infection despite a previous negative TB screening test [15]. This finding has also been reported for patients with non-rheumatic diseases. Celine Debeuckelaere et al. reported two patients with chronic inflammatory bowel disease (IBD) that developed a TB infection after treatment with anti-TNF-alpha agents, despite a negative screening test [25]. Also, a Korean study reported de novo TB infection in 3.1% of IBD patients after anti-TNF-alpha therapy [26].
A panel of experts recommend that annual TB screening test should be considered in patients with RA, AS, psoriatic arthritis (PsA), or psoriasis under treatment with anti-TNF-alpha agents if they travel or work in situations where TB exposure is likely regardless of negative screening test at baseline [13]. However, this has not been adequately appreciated in the current guidelines.
There are two predominant screening tests for TB including TST and IGRA. Despite well-known false-negative and false-positive TST results, the standard screening test is still TST along with a comprehensive medical history and chest X-ray [27]. Furthermore, TST is simpler, has lower costs, and is a widely available test. Therefore, in our study we did not perform IGRA and only TST was conducted as a screening test. Meanwhile, we attempted to diminish the disadvantages of TST; for example, TST was administrated meticulously by an expert and under supervision to reduce the negative impact of misperformance. We did not have any false-positive results as both TST-positive patients (before and after treatment) were confirmed to have active TB by further evaluations. Nonetheless, we could not roll out false-negative TST in our patients due to their immunosuppression treatment. Oral prednisolone is reported to have some impact on TST results; however, this impact is predominantly dose dependent [28]. Kleinert et al. and Ponce de Leon et al. demonstrated that 7.5–10 mg/day may impair TST results [29]. However, majority of our patients received prednisolone with a dose of equal or less than 5 mg/day. Regarding the patient who presented positive TST after treatment, it is unlikely that the negative TST before treatment was due to immunosuppression because he was not under treatment with immunosuppressive drugs and he received the medications with the same dose along with infliximab.
A conversion in TST, defined as a change from negative to a positive test, can occur when a new or enhanced hypersensitivity arise due to de novo TB infection or non-TB mycobacteria, including BCG vaccination [19]. This reaction has been variedly reported to occur 3 to 7 weeks after exposure [19]. In our study, positive TST was seen after 2 weeks of baseline TST (2 weeks after initiation of treatment). This could be due to the booster effect; however, considering that we conducted a second TST 3 days before treatment to roll out this phenomenon, a booster effect was also unlikely to be considered for our patient.
TNF-alpha has an important role in both the host immune response to TB infection and in its immunopathology [30]. It is produced by a variety of immune cells in response to various pathogens, such as lipopolysaccharide or viral and bacterial infections [31]. TNF-alpha in response to TB infection brings about several positive effects. The main receptor of TNF-alpha, acting against TB infection is TNF receptor 1 (TNFR1) [32]. In vitro studies have demonstrated that TNFR1 is essential in both granuloma formation and in susceptibility to intracellular pathogens during TB infection. This results in controlling the mycobacteria and preventing their dissemination [30]. Therefore, it is conceivable that inhibition of this mediator by anti-TNF-alpha agents leads to poor immune reaction potency against TB infection.
The global prevalence of RA is more than AS; but in our study 66% of patients who received infliximab were AS. The main reason was our center’s strategy for treatment of RA. We use biologics after failing combination therapy with three DMARDs for controlling the disease activity. However, some rheumatologists use rituximab as the first biologic for treating seropositive RA.
Our study had some limitations. It was better to perform IGRA and chest X-ray for more comprehensive screening of the patients. But due to our center’s protocol and some aforementioned reasons we only performed TST. Moreover, due to the relatively small number of patients in our study we found only one positive TST after initiation of treatment. It could be possible to detect more positive cases in a larger sample size.
Conclusion
Our study demonstrated the possibility of TST conversion (positive TST) after the administration of infliximab. Therefore, it is important to consider re-screening TB in patients receiving infliximab after initiation of the treatment even if the screening tests were negative before treatment.
Acknowledgements
We would like to thank the members of the Connective Tissue Diseases Research Center of Tabriz University of Medical Sciences for their sincere collaboration.
Abbreviations
- TNF
Anti-tumor necrotizing factor
- PPD
Purified protein derivative
- TB
Tuberculosis
- TST
Tuberculin skin test
- TNFR1
TNF receptor 1
Authors’ contributions
Conceived the idea: MH and AA. Designed the study methodology: AA, AK, AE, and YH. Conducted the study: AA, MF, and AK. Analyzed the data: MF and AE. Interpreted the results: AA, MH, and MF. Composed the early draft: AA and MF. Revised and edited the final manuscript: MH and AE. Approved the manuscript: MH, AA, AK, AE, MF, and YH.
Funding
This study was funded by the Tabriz University of Medical Sciences, Tabriz, Iran.
Availability of data and materials
All Data and material are available upon request.
Ethics approval and consent to participate
Ethical clearance was sought from medical ethics committee of Tabriz University of Medical Sciences, Tabriz, Iran. Written informed consent was obtained from the participants.
Consent for publication
All authors have provided formal consent to publish this work.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chopra A. Disease burden of rheumatic diseases in India: COPCORD perspective. Indian J Rheumatol. 2015;10(2):70–77. doi: 10.1016/j.injr.2015.04.002. [DOI] [Google Scholar]
- 2.Davatchi F, Jamshidi AR, Banihashemi AT, Gholami J, Forouzanfar MH, Akhlaghi M, et al. WHO-ILAR COPCORD study (stage 1, urban study) in Iran. J Rheumatol. 2008;35(7):1384. [PubMed] [Google Scholar]
- 3.Singh JA, Saag KG, Bridges Jr. Sl, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26. [DOI] [PubMed]
- 4.Sadat-Ebrahimi SR, Parnianfard N, Vahed N, Babaei H, Ghojazadeh M, Tang S, et al. An evidence-based systematic review of the off-label uses of lisinopril. Br J Clin Pharmacol. 2018;84(11):2502. doi: 10.1111/bcp.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barone M, Notarnicola A, Lopalco G, Viggiani MT, Sebastiani F, Covelli M, et al. Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology. 2015;62(1):40–46. doi: 10.1002/hep.27716. [DOI] [PubMed] [Google Scholar]
- 6.Moots RJ, Naisbett-Groet B. The efficacy of biologic agents in patients with rheumatoid arthritis and an inadequate response to tumour necrosis factor inhibitors: a systematic review. Rheumatology. 2012;51(12):2252–2261. doi: 10.1093/rheumatology/kes217. [DOI] [PubMed] [Google Scholar]
- 7.Rubbert-Roth A. Assessing the safety of biologic agents in patients with rheumatoid arthritis. Rheumatology. 2012;51(suppl_5):v38–v47. doi: 10.1093/rheumatology/kes114. [DOI] [PubMed] [Google Scholar]
- 8.Salvana EMT, Salata RA. Infectious complications associated with monoclonal antibodies and related small molecules. Clin Microbiol Rev. 2009;22(2):274–290. doi: 10.1128/CMR.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton CD. Infectious complications of treatment with biologic agents. Curr Opin Rheumatol. 2004;16(4):393–398. doi: 10.1097/01.bor.0000127594.92432.7c. [DOI] [PubMed] [Google Scholar]
- 10.Ochoa C, Rajaram P, Tanukonda S, Sadikot RT. Infectious Complications In Patients Receiving Biologic Therapy. A44 DRUG INDUCED AND RARE LUNG DISEASE: American Thoracic Society. 2016. pp. A1592–A159A. [Google Scholar]
- 11.Cantini F, Nannini C, Niccoli L, Petrone L, Ippolito G, Goletti D. Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-TNF-targeted biologics. Mediat Inflamm. 2017;2017:8909834. doi: 10.1155/2017/8909834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantini F, Niccoli L, Capone A, Petrone L, Goletti D. Risk of tuberculosis reactivation associated with traditional disease modifying anti-rheumatic drugs and non-anti-tumor necrosis factor biologics in patients with rheumatic disorders and suggestion for clinical practice. Expert Opin Drug Saf. 2019;18(5):415–425. doi: 10.1080/14740338.2019.1612872. [DOI] [PubMed] [Google Scholar]
- 13.Cantini F, Nannini C, Niccoli L, Iannone F, Delogu G, Garlaschi G, et al. Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev. 2015;14(6):503–509. doi: 10.1016/j.autrev.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Iannone F, Cantini F, Lapadula G. Diagnosis of latent tuberculosis and prevention of reactivation in rheumatic patients receiving biologic therapy: international recommendations. J Rheumatol Suppl. 2014;91:41–46. doi: 10.3899/jrheum.140101. [DOI] [PubMed] [Google Scholar]
- 15.Mobini M, Niksolat F, Ghasemian R, Sharifpour A, Valipour S. De novo tuberculosis during anti-tumor necrosis factor-alpha therapy in a rheumatoid arthritis patient with negative initial screening. J Mazandaran Univ Med Sci. 2017;26(144):382–388. [Google Scholar]
- 16.Slouma M, Mahmoud I, Saidane O, Bouden S, Abdelmoula L. Latent tuberculosis infection screening prior to biological treatment in Tunisian patients. Therapies. 2017;72(5):573–578. doi: 10.1016/j.therap.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Seo GY, Lee JS, Kim T-H, Yoo D-H. Positive conversion of tuberculin skin test and performance of interferon release assay to detect hidden tuberculosis infection during anti-tumor necrosis factor agent trial. J Rheumatol. 2009;36(10):2158. doi: 10.3899/jrheum.090150. [DOI] [PubMed] [Google Scholar]
- 18.Cohn DL, O’Brien RJ, Geiter LJ, Gordin F, Hershfield E, Horsburgh C. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2000;49(6):1–54. [Google Scholar]
- 19.Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J. 2012;3(1):2–6. doi: 10.4103/2229-5178.93479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-Lu JE, Cárcamo CP, García PJ, Bussalleu A, Bernabé-Ortiz A. Tuberculin skin test conversion among health sciences students: a retrospective cohort study. Tuberculosis. 2013;93(2):257–262. doi: 10.1016/j.tube.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murdaca G, Spanò F, Contatore M, Guastalla A, Penza E, Magnani O, et al. Infection risk associated with anti-TNF-α agents: a review. Expert Opin Drug Saf. 2015;14(4):571–582. doi: 10.1517/14740338.2015.1009036. [DOI] [PubMed] [Google Scholar]
- 22.Murdaca G, Colombo BM, Puppo F. Anti-TNF-α inhibitors: a new therapeutic approach for inflammatory immune-mediated diseases: an update upon efficacy and adverse events. London, England: SAGE Publications Sage UK; 2009. [DOI] [PubMed] [Google Scholar]
- 23.Murdaca G, Colombo BM, Cagnati P, Gulli R, Spanò F, Puppo F. Update Upon Efficacy and Safety of TNF-α Inhibitors. Expert Opin Drug Saf. 2012;11(1):1–5. [DOI] [PubMed]
- 24.Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Cöster L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheumatism. 2005;52(7):1986–1992. doi: 10.1002/art.21137. [DOI] [PubMed] [Google Scholar]
- 25.Debeuckelaere C, De Munter P, Van Bleyenbergh P, De Wever W, Van Assche G, Rutgeerts P, et al. Tuberculosis infection following anti-TNF therapy in inflammatory bowel disease, despite negative screening. J Crohn's Colitis. 2014;8(6):550–557. doi: 10.1016/j.crohns.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Byun JM, Lee CK, Rhee SY, Kim H-J, Kim J-W, Shim J-J, et al. The risk of tuberculosis in Korean patients with inflammatory bowel disease receiving tumor necrosis factor-α blockers. J Korean Med Sci. 2015;30(2):173–179. doi: 10.3346/jkms.2015.30.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α–neutralizing agent. N Engl J Med. 2001;345(15):1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 28.Bélard E, Semb S, Ruhwald M, Werlinrud AM, Soborg B, Jensen FK, et al. Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflamm Bowel Dis. 2011;17(11):2340–2349. doi: 10.1002/ibd.21605. [DOI] [PubMed] [Google Scholar]
- 29.Kleinert S, Kurzai O, Elias J, Marten K, Engelke C, Feuchtenberger M, et al. Comparison of two interferon-γ release assays and tuberculin skin test for detecting latent tuberculosis in patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2010;69(4):782–784. doi: 10.1136/ard.2009.113829. [DOI] [PubMed] [Google Scholar]
- 30.Gardam MA, Keystone EC, Menzies R, Manners S, Skamene E, Long R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis. 2003;3(3):148–155. doi: 10.1016/S1473-3099(03)00545-0. [DOI] [PubMed] [Google Scholar]
- 31.Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, et al. Infection of human macrophages and dendritic cells with mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166(12):7033–7041. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73(3):457–467. doi: 10.1016/0092-8674(93)90134-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All Data and material are available upon request.