Abstract
Introduction
Impulse oscillometry (IOS) employs high frequency sinusoidal or impulse pressure and flow waveforms to interrogate the mechanical properties of the respiratory system. It has special applications to preschool and younger children who may have difficulty performing the repetitive forced expiratory maneuvers required for spirometry.
Case presentation
We present a case illustrating improvements of respiratory system mechanics measured by IOS in a 6‐year‐old child with cystic fibrosis (CF) who demonstrated clinical and radiological improvement after a course of therapy with hospitalization and intravenous antibiotics, and initiation of a cystic fibrosis transmembrane regulator (CFTR) protein corrector/potentiator agent. We also report a new finding: observed lower than expected reactance at low compared to high frequencies (“reactance inversion”).
Conclusion
Reactance inversion may reflect parallel pathway inhomogeneities in resistance and elastance or intrabreath airway inertance changes in young children with CF. Further study is needed in children with airway obstruction due to asthma, cystic fibrosis, and chronic lung disease of infancy to demonstrate the prevalence of this finding and whether it is specific to a measurement device.
Keywords: Cystic fibrosis, Exacerbation, Impulse oscillometry, Reactance, Reactance inversion
INTRODUCTION
Impulse oscillometry (IOS) employs high frequency sinusoidal or impulse pressure and flow waveforms to interrogate the mechanical properties of the respiratory system. It has special applications to preschool and younger children who may have difficulty performing the repetitive forced expiratory maneuvers required for spirometry.1 Because the signals are generated at frequencies many times that of the normal breathing frequency of the subject, tests can be acquired in a very short period of time, on the order of 15–20 seconds and in as few as 5 breaths in an adult and perhaps twice that in a child. While the results of any individual can be compared to normal population values, the technique is especially useful in assessing the effects of interventions, e.g., bronchodilator administration, within a subject. There are a few reports of its use in children with cystic fibrosis (CF) before and after treatment of a pulmonary exacerbation with oral or intravenous (IV) antibiotics,2, 3, 4 but results are varied. Here we present a case of its use in a child with CF who demonstrated clinical, radiological and physiological improvement after a course of therapy with hospitalization and IV antibiotics, and initiation of a cystic fibrosis transmembrane regulator (CFTR) protein corrector/potentiator agent. We also draw attention to an unusual shape of the reactance curve at low frequencies.
CASE REPORT
A 6‐year‐old boy from the United Arab Emirates with CF due to homozygous deltaF508 mutations in the CFTR gene was admitted to the Children's Hospital of Philadelphia after 6 months of unsuccessful outpatient treatment of a pulmonary exacerbation with increased airway clearance with manual and chest vest physical therapy, albuterol and hypertonic saline aerosols, and oral antibiotics including ciprofloxacin and inhaled tobramycin. Sputum cultures were positive for methicillin sensitive and methicillin resistant Staphylococcus aureus, Stenotrophomonas maltophilia, and mucoid and non‐mucoid Pseudomonas aeruginosa. Spirometry was attempted on admission, but the child was unable to cooperate with testing sufficiently to produce technically adequate flow volume curves. He was, however, able to cooperate with IOS testing by impulse oscillometry (Carefusion Masterscreen). He was treated with IV tobramycin and ceftazidime and oral trimethoprim‐sulfamethoxazole (TMP/SMZ), 4 times daily airway clearance with the chest oscillating vest, albuterol and 7% hypertonic saline aerosols, recombinant human DNAse and directed cough techniques. Treatment with CFTR protein corrector/potentiator tezacaftor/ivacaftor was also started. Because of insufficient clinical improvement after 2 weeks of therapy, antibiotics were changed with the substitution of clindamycin for TMP/SMZ, and of piperacillin/tazobactam for ceftazidime for another 2 weeks. During the course of the 4‐week hospitalization, his appetite, cough, malaise, and chest physical examination improved with clearing of crackles and rhonchi. Admission and discharge radiographs showed improved lung markings and hyperinflation (Figure 1A–D). Resistance and reactance curves as a function of oscillatory frequency (admission data shown in Figures 2A and 3A) demonstrated acceptable within test coefficients of variation [cv =7% and 3% for resistance at 10 Hz (R10) and reactance at 10 Hz (X10), respectively]. However the reactance curve exhibited a curious “reactance inversion,” that is, at low frequency the curve inverted upward. This was a consistent finding on repeated measures, both within a testing session and between the admission and discharge test (Figures 2 and 3, right panels). IOS testing on admission and discharge (Table 1) demonstrated improvement in respiratory system R10, respiratory system reactance at 10 and 20 Hz (X10 and X20), and the respiratory system resonance frequency (f0).
Figure 1.
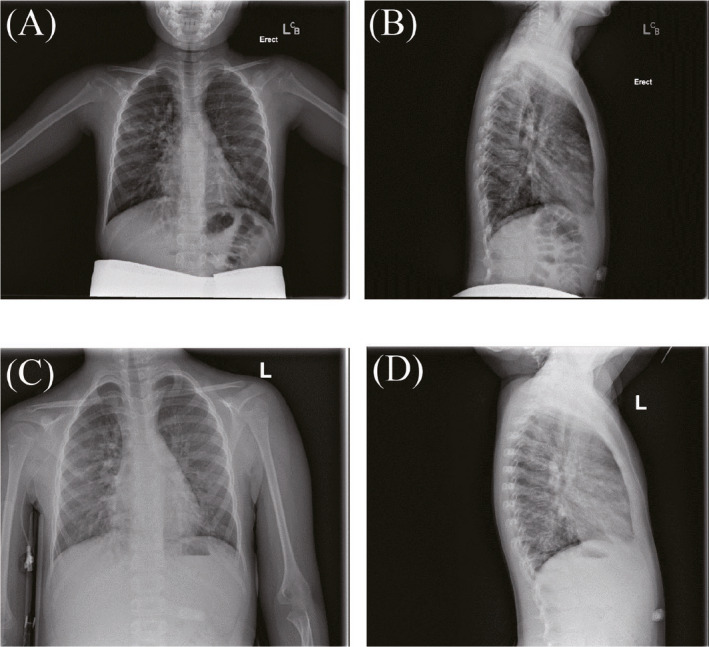
(A) Admission posterior anterior (PA) film; (B) Admission lateral film; (C) Discharge PA film; (D) Discharge lateral film
Figure 2.
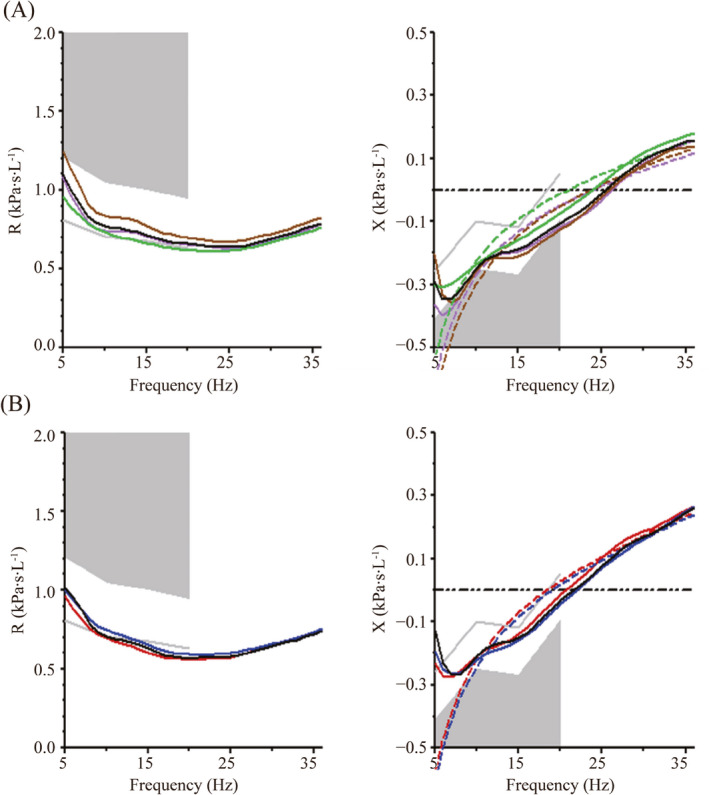
Impulse oscillometry tracings from patient on admission (A) and discharge (B). Resistance (R) is on the left panel, reactance (X) is on the right panel. In both panels, colored lines represent different trials (limited to 3 for simplicity) and the black solid line is the mean of all trials. Note that in the reactance panel, the respiratory system reactance (Xrs) consistently curves upward at low frequencies (< about 7 Hz) (“reactance inversion.”). This was observed both on admission (A) and on discharge (B). The dashed lines represent the theoretical exponentially greater reactance at low frequencies if reactance inversion were not present (see text)
Figure 3.
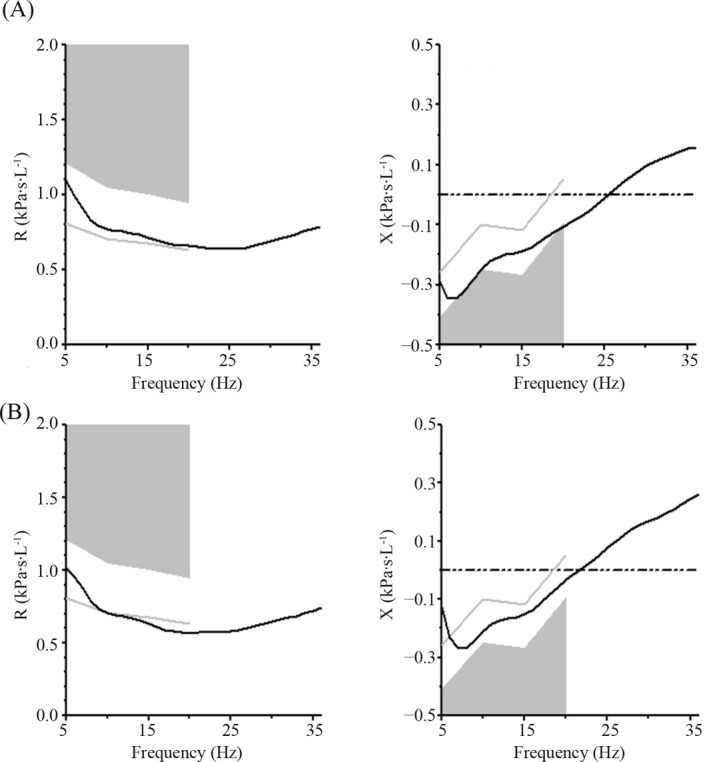
Impulse oscillometry tracings on admission (A) and discharge (B). For clarity, only the mean curves of all trials shown in Figure 2 are displayed. Note that in both the resistance and reactance curves, the actual curve is further away from the abnormal gray shaded area on discharge compared to on admission. In addition, the resonant frequency (the point at which the reactance curve crosses the zero axis) is shifted to a lower value on discharge compared to on admission
Table 1.
Changes in respiratory system resistance, reactance and resonance frequency during course of hospitalization. Reductions in respiratory system resistance at 10 Hz (R10), resonance frequency (f0), and the absolute values of reactance at 10 and 20 Hz (X10 and X20) represent improvement
| Variables | R10 (kPa∙s∙L−1) | R10% pred (%) | X10 (kPa∙s∙L−1) | X10% pred (%) | X20 (kPa∙s∙L−1) | X20% pred (%) | f0 (Hz) | f0% pred (%) |
|---|---|---|---|---|---|---|---|---|
| Admission | 0.77 | 110 | −0.28 | 280 | −0.12 | a | 26 | 152 |
| Discharge | 0.71 | 101 | −0.24 | 240 | −0.09 | a | 22 | 129 |
| % change | −8 | NA | −14 | NA | −25 | NA | −15 | NA |
Cannot be calculated due to a positive predicted value of +0.05; NA, Not applicable.
DISCUSSION
This preschool child showed clinical and radiographic improvements, as well as improvement in all IOS parameters after initiation of IV antibiotics for treatment of a pulmonary exacerbation of CF, as well as initiation of genetics‐based protein corrector/potentiator therapy. Unlike spirometry, IOS has the advantage of not requiring forced expiratory maneuvers, which often cannot be performed by preschool children. Since IOS is performed during tidal breathing, children are often able to perform it reproducibly, as in this case (Figures 2 and 3). IOS has been used to evaluate lung function in children with CF with measurements of respiratory system resistance to airflow and reactance (primarily a measure of stiffness at frequencies less than resonance).1 Results have varied in different studies, with some showing differences in IOS results depending on baseline clinical status and others not.2, 5, 6, 7, 8, 9, 10 Furthermore, IOS can reflect clinical improvement during the course of treatment of pulmonary exacerbations of CF.2, 3, 4 In addition to improvement clinically and radiographically, our patient also had improvement in all oscillometry functions that were measured.
A noteworthy feature of this case is the unusual shape of the IOS reactance curve at lower frequencies (Figure 2). Models of elastic behavior predict that at frequencies less than resonance, negative values of respiratory system reactance (Xrs) should decrease hyperbolically as frequency decreases. In this portion of the curve, the negative sign is a convention that results from pressure waveforms being 180 degrees out of phase between elastic and inertive elements of the respiratory system. Since elastic opposition to flow dominates at low frequencies, whereas inertive opposition to flow dominates at high frequencies, these two oppositions cancel each other out at the resonance frequency, where Xrs = 0.1 While the absolute magnitude of the reactance is an easier way to think of the opposition to flow at any given frequency, the convention explained above gives rise to increasing negative values as the elastic opposition to volume change increases. Below resonance, where elastic forces predominate, the reactance should decrease with decreases in frequency because at lower frequencies, units have more time to fill and empty, and the tidal volumes increase, meaning more elastic distension. This gives rise to the predicted hyperbolically increasing absolute magnitude of Xrs, but hyperbolically decreasing Xrs, with decreasing frequency (dashed line in Figure 2). This is analogous to the capacitive reactance of an electrical alternating current circuit, which also increases in magnitude a s frequency decreases, because the capacitor has more time to charge. To the contrary, our patient demonstrated the negative reactance at low frequencies actually becoming less negative as frequency decreased (“reactance inversion”) (solid black lines in Figure 2). This was a consistent finding, exhibited both within a testing session on multiple trials, and between the admission and discharge testing sessions (Figure 2A–B). This phenomenon has been sporadically observed before, and several explanations have been given. One is that in small children, the gas contained in the lungs acts like a “Helmholz resonator” (Hans Juergen Smith, personal communication). Another is that there is a low frequency leak. We do not think this is the case in our subject as no leak was observed, the volume time plot did not drift, and there was appropriate inter‐trial reproducibility of X and R. We propose a 3rd explanation for the unusual shape of the Xrs curve in this patient‐ parallel pathway differences in time constants in children with heterogeneous lung disease such as CF. Obstructed units with high time constants may not have sufficient time to distend at high frequencies, resulting in lower volume change (greater elastic reactance) for given pressure swings at the mouth. Conversely at lower frequencies, units have more time to fill, leading to greater small airway and alveolar recruitment and the elastic reactance would be diminished, as seen in our patient. While this result is consistent with the findings of Sakarya et al,2 who similarly, showed less negative reactance values at X5 than at X10 during exacerbations of CF lung disease, it is still puzzling, as elastic and electrical models do not predict an upward turn of the Xrs curve with lower frequency for the reasons discussed above, even though the curve could be distorted to somewhat less negative values by recruitment at low frequency. Since the reactance curve is the sum of two components, an elastance curve which has a negative sign and an inertive curve which has a positive sign, it is possible that increases in inertance, even at lower frequencies, may play a role, in upward deflection of the Xrs curve. Airway narrowing, such as occurs during expiration, is predicted to increase airway inertance, and this could deflect the curve upward. The lower frequency, higher oscillatory volume portion of the curve may be more likely to reflect dynamic airway narrowing. Finally, it is possible that reactance inversion is device specific, for example, specific to the impulse oscillometry that we employed, and this suggests that inter‐device comparative studies should be done.
In conclusion, our case report is an example of the usefulness of tracking IOS during the course of a pulmonary exacerbation in preschool children. Low frequency reactance inversion, demonstrated here, is a poorly understood phenomenon which we propose represents a physiologic process related to parallel pathway inhomogeneity or dynamic airway narrowing in CF lung disease. This needs further investigation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Jason Bates for his insightful discussions with us about these findings.
Allen JL, Ren CL, McDonough J, Clem CC. “Reactance inversion” at low frequencies in a child undergoing treatment of a cystic fibrosis exacerbation. Pediatr Invest. 2019;3:257‐260. 10.1002/ped4.12169
Funding source
Morse and Clare Foundations
REFERENCES
- 1. Allen JL. Input oscillometry and the forced oscillation technique for assessing lung function in preschool children with asthma. Pediatr Invest. 2018;2:37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakarya A, Uyan ZS, Baydemir C, Anik Y, Erdem E, Gokdemir Y, et al. Evaluation of children with cystic fibrosis by impulse oscillometry when stable and at exacerbation. Pediatr Pulmonol. 2016;51:1151‐1158. [DOI] [PubMed] [Google Scholar]
- 3. Buchs C, Coutier L, Vrielynck S, Jubin V, Mainguy C, Reix P. An impulse oscillometry system is less efficient than spirometry in tracking lung function improvements after intravenous antibiotic therapy in pediatric patients with cystic fibrosis. Pediatr Pulmonol. 2015;50:1073‐1081. [DOI] [PubMed] [Google Scholar]
- 4. Ren CL, Brucker JL, Rovitelli AK, Bordeaux KA. Changes in lung function measured by spirometry and the forced oscillation technique in cystic fibrosis patients undergoing treatment for respiratory tract exacerbation. Pediatr Pulmonol. 2006;41:345‐349. [DOI] [PubMed] [Google Scholar]
- 5. Ren CL, Rosenfeld M, Mayer OH, Davis SD, Kloster M, Castile RG, et al. Analysis of the associations between lung function and clinical features in preschool children with cystic fibrosis. Pediatr Pulmonol. 2012;47:574‐581. [DOI] [PubMed] [Google Scholar]
- 6. Ramsey KA, Ranganathan SC, Gangell CL, Turkovic L, Park J, Skoric B, et al. Impact of lung disease on respiratory impedance in young children with cystic fibrosis. Eur Respir J. 2015;46:1672‐1679. [DOI] [PubMed] [Google Scholar]
- 7. Kerby GS, Rosenfeld M, Ren CL, Mayer OH, Brumback L, Castile R, et al. Lung function distinguishes preschool children with CF from healthy controls in a multi‐center setting. Pediatr Pulmonol. 2012;47:597‐605. [DOI] [PubMed] [Google Scholar]
- 8. Gangell CL, Hall GL, Stick SM, Sly PD, AREST CF . Lung function testing in preschool‐aged children with cystic fibrosis in the clinical setting. Pediatr Pulmonol. 2010;45:419‐433. [DOI] [PubMed] [Google Scholar]
- 9. Gangell CL, Horak F Jr., Patterson HJ, Sly PD, Stick SM, Hall GL. Respiratory impedance in children with cystic fibrosis using forced oscillations in clinic. Eur Respir J. 2007;30:892‐897. [DOI] [PubMed] [Google Scholar]
- 10. Brennan S, Hall GL, Horak F, Moeller A, Pitrez PM, Franzmann A, et al. Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax. 2005;60:159‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]


