Abstract
Preschool children with asthma present a challenge in lung function testing, as they cannot readily cooperate with spirometry. The forced oscillation technique (FOT) measures passive pressures and flows delivered by a loudspeaker to a facemask, at frequencies much higher than those occurring physiologically. This in turn allows for rapid collection of data from a spontaneously breathing child in a timespan of seconds. However, at very rapid oscillatory flow rates, the mechanical properties opposing flows into and out of the respiratory system (collectively termed the respiratory system impedance, and comprised of elastic, resistive and inertial components) are very different from at normal breathing frequencies, with elastic properties being less important and inertial properties being more important. An understanding of how the respiratory system behaves at high frequencies is essential to understanding the physiological basis of this technique. This article presents a way to understand these oscillatory mechanics of the respiratory system. It then describes studies of the FOT in normal preschool children and in children with asthma. The technique can also measure the separate contributions of the central and peripheral airways, as well as assess for changes after bronchodilator administration. The FOT holds promise for the objective measurement of lung function in children who are too young to reliably perform spirometry.
Keywords: Asthma, Respiratory tract
INTRODUCTION
Asthma is increasingly prevalent in children in the United States, China, and internationally, and most often has its roots in early childhood. In the US, half of all children wheeze by the age of 3 years, and nearly half of these will be diagnosed with asthma by the age of 6 years.1 Despite the importance of better understanding this window of asthma evolution in children between the ages of 3 and 6 years, there have not been extensive tests of lung function in this age range. This is because accurate performance of spirometry, the most commonly employed lung function test, is highly effort dependent. Preschool children present a particular challenge in lung function testing, as they cannot readily cooperate with sustained forced expiratory maneuvers. Mayer et al2 showed that only about 60% of 3‐year‐old children, 75% of 4‐year olds and 85% of 5‐year olds are able to successfully complete spirometry. This makes it difficult to objectively assess asthma control in the preschool age child. The forced oscillation technique (FOT) measures pressures and flow rates at frequencies much higher than those occurring physiologically, measured in cycles per second rather than breaths per minute. This allows rapid collection of large amounts of data in a matter of seconds, on a spontaneously breathing child breathing at normal breathing frequencies, by superimposing a rapidly oscillating pressure and flow signal. Because of this, interest has been developed recently in using FOT to assess lung function in preschool children with asthma.
At very high oscillatory flow rates, the relevant mechanical properties of the lung are very different from those at physiologic respiratory rates. In this article we shall review the physics of lung mechanics at high frequencies, and how this can be applied to the measurement of lung function in preschool children. In order to understand the mechanical properties that the forced oscillation technique Impedance is frequency dependent measures, it is first necessary to review some principles of respiratory system oscillation mechanics. We will then review some representative clinical studies of oscillation mechanics in preschool children with asthma.
OSCILLATION MECHANICS
Oscillation mechanics theory has been thoroughly reviewed by Fredberg and Peslin.3 The major impediments to lung and respiratory system expansion are their elastic properties (related to volume change), resistive properties (related to flow rate, i.e. the first derivative of volume with respect to time) and inertive properties (related to acceleration of gas flow and lung/chest wall, i.e., the second derivative of volume with respect to time). The pressure necessary to expand the lung is given by the Equation of Motion:
P = E × V + R × V’ + I × V’’
Where
P is pressure
E is elastance, or stiffness (the reciprocal of C, compliance)
R is resistance
I is inertance (or mass)
V is volume
V’ is dV/dt, or flow, and
V’’ is dV’/dt, or acceleration
The total pressure cost of flow, ∆P/∆V’ is termed the impedance, Z, and has elastic, resistive and inertive components. These components are frequency dependent, I and E to a greater extend, and R to a lesser extent (Figure 1).
Figure 1.
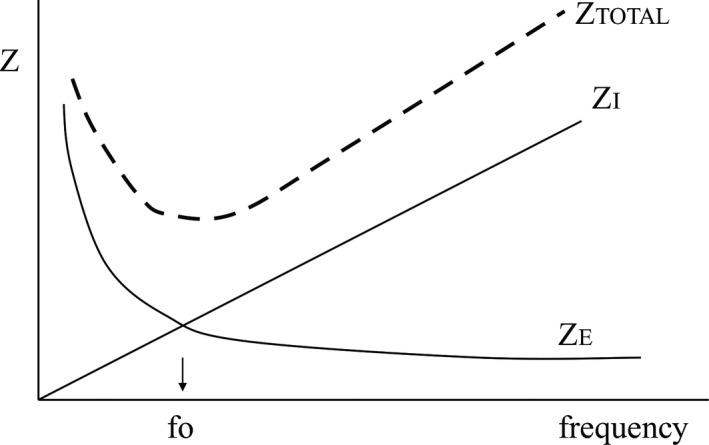
Dependence of total impedance (Z) on oscillatory frequency, and the contributions of elastic impedance, ZE, and inertive impedance, ZI. fo = resonant frequency
As frequency increases, the impedance due to the inertive elements goes up because of the increasing acceleration and deceleration of gas, lung and chest wall masses. Conversely, as frequency increases, the impedance due to the elastic elements of the lung decreases because for a given minute ventilation, as frequency increases tidal volume decreases. Total impedance is comprised of the resistive, inertive and elastic impedances. Impedance is minimum at the “resonant frequency,” fo, that frequency at which inertive and elastic impedance are equal. Increases in inertance lower the resonant frequency, while increases in elastance (decreases in compliance) increase the resonant frequency (Figure 2), which also shows the equation by which the resonant frequency is calculated.
Figure 2.
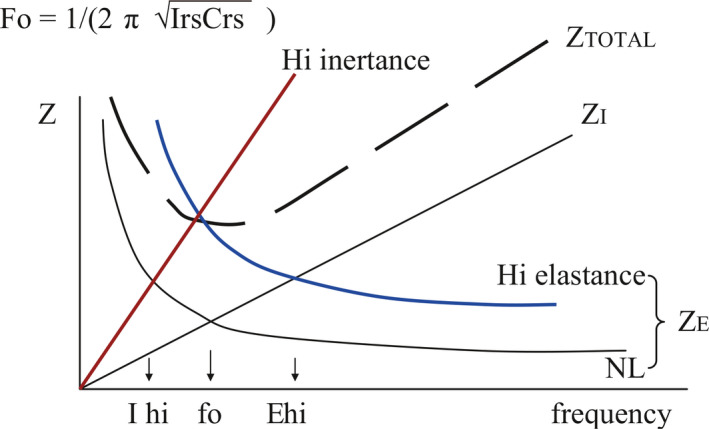
Effect of increases in elastic impedance, ZE, and inertive impedance, ZI, on the resonant frequency, fo.
At the resonant frequency, the inertive and elastic components cancel each other out because of their pressure phase relationships (Figure 3). Pressure and flow swings in a resistive element are in phase with each other. Pressure swings in an inertive element are 180 degrees out of phase with the pressure swings in an elastic element. This is because as flow enters an elastic element, a back pressure develops as a consequence. However, pressure is necessary to produce flow into an inertive element. Thus, during oscillatory flow, the pressure swings precede the flow swings by 90 degrees in the inertive element, but follow the flow swings by 90 degrees in the elastic element. When these impedances are of equal importance (at the resonant frequency), because the pressure swings in these two elements are 180 degrees out of phase, they cancel each other out and the impedance is at a minimum.
Figure 3.
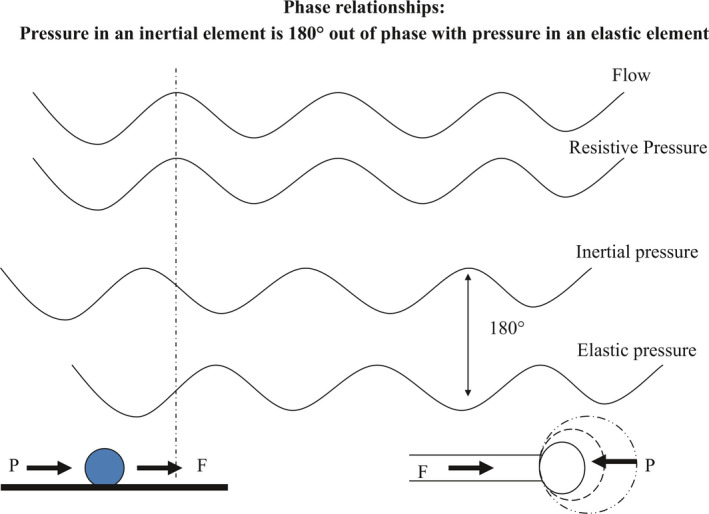
Phase relationships in inertive and elastic elements: Inertial pressure leads flow by 90 degrees (solid ball on left represents a mass); elastic pressure lags flow by 90 degrees (expanding balloon on right represents an elastic element). Thus inertive and elastic pressures are 180 degrees out of phase, and cancel each other out when they are equal in magnitude (at the resonant frequency)
Another way to look at this is that oscillatory pressure through the elastic and inertive elements can be subtracted arithmetically, since they are 180 degrees out of phase with each other; these two terms comprise the “reactance”, X (Figure 4). Because the pressure in the reactance element is out of phase with the pressure in the resistive element by 90 degrees, they cannot be added arithmetically, but as a vector, to give the total impedance, Z, which has both magnitude and phase.
Figure 4.
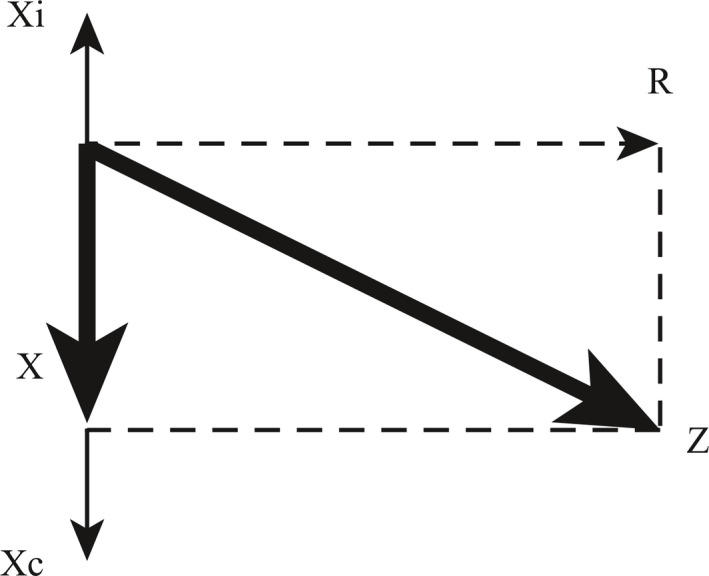
Planar representation of phase relationships between resistive (R), elastic/compliant (Xc) and inertive pressures (Xi). The arithmetic sum of elastic and inertive pressure at any given moment is termed the “reactance,” X. X is 90 degrees out of phase with R. The total impedance, Z, is the vector sum of the reactance and resistance, and has both a magnitude and a phase angle.
A more accurate way to redraw Figure 1, in order to take into account both the magnitude and the phase relationships described in Figures 3 and 4, is shown in Figure 5. In Figure 5, which graphs reactance as a function of frequency, the inertive impedance and elastic impedance have opposite signs reflecting that their pressure swings are opposite in phase (Figure 3). They are seen to be equal and opposite at the resonant frequency. Further, elastic impedance is seen to dominate at low frequencies, while inertive impedance dominates at high frequencies.
Figure 5.
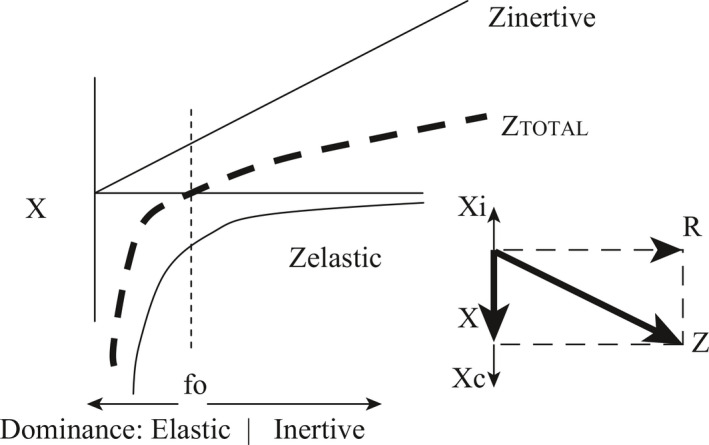
Reactance as a function of frequency, taking into account both the magnitude and phase of the inertive and elastic components. The resonant frequency, fo, occurs at the frequency at which inertive and elastic pressures are equal and opposite, cancelling each other out.
Differences in respiratory system inertance and compliance between infants, children and adults (higher compliance and inertance in adults) explain the lower resonant frequencies in larger humans (see equation, Figure 2). Resonant frequency is approximately proportional to body mass to the (‐)1/3 power.4
MEASUREMENT OF LUNG MECHANICS BY INPUT OSCILLOMETRY
During input oscillometry, a facemask is attached to the patient's mouth and nose and low‐pressure high frequency flow oscillations are introduced at the airway opening (Figure 6).
Figure 6.
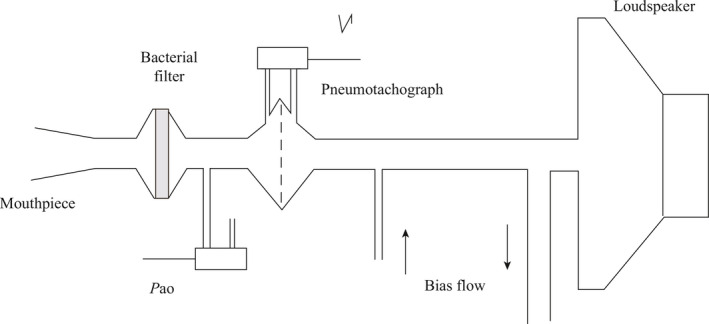
Apparatus for the measurement of forced oscillatory impedance. V’ = flow; Pao = pressure measured at the airway opening (mouth). (Reprinted with permission from Ref.7 Oostveen 2003)
Standards for the performance of input oscillometry in children have recently been described.5, 6, 7 By measuring the impedance, Z, of the respiratory system, inferences can be made about the elastic properties, the resistive properties and the inertive properties of the system, as described under Oscillation mechanics, above. Patients with asthma have characteristic changes in their impedance/frequency profiles, including increased reactance at low frequencies, indicative of increased dynamic elastance compatible with small airway disease and bronchospasm. Low frequency oscillations penetrate more deeply into the lung, since the time constants of the smallest airways and alveoli are comparable to these lower frequencies. At higher frequencies, the relatively long time constants prevent the small airways from oscillating in response to pressure oscillations. Thus measurements below the resonant frequency can be thought of as reflecting properties of the peripheral airways, whereas measurements above the resonant frequency are m ore reflective of properties of t he central airways. In addition, t he “ low frequency reactance area,” that area bounded by the triangle formed by the impedance curve, the y axis and the x axis below the resonant frequency has been proposed as another way of describing peripheral airway function (Figure 7).5, 8
Figure 7.
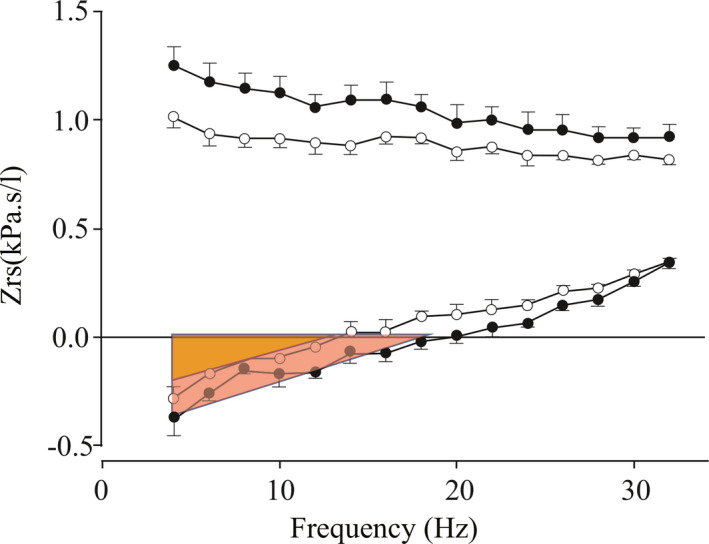
Impedance, Zrs, in a healthy child showing both the resistive component (top) and the reactance component (bottom) before (closed circles) and after (open circles) bronchodilator administration. Compare to Fig. 5. After bronchodilator administration, the resonant frequency falls, and the low frequency reactance area (red triangle) decreases (orange triangle). (Modified with permission from Ref.5 Beydon 2007)
When elastance is high, the resonance frequency is shifted upward. After bronchodilators in normal subjects, the resistance and elastance decreases, the resonant frequency shifts downward5 and the low frequency reactance area becomes smaller (red triangle > orange triangle Figure 7).
These responses are exaggerated in subjects with asthma.8 There are numerous studies of impedance and bronchodilator responsiveness assessed by the FOT in preschool children with asthma, and a detailed summary of all of them is beyond the scope of this article. However, a summary of some representative studies describing oscillation mechanics in normal children and children with asthma follows.
CLINICAL STUDIES OF OSCILLATION MECHANICS IN NORMAL PRESCHOOL CHILDREN
Changes in oscillation mechanics with growth have been reviewed. With increasing age and height, airways grow in size, airway and lung resistance decrease (Figure 8).7
Figure 8.
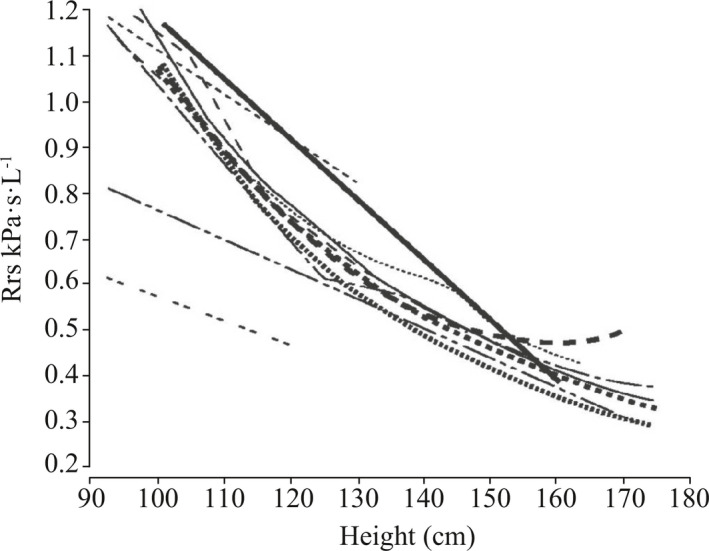
Changes in respiratory system oscillatory resistance (Rrs) with growth (Studies summarized in and reprinted with permission from Ref.7 Oostveen 2003)
Increasing lung compliance (decreased elastance) with growth translates into a less negative reactance at low frequencies below resonance, e.g., X5 (refer to Figure 5).9 Resonance frequency also decreases with growth, corresponding to a decrease in respiratory system elastance. Separate working groups of the American Thoracic Society and the European Respiratory Society have each published detailed recommendations for the technical performance of FOT in children, and for the interpretation of results,5, 7 as well as summarized reference values.
OSCILLATION MECHANICS IN CHILDREN WITH ASTHMA
Several studies have shown no difference between impedance characteristics of children with asthma when well and normal controls.10, 11, 12 These results are not surprising, as children with reversible airflow obstruction would be expected to have relatively normal lung function when well. On the other hand, impedance measurements such as Rrs, resonance frequency, and the low frequency reactance area seem highly sensitive to changes after bronchodilator administration in both normal children and asthmatics.5, 7, 10, 11, 12, 13, 14, 15 Criteria for reversibility that have been proposed include a change in Rrs of >1‐2 intra‐subject standard deviations, >2‐4 intrasubject coefficients of variation,5, 16 or a percent change from baseline of 20%‐40%.5, 10
The magnitude of bronchodilator response may11, 14 or, surprisingly, may not10, 12, 13 differ between asthmatics and controls. Hellinckx reported an average decreases in Rrs5 of 12% after bronchodilator administration in both asthmatics and controls.10 It is unclear why FOT fails, in some studies, to show differences in airway reactivity between asthmatics and controls, as this is a hallmark of asthma in most spirometric studies of asthma in adults. It may be a distinguishing characteristic that only appears with increasing age or the full emergence of asthma as a distinct phenotype.
However, other studies have found differences in bronchodilator responsiveness between asthmatics and controls. The sensitivity and specificity of bronchodilator responsiveness in distinguishing between children with and without asthma has been reported to be between 75%‐85% and 60%‐65%, respectively.17, 18
Another distinguishing characteristic between asthmatic and non‐asthmatic children may be variability of resistance throughout the respiratory cycle. A novel 3‐dimensional descriptive display of reactance and resistance has been described that allows visualization of changes in resistance between inspiration and expiration; in children with asthma there is an exaggerated increase in resistance during expiration which may be related to dynamic airway collapse (Figure 9).19
Figure 9.
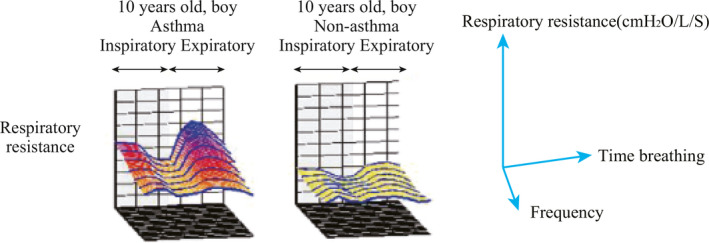
Changes in oscillatory respiratory system resistance during the breathing cycle in two boys, with and without asthma. (Reprinted with permission from Ref.19 Murakami 2014)
In one study, measurement of oscillation mechanics was able to distinguish between 4‐year‐old children with different wheezing phenotypes;14 children with persistent wheeze had greater respiratory system resistance and low frequency reactance area when better than children with transient wheeze, who in turn had greater resistance than children who never wheezed (Figure 10).14
Figure 10.
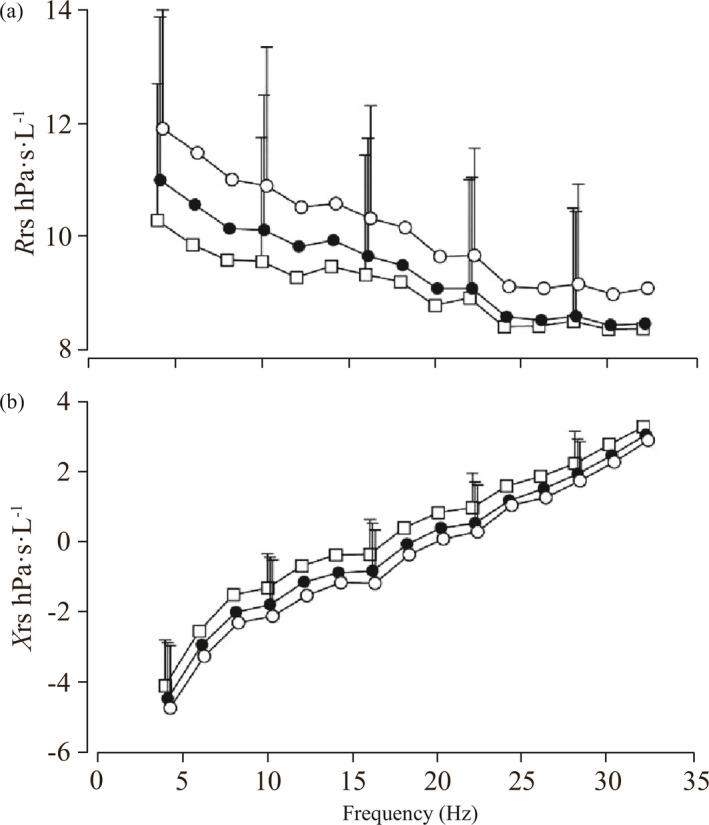
Respiratory system resistance (a) and reactance (b) in non‐wheezing children (squares), children with early transient wheeze (closed circles) and children with persistent wheezing (open circles). (Reprinted with permission from Ref.14 Oostveen 2010)
After bronchodilators, all children's resistance reduced to similar levels, i.e., the bronchodilator response was greater in the persistent and transient wheezers than in the normal controls.
Children with asthma who have abnormal impedance measurements have higher risk of developing poor asthma control.20 In addition, Robinson has shown that high between‐test variability of impedance measurements can be a marker of both asthma severity and poor control.21
Few studies have evaluated the utility of FOT in assessing lung function in the emergency department during asthma exacerbations. Ducharme and Davis showed that FOT was easier to obtain than spirometry in asthmatic children cared for in the ED, especially preschoolers, and that measures of resistance by FOT correlated well with other measures of asthma severity such as respiratory and heart rates. They also found that changes in resistance following treatment correlated with clinical improvement and suggested a change of resistance at of >19% as indicative of improvement.16
CONCLUSIONS
The forced oscillation technique, by virtue of its ability to rapidly accumulate physiological data at many cycles per second, and without forced expiratory effort on the part of the subject, is able to measure lung function in very short bursts of time, which is ideally suited to the attention span of 3‐5 years old children. An understanding of principles of how the respiratory system behaves at high frequency (oscillation mechanics) is crucial to the interpretation of such studies at baseline and after bronchodilator administration in both the healthy and asthmatic states.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Biography
Julian L. Allen Dr. Allen is Chief of the Division of Pulmonary Medicine at the Children's Hospital of Philadelphia (CHOP). CHOP is the nation's first hospital devoted exclusively to the care of children. Founded in 1855, CHOP is a tertiary and quaternary hospital, serving a population of over 10 million in Philadelphia, the Greater Delaware Valley, New Jersey, nationally and internationally. The institution has a long research tradition and was the first children's hospital to initiate a research department devoted not only to clinical investigation, but also to basic research. CHOP has fully approved pediatric subspecialty fellowships in all fields. CHOP attracts applicants both from its own pediatric residency program and nationwide, and all its subspecialty fellows graduate with strong laboratory or clinical research training. CHOP laboratory research space encompasses over 900 000 square feet. Recent additions to the CHOP Main Campus include the Colket Translational Research Building (CTRB), a new research building across the street from the Abramson Pediatric Research Center (ARC), and the Buerger Center for Advanced Pediatric Care. The Division of Pulmonary Medicine cares for children through seven different programs: Asthma, Sleep Medicine, Cystic Fibrosis Center, Technology Dependence Center, Lung Transplantation/Interstitial Lung Disease/Primary Ciliary Dyskinesia, Aerodigestive and Thoracic Insufficiency. In addition, each of these programs has clinical, physiological and translational research components.
Allen JL. Input oscillometry and the forced oscillation technique for assessing lung function in preschool children with asthma. Pediatr Invest. 2018;2:37‐43. 10.1002/ped4.12022
REFERENCES
- 1. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980‐present. J Allergy Clin Immunol. 2003;111:661‐675. [DOI] [PubMed] [Google Scholar]
- 2. Mayer OH, Jawad AF, McDonough J, Allen J. Lung function in 3‐5‐year‐old children with cystic fibrosis. Pediatr Pulmonol. 2008;43:1214‐1223. [DOI] [PubMed] [Google Scholar]
- 3. Fredberg J, Peslin R. Oscillation mechanics of the respiratory system. Handbook of Physiology, Section 3: The Respiratory System. American Physiological Society. 1986; Volume III: Mechanics of Breathing, Part 1. [Google Scholar]
- 4. Williams SP, Fullton JM, Tsai MJ, Pimmel RL, Collier AM. Respiratory impedance and derived parameters in young children by forced random noise. J Appl Physiol: Respirat Environ Exercise Physiol. 1979;47:169‐174. [DOI] [PubMed] [Google Scholar]
- 5. Beydon N, Davis SD, Lombardi E, et al. American Thoracic Society/European Respiratory Society Statement: Pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304‐1345. [DOI] [PubMed] [Google Scholar]
- 6. Rosenfeld M, Allen J, Bert HG, et al. on behalf of the American Thoracic Society Assembly on Pediatrics Working Group on Infant Preschool Lung Function Testing . An Official American Thoracic Society Workshop Report: Optimal Lung Function Tests for Monitoring Cystic Fibrosis, Bronchopulmonary Dysplasia, and Recurrent Wheezing in Children Less Than 6 Years of Age. Ann Am Thorac Soc. 2013;10:S1‐S11. [DOI] [PubMed] [Google Scholar]
- 7. Oostveen E, MacLeod D, Lorino H, et al. on behalf of the ERS Task Force on Respiratory Impedance Measurements . The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22:1026‐1041. [DOI] [PubMed] [Google Scholar]
- 8. Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther. 2001;14:341‐350. [DOI] [PubMed] [Google Scholar]
- 9. Hall GL, Sly PD, Fukushima T, et al. Respiratory function in healthy young children using forced Oscillations. Thorax. 2007;62:521‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hellinckx J, De Boeck K, Bande‐Knops J, van der Poel M. Demedts. Bronchodilator response in 3‐6.5 years old healthy and stable asthmatic children. Eur Respir J. 1998;12:438‐443. [DOI] [PubMed] [Google Scholar]
- 11. Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4‐year‐old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:317‐322. [DOI] [PubMed] [Google Scholar]
- 12. Friedman NL, McDonough JM, Zhang X, Hysinger EB, Adams KM, Allen JL. Bronchodilator Responsiveness Assessed by the Forced Oscillometry (FOT) and Multiple Breath Washout (MBW) Techniques in Preschool Children with Asthma. Am J Respir Crit Care Med. 2016;193:A4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thamrin C, Gangell CL, Kanokporn U, et al. Assessment of bronchodilator responsiveness in preschool children using forced oscillations. Thorax. 2007;62:814‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oostveen E, Dom S, Desager K, Hagendorens M, De Backer W, Weyler J. Lung function and bronchodilator response in 4‐year‐old children with different wheezing phenotypes. Eur Respir J. 2010;35:865‐872. [DOI] [PubMed] [Google Scholar]
- 15. Ortiz G, Menendez R. The effects of inhaled albuterol and salmeterol in 2‐ to 5‐year‐old asthmatic children as measured by impulse oscillometry. J Asthma Asthma. 2002;39:531‐536. [DOI] [PubMed] [Google Scholar]
- 16. Ducharme FM, Davis GM. Respiratory resistance in the emergency department: a reproducible and responsive measure of asthma severity. Chest. 1998;113:1566‐1572. [DOI] [PubMed] [Google Scholar]
- 17. Nielsen KG, Bisgaard H. Discriminative capacity of bronchodilator response measured with three different lung function techniques in asthmatic and healthy children aged 2‐5 years. Am J respire Crit Care Med. 2001;164:554‐559. [DOI] [PubMed] [Google Scholar]
- 18. Shin YH, Jang SJ, Yoon JW, et al. Oscillometric and spirometric bronchodilator response in preschool children with and without asthma. Can Respir J. 2012;19:273‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murakami K, Habukawab C, Kurosawac H, Takemurad T. Evaluation of airway responsiveness using colored three‐dimensional analyses of a new forced oscillation technique in controlled asthmatic and nonasthmatic children. Respir Investig. 2014;52:57‐64. [DOI] [PubMed] [Google Scholar]
- 20. Schulze J, Biedebach S, Christmann M, Herrmann E, Voss S, Zielen S. Impulse Oscillometry as a Predictor of Asthma Exacerbations in Young Children. Respiration. 2016;91:107‐114. [DOI] [PubMed] [Google Scholar]
- 21. Robinson PD, Brown NJ, Turner M, Van Asperen P, Selvadurai H, King GG. Increased day‐to‐day variability of forced oscillatory resistance in poorly controlled or persistent pediatric asthma. Chest. 2014;146:974‐981. [DOI] [PubMed] [Google Scholar]


