ABSTRACT
Prenatal micronutrients in pregnant women’s diets, including supplements, have an essential role in fetal brain development and may reduce the risk of mental disorders in offspring. Folic acid, vitamin D, omega‐3 fatty acids, and choline have been investigated for this purpose. Folic acid supplementation throughout pregnancy has well‐established positive effects. Vitamin D, administered to the mother before birth or to the newborn, has also been shown to reduce the risk of neurodevelopmental disorders. Omega‐3 fatty acids during pregnancy have a more uncertain role, with recent trials questioning a beneficial effect on cognition and attention deficit disorder, despite positive effects on prematurity and neonatal wheezing prevention. Choline supplementation is associated with positive effects on cognition and behavior, including early behaviors associated with the development of autism and schizophrenia. There is no experience yet with COVID‐19, but adverse effects on fetal brain development of most common coronaviruses are mitigated by higher choline levels. Maternal dietary supplementation of nutrients is a benign and inexpensive intervention in pregnancy to prevent life‐long disability from mental illness. Use of dietary supplements in poorer, rural areas of China is below recommendations. Physicians, midwives, and public health officials in China can promote prenatal nutrient supplementation to reduce the future burden of mental illnesses that might be prevented before birth.
Keywords: Folic acid, Vitamin D, Omega‐3 fatty acids, Choline, Pregnancy, Coronavirus
Introduction
Prenatal development of the fetus has long‐lasting consequences for the future health of the child. Children who are born small for gestational age have increased risk for a variety of future illnesses, including cardiovascular, pulmonary, and mental disorders. 1 , 2 Poor fetal development is associated with decreased executive function, ability to focus attention and control impulses; poorer educational achievement; and depressive symptoms. 3 , 4 Many children who show evidence of poor fetal development at birth are subsequently diagnosed with attention deficit disorder (ADD) or autism spectrum disorder (ASD) in childhood. 5 , 6
Epidemiological evidence from the 1944–1995 Dutch Hunger Winter and the 1959–1961 Chinese famines is the most dramatic example of the effect of adverse maternal factors during pregnancy on the child’s future mental wellbeing. Children conceived or in early gestation during the famines have a 2‐fold increased risk of schizophrenia in adulthood. 7 , 8 Xu et al 9 provided further evidence that confirms these results from population in Guangxi, China.
Common factors adversely impacting fetal development include maternal infections, substance abuse, stress, and depression. COVID‐19 infection in pregnancy is currently being assessed, but the virus does not seem to invade the amniotic space or be transmitted directly to the fetus. 10 However, viruses that do not directly infect the fetus may nonetheless increase the risk of future mental illness by causing maternal inflammation that adversely affects the placenta’s ability to support fetal development. 11 , 12 , 13 , 14
Positive steps that protect fetal brain development and mitigate the effect of these risks include increasing the maternal level of micronutrients, specifically folic acid, vitamin D, and choline, promoting full‐term development through nutrients such as docosahexaenoic acid (DHA), as well as evaluation and treatment of maternal stress and depression. In this article, we review the evidence for these interventions with specific recommendations for expectant mothers, clinicians, and public health authorities responsible for population‐wide preventive initiatives. Methodologically, we use a recent review of prenatal prevention of mental illness 15 supplemented with a Medline search [key words (folic acid OR vitamin D OR choline OR omega‐3 fatty acids) AND pregnancy AND China].
Folic acid
Folic acid is a classic example of the positive effects of prenatal micronutrient supplementation on preventing serious fetal developmental problems. Neural tube defects, such as mental illnesses have various causes including maternal infection and familial, most likely genetic factors. After initial observations of folic acid deficiency, a randomized controlled trial evaluating folic acid supplementation was conducted with women who already had one child with a neural tube deficit and had decided to have another child. The study found that supplements of folic acid in early pregnancy reduced the risk of neural tube defects. 16 As a result of this study, folic acid supplementation in pregnancy has become one of the most important public health advances of recent years.
Most studies of prenatal folic acid are now retrospective because of general acknowledgement of its positive effects. These studies compare the effects of folic acid in women who did and did not decide to follow supplementation recommendations. Studies have found that preconception initiation is superior to initiation after 10 weeks gestation; supplementation is more effective than good diet, although the effects are additive; and the positive effects of supplementation extend beyond neural tube deficits to include facial clefts and other midline defects, and general cognition. 17 , 18 One of the few remaining randomized studies reported that folate supplementation during pregnancy, rather than fish oil and fish oil plus 5‐methyltetrahydrofolate supplementation, improves children’s ability to solve response conflicts. 19
The positive data led public health authorities in many countries to mandate folic acid supplementation of processed foods, which has led to more recent studies showing less impressive effects. No adverse effects of taking recommended folic acid supplements, in the context of food supplementation, have been found. 20
Folate supplementation in early pregnancy might also reduce the risk of mental health problems in children. 21 Folate is important in neurogenesis, cell growth and proliferation, and myelination. It has been associated with catecholamine neurotransmitters and serotonin synthesis. 22 Higher birth weights in children of mothers who had folic acid supplementation also may mediate the effect. 23 , 24
The current recommendation from the U.S. Prevention Studies Task Force is a daily supplement containing 0.4 to 0.8 mg (400–800 μg) of folic acid, initiated pre‐conception or as soon as possible in gestation. 25 Women who must take valproate and other anti‐epileptics for seizure control, despite potentially harmful fetal effects, take higher doses 26 . In a Chinese Ministry of Health study in 21 counties in southern (Zhejiang and Jiangsu provinces) and northern (Hebei province) China in 1993–1996, only 41% of pregnant women used folic acid supplements. 27 , 28 , 29
Vitamin D
Vitamin D is ingested or produced by skin exposure to ultraviolet light. 30 Emerging data suggest that gestational 25‐hydroxyvitamin D (25(OH)D) status is associated with brain development in childhood and adolescence. 31 , 32 , 33 In humans, 25(OH)D levels during pregnancy are positively associated with neurocognitive development. 34 , 35 A large prospective study found that early childhood language development is positively associated with gestational 25(OH)D levels. 36
An increasing amount of evidence links gestational vitamin D deficiency with neurodevelopmental disorders such as schizophrenia and ASD. 37 , 38 A Dutch birth cohort study reported an association between developmental vitamin D deficiency and autism traits. 39 Vitamin D supplements either prenatally or initiated within in the first month after birth may reduce the incidence of schizophrenia. 40 , 41 , 42 Some studies have linked ASD with neuronal migration, which starts at 6 weeks’ gestation and ends around 24 weeks’ gestation. 43 Expression of vitamin D receptor in the mammalian brain occurs as early as day 12 of gestation, and then increases throughout pregnancy. 44 Neonatal vitamin D deficiency might be associated with altered neuronal migration. A general mechanism might be alterations in the calcium flux associated with vitamin D‐sensitive ion channels. 45 The active form of vitamin D (1,25‐dihydroxyvitamin D) affects the function of voltage‐gated calcium channels. 46 Genes that code for the subunits of calcium channels are associated with the risk of schizophrenia and ASD. 47 In addition to these effects in the brain, cord‐blood levels of 25(OH)D negatively associated with risk of respiratory infection and childhood wheezing. 48
Currently manufactured prenatal multivitamins contain vitamin D in safe, recommended amounts and consider the amounts obtained via diet and sunlight. The usual recommended dose is 600 IU/day (Table 1). 49 The most robust effects of supplements have been found in Northern European and Nordic countries, where winter sunlight is restricted. Summer gestations in these countries are less likely to benefit from vitamin D supplements. In southern China between 2016 and 2019, 35% of pregnant women were vitamin D deficient. Deficiency was significantly associated with pre‐term birth. 50 For example, in 2014 and 2015, 90% of pregnant women in Guizhou were deemed vitamin D insufficient 51 and low birth weight neonates and other birth complications were observed. 52 In the China‐Anhui Birth Cohort Study, the incidence of small for gestational age was significantly reduced if mothers took recommended vitamin D supplements for at least 2 months. 53
TABLE 1.
Vitamin D daily intake recommendations for pregnant women
| Society | Recommended daily intake (IU) |
|---|---|
| Chinese Nutrition Society | 600 |
| European Food Safety Authority | 600 |
| Global Consensus Recommendations on Prevention and Management of Nutritional Rickets | 600 |
Choline
Choline, a micronutrient grouped with B vitamins, is an essential nutrient involved in many metabolic pathways, including the rapid cellular growth during fetal brain development. Choline is required for the biosynthesis of phosphatidylcholine, the main component of cell membranes. There is higher demand for choline during pregnancy because of accelerated one‐carbon metabolism and the formation of new membranes as cells undergo division. 54 The neurotransmitter, acetylcholine, is produced directly from free choline in cholinergic neurons. 55 Earlier in fetal development, choline acts as the agonist at cholinergic receptors before they receive cholinergic synapses. 56 Animal studies indicate that perinatal choline supplementation or restriction fundamentally changes fetal brain development, particularly in the hippocampus, which can result in changes in attention and spatial memory abilities during adulthood. 57 CHRNA7, the gene for the a7‐nicotinic acetylcholine receptor, which is activated by choline, has been associated with schizophrenia, ASD, and attention deficit‐hyperactivity disorder. 58 However, mouse CHRNA7 null mutants do not benefit from choline supplementation during gestation. 56
The demands of pregnancy cause a pronounced reduction of choline pools, as first studied in animal models. 59 M any pregnant w omen cannot maintain adequate choline levels through dietary intake and endogenous synthesis. Nucleotide variants in PEMT, the gene for phosphatidylethanolamine N‐methyltransferase, are associated with lower choline levels. This gene is also associated with schizophrenia in Asians. 60 Compounding the biological mechanisms, most pregnant women in North America have inadequate dietary consumption of choline, despite recommendations of a minimum of 500 mg/d. Egg yolks, liver, and red meats are the richest sources of choline, and soybeans, fish, and other meats also contain choline. Higher choline levels during gestation and increased dietary intake have been associated with improved cognition lasting as long as 8 years after birth. 61 Dietary intake and gestational choline levels in China have not been studied.
Four double‐blind, placebo‐controlled trials have investigated the effect of choline supplementation during gestation; the timing and dosage of supplementation varied in the studies. 62 , 63 , 64 , 65 The Ross et al 65 trial used high doses of phosphatidylcholine, which is the form found in most foods. An advantage of using phosphatidylcholine is that it is not metabolized by most intestinal bacteria, which can metabolize choline to trimethyl‐urea or the cardiotoxic trimethyl‐amineoxide. They found positive effects of early gestational phosphatidylcholine (6300 mg, equivalent to 900 mg free choline) on the development of cerebral inhibition, measured electrophysiologically in the newborns. This amount triples the minimal amount recommended in the diet. An early benefit on cerebral inhibition was found in the phosphatidylcholine‐supplemented group compared with the placebo group. The positive effects extended to 3.5 years of age; early cerebral inhibition was associated with fewer problems in attention and social behavior. 65 It is possible that the benefits of supplementation could last even longer because these problems in childhood are associated with later mental illnesses, including schizophrenia. 66 In the Ross et al trial, positive outcomes were related to variants in the child’s CHRNA7 genotype. Benefits were also found in women at higher risk for an offspring with mental illness, including women who had mental illnesses themselves.
Other trials have found beneficial effects of choline supplementation in women who were heavy drinkers of alcoholic beverages and in average‐risk women. 63 , 64 One trial failed to find a benefit of choline supplementation on infant cognitive function; this study used a single cognitive test to evaluate the benefit in offspring. 62 None of the four trials have found adverse side effects.
Choline is the only micronutrient to be evaluated specifically in women who have early ges tational infections. 14 When women are infected before 16 weeks’ gestation, Zika virus and cytomegalovirus invade the fetal brain, where the damage can be catastrophic. Conversely, most common viral and bacterial infectious agents, including influenza and coronaviruses, do not invade the fetal brain when infections occur in early pregnancy. These pathogens elicit a maternal inflammatory response that is thought to be most damaging to the placenta. Cytokine‐activated macrophages, called Hofbauer cells, invade the chorionic villi, which they treat as a foreign body. The resultant compromise in placental function causes problems in ongoing fetal development. The most vulnerable gestational period, between 12 and 18 weeks, is when neurogenesis occurs. In this period, the immature neurons migrate into the embryonic cerebral layers and begin differentiation into mature neurons, which includes development of neurotransmitter receptors and formation of synaptic connections. 67
Higher choline levels during early gestation mitigate the effects of these common bacterial and viral infections. Inhibitory cerebral neurons appear to be the most sensitive to the effects of inflammation. Choline’s beneficial effects are initially observed as enhanced cerebral inhibition in newborns with infected mothers. By 3 months of age, the infants whose mothers were infected by common viruses have higher self‐regulation as assessed on the Infant Behavior Questionnaire–Revised (IBQ‐R), a standard instrument for rating behavior in infants up to 1 year of age that has been studied in both the United States and China. 68 The Self‐Regulation Index is associated with later social behavior and reading readiness up to 4 years of age. 69 , 70 Comparing with betaine supplementation, choline supplementation significantly changed the percentage of scores on the Self‐Regulation Index and sub‐components in 3‐month‐old infants whose mothers had viral infection during early pregnancy (Table 2). Infants whose mothers had higher choline levels (at least one standard deviation above the mean) early in pregnancy had fewer problems in distractibility and better ability to regulate their behavior in several dimensions than those with lower choline levels. These choline levels can be achieved by diets rich in eggs and meats or by a daily supplement containing 500–1000 mg choline or 3500–7000 mg phosphatidylcholine. 62 , 63 , 64 , 65
TABLE 2.
The percentage change of scores on behavior ratings in 3‐month‐old infants whose mothers take choline supplementation after viral infection in early pregnancy compared with betain supplementation
| Behavior (n = 33) | Effect of 16 weeks maternal with gestation choline >8.4 μM |
|---|---|
| Distractibility † | –37% |
| Pleasure in quiet play † | 25% |
| Soothability † | 32% |
| Recovery from distress † | 34% |
| Self‐Regulation Index † | 23% |
A re‐analysis of data that were published in our previous study. 14
Infant Behavioral Questionnaire‐R‐short form P < 0.01.
Common viral infections experienced by women in the study were likely coronaviruses, based on their having symptoms of a severe cold. If COVID‐19, like other coronaviruses, primarily causes a maternal inflammatory response that affects the placenta,71 then choline supplementation along with other prenatal micronutrients and a good diet, will likely be helpful in reducing future mental problems in the offspring of infected women. Of course, women should be counseled to avoid infection if possible. To date, COVID‐19‐infected pregnancies have not been found to involve infection of the fetus. 10
Prenatal studies of choline supplementation directed to CHRNA7 and its nicotinic receptor were initiated with the aim of preventing brain developmental problems associated with CHRNA7 gene variants. CHRNA7 polymorphisms are associated with schizophrenia, and microdeletions that include CHRNA7 are considered to be a genetic risk factor. 56 Schizophrenia is impacted by many risk factors, some prenatal and some postnatal.
However, epidemiological evidence points to prenatal risk factors including maternal infection and inflammation playing a major role. 11 To the extent that higher choline levels mitigate these risk factors, prenatal choline supplementation may be one factor in preventing future cases of schizophrenia.
Higher choline levels have other positive effects as well as those discussed above. For example, dietary choline intake and midgestation maternal serum total choline have been found to have inversely associated with the risk of neural tube defects in offspring. 72 , 73
The American Medical Association recommends prenatal choline supplementation, but commercially available prenatal vitamins do not currently contain adequate amounts of choline, at least not in the United States. It is estimated the choline intake of individuals in the United States, including children, men, women, and pregnant women, is far below the recommended adequate intake. 74 The current FDA recommendation for pregnant women is 550 mg/d. 75 On the basis of our review of the current research, we recommend a supplement contain 1000 mg choline or 7200 mg phosphatidylcholine per day. The safe upper limit, according to the FDA, is 36 000 mg of phosphatidylcholine. Although it is possible to achieve adequate amounts from diet, as it is for all the micronutrients, the higher levels that are maximally effective for preventive efforts generally require supplementation. In China, to our knowledge, there are no data or recommendations about choline intake.
Omega‐3 fatty acids
The n‐3 long‐chain polyunsaturated fatty acid (LCPUFA), docosahexaenoic acid (DHA 22:6n‐3), is rapidly accumulated by the fetus during the last trimester of pregnancy. During this last trimester, the fetal brain undergoes a significant growth spurt, and nearly 70 mg DHA is needed per day. Low maternal intake of DHA is associated with lower verbal IQ, diminished prosocial behavior, suboptimal fine motor ability, and impaired social and verbal development. 76 Supplementation with fish oil rich in DHA during pregnancy has been found to increase the offspring’s neurocognitive functioning. 77 , 78 , 79 However, not all studies have had positive findings. 80 , 81 A double‐blind supplementation trial found a lack of benefit of prenatal DHA supplementation on IQ at the seven‐year follow‐up. 82 Fatty acid desaturase (FASD2) polymorphisms have been found to be associated with lower n‐3 LCPUFA levels in a genome‐wide association study 83 and with blood EPA and DHA levels during pregnancy. 84 Genetic effects might partially explain the inconsistent effects observed. Another possible mechanism is that DHA lowers the incidence of premature birth, which, in turn, is associated with lower IQ scores. 85
The risk of schizophrenia is increased in mothers with higher overall levels of fatty acids, and autistic traits are associated with higher levels of n‐6 LCPUFA. 86 , 87 Thus, it is doubtful that omega‐3 fatty acid supplementation would have a positive impact on mental illness. Positive effects of supplementation with fish oil in the third trimester of pregnancy include reduction in persistent wheeze or asthma and infections of the lower respiratory tract in offspring. 88 Current recommendations for intake of omega‐3 fatty acids during pregnancy from dietary sources such as fish are 200–300 mg/d (Table 3).
TABLE 3.
Dietary Omega‐3 fatty acids daily intake recommendations during pregnancy
| Society | Recommended daily intake (mg) |
|---|---|
| European Commission | 200 |
| International Society for the Study of Fatty Acids and Lipids | 300 |
| National Institutes of Health U.S. | 200 |
| World Association of Perinatal Medicine | 300 |
| Chinese Nutrition Society | 200 |
A study in China found higher DHA and eicosapentanoic acid dietary intake in pregnant women from inland and river/lake regions compared with women from coastal regions. Women without access to marine fish may need fish oil supplements for adequate intake. However, there were no differences in umbilical cord erythrocyte membrane levels at birth in the neonates from the three regions. 89
Mental illnesses in pregnancy
Women who experience mental health issues during pregnancy often have sub‐optimal birth outcomes, including increased mortality and morbidity, shorter gestation, and lower birth weight. 90 Depression is one of the most common mental health conditions experienced by women during pregnancy and the childbearing years. The prevalence of major depressive disorder during pregnancy is 12.7%, while 37% of women report experiencing depression symptoms at some point during pregnancy. 91 Depression during pregnancy may not only have physiological consequences, including low birth weight, intrauterine growth restriction, and preterm birth, but may also expose women to an increased risk of poor psychological postnatal adjustment and postnatal depression. 92 A meta‐analysis suggested that psychotherapy might benefit the mental health of mothers which has positive impacts on their children and parenting skills. 93 Antidepressants generally have more robust effects than psychotherapy, and do not adversely affect fetal development. 94 , 95 Assertions of risk for future autism spectrum disorders have discouraged clinicians from prescribing antidepressants to depressed pregnant women; however, the risk may be due to maternal depression itself rather than its treatment. 96
Conclusion
Widespread dietary supplementation with the nutrients discussed in this paper (Figure 1), most of which are benign and inexpensive, is a potential intervention in pregnancy to prevent life‐long disability from mental illness for individuals and families, and to lessen the burden of mental health on society. Currently, folic acid supplementation is widely promoted to prevent neural tube defects; however, the uptake of this recommendation remains less than 70%. 97 Multivitamins with folic acid, which contain vitamins A, B12, and D are also available. Choline supplements are available, but there are currently no public health recommendations or advocacy promoting their use. Public health officials, especially in China, should consider the value of prenatal nutrient supplementation for reducing the burden of future mental disorders.
FIGURE 1.
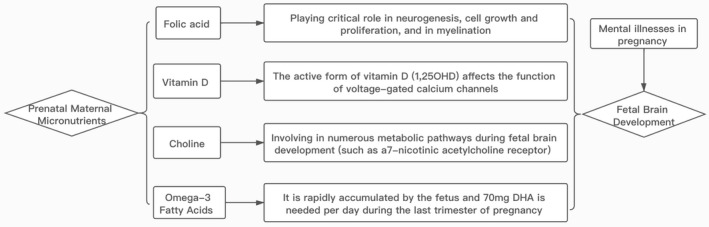
The association between the maternal nutrients and fetal brain development
CONFLICT OF INTEREST
None.
Li Y, Freedman R. Prospects for improving future mental health of children through prenatal maternal micronutrient supplementation in China. Pediatr Invest. 2020;4:118–126. 10.1002/ped4.12199
REFERENCES
- 1. Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the predictive adaptive response hypothesis. J Physiol. 2014;592:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schlotz W, Phillips DI. Fetal origins of mental health: Evidence and mechanisms. Brain Behav Immun. 2009;23:905–916. [DOI] [PubMed] [Google Scholar]
- 3. Aizer A, Currie J. The intergenerational transmission of inequality: Maternal disadvantage and health at birth. Science. 2014;344:856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costello EJ, Worthman C, Erkanli A, Angold A. Prediction from low birth weight to female adolescent depression: A test of competing hypotheses. Arch Gen Psychiatry. 2007;64:338–344. [DOI] [PubMed] [Google Scholar]
- 5. Raikkonen K, Pesonen AK, Roseboom TJ, Eriksson JG. Early determinants of mental health. Best Pract Res Clin Endocrinol Metab. 2012;26:599–611. [DOI] [PubMed] [Google Scholar]
- 6. Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, et al. Preterm birth and poor fetal growth as risk factors of attention‐deficit/hyperactivity disorder. Pediatrics. 2015;136:e599–608. [DOI] [PubMed] [Google Scholar]
- 7. Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Arch Gen Psychiatry. 1992;49:983–988. [DOI] [PubMed] [Google Scholar]
- 8. Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. [DOI] [PubMed] [Google Scholar]
- 9. Xu MQ, Sun WS, Liu BX, Feng GY, Yu L, Yang L, et al. Prenatal malnutrition and adult schizophrenia: Further evidence from the 1959–1961 Chinese famine. Schizophr Bull. 2009;35:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edwards MJ. Hyperthermia in utero due to maternal influenza is an environmental risk factor for schizophrenia. Congenit Anom (Kyoto). 2007;47:84–89. [DOI] [PubMed] [Google Scholar]
- 13. Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz‐Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freedman R, Hunter SK, Law AJ, Wagner BD, D’Alessandro A, Christians U, et al. Higher gestational choline levels in maternal infection are protective for infant brain development. J Pediatr. 2019;208:198–206.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freedman R, Hunter SK, Hoffman MC. Prenatal primary prevention of mental illness by micronutrient supplements in pregnancy. Am J Psychiatry. 2018;175:607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MRC Vitamin Study Research Group . Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 17. Veeranki SP, Gebretsadik T, Mitchel EF, Tylavsky FA, Hartert TV, Cooper WO, et al. Maternal folic acid supplementation during pregnancy and early childhood asthma. Epidemiology. 2015;26:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilcox AJ, Lie RT, Solvoll K, Taylor J, McConnaughey DR, Abyholm F, et al. Folic acid supplements and risk of facial clefts: National population based case‐control study. BMJ. 2007;334:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catena A, Munoz‐Machicao JA, Torres‐Espinola FJ, Martinez‐Zaldivar C, Diaz‐Piedra C, Gil A, et al. Folate and long‐chain polyunsaturated fatty acid supplementation during pregnancy has long‐term effects on the attention system of 8.5‐y‐old offspring: A randomized controlled trial. Am J Clin Nutr. 2016;103:115–127. [DOI] [PubMed] [Google Scholar]
- 20. Viswanathan M, Treiman KA, Kish‐Doto J, Middleton JC, Coker‐Schwimmer EJ, Nicholson WK. Folic acid supplementation for the prevention of neural tube defects: An updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;17:190–203. [DOI] [PubMed] [Google Scholar]
- 21. Roza SJ, van Batenburg‐Eddes T, Steegers EA, Jaddoe VW, Mackenbach JP, Hofman A, et al. Maternal folic acid supplement use in early pregnancy and child behavioural problems: The Generation R Study. Br J Nutr. 2010;103:445–452. [DOI] [PubMed] [Google Scholar]
- 22. Fernstrom JD. Can nutrient supplements modify brain function? Am J Clin Nutr. 2000;71:1669S–1675S. [DOI] [PubMed] [Google Scholar]
- 23. Iyengar L, Rajalakshmi K. Effect of folic acid supplement on birth weights of infants. Am J Obstet Gynecol. 1975;122:332–336. [DOI] [PubMed] [Google Scholar]
- 24. Scholl TO, Johnson WG. Folic acid: Influence on the outcome of pregnancy. Am J Clin Nutr. 2000;71:1295S–1303S. [DOI] [PubMed] [Google Scholar]
- 25. US Preventive Services Task Force , Bibbins‐Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:183–189. [DOI] [PubMed] [Google Scholar]
- 26. Bjørk M, Riedel B, Spigset O, Veiby G, Kolstad E, Daltveit AK, et al. Association of folic acid supplementation during pregnancy with the risk of autistic traits in children exposed to antiepileptic drugs in utero. JAMA Neurol. 2018;75:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Li Z, Ye R, Liu J, Ren A. Periconceptional folic acid supplementation and sex difference in prevention of neural tube defects and their subtypes in China: Results from a large prospective cohort study. Nutr J. 2018;17:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu M, Chen J, Liu J, Zhang S, Wang Q, Shen H, et al. Socioeconomic inequality in periconceptional folic acid supplementation in China: A census of 0.9 million women in their first trimester of pregnancy. BMC Pregnancy Childbirth. 2017;17:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu D, Cheng Y, Dang S, Wang D, Zhao Y, Li C, et al. Maternal adherence to micronutrient supplementation before and during pregnancy in Northwest China: A large‐scale population‐based cross‐sectional survey. BMJ Open. 2019;9:e028843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holick MF. Defects in the synthesis and metabolism of vitamin D. Exp Clin Endocrinol Diabetes. 1995;103:219–227. [DOI] [PubMed] [Google Scholar]
- 31. Bourre JM, Galea F. An important source of omega‐3 fatty acids, vitamins D and E, carotenoids, iodine and selenium: A new natural multi‐enriched egg. J Nutr Health Aging. 2006;10:371–376. [PubMed] [Google Scholar]
- 32. Bryan J, Osendarp S, Hughes D, Calvaresi E, Baghurst K, van Klinken JW. Nutrients for cognitive development in school‐aged children. Nutr Rev. 2004;62:295–306. [DOI] [PubMed] [Google Scholar]
- 33. Hawes JE, Tesic D, Whitehouse AJ, Zosky GR, Smith JT, Wyrwoll CS. Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav Brain Res. 2015;286:192–200. [DOI] [PubMed] [Google Scholar]
- 34. Weinert LS, Silveiro SP. Maternal‐fetal impact of vitamin D deficiency: A critical review. Matern Child Health J. 2015;19:94–101. [DOI] [PubMed] [Google Scholar]
- 35. Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2013;129:485–493. [DOI] [PubMed] [Google Scholar]
- 36. Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Volgyi E, et al. Gestational vitamin 25(OH)D status as a risk factor for receptive language development: A 24‐month, longitudinal, observational study. Nutrients. 2015;7:9918–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeLuca GC, Kimball SM, Kolasinski J. Ramagopalan SV, EbersGC. Review: The role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39:458–484. [DOI] [PubMed] [Google Scholar]
- 38. Mazahery H, Camargo CA Jr, Conlon C, Beck KL, Kruger MC, von Hurst PR. Vitamin D and autism spectrum disorder: A literature review. Nutrients. 2016;8:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vinkhuyzen AAE, Eyles DW, Burne THJ, Blanken LME, Kruithof CJ, Verhulst F, et al. Gestational vitamin D deficiency and autism‐related traits: The Generation R Study. Mol Psychiatry. 2018;23:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: A pilot study using banked sera. Schizophr Res. 2003;63:73–78. [DOI] [PubMed] [Google Scholar]
- 41. McGrath J, Saari K, Hakko H, Jokelainen J, Jones P, Jarvelin MR, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: A Finnish birth cohort study. Schizophr Res. 2004;67:237–245. [DOI] [PubMed] [Google Scholar]
- 42. McGrath JJ, Eyles DW, Pedersen CB, Anderson C, Ko P, Burne TH, et al. Neonatal vitamin D status and risk of schizophrenia: A population‐based case‐control study. Arch Gen Psychiatry. 2010;67:889–894. [DOI] [PubMed] [Google Scholar]
- 43. Reiner O, Karzbrun E, Kshirsagar A, Kaibuchi K. Regulation of neuronal migration, an emerging topic in autism spectrum disorders. J Neurochem. 2016;136:440–456. [DOI] [PubMed] [Google Scholar]
- 44. Eyles D, Burne T, McGrath J. Vitamin D in fetal brain development. Semin Cell Dev Biol. 2011;22:629–636. [DOI] [PubMed] [Google Scholar]
- 45. Zanatta AP, Zanatta L, Goncalves R, Zamoner A, Silva FR. Rapid responses to reverse T3 hormone in immature rat Sertoli cells: Calcium uptake and exocytosis mediated by integrin. PLoS One. 2013;8:e77176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Latimer CS, Brewer LD, Searcy JL, Chen KC, Popovic J, Kraner SD, et al. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci USA. 2014;111:E4359–E4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Casamassima F, Hay AC, Benedetti A, Lattanzi L, Cassano GB, Perlis RH. L‐type calcium channels and psychiatric disorders: A brief review. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1373–1390. [DOI] [PubMed] [Google Scholar]
- 48. Camargo CA Jr, Rifas‐Shiman SL, Litonjua AA, Rich‐Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: An evidence‐based review. J Am Board Fam Med. 2009;22:698–706. [DOI] [PubMed] [Google Scholar]
- 50. Yu L, Guo Y, Ke HJ, He YS, Che D, Wu JL. Vitamin D status in pregnant women in Southern China and risk of preterm birth: A large‐scale retrospective cohort study. Med Sci Monitor. 2019;25:7755–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hong‐Bi S, Yin X, Xiaowu Y, Ying W, Yang X, Ting C, et al. High prevalence of vitamin D deficiency in pregnant women and its relationship with adverse pregnancy outcomes in Guizhou, China. J Int Med Res. 2018;46:4500–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Li H, Zheng M, Wu Y, Zeng T, Fu J, et al. Maternal vitamin D deficiency increases the risk of adverse neonatal outcomes in the Chinese population: A prospective cohort study. PLoS One. 2018;13:e0195700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tao RX, Meng DH, Li JJ, Tong SL, Hao JH, Huang K, et al. Current recommended vitamin D prenatal supplementation and fetal growth: Results from the China‐Anhui Birth Cohort Study. J Clin Endocrinol Metab. 2018;103:244–252. [DOI] [PubMed] [Google Scholar]
- 54. Caudill MA. Pre‐ and postnatal health: Evidence of increased choline needs. J Am Diet Assoc. 2010;110:1198–1206. [DOI] [PubMed] [Google Scholar]
- 55. Ulus IH, Wurtman RJ, Mauron C, Blusztajn JK. Choline increases acetylcholine release and protects against the stimulation‐induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989;484:217–227. [DOI] [PubMed] [Google Scholar]
- 56. Freedman R. α7‐nicotinic receptor agonists for cognitive enhancement in schizophrenia. Ann Rev Med. 2014;65:245–261. [DOI] [PubMed] [Google Scholar]
- 57. Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res Dev Brain Res. 2000;123:25–32. [DOI] [PubMed] [Google Scholar]
- 58. Freedman R, Ross RG. Prenatal choline and the development of schizophrenia. Shanghai Arch Psychiatry. 2015;27:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zeisel SH, Mar MH, Zhou Z, da Costa KA. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–3054. [DOI] [PubMed] [Google Scholar]
- 60. Liu Y, Zhang H, Ju G, Zhang X, Xu Q, Liu S, et al. A study of the PEMT gene in schizophrenia. Neurosci Lett. 2007;424:203–206. [DOI] [PubMed] [Google Scholar]
- 61. Boeke CE, Gillman MW, Hughes MD, Rifas‐Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol. 2013;177:1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheatham CL, Goldman BD, Fischer LM, da Costa KA, Reznick JS, Zeisel SH. Phosphatidylcholine supplementation in pregnant women consuming moderate‐choline diets does not enhance infant cognitive function: A randomized, double‐blind, placebo‐controlled trial. Am J Clin Nutr. 2012;96:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caudill MA, Strupp BJ, Muscalu L, Nevins JEH, Canfield RL. Maternal choline supplementation during the third trimester of pregnancy improve s infant information processing speed: A randomized, double‐blind, controlled feeding study. FASEB J. 2018;32:2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, et al. Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: A randomized, double‐blind, placebo‐controlled clinical trial. Alcohol Clin Exp Res. 2018;42:1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ross RG, Hunter SK, Hoffman MC, McCarthy L, Chambers BM, Law AJ, et al. Perinatal phosphatidylcholine supplementation and early childhood behavior problems: Evidence for CHRNA7 moderation. Am J Psychiatry. 2016;173:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rossi A, Pollice R, Daneluzzo E, Marinangeli MG, Stratt P. Behavioral neurodevelopmental abnormalities and schizophrenic disorder: A retrospective evaluation with the Child Behavior Checklist (CBCL). Schizophrenia Res. 2000;44:121–128. [DOI] [PubMed] [Google Scholar]
- 67. Zecevic N, Hu F, Jakovcevski I. Cortical interneurons in the developing human neocortex Dev Neurobiol. 2011;71:18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behav Develop. 2003;26:64–86. [Google Scholar]
- 69. Gartstein MA, Putnam SP, Kliewer R. Do infant temperament characteristics predict core academic abilities in preschool‐aged children? Learn Individ Diff. 2016;45:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Slobodskaya HR, Kozlova EA. Early temperament as a predictor of later personality. Pers Individ Dif. 2016;99:127–132. [Google Scholar]
- 71. Centers for Disease Control and Prevention (U.S.) . Coronavirus disease 2019 (COVID‐19) and pregnancy. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/pregnancy-faq.html. Accessed March 6, 2020.
- 72. Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–109. [DOI] [PubMed] [Google Scholar]
- 73. Shaw GM, Finnell RH, Blom HJ, Carmichael SL, Vollset SE, Yang W, et al. Choline and risk of neural tube defects in a folate‐fortified population. Epidemiology. 2009;20:714–719. [DOI] [PubMed] [Google Scholar]
- 74. Wu BTF, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One. 2012;7:e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Food and Drug Administration U.S. Nutrition Labeling Requirements. http://s3.amazonaws.com/public-inspection. federalregister.gov/2016-11867.pdf (pages 903–904). Accessed March 1, 2020.
- 76. Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet. 2007;369:578–585. [DOI] [PubMed] [Google Scholar]
- 77. Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–1267. [DOI] [PubMed] [Google Scholar]
- 78. Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: A randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F45–F50. [DOI] [PubMed] [Google Scholar]
- 79. Judge MP, Harel O, Lammi‐Keefe CJ. Maternal consumption of a docosahexaenoic acid‐containing functional food during pregnancy: Benefit for infant performance on problem‐solving but not on recognition memory tasks at age 9 mo. Am J Clin Nutr. 2007;85:1572–1577. [DOI] [PubMed] [Google Scholar]
- 80. Bakker EC, Ghys AJ, Kester AD, Vles JS, Dubas JS, Blanco CE, et al. Long‐chain polyunsaturated fatty acids at birth and cognitive function at 7 y of age. Eur J Clin Nutr. 2003;57:89–95. [DOI] [PubMed] [Google Scholar]
- 81. Ghys A, Bakker E, Hornstra G, van den Hout M. Red blood cell and plasma phospholipid arachidonic and docosahexaenoic acid levels at birth and cognitive development at 4 years of age. Early Hum Dev. 2002;69:83–90. [DOI] [PubMed] [Google Scholar]
- 82. Gould JF, Treyvaud K, Yelland LN, Anderson PJ, Smithers LG, McPhee AJ, et al. Seven‐year follow‐up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA. 2017;317:1173–1175. [DOI] [PubMed] [Google Scholar]
- 83. Lemaitre RN, Tanaka T, Tang W, Manichaikul A, Foy M, Kabagambe EK, et al. Genetic loci associated with plasma phospholipid n‐3 fatty acids: A meta‐analysis of genome‐wide association studies from the CHARGE Consortium. PLoS Genet. 2011;7:e1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Steer CD, Hibbeln JR, Golding J, Davey Smith G. Polyunsaturated fatty acid levels in blood during pregnancy, at birth and at 7 years: Their associations with two common FADS2 polymorphisms. Hum Mol Genet. 2012;21:1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan JA. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA. 2010;304:1675–1683. [DOI] [PubMed] [Google Scholar]
- 86. Harper KN, Hibbeln JR, Deckelbaum R, Quesenberry CP Jr, Schaefer CA, Brown AS. Maternal serum docosahexaenoic acid and schizophrenia spectrum disorders in adult offspring. Schizophr Res. 2011;128:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Steenweg‐de Graaff J, Tiemeier H, Ghassabian A, Rijlaarsdam J, Jaddoe VW, Verhulst FC, et al. Maternal fatty acid status during pregnancy and child autistic traits: The Generation R Study. Am J Epidemiol. 2016;183:792–799. [DOI] [PubMed] [Google Scholar]
- 88. Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, et al. Fish oil‐derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530–2539. [DOI] [PubMed] [Google Scholar]
- 89. Zhang J, Wang C, Gao Y, Man Q, Song P, Meng L, et al. Different intakes of n‐3 fatty acids among pregnant women in 3 regions of China with contrasting dietary patterns are reflected in maternal but not in umbilical erythrocyte phosphatidylcholine fatty acid composition. Nutr Res. 2013;33:613–621. [DOI] [PubMed] [Google Scholar]
- 90. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta‐analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee AM, Lam SK, Sze Mun Lau SM, Chong CS, Chui HW, Fong DY. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. 2007;110:1102–1112. [DOI] [PubMed] [Google Scholar]
- 92. Staneva AA, Bogossian F, Wittkowski A. The experience of psychological distress, depression, and anxiety during pregnancy: A meta‐synthesis of qualitative research. Midwifery. 2015;31:563–573. [DOI] [PubMed] [Google Scholar]
- 93. Cuijpers P, Weitz E, Karyotaki E, Garber J, Andersson G. The effects of psychological treatment of maternal depression on children and parental functioning: A meta‐analysis. Eur Child Adolesc Psychiatry. 2015;24:237–245. [DOI] [PubMed] [Google Scholar]
- 94. Wisner KL, Sit DK, Hanusa BH, Moses‐Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: Impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wisner KL, Bogen DL, Sit D, McShea M, Hughes C, Rizzo D, et al. Does fetal exposure to SSRIs or maternal depression impact infant growth? Am J Psychiatry. 2013;170:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Oberlander TF, Zwaigenbaum L. Disentangling maternal depression and antidepressant use during pregnancy as risks for autism in children. JAMA. 2017;317:1533–1534. [DOI] [PubMed] [Google Scholar]
- 97. Tang L, Lee AH, Yau KKW, Hui YV, Binns CW. Consumption of dietary supplements by Chinese women during pregnancy and postpartum: A prospective cohort study. Matern Child Nutr. 2017;13:e12435. [DOI] [PMC free article] [PubMed] [Google Scholar]


