ABSTRACT
Importance
Octreotide is an off‐label medicine for congenital hyperinsulinism (CHI), but is currently widely used for treatment of patients with CHI. Thus far, variable efficacy and adverse effects have been reported for octreotide.
Objective
The present study evaluated the efficacy and safety of a subcutaneous octreotide injection for treatment of diazoxide‐unresponsive CHI in China.
Methods
This study was a retrospective review of children with diazoxide‐unresponsive CHI who were treated with a subcutaneous octreotide injection. The efficacy and side effects of the treatment were assessed.
Results
Twenty‐five Chinese children (15 boys) were involved in the study. Their median age at diagnosis was 8 weeks (range, 1–24 weeks) and median age at the final follow‐up was 1.8 years (range, 0.3–3.3 years). Octreotide therapy effectively increased blood glucose levels in all patients. The intravenous glucose infusion rate was reduced in all patients. Twenty‐one patients gradually discontinued the intravenous glucose infusion while receiving octreotide combined with frequent carbohydrate/glucose‐rich feeding. Among patients with a monoallelic ATP‐sensitive potassium (KATP) channel mutation, 50.0% showed gradual remission during follow up, indicating that the octreotide treatment may be a feasible alternative to surgery, especially for patients with monoallelic KATP‐channel mutations. Transient elevation of liver enzymes occurred in 20.0% of patients, while asymptomatic gallbladder pathology occurred in one patient. The growth rates of these patients were normal (height standard deviation score was 0.3 ± 1.5 at the final follow‐up).
Interpretation
Octreotide was a well‐tolerated, effective therapy for most children with diazoxide‐unresponsive CHI.
Keywords: Octreotide, Congenital hyperinsulinism, Efficacy, Safety
INTRODUCTION
Congenital hyperinsulinism (CHI) is the most common cause of persistent hypoglycemia during the neonatal and infancy periods. Its pathophysiology involves inappropriate insulin secretion from pancreatic beta cells due to key genetic defects.
Diazoxide is an ATP‐sensitive potassium (KATP) channel agonist and the first‐line drug treatment for CHI. However, diazoxide is often ineffective in children with KATP‐channel mutations. 1 Defects in the KATP‐channel, (including two subunits of the pancreatic KATP‐channel, SUR1 and Kir6.2, which are encoded by ABCC8 or KCNJ11 mutations, respectively) are the most common causes of CHI and are present in nearly 50% of affected cases. 2 , 3 There are two histological forms of CHI, diffuse and local. In the diffuse form, all β cells are affected in the pancreas; this form is caused by biallelic, recessive or monoallelic, dominantly inherited mutations in the KATP‐channel. In the focal form, abnormal β cells are confined to a restricted area of the pancreas; this form is generally caused by inheritance of a paternal monoallelic recessive mutation in the KATP channel combined with somatic loss of the maternal allele, whereby pancreas β cells exhibit dysregulated insulin secretion that provides them with a growth advantage. 4
Half of children with diazoxide‐unresponsive CHI have focal KATP‐hyperinsulinism, and surgery has been reported to be curative for these patients. 5 , 6 18‐Fluoro‐L‐dihydroxyphenylalanine (18F‐DOPA) positron emission tomography computed tomography (PET‐CT) scans are useful for detection of islet lesions, 7 but this technique is not readily available in China. Therefore, treatment of patients with diazoxide‐unresponsive CHI represents a major challenge in clinical practice.
Octreotide is a long‐acting somatostatin analog that inhibits insulin release from pancreatic β‐cells; thus, it has been used as a second‐line drug treatment for CHI since 1990. 8 The efficacy and side effects of octreotide for treatment of diazoxide‐unresponsive CHI have been reported in several studies; however, the outcomes differ considerably among these studies. Yorifuji et al 9 reported that 88.0% of patients with diazoxide‐unresponsive CHI who received octreotide treatment were able to maintain blood glucose levels > 3.33 mmol/L; conversely, Demirbilek et al 10 reported that only 42.8% of patients treated with octreotide were able to maintain normoglycemia. In this study, we retrospectively analyzed patients with diazoxide‐unresponsive CHI who were treated with a subcutaneous octreotide injection.
METHODS
Ethical approval
The Ethics Committee of Beijing Children’s Hospital of Capital Medical University approved this study protocol, and written informed consent was obtained from the patients’ guardians for inclusion in the study.
Patients and treatment
This retrospective study was performed to accumulate real‐world data regarding the efficacy and safety of octreotide. Patients with diazoxide‐unresponsive CHI who were treated with octreotide between November 2013 and December 2017 were included in this study. Unresponsiveness to diazoxide in a patient with CHI was defined as the inability to stabilize blood glucose > 3.3 mmol/L during feeding and fasting periods at a maximum dose of diazoxide (15 mg·kg−1·day−1) for 5 days without an intravenous infusion of dextrose. 11 Octreotide treatment was initiated at 5 μg·kg−1·day−1; the dose was then increased in increments of 2–5 μg·kg−1·day−1 every 2 days until the maximum dose of 25 μg·kg−1·day−1 was reached, with the goal of maintaining blood glucose levels > 3.3 mmol/L. Additional treatments (intravenous glucose or frequent carbohydrate/glucose‐rich feeding) were administered as needed to maintain normoglycemia. The glucose infusion rate (GIR) was minimized at a blood glucose level > 6.0 mmol/L and continued until the discontinuation of glucose. The octreotide efficacy endpoints included the reduction of the GIR, discontinuation of glucose infusion, and blood glucose level. Blood glucose was checked every 2 hours using a portable glucometer while patients were hospitalized. After discharge, all patients were followed up at intervals of 3–6 months.
Clinical and laboratory data collection
We collected the following information: gestational age, birth weight, age at onset, age at diagnosis, and insulin and C peptide levels when blood glucose was < 2.8 mmol/L. Side effects of octreotide treatment were recorded for each patient; these included abdominal symptoms, growth rate, laboratory results regarding liver function, and abdominal ultrasound finding.
Genetic analysis
We screened all patients for mutations using next generation sequencing on the Ion Torrent platform (Thermo Fisher Scientific, Waltham, MA, USA). We designed a custom panel for target enrichment that included genetic and molecular causes of CHI (including the following previously described genes: ABCC8, KCNJ11, GLUD1, GCK, HADH1, UCP2, HNF4A, HNF1A, PGM1, PMM2, HK1, SLC16A1, FOXA2, and CACNA1D). 12 The Human Gene Mutation Database and the NCBI database were searched to determine whether new variants sites were present in the sequences. The pathogenicity of genetic variants was assessed using the American College of Medical Genetics criteria. 13
Statistical analysis
All statistical analyses were performed using SAS JMP version 11.0 (SAS Institute, Inc., Cary, NC, USA). Normality was checked using the Shapiro‐Wilk tests. Normally distributed data are presented as means and standard deviations. Data that were non‐normally distributed are presented as medians and ranges. Differences between groups were determined using the Wilcoxon nonparametric test and the chi‐square test.
RESULTS
Twenty‐five patients (15 boys) were included in this study; the median age at diagnosis was 8 weeks (range, 1–24 weeks), median age at onset was 1 day (range, 1–150 days), and median age at the final follow‐up was 1.8 years (range, 0.3–3.3 years). The median levels of insulin and C peptide were 15.7 μIU/mL (range, 2.5–110.6 μIU/mL) and 3.5 ng/mL (range, 1.2–8.1 ng/mL), respectively. The GIR was 10.2 ± 3.5 mg·kg−1 min−1 at diagnosis and subsequently decreased in all patients. The intervals of octreotide injection were modified from 8 hours to 6 hours because hypoglycemia occurred before octreotide injection when the 8‐hour interval was used. Glucose infusions were discontinued in 21 patients (84.0%) and replaced with an octreotide injection every 6 hours and frequent carbohydrate‐ or glucose‐rich feeding. The median dose of octreotide was 10.0 μg·kg−1·day−1 (range, 1.2–20.0 μg·kg−1·day−1), and blood glucose was stabilized at 7.3 ± 2.0 days. Octreotide was introduced at the median age of 8.5 weeks (range, 4.0–24.0 weeks); the median duration of octreotide treatment was 8.0 months (range, 0.5–25.0 months). Twenty‐one patients (Patients 1–21) were weaned off the glucose infusion; 12 (48.0%) of these patients (Patients 1–12) maintained blood glucose > 3.3 mmol/L while receiving octreotide treatment. The remaining nine patients (Patients 13–21) had occasional slight hypoglycemia (blood glucose > 2.8 mmol/L and < 3.3 mmol/L) while receiving octreotide treatment. At the final follow‐up, 12 children had blood glucose > 3.3 mmol/L with glucose discontinuation. Five of these patients exhibited spontaneous remission; moreover, partial pancreatectomy cured two patients with focal 18F‐DOPA uptake. Five of these patients continued octreotide injections in combination with frequent carbohydrate/glucose‐rich feeding. Among the nine patients with occasional slight hypoglycemia, three exhibiteds pontaneous remission and four with focal 18F‐DOPA uptake underwent curative partial pancreatectomy. Octreotide injections were discontinued in these four patients; normoglycemia was maintained during feeding and fasting periods, including overnight fasting. Four patients (Patients 22–25) (16.0%) could not discontinue intravenous glucose infusions while receiving octreotide treatment; for these patients, the GIR was reduced by 10%–20%. In patients 23 and 24, glucose infusion was discontinued after successful identification and resection of focal lesions; Patient 24 had a maternal mutation of KCNJ11 and the other did not. The remaining two patients (Patients 22 and 25) had biallelic mutations in ABCC8. These patients required continuous intravenous glucose infusion to maintain euglycemia; notably, a high GIR (20 mg·kg−1·min−1) was needed to maintain normoglycemia in Patient 22. We advised both patients’ families to allow the patients to undergo a 18F‐DOPA PET‐CT scan and pancreatectomy; however, both families refused and chose to return home. Patient 22 died a few hours after discharge, and Patient 25 had frequent hypoglycemia (< 2. 8 mmol/L) while receiving octreotide treatment. The detailed findings for all 25 patients are shown in Tables 1 and 2.
Table 1.
Clinical features and follow‐up of 25 patients with diazoxide‐unresponsive congenital hyperinsulinism who were treated with subcutaneous octreotide injection
| Case | Gender | Age of onset (days) | Age of diagnosis (weeks) | Age at final follow‐up (years) | GIR (mg·kg−1·min−1) | Dose of octreotide (μg·kg−1·day−1) | Duration of octreotide (months) | Time for stabilized blood glucose (days) | Current clinical status | 18F‐DOPA PET‐CT | Height SDS | Elevation of liver function |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 90 | 14 | 1.0 | 6.0 | 10.0 | 6.0 | 5 | Continued on octreotide | D | 1.3 | N |
| 2 | Male | 1 | 4 | 2.3 | 6.3 | 5.0 | 15.0 | 9 | Continued on octreotide | D | −1.6 | P |
| 3 | Female | 135 | 20 | 1.3 | 7.6 | 11.6 | 5.0 | 7 | Remission (10 months) | ND | −0.7 | N |
| 4 | Female | 1 | 6 | 0.9 | 12.0 | 7.5 | 1.5 | 10 | Cured by partial resection | F | −2.0 | N |
| 5 | Female | 1 | 12 | 3.3 | 12.0 | 8.1 | 14.0 | 7 | Remission (17 months) | D | 2.3 | N |
| 6 | Female | 3 | 9 | 2.9 | 12.7 | 1.2 | 9.0 | 6 | Cured by partial resection | F | 0.1 | N |
| 7 | Male | 124 | 18 | 2.7 | 6.0 | 3.8 | 13.0 | 7 | Remission (17 months) | D | −0.5 | N |
| 8 | Male | 90 | 22 | 2.6 | 5.2 | 10.0 | 25.0 | 7 | Remission (30 months) | D | 0.5 | P |
| 9 | Female | 150 | 23 | 2.4 | 6.0 | 6.0 | 3.0 | 5 | Remission (8 months) | ND | 0.3 | N |
| 10 | Male | 1 | 10 | 2.1 | 11.0 | 9.0 | 22.0 | 7 | Continued on octreotide | ND | 0.4 | N |
| 11 | Male | 1 | 4 | 1.0 | 9.0 | 20.0 | 12.0 | 12 | Continued on octreotide | F | 2.1 | P |
| 12 | Male | 3 | 1 | 0.6 | 15.0 | 7.5 | 3.0 | 6 | Continued on octreotide | ND | 0.5 | N |
| 13 | Male | 2 | 6 | 1.9 | 15.0 | 12.5 | 9.0 | 9 | Cured by partial resection | F | −0.7 | N |
| 14 | Female | 2 | 16 | 2.2 | 12.0 | 11.5 | 3.0 | 7 | Cured by partial resection | F | 0.8 | P |
| 15 | Male | 1 | 24 | 1.3 | 9.0 | 6.5 | 8.0 | 7 | Remission (14 months) | ND | 0.8 | N |
| 16 | Male | 1 | 8 | 0.7 | 10.0 | 11.8 | 6.5 | 9 | Continued on octreotide | D | −1.7 | N |
| 17 | Male | 1 | 8 | 2.3 | 8.0 | 20.0 | 10.0 | 9 | Cured by partial resection | F | 1.0 | N |
| 18 | Male | 1 | 5 | 0.8 | 8.3 | 10.0 | 9.0 | 5 | Continued on octreotide | D | 2.1 | N |
| 19 | Female | 1 | 6 | 1.8 | 10.0 | 5.2 | 17.0 | 3 | Remission (18 months) | ND | 0.8 | N |
| 20 | Female | 1 | 7 | 2.0 | 10.0 | 20.0 | 7.0 | 9 | Remission (9 months) | D | 2.4 | N |
| 21 | Male | 1 | 5 | 0.3 | 10.0 | 12.5 | 3.0 | 8 | Continued on octreotide | ND | 0.0 | N |
| 22 | Male | 1 | 5 | NA | 20.0 | 25.0 | 2.0 | NA | Died soon after discharge | ND | NA | P |
| 23 | Male | 2 | 15 | 2.8 | 10 | 25 | 0.5 | NA | Cured by partial resection | F | 0.2 | N |
| 24 | Female | 1 | 5 | 1.0 | 10 | 15 | 6 | NA | Cured by partial resection | F | 1.7 | N |
| 25 | Male | 10 | 12 | 1.3 | 9 | 21.8 | 12 | NA | Continued on octreotide | ND | −3.0 | N |
GIR, glucose infusion rate;18F‐DOPA, 18‐Fluoro‐L‐dihydroxyphenylalanine; PET‐CT, positron emission tomography computed tomography; SDS, standard deviation scores; F, focal uptake of 18F‐DOPA; D, diffuse uptake of 18F‐DOPA; ND, not done; N, negative; P, positive; NA, not applicable.
Table 2.
Gene characteristics of 25 patients with diazoxide‐unresponsive congenital hyperinsulinism who were treated with subcutaneous octreotide injection
| Case | Gene analysis | Inherited from father | ACMG evaluation | Inherited from mother | ACMG evaluation |
|---|---|---|---|---|---|
| 1 | Negative | ||||
| 2 | ABCC8 | c.3736T>C, p.W1246R | Likely pathogenic (PM2+PM3+PP3+PP4) | c.536A>G, p.Y119C | Pathogenic (PS1+PM2+PM3+PP3+PP4) |
| 3 | KCNJ11 | c.895C>T, p.Q299X | Pathogenic (PVS1+PM2+PP4) | ||
| 4 | ABCC8 | c.1176+1G>A | Pathogenic (PVS1+PM2+PP4) | ||
| 5 | KCNJ11 | c.703C>T, p.Q235X | Pathogenic (PVS1+PM2+PP4) | ||
| 6 | ABCC8 | c.428G>A, p.W143X | Pathogenic (PVS1+PS1) | ||
| 7 | ABCC8 | c.331G>A, p.G111R | Likely pathogenic (PS1+PM2+PP3+PP4) | ||
| 8 | ABCC8 | c.3663_3664insG, p.F 1222Vfs*184 | Pathogenic (PVS1+PM2+PP4) | ||
| 9 | ABCC8 | c.3641G>C, p.R1214S | Uncertain (PM2+PP3+PP4) | ||
| 10 | Negative | ||||
| 11 | ABCC8 | c.149‐2A>C(IVS1) | Uncertain (PM2+PP4) | ||
| 12 | ABCC8 | c.2924‐9C>T | Uncertain (PM2+PP4) | c.4353T>C, p.L1451P | Uncertain (PM2+PP4+PP3) |
| 13 | ABCC8 | c.850dupG, p.A284fs | Pathogenic (PVS1+PM2+PP4) | ||
| 14 | ABCC8 | c.331G>A, p.G111R | Likely pathogenic (PS1+PM2+PP4+PP3) | ||
| 15 | ABCC8 | c.1585G>A, p.E529K | Likely pathogenic (PS1+PM2+PP3+PP4) | ||
| 16 | ABCC8 | c.331G>A, p.G111R | Likely pathogenic (PS1+PM2+PP3+PP4) | ||
| 17 | ABCC8 | c.4612C>T, p.R1538X | Pathogenic (PVS1+PM2+PP4) | ||
| 18 | ABCC8 | c.3124_c.3126delACCinsCAGCCAGGAACG, p.T1042Qfs*75; | Pathogenic (PVS1+PM2+PM3+PP4) | c.2832_c.2833insA, p.E945Rfs*25 | Pathogenic (PVS1+PM2+PM3+PP4) |
| 19 | ABCC8 | IVS11+2T>C | Uncertain (PM2+PP4) | ||
| 20 | ABCC8 | c.2144T>G, p.V715K | Likely pathogenic (PS1+PM2+PP3+PP4) | ||
| 21 | ABCC8 | c.331G>A, p.G111R | Pathogenic (PS1+PM2+PP3+PP4) | c.1792C>T, p.R598X | Pathogenic (PVS1+PS1+PM2+PP4) |
| 22 | ABCC8 | c.2506C>T, p.R836X | Pathogenic (PVS1+PS1) | c.3540C>G, p.Y1180X | Pathogenic (PVS1+PM2+PM3+PP4) |
| 23 | Negative | ||||
| 24 | KCNJ11 | c.413T>A, p.V138E | Uncertain (PM2+PP4+PP3) | ||
| 25 | ABCC8 | c.3632T>C, p.L1211P; | Uncertain (PM2+PP4+PP3) | c.1412C>T, p.A471V | Uncertain (PM2+PP4+PP3) |
ACMG, American College of Medical Genetics; PM, moderate pathogenicity; PP, supporting pathogenicity; PVS, very strong pathogenicity; PS, strong pathogenicity.
Twenty‐two of the 25 (88.0%) patients exhibited KATP‐channel mutations (19 ABCC8, three KCNJ11), while three patients did not have mutations. Glucose infusions were discontinued in 19 patients with KATP‐channel mutations (two KCNJ11 monoallelic, 13 ABCC8 monoallelic, four biallelic ABCC8) and in two patients who did not have gene mutations; four patients could not be weaned off the glucose infusion (Table 2). Octreotide responsiveness (i.e. the rate of glucose discontinuation) was similar between the patients who had mutations and those who did not (P = 0.41). The proportions of patients who underwent glucose discontinuation were 93.8% (15/16) and 66.7% (4/6) of patients with a monoallelic KATP‐channel mutation and patients with a biallelic mutation, respectively (P = 0.12); the mean octreotide dosage was similar between the two groups (10.0 μg·kg−1·day−1 vs. 8.8 μg·kg−1·day−1, P = 0.76).
In clinical practice, we usually recommend the patients with diazoxide‐unresponsive CHI to undergo 18F‐DOPA PET‐CT scan; 16 patients underwent 18F‐DOPA PET‐CT scans in Hong Kong Sanatorium and Hospital or Shanghai Huashan Hospital, the only two hospitals in China where 18F‐DOPA PET‐CT equipment is available. Eight patients exhibited focal uptake of 18F‐DOPA, and eight patients exhibited diffuse uptake of 18F‐DOPA. There were no significant differences in the rate of glucose discontinuation between the different PET‐CT signal patterns (75.0% in patients with local uptake vs. 100.0% in patients with diffuse uptake; Table 1).
Liver enzyme elevation was observed in 20.0% of patients within 2 weeks of octreotide treatment; this was spontaneously resolved over the subsequent 2 weeks. Asymptomatic gallbladder pathology occurred in one patient (gallstone) during the second week of octreotide treatment and persisted. The height standard deviation score (SDS) was 0.3 ± 1.5 (range, −3.0 to 2.4) at the final follow‐up; one patient was diagnosed with short stature with a height −3.0 SDS due to low birth weight (born at 36 weeks’ gestation: birth weight, 1800 g) with insufficient catch‐up growth.
At the final follow‐up, eight (50.0%) of the 16 patients with a monoallelic KATP‐channel mutation exhibited remission without surgery (Figure 1), and the median age at remission was 15.5 months (8.0–30.0 months). No spontaneous remission was observed in patients with a biallelic KATP‐channel mutation.
Figure 1.
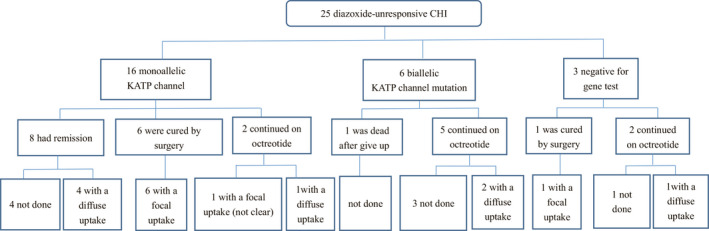
Gene mutation analysis, 18F‐DOPA uptake, and prognosis for 25 patients with diazoxide‐unresponsive congenital hyperinsulinism who were treated with subcutaneous octreotide injection. “Focal uptake” indicates focal uptake of 18F‐DOPA; “Diffuse uptake” indicates diffuse uptake of 18F‐DOPA; “Not done” indicates that 18F‐DOPA PET‐CT scans were not performed in these patient. CHI, congenital hyperinsulinism; KATP, ATP‐sensitive potassium; 18F‐DOPA, 18‐Fluoro‐L‐dihydroxyphenylalanine; PET‐CT, positron emission tomography computed tomography.
DISCUSSION
The treatment of diazoxide‐unresponsive CHI is a major challenge in clinical practice. Octreotide is an off‐label medicine that is currently widely used. 14 Our study showed that octreotide was effective in most patients with diazoxide‐unresponsive CHI, and that 84.0% of patients were able to be weaned off glucose infusion. Octreotide treatment was well tolerated in this study cohort, and it may be useful as the second choice for treatment of CHI, especially for patients in China where 18F‐DOPA PET‐CT scans are not readily available.
Efficacy of octreotide
In this study, blood glucose was stabilized > 3.3 mmol/L in 48.0% of patients with diazoxide‐unresponsive CHI who received octreotide treatment; however, Yorifuji et al 9 reported that 88.0% of patients maintained blood glucose levels > 3.33 mmol/L after weaning off intravenous dextrose. Nevertheless, 84.0% of patients in our study maintained blood glucose > 2.8 mmol/L. The SCORCH prospective trial and observational registry study, reported by Hosokawa et al, revealed that 60% and 64.7% of patients with diazoxide‐unresponsive CHI exhibited GIR reduction > 50% at 4 weeks, respectively, the GIR was reduced by 10%–20% when fasting glucose was > 11.1 mmol/L on two consecutive occasions. 15 Our study combined blood glucose values and dextrose weaning as efficacy criteria to clarify the effectiveness of octreotide treatment. Demirbilek et al 10 reported that octreotide treatment was also effective in post‐surgery patients who underwent near total pancreatectomy (14 patients) and focal resection (two patients).
Factors that affect octreotide efficacy
The present study investigated factors that affected octreotide efficacy. The proportions of patients who were weaned off intravenous glucose infusion were 93.8% and 66.7% of patients with monoallelic and biallelic KATP‐channel mutations, respectively; with no significant difference, and the dose of octreotide between the two groups was similar. Demirbilek et al 10 reported similar rates of octreotide responsiveness in patients with monoallelic (four of 10) and biallelic (six of 15) KATP‐channel mutations. Some parents did not choose 18F‐DOPA PET‐CT when their children carried biallelic KATP‐channel mutations, which were predicted to cause diffuse uptake of 18F‐DOPA. Therefore, it was difficult to determine the relationship between octreotide efficacy and 18F‐DOPA uptake.
The efficient dose of octreotide varied among patients. The efficient median dosage was 10 μg·kg−1·day−1, and blood glucose was stabilized at 7.3 ± 2.0 days.
On follow‐up, 50.0% of patients with monoallelic KATP‐channel mutations exhibited spontaneous remission at a median age of 15.5 months (range, 8.0–30.0 months); this indicates that octreotide treatment is a feasible alternative to surgery, especially for patients with monoallelic KATP‐channel mutations. Yorifuji et al 9 reported that the spontaneous remission rate in patients who received octreotide treatment was 25.0% (three of 12); these three patients had monoallelic KATP‐channel mutations. Salomon‐Estebanez et al 16 reported that seven patients receiving octreotide therapy exhibited remission during follow‐up; three patients had a biallelic KATP mutation, four patients had a monoallelic KATP mutation, and the mean age at spontaneous remission was 5.9 years (range, 1.6–9.0 years). We did not observe spontaneous remission in patients with a biallelic KATP channel mutation at the final follow‐up (median age 0.8 years, range 0.3–2.3 years). We plan to continue following these patients to determine whether they exhibit remission.
Notably, monthly injections of long‐acting somatostatin analogues are reportedly an effective treatment option for most children with diazoxide‐unresponsive CHI, without serious side effects. 17 Thus, long‐acting somatostatin analogues may constitute an alternative approach for treatment of diazoxide‐unresponsive CHI if these drugs were available in China.
Side effects of octreotide
The side effects of octreotide were mild among patients in our study. Liver enzyme elevation occurred in 20.0% of patients within 2 weeks of octreotide treatment; this increase spontaneously resolved over the subsequent 2 weeks or during treatment with symptomatic medication. Asymptomatic gallstones occurred in one patient during the second week of octreotide treatment and persisted. Demirbilek et al 10 reported higher occurrences of liver enzyme elevation (46.4%) and gallbladder pathology (sludge or gallstones) (32.0%), but these symptoms were mild and did not require discontinuation of octreotide therapy. Another cohort study showed only transient gastrointestinal symptoms, including poor appetite, constipation or changes in stool color, (e.g. whitish stool). 9 Some case reports described the occurrence of octreotide‐induced hepatitis with an increase of liver transaminases to 1000 IU/L, 18 , 19 , 20 but this effect has not been observed in large sample cohort studies. Necrotizing enterocolitis, which is a severe side effect of octreotide therapy, has been reported in patients with CHI. 21 However, necrotizing enterocolitis was not observed in our cohort. Necrotizing enterocolitis is associated with some risk factors, such as sepsis, prematurity, history of cardiac anomalies, surgery and fluid overload; 22 the dose of octreotide is presumed to influence the occurrence of necrotizing enterocolitis. 21 The median dose of octreotide in our patients was 10 μg·kg−1·day−1, which may partially explain the low incidence of necrotizing enterocolitis in our study. Decisions by clinicians who performed treatment for children with CHI are influenced by the side effects. A notable side effect that concerns clinicians is the effect of octreotide on growth rates. Our data demonstrated a normal height SDS for nearly all patients, except one who had a low birth weight; Demirbilek et al 10 reported similar results.
In conclusion, our cohort study demonstrated that octreotide treatment was well tolerated among children with diazoxide‐unresponsive CHI, and octreotide may be safe and effective as an alternative therapy for treatment of diazoxide‐unresponsive CHI.
Funding Source
The study was funded by National Key Research and Development Program of China (2016YFC1305304), Beijing Children’s Hospital Young Investigator Program (No. BCHYIPA‐2016‐06), Beijing Municipal Administration of Hospital Clinical Medicine Development of Special Funding Support (No. ZYLX201821).
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
We thank the patients and their parents for their support during this study.
Cao B, Wu D, Su C, Chen J, Liang X, Liu M, et al. Efficacy and safety of octreotide treatment for diazoxide‐unresponsive congenital hyperinsulinism in China. Pediatr Invest. 2020;4:29–36. 10.1002/ped4.12175
REFERENCES
- 1. Touati G, Poggi‐Travert F, Ogier de Baulny H, Rahier J, Brunelle F, Nihoul‐Fekete C, et al. Long‐term treatment of persistent hyperinsulinaemic hypoglycaemia of infancy with diazoxide: a retrospective review of 77 cases and analysis of efficacy‐predicting criteria. Eur J Pediatr. 1998;157:628–633. [DOI] [PubMed] [Google Scholar]
- 2. Gong C, Huang S, Su C, Qi Z, Liu F, Wu D, et al. Congenital hyperinsulinism in Chinese patients: 5‐yr treatment outcome of 95 clinical cases with genetic analysis of 55 cases. Pediatr Diabetes. 2016;17:227–234. [DOI] [PubMed] [Google Scholar]
- 3. Snider KE, Becker S, Boyajian L, Shyng SL, MacMullen C, Hughes N, et al. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013;98:E355–E363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glaser B, Ryan F, Donath M, Landau H, Stanley CA, Baker L, et al. Hyperinsulinism caused by paternal‐specific inheritance of a recessive mutation in the sulfonylurea‐receptor gene. Diabetes. 1999;48:1652–1657. [DOI] [PubMed] [Google Scholar]
- 5. Laje P, Stanley CA, Palladino AA, Becker SA, Adzick NS. Pancreatic head resection and Roux‐en‐Y pancreatic cojejunostomy for the treatment of the focal form of congenital hyperinsulinism. J Pediatr Surg. 2012;47:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suchi M, Thornton PS, Adzick NS, MacMullen C, Ganguly A, Stanley CA, et al. Congenital hyperinsulinism: intraoperative biopsy interpretation can direct the extent of pancreatectomy. Am J Surg Pathol. 2004;28:1326–1335. [DOI] [PubMed] [Google Scholar]
- 7. Laje P, States LJ, Zhuang H, Becker SA, Palladino AA, Stanley CA, et al. Accuracy of PET/CT scan in the diagnosis of the focal form of congenital hyperinsulinism. J Pediatr Surg. 2013;48:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thornton PS, Alter CA, Katz LE, Baker L, Stanley CA. Short‐ and long‐term use of octreotide in the treatment of congenital hyperinsulinism. J Pediatr. 1993;123:637–643. [DOI] [PubMed] [Google Scholar]
- 9. Yorifuji T, Kawakita R, Hosokawa Y, Fujimaru R, Matsubara K, Aizu K, et al. Efficacy and safety of long‐term, continuous subcutaneous octreotide infusion for patients with different subtypes of KATP‐channel hyperinsulinism. Clin Endocrinol (Oxf). 2013;78:891–897. [DOI] [PubMed] [Google Scholar]
- 10. Demirbilek H, Shah P, Arya VB, Hinchey L, Flanagan SE, Ellard S, et al. Long‐term follow‐up of children with congenital hyperinsulinism on octreotide therapy. J Clin Endocrinol Metab. 2014;99:3660–3667. [DOI] [PubMed] [Google Scholar]
- 11. De Leon DD, Stanley CA. Congenital hypoglycemia disorders: new aspects of etiology, diagnosis, treatment and outcomes: highlights of the proceedings of the congenital hypoglycemia disorders symposium, Philadelphia April 2016. Pediatr Diabetes. 2017;18:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stanley CA. Perspective on the genetics and diagnosis of congenital hyperinsulinism disorders. J Clin Endocrinol Metab. 2016;101:815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier‐Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Welters A, Lerch C, Kummer S, Marquard J, Salgin B, Mayatepek E, et al. Long‐term medical treatment in congenital hyperinsulinism: a descriptive analysis in a large cohort of patients from different clinical centers. Orphanet J Rare Dis. 2015;10:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosokawa Y, Kawakita R, Yokoya S, Ogata T, Ozono K, Arisaka O, et al. Efficacy and safety of octreotide for the treatment of congenital hyperinsulinism: a prospective, open‐label clinical trial and an observational study in Japan using a nationwide registry. Endocr J. 2017;64:867–880. [DOI] [PubMed] [Google Scholar]
- 16. Salomon‐Estebanez M, Flanagan SE, Ellard S, Rigby L, Bowden L, Mohamed Z, et al. Conservatively treated Congenital Hyperinsulinism (CHI) due to K‐ATP channel gene mutations: reducing severity over time. Orphanet J Rare Dis. 2016;11:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van der Steen I, van Albada ME, Mohnike K, Christesen HT, Empting S, Salomon‐Estebanez M, et al. A multicenter experience with long‐acting somatostatin analogues in patients with congenital hyperinsulinism. Horm Res Paediatr. 2018;89:82–89. [DOI] [PubMed] [Google Scholar]
- 18. Avatapalle B, Padidela R, Randell T, Banerjee I. Drug‐induced hepatitis following use of octreotide for long‐term treatment of congenital hyperinsulinism. BMJ Case Rep. 2012;2012. pii: bcr2012006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ben‐Ari J, Greenberg M, Nemet D, Edelstein E, Eliakim A. Octreotide‐induced hepatitis in a child with persistent hyperinsulinemia hypoglycemia of infancy. J Pediatr Endocrinol Metab. 2013;26:179–182. [DOI] [PubMed] [Google Scholar]
- 20. Koren I, Riskin A, Barthlen W, Gillis D. Hepatitis in an infant treated with octreotide for congenital hyperinsulinism. J Pediatr Endocrinol Metab. 2013;26:183–185. [DOI] [PubMed] [Google Scholar]
- 21. Laje P, Halaby L, Adzick NS, Stanley CA. Necrotizing enterocolitis in neonates receiving octreotide for the management of congenital hyperinsulinism. Pediatr Diabetes. 2010;11:142–147. [DOI] [PubMed] [Google Scholar]
- 22. Ackermann AM, Palladino AA. Managing congenital hyperinsulinism: improving outcomes with a multidisciplinary approach. Res Rep Endocr Disord. 2015;5:103–117. [Google Scholar]


