Abstract
Importance
Nasal nitric oxide (nNO) testing is a method used in the diagnosis of primary ciliary dyskinesia (PCD). It has not been evaluated in Chinese population.
Objective
To establish a reference nNO value to assist in the diagnosis of PCD in Chinese children.
Methods
nNO values were measured in children with PCD (n = 36), cystic fibrosis (CF) (n = 20), asthma (n = 45), post‐infectious bronchiolitis obliterans (BO) (n = 41) and non‐PCD/non‐CF bronchiectasis (n = 32). The receiver operating characteristic nNO value for the diagnosis of PCD was plotted and the area under the curve was calculated.
Results
nNO values were significantly lower in children with PCD (median 25.66 nL/min) than in children with asthma (186.26 ± 58.95 nL/ min), BO (143.47 ± 49.71 nL/min) and non‐PCD/non‐CF bronchiectasis (173.13 ± 63.80 nL/min), but not in children with CF (90.90 ± 43.20 nL/min). Notably however, no CF patient had an nNO value < 45 nL/min. A cut‐off of 76 nL/min yielded the best sensitivity of 86.1%, and specificity of 91.4%, with an area under the curve of 0.920 (95% confidence interval 0.859–0.981) for the diagnosis of PCD. If CF was ruled out the specificity increased to nearly 100%.
Interpretation
nNO testing is able to discriminate between patients with PCD and those with CF, asthma, post‐infectious BO and non‐PCD/non‐CF bronchiectasis. A cut‐off of 76 nL/min could be further examined in patients suspected of PCD, to establish an nNO reference value for PCD screening in Chinese children.
Keywords: Nasal nitric oxide, Primary ciliary dyskinesia
INTRODUCTION
Primary ciliary dyskinesia (PCD) is an inherited disorder characterized by defects of motile cilia that are associated with impaired ciliary motion.1 Nasal nitric oxide (nNO) testing has been investigated for the screening of PCD in suspected patients, and is recommended by both the American Thoracic Society and the European Respiratory Society (ATS/ERS).2, 3 In recent studies the sensitivity of nNO testing with regard to PCD diagnosis ranged from 0.9 to 1.0, the specificity ranged from 0.75 to 0.97, and nNO testing was able to separate PCD patients from patients with other chronic respiratory diseases.4, 5
Almost all previous studies investigating nNO testing and PCD have been conducted in western countries. In view of the possible differences between Chinese and occidental populations with regard to genotypes and clinical manifestations, the value of nNO testing in PCD diagnosis needs to be evaluated in Chinese children. In the current study, nNO was measured in children with PCD, cystic fibrosis (CF), asthma, post‐infectious bronchiolitis obliterans (BO), and non‐PCD/non‐CF bronchiectasis to estimate the value of nNO testing in PCD diagnosis.
The primary aims of the study were to determine whether nNO testing could separate PCD patients from patients with the four other aforementioned diseases, and to identify a potentially useful nNO cut‐off for PCD for further investigation.
METHODS
The current study was approved by the Ethics Committee of Beijing Children's Hospital (file number 2017‐26).
Participants
Children with PCD (n = 36), CF (n = 20), asthma (n = 45), post‐infectious BO (n = 41) and non‐PCD/non‐CF bronchiectasis (n = 32) were recruited and followed up at Beijing Children's Hospital in China.
Patients with PCD, CF, and post‐infectious BO included in the study were diagnosed between January 2012 and December 2018, and were followed up at least twice either as outpatients or during hospitalization between January 2017 and May 2019, including nNO testing. Patients with asthma and non‐PCD/non‐CF bronchiectasis were diagnosed between January 2017 and May 2019. All the nNO tests were performed between January 2017 and May 2019.
PCD was diagnosed via “hallmark” abnormal transmission electron microscopy (TEM) changes (including absence of outer dynein arms, combined absence of inner and outer dynein arms, and inner dynein arm absence combined with microtubular disarrangement) or a bi‐allelic causal mutation in PCD genotyping in accordance with ATS/ERS guidelines.2, 3 Whole exon sequencing via second generation sequencing techniques was used to detect PCD mutations of the patients, 6 had both “hallmark” TEM abnormalities and genetic mutation, 9 had “hallmark” TEM abnormalities alone, and 21 had genetic mutation alone (see supporting information). Loss‐of‐function variants including nonsense, frameshift, and splice site variants were considered pathogenic mutations. Mutations that had not been previously reported were tested using PROVEAN and SIFT software. Bi‐allelic disease‐causing mutations in autosomal recessive PCD were regarded as pathogenic.
CF was diagnosed based on a suggestive history, an abnormal sweat test and determination of a characteristic genomic defect. Asthma was diagnosed based on the Global Strategy for Asthma Management and Prevention. Post‐infectious BO was diagnosed based on clinical history, high‐resolution computed tomography (HRCT), and spirometry. Non‐PCD/non‐CF bronchiectasis was diagnosed via HRCT and exclusion of CF and PCD.
All children included were aged ≥ 4 years and exhibited no evidence of mental development retardation. Patients were also required to be free of symptoms suggesting exacerbation of respiratory or ear‐nose‐throat system conditions on the day of nNO measurement.
Measurement of nNO
nNO measurement was conducted successfully in all patients included in the study.
NO in ambient air was measured before nNO testing, to ensure it was less than 5 ppb. nNO levels were then measured using a chemiluminescence NO analyzer, CLD 88 sp with DENOX 88 (Sweden) in accordance with ATS/ERS recommendations.6 A nasal sampling tube with an aspiration rate of 0.3 L/min was placed into one nostril while air was directed into the other nostril. Children first inhaled air via a mouthpiece with a filter until total lung capacity was nearly reached. Inhalation was followed by exhalation via mouthpiece against resistance produced by the device, lasting over 10 s with a plateau of NO values of at least 4 s detected automatically. Exhalation was maintained at a flow of 50 ± 5 mL/s via visual real‐time adjustment for velum closure. The NO values of both nostrils were tested, and the higher one was recorded. nNO production (nL/min) was calculated by multiplying nNO concentration (ppb) by the sampling flow rate (0.3 L/min).
Statistical analysis
Statistical analysis was preformed using SPSS statistics 19, USA. The Kolmogorov‐Smirnov test was used to assess the normality of sex, age, and nNO value date distributions in the five disease groups. nNO value were expressed as mean ± standard deviation (SD) if the data‐set was normally distributed, or as median, 25th and 75th percentiles if it was not. Levene's test was used to assess the homogeneity of variance of nNO values. The Kruskal‐Wallis test was used to compare the nNO values in the five groups. P < 0.05 was considered statistically significant. Receiver operating characteristic curves were plotted and areas under the curves calculated.
RESULTS
Demographic data and nNO values in the five disease groups are shown in Table 1. There were no significant differences in age or sex between the five disease groups, but there was a statistically significant difference in nNO values (χ2 = 82.448, P < 0.001). In post‐hoc tests pairwise comparisons nNO values in the PCD group differed significantly from nNO values in the asthma (P < 0.001), BO (P < 0.001) and non‐PCD/non‐CF bronchiectasis (P < 0.001) groups. nNO values in the PCD group did not differ significantly from nNO values in the CF group (P = 0.182).
Table 1.
Demographic data and nNO values in patients in five disease groups
| Diseases | Patients number | Age (year) (mean ± SD) | Sex (male), n (%) | nNO (nL/min) (mean ± SD) |
|---|---|---|---|---|
| Asthma | 45 | 9.29 ± 2.56 | 26 (57.8) | 186.26 ± 58.95 |
| BO | 41 | 8.12 ± 2.78 | 25 (61.0) | 143.47 ± 49.71 |
| CF | 20 | 9.10 ± 3.24 | 8 (40.0) | 90.90 ± 43.20 |
| non‐PCD/non‐CF bronchiectasis | 32 | 10.24 ± 2.67 | 19 (55.9) | 173.13 ± 63.80 |
| PCD | 36 | 8.89 ± 2.53 | 25 (69.4) | P25: 13.84 Median:25.66 P75: 60.60 |
nNO, nasal nitric oxide; BO, bronchiolitis obliterans; CF, cystic fibrosis; PCD, primary ciliary dyskinesia.
In the PCD group nNO values were < 45 nL/min in 24/36 patients (66.7%), but in the CF group no nNO values were < 45 nL/min. A cut‐off of 45 nL/min separated PCD patients from other patients, with a sensitivity of 66.7% and specificity of 100%.
A cut‐off of 76 nL/min yielded the best sensitivity of 86.1% and specificity of 93.9% with an area under the curve of 0.926 (95% confidence interval 0.868–0.984). If CF was ruled out, specificity increased to nearly 100% (Figures 1 and 2).
Figure 1.
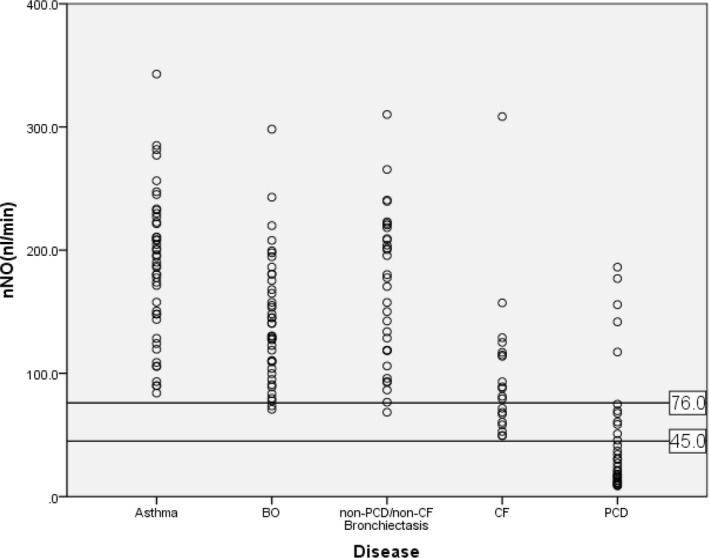
nNO values in children with five chronic respiratory diseases. nNO, nasal nitric oxide; BO, bronchiolitis obliterans; CF, cystic fibrosis; PCD, primary ciliary dyskinesia
Figure 2.
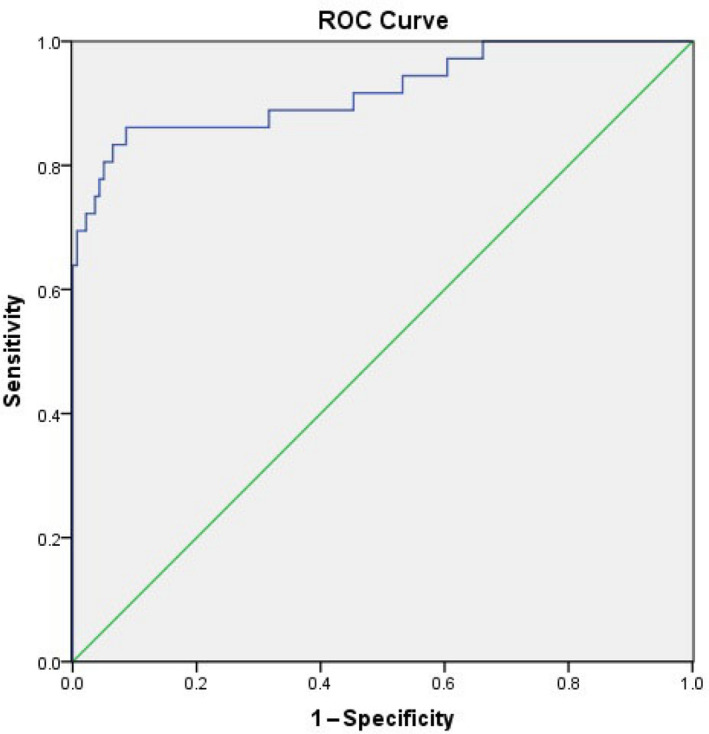
Receiver operating characteristics curve of nasal nitric oxide testing and the diagnosis of primary ciliary dyskinesia
The five PCD patients had nNO values > 76 nL/min, and all 5 yielded a positive genotype result, or exhibited hallmark TEM change, or both (Table 2). TEM revealed central microtubular disorganization in 4/5, and the remaining patient exhibited mutation of the HYDIN gene, which encodes the central apparatus and radial spokes. These 5 patients shared no other common characteristics.
Table 2.
Diagnostic Characteristics of five primary ciliary dyskinesia patients with nNO values > 76 nL/min
| Patient | Sex | Mutation locus | TEM abnormality | Situs inversus | nNO (nL/min) |
|---|---|---|---|---|---|
| No.1 | Male | HYDIN | Cilia not found | No | 144.9 |
| No.2 | Male | Not done | Outer and inner dynein arm dispparatus,Central disorganization | Yes | 119.7 |
| No.3 | Male | RSPH4A | Central dispparatus with disorganization | No | 155.7 |
| No.4 | Male | HYDIN | Central dispparatus with disorganization | No | 165.0 |
| No.5 | Female | HYDIN | Central disorganization | Yes | 186.3 |
nNO, nasal nitric oxide; TEM, transmission electron microscopy.
DISCUSSION
In clinical practice the five chronic respiratory diseases included in the current study share similar clinical presentation, and can co‐exist with one another. Thus distinguishing between them is difficult in some circumstances. Some previous studies have detected lower nNO values in PCD patients than in patients with other conditions such as asthma, CF, idiopathic bronchiectasis, and lone sinusitis.7, 8 If nNO values in Chinese children with PCD are significantly lower than they are in Chinese children with other chronic respiratory diseases, nNO testing may be a useful and simple tool for pediatricians in China to use to diagnose PCD.
For the measurement of nNO, a chemiluminescence analyzer used in conjunction with a velum closure technique (breath holding or oral exhalation against resistance) is regarded as the gold standard and is recommended in PCD ERS/ATS guidelines. In children aged < 6 years suspected of having PCD it is suggested that nNO measurement be conducted using tidal breathing as part of the diagnostic work‐up. In the present study, because all the patients were aged > 4 years and cooperated well, expiration against resistance was used to ensure velum closure during the testing instead of tidal breathing.
nNO testing is one of the methods recommended for screening for primary ciliary dyskinesia. Hypothesized reason for low nNO values in PCD patients include increased nNO metabolism,9 reduced production of NO by airway epithelial cells,10, 11 and abnormalities in the paranasal sinuses leading to obstruction of NO or reduced production at that site.12 It has been reported that a normal range of nNO values in children aged > 6 years is 314.3 nL/min (SD at 80.5 nL/min).13 A cut‐off of < 77 nL/min for PCD diagnosis has been recommended, and reportedly yielded a sensitivity of 98% and specificity of > 99% if CF was ruled out.3 Cut‐offs in other studies, range from 7.2 nL/min to 126 nL/min, sensitivities range from 89% to 100%, and specificities range from 75% to 100%.4 Corbelli et al14 suggested that a nNO value above a cut‐off level of 105 ppb (or 126 nL/min) excluded PCD with 100% certainty. In the current study a cut‐off of 76 nL/min yielded a sensitivity of 83.9% and specificity of 94.4% in cooperative children aged > 4 years. The relatively low sensitivity in the present study may be due to a difference in genetic distribution of PCD between Chinese children and western children. DNAI1 and DNAH5 are estimated to account for > 30% of all PCD cases in western populations15 but they accounted for only 8.3% in the present study, while patients with mutations in genes encoding the central apparatus and radial spokes (HYDIN, RSPH4A and RSPH9) accounted for 30% (see supplementary data). Further investigation of the distribution of PCD gene mutations in the Chinese population is needed. If significant differences in gene mutations exist and are responsible for more than 10% of PCD cases missed by nNO testing alone in the Chinese population, as suggested by the results of the present study, the value of nNO testing for PCD diagnosis in the Chinese population also requires further investigation.
In some previous studies nNO was low or normal in CF patients, although not as low as in PCD patients.7, 8 In the present study nNO values in CF patients were lower than they were in patients with asthma, post‐infectious BO, and non‐PCD/non‐CF bonchiectasis, but they were not significantly lower than in patients with PCD. No CF patient had an nNO value < 45 nL/min, and if this observation is reproducible in future studies it may assist in distinguishing CF from PCD. It is notable that nNO testing is not part of routine neonatal screening in China, and that while nNO testing is a useful test, the sweat test and genetic testing would still be required for definitive differentiation between CF and PCD.
The reason for a “normal” nNO value in some PCD patients is unknown. In a study conducted at a PCD center in Denmark, 6.8% of PCD patients had nNO values above a designated cut‐off, and there was a significant association between complete development of paranasal sinuses and nNO levels > 250 ppb with flow rate at 0.3 L/min or 75 nL/min.16 Pifferi et al17 reported that 24% of PCD patients had an nNO value > 250 ppb and no common ciliary ultrastructural defect was found in these patients, and none had chronic sinusitis. In a RSPH9‐associated primary ciliary dyskinesia case series18 reported in cyprus, 3/7 subjects (42.9%) had nNO levels > 77 nL/min. In the present study nNO value above a cut‐off of 76 nL/min were detected in 5/36 (13.9%) PCD patients. All 5 exhibited either central microtubular disorganization in TEM testing or genetic mutation that could cause central microtubular disorganization. In the rest of the PCD patients, 7 exhibited central microtubular disorganization in TEM testing or gene mutation that could cause central microtubular disorganization, meaning that approximately 41.7% of patients with center microtubular disorganization had nNO levels > 76 nL/min, similar to the observation in the aforementioned cyprian case series.18 Whether central apparatus disorganization is involved in the high nNO associated with PCD remains to be determined.
The present study suggests an nNO cut‐off of 76 nL/min for a putative diagnosis of PCD, which yielded a sensitivity of 86.1% and specificity of 93.9%. Notably however, because nNO values were normal in 13.9% of PCD patients, and low nNO values were detected in CF patients, nNO testing alone can not confirm or exclude a diagnosis of PCD.
The present study suggests that nNO measurement may be an informative PCD screening test in children aged > 4 years. A possible cut‐off of 76 nL/min for PCD diagnosis in Chinese children with a suspicious clinical history may be useful. But nNO testing alone can not confirm nor exclude a diagnosis of PCD. More research should be conducted to investigate the diagnostic efficacy of nNO testing with respect to PCD in the Chinese population.
CONFLICT OF INTEREST
The authors have indicated no conflicts of interest.
Supporting information
Zhang X, Wang X, Li H, Wang W, Zhao S. The value of nasal nitric oxide measurement in the diagnosis of primary ciliary dyskinesia. Pediatr Invest. 2019;3:209‐213. 10.1002/ped4.12160
REFERENCES
- 1. Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, et al. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11:473‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2017;49:1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adam JS, Stephanie DD, Deepika P, Michele M, Margaret R, Sharon DD, et al. Diagnosis of primary ciliary dyskinesia. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;197:24‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kouis P, Papatheodorou SI, Yiallouros PK. Diagnostic accuracy of nasal nitric oxide for establishing diagnosis of primary ciliary dyskinesia: a meta‐analysis. BMC Pulm Med. 2015;15:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapiro AJ, Josephson M, Rosenfeld M, Yilmaz O, Davis SD, Polineni D, et al. Accuracy of nasal nitric oxide measurement as a diagnostic test for primary ciliary dyskinesia. A systematic review and meta‐analysis. Ann Am Thorac Soc. 2017;14:1184‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Thoracic Society, European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912‐930. [DOI] [PubMed] [Google Scholar]
- 7. Wodehouse T, Kharitonov SA, Mackay IS, Barnes PJ, Wilson R, Cole PJ. Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. Eur Respir J. 2003;21:43‐47. [DOI] [PubMed] [Google Scholar]
- 8. Walker WT, Liew A, Harris A, Cole J, Lucas JS. Upper and lower airway nitric oxide levels in primary ciliary dyskinesia, cystic fibrosis and asthma. Respir Med. 2013;107:380‐386. [DOI] [PubMed] [Google Scholar]
- 9. Zihlif N, Paraskakis E, Tripoli C, Lex C, Bush A. Markers of airway inflammation in primary ciliary dyskinesia studied using exhaled breath condensate. Pediatr Pulmonol. 2006;41:509‐514. [DOI] [PubMed] [Google Scholar]
- 10. Narang I, Ersu R, Wilson NM, Bush A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax. 2002;57:586‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grasemann H, Gartig SS, Wiesemann HG, Teschler H, Konietzko N, Ratjen F. Effect of L‐arginine infusion on airway NO in cystic fibrosis and primary ciliary dyskinesia syndrome. Eur Respir J. 1999;13:114‐118. [DOI] [PubMed] [Google Scholar]
- 12. Pifferi M, Bush A, Caramella D, Di Cicco M, Zangani M, Chinellato I, et al. Agenesis of paranasal sinuses and nasal nitric oxide in primary ciliary dyskinesia. Eur Respir J. 2011;37:566‐571. [DOI] [PubMed] [Google Scholar]
- 13. Struben VM, Wieringa MH, Mantingh CJ, Bommelje C, Don M, Feenstra L, et al. Nasal NO: normal values in children age 6 through to 17 years. Eur Respir J. 2005;26:453‐457. [DOI] [PubMed] [Google Scholar]
- 14. Corbelli R, Bringolf‐Isler B, Amacher A, Sasse B, Spycher M, Hammer J. Nasal nitric oxide measurements to screen children for primary ciliary dyskinesia. Chest. 2004;126:1054‐1059. [DOI] [PubMed] [Google Scholar]
- 15. Horani A, Ferkol TW, Dutcher SK, Brody SL. Genetics and biology of primary ciliary dyskinesia. Paediatr Respir Rev. 2016;18:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marthin JK, Nielsen KG. Choice of nasal nitric oxide technique as first‐line test for primary ciliary dyskinesia. Eur Respir J. 2011;37:559‐565. [DOI] [PubMed] [Google Scholar]
- 17. Pifferi M, Bush A, Caramella D, Di Cicco M, Zangani M, Chinellato I, et al. Agenesis of paranasal sinuses and nasal nitric oxide in primary ciliary dyskinesia. Eur Respir J. 2011;37:566‐571. [DOI] [PubMed] [Google Scholar]
- 18. Yiallouros PK, Kouis P, Pirpa P, Michailidou K, Loizidou MA, Potamiti L, et al. Wide phenotypic variability in RSPH9‐associated primary ciliary dyskinesia: review of a case‐series from Cyprus. J Thorac Dis. 2019;11:2067‐2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


