Abstract
Introduction
Rapid‐onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD) syndrome is an exceptionally rare clinical entity with significant morbidity and high mortality with challenging‐to‐treat hypoventilation.
Case presentation
An 11‐year‐old morbidly obese Chinese female presented with a putative diagnosis of ROHHAD associated with a left psoas ganglioneuroma. Initial polysomnography showed severe obstructive sleep apnea and hypoventilation. She was not adherent to prescribed non‐invasive positive pressure ventilation (NIPPV). Echocardiography demonstrated evidence of pulmonary hypertension, likely secondary to chronic hypoventilation. With behavioral modification and trial of average volume‐assured pressure support (AVAPS), adherence improved with eventual improvement of her pulmonary hypertension.
Conclusion
AVAPS may improve ventilation and NIPPV adherence in central hypoventilation disorders such as ROHHAD, reducing risk of morbidity and mortality.
Keywords: Hypoventilation, Obstructive, Polysomnography, Positive pressure ventilation, Pulmonary hypertension, ROHHAD, sleep Apnea
INTRODUCTION
Rapid‐onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD) is a rare diagnostic entity. It is frequently associated with severe morbidity and mortality due to unrecognized hypoventilation and altered autonomic tone.1, 2, 3, 4, 5, 6 As the acronym implies, it is characterized by precipitous weight gain over several months in young children, hypothalamic dysfunction that may influence multiple endocrinologic pathways, sleep related hypoventilation, and features of dysautonomia such as impaired thermoregulation.1, 2, 3, 4, 5, 6 Due to its rarity, the unknown and debated etiology, and absence of a singular genetic, clinical, or other confirmatory test, ROHHAD poses significant clinical challenges to practitioners. Scrutinizing a patient's presenting symptoms to make the diagnosis of ROHHAD requires clinical equanimity and warrants close follow‐up and management of hypoventilation and autonomic dysfunction. We present a patient with a putative diagnosis of ROHHAD with complications from her sleep disordered breathing. We also review the cardinal features, further corroboratory diagnostic evaluations, and management of sleep related hypoventilation in this patient.
CASE REPORT
An 11‐year‐old morbidly obese (body mass index 51.7 kg/m2) Chinese female presented for evaluation of sleep‐disordered breathing. Her past medical history was significant for dramatic weight gain starting at 3‐years of age and persisting in spite of dietary and exercise interventions (Figure 1). The family complained that the patient would frequently sweat at night and have temperature instability. At 8‐years endocrinology evaluation uncovered evidence of central hypothyroidism [free thyroxine 0.6 ng/dL (normal 0.8–1.9), thyroid stimulating hormone (TSH) 0.58 μU/mL (normal 0.35–5.50)] and insulin resistance [two‐hour oral glucose tolerance test 153 mg/dL (normal < 140)]. This prompted levothyroxine and metformin initiation. There was no clear evidence of other hormonal or cortisol dysfunction. Brain imaging was normal. PHOX2B gene sequencing test was normal. Genetic testing for disorders of early‐onset obesity were negative, including karyotype, chromosomal microarray, and targeted testing for MC4R, POMC, LEP, LEPR, PCSK1, and Prader‐Willi syndrome.
Figure 1.
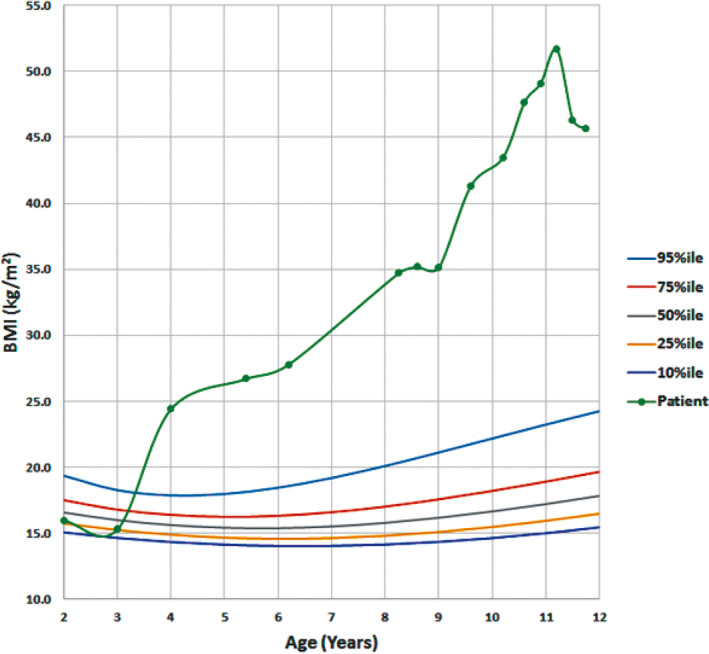
Growth charts for the patient showing extreme weight gain starting at approximately 3 years of age and continuing to present day in spite of medical interventions. Peak weight was at time of presentation to our clinic with evidence of weight loss with better adherence to medical management. BMI, body mass index
Due to complaints of snoring, a polysomnogram (PSG) was performed at 9‐years that showed severe obstructive sleep apnea (OSA) with hypoventilation [obstructive apnea hypopnea index (OAHI) of 21.4 events/h, SpO2 nadir 81%, and 41% of total sleep time with CO2 greater than 50 mmHg]. The patient was prescribed continuous positive airway pressure (CPAP) 10 cmH2O. She was noted to have scoliosis and left leg‐length discrepancy at age 10‐years. Imaging demonstrated an ill‐defined, enhancing left paraspinal mass involving the left psoas and quadratus muscles with intraspinal extension extending from the L2 vertebral level with moderate thecal sac stenosis (Figure 2). Needle biopsy found the mass to be a grade 1 ganglioneuroma. Neurosurgical intervention was deferred due to its benign pathology and surgical risk of permanent weakness. Periodic imaging was planned and subsequently showed stable findings.
Figure 2.
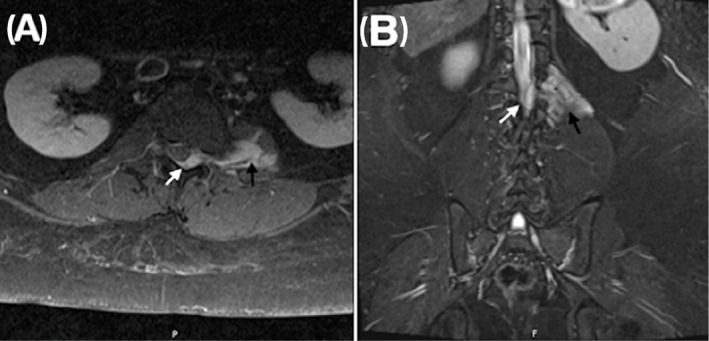
Contrast‐enhanced axial (A) and coronal (B) T1 MRI images demonstrating a left psoas‐based enhancing lesion (black arrows) invading the L1‐L2 neural foramina with displacement of the spinal cord laterally and thecal stenosis (white arrows)
At the time of presentation to our institution, she was poorly adherent to CPAP therapy. Given the concern for her underlying diagnosis, significant hypoventilation on her prior study and her poor adherence, the following studies were requested: a titration PSG, hypercapnic ventilatory response (HCVR) testing, and echocardiography. The baseline PSG recapitulated severe OSA (OAHI 26.0 events/h) with significant hypercapnia despite titration to high CPAP settings, warranting bi‐level positive airway pressure (BPAP) initiation. HCVR testing was performed due to the etiological ambiguity of the patient's hypoventilation (although a cardinal feature of ROHHAD, her hypoventilation may have only been secondary to her severe OSA). In spite of normal waking end‐tidal CO2 values, daytime HCVR testing demonstrated blunted response to increasing CO2 concentrations (Figure 3), thereby solidifying the ROHHAD diagnosis. Echocardiogram while awake showed right‐sided pressures approximately half that of systemic pressures and septal flattening, consistent with severe pulmonary hypertension. She was initiated on sildenafil.
Figure 3.
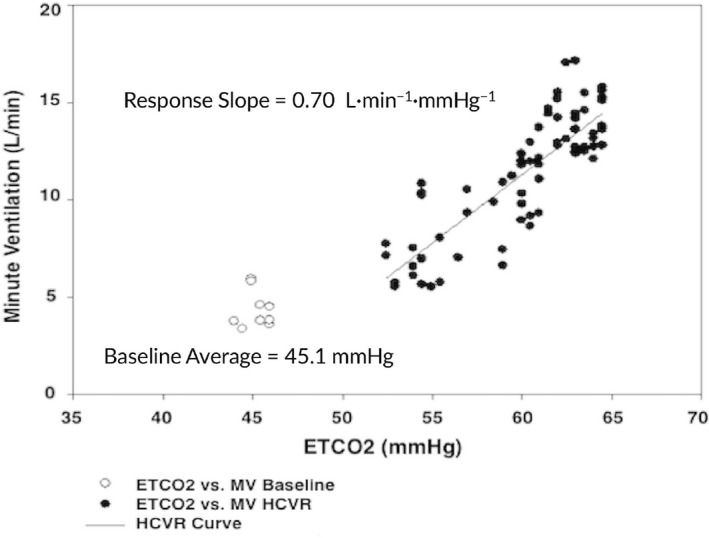
Hypercapneic ventilatory response curve (HCVR) of the patient demonstrated a decreased slope of 0.7 L·min−1·mmHg−1 PETCO 2 whereas a normal slope is 1.4 ±0.6 L·min−1·mmHg−1 PETCO 2.7 MV, minute ventilation; PETCO2, end‐tidal CO 2
Repeat titration PSGs at BPAP settings of inspiratory positive airway pressure (IPAP) 18 cmH2O, expiratory positive airway pressure (EPAP) 14 cmH2O, and back‐up rate of 12 breaths per minute showed persistently elevated OAHI, average end‐tidal and transcutaneous CO2 values of 50 mmHg. She was placed on BPAP with Average volume‐assured pressure support (AVAPS) function at settings of maximum IPAP 26 cmH2O, minimum IPAP 16 cmH2O, minimum EPAP 11 cmH2O, back‐up rate 12 breaths per minute, and exhaled tidal volume 500 mL. This resolved significant sleep‐disordered breathing (OAHI 2.9 events/h) and lowered average end‐tidal and transcutaneous CO2 to 43 mmHg. On AVAPS her 90th percentile IPAP was 16.5 cmH2O and her 90th percentile EPAP was 11.0 cmH2O. In addition to this, behavioral modifications were successfully implemented to help promote adherence to NIPPV. With excellent adherence to AVAPS and treatment with sildenafil for five months, repeat echocardiography demonstrated improvement of her pulmonary hypertension. The patient has otherwise been doing well with significant weight loss since her initial evaluation (Figure 1).
DISCUSSION
Making a diagnosis of ROHHAD is challenging due to the lack of a single confirmatory test; investigations for genetic etiologies have not been illuminating.1, 3 Diagnosis is made based on clinical presentation. ROHHAD was originally termed late onset central hypoventilation syndrome with hypothalamic dysfunction and renamed in 2007 due to the sequence of presenting symptoms. While the acronym details its cardinal features, presentation can be quite varied. Typically, children have normal development until 2 to 4 years of age. Rapid‐onset obesity is often the first recognized symptom at a median age of 3 years.1 A dysfunctional hypothalamic axis may manifest as premature puberty, polyuria, polydipsia, growth hormone deficiency, hyperprolactinemia, and/or hypothyroidism. Autonomic dysregulation has broad presentations including ophthalmologic abnormalities (with pupillary dysfunction being the most common autonomic feature), abnormal thermoregulation, excessive sweating, gastrointestinal dysmotility, and decreased heart rate variability.1, 2, 3, 4, 5 Sleep‐related hypoventilation presents at a median of 6.2 years and is typically confirmed by PSG.1, 4 Some patients with ROHHAD present initially with acute hypercapnic respiratory failure following a minor respiratory illness or general anesthesia because most patients are not able to elicit signs of hypercapnia and/or hypoxemia. Close monitoring with continuous pulse oximetry and end‐tidal or transcutaneous CO2 monitoring is therefore imperative.
Children with ROHHAD may have co‐existing OSA which further complicates their presentation. In such cases, HCVR testing may help support the diagnosis.2 Our patient demonstrated a diminished response to hypercapnia (Figure 3). This helped to solidify the ROHHAD diagnosis, particularly as obese adolescents with and without OSA have been shown to have a higher ventilatory response to hyperoxic hypercapnic gas mixtures compared to lean BMI controls.8
ROHHAD is distinct from congenital central hypoventilation syndrome (CCHS) which most commonly presents in infancy with recurrent cyanosis, apnea, and hypercapnic respiratory failure and is associated with PHOX2B gene mutations.5 Beyond the blunted response to hypercapnia and features of dysautonomia, ROHHAD and CCHS also share features of a neurocristopathy, due to frequent association with neural crest tumors. The most common tumors in ROHHAD syndrome are abdominal or mediastinal ganglioneuromas and are seen in almost 35% of patients.4, 5, 6, 7 ROHHAD patients may demonstrate significant developmental regression or challenging behaviors;1, 4, 5, 6, 7 fortunately our patient had no such neurobehavioral challenges and was cognitively normal.
When a diagnosis of ROHHAD is considered, or confirmed, vigilance and aggressive management is critical due to high risks of cardiorespiratory complications and death. The goal to optimize oxygenation and ventilation may be achieved by positive pressure ventilation via tracheostomy or NIPPV via mask interface. Younger children often require tracheostomy and mechanical ventilation but can be decannulated successfully to NIPPV. Given the risks and complications associated with long term tracheostomy as well as psychosocial burden in older children, NIPPV can be prescribed if the child can tolerate it. The most common mode of NIPPV is BPAP. As this treatment is lifelong, the respiratory support must be tailored to the needs of the growing child. Newer automatic titration modes of BPAP, including AVAPS, could be effective in treating sleep‐related hypoventilation. This mode acts by providing a consistent tidal volume and preventing nightly, even breath‐to‐breath, fluctuations in CO2.9 AVAPS has been reported to be effectively utilized in the treatment of CCHS.9 Our report is the first known description of successful treatment of sleep related hypoventilation using AVAPS in a child with ROHHAD.
Frequent titration PSGs should be performed to ensure adequate treatment of their sleep disordered breathing, aiming to keep their SpO2 > 95% and CO2 values between 35 and 45 mmHg. If hypoventilation is untreated or under‐treated, it can lead to severe sequelae including pulmonary hypertension. Utilization of phosphodiesterase‐5 inhibitors, like sildenafil and tadalafil, in conjunction with excellent adherence to AVAPS was presumed essential for normalizing our patient's right heart pressures. In the consideration of pulmonary hypertension risks and treatment, patients with ROHHAD may be offered various anorexigenic medications to aid in weight loss and caution should be used in consideration of phentermine and/or fenfluramine due to their risks of pulmonary hypertension.10
ROHHAD is a rare disorder with significant morbidity and mortality. Diagnosis may be elusive and is made based on clinical presentation. Aggressive management of sleep related hypoventilation is critical to avoid complications such as pulmonary hypertension. While some patients may require a tracheostomy for long term ventilation, newer modes of NIPPV such as AVAPS may be effective in treating their sleep disordered breathing.
CONFLICT OF INTEREST
All authors have reviewed the manuscript and have no conflicts of interest to report.
Stowe RC, Afolabi‐Brown O. Pulmonary hypertension and chronic hypoventilation in ROHHAD syndrome treated with average‐volume assured pressure support. Pediatr Invest. 2019;3:253‐256. 10.1002/ped4.12168
REFERENCES
- 1. Ize‐Ludlow D, Gray JA, Sperling MA, Berry‐Kravis EM, Milunsky JM, Farooqi IS, et al. Rapid‐onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation presenting in childhood. Pediatrics. 2007;120:e179‐e188. [DOI] [PubMed] [Google Scholar]
- 2. Carroll MS, Patwari PP, Kenny AS, Brogadir CD, Stewart TM, Weese‐Mayer DA. Rapid‐onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD): response to ventilatory challenges. Pediatr Pulmonol. 2015;50:1336‐1345. [DOI] [PubMed] [Google Scholar]
- 3. Barclay SF, Rand CM, Borch LA, Nguyen L, Gray PA, Gibson WT, et al. Rapid‐onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD): exome sequencing of trios, monozygotic twins and tumours. Orphanet J Rare Dis. 2015;10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee JM, Shin J, Kim S, Gee HY, Lee JS, Cha DH, et al. Rapid‐onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neuroendocrine tumors (ROHHADNET) syndrome: a systematic review. Biomed Res Int. 2018;1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cielo CM, Marcus CL. Central hypoventilation syndromes. Sleep Med Clin. 2014;9:105‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell WG, Blaes F. Cancer and autoimmunity: paraneoplastic neurological disorders associated with neuroblastic tumors. Semin Pediatr Neurol. 2017;24:180‐188. [DOI] [PubMed] [Google Scholar]
- 7. Jacobson LA, Rane S, McReynolds LJ, Steppan DA, Chen AR, Paz‐Priel I. Improved behavior and neuropsychological function in children with ROHHAD after high‐dose cyclophosphamide. Pediatrics. 2016;138:e20151080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan H, Pinto SJ, Huang J, McDonough JM, Ward MB, Bradford RM, et al. Ventilatory responses to hypercapnia during wakefulness and sleep in obese adolescents with and without obstructive sleep apnea syndrome. Sleep. 2012;35:1257‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vagiakis E, Koutsourelakis I, Perraki E, Roussos C, Mastora Z, Zakynthinos S, et al. Average volume‐assured pressure support in a 16‐year‐old girl with congenital central hypoventilation syndrome. J Clin Sleep Med. 2010;6:609‐612. [PMC free article] [PubMed] [Google Scholar]
- 10. Rich S, Rubin L, Walker AM, Schneeweiss S, Abenhaim L. Anorexigens and pulmonary hypertension in the United States: results from the surveillance of North American pulmonary hypertension. Chest. 2000;117:870‐874. [DOI] [PubMed] [Google Scholar]


