Abstract
The nature of muscle-bone crosstalk has been historically considered to be only mechanical, where the muscle is the load applier while bone provides the attachment sites. However, this dogma has been challenged with the emerging notion that bone and muscle act as secretory endocrine organs affect the function of each other. Biochemical crosstalk occurs through myokines such as myostatin, irisin, interleukin (IL)-6, IL-7, IL-15, insulin-like growth factor-1, fibroblast growth factor (FGF)-2, and β-aminoisobutyric acid and through bone-derived factors including FGF23, prostaglandin E2, transforming growth factor β, osteocalcin, and sclerostin. Aside from the biochemical and mechanical interaction, additional factors including aging, circadian rhythm, nervous system network, nutrition intake, and exosomes also have effects on bone-muscle crosstalk. Here, we summarize the current research progress in the area, which may be conductive to identify potential novel therapies for the osteoporosis and sarcopenia, especially when they develop in parallel.
Keywords: bone, crosstalk, muscle, myokines, osteoporosis, sarcopenia
1 |. INTRODUCTION
Skeletal muscle and bone are the two major components of the musculoskeletal system. They have a close mechanical relationship, where bone acts as a lever and muscle acts as a pulley to move the organism.1 Bone can adjust their mass and structure according to changes in mechanical load applied by the muscle. This mechanical perspective implies that a decline in muscle function causes a decrease in the loading of bone, which ultimately results in bone loss. However, the reduction in bone mass does not fully explain the occurrence of sarcopenia, nor does muscle atrophy account for the totality of osteoporosis,2 although sarcopenia and osteoporosis often develop in parallel in many patients. In the past decade, several lines of studies have shown that the interaction of bone and muscle goes beyond mechanical. For example, in a mouse open tibial fracture model, the bone repair is significantly enhanced in the fracture area surrounded with muscle flaps. Conversely, the fracture healing will be delayed if the muscle is severely damaged.3 Moreover, some defective muscle phenotypes in mice with osteoblast/osteocyte-deficient connexin43 (Cx43) were partially rescued by subcutaneous injection of the bone-specific factor undercarboxylated osteocalcin (glu-OC).4 These findings support the notion that muscle and bone communication occurs through the secretion of biochemical factors. In addition to the mechanical and biochemical coupling, however, other factors that affect crosstalk between bone and muscle, including aging, circadian rhythm, nervous system network, nutrition intake, and exosomes should not be overlooked. In this review, we summarize the roles of various factors (Figure 1) affecting muscle-bone crosstalk and highlight potential therapeutic targets based on the bone-muscle crosstalk.
FIGURE 1.
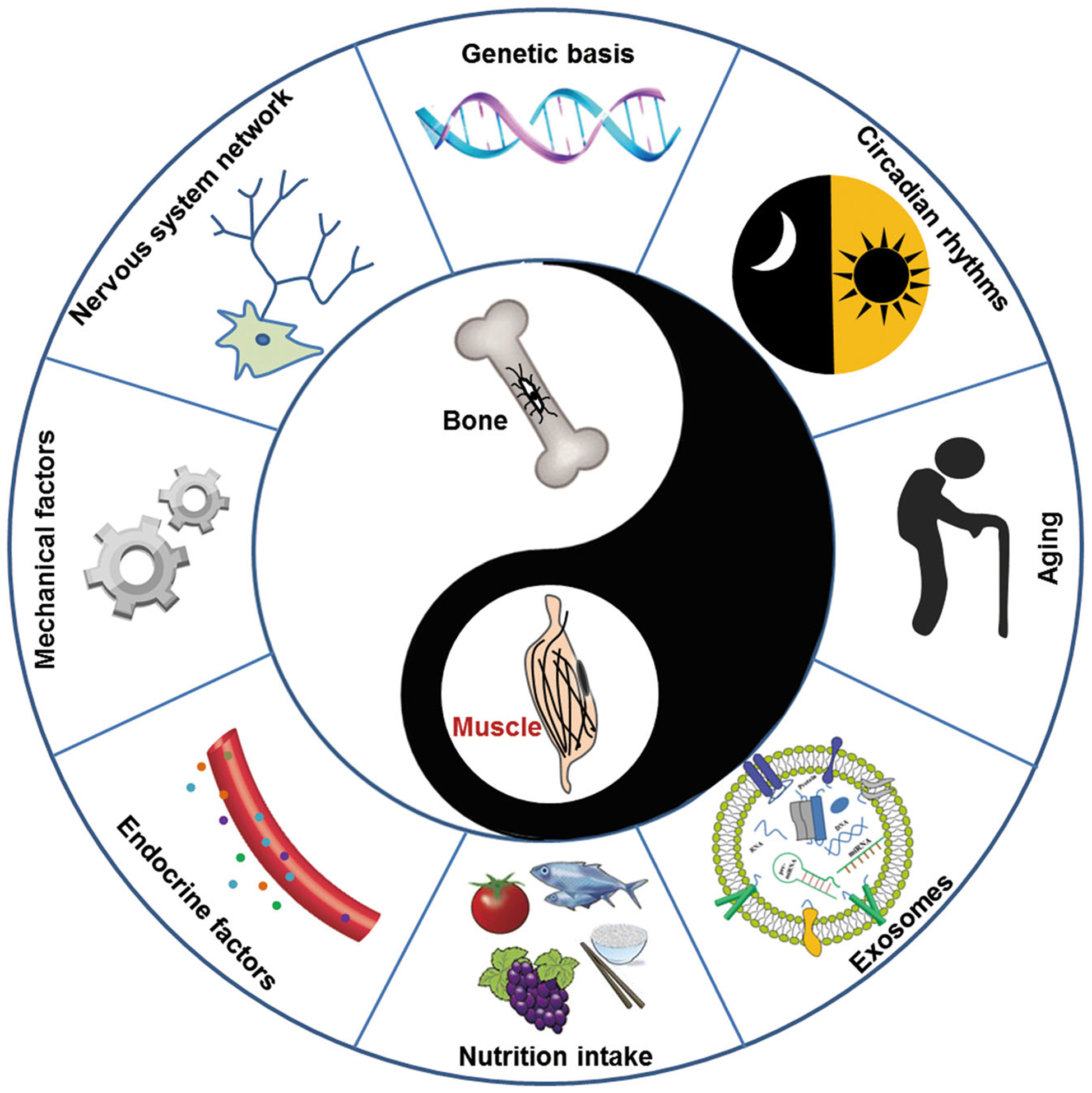
Factors affecting crosstalk between bone and muscle. The synergistic mechanical and biochemical communication between bone and muscle are important for their functional optimization. In addition, some other factors including genetic basis, aging, circadian rhythms, nervous system network, nutrition intake, and exosomes can also affect the bone-muscle crosstalk
2 |. MUSCLE-BONE BIOMECHANICAL CROSSTALK
Historically, the mechanical interaction between bone and muscle has been well described. Bone provides attachment sites for muscle, and skeletal muscle imparts a force on the bone to facilitate locomotion of the organism. The muscle attaches to the bone near the axes of motion, which will result in smaller lever arms. Therefore, large muscle forces must be generated for the motion-required torque. Notably, muscle-derived force is also the main source of mechanical loads that generate the strain in the bone. The mechanical action of muscle on bone begins during embryogenesis. For example, intrauterine muscle contraction determines the specific round outline of each bone and joint development. By contrast, the circumferential shape and mechanical integrity of bone are lost without mechanical loading.5 Another piece of evidence that muscle-derived force affects bone is seen in spaceflight, where astronauts lose both bone and muscle due to lack of gravity. Importantly, when they return to the ground, their muscle mass recovery happens much earlier than that of bone, suggesting that the recovery of bone loss may require support from muscle contraction. Except for the load transfer, muscle and bone also exhibit codependent hypertrophic and hypotrophic adaptations.1 To help understand the mechanical interaction behind these adaptations, some animal models have been developed. In these animal models, the changes in bone are assessed after disrupting the muscle-derived forces. A spinal cord injury model using surgical intervention to induce muscle paralysis is used to simulate the musculoskeletal consequences of neural damage. Although rapid bone loss caused by muscle paralysis is observed, it is hard to isolate the mechanical link between the two tissues since bone also affected by its own neural changes. Other alternative approaches, such as tail suspension and intramuscular injection of botulinum toxin (Botox), appear to hold the potential to isolate the effects of mechanical stimuli on the two tissues despite each of them has some disadvantages. For example, the animal model using rodent tail suspension to mimic space-induced weightlessness have been developed to explore the negative effects of the unloading of bone and muscle that occurs during spaceflight.6 However, muscles can still contract and changes in other systems (such as renal and cardiovascular) can also be induced with tail suspension. Botox blocks the neuromuscular signaling through inhibiting the release of acetylcholine, thereby impairs muscle function.7 At the same time, the neuromuscular proprioceptive signals are also impaired. Further, tail suspension has been combined with Botox injection in the study by Warden et al8 to explore the direct mechanical relationship between the two tissues. In their study, they tried to reduce the resistance required for muscle contraction and inhibit the ability of muscle activation, which would result in the loads near zero level. Indeed, the combined treatment has greater detrimental effects on bone than that presented by tail suspension and Botox alone, suggesting muscle exerts a direct impact on bone. From the aspects of pathology, however, sarcopenia alone is hard to fully explain the totality of osteoporosis and vice versa despite they often develop in parallel. Several lines of evidence have demonstrated that mechanical loading promotes the production of various factors linking muscle to bone, including fibroblast growth factor-2 (FGF-2), IL-6, IL-15, insulin-like growth factor-1 (IGF-I), and irisin,9,10 which will be described in details below. Thus, it is assumed that biochemical communication may exist between muscle and bone in addition to mechanical interaction.
3 |. MUSCLE-BONE BIOCHEMICAL CROSSTALK AND SOLUTE FACTORS
It has been proposed that muscle and bone biochemical crosstalk occurs via solute factors (Tables 1 and 2), thereby affecting the function of each other. In addition, the pleiotropic genes of bone and muscle imply that overlapping genetic determinants and common signaling pathways play an important role in the muscle-bone crosstalk.
TABLE 1.
Muscle-to-bone signaling
| Effect on bone | ||
|---|---|---|
| Myokines | Bone formation (BF) | Bone resorption (BR) |
| Myostatin | Inhibits bone formation11 | Promotes osteoclastogenesis12 |
| IL-6 | Increases osteoblast differentiation13 | Increases osteoclastogenesis14 |
| IL-7 | Inhibits bone formation15 | Upregulates RANKL expression15 |
| IL-15 | Not determined | Promotes preosteoclast differentiation and decreases bone resorption16,17 |
| IGF-1 | Stimulates bone formation18 | Not determined |
| FGF-2 | Stimulates bone formation19 | Not determined |
| Irisin | Promotes osteoblast differentiation20,21 | Increase bone resorption22 |
Abbreviations: IL-6, interleukin-6; IGF-1, insulin-like growth factor-1; FGF-2, fibroblast growth factor-2; RANKL, receptor activator of nuclear factor-κB ligand.
TABLE 2.
Bone-to-muscle signaling
| Bone-derived factors | Effect on muscle | References |
|---|---|---|
| FGF23 | Altered myoblasts differentiation | Kido et al23 |
| Osteocalcin | Increased insulin sensibility, energy metabolism, and protein synthesis | Lee et al,24 Mera et al25,26 |
| PGE2 | Accelerated proliferation and differentiation of myoblasts | Mo et al27,28 |
| Wnt-3a | Enhanced C2C12 cell differentiation | Huang et al29 |
| Sclerostin | Altered myoblasts differentiation | Huang et al29 |
| TGF-β | Increased oxidative stress and decreased muscle function | Regan, et al,30 Waning et al31 |
Abbreviations: FGF23, fibroblast growth factor-23; PGE2, prostaglandin E2; TGF-β, tumor growth factor-β.
3.1 |. Myokines and their impacts on bone metabolism
It is well accepted that skeletal muscle is able to secret factors to regulate bone metabolism. Nowadays, these muscle secreted factors are called “myokines”, a term first coined by Pedersen and colleagues in 2010.32 The currently well-defined list of myokines includes myostatin, interleukin (IL)-6, IL-7, IL-15, IGF-1, FGF-2, irisin, and BAIBA (Table 1).
3.1.1 |. Myostatin
Myostatin, the first identified myokine, belongs to tumor growth factor-β (TGF-β) superfamily.33 It is also termed growth and differentiation factor (GDF)-8, which acts as the most potent negative regulator of muscle cell proliferation and differentiation.33 Myostatin null mice display massive muscle hypertrophy and surprisingly a significant increase in bone mineral density (BMD), which provides direct evidence of muscle to bone biochemical communication.19,34 ActRIIB, a soluble myostatin decoy receptor, has been found on the cell membrane of osteoblasts, and its inhibition increases bone formation in mice,11 which suggests that myostatin exerts a negative effect on osteoblasts differentiation. In contrast, myostatin acts as a positive regulator of osteoclast formation induced by receptor activator of-nuclear factor-κB ligand (RANKL) through activation of the nuclear factor of activated T cells (NFATc1) signaling pathway.12 Therefore, myostatin regulates negatively bone mass by reducing bone formation and increasing bone resorption.
3.1.2 |. Interleukins
IL-6, a pro-inflammatory cytokine, is expressed in many cells, such as fibroblasts, macrophages, and vascular endothelial cells.35 It was originally detected in the circulation after physical activity.36 Except for the effects on glucose uptake and fatty acid oxidation, IL-6 can also stimulate bone resorption after binding to its soluble receptor, gp130. Mechanically loaded myotubes promote osteoclasts formation through the secretion of IL-6 in vitro.14 In addition, IL-6 increases early osteoblastic differentiation and its deletion leads to a decrease in bone mass in mice,13 which implies that IL-6 may negatively regulate bone formation. Thus, IL-6 may have stimulatory effects both on bone formation and resorption. IL-7, abundantly secreted by muscle, is widely considered to be an osteoclastogenic cytokine. Indeed, ovariectomy (OVX)-induced overexpression of IL-7 not only increases bone resorption but also reduces bone formation,15 suggesting IL-7 may play an important role in regulating bone metabolism. IL-15, another anabolic factor in skeletal muscle, also directly acts as bone remolding through stimulating preosteoclast differentiation.16 Elevation of IL-15 levels in circulation following resistance exercise leads to raised bone mass and reduced fat mass,37 which indicates that IL-15 may affect both bone metabolism and fat mass.
3.1.3 |. Growth factors
IGF-1 is an important growth factor for skeletal development and muscle growth. Hamrick et al18 found that IGF-1 is localized on the skeletal muscle close to the periosteum, and its receptor IGF-1R is richly expressed in the periosteum. Paracrine crosstalk in the surface of muscle and periosteum may be another direct support for the muscle-bone biochemical communication. In the period of bone repair, muscle-derived IGF-1 may signal to the osteoprogenitor cells in the periosteum expressing IGF-1R to increase bone formation. In addition, IGF binding proteins (IGFBRs) has been shown to regulate the action of IGF-1. Most of IGFBRs, such as IGFBR2 and IGFBR5, are expressed in the skeletal muscle. However, there are some controversies about the effects of IGFBR1 and IGFBR5 on bone metabolism. For example, IGFBR2 has a negative effect on the IGF-1-mediated bone anabolic action while the circulating IGFR-1 level in human is one of the potent negative predictors of muscle mass, which is related positively with trabecular BMD.38 IGFBR5 are related to the bone anabolic action mediated by IGF-1 although the mechanism is still unclear.19
Like IGF-1, FGF-2 is actively secreted in the muscle-bone interface and has a central role in the regulation of cell proliferation. Notably, due to its specific sequence of structure, FGF-2 is less likely to export out of cells via the classic exocytotic pathway. Thus, mechanically-induced plasma membrane disruption is one possible mechanism by which FGF-2 is released from myocytes both in vivo39 and in vitro.40 FGF-2 is involved with fracture repair, bone formation, and cartilage regeneration after injury or strong exercise.19 Additionally, FGF-2 also has an osteogenic effect in the estrogen-deficient mouse model.
3.1.4 |. Irisin
Irisin, a cleaved product of fibronectin type III domain containing 5 (FNDC5), is actively produced from the skeletal muscle after exercise. It can stimulate the “browning response” in white adipose tissues aside from its effect on muscle.41 Some studies have shown that high dose of irisin up-regulated thermogenesis gene expression, such as uncoupling protein-1. However, a low dose of irisin raised cortical bone mineral density.42 On one hand, irisin upregulates the expression of osteogenic genes such as osteopontin (OPN) and sclerostin (sost) in bone; on the other hand, irisin acts on osteoblasts by increasing the differentiation and activities of bone-forming cells in vitro.43 In human, there exists a negative correlation between irisin level and bone fracture risk for postmenopausal women. Recently, the FNDC5 knock-out mice have been shown to exhibit significant resistance to OVX-induced bone loss compared to the sham group.22 These findings indicate that irisin may exert anabolic effects on bone as well as an interaction between irisin and estrogen signaling. However, the exact mechanism for the effects of irisin on bone metabolism remains to be clarified.
3.1.5 |. BAIBA
BAIBA, a non-protein amino acid, was initially discovered in human urine.44 The secretion of BAIBA is increased with muscle contraction and it has two forms of enantiomers: D-BAIBA and L-BAIBA. Recently, Kitase et al45 showed that L-BAIBA but not its D-isoform can preserve osteocytes viability under oxidative stress through binding to its receptor, Mas-related G-protein receptor Type D (MRGPRD). Interestingly, MRGPRD is highly expressed in young osteocytes while significantly decreased with age. As such, this protective capacity seems to be impaired due to the lower level of MRGPRD expression in old osteocytes, which directly results in bone loss with age. To date, it is unclear whether this regulation is only specific to L-BAIBA. Similarly, some unidentified muscle-derived factors also have osteocyte-protective effects. For instance, a study by Jähn et al46 showed that the unknown factors from C2C12 myotubes that could protect MLO-Y4 osteocytes against cell death induced by dexamethasone. However, the role of BAIBA in bone metabolism remains poorly understood.
3.2 |. Bone-derived factors and their actions on muscle
Emerging data support the relatively new concept that bone functions as an endocrine organ. The list of bone-derived factors continues to grow, and include osteocalcin, FGF23, sclerostin, prostaglandin E2 (PGE2), dentin matrix protein-1 (Dmp1), RANKL, endopeptidases on the X chromosome (PHEX), Wnt-3a, and TGF-β. Herein, we will describe the effects of these factors on muscle (Table 2).
Osteocalcin (OC) is an osteoblast-specific non-collagenous protein.47,48 In general, there are two forms of OC found in the circulation: γ-carboxylated osteocalcin (gla-OC) and uncarboxylated (glu-OC). However, only the latter acts as an endocrine hormone.49 Glu-OC plays an important role in the regulation of male fertility, insulin sensibility,24 energy metabolism,50 and recently it has been shown to signal to the muscle via the Gprc6a receptor. Studies from the Karsenty group showed a significant decline in muscle mass in the Gprc6a-deficient mice. In contrast, mice with deletion of tyrosine phosphatase (Esp−/−), which inhibits osteocalcin function, have been shown to increase muscle mass. In addition, osteocalcin supplementation raised the exercise capacity in young mice and reversed the age-related decline in aerobic endurance. Osteocalcin signaling in myofibers is also the main reason for the aerobic exercise-induced release of myokine IL-6.25,26,51 Interestingly, chronic delivery of osteocalcin not only increases muscle function but also improves muscle mass in older mice.25,26 Another evidence for the effects of osteocalcin on muscle is that muscle phenotypes (i.e., muscle mass, grip strength, fiber numbers, myosin heavy chain iso-forms, and maximum contraction force) were altered in mice with osteoblast/osteocyte specific deletion of Cx43 gene (Gja1).4 Importantly, these cKO mice were shown to a significant reduction in circulating OC but no change for the insulin and glucose levels. Treatment of these mice with synthetic osteocalcin rescued some of the abnormalities in the muscle, such as the reduced muscle cross-sectional area and grip strength. It at least implies that the bone-derived osteocalcin is necessary to maintain muscle mass and function. Evidence from the results in vitro showed that ucOC promotes myoblast proliferation through PI3K/Akt/p38 MAPK pathways and myogenic differentiation via Gprc6a-Erk1/2 signaling.52 Taken together, these findings strongly suggest that osteocalcin, especially for its active form (ucOC), plays an important role in bone-to-muscle biochemical signaling.
FGF-23, secreted both by osteoblast and osteocytes, is the first hormone found in bone tissue and has important roles in regulating the systemic phosphate and Vitamin D levels.53 Some human phosphate metabolism diseases can cause an altered level of FGF23. For instance, X-linked hypophosphatemia is caused by PHEX gene mutation,54 however, the mutation in Dmp1 has been shown to be responsible for autosomal recessive hypophosphatemic rickets. Unexpectedly, in addition to phosphate metabolism, FGF23-deficient mice are characterized with hypoglycemia and increased insulin sensitivity.55 Besides, in the mouse model of hypophosphatemic rickets, DMP1−/− mice, the force production of extensor digitorum longus and soleus muscles was significantly reduced but that of in cardiac muscle was not altered.30,56 Faul et al57 reported that FGF23 appears to induce left ventricular hypertrophy through the calcineurin/NFAT signaling pathway, which suggests that FGF23 can target the cardiomyocyte in a paracrine manner. These studies indicate that bone-derived FGF23 could affect the muscle function apart from phosphate metabolism.
Transforming growth factor (TGF-β) is mainly derived from the bone and stored within the mineralized bone matrix. TGF-β superfamily consists of many members including activin, myostatin, GDF-8, GDF-11, and TGF-β. Of note, both activin and TGF-β can signal to muscle but their mechanism differs. Activin causes strongly muscle wasting with a reduction in muscle mass and force production using an adenovirus vector in mice. However, mice treated with TGF-β showed a reduction in raw and specific muscle force production but the muscle mass remained unchanged.58 Waning et al31 demonstrated that bone-derived TGF-β results in muscle weakness in the setting of osteolytic cancer through an increase in muscle oxidative stress and calcium mishandling.
Sclerostin, mainly secreted by mature osteocytes, serves as a suppressor of bone formation via the canonical Wnt/β-catenin pathway. Wnt-3a was found to promote differentiation of C2C12 myoblast through up-regulating the expression of MyoD and Myogenin. Further, Huang et al29 showed that sclerostin inhibits the effect of Wnt-3a on the C2C12 myoblast differentiation. Thus, these findings imply that sclerostin may also affect myoblast proliferation and differentiation. Sclerostin is coded by SOST gene and its mutations will increase bone mass and thus cause sclerosteosis with low sclerostin levels,59 suggesting that sclerostin antibody holds the potential for osteoporosis treatment. Osteocytes are mechanosensitive cells that coordinate the adaptive response of the bone to mechanical stimulis.60 It has been proposed that the secretion of sclerostin increases in response to bedrest or unloading. Instead, level of sclerostin is reduced after muscle or bone loading.61,62 Interestingly, the sclerostin–deficient mice has larger trabecular bone volume and lower muscle mass than WT mice,63 suggesting sclerostin deficiency has an adverse effect on muscle. However, the role of sclerostin in the bone-muscle crosstalk remains to be further studies.
PGE2, a signaling molecule synthesized by arachidonic acid through the cyclooxygenase, is related to multiple physiological processes such as inflammation, muscle regeneration, and cancer development.28 Large amounts of PGE2 in osteocytes can be released from osteocytes through Cx43 hemichannels in response to fluid shear stress. As such, PGE2 can directly activate the β-catenin pathway via increasing PI3K level in osteocytes.64 In addition, the Runx2 and Osterix mRNA expression, as well as mineralization, are increased when PI3K is inhibited in skeletal muscle even though alkaline phosphatase (ALP) activity is reduced.65 PGE2 also can promote osteocyte survival66 and bone formation.67 Besides, Brotto’s lab previously demonstrated that PGE2 accelerates C2C12 myoblast proliferation27 and differentiation,28 suggesting the PGE2 signaling from bone may be important for muscle myogenesis. However, more in vivo studies are needed to explain the profound role of PGE2 in bone-muscle communication.
3.3 |. Pleiotropic genes in the bone-muscle crosstalk
Heritability studies have demonstrated that genetic determinants are responsible for 60%−70% of bone and muscle phenotypes.68 Shared genes factors that affect muscle and bone also called “pleiotropic” genes. Bivariate genome-wide association studies (GWAS) has been used to identify pleiotropic candidate genes related to traits in both bone and muscle.69 These findings have produced a series of potential pleiotropic genes for bone and muscle although further verified experiments are needed. Some well-known genes, such as myostatin, proliferator-activated receptor gamma coactivator 1-α (PGC-1α), myocyte enhancer factor-2 C (Mef-2C) and methyltransferase-like 21C (METTL21C), are included in GWAS and they are associated to muscle loss and osteoporosis concurrently.70 PGC-1α is induced by physical activity and plays an important role in regulating the mitochondrial biogenesis and oxidative metabolism in the muscle.71The Mef-2C gene is involved in the development of cardiac and skeletal muscle and interacts with myogenic regulatory factors (MRFs), such as MyoD and Myf5. Mice lacking Mef-2C in osteocytes showed an increase in bone mineral density. These findings seem to indicate the important role of the Mef-2C in muscle and bone. METTL21C belongs to the methyltransferase superfamily and it is abundantly expressed in muscle. In vitro studies showed that METTL21C has important roles both in osteocyte survival and myoblastic differentiation, which may be linked with the nuclear factor-κB signaling pathway.72 Therefore, identification of significant pleiotropic genes in both bone and muscle will deepen the understanding of the mechanisms of bone-muscle crosstalk.
4 |. OTHER FACTORS AFFECTING BONE-MUSCLE CROSSTALK
4.1 |. Aging
Aging profoundly affects the mass of both bone and skeletal muscle. The term sarcopenia was originally coined by Rosenberg73 to describe the progressive loss of muscle mass and function with aging. Concomitant with the lower muscle mass, aging also results in a loss of bone density and strength, which refers to osteoporosis. It is estimated that by 2050, the population over the age of 60 will double in the world.74 As the size of the older population increases, so does the occurrence of age-related osteoporosis and sarcopenia, which often develop equally in the same patients.75 Importantly, both sarcopenia and sarcopenia in aging adults are linked to the high risk of bone fracture. According to a study on Europeans living in the middle-aged and elderly community, men with sarcopenia had lower BMD and they were more likely to suffer from osteoporosis than those without sarcopenia.76 Another study, including 1308 men and 1171 women over the age of 65, showed a significant association between lower muscle mass and a higher propensity for osteoporosis.77 The molecular mechanisms that cause both muscle and bone loss are still unclear, but the clinic data suggest that therapeutic approaches targeting either sarcopenia or osteoporosis alone may not be sufficient to effectively prevent fracture. Novel approaches for treatments are advised to target both tissues simultaneously in the future.
4.2 |. Circadian rhythm
Circadian rhythm exists in every cell in the body, bone and muscle are included. The mechanism of circadian rhythms in mammals is called the molecular clock. It mainly consists of a series of interconnected transcriptional-translational feedback loops.78 To date, only one study reported that circadian rhythms in muscle have an essential role in maintaining its own cellular physiology and bone health.79 Bmal1 is a core molecular clock protein and its deletion in skeletal muscle altered significantly expression of several myokines, including myostatin, IGFBP5, TGFβ1, FNDC5/Irisin, and GDF11.80 However, it makes the mechanism for bone-muscle crosstalk difficult at this level due to very limited investigation of the role of the molecular clock in bone tissue. Recently, a study showed that the deletion of Mbtps1 protease in osteocytes leads to an increase in mass and contractile force of soleus muscles.81 Further, many myogenic factors (ie Myh3 and Myh8) in these larger and functionally improved muscles are regulated DEC1 and DEC2, two circadian core transcriptional repressors. These exciting findings suggest that the molecular clock may play an important role for in bone-muscle crosstalk.
4.3 |. Nervous system network
The nervous system network has been shown to affect bone-muscle crosstalk. Studies support the notion that the sympathetic nervous system may involve in the regulation of bone by leptin signaling. Deletion of β2-adrenergic receptors (β2AR) in osteoblasts/osteoclasts results in increased bone volume in mice.82 Consistent with this, the neuropeptide Y receptors-deficient mice show an increase in bone anabolism.83 Other important pathways regulating bone include cannabinoid system, neuromedin U and melanocortins.84 Similarly, β2AR signaling also affects skeletal muscle growth in healthy individuals. β2AR agonists have been shown to improve muscle function and reduce muscle atrophy in mice.85 Thus, β2AR plays an important role in the regulation of muscle and bone. Emerging studies have focused on biochemical communication of bone and muscle, which may be also coregulated by the nervous system network.
4.4 |. Nutrition intake
Nutrition intake is often considered as an important factor affecting both bone and muscle since nutritional disorders are prevalent among elderly people. In general, long-term unbalance between the rate of protein synthesis and protein degradation sustains the age-related loss of muscle and bone. Lower protein intake leads to greater bone loss in the elderly, which suggests the protein intake is necessary to maintain bone mass. Meanwhile, decreased protein synthesis is related to muscle weakness in patients with inadequate protein intake. Several lines of studies have indicated that nutritional deficiencies and anorexia of aging result in pathogenesis of osteoporosis86 and sarcopenia. The primary nutrients that are linked to sarcopenia and osteoporosis in the old adults include vitamin D and antioxidants.87 A high percentage of old population is vitamin D deficient due to low dietary intake, reduced sunshine exposure, and impaired hydroxylation in the kidneys and liver.88 Vitamin D deficiency leads to bone loss although the exact mechanism currently less well understood. A meta-analysis confirmed that vitamin D dietary supplementation (700–1000IU/day) may raise muscle performance and reduce the bone fracture by 19% in community-living elderly with low vitamin D level.89 It is well-established that antioxidant intake is corrected with sarcopenia, bone remolding, physical function. For example, supplementation with antioxidant lycopene appears to reduce bone resorption and oxidative stress.90 However, foods may be a favored source of antioxidant since their content of multiple antioxidant substances, vitamins and fibers.91 So, the old persons should be advised to take antioxidant supplementation to prevent the sarcopenia and osteoporosis. Thus, insufficient nutrition may affect the interaction between muscle and bone.
4.5 |. Exosomes
Exosomes, a subset of extracellular vesicles with a size range of 30–100 nm, are enriched in proteins, mRNA, and microRNA (miRNA). Exosomes play an important role in cell-to-cell communication.92 miRNA, a single-stranded non-coding RNA, binds to the target mRNA and mainly function as a gene repressor. It has been proposed that miRNA may regulate the function of osteoblasts and osteoclasts.93 During osteoblast differentiation, the miRNA-218 promotes the differentiation of bone marrow cells through the down-regulation of Wnt signaling inhibitors sclerostin, dickkopf2, and the secretion of frizzled-related protein 2.94 Additionally, osteoclast-derived miR-214–3p can be transferred to osteoblasts to inhibit bone formation.95 Recent findings have shown that myostatin directly affects osteocyte function and inhibits osteoblastic differentiation through the suppression of osteocyte-derived miR-218.96 These studies suggest that exosomes may play an important role in the muscle-bone communication.
5 |. TARGETING THE MUSCLE-BONE CROSSTALK AS A POTENTIAL THERAPEUTIC STRATEGY FOR SARCO-OSTEOPOROSIS
Age-related and pathological sarco-osteoporosis (combined sarcopenia and osteoporosis) is of a great threat to human health, which results in raised fracture risk in patients. So far, the therapeutic approaches to prevent such fractures only have focused on the bone, however, our increasing insight into muscle-bone crosstalk may represent another new treatment paradigm of these conditions. It implies the novel therapeutic approaches should target both bone and muscle. To our knowledge, the activin signaling pathway is considered to be a feasible therapeutic target for sarco-osteoporosis since it is a potent negative regulator of bone and muscle mass. Administration of myostatin inhibitors raises both muscle and bone mass in young adult mice.97 Mice treated with ActRIIB-Fc, a soluble myostatin receptor, also promotes bone formation except for the expected positive effects on muscle, suggesting it has a direct action on osteoblast activity. In a double-blind trial, a single injection of ActRIIB decoy receptor resulted in a significant increase in lean mass of 48 postmenopausal women after one month. At the same time, single-dose treatment of ActRIIB also raised the level of serum biomarkers for bone turnover, such as ALP and C-terminal telopeptides (CTX).98 The dual beneficial effects of these anabolic agents on muscle and bone represent a potential approach to prevent age-related sarcopenia and osteoporosis. However, more clinical studies of various inhibitors in activin pathway are needed to establish their long-term safety and efficacy.
Overall, the development of drugs targeting both muscle and bone is on the way. More clinical trials for the mentioned molecules are currently conducted. Among these molecules, irisin and osteocalcin may be promising therapeutic targets for sarcopenia and osteoporosis in future trials. May be the next 10 years will tell.
6 |. CONCLUSION
Bone and muscle are intimately coupled in both form and function from embryogenesis, growth, and development, and into aging. That is to say that bone and muscle communication is necessarily bi-directional. Numerous factors impact the relationship, including genetic determinants, mechanical factors, endocrine/paracrine factors, and other factors (eg, aging, circadian rhythm, nervous system network, nutrition intake, and exosomes). Therapeutic approaches to treat sarcopenia and osteoporosis based on the “muscle-bone crosstalk” concept are an area of extremely active research, with currently many focused on targeting the myostatin/activin signaling pathway. In this review, we summarize the roles of various factors affecting muscle-bone crosstalk and potential therapeutic approaches. Understanding the muscle-crosstalk is conductive to identify novel targets to treat age-related bone and muscle diseases, especially for sarcopenia and osteoporosis.
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China (81772409).
Funding information
National Natural Science Foundation of China, Grant/Award Number: 81772409
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Avin KG, Bloomfield SA, Gross TS, Warden SJ. Biomechanical aspects of the muscle-bone interaction. Curr Osteoporos Rep. 2015;13:1–8. 10.1007/s11914-014-0244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brotto M, Bonewald L. Bone and muscle: Interactions beyond mechanical. Bone. 2015;80:109–114. 10.1016/j.bone.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utvåg SE, Grundnes O, Rindal D, Reikerås O. Influence of extensive muscle injury on fracture healing in rat tibia. J Orthop Trauma. 2003;17(6):430–435. 10.1097/00005131-200307000-00007 [DOI] [PubMed] [Google Scholar]
- 4.Shen H, Grimston S, Civitelli R, Thomopoulos S. Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice. J Bone Miner Res. 2015;30(4):596–605. 10.1002/jbmr.2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amnon S, Tomer S, Chagai R, Ron S, Elazar Z. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development. 2011;138(15):3247–3259. 10.1242/dev.063768 [DOI] [PubMed] [Google Scholar]
- 6.Globus RK, Morey-Holton E. Hindlimb unloading: rodent analog for microgravity. J Appl Physiol. 2016;120(10):1196–1206. 10.1152/japplphysiol.00997.2015 [DOI] [PubMed] [Google Scholar]
- 7.Kao I, Drachman DB, Price DL. Botulinum toxin: mechanism of presynaptic blockade. Science. 1976;193(4259):1256–1258. 10.1126/science.785600 [DOI] [PubMed] [Google Scholar]
- 8.Warden SJ, Galley MR, Richard JS, et al. Reduced gravitational loading does not account for the skeletal effect of botulinum toxin-induced muscle inhibition suggesting a direct effect of muscle on bone. Bone. 2013;54(1):98–105. 10.1016/j.bone.2013.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawao N, Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem. 2015;116(5):687–695. 10.1002/jcb.25040 [DOI] [PubMed] [Google Scholar]
- 10.Kawao N, Moritake A, Tatsumi K, Kaji H. Roles of irisin in the linkage from muscle to bone during mechanical unloading in mice. Calcif Tissue Int. 2018;103:24–34. 10.1007/s00223-018-0387-3 [DOI] [PubMed] [Google Scholar]
- 11.Jung-Jun P, Berggren JR, Hulver MW, Houmard JA, Hoffman EP. GRB14, GPD1, and GDF8 as potential network collaborators in weight loss-induced improvements in insulin action in human skeletal muscle. Physiol Genomics. 2006;27(2):114–121. 10.1152/physiolgenomics.00045.2006 [DOI] [PubMed] [Google Scholar]
- 12.Berno D, Michelle F, Daniela B, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. 2015; 21(9):1085–1090. 10.1038/nm.39171085-1090 [DOI] [PubMed] [Google Scholar]
- 13.Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18:992–994. 10.1096/fj.03-1259fje [DOI] [PubMed] [Google Scholar]
- 14.Juffer P, Jaspers RT, Klein-Nulend J, Bakker AD. Mechanically loaded myotubes affect osteoclast formation. Calcif Tissue Int. 2014;94(3):319–326. 10.1007/s00223-013-9813-8 [DOI] [PubMed] [Google Scholar]
- 15.Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110(11):1643–1650. 10.1172/JCI15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djaafar S, Pierroz DD, Chicheportiche R, Zheng XX, Ferrari SL, Ferrari-Lacraz S. Inhibition of T cell-dependent and RANKL-dependent osteoclastogenic processes associated with high levels of bone mass in interleukin-15 receptor-deficient mice. Arthritis Rheumatol. 2010;62(11):3300–3310. 10.1002/art.27645 [DOI] [PubMed] [Google Scholar]
- 17.Feng S, Madsen SH, Viller NN, et al. Interleukin-15-activated natural killer cells kill autologous osteoclasts via LFA-1, DNAM-1 and TRAIL, and inhibit osteoclast-mediated bone erosion in vitro. Immunology. 2015;145(3):367–379. 10.1111/imm.12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;2010(10):64–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. Bonekey Rep. 2012;1:60 10.1038/bonekey.2012.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colaianni G, Grano M. Role of Irisin on the bone-muscle functional unit. Bonekey Rep. 2015;4:765 10.1038/bonekey.2015.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colaianni G, Mongelli T, Cuscito C, et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep. 2017;7(1):2811 10.1038/s41598-017-02557-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Wrann CD, Jedrychowski M, et al. Irisin Mediates Effects on Bone and Fat via alphaV Integrin Receptors. Cell. 2018;175(7):1756–1768. 10.1016/j.cell.2018.10.025e1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kido S, Hashimoto Y, Segawa H, Tatsumi S, Miyamoto K-I. Muscle atrophy in patients with ckd results from FGF23/klotho-mediated suppression of insulin/IGF-I signaling. Kidney Res Clin Pract. 2012;31(2):A44 10.1016/j.krcp.2012.04.435 [DOI] [Google Scholar]
- 24.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. 10.1016/j.cell.2007.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mera P, Laue K, Ferron M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016a;23(6):1078–1092. 10.1016/j.cmet.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. 2016b;5(10):1042–1047. 10.1016/j.molmet.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mo C, Zhao R, Vallejo J, et al. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle. 2015;14(10):1507–1516. 10.1080/15384101.2015.1026520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2 from clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012;6(3):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Romero-Suarez S, Lara N, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-Catenin pathway. JBMR Plus. 2017;1:86–100. 10.1002/jbm4.10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regan JN, Mikesell C, Reiken S, et al. Osteolytic breast cancer causes skeletal muscle weakness in an immunocompetent syngeneic mouse model. Front Endocrinol. 2017;8:358 10.3389/fendo.2017.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waning DL, Mohammad KS, Reiken S, et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21(11):1262–1271. 10.1038/nm.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(Pt2):337–346. 10.1242/jeb.048074 [DOI] [PubMed] [Google Scholar]
- 33.Allen DL, Cleary AS, Speaker KJ, et al. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol-Endoc M. 2008;294(5):E918–E927. 10.1152/ajpendo.00798.2007 [DOI] [PubMed] [Google Scholar]
- 34.Bonewald L Use it or lose it to age: A review of bone and muscle communication. Bone. 2018;120:212–218. 10.1016/j.bone.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005; 54(Suppl 2):S114–S124. 10.2337/diabetes.54.suppl_2.S114(2) [DOI] [PubMed] [Google Scholar]
- 36.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008; 88(4):1379–1406. 10.1152/physrev.90100.2007 [DOI] [PubMed] [Google Scholar]
- 37.Quinn LS, Anderson BG, Lena SB, Stroud AM, Argilés JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol-Endoc M. 2009;296(1):E191–E202. 10.1152/ajpendo.90506.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebrasseur NK, Achenbach SJ, Amin S, Khosla S. Skeletal muscle mass is associated with bone geometry and micro-structure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res. 2012;2012(27):2159–2169. 10.1002/jbmr.1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke MS, Khakee R, Mcneil PL. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci. 1993;1993(106):121–133. 10.1016/0092-8674(91)90616-7 [DOI] [PubMed] [Google Scholar]
- 40.Clarke MS, Feeback DL. Mechanical load induces sarcoplasmic wounding and FGF release in differentiated human skeletal muscle cultures. FASEB J. 1996;1996(10):502–509. 10.1096/fasebj.10.4.8647349 [DOI] [PubMed] [Google Scholar]
- 41.Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302–309. 10.1016/j.cmet.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colaianni G, Cuscito C, Mongelli T, et al. Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol. 2014;2014:902186–902188. 10.1155/2014/902186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crumpler HR, Dent CE, Harris H, Westall RG. β-aminoisobutyric acid (α-methyl-β-alanine): A new amino-acid obtained from human urine. Nature. 1951;167:307–308. [DOI] [PubMed] [Google Scholar]
- 45.Kitase Y, Vallejo JA, Gutheil W, et al. beta-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018;22(6):1531–1544. 10.1016/j.celrep.2018.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jähn K, Lara-Castillo N, Brotto L, et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of β-catenin. Eur Cells Mater. 2012; 2012(24):197–210. 10.22203/eCM.v024a14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanazawa I Osteocalcin as a hormone regulating glucose metabolism. World J Diabetes. 2015;6(18):1345–1354. 10.4239/wjd.v6.i18.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizokami A, Kawakubo-Yasukochi T, Hirata M. Osteocalcin and its endocrine functions. Biochem Pharmacol. 2017;132:1–8. 10.1016/j.bcp.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 49.Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone. 2016;82:42–49. 10.1016/j.bone.2015.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50(2):568–575. 10.1016/j.bone.2011.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karsenty G, Mera P. Molecular bases of the crosstalk between bone and muscle. Bone. 2017;115:43–49. 10.1016/j.bone.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Gao F, Wen L, et al. Osteocalcin induces proliferation via positive activation of the PI3K/Akt, P38 MAPK pathways and promotes differentiation through activation of the GPRC6A-ERK1/2 pathway in C2C12 myoblast cells. Cell Physiol Biochem. 2017;43(3):1100–1112. 10.1159/000481752 [DOI] [PubMed] [Google Scholar]
- 53.Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318(9):1040–1048. 10.1016/j.yexcr.2012.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Econs MJ, Friedman NE, Rowe PSN, et al. A PHEX gene mutation is responsible for adultonset Vitamin D-resistant hypophosphatemic osteomalacia: evidence that the disorder is not a distinct entity from X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. 1998;83:3459–3462. 10.1210/jcem.83.10.5167 [DOI] [PubMed] [Google Scholar]
- 55.Hesse M, Froehlich L, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. 10.1016/j.matbio.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 56.Wacker MJ, Touchberry CD, Silswal N, et al. Skeletal muscle, but not cardiovascular function, is altered in a mouse model of autosomal recessive hypophosphatemic rickets. Front Physiol. 2016;7:173 10.3389/fphys.2016.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. 10.1172/JCI46122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve. 2012;45(1):55–59. 10.1002/mus.22232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Lierop AH, Hamdy NAT, van Bezooijen RL, Löwik CW, Papapoulos SE. The role of sclerostin in the pathophysiology of sclerosing bone dysplasias. Clin Rev Bone Mineral Metab. 2011;10(2):108–116. 10.1007/s12018-011-9123-5 [DOI] [Google Scholar]
- 60.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell. and more. Endocr Rev. 2013;34(5):658–690. 10.1210/er.2012-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–5875. 10.1074/jbc.M705092200 [DOI] [PubMed] [Google Scholar]
- 62.Spatz JM, Fields EE, Yu EW, et al. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97(9):E1736–E1740. 10.1210/jc.2012-1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krause A, Speacht T, Govey P, et al. Sarcopenia and increased body fat in sclerostin deficient mice. J Bone Miner Res. 2014;29:S8–S9. [Google Scholar]
- 64.Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of β-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone. 2010;47(5):872–881. 10.1016/j.bone.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Payne KA, Meszaros LB, Phillippi JA, Huard J. Effect of phosphatidyl inositol 3-kinase, extracellular signal-regulated kinases 1/2, and p38 mitogen-activated protein kinase inhibition on osteogenic differentiation of muscle-derived stem cells. Tissue Eng Part A. 2010;16(12):3647–3655. 10.1089/ten.tea.2009.0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitase Y, Barragan L, Qing H, et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25(12):2657–2668. 10.1002/jbmr.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harada SI, Balena R, Rodan GA, Rodan SB. The role of prostaglandins in bone formation. Connect Tissue Res. 1995;31(4):279–282. 10.3109/03008209509010823 [DOI] [PubMed] [Google Scholar]
- 68.Karasik D, Kiel DP. Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner Res. 2010;23:788–802. 10.1359/jbmr.080218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karasik D, Zhou Y, Cupples LA, et al. Bivariate genome-wide linkage analysis of femoral bone traits and leg lean mass: Framingham study. J Bone Miner Res. 2009;24:710–718. 10.1359/JBMR.081222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karasik D, Cohen-Zinder M. The genetic pleiotropy of musculoskeletal aging. Front Physiol. 2012;3(3):303 10.3389/fphys.2012.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–469. 10.1038/nature07206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang J, Hsu YH, Mo C, et al. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-kappaB signaling pathway. J Bone Miner Res. 2014;29(7):1531–1540. 10.1002/jbmr.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50(5):1231–1233. 10.1093/ajcn/50.5.1231 [DOI] [Google Scholar]
- 74.Miljkovic N, Lim JY, Miljkovic I, Frontera WR. Aging of skeletal muscle fibers. Ann Rehabil Med. 2015;39(2):155–162. 10.5535/arm.2015.39.2.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reginster JY, Beaudart C, Buckinx F, Bruyere O. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care. 2016;19(1):31–36. 10.1097/MCO.0000000000000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verschueren S, Gielen E, O’Neill TW, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporosis Int. 2013;24:87–98. 10.1007/s00198-012-2057-z [DOI] [PubMed] [Google Scholar]
- 77.Kim S, Won WC, Kim BS, Choi HR, Moon MY. The association between the low muscle mass and osteoporosis in elderly Korean people. J Korean Med Sci. 2014;29(7):995–1000. 10.3346/jkms.2014.29.7.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–179. 10.1038/nrg.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schroder EA, Harfmann BD, Zhang X, et al. Intrinsic muscle clock is necessary for musculoskeletal health. J Physiol. 2016;593(24):5387–5404. 10.1113/JP271436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hodge BA, Wen Y, Riley LA, et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle. 2015;5(1):17 10.1186/s13395-015-0039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorski JP, Huffman NT, Vallejo J, et al. Deletion of Mbtps1 (Pcsk8, S1p, Ski-1) gene in osteocytes stimulates soleus muscle regeneration and increased size and contractile force with age. J Biol Chem. 2016;291(9):4308–4322. 10.1074/jbc.M115.686626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–317. 10.1016/S0092-8674(02)01049-8 [DOI] [PubMed] [Google Scholar]
- 83.Baldock PA, Allison SJ, Lundberg P, et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J Biol Chem. 2007;282(26):19092–19102. 10.1074/jbc.M700644200 [DOI] [PubMed] [Google Scholar]
- 84.Houweling P, Kulkarni RN, Baldock PA. Neuronal control of bone and muscle. Bone. 2015;80:95–100. 10.1016/j.bone.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 85.Harcourt LJ, Schertzer JD, Ryall JG, Lynch GS. Low dose formoterol administration improves muscle function in dystrophic mdx mice without increasing fatigue. Neuromuscular Disord. 2007;17(1):47–55. 10.1016/j.nmd.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 86.Rizzoli R Nutrition: its role in bone health. Best Pract Res CL EN. 2008;2008(22):813–829. 10.1016/j.beem.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 87.Robinson S, Cooper C, Aihie SA. Nutrition and sarcopenia: a review of the evidence and implications for preventive strategies. J Aging Res. 2012;2012(9):510801 10.1155/2012/510801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Janssen HJ P, Samson MM, Verhaar HJJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–615. 10.1016/j.nuclphysb.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 89.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692–b3692. 10.1136/bmj.b3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mackinnon ES, Rao AV, Josse RG, Rao LG. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopep-tide of type I collagen in postmenopausal women. Osteoporos Int. 2011;22(4):1091–1101. 10.1007/s00198-010-1308-0 [DOI] [PubMed] [Google Scholar]
- 91.Ferrucci L, Baroni M, Ranchelli A, et al. Interaction between bone and muscle in older persons with mobility limitations. Curr Pharm Des. 2014;20(19):3178–3197. 10.2174/13816128113196660690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–1920. 10.1016/j.jprot.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 93.Lian JB, Stein GS, van Wijnen AJ, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8(4):212–227. 10.1038/nrendo.2011.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hassan MQ, Yukiko M, Hanna T, et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem. 2012;287(50):42084–42092. 10.1074/jbc.M112.377515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li D, Liu J, Guo B, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872 10.1038/ncomms10872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qin Y, Peng Y, Zhao W, et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J Biol Chem. 2017;292(26):11021–11033. 10.1074/jbc.M116.770941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bialek P, Parkington J, Li X, et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone. 2014;60(3):162–171. 10.1016/j.bone.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 98.Attie KM, Borgstein NG, Yang Y, et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve. 2013;47(3):416–423. 10.1002/mus.23539 [DOI] [PubMed] [Google Scholar]


