Abstract
Objective:
To review palbociclib, a novel small-molecule inhibitor of cyclin-dependent kinases 4 and 6, and its current place in therapy for the treatment of hormone receptor (HMR)-positive, human epidermal growth factor receptor 2 (Her2)-negative advanced breast cancer.
Study Selection and Data Abstraction:
Four phase I trials, 2 phase II trials, and 1 phase III trial were identified from May 2004 to May 2015 using PubMed, American Society of Clinical Oncology (ASCO) abstracts, and European Society of Medical Oncology (ESMO) abstracts.
Data Synthesis:
In the first-line setting, the phase II PALbociclib: Ongoing trials in the Management of breast cAncer (PALOMA)-1 trial randomized patients to receive letrozole alone or letrozole plus palbociclib 125 mg daily for 3 weeks, followed by 1 week off, as initial therapy for advanced breast cancer. The investigator-assessed median progression-free survival (PFS) was 20. 2 months for the combination versus 10.2 months for letrozole alone (hazard ratio [HR] = 0.488; 95% CI = 0.319–0.748; 1-sided P = 0.0004). The ensuing Food and Drug Administration approval of palbociclib was given a “breakthrough therapy” designation, where preliminary evidence suggests substantial improvement over existing therapies for a serious or life-threatening disease. A confirmatory phase III trial, PALOMA-2, is under way. In patients who were previously treated with endocrine therapy for advanced breast cancer, the phase III PALOMA-3 trial randomized patients to fulvestrant plus palbociclib versus fulvestrant plus placebo. The investigator-assessed median PFS at the time of a preplanned analysis was 9.2 months with palbociclib-fulvestrant compared with 3.8 months with placebo-fulvestrant (HR = 0.42; 95% CI = 0.32–0.56; P < 0.001).
Conclusions:
Palbociclib, the first-in-class CDK4/6 inhibitor, significantly extended PFS in combination with endocrine therapy in the first and subsequent lines of treatment for HMR-positive, Her2-negative advanced breast cancer.
Keywords: palbociclib, CDK inhibitor, PD-0332991, letrozole, fulvestrant, advanced breast cancer
Background
Breast cancer is the most commonly diagnosed malignancy in women. In 2015, it is expected that 231 840 women will be diagnosed with breast cancer, and 40 290 women will die from the disease in the United States.1 Clinical treatment decisions are driven by the expression of estrogen receptors, progesterone receptors, or both (ie, HMR-positive); the amplification of the human epidermal growth factor receptor 2 (Her2) oncogene (ie, Her2-positive); or lack of either HMR or Her2 alteration (ie, triple negative).2
Despite the profound benefits of screening, early detection, and effective treatment modalities such as surgery, radiation, chemotherapy, biological therapy, and endocrine therapy, all of which may lower the risk of breast cancer recurrence, more than 20% of patients diagnosed with early-stage breast cancer will eventually develop metastatic disease depending on tumor and patient characteristics. The majority of patients (75%) diagnosed with metastatic breast cancer are HMR positive, and the prognosis remains generally more favorable compared with patients with other types of metastatic breast cancer, with a median overall survival (OS) of 24 to 36 months from the time of their diagnosis.3 The goal of treatment is palliative; it is aimed at maintaining quality of life while prolonging survival and postponing the need for chemotherapy. For patients who are relatively asymptomatic or who have minimal disease burden without life-threatening symptoms, endocrine therapy remains the cornerstone of treatment for HMR-positive breast cancer, given its favorable toxicity profile and similar survival rates in hormone-sensitive disease as compared with chemotherapy.4
Current Therapies for the First-Line Treatment of Postmenopausal, HMR-Positive, Advanced Breast Cancer
In postmenopausal women, the sole source of estrogen production is through the conversion of androgen precursors such as testosterone and androstendione into estradiol or estrone via the aromatase enzyme. The third-generation aromatase inhibitors (AIs), anastrozole, letrozole, and exemestane, have all shown marginally better efficacy and tolerability compared with tamoxifen and are currently considered standard first-line treatment for advanced breast cancer in postmenopausal women with HMR-positive disease.5–7 Time to progression (TTP) or progression-free survival (PFS) end points for the AIs were as long as 11 months compared with 6 months with tamoxifen in these trials, although no OS advantages were seen.8
Previous trials had shown that fulvestrant, a selective estrogen receptor downregulator, is at least as effective as anastrozole as a second-line treatment for advanced breast cancer following antiestrogen therapy.9 Subsequently, the phase II FIRST trial10,11 compared the efficacy of fulvestrant with anastrozole in the first-line setting of HMR-positive advanced breast cancer. The primary end point of clinical benefit rate (CBR), which includes the rate of complete response, partial response, and stable disease, was similar between the 2 groups, although long-term follow-up revealed that the median TTP was significantly longer in the fulvestrant versus anastrozole arm—23.4 versus 13.1 (P = 0.01) months, respectively—representing a potential improvement over previously reported TTP with an AI.11
The combination of an AI (anastrozole) and fulvestrant as initial therapy has produced mixed results. The FACT trial12 compared fulvestrant plus anastrozole with anastrozole alone. Median TTPs were 10.8 and 10.2 months in the combination versus the standard arm, respectively (P = 0.91). The majority of patients in this trial had received previous adjuvant endocrine therapy. A second trial (SWOG 0226)13 had the same design with a similar primary end point. Results revealed a median PFS of 15 and 13.5 months in the combination versus the standard arm, respectively (P = 0.007). A subset analysis suggested that patients with no prior endocrine therapy derived the greatest benefit.
For the first-line treatment of postmenopausal, HMR-positive advanced breast cancer in patients who have not had previous endocrine therapy in the preceding 12 months, the National Comprehensive Cancer Network 2015 breast cancer guidelines suggest the use of an AI, fulvestrant, or tamoxifen. The greatest benefit will likely be seen in patients who have not had previous endocrine therapy or who had received previous endocrine therapy in the distant past.14 However, all patients will eventually experience disease progression because of de novo or acquired resistance to endocrine therapy.15 More effective therapies that can overcome endocrine resistance or act synergistically with existing drugs are an area of active research.
Palbociclib (PD-0332991, Ibrance®, Pfizer Inc.) is a first-in-class, oral, reversible, small-molecule inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), which play an important role in the regulation of the cell cycle.16 In February 2015, the Food and Drug Administration (FDA) granted accelerated approval of palbociclib for the treatment of postmenopausal women with HMR-positive, Her2-negative advanced breast cancer as initial endocrine-based therapy in combination with letrozole.17 The primary aim of this review is to summarize the mechanism of action, pharmacology, and ongoing clinical development of palbociclib in patients with breast cancer.
Data Sources
A literature search was conducted through PubMed using the search terms palbociclib, PD-0332991, and breast cancer. Studies included were limited to those published in English. Abstracts presented at the American Society of Clinical Oncology meetings (January 2004 to May 2015) and at the European Society of Medical Oncology meetings (January 2004 to May 2015) were reviewed for data from ongoing studies. ClinicalTrials.gov was searched for a comprehensive listing of ongoing and completed studies. The manufacturer’s prescribing information was used to supplement published data. References of selected articles were reviewed for additional literature.
Pharmacology
Under normal conditions, the cell cycle functions as a highly regulated process consisting of G0 (quiescence), G1 (pre-DNA synthesis), S (DNA synthesis), G2 (pre-division), and M (mitosis) phases. The progression from the G1 to S phase is a key checkpoint protecting against abnormal replication that is regulated by CDKs 4/6 (Figure 1). In the early G1 phase, the interactions between CDKs 4/6 and cyclin D1 results in the hyperphosphorylation of retinoblastoma (Rb), a tumor suppressor protein, resulting in the release of E2F transcription factors that allow progression into the S phase. Endogenous inhibitors of CDK, such as p16 and p21, also regulate this process. Dysregulation occurs in cancer through a variety of mechanisms, including loss of function of Rb, amplification of cyclin D1, amplification of CDK, and loss of p16.18–21
Figure 1.
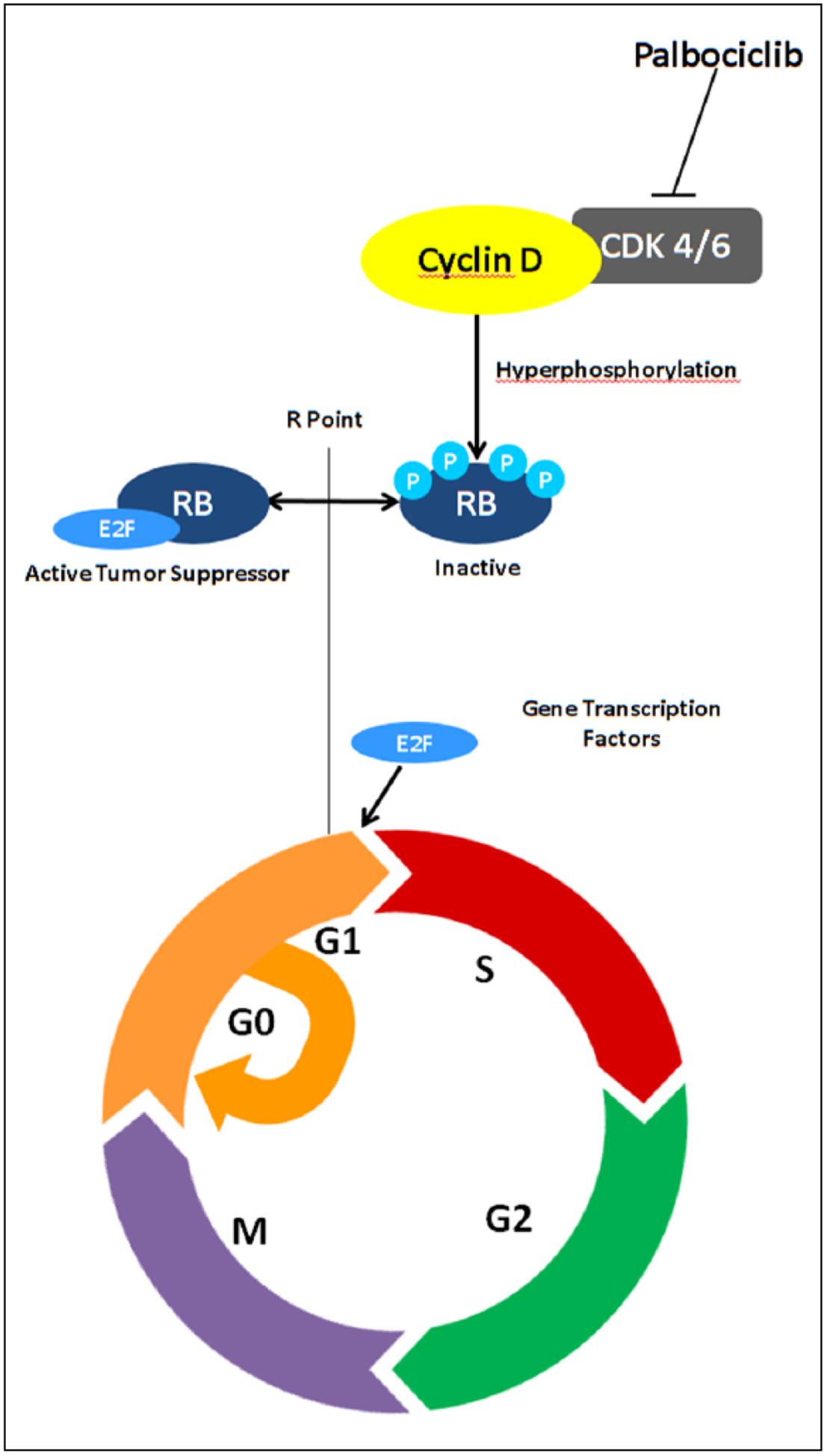
Mechanism of action of palbociclib.a
aAdapted from Dickson.21
Palbociclib is a highly selective inhibitor of CDKs 4/6, thus blocking Rb phosphorylation and thereby inducing G1 arrest. An in vitro study of 45 breast cancer cell lines and 3 control breast epithelial lines found luminal HMR-positive and Her2-amplified cell lines to be most sensitive to growth inhibition by palbociclib. Synergy was seen in combination with tamoxifen or with trastuzumab, respectively, including the ability to enhance sensitivity in tamoxifen-resistant cells. The growth inhibition was characterized as cytostatic with no lethality or apoptosis observed.18 In contrast, an in vivo study of breast cancer xenografts in mice found that the combination of palbociclib with either carboplatin or doxorubicin reduced their antitumor activity compared with administration of these cytotoxic agents alone. The authors concluded that the quiescence induced by palbociclib reduced the efficacy of DNA-damaging chemotherapy.22 Preclinical studies suggest that palbociclib enhances the efficacy of cytostatic agents, such as endocrine therapy or Her2-targeted therapy, but decreases the efficacy of cytotoxic chemotherapy.
Pharmacokinetics
The mean bioavailability of palbociclib 125 mg is 46% and reaches the maximum serum concentration (Cmax) between 6 and 12 hours following oral administration. Compared with overnight-fasted conditions, administration with food increased the average area under the curve (AUC) and Cmax by 21% and 38%, respectively, and reduced interpatient variability in palbociclib exposure. Furthermore, whereas higher solubility is achieved at pH ≤ 4, no clinically relevant effects on absorption are expected with concurrent proton pump inhibitors, H2-antagonists, or local antacids under fed conditions.23
Palbociclib is 85% bound to human plasma proteins with a volume of distribution of 2583 L.23 Preclinical models suggest low central nervous system (CNS) penetration with intact blood-brain barrier models as a result of efflux pump activity.24 However, the integrity of the blood-brain barrier may be compromised in the setting of CNS disease, which may affect palbociclib exposure, as evident in one case report of an intracranial teratoma responding to palbociclib and a preclinical study of glioblastoma multiforme xenografts showing measurable drug levels in CNS tissue.25,26 Palbociclib is extensively metabolized in the liver by CYP3A and SULT2A1. Palbociclib metabolites are primarily excreted renally (17.5%) and fecally (74.1%). It has a long elimination half-life of 29 hours.23
Dosing
Two phase I, open-label, dose-finding studies of palbociclib monotherapy were completed in Rb-positive advanced solid tumors and Non-Hodgkins lymphoma patients to identify the maximum tolerated dose (MTD). Schwartz et al27 studied a once-daily dose for 2 weeks on treatment followed by 1 week off schedule in 33 patients. The MTD was 200 mg, with doses ranging from 100 to 200 mg. Flaherty et al28 studied a once-daily dose for 3 weeks on treatment followed by 1 week off schedule in 41 patients and identified a MTD of 150 mg, with doses ranging from 25 to 150 mg. Based on the safety profile, palbociclib 125 mg once daily for 3 weeks on treatment followed by 1 week off schedule was used for phase II development.
In clinical practice, the recommended dose is a palbociclib 125-mg capsule taken by mouth once daily with food for 21 consecutive days, followed by 7 days off treatment, with letrozole 2.5 mg tablet taken once daily continuously to comprise a cycle of 28 days.23 A phase I trial of palbociclib with letrozole found no interactions and nonoverlapping toxicities.29
No data are available on safety with creatinine clearance (CrCl) <30 mL/min or with total bilirubin >1.5× the upper limit of normal (ULN). No dose adjustment is needed for patients with mild (60 mL/min ≤ CrCl <90 mL/min) or moderate (30 mL/min ≤ CrCl <60 mL/min) renal impairment or mild (total bilirubin ≤ ULN and AST > ULN or total bilirubin >1.0 to 1.5× ULN and any AST) hepatic impairment.23
Pregnancy and lactation risk cannot be ruled out based on the mechanism of action and embryo-fetal toxicities observed in animal studies of rats and rabbits with maternal exposures ≥4× the AUC observed in human clinical exposure. Women of reproductive potential should be advised to use effective contraception during therapy and for at least 2 weeks after the last dose.23
Drug-Drug Interactions
Drug interaction studies with palbociclib have been completed in humans and in vitro. Data from a drug interaction trial in healthy volunteers demonstrated that coadministration with itraconazole 200 mg daily increased palbociclib AUC and Cmax by 87% and 34%, respectively. The manufacturer, therefore, recommends avoiding concomitant strong CYP3A inhibitors, including grapefruit and grapefruit juice. If therapy with a strong inhibitor is medically necessary, palbociclib should be given at a reduced dose of 75 mg. Palbociclib can be resumed at full dose on discontinuation of the strong inhibitor after waiting 3 to 5 half-lives of the inhibitor to ensure that it has cleared the system. Coadministration of strong and moderate CYP3A inducers should be avoided because of decreased palbociclib exposure. Data from a drug interaction trial in healthy individuals with coadministration of rifampin 600 mg daily decreased palbociclib AUC and Cmax by 85% and 70%, respectively. Finally, increased monitoring and possible dose reduction of CYP3A substrates with narrow therapeutic window should be considered because of increased exposure with concurrent palbociclib.23
Adverse Events
Adverse events reported at a rate of >10% included neutropenia, leukopenia, thrombocytopenia, anemia, upper-respiratory infections, epistaxis, nausea, vomiting, diarrhea, stomatitis, decreased appetite, alopecia, asthenia, and peripheral neuropathy.23
In PALbociclib: Ongoing trials in the Management of breast cAncer (PALOMA-1) and PALOMA-3, grade 3 and 4 neutropenia occurred in 54% and 62% of patients on palbociclib respectively. No febrile neutropenia episodes were observed in PALOMA-1, but occurred in 2 patients (0.6%) on palbociclib-fulvestrant and 1 patient (0.6%) on placebo-fulvestrant in PALOMA-3.16,30 A higher rate of infections has been observed, especially upper-respiratory tract infection.23 The median time to onset of any grade of neutropenia was 15 days (13–117 days). Median duration of ≥grade 3 neutropenia was 7 days.23 In phase I studies, an exploratory analysis of absolute neutrophil counts and thrombocytopenia during the first 2 cycles noted that the observed nadirs in percentage decrease from baseline were equivalent in each cycle and, thereby, characterized as reversible and noncumulative.27,28 Preclinical studies note that unlike cytotoxic chemotherapy, palbociclib induces a reversible quiescence in healthy bone marrow progenitor cells without decreasing total marrow cellularity or altering viability.31 Therefore, the paucity of febrile neutropenia compared with the high rate of uncomplicated grade 3 and 4 neutropenia has been attributed to these cells being functional in the setting of infection even if their replication is suppressed.32
In addition, there was a 5% incidence in pulmonary embolism (PE) in the palbociclib arm compared with 0% in the letrozole arm. The prescribing information does not list prior venous thromboembolism as a contraindication, and it does not specify whether palbociclib therapy should be discontinued on development of a PE on therapy.23
Monitoring and Dose Adjustments
Complete blood counts should be monitored at baseline, every 2 weeks for the first 2 cycles, then monthly thereafter. Criteria for dose reductions for hematological toxicities are detailed in Table 1. Palbociclib is available as 125-, 100-, and 75-mg capsules, which correspond to each dose level. Per the prescribing information, if a dose reduction below 75 mg per day is required, then treatment should be discontinued.23
Table 1.
Dose Modification Recommendations.23
| Grade of Hematological Toxicities | Dose Modification |
|---|---|
Platelets < LLN to 75K/mm3 |
|
Platelets <75K-50K/mm3 |
|
Platelets < 50K-25K/mm3 |
|
Fever ≥ 100.4°F ± infection |
|
Platelets < 25K |
|
Abbreviations: ANC, absolute neutrophil count; LLN, lower limit of normal.
Clinical Trial Results
The FDA approval of palbociclib was based on designation as a “breakthrough therapy,” where preliminary evidence suggested substantial improvement over existing therapies for a serious or life-threatening disease.17 Consequently, clinical trial data evaluating the efficacy and safety of palbociclib are limited.
The pivotal trial, PALOMA-1 (TRIO-18; Study 1003; A5481003; and NCT00721409), was a phase I/II, open-label, randomized, parallel-arm, international, multicenter clinical trial comparing palbociclib plus letrozole with letrozole alone. The study population consisted of postmenopausal women with HMR-positive, Her2-negative, locally advanced or metastatic breast cancer. Patients were required to have Eastern Cooperative Oncology Group performance status 0 to 1 and adequate organ function. Patients were excluded if they received prior lines of therapy for advanced disease, adjuvant letrozole within 12 months prior to study entry, and brain metastasis. The primary end point was investigator-assessed PFS. The secondary end points included objective response rate, CBR, duration of response, OS, and safety.16
The phase I portion conducted by Slamon et al29 was designed to assess safety and tolerability of palbociclib plus letrozole, exclude a pharmacokinetic interaction with the combination, and select dosing for the phase II portion. Cycle 1 consisted of palbociclib 125 mg monotherapy for 2 weeks on followed by 1 week off. With cycle 2 and beyond, palbociclib 125 mg was given for 3 weeks on followed by 1 week in combination with letrozole taken continuously for 28 days. A total of 12 patients were enrolled. The most common side effects included neutropenia, leucopenia, and fatigue. No pharmacokinetic interactions were identified with the combination. In terms of efficacy, 9 patients had stable disease for ≥6 months, and 3 patients had a partial response.
The phase II portion conducted by Finn et al16 was divided into 2 cohorts: cohort 1 consisted of an unselected population with the goal of providing an exploratory analysis of initial safety and efficacy, whereas cohort 2 consisted of a biomarker-enriched population with Cyclin D1 gene amplification and/or loss of p16 with the goal of analyzing the primary end point of investigator-assessed PFS. The planned enrollment was 60 patients in cohort 1 and 150 patients in cohort 2 to be analyzed when 114 patients in cohort 2 progressed (termed PFS event) based on the assumption that PFS would improve from 9 months to 13.5 months, with an 80% power to detect a hazard ratio (HR) of 0.67. However, an unplanned interim analysis of cohort 1 was performed at 31 PFS events when investigators observed that twice as many patients in the control group were coming off study because of progression. The preliminary findings showed meaningful activity with palbociclib-letrozole compared with letrozole alone and did not demonstrate improved outcomes based on biomarker selection. Therefore, the protocol was amended to combine cohorts 1 and 2 in determining the primary end point of median PFS. Following a planned, second interim analysis, the investigators observed a substantial decline in the rate of PFS events in the combination arm compared with the letrozole arm, prompting them to move up the final analysis to 95 PFS from 114 PFS events. This would provide a 98% power to detect a HR of 0.50 or a 75% power to detect a HR of 0.67, with a 1-sided α of 0.10. At the time enrollment was stopped, 165 patients were on the study, 66 in cohort 1 and 99 in cohort 2. The investigator-assessed median PFS was 20.2 months for the combination versus 10.2 months for letrozole alone (HR = 0.488; 95% CI = 0.319–0.748; 1-sided P = 0.0004). For cohort 1, the median PFS was 26.1 months with the combination versus 5.7 months with letrozole alone (HR = 0.299; 95% CI = 0.156–0.572; 1-sided P < 0.001). For cohort 2, the median PFS was 18.1 months with the combination versus 11.1 months with letrozole alone (HR = 0.508; 95% CI = 0.303–0.853; 1-sided P = 0.0046). The median OS was 37.5 months for the combination versus 33.3 months (HR = 0.813; 95% CI = 0.492–1.345; 2-sided P = 0.42). Despite that, cross-over to the palbociclib arm at progression was not permitted; the study was not powered to detect an OS difference; and few deaths had occurred at the time of the final analysis. Study limitations included open-label design, investigator-assessed PFS as the primary end point with lack of central radiology review, and multiple modifications to the statistical analysis that could increase risk for type 1 error and affect the robustness of the analysis.
Another pivotal trial, PALOMA-3, assessed palbociclib in combination with fulvestrant in the second-line setting.30 This was a phase III, double-blind, placebo-controlled trial in postmenopausal and premenopausal women with locally advanced or metastatic, HMR-positive, Her2-negative breast cancer with relapse (within 12 months of stopping adjuvant endocrine therapy or within 1 month of stopping endocrine therapy for metastatic disease) or progression during endocrine therapy. One prior line of chemotherapy for metastatic disease was permitted. A total of 521 patients were assigned in a 2:1 ratio to receive palbociclib 125 mg for 3 weeks on followed by 1 week off or placebo in combination with fulvestrant 500 mg on day 1, day 14, day 28, and then every 28 days. Premenopausal or perimenopausal women were started on goserelin at least 4 weeks prior to randomization. Fulvestrant ± goserelin were continued if palbociclib or placebo were held. Unlike PALOMA-1, this study included an additional dose level of palbociclib 75 mg for 2 weeks on followed by 2 weeks off. The primary end point was investigator-assessed median PFS, with secondary end points of OS, objective response rate, CBR, and safety. At the time of the planned interim analysis after 195 PFS events, median PFS was reported as 9.2 months with palbociclib-fulvestrant compared with 3.8 months with placebo-fulvestrant (HR = 0.42; 95% CI = 0.32–0.56; P < 0.001). Approximately 40% of patients were randomly selected for central imaging review by blinded independent review, with results consistent with the investigator-assessed median PFS. At the time of the analysis, OS could not be evaluated. Cross-over to the palbociclib arm at progression was not permitted. The most common grade 4 adverse effects were neutropenia, leukopenia, anemia, thrombocytopenia, and fatigue. Febrile neutropenia, which had not been observed in PALOMA-1, was observed at an incidence of 0.6% in both arms. PE was noted in 3 patients (0.9%) receiving palbociclib versus zero patients on placebo.
Two small trials have explored the use of palbociclib as monotherapy or in combination with chemotherapy in the third-line setting. DeMichele et al32 conducted a phase II study in Rb-positive metastatic breast cancer patients, including HMR-positive/Her2-negative, HMR-positive/Her2-positive, and triple-negative subtypes. Palbociclib 125-mg monotherapy was given for 3 weeks on followed by 1 week off every 28 days. Of the 37 patients enrolled, clinical benefit was achieved in 7 (19%) patients, all of whom were HMR positive and had progressed through ≥2 prior lines of hormonal therapy. The authors reported that all 4 triple-negative patients rapidly progressed on treatment. Clark et al33 conducted a phase I trial of palbociclib in combination with paclitaxel in Rb-positive metastatic breast cancer with ≤3 prior lines of cytotoxic therapy for metastatic breast cancer. Paclitaxel 80 mg/m2 was given weekly on a days 1, 8, 15, and 28 every 28 days for 3 cycles followed by a day 1, 8, 15 every 28-day schedule, with the option to discontinue paclitaxel after 6 cycles. Palbociclib was given on days 2 to 6, 9 to 14, and 16 to 20 of a 28-day cycle in a dose-escalating fashion using a 3 + 3 design. A total of 15 patients were enrolled, of whom 11 patients received clinical benefit. Although the investigators concluded that the combination was safe, they reported that it was often complicated by grade 3 and 4 neutropenia, leading to dose interruptions and dose reductions.
Significant efforts were made to identify predictive biomarkers in the clinical development of palbociclib. Gene expression analysis in preclinical development identified that higher levels of Rb and Cyclin D1 and lower levels of p16 were observed in palbociclib-sensitive cell lines.18 Consequently, the phase I dose-finding studies only included patients with Rb-positive tumor expression and purposely excluded small-cell lung cancer and retinoblastoma because of low expression.27,28 This also applies to the small trials investing palbociclib as salvage therapy in metastatic breast cancer.32,33 The PALOMA-1 trial incorporated a cohort of patients with amplification of cyclin D1 and/or loss of p16.16 However, there are no predictive biomarkers to date that were validated in clinical trials.
Clinical Trials in Progress
Currently palbociclib-based combination therapies are being studied as front-line and second-line treatments for advanced breast cancer as well as adjuvant and neoadjuvant therapy for early-stage breast cancer (Table 2).34 Ribociclib (LEE011) and abemaciclib (LY2835219) are other CDK4/6 inhibitors currently in clinical development.21
Table 2.
Ongoing or Planned Phase III Studies of Palbociclib in HMR-positive, Her2-Negative Breast Cancer.34
| Study and NCT Identifier | Setting | Menopausal Status | Estimated Number of Patients | Randomization | Treatment Arms | Primary End Point |
|---|---|---|---|---|---|---|
| PALOMA-2: NCT01740427 | MBC, first-line | Postmenopausal | 650 | 2:1 | Palbociclib + letrozole vs placebo + letrozole | PFS |
| PALOMA-4: NCT02297438 | MBC, first-line | Postmenopausal, Asian women | 330 | Unavailable | Palbociclib + letrozole vs placebo + letrozole | PFS |
| PEARL: NCT02028507 | MBC, second-line | Postmenopausal | 348 | 1:1 | Palbociclib + exemestane vs capecitabine | PFS |
| PENELOPE-B: NCT01864746 | Adjuvant, residual disease | Premenopausal, Postmenopausal | 800 | 1:1 | Palbociclib vs placebo | iDFS |
| PALLET: NCT02296801 | Neoadjuvant, tumor ≥2 cm | Postmenopausal | 306 | 3:2:2:2 |
|
ΔKi67 cCR |
Abbreviations: cCR, clinical complete response; iDFS, invasive disease-free survival; Her2, human epidermal growth factor receptor 2; HMR, hormone receptor; Ki67, cell marker for proliferation; MBC, locally advanced or metastatic breast cancer; PFS, progression-free survival.
Place in Therapy and Formulary Considerations
Accelerated approval for palbociclib as first-line therapy for postmenopausal women with HMR-positive advanced breast cancer was based on a single, randomized, phase II study.17 A confirmatory phase III trial, PALOMA-2, is ongoing.34 While we await these results, the use of palbociclib plus letrozole as first-line therapy is a reasonable option. If an AI monotherapy has already been used as first-line therapy, one can consider using palbociclib off-label in a subsequent line. Data from the PALOMA-3 trial support the use of palbociclib in combination with fulvestrant following failure of prior endocrine therapy and/or one line of chemotherapy for advanced disease. This study also supported the use of palbociclib in premenopausal women receiving ovarian suppression with goserelin. At this time point, a decision regarding the place of palbociclib in the treatment sequence depends on physician and patient preference because there is no head-to-head comparison of first-line versus subsequent-line use. This sequence would reserve the combination of everolimus plus exemestane for use in the second- or subsequent-line setting after progression on non-steroidal AI therapy, thus optimizing its niche for endocrine-resistant disease, as demonstrated in the BOLERO-2 trial.35
For health plans providing prescription benefits based on efficacy, cost, and adverse effects, the potential addition of palbociclib represents an important decision. Palbociclib is the first CDK inhibitor to receive FDA approval, so the addition of this drug to the formulary is reasonable, given compelling data to support its use in combination with endocrine therapy. However, none of the following can claim an OS advantage: palbociclib plus letrozole, palbociclib plus fulvestrant, an AI alone, fulvestrant alone or in combination with an AI, or everolimus plus exemestane.
In the emerging era of precision medicine, where molecular profiling of tumors is completed, it is possible that palbociclib will be requested for use to treat other malignancies based on patient-specific biopsy results that have identified cyclin D1 amplification, CDK amplification, or p16 loss. Additional studies are needed to evaluate whether this or other genomic alterations can identify patients who will benefit most from this therapy.
Patient Acceptance
For patients receiving palbociclib plus letrozole for the first-line treatment of HMR-positive advanced breast cancer, therapy will continue until disease progression or unacceptable toxicity. Given that the median duration of therapy in the PALOMA-1 trial was 20 months, with an upper range of 27 months, many patients will take this drug for an extended period of time. Fortunately, the drug appears to have a manageable side-effect profile, with neutropenia, fatigue, and mild GI disturbances being the most commonly reported problems. Dose intensity in this trial was 94% with palbociclib plus letrozole.16 Dose intensity in the PALOMA-3 trial was 97.1% in the palbociclib plus fulvestrant arm.30 Together, these results suggest good tolerability and an infrequent need for dose reductions. It is imperative that ongoing data are collected regarding long-term toxicity and compliance. Palbociclib is available through selected specialty pharmacies via a limited distribution plan.23
Summary
Identifying novel targeted therapies to improve clinical outcomes in HMR-positive advanced breast cancer not only offers a new option for these patients but also delays the initiation of chemotherapy and hence improves quality of life. Recent approval of the novel CDK 4/6 inhibitor, palbociclib, in combination with letrozole, represents a compelling, new, initial treatment option for HMR-positive, HER2-negative advanced breast cancer. Ongoing randomized trials must validate its potential use as first-line therapy. Results from the recently completed PALOMA-3 trial may possibly expand its FDA-approved indication.
Supplementary Material
Funding
The authors declared no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.National Cancer Institute. SEER database. http://seer.cancer.gov/statfacts/html/breast.html. Accessed March 4, 2015.
- 2.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, Bischoff J, Brain E, et al. A review of the treatment of endocrine responsive metastatic breast cancer in postmenopausal women. Cancer Treat Rev. 2013;39:457–465. doi: 10.1016/j.ctrv.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Buzdar AU. Endocrine therapy in the treatment of metastatic breast cancer. Semin Oncol. 2001;28:291–304. [DOI] [PubMed] [Google Scholar]
- 5.Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18:3758–3767. [DOI] [PubMed] [Google Scholar]
- 6.Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–2109. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 7.Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–4890. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurlimann B, Hess D, Koberle D, et al. Anastrozole (“Arimidex”) versus tamoxifen as first-line therapy in postmenopausal women with advanced breast cancer: results of the double-blind cross-over SAKK trial 21/95—a sub-study of the TARGET (Tamoxifen or “Arimidex” Randomized Group Efficacy and Tolerability) trial. Breast Cancer Res Treat. 2004;85:247–254. doi: 10.1023/B:BREA.0000025420.78346.f9. [DOI] [PubMed] [Google Scholar]
- 9.Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer. 2003;98:229–238. doi: 10.1002/cncr.11468. [DOI] [PubMed] [Google Scholar]
- 10.Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27:4530–4535. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 11.Robertson JF, Lindemann JP, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized “FIRST” study. Breast Cancer Res Treat. 2012;136:503–511. doi: 10.1007/s10549-012-2192-4. [DOI] [PubMed] [Google Scholar]
- 12.Bergh J, Jonsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30:1919–1925. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 13.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Breast cancer (version 1.2015). http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed March 4, 2015.
- 15.Fedele P, Orlando L, Schiavone P, et al. Recent advances in the treatment of hormone receptor positive HER2 negative metastatic breast cancer. Crit Rev Oncol Hematol. 2015;94:291–301. doi: 10.1016/j.critrevonc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. Palbociclib. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm432886.htm. Accessed March 4, 2015.
- 18.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 20.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 21.Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res. 2014;20:3379–3383. doi: 10.1158/1078-0432.CCR-13-1551. [DOI] [PubMed] [Google Scholar]
- 22.Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476–487. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrance (R) (palbociclib) [prescribing information]. New York, NY: Pfizer Laboratories, Inc; 2015. [Google Scholar]
- 24.Parrish KE, Pokorny JL, Mittapalli RK, et al. BBB efflux pump activity limits brain penetration of palbociclib (PD0332991) in glioblastoma. Mol Cancer Ther. 2013;12(11, suppl):C81. [Google Scholar]
- 25.Schultz KA, Petronio J, Bendel A, Patterson R, Vaughn DJ. PD0332991 (palbociclib) for treatment of pediatric intracranial growing teratoma syndrome. Pediatr Blood Cancer. 2015;62:1072–1074. doi: 10.1002/pbc.25338. [DOI] [PubMed] [Google Scholar]
- 26.Michaud K, Solomon DA, Oermann E, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–3238. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz GK, LoRusso PM, Dickson MA, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br J Cancer. 2011;104:1862–1868. doi: 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, doseescalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18:568.576. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- 29.Slamon DJ, Hurvitz SA, Applebaum S, et al. Phase I study of PD 0332991, cyclin-D kinase (CDK) 4/6 inhibitor in combination with letrozole for first-line treatment of patients with ER-positive, Her2-negative breast cancer [abstract 3060]. J Clin Oncol. 2010;28(suppl):15s. [Google Scholar]
- 30.Turner NC, Ro J, Andre F, et al. Palbociclib in hormonereceptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 31.Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120:2528–2536. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21:995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- 33.Clark AS, O’Dwyer PJ, Heitjan D, et al. A phase I trial of palbociclib and paclitaxel in metastatic breast cancer [abstract 527]. J Clin Oncol. 2014;32(suppl):5s. [Google Scholar]
- 34.Clinicaltrials.gov. https://clinicaltrials.gov/ct2/results?term=palbociclib+AND+breast+cancer&Search=Search. Accessed March 4, 2015.
- 35.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


