Clinical symptoms of active tuberculosis (TB) can range from a simple cough to more severe reactions, such as irreversible lung damage and, eventually, death, depending on disease progression. In addition to its clinical presentation, TB has been associated with several other disease-induced systemic complications, such as hyponatremia and glucose intolerance. Here, we provide an overview of the known, although ill-described, underlying biochemical mechanisms responsible for the clinical and systemic presentations associated with this disease and discuss novel hypotheses recently generated by various omics technologies.
KEYWORDS: clinical symptoms, disease mechanisms, omics, systemic complications, tuberculosis
SUMMARY
Clinical symptoms of active tuberculosis (TB) can range from a simple cough to more severe reactions, such as irreversible lung damage and, eventually, death, depending on disease progression. In addition to its clinical presentation, TB has been associated with several other disease-induced systemic complications, such as hyponatremia and glucose intolerance. Here, we provide an overview of the known, although ill-described, underlying biochemical mechanisms responsible for the clinical and systemic presentations associated with this disease and discuss novel hypotheses recently generated by various omics technologies. This summative update can assist clinicians to improve the tentative diagnosis of TB based on a patient’s clinical presentation and aid in the development of improved treatment protocols specifically aimed at restoring the disease-induced imbalance for overall homeostasis while simultaneously eradicating the pathogen. Furthermore, future applications of this knowledge could be applied to personalized diagnostic and therapeutic options, bettering the treatment outcome and quality of life of TB patients.
INTRODUCTION
Active pulmonary tuberculosis (TB), a highly contagious disease caused by Mycobacterium tuberculosis infection, is currently the leading cause of death from a single infectious pathogen. The latest reports indicate that approximately 7 million new and relapse TB cases were reported globally in 2018, raising the total incidence to 10 million, and of these cases, almost 900,000 were HIV positive. In the same year, global mortality reached 1.5 million, including 251,000 HIV-positive cases. Additionally, although 85% of the new cases reported in 2017 were successfully cured, 18% of previously treated cases presented with drug-resistant TB in 2018 (1).
The clinical symptoms of pulmonary TB develop slowly and are nonspecific. These may include a prolonged cough with mucus, pleuritic chest pain, hemoptysis, dyspnea, wheezing, weakness or progressive fatigue, cachexia/weight loss, loss of appetite (resulting in anorexia), chills/fever, night sweats, and malaise (2–5). Apart from these well-known clinical symptoms, several other secondary systemic complications have also been linked to TB, including increased oxidative stress, hyponatremia (6), hypocholesterolemia (7), glucose intolerance (8), hematological manifestations (9), vitamin D deficiency (10), and an altered host microbiota (11). The overall underlying biochemical mechanisms leading to these TB-related symptoms and systemic complications are rather ill described, and research on this topic is quite scarce and, if available, relatively outdated. Recent technological advancements and evolving bioinformatics systems have led to the development of more powerful research tools; hence, various research groups have applied research approaches, such as metabolomics, to gain new insights into TB disease (12). Such studies pertaining to the characterization of the metabolome of TB patients have, mostly unintentionally, brought about a new perspective to the underlying disease mechanisms. Here, we describe and summarize the current understanding (and the lack thereof) of the biochemical mechanisms leading to the clinical presentations and systemic complications associated with active pulmonary TB. We also discuss the contribution of new research approaches toward this existing pool of knowledge and how it can be used to fill the remaining knowledge gaps.
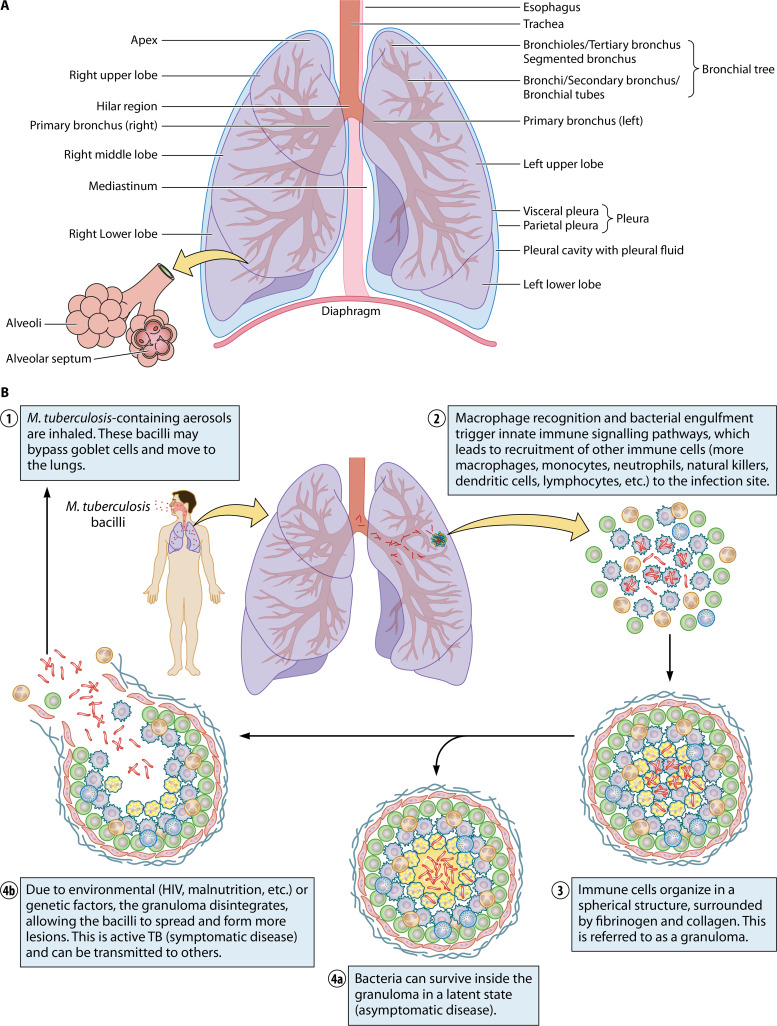
A glossary of the medical terms referred to in this review is provided in Table 1, and a graphical representation of the various lung regions, as specified in this glossary, is provided in Fig. 1A.
TABLE 1.
Glossary
| Terminology | Definition |
|---|---|
| Anemia | A decrease in the number or quality of red blood cells (RBC) or the level of hemoglobin in the body. This may typically occur due to decreased RBC production, increased RBC destruction, or blood loss. Although various forms of anemia exist, this review mentions only normocytic normochromic anemia (the hemoglobin content of the RBC is within normal limits) and macrocytic anemia (enlarged RBC). |
| Anorexia | A medical condition or infection resulting in a lack of or a loss of appetite for food, leading to considerable weight loss. |
| Aspergilloma/mycetoma | A fungal clump of the Aspergillus genus present in a body cavity—the lung in the case of TB. Aspergillomas occur as a complication of cavitary scarring caused by M. tuberculosis infection, resulting in symptoms of cough, sputum hyperproduction, and hemoptysis. |
| Bronchial stenosis | Uniform thickening of the bronchial wall, resulting in narrowed bronchial tubes and involvement of a long segment of the bronchi. |
| Bronchiectasis | Condition in which the bronchial tubes are permanently damaged, widened, and thickened, allowing bacteria and mucus to build up and pool in the lungs. This complication commonly occurs in the apical or posterior segments of the upper lobes of the lungs and is associated with post-primary TB. |
| Bronchoesophageal fistula | A fistulous communication (abnormal connection between two hollow spaces) between the bronchus and esophagus that typically arises due to the granulomatous inflammatory processes of the lymph nodes and that is indicated by air-fluid levels in the pleural space. |
| Broncholithiasis | Presence of calcified necrotic material in the bronchi, originating from the lymph nodes and resulting in airway obstruction. |
| Cachexia (wasting syndrome) | Increased weight loss, muscle atrophy, fatigue, weakness, and anorexia in an individual who is not intentionally trying to lose weight but rather which is due to a disease. |
| Dyspnea | Difficult or labored breathing. |
| Empyema | A rare TB complication characterized by a collection of thick pus and a calcified visceral pleura. Empyema is more common in patients with a history of artificial pneumothorax (surgical treatment to collapse the lung) or thoracoscopy (a minimally invasive procedure to visualize the chest cavity). A combination of pleural effusions and cavitation may indicate tuberculous empyema. |
| Erythema nodosum | An acute, subcutaneous red nodular rash that usually occurs 3–8 wk after infection, i.e., at the time of development of specific immunity. This hypersensitivity phenomenon may be due to increased circulating levels of immune complexes and has been documented in <12% of all TB cases. |
| Erythrocyte sedimentation rate | A hematology (blood) test to determine how quickly erythrocytes descend to the bottom of a standardized test tube. This reaction is typically slow, and an increased erythrocyte sedimentation rate can indicate inflammation. |
| Erythrocytes | Red blood cells that contain hemoglobin and that transport oxygen and carbon dioxide to and from tissue. Also see “erythropoiesis” and “erythropoietin response.” |
| Erythropoiesis | Red blood cell (erythrocyte) production/formation. Also see “erythrocytes” and “erythropoietin response.” |
| Erythropoietin response | Erythropoietin is a hormone primarily produced by the kidneys, which plays a key role in red blood cell production. An increase in this response leads to increased erythropoiesis, whereas a decrease leads to anemia. Also see “erythrocytes” and “erythropoiesis.” |
| Fatigue | Fatigue may include weakness, a lack of energy, constant tiredness or exhaustion, a lack of motivation, and/or difficulty concentrating. |
| Fibrosing mediastinitis (sclerosing mediastinitis; mediastinal fibrosis) | The proliferation of dense fibrous tissue within the mediastinum (the membrane partition between the lungs that contains the heart and its vessels, esophagus, trachea, phrenic and cardiac nerves, thoracic duct, thymus, and the lymph nodes of the central chest), resulting in compression. |
| Hematological manifestations | Blood/serum TB abnormalities. |
| Hematopoiesis | Differentiation processes leading to the formation of all blood cells originating from hematopoietic stem cells. In adults, hematopoiesis occurs mainly in the bone marrow, although it can also occur in the liver, thymus, and spleen. |
| Hemoptysis | Coughing up blood originating from the respiratory tract below the glottis. |
| Hyperkalemia | Abnormally increased levels of potassium in the blood. |
| Hypoalbuminemia | A decrease in albumin concentrations in the blood resulting from either decreased production, increased loss, increased use, or an abnormal distribution. Hypoalbuminemia has been associated with infection (sepsis), among other conditions. |
| Hypochloremia | Abnormally decreased levels of the chloride ion in the blood. |
| Hypoferremia | Abnormally decreased iron levels in the blood. |
| Hyponatremia | Decreased blood sodium levels due to production of an antidiuretic hormone-like compound. |
| Leukocytosis | An increase in leukocytes/white blood cells, typically due to an inflammatory response, commonly associated with an underlying infection. |
| Leukopenia | A decrease in leukocytes/white blood cells, resulting in an increased risk of infection. Also see “monocytopenia.” |
| Lymphocytopenia | A decrease in lymphocytes, white blood cells important for immune system functioning. |
| Lymphocytosis | An increase in lymphocytes, white blood cells important for immune system functioning. |
| Malaise | An overall feeling of discomfort or illness. The exact cause is challenging to identify. |
| Monocytopenia | This is a form of leukopenia associated with a decrease in monocytes. Also see “leukopenia.” |
| Monocytosis | An increase in monocytes, i.e., white blood cells that differentiate into macrophages and dendritic cells in the immune system. |
| Necrosis | Cell or tissue death due to either disease, injury, or an insufficient blood supply. |
| Neutropenia | A decrease in neutrophils, i.e., white blood cells that fight against bacterial and fungal infections. |
| Neutrophilia | An increase in neutrophils, i.e., white blood cells that fight against bacterial and fungal infections. |
| Oncotic pleural pressure | Osmotic pressure exerted by proteins in the plasma, which tends to draw water into the circulatory system. |
| Pancytopenia | An overall reduction in white blood cells, red blood cells, and platelets. |
| Pericardial effusion | An abnormal accumulation of fluid around the heart, in the pericardial cavity. |
| Phlyctenular conjunctivitis | An inflammation/redness confined to the lining of the eye’s sclera and below the eyelid (conjunctiva), accompanied by small, red nodules of lymphoid tissue (phlyctenulae). |
| Pleural effusion | An abnormal collection of fluid in the pleural space. |
| Pleurisy (pleuritic chest pain) | The occurrence of pain when breathing, coughing, and/or sneezing. Some cases may present with a cough, fever, and/or shortness of breath if the individual tries to minimize breathing. |
| Pulmonary cavitation | Process starting with caseous lung necrosis, resulting in caseous pneumonia. This further causes alveolar, septal, bronchus, and vessel destruction, forming cavities when the caseous pneumonia regions liquefy, the contents of which are released during coughing. A cavity is composed of a layer of necrosis overlying lipid pneumonia and may be surrounded by a collagen capsule following tissue repair. |
| Pulmonary fibrosis | Thickening, scarring, and stiffening of connective tissue, usually due to long-term injury. Architectural changes occur when lung tissue is replaced with collagenous tissue. |
| Reactive polyarthritis | A type of inflammatory joint disease (arthritis) due to bacterial infection which simultaneously affects five or more joints. |
| Reticulocytosis | An increase in reticulocytes, i.e., immature red blood cells. This is typically due to an overly active attempt (by the bone marrow) to replace red blood cell loss, as in the case of anemia. |
| Reticuloendothelial (transfer) | The reticuloendothelial system refers to circulating phagocytic cells (i.e., monocytes and macrophages) involved in the host immune response. These cells phagocytose foreign materials and particles, removing such complexes from the circulation and tissue. Reticuloendothelial cells primarily acquire iron from heme via phagocytosis and the breakdown of aging red blood cells in order to return iron to the circulation. |
| Thrombocytopenia | A decrease in blood platelets (thrombocytes), which help blood clotting and prevent bleeding. |
| Thrombocytosis | An increase in blood platelets (thrombocytes), which help blood clotting and prevent bleeding. |
| Tuberculous bronchopneumonia | Inflammation of the bronchi due to TB. |
| Wheezing | A high-pitched whistling sound made while breathing, which is often an indication of dyspnea. |
FIG 1.
(A) Basic anatomy of the lungs. (B) The pathophysiology of M. tuberculosis infection. Following M. tuberculosis infection, the bacilli reach the lungs (step 1), provoking a host immune response (step 2). This in turn leads to granuloma formation (step 3), which typically suppresses the infection in its latent state (step 4a). However, reactivation can occur, resulting in an active disease state in which the disease can spread to other individuals (step 4b).
BASIC PATHOPHYSIOLOGY OF PULMONARY TB
The basic pathophysiology of pulmonary TB is illustrated in Fig. 1B. TB is transmitted from a patient with active disease to an uninfected individual through small M. tuberculosis-containing aerosol droplets (typically, 1 to 5 μm in diameter), which are expectorated by coughing, sneezing, or talking/singing (13). These droplets travel through the respiratory tract, where most of the bacilli become trapped by mucus-secreting goblet cells, which are tasked with blocking entry and/or removing foreign entities. In some cases, however, these droplets can bypass this first-line mucociliary defense system, allowing them to reach the upper aerated parts of the lungs. At this point during infection, the host’s second-line/innate immune mechanisms may be recruited, whereby alveolar macrophages engulf the infecting bacilli and attempt to destroy these using various proteolytic enzymes and cytokines, such as tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ). This reaction signals T lymphocyte transfer to the site of infection, initiating a cell-mediated immune response, which may either eliminate the infecting organism or result in granuloma formation (13, 14). A granuloma is a well-known pathological occurrence characterizing pulmonary TB and can be defined as an amorphous mass of immune cells (macrophages, monocytes, neutrophils, natural killer cells, etc.) aimed at restricting microbial spread (15, 16). During early development, the granuloma is highly vascularized (via vascular endothelial growth factor), with the blood vessels having an extensive lymphocytic cuff. As the granuloma develops, macrophages differentiate into various morphotypes (such as epithelioid cells, multinucleated giant cells, and foamy macrophages), resulting in a stratified structure with a layer of lymphocytes aggregated outside a fibrous cuff surrounding a macrophage-rich layer (17). This signifies a stable granuloma that, although unable to eliminate the pathogen, contains the bacilli and that suppresses progression to active disease in immunocompetent individuals. However, M. tuberculosis still proliferates in the healed lesions since the bacilli avoid death by modulating the host immune system and blocking phagolysosomal fusion. Indeed, this process creates a hospitable niche within these phagosomes for the bacilli to persist in a nonreplicating or slowly replicating state, where they may survive for decades (18, 19). In this case, the host is asymptomatic and noninfectious (and is referred to as having latent TB), and the lesions heal within 6 to 8 weeks. Current reports indicate that one-third of the global population is infected with M. tuberculosis in this manner, and most of these individuals (±90%) never manifest any signs of active disease in their lifetime. Some granulomas show an increased accumulation of the caseum in its center, subsequently losing its rigid integrity and rupturing via liquefactive necrosis, not only releasing the contagious bacteria but also forming a cavity in the airway wall, contributing to the lung damage observed in TB patients (2). The bacteria are then able to spread throughout the lungs and possibly the entire body, and active TB, the symptomatic and highly infectious state of the disease, typically develops. Since lung histology during active disease usually indicates granulomas in all stages of development, disease progression from a latent state to an active state is determined, locally, at the level of the granuloma (17). Although a detailed discussion of granuloma formation is beyond the scope of this review, numerous publications related to this topic are available and discuss both the host-driven processes and the role of the pathogen (17, 20–23). In addition, research suggests that the pathogen-induced dysregulation of host lipid synthesis and the utilization thereof play a significant role in disease transition, with foamy macrophages contributing to both bacterial persistence and tissue pathology, resulting in cavitation (17). Thus, the severity and progression of active disease are, at least in part, determined by the effectiveness of the host immune response to limit bacterial replication. Although HIV coinfection is considered the primary cause of TB activation, other conditions, such as malnutrition, chronic renal failure, uncontrolled diabetes mellitus, sepsis, malignancy, chemotherapy, uncontrolled alcohol use, smoking, drug addiction, and the immunosuppressive medication administered following organ transplants, may also trigger disease conversion from latent to active TB (4, 13, 24). Unfortunately, the exact factors (whether environmental or otherwise) and mechanisms contributing to disease transition remain enigmatic.
Primary versus Post-Primary Active Pulmonary TB: a Radiological View
Active/progressive pulmonary TB is classified as either primary or post-primary pulmonary TB (the latter is also known as secondary or reactivation TB), and various studies have confirmed that susceptibility to these is governed by different genes (25). Although both of these disease states have similar radiological presentations, primary TB is more common in infants and children (26) than in adults (23% to 34%) (24). Progressive primary TB describes patients diagnosed with active TB for the first time; hence, these patients have not had any prior M. tuberculosis infection and are unable to contain the disease in its latent form due to unknown factors. Since progressive primary pulmonary TB has a nonspecific radiological appearance, with cavitation presenting in only 10% to 30% of all cases and in inconsistent parts of the lungs, it is often misdiagnosed. In most cases, the infection becomes localized and forms a necrotic granuloma at the initial site of infection (as discussed above), along with hilar and contiguous mediastinal (paratracheal) lymphadenopathy (i.e., enlarged lymph nodes, located in the throat), which is a radiological hallmark of primary TB (2, 15, 27). Over time, calcium is deposited in the granuloma as the host attempts to heal these, resulting in calcification. Granulomatous diseases, such as TB, are the most common etiology of lymph node calcification. In cases in which a calcified granuloma (known as Ghon’s focus/lesion) presents in combination with a calcified/infected lymph node, it is referred to as a Ghon or Ranke complex, which is often subclinical and usually presents subpleurally in the lower lobes of the lungs (2, 26, 28). If these nodes are large enough, they compress adjacent airways, which results in distal atelectasis (a complete or partial collapsed lung). In primary TB cases, pleural effusion is often observed in combination with parenchymal and/or nodal abnormalities and may develop 3 to 6 months after initial infection due to a hypersensitivity response to tuberculoprotein, although this is more common in adults (30% to 40%) than in children (5% to 10%) (2, 29). These pleural effusions are usually unilateral and result from hematogenous bacterial invasion of the pleural space (30). Four entities of primary TB, gangliopulmonary, tuberculous pleuritic, miliary, and tracheobronchial TB, have been described, and each presents with different radiological findings (24).
Post-primary TB is diagnosed when a patient presents with active TB for at least a second time (due to either endogenous reactivation or exogenous reinfection) as a result of decreased immunity and a subsequent inability to contain the bacilli (2, 31). Progressive post-primary TB denotes approximately 80% of all clinical cases and presents with a patchy appearance with unclear linear and nodular opacities (coin lesions). Post-primary TB does not involve lymph nodes or other organs and, hence, is typically confined to the lungs, developing either in the apical or the posterior regions of the upper lobes (83% to 85%)—since these areas are highly oxygenated, promoting proliferation and allowing M. tuberculosis to thrive—or in the superior regions of the lower lobes (11% to 14%). Unlike primary TB, post-primary pulmonary TB is more likely to present with cavitation (40% to 45%), and hence, it is often referred to as cavitary TB (2, 17, 27). TB lesions (i.e., caseous granulomas) are hypoxic, causing progressive lung destruction and severe necrosis via the upregulation of host collagenases, such as matrix metalloproteinase 1 (32). The formation of these large air spaces/cavities allows semiliquid material to be discharged into the bronchial tree, which then spreads to other parts of the lungs, resulting in tuberculous bronchopneumonia (33). Furthermore, with the development of air-fluid levels, the bacilli disseminate, allowing bronchogenic migration/dissemination along the airways, which is relatively common in most cases and which results in clear nodules (2 to 4 mm). A tuberculous bronchitis with bronchial ulceration may also occur when a lymph node perforates into a bronchus (2, 24). Although they are less common in these cases, granuloma formation (5%) and lobar lesions are also recognized radiological patterns, with other complications, such as pleural effusion (16% to 18%), empyema (4%), and fibrosis (41%), occurring in some cases (2), as will be discussed below. Radiologically, post-primary TB typically manifests as parenchymal disease and cavitation, tracheobronchial TB, tuberculous pleuritis, or other complications (24).
Since early diagnosis is generally associated with increased therapeutic efficacy, a radiologist should be able to recognize the classical and atypical features of both primary and post-primary TB. This is especially important when this disease is suspected in the elderly or in immunocompromised individuals, not only in whom treatment is already complicated but also in whom the disease is more likely to have atypical presentations. For a detailed radiological description of the different primary and post-primary disease states, the authors recommend the work of Curvo-Semedo et al. (24).
SYMPTOMS OF PULMONARY TB
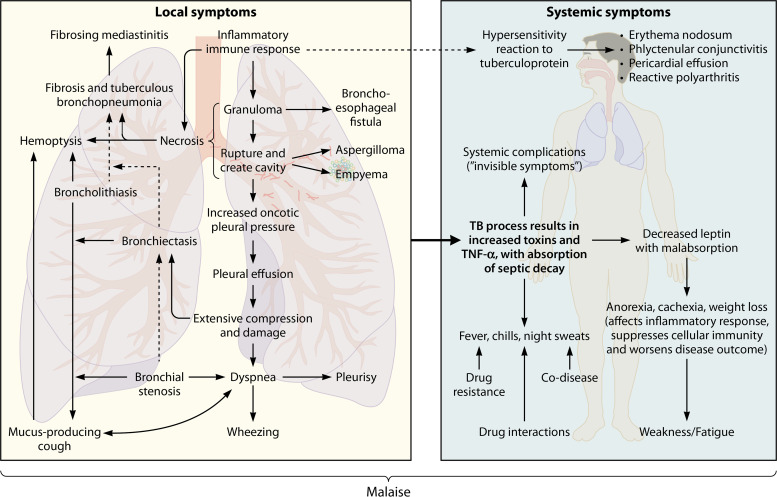
A “symptom” can be defined as any physical or mental indication/feeling of a particular disease, experienced by a patient, that differs from the norm in terms of structure, function, and/or sensation. In this review, local TB symptoms are related to the site of infection (i.e., the lungs), whereas systemic symptoms refer to reactions related to the disease which involve the entire body, such as fever. A systemic complication, on the other hand, can be seen as an invisible symptom to some extent. These manifestations are not typically experienced by the patient, nor are they visible during physical examinations, and they do not form part of the diagnosis of the disease but have a major impact on the overall homeostasis of the body. Figure 2 is an illustration of the symptoms and complications associated with pulmonary TB. It is important to note that the clinical presentation depends on various factors, such as the immune status of the patient, progression of the disease, and the presence of other coinfections, among others. Although the effects of coinfections and comorbidities on the pathophysiology of TB are mentioned in certain subsections below, a detailed discussion of these interactions falls beyond the scope of this review.
FIG 2.
Symptoms and complications associated with active pulmonary TB and their interaction(s) with each other. Active pulmonary TB typically involves local symptoms related to the site of infection (i.e., the lungs), although various systemic symptoms and complications involving the entire body also occur.
Local Symptoms of Pulmonary TB
During active TB, the excessive inflammatory immune response seems to promote lung damage/necrosis via the elevated release of immune mediators, such as transcription factors, cytokines, and chemokines. These either drive the expression of tissue-degrading enzymes (i.e., proteases, such as matrix metalloproteinases) or directly mediate granuloma formation (19), typically forming 6 to 12 weeks after the initial infection (27).
Previous studies suggest that post-primary TB begins as either lipoid pneumonia or a slowly expanding caseous granuloma, often in combination with a microvascular occlusion characteristic of delayed-type hypersensitivity (34). If a granuloma ruptures (hence creating a cavity and leading to the severe lung destruction observed in these patients), the bacilli invade the pleural space and interact with T cells, resulting in the above-mentioned delayed hypersensitivity reaction to mycobacterial proteins and a dramatic increase in fluid in the pleural space (2, 27, 35). This reaction is believed to result in the increased permeability of pleural capillaries to serum proteins, leading to a further increase in oncotic (osmotic) pleural pressure (35), hence compressing the lung as a result of pleural effusion. Pleural effusions can be classified as either transudative (occurring due to hydrostatic pressure or low oncotic pressure) or exudative (occurring due to inflammation and increased capillary permeability), with the latter being associated with TB. Wang et al. (36) applied a 1H nuclear magnetic resonance (NMR)-based metabolomics approach to investigate the etiology of tuberculous pleural effusions. They identified significantly altered levels of metabolites in TB patients with pleural effusion, including decreased levels of citric acid, lactate, creatine, and acetic acid (due to bacterial energy and carbon utilization), as well as increased levels of lipids (associated with the bacterial cell wall) and threonine (either released from host tissue, to meet the needs for gluconeogenesis and host immunity, or produced directly by M. tuberculosis). These novel insights may aid in the diagnosis of tuberculous pleural effusions, which exhibit morphological similarities to the morphologies observed with malignant and transudative pleural effusions.
The extensive lung compression caused by pleural effusion additionally leads to severe inflammation, dyspnea, pleurisy, cough, shortness of breath, and chronic bronchitis in cases with extensive lung damage (2, 19, 27). Interestingly, a metabolomics study done by Che et al. (37) identified decreased serum 5-oxoproline levels in patients with active TB which were associated with cavity formation and, therefore, with pathological lung damage. This metabolite marker can be useful to assess lung damage in TB patients in order to provide preventative care and, in doing so, assist with quality of life post-TB treatment. In rare cases, the granulomatous inflammatory process of the lymph nodes may also lead to the development of an acquired bronchoesophageal fistula (2, 26, 27). Other complications associated with progressive lung destruction and the development of cavities or enlarged air spaces and, hence, with hemolysis may include aspergilloma (<10%) (2, 27) and empyema (4%) (26). Furthermore, bronchial stenosis is a known complication in TB patients (27) and may contribute to the asthma-like symptoms, dyspnea, and wheezing (38). Unfortunately, the exact mechanisms by which bronchial stenosis occurs during TB are not well understood.
As dyspnea develops, an abnormal amount of phlegm (sticky mucus) is produced, which causes wheezing and progressive coughing (in 85% of all pulmonary cases), usually with the excretion of this phlegm, often lasting 3 weeks or more (2). In most TB cases, broncholithiasis occurs due to the extrusion of an adjacent calcified lymph node, further worsening the coughing and directly resulting in lung destruction, which eventually leads to hemoptysis (27, 39), with the latter causing death in 5% to 14% of all patients. This extensive lung damage may also bring about bronchiectasis (71% to 86%), which further adds to the mucus buildup in the lungs and hemoptysis (27). Although the exact mechanism of mucus hyperproduction has not yet been investigated in TB patients, Cohn et al. (40) studied the role of various lymphocytes (e.g., T helper cells) and their associated cytokines in individuals suffering from this occurrence as a result of asthma. In this study, they were able to link mucus hyperproduction to the action of T helper 2 (Th2) lymphocytes in the absence of interleukin-4 (IL-4), IL-5, eosinophils, and mast cells but in conjunction with IL-4 receptor alpha signaling. Furthermore, Th2 cells without IL-13 were unable to stimulate mucus production, even in the presence of airway inflammation. IL-9 also stimulated mucus production via an IL-13-mediated pathway, and IL-13 was additionally shown to act directly on structural lung cells, most likely specialized epithelial cells (i.e., goblet cells) responsible for mucus production. Therefore, in asthma cases, mucus hyperproduction is stimulated by airway inflammation in combination with Th2 lymphocytes and via a single IL-13-mediated pathway. Previous studies have identified a similar increase in the activity of these cytokines in TB patients (41, 42), suggesting that this mechanism of mucus hyperproduction proposed for asthma may also be present in the case of active TB.
As the damaged lung tissue starts to heal, fibrosis occurs, which results in architectural changes. This, especially in combination with infiltrating granulomas, can further lead to fibrosing mediastinitis (although this is rare) (43) and airflow obstruction, resulting in the previously discussed pulmonary symptoms. Fibrosis also contributes to post-TB pulmonary impairment/dysfunction (19).
Systemic Symptoms of Pulmonary TB
Apart from the manifestation of various local clinical symptoms of pulmonary TB, which can directly be linked to the lung infection and the resulting lung damage, the disease is also associated with several systemic symptoms, such as fever. The previously discussed tuberculous process itself (resulting in toxin production and increased TNF-α secretion) and/or the secondary inflammatory processes (e.g., the absorption of toxins and/or septic decay from damaged bronchi and pulmonary cavities) may result in TB-related fever (occurring in 60% to 85% of all cases) (44). Fever occurs in three distinct phases. (i) The heat production phase stimulates heat retention via cutaneous vasoconstriction, while shivering generates additional heat. This causes the hypothalamus to increase the body temperature set point, after which (ii) homeostasis between heat production and heat loss is reached and shivering ceases. The set point lowers back to normal and (iii) cutaneous vasodilatation results in heat loss in the form of sweating (45). Although it is largely unclear why TB-related fever occurs primarily during the night (hence, the term “night sweats”), this occurrence has been associated with the body’s circadian rhythm: the body temperature is normally lower in the predawn hours (36.1°C) and rises in the afternoon (>37.4°C). Moreover, since cortisol is a glucocorticoid/steroid hormone which modulates both the innate and the acquired immune responses and in doing so suppresses fever (45, 46), the decreased secretion of this hormone at night (47) may be the source of TB-related night sweats. Rosha (44) linked TB-related fever to direct complications (22.5%), TB hypersensitivity (12.5%), drug resistance (10%), drug reactions (22.5%), as well as other codiseases, such as other lung infections, malaria, filariasis (a parasitic disease), and amoebic liver abscess (32.5%). Thus, it would be impractical to link fever to a single cause.
The host immune response may also trigger the development of cachexia (also known as wasting syndrome), a hallmark of pulmonary TB and the reason that TB was previously known as “consumption.” Cachexia is characterized by severe, disproportionate muscle wasting and may result from malabsorption or prolonged anorexia (due to a loss of appetite). Although the exact underlying pathophysiological mechanism by which cachexia manifests is poorly understood, it appears to be related to the acute inflammatory host response associated with M. tuberculosis infection, resulting in the activation of numerous previously mentioned cytokines, namely, IL-1, IL-2, TNF-α, and IFN-γ (48–50). TNF-α can act directly on muscle cells, stimulating protein loss, facilitated by nuclear transcription factor κB (NF-κB). This, in turn, increases ubiquitin/proteasome pathway activity, which is mainly involved in disease-related muscle protein degradation, thereby promoting muscle weakness and the consequent weight loss and fatigue. Intermediate steps in this process include the stimulation of TNF-α receptor type 1 (TNFR1) and the increased production of reactive oxygen species (ROS) (48, 51). Cytokine activation also results in decreased MyoD protein (a transcription factor involved in muscle development) and inhibits mRNA activation for myosin synthesis, resulting in myosin proteolysis. Previous studies have indicated significantly increased serum TNF-α concentrations in chronic obstructive pulmonary disease (COPD) patients with cachexia, as well as upregulated NF-κB and inducible nitric oxide synthase in skeletal muscles, leading to impaired protein synthesis (48). Moreover, O’Connell et al. (52) used a 1H NMR-based metabolomics approach to investigate the serum metabolic fingerprint of cachexia in cancer patients. The major metabolic changes associated with cachexia included increased concentrations of both very-low-density lipoprotein and low-density lipoprotein, in conjunction with decreased serum glucose levels. Apart from elucidating these metabolic alterations, this study demonstrated the potential of metabolomics as a diagnostic tool for cachexia. Since individuals with COPD and cancer, like individuals with TB, also suffer from weight loss associated with muscle weakness, further investigations aimed at identifying the proposed mechanism of cachexia in TB patients will be of great value. This may ultimately help guide medical interventions to prevent TB-related weight loss.
In addition to the mechanisms suggested above, the severe weight loss seen in TB patients is potentially related to the plasma concentrations of leptin, the hormone involved in weight regulation and cell-mediated immunity (53, 54). Various studies have shown significantly decreased blood leptin concentrations in TB patients compared to controls. Body fat mass and inflammation independently affect leptin concentrations: fat is positively correlated with leptin, while C-reactive protein (CRP) and TNF-α production are inversely associated with leptin. Moreover, CRP and leptin are independently associated with a loss of appetite. Thus, a prolonged host immune response suppresses leptin production, and since leptin is involved in cell-mediated immunity, a decrease in leptin concentrations may contribute to TB disease severity, especially in the case of cachexia (50, 54, 55). In addition to decreased body fat and increased inflammation, the TB-induced variation in the concentrations of several hormones (including insulin and cortisol) may also potentially mediate and influence leptin production and weight loss. Moreover, research shows that these abnormal leptin concentrations normalized with treatment (50, 56) and that TNF-α levels can be reduced with the administration of thalidomide (α-N-phthalimidoglutarimide), which has been shown to accelerate weight gain in TB patients (57).
Finally, the hypersensitivity reaction to tuberculoprotein observed in some TB cases can be portrayed as either erythema nodosum, phlyctenular conjunctivitis, increased dermal protein hypersensitivity, pleural or pericardial effusion, or reactive polyarthritis, even before evidence of active disease. However, the simultaneous occurrence of more than one of these severe reactions in a single patient is rare (58).
Systemic Complications of Pulmonary TB
Active pulmonary TB has long been associated with several less obvious, sometimes invisible deviations in normal systemic parameters. Although, unlike the clinical symptoms, patients are most likely unaware of these complications, the biochemical mechanisms leading to these manifestations might hold the key to unlocking various mysteries regarding TB vaccination, as well as diagnostic and treatment protocols. The following sections give a brief overview of the current understanding of the mechanisms responsible for some of these complications, including increased oxidative stress, hyponatremia, hypocholesterolemia, vitamin D deficiency, glucose intolerance, hematological manifestations, and an altered microbiota.
Increased oxidative stress.
Increased oxidative stress is a well-known, although unspecific, occurrence in TB patients (59–61). During the initial stages of M. tuberculosis infection, a burst of host-generated ROS (via phagocytic cells, such as macrophages) and reduced glutathione production significantly impact the control of the disease. Previous studies have demonstrated that reduced glutathione levels not only contribute to decreased intracellular bacterial survival but also downregulate the overexpression of proinflammatory cytokines, such as Th2 cells (62). When comparing the sputum metabolome profiles of TB-positive and TB-negative individuals, metabolite variations indicated an increased production of hydrogen peroxide via glucose oxidation, as well as pronounced oxidative stress, during active disease (63). The increased oxidative stress has also been associated with DNA damage in these patients (64). Combrink et al. (65) further showed an overall reduction in oxidative stress levels in TB patients throughout treatment, which, in turn, caused a time-dependent induction and inhibition of several enzymes. A better understanding of the underlying cause(s) of TB-induced oxidative stress could perhaps aid in the development of improved treatment protocols, which could eliminate the bacteria and alleviate ROS-related complications simultaneously.
Hyponatremia.
The association between pulmonary TB and hyponatremia has been evident for many years (66, 67). Recent studies observed hyponatremia in more than 50% of all TB cases, an occurrence that is especially prevalent among older patients (68), with mild and asymptomatic hyponatremia being the most common. The severity of hyponatremia was shown to be directly proportional to sputum positivity and extensive involvement of the pulmonary parenchymal lesions (6, 68). Hyponatremia typically occurs as a result of water retention, when water excretion does not match oral or intravenous water intake. Various studies have indicated normal adrenal and renal functions in hyponatremic TB patients, suggesting that the underlying cause might be attributed to a syndrome of inappropriate antidiuretic hormone secretion (SIADH) (67, 69). Under normal circumstances, antidiuretic hormone (ADH) is secreted by the pituitary gland to stimulate increased renal water retention when body fluid osmolality increases. In the case of SIADH, ADH is continuously secreted, despite a normal or increased plasma volume, leading to impaired water excretion and, hence, hyponatremia. Vorherr et al. (70) were the first to demonstrate the link between ADH and hyponatremia in TB by indicating antidiuretic activity in the active inflammatory zone of the lungs. This activity was absent in uninvolved lung tissue, M. tuberculosis cultures, and culture media containing metabolites of M. tuberculosis. The group accordingly hypothesized that tuberculous lung tissue might either produce ADH, similar to what is seen in bronchogenic carcinomas, or passively adsorb plasma ADH. This suggestion was supported when Lee et al. (71) provided evidence of ectopic ADH production in a TB patient that was triggered by a nonneoplastic infective/inflammatory condition and that resolved after successful treatment. To date, the mechanisms and the underlying cause(s) of the observed hyponatremia in TB patients remain poorly understood; however, the presence thereof should be considered when treating this infection. In addition to hyponatremia, Olalekan et al. (72) also identified hyperkalemia and hypochloremia in TB patients receiving treatment. Further insights into these electrolyte imbalances and the mechanisms causing these imbalances may shed new light on the predisposition, symptoms, and variations in treatment outcomes seen in these patients.
Hypocholesterolemia.
In a study done in 1979, serum cholesterol concentrations were significantly lower in TB patients than in their healthy counterparts (7). Since then, several studies have been done to validate and/or explain this phenomenon. In a recent metabolomics study, Feng et al. (73) detected decreased serum behenic acid (a fatty acid which has been shown to increase serum cholesterol levels in humans) in TB-positive patients compared to healthy individuals. This was attributed to the direct involvement of cholesterol in the activation of lymphocytes (i.e., CD4 T cells, CD8 T cells, and γδ T cells), as well as in the promotion of IFN-γ and TNF-α release during TB infection. As part of the cell membrane of macrophages, cholesterol is also involved in the process of M. tuberculosis phagocytosis, whereas in the lymphocyte membrane, this compound plays an important role in the differentiation and proliferation of cytotoxic cells, further explaining the decreased cholesterol levels observed in TB patients. Apart from its direct involvement in the immune response, the decrease in systemic cholesterol levels has also been associated with a nutritional contribution toward M. tuberculosis growth and maintenance, which is essential for the intracellular survival of this pathogen. The control of M. tuberculosis infection relies heavily on the activation of macrophages via IFN-γ. This activation not only triggers the release of toxic oxidative radicals (mentioned above) but also deprives intracellular bacteria of essential nutrients (74). Although this response to infection decelerates M. tuberculosis replication, the bacilli can persist in this state due to their ability to use lipids (including sterols) as an alternative energy source (14). This concept is supported by the recent identification of the functionality of a gene cluster (mce4), previously recognized to be crucial for bacterial survival in prolonged infection, as a cholesterol import system that allows M. tuberculosis to obtain carbon and energy from the cholesterol present in host membranes (75). Various uncharacterized virulence genes have also recently been identified to have sterol catabolic functions (76, 77), proving that host-derived cholesterol is a crucial nutrient for bacterial survival in vivo. Furthermore, Martens et al. (78) were able to link elevated serum cholesterol levels in mice with increased susceptibility to TB. The rate and severity of tissue necrosis, inflammation, and foamy macrophage formation increased with the level of hypercholesterolemia. These findings suggest that individuals with high serum cholesterol levels might be more vulnerable to TB, although further research is needed to explain the different roles that the various lipoproteins may play in this susceptibility.
Although hypercholesterolemia is typically considered a more predominant health threat, preterm delivery and lower birth weights of infants have been linked to maternal hypocholesterolemia (79). Lowered serum cholesterol levels have also been associated with depression (80), increased suicidal risk (81), and cerebral hemorrhage in men (82).
Vitamin D deficiency.
Lowered serum vitamin D levels have long been associated with TB patients (10), and although various studies have indicated that a vitamin D deficiency increases the risk for the activation of latent TB, others have stated the contrary, posing that the actual M. tuberculosis infection lowers vitamin D levels. Supporting the latter is the fact that the active form of vitamin D, 1,25(OH)2D, acts as a modulator for macrophage function, thereby suppressing M. tuberculosis survival and growth (16). Liu et al. (83) showed that infection-induced activation of Toll-like receptors in macrophages leads to an upregulated expression of the vitamin D receptor and the vitamin D-1-hydroxylase genes, which in turn leads to the induction of cathelicidin, an antimicrobial peptide which is crucial in the killing of intracellular M. tuberculosis bacilli. In addition to this well-known antimicrobial effect of vitamin D, it has also recently been shown that higher levels of vitamin D lead to the abolishment of infection-induced accumulation of lipid droplets within the macrophages by downregulating the adipogenic function of the proadipogenic peroxisome proliferator-activated receptor gamma (PPARγ). This function deprives M. tuberculosis of lipids, which is thought to be the major source of nutrients for this pathogen within macrophages (as previously mentioned), thereby inhibiting its growth (84). Lower serum vitamin D levels can therefore increase the risk of active TB, an observation supported by the fact that TB incidence is higher in colder seasons (when sun exposure and the consequent vitamin D synthesis are less), as well as in individuals with lower vitamin D levels due to either uremic and chronic kidney disease, diet, smoking, ethnicity, or age (10, 85). Although the addition of vitamin D to anti-TB therapy seems to be the next logical step, clinical trials have shown that this supplementation indeed does not reduce the time to sputum culture conversion, which is a surrogate outcome marker for successful treatment of this disease (86, 87). Further studies are therefore required to establish the potential role of vitamin D in TB prevention and cure.
Glucose intolerance.
In the early years of the 20th century, an American physician, Howard Root, wrote a treatise describing the association between diabetes mellitus (DM) and TB after studying 245 patients carrying both burdens (88). His findings have since been confirmed in numerous epidemiological studies done in various parts of the world (8, 89–92), all of which concluded that people with DM have an approximately 3-fold greater risk of developing active TB than those without DM (93). Despite the rising incidence of the convergence of the two epidemics, the basis of this relationship remains poorly understood. When studying this correlation in human subjects, Gomez et al. (94) observed a reduced association of M. tuberculosis with monocytes in DM cases. This suggests an altered route of entry for the pathogen, which may influence the downstream activation of signaling pathways in the monocytes of these patients, thereby enhancing the possibility of pathogen survival. However, although such studies confirm that DM has an unavoidable effect on the development of TB, investigators still debate the directionality of this association, given that TB induces interim glucose intolerance, which dissolves after successful TB treatment (95–98). Various hypotheses explaining the increased prevalence of glucose intolerance among TB patients have been put forward. One of these, malnutrition, was suggested because of its well-known connotation with glucose metabolism disorders. There is also an established association between malnutrition and susceptibility to TB. However, upon investigation, similar body mass index levels were identified in TB patients with normal and abnormal glucose tolerance outcomes (97). Others have proposed that occult glucose intolerance predisposes to TB (99) or that the observed glucose intolerance is a reflection of a prediabetic state and not simply a reaction to M. tuberculosis infection (100). These suggestions, however, still need to be proven by well-controlled studies. The increased prevalence of glucose intolerance has also been attributed to the development of stress diabetes in newly diagnosed TB patients (97). This hypothesis relies on the fact that stress hormones, i.e., epinephrine, glucagon, and cortisol, are stimulated in response to an acute infection, which in turn raises the blood glucose levels (101). This proposal is supported by a metabolomics study in which increased levels of normetanephrine, a derivative of norepinephrine, and d-gluconic acid δ-lactone, a metabolite associated with diabetes, were detected in the sputum of TB patients but not in that of the controls (63). When investigating the matter from a different perspective, Karachunskiĭ et al. (102) observed a relative insulin deficiency and a higher secretory function of the pancreatic insular apparatus, which decreased its functional reserves as the disease progressed in pulmonary TB patients with secondary DM. These findings were recently endorsed by the identification of an altered amino acid metabolism and increased levels of urinary fatty acids, DNA breakdown products, and bacterial components in TB patients, which may be directly linked to reduced insulin levels in these individuals (64).
When considering all of the findings presented above, it is clear that the association between DM and TB is highly complex and that the occurrence can most probably be linked to a variety of intertwined mechanisms and reactions. Also, since a similar temporary glucose intolerance has been identified in pneumonia patients, these hypotheses may not be unique to TB but, rather, may be a reaction to general lung infection (97). However, irrespective of the underlying processes, it is evident that the addition of antidiabetic drugs, such as metformin, to the conventional TB treatment regimen should be considered. Singhal et al. (103) showed that this cotherapy in TB patients reduced the intracellular growth of M. tuberculosis by stimulating the production of ROS, facilitating phagolysosomal fusion by enhancing the host immune response; lowered the TB-induced tissue pathology and inflammation; decreased the TB severity; increased the efficiency of anti-TB drugs; improved the clinical outcome of the patients; and reduced the incidence of latent TB.
Hematological manifestations.
TB has been associated with various hematological abnormalities, occurring in 10% of all TB cases, of which normocytic normochromic anemia (up to 94%) is indicated to be the most common in all forms of TB (i.e., pulmonary and disseminated) (9, 104). Anemia can be classified as either anemia of chronic disease, anemia related to metabolic deficiencies, autoimmune-related anemia, or anemia resulting from bone marrow complications. In anemia of chronic disease, i.e., during TB, reticuloendothelial transfer of iron to the nucleus of erythrocytes is inhibited. Additionally, the severe inflammation activates reticuloendothelial cells, which not only sequester iron, leading to hypoferremia and iron-limited erythropoiesis, but also accelerates erythrocyte destruction, thereby promoting a compensatory erythropoietin response. Furthermore, during phagocytosis, lactoferrin, a glycoprotein, is released from normal white cell granules and binds iron, rendering it unavailable for binding to transferrin, subsequently impairing normal iron transfer, thereby causing anemia. Also, various metabolic deficiencies seen in TB patients are associated with macrocytic anemia, of which folate and vitamin B12 deficiencies are well-known. These may occur due to poor nutritional intake or due to increased bacterial utilization during active disease and have been associated with increased disease progression. Interestingly, anemia is also evident during anti-TB therapy, which has been ascribed to a decrease in inflammation and, hence, an increase in iron availability, allowing for the resumption of normal hematopoiesis, causing reticulocytosis (9). Nonetheless, the observed hematological abnormalities revert to normal following treatment, if the inflammation subsides (104).
Other hematological abnormalities associated with pulmonary TB include an elevated erythrocyte sedimentation rate (ESR), hypoalbuminemia, leukopenia, leukocytosis, neutropenia, neutrophilia, lymphocytopenia, lymphocytosis, monocytopenia, monocytosis, and pancytopenia. Pancytopenia is typically observed only in disseminated TB, in which case thrombocytopenia is also more common; thrombocytosis was shown to be more prevalent in pulmonary TB cases and has been linked to markers of inflammation, as estimated by the ESR (9, 104–106). For a detailed review of hematological abnormalities, the authors recommend the work of Balepur and Schlossberg (9).
Considering the significant disease burden of TB patients, those with other coinfections are more prone to increased mortality. To this end, Javed et al. (106) investigated the hematological profiles of pulmonary TB patients without any comorbidity versus those of patients with codiseases consisting of hepatitis C, HIV infection, diabetes, and/or myocardial infarction alone or in combination. They indicated that the alterations in the white blood cell count and the levels of neutrophils, lymphocytes, monocytes, and eosinophils are much more pronounced during comorbidities. Similarly, Abay et al. (107) assessed the hematological manifestations in pulmonary TB patients with and without HIV infection and indicated significantly worse cases of anemia, leukopenia, neutropenia, lymphopenia, and thrombocytopenia in the coinfected group. An increased ESR was observed in both groups. Since any comorbidity drastically influences anti-TB therapy outcome and quality of life, these not only should be confirmed as soon as possible (before anti-TB therapy), but also the associated hematological manifestations may possibly be used as indicators to assess the diagnosis, follow-up care, and monitoring of pulmonary TB patients.
Altered microbiota.
In recent years, various studies have emphasized the importance of resident microbiota (referring to all microorganisms that live in or on mammals) to human health. Alterations in the microbiota composition have been associated with various pathogenic aspects of diseases, such as obesity (108), type 2 diabetes (109), rheumatic diseases (110), bacterial vaginosis (111), and several other bacterial and viral infections (112–114). A few studies have focused on alterations to the microbiota in patients with pulmonary TB. Cui et al. (11) found that the microbiota in the sputum of TB patients is more diverse (including 24 phyla) and contains more foreign and less normal bacteria than the microbiota in the sputum of healthy individuals (17 phyla). They speculated that the severe host immune response triggered by the initial M. tuberculosis infection may also eliminate some normal bacteria while at the same time may damage lung tissue, thereby providing an environment favoring the invasion of foreign bacteria. These foreign bacteria can then assist with the further destruction of the lungs, especially in patients with active TB. In contrast, Cheung et al. (115) found the diversity of sputum microbiota to be similar in TB patients and healthy controls, although Proteobacteria and Bacteroidetes were more predominant in patient samples and Firmicutes were more predominant in controls. Results from a different study (116) also contradicted those found by Cui et al. (11). Here, the diversity of the microbiota in the sputum of healthy controls was shown to be higher than that in the sputum of TB patients. This group additionally identified a change in the abundance of some genera in recurrent TB patients from that in newly infected TB patients and also found Pseudomonas and the ratio of Pseudomonas to Mycobacterium to be more abundant in patients with a poor treatment outcome than in those with a successful outcome, posing that the microbiota composition should be considered when developing new treatment regimes. In an attempt to elaborate on these studies, Botero et al. (117) evaluated the bacterial and fungal communities in sputum, oropharynx, and nasal respiratory tract samples collected from TB patients. The community structures (bacterial and fungal) were similar in the sputum and oropharynx samples, and the community structures in both of these sets of samples differed from the community structures in nasal samples, suggesting that, in future, oropharynx samples could be used to investigate TB-induced microbiota variations.
Apart from the microbiota composition at the site of infection (in this case, the respiratory system), the gut microbiota has also been strongly associated with the host immune system (118). To determine the effect of TB on the gut microbiota, Winglee et al. (119) studied fecal samples from mice infected with M. tuberculosis from the time of preinfection until death. A significant difference in the microbial composition (decreased diversity) was observed as early as 6 days postinfection, possibly due to host immunological changes. More specifically, a significant change in abundance was observed for Clostridiales (which has been shown to induce regulatory T cells) and Bacteroidales (which plays a role in the anti-inflammatory response and various other aspects of the immune system). Although no study to date has focused on the gut microbiota in human TB patients, a recent metabolomics study observed an altered phenylalanine metabolism in TB patients, which the investigators ascribed to dysregulated gut microbiota activity (120). Not only was this altered phenylalanine metabolism supported by Luies and Loots (64), but they additionally linked a gut microbiome imbalance (specifically, changes in α-resorcylic acid and dihydroferulic acid) at the time of diagnosis with an eventual poor treatment outcome (121, 122). It should also be noted that the TB-related variation in the gut microbiota may potentially be influenced by diet, body mass, and environmental factors (120).
CONCLUSION
Considering the information presented above, it is evident that although proof and hypotheses explaining the mechanisms responsible for some of the symptoms and complications of pulmonary TB exist, this field is still in need of extensive elaborative research. In the past 20 years, newer omics research techniques have contributed substantially to our understanding of various aspects of TB disease and host response mechanisms. Further applications of these and other methodologies to better understand TB symptoms and systemic complications may add a fresh perspective on the disease presentation and the underlying causes leading to this echo. This new knowledge may, in turn, pave the way for the development of more effective treatment procedures; for example, since most successfully cured TB patients have remaining lung damage, targeted treatment to prevent such symptoms will drastically impact their quality of life. However, for such targeted treatment approaches to be effective, knowledge of the exact immune pathology mechanisms contributing to lung damage is necessary. Moreover, given the complexity and often late diagnosis of TB, the findings of such research may perhaps initiate a complete renovation of the currently used diagnostic methods, possibly using clinical and/or secondary systemic complications as indicators to assess or aid the diagnosis, follow-up care, and monitoring of pulmonary TB patients. It is, however, of utmost importance that researchers take individual variation and atypical disease presentation into account when investigating these mechanisms. The clinical symptoms of pulmonary TB are often nonspecific, and in approximately 5% of all adult cases and 60% of all pediatric cases, signs of the disease are completely absent. Studies aimed at describing the prevalence of TB symptoms have reported inconsistent results, with the prevalence of a prolonged cough ranging from 42% to 89% and that of a prolonged fever ranging from 23% to 68%, for example. These variations can most likely be ascribed to the specific study populations included in the different investigations, seeing that a significant association between Asian ethnicity and a lack of symptoms has been identified. In addition, a lower prevalence of fever, sweating, and hemoptysis, accompanied by a greater prevalence of dyspnea and concomitant conditions, such as cardiovascular disorders, COPD, diabetes, and malignancies, has also been identified in older TB patients (patients ≥60 years old) than in their younger counterparts. Sex has furthermore been classified as an indicator of symptomatic variation, with a persistent cough, sputum expectoration, and hemoptysis being less common among women than among men. The identification and characterization of individual variation and atypical presentations of pulmonary TB may help clinicians to develop patient subgroups, which in turn may be a valuable tool when performing symptom-based diagnostics. A better description of these factors may also lead to the development of more personalized treatment protocols, which could alleviate symptoms more quickly and more effectively and perhaps lead to shortened treatment periods with fewer cases of treatment failure and relapse. Although practically impossible, the ideal research sample cohort would therefore have to include a hefty number of patients, including those from various age, ethnicity, sex, and other potentially clustering groups. The combination of globally collected results in a comprehensive database would be an alternative option. However, irrespective of the approach followed, it is evident that more focus should be placed on the elucidation of the underlying biochemical processes which initiate the clinical symptoms and other systemic complications of pulmonary TB.
ACKNOWLEDGMENTS
L.L. conceptualized the review and designed/constructed the figures and table; L.L. and I.D.P. evaluated current literature for drafting the paper, followed by critical review. Both authors approved the final version to be submitted.
We declare that there are no conflicts of interest and that the manuscript and the work described herein are unpublished and have not been submitted for publication elsewhere.
We have no specific funding to report.
Biographies

Laneke Luies completed her Ph.D., entitled “Characterising Tuberculosis and Treatment Failure Thereof Using Metabolomics,” in 2017 at the North-West University (NWU) in Potchefstroom, South Africa. Thereafter, Dr. Luies started two postdoctoral research fellowships at the University of Cape Town (2017) and the NWU (2018) and is currently employed at the Focus Area: Human Metabolomics (at the NWU) as a senior subject specialist (since 2019). Over the past 9 years, she has published numerous papers in high-impact, peer-reviewed international journals relating to her TB research, with a focus in TB metabolomics, specifically to elucidate new biochemical mechanisms allowing for a better understanding of TB and the host-pathogen interactions and adaptations, as well as treatment failure.

Ilse du Preez completed her Ph.D., entitled “A Metabolomics Approach for Characterising Tuberculosis,” in 2013 at the North-West University (NWU) in Potchefstroom, South Africa. Thereafter, she continued working in the field of TB metabolomics as a postdoctoral fellow at the same institution and published several papers on this topic in high-impact international journals. Dr. du Preez is presently employed at the Centre for Human Metabolomics (NWU) as a senior subject specialist, where she functions as the manager of the Contract Research Services division. For the past 13 years, her research has been focused on a major South African burden, TB, specifically, the underlying metabolic mechanisms of the disease.
REFERENCES
- 1.World Health Organization. 2019. Global tuberculosis report 2019. WHO Press, Geneva, Switzerland. [Google Scholar]
- 2.Leung AN. 1999. Pulmonary tuberculosis: the essentials. Radiology 210:307–322. doi: 10.1148/radiology.210.2.r99ja34307. [DOI] [PubMed] [Google Scholar]
- 3.Bakhsi S. 2006. Tuberculosis in the United Kingdom: a tale of two nations. Troubador Publishing Ltd, Leicester, United Kingdom. [Google Scholar]
- 4.Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H, Menzies D, Raviglione M. 2016. Tuberculosis. Nat Rev Dis Primers 2:16076. doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 5.Wong S, Yeung S. 2001. Erythema nodosum as the first presenting complaint of asymptomatic pulmonary tuberculosis. Hong Kong J Emerg Med 8:166–168. doi: 10.1177/102490790100800309. [DOI] [Google Scholar]
- 6.Bokam BR, Badikillaya VU. 2017. Prevalence of hyponatremia in pulmonary tuberculosis—a pilot study from a tertiary care center in south India. Int J Med Sci Public Health 6:75–79. doi: 10.5455/ijmsph.2017.20062016560. [DOI] [Google Scholar]
- 7.Taylor GO, Bamgboye AE. 1979. Serum cholesterol and diseases in Nigerians. Am J Clin Nutr 32:2540–2545. doi: 10.1093/ajcn/32.12.2540. [DOI] [PubMed] [Google Scholar]
- 8.Perez A, Brown HS III, Restrepo BI. 2006. Association between tuberculosis and diabetes in the Mexican border and non-border regions of Texas. Am J Trop Med Hyg 74:604–611. doi: 10.4269/ajtmh.2006.74.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balepur SS, Schlossberg D. 2016. Hematologic complications of tuberculosis. Microbiol Spectr 4:TNMI7-0004-2016. doi: 10.1128/microbiolspec.TNMI7-0004-2016. [DOI] [PubMed] [Google Scholar]
- 10.Nnoaham KE, Clarke A. 2008. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol 37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 11.Cui Z, Zhou Y, Li H, Zhang Y, Zhang S, Tang S, Guo X. 2012. Complex sputum microbial composition in patients with pulmonary tuberculosis. BMC Microbiol 12:276–283. doi: 10.1186/1471-2180-12-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du Preez I, Luies L, Loots DT. 2019. The application of metabolomics toward pulmonary tuberculosis research. Tuberculosis (Edinb) 115:126–139. doi: 10.1016/j.tube.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Knechel NA. 2009. Tuberculosis: pathophysiology, clinical features, and diagnosis. Crit Care Nurse 29:34–43. doi: 10.4037/ccn2009968. [DOI] [PubMed] [Google Scholar]
- 14.Philips JA, Ernst JD. 2012. Tuberculosis pathogenesis and immunity. Annu Rev Pathol 7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 15.Behr MA, Waters WR. 2014. Is tuberculosis a lymphatic disease with a pulmonary portal? Lancet Infect Dis 14:250–255. doi: 10.1016/S1473-3099(13)70253-6. [DOI] [PubMed] [Google Scholar]
- 16.Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, Behar SM. 2014. In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol 12:289–299. doi: 10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell DG, Cardona P-J, Kim M-J, Allain S, Altare F. 2009. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol 10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warner DF. 2015. Mycobacterium tuberculosis metabolism. Cold Spring Harb Perspect Med 5:a021121. doi: 10.1101/cshperspect.a021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravimohan S, Kornfeld H, Weissman D, Bisson GP. 2018. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 27:170077. doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlers S, Schaible UE. 2013. The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Front Immunol 3:411. doi: 10.3389/fimmu.2012.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndlovu H, Marakalala MJ. 2016. Granulomas and inflammation: host-directed therapies for tuberculosis. Front Immunol 7:434. doi: 10.3389/fimmu.2016.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah KK, Pritt BS, Alexander MP. 2017. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis 7:1–12. doi: 10.1016/j.jctube.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell DG. 2007. Who puts the tubercle in tuberculosis? Nat Rev Microbiol 5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 24.Curvo-Semedo L, Teixeira L, Caseiro-Alves F. 2005. Tuberculosis of the chest. Eur J Radiol 55:158–172. doi: 10.1016/j.ejrad.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Alcaïs A, Fieschi C, Abel L, Casanova J-L. 2005. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med 202:1617–1621. doi: 10.1084/jem.20052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Backer A, Mortele K, De Keulenaer B, Parizel P. 2006. Tuberculosis: epidemiology, manifestations, and the value of medical imaging in diagnosis. JBR-BTR 89:243–250. [PubMed] [Google Scholar]
- 27.Andreu J, Caceres J, Pallisa E, Martinez-Rodriguez M. 2004. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol 51:139–149. doi: 10.1016/j.ejrad.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Restrepo R, Palani R, Matapathi UM, Wu Y-Y. 2010. Imaging of round pneumonia and mimics in children. Pediatr Radiol 40:1931–1940. doi: 10.1007/s00247-010-1767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller NL, Franquet T, Lee KS, Silva C. 2007. Imaging of pulmonary infections. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 30.Cruz AT, Starke JR. 2007. Clinical manifestations of tuberculosis in children. Paediatr Respir Rev 8:107–117. doi: 10.1016/j.prrv.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Madkour MM, Abusabaah Y, Mousa AB, Al Masoud A. 2004. Post-primary pulmonary tuberculosis, p 313–327. In Tuberculosis. Springer, New York, NY. [Google Scholar]
- 32.Belton M, Brilha S, Manavaki R, Mauri F, Nijran K, Hong YT, Patel NH, Dembek M, Tezera L, Green J, Moores R, Aigbirhio F, Al-Nahhas A, Fryer TD, Elkington PT, Friedland JS. 2016. Hypoxia and tissue destruction in pulmonary TB. Thorax 71:1145–1153. doi: 10.1136/thoraxjnl-2015-207402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. 2005. Lung remodeling in pulmonary tuberculosis. J Infect Dis 192:1201–1210. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 34.Hunter RL, Jagannath C, Actor JK. 2007. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis (Edinb) 87:267–278. doi: 10.1016/j.tube.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Ferrer J. 1997. Pleural tuberculosis. Eur Respir J 10:942–947. [PubMed] [Google Scholar]
- 36.Wang C, Peng J, Kuang Y, Zhang J, Dai L. 2017. Metabolomic analysis based on 1H-nuclear magnetic resonance spectroscopy metabolic profiles in tuberculous, malignant and transudative pleural effusion. Mol Med Rep 16:1147–1156. doi: 10.3892/mmr.2017.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Che NY, Cheng JH, Li HJ, Zhang ZG, Zhang XX, Ding ZX, Dong FT, Li CY. 2013. Decreased serum 5-oxoproline in TB patients is associated with pathological damage of the lung. Clin Chim Acta 423:5–9. doi: 10.1016/j.cca.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Andrews C. 1935. Bronchial stenosis in pulmonary tuberculosis. Can Med Assoc J 33:36–41. [PMC free article] [PubMed] [Google Scholar]
- 39.Seo JB, Song K-S, Lee JS, Goo JM, Kim HY, Song J-W, Lee IS, Lim T-H. 2002. Broncholithiasis: review of the causes with radiologic-pathologic correlation. Radiographics 22:S199–S213. doi: 10.1148/radiographics.22.suppl_1.g02oc07s199. [DOI] [PubMed] [Google Scholar]
- 40.Cohn L, Whittaker L, Niu N, Homer RJ. 2002. Cytokine regulation of mucus production in a model of allergic asthma, p 201–220. Wiley Online Library, Hoboken, NJ. [PubMed] [Google Scholar]
- 41.Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, Bah B, Benagiano M, Diallo A, Manetti R, Manneh K, Gustafson P, Bennett S, D'Elios MM, McAdam K, Prete GD. 2002. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol 32:1605–1613. doi:. [DOI] [PubMed] [Google Scholar]
- 42.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. 2007. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Tan R, Martires J, Kamangar N. 2016. Tuberculosis-associated fibrosing mediastinitis: case report and literature review. J Clin Imaging Sci 6:32–35. doi: 10.4103/2156-7514.188958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosha D. 2002. Prolonged fever during the treatment of pulmonary tuberculosis. Med J Armed Forces India 58:127–129. doi: 10.1016/S0377-1237(02)80045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalal S, Zhukovsky DS. 2006. Pathophysiology and management of fever. J Support Oncol 4:9–16. [PubMed] [Google Scholar]
- 46.Morey JN, Boggero IA, Scott AB, Segerstrom SC. 2015. Current directions in stress and human immune function. Curr Opin Psychol 5:13–17. doi: 10.1016/j.copsyc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Born J, Kern W, Bieber K, Fehm-Wolfsdorf G, Schiebe M, Fehm HL. 1986. Night-time plasma cortisol secretion is associated with specific sleep stages. Biol Psychiatry 21:1415–1424. doi: 10.1016/0006-3223(86)90333-1. [DOI] [PubMed] [Google Scholar]
- 48.Morley JE, Thomas DR, Wilson M-M. 2006. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 49.Plata-Salamán CR. 1996. Anorexia during acute and chronic disease. Nutrition 12:69–78. doi: 10.1016/s0899-9007(96)90702-9. [DOI] [PubMed] [Google Scholar]
- 50.Chang SW, Pan WS, Beltran DL, Baldelomar LO, Solano MA, Tuero I, Friedland JS, Torrico F, Gilman RH. 2013. Gut hormones, appetite suppression and cachexia in patients with pulmonary TB. PLoS One 8:e54564. doi: 10.1371/journal.pone.0054564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reid MB, Li Y-P. 2001. Tumor necrosis factor-α and muscle wasting: a cellular perspective. Respir Res 2:269–272. doi: 10.1186/rr67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Connell TM, Ardeshirpour F, Asher SA, Winnike JH, Yin X, George J, Guttridge DC, He W, Wysong A, Willis MS, Couch ME. 2008. Metabolomic analysis of cancer cachexia reveals distinct lipid and glucose alterations. Metabolomics 4:216–225. doi: 10.1007/s11306-008-0113-7. [DOI] [Google Scholar]
- 53.Yüksel İ, Şencan M, Sebila Dökmetaş H, Dökmetaş İ, Ataseven H, Yönem Ö. 2003. The relation between serum leptin levels and body fat mass in patients with active lung tuberculosis. Endocr Res 29:257–264. doi: 10.1081/ERC-120025033. [DOI] [PubMed] [Google Scholar]
- 54.Van Crevel R, Karyadi E, Netea MG, Verhoef H, Nelwan RH, West CE, Van der Meer JW. 2002. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab 87:758–763. doi: 10.1210/jcem.87.2.8228. [DOI] [PubMed] [Google Scholar]
- 55.Buyukoglan H, Gulmez I, Kelestimur F, Kart L, Oymak FS, Demir R, Ozesmi M. 2007. Leptin levels in various manifestations of pulmonary tuberculosis. Mediators Inflamm 2007:64859. doi: 10.1155/2007/64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mexitalia M, Dewi YO, Pramono A, Anam MS. 2017. Effect of tuberculosis treatment on leptin levels, weight gain, and percentage body fat in Indonesian children. Korean J Pediatr 60:118–123. doi: 10.3345/kjp.2017.60.4.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tramontana JM, Utaipat U, Molloy A, Akarasewi P, Burroughs M, Makonkawkeyoon S, Johnson B, Klausner JD, Rom W, Kaplan G. 1995. Thalidomide treatment reduces tumor necrosis factor alpha production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med 1:384–379. doi: 10.1007/BF03401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sajid A, Ikram MA, Khalid T. 2011. Erythema nodosum: a cutaneous sign of tuberculosis. JUMDC 2:37–39. [Google Scholar]
- 59.Jamaati H, Mortaz E, Pajouhi Z, Folkerts G, Movassaghi M, Moloudizargari M, Adcock IM, Garssen J. 2017. Nitric oxide in the pathogenesis and treatment of tuberculosis. Front Microbiol 8:2008. doi: 10.3389/fmicb.2017.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamichhane G. 2011. Mycobacterium tuberculosis response to stress from reactive oxygen and nitrogen species. Front Microbiol 2:176. doi: 10.3389/fmicb.2011.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicholson S, Bonecini-Almeida MG, Lapa e Silva JR, Nathan C, Xie Q-W, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho JL. 1996. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med 183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Islamoglu H, Cao R, Teskey G, Gyurjian K, Lucar S, Fraix M, Sathananthan A, Chan J, Venketaraman V. 2018. Effects of ReadiSorb L-GSH in altering granulomatous responses against Mycobacterium tuberculosis infection. J Clin Med 7:40–64. doi: 10.3390/jcm7030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du Preez I, Loots DT. 2013. New sputum metabolite markers implicating adaptations of the host to Mycobacterium tuberculosis, and vice versa. Tuberculosis (Edinb) 93:330–337. doi: 10.1016/j.tube.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Luies L, Loots DT. 2016. Tuberculosis metabolomics reveals adaptations of man and microbe in order to outcompete and survive. Metabolomics 12:55. doi: 10.1007/s11306-016-0979-8. [DOI] [Google Scholar]
- 65.Combrink M, Du Preez I, Ronacher K, Walzl G, Loots DT. 2019. Time-dependent changes in urinary metabolome before and after intensive phase tuberculosis therapy: a pharmacometabolomics study. OMICS 23:560–572. doi: 10.1089/omi.2019.0140. [DOI] [PubMed] [Google Scholar]
- 66.Winkler AW, Crankshaw OF. 1938. Chloride depletion in conditions other than Addison’s disease. J Clin Invest 17:1–6. doi: 10.1172/JCI100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sims E, Welt LG, Orloff J, Needham JW. 1950. Asymptomatic hyponatremia in pulmonary tuberculosis. J Clin Invest 29:1545–1557. doi: 10.1172/JCI102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jafari NJ, Izadi M, Sarrafzadeh F, Heidari A, Ranjbar R, Saburi A. 2013. Hyponatremia due to pulmonary tuberculosis: review of 200 cases. Nephrourol Mon 5:687–691. doi: 10.5812/numonthly.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shalhoub RJ, Antoniou LD. 1969. The mechanism of hyponatremia in pulmonary tuberculosis. Ann Intern Med 70:943–962. doi: 10.7326/0003-4819-70-5-943. [DOI] [PubMed] [Google Scholar]
- 70.Vorherr H, Massry SG, Fallet R, Kaplan L, Kleeman CR. 1970. Antidiuretic principle in tuberculous lung tissue of a patient with pulmonary tuberculosis and hyponatremia. Ann Intern Med 72:383–387. doi: 10.7326/0003-4819-72-3-383. [DOI] [PubMed] [Google Scholar]
- 71.Lee J-C, Yu F-L, Lin M-H, Huang G-S, Chang C-Y, Cheng C-L, Wang G-C. 2010. Utility of immunochromatographic assay for detecting Mycobacterium tuberculosis from positive BACTEC MGIT 960 cultures. J Biomed Lab Sci 22:e9 http://www.labmed.org.tw/upfiles/issues/201076151143.pdf. [Google Scholar]
- 72.Olalekan AW, Oluwaseun FA, Oladele H-W, Akeem AD. 2015. Evaluation of electrolyte imbalance among tuberculosis patients receiving treatments in southwestern Nigeria. Alexandria J Med 51:255–260. doi: 10.1016/j.ajme.2014.10.003. [DOI] [Google Scholar]
- 73.Feng S, Du YQ, Zhang L, Zhang L, Feng RR, Liu SY. 2015. Analysis of serum metabolic profile by ultra-performance liquid chromatography-mass spectrometry for biomarkers discovery: application in a pilot study to discriminate patients with tuberculosis. Chin Med J (Engl) 128:159–168. doi: 10.4103/0366-6999.149188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Appelberg R. 2006. Macrophage nutriprive antimicrobial mechanisms. J Leukoc Biol 79:1117–1128. doi: 10.1189/jlb.0206079. [DOI] [PubMed] [Google Scholar]
- 75.Pandey AK, Sassetti CM. 2008. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A 105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griffin JE, Gawronski JD, DeJesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang JC, Miner MD, Pandey AK, Gill WP, Harik NS, Sassetti CM, Sherman DR. 2009. igr genes and Mycobacterium tuberculosis cholesterol metabolism. J Bacteriol 191:5232–5239. doi: 10.1128/JB.00452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. 2008. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun 76:3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, Muenke M. 2007. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics 120:723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 80.Steegmans P, Fekkes D, Hoes AW, Bak A, van der Does E, Grobbee DE. 1996. Low serum cholesterol concentration and serotonin metabolism in men. BMJ 312:221. doi: 10.1136/bmj.312.7025.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim Y-K, Myint A-M. 2004. Clinical application of low serum cholesterol as an indicator for suicide risk in major depression. J Affect Disord 81:161–166. doi: 10.1016/S0165-0327(03)00166-6. [DOI] [PubMed] [Google Scholar]
- 82.Okumura K, Iseki K, Wakugami K, Kimura Y, Muratani H, Ikemiya Y, Fukiyama K. 1999. Low serum cholesterol as a risk factor for hemorrhagic stroke in men. Jpn Circ J 63:53–58. doi: 10.1253/jcj.63.53. [DOI] [PubMed] [Google Scholar]
- 83.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 84.Salamon H, Bruiners N, Lakehal K, Shi L, Ravi J, Yamaguchi KD, Pine R, Gennaro ML. 2014. Cutting edge: vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. J Immunol 193:30–34. doi: 10.4049/jimmunol.1400736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan T. 2000. Vitamin D deficiency and susceptibility to tuberculosis. Calcif Tissue Int 66:476–478. doi: 10.1007/s002230010095. [DOI] [PubMed] [Google Scholar]
- 86.Martineau A, Nanzer A, Satkunam K, Packe G, Rainbow S, Maunsell Z, Timms P, Venton T, Eldridge S, Davidson R. 2009. Influence of a single oral dose of vitamin D2 on serum 25-hydroxyvitamin D concentrations in tuberculosis patients. Int J Tuberc Lung Dis 13:119–125. [PubMed] [Google Scholar]
- 87.Daley P, Jagannathan V, John KR, Sarojini J, Latha A, Vieth R, Suzana S, Jeyaseelan L, Christopher DJ, Smieja M, Mathai D. 2015. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 15:528–534. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 88.Root HF. 1934. The association of diabetes and tuberculosis. N Engl J Med 210:127–147. doi: 10.1056/NEJM193401182100304. [DOI] [Google Scholar]
- 89.Kim S, Hong Y, Lew W, Yang S, Lee E. 1995. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis 76:529–533. doi: 10.1016/0962-8479(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 90.Buskin SE, Gale JL, Weiss NS, Nolan CM. 1994. Tuberculosis risk factors in adults in King County, Washington, 1988 through 1990. Am J Public Health 84:1750–1756. doi: 10.2105/ajph.84.11.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenman KD, Hall N. 1996. Occupational risk factors for developing tuberculosis. Am J Ind Med 30:148–154. doi:. [DOI] [PubMed] [Google Scholar]
- 92.Coker R, McKee M, Atun R, Dimitrova B, Dodonova E, Kuznetsov S, Drobniewski F. 2006. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ 332:85–87. doi: 10.1136/bmj.38684.687940.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeon CY, Murray MB. 2008. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gomez DI, Twahirwa M, Schlesinger LS, Restrepo BI. 2013. Reduced Mycobacterium tuberculosis association with monocytes from diabetes patients that have poor glucose control. Tuberculosis (Edinb) 93:192–197. doi: 10.1016/j.tube.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh M, Biswas S, Shah A. 1984. Impaired glucose tolerance in active pulmonary tuberculosis. Indian J Tuberc 31:118–121. [Google Scholar]
- 96.Oluboyo P, Erasmus R. 1990. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle 71:135–138. doi: 10.1016/0041-3879(90)90010-6. [DOI] [PubMed] [Google Scholar]
- 97.Basoglu O, Bacakoglu F, Cok G, Saymer A, Ates M. 1999. The oral glucose tolerance test in patients with respiratory infections. Monaldi Arch Chest Dis 54:307–310. [PubMed] [Google Scholar]
- 98.Jain M, Baghel P, Agrawal R. 2006. Study of impaired glucose tolerance in pulmonary tuberculosis. Indian J Community Med 31:137–139. [Google Scholar]
- 99.Bloom JD. 1969. Glucose intolerance in pulmonary tuberculosis 1, 2. Am Rev Respir Dis 100:38–41. [DOI] [PubMed] [Google Scholar]
- 100.Zack MB, Fulkerson LL, Stein E. 1973. Glucose intolerance in pulmonary tuberculosis. Am Rev Respir Dis 108:1164–1169. [DOI] [PubMed] [Google Scholar]
- 101.Guptan A, Shah A. 2000. Tuberculosis and diabetes: an appraisal. Indian J Tuberc 47:3–8. http://medind.nic.in/ibr/t00/i1/ibrt00i1p3.pdf. [Google Scholar]
- 102.Karachunskiĭ M, Balabolkin M, Beglarian N. 1995. Changes in carbohydrate metabolism in patients with tuberculosis. Vestn Ross Akad Med Nauk 1995:18–21. (In Russian.) [PubMed] [Google Scholar]
- 103.Singhal A, Jie L, Kumar P, Hong GS, Leow MK, Paleja B, Tsenova L, Kurepina N, Chen J, Zolezzi F, Kreiswirth B, Poidinger M, Chee C, Kaplan G, Wang YT, De Libero G. 2014. Metformin as adjunct antituberculosis therapy. Sci Transl Med 6:263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- 104.Singh K, Ahluwalia G, Sharma S, Saxena R, Chaudhary V, Anant M. 2001. Significance of haematological manifestations in patients with tuberculosis. J Assoc Physicians India 49:788, 790–794. [PubMed] [Google Scholar]
- 105.Renshaw AA, Gould EW. 2013. Thrombocytosis is associated with Mycobacterium tuberculosis infection and positive acid-fast stains in granulomas. Am J Clin Pathol 139:584–586. doi: 10.1309/AJCPCM1CKASVBMBP. [DOI] [PubMed] [Google Scholar]
- 106.Javed I, Javed MT, Mahmood Z, Riaz M, Iqbal R, Rasul A. 2018. Hematological profiling of tuberculosis-infected and co-morbid patients: a study carried out in central Punjab, Pakistan. Eur J Inflamm 16:1–6. doi: 10.1177/2058739218818684. [DOI] [Google Scholar]
- 107.Abay F, Yalew A, Shibabaw A, Enawgaw B. 2018. Hematological abnormalities of pulmonary tuberculosis patients with and without HIV at the University of Gondar Hospital, northwest Ethiopia: a comparative cross-sectional study. Tuberc Res Treat 2018:5740951. doi: 10.1155/2018/5740951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Delzenne NM, Cani PD. 2011. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr 31:15–31. doi: 10.1146/annurev-nutr-072610-145146. [DOI] [PubMed] [Google Scholar]
- 109.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto J-M, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. 2012. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 110.Yeoh N, Burton JP, Suppiah P, Reid G, Stebbings S. 2013. The role of the microbiome in rheumatic diseases. Curr Rheumatol Rep 15:314–324. doi: 10.1007/s11926-012-0314-y. [DOI] [PubMed] [Google Scholar]
- 111.Ling Z, Liu X, Chen X, Zhu H, Nelson KE, Xia Y, Li L, Xiang C. 2011. Diversity of cervicovaginal microbiota associated with female lower genital tract infections. Microb Ecol 61:704–714. doi: 10.1007/s00248-011-9813-z. [DOI] [PubMed] [Google Scholar]
- 112.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. 2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sekirov I, Finlay BB. 2009. The role of the intestinal microbiota in enteric infection. J Physiol 587:4159–4167. doi: 10.1113/jphysiol.2009.172742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilks J, Golovkina T. 2012. Influence of microbiota on viral infections. PLoS Pathog 8:e1002681. doi: 10.1371/journal.ppat.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheung MK, Lam WY, Fung WYW, Law PTW, Au CH, Nong W, Kam KM, Kwan HS, Tsui S. 2013. Sputum microbiota in tuberculosis as revealed by 16S rRNA pyrosequencing. PLoS One 8:e54574. doi: 10.1371/journal.pone.0054574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu J, Liu W, He L, Huang F, Chen J, Cui P, Shen Y, Zhao J, Wang W, Zhang Y, Zhu M, Zhang W, Zhang Y. 2013. Sputum microbiota associated with new, recurrent and treatment failure tuberculosis. PLoS One 8:e83445. doi: 10.1371/journal.pone.0083445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Botero LE, Delgado-Serrano L, Cepeda ML, Bustos JR, Anzola JM, Del Portillo P, Robledo J, Zambrano MM. 2014. Respiratory tract clinical sample selection for microbiota analysis in patients with pulmonary tuberculosis. Microbiome 2:29–35. doi: 10.1186/2049-2618-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winglee K, Eloe-Fadrosh E, Gupta S, Guo H, Fraser C, Bishai W. 2014. Aerosol Mycobacterium tuberculosis infection causes rapid loss of diversity in gut microbiota. PLoS One 9:e97048. doi: 10.1371/journal.pone.0097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Das MK, Bishwal SC, Das A, Dabral D, Badireddy VK, Pandit B, Varghese GM, Nanda RK. 2015. Deregulated tyrosine-phenylalanine metabolism in pulmonary tuberculosis patients. J Proteome Res 14:1947–1956. doi: 10.1021/acs.jproteome.5b00016. [DOI] [PubMed] [Google Scholar]
- 121.Luies L, Van Reenen M, Ronacher K, Walzl G, Loots DT. 2017. Predicting tuberculosis treatment outcome using metabolomics. Biomark Med 11:1057–1067. doi: 10.2217/bmm-2017-0133. [DOI] [PubMed] [Google Scholar]
- 122.Luies L, Mienie J, Motshwane C, Ronacher K, Walzl G, Loots DT. 2017. Urinary metabolite markers characterizing tuberculosis treatment failure. Metabolomics 13:124–133. doi: 10.1007/s11306-017-1261-4. [DOI] [Google Scholar]