Abstract
Background
This study investigated the effects of various doses of S-ketamine on depression and pain management of cervical carcinoma patients with mild/moderate depression.
Material/Methods
This randomized, double-blind, controlled study included 417 cervical carcinoma patients who received laparoscopic modified radical hysterectomy from April 2015 to July 2018 and who also had mild/moderate depression symptoms based on HAMD-17 scores (8~24). All patients were randomized into 4 groups: 1) the control group, 2) the racemic ketamine group, 3) the high-dose S-ketamine group; and 4) the low-dose S-ketamine group. Pain was assessed using the Visual Analogue Score (VAS), and depression was assessed using theHAMD-17 score. Serum levels of BDNF and 5-HT were measured.
Results
The 4 groups of patients showed no significant differences in operation time, bleeding volume, hospitalization duration, or complications. The high-dose S-ketamine group showed significantly lower VAS and HAMD-17 scores than all other groups at 1 day and 3 days postoperatively, but no differences were observed in the low-dose S-ketamine group and the racemic ketamine group. The high-dose S-ketamine group showed significantly higher serum BDNF and 5-HT levels at 1 day and 3 days after surgery. However, 1 week after surgery, no difference was observed in any of the treatment groups.
Conclusions
At subanesthetic dose, both 0.5 mg/kg and 0.25 mg/kg S-ketamine improved short-term depression and pain for cervical carcinoma patients after surgery, and the effects were better than with the same dose of racemic ketamine.
MeSH Keywords: Depression, Ketamine, Racepinephrine
Background
Cervical cancer is one of the most common gynecological malignant tumors and is the second most common cancer in women [1,2]. It is reported that over 500 000 new cases are diagnosed and over 200 000 women die every year due to cervical cancer worldwide, with especially high rates in developing countries [3,4]. Moreover, since most cervical cancer patients are at an advanced disease stage when diagnosed, the prognosis and 5-year survival rate are both poor [5,6]. Cervical cancer not only threatens women’s lives, but also imposes a huge psychological burden.
It is reported that female patients with malignant tumors such as breast cancer or cervical cancer are at higher risk of developing depression [7]. Research shows that 20~45% of breast cancer patients who undergo surgery develop postoperative depression [8]. For cervical cancer, a Chinese study showed 54.9% patients had depression and 49.7% patients had anxiety [9]. Another study also found that 52.2% of cervical cancer patients had depression and 65.6% had anxiety [10]. Treatment of depression in cervical cancer patients is often ineffective.
Ketamine, a widely used anesthetic, is also used in treatment of depression [11]. The most commonly used ketamine in clinical practice is racemic ketamine, but its use is associated with many complications, such as psychiatric adverse effects and neurotoxicity [12,13]. In recent years, the better efficiency and fewer complications of S-ketamine have attracted attention [14,15], but there has been no published research on the effects of S-ketamine in treatment of cervical carcinoma patients with depression.
The most important brain regions associated with depression are the amygdala and hippocampus [16]. The amygdala is an important brain region that converts negative information such as anxiety and depression into neural signals in the brain, and is closely involved in behavior and emotion. Changes in hippocampal structure and synaptic remodeling are closely related to the onset of depression and the effect of antidepressants. Depression is often caused by reduced levels of monoamine neurotransmitters in the brain, such as serotonin (5-HT) (17). 5-HT is a very important neurotransmitter in the brain, and its levels are closely correlated with the onset of depression. In addition, the occurrence of depression is closely correlated with brain-derived neurotrophic factor (BDNF) and its signaling pathway [18]. BDNF is mainly distributed in the hippocampus, striatum, amygdala, and cortex. BDNF can prevent neuron injury and apoptosis, promote neuron regeneration and differentiation, and maintain normal physiological function of neurons. Therefore, in the present study, we used physiological indexes of 5-HT and BDNF to assess effect of S-ketamine on depression and pain of cervical carcinoma patients who had mild/moderate depression after laparoscopic total hysterectomy. In this randomized, double-blind, controlled research, we observed that at subanesthetic doses, S-ketamine improved short-term depression and pain better than the same doses of racemic ketamine. Our results provide clinical data for improving treatment of depression using S-ketamine in cervical carcinoma patients.
Material and Methods
Patients
This was a randomized, double-blind, controlled trial that enrolled 417 cervical carcinoma patients who received laparoscopic modified radical hysterectomy from April 2015 to July 2018. All patients were at TNM I~II stage. For all patients, the diagnosis was confirmed by histological analysis. Patients were consecutively enrolled during the study period. All patients were evaluated by Hamilton depression rating scale depression (HAMD)-17 score and only patients with HAMD-17 8~24 and with American Society of Anesthesiologists (ASA) score I~II before surgery were included. Exclusion criteria were: HAMD score ≤7 or ≥24 before the study, psychiatric disorders such as mania and schizophrenia, and severe liver, renal, cardiovascular, or systematic inflammatory diseases. Written informed consent was obtained from all patients. The study was approved by Ethics Committee of the Seventh People’s Hospital of Shanghai University of TCM.
Analgesia
The analgesia induction was guided by bispectral index monitoring of (BIS) by intravenous injection of 1.5–2.5 mg/kg propofol, 0.5 mg/kg midazolam, 0.5–0.6 mg/kg cisatracurium, and 2–4 μg/kg fentanyl using a TCI III-B syringe pump (Guangxi VERYARK Technology, Nanning, China). Endotracheal intubation and mechanical ventilation were performed and the end-respiratory partial pressure of carbon dioxide (PetCO2) was monitored. Anesthesia maintenance was achieved by target-controlled infusion of 1.5~4.0 μg/ml propofol, 4–8 μg/(kg·h) remifentanil, and 4–12 μg/kg/min cisatracurium. The PetCO2 was maintained within 35–40 mmHg, and BIS was maintained within 40–60.
Patients were randomly divided into 4 groups using SPSS software: 1) the control group (n=105), in which patients received 50 ml normal saline by intravenous injection after 1 h of analgesia; 2) the racemic ketamine group (n=104), in which patients received 50 ml 0.5 mg/kg racemic ketamine (No. KH080601, Jiangsu Hengrui Pharmaceutical Co., Jiangsu, China) by intravenous injection after 1 h of analgesia; 3) the high-dose S-ketamine group (n=104), in which patients received 50 ml 0.5 mg/kg S-ketamine (No. 20060403, Jiangsu Hengrui Pharmaceutical Co., Jiangsu, China) by intravenous injection after 1 h of analgesia; and 4) the low-dose S-ketamine group (n=104), in which patients received 50 ml 0.25 mg/kg S-ketamine by intravenous injection after 1 h of analgesia.
All analgesia and surgical procedures were performed by the same team. The anesthesiologists were blinded to the medications (normal saline, racemic ketamine, or various doses of S-ketamine). During skin suturing, 1 μg/kg fentanyl and 4 mg ondansetron were used for analgesia and vomiting control, respectively. After surgery, all patients received patient-controlled intravenous analgesia (PCIA) containing 0.75–1 μg/kg sufentanil, 2 mg butorphanol, and 16 mg ondansetron for 24 h.
Data measurement
Clinical characteristics were collected. Data on operation time, blood loss, hospitalization time, and 1-month complication rates were also recorded. Pain condition was evaluated by the Visual Analogue Score (VAS) at 1, 2, 3, 5, and 7 days after surgery. Depression was assessed using HAMD-17 at 1, 2, 3, 5, and 7 days after surgery. The follow-up lasted for 1 month.
Measurement of serum levels BDNF
Measurement of serum BDNF and 5-hydroxytryptamine (HT) were performed by ELISA (Abcam and IBL, respectively) at 1, 2, 3, 5, and 7 days postoperatively.
Statistical analysis
Data are expressed by mean±SD. The chi-square test was used for comparing rates. Comparisons among the 4 groups were performed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test. P value <0.05 was considered to be statistically significant. All calculations were made using SPSS 22.0 (SPSS Inc., Chicago, USA).
Results
Patients’ characteristics
As shown in Table 1, we enrolled 105 patients in the control group, mean age 46.27±10.83 years; 104 patients in the racemic ketamine group, mean age 47.07±10.08 years; 104 patients in the high-dose S-ketamine (0.5 mg/kg) group, mean age 48.53±10.0 years; and 104 cases in the low-dose S-ketamine (0.25 mg/kg) group, mean age 48.11±10.38 years. The mean HAMD score in the control group before surgery was 15.78±4.81; 16.20±4.86 in the racemic ketamine group; 16.69±4.96 in the low-dose S-ketamine group; and 15.75±4.58 in the high-dose S-ketamine group. No patients were lost to follow-up or withdrew from the study. No significant difference was observed in baseline characteristics among the 4 groups.
Table 1.
Clinical characteristics of all patients.
| Variables* | High-dose S-ketamine, n=104 | Low-dose S-ketamine, n=104 | Racemic ketamine, n=104 | Control, n=105 | P value |
|---|---|---|---|---|---|
| Age, year | 48.53±10.0 | 48.11±10.38 | 47.07±10.08 | 46.27±10.83 | 0.383 |
| BMI, kg/m2 | 21.89±2.37 | 22.11±2.37 | 21.91±2.33 | 22.63±2.39 | 0.082 |
| VAS score | 1.11±0.11 | 1.09±0.12 | 1.08±0.11 | 1.09±0.11 | 0.428 |
| HAMD score | 15.75±4.58 | 16.69±4.96 | 16.20±4.86 | 15.78±4.81 | 0.458 |
| BDNF, ng/mL | 23.24±1.29 | 23.08±1.29 | 23.23±1.36 | 23.23±1.18 | 0.775 |
| 5-HT, ng/mL | 228.83±17.70 | 228.10±16.84 | 226.40±16.91 | 222.87±17.22 | 0.059 |
All data in this table are from ANOVA followed by Tukey post hoc test.
Comparison of clinical outcomes
We compared intraoperative and postoperative outcomes: operation time, bleeding volume, hospitalization time, 1-month complication rate, and VAS scores at 1, 2, 3, 5, and 7 days after surgery. No significant difference was observed in operative time, bleeding, hospitalization time, or 1-month complication rate among the 4 groups (Table 2). In all treatment groups, the VAS scores at 1, 2, and 3 days were remarkably lower than in the control group (P<0.05, Figure 1). The high-dose S-ketamine (0.5 mg/kg) group showed the lowest VAS scores, but no significant difference was observed between the low-dose S-ketamine (0.25 mg/kg) group and the racemic ketamine group. Besides, after 5 and 7 days, the VAS scores were reduced to the baseline in all groups. These results indicate that S-ketamine had better efficacy in reducing short-term postoperative pain than the same dose of racemic ketamine.
Table 2.
Intraoperative and postoperative outcomes in the 4 groups.
| Variables | High S-ketamine, n=104 | Low S-ketamine, n=104 | Racemic ketamine, n=104 | Control, n=105 | P* |
|---|---|---|---|---|---|
| Mean operation time, min | 144.78±26.76 | 147.75±25.70 | 148.35±24.71 | 144.07±25.22 | 0.541 |
| Mean bleeding volume, ml | 259.02±71.58 | 269.43±70.69 | 270.16±66.85 | 258.66±68.35 | 0.458 |
| Hospitalization time, days | 16.56±5.41 | 16.75±5.03 | 16.61±5.13 | 16.25±5.32 | 0.916 |
| Complication, n (%) | 1.000 | ||||
| Nausea | 19 (18.27) | 17 (16.35) | 20 (19.23) | 18 (17.14) | |
| Dizzy | 14 (13.46) | 12 (11.54) | 13 (12.50) | 12 (11.43) | |
| Vomit | 9 (8.65) | 8 (7.69) | 10 (9.61) | 8 (7.62) |
Continuous data were compared using ANOVA followed by Tukey post hoc test, and rates were compared by chi-square test.
P values were obtained by ANOVA.
Figure 1.
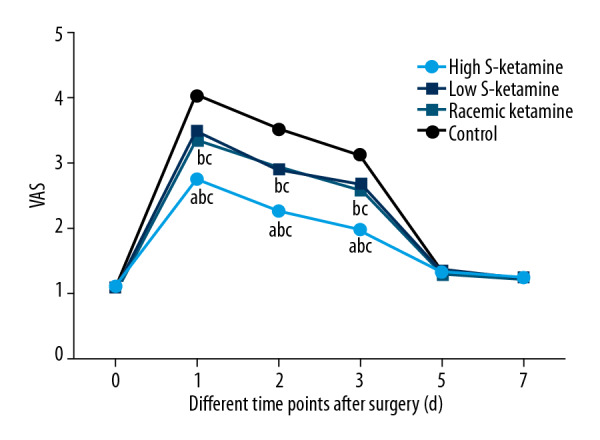
VAS scores at 1, 2, 3, 5, and 7 days after surgery. a P<0.05 vs. low-dose S-ketamine group; b P<0.05 vs. racemic ketamine group; c P<0.05 vs. control ketamine group.
Comparison of HAMD-17 scores
To further evaluate effects of S-ketamine on depression, HAMD-17 scores were assessed. In all treatment groups, HAMD-17 scores were markedly lower at 1, 2, and 3 days than in the control group (P<0.05, Figure 2). The high-dose S-ketamine (0.5 mg/kg) group had the lowest HAMD-17 scores (P<0.05, Figure 2), but no significant difference was found in the low-dose S-ketamine (0.25 mg/kg) group and the racemic ketamine group. No significant difference was found at 5 days after surgery among the 4 groups, suggesting that S-ketamine had better efficacy in reducing short-term depression compared with the same dose of racemic ketamine.
Figure 2.
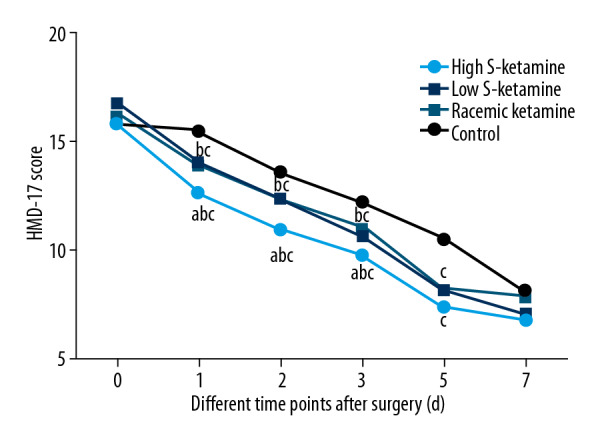
HAMD scores at 1, 2, 3, 5, and 7 days after surgery. a P<0.05 vs. low-dose S-ketamine group; b P<0.05 vs. racemic ketamine group; c P<0.05 vs. control ketamine group.
BDNF and 5-HT in different groups
Finally, BDNF and 5-HT levels at 1, 2, 3, 5, and 7 days after surgery were determined. All 3 treatment groups showed higher BDNF and 5-HT levels at 1, 2, and 3 days after surgery than the control group (P<0.05, Table 3), and the high-dose S-ketamine (0.5 mg/kg) group showed the highest level (P<0.05). No significant difference was observed at 5 days after of surgery among the 4 groups, and no significant difference was found between the low-dose S-ketamine (0.25 mg/kg) group and the racemic ketamine group, further confirming the effects of S-ketamine on improvement of depression.
Table 3.
Serum levels of BDNF and 5-HT at various timepoints after surgery.
| Variables | High-dose S-ketamine, n=104 | Low-dose S-ketamine, n=104 | Racemic ketamine, n=104 | Control, n=105 | P value |
|---|---|---|---|---|---|
| BDNF | |||||
| Before | 23.24±1.29 | 23.08±1.29 | 23.23±1.36 | 23.23±1.18 | 0.775 |
| 1 d | 22.58±1.52b,c,d | 20.36±1.38a,d | 20.17±1.32a,d | 19.11±1.16a,b,c | <0.001 |
| 2 d | 23.86±1.35b,c,d | 21.55±1.42a,d | 21.63±1.26a,d | 20.04±1.15a,b,c | <0.001 |
| 3 d | 24.95±1.54b,c,d | 23.52±1.18a,d | 23.36±1.21a,d | 21.20±1.03a,b,c | <0.001 |
| 5 d | 24.55±2.00d | 24.71±1.52d | 24.64±1.71d | 23.46±1.49a,b,c | <0.001 |
| 7 d | 27.01±1.36 | 26.47±1.14 | 26.59±1.17 | 26.61±1.15 | 0.035 |
| 5-HT | |||||
| Before | 228.83±17.70 | 228.10±16.84 | 226.40±16.91 | 222.87±17.22 | 0.059 |
| 1 d | 252.38±15.89b,c,d | 246.13±14.48a,d | 248.25±13.32a,d | 227.04±15.69a,b,c | <0.001 |
| 2 d | 261.98±17.65b,c,d | 247.81±18.57a,d | 247.51±17.93a,d | 232.16±19.31a,b,c | <0.001 |
| 3 d | 270.39±23.61b,c,d | 255.37±18.57a,d | 250.13±18.58a,d | 235.94±21.48a,b,c | <0.001 |
| 5 d | 335.32±20.30d | 336.47±21.63d | 339.03±23.24d | 305.02±21.48a,b,c | |
| 7 d | 347.05±26.23 | 353.41±26.47 | 345.41±27.66 | 345.24±24.85 | 0.085 |
P<0.05, compared with the high-dose S-ketamine group;
P<0.05, compared with the low-dose S-ketamine group;
P<0.05, compared with the racemic ketamine group;
P<0.05, compared with the control group.
Continuous data were compared using ANOVA followed by Tukey post hoc test.
Discussion
Although treatment methods have been improved and understanding of the molecular mechanisms underlying depression has increased, the treatment of postoperative depression remains a clinical challenge [19]. Female patients with gynecological cancer such as cervical carcinoma and breast cancer have a high risk for preoperative or postoperative depression due to the diseases, which can reduce their quality of life [20]. Several studies have assessed treatment of depression for cervical or breast cancer patients. Kaur et al. reported application of Jacobson’s progressive muscle relaxation technique, counseling, and home care to reduce depression and anxiety [21]. Ergun et al. demonstrated that exercise can benefit breast cancer patients with depression [22]. However, no study has reported on effects of S-ketamine on depression after radical hysterectomy and compared it to racemic ketamine. In the present study, we reported for the first time that the subanesthetic dose of S-ketamine can improve pain and depression of cervical cancer patients with mild/moderate depression before surgery, and the effects were better than with racemic ketamine.
S-ketamine has been used in anesthesia, analgesia, and depression in many diseases. Segmiller et al. demonstrated that repeated S-ketamine infusions could improve resistant depression [23]. A case series found oral treatment with S-ketamine (1.25 mg/kg) improved 50% depression symptoms [24]. In a randomized pilot study, S-ketamine used as an anesthetic adjuvant in electroconvulsive therapy for treatment of depression [25]. In the present research, we also found 0.5 mg/kg and 0.25 mg/kg doses of S-ketamine improved pain and reduced depression, consistent with previous research.
Several previous studies compared S-ketamine and racemic ketamine. An early study reported that S-ketamine had a remarkably higher first-order elimination rate, as well as significantly lower elimination half-life and mean residence time [26]. In a case report, Paul et al. observed no significant difference between S-ketamine and racemic ketamine in antidepressant effects, but S-ketamine showed better tolerance [27]. It was also found that S-ketamine could achieve the same sedation efficiency at only 60% of the dosage of racemic ketamine [28]. Here, we showed that S-ketamine had better effects in improvement of pain and depression than racemic ketamine in cervical cancer patients with mild/moderate depression.
Conclusions
In this randomized, double-blind, controlled research, we found that a subanesthetic dose of S-ketamine had better effects on pain and depression than racemic ketamine in cervical carcinoma patients with mild/moderate depression. Our results strengthen the clinical evidence supporting the value of S-ketamine in treatment of depression in cervical cancer patients.
Footnotes
Conflict of interest
None.
Source of support: Shanghai Municipal Commission of Health and Family Planning (201840189)
References
- 1.International Agency for Research on Cancer, World Health Organization. Cervical cancer: Estimated incidence, mortality and prevalence worldwide in 2012. Archived at: http://www.webcitation.org/6zIbaJC1q,.2019.
- 2.Small W, Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer. 2017;123(13):2404–12. doi: 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- 3.Finocchario-Kessler S, Wexler C, et al. Cervical cancer prevention and treatment research in Africa: A systematic review from a public health perspective. BMC Womens Health. 2016;16:29. doi: 10.1186/s12905-016-0306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez MS, Baker ES, Maza M, et al. Cervical cancer prevention and treatment in Latin America. BMC Womens Health. 2016;16(1):1–25. [Google Scholar]
- 5.Sun F, Xiong Y, Zhou XH, et al. Acylglycerol kinase is over-expressed in early-stage cervical squamous cell cancer and predicts poor prognosis. Tumor Biol. 2016;37(5):6729–36. doi: 10.1007/s13277-015-4498-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Gao B, Li R, et al. Expression levels of resistant genes affect cervical cancer prognosis. Mol Med Rep. 2017;15(5):2802. doi: 10.3892/mmr.2017.6328. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz Y, Akyol M, Alacacioglu A, et al. Sexual satisfaction, anxiety, depression and quality of life amoung Turkish gynecological cancer patients. Ann Oncol. 2016;27(Suppl 6):vi306. [Google Scholar]
- 8.Fann JR, Thomasrich AM, Katon WJ, et al. Major depression after breast cancer: A review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30(2):112–26. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Yang YL, Liu L, Wang Y, et al. The prevalence of depression and anxiety among Chinese adults with cancer: A systematic review and meta-analysis. BMC Cancer. 2013;13(1):393. doi: 10.1186/1471-2407-13-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YL, Liu L, Wang XX, et al. Prevalence and associated positive psychological variables of depression and anxiety among Chinese cervical cancer patients: A cross-sectional study. PLoS One. 2014;9(4):e94804. doi: 10.1371/journal.pone.0094804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aan hRM, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–45. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Zuo D, Lin L, Liu Y, et al. Baicalin attenuates ketamine-induced neurotoxicity in the developing rats: Involvement of PI3K/Akt and CREB/BDNF/Bcl-2 pathways. Neurotox Res. 2016;30(2):159–72. doi: 10.1007/s12640-016-9611-y. [DOI] [PubMed] [Google Scholar]
- 13.Perry EB, Cramer JA, Cho HS, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology. 2007;192(2):253–60. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Goffer Y, Xu D, et al. A Single sub-anesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115(4):812. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casoni D, Spadavecchia C, Adami C. S-ketamine versus racemic ketamine in dogs: Their relative potency as induction agents. Vet Anaesth Analg. 2015;42(3):250–59. doi: 10.1111/vaa.12200. [DOI] [PubMed] [Google Scholar]
- 16.Mervaala E, Föhr J, Könönen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30(1):117–25. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 17.Xie RH, Xie HY, Krewski D, He GP. Plasma concentrations of neurotransmitters and postpartum depression. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(3):274–81. doi: 10.11817/j.issn.1672-7347.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Ye Y, Wang G, Wang H, Wang X. Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci Lett. 2011;503(1):15–19. doi: 10.1016/j.neulet.2011.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Ghoneim MM, O’Hara MW. Depression and postoperative complications: An overview. BMC Surgery. 2016;16(1):5. doi: 10.1186/s12893-016-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodhead C, Cunningham R, Ashworth M, et al. Cervical and breast cancer screening uptake among women with serious mental illness: A data linkage study. BMC Cancer. 2016;16(1):819. doi: 10.1186/s12885-016-2842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur M, Agnihotri M, Das K, et al. Effectiveness of an interventional package on the level of anxiety, depression, and fatigue among patients with cervical cancer. Asia Pac J Oncol Nurs. 2018;5(2):195–200. doi: 10.4103/apjon.apjon_56_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ergun M, Eyigor S, Karaca B, et al. Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. Eur J Cancer Care. 2013;22(5):626–37. doi: 10.1111/ecc.12068. [DOI] [PubMed] [Google Scholar]
- 23.Felix S, Tobias R, Andrea L, et al. Repeated S-ketamine infusions in therapy resistant depression: A case series. J Clin Pharmacol. 2013;53(9):996–98. doi: 10.1002/jcph.122. [DOI] [PubMed] [Google Scholar]
- 24.Paslakis G, Gilles M, Meyerlindenberg A, Deuschle M. Oral administration of the NMDA receptor antagonist S-ketamine as add-on therapy of depression: A case series. Pharmacopsychiatry. 2010;43(01):33–35. doi: 10.1055/s-0029-1237375. [DOI] [PubMed] [Google Scholar]
- 25.Kaija JR, Wojciech C, Olli K, et al. Effects of S-ketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: A randomized pilot study. J ECT. 2013;29(3):158–61. doi: 10.1097/YCT.0b013e318283b7e9. [DOI] [PubMed] [Google Scholar]
- 26.Larenza MP, Knobloch M, Landoni MF, et al. Stereoselective pharmacokinetics of ketamine and norketamine after racemic ketamine or S-ketamine administration in Shetland ponies sedated with xylazine. Vet J. 2008;177(3):432–35. doi: 10.1016/j.tvjl.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Paul R, Schaaff N, Padberg F, et al. Comparison of racemic ketamine and S-ketamine in treatment-resistant major depression: Report of 2 cases. World J Biol Psychiatry. 2009;10(3):241–44. doi: 10.1080/15622970701714370. [DOI] [PubMed] [Google Scholar]
- 28.Olga MJ, Rima B, Anna F, et al. Comparison of the effects of racemic ketamine and S-ketamine for anesthesia in Rheem gazelles (Gazella subgutturosa marica) and Subgutturosa gazelles (Gazella subgutturosa subgutturosa) Am J Vet Res. 2011;72(9):1164–70. doi: 10.2460/ajvr.72.9.1164. [DOI] [PubMed] [Google Scholar]


