Abstract
Background
This study aimed to develop a predictive nomogram for midterm to long-term prognosis in patients with papillary renal cell carcinoma (RCC) based on data from the US Surveillance, Epidemiology, and End Results (SEER) program.
Material/Methods
Clinical pathology data and follow-up information were obtained from the SEER database for patients with papillary RCC between 1997–2014. Univariate and multivariate Cox regression models evaluated the independent prognostic factors, and the nomogram was constructed to predict the 3-year, 5-year, and 10-year survival rates. Multiple parameters were estimated to evaluate the predictive values, including the concordance indices (C-indices), calibration plots, area under the receiver operator characteristics (ROC) curve, net reclassification improvement (NRI), integrated discrimination improvement (IDI), and decision curve analysis (DCA).
Results
The study included 13,926 patients with papillary RCC. Univariate and multivariate Cox regression analysis developed the nomogram that relied on the predictive variables of age, Fuhrman grade, TNM stage, surgery of the primary site, lymphadenectomy, and marital status. The C-indices of the novel model in the validation cohort were more satisfactory than those of the TNM classification. Accurate discrimination and calibration by the nomogram were identified in both cohorts. The NRI and IDI supported prediction improvements, and the DCA supported the nomogram’s clinical significance.
Conclusions
A nomogram was developed to evaluate the prognosis of papillary RCC and to identify the patients who required specialized treatment. However, external validation of the predictive nomogram is required that also includes patients from other countries.
MeSH Keywords: Carcinoma, Renal Cell; Nomograms; SEER Program
Background
Among malignant tumors, renal cell carcinoma (RCC) is highly prevalent in the United States (US) [1]. The incidence of RCC has shown an increasing trend over the last 30 years worldwide [2]. Papillary (RCC) was reported as the second most common type of RCC [3]. Similar to clear cell renal carcinoma, RCC originating from proximal convoluted tubular epithelial cells, accounted for approximately 7–14% of cases of RCC cases [4–6].
The special clinical manifestations, pathological morphology, and biological behaviors of papillary RCC are different from the other types [3,7,8]. Although the prognosis of papillary RCC can be improved upon pretreatment, patients with distant metastasis and higher histopathological grade have poor prognosis and most need adjuvant therapy after surgery [9,10]. It is worth noting that the treatment of papillary RCC still remains controversial [3,9–12].
To date, the TNM staging system is a widely applied evaluation and treatment guideline used for cancer therapy worldwide. It has become the standard method for staging malignant tumors by most clinicians and medical scientists [13]. A serious weakness underlying the system, nevertheless, is that the TNM classification system only considers limited factors, namely tumor size (T), metastasis (M) and regional lymph nodes (N). According to previous studies, other clinicopathological factors, such as age, sex, tumor grade, surgical mode, lymphadenectomy, and chemotherapy, which might have a significant impact on the prognosis of papillary RCC were not included in the TNM system [13,14]. Neglecting these factors reduced the accuracy of predicting survival by the system [14–16]. Based on the clinical uniqueness of papillary RCC, a reliable and accurate prognostic model was urgently needed in clinical practice.
Nomograms are often used clinically for deciding on a patent’s tumor prognosis [17,18]. Therefore, this study aimed to develop a predictive nomogram for midterm to long-term prognosis in patients with papillary renal cell carcinoma (RCC) based on data from the US Surveillance, Epidemiology, and End Results (SEER) program.
Material and Methods
Data source and data processing
We extracted data of patients with papillary renal cell carcinoma (RCC) (ICD-O-3 site code 8050 and 8052) data between 1997–2014 using the US Surveillance, Epidemiology, and End Results (SEER) program and SEER*Stat software. The datasets analyzed in this study are available on the SEER database (http://seer.cancer.gov/). The demographic and clinical data obtained included survival time, vital status, race, follow-up time, the American Joint Committee on Cancer (AJCC) classification, Fuhrman grade, marital status, and cause of death. After excluding cases with unknown or missing clinicopathological information and negative diagnostic identification, there were 13,926 cases included in this study. The variables identified included the age at diagnosis, gender, race, TNM stage, Fuhrman grade, surgery of the primary site, lymphadenectomy, marital status, and chemotherapy. The patients’ ages were categorized into six groups: <40 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, and ≥80 years. The patients included white, African-American, and other ethnic groups.
The Fuhrman grades were as follows: grade I (well-differentiated), grade II (moderately-differentiated), grade III (poorly-differentiated), grade IV (undifferentiated), and unknown. According to the SEER Kidney Surgery Codes 2018, the operation method was categorized into three groups: non-surgery (code 0), partial nephrectomy (PN, code 10-30), and nephrectomy (code 40-90), Marital status included single and married, and chemotherapy was divided no/unknown and yes. All data from the SEER database were free, and the Second Hospital of Jilin University authorized the use of patient information. All authors signed the SEER Research Data Agreement to protect the patients’ privacy, consistent with the ethical principles. Since the SEER program’s data are anonymous, and cancer is a reportable disease in every state, the requirement for informed consent was waived.
Nomogram construction and statistical analysis
Univariate analysis by the Cox regression method was adopted to screen OS-related variables. Variables presenting P<0.05 were selected for multivariate regression. Based on a Cox proportional-hazards model, a multivariate analysis was conducted to evaluate independent risk factors of papillary RCC. The hazard ratios (HR) and the 95% confidence interval (CI) were presented for the results. A P-value <0.05 represented eligibility to be included in our prognosis model. However, individual statistical significance may not accurately reflect the significance in clinical practice. Therefore, the related clinicopathological variables should also be considered. Based on these independent risk factors, a new nomogram model was developed with a graphical tool to estimate the overall survival (OS) rates of papillary RCC at 3, 5, and 10 years, respectively.
Model validation and performance evaluation
Overall, 13,926 patients were randomly subdivided into a training cohort (n=6,964) and validation cohort (n=6,962). For performance evaluation, receiver operating characteristic (ROC) curves were constructed [19], while obtaining the area under the curve (AUC) and corrected concordance index (C-index). The nomogram was validated internally (training cohort) and externally (validation cohort), while 1000 bootstrap resamples were constructed, and a calibration curve was adopted. The accuracy and discrimination of the nomogram was inversely associated with the distance between the calibration curve and the nearest bisector. Additionally, net reclassification improvement (NRI) and relative integrated discrimination improvement (IDI) were analyzed to evaluate nomogram improvements compared with the TNM according to the AJCC (sixth edition) for the 3-year, 5-year, and 10-year prediction of overall survival (OS) [20]. Finally, the decision curve analysis (DCA) for clinical significance was conducted [21]. All statistical analysis and graphs were completed by R software version x 64 3.6.1, R studio, and SPSS version 24.0. The P-value <0.05 represented positive statistical significance in this study.
Results
Demographic clinicopathological features of patients
Based on our inclusion and exclusion criteria, 13,926 patients with papillary RCC in the SEER database, meeting the criteria of our study, were included. All follow-up periods of the patients were complete. The detailed findings of the descriptive analysis are shown in Table 1. The majority of patients were men (10,658; 76.53%), older than 40 years (27,264; 77.56%), married (9,163; 65.80%), and Caucasian (9,707; 69.70%). Most patients underwent nephrectomy or PN (12,489; 95.52%). Moreover, a small percentage of patients underwent lymphadenectomy (1,229; 8.83%). Most patients did not receive chemotherapy (13,492; 96.89%).
Table 1.
Clinicopathological characteristics of 13,926 patients with papillary renal cell carcinoma (RCC) from the database of the Surveillance, Epidemiology, and End Results (SEER) program.
| Cases evaluated | Number |
|---|---|
| Age, years | |
| ≤40 | 484 (3.48%) |
| 40–49 | 1437 (10.32%) |
| 50–59 | 3389 (24.34%) |
| 60–69 | 4575 (32.85%) |
| 70–79 | 3042 (21.84%) |
| >80 | 799 (7.17%) |
| Gender | |
| Female | 3268 (23.47%) |
| Male | 10658 (76.53%) |
| Race | |
| White | 9707 (69.7%) |
| African-American | 3807 (27.34%) |
| Others | 412 (2.96%) |
| Grade | |
| I | 1400 (10.34%) |
| II | 5902 (42.38%) |
| III | 3533 (25.37%) |
| IV | 434 (3.12%) |
| Unknown | 2617 (18.79%) |
| T | |
| T1 | 10656 (76.52%) |
| T2 | 1511 (10.85%) |
| T3 | 1548 (11.12%) |
| T4 | 98 (0.70%) |
| TX | 113 (0.80%) |
| N | |
| N0 | 13133 (94.31%) |
| N1 | 326 (2.34%) |
| N2 | 241 (1.73%) |
| NX | 226 (1.62%) |
| M | |
| M0 | 13303 (95.53%) |
| M1 | 520 (3.73%) |
| MX | 103 (0.74%) |
| Surgery of primary site | |
| None | 586 (4.48%) |
| Partial nephrectomy | 6245 (47.76%) |
| Total nephrectomy | 6244 (47.776%) |
| Lymphadenectomy, num | |
| 0 | 12697 (91.17%) |
| 1–3 regional lymph nodes | 728 (5.23%) |
| ≥4 regional lymph nodes | 501 (3.60%) |
| Chemotherapy | |
| No/Unknown | 13492 (96.89%) |
| Yes | 434 (3.11%) |
| Marital status | |
| Single | 4763 (34.20%) |
| Married | 9163 (65.80%) |
SEER – Surveillance, Epidemiology, and End Results; papillary RCC – papillary renal cell carcinoma.
Univariate and multivariate Cox regression analysis
Univariate and multivariate models were established in sequence to select factors that could significantly influence OS. Hazard ratios (HR) were assessed to quantify the influence on OS (Table 2). Univariate analysis showed that age ≥60 years (HR, 1.140; 95% CI, 0.781–1.664; P<0.05), Fuhrman grade III (HR, 1.590; 95% CI, 1.335–1.894; P<0.05), IV (HR, 3.685; 95% CI, 2.925–4.643; P<0.05), unknown (HR, 1.630; 95% CI, 1.361–1.952; P<0.05), T2 (HR, 1.779; 95% CI, 1.564–2.204; P<0.05), T3 (HR, 2.737; 95% CI, 2.434–3.077; P<0.05), T4 (HR, 7.103; 95% CI, 5.139–9.817; P<0.05), TX (HR, 4.824; 95% CI, 3.491–6.666; P<0.05), M1 (HR, 11.212; 95% CI, 9.711–12.945; P<0.05), PN (HR, 0.131; 95% CI, 0.110–0.156; P<0.05), nephrectomy (HR, 0.329; 95% CI, 0.281–0.384; P<0.05), lymphadenectomy of 1–3 regional lymph nodes (HR, 1.844; 95% CI, 1.565–2.713; P<0.05), lymphadenectomy of ≥4 regional lymph nodes (HR, 2.138; 95% CI, 1.778–2.507; P<0.05), chemotherapy (HR, 7.244; 95% CI, 6.180–8.491; P<0.05) could be considered as independent OS predictors for patients with papillary RCC. The findings of multivariate analyses for the training cohort are also shown in Table 2. All variables, except race, sex, and lymphadenectomy, showed positive statistical significance. However, chemotherapy is a risk factor for survival, which is inconsistent with previous studies [22–24]. We consider that this may be due to retrospective bias, so it was not included in the model. Overall, the prognostic variables with statistical significance had the potential to predict the OS associated with papillary RCC effectively.
Table 2.
Univariate and multivariate Cox regression model analysis of overall survival (OS) in the training cohort.
| Variables | Univariate Cox model | P-value | Multivariate Cox model | P-value | ||
|---|---|---|---|---|---|---|
| Overall survival | Overall survival | |||||
| HR | 95% CI | HR | 95% CI | |||
| Age (years)) | ||||||
| <40 | 1 (Reference) | 1 (Reference) | ||||
| 40–49 | 1.14 | 0.781–1.664 | 0.498 | 1.335 | 0.914–1.952 | 0.135504 |
| 50–59 | 1.425 | 1.005–2.020 | 0.047* | 1.730 | 1.219–2.458 | 0.002169** |
| 60–69 | 2.025 | 1.439–2.848 | 5.08e-05*** | 2.513 | 1.783–3.542 | 1.41e-07*** |
| 70–79 | 3.319 | 2.360–4.669 | 5.57e-12*** | 3.977 | 2.821–5.607 | 3.39e-15*** |
| ≥80 | 6.045 | 4.256–8.587 | <2e-16*** | 5.964 | 4.189–8.491 | <2e-16*** |
| Gender | ||||||
| Female | 1 (Reference) | |||||
| Male | 1.06 | 0.9522–1.181 | 0.286 | |||
| Race | ||||||
| White | 1 (Reference) | |||||
| Black/African-American | 1.044 | 0.945–1.154 | 0.4 | |||
| Others | 0.8347 | 0.622–1.120 | 0.228 | |||
| Grade | ||||||
| I | 1 (Reference) | 1 (Reference) | ||||
| II | 1.057 | 0.525 | 1.093 | 0.920–1.300 | 0.312058 | |
| III | 1.59 | 1.335–1.894 | 1.95e-07*** | 1.341 | 1.120–1.605 | 0.001410** |
| IV | 3.685 | 2.925–4.643 | <2e-16*** | 1.991 | 1.562–2.536 | 2.53e-08*** |
| Unknown | 1.63 | 1.361–1.952 | 1.15e-07*** | 1.280 | 1.065–1.539 | 0.008616** |
| T | 1.63 | |||||
| T1 | 1 (Reference) | 1 (Reference) | ||||
| T2 | 1.779 | 1.564–2.024 | <2e-16*** | 1.262 | 1.103–1.444 | 0.000724*** |
| T3 | 2.737 | 2.434–3.077 | <2e-16*** | 1.549 | 1.356–1.770 | 1.20e-10*** |
| T4 | 7.103 | 5.139–9.817 | <2e-16*** | 1.405 | 0.983–2.008 | 0.062421 |
| TX | 4.824 | 3.491–6.666 | <2e-16*** | 1.649 | 1.152–2.359 | 0.006237** |
| N | ||||||
| N0 | 1 (Reference) | 1 (Reference) | ||||
| N1 | 6.383 | 5.312–7.670 | <2e-16*** | 2.182 | 1.729–2.753 | 5.01e-11*** |
| N2 | 7.936 | 6.494–9.698 | <2e-16*** | 2.207 | 1.554–2.643 | 1.83e-07*** |
| NX | 1.598 | 1.180–2.163 | 0.00242** | 1.184 | 0.834–1.679 | 0.344867 |
| M | ||||||
| M0 | 1 (Reference) | 1 (Reference) | ||||
| M1 | 11.212 | 9.711–12.945 | <2e-16*** | 3.989 | 3.268–4.869 | <2e-16*** |
| MX | 1.547 | 0.984–2.431 | 0.059 | 0.991 | 0.587–1.674 | 0.972989 |
| Surgery of primary site | ||||||
| None | 1 (Reference) | |||||
| Partial nephrectomy | 0.131 | 0.110–0.156 | 0.316 | 0.256–0.389 | <2e-16*** | |
| Total nephrectomy | 0.329 | 0.281–0.384 | 0.612 | 0.502–0.746 | 1.14e-06*** | |
| Lymphadenectomy, num | ||||||
| 0 | 1 (Reference) | 1 (Reference) | ||||
| 1–3 regional lymph nodes | 1.844 | 1.565–2.173 | 2.82e-13*** | 1.024 | 0.848–1.235 | 0.807307 |
| ≥4 regional lymph nodes | 2.138 | 5.84e-16*** | 0.987 | 0.793–1.228 | 0.906166 | |
| Chemotherapy | ||||||
| No/Unknown | 1 (Reference) | 1.778–2.570 | 1 (Reference) | |||
| Yes | 7.244 | 6.180–8.491 | <2e-16*** | 1.448 | 1.170–1.791 | 0.000662*** |
| Marital status | ||||||
| Single | 1 (Reference) | 1 (Reference) | ||||
| Married | 0.7713 | 0.704–0.846 | 3.03e-08*** | 0.752 | 0.684–0.825 | 2.17e-09*** |
SEER – Surveillance, Epidemiology, and End Results; HR – hazard ratio; CI – confidence interval. p<0.1;
p<0.05;
p<0.01;
p<0.001.
Nomogram construction for 3-year, 5-year, and 10-year OS
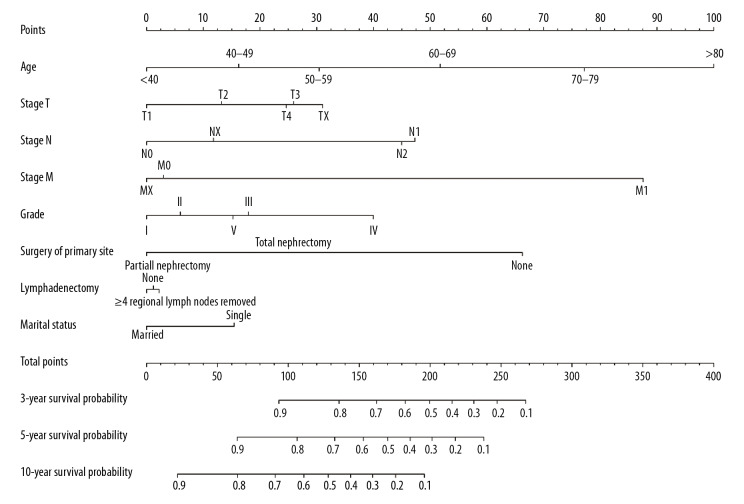
The results of univariate and multivariate Cox regression analyses are presented in Table 2. Combined with clinical practice, the following variables were considered independent risk factors: age, gender, TNM classification, Fuhrman grade, surgery of primary sites, and marital status. On the basis of these factors, the nomogram was established for estimating the 3-year, 5-year, and 10-year OS for the training cohort, shown in Figure 1, where linear parallel lines show the nomogram model.
Figure 1.
Nomograms for 3-year, 5-year, and 10-year overall survival (OS) for patients with papillary renal cell carcinoma (RCC). OS – overall survival; RCC – renal cell carcinoma.
Validation of the nomogram
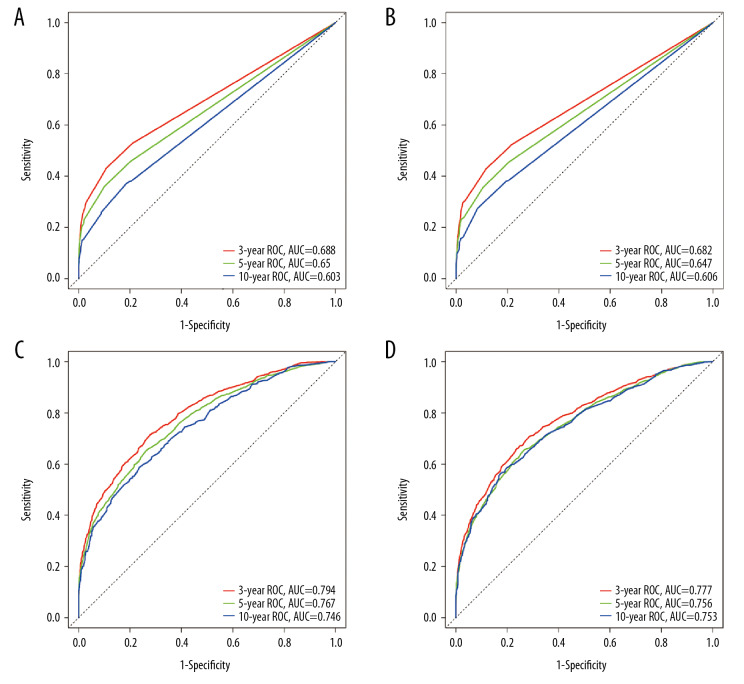
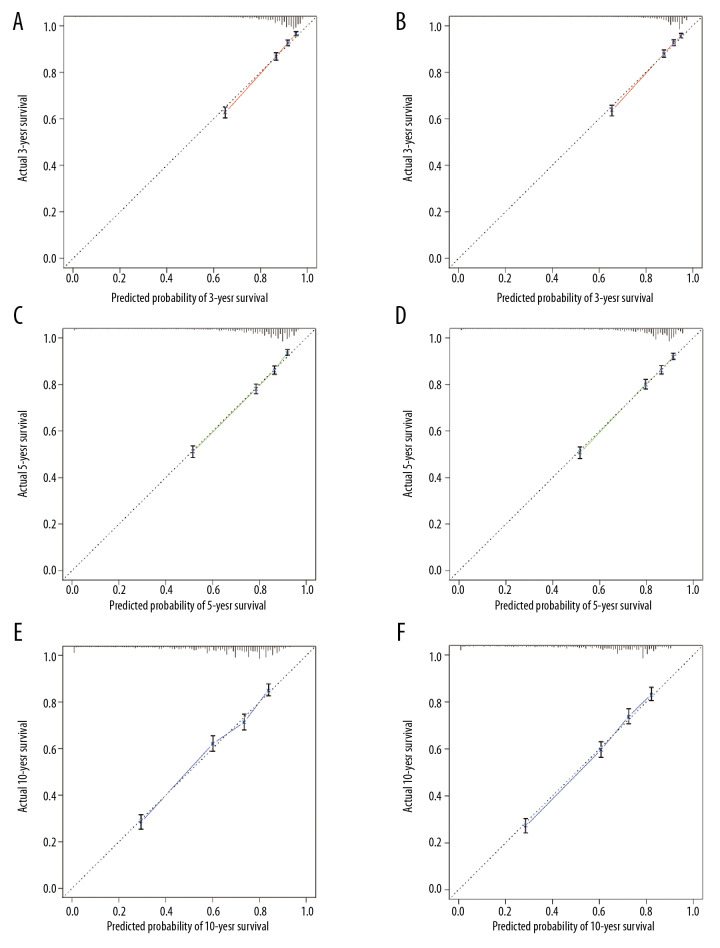
Internal validation using the training and validation cohorts showed the C-indices (0.752, 95% CI, 0.740–0.746; 0.747, 95% CI, 0.735–0.759, respectively), indicating improved predictive efficacy compared with TNM stage (0.643, 95% CI, 0.629–0.657; 0.642, 95% CI, 0.630–0.654, respectively), which specifically indicated the good discriminative ability of the nomogram (Figure 2). The calibration plots showed the nomogram’s significance in training and validation cohorts (Figure 3). The nomogram showed enhanced discrimination and accuracy in predicting values for the 3-year, 5-year, and 10-year OS.
Figure 2.
Receiver operating characteristic (ROC) plots were constructed to compare the TNM classification and the newly established nomogram, by the area under the ROC curve (AUC). (A) From the training cohort. (B) From the validation cohort. A and B are based on the TNM classification. (C) From the training cohort. (D) From the validation cohort. C and D are based on the nomogram.
Figure 3.
The calibration curves for the 3-year, 5-year, and 10-year overall survival (OS) for the training cohort (A, C, E) and validation cohort (B, D, F).
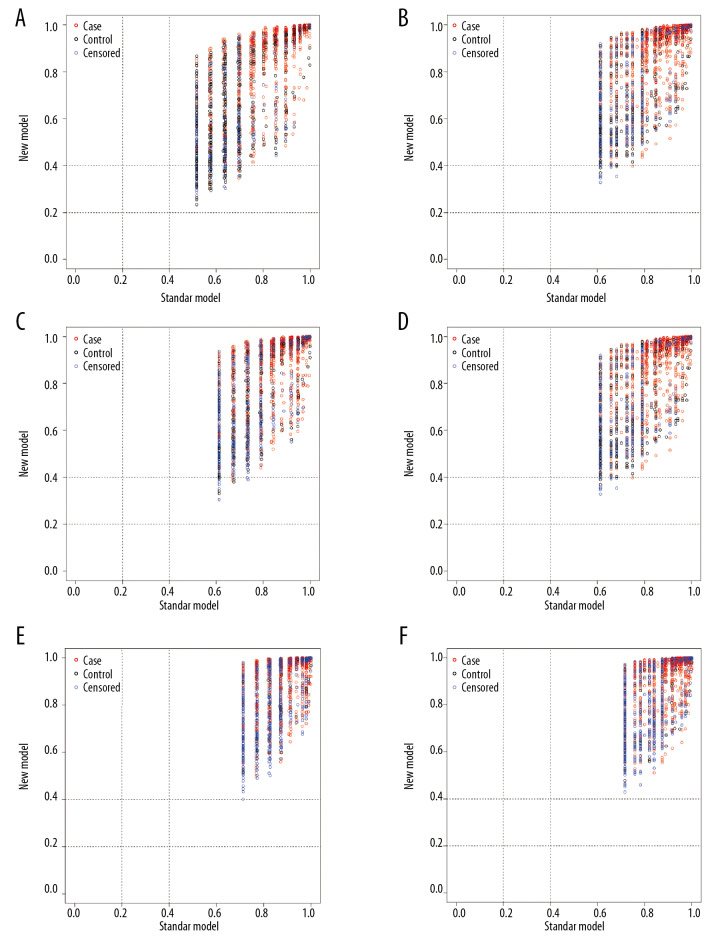
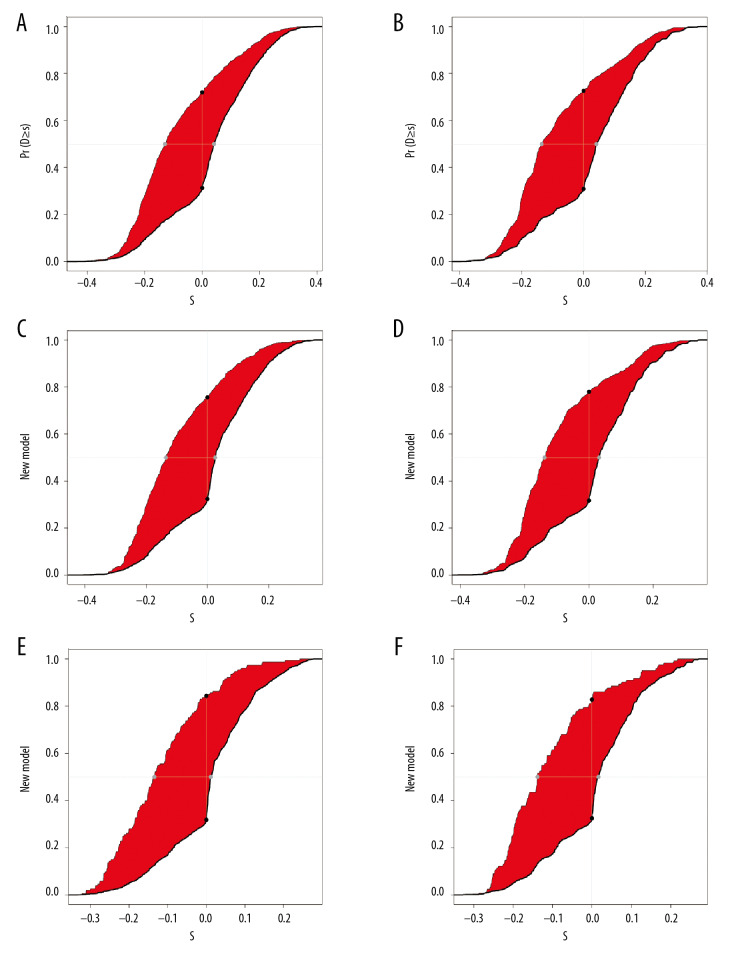
The NRI and IDI values for the 3-year, 5-year, and 10-year OS in the training and validation cohorts were calculated, respectively. The results are listed in Table 3, Figures 4, and 5. The results suggest that the new model had significantly improved accuracy and reliability for cancer prediction compared with the TNM staging system.
Table 3.
NRI and IDI for 3-, 5-, and 10-year overall survival in training cohort and validation cohort.
| Training cohort | Validation cohort | |||
|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |
| NRI for 3 years | 0.196 | 0.418–0.252 | 0.134 | 0.117–0.221 |
| NRI for 5 years | 0.036 | 0.007–0.069 | 0.032 | 0.007–0.062 |
| NRI for 10 years | 0.000 | 0–0.003 | 0.000 | 0–0.002 |
| IDI for 3 years | 0.125 | 0.106–0.141 | 0.121 | 0.117–0.221 |
| IDI for 5 years | 0.127 | 0.109–0.141 | 0.126 | 0.109–0.141 |
| IDI for 10 years | 0.133 | 0.115–0.153 | 0.123 | 0.106–0.147 |
NRI – net reclassification improvement; IDI – integrated discrimination improvement; CI – confidence interval.
Figure 4.
The net reclassification improvement (NRI) plots for the 3-year, 5-year, and 10-year overall survival (OS) for the training cohort (A, C, E) and validation cohort (B, D, F).
Figure 5.
The integrated discrimination improvement (IDI) plots for the 3-year, 5-year, and 10-year overall survival (OS) for the training cohort (A, C, E) and validation cohort (B, D, F).
Potential application of the nomogram in clinical practice
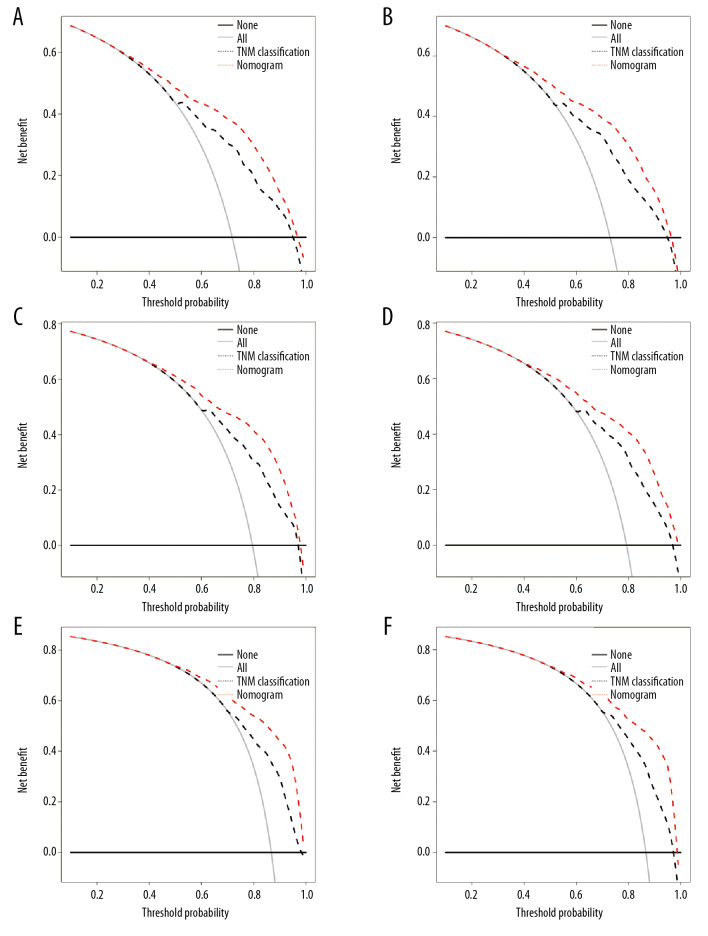
To further explore the application of the new model for decision making in clinical practice, the DCA in the training and validation cohorts were mapped based on the above analysis. For most patients, the new model provided increased net benefits relative to those provided by the TNM staging system, which is an advanced and efficient tool for decision making in clinical practice (Figure 6).
Figure 6.
The decision curve analysis (DCA) for the training cohort (A, C, E) and validation cohort (B, D, F) for the 3-year, 5-year, and 10-year overall survival (OS). In the figure, the red dotted line represents the new nomogram model. The black dotted line represents the TNM classification. All – assume all patients with papillary RCC survive; None – assume no patient with papillary RCC survived; DCA – decision curve analysis.
Discussion
This study developed a predictive nomogram for midterm to long-term prognosis in patients with papillary renal cell carcinoma (RCC) based on data from the US Surveillance, Epidemiology, and End Results (SEER) program and included a large number of clinical samples. A series of variables were screened by clinical significance to construct and validate the performance of the model. The new model may provide the basis for future clinical decisions. RCC accounts for about 85% of adult renal malignancies [8,15]. RCC is the most common type of renal malignancy, and papillary RCC accounts for 10–20% of all RCC cases [15]. However, compared with other types of RCC, papillary RCC lacks specific clinical manifestations and related symptoms and is also difficult to identify by preoperative imaging, and its biological behavior is still not fully understood [25,26]. According to previous studies, the prognosis of patients with papillary RCC is generally better than for other types of RCC. Patard et al. showed that the 5-year survival rates of RCC, papillary RCC, and chromophobe RCC were 73.2%, 79.4%, and 87.9%, respectively [27]. However, there is a lack of a concise and effective tool to predict prognosis in patients with papillary RCC individually in clinical practice, apart from the TNM staging system.
A nomogram is a graphic tool widely used as a predictive model that integrates biological, clinical, and social variables to estimate the risk of specific diseases [28,29]. Nevertheless, considering that numerous prognostic factors of papillary RCC have been identified in clinical practice, the high variability in sequelae observed in individual patients is not expected to be predictable based on any single factor. Compared with the conventional TNM tumor staging system, the nomogram showed enhanced accuracy in predicting survival outcomes, which benefited both clinicians and patients in counseling and personalized treatment [30,31]. Currently, no prognostic nomogram has been constructed for patients with papillary RCC. Therefore, it is necessary to construct a more reliable and precise prognostic model.
In this study, a new nomogram was established to estimate the prognosis of patients with papillary RCC, assisting in the development of therapeutic strategies for patients with papillary RCC. The annual incidence of papillary RCC was relatively rare, indicating that single-center studies were generally unable to reach reliable conclusions based on significant sample sizes [32]. Consequently, the scope of this study relied on the SEER program, a large sample database that was initially established by eight registries in 1973, with other institutes enrolling in the last few decades. At this time, the database covered 18 geographically different areas containing 26% of the US population, displaying the population’s diversity comprehensively [33,34]. For establishing reliable results, data for 13 926 patients diagnosed with papillary RCC from 1997 to 2014 were extracted from the SEER database. Among the included cases, most (61.86%) were ≥60 years old. Our sampling pool was consistent with that in the previous research, considering that most of the patients were elderly, male, white, married, and treated with surgery [2,5,35]. Also, older patients had a relatively high incidence of papillary RCC, in line with previous work, and accordingly, the prognosis was generally worse than that of younger people [7,36]. These results are shown graphically in our nomogram. As the age increased, the survival rates for papillary RCC remained quite dismal. In clinical practice, the TNM classification was the conventional method for evaluating the prognosis of cancers [26,28]. The TNM categories contributed to the prognosis and treatment of cancers. Depending on the enlarged tumor size, multiple occurrences of metastatic lymph nodes, and the onset of distant metastasis, the risk of mortality could be increased to a large extent [37], which has been supported by previous studies [8,15]. However, besides the TNM system, various risk factors for poor prognosis of cancer were also suggested. For example, the impact of mental health has garnered increasing attention in recent years [38]. Specifically, marital status significantly affected OS in this study. Married patients tended to receive more support and emotional comfort from family, which possibly led to a better prognosis [38,39].
The Fuhrman grade was another independent factor for OS, as shown by the new model presented in this stud, High-grade surgery was a protective factor, indicating better prognostic outcome; conversely, patients with lower-grade surgery tended to have worse prognostic outcomes. In contrast to previous research, tumor grade was not identified as a significant independent risk in prognosis [14,18]. In 2005, John et al. found that papillary RCC did not cause significantly different outcomes during follow-up [18]. The conflicting data may be caused by different research methodologies, differences in data sources, study duration, and inclusion and exclusion criteria. Therefore, in-depth research based on an extended sample size would be required in the future.
In line with previous studies, patients with papillary RCC were less likely to show disease-specific symptoms, compared with patients with clear cell and chromophobe RCC [28]. Benefitting from improved biological and imaging technology, patients with minimal metastatic disease had a chance to be identified early, thus improving the prognosis of papillary RCC. In this study, patients who received PN presented with increased OS compared with those who received radical nephrectomy (RN). Many retrospective studies suggest that patients undergo nephron-sparing surgery (NSS) related to RN [40,41]. Also, NSS was recommended as the standard surgical approach for T1a (TNM classification) for renal tumors [42]. RN was widely utilized for cT1b tumors, while it was also a recommended therapy for T2a (TNM classification) and other tumors with relatively large sizes [32,33]. Mir et al. considered that young patients undergoing PN had reduced masses, which might result in improved prognosis. However, patients with solitary RCC who received surgical treatment had a better prognostic outcome; this highlights the necessity of surgical treatment for better renal function outcomes [43]. A decrease in renal function could contribute to an increased risk of severe cardiovascular disease, as well as all-cause mortality [44]. In a meta-analysis, Kim et al. [45] analyzed the risk of severe chronic renal failure and all-cause mortality for patients undergoing NSS. Consequently, there were 61% and 19% reductions, respectively. Also, based on the preservation of renal function and the reduced risks of cardiovascular events and other adverse effects, such as new-onset hypertension, cerebrovascular disease, and diabetes, the prognosis of patients could be effectively improved with the surgical procedure.
However, routine lymph node dissection and the scope of cleansing in RN are still controversial. Generally, RN regional lymph node dissection should include the renal hilar lymph nodes, para-aortic and inferior vena cava, up to the level of the upper pole of the kidney, and down to the level of the infrarenal pole for enlarged lymph node dissection and the need for the aorta after the regional lymph node dissection. At the vena cava, the tissue is removed up to the angle of the diaphragm and down to the bifurcation of the abdominal aorta. In this study, although regional lymph node dissection was not significantly associated with OS, considering the clinical experiences and follow-up results, we supposed that regional lymph node dissection might have a significant impact on prognosis; thus, it was included as a crucial prognostic factor in our new model. Nevertheless, Michael et al. suggested that lymph node dissection could help patients with RCC who have only lymph node metastasis without distant metastasis, by significantly improving the patient’s 5-year survival rate [46]. For patients with both lymph nodes and distant metastases, lymph node dissection could effectively reduce tumor cells. The function, however, was reported with increased clinical significance in regional lymph node dissection and enlarged lymph node dissection. Lymph node dissection could reduce the number of tumor cells in patients with lymph nodes and distant metastases. However, more reports indicated that the 5-year survival rates associated with regional lymph node dissection and enlarged lymph node dissection were statistically indistinguishable [47]. Manohar et al. reported that the metastatic rate of T3–T4 renal cancer lymph nodes were 20%, and regional lymph node dissection was recommended [48]. For this reason, although considerable controversy was ongoing regarding the scope of lymph node dissection, there was a consensus that surgery could provide certain improvements in prognosis.
Finally, the newly constructed nomogram model in our study included multiple factors, such as age at diagnosis, gender, race, TNM classification, Fuhrman grade, surgery of the primary site, lymphadenectomy, and marital status, which were readily accessible for information collection in clinical practice. Figure 6 shows the results of DCA, indicating that the abscissa and ordinate were the threshold probability and net benefit rate, respectively [21,49,50]. So far, IDI, NRI, and DCA were adopted to verify the accuracy and predictive abilities of nomograms for papillary RCC. In conclusion, the nomogram was validated to accurately predict the 3-year, 5-year, and 10-year OS for patients with papillary RCC.
This study had several limitations. First, the nomogram relied on retrospective data from the SEER database, which could potentially lead to selection bias. Second, some candidate prognostic variables were unavailable, including subtype of papillary RCC (type I and type II), surgical margin status, vascular invasion, surgical treatment details, and other non-surgical treatments, such as radiation therapy and targeted therapy [14,51]. These unavailable data in the SEER dataset should be highlighted for the follow-up research. Furthermore, considering the retrospective nature of our research, our results should be further validated with a clinical trial or prospective cohort. Finally, although the accuracy exceeded that of the TNM classification, our nomogram was not optimized.
Conclusions
This study aimed to develop a predictive nomogram for midterm to long-term prognosis in patients with papillary renal cell carcinoma (RCC) based on data from the US Surveillance, Epidemiology, and End Results (SEER) program. The nomogram that was developed evaluated the prognosis of papillary RCC and identified the patients who required specialized treatment. However, external validation of the predictive nomogram is required that also includes patients from other countries.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Chow WH, Devesa SS, Warren JL, Fraumeni JF. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Chow W-H, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J. 2008;14:288–301. doi: 10.1097/PPO.0b013e3181867628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrader AJ, Rauer-Bruening S, Olbert PJ, et al. Incidence and long-term prognosis of papillary renal cell carcinoma. J Cancer Res Clin Oncol. 2009;135:799–805. doi: 10.1007/s00432-008-0515-y. [DOI] [PubMed] [Google Scholar]
- 4.Novara G, Martignoni G, Artibani W, Ficarra V. Grading systems in renal cell carcinoma. J Urol. 2007;177:430–36. doi: 10.1016/j.juro.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic Subtyping of adult renal epithelial neoplasms – An experience of 405 cases. Am J Surg Pathol. 2002;26:281–91. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Amin MB, Corless CL, Renshaw AA, et al. Papillary (chromophil) renal cell carcinoma: Histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J Surg Pathol. 1997;21:621–35. doi: 10.1097/00000478-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Cheville JC, Lohse CM, Zincke H, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–24. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Margulis V, Tamboli P, Matin SF, et al. Analysis of clinicopathologic predictors of oncologic outcome provides insight into the natural history of surgically managed papillary renal cell carcinoma. Cancer. 2008;112:1480–88. doi: 10.1002/cncr.23322. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–81. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 10.Dralle H, Musholt TJ, Schabram J, et al. German Association of Endocrine Surgeons practice guideline for the surgical management of malignant thyroid tumors. Langenbecks Arch Surg. 2013;398:347–75. doi: 10.1007/s00423-013-1057-6. [DOI] [PubMed] [Google Scholar]
- 11.Daugherty M, Sedaghatpour D, Shapiro O, et al. The metastatic potential of renal tumors: Influence of histologic subtypes on definition of small renal masses, risk stratification, and future active surveillance protocols. Urol Oncol. 2017;35(4):153.e15–20. doi: 10.1016/j.urolonc.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Shuch B, Amin A, Armstrong AJ, et al. Understanding pathologic variants of renal cell carcinoma: Distilling therapeutic opportunities from biologic complexity. Eur Urol. 2015;67:85–97. doi: 10.1016/j.eururo.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Gettman MT, Blute ML, Spotts B, et al. Pathologic staging of renal cell carcinoma: Significance of tumor classification with the 1997 TNM staging system. Cancer. 2001;91:354–61. doi: 10.1002/1097-0142(20010115)91:2<354::aid-cncr1009>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Leibovich BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183:1309–15. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Cairns P. Renal cell carcinoma. Cancer Biomark. 2011;9:461–73. doi: 10.3233/CBM-2011-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linehan WM, Spellman PT, Ricketts CJ, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374:135–45. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015;16:E173–80. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cindolo L, Patard JJ, Chiodini P, et al. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy – A multicenter European study. Cancer. 2005;104:1362–71. doi: 10.1002/cncr.21331. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S. The need for reorientation toward cost-effective prediction: Comments on ‘Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond’ by M. J. Pencina et al., Statistics in Medicine. Stat Med. 2008;27(2):199–206. doi: 10.1002/sim.2995. [DOI] [PubMed] [Google Scholar]
- 21.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 23.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 24.Yap NY, Khoo WT, Perumal K, et al. Practical updates in medical therapy for advanced and metastatic renal cell carcinoma. Urological Sci. 2018;29:120–28. [Google Scholar]
- 25.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y, Huang H, Kang M, et al. Combined Ki67 and ERCC1 for prognosis in non-keratinizing nasopharyngeal carcinoma underwent chemoradiotherapy. Oncotarget. 2017;8:88552–62. doi: 10.18632/oncotarget.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianco FJ., Jr Nomograms and medicine. Eur Urol. 2006;50:884–86. doi: 10.1016/j.eururo.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 29.Lerner SP, Bochner B, Kibel AS. The use and abuse of data: Nomograms and talking to patients about clinical medicine. Urol Oncol-Semin Orig Investig. 2007;25:333–37. doi: 10.1016/j.urolonc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Brateanu A, Yu C, Kattan MW, et al. A nomogram to predict the probability of passing the American Board of Internal Medicine examination. Med Educ Online. 2012;17:18810. doi: 10.3402/meo.v17i0.18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao J, Yuan P, Wang L, et al. Clinical nomogram for predicting survival of esophageal cancer patients after esophagectomy. Sci Rep. 2016;6:26684. doi: 10.1038/srep26684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow W-H, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–57. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JL, Dalkin BL, True LD, et al. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. 2010;183:2213–18. doi: 10.1016/j.juro.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang G, Wu Y, Zhang J, et al. Nomograms for predicting long-term overall survival and disease-specific survival of patients with clear cell renal cell carcinoma. Onco Targets Ther. 2018;11:5535–44. doi: 10.2147/OTT.S171881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–45. [PubMed] [Google Scholar]
- 36.Mejean A, Hopirtean V, Bazin JP, et al. Prognostic factors for the survival of patients with papillary renal cell carcinoma: Meaning of histological typing and multifocality. J Urol. 2003;170:764–67. doi: 10.1097/01.ju.0000081122.57148.ec. [DOI] [PubMed] [Google Scholar]
- 37.Uhlman DL, Nguyen P, Manivel JC, et al. Epidermal growth factor receptor and transforming growth factor alpha expression in papillary and nonpapillary renal cell carcinoma: Correlation with metastatic behavior and prognosis. Clin Cancer Res. 1995;1:913–20. [PubMed] [Google Scholar]
- 38.Zhou H, Zhang Y, Song Y, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Clin Res Hepatol Gastroenterol. 2017;41:476–86. doi: 10.1016/j.clinre.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Zhang G, Wu Y, Zhang J, et al. Nomograms for predicting long-term overall survival and disease-specific survival of patients with clear cell renal cell carcinoma. Onco Targets Ther. 2018;11:5535–44. doi: 10.2147/OTT.S171881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: Effect on overall and noncancer mortality. Cancer. 2009;115:1465–71. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 41.Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors – is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–24. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–22. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 44.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 2006;7:735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SP, Murad MH, Thompson RH, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol. 2012 doi: 10.1016/j.juro.2012.10.026. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Harper JD, Spencer ES, Porter MP, Gore JL. Adoption of laparoscopic radical nephrectomy in the state of Washington. Urology. 2012;79:326–31. doi: 10.1016/j.urology.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 47.Gabr AH, Elsayed ER, Gdor Y, et al. Obesity and morbid obesity are associated with a greater conversion rate to open surgery for standard but not hand assisted laparoscopic radical nephrectomy. J Urol. 2008;180:2357–62. doi: 10.1016/j.juro.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 48.Ganpule AP, Sharma R, Thimmegowda M, et al. Laparoscopic radical nephrectomy versus open radical nephrectomy in T1-T3 renal tumors: An outcome analysis. Indian J Urol. 2008;24:39–43. doi: 10.4103/0970-1591.38602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, et al. Long-term bleeding risk prediction in ‘real world’ patients with atrial fibrillation: Comparison of the HAS-BLED and ABC-Bleeding risk scores. The Murcia Atrial Fibrillation Project. Thromb Haemost. 2017;117(10):1848–58. doi: 10.1160/TH17-07-0478. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Fernandez A, Roldan V, Rivera-Caravaca JM, et al. Does von Willebrand factor improve the predictive ability of current risk stratification scores in patients with atrial fibrillation? Sci Rep. 2017;7:41565. doi: 10.1038/srep41565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herrmann E, Trojan L, Becker F, et al. Prognostic factors of papillary renal cell carcinoma: Results from a multi-institutional series after pathological review. J Urol. 2010;183:460–66. doi: 10.1016/j.juro.2009.10.026. [DOI] [PubMed] [Google Scholar]