Abstract
Purpose
Metabolic and bariatric surgery (MBS) is increasingly performed in patients with previous solid organ transplantation (PSOT). In addition, controversy remains about whether racial disparity in outcomes following MBS exists. Therefore, the aim of this analysis was to determine if race independently predicts outcomes in MBS patients with PSOT.
Materials and Methods
Patients with PSOT undergoing sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) were identified in the 2017 Metabolic and Bariatric Surgery Accreditation Quality and Improvement Project (MBSAQIP) database. Patients were stratified by race (Black and White). Propensity score matching was utilized to adjust for multiple demographic variables. Multivariable logistic regression analyses were performed for overall and bariatric-related morbidity.
Results
Of 335 MBS patients with PSOT, 250 (75%) were white and 85 (25%) were black patents. Procedure-type and surgical approach (p > 0.1) were similarly distributed. Black patients were more likely (p < 0.05) to have hypertension dialysis-dependent chronic kidney disease, and be on chronic steroids). Mortality and morbidity were similar. Black patients had significantly (p < 0.05) higher rates of renal failure, pulmonary complications, and emergency department visits in unmatched analysis. After propensity score matching, 82 patients in each cohort were identified and were similar at baseline (p > 0.5). In the matched analysis, black patients had higher overall (17% vs. 10%, p = 0.12) and bariatric-related morbidity (14% vs. 7.2%, p = 0.05). In addition, black patients had significantly (p < 0.05) higher rates of postoperative pneumonias, progressive renal insufficiency, and emergency department visits. On multivariable regression analysis, black race did not independently predict overall or bariatric-related morbidity.
Conclusion
MBS in racial cohorts with PSOT is safe, with very low rates of overall morbidity and mortality. Black race trended toward increased postoperative morbidity. Larger cohort studies are needed to validate our findings.
Electronic supplementary material
The online version of this article (10.1007/s11695-020-04813-9) contains supplementary material, which is available to authorized users.
Keywords: Bariatric surgery, Prior solid organ transplant, Outcomes, Racial disparity
Introduction
Obesity is an increasing epidemic worldwide [1, 2] that is associated with significant morbidity. Metabolic and bariatric surgery (MBS) has been proven as a safe and effective treatment option for severe obesity that is associated with durable weight loss, improvement and resolution of comorbid conditions [3–6], reduced cancer risk, increased longevity [7, 8], and improved quality of life [9, 10].
Given the increasing safety profile of MBS, there is an increasing interest in its role in higher-risk patient cohorts, including transplant candidates and patients. This increasing practice trend is driven by data showing that 2–12% of patients awaiting solid organ transplantation meet the criteria for severe obesity (body mass index (BMI) > 35 kg/m2) [11] and weight loss prior to organ transplantation results in better perioperative outcomes and improved graft survival [12, 13]. While limited to small case series and single-institution experience, there is growing evidence suggesting that MBS can be a safe and effective weight loss treatment option in patients with previous solid organ transplant (PSOT) [11–16].
While obesity has increased among all demographics in the USA, the prevalence and health impact disproportionately impact racial and ethnic minority patients [1–3, 17–20] who continue to be an underrepresented cohort of MBS patients. Even though MBS is overall safe and effective [3–10], there remains a controversy regarding the safety profile and effectiveness of MBS surgery in racial and ethnic cohorts, with some studies reporting no outcome differences [21–23] while others report higher rates of adverse outcomes in racial and ethnic minority patients [24, 25].
No published data exists on outcomes in racial and ethnic MBS patient cohorts with PSOT. Therefore, the goal of this study goal was to compare outcomes between black and white MBS patients with a history of PSOT to determine if race was an independent predictor of perioperative outcomes.
Material and Methods
Data Source
A retrospective analysis of the 2017 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) Participant Use Files (PUF) database was performed, and outcomes between racial cohorts of MBS patients with PSOT were compared. The MBSAQIP is responsible for the accreditation of bariatric surgical facilities. Requirements for certification includes reporting bariatric surgical outcomes to the MBSAQIP Participant Use Data File (PUF), a Health Insurance Portability and Accountability Act (HIPAA)-compliant data file registry containing prospectively entered, risk-adjusted, clinically rich data using standardized definitions for preoperative, intraoperative, and postoperative variables that are specific to metabolic and bariatric surgical care. Data points are abstracted at participating institutions by certified reviewers who are audited for accuracy of performance. The 2017 file included data on a composite variable of previous solid organ transplantation (PSOT), including a history of heart, lung, liver, renal, pancreas, and bowel transplantation. This is a de-identified, nationally available, clinical database; therefore, neither institutional review board (IRB) approval nor patient consent was required for our study.
Case Selection and Inclusion Criteria
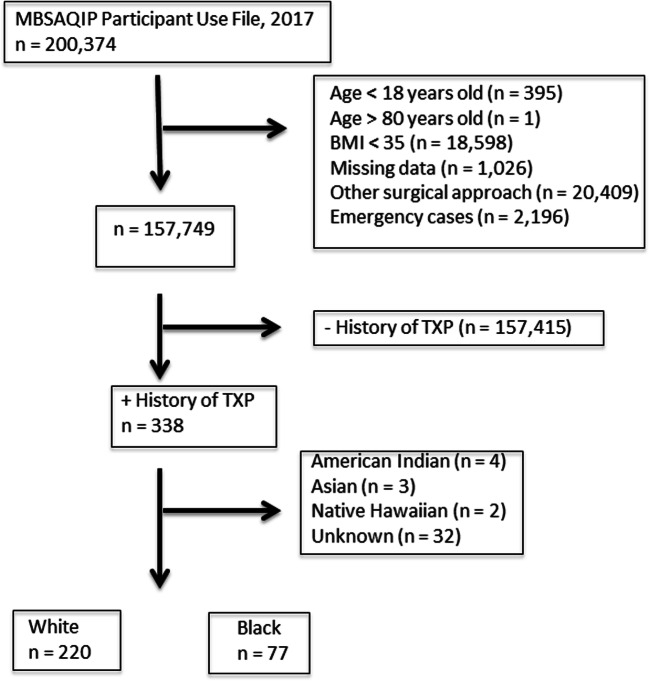
Our inclusion and exclusion criteria are detailed in Fig. 1. Patients with PSOT undergoing primary gastric bypass (RYGB) or sleeve gastrectomy (SG) cases were identified through Current Procedural Terminology (CPT) codes 43644, 43645, and 43775. Exclusions included bariatric procedure other than a RYGB or SG, bariatric procedures designated as emergency, open cases, surgical approach other than robotic-assisted or conventional laparoscopy, and other races including American Indian or Alaska Native, Asian, Native Hawaiian, or Other Pacific Islands and Unknown. Selected cases were further stratified by race (non-Hispanic black vs. non-Hispanic white).
Fig. 1.
Inclusion and exclusion flow diagram. MBSAQIP = Metabolic and Bariatric Surgery Accreditation and Quality Improvement Project, BMI = body mass index, TXP = transplantation
Data Collection, Matching, and Statistical Analysis
Descriptive statistics were collected and compared between groups, including demographics, health summary status, preoperative comorbidities, and operative characteristics. Primary outcome measures included 30-day mortality and morbidity. Secondary outcome measures included other 30-day adverse outcomes (reoperation, readmission, and reintervention), postoperative complication, aggregate complications (Appendix Table 7), and hospital outcomes (operative duration, conversion, and hospital length of stay). Propensity score–matched analyses were performed to adjust for intergroup biases. The matching ratio was one-to-one. A logistic regression model was generated on patient demographics and variables with a p < 0.1 on univariate analysis between racial cohorts with prior solid organ transplantation. Variables included in our regression model were Hispanic ethnicity, hypertension (HTN), chronic steroid use, obstructive sleep apnea (OSA), dialysis-dependent chronic kidney disease (CKD), and renal insufficiency. A propensity score from 0 to 1 was generated from this model and assigned to each subject. A nearest neighbor 1:1 variable ratio with propensity scores that fell within a caliper of 0.02 was then used to generate matched cohorts hypothesized to be balanced of potentially confounding baseline characteristics.
Table 7.
Composite complication methodology
| Aggregate variable | Composite variables |
|---|---|
| Leak | Reoperation with suspected reason: leak |
| Readmission with suspected reason: leak | |
| Intervention with suspected reason: leak | |
| Drain present over 30 days | |
| Complication: organ space SSI | |
| Bleeding | Reoperation with suspected reason: bleeding |
| Readmission with suspected reason: bleeding | |
| Intervention with suspected reason: bleeding | |
| Cardiac/CVA | Reoperation with suspected reason: cardiac NOS, CVA, or MI |
| Readmission with suspected reason: cardiac NOS, CVA, or MI | |
| Intervention with suspected reason: cardiac NOS, CVA, or MI | |
| Complication of CVA | |
| Complication of MI | |
| Pulmonary | Reoperation with suspected reason: shortness of breath, pneumonia, or other respiratory failure |
| Readmission with suspected reason: shortness of breath, pneumonia, or other respiratory failure | |
| Intervention with suspected reason: shortness of breath, pneumonia, or other respiratory failure | |
| Complication: on ventilator > 48 h | |
| Complication: unplanned intubation | |
| Complication: pneumonia | |
| Renal | Reoperation with suspected reason: renal insufficiency |
| Readmission with suspected reason: renal insufficiency | |
| Intervention with suspected reason: renal insufficiency | |
| Complication: progressive renal insufficiency | |
| Complication: acute renal failure | |
| DVT or PE | Reoperation with suspected reason: vein thrombosis requiring therapy or pulmonary embolism |
| Readmission with suspected reason: vein thrombosis requiring therapy or pulmonary embolism | |
| Intervention with suspected reason: vein thrombosis requiring therapy or pulmonary embolism | |
| Complication: vein thrombosis requiring therapy | |
| Complication: pulmonary embolism | |
| Complication: anticoagulation initiated of presumed/confirmed vein thrombosis/PE | |
| Wound infection | Reoperation with suspected reason: wound infection or other abdominal sepsis |
| Readmission with suspected reason: wound infection or other abdominal sepsis | |
| Intervention with suspected reason: wound infection or other abdominal sepsis | |
| Complication: post-op superficial incisional SSI occurrence | |
| Complication: post-op deep incisional SSI occurrence | |
| Other infection | Reoperation with suspected reason: infection/fever |
| Readmission with suspected reason: infection/fever, | |
| Intervention with suspected reason: infection/fever | |
| Complication: post-op sepsis occurrence | |
| Complication: post-op septic shock occurrence | |
| Complication: post-op pneumonia occurrence | |
| Complication: post-op urinary tract infection occurrence | |
| Overall morbidity |
Mortality within 30 days Need for intervention within 30 days Need for readmission within 30 days Need for reoperation within 30 days Unplanned ICU transfer within 30 days |
| Aggregate-related reoperation | Any reoperation designated as related to metabolic/bariatric by variable REOP_RELATED_BAR1. To REOP_RELATED_BAR.13 |
| Aggregate-related readmission | Any readmission designated as related to metabolic/bariatric by variable READ_RELATED_BAR1. To READ_RELATED_BAR.11 |
| Aggregate-related intervention | Any intervention designated as related to metabolic/bariatric by variable INVT_RELATED_BAR1. To INTV_RELATED_BAR.5 |
| Bariatric surgery–related morbidity |
Death related to bariatric surgery Aggregate reoperation related to metabolic/bariatric surgery Aggregate readmission related to metabolic/bariatric surgery Aggregate intervention related to metabolic/bariatric surgery |
A backward method multivariable logistic regression was performed for overall and bariatric-related morbidity. Factors with a difference (p < .05) on univariate analysis between the two racial cohorts were included in the model: race (Black vs. White), Hispanic ethnicity, hypertension, chronic steroid use, and dialysis-dependent CKD in our multivariate logistic regression analyses. A procedure-specific multivariable regression analysis was performed for sleeve gastrectomy and gastric bypass cases independently. For each procedure, race and variables that were significantly (p < .05) on univariate analysis were included in the multivariate regression models. For SG cases, variables included in the regression algorithm were race, Hispanic ethnicity, gender, and OSA. For RYGB, variables included race, ethnicity, body mass index (BMI), and renal insufficiency.
For univariate unmatched and matched analyses, primary and secondary outcomes were compared by Pearson Chi-Square test for categorical variables and Mann-U-Whitney for continuous variables. Continuous data is expressed as median and interquartile range (IQR) and categorical data is expressed as frequency and percentage. All statistical analyses were performed with SPSS version 26 (IBM Corporation, Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC). A p value of < 0.05 was considered statistically significant.
Results
Patient Demographics
Of the 200,374 cases in the 2017 MBSAQIP database, 0.3% (n = 614) had a PSOT. Following exclusions, 335 patients with a prior solid organ transplant were analyzed, including 250 white and 85 black patients. Thirty-four percent (n = 208) of the transplant cohort was excluded for CPT codes associated with procedures other than RYBG and SG. Characteristics of the unmatched racial cohorts are detailed in Table 1. Patient demographics (age, gender, and body mass index), operative characteristics (procedure type (RYGB vs. SG), surgical approach (conventional laparoscopy vs. robotic-assisted)), and most preoperative comorbidities were similar between racial cohorts. Black patients had significantly higher rates of hypertension (77% vs. 64%, p = 0.03), chronic steroid use (52% vs. 38%, p = 0.03), and dialysis-dependent chronic kidney disease (15% vs. 6.8%, p = 0.02).
Table 1.
Unmatched patient characteristics
| Total (n = 335) |
White (n = 250) |
Black (n = 85) |
p Value | Effect size | |
|---|---|---|---|---|---|
| Continuous variables, median | |||||
| Age, years | 49 (41–58) | 49 (41–59) | 48 (41–57) | 0.317 | 0.17 |
| 42.1 (38.5- | 41.6 (38.5- | 42.9 (38.6- | |||
| BMI, kg/m2 | 46.2) | 46.2) | 46.2) | 0.467 | 0.14 |
| Categorical variables, n, (%) | |||||
| Gender, female | 237 (71) | 173 (69) | 64 (75) | 0.286 | 1.26 |
| Ethnicity (Hispanic) | 46 (14) | 45 (18) | 1 (1.2) | < 0.001 | 13 |
| ASA class > 3 | 297 (89) | 218 (87) | 79 (93) | 0.149 | 1.93 |
| Operation type | 0.141 | 1.42 | |||
| Sleeve | 252 (75) | 183 (73) | 69 (81) | ||
| Gastric bypass | 83 (25) | 67 (27) | 16 (19) | ||
| Surgical approach | 0.454 | 0.92 | |||
| Laparoscopic | 309 (92) | 229 (92) | 80 (94) | ||
| Robotic | 26 (8) | 21 (8) | 5 (6) | ||
| History of myocardial infarction | 9 (2.7) | 8 (3.2) | 1 (1.2) | 0.458 | 2.32 |
| History of PCI | 16 (4.8) | 13 (5.2) | 3 (3.5) | 0.533 | 1.37 |
| History of Cardiac Surgery | 23 (6.9) | 20 (8.0) | 3 (3.5) | 0.159 | 2.02 |
| Hypertension | 225 (67) | 160 (64) | 65 (77) | 0.034 | 1.83 |
| Diabetes mellitus | 117 (35) | 92 (37) | 25 (29) | 0.217 | 0.92 |
| Hyperlipidemia | 135 (40) | 103 (41) | 32 (38) | 0.564 | 1.12 |
| Gastroesophageal reflux disease | 143 (43) | 112 (45) | 31 (37) | 0.180 | 1.30 |
| COPD | 4 (1.2) | 4 (1.6) | 0 (0) | 0.241 | 0.74 |
| Obstructive sleep apnea | 118 (35) | 95 (38) | 23 (27) | 0.068 | 0.89 |
| Oxygen dependent | 4 (1.2) | 3 (1.2) | 1 (1.2) | 0.986 | 1.02 |
| Smoker | 11 (3.3) | 8 (3.2) | 3 (3.5) | 0.883 | 1.11 |
| Renal insufficiency | 47 (14) | 30 (12) | 17 (20) | 0.067 | 1.83 |
| Dialysis | 30 (9) | 17 (6.8) | 13 (15) | 0.018 | 2.50 |
| DVT | 14 (4.2) | 9 (3.6) | 5 (5.9) | 0.364 | 1.67 |
| Pulmonary embolism | 11 (3.3) | 6 (2.4) | 5 (5.9) | 0.120 | 2.54 |
| IVC filter | 6 (1.8) | 3 (1.2) | 3 (3.5) | 0.162 | 1.50 |
| Anticoagulation | 24 (7.2) | 15 (6) | 9 (11) | 0.156 | 1.72 |
| Chronic steroids | 140 (42) | 96 (38) | 44 (52) | 0.031 | 1.72 |
| Limited ambulation status | 9 (2.7) | 5 (2.0) | 4 (4.7) | 0.239 | 2.42 |
| Independent functional status | 329 (98) | 247 (99) | 82 (97) | 0.170 | 2.01 |
| Prior bariatric/foregut surgery | 23 (6.9) | 19 (7.6) | 4 (4.7) | 0.362 | 1.49 |
IQR interquartile range, BMI body mass index (kg/m2), ASA American society of anesthesiologists, PCI percutaneous coronary intervention, COPD chronic obstructive pulmonary disease, DVT deep vein thrombosis, IVC inferior vena cava, Bold statistically significant, p < 0.05
Outcomes Following Unmatched Analysis
Primary and secondary outcome measures were compared in the unmatched racial cohorts and are detailed in Table 2. Overall mortality and morbidity were 0.3% and 12%, respectively. All-cause mortality (0% vs. 0.4%, p = 1.0) and morbidity (17% vs. 10%, p = 0.1) were similar between black and white patients. Bariatric-related morbidity was twofold higher in black patients (14% vs. 7.2%) and trended towards significance (p = 0.05). In unmatched cohort analysis, black patients had higher rates of postoperative pneumonia (p = 0.02), acute renal failure (p = 0.02), aggregate pulmonary complications (p = 0.02), and emergency (ED) department visit (p = 0.03). All other outcome measures were similar (Table 2).
Table 2.
Unmatched perioperative outcomes
| Total (n = 335) |
White (n = 250) |
Black (n = 85) |
p Value | |
|---|---|---|---|---|
| Hospital outcomes, median (IQR) | ||||
| Operative time, minutes | 97 (64–136) | 100 (63–135) | 94 (64–138) | 0.853 |
| Length of stay, days | 2 (1–2) | 2 (1–2) | 2 (1–3) | 0.091 |
| 30-day adverse outcomes and postoperative complications, n, (%) | ||||
| Overall mortality | 1 (0.3) | 1 (0.4) | 0 (0) | 1.000 |
| Bariatric-related mortality | 1 (0.3) | 1 (0.4) | 0 (0) | 1.000 |
| Overall morbidity | 39 (12) | 25 (10) | 14 (17) | 0.108 |
| Bariatric-related morbidity | 30 (9) | 18 (7.2) | 1 (14) | 0.054 |
| Reoperation | 6 (1.8) | 4 (1.6) | 2 (2.4) | 0.651 |
| Reoperation related | 6 (1.8) | 4 (1.6) | 2 (2.4) | 0.651 |
| Readmission | 33 (10) | 21 (8.4) | 12 (14) | 0.126 |
| Readmission related | 27 (8.1) | 16 (6.4) | 11 (13) | 0.056 |
| Postoperative intervention | 9 (2.7) | 5 (2.0) | 4 (4.7) | 0.183 |
| Postoperative intervention related | 9 (2.7) | 5 (2.0) | 4 (4.7) | 0.183 |
| Unplanned ICU admission | 10 (3.0) | 5 (2.0) | 5 (5.9) | 0.069 |
| Perioperative transfusions | 9 (2.7) | 7 (2.8) | 2 (2.4) | 0.826 |
| Myocardial infarction | 1 (0.3) | 1 (0.4) | 0 (0) | 0.559 |
| Pulmonary embolism | 2 (0.6) | 1 (0.4) | 1 (1.2) | 0.422 |
| Pneumonia | 2 (0.6) | 0 (0) | 2 (2.4) | 0.015 |
| Superficial SSI | 4 (1.2) | 4 (1.6) | 0 (0) | 0.598 |
| Deep incisional SSI | 1 (0.3) | 1 (0.4) | 0 (0) | 0.559 |
| Organ space infection | 4 (1.2) | 4 (1.6) | 0 (0) | 0.241 |
| Sepsis | 1 (0.3) | 1 (0.4) | 0 (0) | 0.559 |
| Progressive renal insufficiency | 4 (1.2) | 4 (1.6) | 0 (0) | 0.241 |
| Acute renal failure | 2 (0.6) | 0 (0) | 2 (2.4) | 0.015 |
| Urinary tract infection | 1 (0.3) | 1 (0.4) | 0 (0) | 0.559 |
| Clostridium difficile | 1 (0.3) | 1 (0.4) | 0 (0) | 0.559 |
| Emergency department visits | 25 (7.5) | 14 (5.6) | 11 (13) | 0.026 |
| Approach converted | 5 (1.5) | 3 (1.2) | 2 (2.4) | 0.449 |
| Aggregate complications, n (%) | ||||
| Bleeding | 1 (0.3) | 1 (0.4) | 0 (0) | 0.559 |
| Anastomotic leak | 7 (2.1) | 7 (2.8) | 0 (0) | 0.119 |
| Cardiovascular | 2 (2.6) | 2 (0.8) | 0 (0) | 0.408 |
| Pulmonary | 2 (2.6) | 0 (0) | 2 (2.4) | 0.015 |
| Renal | 6 (1.8) | 4 (1.6) | 2 (2.4) | 0.651 |
| Venous thromboembolic | 5 (1.5) | 3 (1.2) | 2 (2.4) | 0.449 |
| Surgical site infections | 10 (3) | 10 (4.0) | 0 (0) | 0.061 |
IQR interquartile range, ICU intensive care unit, SSI surgical site infection, Bold statistically significant, p < 0.05
Outcomes Following Propensity Score Matching and Multivariate Logistic Regression Analysis
One-to-one propensity score analysis compared 82 black and 82 white MBS patients with PSOT that were statistically similar (p > 0.1) at baseline (Supplementary Table 1). Perioperative outcomes after propensity score matching are outlined in Table 3. There was no mortality in either cohort. Overall (17% vs. 10%, p = 0.2) and bariatric-related morbidity (15% vs. 8.5%, p = 0.2) were statistically similar between black and white patients. All other outcome measures were also similar between racial cohorts. Multivariate regression analyses were performed to identify independent predictors of overall and bariatric-related morbidity as shown in Table 4. Black race was not found to be an independent predictor of overall morbidity (OR 1.6, p = 0.2) or bariatric-related morbidity (OR 1.78, p = 0.2). Both overall and bariatric-related morbidities were independently associated with chronic steroid use.
Table 3.
Propensity score–matched perioperative outcomes
| Total (n = 164) |
White (n = 82) |
Black (n = 82) |
p Value | |
|---|---|---|---|---|
| Hospital outcomes, median (IQR) | ||||
| Operative time, minutes | 95 (63–139) | 95 (57–151) | 95 (65–137) | 0.732 |
| Length of stay, days | 2 (1–3) | 2 (1–2) | 2 (1–3) | 0.649 |
| 30-day adverse outcomes and postoperative complications n, (%) | ||||
| Overall morbidity | 22 (13) | 8 (10) | 14 (17) | 0.169 |
| Bariatric-related morbidity | 19 (12) | 7 (8.5) | 12 (15) | 0.222 |
| Reoperation | 3 (1.8) | 1 (1.2) | 2 (2.4) | 0.560 |
| Reoperation related | 3 (1.8) | 1 (1.2) | 2 (2.4) | 0.560 |
| Readmission | 19 (12) | 7 (8.5) | 12 (15) | 0.222 |
| Readmission related | 18 (11) | 7 (8.5) | 11 (13) | 0.318 |
| Postoperative intervention | 5 (3.0) | 1 (1.2) | 4 (4.9) | 0.173 |
| Postoperative intervention related | 5 (3.0) | 1 (1.2) | 4 (4.9) | 0.173 |
| ICU admission | 8 (4.9) | 3 (3.7) | 5 (6.1) | 0.468 |
| Perioperative transfusions | 6 (3.7) | 4 (4.9) | 2 (2.4) | 0.405 |
| Myocardial infarction | 1 (0.6) | 1 (1.2) | 0 (0) | 0.316 |
| Pulmonary embolism | 1 (0.6) | 0 (0) | 1 (1.2) | 0.316 |
| Pneumonia | 2 (1.2) | 0 (0) | 2 (2.4) | 0.155 |
| Deep incisional SSI | 1 (0.6) | 1 (1.2) | 0 (0) | 0.316 |
| Organ space infection | 1 (0.6) | 1 (1.2) | 0 (0) | 0.316 |
| Progressive renal insufficiency | 3 (1.8) | 3 (3.7) | 0 (0) | 0.080 |
| Acute renal failure | 2 (1.2) | 0 (0) | 2 (2.4) | 0.155 |
| Urinary tract infection | 1 (0.6) | 1 (1.2) | 0 (0) | 0.316 |
| Clostridium difficile | 1 (0.6) | 1 (1.2) | 0 (0) | 0.316 |
| Emergency department visits | 14 (8.5) | 4 (4.9) | 10 (12) | 0.094 |
| Approach converted | 2 (1.2) | 1 (1.2) | 1 (1.2) | 1.000 |
| Aggregate complications, n (%) | ||||
| Bleeding | 0 (0) | 0 (0) | 0 (0) | – |
| Anastomotic leak | 4 (2.4) | 4 (4.9) | 0 (0) | 0.120 |
| Cardiovascular | 1 (0.6) | 1 (1.2) | 0 (0) | 0.316 |
| Pulmonary | 2 (1.2) | 0 (0) | 2 (2.4) | 0.155 |
| Renal | 5 (3.0) | 3 (3.7) | 2 (2.4) | 0.650 |
| Venous thromboembolic | 4 (2.4) | 2 (2.4) | 2 (2.4) | 1.000 |
| Surgical site infections | 3 (1.8) | 3 (3.7) | 0 (0) | 0.080 |
IQR interquartile range, ICU intensive care unit, SSI surgical site infection
Table 4.
Multivariable regression for risk factors associated with overall and bariatric-related morbidity
| Odds ratio | 95% CI | p Value | |
|---|---|---|---|
| Overall morbidity | |||
| Race (Black vs. White) | 1.60 | 0.76–3.82 | 0.218 |
| Preoperative steroid use | 2.40 | 1.17–4.92 | 0.017 |
| Preoperative dialysis | 1.04 | 0.33–3.26 | 0.941 |
| Hispanic | 1.00 | 0.35–2.83 | 0.993 |
| Hypertension | 0.95 | 0.43–2.07 | 0.890 |
| Bariatric-related morbidity | |||
| Race (Black vs. White) | 1.78 | 0.79–4.02 | 0.168 |
| Preoperative steroid use | 2.25 | 1.05–4.84 | 0.038 |
| Preoperative dialysis | 1.48 | 0.47–4.71 | 0.506 |
| Hypertension | 1.32 | 0.53–3.32 | 0.556 |
| Hispanic | 0.81 | 0.22–2.93 | 0.747 |
Bold statistically significant, p < 0.05
Procedure-Specific Analysis
Subgroup analyses of SG (n = 252) and RYGB (n = 83) cohorts were performed. Descriptive statistics of these procedure-specific analyses are detailed in Supplementary Table 2. For SG cases, female sex (p = 0.04) was more prevalent in black patients and preoperative obstructive sleep apnea (p = 0.03) was more prevalent in white patients. For RYGB cases, preoperative BMI (p = 0.03) and renal insufficiency (19% vs. 3%, p = 0.05) were higher in black patients. For both SG and RYGB, all other patient demographics and preoperative comorbidities were similar between racial cohorts. Outcomes of these unmatched procedure-specific subgroup analyses are reported in Supplementary Table 3. Mortality was 0–0.5% (p > 0.5). There were no procedure-specific statistically significant racial differences in overall or bariatric-related morbidity; however, morbidity was higher in black patients for both SG and RYGB cases. For SG, there was a higher rate of aggregate pulmonary complications (2.9% vs. 0%, p = 0.07) and ED visits (10% s. 3.8%, p = 0.05), though it did not reach statistical significance. For RYGB cases, there was an 8.6-fold higher likelihood of ICU admission for black patients; however, the difference was not statistically significant (p = 0.09). Procedure-specific multivariate regression analyses were performed and results are detailed in Table 5. For SG cases, black race was associated with a higher likelihood of overall (OR 2.3, CI 0.92–5.73, p = 0.07) and bariatric-related morbidity (OR 2.78, CI 0.98–7.89, p = 0.06), but not significantly so. Obstructive sleep apnea also independently correlated with bariatric-related morbidity (OR 2.95, CI 1.06–8.24, p = 0.04). While overall (OR 2.08) and bariatric related (OR 2.52) morbidity were also higher in black patients after RYGB, the differences were not significant (Table 6).
Table 5.
Multivariable regression for risk factors associated with overall and bariatric-related morbidity in SG patients
| Odds ratio | 95% CI | p Value | |
|---|---|---|---|
| Overall morbidity | |||
| Race (Black vs. White) | 2.30 | 0.92–5.73 | 0.074 |
| Obstructive sleep apnea | 2.39 | 0.99–5.77 | 0.052 |
| Hispanic | 1.16 | 0.31–4.36 | 0.825 |
| Male (vs. Female) | 1.10 | 0.44–2.75 | 0.836 |
| Bariatric-related morbidity | |||
| Race (Black vs. White) | 2.78 | 0.98–7.89 | 0.055 |
| Obstructive sleep apnea | 2.95 | 1.06–8.24 | 0.039 |
| Hispanic | 1.11 | 0.23–5.44 | 0.898 |
| Male (vs. Female) | 0.94 | 0.32–2.76 | 0.906 |
SG sleeve gastrectomy, Bold statistically significant, p < 0.05
Table 6.
Multivariable regression for risk factors associated with overall and bariatric-related morbidity in RYGB patients
| Odds ratio | 95% CI | p Value | |
|---|---|---|---|
| Overall morbidity | |||
| Race (Black vs. White) | 2.08 | 0.48–9.09 | 0.330 |
| Hispanic | 0.99 | 0.18–5.45 | 0.993 |
| Preoperative renal insufficiency | 0.88 | 0.08–9.61 | 0.917 |
| BMI | 0.65 | 0.11–3.72 | 0.626 |
| Bariatric-related morbidity | |||
| Race (Black vs. White) | 2.52 | 0.55–11.5 | 0.233 |
| Preoperative renal insufficiency | 1.01 | 0.09–11.4 | 0.992 |
| Hispanic | 0.55 | 0.06–5.01 | 0.593 |
| BMI | 0.50 | 0.08–2.98 | 0.449 |
| Race (Black vs. White) | 2.52 | 0.55–11.5 | 0.233 |
RYGB Roux-en-Y gastric bypass, BMI body mass index, Bold statistically significant, p < 0.05
Discussion
This is the first and largest study reporting on disparity in outcomes following metabolic and bariatric surgery in racial cohorts with a history of prior solid organ transplantation. In this analysis, we have reinforced the safety of MBS in transplant patients, showing a low rate of overall mortality (0.3%), overall morbidity (12%), bariatric-related mortality (0.3%), and bariatric-related morbidity (9%). This is consistent with the published literature on perioperative outcomes following MBS. The systematic review of randomized and observational studies (n = 161,756) by Chang et al. [3] reported mortality and morbidity rates of 0.22–0.35% and 9.8%, respectively, following metabolic and bariatric surgery. Following propensity score–matched analyses in our study, there were no statistically significant differences in hospital outcomes, 30-day adverse outcomes, and postoperative or aggregate complications between racial cohorts. Nonetheless, the rates of readmission (1.8-fold), reoperation (2-fold), reintervention (4-fold), and emergency department (ED) visits (2.4-fold) were higher in black patients. On multivariate regression analysis, black race was not found to be an independent predictor of all-cause or bariatric-related morbidity. While race was not an independent predictor of outcomes in this cohort of MBS patients with prior solid organ transplantation, chronic steroid use was.
The nonsignificant differences in outcomes noted between black and white patients in this study are consistent with prior studies reporting a lack of significant correlation between race and MBS outcomes [21–23]. However, the nonsignificantly higher rates of some adverse outcomes (reoperation, readmission, reintervention) in black patients in our study are concerning and need further exploration with larger patient cohorts. It is possible that these differences represent racial disparity in outcomes, but given the small sample sizes, the study had inadequate power to show a statistically significant difference.
Upon multivariable regression analysis of SG and RYGB cases independently, black race was associated with a high odds ratio (OR > 2) for both overall and bariatric-related morbidity, more so with SG. However, these differences did not reach statistical significance. In our study, we also notice a trend toward a higher rate of ED visits (2.6-fold) in black SG patients. The reasons for this finding remain unclear and could not be elucidated from the database used for our study. In the published literature, the most common reported reasons for ED visits including abdominal pain, nausea/vomiting, dehydration, wound concerns, and compliance issues [26–29]. In a recent prospective cohort study of patients in the Michigan Bariatric Surgery Collaborative (MBSC) database, Stevens et al. [26] found that most ED presenting symptoms following bariatric surgery were of low acuity and non-life-threatening. Some studies have highlighted educational level as a possible contributor to poor compliance, resulting in higher readmission rates. Mahoney et al [30] evaluated grade level education (survey) and health literacy using the Rapid Estimate of Adult Literacy in Medicine-Short Form (REALM-SF) health literacy test and found that educational level less than or equal to 12th grade was associated with a threefold higher rate of ED visits and readmissions following bariatric surgery. No correlation was found between health literacy and ED visits. Others have reported patient demographics, such as younger age and female sex as potential predictors of ED visits [28]. Given the higher associated healthcare cost from ED visits and readmissions, there is increasing interest to improve this MBS quality metric. Further studies to differentiate reasons for ED visits and readmissions between racial cohorts and high-risk patient cohorts (e.g., prior transplantation) may further our understanding of this quality metric and highlight opportunities for improvement, efficiency, and cost-savings. In contrast to numerous studies showing a higher rate of adverse outcomes following RYGB compared with SG, in our procedure-specific analyses of racial bariatric surgery cohorts with prior solid organ transplantation, there were no significant differences in outcomes between RYGB and SG cases.
While our study demonstrated that outcomes in this cohort of MBS patients are not mediated by race, there are several study limitations to consider. First, this is a retrospective analysis that is subject to the inherent biases of such an analysis. Second, a significant portion of transplant cases were excluded, primarily because of CPT codes not specific to SG or RYGB. Even though this represents the largest study in this cohort of patients, generalizability is limited by the overall small sample size analyzed. In addition, several granular data points are not included in the database and therefore could not be accounted for in our analysis. Such data points include the type of solid organ transplantation performed, timeframe from transplantation to bariatric surgery, and the immunosuppressive regimen of each patient. The timeframe between organ transplantation and metabolic and bariatric surgery may impact intraoperative findings, operative course, and ultimately perioperative outcomes. The inability to account for these confounders may have an impact on our findings.
Conclusion
Metabolic and bariatric surgery in racial cohorts of post-solid organ transplantation patients is safe, with very low rates of morbidity and mortality. While some adverse outcomes were higher in black patients, race was not found to be an independent predictor of adverse outcomes following SG or RYGB in patients with prior solid organ transplantation. However, there were trends toward higher rates of adverse outcomes, including overall and bariatric-related morbidity, in both propensity score–matched and multivariate regression analyses. Given the sample size limitation of our study, additional analyses of larger cohorts are needed to further explore the impact of race on outcomes in bariatric surgery patients with prior organ transplantation.
Electronic supplementary material
(DOCX 17 kb).
(DOCX 20 kb).
(DOCX 18 kb).
Appendix
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
This is a retrospective study of nationally available de-identified data. For this type of study formal consent is not required.
Informed Consent Statement
Informed consent does not apply.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hales C, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the Unites States, 2013-2016. JAMA. 2018;319(23):2319–2429. doi: 10.1001/jama.2018.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang SH, Stoll CRT, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J, Zhou X, Li L, Li S, Tan J, Li Y, Sun X. The long-term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non-randomized evidence. Obes Surg. 2015;25:143–158. doi: 10.1007/s11695-014-1460-2. [DOI] [PubMed] [Google Scholar]
- 5.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR, STAMPEDE Investigators Bariatric surgery versus intensive medical therapy for diabetes—5-year outcome. NEJM. 2017;376(7):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B, Pothier CE, Brethauer S, Nissen S, Gupta M, Kirwan JP, Schauer PR. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36(8):2175–2182. doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christou NV. Impact of obesity and bariatric surgery on survival. World J Surg. 2009;33(10):2022–2027. doi: 10.1007/s00268-009-0050-2. [DOI] [PubMed] [Google Scholar]
- 8.Fouse T, Brethauer S. Resolution of comorbidities and impact on longevity following bariatric and metabolic surgery. Surg Clin North Am. 2016;96(4):717–732. doi: 10.1016/j.suc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Hachem A, Brennan L. Quality of life outcomes of bariatric surgery: a systematic review. Obes Surg. 2016;26(2):395–409. doi: 10.1007/s11695-015-1940-z. [DOI] [PubMed] [Google Scholar]
- 10.Mazer LM, Azagury DE, Morton JM. Quality of life after bariatric surgery. Curr Obes Rep. 2017;6(2):204–210. doi: 10.1007/s13679-017-0266-7. [DOI] [PubMed] [Google Scholar]
- 11.Yemini R, Nesher E, Winkler J, Carmeli I, Azran C, Ben David M, Mor E, Keidar A. Bariatric surgery in solid organ transplant patients: long-term follow-up results of outcome, safety, and effect on immunosuppression. Am J Transplant. 2018;18(11):2772–2780. doi: 10.1111/ajt.14739. [DOI] [PubMed] [Google Scholar]
- 12.Khoraki J, Katz MG, Funk LM, Greenberg JA, Fernandez LA, Campos GM. Feasibility and outcomes of laparoscopic sleeve gastrectomy after solid organ transplantation. Surg Obes Relat Dis. 2016;12(1):75–83. doi: 10.1016/j.soard.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Lin MY, Tavakol MM, Sarin A, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis. 2013;9(5):653–658. doi: 10.1016/j.soard.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Bennett WM, McEvoy KM, Henell KR, et al. Kidney transplantation in the morbidly obese: complicated but still better than dialysis. Clin Transpl. 2011;25(3):401–405. doi: 10.1111/j.1399-0012.2010.01328.x. [DOI] [PubMed] [Google Scholar]
- 15.Elli EF, Gonzalez-Heredia R, Sanchez-Johnsen L, Patel N, Garcia-Roca R, Oberholzer J. Sleeve gastrectomy surgery in obese patients post-organ transplantation. Surg Obes Relat Dis. 2016;12(3):528–534. doi: 10.1016/j.soard.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Kienzl-Wagner K, Weissenbacher A, Gehwolf P, Wykypiel H, Öfner D, Schneeberger S. Laparoscopic sleeve gastrectomy: gateway to kidney transplantation. Surg Obes Relat Dis. 2017;13(6):909–915. doi: 10.1016/j.soard.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Obesity. 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 18.Zhang, Rodriguez-Monguio Racial disparities in the risk of developing obesity-related diseases: a cross-sectional study. Ethn Dis. 2012;22(3):308–316. [PubMed] [Google Scholar]
- 19.Cohen, et al. A pooled analysis of body mass index and mortality among African Americans. PLoS One. 2014;9(11):e111980. doi: 10.1371/journal.pone.0111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang SH, Yu YC, Carlsson NP, Colditz GA. Racial disparity in life expectancies and life years lost associated with multiple obesity-related conditions. Obesity. 2017;25(5):950–957. doi: 10.1002/oby.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elli FE, et al. Bariatric surgery outcomes in ethnic minorities. Surgery. 2016;160:805–812. doi: 10.1016/j.surg.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Flum DR, et al. Longitudinal assessment of bariatric surgery (LABS) consortium, perioperative safety in the longitudinal assessment of bariatric surgery. NEJM. 2009;361(5):445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng J, Seip R, Stone A, Ruano G, Tishler D, Papasavas P. Ethnic variation in weight loss, but no co-morbidity remission, after laparoscopic gastric banding and Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2015;11:94–100. doi: 10.1016/j.soard.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Turner PL, Oyetunji TA, Gantt G, Chang DC, Cornwell EE, Fullum TM. Demographically associated variations in outcomes after bariatric surgery. Am J Surg. 2011;201:475–480. doi: 10.1016/j.amjsurg.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Weller WE, Rosati C, Hannan E. Predictors of in-hospital postoperative complications among adults undergoing bariatric surgery in New York State, 2003. Obes Surg. 2006;16:702–708. doi: 10.1381/096089206777346790. [DOI] [PubMed] [Google Scholar]
- 26.Stevens H, Wells E, Ross R, Stricklen A, Ghaferi AA. Patient perspectives on emergency department self-referral after bariatric surgery. Surg Obes Relat Dis. 2018;14(5):674–681. doi: 10.1016/j.soard.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Mackenzie J, Zhai Y, O’Loughlin J, Kholer R, Morrow E, Glasgow R, Volckmann E, Ibele A. Preventing returns to the emergency department following bariatric surgery. Obes Surg. 2017;27(8):1986–1992. doi: 10.1007/s11695-017-2624-7. [DOI] [PubMed] [Google Scholar]
- 28.Macht R, George J, Ameli O, Hess D, Cabral H, Kazis L. Factors associated with bariatric postoperative emergency department visits. Surg Obes Relat Dis. 2016;12(10):1826–1831. doi: 10.1016/j.soard.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Altieri MS, Yang J, Groves D, Obeid N, Park J, Talamini M, Pryor A. Sleeve Gastrectomy: the first 3 years: evaluation of emergency department visits, readmissions, and reoperations for 14,080 patients in New York State. Surg Endosc. 2018;32(3):1209–1214. doi: 10.1007/s00464-017-5793-5. [DOI] [PubMed] [Google Scholar]
- 30.Mahoney ST, Tawfik-Sexton D, Strassle PD, Farrell TM, Duke MC. Effects of education and health literacy on postoperative hospital visits in bariatric surgery. J Laparoendosc Adv Surg Tech A. 2018;28(9):1100–1104. doi: 10.1089/lap.2018.0093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 17 kb).
(DOCX 20 kb).
(DOCX 18 kb).