To the Editor: The COVID-19 pandemic caused by the severe acute respiratory syndrome virus (SARS-CoV-2) has led to uncertainty regarding the safety of immunosuppressive psoriasis therapy. We recently reported a meta-estimate that showed a 30% to 60% increase in the risk of respiratory tract infections (RTIs) in patients with psoriasis receiving interleukin (IL) 17 biologics compared to placebo.1 Similar to IL-17, IL-23 is a key cytokine in the maintenance of T-helper 17 cells, which mediate protection against pathogens. Although not believed to be a central cytokine in the defense of viral infections, reduced levels of IL-23 may contribute to impairment of mucosal barrier immunity, resulting in an increased risk of respiratory infections.2 Biologics that specifically target IL-23 have high efficacy for psoriasis and a favorable risk-benefit profile.3 However, data on the use of IL-23 inhibitors and their impact on the incidence and outcomes of novel SARS-CoV-2 infection are limited. A study from northern Italy found that patients with psoriasis receiving biologics had a higher risk for becoming infected with SARS-CoV-2 and hospitalization for COVID-19 but saw no difference in mortality or use of a ventilator.4 A case report observed improvement in COVID-19 illness after guselkumab injection.5
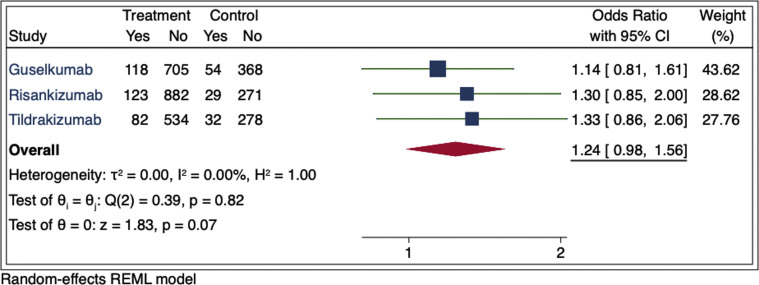
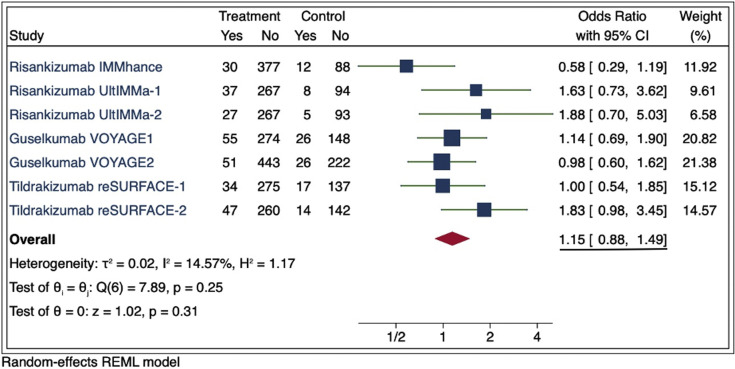
To evaluate the risk of RTIs associated with IL-23 biologic use, we analyzed data reported in US Food and Drug Administration–approved phase 3 placebo-controlled clinical trials for guselkumab, risankizumab, and tildrakizumab from US Food and Drug Administration prescribing information (PI) and clinicaltrials.gov. To ensure uniformity, terms were reviewed to include only those consistent with RTI. RTI events were divided by the total number of patients at risk in each study and compared to the placebo group by a meta-estimate. In the PI, we found an increased risk of RTI in the IL-23 groups compared to placebo, but this did not achieve statistical significance (odds ratio, 1.24; 95% confidence interval, 0.98-1.56; P = .07) (Fig 1 ). Because limited terms consistent with RTI were reported in the PI, we broadened our search to include additional RTI adverse events reported by the same trials in clinicaltrials.gov. Our risk assessment showed similar results, with attenuation in the association and no statistical significance (odds ratio, 1.15; 95% confidence interval, 0.88-1.49) (Fig 2 ). Sensitivity analyses were conducted, including additional adverse events reported in phase 2 trials included in the PI, which yielded similar findings.
Fig 1.
Meta-estimate of respiratory tract infections from prescribing information adverse events tables (includes “upper respiratory tract infections,” “nasopharyngitis,” “respiratory tract infection (viral, bacterial and unspecified),” “influenza,” “sinusitis (including acute),” “pharyngitis (including viral),” “tonsillitis,” and “rhinitis”). Doses used in this meta-estimate were guselkumab 100 mg, risankizumab 150 mg, and tildrakizumab 100 mg for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. The size of the square corresponds to the relative weight assigned in the pooled analysis, and the horizontal lines indicate the 95% CI. The diamond denotes the overall effect size, and the lateral tips of the diamond indicate the associated CI. CI, Confidence interval; REML, restricted maximum likelihood.
Fig 2.
Meta-estimate of respiratory tract infections from clinicaltrials.gov in phase 3 randomized controlled trials that were submitted for US Food and Drug Administration approval (includes “upper respiratory tract infections,” “viral upper respiratory tract infections,” “influenza,” “chylothorax,” “sinusitis,” “bronchitis,” “tonsillitis,” “nasopharyngitis,” and “pneumonia”). Doses used in this meta-estimate were guselkumab 100 mg, risankizumab 150 mg, and tildrakizumab 100 mg for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. The size of the square corresponds to the relative weight assigned in the pooled analysis, and the horizontal lines indicate the 95% confidence interval. The diamond denotes the overall effect size, and the lateral tips of the diamond indicate the associated CI. reSURFACE-2 adverse events were verified from the sponsor because clinicaltrials.gov adverse events were reported in the sum of the extended trial period. CI, Confidence interval; REML, restricted maximum likelihood.
In summary, we did not observe a statistically significant signal for RTI in patients with psoriasis treated with IL-23 biologics. Although we did observe a statistically significant increased risk of RTI using the same analysis approach with IL-17 biologics, we note that the confidence intervals overlap with our current analysis, and therefore, we cannot conclude with certainty that there is a true difference in RTI risk between biologics that target IL-17 versus IL-23. There were greater than 900 more patients treated with IL-17 biologics, and therefore, sample size may explain the different statistical results. Because of the highly variable course of COVID-19 and the potential differences between COVID-19 and other pathogens, large studies with appropriate epidemiologic methods are still needed to determine if IL-17/23–targeted biologics meaningfully alter the risk of COVID-19 in patients with psoriasis.
Footnotes
Funding sources: Supported in part by a grant (P30AR069589-03) from the National Institutes of Health (to Dr Gelfand).
Disclosure: Drs Syed and Wan are supported in part by a grant from Pfizer. Dr Winthrop receives grants from Bristol Myers Squibb and Pfizer and is a consultant for UCB, AbbVie, Lilly, Bristol Myers Squibb, Pfizer, GlaxoSmithKline, and Roche. Dr Gelfand served as a consultant for Bristol Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Janssen Biologics, Novartis Corp, Regeneron, UCB (data safety and monitoring board), Sanofi, Pfizer Inc, receiving honoraria; receives research grants (to the Trustees of the University of Pennsylvania) from AbbVie, Janssen, Novartis Corp, Sanofi, Celgene, Ortho Dermatologics, and Pfizer Inc; has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Company and Ortho Dermatologics; is a copatent holder of resiquimod for the treatment of cutaneous T-cell lymphoma; and is a deputy editor for the Journal of Investigative Dermatology, receiving honoraria from the Society for Investigative Dermatology. Dr Shin has no conflicts of interest to declare.
IRB approval status: Not applicable.
Reprints not available from the author(s).
References
- 1.Wan M.T., Shin D.B., Winthrop K.L., Gelfand J.M. The risk of respiratory tract infections and symptoms in psoriasis patients treated with IL-17-pathway inhibiting biologics: a meta-estimate of pivotal trials relevant to decision-making during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:677–679. doi: 10.1016/j.jaad.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yannam G.R., Gutti T., Poluektova L.Y. IL-23 in infections, inflammation, autoimmunity and cancer: possible role in HIV-1 and AIDS. J Neuroimmune Pharmacol. 2012;7:95–112. doi: 10.1007/s11481-011-9315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamata M., Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int J Mol Sci. 2020;21:1690. doi: 10.3390/ijms21051690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damiani G., Pacifico A., Bragazzi N.L., Malagoli P. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020:e13475. doi: 10.1111/dth.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benhadou F., Del Marmol V. Improvement of SARS-CoV2 symptoms following guselkumab injection in a psoriatic patient. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16590. [DOI] [PMC free article] [PubMed] [Google Scholar]