Abstract
Background
Repurposing broad-spectrum antivirals is an immediate treatment opportunity for 2019 coronavirus disease (COVID-19). Favipiravir is an antiviral previously indicated for influenza and Ebola, which has shown some promise in early trials for treatment of COVID-19. We aim to review existing favipiravir safety evidence, which is vital to informing the potential future use of favipiravir in COVID-19.
Methods
A search was conducted across EMBASE and MEDLINE databases, supplemented by relevant grey-literature and ClinicalTrials.gov. All studies assessing the use of favipiravir in humans by 27 March 2020 were considered for inclusion. Further analysis of available safety data from phase 2 and 3 studies was undertaken. Data extracted were adverse events (AEs) grade 1–4, serious AEs and discontinuation for AEs. Specific AEs of interest highlighted in early-phase studies, including gastrointestinal AEs and hyperuricaemia, were also examined.
Results
Twenty-nine studies were identified as potential sources of evidence of the clinical safety of favipiravir. Six were phase 2 and 3 studies reporting relevant safety data for statistical comparison, representing a total of 4299 participants, an estimated 175 person-years-of-follow-up (PYFU). Comparator drugs were oseltamivir, umifenovir, lopinavir/ritonavir or placebo. Study follow-up was between 5 and 21 days. The proportions of grade 1–4 AEs on favipiravir was 28.2% vs 28.4% (P = n.s.) in the comparison arms. The proportion of discontinuations due to AEs on favipiravir was 1.1% vs 1.2% (P = n.s.) in the comparison arms. For serious AEs the proportion was 0.4% in both arms (P = n.s.). There were significantly fewer gastrointestinal AEs occurring on favipiravir vs comparators [8.7% vs 11.5%; P = 0.003]. Favipiravir showed significantly more uric acid elevations than comparators [5.8% vs 1.3%; P<0.0001].
Conclusions
Favipiravir demonstrates a favourable safety profile regarding total and serious AEs. However, safety concerns remain: hyperuricaemia, teratogenicity and QTc prolongation have not yet been adequately studied. Favipiravir may be safe and tolerable in short-term use, but more evidence is needed to assess the longer-term effects of treatment. Given the limitations of the evidence and unresolved safety concerns, caution is warranted in the widespread use of favipiravir against pandemic COVID-19.
Background
As 2019 coronavirus disease (COVID-19) incidence and mortality rapidly climb, treatment options are limited. New therapeutic options are needed now but are limited by a lack of evidence and require time to develop. Repurposing existing pharmaceuticals provides an immediate treatment opportunity. While there is no licensed treatment that specifically acts against COVID-19, medications such as broad-spectrum antivirals are being employed as experimental adjuncts to supportive care [1]. Potential drugs that may be repurposed include antimalarial hydroxychloroquine, antiretrovirals lopinavir/ritonavir and darunavir/ritonavir, and influenza drugs oseltamivir, remdesivir and favipiravir [2–4]. These drugs are now being trialled globally in different combinations for the treatment of COVID-19. Because of the pandemic status of COVID-19, detailed safety analysis and access to generic treatment production are vital.
None of these therapeutics have yet been definitively proven effective for COVID-19, but several have shown promise in early-stage investigations. One such treatment option is RNA polymerase inhibitor favipiravir, designed to treat influenza and trialled for Ebola among other diseases.
An observational study carried out in Shenzhen in February 2020 showed a significantly faster mean time to viral clearance of favipiravir than lopinavir/ritonavir [4 days vs 11 days (P<0.001)] [5]. These results were supported by an early Chinese randomised clinical trial (RCT), where favipiravir treatment led to a significantly greater recovery rate in non-critical COVID-19 patients than umefenovir (71.4% vs 55.9% [P<0.05]) [6]. Although neither were effective in boosting recovery rate for critically ill patients in this same trial [5.6% vs 0.0% (P = n.s.)] [6], these early results have led to further trials being instigated in China and Italy.
Given the potential for the use of favipiravir in the treatment of COVID-19, safety analysis is vital to inform both ongoing clinical trials and future widespread use. However, existing safety data for favipiravir for COVID-19, as well as prior indications, are limited and difficult to access. We aimed to conduct a review of the existing evidence regarding the safety of the clinical use of favipiravir in the treatment of COVID-19, or any prior indication. We also aimed to identify gaps in the evidence where they exist, in order to inform further crucial research into this potential treatment for the COVID-19 pandemic.
Methods
A review of favipiravir clinical research was conducted in accordance with the Cochrane framework for systematic reviews, following the PRISMA statement reporting method for systematic reviews and meta-analyses [7]. A search was conducted using EMBASE and MEDLINE databases via Ovid (full search terms are available from the authors upon request). The search was supplemented by relevant grey literature from ClinicalTrials.gov and augmented by consultation with clinicians. The search was concluded on 27 March 2020.
All studies assessing the use of favipiravir in healthy volunteers, or for the treatment of Ebola virus, influenzas and COVID-19 were included in an overall summary to comprehensively summarise all available evidence of the safety of the use of the drug in humans.
Further analysis was undertaken of all available safety data from all phase 2 and 3 studies with adverse event (AE) reporting. Data extracted were AEs grade 1–4, serious AEs and discontinuation for AEs. Data were also extracted on specific AEs of interest, as highlighted in early-phase safety studies, for gastrointestinal AEs and hyperuricaemia. Statistical comparison of event proportions, and further literature searches to summarise evidence of teratogenicity and QTc prolongation were also carried out.
Results
Thirty-two studies were identified as potential sources of favipiravir clinical safety data (all of which are described in Table 1), but only seven studies were published [6,8–13], the remaining 25 being unpublished [14,15]. Attempts were made to contact the original investigators to request data, and two requests successfully yielded data, as well as a further 19 studies for which results were identified in a Japanese drug safety bureau report [14] and one study that had results listed on ClinicalTrials.gov [15]. Three trials were ultimately excluded as the results were inaccessible [16–19]; therefore 29 trials could be included in this review (Figure 1).
Table 1.
Summary of the characteristics of all studies identified as providing evidence of the safety of the use of favipiravir in humans. Trials are organised into those phase 2 and 3 studies reporting safety data, trials with further safety evidence, studies for which results were inaccessible (grey) and ongoing trials (blue)
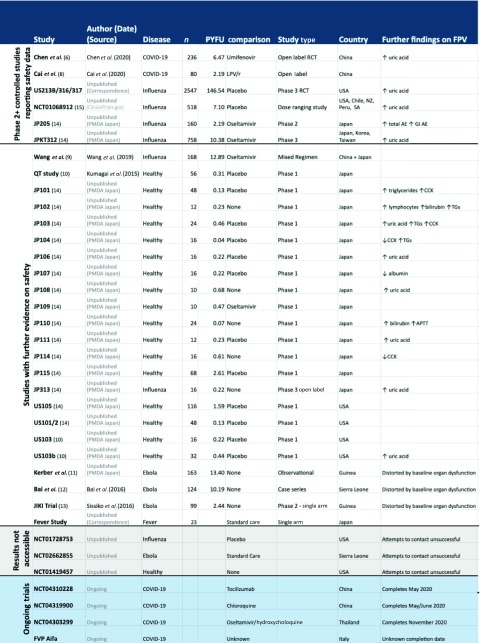
|
FPV: favipiravir; PYFU: person-years-of-follow-up; TG: triglycerides; CCK: creatinine phosphate kinase
Figure 1.
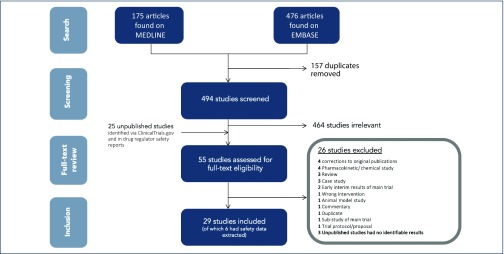
Summary of the characteristics of all studies included in this review, organised into protease and non-protease inhibitor comparator drug regimen subgroups.
Twenty-nine studies contained evidence regarding the safety of favipiravir. Doses of favipiravir ranged widely across studies, from 400 mg up to 6000 mg loading doses, with the more common regimens being 1200 mg per day, split into twice or three times daily doses. Comparison was most commonly made to a placebo control (14 studies), although nine studies had no comparison arm. Active comparisons were used in six trials (detailed in Table 1).
Of these 29 studies, six were phase 2 and 3 and provided safety data that allowed comparison of AE numbers between treatment and control arms for analysis [6,8,14,15,20,21] (Table 2). The data represent 4299 participants with 175 person-years-of-follow-up (PYFU). The vast majority of these data (147 PYFU) come from the pooled safety findings of the US213B/316/317 trials [20,21], which used placebo control. Only grade 1–4 and gastrointestinal AEs were reported consistently across all six studies. Comparisons, including oseltamivir, umifenovir, lopinavir/ritonavir and placebo, are detailed in Table 2.
Table 2.
Summary of the safety data extracted from the six phase 2 and 3 controlled studies with adverse event reporting. Extracted data for six reported safety endpoints is displayed for each included study
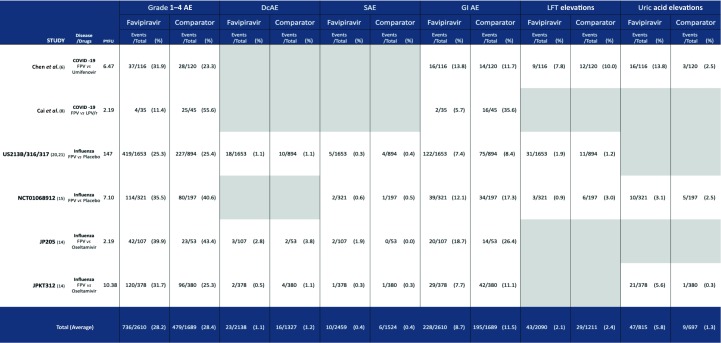
|
AE: adverse events; DcAE: discontinuations due to adverse events; SAE: serious adverse events; GI: gastrointestinal; FPV: favipiravir; LFT: liver function tests; LPV/r: lopinavir /ritonavir; PYFU: person-years-of-follow-up.
Of these six main studies, two were in COVID-19 patients in China and four were in influenza patients across North and South America, and Asia. Trials ranged from 5 to 21 days in length. All studies reported similar baseline participant demographics between trial arms. On pooling trial participant demographics across included studies (where reported), trial populations included 55% women and 34% non-white participants with a mean age of 43 years.
Twenty-three smaller, early-stage studies provided further descriptive evidence on safety. No serious AEs were reported in any of these 23 studies. The main concern raised was elevations in blood uric acid levels, seen in 6 of 23 early studies. Three studies of favipiravir as a last-resort treatment in Ebola patients were conducted; these studies contained limited interpretable safety data due to high baseline organ dysfunction and mortality rates.
One study looked specifically at the effects of different doses of favipiravir [15], and Table 3 shows the numbers of each AE type occurring in each study arm, split into low dose, high dose and placebo comparison. The low-dose regimen was 2000 mg/day loading dose, followed by 800 mg/day. The high-dose regimen was 2400 mg/day loading, followed by 1600 mg/day.
Table 3.
Table displaying the results of the dose ranging study (NCT01068912) [11], broken down into events occurring on low and high doses of favipiravir, compared with placebo
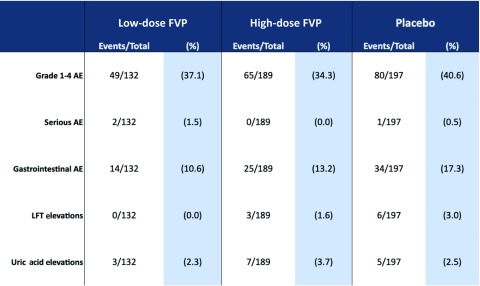
|
AE: adverse events; LFT: liver function test; FPV: favipiravir.
Similar proportions of AEs occurred between low and high doses overall, with the highest proportions occurring in the placebo arm for grade 1–4 AE, gastrointestinal AEs and liver function test (LFT) elevations. These results suggest no particular dose-dependent trend in increased incidence of AE on favipiravir for these specific safety endpoints. However, patient follow-up was only 5 days, so no evidence is provided regarding longer-term AEs.
Figure 2 shows the proportions of each type of AE occurring across all pooled participants in the six main phase 2 and 3 trials with a control arm. Safety was similar between favipiravir and control across endpoints and studies. The proportion of grade 1–4 AEs on favipiravir was 28.2% vs 28.4% in the comparison arm (P = n.s.). The proportion of discontinuations due to AEs on favipiravir was 1.1% vs 1.2% in the comparison arm (P = n.s.). The proportion of LFT elevations on favipiravir was 2.1% vs 2.4% in the comparison arm (P = n.s.). For serious AEs the proportion was 0.4% in both arms (P = n.s.). There was a statistically significant difference in the proportion of gastrointestinal AEs [8.7% vs 11.5% (P = 0.003)], with fewer events occurring on favipiravir vs comparison arms.
Figure 2.
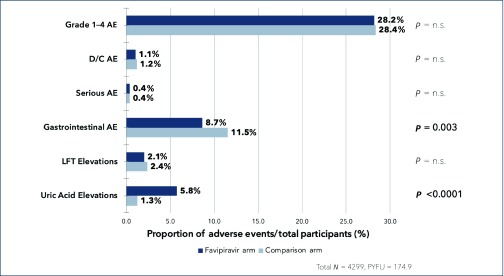
Summary results of the event proportions occurring across reported safety endpoints. AE: adverse events; D/C AE: discontinuations due to adverse events; SAE: serious adverse events; LFT: liver function test; PYFU: person-years-of-follow-up
The only endpoint for which favipiravir had a higher proportion of AEs was uric acid elevations, with a statistically significant difference between favipiravir and comparison arms [5.8% vs 1.3% (P<0.0001)].
Discussion
This analysis reviews all existing, accessible clinical data on the safety of favipiravir and summarises available data from the six larger, phase 2 and 3 controlled trials that have been carried out. These six studies account for 4299 participants and an estimated 175 PYFU. Favipiravir demonstrates a lower proportion of grade 1–4 AEs and gastrointestinal AEs, and an overall comparable safety profile to comparators, with the vast majority of data based on placebo comparison.
However, favipiravir was shown to be associated with hyperuricaemia. This finding was demonstrated in many of the reviewed trials as well as in the overall pooled safety data. Other existent safety concerns, such as QTc prolongation and the potential for teratogenicity, remain unresolved.
Limitations
Our analysis is limited to the publicly available body of literature. The results of three unpublished studies revealed by our literature search remain inaccessible. Sixteen included studies were phase 1 trials in healthy patients with small numbers and very short follow-up. Even the larger patient-number phase 2 and 3 studies that have been carried out had short follow-up periods of 5–21 days, although this is consistent with the typical duration of treatment for COVID-19. More evidence would be needed regarding the longer-term effects of treatment should favipiravir be considered for preventative use.
The generalisability of these findings is also limited to the settings and populations in which included trials were carried out, with a large proportion of the participants from the included studies being young. This means findings may be less applicable to older patients with COVID-19.
QTc prolongation
Early concerns over QTc interval prolongation were raised in laboratory and pharmacodynamic studies [14]. One recent Japanese study in healthy adults (n = 56) which used moxifloxacin as a positive control to enable higher powered statistical analysis showed no detectable effect of favipiravir on QT intervals [10]. However, more evidence is required to establish risk in larger numbers of patients, over longer follow-up periods, within specific relevant disease populations.
Uric acid
Favipiravir was previously found to transiently increase uric acid levels in phase 1–3 safety studies [14], with evidence of a dose-dependent increasing trend. The present review finds the same trend across multiple studies. Notably, two phase 3 RCTs (n = 2547) reported increases in uric acid levels that returned to normal serum concentrations before the 21-day trial cut-off. There has been no evidence that hyperuricaemia caused by favipiravir leads to clinical manifestations; however, longer trial follow-up periods would be required to fully assess this risk.
Teratogenicity
There is evidence that favipiravir has teratogenic potential. Doses equivalent to proposed human regimens were tested in animal models and demonstrated delayed development or embryonic death in the first trimester in four separate animal species [14]. Given this potential AE, no human study has included pregnant or lactating women; therefore this review could provide no further analysis on the issue. Participants in existing trials were required to avoid unprotected sexual intercourse for 90 days from the end of phase 3 studies. Despite these precautions, seven subjects became pregnant during this period, but limited fetal outcome information is available for those pregnancies. To assess the drug's effect on testicular function, a safety study (US105) was carried out and no adverse effect on sperm count or motility could be demonstrated [14].
To mitigate the undefined risk of teratogenicity, the Japanese drug safety bureau approval advises that favipiravir be given a strong warning against use in women of reproductive age and recommends precautionary statements on packaging and prescription alerts. The bureau also recommends that favipiravir should be avoided where alternative drugs could be used [14]. Given the teratogenic potential, caution is warranted in the consideration of favipiravir for widespread use in the treatment, or even prevention, of pandemic COVID-19.
Drug safety reporting and licensing
Safety data for favipiravir is limited to its use for prior indications. Other drugs designed for infectious diseases with pandemic potential, such as oseltamivir or zanamivir for influenza, typically have relatively little safety information at licensing. The European Medicines Agency (EMA) assessment report for zanamivir contained safety data from only 611 phase 2/3 trial participants [22]. Similarly, oseltamivir was approved by the EMA with data from only 1504 trial participants [23]. However, in contrast to current knowledge of favipiravir, data were available on oseltamivir as prophylaxis in 1230 participants [23]. Furthermore, although oseltamivir was shown to cross the placenta at higher doses in animal models, there was no evidence of animal or human teratogenicity prior to licensing, with 115 pregnancy outcomes available in the Roche database [24]. Exemplifying the limited safety data on favipiravir, the WHO VigiAccess tool for AE reporting contains only 11 records despite favipiravir having been licensed in Japan since 2014 [25].
Conclusions
Reviewed existing clinical evidence suggests that favipiravir is relatively safe regarding total AEs, as well as serious AEs and less serious gastrointestinal AEs. However, increases in blood uric acid remain a safety concern, demonstrated on pooled analysis of larger studies, with some evidence of an increasing dose-dependent trend. Further safety concerns, such as the potential for teratogenicity and QTc prolongation, have not yet been adequately studied.
There is evidence to support the safety and tolerability of favipiravir in short-term use. However, more evidence is needed to assess the longer-term effects of treatment. Given the limits of the evidence and the remaining specific safety concerns, caution is warranted in the widespread use of favipiravir against pandemic COVID-19.
Conflicts of interest
VP and TP have no conflicts of interest to declare.
AH received a consultancy payment from Merck for a clinical trial review, not connected with this project.
Funding
Funding received from the International Treatment Preparedness Coalition (IPTC) as part of the Unitaid-supported project: ‘Affordable Medicines for Developing Countries’.
References
- 1. Kalil AC. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 2020; 10.1001/jama.2020.4742. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2. Mitja O, Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health 2020. 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020; 19 ( 3 ): 149– 150. [DOI] [PubMed] [Google Scholar]
- 4. Rismanbaf A. Potential treatments for COVID-19; a narrative literature review. Arch Acad Emerg Med 2020; 8 ( 1 ): e29. [PMC free article] [PubMed] [Google Scholar]
- 5. Yu Xuan. Initial clinical results announced for favipiravir treatment of novel coronavirus pneumonia – viral clearance in four days. Biodiscover 2020. [Chinese] Available at: m.biodiscover.com/news/company/736154.html ( accessed April 2020).
- 6. Chen C, Huang J, Cheng Z et al. . Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv 2020; 10.1101/2020.03.17.20037432. Pre-print. [DOI] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009; 6 ( 7 ): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai Q, Yang M, Liu D et al. . Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering 2020. 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Fan G, Salam A et al. . Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J Infect Dis 2019; 10.1093/infdis/jiz656. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10. Kumagai Y, Murakawa Y, Hasunuma T et al. . Lack of effect of favipiravir, a novel antiviral agent, on the QT interval in healthy Japanese adults. Int J Clin Pharmacol Ther 2015; 53 ( 10 ): 866– 674. [DOI] [PubMed] [Google Scholar]
- 11. Kerber R, Lorenz E, Duraffour S et al. . Laboratory findings, compassionate use of favipiravir, and outcome in patients with ebola virus disease, Guinea, 2015 – a retrospective observational study. J Infect Dis 2019; 220 ( 2 ): 195– 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai C-Q, Mu J-S, Kargbo D et al. . Clinical and virological characteristics of ebola virus disease patients treated with favipiravir (T-705) – Sierra Leone, 2014. Clin Infect Dis 2016; 63 ( 10 ): 1288– 1294. [DOI] [PubMed] [Google Scholar]
- 13. Sissoko D, Laouenan C, Folkesson E et al. . Experimental treatment with favipiravir for ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med 2016; 13 ( 3 ): e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pharmaceuticals and Medical Devices Agency , Report on the deliberation results – avigan. Japan; Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, 2011. Available at: www.pmda.go.jp/files/000210319.pdf ( Accessed April 2020).
- 15. ClinicalTrials.gov Dose-finding study of Favipiravir in the Treatment of Uncomplicated Influenza: Identifier: NCT01068912. Bethesda (MD): US National Library of Medicine 2010. February 15 Available at: clinicaltrials.gov/ct2/show/NCT01068912?term=Favipiravir&draw=2&rank=10 ( Accessed April 2020).
- 16. AdisInsight Clinical Study of Favipiravir for Patients with Severe Fever with Thrombocytopenia Syndrome. Available at: adisinsight.springer.com/trials/700272660 ( Accessed April 2020).
- 17. ClinicalTrials.gov T-705a Multicenter Study in Adult Subjects with Uncomplicated Influenza: Identifier: NCT01728753. Bethesda (MD): US National Library of Medicine 2012. November 20 Available at: clinicaltrials.gov/ct2/show/NCT01728753 ( Accessed April 2020).
- 18. ClinicalTrials.gov Efficacy of Favipiravir against Severe Ebola Virus Disease: Identifier: NCT02662855. Bethesda (MD): US National Library of Medicine 2016. January 26 Available at: clinicaltrials.gov/ct2/show/NCT02662855?term=Favipiravir&draw=2&rank=8 ( Accessed April 2020).
- 19. ClinicalTrials.gov Pharmacokinetics of Favipiravir in Volunteers with Hepatic Impairment: Identifier: NCT01419457. Bethesda (MD): US National Library of Medicine 2011. August 18 Available at: clinicaltrials.gov/ct2/show/NCT01419457?term=Favipiravir&draw=2&rank=5 ( Accessed April 2020).
- 20. ClinicalTrials.gov Phase 3 Efficacy and Safety Study of Favipiravir for Treatment of Uncomplicated Influenza in Adults – T705US316: Identifier: NCT02026349. Bethesda (MD): US National Library of Medicine 2014. January 3 Available at: clinicaltrials.gov/ct2/show/NCT02026349.
- 21. ClinicalTrials.gov Phase 3 Efficacy and Safety Study of Favipiravir for Treatment of Uncomplicated Influenza in Adults: Identifier: NCT02008344. Bethesda (MD): US National Library of Medicine 2013. December 11 Available at: clinicaltrials.gov/ct2/show/NCT02008344.
- 22. CHMP Committee for Medicinal Products for Human Use (CHMP) Assessment Report. 2019. DECTOVA – Zanamivir. European medicines agency 2019 Available from: www.ema.europa.eu/en/documents/assessment-report/dectova-epar-public-assessment-report_en.pdf ( Accessed April 2020).
- 23. European Medicines Agency Tamiflu (Oseltamivir) European Public Assessment Report – Scientific Discussion. 2005. Available at: www.ema.europa.eu/en/documents/scientific-discussion/tamiflu-epar-scientific-discussion_en.pdf ( Accessed April 2020).
- 24. Donner B, Niranjan V, Hoffmann G. Safety of oseltamivir in pregnancy: A review of preclinical and clinical data. Drug Saf 2010; 33 ( 8 ): 631– 642. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization VigiAccess: WHO Collaborating Centre for Intenational Drug Monitoring. Available at: www.vigiaccess.org/ ( Accessed April 2020).


