Abstract
Backround and aims
After the emergence of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in the last two decades, the world is facing its new challenge in SARS-CoV-2 pandemic with unfathomable global responses. The characteristic clinical symptoms for Coronavirus (COVID-19) affected patients are high fever, dry-cough, dyspnoea, lethal pneumonia whereas some patients also show additional neurological signs such as headache, nausea, vomiting etc. The accumulative evidences suggest that SARS-CoV-2 is not only confined within the respiratory tract but may also invade the central nervous system (CNS) and peripheral nervous system (PNS) inducing some fatal neurological diseases. Here, we analyze the phylogenetic perspective of SARS-CoV-2 with other strains of β-Coronaviridae from a standpoint of neurological spectrum disorders.
Methodology
A Pubmed/Medline, NIH Lit Covid, Cochrane library and some open data bases (BioRxiv, MedRxiv,preprint.org and others) search were carried out by using keywords relevant to our topic of discussion. The extracted literatures are scrutinized by the authors.
Results
58 literatures including original articles, case reports and case series were selected by the authors to analyze the differential distribution of neurological impairments in COVID-19 positive patients along with angiotensin-converting enzyme-2 (ACE2) expression dynamics in neuronal and non-neuronal tissue in CNS and PNS with neuroinvasive potential of SARS-CoV2.
Conclusion
We discuss the need for modulations in clinical approach from a neurological point of view, as a measure towards reducing disease transmission, morbidity and mortality in SARS-CoV2 positive patients.
Keywords: COVID-19, ACE2, SARS-CoV2, Neurological disorders, Phylogenetic perspective, Coronavirus, CNS, PNS
1. Introduction
The recent worldwide outbreak was caused by a novel virus named 2019 novel coronavirus (2019-nCoV) by the Chinese researchers and later it was named as Severe-Acute- Respiratory-Syndrome-2 (SARS-CoV2) by International Committee on Taxonomy of Viruses (ICTV) [1]. SARS-CoV2, first reported in Wuhan city, Hubei province of China in December 2019, soon spread nationwide and rapidly spilled all over the world. Currently, it has spread to 203 countries/territories worldwide, with >7,905,723 infected cases and>432,973 casualties till date (www.worldometers.info/coronavirus/).
SARS-CoV2 shares a significant amount of homology with SARS-CoV-like bat viruses which suggest that bat could be a key reservoir. The human to human transfer is confirmed based on world wide data but the intermediate host is still unknown [1]. The first case report in Huanan Seafood Wholesale market of Wuhan suggested that initially SARS-CoV-2 showed animal-to-human transmission but subsequent cases confirmed about human-to-human transmission [2].
SARS-CoV-2 spreads rapidly and changes character continuously. Although mortality rate is low but morbidity rate is extremely high. Acute respiratory distress syndrome (ARDS) is considered as significant clinical manifestation. Based on various case reports, neurological symptoms are categorized majorly in four groups - (a) Acute cerebrovascular disease-related symptoms (b) Intracranial infection-related symptoms (c) Peripheral nervous system symptoms and (d) Neuromuscular symptoms [3]. Besides these, headache, reported in as much as 34% of COVID-19 patients in Zhejiang, China [4], and profound significant anosmia (Ear, Nose and Throat surgery society, ENT UK; https://www.entuk.org/sites/default/files/files/Loss of sense of smell as marker of COVID.pdf) were commonly observed in SARS-CoV2 infected patients during early stage of infection. Interestingly, a report by Li et al. (2016) had indicated that a very low dose of nasal viral administration with MERS-CoV, infected the CNS but the virus was not detected in other tissues including lungs [5] and similar infection pattern was also established by SARS-CoV [6]. This may indicate that SARS-CoV2, sharing a high identity with SARS-CoV and MERS-CoV, at early stages or at low doses of viral invasion can target the CNS first, even before the lungs. Nevertheless, this emphasizes the importance for further investigation of the probable neuroinvasive property of SARS-CoV2 for a complete understanding of COVID-19 impact.
As indicated by Desforges (2019), the mechanisms by which human coronavirus target the CNS are either by direct viral invasion in CNS cells or by virus-induced dysfunctional host-immune response [7]. Thus, it may be expected that upon infection with SARS-CoV2, undetected neurological disorders may be triggered or the virus may exacerbate pre-existing neurological disorders whose etiology remained poorly understood. Mouse hepatitis virus already provides a well-described corona virus involvement in short- and long-term neuropathologies [8,9]. All these may indicate a frequent involvement of SARS-CoV2 in aggravating or even inducing neurological damage where more than short-term, the long-term effect on the CNS should be brought into consideration during treatment of COVID-19.
2. Search methodology for the review
A first round PubMed/Medline, Cochrane library, NIH Lit Covid,NCBI, open database (BioRxiv,Medrixv,pre-print.org) were carried out using keywords (phylogenetic perspective,SARS-CoV-2,SARS,MERS) in Title/Abstract which yielded approximately 50 results. Similarly a second search was done using keywords (ACE2 receptor, COVID-19, brain) which yielded 13 results. Third round search included keywords (neuroimmunological response, COVID-19) and 25 results were found. Finally the last round search was completed using keywords (neuroinvasive potential, neurological disorders,SARS-CoV-2) which yielded 297 results. The complete literature search was done by GS and SS. The abstracts obtained were scrutinized by SD and DB. The full texts were analyzed by RM and DL in case the abstracts found relevant. For articles without abstracts or with less detailed abstracts the full text was directly analyzed by SD. Once the findings were compiled, they were reviewed by SB, DL and RM.
3. Initial attachment, entry and clinical features of SARS-CoV2
The initial attachment of the virion particles to the host cell starts with interactions between the spike (S) protein and the host’s receptor. The various sites of receptor binding domains (RBD) within the S1 region of a coronavirus S protein varies depending upon the virus itself, with some having the RBD at the C-terminus of S1 (SARS-CoV, SARS-CoV-2) while others (Murine Hepatitis Virus strain-1/MHV-1) have the RBD at the N-terminus of S1 [10,11]. The S-protein-receptor interaction is the primary interaction which determines how a coronavirus can infect a host species and also governs the tissue tropism of the virus. Many coronaviruses utilize peptidases as their cellular receptor. It is unclear why peptidases are used, as entry occurs even in the absence of the enzymatic domain of these proteins [12]. Many α-coronaviruses utilize aminopeptidase-N (APN) as their receptor. SARS-CoV, HCoV-NL63 and SARS-COV2 use ACE2 as their receptor, MHV enters through CEACAM1 (Carcinoembyonic Antigen-Related Cell Adhesions Molecules), MERS- CoV binds to dipeptidyl-peptidase 4 (DPP4) to gain entry into human cells [13].
The next step may be the replication and transcription of the viral genome after endocytosis into the cell many set of genes that help during replication like the non-structural protein genes nsp1-nsp16 are then transcribed inside the host cell [14]. Following translation of protein, assembly takes place within major cellular vesicles, utilized for assembly of structural proteins such as nucleoprotein (N), membrane protein (M) and the S protein. Virions are released afterwards [15]. Accumulative evidences suggest that the incubation time could be generally within 3–7 days and extend up to 2 weeks [2]. The characteristic clinical symptoms for the COVID-19 affected patients are high fever, dry cough, dyspnoea, lethal pneumonia whereas some patients show some additional neurological signs such as headache, disturbed consciousness and also acute cerebrovascular disease-related symptoms and neuromuscular symptoms [3,16].
4. The phylogenetic homology and its correlation with the possibilities of developing neurological impairments in SARS-CoV2 infected patients
The genome sequences of all the reported SARS-CoV2 share a similarity of around 99% or more (NCBI database). Sequencing studies followed with Genome Wide Association Studies (GWAS), suggest that the strain primarily isolated in Wuhan, China has a spectacular similarity with the bat derived coronavirus namely bat-SL-CoVZC45 and bat-SL-CoVZXC21 [17]. In a recent phylogenetic network analysis study of 160 human SARS-CoV2 genomes, three major variants have been traced, named: A, B and C; as per their dissimilarities in amino acid sequence with type-A being the ancestral type. Phylogeographic patterns show, type A and C in considerable proportion outside East Asia, mainly in Europeans and Americans; while type B remains the most common in East Asia and B’s ancestral genome somehow shows resilience against spreading outside this area without mutating into derived B types, inclining towards founder effects or immunological or environmental resistance outside East Asia [18].
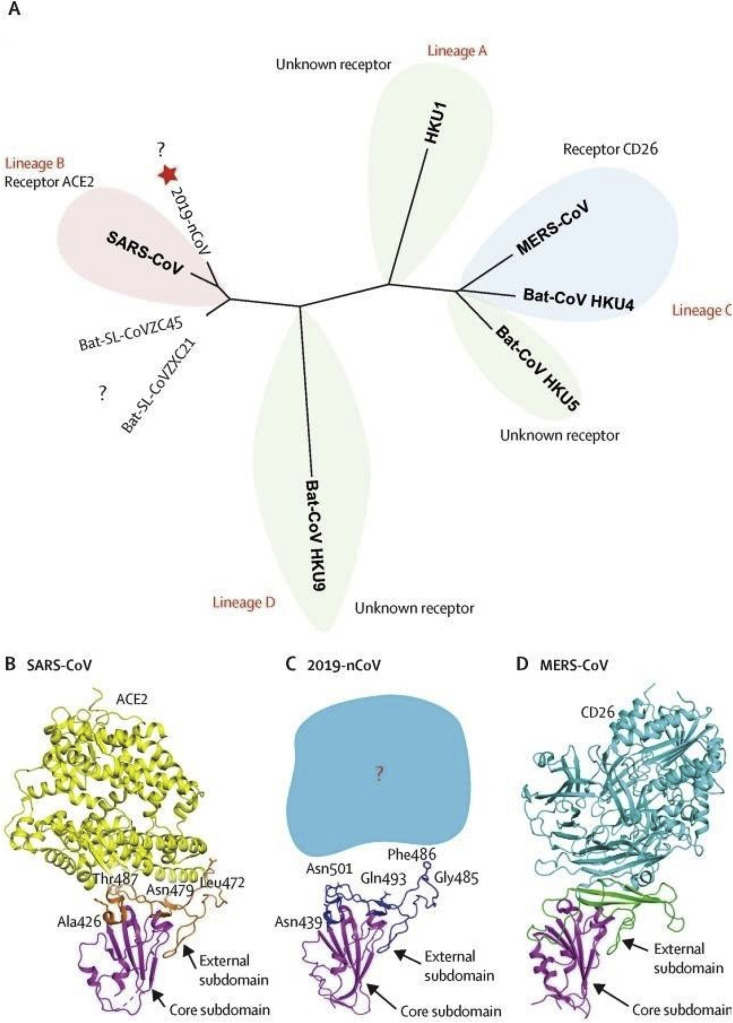
On the other hand, the virus shows an identity match on the BLAST platform with the SARS-CoV (79%) and distantly with the MERS-CoV (50%). Although SARS-CoV2 was genetically distinct from SARS-CoV and more similar to the bat counterpart of the coronavirus, homology modelling based studies have shown similar receptor binding domain structure for binding with ACE2 receptor irrespective of having key differences in the amino acid sequence between SARS-CoV and SARS-CoV2 (Fig. 1 ) [17].
Fig. 1.
A. Phylogenetic network showing receptor binding domains from various betacoronaviruses. The star denotes nCoV-19 and the question marks means unknown receptor used by the viruses.B,C,D depict structural comparison of the receptor binding domain of SARS- CoV,nCoV-19,MERS-CoV binding to their own receptors respectively. Magenta colour represents core domain and the external subdomains of SARS-CoV,nCoV-19,MERS CoV are represented by orange, blue and green respectively [9].
Many proteins are encoded by the ss (+) m-RNA of the SARS-CoV2 and SARS-CoV along with the S1 spike proteins that aid in the attachment of the virion to the cell membrane with interaction to the ACE2 receptor of the host cell [19,20]. Pairwise sequence alignment studies along with BLASTN (Nucleotide BLAST) search of the RBD and partial S1 protein revealed that SARS-CoV2 is very much similar to the SARS-COV and bat version of the Beta-Coronaviridae [21]. SWISS-PROT docking studies also revealed an increased affinity of the SARS-CoV2 to the ACE2 receptor than SARS-COV and its counterparts of the bat versions [21].
SARS-CoV is primarily considered a respiratory pathogen in humans, still the virus has been detected in the brain tissue of infected patients. Autopsy samples from eight patients with SARS-CoV also revealed the presence of the virus in brain samples by immunohistochemistry, electron microscopy, and real-time reverse transcription-PCR [22]. In some patients of neurological sequelae, SARS-COV was detected in samples of cerebrospinal fluids using reverse transcriptase-PCR (RT-PCR) during the phase of acute illness [23]. The above results are consistent with well described high propensity of other CoVs to infect CNS and neuronal tissue cells in general. Human CoV (HCoV)-OC43 and HCoV-229E which primarily cause the common cold, are also detected in the human brain [24]. Neuroinvasive propensity of other different Beta-CoVs were also reported such as MERS-CoV [5], porcine and mouse CoV [25]. Recent studies on 214 COVID-19 patients further displayed that about 88% (78/88) among the severe patients displayed neuronal tissue manifestations including acute cerebrovascular diseases with impaired consciousness [26].
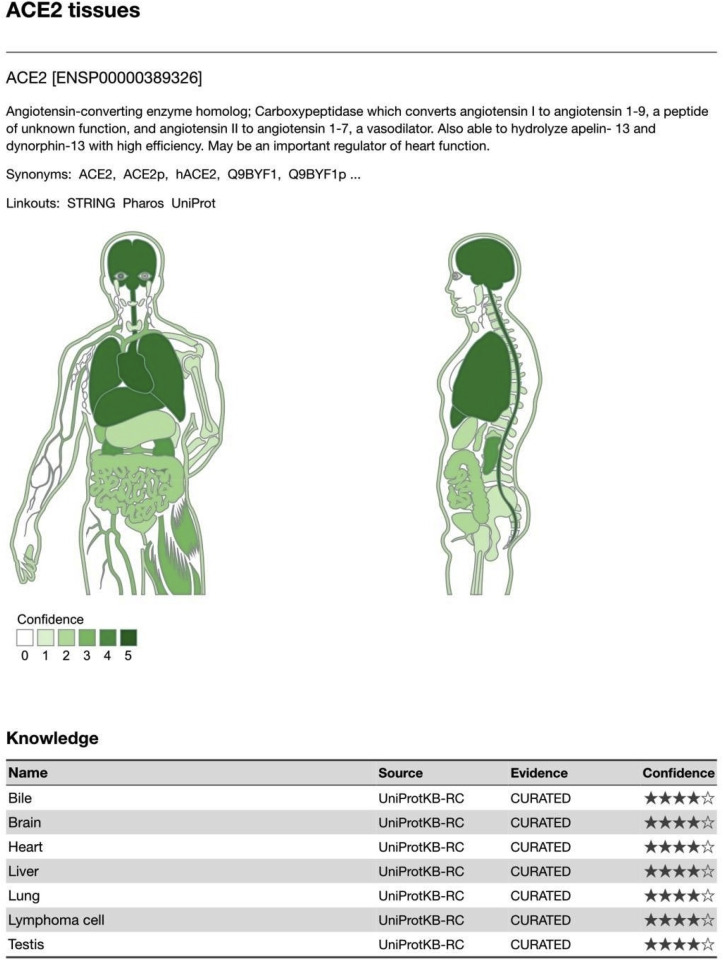
5. ACE-2 receptor expression and neurotropism of SARS-CoV2
Tissue specific expression of HUMANE ACE2 is also found in nervous tissue (ACE2 [ENSP00000389326]) (TISSUE2.0). In this segment we are reviewing the possible neurovirulence of SARS-CoV2 and relate it to the expression of ACE2 in brain (Fig. 2 ). Binding of SARS-CoV2 with ACE2 receptor leads to significant drop of ACE2 level, causing a spike in ACE-1 mediated neuro-inflammation, neuronal apoptosis, and neurodegeneration, leading towards COVID-19 mediated neurological impairments [27]. In order to determine the neuro-invading potential of SARS-CoV2 the neurological expression of ACE2 receptor has to be reported. Studies have shown that ACE2 receptors are highly expressed in regions like Sub-Fornical organ, Rostral ventrolateral medulla (RVLM), paraventricular nucleus (PVN) and Nucleus of Tractus solitarius (NTS) [6]. SARS-CoV2 can enter the brain via two pathways: (1)- haematogenous spread and (2)- retrograde neuronal transport via the olfactory nerve, the former being the more common amongst the two. However, in order to enter the milieu by haematogenous route the virus has to cross the blood brain barrier (BBB). The phylogenetic relationship ensures the structural and infection pathway based similarity between SARS-CoV and SARS-CoV2 [28,29]. ACE2 is expressed in human airway epithelia, lung parenchyma, vascular endothelia, kidney cells, small intestine cells and it mediates the entry of SARS-CoV into human host cells [[30], [31], [32]] whereas MERS-CoV enter the host cells via DPP4 which is present in lower respiratory tract, kidney, small intestine, liver and the cells of the immune system [33,34]. Besides, SARS-CoV and MERS-CoV particles are also found in brain but the exact routes of their entry into CNS are still not reported. Haematogenous or lymphatic routes seem impossible, especially at the early stage of infection whereas some other literatures suggest about the invasion of Coronavirus via peripheral nerve terminals and then they gain access to CNS via a synapse connected route [[35], [36], [37]]. Henceforth considering higher amount of similarity, SARS-CoV2 also possesses a similar neuroinvasive potential which holds an importance for the development of a treatment strategy [38]. Accumulative evidences suggest that glial cells and neurons possess a significant amount of ACE2 receptors which may be a potential target for COVID-19 [19].
Fig. 2.
Based on the manually curated knowledge in UniProtKB and via automatic text mining of the biomedical literature,tissue associations are derived. The confidence of each association is signified by stars, where ★★★★★ is the highest confidence and ★☆☆☆☆ is the lowest. Each tissue–gene association is represented on a text-mining score, which is proportional to 1) the absolute number of comentionings and 2) the ratio of observed to expected comentionings (i.e. the enrichment). These scores are normalized to z-scores by comparing them to a random background. This is represented by stars, each star corresponding to two standard deviations above the mean of the background distribution [TISSUE2.0].
ACE2 transforms Angiotensin-II into Angiotensin-(1–7), thus turning the vasoconstrictor into a vasodilator peptide though upregulation of Nitric Oxide Synthase (NOS) activity [39]. It has been found that the presence of SARS-CoV2 in general circulation enables it to pass into cerebral circulation where the sluggish movement of the blood within the microcirculation may facilitate the interaction between viral spike proteins and ACE2 expressed in the capillary endothelium. Subsequently, viral particles budding from the capillary endothelium may damage the endothelial lining and gain access to the brain [21].
Interestingly,the movement of SARS-CoV2 to the brain via the cribriform plate close to the olfactory bulb can be an additional pathway to reach brain which can further create hyposmia among the COVID-19 affected patients [21]. Increased ACE2 receptors in buccal mucosa (more in tongue than gingiva) may also result in symptoms like altered taste sensation [40]. Henceforth, a thorough epidemiological study should be performed to establish the neurovirulence of SARS-CoV-2 in order to prioritize and individualize the treatment protocols. The presence of ACE2 receptors on the surface of spinal cord neurons supports the cause of acute myelitis as a post inflectional neurological complication [41,42].
6. Immunological interplay may amplify possibilities of SARS-CoV2 mediated neuroinfection and subsequent neurological impairments
According to different literatures ‘Cytokine storm’ is one of the characteristic evidences of COVID-19 infection [43]. Astrocytes, which form the BBB, and microglia (CNS macrophages) together constitute an integral part of the immuno-surveillance of the brain. Previously neurotropic strains of CoV like MHVA59 have been found to invade the brain via astrocytes. The cytokine cascade that follows potentiates the invasion of the virus into the brain. TNF-α, IL-12 (p40-subunit) plays a major role in it apart from IL- 6, IL-15 & IL-1β [44]. Consequent pro-inflammatory state is persistent with the neurovirulence of the virus.
Studies in children with COVID-19 have shown that GM-CSF levels were significantly higher in the cerebrospinal fluid (CSF) in SARS-CoV2 patients with CNS impairments than in the serum sample of SARS-CoV2 patients with classical respiratory illness [45]. Cases of SARS-CoV causing encephalitis in patients and the large homologue sequences it shares with SARS-CoV2 makes SARS-CoV2 equally capable of posing a threat to the CNS.
Intranasal inoculation of MERS-CoV into h-DPP4 transgenic mice precipitating paralysis is also reported [46]. A recent retrospective study in China involving COVID-19 patients revealed that these patients had reduced platelet and lymphocyte counts [26]. Besides this functional exhaustion, antiviral lymphocytes also play a role in COVID-19 as SARS-CoV2 increased NKG2A expression in T lymphocytes and Natural Killer (NK) cells [47]. Increased NKG2A expression in cytotoxic-T-lymphocytes (Tc) and NK cells cannot secrete TNF-α, Granzyme-b, interferon gamma (IFN-γ), IL2. As immune patrolling of the brain is largely dependent on CD8+T lymphocytes due to the lack of major histocompatibility antigen this may aid the invading power of the virus [48]. Hence, lymphopenia may be a predisposition towards SARS-CoV2 mediated neuroinfection or SARS-CoV2 positive patients at risk of developing neuro-infection.
Case report of Acute Necrotizing Encephalopathy (ANE) comes up with an evidence that intracranial cytokine storm may have happened that led to the breakdown of BBB. Huang et al. reported that COVID-19 provokes the release of inflammatory cytokines including IL-2, IL-6, IL-7, IL-10, TNF- alpha and granulocyte colony-stimulating factor and that TNF-alpha, IL-6 and C3 of the complement system are the main factors in stimulating the immune system [49]. Consecutively, these cytokines can drive neuronal hyper-excitability via activation of glutamate receptors and play a role in development of acute seizures [43].
7. Neurological disorders with a higher risk factor towards developing COVID-19
Epidemiological data suggest an onset of neurological symptoms among the COVID-19 patients. According to Association of British Neurologists (ABN), numerous risk factors associated with neurological conditions and treatment affect susceptibility to COVID-19 infection. A limited evidence suggests individuals taking Azathioprine, Mycophenolate-mofetil, methotrexate with or without Prednisone, Infliximab, Rituximab, Ocrelizumab are at increased risk of viral infections. Beside this, some neurological conditions require immunotherapies at a regular interval (e.g. monthly infusions of natalizumab for multiple sclerosis) but the frequent attendance may be incompatible with social distance during pandemic time period [50]. A number of reports suggest that some neurological conditions such as Multiple Sclerosis, Duchene/Becker dystrophy, Congenital muscular dystrophy, Spinal muscular atrophy, Autoimmune Encephalitis, Cerebral vasculitis, Neurosarcoidosis etc show high to moderate amount of risk to develop COVID-19 and on the other hand, some other neurological conditions such as Ocular myasthenia, Glycogen Storage Disorder show lower amount of susceptibility to develop COVID-19 [50].
8. Neuro-COVID19 from a clinical point of view
An early study published from China reported, neurological manifestations may be detected in more than 1/3rd of COVID-19 cases and severely affected patients are more likely to present features of neuro-invasion [26]. Neurological consequences of COVID-19 relate to both CNS as well as PNS involvement. CNS involvement has been reported to present in the form headache, dizziness, ataxia, impaired consciousness and seizures; while the observed features of PNS involvement are neuralgia, hyposmia, hypogeusia and hypopsia. Besides, skeletal muscle damage has also been recorded in literature which occasionally may amount to rhabdomyolysis and resultant renal shutdown [26]. With gradual subsidence of the pandemic, post-infectious complications are supposed to draw more clinical attention. We present below a brief summary of reported neurological manifestations of this ailment from the perspective of a clinician.
The tally of CNS features includes some unusual manifestations for a viral infection; one of which is stroke. A monocentric study from Wuhan documented that 5.7% of neurological manifestations in COVID-19 can be attributed to acute stroke [51]. Ischemic stroke was much more frequent than hemorrhagic type. The increased propensity of this virus to cause stroke, particularly of ischemic type, may be linked to the cytokine storm that it gives rise to. However, it is speculated that the ACE2 receptor tropism of the viral particle may contribute to rise in blood pressure, particularly affecting intra-cerebral vessels, resulting in cerebral haemorrhages. Apart from cerebro-vascular events, impaired consciousness has been noted in several of the cases so far and the etiological spectrum includes viral meningitis, encephalitis, hypoxic brain damage and infectious toxic encephalopathy. The Wuhan study finds that approximately 7.5% patients may present impaired consciousness and the underlying reasons may be variable [26]. Acute necrotizing hemorrhagic encephalopathy (ANE) leading to impaired consciousness as an initial manifestation of COVID-19 has been described in a recent report [52]. This particular case consolidates the assumption that CNS involvement is possible even without direct viral invasion. Previous studies have shown ANE to be associated with influenza and many other viral infections but the above mentioned report is the first one to demonstrate a possible association between ANE and COVID-19. According to another report, a 74 year old man with past medical history of atrial fibrillation, cardioembolic stroke, Parkinson’s disease, chronic obstructive pulmonary disease (COPD) and recent cellulitis came to the emergency room with encephalopathy and subsequently developed fever with cough [53]. Later X-ray thorax revealed multifocal airspace opacities and ground-glass shadows, characteristic of COVID-19. One more recent report describes a 30 years old healthy female presenting tonic-clonic seizure without a history of substance abuse. She complained of dry cough five days prior to admission and later was found positive for SARS-CoV-2 [43]. Seizure is a known after effect of viral encephalitis. Alternatively, cytokine storm which is an important pathological hallmark of COVID-19 may also cause seizure. A striking case report of a 79-year-old patient without a travel history, presenting with syncope along with normal chest radiograph brings to attention another atypical CNS presentation [54]. Therefore it is understood that CNS manifestations in COVID-19, including atypical ones, are not infrequent. Underestimation of the problem is possible, however, owing to scarcity of published literature at this point. A more pressing concern is the CNS symptoms to appear ahead of the typical respiratory symptoms that the virus is known for.
Seen in Wuhan report, PNS involvement of COVID-19 has a predilection towards cranial nerves as evidenced by hyposmia, hypogeusia and hypopsia [26]. While loss of smell sensation has been widely reported so far, consideration is also raised if hyposmia in this ailment reflects olfactory bulb involvement rather than mere peripheral neuropathy. On the same note, hypopsia, if considered a manifestation of optic neuropathy, would also point towards CNS involvement because optic nerve is thought to be an extension of CNS according to classic neuro-anatomy teaching. Neuropathic pain, another symptom described in the Wuhan report under PNS sub-section, was not explored in detail with respect to its distribution. Further elaboration of neuralgia in future studies therefore will be of importance to advance our understanding of its pathogenesis. Additional evidences of PNS involvement, however, can be gathered from two very recent single case reports. One of them is a report from China that describes a 61-year-old woman presenting acute weakness in both the legs and severe fatigue but no flu like symptoms such as fever and cough. Initially she was diagnosed as GBS but after a few days she was positive for COVID-19. Although causality was not claimed by the authors, this particular case once more exemplifies that neurological manifestations may precede the so-called respiratory symptoms [55]. A similar case of myelitis has been documented following SARS-CoV-2 infection and appearance of limb weakness was co-incident with the febrile illness pointing towards para-infectious etiopathology [56]. However, it has been discussed here in this section keeping in mind the presentation as quadriparesis which often draws differentials from disorders of peripheral neuroaxis. Supposedly, PNS manifestations are less frequently observed thus far, yet they can precede the respiratory symptoms of COVID-19.
The atypical temporal relation between appearance of neurological and pulmonary symptoms is therefore observable in both CNS and PNS manifestations. In situations like these, a neurologist runs the risk of not only getting an inadvertent exposure but also spreading the virus. Horizontal infections are something that the world is worried about at the moment. We believe that some patients presenting to the neurological facility may be the so-called ‘silent spreaders’ by virtue of their atypical clinical trajectory. Recognizing this important issue at the earliest may help us all to come in grips with the transmission chain.
Since the onset of SARS-CoV2 outbreak, if there is one pulmonary manifestation that has received maximum focus, it is ARDS. Evidences suggest a significant percentage of ARDS survivors may suffer long-term cognitive impairment [57]. Several factors, including mechanical ventilation, have been observed to cause decline in higher brain functions following acute respiratory distress syndrome (ARDS). Acute injury to blood-brain barrier has been implicated as the underlying mechanism for cognitive impairment following ARDS. Effect of such injury may be amplified if there is pre-existing cognitive impairment that corresponds to chronic blood-brain barrier damage. Patients with brain injury, on the other hand, have been found to develop neurogenic pulmonary oedema. Therefore, it is postulated that the so-called brain-lung axis works both ways. The above observations are particularly relevant in the present circumstances given the need for mechanical ventilation in majority of the severely affected COVID-19 patients. As the pandemic continues to unfold, the number of people getting off mechanical ventilation will rise and long-term cognitive outcomes will come into view. It can be anticipated that not only we shall witness cognitive decline lasting for months in this group of patients, but also some of them may progress to premature onset of dementia.
Chronic neurology patients are anticipated to pose unique challenge to neurologists amidst this pandemic. Firstly, they are more predisposed than general population to acquire the virus. Secondly, treatment plan needs to be individualized for patients on long term immune suppression because evidence-based guidelines are lacking at present [58]. Therefore, a more patient-tailored approach has to be adapted by the neurologists in present circumstances. Thirdly, in the midst of social distancing teleneurology is going to become extremely relevant in coming days. Rapid acceptance both on part of the physician and patients will be of help.
9. Conclusion
In our review we have tried to establish the neurovirulence of SARS-CoV2 beyond its classical manifestations in cardiorespiratory system with critically ill patients being more susceptible to it. The extent of neuroinvasion of the virus depends on its ability to disrupt the BBB, infect neuroglial cells and on the cytokine mediated inflammatory response as a sequela of it. We have hypothesized that higher phylogenetic similarities between SARS-CoV and SARS-CoV-2 and significant homology between the receptor binding domain (RBD), with simultaneous evidences suggesting higher degree of ACE2 expression in neuronal and neuroglial cells can enhance the virion attachment, augmenting the neurovirulence of SARS-CoV2. Certain case reports along with supported articles have also suggested the development of neurological disorders in patients during the ongoing outbreak of COVID-19. It would also be worthwhile to investigate if phylogeographically distributed, mutational variances of SARS-CoV-2 strains (A, B and C) are associated with modulations in disease spreading and diversity in clinical presentations in neurological spectrum.
Neurological manifestations are being observed in COVID-19 in a considerable number of cases and occasionally they are seen to precede the typical symptoms of fever and cough. Neurologists must practice adequate precaution under such circumstances so as to prevent inadvertent exposure; facilitate early diagnosis and isolation; and break the chain of transmission. Chronic neurology patients, particularly those on immune-suppression, would deserve particular attention from therapeutic perspective. It can be assumed that due to scarcity of reporting at the moment, neurological consequence of SARS-CoV2 is being underestimated. As we make our way through this pandemic a greater number of descriptions are supposed to come up and a clearer view of the subject will be available.
Conflicts of interest
Authors declare no conflict of interest.
References
- 1.Shereen M.A., et al. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cascella M., et al. 2020. Features, evaluation and treatment coronavirus (COVID-19) [PubMed] [Google Scholar]
- 3.Liu K., et al. Neurological manifestations of the coronavirus (SARS-CoV-2) pandemic 2019-2020. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323177. [DOI] [PubMed] [Google Scholar]
- 4.Xu X.W., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K., et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steardo L., et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020 doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desforges M., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1) doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender S.J., Weiss S.R. Pathogenesis of murine coronavirus in the central nervous system. J Neuroimmune Pharmacol. 2010;5(3):336–354. doi: 10.1007/s11481-010-9202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley T.J., Weiss S.R. Murine coronavirus neuropathogenesis: determinants of virulence. J Neurovirol. 2010;16(6):427–434. doi: 10.1007/BF03210848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo H., Yamada Y.K., Taguchi F. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J Virol. 1994;68(9):5403–5410. doi: 10.1128/jvi.68.9.5403-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng P.K., et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raj V.S., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snijder E.J., et al. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003;331(5):991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manji H., et al. Neurology in the time of covid-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323414. [DOI] [PubMed] [Google Scholar]
- 17.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forster P., et al. Proc Natl Acad Sci U S A; 2020. Phylogenetic network analysis of SARS-CoV-2 genomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palasca O., et al. Database; Oxford: 2018. Tissues 2.0: an integrative web resource on mammalian tissue expression. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netland J., et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baig A.M., et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 22.Gu J., et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau K.K., et al. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbour N., et al. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X., et al. Hepatitis E virus infects neurons and brains. J Infect Dis. 2017;215(8):1197–1206. doi: 10.1093/infdis/jix079. [DOI] [PubMed] [Google Scholar]
- 26.Mao L., et al. JAMA Neurol; 2020. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiappelli F. Towards neuro-CoViD-19. Bioinformation. 2020;16(4):288–292. doi: 10.6026/97320630016288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Y., et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;8:15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulswit R.J., de Haan C.A., Bosch B.J. Coronavirus spike protein and tropism changes. Adv Virus Res. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donoghue M., et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 31.Harmer D., et al. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1-2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 32.Hamming I., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattern T., et al. Expression of CD26 (dipeptidyl peptidase IV) on resting and activated human T-lymphocytes. Scand J Immunol. 1991;33(6):737–748. doi: 10.1111/j.1365-3083.1991.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 34.Boonacker E., Van Noorden C.J. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003;82(2):53–73. doi: 10.1078/0171-9335-00302. [DOI] [PubMed] [Google Scholar]
- 35.Li Y.C., et al. Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res. 2012;163(2):628–635. doi: 10.1016/j.virusres.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y.C., et al. Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J Comp Neurol. 2013;521(1):203–212. doi: 10.1002/cne.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andries K., Pensaert M.B. Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am J Vet Res. 1980;41(9):1372–1378. [PubMed] [Google Scholar]
- 38.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J., et al. Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ Res. 2017;121(1):43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemoto W., et al. Effect of spinal angiotensin-converting enzyme 2 activation on the formalin-induced nociceptive response in mice. Eur J Pharmacol. 2020;872:172950. doi: 10.1016/j.ejphar.2020.172950. [DOI] [PubMed] [Google Scholar]
- 42.Ogata Y., et al. Anti-hypersensitive effect of angiotensin (1-7) on streptozotocin-induced diabetic neuropathic pain in mice. Eur J Pain. 2019;23(4):739–749. doi: 10.1002/ejp.1341. [DOI] [PubMed] [Google Scholar]
- 43.Karimi N., Razavi A.S., Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3) [Google Scholar]
- 44.Li Y., et al. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol. 2004;78(7):3398–3406. doi: 10.1128/JVI.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y., et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59(3):163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao G., et al. Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with Middle East respiratory syndrome-coronavirus. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0145561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng M., et al. Cell Mol Immunol; 2020. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y., et al. Brain Behav Immun; 2020. Nervous system involvement after infection with COVID-19 and other coronaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Association of British Neurologists Guidance on COVID-19 for people with neurological conditions, their doctors and carers. 2020. https://cdn.ymaws.com/www.theabn.org/resource/collection/6750BAE6-4CBC-4DDB-A684-116E03BFE634/ABN_Neurology_COVID-19_Guidance_22.3.20.pdf [Google Scholar]
- 51.Li Y., et al. 2020. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Preprints with THE LANCET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poyiadji N., et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filatov A., et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3) doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tape C., et al. COVID-19 in a patient presenting with syncope and a normal chest X-ray. R I Med J. 2013;103(3):50–51. 2020. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao H., et al. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao K., et al. 2020. Acute myelitis after SARS-CoV-2 infection: a case report. medRxiv. [DOI] [Google Scholar]
- 57.Sasannejad C., Ely E.W., Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352. doi: 10.1186/s13054-019-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brownlee W., et al. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020 doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]