Abstract
Although invasive pulmonary aspergillosis (IPA) is typically described in immunocompromised host, patient with severe influenzae can develop IPA. Similarly, patients with severe COVID-19 complicated with IPA are increasingly reported. Here, we describe a case of invasive aspergillosis with triazole-resistant A. fumigatus (TR34/L98H mutation) in a 56-year-old patient with COVID-19 in intensive care unit. This report highlights the need to define the available tools for diagnosis of invasive aspergillosis in severe COVID-19 patients.
Keywords: Aspergillosis, COVID-19, ICU, Triazole-resistance, Coronavirus, SARS-CoV2
Highlights
-
•
Association between COVID-19 and invasive aspergillosis.
-
•
Early screening for aspergillosis should be performed in respiratory specimen.
-
•
This case emphasizes the need to screen isolates for pan-azole resistance.
1. Introduction
Aspergillus fumigatus is an opportunistic fungal pathogen that causes invasive pulmonary aspergillosis (IPA) in severely immunocompromised host. However, in the past two decades, IPA was reported in critically ill patients without any classical immunosuppression factors [1]. These patients did not meet the host criteria of the European Organization for the Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) [2]. Blot et al. defined new criteria to classify intensive care unit (ICU) patients suspect of IPA as “putative” aspergillosis. Early diagnosis of IPA in immunocompromised patients remains a real challenge. In severe patients [3], the diagnostic process is even more difficult, as patients present with atypical and unspecific clinical pictures. Malnutrition, diabetes mellitus, pulmonary disorders and corticosteroid use were found as underlying predisposing conditions for the development of IPA in ICU patients [4]. Among pulmonary disorders, severe influenza pneumonia was clearly identified as an independent risk factor for IPA. Indeed, the incidence of IPA in ICU patients admitted for severe influenza reaches up to 19% [5]. Consequently, occurrence of IPA in acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2 is highly expected. In ICU-hospitalized patients with severe COVID-19, preliminary reports showed 19–33% of COVID-19-associated pulmonary aspergillosis (CAPA) [6,7]. Azole antifungal therapy is the first line of treatment for IPA, but due to emerging azole-resistance amongst A. fumigatus, local prevalence of azole-resistance needs to be taken into account when initiating antifungal therapy. Susceptibility testing is of huge importance to detect azole-resistance early in the course of disease to enable effective treatment. We report a case of CAPA due to a triazole-resistant A. fumigatus.
2. Case presentation
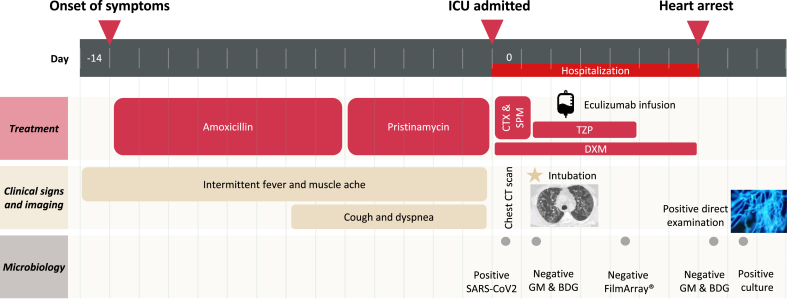
A 56-year-old man, janitor with a history of diabetes mellitus, hypertension, hyperlipidemia, and obesity (body mass index: 31 kg/m2) was admitted (day 0) to the intensive care unit (ICU) of Saint-Louis Hospital (Paris, France) for ARDS. His medication consisted of an antihypertensive association with angiotensin II receptor blockers and calcium channel blockers, metformin, glimepiride, atorvastatin. He has been under inhaled fluticasone propionate/salmeterol for the suspicion of early stage chronic obstructive pulmonary disease (COPD). He reported intermittent fever (>38.3 °C), muscle ache for 2 weeks. Nonproductive cough and progressively increasing dyspnea started 7 days ago. He received 8 days of amoxicillin and 5 days of pristinamycin for the suspicion of bacterial pneumonia. Time course of the patient is detailed in Fig. 1.
Fig. 1.
Time course of the patient with COVID-19-associated pulmonary aspergillosis. First day of hospitalization is considered day 0. CTX, cefotaxime; SPM, spiramycin; DXM, dexamethasone; TZP, piperacillin-tazobactam; GM, serum galactomannan; BDG, serum β-D-Glucan.
On examination, the heart rate was 99 beats/min, respiratory rate 34 breaths/min, blood pressure 132/92 mmHg, temperature 38 °C and oxygen saturation 95% on high flow nasal oxygenation. Arterial blood gas on 100% oxygen showed a pH of 7.41, pCO2 27.9 mmHg, pO2 of 87.9 mmHg (PaO2/FiO2: 87.9). White blood-cell count was 10,720 cells per mm3 (normal range, 4,000 to 10,000) with 82.6% neutrophils (8,900 cells per mm3) and 12.7% lymphocytes (1,370 cells per mm3), lactate dehydrogenase blood level was 882 U per liter (normal range, 240 to 480), CRP 206 mg per deciliter (normal value < 5), ferritin 556 ng per milliliter (normal range, 30 to 400) and D-Dimer 2390 ng per milliliter (normal value < 500). In addition, interleukin-6 level was 93.6 pg per milliliter (normal value < 7). His Sepsis-related Organ Failure Assessment (SOFA) score at ICU admission was 8. He was diagnosed with hypoxemic respiratory failure (PaO2/FiO2 <100) and rapidly required mechanical ventilation and norepinephrine. He also presented with diabetic ketoacidosis (pH 7.3, glucose level 20.5 mmol per liter, ketonemia and bicarbonate ions level 17.2 mmol per liter) requiring continuous insulin infusion, and acute kidney failure without criteria for renal replacement therapy (creatinine level 2.76 mg per deciliter, 221 μmol per liter).
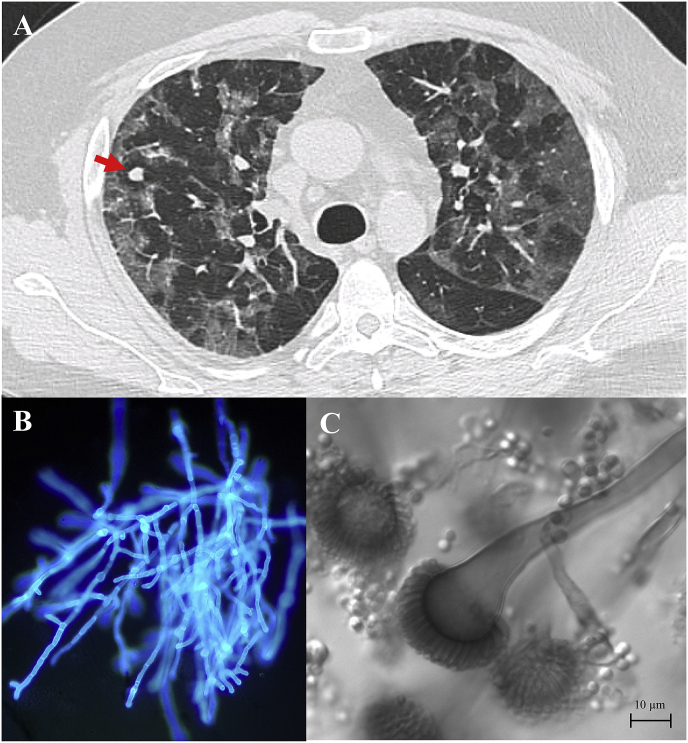
Chest computed tomography (CT) showed multiple bilateral ground-glass opacities with a crazy paving pattern characteristic of COVID-19 (Fig. 2A). In addition, 3 pulmonary nodules (7mm, 1mm, 1mm) were visualized in the right upper lobe. Nasopharyngeal swab real-time reverse-transcriptase PCR (RealStar® SARS-CoV-2 Kit, Altona Diagnostics) performed at the admission was positive for SARS-CoV-2 on the 2 targets (E and S genes) with quantification cycle (Cq) values of 23.3 and 22.8 respectively. He received a 7-day course of dexamethasone (20 mg) and 1 infusion of eculizumab (1200 mg) on day 2 (clinicaltrials.gov, NCT04346797). The patient was also treated with broad-spectrum antibiotic regimen, including piperacillin-tazobactam, cefotaxime and spiramycin which were stopped upon negative tracheal aspirate BioFire® FilmArray® pneumonia plus panel (BioFire Diagnostics, Biomerieux) on day 4.
Fig. 2.
(A) Chest CT on day 14 of symptom onset, which demonstrates bilateral ground glass opacities with a 7mm nodule in the right upper lobe. (B) Direct examination of the sample with calcofluor white, original magnification x200. (C) Strain culture of Aspergillus fumigatus isolates from tracheal aspirate.
At admission, screening for fungal infections with serum galactomannan index (Platelia® Aspergillus enzyme immunoassay, Biorad) and serum β-D-Glucan (Fungitell®, Cape Code diagnostics) was negative (0.07 and 10.4 pg per milliliter, respectively).
On day 6, his condition worsened with increasing vasopressor and oxygen need. Tracheal aspirate showed branching hyphae on direct examination with calcofluor white (Fig. 2B). Culture on Malt Extract Agar (VWR, Avantor) and Sabouraud Dextrose Agar with Chloramphenicol media (Bio-Rad) isolated A. fumigatus (20 CFU per 50 μL) after 1 day at 30 °C (Fig. 2C). Specific A. fumigatus quantitative PCR [8] (Af qPCR) performed on tracheal aspirate was positive with Cq value of 26.3. Bacterial culture on chocolate (PolyViteX®, BioMérieux) and blood (Columbia agar + 5% sheep blood, BioMérieux) agar media remained negative after 5 days of incubation. Concomitantly, serum galactomannan index (0.05), serum β-D-Glucan (<7.8 pg per milliliter) and serum qPCR-Af were negative. On the same day, transthoracic echocardiogram showed hypertrophy and dilation of the right ventricle. D-dimer level increased >20000 ng per milliliter with fibrinogen 3.85 g per liter. The patient evolved unfavorably and died of a cardiac arrest suspect of massive pulmonary embolism. In this case, we were not able to efficiently treat the patient as the evolution was unfortunately too rapid. Autopsy was prohibited due to SARS-CoV-2 infection.
Antifungal susceptibility testing using EUCAST method and according to EUCAST clinical breakpoints [9] showed the following minimal inhibitory concentrations (MIC) of posaconazole (0.5 μg per milliliter, resistant MIC >0.25), itraconazole (>8 μg per milliliter, resistant MIC >1), voriconazole (2 μg per milliliter, resistant MIC >1) and isavuconazole (4 μg per milliliter, resistant MIC >1). DNA was extracted and cyp51A gene was sequenced as previously described [10]. TR34/L98H mutation was identified.
3. Discussion
Here, we report the case of a patient with severe SARS-CoV-2 infection complicated with a putative IPA. The isolated strain of A. fumigatus was triazole-resistant and TR34/L98H mutation was recovered from sequencing cyp51A gene. Triazole-resistance frequency varies considerably among geographic regions. The prevalence is less than 1% in France [10] and reaches 10% in the Netherlands [11], complicating the management and worsening the prognosis in patients treated for aspergillosis [12]. In this case, the patient was azole-naive which precludes the resistance development through azole therapy. In Europe, wood from the sawmill, wood chippings, flower bulb waste, green waste materials and soil from market garden were described as environmental hotspots for triazole-resistance selection of A. fumigatus [[13], [14], [15]]. Our patient worked as a janitor, performing various tasks such as gardening and may have manipulated organic matter containing triazole-resistant isolates of A. fumigatus. Hospital-acquired infection is unlikely since nosocomial infection with A. fumigatus are defined as infection occurring after 7 days of admission [16] while the strain was recovered at day 6 in our patient. This report emphasizes the need to screen isolates for pan-azole resistance on a routine basis that can be performed by broth microdilution reference method [9] or less time-consuming method such as gradient concentration strips [17], agar supplemented with various concentrations of azoles [18] or PCR screening for specific mutations [19].
CAPA was only recently described [20,21]. Misclassification between Aspergillus colonization and putative IPA may impact the management of the patients and their outcome. Depending on the chosen classification, our case could be characterized differently as infection or colonization. For this purpose, guidelines were compared to solve this issue (Table 1). (i) EORTC/MSGERC classification from 2019 is not suitable due to the lack of host factors in our patient. (ii) According to Blot et al. criteria, this case is classified as a putative aspergillosis. (iii) Using Schauwvlieghe et al. criteria for IAPA, this case would be considered as a colonization rather than an infection as only positive culture from BAL is an accepted criterion. However, fungal load in the patient tracheal aspirate was significant (20 CFU per μliter) at day 1 of culture and the direct examination was positive. (iv) Verweij et al. [22] recently proposed a consensus case definition of IAPA and CAPA which classifies our case as colonization. A positive tracheal aspirate is an accepted criterion if cavitary infiltrates are observed on CT imaging while our patient's chest CT showed ground glass opacities with nodules.
Table 1.
Categorization of reported case of pulmonary aspergillosis according to various guidelines.
| Donnelly et al., 2019 | Blot et al., 2012 | Schauwvlieghe et al., 2018 | Verweij et al., 2020 | |
|---|---|---|---|---|
| Host factor | no | Glucocorticoid treatment | NA | COVID-19 |
| Clinical criteria | NA | Dyspnea Worsening respiratory insufficiency |
Dyspnea Worsening respiratory insufficiency |
NA |
| Imaging criteria | Dense well-circumscribed lesion | Abnormal chest CT scan | Any infiltrate on chest CT scan | Infiltrate on chest CT scan |
| Mycology criteria | Aspergillus-positive culture on tracheal aspirate | Aspergillus-positive culture in lower respiratory tract | NFR (only BAL required) | Aspergillus-positive culture on tracheal aspiratea |
| Diagnosis | Colonization | Putative invasive aspergillosis | Colonization | Colonization |
NA, Not applicable; CT scan, computed tomography scan.
BAL, bronchoalveolar lavage.
Tracheal aspirate accepted if cavitary infiltrates are observed on CT scan.
Patient classification as Aspergillus colonization or infection remains variable depending on the classification used, which is well illustrated herein, hence the difficulty to compare different studies and the need to find consensus on these definitions. This difficulty is further increased by the complexity of the aspergillosis diagnosis in ICU. In patients with COVID-19 hospitalized in ICU, medical imaging of the lungs is less specific than in neutropenic patients due to underlying COVID-19 damage. Furthermore, serum galactomannan lacks sensitivity in non-neutropenic patients [23] which could also be expected with serum quantitative PCR [8]. Both were negative in this patient. In previous studies only 3/20 patients (0 to 40%) with CAPA had positive serum galactomannan [3,6,7]. The lack of accurate marker may be an argument for colonization, showing the absence of angioinvasion. More studies compiling available cases are needed to select appropriate markers to define viral ARDS-associated pulmonary aspergillosis. Post-mortem autopsy may assist in identifying colonization or proven infection, unfortunately in this case it was not performed because of the risk of contamination. Here, the patient death was likely not related to aspergillosis, but clinically attributed to presumed pulmonary embolism.
More studies are needed to evaluate the impact of Aspergillus colonization or putative IPA on patients’ outcome. It remains unclear whether the presence of Aspergillus is a prognosis marker of severity or is responsible for the clinical worsening of the patient potentially leading to death.
Declaration of competing interest
There was no funding for this manuscript. All authors declare that they have no conflicts of interest regarding this manuscript.
Acknowledgements
We acknowledge Dea Garcia-Hermoso et Cécile Gautier for assistance in antifungal susceptibility testing.
References
- 1.Blot S.I., Taccone F.S., Van Den Abeele A.M., Bulpa P., Meersseman W., Brusselaers N. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012;186(1):56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E. Revision and update of the consensus definitions of invasive fungal disease from the European organization for Research and treatment of cancer and the mycoses study Group education and Research Consortium. Clin. Infect. Dis. 2019;46 doi: 10.1093/cid/ciz1008. 1813–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19 associated pulmonary aspergillosis. Am. J. Respir. Crit. Care Med. 2020;202(1):132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garnacho-Montero J., Amaya-Villar R., Ortiz-Leyba C., León C., Alvarez-Lerma F., Nolla-Salas J. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit. Care. 2005;9(3):R191. doi: 10.1186/cc3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. 2018;6(10):782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 6.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020;8(6):e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alanio A., Menotti J., Gits-Muselli M., Hamane S., Denis B., Rafoux E. Circulating Aspergillus fumigatus DNA is quantitatively correlated to galactomannan in serum. Front. Microbiol. 2017;8:2040. doi: 10.3389/fmicb.2017.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs for antifungal agents. 2020. http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ Version 10.0.
- 10.Alanio A., Sitterlé E., Liance M., Farrugia C., Foulet F., Botterel F. Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. J. Antimicrob. Chemother. 2011;66(2):371–374. doi: 10.1093/jac/dkq450. [DOI] [PubMed] [Google Scholar]
- 11.Lestrade P.P.A., Buil J.B., Beek MT Van Der, Kuijper E.J., Dijk K Van, Kampinga G.A. Paradoxal trends in azole-resistant Aspergillus fumigatus in a national multicenter surveillance program, The Netherlands, 2013–2018. Emerg. Infect. Dis. 2020;26(7):1447–1455. doi: 10.3201/eid2607.200088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verweij P.E., Ananda-Rajah M., Andes D., Arendrup M.C., Brüggemann R.J., Chowdhary A. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updates. 2015;21–22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Schoustra S.E., Debets A.J.M., Rijs A.J.M.M., Zhang J., Snelders E., Leendertse P.C. Environmental hotspots for azole resistance selection of aspergillus fumigatus, The Netherlands. Emerg. Infect. Dis. 2019;25(7):1347–1353. doi: 10.3201/eid2507.181625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeanvoine A., Rocchi S., Reboux G., Crini N., Crini G., Millon L. Azole-resistant Aspergillus fumigatus in sawmills of eastern France. J. Appl. Microbiol. 2017;123(1):172–184. doi: 10.1111/jam.13488. [DOI] [PubMed] [Google Scholar]
- 15.Rocchi S., Ponçot M., Morin-Crini N., Laboissière A., Valot B., Godeau C. Determination of azole fungal residues in soils and detection of Aspergillus fumigatus-resistant strains in market gardens of Eastern France. Environ. Sci. Pollut. Res. 2018;25(32):32015–32023. doi: 10.1007/s11356-018-3177-6. [DOI] [PubMed] [Google Scholar]
- 16.Nicolle M.C., Benet T., Vanhems P. Aspergillosis: nosocomial or community-acquired? Med. Mycol. 2011;49(suppl. 1):s24–29. doi: 10.3109/13693786.2010.509335. [DOI] [PubMed] [Google Scholar]
- 17.Dellière S., Verdurme L., Bigot J., Dannaoui E., Senghor Y., Botterel F. Comparison of the MICS obtained by gradient concentration strip and EUCAST methods for four azole drugs and amphotericin B against azole-susceptible and -resistant Aspergillus section Fumigati clinical isolates. Antimicrob. Agents Chemother. 2020;64(3) doi: 10.1128/AAC.01597-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buil J.B., van der Lee H.A.L., Rijs A.J.M.M., Zoll J., Hovestadt J.A.M.F., Melchers W.J.G. Single-center evaluation of an agar-based screening for azole resistance in Aspergillus fumigatus by using VIPcheck. Antimicrob. Agents Chemother. 2017;61(12) doi: 10.1128/AAC.01250-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dannaoui E., Gabriel F., Gaboyard M., Lagardere G., Audebert L., Quesne G. Molecular diagnosis of invasive aspergillosis and detection of azole resistance by a newly commercialized PCR kit. J. Clin. Microbiol. 2017;55(11):3210–3218. doi: 10.1128/JCM.01032-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verweij P.E., Gangneux J.-P., Bassetti M., Brüggemann R.J.M., Cornely O.A., Koehler P. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1(2):e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangneux J.P., Bougnoux M.E., Dannaoui E., Cornet M., Zahar J.R. Invasive fungal diseases during COVID-19: we should be prepared. J. Mycol. Med. 2020;30(2) doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., Azoulay E., Bassetti M., Blot S. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020:1–12. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordonnier C., Botterel F., Ben Amor R., Pautas C., Maury S., Kuentz M. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin. Microbiol. Infect. 2009;15(1):81–86. doi: 10.1111/j.1469-0691.2008.02122.x. [DOI] [PubMed] [Google Scholar]