Abstract
Diffuse alveolar hemorrhage (DAH) is a severe and potentially life-threatening disease manifestation. In addition to autoimmune diseases such as antineutrophil cytoplasmic antibody-associated vasculitis and anti-glomerular basement membrane syndrome, pulmonary viral infections are known to be culprits of DAH. Health-care providers worldwide in the coronavirus disease 2019 pandemic have been confronted with an unprecedented number of viral lung infections, with great variance in symptoms and severity. Hemoptysis, the key symptom of DAH, is a rare complication. We present two cases of immunocompromised patients with rapidly developing hypoxemic respiratory failure and evidence of DAH in the context of severe acute respiratory syndrome coronavirus 2 infection.
Key Words: coronavirus disease, COVID-19, diffuse alveolar hemorrhage
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DAH, diffuse alveolar hemorrhage; EGPA, eosinophilic granulomatosis with polyangiitis; GBM, glomerular basement membrane
Case Report: Patient No. 1
Patient No. 1 is a 79-year-old white man who was admitted to our hospital with general weakness, malaise, and nonproductive cough. The symptoms had been present for 8 days prior to admission. A diagnosis of autoimmune aortitis of the abdominal aorta had been established 18 years prior to admission; today, this would have been classified as single organ vasculitis according to the 2012 Chapel Hill Consensus definition.1 The patient had been receiving serial immunosuppressive treatments with glucocorticoids either alone or in combination with azathioprine or 6-mercaptopurine, as well as six courses of IV cyclophosphamide, until 14 months prior to the current presentation.
At this point in time, the patient was diagnosed with high-grade urothelial carcinoma of the bladder staged as T2N0M0 (with infiltration of the bladder musculature) after having presented with macrohematuria. He underwent transurethral resection of the bladder and received intermittent radiotherapy with a maximum dose of 59.4 Gy and simultaneous chemotherapy with 5-fluorouracil and mitomycin. During the 8 months prior to admission, the patient was without antineoplastic or immunosuppressive treatment.
The patient’s further medical history comprised chronic inflammatory demyelinating polyneuropathy, COPD, coronary artery disease, type 2 diabetes mellitus, chronic kidney disease stage 3a, Parkinson disease, osteoporosis, and intermittent atrial fibrillation.
On admission on March 6, 2020, the patient’s body temperature was 36.8°C, BP was 130/80 mm Hg, and pulse was 80 beats/min. His respiratory rate was 16 breaths/min, and peripheral oxygen saturation was 94% while breathing ambient air. The WBC count revealed mild leukocytopenia with absolute lymphopenia of 370 lymphocytes/mm3 (reference range, 1,090-2,990 lymphocytes/mm3), mild thrombocytopenia with 102,000/mm3 (reference range, 150,000-350,000/mm3), a C-reactive protein (CRP) level of 75.1 mg/L (reference range, < 8.2 mg/L), and a lactate dehydrogenase level of 418 U/L (reference range, < 248 U/L). Antineutrophil cytoplasmic antibodies (ANCAs), antinuclear antibodies, and anti-glomerular basement membrane (anti-GBM) antibodies were negative. A chest radiograph was unremarkable.
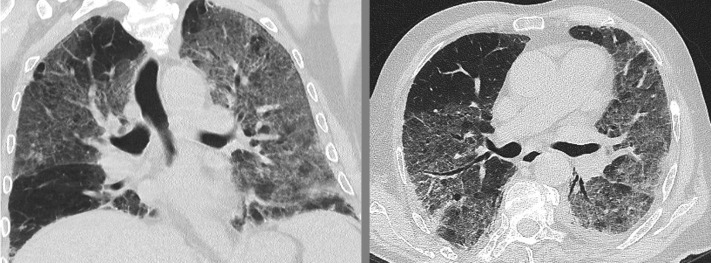
Blood cultures were drawn. The patient was given 2 L of oxygen per minute per nasal cannula and was admitted for further evaluation. A CT scan of the chest revealed extensive ground-glass opacities suggestive of acute interstitial pneumonia (Fig 1 ). Within 4 days of admission, the patient developed a body temperature of 38.3°C, and his respiratory situation deteriorated, now presenting with a peripheral oxygen saturation of 89% under 2 L of oxygen per minute as well as hemoptysis. Blood gas analysis confirmed hypoxemia with a Pao 2 of 61 mm Hg and hyperventilation with a Paco 2 of 31 mm Hg. We administered IV piperacillin with tazobactam, cotrimoxazole, and oral roxithromycin. Bronchoscopy revealed signs of chronic bronchitis with extensive and progressively hemorrhagic bronchial fluid from successive aliquots during the BAL, consistent with DAH. BAL revealed a total cell count of 10 × 105 cells/mL, mostly comprising alveolar macrophages (80%) and 1,000 erythrocytes/mm³. Gram stains and PCRs for Legionella species, Chlamydia species, Mycoplasma species, cytomegalovirus, herpes simplex viruses 1 and 2, influenza viruses A and B, respiratory syncytial virus, pneumocystis jirovecii, and Mycobacteria other than TB were negative.
Figure 1.
CT imaging of the chest in patient No. 1. Extensive bilateral ground-glass opacities of the entire left lung and the right upper and middle lobe can be seen.
We transferred the patient to the ICU, where noninvasive ventilation was commenced. Eventually, real-time PCR for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)2 from a nasopharyngeal swab was positive while other possible causes of pneumonia were excluded. Within the next 24 h, the patient’s condition continuously worsened to the point where invasive mechanical ventilation would have been necessary. According to the patient’s will, we refrained from intubation and provided palliative care under which he died shortly thereafter.
Diagnosis: DAH due to pulmonary SARS-CoV-2 infection
Case Report: Patient No. 2
Patient No. 2 is a 70-year-old white man who had been in his usual state of health until 6 weeks prior to admission. At this time, he was referred from his general practitioner to another hospital with malaise and intermittent, daily fever (usually in the second half of the day), rising up to 39.0°C. No specific symptoms were otherwise present, and he had no travel history or sick contacts. Apart from nucleus pulposus prolapse of the lumbar spine, there was no remarkable medical history.
Elevated laboratory markers of inflammation were found with a maximum CRP of 233 mg/L (reference range, < 8.2 mg/L) and an erythrocyte sedimentation rate > 100 mm/h. An extensive medical evaluation was conducted by CT imaging of the thorax, abdomen, and pelvis, blood cultures, MRI of the spine, transthoracic and transesophageal echocardiography, bone marrow aspiration and biopsy and 15-fluoro-deoxyglucose-PET/CT imaging. None of the findings were remarkable. ANCA, antinuclear antibodies, anti-GBM antibodies, serologies of HIV, cytomegalovirus, Epstein-Barr virus, leishmaniasis, Brucella species, leptospirosis, Rickettsia species, and Treponema pallidum were negative. Complement factors C3 and C4 were normal. Two courses of antibiotic treatment with ampicillin/clavulanic acid and piperacillin/tazobactam were ineffective.
On admission to our hospital, the patient continued to develop daily, intermittent fever. His BP was 130/70 mm Hg, his pulse was 77 beats/min, and his temperature was 37.7°C. On examination, an erythematous rash was detected on the ventral thorax that was independent of the fever episodes; bilateral pretibial and perimalleolar edema was also detected. Blood analysis revealed a leukocytosis of 21,000/mm3 with a neutrophil count of 70% and hypereosinophilia of 4.290 eosinophils/mm3 (reference range, 0.03-0.44/mm3), a CRP level of 188 mg/L, an erythrocyte sedimentation rate of 108 mm/h, a lactate dehydrogenase level of 184 U/L, and a pro-brain natriuretic peptide level of 5,116 pg/mL (reference range, 0-125 pg/mL). The initially impaired kidney function with a creatinine level of 1.8 mg/dL (reference range, 0.41-1.44 mg/dL) rapidly deteriorated to 3.5 mg/dL, and peripheral edema progressed. Results of an ultrasound-guided kidney biopsy revealed extensive tubular-interstitial infiltration of eosinophils showing unremarkable glomeruli, with no evidence of vasculitis or granulomatous disease. A cardiac MRI without gadolinium enhancement due to compromised renal function revealed evidence of myocardial thickening (septum diameter, 1.3 cm) and signs of infiltrative myocardial disease.
After repeat bone marrow aspiration and biopsy provided no evidence of systemic mastocytosis or myeloproliferative disease, and genetic analyses for bcr-abl, JAK2, and FIP1L1-PDGFRA/B were negative, we established a diagnosis of idiopathic hypereosinophilic syndrome (iHES), and the patient was started on 500 mg IV prednisolone per day. At this point in time, patient No. 1 was admitted to the same room as patient No. 2.
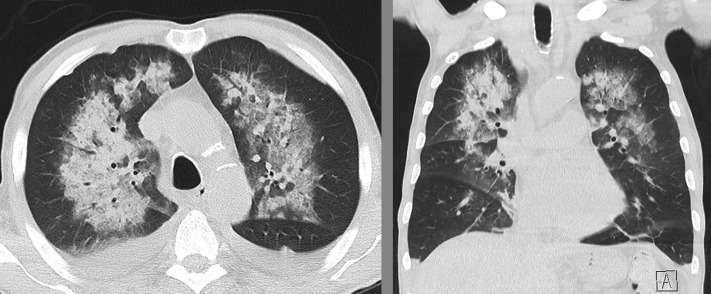
After 3 days of IV prednisolone at a dose of 500 mg/d, CRP levels dropped to 35 mg/L, eosinophil counts normalized, both fever and rash dissolved, and the patient’s general health improved. On day 4, after having had 4 days of contact with patient No. 1, he developed massive hemoptysis and hypoxemic respiratory failure. A CT scan of the chest revealed bilateral ground-glass opacities with peribronchovascular consolidations consistent with DAH (Fig 2 ). The patient was referred to our ICU. A BAL was performed confirming alveolar bleeding by progressive hemorrhagic return in sequential aliquots. Gram stains and a broad infective PCR panel (similar to patient No. 1) returned negative. After a nasopharyngeal swab was positive for SARS-CoV-2 (PCR), we diagnosed COVID-19, most likely as a result of an in-hospital transmission from patient No. 1.
Figure 2.
CT imaging of the chest in patient No. 2. Diffuse bilateral, perihilar and peribronchovascular consolidations, and ground-glass opacities consistent with alveolar hemorrhage can be seen.
After tapering of glucocorticoid therapy and supportive therapy, the patient gradually recovered.
Diagnosis: DAH due to pulmonary SARS-CoV-2 infection. Idiopathic hypereosinophilic syndrome
Discussion
DAH is a severe and potentially life-threatening disease manifestation. The clinical presentation consists of hypoxemic respiratory failure, hemoptysis, anemia, and diffuse pulmonary infiltrates on imaging. The leading known etiologies are autoimmune diseases such as ANCA-associated vasculitis, anti-GBM syndrome, and connective tissue disorders such as systemic lupus erythematosus. A plethora of non-autoimmune conditions have also been recognized to cause DAH such as idiopathic pulmonary hemosiderosis, drug reactions, radiation exposure, and radiotherapy, as well as infections.
Given the COVID-19 pandemic, health-care providers worldwide are increasingly confronted with patients presenting with airway and pulmonary symptoms. Hemoptysis has been described as a rather rare presentation of COVID-19. To the best of our knowledge, DAH has thus far not been reported as a manifestation of COVID-19.
Disease severity of COVID-19 seems to vary. According to epidemiologic data from China, the frequency of severe or critical diseases is reportedly 15% to 19%.3 , 4 As of March 22, 2020, the worldwide fatality rate of COVID-19 was 4.2% according to the figures published by the World Health Organization (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). Age > 50 years, comorbidities, and lymphocytopenia have been recognized as independent risk factors for severe courses of the disease.5 Both of the patients presented here fulfilled all of these risk factors. Both patients were immunocompromised by immunosuppressive treatments and, in patient No. 1, additionally by antineoplastic treatment in the past and extensive comorbidities. The foremost findings on chest CT imaging in COVID-19 are ground-glass opacities (mostly in the lung periphery), septal thickening, crazy paving pattern, and consolidations with a positive air bronchogram. Reversed halo sign, cysts, and nodules are considered to be rare.6, 7, 8
In June 2020, a time when the first wave of the COVID-19 pandemic was calming down, a patient presenting with a chest CT scan as seen in patient No. 1 would immediately be identified as highly suspicious for COVID-19, and appropriate measures of health care would be applied immediately. Patient No. 2, however, would still invite a broad differential diagnosis. Interestingly, both cases had alveolar hemorrhage.
Guan et al3 reported hemoptysis to be a rare symptom of COVID-19, ranging from 0.6% in nonsevere cases to 2.3% in severe cases. It might be speculated that DAH is underrecognized in COVID-19 because the use of bronchoscopy is supposed to be restricted in these patients due to the high risk for health-care workers involved in the procedure.
DAH is not a specific CT pattern but a clinical and pathological syndrome consisting of anemia, hemoptysis, hypoxemic respiratory failure, diffuse radiographic infiltrates, and an increase of intraalveolar RBCs and hemosiderin-laden macrophages.9 Both of the study patients met these criteria, and in both instances we were able to prove intraalveolar bleeding by bronchoscopy. Alternative causes, especially autoimmunologic ones, were excluded.
In the case of patient No. 2 presenting with an inflammatory disease with hypereosinophilia, renal, and suspected myocardial involvement, DAH would generally be highly suspicious for vasculitis, especially for eosinophilic granulomatosis with polyangiitis (EGPA).10 However, asthma, a decisive diagnostic feature of EGPA according to the 1990 American College of Rheumatology criteria and the 2012 Chapel Hill definition, was missing in this patient.1 , 11 Furthermore, this case’s renal involvement was due to tubulointerstitial eosinophilic infiltration without vasculitis or granulomas, which is not a typical renal feature of EGPA in which one would expect necrotizing pauci-immune glomerulonephritis. In a large epidemiologic study of patients with EGPA conducted by the French vasculitis group, all patients with histologically proven renal involvement had glomerulonephritis.12 Moreover, renal involvement in EGPA is significantly more common in ANCA-positive individuals.13 Patient No. 2 was ANCA negative. The fourth and perhaps strongest argument against DAH being an expression of EGPA in patient No. 2 is the timing of events: DAH occurred on the fourth day of high-dose IV glucocorticoid therapy, at a time when CRP levels had dropped to the lowest point in 8 weeks, and eosinophils were absent. Simultaneously, at the onset of DAH in patient No. 2, he (unfortunately and unwillingly) had been exposed to patient No. 1 for at least 96 h, which is consistent with the reported incubation time of SARS-CoV-2.14
Severe pulmonary infections are recognized as possible triggers for DAH, even in immunocompetent hosts. Among these, viral diseases such influenza A play a major role.15 Furthermore, according to the current understanding of its mechanism of disease, SARS-CoV-2 causes pulmonary endothelialitis leading to thrombotic microangiopathy, thrombosis, and hemorrhage, as shown in a recent autopsy study.16 This mode of action resembles that of vasculitic diseases and might explain why DAH can be a complication of both viral infection and ANCA vasculitis or anti-GBM disease.
We therefore argue that in both reported instances, DAH was caused by SARS-CoV-2 infection. Patient No. 1 acquired the disease due to his high susceptibility (age, previous immunosuppression and radiochemotherapy, and multiple comorbidities) from an unknown source. Due to the atypical and initially nonsevere presentation of patient No. 1, his negative travel and contact history, and a low prevalence of SARS-CoV-2 in Germany at the time of the onset of symptoms in February 2020, COVID-19 was not suspected at the time of admission. This led to the spreading of the disease to patient No. 2, whose immunologic defenses, especially lymphocytes and eosinophils, were low as a result of the treatment with high-dose prednisolone.
Conclusions
According to the data presented, DAH should be taken into consideration as a complicating feature of severe COVID-19.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
References
- 1.Jennette J.C., Falk R.J., Bacon P.A. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;2019:3–6. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 6.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W. Imaging changes in severe COVID-19 pneumonia. Intensive Care Med. 2020;46(4):583–585. doi: 10.1007/s00134-020-05976-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 9.Lara A.R., Schwarz M.I. Diffuse alveolar hemorrhage. Chest. 2010;137(5):1164–1171. doi: 10.1378/chest.08-2084. [DOI] [PubMed] [Google Scholar]
- 10.Hellmich B., Holl-Ulrich K., Merz H., Gross W.L. Hypereosinophiles Syndrom und Churg-Strauss-Syndrom: Ist die Differenzierung der Syndrome klinisch relevant? Internist. 2008;49(3):286–296. doi: 10.1007/s00108-007-2009-4. [DOI] [PubMed] [Google Scholar]
- 11.Masi A.T., Hunder G.G., Lie J.T. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss Syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33(8):1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 12.Comarmond C., Pagnoux C., Khellaf M. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65(1):270–281. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 13.Sinico R.A., Di Toma L., Maggiore U. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005;52(9):2926–2935. doi: 10.1002/art.21250. [DOI] [PubMed] [Google Scholar]
- 14.Linton N.M., Kobayashi T., Yang Y. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9(2):538–546. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Von Ranke F.M., Zanetti G., Hochhegger B., Marchiori E. Infectious diseases causing diffuse alveolar hemorrhage in immunocompetent patients: a state-of-the-art review. Lung. 2013;191(1):9–18. doi: 10.1007/s00408-012-9431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]