Highlights
-
•
Fetuses of COVID-19 patients are under the effect of maternal pyrexia, maternal inflammatory response and the “cytokine storm”.
-
•
Cardiotocograph showed a raised baseline FHR (>10 %), loss of accelerations and cycling, late decelerations and ZigZag pattern.
-
•
Perinatal outcomes appear to be favourable.
-
•
Clinicians should optimise the maternal environment prior to rapidly transferring the mother for an operative delivery for a “pathological CTG”.
Keywords: COVID-19, CTG, Cardiotocograph, Cytokine storm, Physiological CTG interpretation, ZigZag pattern
Abstract
Objective
To determine the cardiotocograph (CTG) changes in women with symptomatic COVID-19 infection.
Study design
12 anonymised CTG traces from 2 hospitals in Spain were retrospectively analysed by 2 independent assessors. CTG parameters were studied based on fetal pathophysiological responses to inflammation and hypoxia that would be expected based on the pathogenesis of COVID-19 patients. Correlation was made with perinatal outcomes (Apgar score at 5 min and umbilical cord pH).
Results
All fetuses showed an increased baseline FHR > 10 percent compared to the initial recording, in addition to absence of accelerations. 10 out of 12 CTG traces (83.3 percent) demonstrated late or prolonged decelerations and 7 out of 12 fetuses (58.3 percent) showed absence of cycling. Not a single case of sinusoidal pattern was observed. ZigZag pattern was found in 4 CTG traces (33 percent). Excessive uterine activity was observed in all CTG traces where uterine activity was monitored (10 out of 12). Apgar scores at 5 min were normal (>7) and absence of metabolic acidosis was found in the umbilical cord arterial pH (pH > 7.0) in the cases that were available (11 and 9, respectively).
Conclusion
Fetuses of COVID-19 patients showed a raised baseline FHR (>10 percent), loss of accelerations, late decelerations, ZigZag pattern and absence of cycling probably due to the effects of maternal pyrexia, maternal inflammatory response and the “cytokine storm”. However, the perinatal outcomes appear to be favourable. Therefore, healthcare providers should optimise the maternal environment first to rectify the reactive CTG changes instead of performing an urgent operative intervention.
Introduction
The parameters recorded on the cardiotocograph (CTG) reflect the activity of fetal autonomic and somatic nervous systems, as well as the oxygenation of the fetal myocardium [1]. A fetus responds to hypoxia by reducing the myocardial workload to maintain a positive aerobic metabolism in the heart, seen as decelerations on the CTG trace [1]. Excessive uterine irritability may reduce utero-placental circulation by compressing the myometrial blood vessels, as well as causing compression of the umbilical cord [2].
CTG changes are also influenced by maternal status. Hypoxic and inflammatory conditions, as well as maternal pyrexia, can cause abnormal fetal heart rate changes. Maternal fever increases her oxygen requirement (thereby reducing utero-placental oxygen transfer), as well as increases placental oxygen consumption given the augmented metabolism of the feto-placental unit. This can result in fetal hypoxic neurological injury.
COVID-19 infection is associated with maternal pyrexia, “cytokine storm” and hypercoagulability, which increases the risk of placental intervillous thrombosis and infarction, as well as maternal hypoxia secondary to adult respiratory distress syndrome (ARDS).
Although, there is currently no evidence to suggest that COVID-19 infection has a direct effect on fetal morbidity and mortality, maternal changes secondary to COVID-19 infection may result in fetal heart rate changes.
Pathogenesis of the COVID-19 infection
Recent studies have suggested that hypoxia and adult respiratory distress syndrome (ARDS) may cause the release of a cytokine storm leading to multi-organ failure. A deadly uncontrolled systemic inflammatory response resulting from the release of large amounts of pro-inflammatory cytokines (IFN-a, IFN-g, IL-1b, IL-6, IL-12, IL-18, IL-33, TNF-a, TGFb, etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10) have also been reported [3]. Moreover, it has been suggested that recruitment of aberrant CD45RA + T cells is the immunologic feature of COVID-19 [4].
Furthermore, it has been postulated that SARS-CoV-2 can dissociate oxy-Hb, carboxy-Hb and glycosylated Hb. This may result in prolonged and progressive hypoxia eventually leading to desaturation and respiratory distress.
Excessive secretion of erythropoietin to stimulate the bone marrow (in order to secrete new red blood cells to compensate for progressive and profound hypoxia) may lead to thrombocytosis and a resultant hypercoagulable state. Evidence of disseminated intravascular coagulation (DIC) significantly higher D-dimer (p < 0.05), fibrin degradation products (FDP) levels (p < 0.05), and prolonged PT (p < 0.05) and APTT (p < 0.05) has been documented [5]. A case with poor neonatal outcome with an Apgar score of 0 and unsuccessful neonatal resuscitation [6] and the adverse effect on the type 2 Pneumocytes have been recently reported [7].
Therefore, COVID-19 results in an immunological response in a pregnant woman whose immune system is already altered as a result of normal physiological changes of pregnancy. Despite the lack of strong evidence that COVID-19 is transferred in sufficient load across the placenta to cause fetal infection or an inflammatory response [[8], [9], [10]], it is likely that there would be a reactive response of the fetus secondary to a maternal inflammatory state. In addition, maternal pyrexia, inflammatory mediators and cytokines may irritate the uterine myometrium leading to a reduction in utero-placental oxygen transfer.
Materials & methods
Retrospective analysis of 12 anonymised CTG traces from pregnant women with symptomatic COVID-19 infection, from two different hospitals in Spain, was performed. (Table 1 ). The CTG traces were independently analysed by two assessors (AGPB and EC) to determine the expected changes in maternal COVID-19 infection (Table 2 ) and correlated with perinatal outcomes (Apgar Score at 5 min, and umbilical cord pH). Poor neonatal outcomes were defined by 5 min Apgar Score < 7, umbilical cord arterial pH < 7.0 and unexpected admission to the neonatal unit.
Table 1.
Analysis of CTG features in women with COVID-19 infection.
| Case | Gestational age | Baseline FHR > 10 % | Variability | Cycling | Accelerations | Decelerations | Sinusoidal | Uterine irritability | Apgar 5 | Cord arterial pH |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | + | N | – | – | Late | – | + | 10 | 7.3 (V) |
| Prolonged | ||||||||||
| 2 | 39.5 | + | N | + | – | Late | – | + | 10 | 7.12 |
| 3 | 36.4 | + | N | – | – | Late | – | Not Recorded | 10 | – |
| 4 | 40.6 | + | Reduced | – | – | Late | – | + | 10 | – |
| 5 | 28 | >200 | – | – | – | – | – | Not Recorded | N/A | N/A |
| 6 | 40 | + | Reduced | – | – | Late | – | + | 10 | 7.14 |
| 7 | 37.3 | + | ZigZag | + | – | Late | – | + | 10 | 7.28 |
| Prolonged | ||||||||||
| 8 | 37.6 | + | N | + | – | Variable | – | + | 10 | 7.23 |
| 9 | 38.3 | + | ZigZag | + | – | Late | – | + | 10 | 7.28 |
| 10 | 40.6 | + | ZigZag | + | – | Late | – | + | 9 | 7.31 |
| 11 | 39.4 | + | Reduced | – | – | Late | – | + | 9 | 7.26 |
| 12 | 39 | + | ZigZag | – | – | Late | – | + | 9 | 7.23 |
FHR = fetal heart rate; N = normal; V = venous.
Table 2.
Anticipated CTG features in COVID-19 patients.
| CTG feature | Normal parameters | COVID-19 | |||
|---|---|---|---|---|---|
| Likely changes in COVID-19 | Underlying reason | Correlation pathogenesis | |||
| Baseline FHR | 110−160 bpm After 40 weeks the upper limit is 150 bpm (strong vagal dominance) | Increased FHR > 10 % bpm or >10 % above expected for gestational age | Fetal reaction to maternal pyrexia | Maternal inflammatory response characterised by pyrexia. | |
| Gross fetal tachycardia (FHR > 180 bpm) | Fetal response to intense maternal inflammation. | "Cytokine Storm" | |||
| Acute fetal bradycardia | Placental intervillous thrombosis or acute maternal hypoxia. | Hypercoagulable state and ARDS leading to maternal hypoxia and reduction in placental circulation. | |||
| Baseline variability | 5−25 bpm | Reduced variability (< 5 bpm) | Fetal CNS depression secondary to maternal hypoxia, medications and the effects of inflammatory cytokines | COVID-19 is associated with ARDS and "Cytokine Storm", releasing TNF-alfa, interferons and interleukins that can cross the placenta and the fetal blood-brain barrier causing depression of fetal CNS. Opiates and other CNS depressants can also cause depressed variability. | |
| Increased variability (> 25 bpm) resulting in the ZigZag pattern. | Fetal autonomic instability secondary to maternal pyrexia and inflammatory cytokines. | Maternal pyrexia may increase fetal core body temperature. The "Cytokine Storm" may cause fetal autonomic instability. | |||
| Cycling | Alternate periods of normal and reduced variability (approx. once in every 50 min) due to active and quiet fetal sleep | Loss of cycling with either the absence of the quiet epoch or persistent reduced variability. | Depression of the fetal CNS due to persistent maternal pyrexia and / or inflammatory mediators that cross the placenta. | Maternal pyrexia and the "Cytokine Storm" | |
| Accelerations | A transient rise in the FHR with the amplitude of 15 bpm lasting for 15 s, at least 2 episodes in 20 min. | Loss of accelerations | Fetus conserve body movements during hypoxia. | ARDS leading to maternal hypoxia and the resultant reduction in placental circulation. | |
| Loss of fetal movements as a result of CNS depression due to inflammatory mediators. | COVID-19 is associated with maternal "Cytokine Storm" and these toxic cytokines may cross the placenta. | ||||
| Increased accelerations | Intrauterine fetal convulsion | Maternal pyrexia may increase fetal core body temperature leading to fetal hyperpyrexia and febrile convulsion. | |||
| Deceleration | Late or "shallow" declarations with delayed recovery to the baseline. | Utero-placental insufficiency due to maternal hypoxia and placental intervillous thrombosis. | ARDS leading to maternal hypoxia and reduction in placental circulation as well as due to the hypercoagulable state. | ||
| A transient decrease in the FHR of at least 15 bpm from the normal baseline lasting a minimum of 15 s. | Atypical variable decelerations | Placental thrombosis can cause long standing utero-placental insufficiency, fetal redistribution and oligohydramnios. | Hypercoagulable state in COVID-19 patients may lead to placental thrombosis and infarction. | ||
| Single prolonged deceleration (>30 bpm drop, for > 3 min) and an acute fetal bradycardia (>30 bpm drop for > 10 min). | Infarction and thrombosis of >50 % of placenta, and/or secondary to an acute reduction in utero-placental perfusion. | ||||
| Hypercoagulability may lead to massive placental thrombosis and infarction | |||||
| ARDS leading to an acute maternal hypoxia and/ or hypotension causing an acute reduction in placental oxygenation. | |||||
| Sinusoidal Pattern | Typical | Smooth undulated oscillations of the FHR baseline with amplitude of 15 bpm and frequency 2−5/min, reduced variability and absence of accelerations. Due to fetal thumb-sucking and physiological glottic movements that occur for upto 30 min. | Persistence of typical sinusoidal pattern for longer than 30 min | Chronic fetal anaemia and acidosis | Destruction of red cells have been reported in patients with COVID-19. This may not only reduce oxygen carrying capacity of maternal blood to the placenta, if a similar effect occurs in a fetus this may lead to a chronic fetal anaemia. |
| Uterine contractions | Atypical | Sharp, “Shark-teeth-like” oscillation of the baseline FHR, persisting for up to 10 min secondary to fetal thumb sucking. | Persistence > 30 min | Acute feto-maternal haemorrhage leading to fetal hypovolemia and hypotension, and the resultant autonomic instability. | DIC has been reported in COVID-19 patients. If similar changes affect the fetoplacental unit, this may result in an acute feto-maternal haemorrhage and fetal hypovolemia. |
| 3−4 in 10 min, lasting <60 s with inter-contraction interval of >90 s. | Excessive uterine activity | Myometrial irritability secondary to maternal pyrexia and inflammation. | Increased temperature and inflammatory response. |
FHR = Fetal heart rate; ADRS = Adult Distress Syndrome; DIC = Disseminated Intravascular Coagulation; bpm = beats per minute.
Theory
Maternal inflammatory response and fever will affect the fetal heart rate (FHR), thereby resulting in changes on the CTG trace. Firstly, based on the fact that maternal interleukines and cytokines have the ability to cross the placenta, it is reasonable to expect reactive fetal tachycardia. The “cytokine storm” is likely to increase the fetal heart rate >180 bpm due to its direct effect on the fetal myocardium, in addition to an inflammatory response. Secondly, the placental metabolism will be increased leading to relative utero-placental insufficiency. This might be seen in the CTG trace as chemo-receptor mediated “late decelerations”.
On the other hand, if inflammatory mediators affect the fetal central nervous system, one would expect to see changes in fetal variability, including the loss of cycling and the ZigZag pattern secondary to autonomic instability [11].
Moreover, due to maternal hypercoagulable state, there may be increased incidence of intrauterine fetal growth restriction (IUGR), and sudden bradycardia secondary to placental or umbilical venous thrombosis.
Lastly, in cases of severe maternal hypoxia or anaemia, sinusoidal patterns may occur.
The anticipated CTG changes based on the pathogenesis of COVID-19 infection are provided in Table 2.
Results
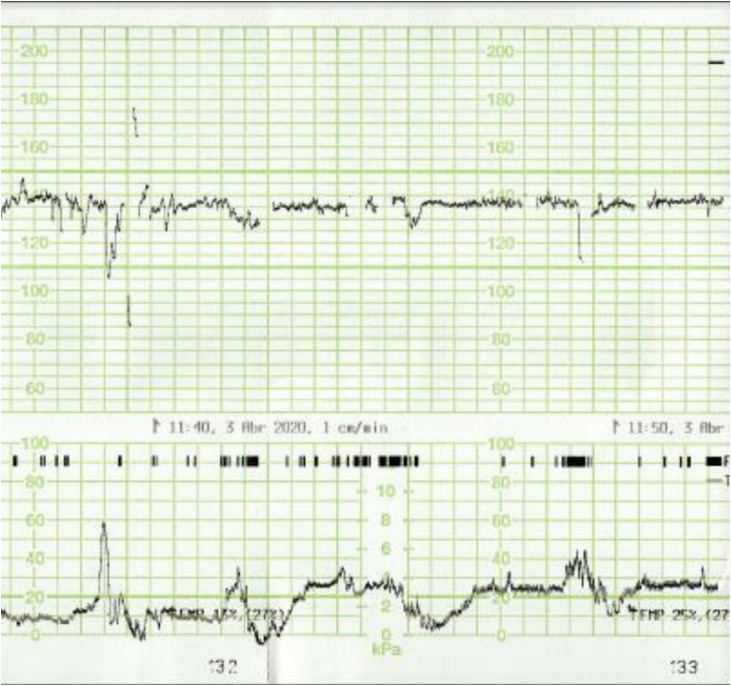
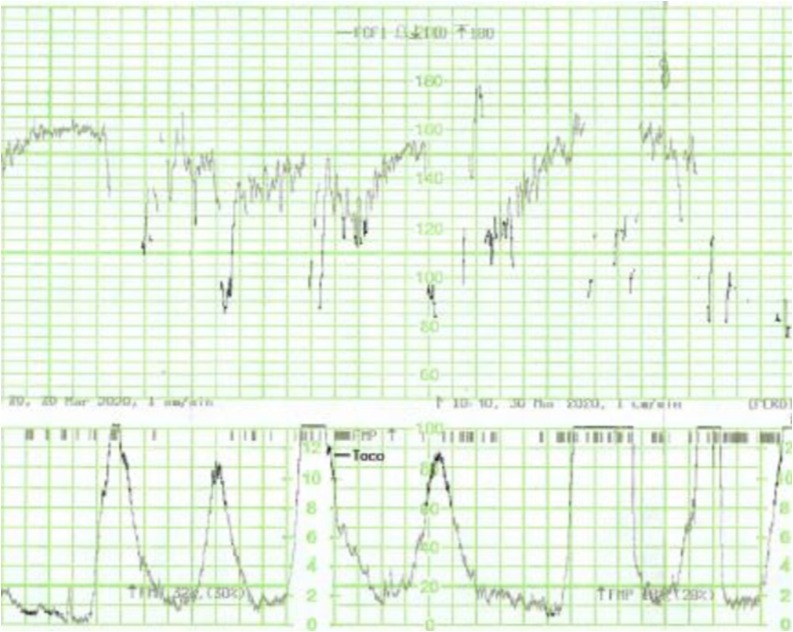
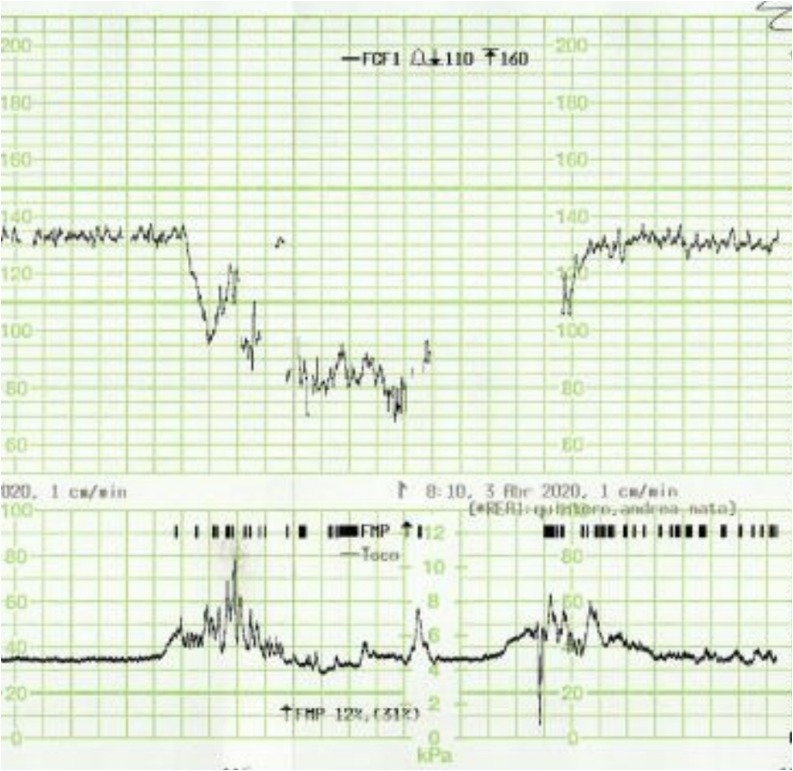
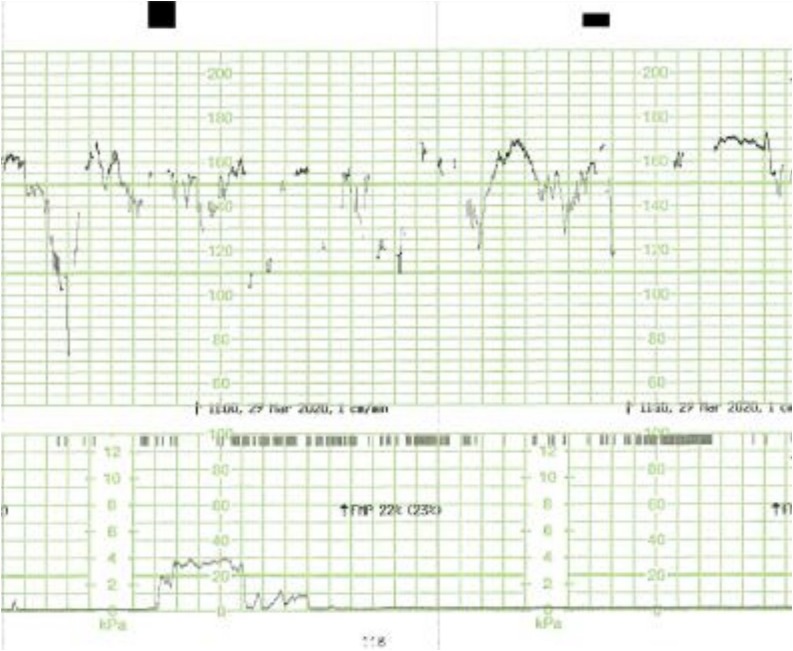
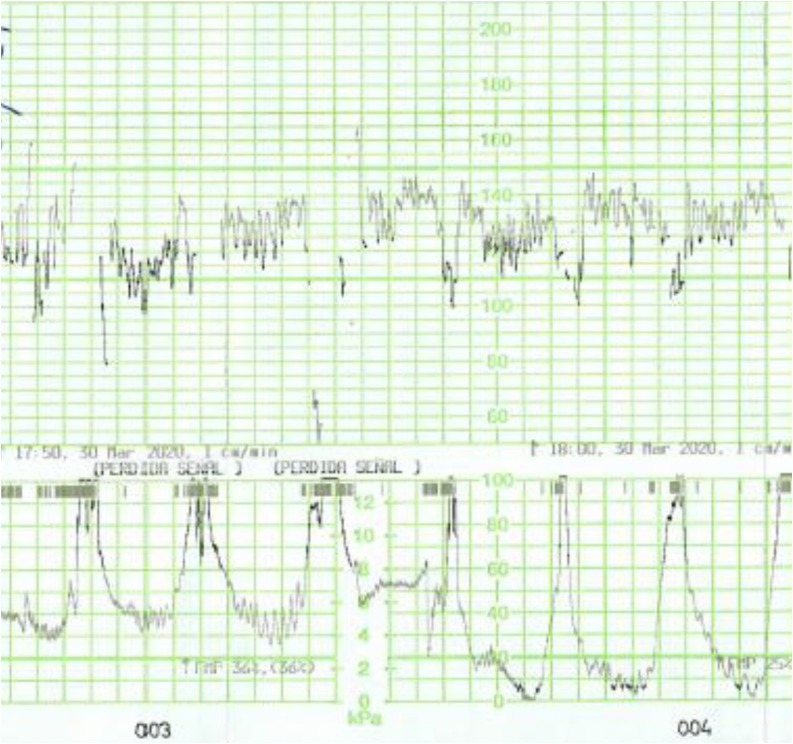
All fetuses showed an increased baseline FHR > 10 % compared to the initial recording and one fetus (No. 5) showed gross fetal tachycardia (>210 bpm). All fetuses showed absence of accelerations (Fig. 1 ). Late (Fig. 2 ) or prolonged decelerations (Fig. 3 ) were observed in 10 out of 12 CTG traces (83.3 %) CTG traces. 7 out of 12 fetuses (58.3 %) showed absence of cycling (Fig. 4 ). Exaggerated or augmented variability >25bpm was found in 4 out of 12 cases (33 %) confirming a ZigZag pattern (Fig. 5 ). None of the CTGs demonstrated a sinusoidal pattern. All CTG traces (100 %) where uterine activity was monitored (10 out of 12 cases) showed evidence of excessive uterine activity. In the cases where delivery was accomplished (11 out of 12), the Apgar scores at 5 min were normal (>7). Umbilical cord arterial pH was available in 9 cases, and none showed evidence of neonatal metabolic acidosis (defined as pH < 7.0).
Fig. 1.
Absence of accelerations in the CTG trace of a patient with COVID-19 infection.
Fig. 2.
Late decelerations in the CTG trace of a patient with COVID-19 infection.
Fig. 3.
Prolonged deceleration in the CTG trace of a patient with COVID-19 infection.
Fig. 4.
Absence of cycling in the CTG trace of a patient with COVID-19 infection.
Fig. 5.
ZigZag pattern (exaggerated variability) in the CTG trace of a patient with COVID-19 infection.
Discussion
To our best knowledge, this is the first study that analysed the CTG changes in maternal COVID-19 infection using pathophysiology according to the International Physiological CTG Guidelines produced by 36 CTG experts from 14 countries in 2018 (https://physiological-ctg.com/guideline.html). As expected, all fetuses showed an increased baseline FHR > 10 % compared to the initial recording, which was suggestive of reactive fetal response to maternal pyrexia and inflammatory response. The gross fetal tachycardia (>210 bpm) seen in one case was most likely secondary to the maternal “cytokine storm” resulting in excessive fetal sympathetic response and/or due to cardiac arrythmia secondary to excessive inflammatory mediators. All fetuses showed absence of accelerations (Fig. 1). This was most likely secondary to the depression of the fetal somatic nervous system by inflammatory mediators or due to ongoing hypoxia as a result of increased maternal or placental oxygen consumption leading to conservation of fetal somatic muscle activity. Late (Fig. 2) or prolonged decelerations (Fig. 3) which were observed in 10 out of 12 CTG traces (83.3 %), were either secondary to increased placental metabolism and resultant reduced utero-placental oxygen transfer (i.e. a relative utero-placental insufficiency), or due to placental intervillous thrombosis secondary to maternal hypercoagulable state in COVID-19 infection.
58.3 % showed absence of cycling (Fig. 4), which was most likely due to the loss of the normal active and quiet sleep epochs secondary to placental transfer of maternal inflammatory mediators, and resultant depressive effect on the fetal brain. Exaggerated or augmented variability >25bpm found in 33 % confirming a ZigZag pattern, was most likely secondary to an autonomic instability as a result of fetal inflammatory response secondary to maternal cytokine storm and pyrexia. None of the CTGs demonstrated a sinusoidal pattern which was suggestive that a chronic fetal anaemia secondary to haemolysis is a rare phenomenon in maternal COVID-19 infection. All CTG traces (100 %) where uterine activity was monitored (10 out of 12 cases) showed evidence of excessive uterine activity, which was most likely secondary to myometrial irritability due to maternal pyrexia and inflammatory response. In the cases where delivery was accomplished (11 out of 12), the Apgar scores at 5 min were normal (>7). Umbilical cord arterial pH was available in 9 cases, and these showed absence of evidence of neonatal metabolic acidosis (pH > 7.0). These indicate that despite the observed abnormal patterns such as absence of accelerations, increased baseline FHR and the presence of late or single prolonged decelerations on the CTG traces, the perinatal outcomes were favourable. This suggest that the observed abnormalities on the CTG trace were reactive changes in the fetus secondary to the pathophysiology o the maternal COVID-19 infection. This may have a significant clinical application as performing an urgent operative delivery or adjunctive tests such as fetal scalp blood sampling for a “pathological CTG trace” in COVID-19 patients should be reviewed. Immediate measures to improve the maternal environment to correct maternal hypoxia, maternal pyrexia and inflammatory response should be undertaken to rectify CTG changes, prior to considering any intervention based on the observed abnormal CTG changes. This is because the primary causes of abnormal CTG changes in maternal COVID-19 infection are present within the maternal compartment. It is also important to anticipate the CTG changes we have highlighted in maternal COVID-19 infections. By doing so unnecessary operative interventions based on CTG guidelines -which are produced to detect intrapartum hypoxia- could be avoided in maternal COVID-19 infection, where the primary pathology is inflammation and not hypoxia.
The main strength of our study is that the authors analysed the CTG features that would be expected in maternal COVID-19 patients based on the knowledge of pathophysiology, instead of relying on standard CTG guidelines (FIGO, NICE, ACOG or National) which are intended to recognise intrapartum hypoxia and not inflammation. COVID-19 infection is currently of immense clinical significance and therefore, we hope the findings in our study will help avoid unnecessary operative interventions for abnormal CTG changes. This may help avoid additional metabolic and stress response to an operative intervention in a woman who is already symptomatic of COVID-19 infection.
The main limitation of our study is the small number. However, symptomatic maternal COVID-19 infection is a novel, relatively rare condition, and therefore, there is an urgent need to inform frontline clinicians of the abnormal CTG changes observed and the associated perinatal outcomes. So that unnecessary operative interventions are avoided in pregnant women with symptomatic COVID-19 infection.
Conclusion
Maternal COVID-19 infection appears to cause changes in the cardiotocograph (CTG) trace due to a combination of effects. These include maternal hypoxia, “cytokine storm”, maternal pyrexia, uterine irritability and diminished transfer of oxygen through the placenta. This can be a consequence of increased placental metabolic demands and / or placental intervillous thrombosis secondary to maternal hypercoagulable state. However, as these are due to the pathology in the maternal environment, despite the abnormal features observed on the CTG trace, the neonatal outcome appears to be favourable. Therefore, when abnormalities are observed on the CTG trace, clinicians should aim to correct the pathology related to COVID-19 within the mother, in order to eliminate the detrimental effects of the maternal environment on the fetal heart rate. Whenever possible, this should be done prior to rapidly transferring the mother for an operative delivery for a “pathological CTG”. It is highly likely that by correcting the adverse maternal environment, the CTG changes secondary to the effects of COVID-19 infection could be reversed. It is hoped that future research may help determine the time taken for the CTG changes to resolve after the correction of adverse maternal environment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
To the team of Puerta de Hierro hospital (Madrid) and Germans Trias i Pujol hospital (Badalona) who kindly provided us with the CTGs.
References
- 1.Pinas A., Chandraharan E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol. 2016 doi: 10.1016/j.bpobgyn.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Chandraharan E., Sabaratnam A. Prevention of birth asphyxia : responding appropriately to cardiotocograph (CTG) traces. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):609–624. doi: 10.1016/j.bpobgyn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;19(xxxx):1–7. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Gao Y., Qiao L., Wang W., Chen D. Inflammatory Response Cells During Acute Respiratory Distress Syndrome in Patients With Coronavirus Disease 2019 (COVID-19) Ann Intern Med. 2020 doi: 10.7326/L20-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terpos E., Ntanasis-Stathopoulos I., Elalamy I. Hematological findings and complications of COVID-19. Am J Hematol. 2020 doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karami P., Naghavi M., Feyzi A. Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020;(April):101665. doi: 10.1016/j.tmaid.2020.101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason R.J. Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J. 2020 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) and Pregnancy: What obstetricians need to know. Am J Obstet Gynecol. 2020;2019 doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;1(212) doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gracia-Perez-Bonfils A., Vigneswaran K., Cuadras D., Chandraharan E. Does the saltatory pattern on cardiotocograph (CTG) trace really exist? The ZigZag pattern as an alternative definition and its correlation with perinatal outcomes. J Matern Fetal Neonatal Med. 2019:1–9. doi: 10.1080/14767058.2019.1686475. [DOI] [PubMed] [Google Scholar]