Abstract
Introduction
‘Repurposing’ existing drugs to treat COVID-19 is vital to reducing mortality and controlling the pandemic. Several promising drugs have been identified and are in various stages of clinical trials globally. If efficacy of these drugs is demonstrated, rapid, mass availability at an affordable cost would be essential to ensuring equity and access especially amongst low- and middle-income economies.
Methods
Minimum costs of production were estimated from the costs of active pharmaceutical ingredients using established methodology, which had good predictive accuracy for medicines for hepatitis C and HIV amongst others. Data were extracted from global export shipment records or analysis of the route of chemical synthesis. The estimated costs were compared with list prices from a range of countries where pricing data were available.
Results
Minimum estimated costs of production were US $0.93/day for remdesivir, $1.45/day for favipiravir, $0.08/day for hydroxychloroquine, $0.02/day for chloroquine, $0.10/day for azithromycin, $0.28/day for lopinavir/ritonavir, $0.39/day for sofosbuvir/daclatasvir and $1.09/day for pirfenidone. Costs of production ranged between $0.30 and $31 per treatment course (10–28 days). Current prices of these drugs were far higher than the costs of production, particularly in the US.
Conclusions
Should repurposed drugs demonstrate efficacy against COVID-19, they could be manufactured profitably at very low costs, for much less than current list prices. Estimations for the minimum production costs can strengthen price negotiations and help ensure affordable access to vital treatment for COVID-19 at low prices globally.
Keywords: SARS-CoV2, COVID-19, drug prices, access to medicines
Introduction
As the SARS-CoV2 pandemic continues to grow, researchers worldwide are urgently looking for new treatments to prevent new infections, cure those already infected or lessen the severity of disease. An effective vaccine may not be widely available until late 2021, even if trials are successful [1].
There are clinical trials in progress to ‘repurpose’ drugs, normally indicated for other diseases, to treat COVID-19 [2,3]. The shortened development timeline and reduced costs to this approach [4] of using already existing compounds is particularly advantageous compared with new drug discovery in a pandemic situation, where time is of the essence.
Antiviral drugs include nucleotide analogue remdesivir, which was previously used experimentally but unsuccessfully against Ebola [5–8], favipiravir, used to treat influenza [9], the HIV protease inhibitor lopinavir/ritonavir (LPV/r) [10,11], the antimalarials chloroquine and hydroxychloroquine [12–14], and the direct-acting antivirals sofosbuvir and daclatasvir [15], which are all potential candidates. Additionally, treatments to improve lung function and reduce inflammation, such as pirfenidone [16,17] and tocilizumab [18,19], are being evaluated in clinical trials.
Most of the clinical trials reported so far are small pilot studies, often non-randomised, making interpretation of current evidence difficult. However, results from randomised trials of these repurposed treatments should become available from May 2020 onwards. If favourable results are shown from these new trials, there is the potential to rapidly upscale production of the most promising drugs. The safety profiles of these drugs have already been established from clinical trials for other diseases, so they could be rapidly deployed to treat COVID-19 before vaccines become available.
Low- and middle-income countries will need access to these treatments at minimum prices to ensure all those in need can be treated. Even in high-income countries, the burden of disease could be so great that access to drugs at minimum costs could also be necessary. The HIV epidemic has been controlled by mass treatment with antiretroviral drugs worldwide, at very low unit costs. Large donor organisations such as the Global Fund for AIDS, TB and Malaria (GFATM) and the President's Emergency Plan for AIDS Relief (PEPFAR) order drugs to treat >20 million people with HIV, at prices close to the cost of production [20,21]. This system allows low- and middle-income countries to access high-quality drugs at affordable prices.
The minimum costs of drug production can be estimated by calculating the cost of active pharmaceutical ingredients (API), which is combined with costs of excipients, formulation, packaging and a profit margin, to estimate the price of ‘final finished product’ (FFP) – the drug ready for use. There are established methods for these calculations, which have reliably predicted the minimum costs of drugs to cure hepatitis C [22,23] and other diseases [24,25]. The purpose of this analysis was to apply the same calculations to the new candidate treatments for COVID-19.
Methods
The leading candidate drugs to treat COVID-19 were selected based on recent reviews and analysis of ongoing clinical trials [2,8,10–12,15,17–19,26–33]. The treatments selected were remdesivir, favipiravir, lopinavir/ritonavir, hydroxychloroquine, chloroquine, azithromycin, pirfenidone, tocilizumab and sofosbuvir/daclatasvir. All are currently being evaluated in randomised trials, with results expected between May and September 2020.
The methods used to estimate minimum costs of production have been described previously [24,25]. Briefly, we analysed the costs of exports of API from India using the online tracking database Panjiva [34], which shows details of all shipments of API with quantities and costs per kilogram. We used all available costing data for each drug API found on Panjiva, excluding shipments <1kg in size, alongside the lowest and highest 15% of results based on prices per kg. A 5% API loss during tableting process was factored into our calculations, and a conversion cost of US $0.01 per tablet was used. A multiplier based on API mass was applied to account for the price of excipients, which are additional substances needed to convert API into FPP. Our estimated API costs presume that production is carried out by a generic provider of APIs, where associated costs of capital investment, overhead and labour are not as high as for production by originator companies.
This method was applied to small-molecule drugs only. In addition, the drug remdesivir, which is administered by IV infusion, was considered separately when estimating formulation costs.
A profit margin of 10% and Indian taxation of 27% on profit was added. These results were cross-checked with a second API database, Pharmacompass [35], which displayed records up to 2016 only. Panjiva was selected to provide real time, up-to-date shipment and cost data. The estimates assume a volume of 1000 kg of API for each compound.
Three drugs did not have available data on API production: remdesivir and favipiravir and tocilizumab. For the first two, API production costs were estimated based on published routes of chemical synthesis [6,7,36,37]. Since remdesivir is administered by IV infusion, the costs of production were adjusted to include the cost of injections, according to an established method used for the World Health Organization (WHO) Essential Medicines List [24]. It was not possible to track the cost of API for the monoclonal antibody tocilizumab; therefore, we tracked list prices in a range of countries, in particular developing economies as a proxy for minimum costs.
The costs of regulatory filings and approvals are often significant add-ons to the initial use of drugs in any specific country. All drugs analysed in this study, except for remdesivir have been approved for treatment for some indications in all countries, but few are approved for treatment of COVID-19. Remdesivir is an investigational drug without any prior regulatory approvals, but it has a known favourable safety profile after clinical trials against Ebola.
Favipiravir has been approved for the treatment of influenza in Japan since 2014 [9], and in China since March 2020. It has also been approved for investigational use (China and Italy) against COVID-19 in March 2020. We have not included the cost or timing associated with regulatory approvals for the use of these drugs. We are assuming in our analysis that the WHO and other influential regulatory agencies will cooperate to define a pathway for use of these drugs which does not include additional financial outlays or filing for marketing approvals.
The minimum costs of production were then compared with published list prices for each drug in a range of countries – USA, UK, France, Sweden, South Africa, India, Bangladesh, Malaysia, Brazil, Turkey, Pakistan, Egypt and China [38–51] – to give a representative sample of prices in countries with different levels of economic development. For consistency, we selected a single data source per country to be used for all searches of drug prices within that country, based on the organisation of data and perceived reliability. Not all drugs analysed in this study were available in our selected countries, and in some countries, online pharmacy sites were used because national databases were not available. Where several prices were available in the same database, the lowest price was selected.
Available clinical trial data for each drug were collected from literature searches, C linicalTrials.gov and the Chinese Clinical Trials Registry. Results from pilot studies were included, together with the planned clinical trial programmes and expected completion dates.
Results
The predicted costs of production, and the highest/lowest available list prices of all drugs analysed are shown in Table 1, and chemical structures for all drugs are shown in Figure 1.
Table 1.
Summary of costs of production and lowest/highest prices
| Drug | Dose | Highest list price | Lowest list price | Estimated cost price (course) | Estimated cost price (day) |
|---|---|---|---|---|---|
| Remdesivir
(10 Days) |
100 mg IV BD (Day 1)
100 mg IV OD (Days 2–9) |
___ | ___ | $9 | $0.93 |
| Favipiravir
(14 Days) |
600 mg BD | ___ | $231 (China) | $20 | $1.45 |
| Lopinavir/ritonavir
(14 Days) |
400/100 BD | $503 (US) | $9 (Global Fund)*
$15 (South Africa) |
$4 | $0.28 |
| Hydroxychloroquine
(14 Days) |
400 mg OD | $19 (China) | $2 (India) | $1 | $0.08 |
| Chloroquine
(14 days) |
155 mg OD | $93 (US) | $0.20 (Bangladesh) | $0.30 | $0.02 |
| Azithromycin
(14 days) |
500 mg OD | $63 (US) | $5 (India) | $1.40 | $0.10 |
| Sofosbuvir/daclatasvir
(14 days) |
400/60 OD | $18,610 (US) | $6 (Pakistan) | $5 | $0.39 |
| Pirfenidone
(28 days) |
801 mg TD | $9606 (US) | $100 (India) | $31 | $1.09 |
| Tocilizumab
(Per dose) |
560 mg BD | $3383 (US) | $510 (Pakistan) | ___ | ___ |
Median price available to a range of low- and middle-income countries. OD: once daily; BD: two times daily; TD: three times daily; IV: intravenous.
Figure 1.
Chemical structures of candidate drugs
Remdesivir
Remdesivir (formerly GS-5374) has been evaluated for treatment of SARS and Ebola [5,7]. There are five randomised trials of remdesivir for SARS-CoV2, with first results expected at the end of April 2020. There is a 10-day course of treatment under evaluation. The dose of remdesivir is 200 mg on the first day with 100 mg per day thereafter. Remdesivir is administered by IV infusion. One metric ton of remdesivir is sufficient API to manufacture 900,000 courses of treatment, without allowance for any losses during formulation.
Based on analysis of the published second-generation route of chemical synthesis and assumed overheads such as occupancy rate per hour and labour of an India-based generic producer, the cost of API was estimated to be $4000/kg [6,7] for 100-kg batches produced without capital investment in dedicated remdesivir production facilities. The 10-day course of treatment would therefore cost $4.80 per person. After adjustment for the cost of formulation (and 20% losses projected during formulation), cost of vials, profit margin and tax, the estimated cost per treatment would be approximately $9 per person as shown in Figure 2a. Daily cost is estimated to be $0.93.
Figure 2.
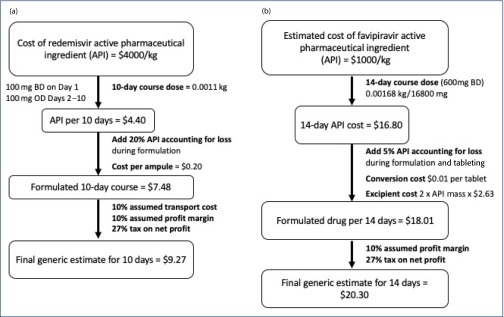
(a) Flowchart to show the calculation of treatment costs for remdesivir. (b) Flowchart to show the calculation of treatment costs for favipiravir. OD: once daily; BD; twice daily.
As an IV infusion, there would be an additional cost for saline, estimated at $4 per infusion [52], equipment such as syringe, sterile water for reconstitution and IV lines, as well as staff-time cost associated with the healthcare professional administrating the infusion. These additional non-drug components are likely to be more expensive than the estimated cost of the drug itself and are not included in our cost estimate.
Favipiravir
The main randomised trial of favipiravir evaluated up to 10 days of treatment, vs another influenza drug, umifenovir, in 240 patients. After 7 days of treatment, the clinical recovery rate was 71% for favipiravir vs 56% for umifenovir (P<0.02). Recovery from fever was also faster for people treated with favipiravir (P<0.001), but there was no difference between arms in auxiliary oxygen therapy or non-invasive mechanical ventilation rates [31].
A second, non-randomised study in China evaluated 14 days of treatment with either favipiravir or LPV/r. The median time to virus clearance was significantly shorter in the favipiravir group (4 days) vs the LPV/r group (11 days, P<0.001) [32].
Favipiravir is dosed 600 mg twice daily. A metric ton of favipiravir, therefore, would provide approximately 59,000 courses of treatment. The cost of API was estimated at about $1000/kg, based on analysis of the route of chemical synthesis [36,37]. This is a simple molecule to synthesise: several very basic steps involved are more akin to processes for manufacturing fine chemicals rather than pharmaceuticals. The structure is a 6-fluoro substituted 3-hydroxypyrazine-2-carboxamide.
Based on a 14-day course of treatment, the cost of the API would be $16.80. After adjustment for loss, formulation, packaging and profit margins, the estimated cost of production is $20 per 14-day treatment cost (Figure 2b), or $1.45 per day. In late February 2020, favipiravir was launched for sale in China for $231 per treatment course, in an announcement by the Shandong Provincial Public Resource Trading Centre [53].
Lopinavir/ritonavir
The standard dose of LPV/r is 400/100 mg twice daily. LPV/r has been evaluated in two published studies of SARS-CoV2 infection [11]. In these studies, there has been no difference in measures of efficacy between LPV/r (given for 14 days) and control treatment. A systematic review by the WHO showed no clear evidence for the efficacy of LPV/r. However, there were very few clinical trials available for this evaluation at the time of the review [10]. It is not clear whether this HIV protease inhibitor will work in the earlier stage of SARS-CoV2 infection, or if used in combination with other drugs. Results from other clinical trials are expected between June and August 2020.
The estimated cost of production is $3.09 per treatment course, based on API cost of $233/kg for lopinavir and $212/kg for ritonavir. After adjustment for loss, formulation, packaging and profit margins, the estimated cost of production of the combined drug is $4 per 14-day course, or $0.28 per day.
Searches in selected national databases and online pharmacies yielded a range of list prices between $503 in the US, to $15 in South Africa per 14-day dose, as shown in Figure 3a. Additionally, LPV/r is also available through the Global Fund for a range of low- and middle-income countries, with a median cost of $9 per 14-day course [54].
Figure 3.
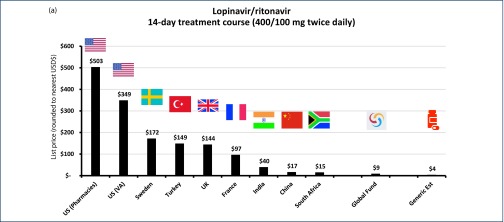
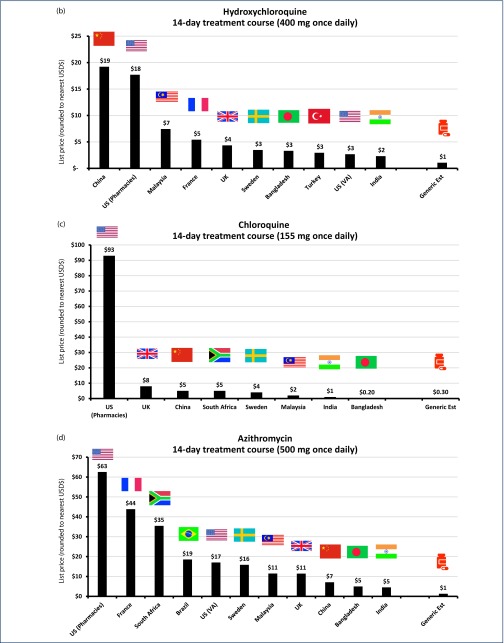
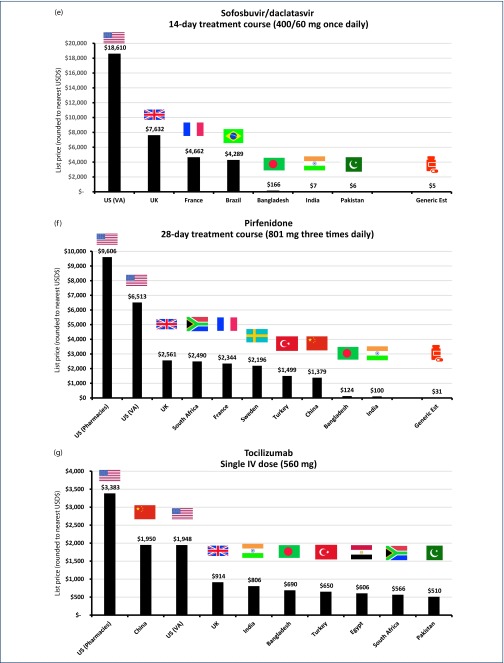
(a) List price cost of lopinavir/ritonavir in selected countries for 14-day treatment (400/100 mg twice daily). (b) List price cost of hydroxychloroquine in selected countries for 14-day treatment (400 mg once daily). (c) List price cost of chloroquine in selected countries for 14-day treatment (155 mg chloroquine base once daily). (d) List price cost of azithromycin in selected countries for 14-day treatment (500 mg once daily). (e) List price cost of sofosbuvir/daclatasvir in selected countries for 14-day treatment (400/60 mg once daily). (f) List price cost of pirfenidone in selected countries for 28-day treatment (801 mg three times daily). (g) List price cost of IV tocilizumab in selected countries for single dose (560 mg).
Hydroxychloroquine and chloroquine
In vitro, hydroxychloroquine is predicted to show superior activity against COVID-19 compared with chloroquine [12]. In a recent parallel-group, a randomised Chinese study in Wuhan of 62 patients found treatment with hydroxychloroquine 400 mg per day led to faster symptomatic improvement times in the treatment arm (n = 31) after 5 days in terms of temperature normalisation and cough remission, and a greater proportion of patients (80.6% vs 54.8%) with improved pneumonia [26].
Similarly, another Chinese randomised trial in Shanghai [27] of 30 patients also used 400 mg per day in the treatment arm but found no statistically significant difference in clinical findings, symptomatic improvements or radiological improvements between the arms by day 5. The authors also highlighted the need for much larger, better powered trials to reach reliable conclusions.
Different dosing protocols are being used for hydroxychloroquine, including 600 mg daily in the small, open-label, non-randomised French study by Gautret et al. (n = 26) that has suggested improved efficacy for hydroxychloroquine [28]. For our analysis, we have therefore chosen 400 mg daily, which was the most commonly used dosage. This is also the upper recommended dose by the British National Formulary for existing indications [38].
API cost-per-kilogram of hydroxychloroquine is $140/kg, with a 14-day dose-equivalent of API costing $0.78. After adjustment for the cost of formulation, packaging and profit margin, the final cost would be $1 per 14-day treatment course ($0.08 per day). Globally, list prices range between $19 per 14-day course in China and only $2 per 14-day course in India (Figure 3b).
For chloroquine, the cost of API is $49/kg from Panjiva data. A 14-day course equivalent of API would therefore cost $0.11 based on the equivalent dose of 155 mg per day of chloroquine base. After adjustment for the cost of loss, formulation, packaging and profit margin, the final cost would be $0.30 per 14-day treatment course of chloroquine, equivalent to $0.02 per day.
Available list prices vary from $93 in the US, to $0.20 per 14-day course in Bangladesh, which is less than our estimated generic treatment cost. It is worth noting that the US price for chloroquine may be considered an outlier, given the next most expensive list price, found in the UK, was only $8 per 14-day course (Figure 3c).
Azithromycin
This macrolide antibiotic has been used as an adjunctive treatment for six patients in the small French pilot study of hydroxychloroquine by Gautret et al. to prevent bacterial superinfection, with all six patients virologically cured by day six [28]. However, this finding is contradicted by a small, open-label study (n = 11) in Paris by Molina et al. [29], who found no strong viral clearance effect associated with hydroxychloroquine/azithromycin combination therapy.
The cost of API derived from Panjiva shipment data for azithromycin is $139/kg. A 14-day course equivalent of API at a dose of 500 mg per day would therefore cost $0.98. After adjustment for the cost of loss, formulation, packaging and profit margin, the final cost would be $0.10 per day or $1.40 per 14-day treatment course. List prices for azithromycin range between $63 per 14-day course in the US, and $5 per 14-day course in India and Bangladesh (Figure 3d).
Sofosbuvir/daclatasvir
Combination treatment with sofosbuvir/daclatasvir, direct-acting antivirals normally used to treat hepatitis C, is being evaluated in Iran for 70 COVID-19 patients with moderate to severe symptoms [15]. The dosage of sofosbuvir/daclatasvir is 400/60 mg daily.
API per kilogram was $700 for sofosbuvir and $600 for daclatasvir. Fourteen-day dose-equivalent of API for the combined drug therefore costs $4.42. After adjusting for the cost of loss, formulation, packaging and profit margin, the final cost would be $5 per 14-day treatment course, or $0.39 per day.
The cost of sofosbuvir/daclatasvir API has been falling significantly in recent years; earlier estimates in 2016 for a 12-week course of treatment were $200 per patient, or $33 per 14 days [23] and in 2017, $47 per 12-week course, or $7.8 per 14 days [55]. Therefore, our new estimates represent a 6.6-fold reduction in the minimum cost of production since 2016.
Globally, 14-day course list prices range between $18,610 in the US and $7 in India, or $6 in neighbouring Pakistan, as shown in Figure 3e [56].
Pirfenidone
There is a randomised trial of pirfenidone vs placebo in progress [17]. There are 292 patients with severe or critical SARS-CoV2 infection being evaluated in this clinical trial, with results expected in May 2020. The dose being evaluated is 801 mg three times daily for 4 weeks.
The cost of API from the Panjiva database was $368/kg, representing a 4-week API cost of $26. After adjustment for costs of loss, formulation, packaging and profit margins, the minimum cost of treatment would be $31 per person, or $1.09 per day.
There is again a large variation between individual countries’ list prices. Pirfenidone is available in the US for $9606 for a 4-week course, but only $124 in Bangladesh and $100 in India for a generic version (Figure 3f). However, even at $100 per month, this is still higher than our API cost-based estimate.
Tocilizumab
There are several large clinical trials of this monoclonal antibody in progress, for patients with late-stage disease [18,19]. As an IV infusion, doses are based on bodyweight (8 mg/kg) with a maximum single dose of 800 mg every 12 hours. We therefore made the assumption of average bodyweight being 70 kg, with a single dose of 560 mg.
No API data were available for tocilizumab; therefore, we were unable to estimate the minimum cost of production. List prices per 560 mg single dose varied from $3383 in the US to $510 in Pakistan (Figure 3g). Across several developing economies with available list prices – India, Bangladesh, Turkey, South Africa, Egypt and Pakistan – the median was $628 per dose.
Several tocilizumab biosimilars are currently under development [57,58]; however, none has yet been approved and launched. The general experience so far of biosimilars has been that they offer health care systems the potential to lower costs significantly [59], with the UK alone expected to save up to GBP200–GBP300 million per year through increased uptake of better-value biological medicines [60].
Discussion
This analysis shows that drugs to treat COVID-19 could be manufactured for very low prices, between $1 and $29 per course. Many of these drugs are already available as generics, at prices close to the cost of manufacture, in low- and middle-income countries. We do not yet know which of these drugs will show significant benefits. However, if promising results emerge from pivotal clinical trials, there is the potential to upscale generic production and provide treatment for millions of people at very low unit prices. There is an established mechanism to do this: donor organisations such as GFATM and PEPFAR already provide mass treatment of HIV, TB and malaria worldwide at prices close to the costs of production.
The drugs in this analysis were not designed against the SARS-CoV2 virus; they were developed to treat other viruses or diseases. Some, such as chloroquine, were developed in the 1950s. Most of the clinical trials of treatments have been funded by national health authorities and donor agencies rather than pharmaceutical companies. Patients with COVID-19 have risked their own health in these clinical trials, often with no clear benefits. Companies should be encouraged to continue their research, with costs of clinical trials supported by public funding. Since the start of the pandemic, the money spent by pharmaceutical companies on research and development of these drugs will be minimal, relative to funding from national health authorities. Where pharmaceutical companies have donated drugs for clinical trials, there are already tax rebate systems in place that will recover the costs of the donated drugs.
For mass production of these drugs, our analysis assumed a profit margin of 10% to companies manufacturing the drugs. This is similar to the pricing structure for HIV, TB and malaria, where generic drug companies still earn acceptable profits while mass producing these drugs at prices close to production costs. Large-volume orders are needed to incentivise generic companies to manufacture drugs at low prices. Other mechanisms exist to optimise drug manufacture. With pooled procurement, a set of countries can order drug supplies together, to take advantage of economies of scale. There can be volume-price guarantees to procure large amounts of drugs at fixed prices for a set number of years. Prequalification of key companies by the WHO can be recognised by any country as an indicator of drug quality, including adherence to good manufacturing practice and the stability, or viability of the drug over its stated shelf life, alongside the bioequivalence of generic drugs vs the original branded versions.
Additionally, the costs of treatment may be higher if combinations of two or three drugs are needed. Other infectious diseases such as HIV, hepatitis C or TB are best treated with two/three-drug combination treatments. Drugs which have not shown efficacy against COVID-19 as monotherapy should not necessarily be discarded: they might still contribute to the efficacy of two- or three-drug combination treatments. Drugs that are not curative but lessen disease severity are also needed. These treatments could lessen the burden on healthcare systems, which could otherwise be overwhelmed by a lack of ventilators or other supportive services.
When these drugs are repurposed to treat COVID-19, we will need to ensure a constant supply of drugs for the original indications, for example, pirfenidone for people with pulmonary fibrosis, or hydroxychloroquine for people with rheumatoid arthritis and systemic lupus erythematosus.
The costs of these treatments will be higher if used for longer-term prevention, for example, in healthcare workers. Randomised trials of chloroquine and hydroxychloroquine for prevention of SARS-CoV2 infection are in progress [12,33] and other candidate drugs could emerge for use as prophylactics.
We highlight four limitations of our study, for consideration in future work. First, this analysis does not include all candidate drugs for COVID-19. There are drugs at earlier stages of development; a wide range of candidate drugs have been identified by machine learning models [3]. These drugs may need further in vitro testing before being introduced into human studies.
Second, treatments like LPV/r and sofosbuvir/daclatasvir have only a small chance of showing significant benefits to patients in ongoing trials, given current evidence.
Third, for newer drugs such as remdesivir, favipiravir and pirfenidone, costs of production could continue to fall over time through economies of scale. This trend has been seen for drugs to treat HIV and hepatitis C. The cost of API for the hepatitis C drug daclatasvir fell by 90% in the 2 years after initial launch, as more generic companies upscaled synthesis of the API with greater competition in the market.
Fourth, many drugs may have been given discounts from the list prices that we have identified for comparison in this analysis following in-country negotiations. Even so, list prices can be over 100-fold higher than the predicted costs of production in some cases.
We propose four main recommendations to ensure that any patient with COVID-19, in any country, can access the drugs they need:
-
1.
Treatments showing efficacy in well-powered clinical trials should be made available worldwide at prices close to the cost of manufacture. All the treatments being evaluated in clinical trials are very cheap to manufacture. Clearly, the mass production of these drugs will need to be economically sustainable. Treatments for HIV, TB and malaria are distributed worldwide by GFATM and PEPFAR, to treat millions of people at prices close the cost of manufacture. The prices paid allow generic companies to make acceptable profits. We should adopt a similar model of drug distribution for COVID-19.
-
2.
There should be parallel manufacture by at least three different companies for each product, sourcing their API from different countries. In the early stages of the SARS-CoV2 epidemic, API production in China was severely disrupted because of quarantine of key workers and delays in transporting key raw materials between factories [61]. India has suspended export of several key drugs because of anticipated local shortages. Production of drugs in a range of countries will protect us from disruption or shortages in individual countries.
-
3.
There should be no intellectual property barriers preventing mass production of these treatments worldwide. We need open ‘technology transfer’ so that the methods used to manufacture the key drugs can be shared with any country deciding to produce the drugs locally.
-
4.
Results and databases from all COVID-19 clinical trials should be fully accessible so others can learn from them. To speed up access to these drugs, countries could rely on recognition of the review and approval of key treatments by regulatory authorities in the US or Europe, or other stringent regulatory authorities. There may not be time for the normal times of regulatory review by all individual countries.
In summary, repurposed drugs may be our only option to treat COVID-19 for the next 12–18 months, until effective vaccines can be developed and manufactured at scale. If repurposed drugs do show efficacy against COVID-19, they could be manufactured at very low unit prices, in the range of $1 to $29 per treatment course. The system of mass production and distribution of drugs to treat HIV, TB and malaria via GFATM and PEPFAR could act as a blueprint for the treatment of SARS-CoV2, to ensure access to effective drugs at low prices worldwide.
Acknowledgements
Conflicts of interest
AH received a consultancy payment from Merck for a clinical trial review that is not connected with this project.
Funding
AH received funding from the International Treatment Preparedness Coalition (ITPC) as part of the Unitaid-supported project ‘Affordable medicines for developing countries’.
References
- 1. Gates B. Responding to Covid-19 – a once-in-a-century pandemic? N Engl J Med 2020; 10.1056/NEJMp2003762. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020; 19 ( 3 ): 149– 150. [DOI] [PubMed] [Google Scholar]
- 3. Beck B, Shin B, Choi Y et al. Predicting commercially available antiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug-target interaction deep learning model. bioRxiv preprint 10.1101/2020.01.31.929547. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pushpakom S, Iorio F, Eyers P et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019; 18 ( 1 ): 41– 58. [DOI] [PubMed] [Google Scholar]
- 5. Sheahan TP, Sims AC, Graham RL et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017. 10.1126/scitranslmed.aa3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Center for Biotechnology Information Methods for Treating Filoviridae. PubChem Database: Patent=US9724360. Available at: pubchem.ncbi.nlm.nih.gov/patent/US9724360 ( Accessed April 2020).
- 7. Siegal D, Hui H, Doerffler E et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazine-4-amino] adenine C-nucleoside (GS-5374) for the treatment of Ebola and emerging viruses. J Med Chem 2017; 60 ( 5 ): 1648– 1661. [DOI] [PubMed] [Google Scholar]
- 8. ClinicalTrials.gov Severe 2019-nCov Remdesivir RCT: Identifier: NCT04257656. Bethesda (MD): US National Library of Medicine, 2020. Feb 06 Available at: clinicaltrials.gov/ct2/show/NCT04257656 ( Accessed April 2020).
- 9. Hayden FG, Shindo N. Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis 2019; 32 ( 2 ): 176– 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ford N, Vitoria M, Rangaraj A et al. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS, or COVID-19: initial assessment. J Int AIDS Soc 2020. 10.1002/jia2.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao B, Wang Y, Wen D et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 10.1056/NEJMoa2001282. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao X, Ye F, Zhang M et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 10.1093/cid/ciaa237. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vardanyan RS, Hruby VJ. Drugs for treating protozoan infections In: Synthesis of Essential Drugs ( Vardanyan RS, Hruby VJ, eds), Elsevier, 2006, pp 559– 582. [Google Scholar]
- 14. World Intellectual Property Office 1. WO2005062723 – An Improved Process for the Preparation of 7-chloro-4-(5-n-ehtyl-n-2-hydroxyethylamine)-2-pentyl] Aminoquinoline and Its Intermediates. WIPO IP Portal. [Patent] WO2005/062723 A2 to Ipca Laboratories Kumar A, Vyas KD, Singh D, Nandavadekar S, Bhise S, Jadhav A Available at: patentscope.wipo.int/search/en/detail.jsf?docId=WO2005062723&tab=PCTBIBLIO ( Accessed April 2020).
- 15. Iran Registry of Clinical Trials Tehran. Identifier: IRCT20200128046294N2 A Prospective Randomized Controlled Clinical Trial Comparing the Therapeutic Efficacy of Sovodak (Sofosbuvir/Daclatasvir) with Standard Care in Patients with Moderate to Severe Coronavirus (COVID-19) Virus. 2020. March 14 Available at: en.irct.ir/trial/46463 ( Accessed April 2020).
- 16. World Intellectual Property Office WO2017/122139 An Improved Process for the Preparation of Pirfenidone. Bollu RB, Mandadapu VPK, Indukuri VSK, Chava S 2017. Available at: patentscope.wipo.int/search/en/detail.jsf?docId=WO2017122139 ( Accessed April 2020).
- 17. ClinicalTrials.gov A Study to Evaluate the efficacy and Safety of Pirfenidone with Novel Coronavirus Infection: Identifier: NCT04282902. Bethesda (MD): US National Library of Medicine 2020. Feb 25 Available at: clinicaltrials.gov/ct2/show/NCT04282902?cond=pirfenidone+coronavirus&draw=2&rank=1 ( Accessed April 2020).
- 18. ClinicalTrials.gov Favipiravir Combined with Tocilizumab for the Treatment of Coronavirus Disease 2019: Identifier: NCT04310228. Bethesda (MD): US National Library of Medicine 2020. March 17 Available at: clinicaltrials.gov/ct2/show/NCT04310228 ( Accessed April 2020).
- 19. ClinicalTrials.gov Tocilizumab in COVID-19 Pneumonia (TOCIVID-19): Identifier: NCT04317092. Bethesda (MD): US National Library of Medicine 2020. March 20 Available at: clinicaltrials.gov/ct2/show/NCT04317092 ( Accessed April 2020).
- 20. Global Fund for AIDS, TB and Malaria Results Report 2019. Available at: www.theglobalfund.org/media/8752/corporate_2019resultsreport_report_en.pdf?u=637146355110000000 ( Accessed April 2020).
- 21. PEPFAR The United States President's Emergency Plan for AIDS Relief. 2019 Annual Report to Congress 2019. Available at: www.state.gov/wp-content/uploads/2019/09/PEPFAR2019ARC.pdf ( Accessed April 2020).
- 22. Hill A, Khoo S, Fortunak J et al. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis 2014; 58 ( 7 ): 928– 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill A, Simmons B, Gotham D et al. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J Virus Erad 2016; 2 ( 1 ): 28– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gotham D, Barber MJ, Hill AM. Estimation of cost-based prices for injectable medicines in the WHO Essential Medicines List. BMJ Open 2019; 9 ( 9 ): e027780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hill AM, Barber MJ, Gotham D. Estimated costs of production and potential prices for the WHO Essential Medicines List. BMJ Glob Health 2018; 3 ( 1 ): e000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Z, Hu J, Zhang Z et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv 2020; 10.1101/2020.03.22.20040758. Pre-print. [DOI] [Google Scholar]
- 27. Chen J, Liu D, Liu L et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang Univ (Med Sci) 2020; 49 ( 1 ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gautret P, Lagier J, Parola P et al. Hydroxychloroquine and azithromycin as a treatment for COVID-19: results of an open-label, non-randomized clinical trial. Int J Antimicrob Agents 2020; 10.1016/j.ijantimicag.2020.105949. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Molina JM, Delaugerre C, Goff JL et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect 2020; 10.1016/j.medmal.2020.03.006. [ Article in French]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020; 14 ( 1 ): 72– 73. [DOI] [PubMed] [Google Scholar]
- 31. Chen C, Huang J, Cheng Z et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv 2020; 10.1101/2020.03.17.20037432. Pre-print. [DOI] [Google Scholar]
- 32. Biodiscover Favipiravir Treatment of COVID-19 Pneumonia Initial Clinical Results Released. [Chinese] Available at: Biodiscover.com/news/company/736154.html ( Accessed by authors March 2020).
- 33. ClinicalTrials.gov Hydroxychloroquine Post Exposure Prophylaxis for Coronavirus Disease (COVID-19): Identifier: NCT04318444. Bethesda (MD): US National Library of Medicine, 2020 March 24. Available at: www.clinicaltrials.gov/ct2/show/NCT04318444 ( Accessed April 2020).
- 34. Panjiva Supply Chain Intelligence. Available at: panjiva.com/ ( Accessed April 2020).
- 35. Pharmacompass Import-Export Database. Available at: www.pharmacompass.com/manufacturers-suppliers-exporters/ ( Accessed April 2020).
- 36. Liu F, Li C, Xiang H et al. A practical and step-economic route to Favipiravir. Chem Pap 2017; 71: 2153– 2158. [Google Scholar]
- 37. Guo Q, Xu M, Guo S et al. The complete synthesis of favipiravir from 2-aminopyrazine. Chem Pap 2019; 73: 1043– 1051. [Google Scholar]
- 38. National Institute for Health and Care Excellence British National Formulary. London: NICE, 2020. Available at: bnf.nice.org.uk/ ( Accessed April 2020). [Google Scholar]
- 39. Drug Prices 315 [Chinese]: Available at: www.315jiage.cn/ ( Accessed by authors March 2020).
- 40. Drugs.com Drug Price Information. Available at: www.drugs.com/price-guide/ ( Accessed April 2020).
- 41. US Department of Veterans Affairs Office of Procurement, Acquisition and Logistics: Pharmaceutical Prices. 2020. 15 March Available at: www.va.gov/opal/nac/fss/pharmPrices.asp ( Accessed April 2020).
- 42. OpenUp Medicine Price Registry. South Africa. Available at: openup.org.za/tools/mpr.html ( Accessed April 2020).
- 43. BrazilianHealth Regulatory Agency Drug Price Lists. Available at: portal.anvisa.gov.br/listas-de-precos ( Accessed April 2020).
- 44. French Ministry of Solidarity and Health Base de Données Publique des Médicaments. Available at: base-donnees-publique.medicaments.gouv.fr/ ( Accessed April 2020).
- 45. Sweden Dental and Pharmaceutical Benefits Agency Drug Database. Available at: www.tlv.se/beslut/sok-i-databasen.html ( Accessed April 2020).
- 46. MedEx A Comprehensive Online Medicine Index of Bangladesh. Available at: medex.com.bd/ ( Accessed April 2020).
- 47. Ministry of Health Malaysia Pharmaceutical Services Programme. Consumer Price Guide. Available at: www.pharmacy.gov.my/v2/en/apps/drug-price ( Accessed April 2020).
- 48. Medindia Drug Price of All the Brand Names. Available at: www.medindia.net/drug-price/index.asp ( Accessed April 2020).
- 49. ilacaBak Turkey. Available at: ilacabak.com/ ( Accessed April 2020).
- 50. Egyption Drug Store Actemra 400 mg/20 mL (Tocilizumab) Vial for IV Infusion. Available at: egyptiandrugstore.com/index.php?route=product/product&product_id=2708 ( Accessed April 2020).
- 51. Sehat Pharmacy. Available at: sehat.com.pk/ ( Accessed April 2020).
- 52. Laplante S, Makhija DU, Munson SH et al. Impact of fluid choice in systemic inflammatory response syndrome patients on hospital cost savings. Pharmacoecon Open 2018; 2 ( 3 ): 325– 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sina News Haizheng Pharmaceutical Favipiravir Price Announced: 398CNY/Box [Chinese] 2020. 23 Febuary Available at: med.sina.com/article_detail_100_2_77966.html ( Accessed by March 2020).
- 54. The Global Fund Price Reference Report – 2013-Present. Available at: public.tableau.com/profile/the.global.fund#!/vizhome/PQRPricelist_English/PriceList ( Accessed April 2020).
- 55. Grillon C, Krishtel P, Mellouk O et al. Treatment advocate tactics to expand access to antiviral therapy for HIV and viral hepatitis C in low- to high-income settings: making sure no one is left behind. J Int AIDS Soc 2018; 21 ( suppl 2 ): e25060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hill AM, Barber M, Khwairakpam G et al. 2019. The Road to Elimination of Hepatitis C: Costs Per Cure Fall to US$32 Per Person. American Association for the Study of Liver Disease: The Liver Meeting. 8–12 November 2019, Boston, USA. Abstract 0559.
- 57. Mycenax Biotech Inc LusiNEX (Tocilizumab, Mycenax In-House). Available at: www.mycenax.com.tw/en/product.php?act=view&id=6 ( Accessed April 2020).
- 58. ClinicalTrials.gov Comparative study of BAT1806 to RoActemra® in Rheumatoid Arthritis Patients with Inadequate Response to Methotrexate: Identifier: NCT03830203. Bethesda (MD): US National Library of Medicine, 2019. Feb 05 Available at: clinicaltrials.gov/ct2/show/NCT03830203 ( Accessed April 2020).
- 59. Smolen JS, Goncalves J, Quinn M et al. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open 2019; 5 ( 1 ): e000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. NHS England Commissioning Framework for Biological Medicines (Including Biosimilar Medicines). 2017. Available at: www.england.nhs.uk/wp-content/uploads/2017/09/biosimilar-medicines-commissioning-framework.pdf ( Accessed April 2020). [Google Scholar]
- 61. Chatterjee P. Indian pharma threatened by COVID-19 shutdowns in China. Lancet 2020; 395 ( 10225 ): 675. [DOI] [PMC free article] [PubMed] [Google Scholar]


