Abstract
Objective
Most cases of coronavirus disease 2019 (COVID-19) are identified as moderate, which is defined as having a fever or dry cough and lung imaging with ground-glass opacities. The risk factors and predictors of prognosis in such cohorts remain uncertain.
Methods
All adults with COVID-19 of moderate severity diagnosed using quantitative RT-PCR and hospitalized at the Central Hospital of Wuhan, China, from 1 January to 20 March 2020 were enrolled in this retrospective study. The main outcomes were progression from moderate to severe or critical condition or death.
Results
Among the 456 enrolled patients with moderate COVID-19, 251/456 (55.0%) had poor prognosis. Multivariate logistic regression analysis identified higher neutrophil count: lymphocyte count ratio (NLR) on admission (OR 1.032, 95% CI 1.042–1.230, p 0.004) and higher C-reactive protein (CRP) on admission (OR 3.017, 95% CI 1.941–4.690, p < 0.001) were associated with increased OR of poor prognosis. The area under the receiver operating characteristic curve (AUC) for NLR and CRP in predicting progression to critical condition was 0.77 (95% CI 0.694–0.846, p < 0.001) and 0.84 (95% CI 0.780–0.905, p < 0.001), with a cut-off value of 2.79 and 25.95 mg/L, respectively. The AUC of NLR and CRP in predicting death was 0.81 (95% CI 0.732–0.878, p < 0.001) and 0.89 (95% CI 0.825–0.946, p < 0.001), with a cut-off value of 3.19 and 33.4 mg/L, respectively.
Conclusions
Higher levels of NLR and CRP at admission were associated with poor prognosis of individuals with moderate COVID-19. NLR and CRP were good predictors of progression to critical condition and death.
Keywords: Coronavirus disease 2019, Moderate, Neutrophil–lymphocyte ratio, Predict, Prognosis
Introduction
As of 19 April 2020, there had been 2 241 359 confirmed cases of coronavirus disease 2019 (COVID-19) worldwide, including 152 551 deaths reported by WHO [1]. The outbreak of COVID-19 has become an international public health emergency [2,3].
The prognosis of individuals with COVID-19 of different severities at admission is significantly different. Most patients with mild or moderate disease who receive basic medical care at Fangcang shelter hospitals, which are large-scale, temporary hospitals rapidly built since 5 February in China, have a better prognosis [4]. Relative to the moderate cases, patients with severe or critical disease have a higher probability of being admitted to intensive care units, have longer stays [5,6] and are more likely to die [7,8].
Identification of which individuals with initially mild or moderate disease will deteriorate into having severe or critical illness is useful, as it would allow for earlier treatment to prevent worsening outcomes and save medical resources for other patients. In this study, we focus on the clinical features and outcomes of patients with moderate COVID-19 treated at a single institution and explore the factors and indicators associated with their prognosis.
Methods
Study design and participants
All adult patients with moderate cases of COVID-19 hospitalized at the Central Hospital of Wuhan from 1 January to 20 March 2020, were enrolled in this retrospective cohort study. This is a tertiary hospital located in the central area of Wuhan, China, and is one of the designated hospitals for treating COVID-19 patients. The data cut-off for this study was 31 March 2020. The flowchart of confirmed patients enrolled in this study is shown in the Supplementary material (Fig. S1). All patients were diagnosed with COVID-19 based on positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) quantitative RT-PCR using throat swab samples, in accordance with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia recommended by the National Health Commission of China (version 7.0) [9]. This study was approved by the Central Hospital of Wuhan Hospital Ethics Committee (No. 2020-75). Written informed consent was waived by the ethics commission of the designated hospital for emerging infectious diseases.
Data collection
Epidemiological, demographic, clinical, laboratory, treatment and outcome data (progression to severe/critical/death) were reviewed and extracted by experienced clinicians from electronic medical records using a standardized data collection form and independently reviewed by two researchers.
Definitions
Fever was defined as an axillary temperature of at least 37.3°C. Disease severity grading (mild, moderate, severe, or critical) of COVID-19 was defined according to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. Mild grade was defined as few symptoms (low fever, fatigue) and without lung CT findings. Moderate grade was defined as fever, respiratory symptoms (dry cough, chest distress and shortness of breath after activities) and lung CT findings (i.e. ground-glass opacity, multiple small patchy shadows and pulmonary consolidation). Severe grade was defined as respiratory frequency ≥30 breaths/min, blood oxygen saturation ≤93%, oxygenation index <300 mmHg and/or lung infiltrates >50% within 24–48 hours. Critical grade was defined as respiratory failure, septic shock and/or multiple organ dysfunction or failure. Poor prognosis refers to progression from moderate to severe grade, critical grade or death.
Statistical analysis
Categorical variables are reported as number (%). Normally distributed continuous data were reported as mean ± standard deviation (SD) and non-normally distributed continuous data were reported as median (interquartile range (IQR)). Categorical data were compared using the χ2 test or Fisher exact test. Independent t tests were used to compare normally distributed continuous data, and the Mann–Whitney U-test or Exact Mann–Whitney rank sum test was used to compare non-normally distributed continuous data. To adjust for the risk factors associated with illness progression in-hospital, univariable and multivariable logistic regression models were used. Considering the total number of prognoses (n = 251) in our study and to avoid overfitting of the model, 12 variables were chosen for multivariable logistic analysis on the basis of univariable logistic analysis results and clinical significance. Multivariable Cox proportional hazards regression analyses were used to further adjust the risk factors associated with survival. Considering the total number of deaths (n = 46) in our study and to avoid overfitting of the model, four variables were chosen for Cox regression analysis on the basis of multivariable logistic analysis results and clinical significance. Receiver operating characteristic (ROC) curves were used to evaluate the potential predictive value of risk factors on prognoses in-hospital. The Hosmer–Lemeshow test was used to calibrate the ROC curves. The Net Reclassification Index (NRI) was used to determine which indicators of ROC curves analysis were better at predicting outcomes, in line with previously published methods [10]. A p value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS (version 19.0) (SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 8.0) (GraphPad, San Diego, CA, USA) software.
Results
Demographics, laboratory, treatment and prognosis characteristics
A total of 456 (100%) moderate cases were recruited in this study (Table 1 ), of which 44.96% (205/456) did not progress and 55.04% (251/456) had poor prognosis in-hospital. Briefly, 33.99% (155/456) of individuals worsened to a severe condition, 10.96% (50/456) of individuals worsened to become critical cases and 9.8% (46/456) of individuals died (Table 2 ). Basic information characteristics are shown in Table 1. The mean patient age was 54.97 years (range 18–99 years), and more than half of patients were female (245/456, 53.73%). Compared with individuals with no progression, individuals with poor prognoses were significantly older and more likely to have co-morbidities.
Table 1.
Demographic, clinical, laboratory findings of patients with moderate coronavirus disease 2019 on admission
| Total (n = 456) | No progression (n = 205) | Poor prognoses (n = 251) | p value | |
|---|---|---|---|---|
| Demographics and clinical characteristics | ||||
| Age (years) | 54.97 ± 18.59 | 48.95 ± 18.17 | 59.89 ± 17.48 | <0.001 |
| ≤45 | 159 (34.88%) | 97 (47.32%) | 62 (24.70%) | |
| 45–65 | 137 (30.04%) | 56 (27.32%) | 81 (32.27%) | |
| ≥65 | 160 (35.08%) | 52 (25.36%) | 108 (43.03%) | |
| Sex | <0.001 | |||
| Male | 211 (46.27%) | 71 (34.63%) | 140 (55.77%) | |
| Female | 245 (53.73%) | 134 (65.37%) | 111 (44.23%) | |
| Systolic pressure (mmHg) | 126.08 ± 23.86 | 126.75 ± 22.75 | 125.54 ± 24.77 | 0.592 |
| Diastolic pressure (mmHg) | 76.09 ± 15.39 | 77.38 ± 13.93 | 75.03 ± 16.44 | 0.104 |
| Co-morbidities | ||||
| Hypertension | 150 (32.89%) | 48 (23.41%) | 102 (40.63%) | <0.001 |
| Diabetes | 70 (15.35%) | 20 (9.76%) | 50 (19.92%) | 0.003 |
| Chronic kidney disease | 19 (4.16%) | 2 (0.97%) | 17 (6.77%) | 0.002 |
| Cardiovascular disease | 52 (11.40%) | 15 (7.32%) | 37 (14.74%) | 0.013 |
| Neural system diseases | 33 (7.23%) | 6 (2.92%) | 27 (10.75%) | 0.001 |
| Pulmonary disease | 18 (3.94%) | 4 (1.95%) | 14 (5.57%) | 0.048 |
| Cancer | 12 (2.63%) | 2 (0.97%) | 10 (3.98%) | 0.046 |
| Signs and symptoms | ||||
| Fever | 297 (65.13%) | 136 (66.34%) | 161 (64.14%) | 0.624 |
| Cough | 241 (52.85%) | 111 (54.14%) | 130 (51.79%) | 0.616 |
| Sputum production | 66 (14.47%) | 28 (13.65%) | 38 (15.13%) | 0.655 |
| Shortness of breath | 102 (22.23%) | 46 (22.24%) | 56 (22.31%) | 0.974 |
| Myalgia or fatigue | 153 (33.55%) | 69 (33.65%) | 85 (33.86%) | 0.963 |
| Diarrhoea | 28 (6.14%) | 10 (4.87%) | 18 (7.17%) | 0.310 |
| Nausea and vomiting | 18 (3.94%) | 7 (3.41%) | 11 (4.38%) | 0.598 |
| Respiratory rate (breaths/min) | 20 (18–20) | 20 (18–20) | 20 (18–21) | 0.448 |
| Oxygen saturation (%) | 98 (96–99) | 98 (97–99) | 96 (97–99) | 0.493 |
| Leucocytes count (× 10⁹/L) | 5.14 (3.95–6.75) | 5.24 (4.03–6.25) | 5.02 (3.90–6.92) | 0.019 |
| <4 | 117 (25.65%) | 46 (22.43%) | 71 (28.28%) | |
| 4–10 | 315 (69.07%) | 149 (72.68%) | 166 (66.13%) | |
| >10 | 24 (5.28%) | 10 (4.89%) | 14 (5.59%) | |
| Neutrophil count (× 10⁹/L) | 3.20 (2.35–4.75) | 3.03 (2.23–4.25) | 3.29 (2.38–5.18) | 0.006 |
| Lymphocyte count (× 10⁹/L) | 1.19 (0.85–1.61) | 1.47 (1.05–1.86) | 1.02 (0.70–1.42) | <0.001 |
| NLR | 2.59 (1.66–4.55) | 2.00 (1.42–3.25) | 3.37 (2.06–5.66) | <0.001 |
| Haemoglobin (g/L) | 126.50 ± 19.58 | 125.38 ± 21.78 | 125.09 ± 20.99 | 0.886 |
| Platelet count ( × 10⁹/L) | 189 (147–246) | 216 (167–255) | 174 (130–231) | <0.001 |
| Albumin (g/L) | 38.89 ± 6.23 | 40.16 ± 5.88 | 37.86 ± 6.33 | <0.001 |
| APTT (seconds) | 28.00 (24.00–21.70) | 26.94 (24.44–30.45) | 29.50 (25.50–33.90) | 0.001 |
| Prothrombin time (seconds) | 11.21 ± 2.74 | 10.85 ± 2.81 | 11.50 ± 2.56 | 0.011 |
| INR | 0.98 (0.93–1.05) | 0.98 (0.92–1.04) | 1.0 (0.94–1.06) | 0.008 |
| D-dimer (μg/L) | 0.52 (0.21–1.31) | 0.4 (0.36–1.04) | 0.66 (0.3–1.66) | <0.001 |
| <1.0 | 314 | 153 | 161 | |
| ≥1.0 | 142 | 52 | 90 | |
| Total bilirubin (mmol/L) | 9.40 (7.1–12.97) | 9.3 (7.2–12.55) | 9.5 (7.0–13.50) | 0.900 |
| Alanine aminotransferase (U/L) | 18.55 (12.5–30.37) | 17.50 (11.5–29.65) | 19.3 (13.4–31.90) | 0.539 |
| Aspartate aminotransferase (U/L) | 20.80 (15.7–29.60) | 18.50 (14.95–23.10) | 24.00 (17.00–36.60) | 0.006 |
| Creatinine (μmol/L) | 66.60 (51.4–79.8) | 61.00 (50.90–73.60) | 70.0 (53.20–85.80) | <0.001 |
| <133 | 436 (95.61%) | 200 (97.56%) | 236 (94.02%) | |
| ≥133 | 20 (4.39%) | 5 (2.44%) | 15 (5.98%) | |
| Potassium (mmol/L) | 3.3 (3.10–4.00) | 3.72 (3.50–4.30) | 3.90 (3.40–4.60) | 0.021 |
| Creatine kinase (U/L) | 63.5 (32.32–111.50) | 52.50 (42.00–100.75) | 74.5 (40.00–139.90) | 0.002 |
| Lactate dehydrogenase (U/L) | 166 (132–213) | 148 (110–180) | 191 (150–237) | <0.001 |
| Procalcitonin (ng/mL) | 0.05 (0.04–0.08) | 0.04 (0.03–0.06) | 0.07 (0.17–0.60) | <0.001 |
| <0.5 | 438 (96.05%) | 204 (99.51%) | 234 (93.22%) | |
| ≥0.5 | 18 (3.95%) | 1 (0.49%) | 17 (6.79%) | |
| C-reactive protein (mg/L) | 0.61 (0.10–3.12) | 0.28 (0.06–1.05) | 2.02 (0.24–4.98) | <0.001 |
| <6.0 | 226 (49.56%) | 141 (68.78%) | 85 (33.86%) | |
| ≥6.0 | 230 (50.44%) | 64 (31.22%) | 166 (66.14%) | |
| Erythrocyte sedimentation rate (mm/h) | 31.00 (14.00–54.75) | 17.00 (10.0–41.25) | 38.00 (13.00–58.5) | <0.001 |
| Interleukin-6 (pg/mL) | 3.83 (1.67–10.41) | 2.43 (1.5–4.99) | 5.94 (2.47–24.07) | <0.001 |
Data are n (%). Normal distributed data are mean ± SD and non-normal distributed data are median (IQR). p values were calculated by Mann–Whitney U-test, χ2 test, or Fisher's exact.
Abbreviations: APTT, activated partial thromboplastin time; INR, international normalized ratio; NLR, neutrophil count/lymphocyte count ratio.
Table 2.
Treatments and outcomes of patients with moderate coronavirus disease 2019
| Total (n = 456) | No progression (n = 205) | Poor prognoses (n = 251) | p value | |
|---|---|---|---|---|
| Treatment | ||||
| Antivirala | 437 (95.83%) | 193 (94.14%) | 244 (97.21%) | 0.103 |
| Antibioticb | 369 (80.92%) | 173 (84.39%) | 196 (78.08%) | 0.088 |
| Glucocorticoids | 226 (49.56%) | 70 (34.14%) | 156 (62.15%) | <0.001 |
| Intravenous immunoglobulin | 145 (31.79%) | 54 (26.31%) | 91 (36.25%) | 0.024 |
| Outcomes | ||||
| Time from illness onset to admission, days | 7 (4.25–14) | 8 (4–14) | 7 (5–11) | 0.135 |
| Severe progression | — | — | 155 (61.75%) | |
| Critical progression | — | — | 50 (19.92%) | |
| Death progression | — | — | 46 (18.33%) | |
Data are n (%). Normal distributed data are mean ± SD and non-normal distributed data are median (IQR).
Antiviral treatments included ribavirin, arbidol and lopinavir/ritonavir.
Antibiotic treatments included cephalosporins and quinolones.
The laboratory data of all moderate cases on admission are shown in Table 1. Numerous variables were significantly associated with outcome, and individuals with poor prognoses generally had lower lymphocyte counts and higher levels of C-reactive protein (CRP) and procalcitonin, and higher neutrophil/lymphocyte ratio (NLR).
Treatment and outcome data are presented in Table 2. As indicated, antiviral treatment (i.e. ribavirin, arbidol and lopinavir/ritonavir) was the most common treatment method for moderate cases (437/456, 95.83%), followed by antibiotic treatment (i.e. cephalosporins and quinolones; 369/456, 80.92%) and glucocorticoid treatment (226/456, 49.56%). Glucocorticoid treatment and intravenous immunoglobulin were more commonly used for individuals with poor prognoses than for those that did not progress. The median time from illness onset to admission was 7 days (IQR 4.25–14 days) in all moderate patients and did not differ significantly between the two groups (p > 0.05).
Risk factors associated with poor prognosis
Table 3 summarizes the results of univariable and multivariable logistic analyses of risk factors associated with progression from moderate to severe or critical condition or death. After adjusting for age, gender, co-morbidities, neutrophil count, lymphocyte count, NLR, CRP and procalcitonin, we found that older age (>45 years) (odds ratio (OR) 1.885, 95% CI 1.094–3.249, p = 0.022), male gender (OR 2.314, 95% CI 1.385–3.287, p < 0.001), higher NLR on admission (OR 1.032, 95% CI 1.042–1.230, p = 0.004) and higher CRP on admission (OR 3.017, 95% CI 1.941–4.690, p < 0.001) were associated with increased OR of poor prognoses. Furthermore, we calculated the OR for the different prognoses in more detail (see Supplementary material, Table S1). Briefly, older age, male gender, NLR and CRP levels at admission >6.0 mg/L were associated with increased OR of severe progression. Male gender, NLR, CRP >6.0 mg/l on admission were associated with increased OR of progression to critical condition. Older age, male gender, NLR, procalcitonin >0.5 ng/mL and CRP >6.0 mg/L on admission were associated with increased OR of death. These results are consistent with our Cox regression analysis (see Supplementary material, Table S2).
Table 3.
Risk factors associated with any in-hospital disease progression
| Univariable regression |
Multivariable regression |
|||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Demographics and clinical characteristics | ||||
| Age (years) | ||||
| ≤45 | 1 (ref) | |||
| 45–65 | 2.263 (1.149–3.608) | 0.001 | 1.885 (1.094–3.249) | 0.022 |
| ≥65 | 3.249 (2.053–5.144) | <0.001 | 2.247 (1.242–4.064) | 0.007 |
| Male (versus female) | 2.380 (1.627–3.483) | <0.001 | 2.134 (1.385–3.287) | 0.001 |
| Hypertension | 0.447 (0.296–0.673) | <0.001 | 0.929 (0.557–1.550) | 0.778 |
| Diabetes | 0.435 (0.249–0.758) | 0.003 | 0.749 (0.392–1.432) | 0.382 |
| Chronic kidney disease | 0.136 (0.031–0.594) | 0.008 | 0.415 (0.078–2.206) | 0.302 |
| Cardiovascular disease | 0.457 (0.243–0.859) | 0.015 | 1.204 (0.554–2.619) | 0.639 |
| Neural system diseases | 0.250 (0.101–0.618) | 0.003 | 0.462 (0.160–1.336) | 0.154 |
| Pulmonary disease | 0.337 (0.109–1.040) | 0.058 | ||
| Cancer | 0.237 (0.051–1.096) | 0.065 | ||
| Laboratory findings | ||||
| Leucocytes count (× 10⁹/L) | ||||
| <4 | 1.012 (0.452–2.691) | 0.830 | ||
| 4–10 | 0.796 (0.343–1.845) | 0.595 | ||
| >10 | 1 (ref) | |||
| Neutrophil count (× 10⁹/L) | 1.169 (1.071–1.276) | <0.001 | 1.097 (0.996–1.208) | 0.062 |
| Lymphocyte count (× 10⁹/L) | 0.443 (0.316–0.620) | <0.001 | 0.789 (0.581–1.072) | 0.129 |
| NLR | 1.251 (1.148–1.362) | <0.001 | 1.132 (1.042–1.230) | 0.004 |
| Platelet count (× 10⁹/L) | 0.997 (0.994–0.999) | 0.002 | ||
| Albumin (g/L) | 0.931 (0.897–0.966) | <0.001 | ||
| APTT (seconds) | 1.031 (1.008–1.055) | 0.008 | ||
| Prothrombin time (seconds) | 1.095 (1.018–1.178) | 0.015 | ||
| INR | 2.840 (1.241–6.50) | 0.013 | ||
| D-dimer (μg/L) | ||||
| <1.0 | 1 (ref) | |||
| ≥1.0 | 1.298 (0.280–6.027) | 0.739 | ||
| Aspartate aminotransferase (U/L) | 1.033 (1.018–1.048) | <0.001 | ||
| Creatinine (μmol/L) | ||||
| <133 | ||||
| ≥133 | 9.330 (0.624–139.57) | 0.106 | ||
| Potassium (mmol/L) | 1.240 (1.128–1.363) | <0.001 | ||
| Creatine kinase (U/L) | 1.005 (1.003–1.008) | <0.001 | ||
| Lactate dehydrogenase (U/L) | 1.008 (1.005–1.010) | <0.001 | ||
| Procalcitonin (ng/mL) | ||||
| <0.5 | 1 (ref) | 1 (ref) | ||
| ≥0.5 | 14.291 (1.955–112.339) | 0.009 | 4.003 (0.442–36.272) | 0.217 |
| C-reactive protein (mg/L) | ||||
| <6.0 | 1 (ref) | 1 (ref) | ||
| ≥6.0 | 4.303 (2.900–6.383) | <0.001 | 3.017 (1.941–4.690) | <0.001 |
| Erythrocyte sedimentation rate (mm/h) | 1.013 (1.005–1.021) | 0.002 | ||
| Interleukin-6 (pg/mL) | 1.035 (1.011–1.059) | 0.004 | ||
Abbreviations: APTT, activated partial thromboplastin time; INR, international normalized ratio; NLR, neutrophil count/lymphocyte count ratio.
Risk factors predicting the prognosis of individuals with moderate COVID-19
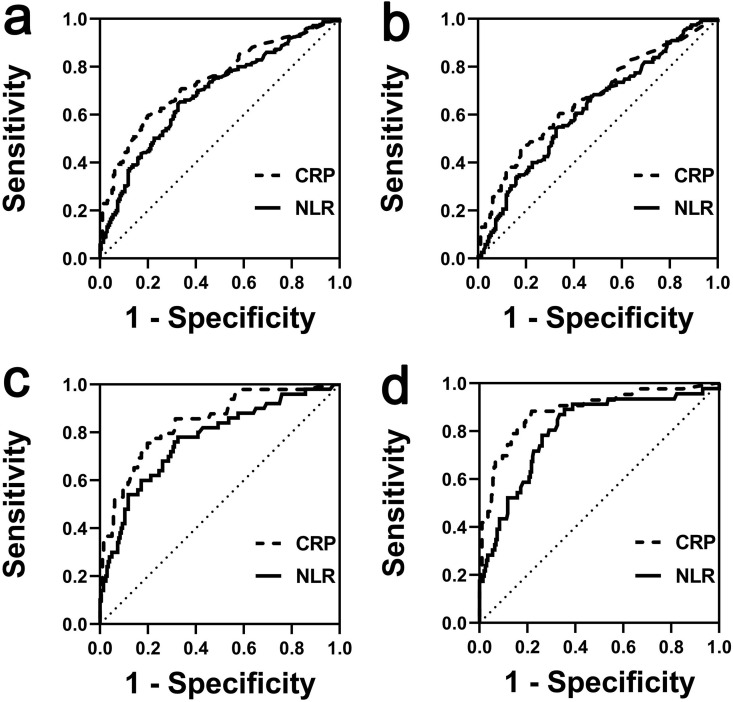
To explore risk factors that can predict the prognosis of individuals with moderate COVID-19, we used ROC curve analysis. The ROC curve of NLR and CRP in predicting the total poor prognoses and severe progression is shown in Figs. 1 a,b. The areas under the curve (AUC) of NLR and CRP in predicting critical progression were 0.77 (95% CI 0.694–0.846, p < 0.001) and 0.84 (95% CI 0.780–0.905, p < 0.001), with cut-off values of 2.79 and 25.95 mg/L, respectively (Fig. 1c). Additionally, the AUC of NLR and CRP in predicting death outcome were 0.81 (95% CI 0.732–0.878, p < 0.001) and 0.89 (95% CI 0.825–0.946, p < 0.001), with cut-off values of 3.19 and 33.4 mg/L, respectively (Fig. 1d). Other exact results of the ROC curve analysis, including sensitivity, specificity, Youden Index, Hosmer–Lemeshow test and NRI, are shown in Table 4 .
Fig. 1.
Receiver operating characteristics (ROC) curves of neutrophil: lymphocyte ratio (NLR) and C-reactive protein (CRP) in patients with moderate coronavirus disease 2019 (COVID-19). (a) ROC curve of NLR and CRP in predicting total poor prognoses; (b) ROC curve of NLR and CRP in predicting severe progression; (c) ROC curve of NLR and CRP in predicting critical progression; (d) ROC curve of NLR and CRP in predicting death. Total poor prognoses, moderate cases progress to severe, critical cases or death.
Table 4.
The parameter results of ROC curve analysis
| AUC (95% CI) | p valuea | Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
Youden index | Cut-off value | p valueb | NRI | |
|---|---|---|---|---|---|---|---|---|
| Prediction for total prognoses | ||||||||
| NLR | 0.69 (0.637–0.734) | <0.001 | 64.54 (0.583–0.706) | 67.49 (0.606–0.739) | 0.32 | 2.59 | 0.035 | — |
| CRP | 0.74 (0.695–0.786) | <0.001 | 61.48 (0.552–0.674) | 78.17 (0.719–0.834) | 0.39 | 10.85 mg/L | 0.009 | — |
| Prediction for severe progression | ||||||||
| NLR | 0.62 (0.565–0.681) | <0.001 | 54.19 (0.463–0.618) | 67.49 (0.608–0.736) | 0.22 | 2.60 | 0.343 | — |
| CRP | 0.67 (0.609–0.725) | <0.001 | 45.39 (0.377–0.533) | 81.73 (0.757–0.865) | 0.28 | 14.15 mg/L | 0.646 | — |
| Prediction for critical progression | ||||||||
| NLR | 0.77 (0.694–0.846) | <0.001 | 76.00 (0.618–0.869) | 68.97 (0.621–0.753) | 0.45 | 2.79 | 0.635 | ref |
| CRP | 0.84 (0.780–0.905) | <0.001 | 78.00 (0.645–0.872) | 67.49 (0.608–0.735) | 0.45 | 25.95 mg/L | 0.134 | 15.52 |
| Prediction for in-hospital death | ||||||||
| NLR | 0.81 (0.732–0.878) | <0.001 | 78.26 (0.637–0.891) | 73.89 (0.673–0.798) | 0.52 | 3.16 | 0.059 | ref |
| CRP | 0.89 (0.825–0.946) | <0.001 | 67.44 (0.525–0.7951) | 0.929 (0.884–0.957) | 0.60 | 33.40 mg/L | 0.121 | 31.75 |
| PCT | 0.89 (0.835–0.962) | <0.001 | 73.81 (0.589–0.847) | 93.62 (0.892–0.963) | 0.67 | 0.85 ng/mL | <0.001 | — |
Abbreviations: AUC, area under the curve; CRP, C-reactive protein; NLR, neutrophil count/lymphocyte count ratio; NRI, net reclassification index.
p value for ROC curve.
p value for Hosmer–Lemeshow test.
Discussion
In this retrospective study, the major symptoms of moderate COVID-19 were fever and cough and these symptoms did not differ between the two outcome groups (Table 1). Therefore, predicting prognosis based on symptoms was not possible. Using comparative and multivariable analyses of basic patient characteristics, we found that co-morbidities in moderate cases are not a risk factor for poor prognosis, which is consistent with recent studies [11]. However, older age, male gender, NLR and CRP levels on admission were significantly associated with poor prognoses in individuals with moderate COVID-19.
In our study, the AUC of both NLR and CRP in predicting progression to critical condition and death was >0.75 (Table 4), which suggests that NLR and CRP may act as predictors of progression. Compared with NLR, the NRI of CRP was >0 in predicting progression to critical condition and death, indicating that CRP is a better predictor, which is consistent with the AUC results. Additionally, although the AUC of procalcitonin in predicting death was also >0.75, the p value of the ROC curve of the Hosmer–Lemeshow test for procalcitonin was <0.001 (Table 4), which suggests poor calibration of the ROC curve. Hence, the difference between the predicted value and the true value cannot be explained by chance. Hence, these results indicate that procalcitonin is not a good predictor of death in individuals with moderate COVID-19 in our study.
Additionally, multivariable logistic analysis revealed that treatments using antibiotics, intravenous immunoglobulin and glucocorticoids were not associated with prognosis (see Supplementary material, Table S3), suggesting that these medications did not improve prognosis when given to individuals with moderate COVID-19. As most COVID-19 cases are mild or moderate and medical resources are limited, these findings are clinically significant for taking appropriate treatment options and using medical resources in a cost-effective way. However, randomized controlled trials are required to confirm the impact of drug treatment on individuals with moderate COVID-19.
There are several limitations of the study. First, this is a single centre, retrospective study. Second, most individuals with moderate COVID-19 that were enrolled in this study were older and had multiple co-morbidities, so were more likely to have adverse outcomes. Hence, the rate of disease progression in our study may not reflect the true rate.
In conclusion, age, gender, NLR and CRP levels at admission are associated with poor prognoses of patients with moderate COVID-19. NLR and CRP levels on admission tend to be good predictors of critical progression and death.
Contributors
BC contributed to writing and editing; JH contributed to writing and checking data; XZ contributed to formal analysis and software; JC contributed to data curation and resources; XL contributed to methodology; YC contributed to formal analysis; GY contributed to conceptualization and supervision; XS contributed to conceptualization and checking data; and AD contributed to supervision and editing. All authors contributed to the review and revision of the manuscript and have read and approved the final version.
Funding
This work was supported by the Natural Science Foundation of Hubei Province of China (2019CFA426).
Transparency declaration
The authors declare that they have no conflicts of interest.
Acknowledgements
None.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.06.033.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization Coronavirus disease (COVID-19) outbreak situation. 2020. https://who.sprinklr.com/ Available from:
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S., Zhang Z., Yang J., Wang J., Zhai X., Barnighausen T. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020 doi: 10.1016/S0140-6736(20)30744-3. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.004. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L., He W., Yu X., Hu D., Bao M., Liu H. Coronavirus Disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.019. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National health Commission of the People's Republic of China Diagnosis and treatment protocol for novel coronavirus pneumonia recommended. 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml Available from:
- 10.Alba A.C., Agoritsas T., Walsh M., Hanna S., Iorio A., Devereaux P.J. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA. 2017;318:1377–1384. doi: 10.1001/jama.2017.12126. [DOI] [PubMed] [Google Scholar]
- 11.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.