Graphical abstract
Keywords: COVID-19, Coronavirus, Protease inhibitors, Peptidomimetics, Antivirals
Abstract
The unprecedented pandemic of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is threatening global health. The virus emerged in late 2019 and can cause a severe disease associated with significant mortality. Several vaccine development and drug discovery campaigns are underway. The SARS-CoV-2 main protease is considered a promising drug target, as it is dissimilar to human proteases. Sequence and structure of the main protease are closely related to those from other betacoronaviruses, facilitating drug discovery attempts based on previous lead compounds. Covalently binding peptidomimetics and small molecules are investigated. Various compounds show antiviral activity in infected human cells.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the ongoing COVID-19 (coronavirus disease 2019) pandemic. Globally, 10 million infections have been confirmed with 500,000 fatalities.1 The novel coronavirus had first been reported in Hubei province of China in late 2019, where it caused a major cluster of atypical pneumonia.2, 3, 4 Despite major efforts to contain the original outbreak, SARS-CoV-2 has since spread worldwide.5, 6, 7, 8 Following the 2002/2003 SARS and 2012 MERS (Middle East respiratory syndrome) epidemics, this marks the third notable coronavirus outbreak in the 21st century.6, 9
Four additional coronaviruses can infect humans, HCoV-229E, HCoV-HKU1, HCoV-NL63 and HCoV-OC43,10 which in stark contrast to the highly contagious and pathogenic SARS-CoV, MERS-CoV and SARS-CoV-2,7, 11, 12 cause only mild respiratory illness like the common cold.13 The case fatality rate (CFR) of COVID-19 is estimated to be lower than for SARS (~10%) and MERS (~35%). However, its basic reproduction number (R 0) is potentially higher than for SARS (~2–3) and MERS (<1). The values for CFR and R 0 of SARS-CoV-2 are still under controversial debate.14, 15 Undetected and asymptomatic infections can challenge the accuracy of these parameters.16, 17, 18, 19 The ongoing COVID-19 pandemic has had an unprecedented impact on individuals and the economy, as travel restrictions, social distancing and quarantine measures were implemented by many countries.20., 21
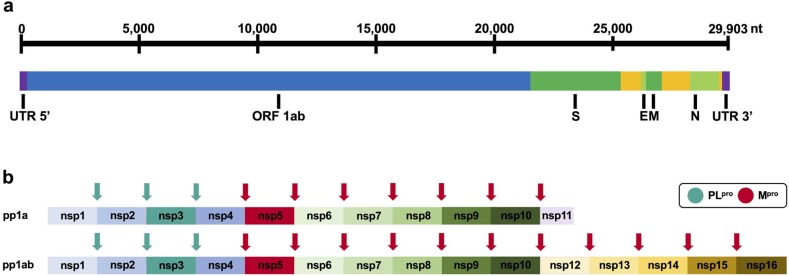
SARS-CoV, MERS-CoV and SARS-CoV-2 belong to the family of Coronaviridae and the genus Betacoronavirus. Coronaviruses are enveloped, positive-sense, single-stranded RNA viruses that feature the largest known RNA virus genomes ranging approximately from 26 to 32 kb,22, 23, 24., 25, 26 containing at least 6 (14 in case of SARS-CoV-2) open reading frames (ORFs).27, 28 The major reading frame ORF 1ab encodes for two overlapping polyproteins (pp1a, pp1ab), which are cleaved into 16 non-structural proteins (nsp1-16) by the main protease Mpro (also referred to as 3CLpro) and the papain-like protease PLpro (Fig. 1 ).27, 29, 30, 31, 32 In addition, the papain-like protease is also a deubiquitinase.33 The remainder of the genome encodes for accessory and structural proteins such as the spike glycoprotein (S), envelope protein (E), membrane protein (M) and the nucleocapsid phosphoprotein (N).27, 29, 30, 31, 32 The first genome sequence of SARS-CoV-2 deposited in Genbank was from the ~30 kb isolate Wuhan-Hu-1 (MN908947),3 which is used for sequence-related analyses in this article.
Fig. 1.
(a) Organisation of the RNA genome of SARS-CoV-2 with selected genes (Wuhan-Hu-1 isolate MN908947). (b) Schematic representation of polyprotein cleavage sites of SARS-CoV-2. The papain-like protease PLpro cleaves at 3 distinct sites. The main protease Mpro (also referred to as 3CLpro) cleaves at 11 distinct sites.
SARS-CoV-2 is closely related to other viruses of the Betacoronavirus genus such as the bat coronavirus BatCoV RaTG13 (~96% sequence identity) and the SARS-CoV (~80% sequence identity).34, 35 SARS-CoV and MERS-CoV are both of zoonotic origin, with bats being their natural reservoir. Transmission to humans can occur via their intermediate hosts palm civets (SARS) and dromedary camels (MERS).36 SARS-CoV-2 is thought to have followed a similar evolutionary transmission cascade.37, 38 The spike glycoprotein (S) plays an important role in host range (tropism) and ‘host jumps’.39, 40 In the case of SARS-CoV and SARS-CoV-2, it recognises the receptor angiotensin-converting enzyme 2 (ACE 2), which both viruses employ for cell entry.41, 42, 43 Comparisons of structural proteins of SARS-CoV-2, such as the spike protein (S), with those from animal coronaviruses indicate the involvement of bats as natural reservoir with a possibility for pangolins as intermediate hosts.38, 44, 45, 46, 47, 48, 49 As the introduction of coronaviruses into human population has been observed on multiple occasions, a better understanding of the naturally circulating viruses is of high interest for pandemic prevention as is antiviral research to prepare for future outbreaks.50, 51
The main protease as drug target
The current COVID-19 pandemic has triggered global efforts for the rapid identification of vaccines and specific antiviral treatments.52, 53, 54, 55 Amongst the coronaviral targets that have been studied in the past, the main protease (Mpro, 3CLpro, nsp5) received major attention,25, 56 particularly following the first SARS-CoV outbreak in the early 2000s.23, 57 Alternative coronaviral targets include the spike protein (S), RNA-dependent RNA-polymerase (RdRp, nsp12), NTPase/helicase (nsp13) and papain-like protease (PLpro, part of nsp3).50, 58 The papain-like protease also recognises the C-terminal sequence of ubiquitin. Therefore, substrate-derived inhibitors of PLpro would be expected to also inhibit host-cell deubiquitinases, making drug-discovery campaigns against PLpro challenging. In stark contrast, the main protease Mpro exclusively cleaves polypeptide sequences after a glutamine residue, positioning the main protease as an ideal drug target because, to the best of our knowledge, no human host-cell proteases are known with this substrate specificity.59, 60, 61
Viral proteases are well validated drug targets that have led to various approved drugs, for example, against chronic infections with human immunodeficiency virus (HIV) or hepatitis C virus (HCV), which employ aspartyl and serine proteases, respectively.62 The SARS-CoV-2 Mpro proteolytically cleaves the overlapping pp1a and pp1ab polyproteins to functional proteins (Fig. 1), which is a critical step during viral replication.29, 63, 64 Replication-essential enzymes such as RdRp or nsp13 cannot fully function without prior proteolytic release,56 positioning Mpro as a key enzyme in the viral replication cycle. Consequently, its inhibition can stall the production of infectious viral particles and thus alleviate disease symptoms.23, 50, 65, 66, 67, 68 Capitalising on knowledge gained on structure and inhibitors of Mpro from previous epidemical coronaviruses, Mpro is one of the most attractive viral targets for antiviral drug discovery against SARS-CoV-2.
Structure and function of the main protease
Early homology models of SARS-CoV-2 Mpro indicated close structural relation to other coronaviral main proteases.69 Amino acid sequence alignments reveal ~99% identity with BatCoV RaTG13 Mpro and ~96% with the previous SARS-CoV Mpro. In contrast, sequence identity with MERS-CoV Mpro is only ~50% (Fig. 2 ).
Fig. 2.
Alignment of the amino acid sequences of crystallised main proteases of SARS-CoV-2 (PDB: 6Y2E), SARS-CoV (PDB: 2BX4) and MERS-CoV (PDB: 5C3N). Domains I, II and III comprise residues 8–101, 102–184 and 201–306, respectively. The catalytic dyads are indicated by asterisks. The alignment was generated using T-Coffee and shaded with Boxshade.
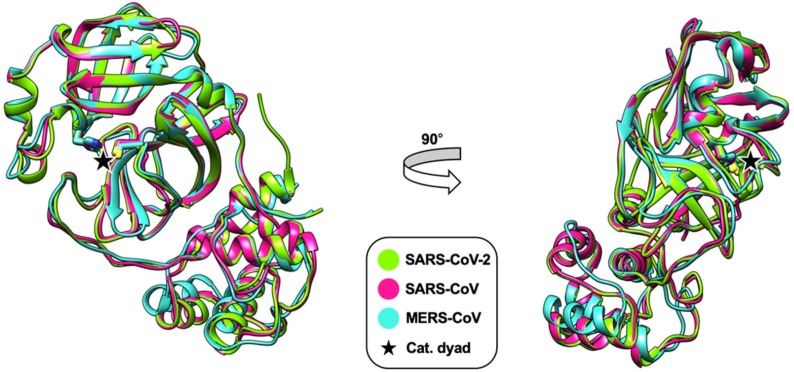
Superimposition of the X-ray crystal structures of the main proteases of SARS-CoV-2, SARS-CoV and MERS-CoV indicates a high degree of structural similarity and conservation of the active site (Fig. 3 ). This might prove valuable for the development of pan-coronaviral drugs and has already been employed for the development of SARS-CoV-2 Mpro inhibitors that were based on previous compounds targeting the SARS-CoV or MERS-CoV main proteases.
Fig. 3.
Superimposition of X-ray crystal structures of the main proteases of SARS-CoV (pink, PDB: 2BX4), MERS-CoV (cyan, PDB: 5C3N) and SARS-CoV-2 (green, PDB: 6Y2E). Only the monomers are shown. Residues of the catalytic dyad are indicated (His41/Cys145 for SARS-CoV and SARS-CoV-2 and His41/Cys148 for MERS-CoV). The root-mean-square deviation (RMSD) of the superimpositions is 0.934 Å for SARS-CoV/MERS-CoV, 0.532 Å for SARS-CoV/SARS-CoV-2 and 0.905 Å for MERS-CoV/SARS-CoV-2. This figure was generated with UCSF Chimera.70.
Mpro is a cysteine protease with a catalytic dyad (cysteine and histidine) in its active centre (Fig. 3). While other cysteine and serine proteases contain a third catalytic residue, a buried water molecule occupies this place in the active site of Mpro.23, 25, 71 The proteolytic process is believed to follow a multi-step mechanism. After the cysteine side chain proton is abstracted by the histidine’s imidazole, the resulting thiolate nucleophile attacks the amide bond of the substrate. The N-terminal peptide product is released by proton abstraction from histidine before the thioester is hydrolysed to release the C-terminal product and restore the catalytic dyad.72, 73
Mpro (nsp5) autocleaves itself between nsp4 and nsp6,74, 75 before processing the overlapping polyproteins pp1a and pp1ab at 11 cleavage sites (Fig. 1).29, 63, 64 While the Mpro monomer is basically inactive, the homodimer is the primary active species with both protomers almost orthogonally aligned to each other (Fig. 4 a).68, 72 Each protomer consists of three domains (Fig. 4b). In case of SARS-CoV and SARS-CoV-2, domains I and II comprise residues 8–101 and 102–184, respectively, and include an antiparallel β-barrel with similarities to trypsin-like serine proteases.57 Domain II is connected to Domain III (residues 201–306) via a longer loop region (residues 185–200). Domain III is characterised by a cluster of five α-helices.57, 59, 76, 77, 78, 79
Fig. 4.
X-ray crystal structure of the Mpro homodimer of SARS-CoV-2 (PDB: 6Y2E). Residues of the catalytic dyad (His41/Cys145) are indicated. (a) Protomers are indicated. (b) Protomer domains are indicated. This figure has been generated with UCSF Chimera.70.
The protomers bind to each other via an N-terminal finger (residues 1–7) located between domains II and III, which is involved in the formation of the substrate-binding site in a cleft between domains I and II.57, 59, 76, 77, 78 It is known that the mutations Ser284Ala, Thr285Ala and Ile286Ala in SARS-CoV Mpro result in a 3.6-fold increase in catalytic activity.80 Two similar mutations (Thr285Ala and Ile286Leu) are present in SARS-CoV-2 Mpro, potentially explaining higher activity observed for SARS-CoV-2 Mpro compared to SARS-CoV Mpro.59 The Thr285Ala mutation is believed to bring domain III of both protomers closer to each other.59, 79
Substrate specificity and inhibitor design
According to the nomenclature introduced by Schechter and Berger,81 Mpro mainly recognises substrate residues ranging from P 4 to P 1′.82 Prime site recognition beyond P 1′ is not conserved (Fig. 5 ). Specificity is mostly determined by P 1, P 2 and P 1′, which show the highest degree of conservation amongst the cleavage sites.63 Glutamine in P 1 is highly conserved in all polyprotein cleavage sites of SARS-CoV, MERS-CoV and SARS-CoV-2 (Fig. 5). In P 2 more hydrophobic amino acids are tolerated with a clear preference for leucine. P 1′ tolerates small residues like serine or alanine.56, 60, 61, 83, 84, 85 Analysis of all polyprotein cleavage sites processed by Mpro for SARS-CoV, MERS-CoV and SARS-CoV-2 illustrates very similar substrate recognition profiles amongst these viruses (Fig. 5). Particularly important is the pronounced preference for glutamine in P 1, strongly informing inhibitor design.84 Since no human host-cell proteases with similar specificity are reported, reduced off-target effects are assumed for peptidomimetic inhibitors.60, 61
Fig. 5.
Polyprotein cleavage sites recognised by Mpro of SARS-CoV-2, SARS-CoV and MERS-CoV. Peptide sequences cover residues P5 to P5′ according to the nomenclature of Schechter and Berger.81 Data were generated from pp1ab polyprotein sequences reported in the UniProt database with the accession codes P0DTD1 (SARS-CoV-2), P0C6X7 (SARS-CoV) and K9N7C7 (MERS-CoV). The consensus sequence over all cleavage sites was plotted using WebLogo.86.
The catalytic dyad of Mpro is located in a cleft between domains I and II (Fig. 4b).23, 71 Despite the minor mutation S46A in close proximity, the active sites of SARS-CoV and SARS-CoV-2 Mpro are highly conserved. The influence of the S46A mutation on shape, size, flexibility and plasticity of the active site and its relevance for inhibitor design is under debate.87, 88
As the monomer of Mpro is principally regarded as inactive, the dimerization interface offers an alternative target site for drug discovery.68, 72 Although strong dimerization inhibitors are yet unavailable, the principle has been proven with the N-terminal octapeptide of SARS-CoV Mpro.89
Inhibitors
Inhibitors of SARS-CoV Mpro have been reviewed comprehensively by Pillaiyar et al. in 2016.72 MERS-CoV inhibitors have been reviewed by Liang et al. in 2018.90 Peptidomimetics and small molecules have been reported with affinities in the micro- to nanomolar range. They often depend on warhead-based design strategies, employing different reactive groups to covalently attack the catalytic cysteine residue. Warheads utilised include Michael acceptors, aldehydes, epoxy ketones and other ketones.72
Although SARS-CoV-2 emerged only very recently, several inhibitors have already been identified and successfully co-crystallised with Mpro (Fig. 6 ).59, 79, 91 They are often derived from previous campaigns which targeted the main proteases of SARS-CoV or MERS-CoV and contain cysteine-reactive warheads (Fig. 6).
Fig. 6.
X-ray co-crystal structures of SARS-CoV-2 Mpro with covalently binding peptidomimetic inhibitors. Mpro is shown as hydrophobicity surface (red indicating hydrophobic and blue hydrophilic surface areas) and grey ribbon with amino acid side chains (UCSF Chimera).70 Inhibitor groups binding to protease subsites are indicated according to the Schechter Berger nomenclature.81 Electrophilic warheads covalently binding to Cys145 are circled. (a) Compound 3 with an α-ketoamide warhead (PDB: 6Y2F). (b) Compound 4 with a Michael acceptor warhead (PDB: 7BQY). (c) Compound 5 with a C-terminal aldehyde warhead (PDB: 6LZE).
The first reported inhibitors were covalently binding peptidomimetics (1–3) addressing the major substrate-recognition motif from P 1′ to P 3.59 They all comprise an α-ketoamide functionality that forms a hemithioacetal with Cys145. α-Ketoamides are already used as viral serine protease warheads in the approved HCV drugs telaprevir and boceprevir.62 Compound 1 has previously been investigated as a broad-spectrum corona- and enteroviral protease inhibitor.60 Like many other Mpro inhibitors, the P 1 side chains of 1–3 employ a γ-lactam as a glutamine mimetic. P 2 comprises hydrophobic cyclohexyl (1, 2) or smaller cyclopropyl (3) groups as leucine mimetics and P 1′ contains cyclopropyl (2) or benzyl (1, 3) residues. Compounds 2 and 3 feature pyridone rings between P 2 and P 3 as well as N-terminal Boc groups, which were associated with increased plasma half-life, kinetic solubility and thermodynamic solubility. Pharmacokinetic profiling of 2 and 3 in mice also indicated favourable lung tropism. Compounds 1 and 3 displayed sub-micromolar Mpro inhibition (Fig. 7 ). Compound 3 is similarly active against the SARS-CoV and MERS-CoV main proteases and inhibits SARS-CoV-2 replication in human Calu3 lung cells.
Fig. 7.
Inhibitors of the SARS-CoV-2 main protease Mpro. IC50 indicates enzymatic inhibition. EC50 indicates antiviral activity in cells.
Compound 4 is another peptidomimetic Mpro inhibitor co-crystallised in complex with SARS-CoV-2 Mpro (Fig. 6b).79 It originated from previous campaigns targeting SARS-CoV Mpro.92 Its Michael acceptor irreversibly modifies Cys145. Compound 4 shows anti-SARS-CoV-2 activity in Vero cells.
Compounds 5 and 6 are the strongest known SARS-CoV-2 Mpro inhibitors with inhibition constants in the two-digit nanomolar range. These small peptidomimetics feature an indole moiety at the N-terminus (P 3) and a C-terminal aldehyde warhead which binds covalently to Cys145, as proven by X-ray crystallography (Fig. 6c).91 Similar peptide-aldehydes have previously been explored as inhibitors of SARS-CoV Mpro.93, 94 Compound 5 and the previously investigated α-ketoamides 1 and 2 are structurally identical in P 1 and P 2; however, 5 inhibits one order of magnitude stronger, which is likely caused by the increased electrophilicity of the aldehyde warhead compared to the more drug-like α-ketoamide. Despite, the high reactivity of the aldehyde function, compounds 5 and 6 displayed sub-micromolar antiviral activity in Vero cells (Fig. 7). In agreement with compound 3, the in vitro activity of 5 and 6 was one order of magnitude weaker than the direct Mpro inhibition in the enzymatic assay. Notably, 5 exhibited only low toxicity in animal models despite its aldehyde warhead.91
A high-throughput screening campaign of a library of approved drugs and clinical candidates revealed six small molecules, ebselen, disulfiram, carmofur, tideglusib, shikonin and PX-12, as inhibitors of SARS-CoV-2 Mpro (Fig. 7).79 Mass spectrometry experiments showed that ebselen, carmofur and PX-12 covalently modify Cys145. Small covalent modifiers like these may bind unspecifically, a characteristic associated with pan-assay interference compounds (PAINS).95 Ebselen showed antiviral activity in Vero cells in the low micromolar range. In case of carmofur, a crystal structure of SARS-CoV-2 Mpro revealed transfer of the hexylurea side chain to Cys145, forming a hexylcarbamothioate interacting with the S2 subsite (PDB: 7BUY).96
Crystal structures of SARS-CoV-2 Mpro in complex with X77 and baicalein (Fig. 7) have been deposited in the protein data bank under the accession codes 6W63 and 6M2N, respectively. Notably, both compounds bind non-covalently to the active site. X77 and derivatives have previously been investigated as low-micromolar inhibitors of SARS-CoV Mpro, where a strong stereochemical bias for the (R) enantiomer of the pyridyl side chain has been observed.97, 98 In the X-ray co-crystal structure of X77, the aforementioned pyridyl side chain acts as a P 1 mimetic. Baicalein is a flavonoid found in Scutellaria baicalensis, a plant used in traditional Chinese medicine.99 Several flavonoids and derivatives had previously been reported to inhibit the activity of SARS-CoV Mpro.72, 100
A fragment screening has produced several crystal structures of fragments bound to SARS-CoV-2 Mpro, including covalent modifiers of Cys145.101 While the majority of fragments bind to the active site, some bind near the dimer interface of SARS-CoV-2 Mpro. These fragments may inform the development of small-molecule inhibitors that are not substrate-derived.
While this manuscript was under peer-review, a study assessing known protease inhibitors for their anti-SARS-CoV-2 Mpro activity was published.102 Amongst the compounds that displayed Mpro inhibition and reduction of cytopathic effect were the α-ketoamides boceprevir (K i = 1.18 μΜ, EC50 = 1.31 μM) and calpain inhibitor XII (K i = 0.13 μΜ, EC50 = 0.49 μM), the peptide-aldehyde calpain inhibitor II (K i = 0.40 μΜ, EC50 = 2.07 μM) and the sulfonate-featured peptide GC-376 (k 2/K i = 40,800 M−1 s−1, EC50 = 3.37 μM). A crystal structure of SARS-CoV-2 Mpro in complex with GC-376 was also reported (PDB: 6WTT).
Conclusion and outlook
The COVID-19 pandemic poses a major challenge to mankind. In view of the magnitude of the current global crisis, numerous attempts to develop vaccines and antiviral treatments are obviously underway. With respect to drug development, the main protease of SARS-CoV-2 stands out as a promising viral target, as it differs significantly from human proteases. Given the conserved structure and specificity of Mpro amongst SARS-CoV, MERS-CoV and SARS-CoV-2, pan-coronaviral main protease inhibitors might become available. However, in line with previously successful examples like HIV or HCV, the development of novel specific protease inhibitors and their approval will take several years. Although this process will likely take too long to impact on the current COVID-19 crisis, protease inhibitors would be worth pursuing as they may provide specific drugs for upcoming coronavirus outbreaks.
Pharmacodynamic and pharmacokinetic properties of peptidomimetic Mpro inhibitors like 2, 3, 5 or 6 already point into the right direction.59, 91 Although peptide-aldehydes have entered clinical trials before (e.g. efegatran),103, 104 5 and 6 are likely to face challenges during further drug development. The α-ketoamide in 1–3 or Michael acceptor in 4 are covalent modifiers with precedents in approved drugs (e.g. telaprevir or afatinib).62, 105 Potential problems associated with limited drug-likeness of peptidomimetics could be circumvented by pursuing alternative small molecules, which might, for example, be accessible from fragment-based drug discovery campaigns.101, 106
Repurposing of known drugs can provide an accelerated route to approval, which is likely the only option to address the current COVID-19 crisis. Small molecules like ebselen or carmofur are Mpro inhibitors with anti-SARS-CoV-2 activity in cells;79 however, their thiol reactivity might prove challenging. Repurposing approved protease inhibitors is an alternative approach.102 Attempts to repurpose the approved combination of HIV protease inhibitors, ritonavir and lopinavir, was unsuccessful in clinical studies, which is not entirely unexpected, given the differences between the proteases of HIV and SARS-CoV-2.107
It is likely that SARS-CoV-2 is not the last human coronavirus emerging from animals. It is therefore important to closely monitor virus populations to understand their replication mechanism early on and investigate druggable targets. A sharp decline in research funding had been noted a few years after the first SARS epidemic.61 Given the long-term nature of drug discovery projects, this has proven disastrous with respect to the current crisis. Now is the best time to progress protease inhibitors to anti-coronaviral drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
C.N. thanks the Australian Research Council for a Discovery Early Career Research Award (DE190100015). S.U. acknowledges a PROMOS scholarship from the German Academic Exchange Service.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.-F.-W., Yuan S., Kok K.-H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y., Peng F., Wang R., et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau H., Khosrawipour V., Kocbach P., et al. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J Travel Med. 2020;27(3):taaa037. doi: 10.1093/jtm/taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Costa V.G., Moreli M.L., Saivish M.V. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch Virol. 2020;165(7):1517–1526. doi: 10.1007/s00705-020-04628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Zheng X., Tong Q., et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92(5):491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie M., Chen Q. Insight into 2019 novel coronavirus - an updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C., Ji F., Wang L., et al. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder-Smith A., Teleman M.D., Heng B.H., Earnest A., Ling A.E., Leo Y.S. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11(7):1142–1145. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Tawfiq J.A., Gautret P. Asymptomatic Middle East respiratory syndrome coronavirus (MERS-CoV) infection: extent and implications for infection control: a systematic review. Travel Med Infect Dis. 2019;27:27–32. doi: 10.1016/j.tmaid.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles' heel of current strategies to control COVID-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilder-Smith A., Freedman D.O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27(2):taaa020. doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khosrawipour V., Lau H., Khosrawipour T., et al. Failure in initial stage containment of global COVID-19 epicenters. J Med Virol. 2020;92:863–867. doi: 10.1002/jmv.25883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Z., Xu Y., Bao L., et al. From SARS to MERS: thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 24.Woo P.C.Y., Huang Y., Lau S.K.P., Yuen K.-Y. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8):1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziebuhr J. Molecular biology of severe acute respiratory syndrome coronavirus. Curr Opin Microbiol. 2004;7(4):412–419. doi: 10.1016/j.mib.2004.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J Gen Virol. 2000;81(4):853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 30.Masters P.S. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziebuhr J., Heusipp G., Siddell S.G. Biosynthesis, purification, and characterization of the human coronavirus 229E 3C-like proteinase. J Virol. 1997;71(5):3992–3997. doi: 10.1128/jvi.71.5.3992-3997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19 doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Báez-Santos Y.M., John St.S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceraolo C., Giorgi F.M. Genomic variance of the 2019-nCoV coronavirus. J Med Virol. 2020;92(5):522–528. doi: 10.1002/jmv.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X., Zai J., Zhao Q., et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020;92:602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV - a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu G., Wang Q., Gao G.F. Bat-to-human: spike features determining 'host jump' of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23(8):468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W., Moore M.J., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease Inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lan J., Ge J., Yu J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y. Unveiling the origin and transmission of 2019-nCoV. Trends Microbiol. 2020;28(4):239–240. doi: 10.1016/j.tim.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nature Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30(7):1346–1351. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y.-Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181(2):223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau S.K.P., Luk H.K.H., Wong A.C.P., et al. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilgenfeld R., Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antiviral Res. 2013;100(1):286–295. doi: 10.1016/j.antiviral.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong G., Bi Y.-H., Wang Q.-H., Chen X.-W., Zhang Z.-G., Yao Y.-G. Zoonotic origins of human coronavirus 2019 (HCoV-19/SARS-CoV-2): why is this work important? Zool Res. 2020;41(3):213–219. doi: 10.24272/j.issn.2095-8137.2020.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang C., Huang S., Zheng F., Dai Y. Controversial treatments: an updated understanding of the coronavirus disease 2019. J Med Virol. 2020 doi: 10.1002/jmv.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lurie N., Saville M., Hatchett R., Halton J. Developing COVID-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 54.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines. 2020;8(2):153. doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiel V., Ivanov K.A., Putics Á., et al. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol. 2003;84(9):2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 57.Yang H., Yang M., Ding Y., et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. PNAS. 2003;100(23):13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C., Liu Y., Yang Y., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L., Lin D., Sun X., et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L., Lin D., Kusov Y., et al. α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J Med Chem. 2020;63(9):4562–4578. doi: 10.1021/acs.jmedchem.9b01828. [DOI] [PubMed] [Google Scholar]
- 61.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281(18):4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agbowuro A.A., Huston W.M., Gamble A.B., Tyndall J.D.A. Proteases and protease inhibitors in infectious diseases. Med Res Rev. 2018;38(4):1295–1331. doi: 10.1002/med.21475. [DOI] [PubMed] [Google Scholar]
- 63.Hegyi A., Ziebuhr J. Conservation of substrate specificities among coronavirus main proteases. J Gen Virol. 2002;83(3):595–599. doi: 10.1099/0022-1317-83-3-595. [DOI] [PubMed] [Google Scholar]
- 64.Du Q.-S., Wang S.-Q., Zhu Y., et al. Polyprotein cleavage mechanism of SARS CoV Mpro and chemical modification of the octapeptide. Peptides. 2004;25(11):1857–1864. doi: 10.1016/j.peptides.2004.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang P.-H. Characterization and inhibition of SARS-coronavirus main protease. Curr Top Med Chem. 2006;6(4):361–376. doi: 10.2174/156802606776287090. [DOI] [PubMed] [Google Scholar]
- 66.Steuber H., Hilgenfeld R. Recent advances in targeting viral proteases for the discovery of novel antivirals. Curr Top Med Chem. 2010;10(3):323–345. doi: 10.2174/156802610790725470. [DOI] [PubMed] [Google Scholar]
- 67.Yang H., Bartlam M., Rao Z. Drug design targeting the main protease, the Achilles' heel of coronaviruses. Curr Pharm Des. 2006;12(35):4573–4990. doi: 10.2174/138161206779010369. [DOI] [PubMed] [Google Scholar]
- 68.Grum-Tokars V., Ratia K., Begaye A., Baker S.C., Mesecar A.D. Evaluating the 3C-like protease activity of SARS-coronavirus: recommendations for standardized assays for drug discovery. Virus Res. 2008;133(1):63–73. doi: 10.1016/j.virusres.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stoermer M. Homology models of coronavirus 2019-nCoV 3CLpro protease. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11637294.v3. [DOI] [Google Scholar]
- 70.Pettersen E.F., Goddard T.D., Huang C.C., et al. UCSF Chimera - a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 71.Ho B.-L., Cheng S.-C., Shi L., Wang T.-Y., Ho K.-I., Chou C.-Y. Critical assessment of the important residues involved in the dimerization and catalysis of MERS coronavirus main protease. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.-H. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem. 2016;59(14):6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang C., Wei P., Fan K., Liu Y., Lai L. 3C-like proteinase from SARS coronavirus catalyzes substrate hydrolysis by a general base mechanism. Biochemistry. 2004;43(15):4568–4574. doi: 10.1021/bi036022q. [DOI] [PubMed] [Google Scholar]
- 74.Xia B., Kang X. Activation and maturation of SARS-CoV main protease. Protein Cell. 2011;2(4):282–290. doi: 10.1007/s13238-011-1034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muramatsu T., Kim Y.-T., Nishii W., Terada T., Shirouzu M., Yokoyama S. Autoprocessing mechanism of severe acute respiratory syndrome coronavirus 3C-like protease (SARS-CoV 3CLpro) from its polyproteins. FEBS J. 2013;280(9):2002–2013. doi: 10.1111/febs.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu M.-F., Kuo C.-J., Chang K.-T., et al. Mechanism of the maturation process of SARS-CoV 3CL protease. J Biol Chem. 2005;280(35):31257–31266. doi: 10.1074/jbc.M502577200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chou C.-Y., Chang H.-C., Hsu W.-C., Lin T.-Z., Lin C.-H., Chang G.-G. Quaternary structure of the severe acute respiratory syndrome (SARS) coronavirus main protease. Biochemistry. 2004;43(47):14958–14970. doi: 10.1021/bi0490237. [DOI] [PubMed] [Google Scholar]
- 78.Ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin Z., Du X., Xu Y., et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 80.Lim L., Shi J., Mu Y., Song J. Dynamically-driven enhancement of the catalytic machinery of the SARS 3C-like protease by the S284–T285-I286/A mutations on the extra domain. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 82.Muramatsu T., Takemoto C., Kim Y.-T., et al. SARS-CoV 3CL protease cleaves its C-terminal autoprocessing site by novel subsite cooperativity. PNAS. 2016;113(46):12997–13002. doi: 10.1073/pnas.1601327113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan K., Ma L., Han X., et al. The substrate specificity of SARS coronavirus 3C-like proteinase. Biochem Biophys Res Commun. 2005;329(3):934–940. doi: 10.1016/j.bbrc.2005.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rut W., Groborz K., Zhang L., et al. Substrate specificity profiling of SARS-CoV-2 Mpro protease provides basis for anti-COVID-19 drug design. BioRxiv. 2020 doi: 10.1101/2020.03.07.981928. [DOI] [Google Scholar]
- 85.Fan K., Wei P., Feng Q., et al. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. J Biol Chem. 2004;279(3):1637–1642. doi: 10.1074/jbc.M310875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crooks G.E., Hon G., Chandonia J.-M., Brenner S.E. WebLogo: a sequence logo generator. Genome Res. 2004;14(6):1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bzówka M., Mitusińska K., Raczyńska A., Samol A., Tuszyński J.A., Góra A. Structural and evolutionary analysis indicate that the SARS-CoV-2 Mpro is a challenging target for small-molecule inhibitor design. Int J Mol Sci. 2020;21(9):3099. doi: 10.3390/ijms21093099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei P., Fan K., Chen H., et al. The N-terminal octapeptide acts as a dimerization inhibitor of SARS coronavirus 3C-like proteinase. Biochem Biophys Res Commun. 2006;339(3):865–872. doi: 10.1016/j.bbrc.2005.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang R., Wang L., Zhang N., et al. Development of small-molecule MERS-CoV inhibitors. Viruses. 2018;10(12):721. doi: 10.3390/v10120721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dai W., Zhang B., Su H., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang H., Xie W., Xue X., et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3(10) doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Gharabli S.I., Shah S.T.A., Weik S., et al. An efficient method for the synthesis of peptide aldehyde libraries employed in the discovery of reversible SARS coronavirus main protease (SARS-CoV Mpro) inhibitors. ChemBioChem. 2006;7(7):1048–1055. doi: 10.1002/cbic.200500533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu L., George S., Schmidt M.F., Al-Gharabli S.I., Rademann J., Hilgenfeld R. Peptide aldehyde inhibitors challenge the substrate specificity of the SARS-coronavirus main protease. Antiviral Res. 2011;92(2):204–212. doi: 10.1016/j.antiviral.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baell J., Walters M.A. Chemistry: chemical con artists foil drug discovery. Nature. 2014;513(7519):481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- 96.Jin Z., Zhao Y., Sun Y., et al. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat Struct Mol Biol. 2020;27(6):529–532. doi: 10.1038/s41594-020-0440-6. [DOI] [PubMed] [Google Scholar]
- 97.Jacobs J., Tokars V., Zhou Y., et al. Discovery, synthesis, and structure-based optimization of a series of N-(tert-butyl)-2-(N-arylamido)-2-(pyridin-3-yl) acetamides (ML188) as potent noncovalent small molecule inhibitors of the severe acute respiratory syndrome coronavirus (SARS-CoV) 3CL protease. J Med Chem. 2013;56(2):534–546. doi: 10.1021/jm301580n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.St. John SE, Mesecar AD. Broad-spectrum non-covalent coronavirus protease inhibitors. US20170313685 United States of America: Purdue Research Foundation 2017.
- 99.Su H., Yao S., Zhao W., et al. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. BioRxiv. 2020 doi: 10.1101/2020.04.13.038687. [DOI] [Google Scholar]
- 100.Jo S., Kim S., Shin D.H., Kim M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Douangamath A., Fearon D., Gehrtz P., et al. Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease. BioRxiv. 2020 doi: 10.1101/2020.05.27.118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma C., Sacco M.D., Hurst B., et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020 doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steinmetzer T., Pilgram O., Wenzel B.M., Wiedemeyer S.J.A. Fibrinolysis inhibitors: potential drugs for the treatment and prevention of bleeding. J Med Chem. 2020;63(4):1445–1472. doi: 10.1021/acs.jmedchem.9b01060. [DOI] [PubMed] [Google Scholar]
- 104.Bengtson J., Adolphson M., Brewer D.L., et al. Multicenter, dose-ranging study of efegatran sulfate versus heparin with thrombolysis for acute myocardial infarction: the promotion of reperfusion in myocardial infarction evolution (PRIME) trial. Am Heart J. 2002;143(1):95–105. doi: 10.1067/mhj.2002.119896. [DOI] [PubMed] [Google Scholar]
- 105.Jackson P.A., Widen J.C., Harki D.A., Brummond K.M. Covalent modifiers: a chemical perspective on the reactivity of α, β-unsaturated carbonyls with thiols via hetero Michael addition reactions. J Med Chem. 2017;60(3):839–885. doi: 10.1021/acs.jmedchem.6b00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmidt M.F., Isidro-Llobet A., Lisurek M., et al. Sensitized detection of inhibitory fragments and iterative development of non-peptidic protease inhibitors by dynamic ligation screening. Angew Chem Int Ed. 2008;47(17):3275–3278. doi: 10.1002/anie.200704594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]