Objective:
In the midst of the severe acute respiratory syndrome coronavirus 2 pandemic, which causes coronavirus disease 2019, there is a recognized need to expand critical care services and beds beyond the traditional boundaries. There is considerable concern that widespread infection will result in a surge of critically ill patients that will overwhelm our present adult ICU capacity. In this setting, one proposal to add “surge capacity” has been the use of PICU beds and physicians to care for these critically ill adults.
Design:
Narrative review/perspective.
Setting:
Not applicable.
Patients:
Not applicable.
Interventions:
None.
Measurements and Main Results:
The virus’s high infectivity and prolonged asymptomatic shedding have resulted in an exponential growth in the number of cases in the United States within the past weeks with many (up to 6%) developing acute respiratory distress syndrome mandating critical care services. Coronavirus disease 2019 critical illness appears to be primarily occurring in adults. Although pediatric intensivists are well versed in the care of acute respiratory distress syndrome from viral pneumonia, the care of differing aged adult populations presents some unique challenges. In this statement, a team of adult and pediatric-trained critical care physicians provides guidance on common “adult” issues that may be encountered in the care of these patients and how they can best be managed in a PICU.
Conclusions:
This concise scientific statement includes references to the most recent and relevant guidelines and clinical trials that shape management decisions. The intention is to assist PICUs and intensivists in rapidly preparing for care of adult coronavirus disease 2019 patients should the need arise.
Keywords: adult critical care, adults in pediatric intensive care unit, coronavirus disease 2019
The worldwide pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 has already resulted in critical care demands overwhelming resources in nations such as Italy (1). This has stressed local healthcare systems requiring new approaches for triage and acute care. With significant resource limitations, especially in differing geographic locales, this pandemic may exhaust existing capacity making it difficult to maintain adequate critical care necessitating adaptations.
Fortunately, COVID-19 disease has been uncommon in children with a reported mean age for most ICU patients between 65 and 70 years (2–9). Many of these patients have comorbidities such as hypertension, type 2 diabetes, coronary vascular disease, cerebrovascular events, and chronic obstructive pulmonary disease (COPD). Patients commonly present on day 5–7 of illness with acute hypoxemic respiratory failure (2, 4, 5, 7, 9) and the frequent ICU complications include shock (30%), acute myocardial injury (22.2%), arrhythmia (44.4%), and acute kidney injury (AKI) (8.3%) (5).
Since COVID-19 is less severe in children (10) with nearly all fatalities in adults (11), one proposed strategy is the use of PICUs to provide surge capacity if adult ICUs are overwhelmed (12–15). For instance, in the United States, there are 534,964 acute care hospital beds (general medical and surgical wards, ICU, step-down, and burn beds) but only 68,558 adult ICU beds; thus use of 5,137 PICU beds may be needed for adult care (6, 15–18).This report aims to prepare PICUs to manage critically ill adults with COVID respiratory failure drawing on the experience of combined adult and pediatric critical care experts while providing confidence that many of these principles are fluent to pediatric intensivists..
GENERAL PRINCIPLES FOR ADULT CRITICAL CARE IN THE PICU
The A, B, C, Ds, and E of Caring for Adults in a PICU
Recent data from New York City shows ~20% of hospitalized patients are between the ages of 20–44 years (19, 20). Some of the complexity of managing adults in a PICU stem from greater comorbidities which can be minimized when selecting for these younger patients at triage. Before making the transition to care for adults in a PICU, one should consider the A, B, C, Ds, and E to assure these patients are cared for safely (21).
“A” represents Accreditation and licensure. Some jurisdictions require notification of changes in ICU bed numbers or patient type. The PICU’s admission, discharge, and transfer criteria also need to be updated prior to accepting the first adult patient.
“B” represents Barriers obstructing the acceptance of adult patients such as space, equipment, supplies, staffing, skill mix, and medications. A multidisciplinary team can usually identify these barriers and mitigate them.
“C” represents Competency. Assuring a competent staff, comfortable in caring for the adult patients and families is essential. Just-in-time education and in-service training with unit-based educators and consultants can assist in addressing competency.
The four “D”s of patient safety for children include Developmental Stage, Differential Epidemiology, Dependence, and Demographic Patterns (21). Development is a natural competency for pediatric providers. Younger adults versus elderly represent distinct developmental stages. Differential epidemiology in adults translates to greater propensity for age-related conditions that are rare in children, like brain or cardiac ischemia. Adults are less “dependent” for care and follow-up than pediatric patients are, but Demographics including the adverse effects of the social determinants of health like poverty, insurance gaps, housing insecurity, and malnutrition which in adults have differing resources for assistance.
Finally, Expectations and Outcomes need to be monitored to assure that the structural and process changes do not result in inadvertent outcomes.
When taken together, the A, B, C, Ds, and E can provide a roadmap for integrating the care of adult patients into the PICU.
Differences Between Adults and Pediatric Advanced Life Support
Cardiopulmonary resuscitation (CPR) and life support algorithms for adults are deliberately similar to pediatric patients. Identical approaches should be taken toward both ventricular fibrillation/pulseless ventricular tachycardia and asystole/pulseless electrical activity (22). The advanced cardiac life support algorithm for symptomatic bradycardia does not include CPR and uses atropine IV (0.5 mg every 3–5 min, maximum 3 mg) as a first-line agent followed by early consideration of epinephrine or dopamine infusions and transcutaneous pacing (23). Tachycardia with hemodynamic instability due to regular rhythms (e.g., atrial flutter) requires synchronized cardioversion with 50–100 J while irregular rhythms (e.g., atrial fibrillation [AF]) require 120–200 J. CPR on adults is similar to pediatrics: push hard (although deeper, > 2 inches), fast (100–120 per min), and allow complete recoil (23). Advanced directives and patient prognosis in determining code status should be considered at PICU admission in every patient and in some cases, the team may determine to limit resuscitation.
Equipment and Supplies
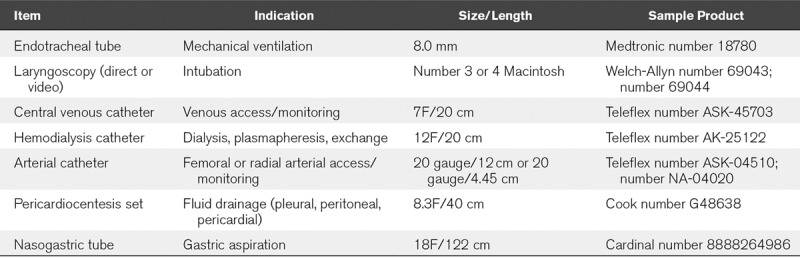
PICU epidemiology favors smaller sizes/heights thus deficits in supplies tend to occur when dealing with taller (> 180 cm) and heavier (> 100 kg) adults. Table 1 provides a list of commonly used supplies to consider for these larger individuals. Central venous catheters for vascular access or dialysis placed in the right internal jugular or subclavian often require ~15–16 cm length which most PICUs stock. We recommend adding 20 cm catheters that are better for adult left sided upper body and femoral approaches.
TABLE 1.
Commonly Used Equipment and Supplies for Larger Adults
Common Adult Consultant Services
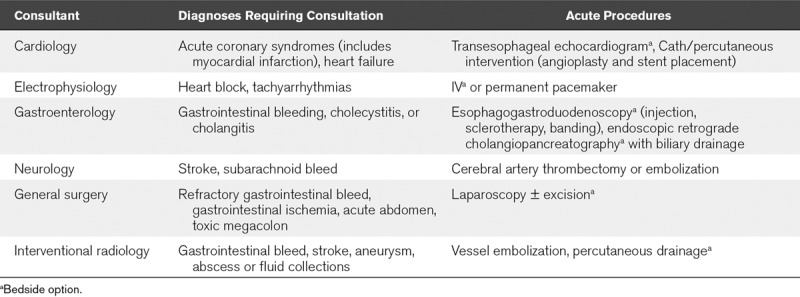
As the COVID pandemic has driven use of telecommunications in lieu of in person meetings, it is our anticipation that most PICUs will have access to a full suite of adult physician consult services. In Table 2, we outline the most likely needed consultations for acute COVID-19 issues. We include procedures which may be performed in the acute setting by the consultant (Table 2), purposely omitting those which do not offer therapeutic potential and thus may be deferred. Likewise, we omit consultative services where pediatric specialists can provide support, or the entire consultation may be performed by telecommunication. For procedures, the consulting physicians and PICU team will need to determine whether the services can be safely rendered within the pediatric facility or require transport to an adult hospital. Many procedures are now feasible at the bedside in adult hospitals such that a similar approach would appear to be less problematic than transport of a highly infectious and critically ill COVID patient across centers.
TABLE 2.
Common Acute Consultations and Procedures Required in Critically Ill Adults
Common Adult Procedural Modifications
Procedures in adult ICUs are similar; therefore, we highlight differences in preparation and execution below and necessary equipment and supplies in Table 1. Endotracheal intubation is generally performed with a Macintosh 3 or 4 blade placing a 7–8.0 mm-cuffed tube. Morbidly obese adults are often best preoxygenated in a reverse Trendelenburg position with the head of bed elevated to drop abdominal weight off the chest. With COVID-19, we recommend the use of video laryngoscopy for rapid sequence intubation (RSI) by the most experienced operator (24) to maximize success and prevent aerosols. Central venous catheters are placed infrequently in the femoral position due to heightened risk of deep venous thrombosis and infection (25). Arterial catheters are used more frequently than in the PICU and often employ a preloaded needle/wire introducer kit. Thoracentesis and lumbar puncture (LP) in a cooperative adult may have more success with the patient sitting upright and from behind with ultrasound guidance. Obese patients often require longer needle lengths than the standard 8.8 cm (3.5 in) for LP (26) with various lengths up to 18 cm available. The length needed can be estimated in cm as 0.077 × body mass index + 0.88 (27).
Adult Multiple Medications at Admission
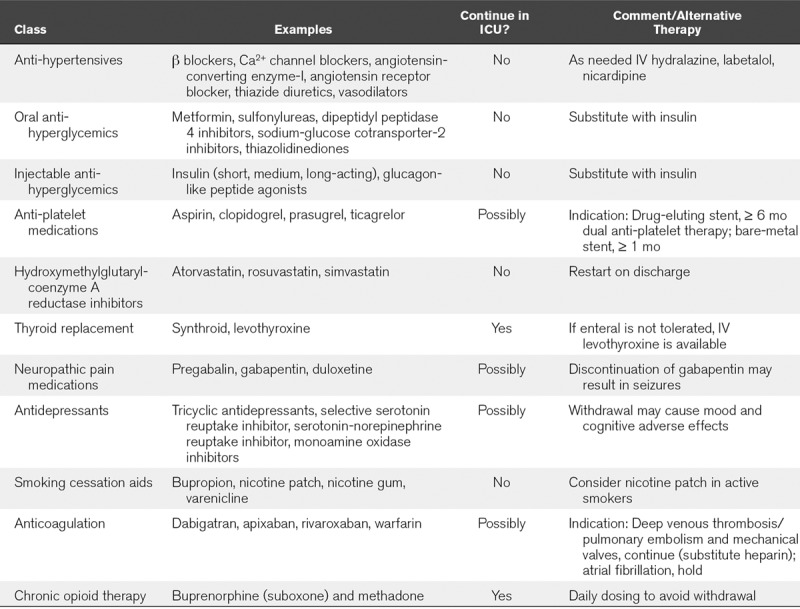
Table 3 provides a list of commonly prescribed medications for adults which may not be commonly stocked in pediatric centers (at least not in large supply) as well as recommendations on whether continuation is critical and whether substitutions can be made with agents more often found in a pediatric formulary.
TABLE 3.
Frequently Encountered Medications in the Adult Population
DISTINCT FEATURES OF RESPIRATORY SUPPORT FOR ACUTE HYPOXEMIC RESPIRATORY FAILURE
Noninvasive Support
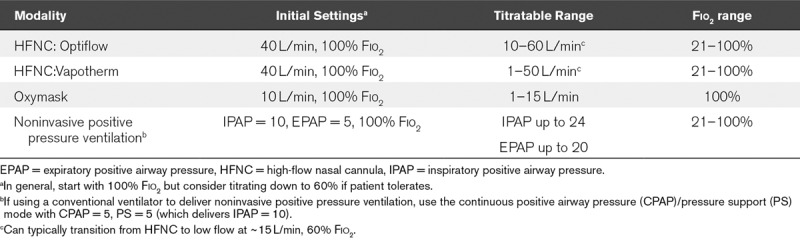
Escalation of respiratory support in adults generally includes nonrebreather mask, venturi or oxymask, high-flow nasal cannula (HFNC), or noninvasive positive pressure ventilation (NIPPV) (28–32). With COVID-19, there is concern for generating infectious aerosols in when using HFNC and NIPPV such that some institutions are avoiding greater than 6 L flow (33), although recent Society of Critical Care Medicine/European Society of Intensive Care Medicine guidelines include both modalities (24). This risk is minimal with good cannula or mask fit on the patient (33, 34) and with use of protective filters (35). Negative pressure isolation rooms mitigate this concern. Oxymask allows titration of oxygen flow but not titration of the Fio2, whereas HFNC and NIPPV allow Fio2 titration and use with inhaled pulmonary vasodilators (16, 36–38). Commonly used settings are listed in Table 4.
TABLE 4.
Advanced Noninvasive Respiratory Support
Intubation and Mechanical Ventilation Strategies
For COVID-19 patients we recommend RSI in a negative pressure room (24, 33, 35, 39). In RSI, bag-valve-masking is minimized and patients receive an induction agent (Propofol at 1.5–2.5 mg/kg, or etomidate 0.3 mg/kg) “immediately” followed by a neuromuscular blocker (succinylcholine at 1.5 mg/kg, rocuronium at 1.2 mg/kg, or cisatracurium at 0.3 mg/kg) and intubation within a minute. Succinylcholine and propofol use in adults is common and offers the advantage of rapid and favorable intubation conditions with less safety concerns compared with other agents (40).
Mechanically ventilated adults are mostly managed with assist-control ventilation, rather than synchronized intermittent mandatory ventilation based on studies showing improved work of breathing, synchrony, and extubation rates (41). Volume modes, such as volume control (VC) or pressure regulated VC (VC+), permit maintenance of lower tidal volumes (4–8 mL/kg) based on predicted body weight and lower plateau pressures (< 30 cm H2O) in acute respiratory distress syndrome (ARDS) (16, 42). In the absence of ARDS, 10 mL/kg is safe (43). Positive end-expiratory pressure (PEEP) is titrated based on Fio2 using validated protocols (44, 45) to levels higher (18–24 cm H2O at Fio2 = 1) than encountered in pediatrics (46). Our experience and that of many centers is that COVID-19 hypoxemia responds well to PEEP increases. However, notable exceptions have been found where lower PEEP is preferred (47). These cases may be the result of pulmonary microthrombi reducing blood flow (47) as COVID-19 patients are recently reported to develop coagulation abnormalities (48). In these cases, higher PEEP may be deleterious by increasing pulmonary vascular resistance. High-frequency oscillation is not used in adults due to randomized trials showing increased mortality (49) and greater need for sedation (49, 50). Assessment for extubation readiness is typically done using a combined spontaneous awakening-spontaneous breathing trial (51) in which all sedation is lifted and the patient is placed on a continuous positive airway pressure of 5 cm H2O and pressure support set at 0–8 cm H2O (52, 53). Success is defined as a rapid shallow breathing index score (respiratory frequency/tidal volume) less than 105 (54) after at least 30 minutes (52, 53) without objective evidence of distress.
Sedation and Analgesia in Adults (Table on Dosing)
Opioid, benzodiazepine, and dexmedetomidine IV infusions and/or boluses are used for sedation in adults and pediatrics in similar dose ranges despite the common practice in adults of using absolute doses (e.g., mg/hr) as opposed to weight-based dosing (e.g., mg/kg/hr). Propofol use is common in adults due to few reports of propofol infusion syndrome (55, 56) with dose range of 5–60 µg/kg/min employed for continuous prolonged sedation or up to 200 µg/kg/min for brief procedures. Multiple randomized trials have failed to demonstrate any optimal adult sedative (57–61). Propofol or dexmedetomidine produces more hypotension than midazolam and opioids but are metabolized more rapidly. Sedation interruptions or closely titrated sedation based on clinical scores (i.e., Richmond Agitation-Sedation Scale) are superior to both or minimal sedation in producing patient comfort and hemodynamic stability (62–64).
Prone Positioning
Prone positioning for at least 12 hours daily in adults with severe ARDS may increase ventilator-free days, reduce in-hospital mortality, and reduce the need for rescue therapies like inhaled nitric oxide and extracorporeal membrane oxygenation (ECMO) (16, 65–68). The Surviving Sepsis guidelines for COVID-19 for moderate to severe ARDS recommend proning within 24 hours of presentation (24). Our collective experience supports impressive responses in oxygenation following proning in COVID-19. Prone patients typically require additional staff for patient manipulation, deep sedation, and often neuromuscular blockade. Care should be taken to minimize complications such as endotracheal tube obstruction, pressure sores, facial edema, and ocular injury (65–68).
COPD Acute Exacerbations Initiated by COVID-19
COPD is a common chronic illness worldwide and leading cause of both morbidity and mortality (69). Patients with COPD are at high risk to develop acute exacerbation of COPD (AECOPD) during the COVID-19 pandemic and early recognition and treatment is essential. The mainstay of treatment for AECOPD are short-acting bronchodilators, short courses of steroids, oxygen therapy to target oxygen saturations of 88–92%, and short courses of antibiotics (5–7 d [70]).
Inhaled bronchodilators (short-acting β2 agonists and muscarinic antagonists) are effective in the treatment of acute exacerbations. Nebulization should be avoided due to risk of viral aerosolization rather these medications should be administered via meter-dose inhalers. Prednisone or IV methylprednisolone (30–50 mg daily) for 5–7 days is recommended (70). NIPPV is the standard of care especially for AECOPD as it has been demonstrated to decrease intubation rates, and overall mortality due to respiratory failure (70, 71). Akin to intubated asthmatics, intubated AECOPD with COVID may require lower respiratory rates and higher tidal volumes to avoid auto-peep and increased intrathoracic pressure, decreased venous return, and hemodynamic compromise.
Venovenous Extracorporeal Life Support
Adults with ARDS may receive a survival and disability benefit from venovenous ECMO when offered within 7 days of initiation of mechanical ventilation (72–74). Venovenous ECMO has been found to be safe and effective, especially in ARDS patients during the H1N1 influenza pandemic (75–77). Evidence from adults with COVID-19 in Japan and South Korea suggest that carefully selected patients with severe ARDS failing conventional treatment can be successfully supported with venovenous ECMO (72, 78, 79). Venovenous ECMO flow rates needed to support oxygenation in adults are generally 60–80 mL/kg/min (80). “Lung rest” ventilation should target Fio2 less than or equal to 60%, PEEP ~10, and plateau pressure ~20–25 (77). COVID-19 appears to cause myocardial injury with increased mortality in these patients (81). Selected adults progressing to cardiovascular failure may benefit from venoarterial ECMO, although this is associated with a higher risk of stroke, bleeding, and renal failure and should only be considered only in experienced, resourced centers (82).
MANAGEMENT OF COMMON ADULT COMORBIDITIES AND COMPLICATIONS
Stroke and Intracranial Hemorrhage
Cerebrovascular accident (CVA) is a leading cause of death in the United Sates with an overall prevalence of 2.5% in those greater than 20 years old (83) (Table 5). Most CVA (85%) is ischemic. Immediate evaluation to stabilize hemodynamics, decipher if intracranial hemorrhage or ischemia is present, and then decide on reperfusion therapy is temporally critical. Sudden loss of focal brain function is a core feature of ischemic stroke onset. Management of CVA includes stabilizing the patient’s airway, breathing, and circulation (ABCs), reversing contributing issues, determining the etiology (for ischemic strokes, consider thrombolysis or endovascular thrombectomy), and preparation for post intervention surveillance/management. Pediatric intensivists should calculate a National Institutes of Health stroke scale score, obtain immediate acute imaging to exclude hemorrhage, assess the degree of brain injury, and identify the vascular lesion responsible for the deficit. Imaging may be difficult given isolation for COVID-19; however, these studies are time critical as thrombolysis must occur in less than 4.5 hours from symptoms (83–89). Imaging includes hyperacute MRI, noncontrast CT, or CT angiography. Reperfusion is the most effective maneuver for salvaging ischemic brain that is not already infarcted and is time sensitive as the benefits of reperfusion for ischemic stroke diminish over time. Recent guidelines for early stroke management are published (90). Consultation with a stroke team (telestroke) is recommended.
TABLE 5.
Summary of Common Complications in Adult Critically Ill Patients
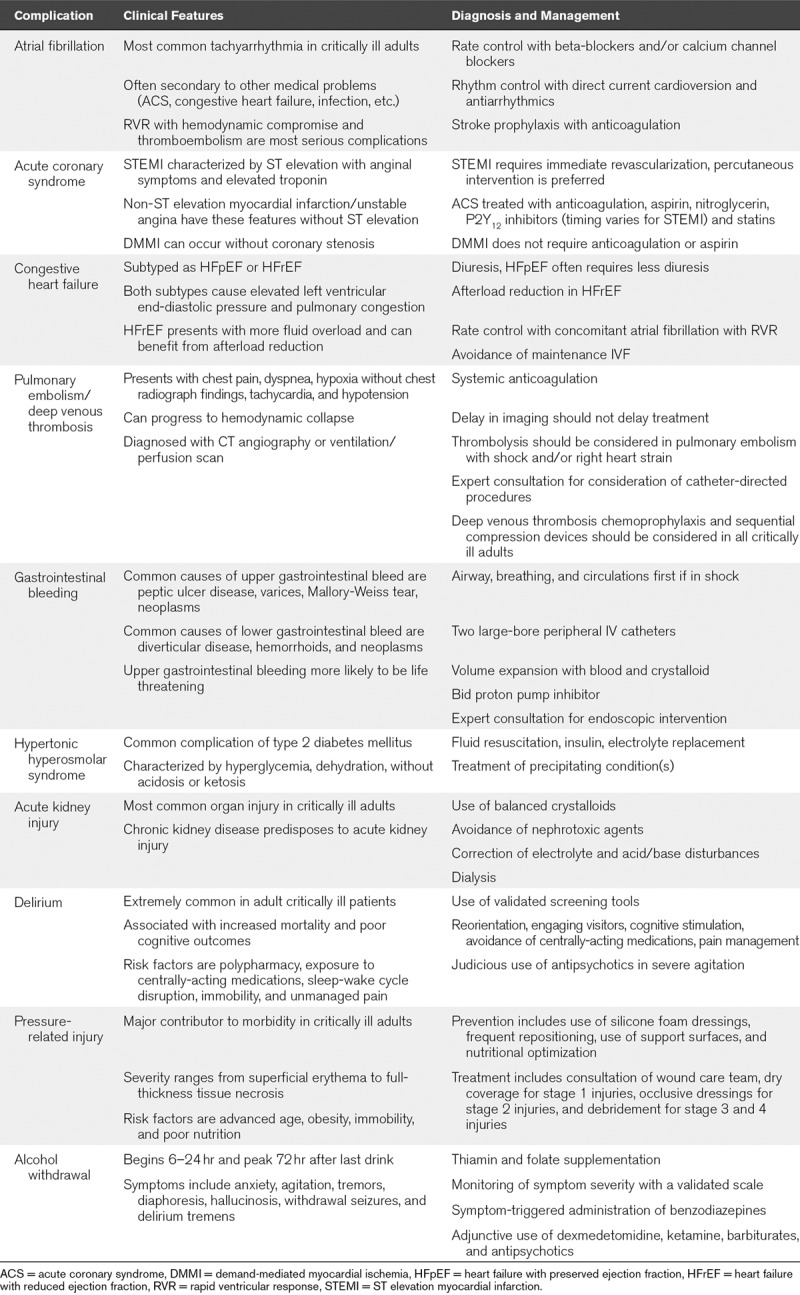
Cardiac Complications
Mounting evidence demonstrates that up to 40% of COVID-19 patients have direct cardiac injury with increases in arrhythmia, myocardial infarction (MI), myocarditis, and acute heart failure (7, 20, 81, 91, 92). Thus, we provide considerations for these common complications with guidance on management.
Acute or New Onset Atrial Fibrillation.
AF is the most common cardiac arrhythmia in adults, more prevalent in men, and prevalence increases with age (93, 94). AF presents as an irregularly irregular pulse which on electrocardiogram (ECG) has RR intervals without repetitive pattern and often absent P waves. AF and resultant tachycardia may compromise cardiac output and result in atrial thrombus formation with potential for embolic stroke. Understanding the immediate etiology for AF is important, as some causes are reversible (i.e., MI, active infection, electrolyte disturbance). Management of AF centers on rate and rhythm control. Rate control to slow the ventricular rate is best achieved via use of beta-blockers (metoprolol or esmolol) or calcium channel blockers (diltiazem). A transesophageal echocardiogram is recommended to evaluate for signs of acute heart failure or left atrial appendage thrombus. To immediately restore normal sinus rhythm direct electric cardioversion within 48 hours of onset is warranted if AF is causing hemodynamic embarrassment. Direct current cardioversion may be more successful with use of amiodarone infusion for 24 hours. In the setting of persistent AF with lower blood pressures, digoxin and amiodarone may be considered for rate control. Management of AF is the subject of a recent guideline update (95).
Acute Coronary Syndromes (Including Demand Ischemia).
Assessment of chest pain and acute coronary syndrome (ACS) must be undertaken immediately. If a patient experiences chest pain, arm pain, dizziness, or new onset arrhythmia a STAT ECG should be ordered to determine if there is ST elevation. Patients experiencing an acute ST elevation myocardial infarction (STEMI) require immediate interventional cardiology consultation to consider percutaneous intervention within 90 minutes. If angiography is deemed unacceptable due to COVID-19 infection risk, thrombolysis is an option (96). In the absence of STEMI, these symptoms with troponin elevation mark unstable angina (USA) or non-STEMI. Treatment of USA/non-ST elevation myocardial infarction (NSTEMI) consists of anticoagulation, aspirin (162–325 mg), β blockade and if needed, oxygen (97). These same treatments applied for USA/NSTEMI are often employed initially in the setting of STEMI until reperfusion occurs. Persistent chest pain may be treated with 0.4 mg sublingual nitroglycerin every 5 minutes or a nitroglycerin drip assuming blood pressure is adequate.
Severe critical illness in adults with limited coronary perfusion may result in troponin elevation due to demand-mediated myocardial ischemia (DMMI). Management of DMMI is to minimize myocardial oxygen demands and patient stress (e.g., β blockade, sedation/paralysis); however, there is no role for aspirin or anticoagulation (98). Bedside echocardiogram or point of care ultrasound to evaluate for focal wall motion abnormality can help distinguish infarction from DMMI. Laboratory evaluation of ACS should include electrolytes (with correction of abnormalities), serial troponins, platelets, and coagulation indices.
MI should be treated with high dose statin therapy (e.g., 80 mg atorvastatin daily). Recommendations from 2019 suggest NSTEMI patients should also receive P2Y12 inhibitor (89). Typically, before administering additional antiplatelet therapy, a cardiology consult is warranted to discuss the timing of angiography.
Congestive Heart Failure.
Acute decompensated heart failure (ADHF) is one of the main causes of respiratory distress in adult patients requiring the ICU (99). Heart failure with preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF) have similarities and differences in management. HFrEF shares similarities to the congestive heart failure (CHF) seen in the PICU. Respiratory distress is typically a result of elevated left ventricular end-diastolic pressure (LVEDP) resulting in pulmonary congestion. Diuresis is helpful in both clinical presentations, although patients in HFrEF generally are more hypervolemic. In general, ICU patients in ADHF do not require maintenance IV fluids.
HFpEF patients have diastolic dysfunction and often present with tachycardia and hypertension; subsequently elevating LVEDP. These respond well to vasodilators and β blockade directed at restoring “normal” range heart rates and blood pressures (100). AF should be rate controlled immediately as it can exacerbate HFpEF. Point of care cardiac ultrasound can assist in identifying patients with reduced ejection fraction (101–104).
Hypertensive patients with HFrEF require afterload reduction to optimize cardiac output and may require low-dose inotropic support. Home medications (angiotensin blockade and β blockers) should be discontinued at admission to the ICU and assessed for continuation after the patient has reached clinical stability. In patients with significant hypervolemia, high venous pressures may contribute to poor renal perfusion and poor diuretic response (“cardiorenal syndrome”). Aggressive diuresis (occasionally dialysis) with inotropic or vasodilator support may be needed to improve oxygenation. Weighing the patient daily may assist in targeting appropriate fluid balance. Myocardial ischemia should be considered as a cause of ADHF and ruled out with serial troponins.
Acute Pulmonary Embolism and Deep Vein Thrombosis Prophylaxis.
Acute pulmonary embolism (PE) is a common and fatal complication of hospitalization that account for over 100,000 deaths in the United States annually. The diagnosis and management of PE is summarized (102–105). PE in the ICU may present as hemodynamic stability or increased hypoxia not explained by new chest radiograph findings. This diagnosis is rarely seen in the PICU and a high index of suspicion should be maintained when caring for adults. Definitive imaging includes CT pulmonary angiography and less commonly ventilation/perfusion scan (102, 106). Treatment is identical to PE for presence on ultrasound of deep vein thrombosis (DVT) in the setting of PE symptoms. The mainstay of therapy is systemic anticoagulation (105) with unfractionated heparin or low molecular weight heparin that should not be withheld due to delay in obtaining imaging especially due to quarantine for COVID-19. Hemodynamic instability including right heart strain should warrant consideration for thrombolysis or acute thrombectomy (103, 104, 106). To prevent DVT, especially given immobility with COVID-19 in the ICU, the use of sequential compression devices and, if not contraindicated, prophylactic anticoagulation (107, 108) is recommended.
Gastrointestinal Bleeding
The common adult conditions causing acute gastrointestinal bleeding (GIB) are distinguished based on whether their origin is in the upper or lower gastrointestinal tract. The most common etiologies of upper gastrointestinal bleeding are peptic ulcer disease, variceal bleeding, Mallory-Weiss tears, and carcinoma (109). The most common cause of lower gastrointestinal bleeding are diverticular disease, angiodysplasia, neoplasms, colitis, and anal lesions like hemorrhoids and fissures (109). In critically ill intubated adults stress ulcer prophylaxis with a proton pump inhibitor (PPI) has a small benefit in preventing GIB (110).
As with pediatric patients experiencing acute GIB, the initial priorities are managing the ABCs particularly hemorrhagic shock. To facilitate transfusion, two large bore (18 gauge) IV catheters should be established and hypotension managed aggressively with IV fluids and the transfusion of blood and blood products as necessary. A PPI should be administered for upper GIB. We recommend pantoprazole 40 mg IV bid as an initial approach with an immediate gastrointestinal consult. Upper endoscopy can be both diagnostic and therapeutic in upper GIB, whereas colonoscopy is primarily diagnostic. A nasogastric tube may be helpful to differentiate the source of bleeding or remove stomach contents and blood prior to endoscopy. This helps to identify a source and allow specific treatments to be provided. If no source is found on the initial endoscopy and the patient remains unstable, additional diagnostic testing including computerized tomography and/or angiography can be pursued while resuscitation continues. Surgery remains an option for those in whom the source remains elusive.
Hypertonic Hyperosmolar Syndrome
Hyperosmolar hyperglycemic state (HHS) is an acute metabolic emergency classically affecting type 2 diabetics. It is distinct from diabetic ketoacidosis (DKA) in that it typically presents with higher levels of hyperglycemia (plasma glucose > 600 mg/dL), a greater degree of dehydration, minimal acidosis (pH > 7.30) and ketosis (111, 112). Treatment principles of HHS are insulin infusion titrated to decrease blood glucose to less than 180 mg/dL (which is the threshold of glucosuria which drives dehydration/electrolyte abnormalities) and aggressive hydration. Total fluid resuscitation requirements are usually much greater than in DKA (111), although in COVID-19 this must be balanced against the risks of volume overload and CHF. Resolution of HHS is indicated by improvement in osmolality, dehydration, and altered mental state (111).
Acute Kidney Injury Superimposed on Chronic Kidney Disease
AKI is the most common organ dysfunction in critically ill adults (34%) and is associated with high in-hospital mortality (62%) (113). Patients with advanced chronic kidney disease or end-stage renal disease may already be on intermittent hemodialysis (iHD) through a tunneled percutaneous hemodialysis catheter or a matured arteriovenous fistula. Temporary catheters can be used for iHD or continuous renal replacement therapy, but an arteriovenous fistula is reserved for iHD.
The prevalence of AKI in COVID-19 is low (7%) similar to that seen in the severe acute respiratory syndrome (SARS) epidemic (6.7%) (7, 114). Like SARS, COVID-19 may cause an acute tubular necrosis (114). Patient in the SARS epidemic who developed AKI had a higher overall mortality compared with those without renal impairment (91.7% vs 8.8%) (114). Management should include avoidance of nephrotoxic agents and use of pH balanced crystalloids (115).
Delirium
Delirium is common among adult ICU patients (prevalence: 16–89%) (116) and caused by an underlying medical condition, intoxication, or medication effect. It is a significant contributor to both morbidity and mortality, including worse long-term cognitive outcomes (117–119). Delirium can occur in agitated, hypoactive, and mixed subtypes, with the overwhelming majority of patients falling into the latter two categories. There are several validated scales for delirium assessment in the ICU, with the Confusion Assessment Method for the ICU being the most widely used (114, 115). Many of the risk factors are modifiable and include exposure to psychoactive or centrally-acting medications, sleep-wake cycle disruption, immobility, polypharmacy, and unmanaged pain (117–119). Nonpharmacologic approaches to these modifiable risk factors include frequent environmental reorientation, cognitive stimulation, minimizing sleep interruptions, engaging familiar visitors, limiting use of sedative medications, and scheduled sedation “holidays.” These strategies have consistently shown improved clinical outcomes in critically ill patients and are now considered standard of care (117). Although there is some evidence suggesting the prophylactic use of certain pharmacologic agents (antipsychotics, dexmedetomidine, ketamine, etc.), this is currently not recommended due to the inconsistency and lower quality of most of the studies and lack of benefit in other patient-centered outcomes (117). For severe agitation posing risk of self-harm or interruption of care, a trial of short-term low-dose antipsychotics (haloperidol, quetiapine, and olanzapine) may be helpful (117).
Skin Breakdown and Pressure Ulcers
Although children can develop pressure-related injury (PI), it affects a higher frequency (~17–24%) of critically ill adults (120). Severity ranges from nonblanchable skin erythema (stage 1) to full-thickness destruction of dermis and subcutaneous tissue (stage 4) (121). Some of the healthcare burden from PI’s is preventable with good risk assessment and implementation of skin care protocols (120). Distinct ICU risk factors include prolonged mechanical ventilation and bedbound status which is often exacerbated by higher prevalences of neuromuscular weakness in adults (122–124), hypotension and vasopressor administration (125) and should be considered along with general risk factors (age, comorbidities, obesity, mobility, and nutrition) when utilizing risk assessment tools like the Braden Scale (125). PI preventative strategies include use of protective silicone foam dressings, frequent repositioning, use of support surfaces, and nutritional optimization. Although the use of silicone foam dressings has proven effective, evidence for the other strategies remains limited (126). Early consultation of a wound care team (if available), coverage with a transparent film for stage 1 injuries, maintaining a moist wound environment with occlusive dressings for stage 2 injuries, and possible debridement for stage 3 and 4 injuries form the basis for preventing PI progression (127). Efforts should be made to efficiently incorporate these strategies into the overall care of the patient in a way that limits patient staff interactions.
Alcohol Withdrawal
About 20–30% of adult ICU patients have an alcohol use disorder and are at risk for developing alcohol withdrawal syndrome (AWS) (128, 129). AWS carries significant morbidity and mortality in hospitalized patients and requires careful management. Without treatment, symptoms begin within 6–24 hours after cessation of drinking and may include anxiety, agitation, tremors, diaphoresis, headache, hallucinosis, withdrawal seizures, and delirium (i.e., delirium tremens). Symptoms may be measured using the Clinical Institutes Withdrawal Assessment Scale for Alcohol and management tailored based on severity of symptoms. A high index of suspicion and preemptive treatment with folate (5 mg daily) and thiamin (100 mg IV daily) is important to avoid Wernicke-Korsakoff syndrome. Withdrawal symptoms are managed first line using titrated doses of benzodiazepines with potential benefit from other therapies such as dexmedetomidine, ketamine, phenobarbital, and antipsychotics (128–130). Propofol may be added in agitated intubated patients.
ETHICAL ISSUES INCLUDING PALLIATIVE CARE AND END OF LIFE DECISION-MAKING
Critically ill adults typically require surrogate decision-making while incapacitated (131) and many have a prepared advanced care document (i.e., durable powers of attorney for healthcare (DPAHC) and living wills) to express healthcare wishes (132, 133). DPAHCs authorize particular person(s) as legally recognized medical decision-makers if the patient lacks capacity. Living wills summarize medical care that a patient would or would not want under specific circumstances such as serious illness or hospitalization. Particularly in a setting of critical resource limitation, an ethical duty to plan compels physicians to identify these advanced directives or identify a surrogate decision-maker, as misapplication of these resources may detract from other patients. Do-not-resuscitate (DNR) orders should be entered in the medical record for patients who do not desire CPR.
Public health ethics, which focuses on overall community good, differs from clinical ethics, which focuses on the good of the individual patient (134, 135). Crisis resource allocation and rationing strategies, often designed to save the most possible lives and the most possible life years, deserve early institutional articulation (136). Such policies may create tension during the care of adults in pediatric settings, as many allocation guidelines give preference to younger patients. Palliative care consultation should be engaged early, which may reduce ICU resource utilization by increasing transition to DNR status without increasing overall mortality (137). Additionally, if crisis resource allocation is used, patients (and/or surrogates) should be proactively informed and palliative care should be provided to those who do not receive ICU resources. Consultation with adult practitioners in cases where limitation of life sustaining therapy is being considered would be prudent.
Finally, adults receiving medical treatment in a pediatric facility will certainly recognize differences in the typical standard of care and should receive transparent communication about these deviations. Hospitals should clearly define and document their triggers for adopting altered standards of care. This approach creates a helpful framework for physicians and also engenders discussions with patients about the care they can expect to receive.
CONCLUSIONS
With significant resource limitations, the COVID-19 pandemic may challenge PICUs to adapt to the care of adult patients after existing capacity is exhausted. Surprisingly, the majority of care delivered to these “big children” will be familiar to the pediatric intensivist. Understanding and preparing for the differences and anticipating complications is important to optimize care in the setting of COVID-19 or other situations where adults may be cared for in a PICU (e.g., adult congenital heart disease).
The authors would like to offer support and expertise to our pediatric critical care colleagues caring for adult patients during this pandemic. As such, we have provided the emails of the combined adult and pediatric critical care medicine authors and will do our best to respond promptly to questions: Kenneth E. Remy, MD, MHSc (kremy@wustl.edu); Philip A. Verhoef, MD, PhD (Philip.a.verhoef@kp.org); Timothy B. Kaselitz, MD, MPH (kaselitzt@upmc.edu); Frank Lodeserto, MD (flodeserto@geisinger.edu); Eliotte L. Hirshberg, MD (ellie.hirshberg@have.utah.edu); Anthony Slonim, MD, PhD (aslonim@renown.org); and Cameron Dezfulian, MD (dezfulianc@upmc.edu).
Footnotes
*See also p. 679.
Dr. Verhoef’s institution received funding from National Institutes of Health National Heart, Lung, and Blood Institute K08 award. Dr. Dezfulian’s institution received funding from Mallinckrodt Pharmaceuticals. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rosenbaum L. Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med 2020. Mar 18. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng F, Liao C, Fan QH, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci 2020. Mar 24. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Gao YH, Lou LL, et al. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J 2020. Mar 26. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. Feb 7. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID-19. JAMA 2020. Mar 11. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong H, Wang Y, Chung HT, et al. Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children. Pediatr Neonatol 2020; 61:131–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. Feb 28. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020. Mar 16. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020. Mar 23. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Biddison LD, Berkowitz KA, Courtney B, et al. ; Task Force for Mass Critical Care; Task Force for Mass Critical Care: Ethical considerations: Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest 2014; 146:e145S–e155S [DOI] [PubMed] [Google Scholar]
- 13.Christian MD, Sprung CL, King MA, et al. ; Task Force for Mass Critical Care; Task Force for Mass Critical Care: Triage: Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest 2014; 146:e61S–e74S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dries D, Reed MJ, Kissoon N, et al. ; Task Force for Mass Critical Care; Task Force for Mass Critical Care: Special populations: Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest 2014; 146:e75S–e86S [DOI] [PubMed] [Google Scholar]
- 15.Einav S, Hick JL, Hanfling D, et al. ; Task Force for Mass Critical Care; Task Force for Mass Critical Care: Surge capacity logistics: Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest 2014; 146:e17S–e43S [DOI] [PubMed] [Google Scholar]
- 16.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 17.Halpern NA, Tan KS, DeWitt M, et al. Intensivists in U.S. acute care hospitals. Crit Care Med 2019; 47:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpern N, Tan K. U.S. ICU resource availability for COVID-19. 2020 Available at: https://sccm.org/Blog/March-2020/United-States-Resource-Availability-for-COVID-19. Accessed March 21, 2020.
- 19.Centers for Disease Control. Cases of Coronavirus Disease (COVID-19) in the U.S. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed on March, 21, 2020
- 20.Shi S, Shen B, Cai Y, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020. Mar 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beal AC, Co JP, Dougherty D, et al. Quality measures for children’s health care. Pediatrics 2004; 113:199–209 [PubMed] [Google Scholar]
- 22.Craig-Brangan KJ, Day MP. Update: 2017/2018 AHA BLS, ACLS, and PALS guidelines. Nursing 2019; 49:46–49 [DOI] [PubMed] [Google Scholar]
- 23.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015; 132:S465–S482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med 2020. Mar 27. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parienti JJ, Mongardon N, Mégarbane B, et al. ; 3SITES Study Group: Intravascular complications of central venous catheterization by insertion site. N Engl J Med 2015; 373:1220–1229 [DOI] [PubMed] [Google Scholar]
- 26.Halpenny D, O’Sullivan K, Burke JP, et al. Does obesity preclude lumbar puncture with a standard spinal needle? The use of computed tomography to measure the skin to lumbar subarachnoid space distance in the general hospital population. Eur Radiol 2013; 23:3191–3196 [DOI] [PubMed] [Google Scholar]
- 27.Nayate AP, Nasrallah IM, Schmitt JE, et al. Using body mass index to predict needle length in fluoroscopy-guided lumbar punctures. AJNR Am J Neuroradiol 2016; 37:572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dailey PA, Harwood R, Walsh K, et al. Aerosol delivery through adult high flow nasal cannula with heliox and oxygen. Respir Care 2017; 62:1186–1192 [DOI] [PubMed] [Google Scholar]
- 29.Frat JP, Coudroy R, Thille AW. Non-invasive ventilation or high-flow oxygen therapy: When to choose one over the other? Respirology 2019; 24:724–731 [DOI] [PubMed] [Google Scholar]
- 30.Frat JP, Ricard JD, Quenot JP, et al. ; FLORALI-2 study group; REVA network: Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: A randomised, multicentre, open-label trial. Lancet Respir Med 2019; 7:303–312 [DOI] [PubMed] [Google Scholar]
- 31.Frat JP, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network: High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372:2185–2196 [DOI] [PubMed] [Google Scholar]
- 32.Lodeserto FJ, Lettich TM, Rezaie SR. High-flow nasal cannula: Mechanisms of action and adult and pediatric indications. Cureus 2018; 10:e3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung JC, Ho LT, Cheng JV, et al. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med 2020; 8:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J 2019; 53 [DOI] [PubMed] [Google Scholar]
- 35.Respiratory care committee of Chinese Thoracic Society: [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020; 17:E020. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed A, Azim A. Difficult tracheal intubation in critically ill. J Intensive Care 2018; 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamb K, Piper D. Southmedic oxyMask™ compared with the hudson RCI(®) non-rebreather mask™: Safety and performance comparison. Can J Respir Ther 2016; 52:13–15 [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Harnois LJ, Markos B, et al. Epoprostenol delivered via high flow nasal cannula for ICU subjects with severe hypoxemia comorbid with pulmonary hypertension or right heart dysfunction. Pharmaceutics 2019; 11:E281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuo MZ, Huang YG, Ma WH, et al. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin Med Sci J 2020. Feb 27. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran DT, Newton EK, Mount VA, et al. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2015:CD002788. doi: 10.1002/14651858.CD002788.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kacmarek RM, Branson RD. Should intermittent mandatory ventilation be abolished? Respir Care 2016; 61:854–866 [DOI] [PubMed] [Google Scholar]
- 42.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: Advances in diagnosis and treatment. JAMA 2018; 319:698–710 [DOI] [PubMed] [Google Scholar]
- 43.Simonis FD, Serpa Neto A, Binnekade JM, et al. ; Writing Group for the PReVENT Investigators: Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: A randomized clinical trial. JAMA 2018; 320:1872–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med 2018; 378:1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. ; EPVent-2 Study Group: Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-FIO2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: A randomized clinical trial. JAMA 2019; 321:846–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modesto I Alapont V, Khemani RG, Medina A, et al. Bayes to the rescue: Continuous positive airway pressure has less mortality than high-flow oxygen. Pediatr Crit Care Med 2017; 18:e92–e99 [DOI] [PubMed] [Google Scholar]
- 47.Gattinoni L, Coppola S, Cressoni M, et al. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020. Mar 30. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020. Apr 8. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young D, Lamb SE, Shah S, et al. ; OSCAR Study Group: High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813 [DOI] [PubMed] [Google Scholar]
- 50.Ferguson ND, Cook DJ, Guyatt GH, et al. ; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group: High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805 [DOI] [PubMed] [Google Scholar]
- 51.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet 2008; 371:126–134 [DOI] [PubMed] [Google Scholar]
- 52.Schmidt GA, Girard TD, Kress JP, et al. Liberation from mechanical ventilation in critically ill adults: Executive summary of an official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline. Chest 2017; 151:160–165 [DOI] [PubMed] [Google Scholar]
- 53.Subirà C, Hernández G, Vázquez A, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: A randomized clinical trial. JAMA 2019; 321:2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 1991; 324:1445–1450 [DOI] [PubMed] [Google Scholar]
- 55.Fong JJ, Sylvia L, Ruthazer R, et al. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med 2008; 36:2281–2287 [DOI] [PubMed] [Google Scholar]
- 56.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia 2007; 62:690–701 [DOI] [PubMed] [Google Scholar]
- 57.Jakob SM, Ruokonen E, Grounds RM, et al. ; Dexmedetomidine for Long-Term Sedation Investigators: Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA 2012; 307:1151–1160 [DOI] [PubMed] [Google Scholar]
- 58.Kawazoe Y, Miyamoto K, Morimoto T, et al. ; Dexmedetomidine for Sepsis in Intensive Care Unit Randomized Evaluation (DESIRE) Trial Investigators: Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: A randomized clinical trial. JAMA 2017; 317:1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA 2007; 298:2644–2653 [DOI] [PubMed] [Google Scholar]
- 60.Riker RR, Shehabi Y, Bokesch PM, et al. ; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group: Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA 2009; 301:489–499 [DOI] [PubMed] [Google Scholar]
- 61.Shehabi Y, Howe BD, Bellomo R, et al. ; ANZICS Clinical Trials Group and the SPICE III Investigators: Early sedation with dexmedetomidine in critically ill patients. N Engl J Med 2019; 380:2506–2517 [DOI] [PubMed] [Google Scholar]
- 62.Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342:1471–1477 [DOI] [PubMed] [Google Scholar]
- 63.Mehta S, Burry L, Cook D, et al. ; SLEAP Investigators; Canadian Critical Care Trials Group: Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: A randomized controlled trial. JAMA 2012; 308:1985–1992 [DOI] [PubMed] [Google Scholar]
- 64.Olsen HT, Nedergaard HK, Strøm T, et al. Nonsedation or light sedation in critically ill, mechanically ventilated patients. N Engl J Med 2020; 382:1103–1111 [DOI] [PubMed] [Google Scholar]
- 65.Gattinoni L, Taccone P, Carlesso E, et al. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med 2013; 188:1286–1293 [DOI] [PubMed] [Google Scholar]
- 66.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 67.Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc 2017; 14:S280–S288 [DOI] [PubMed] [Google Scholar]
- 68.Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: Systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599 [DOI] [PubMed] [Google Scholar]
- 69.Buist AS, McBurnie MA, Vollmer WM, et al. ; BOLD Collaborative Research Group: International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet 2007; 370:741–750 [DOI] [PubMed] [Google Scholar]
- 70.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195:557–582 [DOI] [PubMed] [Google Scholar]
- 71.Ram FS, Picot J, Lightowler J, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004; 1:CD004104. [DOI] [PubMed] [Google Scholar]
- 72.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet: Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018; 378:1965–1975 [DOI] [PubMed] [Google Scholar]
- 73.Peek GJ, Clemens F, Elbourne D, et al. CESAR: Conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res 2006; 6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009; 374:1351–1363 [DOI] [PubMed] [Google Scholar]
- 75.Davies A, Jones D, Bailey M, et al. ; Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators: Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009; 302:1888–1895 [DOI] [PubMed] [Google Scholar]
- 76.Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668 [DOI] [PubMed] [Google Scholar]
- 77.Sen A, Callisen HE, Alwardt CM, et al. Adult venovenous extracorporeal membrane oxygenation for severe respiratory failure: Current status and future perspectives. Ann Card Anaesth 2016; 19:97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henry BM. COVID-19, ECMO, and lymphopenia: A word of caution. Lancet Respir Med 2020; 8:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med 2020. Mar 20. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jayaraman AL, Cormican D, Shah P, et al. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: Techniques, limitations, and special considerations. Ann Card Anaesth 2017; 20:S11–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020. Mar 25. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kon ZN, Bittle GJ, Pasrija C, et al. Venovenous versus venoarterial extracorporeal membrane oxygenation for adult patients with acute respiratory distress syndrome requiring precannulation hemodynamic support: A review of the ELSO registry. Ann Thorac Surg 2017; 104:645–649 [DOI] [PubMed] [Google Scholar]
- 83.Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019; 139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 84.Ekker MS, Boot EM, Singhal AB, et al. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol 2018; 17:790–801 [DOI] [PubMed] [Google Scholar]
- 85.Furlan AJ, Katzan IL, Caplan LR. Thrombolytic therapy in acute ischemic stroke. Curr Treat Options Cardiovasc Med 2003; 5:171–180 [DOI] [PubMed] [Google Scholar]
- 86.Katzan I, Speck M, Uchino K, et al. The stroke 8: A daily checklist for inpatient stroke management. Crit Pathw Cardiol 2015; 14:1–6 [DOI] [PubMed] [Google Scholar]
- 87.Maldonado NJ, Kazmi SO, Suarez JI. Update in the management of acute ischemic stroke. Crit Care Clin 2014; 30:673–697 [DOI] [PubMed] [Google Scholar]
- 88.Russman AN, Katzan IL. Acute stroke treatment in the community: Improving our performance and expanding our options. Semin Neurol 2005; 25:337–344 [DOI] [PubMed] [Google Scholar]
- 89.Schüpke S, Neumann FJ, Menichelli M, et al. ; ISAR-REACT 5 Trial Investigators: Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 2019; 381:1524–1534 [DOI] [PubMed] [Google Scholar]
- 90.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council: 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49:e46–e110 [DOI] [PubMed] [Google Scholar]
- 91.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis 2020. Mar 10. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes 2020; 13:e006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morin DP, Bernard ML, Madias C, et al. The state of the art: Atrial fibrillation epidemiology, prevention, and treatment. Mayo Clin Proc 2016; 91:1778–1810 [DOI] [PubMed] [Google Scholar]
- 94.Omae T, Inada E. New-onset atrial fibrillation: An update. J Anesth 2018; 32:414–424 [DOI] [PubMed] [Google Scholar]
- 95.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019; 140:e125–e151 [DOI] [PubMed] [Google Scholar]
- 96.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140 [DOI] [PubMed] [Google Scholar]
- 97.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64:e139–e228 [DOI] [PubMed] [Google Scholar]
- 98.DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation 2019; 140:1661–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med 2005; 353:2788–2796 [DOI] [PubMed] [Google Scholar]
- 100.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136:e137–e161 [DOI] [PubMed] [Google Scholar]
- 101.Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020; 22:391–412 [DOI] [PubMed] [Google Scholar]
- 102.Douma RA, Kamphuisen PW, Büller HR. Acute pulmonary embolism. Part 1: Epidemiology and diagnosis. Nat Rev Cardiol 2010; 7:585–596 [DOI] [PubMed] [Google Scholar]
- 103.Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primers 2018; 4:18028. [DOI] [PubMed] [Google Scholar]
- 104.van Es J, Douma RA, Gerdes VE, et al. Acute pulmonary embolism. Part 2: Treatment. Nat Rev Cardiol 2010; 7:613–622 [DOI] [PubMed] [Google Scholar]
- 105.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352 [DOI] [PubMed] [Google Scholar]
- 106.Pelliccia F, Schiariti M, Terzano C, et al. Treatment of acute pulmonary embolism: Update on newer pharmacologic and interventional strategies. Biomed Res Int 2014; 2014:410341. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2018; 2:3198–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qaseem A, Chou R, Humphrey LL, et al. ; Clinical Guidelines Committee of the American College of Physicians: Venous thromboembolism prophylaxis in hospitalized patients: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2011; 155:625–632 [DOI] [PubMed] [Google Scholar]
- 109.Kim BS, Li BT, Engel A, et al. Diagnosis of gastrointestinal bleeding: A practical guide for clinicians. World J Gastrointest Pathophysiol 2014; 5:467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Young PJ, Bagshaw SM, Forbes AB, et al. ; PEPTIC Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group, Alberta Health Services Critical Care Strategic Clinical Network, and the Irish Critical Care Trials Group: Effect of stress ulcer prophylaxis with proton pump inhibitors vs histamine-2 receptor blockers on in-hospital mortality among ICU patients receiving invasive mechanical ventilation: The PEPTIC randomized clinical trial. JAMA 2020323:616–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009; 32:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: A historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 2014; 37:3124–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang CH, Fan PC, Chang MY, et al. Acute kidney injury enhances outcome prediction ability of sequential organ failure assessment score in critically ill patients. PLoS One 2014; 9:e109649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chu KH, Tsang WK, Tang CS, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 2005; 67:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Semler MW, Self WH, Wanderer JP, et al. ; SMART Investigators and the Pragmatic Critical Care Research Group: Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018; 378:829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brummel NE, Girard TD. Delirium in the critically ill patient. Handb Clin Neurol 2019; 167:357–375 [DOI] [PubMed] [Google Scholar]
- 117.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 118.Kotfis K, Marra A, Ely EW. ICU delirium - a diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiol Intensive Ther 2018; 50:160–167 [DOI] [PubMed] [Google Scholar]
- 119.Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med 2014; 370:444–454 [DOI] [PubMed] [Google Scholar]
- 120.Chaboyer WP, Thalib L, Harbeck EL, et al. Incidence and prevalence of pressure injuries in adult intensive care patients: A systematic review and meta-analysis. Crit Care Med 2018; 46:e1074–e1081 [DOI] [PubMed] [Google Scholar]
- 121.Coyer F, Tayyib N. Risk factors for pressure injury development in critically ill patients in the intensive care unit: A systematic review protocol. Syst Rev 2017; 6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014; 370:1626–1635 [DOI] [PubMed] [Google Scholar]
- 123.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: A major cause of muscle weakness and paralysis. Lancet Neurol 2011; 10:931–941 [DOI] [PubMed] [Google Scholar]
- 124.Latronico N, Peli E, Botteri M. Critical illness myopathy and neuropathy. Curr Opin Crit Care 2005; 11:126–132 [DOI] [PubMed] [Google Scholar]
- 125.Cox J. Pressure injury risk factors in adult critical care patients: A review of the literature. Ostomy Wound Manage 2017; 63:30–43 [PubMed] [Google Scholar]
- 126.Tayyib N, Coyer F. Effectiveness of pressure ulcer prevention strategies for adult patients in intensive care units: A systematic review. Worldviews Evid Based Nurs 2016; 13:432–444 [DOI] [PubMed] [Google Scholar]
- 127.Reddy M, Gill SS, Kalkar SR, et al. Treatment of pressure ulcers: A systematic review. JAMA 2008; 300:2647–2662 [DOI] [PubMed] [Google Scholar]
- 128.Dixit D, Endicott J, Burry L, et al. Management of acute alcohol withdrawal syndrome in critically ill patients. Pharmacotherapy 2016; 36:797–822 [DOI] [PubMed] [Google Scholar]
- 129.Ferreira JA, Wieruszewski PM, Cunningham DW, et al. Approach to the complicated alcohol withdrawal patient. J Intensive Care Med 2017; 32:3–14 [DOI] [PubMed] [Google Scholar]
- 130.Woods AD, Giometti R, Weeks SM. The use of dexmedetomidine as an adjuvant to benzodiazepine-based therapy to decrease the severity of delirium in alcohol withdrawal in adult intensive care unit patients: A systematic review. JBI Database System Rev Implement Rep 2015; 13:224–252 [DOI] [PubMed] [Google Scholar]
- 131.Emanuel EJ, Emanuel LL. Proxy decision making for incompetent patients. An ethical and empirical analysis. JAMA 1992; 267:2067–2071 [PubMed] [Google Scholar]
- 132.Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med 2010; 362:1211–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yadav KN, Gabler NB, Cooney E, et al. Approximately one in three US adults completes any type of advance directive for end-of-life care. Health Aff (Millwood) 2017; 36:1244–1251 [DOI] [PubMed] [Google Scholar]
- 134.Thambu D. Allocating scarce life support in a public health emergency. Indian J Med Ethics 2010; 7:183–184 [DOI] [PubMed] [Google Scholar]
- 135.White DB, Katz MH, Luce JM, et al. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med 2009; 150:132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020. Mar 23. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 137.Ma J, Chi S, Buettner B, et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med 2019; 47:1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]


