Abstract
This meta-analysis is aimed to investigate the association between the neutrophil-to-lymphocyte ratio (NLR) and obstructive sleep apnea (OSA). The PubMed, Web of Science, Google Scholar, and Cochrane Library databases were searched to collect all relevant articles. The pooled standardized mean difference (SMD) with a 95% confidence interval (CI) was calculated using the random effects model. In addition, subgroup analysis and meta-regression analysis were performed. Eleven eligible articles containing 2,259 patients with OSA were included in this study. Pooled outcomes revealed that the NLR was significantly higher in patients with OSA than in controls (SMD 0.62, 95% CI 0.29–0.94, P = 0.002). In subgroup analyses, differences in the NLR between patients and controls increased with worsening OSA grades. Furthermore, meta-regression analysis showed that differences in mean BMI exerted a significant effect on differences in the NLR (P = 0.0003). In summary, our meta-analysis demonstrated that the NLR in OSA patients was significantly higher than that in controls, and the difference was larger in patients with severe OSA. These results indicate that the NLR may be a reliable marker for detecting systemic inflammation and predicting disease severity in patients with OSA.
Subject terms: Sleep disorders, Biomarkers, Biomarkers
Introduction
Obstructive sleep apnea (OSA) is a chronic inflammatory disorder characterized by recurrent episodes of partial or complete upper airway obstruction during sleep1,2, affecting 3%–9% of the general population3. OSA is a serious health problem associated with cardiovascular diseases, neurological diseases, and various types of mortality2,4–6. Although the precise underlying mechanisms are not fully understood, systemic inflammation has been proposed as a key factor in the pathogenesis of cardiovascular complications in OSA patients7,8. Therefore, investigating the inflammation process is crucial for the management of OSA.
Recently, several markers of systemic inflammation that are obtainable from routine blood tests have attracted attention because of their wide availability and low cost. Among them, the neutrophil-to-lymphocyte ratio (NLR) has been recognized as a reliable measure of systemic inflammation with prognostic value in various chronic diseases9–12, likely because chronic systemic inflammation activates white blood cells during disease progression.
Previous studies have investigated the relationship between the NLR and OSA but with inconsistent and controversial results13–22. Some researchers have observed significantly elevated NLRs in patients with OSA compared to control groups13,16–20,22, whereas others have not14,15,21. The incongruity of these results might have been due to multiple factors, including the study design, statistical power of the study, and genetic heterogeneity of the study population. Therefore, we conducted a comprehensive and systematic review and meta-analysis of related studies to determine the associations between the NLR and the presence and severity of OSA.
Methods
Search strategy
We conducted this systematic review in adherence to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses23. A systematic search of publications in the PubMed, Web of Science, Google Scholar, and Cochrane Library electronic databases (until March 24, 2019) was conducted using the following MeSH terms and keywords: “sleep apnea syndromes”[MeSH], “obstructive sleep apnea”, “sleep apnea”, “OSA”, “sleep-disordered breathing”, “sleep disorders”, “neutrophil to lymphocyte ratio”, “neutrophil lymphocyte ratio”, “neutrophil-to-lymphocyte ratio”, and “NLR”. Supplementary Table 1 describe the full search strategy.
Eligibility criteria, study selection, and quality assessment
The PICOS (Population, Intervention, Comparator, Outcome and Study design) approach was utilized to define study eligibility. (1) P: adult patients with OSA (defined as apnea–hypopnea index (AHI) of ≥ 5/hour in sleep studies), (2) I: assessment of NLR, (3) C: subjects without OSA or patients with OSA after continuous positive airway pressure (CPAP) treatment (4) O: the NLR value, (5) S: observational studies including cross-sectional studies, cohort studies, case–control studies, or case series. In addition, studies were eligible if they were written in English and full-text publications. Abstracts were independently screened for relevance by two investigators. The full text of relevant articles was reviewed independently by two authors. The references of the retrieved articles were also searched to identify additional studies. Any disagreement between the reviewers was resolved by a third investigator.
The Newcastle–Ottawa scale (NOS) was used to assess the quality of each study based on the following components: selection of the cohort, comparability of cohorts on the basis of the design or analysis, how the exposure was ascertained, and how the outcomes of interest were assessed24. Two researchers independently evaluated the quality of each study. Disagreement between the researchers was resolved through consensus. Studies achieving six or more stars were considered to be of high quality.
Data extraction
The extracted information was as follows: author name, publication year, country, study design, age, sex, mean body mass index (BMI), sample number, OSA severity, and NLR. When the data were only graphically represented18, NLR values were extracted from the graph using Adobe Acrobat’s measuring tools (Adobe Systems Incorporated, San Francisco, CA, USA).
Statistical analysis
The meta-analysis of enrolled studies was performed using R 3.4.3 version statistical software (R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.). The difference in the NLR between the OSA and control groups was assessed using the standardized mean difference (SMD). Heterogeneity across the enrolled studies was calculated using the I2 test, where I2 > 50% indicated significant heterogeneity between studies and prevented reliance on combined study results. In these cases, the random effects model was used to generate pooled effects. By contrast, outcomes without a significant level of heterogeneity (I2 < 50) were analyzed using the fixed effects model. Subgroup analysis was performed to evaluate the effects of disease severity, BMI, country, and sex. We also conducted meta-regression analysis to identify possible sources of heterogeneity. Differences in mean age (years), proportion of male patients (%), and mean BMI were the sources of heterogeneity assessed. A funnel plot and Egger test were used to detect publication bias25. Additionally, the Duval and Tweedie trim-and-fill method was used to adjust for missing studies and correct the overall effect size according to publication bias26. Sensitivity analyses were performed to estimate the influence of each study on the overall meta-analysis results27.
Results
Literature search and study characteristics
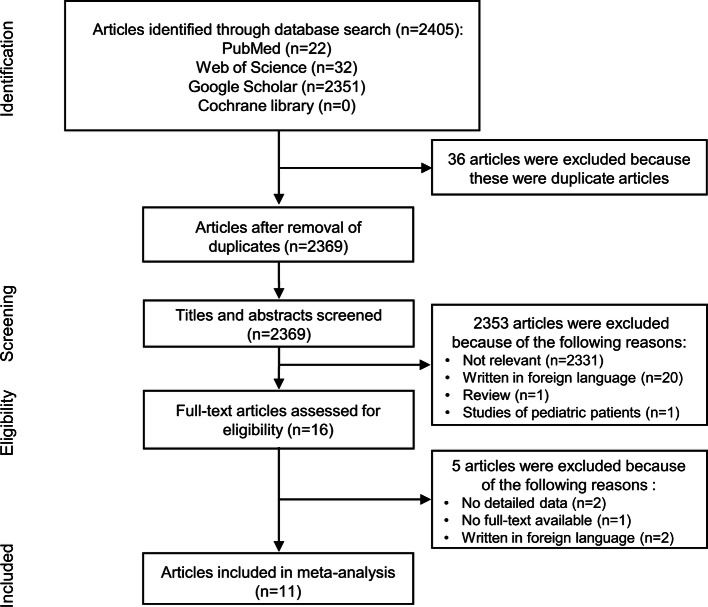
The database search using the aforementioned keywords and search strategies returned 2,405 articles, 36 of which were excluded because of duplication. After the title and abstract screening, we excluded 2,353 articles and read the full text of only 16 articles. Finally, 11 articles involving 2,259 OSA patients were included in our meta-analysis13–22,28. The process of study selection is presented in Fig. 1. Among the 11 studies, nine were conducted in Turkey and two were conducted in East Asia (Japan and China). The NOS scores of enrolled studies ranged from 6 to 7, and the publication year ranged from 2015 to 2019. All enrolled studies were case series and hospital-based studies. Eight articles13–15,17–19,21,22 provided data based on OSA severity (mild: 5 ≤ AHI < 15; moderate: 15 ≤ AHI < 30; and severe: 30 ≤ AHI) and one article20 provided data based on BMI (normal weight: BMI < 25; overweight: 25 ≤ BMI ≤ 30; and obesity: 30 < BMI). Two studies18,28 provided NLR value before and after CPAP treatment. The characteristics of the enrolled studies are summarized in Table 1.
Figure 1.
Flow diagram of study search and selection.
Table 1.
Characteristics of enrolled studies.
| First author | Year | Country | Study design | OSA severity | OSA | NOS | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (mean ± SD) | Sex (M/F) | BMI (mean) | NLR (mean ± SD) | ||||||
| Kivanca | 2018 | Turkey | RCS | Control | 52 | 41 ± 12 | 35/17 | 29 | 1.90 ± 1.03 | 6 |
| Mild | 53 | 43 ± 10 | 44/10 | 29 | 2 ± 1 | |||||
| Moderate | 65 | 50 ± 11 | 47/28 | 31.3 | 1.8 ± 1 | |||||
| Severe | 130 | 51 ± 11 | 101/29 | 34 | 1.8 ± 0.7 | |||||
| Fana | 2019 | China | RCS | Control | 135 | 46.3 ± 12.04 | 135/0 | 24.0 | 1.72 ± 0.74 | 7 |
| Mild | 185 | 44.9 ± 11.1 | 185/0 | 25.4 | 1.75 ± 0.95 | |||||
| Moderate | 171 | 46.0 ± 11.17 | 171/0 | 26.7 | 1.78 ± 0.67 | |||||
| Severe | 596 | 43.5 ± 10.93 | 596/0 | 29.1 | 1.76 ± 0.82 | |||||
| Korkmaza | 2015 | Turkey | RCS | Control | 40 | 43.3 ± 11.14 | 14/26 | 29.27 | 1.80 ± 0.64 | 6 |
| Mild | 27 | 44.96 ± 9.25 | 18/9 | 29.15 | 1.78 ± 0.57 | |||||
| Moderate | 37 | 47.24 ± 9.12 | 21/16 | 31.97 | 1.57 ± 0.54 | |||||
| Severe | 43 | 49.35 ± 9.79 | 26/17 | 32.6 | 1.61 ± 0.56 | |||||
| Yeniguna | 2015 | Turkey | PCS | Control | 38 | 48.08 ± 8.82 | 20/18 | 30.54 | 1.70 ± 0.71 | 7 |
| Mild | 34 | 46.75 ± 8.06 | 24/10 | 33.97 | 1.69 ± 0.69 | |||||
| Moderate | 30 | 53.64 ± 12.60 | 14/16 | 33.53 | 2.44 ± 1.44 | |||||
| Severe | 34 | 52.94 ± 12.21 | 18/16 | 36.15 | 3.37 ± 1.21 | |||||
| Uygura | 2016 | Turkey | RCS | Control | 118 | 50.3 ± 11.7 | 61/57 | 29.4 | 1.81 ± 0.50 | 7 |
| Mild | 57 | 53.7 ± 10.8 | 36/21 | 30.8 | 2.39 ± 0.60 | |||||
| Moderate | 53 | 51.8 ± 12.1 | 30/23 | 31.6 | 3.34 ± 0.90 | |||||
| Severe | 61 | 54.5 ± 12.7 | 39/22 | 32.1 | 4.18 ± 1.10 | |||||
| Oyamaa | 2016 | Japan | PCS | Control | 5 | 50 ± 14.6 | 2/3 | 21.9 | 1.43 ± 0.36 | 7 |
| Mild | 14 | 61.8 ± 17.3 | 9/5 | 22.4 | 1.66 ± 0.72 | |||||
| Moderate | 26 | 63.8 ± 9.4 | 18/8 | 26.3 | 1.94 ± 0.80 | |||||
| Severe | 50 | 59.3 ± 13.1 | 43/7 | 28.7 | 2.55 ± 1.62 | |||||
| Moderate-severe (pre-CPAP) | 29 | 62.3 ± 9.5 | 24/5 | 27.3 | 1.85 ± 0.71 | |||||
| Moderate-severe (post-CPAP) | 29 | 62.3 ± 9.5 | 24/5 | 27.3 | 1.62 ± 0.58 | |||||
| Sunbul | 2015 | Turkey | RCS | Control | 65 | 48.7 ± 10.2 | 42/23 | 26.9 | 1.49 ± 0.48 | 7 |
| All | 130 | 49.87 ± ND | 91/39 | 34.42 | 1.87 ± 0.80 | |||||
| Bozkuşb | 2018 | Turkey | RCS | Control | 42 | 44.02 ± 11.35 | 25/17 | 23.31 | 1.55 ± 0.16 | 7 |
| All (normal weight) | 36 | 42 ± 11.24 | 20/16 | 23.34 | 1.59 ± 0.15 | |||||
| All (overweight) | 38 | 43 ± 7.16 | 21/17 | 27.82 | 1.83 ± 0.30 | |||||
| All (obesity) | 39 | 43.69 ± 7.42 | 25/14 | 36.29 | 2.98 ± 0.29 | |||||
| Koseoglua | 2015 | Turkey | RCS | Control | 48 | 43.08 ± 8.88 | 29/19 | 27.06 | 2.02 ± 0.85 | 6 |
| Mild | 67 | ND | ND | ND | 1.97 ± 1.25 | |||||
| Moderate | 61 | ND | ND | ND | 1.87 ± 0.66 | |||||
| Severe | 108 | ND | ND | ND | 1.85 ± 0.64 | |||||
| Günbatara | 2015 | Turkey | RCS | Control | 26 | 44.7 ± 10.4 | 21/5 | 27.15 | 1.73 ± 0.68 | 6 |
| Moderate | 22 | 49.3 ± 10.6 | 16/6 | 32.4 | 1.95 ± 0.90 | |||||
| Severe | 63 | 51.02 ± 11.1 | 50/13 | 33.5 | 2.29 ± 1.40 | |||||
| Özdemir | 2019 | Turkey | PCS | Moderate-severe (pre-CPAP) | 29 | 46 ± 10.11 | 18/11 | 34.85 | 1.56 ± 0.47 | 6 |
| Moderate-severe (post-CPAP) | 29 | 46 ± 10.11 | 18/11 | 35.51 | 1.63 ± 0.58 | |||||
OSA obstructive sleep apnea, NOS Newcastle–Ottawa scale, PCS prospective case series, RCS retrospective case series, ND not determined, SD standard deviation, BMI body mass index, NLR neutrophil-to-lymphocyte ratio, M male, F female.
aThese articles divided patients into three groups according to disease severity (mild: 5 ≤ apnea–hypopnea index (AHI) < 15; moderate: 15 ≤ AHI < 30; and severe: 30 ≤ AHI).
bThis article divided patients into three groups according to body mass index (BMI) (normal weight: BMI < 25; overweight: 25 ≤ BMI ≤ 30; and obesity: 30 < BMI).
Associations between the NLR and the presence of OSA
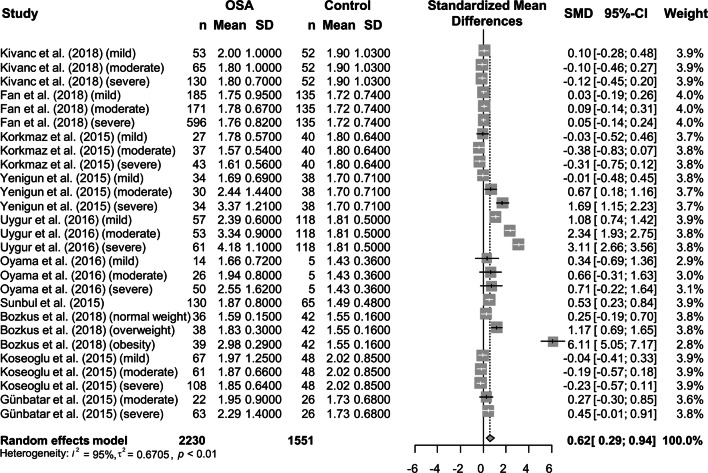
Since 10 studies13–22 fully provided NLR values of control subjects and patients with OSA, we included those studies for the meta-analysis to investigate association between NLR and the presence of OSA. We used the random effects model due to significant heterogeneity between the enrolled studies (I2 = 95%). A significant difference in the NLR was observed between the OSA and control groups (SMD = 0.62, 95% confidence interval (CI) = 0.29–0.94, P = 0.002) (Fig. 2).
Figure 2.
Comparison of the neutrophil-to-lymphocyte ratio between obstructive sleep apnea patients and controls. Calculation was based on the random effects model. The results were expressed as the standardized mean difference and 95% confidence intervals. OSA, obstructive sleep apnea; SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.
Subgroup analysis
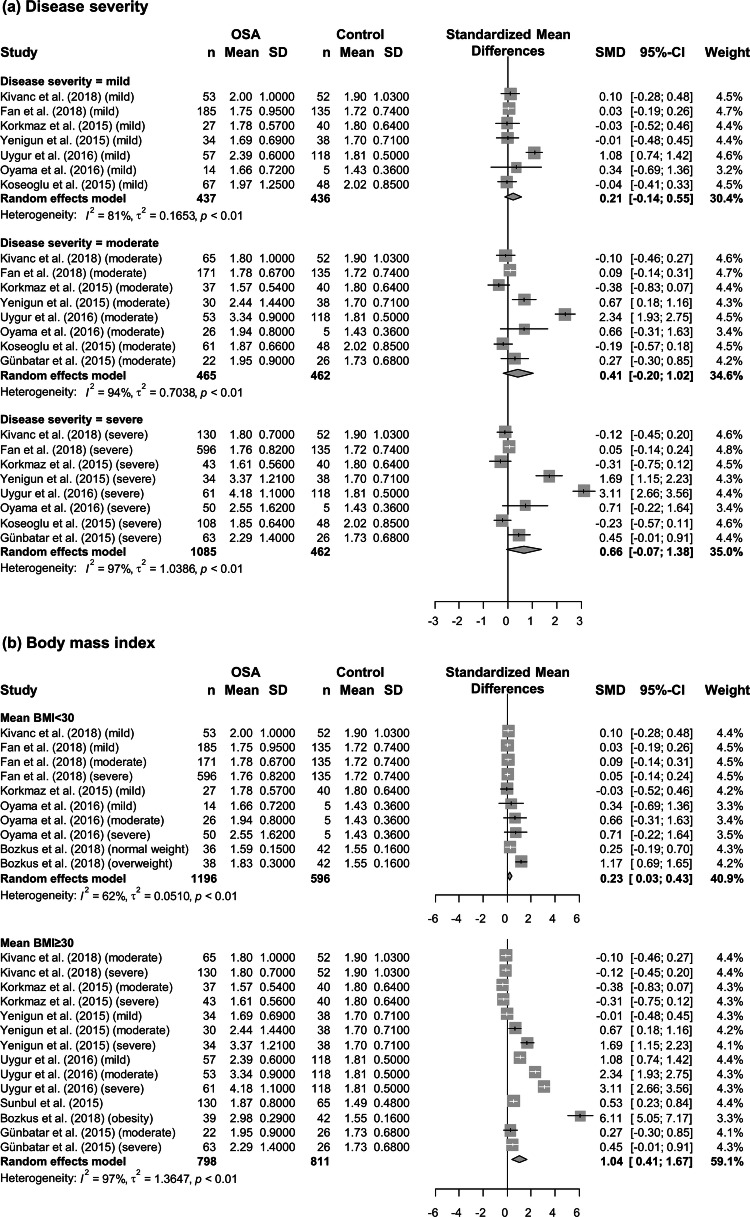
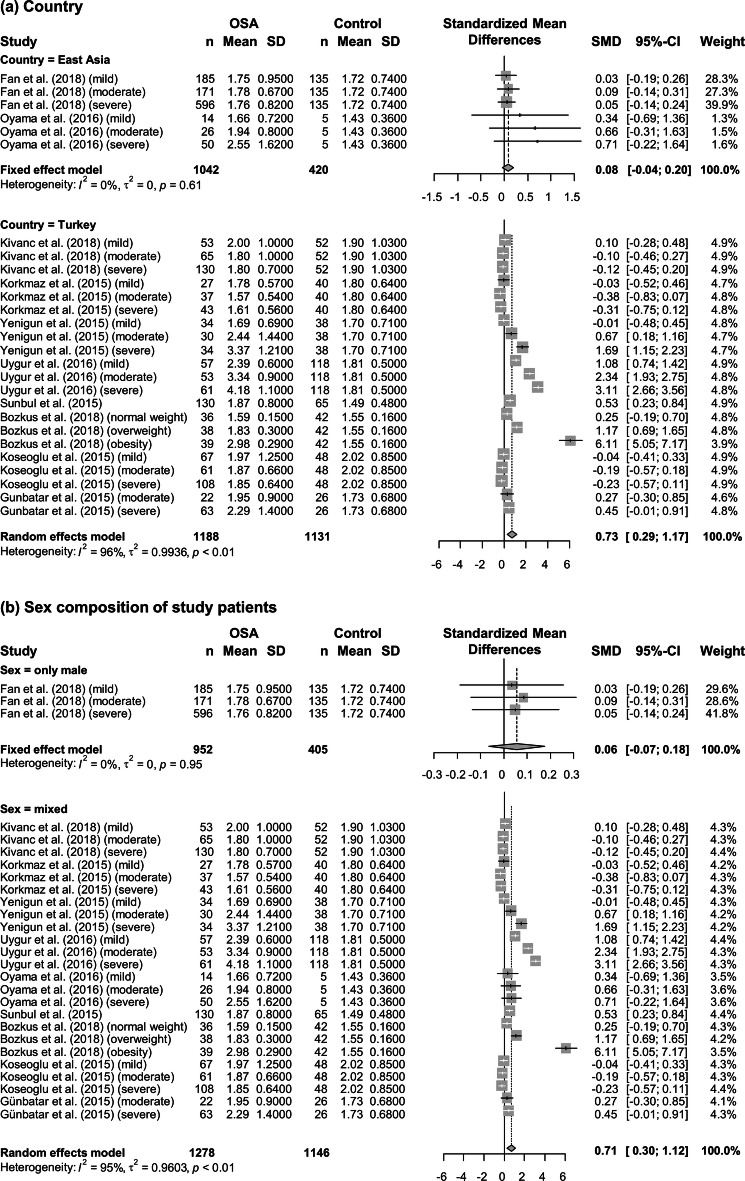
To identify possible sources of heterogeneity, subgroup analysis was performed according to the severity of OSA, BMI, country, and sex. When studies were stratified by disease severity, differences in the NLR between patients and controls increased gradually with OSA severity. The NLR in patients with mild, moderate, and severe OSA was significantly higher than that in controls by 0.21 (95% CI = − 0.14 to 0.55, P < 0.01), 0.41 (95% CI = − 0.20 to 1.02, P < 0.01), and 0.66 (95% CI = − 0.07 to 1.38, P < 0.01), respectively (Fig. 3a). Furthermore, both non-obese (BMI < 30; SMD = 0.23, 95% CI = 0.03–0.43, P < 0.01) and obese patients (BMI ≥ 30; SMD = 1.04, 95% CI = 0.41–1.67, P < 0.01) showed significantly higher NLR compared to controls (Fig. 3b). In subsequent subgroup analysis according to country, the difference in the NLR between patients and controls was not statistically significant in studies from East Asia (SMD = 0.08, 95% CI = − 0.04 to 0.20, P = 0.61), whereas it was statistically significant in studies from Turkey (SMD = 0.73, 95% CI = 0.29–1.17, P < 0.01) (Fig. 4a). When we analyzed data according to the sex composition of study patient groups, a statistically significant difference in the NLR between patients and controls was observed in studies with mixed sex patient groups (SMD = 0.71, 95% CI = 0.30–1.12, P < 0.01), but not in studies with only male patients (SMD = 0.06, 95% CI = − 0.07 to 0.18, P = 0.95) (Fig. 4b).
Figure 3.
Subgroup analysis based on disease severity evaluated using the apnea–hypopnea index and body mass index. (a) Studies were divided into three subgroups (mild: 5 ≤ apnea–hypopnea index (AHI) < 15; moderate: 15 ≤ AHI < 30; and severe: 30 ≤ AHI) according to disease severity. (b) Studies were divided into two subgroups (body mass index (BMI) < 30 and BMI ≥ 30) according to the mean BMI of patients. Calculation for each subgroup was based on the random effects model. The results were expressed as the standardized mean difference and 95% confidence intervals. OSA, obstructive sleep apnea; SD, standard deviation; SMD, standardized mean difference; CI, confidence interval; BMI, body mass index.
Figure 4.
Subgroup analysis based on country and sex composition of study patients. (a) Studies were divided into two subgroups (East Asia and Turkey) according to the country in which the research was conducted. Calculation for studies from East Asia and Turkey was based on the fixed and random effects models, respectively. (b) Studies were divided into two subgroups (male and mixed sex) according to the sex composition of the study patients. Calculation for studies with only male patient groups and studies with mixed-sex patient groups was based on the fixed and random effects models, respectively. The results were expressed as the standardized mean difference and 95% confidence intervals. OSA, obstructive sleep apnea; SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.
Meta-regression analysis
We conducted a meta-regression analysis to further assess the effect of confounding factors on differences in the NLR between OSA patients and controls. The outcome variable was the SMD of the NLR, and the covariates included differences in age (years), BMI, and the proportion of male patients (%). Only the difference in BMI (P = 0.0003) was found to exert a significant effect on the difference in the NLR, whereas differences in age (P = 0.3849) and the proportion of male patients (%) (P = 0.1275) showed no significant effect (Supplementary Fig. 1a–c).
Publication bias
Visual inspection of funnel plots revealed the existence of asymmetry (Supplementary Fig. 2). Egger’s regression test also suggested evidence of publication bias (t = 2.2406, P = 0.0372). However, the trim-and-fill method showed that no study required to ensure symmetry and the adjusted SMD was not changed (adjusted SMD = 0.62, 95% CI 0.29–0.94, P = 0.002).
Sensitivity analysis
Stability of the results was evaluated through sensitivity analysis. The corresponding pooled SMD values were not substantially altered when single studies were sequentially removed, with an effect size ranging between 0.46 and 0.66, suggesting that the results of the meta-analysis were stable (Supplementary Fig. 3).
The effect of CPAP treatment on NLR
In our primary search results, two studies18,28 compared NLR before and after CPAP treatment. We performed a meta-analysis of those studies to investigate the effect of CPAP treatment on NLR. There was no significant difference in NLR before and after treatment with CPAP (P = 0.20) (Supplementary Fig. 4).
Discussion
Although the association between the NLR and OSA has drawn considerable attention and has been widely investigated, previous results have been controversial. In the present study, we comprehensively analyzed the results of 10 studies through a meta-analysis and found that the NLR value was higher in OSA patients than in control subjects. These results indicate that neutrophilic inflammation may play a key role in the pathogenesis of OSA.
In recent years, both local and systemic inflammation as well as changes in immune response have been reported in patients with OSA. Previous studies have indicated that intermittent hypoxia selectively activates nuclear factor-κB (NF‑κB) pathways29,30. In addition, the levels of several inflammatory markers, including C-reactive protein, interleukin-6, and tumor necrosis factor, have been widely recognized as being higher in OSA patients31–33, suggesting that inflammatory activation is critical in the pathogenesis of OSA. CPAP treatment significantly reduces elevated inflammatory markers in OSA patients34. Several studies have also demonstrated that systemic inflammation may contribute to a higher risk of cardiovascular diseases in patients with OSA7,35 and elevated levels of inflammatory markers are associated with a higher cardiovascular risk36.
Because neutrophils and lymphocytes play a major role in inflammatory responses through the release of various inflammatory mediators, their absolute counts or relative values may reflect the status of systemic inflammation. NLR is superior to other individual leukocyte parameters (e.g. neutrophil, lymphocyte, and total leukocyte counts) in terms of stability, because it is a ratio of two different immune pathways37,38. Hence, the NLR has been proposed as a novel inflammatory marker associated with many chronic diseases, including coronary heart disease, hypertension, pulmonary thromboembolism, psoriasis, and various malignancies12,39–41. A previous study also reported an association between an elevated NLR and mortality in a group of non-ST-elevation myocardial infarction patients42. Similarly, our meta-analysis demonstrated that the NLR was associated with OSA. However, NLR may be influenced by certain factors, including bacterial infection43 and medications44,45. Therefore, the NLR is a particularly reliable marker in patients without conditions that may affect inflammatory response. The mechanism underlying the elevation of the NLR in OSA patients may be related to hypoxia-induced chronic inflammation. Neutrophils from patients with moderate and severe OSA demonstrate prolonged survival, which is associated with higher NF‑κB levels and worse balance of pro-apoptotic and anti-apoptotic proteins30,46,47. In the case of lymphocytes, some researchers have proposed that the decrease in lymphocytes reflects a higher degree of physiological stress48,49.
In subgroup analyses, we found that the difference in the NLR between patients and controls increased gradually with the severity of OSA. These findings strongly suggest that the NLR may reflect disease severity, and the activation of the inflammatory process is linked to the disease activity of OSA. In addition, considering that the difference in the NLR between patients and controls was larger in obese patients and the difference in BMI was correlated with the NLR in meta-regression analysis, obesity may play a key role in systemic inflammation in OSA patients via unknown mechanisms. Furthermore, several investigators reported that the NLR value was higher in obese individuals than in control subjects with normal weight50,51. Therefore, comparison with BMI-matched controls is needed to avoid the confounding effect of obesity on NLR. Notably, no statistically significant differences in the NLR were observed between patients and controls in studies with only male patients and studies from East Asia. Previous studies using a large US national data set reported that females and non-Hispanic black were associated with lower NLR52,53, indicating that gender and ethnicity may affect NLR value. In addition, given that racial difference in inflammatory responses has been proposed54, increase of inflammatory biomarker levels in patients with OSA may be different between races. Similarly, differential inflammatory responses between male and female subjects might explain the discrepancy in the results among each subgroup according to sex composition. It has been described that females show a more pronounced response to inflammatory stimuli55. Furthermore, pro-inflammatory cytokine responses and NF-κB activation of mononuclear cells following sleep disturbance were more exaggerated in females than in males56,57. However, concluding a relationship between these parameters and the NLR in OSA patients may be premature because the results were derived from few studies. Future studies with a large number of patients may be required to obtain more definitive results.
This study had several limitations. First, there might be a referral bias because all the studies enrolled in this meta-analysis were hospital-based studies. Population-based studies may provide more accurate information on the general population, compared to hospital-based studies. Second, we excluded articles in foreign languages, which may have biased the results. Third, the medication status and co-morbidities of OSA patients were unknown, which may represent uncontrollable factors in our analysis. Fourth, all enrolled studies were case series, which generally have a lower level of evidence than prospective cohort studies. Fifth, a potential bias may be derived from the geographical background of included studies. The majority of included studies investigated Turkish population. Further studies conducted in other countries are required to validate our results. Despite these limitations, this meta-analysis has several considerable strengths. First, we included the largest number of studies available. Second, our study was the first to conduct analyses to investigate the effects of potential modifiers.
In summary, our meta-analysis demonstrated that the NLR of patients with OSA was significantly higher than that of controls. Furthermore, the difference in the NLR between patients and controls gradually increased with the severity of OSA. These findings indicate that the NLR may be a reliable marker for detecting systemic inflammation and predicting disease activity in patients with OSA.
Supplementary information
Acknowledgements
This study was supported by a Faculty Research Grant of Yonsei University College of Medicine (6-2018-0167) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1A02049236) to H.-J.C. This research was also supported by the Bio & Medical Technology Development Program of NRF funded by the ministry of Science, ICT & Future Planning (NRF-2016M3A9D5A01952414).
Author contributions
M.-S.R., J.-H.Y., and H.-J.C. designed the study. M.-S.R. and H.-J.C. carried out the literature search and extracted data. M.-S.R. and C.-H.K. analyzed the data. M.-S.R. and H.-J.C. wrote the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67708-w.
References
- 1.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy P, et al. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers. 2015;1:15015. doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am. J. Respir. Crit. Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.McNicholas WT, Bonsigore MR, Management Committee of, E. C. A. B. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur. Respir. J. 2007;29:156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 7.Williams A, Scharf SM. Obstructive sleep apnea, cardiovascular disease, and inflammation—is NF-kappaB the key? Sleep Breath. 2007;11:69–76. doi: 10.1007/s11325-007-0106-1. [DOI] [PubMed] [Google Scholar]
- 8.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–636. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 9.Celikbilek M, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J. Clin. Lab. Anal. 2013;27:72–76. doi: 10.1002/jcla.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paliogiannis P, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur. Respir. Rev. 2018;27:170113. doi: 10.1183/16000617.0113-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mertoglu C, Gunay M. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab. Syndr. 2017;11(Suppl 1):S127–S131. doi: 10.1016/j.dsx.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Paliogiannis P, et al. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: a systematic review and meta-analysis. Clin. Exp. Med. 2019;19:37–45. doi: 10.1007/s10238-018-0538-x. [DOI] [PubMed] [Google Scholar]
- 13.Günbatar H, et al. The relationship between neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with obstructive sleep apnea syndrome. Dicle Tıp Dergisi. 2015;42:289–293. [Google Scholar]
- 14.Korkmaz M, Korkmaz H, Küçüker F, Ayyıldız SN, Çankaya S. Evaluation of the association of sleep apnea-related systemic inflammation with CRP, ESR, and neutrophil-to-lymphocyte ratio. Med. Sci. Monit. 2015;21:477–481. doi: 10.12659/MSM.893175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koseoglu S, et al. Relationship between neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and obstructive sleep apnea syndrome. Adv. Clin. Exp. Med. 2015;24:623–627. doi: 10.17219/acem/47735. [DOI] [PubMed] [Google Scholar]
- 16.Sunbul M, et al. The association of neutrophil to lymphocyte ratio with presence and severity of obstructive sleep apnea. Bratisl. Lek. Listy. 2015;116:654–658. doi: 10.4149/bll_2015_125. [DOI] [PubMed] [Google Scholar]
- 17.Yenigun A, Karamanli H. Investigation of the relationship between neutrophil-to-lymphocyte ratio and obstructive sleep apnoea syndrome. J. Laryngol. Otol. 2015;129:887–892. doi: 10.1017/S0022215115001747. [DOI] [PubMed] [Google Scholar]
- 18.Oyama J, et al. The relationship between neutrophil to lymphocyte ratio, endothelial function, and severity in patients with obstructive sleep apnea. J. Cardiol. 2016;67:295–302. doi: 10.1016/j.jjcc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Uygur F, et al. The neutrophil-to-lymphocyte ratio in patients with obstructive sleep apnoea syndrome and its relationship with cardiovascular disease. Heart Lung. 2016;45:121–125. doi: 10.1016/j.hrtlng.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Bozkuş F, et al. Does the neutrophil-to-lymphocyte ratio have any importance between subjects with obstructive sleep apnea syndrome with obesity and without obesity? Tuberk. Toraks. 2018;66:8–15. doi: 10.5578/tt.66535. [DOI] [PubMed] [Google Scholar]
- 21.Kıvanc T, Kulaksızoglu S, Lakadamyalı H, Eyuboglu F. Importance of laboratory parameters in patients with obstructive sleep apnea and their relationship with cardiovascular diseases. J. Clin. Lab. Anal. 2018;32:e22199. doi: 10.1002/jcla.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan ZQ, Lu XX, Long H, Li TP, Zhang YH. The association of hemocyte profile and obstructive sleep apnea. J. Clin. Lab. Anal. 2019;33:e22680. doi: 10.1002/jcla.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 27.Lubin JH, Boice JD., Jr Lung cancer risk from residential radon: meta-analysis of eight epidemiologic studies. J. Natl. Cancer Inst. 1997;89:49–57. doi: 10.1093/jnci/89.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Ozdemir C, Sokucu S, Aydin S, Onur ST, Kara K. Response of blood parameters to CPAP treatment in patients with obstructive sleep apnea. Noro Psikiyatr Ars. 2019;56:182–185. doi: 10.29399/npa.23011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 30.Htoo AK, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10:43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 31.Li QS, Zheng X. Tumor necrosis factor alpha is a promising circulating biomarker for the development of obstructive sleep apnea syndrome: a meta-analysis. Oncotarget. 2017;8:27616–27626. doi: 10.18632/oncotarget.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motamedi V, et al. Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Med. 2018;43:71–76. doi: 10.1016/j.sleep.2017.11.1121. [DOI] [PubMed] [Google Scholar]
- 33.Nadeem R, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J. Clin. Sleep Med. 2013;9:1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning Y, et al. Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Sleep Breath. 2019;23:77–86. doi: 10.1007/s11325-018-1662-2. [DOI] [PubMed] [Google Scholar]
- 35.Kokturk O, Ciftci TU, Mollarecep E, Ciftci B. Elevated C-reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndrome. Int. Heart J. 2005;46:801–809. doi: 10.1536/ihj.46.801. [DOI] [PubMed] [Google Scholar]
- 36.Danesh J, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 37.Azab B, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am. J. Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 38.Azab B, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann. Surg. Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, et al. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am. J. Hypertens. 2015;28:1339–1346. doi: 10.1093/ajh/hpv034. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Ma J, Jiang Z, Ming L. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: a systematic review and meta-analysis. Int. Angiol. 2018;37:4–11. doi: 10.23736/S0392-9590.17.03848-2. [DOI] [PubMed] [Google Scholar]
- 41.Stotz M, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable as well as inoperable pancreatic cancer. Br.J. Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gul M, et al. Predictive value of neutrophil to lymphocyte ratio in clinical outcomes of non-ST elevation myocardial infarction and unstable angina pectoris: a 3-year follow-up. Clin. Appl. Thromb. Hemost. 2014;20:378–384. doi: 10.1177/1076029612465669. [DOI] [PubMed] [Google Scholar]
- 43.Naess A, Nilssen SS, Mo R, Eide GE, Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. 2017;45:299–307. doi: 10.1007/s15010-016-0972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fici F, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J. Cardiovasc. Pharmacol. 2013;62:388–393. doi: 10.1097/FJC.0b013e31829f716a. [DOI] [PubMed] [Google Scholar]
- 45.Karaman M, et al. The comparative effects of valsartan and amlodipine on vWf levels and N/L ratio in patients with newly diagnosed hypertension. Clin. Exp. Hypertens. 2013;35:516–522. doi: 10.3109/10641963.2012.758734. [DOI] [PubMed] [Google Scholar]
- 46.Dyugovskaya L, Polyakov A, Lavie P, Lavie L. Delayed neutrophil apoptosis in patients with sleep apnea. Am. J. Respir. Crit. Care Med. 2008;177:544–554. doi: 10.1164/rccm.200705-675OC. [DOI] [PubMed] [Google Scholar]
- 47.Dyugovskaya L, Polyakov A, Cohen-Kaplan V, Lavie P, Lavie L. Bax/Mcl-1 balance affects neutrophil survival in intermittent hypoxia and obstructive sleep apnea: effects of p38MAPK and ERK1/2 signaling. J. Transl. Med. 2012;10:211. doi: 10.1186/1479-5876-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibson PH, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am. J. Cardiol. 2010;105:186–191. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am. J. Cardiol. 1997;79:812–814. doi: 10.1016/s0002-9149(96)00878-8. [DOI] [PubMed] [Google Scholar]
- 50.Aydin M, et al. Neutrophil/lymphocyte ratio in obese adolescents. North Clin. Istanb. 2015;2:87–91. doi: 10.14744/nci.2015.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vehapoglu A, Turkmen S, Goknar N, Ozer OF. Reduced antioxidant capacity and increased subclinical inflammation markers in prepubescent obese children and their relationship with nutritional markers and metabolic parameters. Redox Rep. 2016;21:271–280. doi: 10.1080/13510002.2015.1133035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howard R, Scheiner A, Kanetsky PA, Egan KM. Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio. Ann. Epidemiol. 2019;38:11 e16–21 e16. doi: 10.1016/j.annepidem.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS ONE. 2014;9:e112361. doi: 10.1371/journal.pone.0112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferguson JF, et al. Race and gender variation in response to evoked inflammation. J. Transl. Med. 2013;11:63. doi: 10.1186/1479-5876-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Eijk LT, et al. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit. Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 56.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav. Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Irwin MR, et al. Sleep loss activates cellular inflammatory signaling. Biol. Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.