Abstract
T-cell-stimulating cytokines have shown promise as monotherapies or in combination with other therapeutic modalities for immunotherapy of cancer. However, their efficacy is limited due to their short half-life, pleiotropic roles, and induction of severe toxicity even at therapeutic doses. To overcome these major therapeutic barriers, cytokine-based products are being further developed to improve their therapeutic index. These approaches include manipulating their activity to preferentially bind to effector immune cells rather than immune-suppressive cells, prolonging their half-life in vivo and modifying them to target tumors. This review focuses on IL-2, IL-15, and IL-10, which have potent effects on immune cells that mediate effective antitumor responses. We will summarize the recent progress of these cytokines in both preclinical studies and selective clinical applications and will discuss our perspectives on the development of new strategies to potentiate cytokine-based immunotherapy.
Keywords: IL-2, IL-15, IL-10, cytokine therapy, cancer
Subject terms: Cytokines, Tumour immunology
Introduction
Immunotherapy has emerged as a new pillar of cancer treatment. Most studies focus on stimulating T cells by blocking checkpoints or enhancing costimulation signals. Cytokines are a group of major factors that regulate both innate and adaptive immune responses in autocrine and paracrine manners throughout the host immune system. Among the cytokines that have shown natural killer (NK) cell- and CD8+ T-cell-mediated immune responses against tumors, interleukin-2 (IL-2), interleukin-15 (IL-15), and interleukin-10 (IL-10) have recently achieved rapid advances in their development as clinical therapeutics for cancer treatment. The application of these cytokine-based therapeutics for clinical study in cancer treatment has shown encouraging results, such as an increase in NK or/and CD8+ T cells that may play key roles in mediating antitumor effects. On the other hand, after systemic administration of IL-2, IL-15, or IL-10, a short half-life and treatment-related adverse events are major barriers to therapeutic efficacy. In this review, we focus on recent progress in the understanding of therapeutic mechanisms and the development of strategies to overcome these barriers to improve antitumor effects with reduced toxicity.
Interleukin-2 (IL-2)
IL-2 is a 15.5-kDa glycoprotein and is mainly produced by activated CD4+ T cells.1–3 Other immune cells, such as activated CD8+ T cells, NK cells, NKT cells, and innate lymphoid cells (ILCs), can also produce IL-2 to a lesser extent.4,5 The IL-2 receptor is a heterocomplex of three subunits, the IL-2Rα, IL-2Rβ, and IL-2Rγ chains, which are also known as CD25, CD122, and CD132, respectively.6,7 To activate intracellular signals, IL-2 must bind with the intermediate-affinity (Kd 10−9 M) IL-2Rβγ heterodimer or the strong-affinity (Kd 10−11 M) IL-2Rαβγ trimer. While low-dose IL-2 primarily stimulates and expands regulatory T cells (Tregs) due to their enriched expression of the high-affinity receptor complex (IL-2Rαβγ), a higher dose of IL-2 allows extra IL-2 to also stimulate CD8+ T cells and NK cells.8–10 Rosenberg et al. showed that systemic administration of a high dose of IL-2 (HD-IL-2) resulted in an overall objective response rate of 14%, with complete responses seen in 5% of patients with metastatic renal cell carcinoma and malignant melanoma.11–14 As such, the use of HD-IL-2 has been approved as a first-line immunotherapeutic agent to treat cancer patients. However, systemic HD-IL-2 treatment has been associated with severe host toxicity, including vascular leak syndrome, due to damage to endothelial cells expressing the IL-2 receptor and unwanted inflammatory responses due to elevated proinflammatory cytokines released by peripheral T and NK cells. As such, patients receiving HD-IL-2 treatment need to do so at specialized hospitals.
Various strategies have been developed to improve IL-2 therapy efficacy and reduce toxicity. For example, one study has demonstrated that injection of a certain monoclonal antibody against IL-2 resulted in the formation of a complex between endogenous IL-2 and this antibody.15 Furthermore, they demonstrated that this effect not only led to the proliferation of memory CD8+ T cells and NK cells that express the intermediate-affinity receptor IL-2Rβγ but also prevented IL-2 from binding to IL-2Rα on Tregs, a major immunosuppressive cell population within the tumor microenvironment (TME). Moreover, this approach was also tested in a preclinical toxicity study, which showed that the complex of IL-2 and the anti-IL2 antibody prevented vascular leak syndromes such as pulmonary edema by blocking IL-2 from binding to IL-2Rα on lung endothelial cells.16 Finally, it was reported very recently that the IL-2/anti-IL2 antibody complex can overcome immune checkpoint blockade resistance in murine melanoma models in a CD8+ T-cell-dependent manner when combined with anti-PD-1 antibodies or in both a CD8+ T-cell- and NK cell-dependent manner when combined with anti-CTLA-4 antibodies.17 It is likely that anti-CTLA-4 antibodies deplete Tregs, allowing much more IL-2 to stimulate effector cells while mitigating immune suppression by Tregs.
Alternatively, an IL-2 “superkine” was generated based on the IL-2/IL-2R binding structure confirmation. This approach aimed to increase the binding affinity for IL-2Rβ while also eliminating the binding affinity for IL-2Rα and maintaining optimal signal transduction.18 In line with the IL-2/anti-IL-2 antibody complex, the IL-2 superkine demonstrated improved antitumor activity with an expansion of CD8+ T and NK cells, a decrease in Tregs and reduced toxicity.18 Later, in an attempt to overcome the short half-life, PEGylated IL-2 with reduced IL-2Rα binding and a prolonged half-life (termed NKTR-214) was generated and tested in both preclinical and clinical studies. In murine melanoma models, NKTR-214 significantly increased the ratio of CD8+ T cells to Tregs and resulted in improved antitumor efficacy compared with recombinant IL-2 (aldesleukin).19 Notably, in murine breast cancer models, the combination of NKTR-214 and anti-CTLA-4 antibodies generated synergistic and durable antitumor effects.19 It was reported very recently that NKTR-214 can also synergize with anti-PD-L1 therapy in a variety of murine tumor models.20 Clinically, preliminary results have reported an increase in NK and CD8+ T cells in the peripheral blood of patients receiving NKTR-214,21 and another study recently reported a decrease in intratumoral Tregs in a small cohort of cancer patients treated with NKTR-214.20 In addition, a 23% (6 out of 26 patients) tumor reduction response rate as well as a well-tolerated safety profile was also reported.22 It is expected that the response rate could increase upon combination with other treatments. However, PEGylated products do not specifically target tumor tissues, and technical improvements must be made to achieve tumor-targeting products with precisely PEGylated sites. Otherwise, variations in clinical outcomes may be obtained due to inconsistently manufactured products. The ongoing clinical trials on the combination therapy of NKTR-214 with immune checkpoints and other therapeutics, as well as its limitations, are reviewed elsewhere.23
To increase IL-2 delivery to tumor sites, along with the consideration that IL-2-Fc fusion can prolong its half-life,24 antibody-based tumor-targeted delivery of IL-2 has been attempted by our group and other groups. In mouse studies, it has been shown that when delivered systemically, a tumor-targeting antibody-IL-2 fusion protein can localize to tumor sites, including lung micrometastases.25 More importantly, the fusion protein alone can induce effective CD8+ T-cell-mediated antitumor immune responses, activate NK cells, and reduce IL-2 retention in peripheral blood, which may lead to lower toxicity.26 Finally, the fusion protein resulted in significantly improved antitumor effects compared with either antibody or cytokine monotherapy and even better effects than the combination of the antibody and cytokine therapies.25–28 A tumor-specific antibody fusion with engineered IL-2 has also been developed and demonstrated similar antitumor efficacy to one containing wild-type IL-2 but with reduced toxicity in both immune-competent mice and cynomolgus monkeys.29 Recently, our group developed a next-generation antibody-IL-2 fusion protein.30 We engineered IL-2 by introducing several mutations that have been reported to reduce its binding to IL-2Rα (F42A)31 and increase its binding to IL-2Rβ (super IL-2).18 The engineered IL-2 was linked to an Fc moiety to generate a super mutant IL2-Fc (termed sumIL-2-Fc). To improve the tumor targeting of sumIL-2-Fc, we generated a heterodimeric sumIL-2 fusion protein that is composed of a sumIL-2 Fc monomer on one arm and an antitumor monoclonal antibody on the other arm, named Ab-sumIL-2. In a mouse study, the prolonged half-life of Ab-sumIL-2 in serum was ~8 h, whereas FDA-approved recombinant IL-2 was cleared from circulation in minutes.15 Notably, Ab-sumIL-2 not only showed superior antitumor efficacy over fusion proteins containing only F42A or super IL-2 but also achieved improved efficacy when combined with surgery, small molecule targeted therapy, and even immune checkpoint blockade. Cold (fewer TILs) tumors often fail to respond to various immunotherapies, and converting cold tumors to hot ones is challenging. In mice bearing the TUBO mammary tumor model, which is considered a cold tumor due to a lower frequency of CD8+ TILs than murine tumor models such as B16F10 and MC38,30 antibody-sumIL-2 generated synergistic antitumor effects with an EGFR tyrosine kinase inhibitor (EGFR-TKI), most likely through an increase in TILs. Furthermore, for the treatment of metastatic diseases, we used a 4T1 murine mammary carcinoma model with spontaneous metastases, which allowed us to mimic the clinical setting by surgically removing primary tumors established in the mammary gland. Interestingly, we observed that the treatment regimen of preadministration of Ab-sumIL-2 followed by surgery prolonged mouse survival compared with Ab-sumIL-2 administered as an adjuvant. This antitumor effect was dependent on both CD4+ and CD8+ T cells. In addition, our results showed that Ab-sumIL-2 increased PD-L1 expression on intratumoral DCs and that the combination of Ab-sumIL-2 and anti-PDL-1 therapy could overcome resistance to immune checkpoint blockade. Since IL-2 plays a pivotal role in T-cell survival and proliferation, it is possible that targeting tumors with Ab-sumIL-2 may help expand TILs that are brake-released intratumorally and/or those that are newly recruited into tumors when combined with immune checkpoint blockades.
One of the prospective uses of immune cell growth cytokines is in combination with cell therapy, as a sufficient number of tumor-reactive cells may require exogenous growth factors to sustain their survival and expansion after cell transfer. With respect to this combination strategy, a recent study showed that the combination of an IL-2-Fc fusion protein, antitumor antibody, and adoptive T-cell transfer induced durable tumor control through activation and expansion of tumor-specific CD8+ T cells.24 Considering that the Ab-sumIL-2 immunocytokine has a longer half-life and less toxicity than unmodified IL-2, tumor-targeted delivery, and preferential binding to CD8+ T cells over Tregs, it is expected that tumor-targeted Ab-sumIL-2 may be further developed as a new agent to improve immunotherapies, including immune checkpoint blockade agents and cell therapies, for cancer patients.
Interleukin-15 (IL-15)
IL-15 has a heterotrimeric receptor including IL-15Rα and IL-2Rβγ, which is also used by IL-2. In contrast to IL-2, IL-15 is mainly produced by monocytes, macrophages, and dendritic cells,32 with a pivotal role in NK cell development and in the homeostasis of memory CD8+ T cells. Its effects on other types of immune cells, such as ILCs, were also identified in recent studies.33 Importantly, IL-15 does not stimulate Tregs because it does not bind to the IL-2Rα chain, which is required for the formation of the high-affinity receptor complex (IL-2Rαβγ) on Tregs to stimulate immunosuppressive signals. Of note, IL-15 associated with IL-15α on the cell surface of monocytes or DCs, rather than the IL-15 monomer, can provide strong signals in trans to NK and CD8+ memory T cells, leading to enhanced antitumor effects.34–36 As such, several versions of the IL-15/IL-15Ra complex have been developed for clinical applications. For example, an IL-15 superagonist, termed ALT-803, consisting of human IL-15 covalently linked to the sushi domain of human IL-15Rα, has been used in combination with anti-PD-1 antibodies in a phase Ib trial for patients with metastatic non-small-cell lung cancer. The preliminary results showed promising antitumor activity in 6 out of 21 patients with a tolerable safety profile.37 In addition, based on the observations of effective antitumor effects in preclinical studies,38–42 clinical trials have been initiated for combinations of recombinant IL-15 (rhIL-15) with both anti-CTLA-4 and anti-PD-1 therapy, with a CD40 agonist and with monoclonal antibodies including anti-CD52 and anti-CD20 antibodies.33 As a whole, collective clinical studies have suggested that the combination of IL-15 with other immune therapeutics to increase NK and CD8+ T-cell-mediated antitumor immunity may improve efficacy, but the risk of IL-15 therapy-induced adverse events should be kept in mind since IL-15 can activate NK and T cells in peripheral blood (reviewed elsewhere).33
To improve IL-15 efficacy with reduced toxicity, several novel strategies have been very recently developed and tested in preclinical studies. For example, since IL-2 and IL-15 share the same receptor, IL-2Rβγ, computational approaches to design proteins mimics of IL-2 and IL-15 that bind human and mouse IL-2Rβγ chains but do not bind IL-2Rα or IL-15Rα were utilized, and the resulting protein was termed Neo-2/15.43 The resulting product can recapitulate the natural signaling function of IL-2 and IL-15 but does not carry the adverse effects associated with IL-2Rα or IL-15Rα binding. This product has shown superior therapeutic activity with reduced toxicity in the treatment of murine tumors, suggesting the possibility of creating superior therapeutic candidates in the future.43 Another study examined the tumor-targeted delivery of an IL-15 superagonist by using CAR-T cells, which were manipulated by protein nanogels to selectively release IL-15 into the TME.44 As such, this approach would not only increase the therapeutic window for cytokine-based therapy but also achieve the combined therapeutic efficacy of both cytokine and T-cell therapy. This strategy may be feasible to utilize in the next generation of cytokines by engineering the cytokine itself, creating a “pro-cytokine” that is only activated in the TME rather than systemically. This would subsequently potentiate intratumoral effects while mitigating the induction of host toxicity.
Interleukin-10 (IL-10)
In contrast to IL-2 and IL-15, IL-10 was initially identified as an inhibitory cytokine that is produced by Th2 cells and can inhibit Th1 cell cytokine production.45,46 Later, it was found that IL-10 can indeed be expressed not only by immune-suppressive Tregs (both Foxp3+ and Foxp3-)47,48 but also by other immune cells.49–52 In addition, the production of IL-10 by normal human epithelial cells and human melanoma cells has also been reported.53,54 Much of the known roles of IL-10 are related to its immune regulatory function. In line with this, it has been demonstrated that the genetic ablation of Il10 or deficiency of the IL-10 receptor (IL-10R) is associated with inflammatory pathology and autoimmune diseases, including inflammatory bowel disease, rheumatoid arthritis, and psoriasis. The IL-10R is a heterodimer of two subunits termed IL-10Rα and IL-10Rβ, also known as IL-10R1 and IL-10R2, respectively. IL-10 initially binds IL-10R1, and the subsequent conformational change mediates IL-10 binding to IL-10R2.55 The phosphorylation and activation of intracellular STAT3 are major signaling events leading to IL-10-mediated anti-inflammatory responses,56,57 although the activation of STAT1 and STAT5 pathways has also been studied.58–60 The deletion of STAT3 in myeloid cells can cause enterocolitis and aberrant inflammation in mice.61,62 Mechanistically, the anti-inflammatory roles of IL-10 include inhibition of proinflammatory cytokine production, limitation of antigen presentation through downregulation of MHCII and costimulatory molecules, and maintenance of Foxp3 expression and the immune-suppressive function of Tregs, among others.56,63–65 As with other IL-10 family cytokines, IL-10-mediated anti-inflammatory mechanisms are still mainly understood in the context of inflammatory disease models,66,67 and the application of IL-10-based treatment has also been mainly used for inflammatory and autoimmune diseases.68
In the context of tumor models, IL-10 has shown a paradox in terms of immunological response, likely resulting from its pleiotropic action on a variety of immune cells, particularly within the complicated TME. Indeed, in most studies of antitumor immunity, endogenous IL-10 is considered a main factor contributing to the immune-suppressive TME.47,69–71 In patients, it has been reported that tumor-derived IL-10 can inhibit T-cell proliferation, likely by impairing the functions of antigen-presenting cells, including DCs and monocytes.72,73 Interestingly, it has been reported that melanoma patient-derived serum amyloid A-1 (SSA-1) protein can induce IL-10 production by immune-suppressive neutrophils, but SSA-1 can also promote the interaction between these neutrophils and invariant NKT cells, resulting in greatly decreased IL-10 secretion and reduced suppressive function of the neutrophils.74 This study suggested that IL-10 may be a key factor in modulating the plasticity of certain immune cell subsets, such as neutrophils, in tumor immunity. Moreover, blockade of IL-10 signaling has been shown to enhance antitumor immunity. For example, in mouse studies, blockade of the IL-10R significantly improved chemotherapeutic efficacy in a CD8+ T-cell-dependent manner.69 In addition, blockade of IL-10 can increase the effects of anti-PD-1 therapy on melanoma patient-derived tumor-antigen-specific CD8+ T-cell expansion and function.70 Finally, it was reported that there is a positive correlation between serum levels of IL-10 and tumor progression.75,76
However, paradoxically, increasing evidence demonstrates that IL-10 can induce antitumor effects in an immune-dependent manner.77–83 Particularly, recent preclinical studies report that systemic delivery of a pegylated form of IL-10 (PEG-IL10), initially designed to prolong the half-life of IL-10, can inhibit tumor growth by enhancing intratumoral CD8+ T-cell proliferation and function.81,82 A phase I clinical trial with PEG-IL10 has also shown encouraging antitumor activity in the treatment of patients with advanced solid tumors.84 Furthermore, the combination of PEG-IL10 treatment and anti-PD-1 therapy was tested as a treatment for patients with solid tumors. Recent preliminary results reported that the combination of PEG-IL10 and anti-PD-1 therapy had a 42% overall response rate among 19 patients. In a subsequent phase Ib trial, the results showed that the objective response rates were 43% among 28 patients with non-small-cell lung cancer and 40% among 35 patients with renal cell carcinoma.85 However, PEG-IL10-induced toxicity has been observed in both preclinical and clinical studies. Systemic administration of PEG-IL10 to treat murine tumors showed an increase in immune cell infiltration and pathological immune responses in several normal organs, possibly due to off-tumor delivery of PEG-IL10.81 Moreover, patients receiving the highest doses of PEG-IL10 showed treatment-related adverse events,84 in line with other clinical studies that showed that systemic administration of rIL-10 promotes proinflammatory cytokine production, potentially limiting therapeutic effects.86,87
To overcome its short half-life and allow tumor-targeted delivery of IL-10, we recently generated a bispecific fusion protein targeting both an oncogenic receptor and IL-10R.88 For proof-of concept studies, we chose the FDA-approved anti-epidermal growth factor receptor (anti-EGFR) antibody cetuximab (Erbitux) for targeted delivery of IL-10 to EGFR+ tumors. We generated an antioncogenic receptor antibody-based fusion protein, termed CmAb-(IL10)2, and tested whether it could overcome the short half-life of rIL-10 while minimizing off-tumor toxicity, evaluated its antitumor effects and elucidated the mechanisms by which CmAb-(IL10)2 improves CD8+ T-cell-mediated antitumor responses. Our results showed that the half-life of CmAb-(IL10)2 is ~40 h. Notably, at least 10% of the initial dose of CmAb-(IL10)2 was detected and retained in serum up to 4 days after administration. By comparison, in mice, PEG-IL10, which was specially designed to prolong the circulation time of IL-10, was detected at less than 10% of the initial dose in serum 24 h after i.v. adminstration.89 Importantly, our results in mouse tumor models suggest that systemic delivery of CmAb-(IL10)2 can target IL-10 to and retain it within tumor tissue, leading to superior antitumor effects over nontargeted IL-10 fusion proteins.88
Ultimately, although IL-10 is generally known as an immunosuppressive cytokine, it has also been shown to promote CD8+ T-cell responses in cancer treatment.68,80–82 We speculate that the dose and administration schedule of exogenous IL-10 and the abundance, activation status, and expression of the IL-10R on CD8+ TILs may contribute to IL-10-mediated antitumor effects. However, it remains elusive how the immune-suppressive effects of IL-10 can contribute to CD8+ T-cell-mediated immune responses against tumors. As such, we further investigated the therapeutic mechanisms of CmAb-(IL10)2 in cancer treatment. We revealed a new mechanism by which CmAb-(IL10)2 suppresses DC-mediated antigen-specific CD8+ T-cell apoptosis, leading to higher levels of intratumoral, tumor-specific CD8+ T cells in a DC-IL10R signal-dependent manner.88 The molecular mechanism linking this role of DCs to T-cell enhancement is that CmAb-(IL10)2 regulates the IL-12/IFN-γ production axis to suppress IFN-γ-mediated apoptosis of antigen-specific CD8+ T cells (as denoted in Fig. 1). Our finding is in support of recent studies showing that apoptosis of CD8+ TILs may be a critical barrier to effective immunotherapy90 and further suggests that IL-10-based treatment may provide a novel strategy to improve immunotherapy for cancer. In support of our findings, we observed that the combination of CmAb-(IL10)2 with anti-CTLA-4 and anti-PD-L1 blockade therapy in the treatment of advanced murine tumors had greatly improved therapeutic efficacy compared with either IL-10 or immune checkpoint blockade therapy alone. Moreover, this improved efficacy was associated with a decrease in tumor-specific CD8+ TIL apoptosis.88 Taken together, these results suggest that immunotherapies aimed at stimulating effector cells to produce high levels of IFN-γ, such as checkpoint blockade therapies that can significantly increase the expression of IFN-γ by TILs,91,92 would be ideal candidates for rational combination with IL-10-based therapies to prevent T-cell apoptosis. In future studies, since anti-CTLA-4 and anti-PD-1 therapies induce different antitumor effects through specific mechanisms,93 it will be necessary to dissect the role of IL-10 in combination with either anti-CTLA-4 or anti-PD-1 antibodies to better design an optimal treatment regimen. A major issue with immune checkpoint blockade therapy, particularly when combining anti-CTLA-4 and anti-PD-L1 strategies together, is the frequency of immune-related adverse events.94,95 Although we did not observe toxicity in mice treated with the combination of CmAb-(IL10)2 and anti-CTLA-4 and anti-PD-L1 antibodies, whether it would have limited toxicity in humans remains to be determined.
Fig. 1.
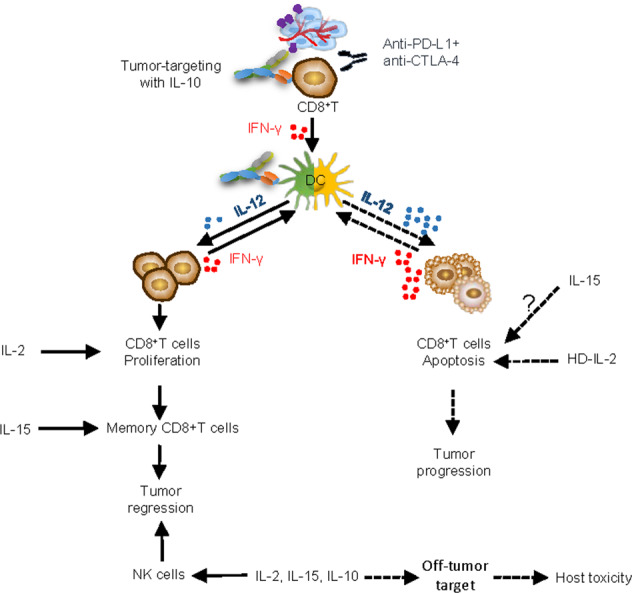
Distinct and cooperative roles of IL-10, IL-2, and IL-15 in antitumor immunity. Tumor-targeted delivery of IL-10 inhibits intratumoral CD8+ T-cell apoptosis, which may offer a strong rationale for combining IL-10-based strategies with immunotherapies that can potently boost T-cell proliferation and function, such as IL-2 and immune checkpoint blockade therapy, and the addition of IL-15 may further boost NK and memory CD8+ T-cell immunity to achieve synergistic antitumor effects. Nontargeted delivery of cytokines induces host toxicity, limiting the therapeutic index
Conclusions and perspectives
Checkpoint blockade and adoptive T-cell transfer immunotherapies for cancer treatment have shown success in the clinic. Strategies aimed at improving cytokine-based therapy along with reducing toxicity are rapidly being developed, mainly through engineering better cytokines and optimizing combination therapy regimens. Clinically, NKTR-214, an engineered IL-2 molecule, has shown evidence of antitumor activity and tolerability in a first-in-human phase I study as a monotherapy in patients with advanced solid tumors.96 This led to the further combination of NKTR-214 with immune checkpoint blockade therapy, which is already under ongoing clinical trials.
Furthermore, our strategy of tumor-targeted delivery of IL-10 potentiates intratumoral CD8+ T-cell-mediated antitumor immunity without inducing host toxicity in mice. We thus expect CmAb-(IL10)2 to be a good candidate for further development, by which it can ultimately be tested in clinical studies as monotherapy or in combination immunotherapy strategies for cancer. The feasibility of generating new fusion proteins by integrating tumor-targeting molecules other than anti-EGFR antibodies or by integrating cytokines other than IL-10 would allow a broad application of this approach for the treatment of different types of tumors. Moreover, our discovery that IL-10 inhibits T-cell apoptosis offers a strong rationale for combining IL-10-based strategies with immunotherapies that can potently boost T-cell proliferation and function, such as IL-2 and immune checkpoint blockade therapy, and the addition of IL-15 may further boost NK and memory CD8+ T-cell immunity to achieve synergistic antitumor effects (as denoted in Fig. 1). Given that the goal of enhancing immunotherapy to increase efficacy while minimizing therapy-induced adverse effects is important, the key next step for further development of cytokine therapy is to fully understand the mechanisms of each factor-induced response. Based on the knowledge obtained, a potential next generation of cytokine-based agents and a strategy for rational combination therapy could be generated and will ultimately provide therapeutic benefits for cancer patients.
Competing interests
The authors declare no competing interests.
Contributor Information
Jian Qiao, Email: Jian.Qiao@UTSouthwestern.edu.
Yang-Xin Fu, Email: Yang-Xin.Fu@UTSouthwestern.edu.
References
- 1.Robb RJ, Smith KA. Heterogeneity of human T-cell growth factor(s) due to variable glycosylation. Mol. Immunol. 1981;18:1087–1094. doi: 10.1016/0161-5890(81)90024-9. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi T, et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983;302:305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- 4.Malek TR. The biology of interleukin-2. Annu. Rev. Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature. 2019;568:405–409. doi: 10.1038/s41586-019-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat. Immunol. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 9.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 10.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat. Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann. Surg. 1989;210:474–484. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fyfe G, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 13.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 14.Alva A, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2016;65:1533–1544. doi: 10.1007/s00262-016-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin. Biol. Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- 16.Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl Acad. Sci. USA. 2010;107:11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caudana P, et al. IL2/Anti-IL2 complex combined with CTLA-4, But Not PD-1, blockade rescues antitumor NK cell function by regulatory T-cell modulation. Cancer Immunol. Res. 2019;7:443–457. doi: 10.1158/2326-6066.CIR-18-0697. [DOI] [PubMed] [Google Scholar]
- 18.Levin AM, et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature. 2012;484:529–533. doi: 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charych DH, et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin. Cancer Res. 2016;22:680–690. doi: 10.1158/1078-0432.CCR-15-1631. [DOI] [PubMed] [Google Scholar]
- 20.Sharma M, et al. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat. Commun. 2020;11:661. doi: 10.1038/s41467-020-14471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parisi G, et al. Persistence of adoptively transferred T cells with a kinetically engineered IL-2 receptor agonist. Nat. Commun. 2020;11:660. doi: 10.1038/s41467-019-12901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marin-Acevedo JA, Soyano AE, Dholaria B, Knutson KL, Lou Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 2018;11:8. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pol, J. G., Caudana, P., Paillet, J., Piaggio, E. & Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med.217, 10.1084/jem.20191247 (2020). [DOI] [PMC free article] [PubMed]
- 24.Zhu EF, et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell. 2015;27:489–501. doi: 10.1016/j.ccell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker JC, Varki N, Gillies SD, Furukawa K, Reisfeld RA. An antibody-interleukin 2 fusion protein overcomes tumor heterogeneity by induction of a cellular immune response. Proc. Natl Acad. Sci. USA. 1996;93:7826–7831. doi: 10.1073/pnas.93.15.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du YJ, et al. Stability of the recombinant antierbB2 scFvFcinterleukin2 fusion protein and its inhibition of HER2overexpressing tumor cells. Int J. Oncol. 2013;42:507–516. doi: 10.3892/ijo.2012.1747. [DOI] [PubMed] [Google Scholar]
- 27.Gutbrodt KL, et al. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci. Transl. Med. 2013;5:201ra118. doi: 10.1126/scitranslmed.3006221. [DOI] [PubMed] [Google Scholar]
- 28.Gutbrodt KL, Casi G, Neri D. Antibody-based delivery of IL2 and cytotoxics eradicates tumors in immunocompetent mice. Mol. Cancer Ther. 2014;13:1772–1776. doi: 10.1158/1535-7163.MCT-14-0105. [DOI] [PubMed] [Google Scholar]
- 29.Gillies SD, et al. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin. Cancer Res. 2011;17:3673–3685. doi: 10.1158/1078-0432.CCR-10-2921. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z, et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat. Commun. 2019;10:3874. doi: 10.1038/s41467-019-11782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mott HR, et al. The solution structure of the F42A mutant of human interleukin 2. J. Mol. Biol. 1995;247:979–994. doi: 10.1006/jmbi.1994.0194. [DOI] [PubMed] [Google Scholar]
- 32.Bamford RN, Battiata AP, Waldmann TA. IL-15: the role of translational regulation in their expression. J. Leukoc. Biol. 1996;59:476–480. doi: 10.1002/jlb.59.4.476. [DOI] [PubMed] [Google Scholar]
- 33.Waldmann, T. A., Miljkovic, M. D. & Conlon, K. C. Interleukin-15 (dys)regulation of lymphoid homeostasis: implications for therapy of autoimmunity and cancer. J. Exp. Med. 217, 10.1084/jem.20191062 (2020). [DOI] [PMC free article] [PubMed]
- 34.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 36.Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine. 2012;59:479–490. doi: 10.1016/j.cyto.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrangle JM, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19:694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M, et al. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc. Natl Acad. Sci. USA. 2009;106:7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, et al. Augmented IL-15Ralpha expression by CD40 activation is critical in synergistic CD8 T cell-mediated antitumor activity of anti-CD40 antibody with IL-15 in TRAMP-C2 tumors in mice. J. Immunol. 2012;188:6156–6164. doi: 10.4049/jimmunol.1102604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu P, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc. Natl Acad. Sci. USA. 2012;109:6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moga E, et al. Interleukin-15 enhances rituximab-dependent cytotoxicity against chronic lymphocytic leukemia cells and overcomes transforming growth factor beta-mediated immunosuppression. Exp. Hematol. 2011;39:1064–1071. doi: 10.1016/j.exphem.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc. Natl Acad. Sci. USA. 2018;115:E10915–E10924. doi: 10.1073/pnas.1811615115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva DA, et al. De novo design of potent and selective mimics of IL-2 and IL-15. Nature. 2019;565:186–191. doi: 10.1038/s41586-018-0830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang L, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 2018;36:707–716. doi: 10.1038/nbt.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today. 1991;12:A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 47.Bedke T, Muscate F, Soukou S, Gagliani N, Huber S. Title: IL-10-producing T cells and their dual functions. Semin. Immunol. 2019;44:101335. doi: 10.1016/j.smim.2019.101335. [DOI] [PubMed] [Google Scholar]
- 48.Saravia J, Chapman NM, Chi H. Helper T cell differentiation. Cell Mol. Immunol. 2019;16:634–643. doi: 10.1038/s41423-019-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhry A, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 51.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 52.Lin X, et al. IL-10-producing regulatory B cells restrain the T follicular helper cell response in primary Sjogren’s syndrome. Cell Mol. Immunol. 2019;16:921–931. doi: 10.1038/s41423-019-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarry A, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J. Clin. Investig. 2008;118:1132–1142. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Itakura E, et al. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod. Pathol. 2011;24:801–809. doi: 10.1038/modpathol.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon SI, Logsdon NJ, Sheikh F, Donnelly RP, Walter MR. Conformational changes mediate interleukin-10 receptor 2 (IL-10R2) binding to IL-10 and assembly of the signaling complex. J. Biol. Chem. 2006;281:35088–35096. doi: 10.1074/jbc.M606791200. [DOI] [PubMed] [Google Scholar]
- 56.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharm. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 57.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief. Funct. Genom. 2013;12:489–498. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J. Immunol. 1995;155:1079–1090. [PubMed] [Google Scholar]
- 59.Wehinger J, et al. IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett. 1996;394:365–370. doi: 10.1016/0014-5793(96)00990-8. [DOI] [PubMed] [Google Scholar]
- 60.Rahimi AA, Gee K, Mishra S, Lim W, Kumar A. STAT-1 mediates the stimulatory effect of IL-10 on CD14 expression in human monocytic cells. J. Immunol. 2005;174:7823–7832. doi: 10.4049/jimmunol.174.12.7823. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi M, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Investig. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsukawa A, et al. Stat3 in resident macrophages as a repressor protein of inflammatory response. J. Immunol. 2005;175:3354–3359. doi: 10.4049/jimmunol.175.5.3354. [DOI] [PubMed] [Google Scholar]
- 63.Conaway EA, de Oliveira DC, McInnis CM, Snapper SB, Horwitz BH. Inhibition of inflammatory gene transcription by IL-10 is associated with rapid suppression of lipopolysaccharide-induced enhancer activation. J. Immunol. 2017;198:2906–2915. doi: 10.4049/jimmunol.1601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiorentino DF, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 65.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, et al. Type 3 innate lymphoid cell-derived lymphotoxin prevents microbiota-dependent inflammation. Cell Mol. Immunol. 2018;15:697–709. doi: 10.1038/cmi.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang, X., Wong, K., Ouyang, W. & Rutz, S. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb. Perspect. Biol.11, 10.1101/cshperspect.a028548 (2019). [DOI] [PMC free article] [PubMed]
- 69.Ruffell B, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun Z, et al. IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res. 2015;75:1635–1644. doi: 10.1158/0008-5472.CAN-14-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilke CM, et al. Dual biological effects of the cytokines interleukin-10 and interferon-gamma. Cancer Immunol. Immunother. 2011;60:1529–1541. doi: 10.1007/s00262-011-1104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int. J. Cancer. 1997;73:309–316. doi: 10.1002/(sici)1097-0215(19971104)73:3<309::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 73.Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J. Immunol. 1999;163:6251–6260. [PubMed] [Google Scholar]
- 74.De Santo C, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat. Immunol. 2010;11:1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 76.Mannino MH, et al. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367:103–107. doi: 10.1016/j.canlet.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Giovarelli M, et al. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J. Immunol. 1995;155:3112–3123. [PubMed] [Google Scholar]
- 78.Berman RM, et al. Systemic administration of cellular IL-10 induces an effective, specific, and long-lived immune response against established tumors in mice. J. Immunol. 1996;157:231–238. [PubMed] [Google Scholar]
- 79.Zheng LM, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J. Exp. Med. 1996;184:579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujii S, Shimizu K, Shimizu T, Lotze MT. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood. 2001;98:2143–2151. doi: 10.1182/blood.v98.7.2143. [DOI] [PubMed] [Google Scholar]
- 81.Mumm JB, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Emmerich J, et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 2012;72:3570–3581. doi: 10.1158/0008-5472.CAN-12-0721. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, et al. Autocrine complement inhibits IL10-dependent T-cell-mediated antitumor immunity to promote tumor progression. Cancer Discov. 2016;6:1022–1035. doi: 10.1158/2159-8290.CD-15-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naing A, et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J. Clin. Oncol. 2016;34:3562–3569. doi: 10.1200/JCO.2016.68.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naing A, et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 2019;20:1544–1555. doi: 10.1016/S1470-2045(19)30514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lauw FN, et al. Proinflammatory effects of IL-10 during human endotoxemia. J. Immunol. 2000;165:2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 87.Tilg H, et al. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut. 2002;50:191–195. doi: 10.1136/gut.50.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiao J, et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8(+) T cell apoptosis. Cancer Cell. 2019;35:901–915.e4. doi: 10.1016/j.ccell.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Mattos A, de Jager-Krikken A, de Haan M, Beljaars L, Poelstra K. PEGylation of interleukin-10 improves the pharmacokinetic profile and enhances the antifibrotic effectivity in CCl(4)-induced fibrogenesis in mice. J. Control Release. 2012;162:84–91. doi: 10.1016/j.jconrel.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 90.Horton BL, Williams JB, Cabanov A, Spranger S, Gajewski TF. Intratumoral CD8(+) T-cell apoptosis is a major component of T-cell dysfunction and impedes antitumor immunity. Cancer Immunol. Res. 2018;6:14–24. doi: 10.1158/2326-6066.CIR-17-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng W, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi LZ, et al. Interdependent IL-7 and IFN-gamma signalling in T-cell controls tumour eradication by combined alpha-CTLA-4+alpha-PD-1 therapy. Nat. Commun. 2016;7:12335. doi: 10.1038/ncomms12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei SC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–1133.e1117. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brahmer JR, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wolchok JD, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bentebibel SE, et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rbetagamma-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 2019;9:711–721. doi: 10.1158/2159-8290.CD-18-1495. [DOI] [PubMed] [Google Scholar]


