Abstract
Background: The mechanisms underlying the initiation of sustained ventricular tachycardia (VT) have not been fully elucidated. The extent to which reentry, abnormal automaticity, and triggered activity play a role in VT differs depending on the etiology of left ventricular dysfunction. By analyzing electrograms from implantable cardioverter defibrillator (ICD), we sought to determine whether there were differences in VT initiation patterns between patients with ischemic and nonischemic cardiomyopathy.
Methods: We analyzed ICD electrograms in patients with ejection fractions < 40% who had sustained VT over a 27‐month period. The trigger for VT onset was classified as a ventricular premature beat (VPB), supraventricular tachycardia, or of “sudden onset.” The baseline cycle length, VT cycle length, coupling interval, and prematurity ratio were recorded for each event. The prematurity ratio was calculated as the coupling interval of the VT initiator divided by the baseline cycle length.
Results: Sixty‐three VT events in 14 patients met the inclusion criteria. A VPB initiated the VT in 58 episodes (92%), 1 episode (2%) was initiated by a supraventricular tachycardia, and 4 episodes (6%) were sudden onset. The prematurity ratio was significantly higher (P < 0.05) in patients with ischemic cardiomyopathy (0.751 ± 0.068) as compared to patients with nonischemic cardiomyopathy (0.604 ± 0.139).
Conclusion: VPBs initiated most sustained VT episodes. A significantly higher prematurity ratio was observed in the ischemic heart disease group. This may represent different mechanisms of VT initiation in patients with ischemic versus nonischemic heart disease.
Keywords: ventricular tachycardia, implantable cardioverter defibrillators, reentry, prematurity ratio
Electrophysiologic mechanisms responsible for initiation and maintenance of ventricular arrhythmias have not been fully elucidated. Reentry, abnormal automaticity, and triggered activity all play a role in the generation of sustained ventricular tachycardia (VT) though the relative contribution of each mechanism is unclear. Reentry, the dominant mechanism in ischemic heart disease, is believed to play a lesser role in patients with nonischemic cardiomyopathy. 1 , 2 , 3 , 4
Prior to widespread implantable cardioverter defibrillator (ICD) use, research of spontaneous VT was limited to 12‐lead electrocardiography and short‐term telemetry monitoring. Advances in ICD technology have revolutionized both the investigation and treatment of life‐threatening arrhythmias. 5 , 6 , 7 ICD capabilities now routinely include automated recording of electrograms prior to, during, and following an arrhythmia. With this information, tachycardia initiation patterns can be analyzed yielding indirect information about tachycardia mechanisms. 8 , 9
The purpose of this study was to investigate whether differences in heart failure etiology influence mode of VT initiation. We hypothesized that the majority of VT episodes would be initiated by ventricular premature beats (VPBs) and that the prematurity of the VPBs would differ based on the etiology of the left ventricular dysfunction.
METHODS
Study Population
Inclusion Criteria
The study group consisted of patients who are followed in Beth Israel Medical Center's ICD clinic and who had their device placed between 1996 and 2003. Patients were also required to have had a coronary angiogram at Beth Israel Medical Center and either a left ventriculogram or an echocardiogram showing a left ventricular ejection fraction (EF) ≤ 40%.
The study period was from February 2001 through May 2003. All electrograms from the included patients' ICDs, stored during that time period, were reviewed. Episodes of spontaneous sustained VT that were terminated by antitachycardia pacing or direct‐current cardioversion were identified. Events that had at least four recorded beats before and four recorded beats after the VT were included in our analysis.
One hundred and eighteen patients met the selection criteria. Seventeen of those patients had a total of 122 episodes of sustained VT during the 28‐month study period. Of these, 63 episodes in 14 patients met the electrocardiogram inclusion criteria. Thirty‐five (59%) of the excluded episodes were from one patient who had 44 episodes, only 9 of which were saved at the time of the event.
ICD and Stored Intracardiac Electrograms Acquisition and Analysis
All included ICDs (11 dual‐chamber and 3 single‐chamber ventricular devices) had diagnostic memory and were capable of recording and storing intracardiac electrograms, allowing for correct classification of the treated arrhythmia and its trigger. 10 Devices were programmed to record atrial and ventricular cycle lengths before, during, and after each arrhythmia. Intracardiac electrograms were recorded only from the onset of the arrhythmia episode.
Interpretation of Intracardiac Electrograms
Stored data were analyzed to identify all sustained VT events. Monomorphic VT was defined by a sudden increase in rate associated with a uniform electrogram with regard to QRS polarity, amplitude, and morphology with a constant cycle length (did not vary more than 10% from the mean cycle length) of more than 240 ms. 10 Atrioventricular dissociation, in those patients with dual chamber devices, was also used as a criterion for VT.
Interpretation of ICD Data
VT episodes were divided into three groups based on the beats preceding the VT; triggered by a VPB, triggered by a supraventricular tachycardia, or sudden onset. VPBs could be single or multiple and were defined as all premature recordings of ventricular origin between baseline rhythm and VT. Sudden onset VT was diagnosed when there were no beats between the baseline rhythm and the onset of VT.
For each event, the baseline cycle length, VT cycle length, and coupling interval of the initiating beat were recorded. The prematurity of the initiating beat of the tachycardia was assessed using the “prematurity ratio,” defined as the coupling interval of the complex initiating VT divided by the baseline cycle length (Fig. 1). The prematurity ratio has been utilized as a mode of analyzing onset of ventricular arrhythmias. 11 , 12 , 13 , 14 All stored data on events that triggered ICD therapy were analyzed independently by two investigators experienced at interpreting ICD interrogation data and blind to the patients' clinical information. The interobserver agreement with regard to determining the initiation of VT was 100%.
Figure 1.
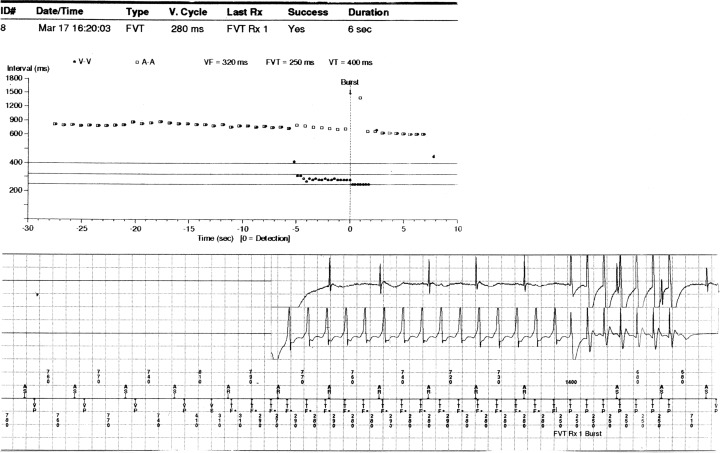
Electrogram of VT initiation and termination. (Baseline CL: 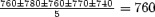 ; coupling interval: 410; prematurity ratio:
; coupling interval: 410; prematurity ratio: 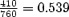 .)
.)
Clinical Data Analysis
All patients had an EF ≤ 40% as determined by ventriculogram or echocardiogram. Patients were categorized as ischemic or nonischemic based on angiographic findings. Ischemic cardiomyopathy was defined as ≥ 70% reduction in the absolute lumen diameter of ≥ 1 major epicardial artery or major branch vessel. We examined the relationship between the VT parameters described above and the patients' heart failure etiology; ischemic or nonischemic.
Statistical Analysis
For every tachycardia episode, we evaluated the relationship between etiology of ventricular dysfunction and each component of the initiation sequence of VT (pattern of onset, baseline cycle length, VT cycle length, coupling interval, and prematurity ratio). The generalized estimating equations (GEE) statistical technique was used to account for the varied number of episodes among patients. A t‐test was used to confirm any differences found with the GEE technique. Data are presented as mean ± standard deviation. A P value of <0.05 was considered statistically significant.
RESULTS
Fourteen patients (12 men and 2 women, mean age 66.4 ± 8.9 years, range 56–81 years) with 63 VT episodes met the inclusion criteria. Eight patients (57%) had ischemic cardiomyopathy and 6 patients (43%) had nonischemic cardiomyopathy.
VT was initiated by a VPB in 58 episodes (92%), by supraventricular tachycardia in 1 episode (2%) and was sudden onset in 4 episodes (6%). Of the 58 VPB triggered events, 50 (86%) were single and 8 (14%) were multiple in number. There were no statistically significant differences between the two cardiomyopathy groups with regard to the category of VT trigger. There were also no significant differences in cycle length or coupling interval between the two groups. However, the prematurity ratio was significantly higher in the ischemic cardiomyopathy group as compared to the nonischemic cardiomyopathy group (0.751 ± 0.068 vs 0.604 ± 0.139, respectively; P < 0.05) (Table 1).
Table 1.
Data Analysis of Electrograms from Ischemic and Nonischemic Cardiomyopathy Patients
| Ischemic (8 patients, 27 episodes) | Nonischemic (6 patients, 36 episodes) | P Value | |
|---|---|---|---|
| Baseline cycle length | 698 ± 157 | 769 ± 97 | 0.35 |
| Coupling interval | 522 ± 124 | 463 ± 126 | 0.4 |
| Prematurity ratio | 0.751 ± 0.068 | 0.604 ± 0.139 | <0.05 |
| VT cycle length | 347 ± 65 | 322 ± 26 | 0.36 |
DISCUSSION
The understanding of mechanisms responsible for the initiation of sustained ventricular arrhythmias remains incomplete. Extensive animal and human studies suggest that reentrant foci, often around areas of prior infarction are the primary driver of VT in ischemic heart disease. 1 , 2 , 4 However, the importance of reentry relative to abnormal automaticity and triggered activity in nonischemic cardiomyopathy is not well established.
The current study demonstrated that virtually all (92%) episodes of sustained monomorphic VT, regardless of etiology of cardiomyopathy, were immediately preceded by VPBs. This predominance of VPB triggers is consistent with findings of prior studies, though our study found the highest reported percentage of initiation by VPB. Taylor et al. 11 reported that 85% of spontaneous sustained VT was initiated by late‐coupled (prematurity ratio > 0.5) VPBs, regardless of underlying structural heart disease. In addition, Saeed et al. 12 and Gorenek et al. 13 reported that VT was initiated by VPBs in 66% and 73% of cases, respectively. Neither study found a correlation between underlying heart disease and VT initiation pattern.
Interestingly, we found that the prematurity ratio of the VT triggers in ischemic cardiomyopathy was significantly higher than in nonischemic cardiomyopathy. This may signify a different mechanism of VT initiation in the two groups. This finding is in contrast with the study by Taylor et al. 11 who found no significant differences between patients with ischemic and nonischemic cardiomyopathy with regard to prematurity ratio. However, Taylor et al. 11 included episodes of polymorphic VT and ventricular fibrillation, whereas the current study included only monomorphic VT.
Study Limitations
There are two main limitations of the present study. The small sample size is the major limitation. Use of multivariate techniques may yield unstable results when the sample size is small and the results should be viewed with caution. Second, electrogram morphology was not recorded for the beats preceding the tachycardia. Consequently, the ventricular origin of premature beats cannot be definitively determined in patients with single‐chamber devices. However, the majority of devices in our study were dual‐chamber and single or coupled supraventricular beats are unlikely to trigger sustained monomorphic VT.
CONCLUSION
In patients with reduced left ventricular systolic function, the majority (92%) of sustained monomorphic VT was initiated by VPBs. The only significant difference found between the ischemic and nonischemic groups was that the prematurity ratio was significantly higher is patients with ischemic cardiomyopathy. This finding may reflect a different mechanism of VT initiation in patients with nonischemic cardiomyopathy. Further research on the precise mechanisms responsible for VT in both ischemic and nonischemic cardiomyopathy is needed.
REFERENCES
- 1. Josephson ME. Electrophysiology of ventricular tachycardia: An historical perspective. J Cardiovasc Electrophysiol 2003;14: 1134 – 1148. [DOI] [PubMed] [Google Scholar]
- 2. Zipes DP. Mechanisms of clinical arrhythmias. J Cardiovasc Electrophysiol 2003;14: 902 – 912. [DOI] [PubMed] [Google Scholar]
- 3. Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation 1995;92: 1034 – 1048. [DOI] [PubMed] [Google Scholar]
- 4. Wellens HJJ, Duren DR, Lie KI. Observations on mechanisms of ventricular tachycardia in man. Circulation 1976;54: 237 – 244. [DOI] [PubMed] [Google Scholar]
- 5. Moss AJ, Zareba W, Hall WJ, et al Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346: 877 – 883. [DOI] [PubMed] [Google Scholar]
- 6. Buxton AE, Lee KL, Fisher JD, et al A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999;341: 1882 – 1890. [DOI] [PubMed] [Google Scholar]
- 7. Kadish A, Dyer A, Daubert JP, et al Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350: 2151 – 2158. [DOI] [PubMed] [Google Scholar]
- 8. Hook BG, Marchlinski FE. Value of ventricular electrogram recordings in the diagnosis of arrhythmias precipitating electrical device shock therapy. J Am Coll Cardiol 1991;17: 985 – 990. [DOI] [PubMed] [Google Scholar]
- 9. Marchlinski FE, Gottlieb CD, Sarter B, et al ICD data storage: Value in arrhythmia management. Pacing Clin Electrophysiol 1993;16: 527 – 534. [DOI] [PubMed] [Google Scholar]
- 10. Maron BJ, Shen WK, Link MS, et al Efficacy of implantable cardioverter‐defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 2004;350: 2151 – 2158. [DOI] [PubMed] [Google Scholar]
- 11. Taylor E, Berger R, Hummel JD, et al Analysis of the pattern of initiation of sustained ventricular arrhythmias in patients with implantable defibrillators. J Cardiovasc Electrophysiol 2000;11: 719 – 726. [DOI] [PubMed] [Google Scholar]
- 12. Saeed M, Link MS, Mahapatra S, et al Analysis of intracardiac electrograms showing monomorphic ventricular tachycardia in patients with implantable cardioverter‐defibrillators. Am J Cardiol 2000;85: 580 – 587. [DOI] [PubMed] [Google Scholar]
- 13. Gorenek B, Kudaiberdieva G, Birdane A, et al Initiation of monomorphic ventricular tachycardia: Electrophysiological, clinical features, and drug therapy in patients with implantable defibrillators. J Electrocardiol 2003;36: 213 – 218. [DOI] [PubMed] [Google Scholar]
- 14. Viskin S, Lesh MD, Eldar M, et al Mode of onset of malignant ventricular arrhythmias in idiopathic ventricular fibrillation. J Cardiovasc Electrophysiol 1997;8: 1115 – 1120. [DOI] [PubMed] [Google Scholar]


