Abstract
The Olive Quick Decline Syndrome by Xylella fastidiosa subspecies pauca is among the most severe phytopathological emergencies nowadays. In few years, the outbreak devastated olive groves in Apulia (Italy), potentially endangering the entire Mediterranean basin. This research aimed to develop a multiple locus VNTR analysis assay, a molecular tool to differentiate between populations of the pathogen. It has already been successfully applied to different X. fastidiosa subspecies from various plant hosts. The previously published TR loci, together with a set of new design, have been tested in silico on the genome of the Apulian De Donno strain. The resulting selection of 37 TR loci was amplified on the genomic DNAs of the Apulian strains AND from representatives of X. fastidiosa subspecies, and directly on DNA extracted from infected plants. The assay clearly discerned among subspecies or even sequence types (ST), but also pointed out variants within the same ST so as to provide more detailed information on the dynamics and pathogen diffusion pathways. Its effective application even on total DNAs extracted from infected tissues of different host plants makes it particularly useful for large-scale screening of infection and for the strengthening of containment measures.
Subject terms: Microbial ecology, Bacterial evolution, Bacteriology, Classification and taxonomy, Genetic markers, Haplotypes, Genetic variation
Introduction
Xylella fastidiosa is a Gram-negative phytopathogenic bacterium belonging to the Xanthomonadaceae family, which is able to infect and cause diseases on 563 plant species1. It is transmitted by several xylem sap-feeding insect species, especially sharpshooters, froghoppers (Hemiptera: Cicadellidae) and spittlebugs (Hemiptera: Cercopidea)2. The bacterium grows in the xylem of the host, where it actively multiplies and forms a biofilm slowly occluding xylem vessels, thus causing water stress and nutritional deficiencies3. X. fastidiosa causes a broad range of symptoms according to the infected host. The first disease attributable to this pathogen was the Pierce’s disease (PD) on grapevine. Over the years, many other diseases have progressively been reported on crops, ornamentals and woody plants4, such as Citrus Variegated Chlorosis (CVC)5, and several leaf scorch diseases e.g. almond leaf scorch (ALS). At present, relying on DNA-DNA hybridization and MLST data2, four X. fastidiosa subspecies have been classified, each one specific for a particular range of host plants and a native zone6–8. These subspecies are: (1) fastidiosa, which causes, among others, PD and ALS in North and Central America, (2) sandyi causing OLS in California, (3) multiplex, associated with scorch diseases of a wide range of trees in North and South America, and (4) pauca, mostly found in South America on citrus and coffee9,10. Two additional subspecies have been proposed: (1) morus, which includes isolates infecting mulberry11, and (2) tashke, from Chitalpa tashkentensis in southwestern USA 12. The evolutionary history and the geographical distribution of X. fastidiosa subspecies in the Americas indicate that the different subspecies evolved in geographic isolation, X. fastidiosa subsp. multiplex and subsp. sandyi in North America, subsp. fastidiosa in Southern-Central America and subsp. pauca in South America2. However, anthropogenic activities have introduced them into new areas. Indeed, the pathogen was reported in 2002 in Taiwan13, in 2013 in Italy14, in 2014 in Iran15, in 2015 in France10, in 2016 in Spain16, and in 2018 in Portugal17 and Israel18. All these new outbreaks raised up the need to accurately estimate the changes of genetic features to understand the dynamics and evolutionary process of populations, as well as the adaptation to different hosts and environments. In response to this, several molecular technologies have been tested on X. fastidiosa. These include non-sequence-based methods, such us restriction fragment length polymorphism (RFLP)19, randomly amplified polymorphic DNA (RAPD)20, and amplified fragment length polymorphism (AFLP)21, but with some limitations due to low reproducibility and potential homoplasy of alleles. Sequenced-based methods targeting specific regions such as 16S rDNA or 16S-23S internal transcribed spacer (ITS) were applied, with some success, as with the new subspecies tashke identified and proposed by Randall et al.12 using these approaches. It has been demonstrated that the introduction of foreign X. fastidiosa strains in new geographical areas and subsequent recombination with endemic strains may be relevant in increasing the genetic variability, shifting the target host and thus, inducing new crop diseases22. In this regard, multilocus sequence typing (MLST), based on the identification of nucleotide sequence differences in seven housekeeping genes, has been applied to study the evolution of X. fastidiosa and its subspecies6, 23. Through this method, it was proposed that the new subsp. morus originated by intersubspecific homologous recombination from X. fastidiosa subsp. fastidiosa and X. fastidiosa subsp. multiplex11. Likewise, MLST analysis of X. fastidiosa subsp. pauca isolated from coffee plants in Costa Rica and subsequently from olive trees in Italy was referred to the ST53 and provided important information about the origin of the outbreak22,24,25. However, despite being a powerful tool, MLST has some limits: relying on only seven core genome genes could be less performing in differentiating between very closely related strains and, moreover, it is not suitable for large scale and routine monitoring because of the costs of sequencing4,10,26,27. The whole genome sequencing is the most informative approach; however, although extremely powerful, this analysis requires highly skilled personnel, is time-consuming and costly, making it poorly suitable for fast and large-scale surveys28. Instead, tools based on markers with an adequate discrimination power like Simple-Sequence Repeat (SSR), also known as variable-number tandem repeat (VNTR), are a good compromise to analyse genetically homogeneous bacteria. The analysis of VNTR loci is the basis for multiple-Locus VNTR analysis (MLVA), where the number of repeats can be determined by PCR amplification using primers complementary to the well-conserved sequences flanking the tandem repeats. This method is rapid, easy to perform, inexpensive and highly reproducible28–30. Indeed, the MLVA analysis has often been adopted by microbiologists to study population structure of several human and animal bacterial pathogens, such as Mycobacterium tuberculosis, Yersinia pestis, Staphylococcus aureus, Salmonella typhimurium, and Mycobacterium bovis31. Among plant pathogens, several studies have already been carried out using SSR markers for X. fastidiosa genotyping. In 2001, Della Coletta Filho et al.32 described the efficacy of a set of 9 SSR markers in comparison with RAPD to evaluate genetic diversity of Brazilian strains of X. fastidiosa subsp. pauca, responsible for citrus variegated chlorosis (CVC) ; in 2005, Lin et al.33 used a genome-wide approach to design a new set of 34 SSR to estimate genetic diversity among 43 isolates of X. fastidiosa collected in California from grape, almond, citrus and oleander, obtaining significant results. A similar approach was used in 2007 by Montero-Astùa et al.34 on different plant species from Costa Rica, Brazil and USA in combination with other techniques to understand the relationship between strains. In 2014, Della Coletta-Filho et al.35 used MLVA to provide information on the genetic diversity of populations in sweet orange, as well as the consequences of vector transmission of X. fastidiosa on their structure. Another successful SSR genotyping has been proposed to analyse the seasonal and annual variation in genetic diversity of this bacterium in two almond orchards in California36. In the same year a combined use of SNP-based assay and multilocus SSR markers was attempted to assess the genetic diversity of X. fastidiosa subsp. pauca infecting citrus and coffee37. More recently, a new MLVA assay with 7 SSR markers was also used to demonstrate that X. fastidiosa subsp. pauca populations from coffee have higher genetic diversity and allelic richness compared with those from citrus38. These researches represented the starting point for this study, focused on X. fastidiosa subsp. pauca, which gained enormous attention after its detection on olive trees in Italy in 201339 and its new pathogenic expression described as Olive Quick Decline Syndrome (OQDS)40. It represents an exemplary model of the introduction of an exotic pathogen in an area where a cultivated species, not coevolved with the pathogen, proves to be extremely susceptible to its attack. This, together with the transmission by non-host-specific xylem-feeding insects and the difficulty to control a pathogen living in the vascular system of the infected plant, has led to an impressive spread of the disease within few years. Despite massive efforts in containment measures, nowadays thousands of hectares of olive groves in Apulia are sevrely affected by the syndrome and the spread is still going on. Again, in this situation, a fine-tuned genotyping of the strains responsible for the outbreak is crucial to understand where the disease comes from, how it moves in the infected areas and to monitor if new variants would appear in this scenario or if the present type undergoes evolutionary forces that can lead to new variants. Today it is acknowledged that the strains infecting olive trees, but also other plant species in Italy, belong to the sequence type ST53, and the most plausible origin of the Olive Quick Decline Syndrome refers to strains infecting imported coffee plants (as ornamentals) from Costa Rica25,39,41,42. It is worth noting that the same ST53 was reported from imported plants in both France and the Netherlands10,41. Thus, we firstly aimed to check in silico the presence of the VNTR loci reported in the literature within the completely edited genome of the De Donno strain (accession CP020870). A further search for new VNTR loci was independently conducted on the same genome, aiming to obtain a final selection of markers to be used in a novel, inclusive MLVA assay capable to generate novel and deeper information about the genetic diversity of this subspecies, with specific reference to the Italian outbreak in Apulia.
Results
In silico analysis of VNTR loci from literature
The in silico check of the 50 TRs and related primers reported in literature on the genome of the De Donno strain of X. fastidiosa subsp. pauca showed several inconsistencies. First of all, numerous markers resulted to be the same, even if reported in the papers with different names and amplified with different primers. In some cases, the tandem repeat is also reported as reverse and opposite sequence. More specifically, SSR20 marker32 is the same as COSS1 marker38, SSR28 marker32 is the same as marker ASSR-1433, SSR30 marker32 is the same as marker OSSR-1933, SSR32 marker32 is the same as COSSR6 marker38, CSSR-17 marker33 is the same as COSSR3 marker38, OSSR-9 marker33 is the same as marker ASSR-2033, OSSR-14 marker33 is the same as CSSR45 marker38, OSSR-16 marker 33 is the same as CSSR-20 marker33, OSSR-17 marker33 is the same as CSSR-7 marker33, CSSR-18 marker33 is the same as GSSR-6 marker 33, and GSSR-12 marker33 is the same as CSSR42 marker38. These correspondences between markers from different studies and their respective positions along the De Donno genome are summarized in Table S1. In these cases only one pair of primers was chosen for the amplification, i.e. the ones whose sequences best fitted X. fastidiosa subsp. pauca strain De Donno (loci highlighted in yellow in Table S2). In addition to these duplications, several other discrepancies have been found in comparison to the De Donno genome, which are marked as nucleotides in red in Table S2. Specifically for markers from the study of Della Coletta-Filho et al.32, it was not possible to detect the reverse primer for SSR26 marker, SSR32 marker contains 2 different tandem repeats, both of 8 bp (the one reported is in green) and one SNP in the forward primer, for SSR36 and SSR40 markers, the TR was not retrievable even if their respective primers were detected with few differences, for both; finally, none of the two primers for the SSR34 marker amplification was found. Regarding the markers described by Lin et al.33, it was not possible to find the forward primer of OSSR-12 marker; OSSR-19 marker has an SNP in the reverse primer; only a single TR was found for CSSR-4, CSSR-6, GSSR-14, GSSR-15, GSSR-19, GSSR-20 markers; TR is absent for CSSR-12 and CSSR-13 markers; the primers of CSSR-16 marker show multiple annealing sequences; a different TR sequence and one SNP were found in the reverse primer for ASSR-16 marker, one SNP was found in the forward and one in the reverse primer of both ASSR-19 and GSSR-4 markers. No discrepancies were found in the 7 VNTR loci described in the paper of Francisco et al.38. After this check, several markers were discarded due to duplication, failure to detect the primer sequence or repeat sequence. Also, all the differences detected between the primer sequences and the corresponding pairing sequences on the De Donno genome were accordingly corrected (nucleotides in green in Table S2) for the synthesis of the primers for this study that are reported in Table 1.
Table 1.
Primer pairs for the amplification of the selected 37 VNTR loci and sequence of the respective tandem repeats
| TR | Locus | Forward primer | Reverse primer | TR sequence |
|---|---|---|---|---|
| 1 | SSR20 | ATGAAGAAGCCAGGATACAT | GCTACACGTGCAACAAC | ATTGCTG |
| 2 | SSR21 | AACACGGATCAAGCTCATG | GGAACACGCAATAGTAAGA | TGTTATC |
| 3 | SSR28 | GTAACGCTGTTATCTCAAT | ATTACGCTTCTTATCGCTGT | GTGTGCCT |
| 4 | OSSR-9 | TAGGAATCGTGTTCAAACTG | TTACTATCGGCAGCAGAC | TTTCCGT |
| 5 | OSSR-16 | GCAAATAGCATGTACGAC | GTGTTGTGTATGTGTTGG | CTGCTA |
| 6 | OSSR-19 | GCTGTGAACTTCCATCAATCC | GCAAGTAGGGGTAAATATGAC | CAGGATCA |
| 7 | OSSR-20 | ATCTGTGCGGCGGTTCTG | CACTTGCGGCGTAGATACTTC | AGGATGCTA |
| 8 | CSSR-7 | CACAGCGAACAGGCATTG | AGCAACCAAGACGGGAAC | CTGTGC |
| 9 | CSSR-10 | GCAACCACAAAGCCGCAG | AGCACCTCTTAGCATCACTGG | CAATGA |
| 10 | CSSR-18 | GTGCTTCCAGAAGTTGTG | GACTGTTCTCTTCGTTCAG | GCCAA |
| 11 | CSSR-19 | TGCTGTGATTGGAGTTTTGC | TCAAACGAATCTGTCCATCAAG | TGGTGAG |
| 12 | ASSR-9 | GGTTGTCGGGCTCATTCC | TTGTCACAGCATCACTATTCTC | CAAGTAC |
| 13 | ASSR-11 | AGAGGCAACGCAGGAACAG | GTGAGTTATATCGGTGCAGCAG | ACGCATC |
| 14 | ASSR-12 | TGCTCATTGTGGCGAAGG | CGCAACGTGCATTCATCG | GATTCAG |
| 15 | ASSR-16 | TTAATCAACAACGCTTATCC | TCGCAGTAGCCAGTATGC | GCTCCA |
| 16 | ASSR-19 | CGCCGACTGTCTATATGAC | TTCGTAGCAATGGCAATGTTG | ACAACG |
| 17 | GSSR_4 | GCGTTACTGGCGACAAGC | GCTCGT(C)TCCTGACCTGTG | ATCC |
| 18 | GSSR_7 | ATCATGTCGTGTCGTTTC | CAATAAAGCACCGAATTAGC | GGCAAC |
| 19 | COSSR6 | TGCTGCGCGATAACCAAGT | CATCCAATCAGCCCTAACCT | GTGATGCG |
| 20 | CSSR45 | ACAGACATCACCGGCATTG | AATGTCGCTGCCAATCCAT | CACACCGAGATGGAC |
| 21 | COSSR4 | CAAGGTGACCGCTAGCCTAT | GCTGTCATTGGGTGATGC | CAATACAC |
| 22 | COSSR5 | ACACTGACACAACAGCCACCA | AATGGTGGGTGTGATGGTTTC | CATACAGA |
| 23 | CSSR42 | ATTACGCTGATTGGCTGCAT | GTTTCATTACGCGGAACAC | TGTTATC |
| 24 | TR4 | CATACGGCAGTTCTGTGTCG | CGGGCAAGCTTTTCCCACCC | CAGCGCAT |
| 25 | TR5 | ATTCCAAGATTTGCGAGTGG | ACGATTCGAACATGGAGGTA | TTCTAG |
| 26 | TR6 | ACATCGGAGGTAGGCTGTGA | ATTGAAGACCCTTTTCAGCC | CGCTTAT |
| 27 | TR7 | GGGTTGGGTCTTTTATTTGC | CATTGACTCTCAACCCTGCTAC | GCTGT |
| 28 | TR8 | GCGGTTTGGTTGTATTGCTT | CTCACATCACGCACCGACGA | GACAGG |
| 29 | TR9 | GGTGTGCCGTGTACATTGAG | TTGCCATCACCGACACCTCT | ATGATCTGA |
| 30 | TR10 | CGTGCTGAAGTCTTGCTTGA | ACTTCACCCTACCCTGCATA | GTAACG |
| 31 | TR12 | AGGGATATAGTGCCGCGATT | TTTTGTGGTCGAACGTGCGG | GGTGTGA |
| 32 | TR15 | ATGCAGCGGTAGTCCCTCTA | CACGATGCCCACGTAGCAGC | GTGTCG |
| 33 | TR18 | TGTCATGACCGTGCTTATGG | TGGTGGTCAAGGCAGCGG | CCGCCGCCGTAACCACCG |
| 34 | TR19 | CTGCCTTGACCACCACCAC | ACAAAGCTCTCTGATCAATCAC | CCACTCCAGCTG |
| 35 | TR21 | CAGGGTGTATGGCCTGAAGT | CCTACCATCCATGCAGCAAC | CAGCACAT |
| 36 | TR23 | CAGGAGCCTCCATGAACAAT | AATGATCCTTGCTGGGTCAG | CTTCAAGAG |
| 37 | TR24 | ATGGCCCAAACATACTCCAA | TGTTCATATCTTGGTCTCAT | GTCCTG |
TRs 1–3 were obtained from Della Coletta-Filho et al., 2001; TRs 4–18 were obtained from Lin et al., 2005; TRs 19–23 were obtained from Francisco et al., 2015; TRs 24–37 are from this study.
New VNTR loci identification
The searching procedure by Tandem Repeat Finder (TRF; https://tandem.bu.edu/trf/trf.html)43 has led to the identification of 25 VNTR loci, which have also undergone an in silico check to ascertain their position on the De Donno genome and to verify any correspondence with loci selected from literature. Again, this correspondence was found for 5 of them, which were consequently discarded. At the end, 45 total VNTR loci were selected (25 from the literature and 20 newly designed) to be used in the following experimental procedures.
PCR amplification of VNTR loci
A first round of PCR was done using only genomic DNAs from two strains, the De Donno strain from olive and the V104 strain isolated from oleander, to validate the efficacy of the entire MLVA assay. The primer pairs related to newly designed VNTR loci (TR3, TR11, TR14, TR16, TR17 and TR25) produced multiple amplicons, indicating the presence of multiple pairing sites for at least one of the two primers and were consequently discarded. Also, SSR40 locus32 invariably produced a 133 bp amplicon, corresponding exactly to the sum of the only flanking regions, thus indicating the absence of the tandem repeat; whereas OSSR-2 locus33 invariably produced a 181 bp amplicon, smaller than the sum of the flanking regions; both were discarded. Table 1 shows the list of 37 loci and the related final primers used in the amplification step. According to the amplicon sizes obtained by capillary electrophoresis, the number of repeats per locus and per strain was calculated. Amplification failure was eventually coded as “0”. Thus, the haplotype of each individual was defined as the ordered sequence of 37 numbers, as reported in Table S3. It has to be noticed that the amplification of two DNAs obtained from infected tissues of Prunus dulcis and Polygala myrtifolia in the province of Lecce (Pd_Le2 and Pm_Le10) provided multiple bands in some loci. Due to these discrepancies, which cannot be properly coded as input data, both samples were excluded from the following analysis.
Data analysis
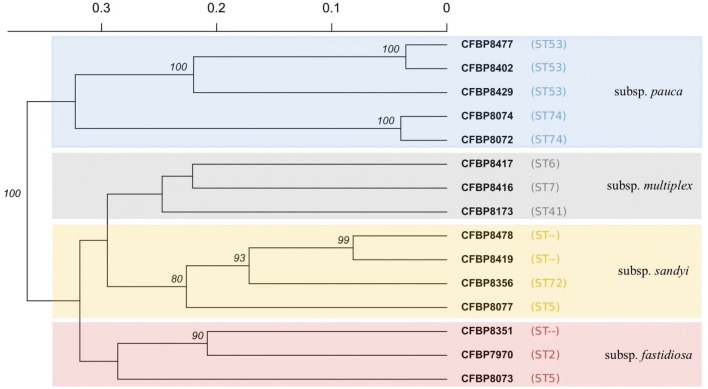
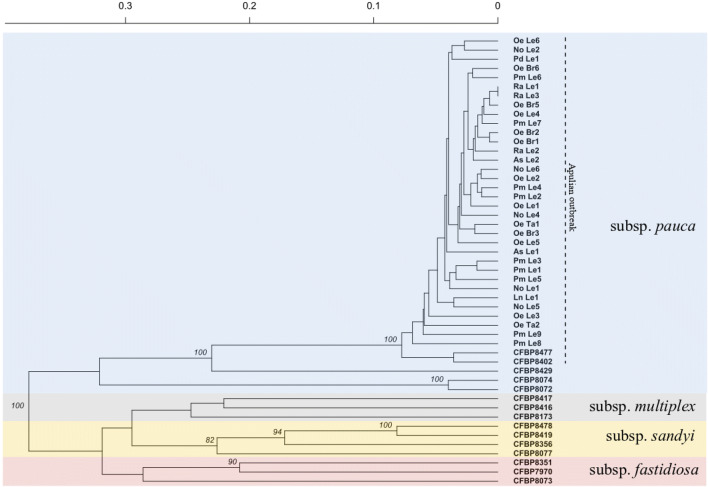
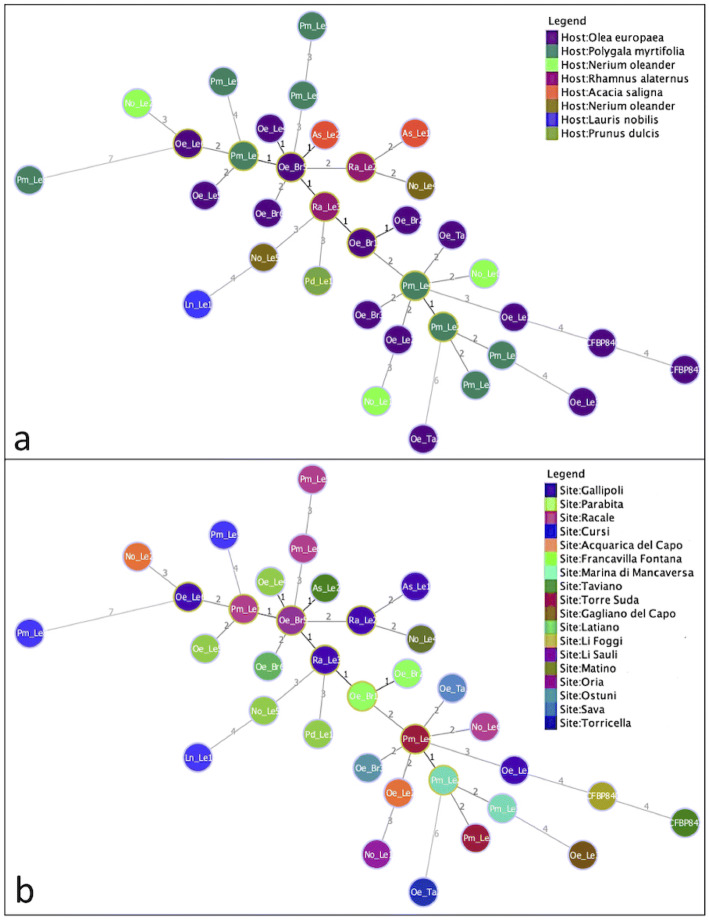
The haplotypes obtained by the amplification of 37 VNTR loci on a total of 51 DNAs are reported in Table S3, which includes the genomic DNAs extracted from 15 strains of the CFBP collection, 9 strains isolated in Apulia and 27 total DNAs as extracted from infected plant samples; the last 2 lines of the Table S3 reveal that multiple repeats have been detected in some loci of the samples Pd_Le2 and Pm_Le10, which were not considered in the subsequent analyses. The first run of data processing was carried out limited to the 15 strains from CFBP collection by hierarchical clustering using Bruvo’s distance (Fig. 1) to check the efficacy of the method to assess genetic differences between subspecies and sequence types (STs). Four main clusters were formed, each corresponding to one subspecies of X. fastidiosa. However, the strains of the same subspecies yet belonging to different STs are separated by genetic distances greater than those measured between strains belonging to the same sequence type, i.e. the two strains of subspecies pauca belonging to ST74 and two, out of three, belonging to ST53. Following the focus of the paper, the data obtained from VNTR loci amplification of DNAs extracted from strains isolated in Apulia and from DNAs extracted from infected tissues of different host plants in the same region were added for the second run of analysis. We were expecting that the very high number of loci analyzed here would highlight even minimal differences between samples. Indeed, among all the individuals, only 2 haplotypes obtained from samples of infected Rhamnus alaternus plants in the same geographic location were found completely identical to each other along the 37 loci. The hierarchical clustering of all the samples (Fig. 2) maintains the same structure as the previous dendrogram, with the addition of all the Apulian specimens to the subspecies pauca cluster. Within this group, the CFBP8429 strain, isolated from Coffea arabica plants in Angers (France) in 2015 and reported in the CFBP database as belonging to the ST53, results significantly separated from all the others ST53 from Italy. Lastly, the 38 DNAs of Apulian origin were analysed independently to better appreciate their relationships, given that their reciprocal genetic variability turned out to be extremely low. Indeed, an identical number of repetitions was obtained in as many as 18 loci out of 37 (48%), and, among the remaining 19 loci, 11 changed sporadically in few samples, whilst only 7 loci (TR7, TR8, TR12, OSSR-16, OSSR19, ASSR-16, and COSSR-4) showed frequent variations in the numbers of repeats. Table S4 reports the position of the latter loci, amplified by the primers used in this study, along with the genome of De Donno strain of X. fastidiosa subsp. pauca, and the incidental inclusion of coding sequences. In Fig. 3, the Minimum Spanning Trees (MST) illustrate how the haplotypes of these samples are linked to each other at best. The MSTs reveal that no specific correlation of the haplotypes with the species of host plants from which they were obtained (Fig. 3a) can be inferred, nor with the specific geographic origin (Fig. 3b).
Figure 1.
Hierarchical clustering of 15 Xylella fastidiosa strains from CFBP based on MLVA results and obtained using Bruvo’s genetic distance, UPGMA as agglomerative algorithm, and 1,000 bootstrap replications. Backgroud colors refers to subspecies. Only bootstrap percentages above 80% are reported.
Figure 2.
Hierarchical clustering of Xylella fastidiosa strains from both CFBP and from the Apulian oubreak, and of total DNAs from infected plants in Apulia, based on MLVA results and obtained using Bruvo’s genetic distance, UPGMA as agglomerative algorithm, and 1,000 bootstrap replications. Backgroud colors refers to subspecies. Only bootstrap percentages above 80% are reported.
Figure 3.
Minimum spanning tree (MST) of Xylella fastidiosa strains. (a) the color of the haplotypes corresponds to the host plant species; (b) the color of the haplotypes corresponds to their geographic origin at locality scale. Numbers on lines connecting two haplotypes represents the number of variable loci out of 37.
Discussion
Xylella fastidiosa is one of the most feared bacterial plant diseases nowadays. Several biological features make its management very challenging, first and foremost for its ability to colonize an astonishing number of plant hosts, often symptomless, as well as for the difficulty in its isolation and detection. A crucial issue to better understand the spread dynamics is the genotyping of the pathogen, which can be carried out by different molecular techniques. MLST is the method of choice for Xylella fastidiosa genotyping at subspecies level and beyond 44, based on the detection of SNPs included in seven constitutive genes45. By this method, it is possible to further categorize the six subspecies of Xylella fastidiosa in 87 different sequence types (STs)8,22,23,26,46. The protocol entails the specific PCR amplification of the genes using DNA extracted from both cultured strains and infected plant tissues, followed by Sanger sequencing, preferably in both strand directions44, that take time to get the final results. This might be a limit if large numbers of samples are to be analysed, as in epidemic monitoring4,10,26,27. According to this method and to our knowledge, all the strains related to the Apulian outbreak in Italy analysed so far are univocally assigned to the genotype ST53 of X. fastidiosa subsp. pauca 11,25,47. MLVA has proven suitable in genotyping Xylella as well, with a specific focus on assessing genetic variability among strains involved in local outbreaks. This distinctive feature is testified in numerous studies on different host plants such as grapevine48, orange35, almond tree36 and coffee38. Only recently, a first study using a panel of 12 SSRs on X. fastidiosa subsp. pauca strains from olive trees in Brazil was published46. This study explored the potential of this method in detecting subtle genetic differences among a selection of samples from the Xylella outbreak in Apulia, relying on its high sensitivity coupled with a mere amplicon dimension measurement. To our knowledge, a new inclusive MLVA assay was developed for the first time, and applied to this scenario. It’s worth emphasizing the importance of a preliminary in silico analysis of markers when obtained from multiple literature sources. Indeed, the screening carried out here revealed that several loci from previous independent studies were the same. This leads to the first noteworthy consideration that, even if lots of repeats can be found along a whole bacterial genome, the effective loci with features suitable for proper genotyping are much less common and numerous. Moreover, a clearer classification of markers to avoid misunderstanding and confusion in their use appears is highly needed. As an example, in Safady et al.46 twelve markers from literature have been used33,38; however, our in silico analysis demonstrated that two of them, i.e. CSSR42 and GSSR12 loci, refer to the same repetition, giving the same result. This kind of misunderstanding can negatively affect results and possibly lead to incorrect conclusions. In silico screening and the first round of PCR testing have almost halved a large number of potential markers (50 from literature and 25 newly designed) to a selection of 37 affordable TR loci. However, this is still a conspicuous set - thoroughly tested - to detect genetic differences among X. fastidiosa DNAs from the Apulian outbreak. Two analytical approaches were independently applied to haplotype data according to their peculiarities. The Bruvo’s genetic distance49 is particularly suitable for genetic markers as tandem repeats because it accounts for repeat length into calculation and is not sensitive to ploidy levels50–52. For these reasons it was used to build the dendrograms of Figs. 1 and 2. The approach proposed by Francisco et al.53, in which the efficacy of eBURST algorithm54 was reinforced with additional rules to better elucidate possible patterns of evolution, was used to analyse the Apulian dataset in detail. Although MLVA is not primarily aimed to ascertain phylogeny, due to its inherent sensitivity in appreciating minimal differences between individuals, this assay has proven proficient to correctly cluster subspecies of Xylella fastidiosa under the recognized phylogeny. However, the use of few representatives doesn’t allow to draw consistent conclusions. When compared with MLST categorization, this assay seems to have a higher discriminatory power, as highlighted by the distinction within STs in the results. Therefore, it is of particular importance that CFBP 8429 strain, isolated from a coffee plant intercepted in Angers (France) in 2015, and belonging to the ST53 (as reported in CFBP database), showed substantial differences, in MLVA analysis, in the number of repetitions compared to the other samples of the same ST53. This led to its independent positioning in hierarchical clustering, with 100% bootstrap support, and in MST. However, we cannot exclude that this discrepancy could be related to misidentification of its ST, so that a larger comparison with isolates of the same subspecies from other geographic regions is recommendable to validate this hypothesis. In this study, the MLVA assay was steadily successful in ascertaining differences within single ST, differentiating between almost all the samples. This could make its utilization valuable in scenarios like the Apulian outbreak, where a highly clonal population of X. fastidiosa subsp. pauca, belonging only to ST53 and whose origin is probably attributable to a single infection, is under investigation. In our data, such clonality is widely confirmed and information capable of differentiating between individuals are relegated to a few loci. As to these most variable loci, looking at their correspondence in the genome sequence of the De Donno strain, it is noticeable that in five out of seven the tandem repeats are included within classified or hypothetical proteins, highlighting their role for the adaptation ability of the pathogen. From a practical point of view, those VNTR loci could be selected to arrange a further MLVA assay capable to resolve close genetic relationships in this scenario. Once their robustness is confirmed, they could be multiplexed to obtain a final assay for large-scale monitoring. However, the small differences revealed within ST53 strains in this analysis don’t show evidence for specific relationships with the species of the host plant or with the geographic origin of the strains. This has not to be necessarily meant as a failure of the method. Most probably, these differences account for the first signal of random variability within the Xylella population in Apulia; they do not reflect yet the effect of any evolutionary pressure toward host-specific variants, nor to the effect of spatial separation toward local independent populations. This is consistent with the multi-host nature of the pathogen and the relatively short time lapse since the first outbreak. Since Xylella fastidiosa is very “fastidious” and time-consuming for isolation and culturing, MLVA assay developed in this study has, similarly to other molecular techniques, the significant advantage to eventually screen the DNA extracted straight from the infected plant material without losing reliability. In this respect, we also postulate that this method could diagnose infections by multiple genotypes in the same plant tissue, such as for the two samples Pd_Le2 and Pm_Le10, in which some loci showed simultaneously multiple amplicons, corresponding to different numbers of repetitions. In conclusion, all the results seem to indicate that this novel MLVA assay has the potential to become a valuable method for thorough monitoring of the Italian outbreak of Xylella fastidiosa subsp. pauca, as well as for any other Xylella fastidiosa epidemics.
Methods
All the 24 strains analysed in this study are reported in Table 2, where subspecies, host plant, geographic origin, time of isolation, and ST classification are also indicated. Fifteen strains (marked with §) were sourced from the CIRM-CFBP (Collection Française de Bactéries associées aux Plantes). These strains were grown on Buffered Charcoal-Yeast Extract (BCYE) medium at 25 °C for 3–4 weeks; 100 mg of bacterial cells were then collected, and DNA was extracted with the Nucleospin Plant kit (Macherey Nagel) according to the manufacturer’s instructions. Similarly, 9 strains (marked with *), were isolated from different host plants in Apulia and their genomic DNAs were extracted from freshly grown strains at CIHEAM-Mediterranean Agronomic Institute of Bari (CIHEAM-IAMB). The remaining 27 samples (marked with ) are instead constituted by total DNAs extracted straight from tissues of plants whose infection by X. fastidiosa was previously assessed. These include DNAs from different host plants in various locations in Apulia, i.e. in the provinces of Lecce, Taranto, and Brindisi (Fig. S155,56). All DNAs were checked by q-PCR to confirm their belonging to X. fastidiosa according to the protocol described in Harper et al.57.
Table 2.
List of X. fastidiosa strains analysed in this study and details about thier subspecies, host plant, geographic origin, time of isolation, and ST classification
| Sample | Subspecies | Host | Country (Region) | Province | Year | ST |
|---|---|---|---|---|---|---|
| CFBP8073§ | fastidiosa/sandyi | Coffea canephora | Mexico | Mexico | 2012 | ST75 |
| CFBP7970§ | fastidiosa | Grapevine | USA (Florida) | Florida | 1987 | ST2 |
| CFBP8351§ | fastidiosa | Vitis vinifera L. | USA (California) | Fresno | 1993 | – |
| CFBP8077§ | sandyi | Nerium oleander | USA (California) | Orange | 1995 | ST5 |
| CFBP8356§ | sandyi | Coffea arabica | France | (intercepted) | 2015 | ST72 |
| CFBP8419§ | sandyi | Coffea arabica | Costarica | Costarica | 2015 | – |
| CFBP8478§ | sandyi | Coffea arabica | France | (intercepted) | 2015 | – |
| CFBP8173§ | multiplex | Prunus sp. | USA (Georgia) | Georgia | 1983 | ST41 |
| CFBP8416§ | multiplex | Polygala myrtifolia | France (Corsica) | Propriano | 2015 | ST7 |
| CFBP8417§ | multiplex | Spartium junceum | France (Corsica) | Alata | 2015 | ST6 |
| CFBP8429§ | pauca | Coffea arabica | France (Loira) | Angers | 2015 | ST53 |
| CFBP8072§ | pauca | Coffea arabica | Equador | Equador | 2012 | ST74 |
| CFBP8074§ | pauca | Coffea arabica | Equador | Equador | 2012 | ST74 |
| CFBP8402§ | pauca | Olea europaea | Italy (Apulia) | Lecce | 2014 | ST53 |
| CFBP8477§ | pauca | Olea europaea | Italy (Apulia) | Lecce | 2015 | ST53 |
| Oe_Le1* | pauca | Olea europaea | Italy (Apulia) | Lecce | 2014 | ST53 |
| No_Le1* | pauca | Nerium oleander | Italy (Apulia) | Lecce | 2016 | ST53 |
| Oe_Le2* | pauca | Olea europaea | Italy (Apulia) | Lecce | 2017 | ST53 |
| No_Le2* | pauca | Nerium oleander | Italy (Apulia) | Lecce | 2017 | ST53 |
| Oe_Le3* | pauca | Olea europaea | Italy (Apulia) | Lecce | 2017 | ST53 |
| Pm_Le1* | pauca | Polygala myrtifolia | Italy (Apulia) | Lecce | 2017 | ST53 |
| Pm_Le2* | pauca | Polygala myrtifolia | Italy (Apulia) | Lecce | 2017 | ST53 |
| Pm_Le3* | pauca | Polygala myrtifolia | Italy(Apulia) | Lecce | 2017 | ST53 |
| Pm_Le4* | pauca | Polygala myrtifolia | Italy(Apulia) | Lecce | 2017 | ST53 |
| Oe_Le4 | pauca | Olea europaea | Italy (Apulia) | Lecce | 2018 | ST53 |
| Pm_Le5 | pauca | Polygala myrtifolia | Italy (Apulia) | Lecce | 2018 | ST53 |
| Pd_Le1 | pauca | Prunus dulcis | Italy (Apulia) | Lecce | 2018 | ST53 |
| Ra_Le3 | pauca | Rhamnus alaternus | Italy(Apulia) | Lecce | 2018 | ST53 |
| Ra_Le1 | pauca | Rhamnus alaternus | Italy (Apulia) | Lecce | 2018 | ST53 |
| Pm_Le6 | pauca | Polygala myrtifolia | Italy (Apulia) | Lecce | 2018 | ST53 |
| No_Le6 | pauca | Nerium oleander | Italy(Apulia) | Lecce | 2018 | ST53 |
| Oe_Le5 | pauca | Olea europaea | Italy (Apulia) | Lecce | 2018 | ST53 |
| Oe_Br5 | pauca | Olea europaea | Italy(Apulia) | Brindisi | 2018 | ST53 |
| Oe_Br1 | pauca | Olea europaea | Italy (Apulia) | Brindisi | 2018 | ST53 |
| Oe_Br2 | pauca | Olea europaea | Italy (Apulia) | Brindisi | 2018 | ST53 |
| Oe_Br6 | pauca | Olea europaea | Italy (Apulia) | Brindisi | 2018 | ST53 |
| Oe_Br3 | pauca | Olea europaea | Italy (Apulia) | Brindisi | 2018 | ST53 |
| Oe_Ta1 | pauca | Olea europaea | Italy (Apulia) | Taranto | 2018 | ST53 |
| Oe_Ta2 | pauca | Olea europaea | Italy (Apulia) | Taranto | 2018 | ST53 |
| Oe_Le6 | pauca | Olea europaea | Italy (Apulia) | Lecce | 2018 | ST53 |
| As_Le1 | pauca | Acacia saligna | Italy (Apulia) | Lecce | 2018 | ST53 |
| Ra_Le2 | pauca | Rhamnus alaternus | Italy (Apulia) | Lecce | 2018 | ST53 |
| As_Le2 | pauca | Acacia saligna | Italy (Apulia) | Lecce | 2018 | ST53 |
| Pm_Le7 | pauca | Polygala myrtifolia | Italy (Apulia) | Lecce | 2018 | ST53 |
| No_Le4 | pauca | Nerium oleander | Italy (Apulia) | Lecce | 2018 | ST53 |
| No_Le5 | pauca | Nerium oleander | Italy (Apulia) | Lecce | 2018 | ST53 |
| Ln_Le1 | pauca | Laurus nobilis | Italy (Apulia) | Lecce | 2018 | ST53 |
| Pm_Le8 | pauca | Polygala myrtifolia | Italy (Apulia) | Lecce | 2018 | ST53 |
| Pm_Le9 | pauca | Polygala myrtifolia | Italy (Apulia) | Lecce | 2018 | ST53 |
| Pd_Le2 | pauca | Prunus dulcis | Italy (Apulia) | Lecce | 2018 | ST53 |
| Pm_Le10 | pauca | Polygala myrtifolia | Italy (Apulia) | Lecce | 2018 | ST53 |
Strains marked with § were obtained from CFBP collection, strains marked with * were isolated from plants in Apulia, whilst indicates total DNAs extracted from infected plant tissues.
In silico analysis of Tandem Repeats previously reported on Xylella fastidiosa
Molecular typing of X. fastidiosa by VNTR markers was already been carried out in the past with significant results. In this study, 9 upstream markers from Della Coletta-Filho et al.32, 34 from Lin et al.33 and 7 from Francisco et al.38 have been considered. The presence of these 50 markers and their respective primers was in silico checked on the completely edited genome of the strain “De Donno” of X. fastidiosa subsp. pauca, deposited in GenBank (accession number: CP020870), using Nucleotide BLAST (for the tandem repeats) or Primer BLAST (for the respective primers) tools in NCBI website.
New VNTR loci identification and primer design
In the meanwhile, a new search, aimed to identify potential new markers to be added to the analysis, was carried out on the same genome using the TRF program43 set with the following parameters: 2 matches, 7 mismatches, 7 indels as alignment Parameters; 50 as Minimum Alignment Score; 250 as Maximum Period Size. A further selection of the results obtained was made imposing the following parameters :> 5 as Period Size, > 2 as Copy Number; > 90% as Percent Matches, Consensus Size as Period Size. This procedure led to the identification of 25 new Tandem Repeats loci for which suitable primers in respective flanking regions were designed using Primer3 with default parameters.
PCR amplification of VNTR loci
All VNTR loci were amplified with single PCR reactions using the primer pairs reported in Table 1. Each reaction contained 12.5 l of GoTaq G2 Green Master Mix Master Mix 2x (Promega Corporation, USA), 1 l of DNA sample (40 ng), 1 l of forward primer and 1 l of reverse primer (10 M final concentration), 9.5 l of molecular grade SDW to the final volume of 25 l. The PCR amplifications were performed with a C1000 thermocycler (Biorad Laboratories Inc., Ca., USA). The following parameters were used for the TR loci identified in this study: initial denaturation for 5 min at 95 C, 40 cycles of denaturation for 30 s at 95 C, annealing for 30 s at temperatures ranging from 47.9 C to 58 C according to primers requirements, and extension for 36 s at 72 C, plus a final elongation step for 5 min at 72 C. For the primers obtained from literature review 32,33,38 the respective protocols were followed.
Capillary electrophoresis
The QIAxcel capillary electrophoresis system (QIAGEN, Milan, Italy) was used to estimate precisely the size of the amplicons. The DNA High Resolution cartridge was used for all samples and the OM800 method was run, as recommended, to achieve maximum accuracy (2-3 bp maximum error) with amplicons ranging in size from 200 to 500 bp. No template controls (SDW) and size markers were included in each run. The results were analysed and interpreted using the ScreenGel v.1.6.0 software (QIAGEN), which gives accurate estimation of both size and concentration of amplicons. Then, the number of tandem repeats at each VNTR locus was calculated by subtracting the flanking regions size from the amplicon size and dividing the remaining by the repeat unit length. In case of loci with a truncated final repeat, the copy number was rounded down to the previous integer. To validate the robustness of the results, the entire PCR and capillary electrophoresis procedure was performed at least twice per sample. To check the accuracy of tandem repeats calculations, a random selection of amplicons was Sanger sequenced, as well as for each case of disputable TR number attribution. A final data matrix (Table S3) of 51 samples and 37 numbers of TR for each locus was produced.
Data processing
The string of integers obtained as above constitutes the haplotype of each strain under investigation. These were reciprocally compared using independently two analytical approaches suitable for this type of data, the hierarchical clustering and the goeBURST algorithm. Data were analysed in two steps: first, only the 15 strains from the CFBP collection were included to check the effectiveness of the method in maintaining the correct subspecies structure and in detecting differences among Sequence Types (STs); then, the remaining 34 DNAs, all related to the Apulian outbreak, were added to assess their relative positioning and grouping.
Hierarchical clustering
The data matrix was imported into R version 3.4.4 (R Core Team, 2018). The genetic distance among individuals was calculated using Bruvo’s distance49, and bootstrapped using the poppr bruvo.boot() function with a cut-off threshold of 80%. The hierarchical clustering was then obtained by hclust() function of the R package stats (R Core Team, 2018) using UPGMA as agglomerative algorithm. The final dendrogram was visualized with the R package factoextra version 1.0.558.
Globally optimized eBURST algorithm (goeBURST–Phyloviz)
The software Phyloviz 2.059 was used for the goeBURST analysis and to build the MST, a tree in which the sum of the distances among all the isolates, as represented by data, is the shortest possible.
Supplementary information
Acknowledgements
This research was financially supported by the Project: “Messa a punto di strategie molecolari per la comparazione di ceppi del batterio Xylella fastidiosa rinvenuti su olivo in Italia ed Argentina” of the Italian Ministry of Foreign Affairs and International Cooperation (MAECI). The research was carried out in the frame of the MIUR (Ministry for education, University and Research) initiative “Department of Excellence” (Law 232/2016). Y.J.R. received a PhD research fellowship from HISPRO - High Studies Program for Iraqi Officials at Italian Academic Institution-cooperation project.
Author contributions
A.M. conceived the experiments and wrote the paper, Y.J.R., M.C.T., M.T., S.C., V.T. and F.V. contributed to the experiments and data analysis, S.T. and A.M. contributed to data interpretation and bioinformatic analysis., A.M., S.T., A.M.D. and G.M.B contributed to scientific discussions. All authors reviewed the manuscript.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-68072-5.
References
- 1.EFSA Update of the Xylella spp. host plant database. EFSA J. 2018;16:5408. doi: 10.2903/j.efsa.2018.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida RPP, Nunney L. How do plant diseases caused by Xylella fastidiosa emerge? Plant Dis. 2015 doi: 10.1094/PDIS-02-15-0159-FE. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins DL. Xylella fastidiosa: Xylem-limited bacterial pathogen of plants. Plant Dis. 1989 doi: 10.1094/PDIS-02-15-0159-FE. [DOI] [Google Scholar]
- 4.Baldi P, La Porta N. Xylella fastidiosa: Host range and advance in molecular identification techniques. Front. Plant Sci. 2017;8:944. doi: 10.3389/fpls.2017.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purcell A. Paradigms: Examples from the bacterium Xylella fastidiosa. Annu. Rev. Phytopathol. 2013;51:339–356. doi: 10.1146/annurev-phyto-082712-102325. [DOI] [PubMed] [Google Scholar]
- 6.Almeida RPP, et al. Genetic structure and biology of Xylella fastidiosa strains causing disease in citrus and coffee in Brazil. Appl. Environ. Microbiol. 2008;74:3690–3701. doi: 10.1128/AEM.02388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris JL, Balci Y. Population structure of the bacterial pathogen Xylella fastidiosa among street trees in Washington DC. PLoS One. 2015;10:e0121297. doi: 10.1371/journal.pone.0121297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunney L, et al. Recent evolutionary radiation and host plant specialization in the Xylella fastidiosa subspecies native to the United States. Appl. Environ. Microbiol. 2013;79:2189–2189. doi: 10.1128/AEM.03208-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaad NW, Postnikova E, Lacy G, Fatmi M, Chang CJ, et al. Xylella fastidiosa subspecies: X. fastidiosa subsp. fastidiosa subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov. Syst. Appl. Microbiol. 2004;27:290–300. doi: 10.1078/0723-2020-00263. [DOI] [PubMed] [Google Scholar]
- 10.Denancé N, et al. Several subspecies and sequence types are associated with the emergence of Xylella fastidiosa in natural settings in France. Plant Pathol. 2017;66:1054–1064. doi: 10.1111/ppa.12695. [DOI] [Google Scholar]
- 11.Nunney L, Schuenzel E, Scally M, Bromley R, Stouthamer R. Large-scale intersubspecific recombination in the plant-pathogenic bacterium Xylella fastidiosa is associated with the host shift to mulberry. Appl. Environ. Microbiol. 2014;80:3025–3033. doi: 10.1128/AEM.04112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randall JJ, et al. Genetic analysis of a novel Xylella fastidiosa subspecies found in the southwestern United States. Appl. Environ. Microbiol. 2009;75:5631–5638. doi: 10.1128/AEM.00609-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su C, et al. Pierce’s disease of grapevines in Taiwan: Isolation, cultivation and pathogenicity of Xylella fastidiosa. J. Phytopathol. 2013;161:389–396. doi: 10.1111/jph.12075. [DOI] [Google Scholar]
- 14.Saponari M, Boscia D, Nigro F, Martelli G. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (southern Italy) J. Plant Pathol. 2013;95:668–668. [Google Scholar]
- 15.Amanifar N, Taghavi M, Izadpanah K, Babaei G. Isolation and pathogenicity of Xylella fastidiosa from grapevine and almond in Iran. Phytopathol. Mediterr. 2014 doi: 10.14601/Phytopathol_Mediterr-12647. [DOI] [Google Scholar]
- 16.Olmo D, et al. First detection of Xylella fastidiosa infecting cherry (Prunus avium) and Polygala myrtifolia plants, in Mallorca Island, Spain. Plant Dis. 2017;101:1820–1820. doi: 10.1094/PDI5-04-17-0590-PDN. [DOI] [Google Scholar]
- 17.EPPO. First report of Xylella fastidiosa subsp. multiplex in Portugal. Report, European Plant Protection Organization (2019).
- 18.EPPO. First report of Xylella fastidiosa in Israel. Report, European Plant Protection Organization (2019).
- 19.Mehta A, Pereira Leite R, Bomura Rosato Y. Assessment of the genetic diversity of Xylella fastidiosa isolated from citrus in Brazil by PCR–RFLP of the 16s r DNA and 16 S-23 S intergenic spacer and rep-PCR fingerprinting. Antonie Van Leeuwenhoek. 2001;79(53–59):2001. doi: 10.1023/A:1010219811555. [DOI] [PubMed] [Google Scholar]
- 20.Lacava P, Araújo W, Maccheroni W, Jr, Azevedo J. RAPD profile and antibiotic susceptibility of Xylella fastidiosa, causal agent of citrus variegated chlorosis. Lett. Appl. Microbiol. 2001;33:302–306. doi: 10.1046/j.1472-765X.2001.01000.x. [DOI] [PubMed] [Google Scholar]
- 21.Kishi L, Wickert E, de Macedo Lemos E. Evaluation of Xylella fastidiosa genetic diversity by fAFPL markers. Rev. Bras. Fruticult. 2008;30:202–208. doi: 10.1590/S0100-29452008000100037. [DOI] [Google Scholar]
- 22.Nunney L, Ortiz B, Russell S, Sanchez R, Stouthamer R. The complex biogeography of the plant pathogen Xylella fastidiosa: Genetic evidence of introductions and subspecific introgression in Central America. Plos One. 2014 doi: 10.1371/journal.pone.0112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan X, et al. Multilocus sequence typing of Xylella fastidiosa causing Pierce’s Disease and oleander leaf scorch in the United States. Phytopathology. 2010 doi: 10.1094/PHYTO-100-6-0601. [DOI] [PubMed] [Google Scholar]
- 24.Elbaino T, et al. Multilocus sequence typing of Xylella fastidiosa, isolated from olive affected by ‘ Olive Quick Decline Syndrome’ in Italy. Phytopathol. Mediterr. 2014;53:533–542. [Google Scholar]
- 25.Loconsole G, et al. Intercepted isolates of Xylella fastidiosa in Europe reveal novel genetic diversity. Eur. J. Plant Pathol. 2016;146:85–94. doi: 10.1007/s10658-016-0894-x. [DOI] [Google Scholar]
- 26.Coletta-Filho HD, Francisco CS, Lopes JRS, Muller C, Almeida RPP. Homologous recombination and Xylella fastidiosa host-pathogen associations in South America. Phytopathology. 2017;107:305–312. doi: 10.1094/PHYTO-09-16-0321-R. [DOI] [PubMed] [Google Scholar]
- 27.Marcelletti S, Scortichini M. Genome-wide comparison and taxonomic relatedness of multiple Xylella fastidiosa strains reveal the occurrence of three subspecies and a new Xylella species. Arch. Microbiol. 2016;198:803–812. doi: 10.1007/s00203-016-1245-1. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Raoult D, Fournier P. Bacterial strain typing in the genomic era. Fems Microbiol. Rev. 2009;33:892–916. doi: 10.1111/j.1574-6976.2009.00182.x. [DOI] [PubMed] [Google Scholar]
- 29.Kremer K, et al. Comparison of methods based on different molecular epidemiological markers for typing of mycobacterium tuberculosis complex strains: Interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 1999;37:2607–2618. doi: 10.1128/JCM.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Belkum A. Tracing isolates of bacterial species by multilocus variable number of tandem repeat analysis (MLVA) Fems Immunol. Med. Microbiol. 2007;49:22–27. doi: 10.1111/j.1574-695X.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 31.Lindstedt B. Multiple-locus variable number tandem repeats analysis for genetic fingerprinting of pathogenic bacteria. Electrophoresis. 2005;26:2567–2582. doi: 10.1002/elps.200500096. [DOI] [PubMed] [Google Scholar]
- 32.Coletta-Filho HD, et al. Differentiation of strains of Xylella fastidiosa by a variable number of tandem repeat analysis. Appl. Environ. Microbiol. 2001 doi: 10.1128/AEM.67.9.4091-4095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin H, et al. Multilocus simple sequence repeat markers for differentiating strains and evaluating genetic diversity of Xylella fastidiosa. Appl. Environ. Microbiol. 2005;71:4888–4892. doi: 10.1128/AEM.71.8.4888-4892.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montero-Astúa M, et al. Isolation and molecular characterization of Xylella fastidiosa from coffee plants in Costa Rica. J. Microbiol. 2008 doi: 10.1007/s12275-008-0072-8. [DOI] [PubMed] [Google Scholar]
- 35.Coletta-Filho HD, Francisco CS, Almeida RPP. Temporal and spatial scaling of the genetic structure of a vector-borne plant pathogen. Phytopathology. 2014 doi: 10.1094/PHYTO-06-13-0154-R. [DOI] [PubMed] [Google Scholar]
- 36.Lin H, Islam M, Cabrera-La Rosa J, Civerolo E, Groves R. Population structure of Xylella fastidiosa associated with almond leaf scorch disease in the San Joaquin Valley of California. Phytopathology. 2015;105(825–832):2015. doi: 10.1094/PHYTO-09-14-0254-R. [DOI] [PubMed] [Google Scholar]
- 37.Montes-Borrego M, Lopes JRS, Jiménez-Díaz RM, Landa BB. Combined use of a new snp-based assay and multilocus SSR markers to assess genetic diversity of Xylella fastidiosa subsp. pauca infecting citrus and coffee plants. Int. Microbiol. 2015 doi: 10.2436/20.1501.01.230. [DOI] [PubMed] [Google Scholar]
- 38.Francisco CS, Ceresini PC, Almeida RPP, Coletta-Filho HD. Spatial genetic structure of coffee-associated Xylella fastidiosa populations indicates that cross infection does not occur with sympatric citrus orchards. Phytopathology. 2017;107:395–402. doi: 10.1094/PHYTO-08-16-0300-R. [DOI] [PubMed] [Google Scholar]
- 39.Cariddi C, et al. Isolation of a Xylella fastidiosa strain infecting olive and oleander in Apulia, Italy. J. Plant Pathol. 2014 doi: 10.4454/JPP.V96I2.024. [DOI] [Google Scholar]
- 40.Saponari M, et al. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Sci. Rep. 2017 doi: 10.1038/s41598-017-17957-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergsma-Vlami M, et al. Assessment of the genetic diversity of Xylella fastidiosa in imported ornamental coffea arabica plants. Plant Pathol. 2017;66:1065–1074. doi: 10.1111/ppa.12696. [DOI] [Google Scholar]
- 42.Martelli G, Boscia D, Porcelli F, Saponari M. The olive quick decline syndrome in south-east Italy: A threatening phytosanitary emergency. Eur. J. Plant Pathol. 2016;144:235–243. doi: 10.1007/s10658-015-0784-7. [DOI] [Google Scholar]
- 43.Benson G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.EPPO Pm 7/24 (4) Xylella fastidiosa. EPPO Bull. 2019;49:175–227. doi: 10.1111/epp.12575. [DOI] [Google Scholar]
- 45.Maiden M, et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safady N, Lopes J, Francisco C, Della Coletta H. Distribution and genetic diversity of Xylella fastidiosa subsp. pauca associated with olive quick syndrome symptoms in southeastern Brazil. Phytopathology. 2019;109:257–264. doi: 10.1094/PHYTO-07-18-0273-FI. [DOI] [PubMed] [Google Scholar]
- 47.EFSA Statement on diversity of Xylella fastidiosa subsp. pauca in Apulia. EFSA J. 2016;14:4542. doi: 10.2903/j.efsa.2016.4542. [DOI] [Google Scholar]
- 48.Lin H, et al. Genetic variation of Xylella fastidiosa associated with grapevines in two major viticultural regions in the united states: California and Texas. J. Plant Pathol. 2013;95:329–337. [Google Scholar]
- 49.Bruvo R, Michiels N, D’Souza T, Schulenburg H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 2004;13:2101–2106. doi: 10.1111/j.1365-294X.2004.02209.x. [DOI] [PubMed] [Google Scholar]
- 50.Clark L, Jasieniuk M. POLYSAT: An R package for polyploid microsatellite analysis. Mol. Ecol. Resour. 2011;11:562–566. doi: 10.1111/j.1755-0998.2011.02985.x. [DOI] [PubMed] [Google Scholar]
- 51.Kamvar ZN, Tabima JF, Grunwald NJ. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ. 2014;1:1–14. doi: 10.7717/peerj.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meirmans P, Van Tienderen P. GENOTYPE and GENODIVE: Two programs for the analysis of genetic diversity of asexual organisms. Mol. Ecol. Notes. 2004;4:792–794. doi: 10.1111/j.1471-8286.2004.00770.x. [DOI] [Google Scholar]
- 53.Francisco A, Bugalho M, Ramirez M, Carrico J. Global optimal e BURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinf. 2009 doi: 10.1186/1471-2105-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feil E, Li B, Aanensen D, Hanage W, Spratt B. e BURST: Inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 2004;186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.code by Richard A. Becker, O. S., version by Ray Brownrigg. Enhancements by Thomas P Minka, A. R. W. R. & Deckmyn., A. maps: Draw Geographical Maps (2018). R package version 3.3.0.
- 56.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2016. [Google Scholar]
- 57.Harper S, Ward L, Clover G. Development of LAMP and real-time PCR methods for the rapid detection of Xylella fastidiosa for quarantine and field applications. Phytopathology. 2010;100:1282–1288. doi: 10.1094/PHYTO-06-10-0168. [DOI] [PubMed] [Google Scholar]
- 58.Kassambara, A. & Mundt, F. Factoextra: Extract and visualize the results of multivariate data analyses [R package Factoextra version 1.0.5] (2016).
- 59.Nascimento M, et al. Phyloviz 2.0: Providing scalable data integration and visualization for multiple phylogenetic inference methods. Bioinformatics. 2017;33:128–129. doi: 10.1093/bioinformatics/btw582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.