Abstract
Pregnancy is a state of immunological balance during which the mother and the developing fetus must tolerate each other while maintaining sufficient immunocompetence to ward off potential threats. The site of closest contact between the mother and fetus is the decidua, which represents the maternal–fetal interface. Many of the immune cell subsets present at the maternal–fetal interface have been well described; however, the importance of the maternal T cells in this compartment during late gestation and its complications, such as preterm labor and birth, has only recently been established. Moreover, pioneer and recent studies have indicated that fetal T cells are activated in different subsets of preterm labor and may elicit distinct inflammatory responses in the amniotic cavity, leading to preterm birth. In this review, we describe the established and proposed roles for maternal T cells at the maternal–fetal interface in normal term parturition, as well as the demonstrated contributions of such cells to the pathological process of preterm labor and birth. We also summarize the current knowledge of and proposed roles for fetal T cells in the pathophysiology of the preterm labor syndrome. It is our hope that this review provides a solid conceptual framework highlighting the importance of maternal and fetal T cells in late gestation and catalyzes new research questions that can further scientific understanding of these cells and their role in preterm labor and birth, the leading cause of neonatal mortality and morbidity worldwide.
Keywords: maternal-fetal interface, decidua, amniotic fluid, adaptive immunity, parturition
Subject terms: Inflammatory diseases, T cells
Introduction
Pregnancy, a state in which the mother must maintain a synergetic relationship with the developing fetus, depends on constant, effective crosstalk between these two genetically dissimilar individuals.1–12 Thus, pregnancy represents a complex state of balance in which the maternal immune system must tolerate the fetus3,13–17 (and vice versa18–21) and concurrently maintain a certain level of immunocompetence to protect against potential threats.22–24 In particular, such distinction necessitates systemic and local immune interactions throughout pregnancy, in which the most intimate site of contact is the maternal–fetal interface (i.e., decidua).25,26 In humans, the maternal–fetal interface comprises the decidualized endometrium, named according to the specific adjacent maternal and fetal tissues: the decidua basalis forms the junction between the uterine endometrium and the placenta, and the decidua parietalis connects the fetal membranes to the uterine wall.26,27 Together, these two unique tissues form the site of specific mechanisms through which maternal and fetal immunity are bridged to promote the maintenance of pregnancy.12,22,25,28–32
The cellular immune repertoire of the maternal–fetal interface comprises numerous innate subsets, including natural killer (NK) cells33–40 and macrophages41–47 as well as adaptive immune cells such as effector T cells,48–56 regulatory T cells (Tregs),3,45,57–60 and a fraction of B cells,38,39,49,61–64 all of which vary throughout gestation.65–68 Cell types that bridge innate and adaptive immune responses, such as invariant NK T cells (iNKT cells)69–71 and innate lymphoid cells (ILCs)67,72–75 have also been described at the maternal–fetal interface. Each of these subsets performs specific roles throughout pregnancy such as supporting placental development,35,68,76 maintaining maternal–fetal tolerance,1–12,45 and participating in the inflammatory processes that accompany labor.46,49,55,64,70,77–82 Consequently, a disruption of these different immune cell subsets is often associated with adverse pregnancy outcome.22,31,68,83 In particular, the premature influx of activated effector T cells55,84–87 or alterations in the functions or proportions of Tregs88–93 in the decidual tissues have been associated with pregnancy complications. Thus, a clearer understanding of the roles that maternal T cells play at the maternal–fetal interface in late gestation, as well as in the physiological and pathological processes of labor, is warranted.
To further explore the cellular immune repertoire of the maternal–fetal interface, recent cutting-edge molecular surveys utilizing next-generation sequencing technologies have provided a deep characterization of the many leukocyte subsets present in the decidual tissues as well as their interaction networks.94–98 Importantly, placental and decidual cell type-specific gene signatures can be monitored in the maternal circulation, providing a noninvasive means of evaluating maternal–fetal crosstalk.95,98 Pertinent to the topic discussed herein, we have shown that activated T cell-specific transcriptional signatures derived from the maternal–fetal interface are modulated during pregnancy, in term labor, and in preterm labor.98–100 This evidence further supports a role for maternal T cells in normal and complicated pregnancies.
Fetal immunity must also be considered in the context of pregnancy and its complications, as the fetus displays an established and functional T-cell repertoire beginning early in gestation.18,101–103 Indeed, pioneer studies showed that the fetal immune system is activated during the onset of preterm labor, which precedes preterm birth.104,105 Recently, this concept has been revisited to establish the contribution of fetal T cells to preterm labor and birth as well as to elucidate the stimuli that might drive such fetal T-cell activation;21,106 however, additional investigation is required in this research area.
In this review, we highlight the established and proposed roles for maternal T cells at the maternal–fetal interface in the process of normal parturition at term. Moreover, we describe the known contributions of maternal T cells to the pathological process of preterm labor and birth. Finally, we summarize current knowledge on the presence and functional status of fetal T cells in the context of preterm labor and birth. We aim to provide a clear summary of the importance of both maternal and fetal T cells in the mechanisms of physiological and pathological parturition, which can shed light on the gaps in knowledge that still remain in order to guide future research.
Maternal T cells at the maternal–fetal interface
Generally speaking, naïve T cells (TN) are present in the circulation, awaiting presentation of their cognate antigen by dendritic cells in secondary lymphoid tissues,107 after which the T cells will proliferate and differentiate to carry out effector functions.108 Once the threat has been cleared, a fraction of antigen-specific T cells will persist in the circulation as memory T cells to provide more rapid responses should the same antigen be detected again.108 Memory T cells can be further divided into central (TCM), effector (TEM), and terminally differentiated effector memory (TEMRA) T cells based on immediate or latent function and the expression of migratory homing receptors.108 In addition, CD4+ T cells (and CD8+ T cells, to some extent) can differentiate into one of several effector subsets including T helper type 1 (Th1), Th2, Th9, and Th17 cells based on the surrounding microenvironment.108,109 Various reports described below have indicated the participation of multiple T-cell subsets in both the normal physiological processes of pregnancy and in its complications such as preterm labor and birth.
Conventional effector T cells had typically been considered a relatively minor component of the cellular immune repertoire at the maternal–fetal interface prior to term parturition, with their increased presence or activation indicating inflammation-related pregnancy pathologies. Indeed, early reports indicated that suppressive mechanisms exist in the decidua that are targeted specifically toward IL-2-dependent cells,110 likely to be driven at least partially by Tregs. Seminal studies have shown that tissue-specific gene silencing mechanisms exist to prevent uncontrolled invasion of effector T cells into the decidua during early pregnancy, thereby preventing premature fetal loss.111 Thus, together with the presence of Tregs and other homeostatic cells such as macrophages,41–46 the decidual tissues represent a tightly controlled microenvironment. More recently, we and others showed that a proportion of the maternal T cells found at the maternal–fetal interface display an exhausted or dysfunctional phenotype at term pregnancy,24,82 demonstrating other mechanisms in which effector T cells are precluded from disrupting normal gestation, which is further described below.
In addition to the abovementioned mechanisms of regulation, the fetal tissues themselves contribute to the modulation of the maternal immune environment through the expression of specific HLA antigens, most notably HLA-G.112 First described as being solely expressed by extravillous trophoblast cells,113–115 soluble isoforms of this protein are found in the placenta, chorion, decidua, and even maternal blood during pregnancy.116 HLA-G interacts with several members of the immunoglobulin-like transcript (ILT) receptor family117–121 [now termed leukocyte Ig-like receptor subfamily B (LILRB)122] that are expressed by multiple subsets of immune cells,117,123 including T cells.124–128 LILRB receptors exhibit immunosuppressive behavior upon their activation and have thus been considered as immune checkpoints for T cells.129 Accordingly, studies have indicated that the interaction of fetal HLA-G with maternal T cells may promote a more tolerant state.130–135 On the other hand, several studies have suggested that altered levels of HLA-G at the maternal–fetal interface or in the maternal circulation could be associated with adverse pregnancy outcomes including preterm birth,136–140 and increased concentrations of soluble HLA-G have been detected in amniotic fluid of women with intra-amniotic infection.141 Yet, the participation of HLA-G in adverse pregnancy outcomes remains unclear.142,143 Interestingly, HLA-G expression may not be exclusive to fetal tissues during pregnancy, as recent reports have shown that subsets of immunosuppressive regulatory-like T cells can express HLA-G themselves144–146 or in some cases can acquire the expression of this molecule through trogocytosis from antigen presenting cells (APCs).147,148 The investigation of whether the latter method of HLA-G “transfer” from APCs to T cells occurs during pregnancy could provide important insight into the mechanisms of maternal–fetal tolerance during this critical period.
T cells at the maternal–fetal interface in term pregnancy
As the end of gestation approaches, conventional T cells constitute an obvious proportion of the immune cellular repertoire at the maternal–fetal interface.48,51,55,62,78,79,81,98,149 A comparative immunohistochemistry-based study reported that the proportion of CD45+CD3+ cells was significantly increased in the human term decidua compared to that in the first trimester, and such an increase was reflected by the elevated numbers of T cells expressing CD4, CD8, TCRαβ, or TCRγδ,48 suggesting that a general increase in decidual T cells occurs in late pregnancy. The comparison of specific T-cell subsets between the two main sites of the maternal–fetal interface at term indicated that the frequencies of TCRγδ+ and CD8+ T cells were greater in the decidua parietalis versus the decidua basalis, whereas the opposite was true for CD4+ T cells.62 In addition to the above observations, several studies have also indirectly confirmed the presence of conventional T cells at the maternal–fetal interface using immunofluorescence microscopy150 or immunophenotyping151 to detect CD4+ and CD8+ T cells in the murine placenta during late pregnancy. These studies indicate that T cells are residents of the maternal–fetal interface in term pregnancy, even before the onset of labor, the coordinated timing of which is critical.
The migration of conventional T cells to the maternal–fetal interface prior to term parturition is controlled at least partially through tissue-specific mechanisms: a series of studies revealed that T-cell chemotactic pathways were upregulated in the decidual tissues from cases of term labor.78–80 Moreover, decidual T cells express activation markers such as CD38 and CD69 at term pregnancy,149 diminishing the possibility of such cells being mere bystanders at the maternal–fetal interface. More recently, in support of the previously described general residence of T cells at the maternal–fetal interface, high-dimensional immunophenotyping studies showed that term decidual tissues host a heterogeneous population of CD4+ and CD8+ TN, TCM, and TEM subsets.55 In addition, decidual effector T cells expressed enhanced levels of effector molecules such as perforin and granzyme B, cytotoxic molecules that function in a cooperative manner to perforate the target cell membrane and initiate apoptosis,152 in women who underwent the physiological process of labor at term.55 This suggests that T-cell activation at the maternal–fetal interface is an intrinsic mechanism associated with the inflammatory process of labor at term, which is in line with in vitro observations showing that the stimulation of decidual T cells results in upregulation of perforin and granzymes.24 Single-cell analyses of the maternal–fetal interface complemented these prior studies by showing upregulation of the cell signature specific to activated T cells in the decidua basalis in cases of term labor compared to term deliveries without labor.98 Together, these data support a role for activated effector maternal T cells at the maternal–fetal interface in physiological parturition at term.
Effector T cells at the maternal–fetal interface in preterm labor and birth
As the presence of functional maternal effector T cells at the maternal–fetal interface is amplified toward the end of pregnancy, a premature increase in the frequency and/or activation of such cells has thus been associated with pathological pregnancy outcomes such as preterm labor and birth. Initial studies established that the invasion of cytotoxic T cells into the decidual tissues, a placental lesion classified as chronic histological chorioamnionitis, can occur in a subset of term deliveries but is more frequently observed in cases of preterm labor and birth, preterm prelabor rupture of membranes (pPROM), and fetal death.84–86 Such associations have been confirmed using flow cytometry55 and single-cell transcriptomic98 approaches to demonstrate the increased presence of effector and activated T cells at the maternal–fetal interface of women with preterm labor and birth. Indeed, these pathological cases are characterized by an influx of TEM expressing perforin and granzyme B into the maternal–fetal interface, which is even greater than that observed in labor at term.55 Further, murine studies have established that the systemic activation of T cells through the administration of an anti-CD3 antibody is sufficient to induce preterm birth55,87 through inflammatory mechanisms that are distinct from those observed in conventional models of preterm birth [e.g., administration of endotoxin (LPS) or progesterone receptor antagonist (RU486)].55 Together, these observations have caused a paradigm shift in regard to how the adaptive immune system is viewed in the context of pregnancy complications.
IL-17-producing T cells were shown to be present at the maternal–fetal interface in early pregnancy,153–157 and a disruption of the balance between Th17 cells and Tregs has been implicated in early pregnancy complications (e.g., spontaneous abortion).158–164 Yet, studies have also suggested that Th17 cells are implicated in late pregnancy disorders such as preeclampsia162,165–170 or preterm labor.171 Cases of preterm labor with chronic inflammation of the placenta showed higher amounts of IL-17 at the maternal–fetal interface and in amniotic fluid compared to preterm labor cases without this lesion, which may be released by the Th17 cells infiltrating the chorioamniotic membranes.171 Moreover, a recent transcriptomic study demonstrated increased expression of Th1- and Th17-related genes together with downregulation of Foxp3 at the maternal–fetal interface of women with preterm labor and pPROM.172 Together, these findings provide a possible link between dysregulated Th17 responses and preterm labor and birth.
Besides dedicated adaptive lymphocytes such as T cells, cells that bridge innate and adaptive immunity [e.g., iNKT cells (discussed below) and ILCs (reviewed in ref. 67)] are also present at the maternal–fetal interface in early and late gestation.173,174 Pregnant mice deficient in iNKT cells were less susceptible to endotoxin-induced preterm birth, indicating that iNKT-cell activation could be detrimental to pregnancy outcomes.69,175 Accordingly, the systemic activation of iNKT cells using α-galactosylceramide resulted in preterm delivery and increased neonatal mortality.70 Such systemic activation of iNKT cells resulted in their expansion at the maternal–fetal interface as well as concomitant reductions in the local numbers of total T cells and Tregs,71 suggesting an inverse relationship between the Tregs and iNKT cells in this compartment. Moreover, such expansion of iNKT cells at the maternal–fetal interface was accompanied by increased numbers of Th17 cells.71 In humans, transcriptomic analysis of decidual lymphocytes revealed elevated expression of CD1d (an established iNKT-cell receptor) in cases of preterm labor and birth compared to labor at term.39 Moreover, immunophenotyping consistently revealed an increased proportion of activated iNKT-like cells in the decidual basalis of women with preterm labor and birth.70 Given that iNKT cells are present at the murine maternal–fetal interface throughout pregnancy, further investigation of this unique decidual subset is required to establish the different roles of iNKT cells in early and late gestation.
Exhausted and senescent T cells at the maternal–fetal interface during pregnancy
Several physiological mechanisms exist to reduce the severity of effector T-cell functions in situations where such responses may cause more harm than good. Prime examples of these mechanisms include T-cell suppression and the more recently described phenomena of T-cell exhaustion and senescence.176,177 Although the endpoints of T-cell exhaustion and senescence are similar (i.e., the control of effector T-cell responses), these two cell fates have been demonstrated to be distinct176 and are the topic of current investigation.178–181
T-cell exhaustion typically occurs in the context of chronic antigen exposure/stimulation that results in a progressive loss of function accompanied by upregulated expression of multiple inhibitory receptors such as TIM-3, PD-1, CTLA-4, and LAG-3.182–184 Clinically, this phenomenon has been described in the context of chronic viral infections and cancers;182–185 yet, recent reports have suggested that this process occurs in pregnancy as well.24,52,82
In contrast to exhaustion, senescent T cells maintain effector functions in the absence of inhibitory receptor expression while losing the ability to proliferate,176 thus reaching a terminal state. Senescence is indicated by the upregulation of markers such as CD57, KLRG-1, and CD45RA together with the downregulation of CD27 and CD28.186–190 Among T cells, recent studies have indicated that the TEMRA subset is at least partially comprised of senescent T cells based on phenotypic and functional data.191 To date, few reports have investigated the presence of senescent T cells during pregnancy,82 evidence that is further discussed below.
Animal studies have revealed that the decidua hosts a population of CD8+PD-1+TIM-3+ T cells in early and mid-pregnancy that display enhanced inflammatory capacity upon blockade of either inhibitory marker.52 In addition, the in vivo blockade of PD-1 or TIM-3 resulted in increased rates of fetal loss, indicating that the expression of inhibitory receptors is important for pregnancy maintenance.52 This was further supported by the observation that CD8+PD-1+TIM-3+ T cells were impaired in decidual tissues from women with miscarriage.52 In the third trimester, decidual cytotoxic T cells expressed a transcriptomic signature indicative of a combination of dysfunction and activation that could be reversed by in vitro stimulation,24 and detailed immunophenotypic analysis revealed that a large proportion of CD4+ and CD8+ TEM expressed an exhausted-like PD-1+TIM-3+ phenotype at the maternal–fetal interface.82 Notably, the proportion of exhausted CD4+ T cells in the decidua parietalis increased with advancing gestational age,82 suggesting that the prolonged exposure to antigens (fetal, microbial, or even self) that occurs during pregnancy necessitates increasing regulation of these cells to avoid aberrant T-cell activation. The process of physiological labor affected exhausted T cells in the decidua basalis and decidua parietalis differently, as a significant reduction in the proportions of exhausted CD4+ and CD8+ T cells was observed in the decidua basalis of women who underwent term parturition compared to non-labor controls.82 These studies confirm that T-cell exhaustion is a physiological phenomenon at the maternal–fetal interface in late gestation; yet, further investigation into the clinical implications of exacerbated T-cell exhaustion in term gestation (delayed parturition, prolonged labor, dystocia, etc.) is needed. In line with this concept, a recent report showed that mice of advanced maternal age undergo prolonged labor and dystocia that is associated with a reduced number of pro-inflammatory T cells at the maternal–fetal interface; yet, the exhausted phenotype of such cells was not investigated.192
Besides exhaustion, a fraction of T cells at the maternal–fetal interface was shown to express surface markers consistent with a senescent phenotype (KLRG-1 and CD57) in late gestation.82 Among both CD4+ and CD8+ populations, a large proportion of senescent T cells was of the TEMRA subset,82 which is in line with previous reports.191 Unlike their exhausted counterparts, senescent T cells at the maternal–fetal interface did not significantly vary with gestational age or with the presence of labor.82 Taken together, these findings suggest that both exhaustion and senescence play a role in regulating the function of effector T cells at the maternal–fetal interface, which future mechanistic studies may demonstrate.
Exhausted and senescent T cells at the maternal–fetal interface in preterm labor and birth
Mechanistic studies demonstrated that the blockade of PD-1 and TIM-3 in early to mid-pregnancy results in fetal loss.52 However, whether a reduction in the number or function of these cells is associated with late pregnancy complications such as preterm labor and birth is still unclear. Among women with preterm labor and birth, those with acute inflammatory lesions of the placenta displayed reduced proportions of exhausted T cells in the decidua basalis.82 These observations suggest that, although T-cell exhaustion is a physiological process in late gestation, adverse events such as pathological inflammation can reactivate these cells. Such a concept is supported by the fact that the in vitro stimulation of exhausted decidual T cells results in the restoration of effector functions.24,82
Investigation focused on senescent T cells is relevant to pregnancy complications given that the process of decidual senescence has been proposed as a new mechanism of disease for preterm labor and birth (reviewed in refs. 193,194). In line with this concept, the presence of acute placental inflammation in preterm gestations, but not at term, was associated with a significantly reduced proportion of senescent T cells in the decidua basalis.82 Senescence has been demonstrated to be reversible in T cells in vitro;195–198 thus, it is possible that the pathological inflammation accompanying specific subsets of preterm labor cases will cause reversal of senescent T cells in the decidual tissues, further propagating inflammation.
Collectively, these studies suggest that the inflammatory responses accompanying preterm labor may reactivate exhausted and senescent T cells at the maternal–fetal interface, leading to an aberrant effector T-cell response that can trigger preterm labor and birth. However, further investigation into how the various etiologies of preterm labor differentially affect exhausted and/or senescent T cells at the maternal–fetal interface is warranted in order to find novel strategies to prevent the adverse neonatal outcomes associated with this syndrome.
Regulatory T cells at the maternal–fetal interface during pregnancy
Regulatory T cells (Tregs) are well known for their critical functions in preventing autoimmunity, driving successful transplantation, and modulating tumor–immune interactions (reviewed in refs. 199,200). Importantly, Tregs are also highly relevant for maternal–fetal tolerance.1–12,45,57–60 Regulatory T cells are classically described as CD4+CD25+Foxp3+ cells that display potent immunosuppressive functions.200 In particular, expression of the transcription factor Foxp3 is essential for the development, differentiation, and suppressive function of Tregs, and is thus a key phenotypic marker for the identification of such cells.201–204 Regulatory T cells are further subdivided into thymic/natural Tregs and peripheral/induced Tregs, which arise through different developmental processes.205,206 Natural Tregs are thought to be mainly involved in the control of autoimmune responses,207–209 while peripheral Tregs appear to be significant in mucosal immunity,210–214 including maternal–fetal tolerance.8,9,215–217 Regulatory T cells exert their suppressive function primarily by direct cell-cell interactions and through the release of anti-inflammatory cytokines such as IL-10,218,219 TGFβ,220,221 and IL-35.222 Such regulatory functions are especially critical for pregnancy establishment and maintenance, as the systemic depletion of Tregs prior to implantation or in early gestation leads to implantation failure or pregnancy loss.4,6–9,223–225 Yet, due to their importance in the establishment and maintenance of maternal–fetal tolerance, few studies have investigated the function of Tregs at the maternal–fetal interface in the third trimester of pregnancy, a central period for the onset of obstetrical disease.226
A role for decidual Tregs late in term gestation was first suggested when it was found that the proportions of CD3+CD4+CD25+ T cells in the decidua basalis and decidua parietalis were reduced in women with spontaneous term labor compared to those with term cesarean sections.49 The authors proposed that this subset may include decidual Tregs that “disappeared” prior to the onset of labor.49 A later study further investigated the CD4+CD25bright and CD4+CD25dim populations in decidual tissues from the second trimester and term deliveries (both cesarean section and vaginal), and showed that these populations did not change with the length of gestation.50 Subsequently, the same CD4+CD25+ population was further characterized by the expression of Foxp3 and other cellular markers associated with Tregs, revealing that the CD4+CD25bright decidual population was largely composed of Tregs with high proportions of Foxp3, CTLA-4, and HLA-DR expression, whereas the CD4+CD25dim population displayed an activated phenotype with high percentages of CD69+ cells.227 Together, these studies provided an initial overview of Tregs at the maternal–fetal interface in normal term deliveries, prompting further discovery.
A subsequent in-depth investigation included decidual samples from women undergoing cesarean section either without any signs of labor, in early labor, or in advanced labor, and confirmed that Tregs are present at the maternal–fetal interface at term.228 Notably, the authors found that the proportions of decidual Tregs declined as labor progressed, suggesting that the process of labor is associated with alterations in Treg populations at the maternal–fetal interface.228 A recent report described three distinct Treg subsets in the decidua defined by the high expression of CD25, PD-1, or TIGIT.60 The authors compared Treg populations in the decidua basalis and decidua parietalis and showed that there are no significant differences in Treg populations between these tissues, although the proportions of the CD25+ and TIGIT+ Treg subsets tended to be greater in the decidua parietalis.60 Moreover, the ability of decidual Tregs to suppress CD4+ effector T-cell proliferation was significantly reduced at term compared to those from the first trimester decidua, suggesting that the functional capacity of decidual Tregs declines, but is not completely reduced, toward the end of gestation.60
Several investigations in mice have also consistently found a Treg population in the decidual tissues prior to term delivery.229–231 Specifically, two of these reports investigating the anti-inflammatory properties of vaginal progesterone229 or human chorionic gonadotropin (hCG)231 showed that both treatments significantly increased the proportion of Tregs at the maternal–fetal interface in the third week of murine pregnancy, suggesting that the observed anti-inflammatory properties of these hormones were at least partially due to modulation of immune cell populations in this compartment (the immune modulatory functions of progesterone and hCG during pregnancy have recently been reviewed in refs. 232,233). Together, human and animal studies support the presence and functionality of Tregs at the maternal–fetal interface in term gestations.
Is there a role for Tregs at the maternal–fetal interface in preterm birth?
The importance of Tregs throughout gestation is underscored by the fact that systemic and local alterations in these cells are consistently observed in women with preeclampsia (as reviewed in refs. 91,93), a clinical condition for which the only current effective treatment is delivery of the placenta.234,235 Indeed, animal studies have demonstrated a preeclamptic-like phenotype resulting from the depletion of Tregs in early gestation.92 Such alterations hold true in late pregnancy, as preeclampsia impacts Treg numbers and function at the human maternal–fetal interface.59,88,89,170,236 Importantly, preeclampsia can have adverse effects on neonatal Treg populations, as evidenced by studies of umbilical cord blood,237,238 further extending the negative outcomes associated with this obstetric disease.
While dysfunctional or reduced Tregs have been implicated in the pathogenesis of preeclampsia, which could secondarily result in preterm delivery, the concept that Tregs could directly contribute to the onset of preterm labor has largely been ignored. Yet, a pioneer study indicated that the depletion of CD25+ cells in late pregnancy did not cause preterm birth.7 However, given that CD25 is a less specific marker for Tregs than Foxp3,239–241 the question of whether alterations in the number or function of decidual Tregs are implicated in the onset of preterm labor is still unclear. Importantly, human and murine data from our laboratory show that Tregs play a central role in the pathophysiology of preterm labor and birth as well as in neonatal well-being, highlighting the importance of these regulatory cells in the last period of pregnancy (Gomez-Lopez N, 2020, unpublished data).
Fetal T cells during pregnancy and preterm labor
Fetal T cells during early and mid-pregnancy
The fetal immune system is exposed to maternal antigens, thus necessitating the development of a tolerogenic state to avoid any potential adverse reactions.18–21 Accordingly, the cellular immunity of the fetus undergoes continuous development, in some cases starting as early as 6 weeks of gestation.18–20,101–103 Notably, maternal microchimerism (i.e., the presence of maternal cells in fetal circulation,242–244 which can persist long after delivery245), drives the induction of fetal Tregs that serve to suppress immune responses against maternal antigens.18,101 Indeed, the large naïve T-cell populations found in the fetus are predisposed to differentiate into Tregs when exposed to maternal antigens, likely driven by the enhanced TGFβ signaling.246 Genetic surveys of fetal naïve T cells showed that multiple epigenetic and transcriptional programs are expressed, similar to those expressed by committed Tregs, confirming this underlying predisposition for naïve T-cell differentiation into Tregs in the fetus.247 The transcription factor Helios was demonstrated to be important for such fetal-specific predisposition, as the ablation of this molecule reduced the expression of genes associated with immunosuppression and increased that of inflammatory mediators.247
Recent studies have made the remarkable observation that fetal T cells display a memory phenotype as early as in the first trimester.102,103 Single-cell and mass cytometry surveys of the first trimester fetal intestine described multiple CD4+ T-cell subsets with a memory-like gene expression profile.102 Moreover, TCR analysis and imaging cytometry revealed CD4+ T-cell clonal expansion and localization with APCs in the fetal intestinal tissues, suggestive of exposure to foreign antigens.102 Another report identified effector memory CD4+ T cells with a PLZF+CD161+ phenotype that displayed effector functions (such as IFNγ production) in the fetal intestine, and such cells were enriched in the cord blood of infants with gastroschisis.103 Moreover, new evidence suggests that, in a limited number of cases, viable bacteria in the fetal intestine at mid-gestation modulate the activation of fetal T cells;248 yet, there is no consistent evidence for a fetal microbiome in late gestation.249 Together, these studies demonstrate the presence and functionality of antigen-experienced fetal T cells during early and mid-pregnancy.
Fetal T cells in preterm labor and birth
The participation of the fetal immune system in the pathogenesis of preterm labor and birth was first indicated by a seminal study showing enhanced activation of leukocytes in the umbilical cord blood of fetuses who were ultimately delivered after preterm labor compared to those who were delivered at term, even in the absence of intra-amniotic infection.104 This study was unique in that the cord blood sampling was performed prior to delivery (obtained via cordocentesis), thus allowing for a rare view of in vivo fetal immunity in humans.104 Importantly, this report also gave rise to the concept that the fetus itself was responding to foreign antigens, including those derived from microbes in the context of intra-amniotic infection-associated preterm labor.105 More recently, the concept that the fetal immune system participates in the host defense mechanisms against microbial invasion of the amniotic cavity was further expanded by showing that women with intra-amniotic infection have abundant innate and adaptive immune cells that are highly functional in amniotic fluid, including T cells.250–256
In recent times, the concept that the fetus responds to maternal alloantigens was revisited by implementing a multifaceted approach that combined human samples (umbilical cord blood obtained at the time of delivery) and animal models.21 This study demonstrated that not only does the cord blood of preterm neonates contain a significant fraction of functional central memory Th1 cells that is largely absent in term neonates, but such T cells also specifically respond to maternal alloantigens in vitro.21 Yet, given the fact that some of these neonates were born to women who presented with pPROM (a clinical condition highly associated with intra-amniotic infection257,258), whether a subset of these neonatal T cells were activated against microbes and/or their products should also be considered. These preterm neonatal T cells also induced myometrial contractility in vitro through the release of Th1 cytokines.21 Finally, the adoptive transfer of activated T cells into murine fetuses resulted in pregnancy loss, providing in vivo confirmation that the presence of activated T cells in the fetus is associated with adverse pregnancy outcome. Similar phenomena were observed in the context of placental malaria infection, in which a large proportion of functional effector memory T cells was found in the cord blood of infants born to infected mothers.259 These fetal T cells responded to malarial antigens in vitro and, importantly, the strength of the proliferative response to such antigens correlated with prospective protection from this infectious disease during childhood.259 Each of these studies provided novel contributions to the understanding of fetal adaptive immunity; yet, both featured T cells that were isolated from the umbilical cord blood, thus providing only indirect measurements of fetal T-cell responses in utero.
In light of these limitations, a recent study investigated the presence and role of fetal T cells in the amniotic cavity.106 Amniotic fluid, which is in direct contact with the fetus throughout pregnancy, contains a notable fraction of immune cells.253 Multiple studies have established that neutrophils and monocytes found in amniotic fluid in the context of local (i.e., intra-amniotic) inflammation/infection are of fetal origin in preterm gestations.260–262 In addition, a recent report indicated that functional ILCs of fetal origin are present in amniotic fluid in the absence of intra-amniotic inflammation/infection.263 Amniotic fluid ILCs expressed a phenotype consistent with that of intra-epithelial localization,263 which suggested that amniotic fluid lymphocytes could originate directly from fetal tissues such as the intestine. In line with these observations, we reported that T cells present in amniotic fluid in preterm gestations are of fetal origin and express surface markers such as CD103 and CD161 that are indicative of a mucosal origin.106 Together, these studies provide evidence that fetal T cells in the amniotic cavity are largely derived from the mucosal tissues, thereby providing clues that can shape future investigations of these cells.
A notable finding of the latter study was that, among the major immune cell populations, only fetal CD4+ T cells were increased in the amniotic fluid of women with preterm labor occurring in the absence of a demonstrable clinical cause (intra-amniotic inflammation/infection), which is commonly known as idiopathic preterm labor.106 The influx of fetal CD4+ T cells into the amniotic cavity was accompanied by modest increases in T-cell specific cytokines, providing further evidence that some cases of idiopathic preterm labor are accompanied by a mild and distinct fetal T-cell response.106 This represents novel evidence that, in cases of idiopathic preterm labor, fetal T-cell activation is not directed against microbial antigens given that the patients included in this study were confirmed to have no viable microbes in the amniotic cavity.106 In support of these findings, umbilical cord blood T cells from neonates born after idiopathic preterm labor displayed enhanced in vitro functionality compared to those from women who delivered at term.106 Finally, the ultrasound-guided intra-amniotic injection of activated neonatal T cells induced preterm birth in mice,106 providing a causal in vivo demonstration of a new mechanism of disease for preterm labor and birth, which is mediated by activated fetal T cells. Yet, it is worth mentioning that the mechanisms leading to premature activation of amniotic fluid fetal T cells in idiopathic preterm labor require further exploration.
Conclusion
In summary, the maternal–fetal interface contains a diverse population of maternal T cells at term, which includes effector T cells, exhausted and senescent T cells, and Tregs. Maternal Tregs may serve to prevent fetus-specific immune responses and control the presence of effector T cells as well as prevent their premature activation. Moreover, the presence of exhausted and senescent T cells at the maternal–fetal interface indicates additional safeguards that exist to further control T-cell responses. Failure of such mechanisms, either through the excessive invasion of effector T cells into the maternal–fetal interface or the dysfunction or reduced presence of Tregs, may lead to the premature activation of the common pathway of parturition resulting in preterm birth (Fig. 1). Importantly, recent studies have now provided firm evidence that fetal T cells, most likely derived from the mucosal tissues, undergo premature activation in a subset of preterm labor cases, most notably in cases that would otherwise be considered idiopathic. Fetal T cells also participate in the host immune defense mechanisms involved in the better-characterized clinical scenario of intra-amniotic infection-associated preterm labor (Fig. 2). Together, these findings implicate the fetus as an entity that can contribute to adverse pregnancy outcome, thus adding a new layer of complexity to the syndrome of preterm labor. It is our hope that this review provides a solid conceptual framework highlighting the importance of maternal and fetal T cells in pregnancy and catalyzes new research questions that can further scientific understanding of these cells and their role in preterm labor and birth.
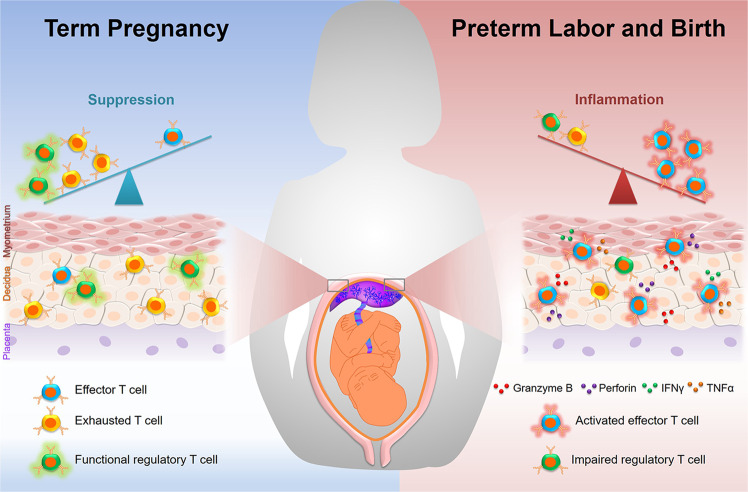
Fig. 1.
Maternal T cells at the maternal–fetal interface in term pregnancy and preterm labor/birth. (Left panel) During normal pregnancy, a suppressive microenvironment exists at the maternal–fetal interface to prevent aberrant maternal immune responses against foreign antigens. This suppressive microenvironment is mainly driven by regulatory T cells and exhausted T cells. (Right panel) Preterm labor is accompanied by inflammation at the maternal–fetal interface, which can be driven or exacerbated by the invasion of activated effector T cells that release pro-inflammatory mediators such as perforin and granzyme B. Moreover, local inflammation may lead to the reactivation of exhausted T cells, restoring effector functions such as the release of pro-inflammatory cytokines and thereby further propagating T-cell responses
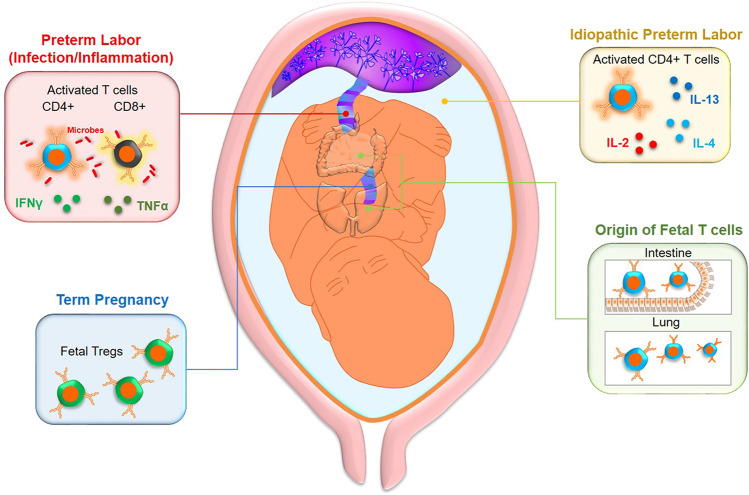
Fig. 2.
Fetal T cells in preterm labor subsets and term gestation. (Upper left panel) Intra-amniotic infection/inflammation-associated preterm labor and birth are accompanied by the activation of conventional CD4+ and CD8+ T cells by microbial products, resulting in the release of pro-inflammatory cytokines. (Upper right panel) A subset of idiopathic preterm labor cases (preterm labor occurring in the absence of clinical intra-amniotic infection/inflammation) are associated with an increased number of activated fetal CD4+ T cells in amniotic fluid together with elevated concentrations of T-cell cytokines. (Lower left panel) In term pregnancy, fetal immunity is skewed toward a tolerant state, as evidenced by an elevated propensity for fetal naïve T cells to differentiate into regulatory T cells (Tregs). (Lower right panel) Fetal T cells in the amniotic cavity express a phenotype similar to that of intra-epithelial lymphocytes in the intestines, suggesting that some T cells in this compartment are derived from the mucosal organs that are in contact with amniotic fluid
Acknowledgements
We gratefully acknowledge Valeria Garcia-Flores, PhD, Yi Xu, PhD, and Marcia Arenas-Hernandez, PhD, for their participation in critical discussions. This research was supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health and the Perinatology Research Branch, Division of Obstetrics and Maternal–Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with federal funds from NICHD/NIH/DHHS under Contract no. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Competing interests
The authors declare no competing interests.
References
- 1.Chaouat G, Voisin GA, Escalier D, Robert P. Facilitation reaction (enhancing antibodies and suppressor cells) and rejection reaction (sensitized cells) from the mother to the paternal antigens of the conceptus. Clin. Exp. Immunol. 1979;35:13–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Bonney EA, Onyekwuluje J. The H-Y response in mid-gestation and long after delivery in mice primed before pregnancy. Immunol. Invest. 2003;32:71–81. doi: 10.1081/imm-120019209. [DOI] [PubMed] [Google Scholar]
- 3.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 4.Zenclussen AC, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy—the contribution of seminal fluid. J. Reprod. Immunol. 2009;83:109–116. doi: 10.1016/j.jri.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc. Natl Acad. Sci. USA. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shima T, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang TT, et al. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J. Immunol. 2014;192:4949–4956. doi: 10.4049/jimmunol.1400498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shima T, et al. Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasma-priming in allogeneic mouse pregnancy. J. Reprod. Immunol. 2015;108:72–82. doi: 10.1016/j.jri.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Bonney EA. Immune regulation in pregnancy: a matter of perspective? Obstet. Gynecol. Clin. North Am. 2016;43:679–698. doi: 10.1016/j.ogc.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J. Dev. Biol. 2010;54:421–430. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol. Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 15.Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv. Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 16.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 17.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat. Rev. Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 18.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivarsson MA, et al. Differentiation and functional regulation of human fetal NK cells. J. Clin. Invest. 2013;123:3889–3901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGovern N, et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature. 2017;546:662–666. doi: 10.1038/nature22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frascoli M, et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci. Transl. Med. 2018;10:eaan2263. doi: 10.1126/scitranslmed.aan2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev. Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 23.van Egmond A, van der Keur C, Swings GM, Scherjon SA, Claas FH. The possible role of virus-specific CD8(+) memory T cells in decidual tissue. J. Reprod. Immunol. 2016;113:1–8. doi: 10.1016/j.jri.2015.09.073. [DOI] [PubMed] [Google Scholar]
- 24.van der Zwan A, et al. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc. Natl Acad. Sci. USA. 2018;115:385–390. doi: 10.1073/pnas.1713957115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaouat G, Kolb JP, Wegmann TG. The murine placenta as an immunological barrier between the mother and the fetus. Immunol. Rev. 1983;75:31–60. doi: 10.1111/j.1600-065x.1983.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 26.Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin. Immunopathol. 2016;38:635–649. doi: 10.1007/s00281-016-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 28.Finn R, St Hill CA, Davis JC, Hipkin LJ, Harvey M. Feto-maternal bidirectional mixed lymphocyte reaction and survival of fetal allograft. Lancet. 1977;2:1200–1202. doi: 10.1016/s0140-6736(77)90439-1. [DOI] [PubMed] [Google Scholar]
- 29.Hunziker RD, Wegmann TG. Placental immunoregulation. Crit. Rev. Immunol. 1986;6:245–285. [PubMed] [Google Scholar]
- 30.Petroff MG. Immune interactions at the maternal-fetal interface. J. Reprod. Immunol. 2005;68:1–13. doi: 10.1016/j.jri.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol. Immunol. 2014;11:571–581. doi: 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.PrabhuDas M, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat. Immunol. 2015;16:328–334. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croy BA, Gambel P, Rossant J, Wegmann TG. Characterization of murine decidual natural killer (NK) cells and their relevance to the success of pregnancy. Cell Immunol. 1985;93:315–326. doi: 10.1016/0008-8749(85)90137-6. [DOI] [PubMed] [Google Scholar]
- 34.Koopman LA, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann AP, Gerber SA, Croy BA. Uterine natural killer cells pace early development of mouse decidua basalis. Mol. Hum. Reprod. 2014;20:66–76. doi: 10.1093/molehr/gat060. [DOI] [PubMed] [Google Scholar]
- 36.Marcellin L, et al. Immune modifications in fetal membranes overlying the cervix precede parturition in humans. J. Immunol. 2017;198:1345–56.. doi: 10.4049/jimmunol.1601482. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, et al. Tim-3 signaling in peripheral NK cells promotes maternal-fetal immune tolerance and alleviates pregnancy loss. Sci. Signal. 2017;10:eaah4323. doi: 10.1126/scisignal.aah4323. [DOI] [PubMed] [Google Scholar]
- 38.Solders M, et al. Maternal adaptive immune cells in decidua parietalis display a more activated and coinhibitory phenotype compared to decidua basalis. Stem Cells Int. 2017;2017:8010961. doi: 10.1155/2017/8010961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinaldi SF, Makieva S, Saunders PT, Rossi AG, Norman JE. Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Mol. Hum. Reprod. 2017;23:708–724. doi: 10.1093/molehr/gax038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi H, et al. Natural cytotoxicity receptors in decidua natural killer cells of term normal pregnancy. J. Pregnancy. 2018;2018:4382084. doi: 10.1155/2018/4382084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt JS, Manning LS, Wood GW. Macrophages in murine uterus are immunosuppressive. Cell Immunol. 1984;85:499–510. doi: 10.1016/0008-8749(84)90262-4. [DOI] [PubMed] [Google Scholar]
- 42.Gustafsson C, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE. 2008;3:e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL. Two unique human decidual macrophage populations. J. Immunol. 2011;186:2633–2642. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svensson J, et al. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011;187:3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 45.Svensson-Arvelund J, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol. 2015;194:1534–1544. doi: 10.4049/jimmunol.1401536. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, et al. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J. Immunol. 2016;196:2476–2491. doi: 10.4049/jimmunol.1502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang X, Du MR, Li M, Wang H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell Mol. Immunol. 2018;15:1027–1037. doi: 10.1038/s41423-018-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vargas M. L., et al. Comparison of the proportions of leukocytes in early and term human decidua. Am. J. Reprod. Immunol.29, 135–140 (1993). [DOI] [PubMed]
- 49.Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, et al. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J. Reprod. Immunol. 2004;62:125–137. doi: 10.1016/j.jri.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Tilburgs T, et al. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(-) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl A):S47–S53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Taglauer ES, Trikhacheva AS, Slusser JG, Petroff MG. Expression and function of PDCD1 at the human maternal-fetal interface. Biol. Reprod. 2008;79:562–569. doi: 10.1095/biolreprod.107.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang SC, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015;6:e1738. doi: 10.1038/cddis.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lissauer D, Kilby MD, Moss P. Maternal effector T cells within decidua: the adaptive immune response to pregnancy? Placenta. 2017;60:140–144. doi: 10.1016/j.placenta.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Powell RM, et al. Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. J. Immunol. 2017;199:3406–3417. doi: 10.4049/jimmunol.1700114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arenas-Hernandez M, et al. Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. J. Immunol. 2019;202:2585–2608. doi: 10.4049/jimmunol.1801350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terzieva A, et al. Early pregnancy human decidua is enriched with activated, fully differentiated and pro-inflammatory gamma/delta T cells with diverse TCR repertoires. Int. J. Mol. Sci. 2019;20:687. doi: 10.3390/ijms20030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki Y, et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 58.Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin. Exp. Immunol. 2004;136:373–378. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsuda S, et al. Clonally expanded decidual effector regulatory T cells increase in late gestation of normal pregnancy, but not in preeclampsia, in humans. Front. Immunol. 2018;9:1934. doi: 10.3389/fimmu.2018.01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salvany-Celades M, et al. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 2019;27:2537–47 e5. doi: 10.1016/j.celrep.2019.04.109. [DOI] [PubMed] [Google Scholar]
- 61.Haller H, et al. An immunohistochemical study of leucocytes in human endometrium, first and third trimester basal decidua. J. Reprod. Immunol. 1993;23:41–49. doi: 10.1016/0165-0378(93)90025-d. [DOI] [PubMed] [Google Scholar]
- 62.Sindram-Trujillo A., Scherjon S., Kanhai H., Roelen D., Claas F. Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am. J. Reprod. Immunol.49, 261–268 (2003). [DOI] [PubMed]
- 63.Bartmann C., et al Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am. J. Reprod. Immunol.71, 109–119 (2014). [DOI] [PubMed]
- 64.Leng Y., et al. Are B cells altered in the decidua of women with preterm or term labor? Am. J. Reprod. Immunol.81, e13102 (2019). [DOI] [PMC free article] [PubMed]
- 65.Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009;82:24–31. doi: 10.1016/j.jri.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J. Leukoc. Biol. 2010;88:625–633. doi: 10.1189/jlb.1209796. [DOI] [PubMed] [Google Scholar]
- 67.Miller D, Motomura K, Garcia-Flores V, Romero R, Gomez-Lopez N. Innate lymphoid cells in the maternal and fetal compartments. Front. Immunol. 2018;9:2396. doi: 10.3389/fimmu.2018.02396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang F, Zheng Q, Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 2019;10:2317. doi: 10.3389/fimmu.2019.02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li LP, Fang YC, Dong GF, Lin Y, Saito S. Depletion of invariant NKT cells reduces inflammation-induced preterm delivery in mice. J. Immunol. 2012;188:4681–4689. doi: 10.4049/jimmunol.1102628. [DOI] [PubMed] [Google Scholar]
- 70.St Louis D, et al. Invariant NKT cell activation induces late preterm birth that is attenuated by rosiglitazone. J. Immunol. 2016;196:1044–1059. doi: 10.4049/jimmunol.1501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez-Lopez N, et al. In vivo activation of invariant natural killer T cells induces systemic and local alterations in T-cell subsets prior to preterm birth. Clin. Exp. Immunol. 2017;189:211–225. doi: 10.1111/cei.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vacca P, et al. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol. 2015;8:254–264. doi: 10.1038/mi.2014.63. [DOI] [PubMed] [Google Scholar]
- 73.Doisne JM, et al. Composition, development, and function of uterine innate lymphoid cells. J. Immunol. 2015;195:3937–3945. doi: 10.4049/jimmunol.1500689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Y., et al. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am. J. Reprod. Immunol.79, e12820 (2018). [DOI] [PMC free article] [PubMed]
- 75.Vazquez J, et al. Transcriptional and functional programming of decidual innate lymphoid cells. Front. Immunol. 2019;10:3065. doi: 10.3389/fimmu.2019.03065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Croy BA, Chantakru S, Esadeg S, Ashkar AA, Wei Q. Decidual natural killer cells: key regulators of placental development (a review) J. Reprod. Immunol. 2002;57:151–168. doi: 10.1016/s0165-0378(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 77.Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J. Soc. Gynecol. Investig. 2006;13:97–103. doi: 10.1016/j.jsgi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J. Reprod. Immunol. 2009;80:122–131. doi: 10.1016/j.jri.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am. J. Obstet. Gynecol. 2011;204:364 e9–16. doi: 10.1016/j.ajog.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Gomez-Lopez N, et al. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am. J. Obstet. Gynecol. 2011;205:235 e15–24. doi: 10.1016/j.ajog.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 81.Gomez-Lopez N. et al. Evidence for a role for the adaptive immune response in human term parturition. Am. J. Reprod. Immunol.69, 212–230 (2013). [DOI] [PMC free article] [PubMed]
- 82.Slutsky R, et al. Exhausted and senescent T cells at the maternal-fetal interface in preterm and term labor. J. Immunol. Res. 2019;2019:3128010. doi: 10.1155/2019/3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arenas-Hernandez M, et al. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol. Immunol. 2016;13:462–473. doi: 10.1038/cmi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim CJ, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod. Pathol. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee J, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013;34:681–689. doi: 10.1016/j.placenta.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 86.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015;213(4 Suppl):S53–S69. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gomez-Lopez N., et al. In vivo T-cell activation by a monoclonal alphaCD3epsilon antibody induces preterm labor and birth. Am. J. Reprod. Immunol.76, 386–390 (2016). [DOI] [PMC free article] [PubMed]
- 88.Sasaki Y, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin. Exp. Immunol. 2007;149:139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J. Reprod. Immunol. 2011;91:76–82. doi: 10.1016/j.jri.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Schober L, et al. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol. Cell Biol. 2012;90:935–944. doi: 10.1038/icb.2012.33. [DOI] [PubMed] [Google Scholar]
- 91.Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev. Clin. Immunol. 2016;12:209–227. doi: 10.1586/1744666X.2016.1105740. [DOI] [PubMed] [Google Scholar]
- 92.Care AS, et al. Reduction in regulatory T cells in early pregnancy causes uterine artery dysfunction in mice. Hypertension. 2018;72:177–187. doi: 10.1161/HYPERTENSIONAHA.118.10858. [DOI] [PubMed] [Google Scholar]
- 93.Robertson SA, et al. Therapeutic potential of regulatory T cells in preeclampsia-opportunities and challenges. Front. Immunol. 2019;10:478. doi: 10.3389/fimmu.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pavlicev M, et al. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res. 2017;27:349–361. doi: 10.1101/gr.207597.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsang JCH, et al. Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics. Proc. Natl Acad. Sci. USA. 2017;114:E7786–E7795. doi: 10.1073/pnas.1710470114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vento-Tormo R, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563:347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suryawanshi H, et al. A single-cell survey of the human first-trimester placenta and decidua. Sci. Adv. 2018;4:eaau4788. doi: 10.1126/sciadv.aau4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pique-Regi R, et al. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife. 2019;8:e52004. doi: 10.7554/eLife.52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tarca AL, et al. Targeted expression profiling by RNA-Seq improves detection of cellular dynamics during pregnancy and identifies a role for T cells in term parturition. Sci. Rep. 2019;9:848. doi: 10.1038/s41598-018-36649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gomez-Lopez N, et al. The cellular transcriptome in the maternal circulation during normal pregnancy: a longitudinal study. Front. Immunol. 2019;10:2863. doi: 10.3389/fimmu.2019.02863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burt T. D. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. Am. J. Reprod. Immunol.69, 346–358 (2013). [DOI] [PMC free article] [PubMed]
- 102.Li N, et al. Memory CD4(+) T cells are generated in the human fetal intestine. Nat. Immunol. 2019;20:301–312. doi: 10.1038/s41590-018-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Halkias J, et al. CD161 contributes to prenatal immune suppression of IFNgamma-producing PLZF+ T cells. J. Clin. Invest. 2019;130:3562–3577. doi: 10.1172/JCI125957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berry SM, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am. J. Obstet. Gynecol. 1995;173:1315–1320. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 105.Romero R, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am. J. Obstet. Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 106.Gomez-Lopez N, et al. Fetal T cell activation in the amniotic cavity during preterm labor: a potential mechanism for a subset of idiopathic preterm birth. J. Immunol. 2019;203:1793–1807. doi: 10.4049/jimmunol.1900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 108.Brummelman J, Pilipow K, Lugli E. The single-cell phenotypic identity of human CD8(+) and CD4(+) T cells. Int. Rev. Cell Mol. Biol. 2018;341:63–124. doi: 10.1016/bs.ircmb.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Saravia J, Chapman NM, Chi H. Helper T cell differentiation. Cell Mol. Immunol. 2019;16:634–643. doi: 10.1038/s41423-019-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Daya S, Rosenthal KL, Clark DA. Immunosuppressor factor(s) produced by decidua-associated suppressor cells: a proposed mechanism for fetal allograft survival. Am. J. Obstet. Gynecol. 1987;156:344–350. doi: 10.1016/0002-9378(87)90281-x. [DOI] [PubMed] [Google Scholar]
- 111.Nancy P, et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 113.Ellis SA, Sargent IL, Redman CW, McMichael AJ. Evidence for a novel HLA antigen found on human extravillous trophoblast and a choriocarcinoma cell line. Immunology. 1986;59:595–601. [PMC free article] [PubMed] [Google Scholar]
- 114.Ellis SA, Palmer MS, McMichael AJ. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA Class I molecule. J. Immunol. 1990;144:731–735. [PubMed] [Google Scholar]
- 115.Kovats S, et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 116.Morales PJ, et al. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J. Immunol. 2003;171:6215–6224. doi: 10.4049/jimmunol.171.11.6215. [DOI] [PubMed] [Google Scholar]
- 117.Colonna M, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Colonna M, et al. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J. Immunol. 1998;160:3096–3100. [PubMed] [Google Scholar]
- 119.Fanger NA, et al. The MHC class I binding proteins LIR-1 and LIR-2 inhibit Fc receptor-mediated signaling in monocytes. Eur. J. Immunol. 1998;28:3423–3434. doi: 10.1002/(SICI)1521-4141(199811)28:11<3423::AID-IMMU3423>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 120.Navarro F, et al. The ILT2(LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur. J. Immunol. 1999;29:277–283. doi: 10.1002/(SICI)1521-4141(199901)29:01<277::AID-IMMU277>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 121.Allan DS, McMichael AJ, Braud VM. The ILT family of leukocyte receptors. Immunobiology. 2000;202:34–41. doi: 10.1016/S0171-2985(00)80050-9. [DOI] [PubMed] [Google Scholar]
- 122.Kang X, et al. Inhibitory leukocyte immunoglobulin-like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell Cycle. 2016;15:25–40. doi: 10.1080/15384101.2015.1121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Borges L, Hsu ML, Fanger N, Kubin M, Cosman D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J. Immunol. 1997;159:5192–5196. [PubMed] [Google Scholar]
- 124.Banham AH, et al. Identification of the CD85 antigen as ILT2, an inhibitory MHC class I receptor of the immunoglobulin superfamily. J. Leukoc. Biol. 1999;65:841–845. doi: 10.1002/jlb.65.6.841. [DOI] [PubMed] [Google Scholar]
- 125.Saverino D, et al. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J. Immunol. 2000;165:3742–3755. doi: 10.4049/jimmunol.165.7.3742. [DOI] [PubMed] [Google Scholar]
- 126.Naji A, Durrbach A, Carosella ED, Rouas-Freiss N. Soluble HLA-G and HLA-G1 expressing antigen-presenting cells inhibit T-cell alloproliferation through ILT-2/ILT-4/FasL-mediated pathways. Hum. Immunol. 2007;68:233–239. doi: 10.1016/j.humimm.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 127.Gustafson CE, et al. Immune checkpoint function of CD85j in CD8 T cell differentiation and aging. Front. Immunol. 2017;8:692. doi: 10.3389/fimmu.2017.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saurabh A, et al. Inhibiting HLA-G restores IFN-gamma and TNF-alpha producing T cell in pleural. Tuberculosis. 2018;109:69–79. doi: 10.1016/j.tube.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 129.Carosella ED, Rouas-Freiss N, Tronik-Le Roux D, Moreau P, LeMaoult J. HLA-G: an immune checkpoint molecule. Adv. Immunol. 2015;127:33–144. doi: 10.1016/bs.ai.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 130.Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am. J. Obstet. Gynecol. 2000;183:682–688. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- 131.Lombardelli L, et al. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4(+) T cells and macrophages. J. Immunol. 2013;191:3651–3662. doi: 10.4049/jimmunol.1300567. [DOI] [PubMed] [Google Scholar]
- 132.Du MR, et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J. Immunol. 2014;192:1502–1511. doi: 10.4049/jimmunol.1203425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Le Bouteiller P. HLA-G in human early pregnancy: control of uterine immune cell activation and likely vascular remodeling. Biomed. J. 2015;38:32–38. doi: 10.4103/2319-4170.131376. [DOI] [PubMed] [Google Scholar]
- 134.Djurisic S., Skibsted L., Hviid T. V. A phenotypic analysis of regulatory T cells and uterine NK cells from first trimester pregnancies and associations with HLA-G. Am. J. Reprod. Immunol. 74, 427–444 (2015). [DOI] [PubMed]
- 135.Melsted WN, Matzen SH, Andersen MH, Hviid TVF. The choriocarcinoma cell line JEG-3 upregulates regulatory T cell phenotypes and modulates pro-inflammatory cytokines through HLA-G. Cell Immunol. 2018;324:14–23. doi: 10.1016/j.cellimm.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 136.Steinborn A, Rebmann V, Scharf A, Sohn C, Grosse-Wilde H. Placental abruption is associated with decreased maternal plasma levels of soluble HLA-G. J. Clin. Immunol. 2003;23:307–314. doi: 10.1023/a:1024592901663. [DOI] [PubMed] [Google Scholar]
- 137.Steinborn A. et al. Early detection of decreased soluble HLA-G levels in the maternal circulation predicts the occurrence of preeclampsia and intrauterine growth retardation during further course of pregnancy. Am. J. Reprod. Immunol.57, 277–286 (2007). [DOI] [PubMed]
- 138.Rizzo R. et al. Soluble human leukocyte antigen-G isoforms in maternal plasma in early and late pregnancy. Am. J. Reprod. Immunol.62, 320–338 (2009). [DOI] [PubMed]
- 139.Stout MJ, et al. Increased human leukocyte antigen-G expression at the maternal-fetal interface is associated with preterm birth. J. Matern. Fetal Neonatal Med. 2015;28:454–459. doi: 10.3109/14767058.2014.921152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beneventi F, et al. Soluble HLA-G concentrations in maternal blood and cervical vaginal fluid of pregnant women with preterm premature rupture of membranes. J. Reprod. Immunol. 2016;116:76–80. doi: 10.1016/j.jri.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 141.Kusanovic JP, et al. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. J. Matern. Fetal Neonatal Med. 2009;22:1151–1166. doi: 10.3109/14767050903019684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Biyik I. Maternal serum soluble HLA-G in complicated pregnancies. J. Matern. Fetal Neonatal Med. 2014;27:381–384. doi: 10.3109/14767058.2013.818126. [DOI] [PubMed] [Google Scholar]
- 143.Lee JY, Kim HM, Kim MJ, Cha HH, Seong WJ. Comparison of single nucleotide polymorphisms in the 3’ untranslated region of HLA-G in placentas between spontaneous preterm birth and preeclampsia. BMC Res. Notes. 2018;11:176. doi: 10.1186/s13104-018-3280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang YH, Zozulya AL, Weidenfeller C, Schwab N, Wiendl H. T cell suppression by naturally occurring HLA-G-expressing regulatory CD4+ T cells is IL-10-dependent and reversible. J. Leukoc. Biol. 2009;86:273–281. doi: 10.1189/jlb.1008649. [DOI] [PubMed] [Google Scholar]
- 145.Amodio G, et al. HLA-G expressing DC-10 and CD4(+) T cells accumulate in human decidua during pregnancy. Hum. Immunol. 2013;74:406–411. doi: 10.1016/j.humimm.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hsu P., Santner-Nanan B., Joung S., Peek M. J., Nanan R. Expansion of CD4(+) HLA-G(+) T Cell in human pregnancy is impaired in pre-eclampsia. Am. J. Reprod. Immunol.71, 217–228 (2014). [DOI] [PubMed]
- 147.LeMaoult J, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 148.Brown R, et al. CD86+ or HLA-G+ can be transferred via trogocytosis from myeloma cells to T cells and are associated with poor prognosis. Blood. 2012;120:2055–2063. doi: 10.1182/blood-2012-03-416792. [DOI] [PubMed] [Google Scholar]
- 149.Abadia-Molina AC, et al. Immune phenotype and cytotoxic activity of lymphocytes from human term decidua against trophoblast. J. Reprod. Immunol. 1996;31:109–123. doi: 10.1016/0165-0378(96)00965-5. [DOI] [PubMed] [Google Scholar]
- 150.Constantin CM, et al. Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J. Immunol. 2007;179:4383–4389. doi: 10.4049/jimmunol.179.7.4383. [DOI] [PubMed] [Google Scholar]
- 151.Norton MT, Fortner KA, Oppenheimer KH, Bonney EA. Evidence that CD8 T-cell homeostasis and function remain intact during murine pregnancy. Immunology. 2010;131:426–437. doi: 10.1111/j.1365-2567.2010.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat. Rev. Immunol. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 153.Ostojic S. et al. Demonstration of the presence of IL-16, IL-17 and IL-18 at the murine fetomaternal interface during murine pregnancy. Am. J. Reprod. Immunol.49, 101–112 (2003). [DOI] [PubMed]
- 154.Wu HX, Jin LP, Xu B, Liang SS, Li DJ. Decidual stromal cells recruit Th17 cells into decidua to promote proliferation and invasion of human trophoblast cells by secreting IL-17. Cell Mol. Immunol. 2014;11:253–262. doi: 10.1038/cmi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakashima A. et al. Circulating and decidual Th17 cell levels in healthy pregnancy. Am. J. Reprod. Immunol.63,104–109 (2010). [DOI] [PubMed]
- 156.Lombardelli L, et al. Interleukin-17-producing decidual CD4+ T cells are not deleterious for human pregnancy when they also produce interleukin-4. Clin. Mol. Allergy. 2016;14:1. doi: 10.1186/s12948-016-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pinget GV, et al. The majority of murine gammadelta T cells at the maternal-fetal interface in pregnancy produce IL-17. Immunol. Cell Biol. 2016;94:623–630. doi: 10.1038/icb.2016.48. [DOI] [PubMed] [Google Scholar]
- 158.Wang WJ, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol. 2010;84:164–170. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 159.Nakashima A., et al Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am. J. Reprod. Immunol. 64, 4–11 (2010). [DOI] [PubMed]
- 160.Lee SK, et al. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011;26:2964–2971. doi: 10.1093/humrep/der301. [DOI] [PubMed] [Google Scholar]
- 161.Lee S. K., Kim J. Y., Lee M., Gilman-Sachs A., Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am. J. Reprod. Immunol.67, 311–318 (2012). [DOI] [PubMed]
- 162.Fu B, Tian Z, Wei H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol. Immunol. 2014;11:564–570. doi: 10.1038/cmi.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu L. et al. IL-7/IL-7R signaling pathway might play a role in recurrent pregnancy losses by increasing inflammatory Th17 cells and decreasing Treg cells. Am. J. Reprod. Immunol.76, 454–464 (2016). [DOI] [PubMed]
- 164.Zhu L. et al. Treg/Th17 cell imbalance and IL-6 profile in patients with unexplained recurrent spontaneous abortion. Reprod. Sci.24, 882–890 (2017). [DOI] [PubMed]
- 165.Santner-Nanan B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 166.Saito S. Th17 cells and regulatory T cells: new light on pathophysiology of preeclampsia. Immunol. Cell Biol. 2010;88:615–617. doi: 10.1038/icb.2010.68. [DOI] [PubMed] [Google Scholar]
- 167.Darmochwal-Kolarz D, et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012;93:75–81. doi: 10.1016/j.jri.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 168.Hsu P, Nanan RK. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front. Immunol. 2014;5:125. doi: 10.3389/fimmu.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Y, et al. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol. Immunol. 2018;15:710–723. doi: 10.1038/cmi.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ding H, et al. Upregulation of CD81 in trophoblasts induces an imbalance of Treg/Th17 cells by promoting IL-6 expression in preeclampsia. Cell Mol. Immunol. 2019;16:302–312. doi: 10.1038/s41423-018-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ito M, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J. Reprod. Immunol. 2010;84:75–85. doi: 10.1016/j.jri.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 172.Lyubomirskaya ES, et al. SNPs and transcriptional activity of genes of innate and adaptive immunity at the maternal-fetal interface in woman with preterm labour, associated with preterm premature rupture of membranes. Wiad. Lek. 2020;73:25–30. [PubMed] [Google Scholar]
- 173.Wang S, Li C, Kawamura H, Watanabe H, Abo T. Unique sensitivity to alpha-galactosylceramide of NKT cells in the uterus. Cell Immunol. 2002;215:98–105. doi: 10.1016/s0008-8749(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 174.Boyson JE, et al. CD1d and invariant NKT cells at the human maternal-fetal interface. Proc. Natl Acad. Sci. USA. 2002;99:13741–13746. doi: 10.1073/pnas.162491699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li L, Yang J, Jiang Y, Tu J, Schust DJ. Activation of decidual invariant natural killer T cells promotes lipopolysaccharide-induced preterm birth. Mol. Hum. Reprod. 2015;21:369–81.. doi: 10.1093/molehr/gav001. [DOI] [PubMed] [Google Scholar]
- 176.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 177.Zhao Y, Shao Q, Peng G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol. Immunol. 2020;17:27–35. doi: 10.1038/s41423-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Buchholz VR, Busch DH. Back to the future: effector fate during T cell exhaustion. Immunity. 2019;51:970–972. doi: 10.1016/j.immuni.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 179.Chen Z, et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity. 2019;51:840–55 e5. doi: 10.1016/j.immuni.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hudson WH, et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8(+) T cells during chronic infection. Immunity. 2019;51:1043–58 e4. doi: 10.1016/j.immuni.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zander R, et al. CD4(+) T cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity. 2019;51:1028–42 e4. doi: 10.1016/j.immuni.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 183.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Catakovic K, Klieser E, Neureiter D, Geisberger R. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun. Signal. 2017;15:1. doi: 10.1186/s12964-016-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J. Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 187.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 188.Brenchley JM, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 189.Plunkett FJ, et al. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J. Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 190.Henson SM, et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J. Clin. Invest. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Verma K, et al. Human CD8+ CD57- TEMRA cells: too young to be called “old”. PLoS ONE. 2017;12:e0177405. doi: 10.1371/journal.pone.0177405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Levenson D. et al. The effects of advanced maternal age on T-cell subsets at the maternal-fetal interface prior to term labor and in the offspring: a mouse study. Clin. Exp. Immunol.10.1111/cei.13437 (2020). [DOI] [PMC free article] [PubMed]
- 193.Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am. J. Obstet. Gynecol. 2018;218(2S):S762–S773. doi: 10.1016/j.ajog.2017.11.567. [DOI] [PubMed] [Google Scholar]
- 194.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Warrington KJ, Vallejo AN, Weyand CM, Goronzy JJ. CD28 loss in senescent CD4+ T cells: reversal by interleukin-12 stimulation. Blood. 2003;101:3543–3549. doi: 10.1182/blood-2002-08-2574. [DOI] [PubMed] [Google Scholar]
- 196.Fauce SR, et al. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J. Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Di Mitri D, et al. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J. Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 198.Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 2014;15:965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Campbell C, Rudensky A. Roles of regulatory T cells in tissue pathophysiology and metabolism. Cell Metab. 2020;31:18–25. doi: 10.1016/j.cmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sakaguchi S, et al. Regulatory T cells and human disease. Annu. Rev. Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 201.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61.. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 202.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 203.Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nat. Rev. Immunol. 2014;14:343–349. doi: 10.1038/nri3650. [DOI] [PubMed] [Google Scholar]
- 204.Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017;17:703–717. doi: 10.1038/nri.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yuan X, Malek TR. Cellular and molecular determinants for the development of natural and induced regulatory T cells. Hum. Immunol. 2012;73:773–782. doi: 10.1016/j.humimm.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Abbas AK, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 207.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 208.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 209.Kumar P, et al. Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation. Cell Mol. Immunol. 2019;16:138–153. doi: 10.1038/cmi.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Curotto de Lafaille MA, et al. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 211.Haribhai D, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Josefowicz SZ, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A. Induced regulatory T cells: their development, stability, and applications. Trends Immunol. 2016;37:803–811. doi: 10.1016/j.it.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 215.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum. Reprod. Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Invest. 2018;128:4224–4235. doi: 10.1172/JCI122182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schjenken J. E. et al. MicroRNA miR-155 is required for expansion of regulatory T cells to mediate robust pregnancy tolerance in mice. Mucosal Immunol.10.1038/s41385-020-0255-0 (2020). [DOI] [PubMed]
- 218.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Annacker O, et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 220.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 223.Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol. Lett. 2006;102:106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 224.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chen T, et al. Self-specific memory regulatory T cells protect embryos at implantation in mice. J. Immunol. 2013;191:2273–2281. doi: 10.4049/jimmunol.1202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tilburgs T, et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 228.Galazka K., et al. Changes in the subpopulation of CD25+ CD4+ and FOXP3+ regulatory T cells in decidua with respect to the progression of labor at term and the lack of analogical changes in the subpopulation of suppressive B7-H4 macrophages—a preliminary report. Am. J. Reprod. Immunol.61, 136–146 (2009). [DOI] [PubMed]
- 229.Furcron AE, et al. Vaginal progesterone, but not 17alpha-hydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal-fetal interface. Am. J. Obstet. Gynecol. 2015;213:846 e1–e19. doi: 10.1016/j.ajog.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gomez-Lopez N, Olson DM, Robertson SA. Interleukin-6 controls uterine Th9 cells and CD8(+) T regulatory cells to accelerate parturition in mice. Immunol. Cell Biol. 2016;94:79–89. doi: 10.1038/icb.2015.63. [DOI] [PubMed] [Google Scholar]
- 231.Furcron AE, et al. Human chorionic gonadotropin has anti-inflammatory effects at the maternal-fetal interface and prevents endotoxin-induced preterm birth, but causes dystocia and fetal compromise in mice. Biol. Reprod. 2016;94:136. doi: 10.1095/biolreprod.116.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Solano ME, Arck PC. Steroids, pregnancy and fetal development. Front. Immunol. 2019;10:3017. doi: 10.3389/fimmu.2019.03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schumacher A, Zenclussen AC. Human chorionic gonadotropin-mediated immune responses that facilitate embryo implantation and placentation. Front Immunol. 2019;10:2896. doi: 10.3389/fimmu.2019.02896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014;10:466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mol BWJ, et al. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 236.Nguyen TA, Kahn DA, Loewendorf AI. Maternal-fetal rejection reactions are unconstrained in preeclamptic women. PLoS ONE. 2017;12:e0188250. doi: 10.1371/journal.pone.0188250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Loewendorf A. I., Nguyen T. A., Yesayan M. N., Kahn D. A. Preeclampsia is characterized by fetal NK cell activation and a reduction in regulatory T cells. Am. J. Reprod. Immunol.74, 258–267 (2015). [DOI] [PMC free article] [PubMed]
- 238.Nguyen TA, Kahn DA, Loewendorf AI. Placental implantation over prior cesarean scar causes activation of fetal regulatory T cells. Immun. Inflamm. Dis. 2018;6:256–263. doi: 10.1002/iid3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kohm AP, et al. Cutting Edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 240.Couper KN, et al. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J. Immunol. 2007;178:4136–4146. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fan MY, et al. Differential roles of IL-2 signaling in developing versus mature tregs. Cell Rep. 2018;25:1204–1213.e4. doi: 10.1016/j.celrep.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Srivatsa B, Srivatsa S, Johnson KL, Bianchi DW. Maternal cell microchimerism in newborn tissues. J. Pediatr. 2003;142:31–35. doi: 10.1067/mpd.2003.mpd0327. [DOI] [PubMed] [Google Scholar]
- 243.Su EC, Johnson KL, Tighiouart H, Bianchi DW. Murine maternal cell microchimerism: analysis using real-time pcr and in vivo imaging. Biol. Reprod. 2008;78:883–887. doi: 10.1095/biolreprod.107.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Stevens AM, Hermes HM, Kiefer MM, Rutledge JC, Nelson JL. Chimeric maternal cells with tissue-specific antigen expression and morphology are common in infant tissues. Pediatr. Dev. Pathol. 2009;12:337–346. doi: 10.2350/08-07-0499.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bakkour S, et al. Analysis of maternal microchimerism in Rhesus monkeys (Macaca mulatta) using real-time quantitative PCR amplification of MHC polymorphisms. Chimerism. 2014;5:6–15. doi: 10.4161/chim.27778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bronevetsky Y, Burt TD, McCune JM. Lin28b regulates fetal regulatory T cell differentiation through modulation of TGF-beta signaling. J. Immunol. 2016;197:4344–4350. doi: 10.4049/jimmunol.1601070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ng M. S. F., Roth T. L., Mendoza V. F., Marson A., Burt T. D. Helios enhances the preferential differentiation of human fetal CD4(+) naive T cells into regulatory T cells. Sci. Immunol.4 (2019). [DOI] [PMC free article] [PubMed]
- 248.Rackaityte E, et al. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 2020;26:599–607. doi: 10.1038/s41591-020-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Theis KR, et al. No consistent evidence for microbiota in murine placental and fetal tissues. mSphere. 2020;5:e00933–19. doi: 10.1128/mSphere.00933-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gomez-Lopez N, et al. Amniotic fluid neutrophils can phagocytize bacteria: a mechanism for microbial killing in the amniotic cavity. Am. J. Reprod. Immunol. 2017;78:e12723. doi: 10.1111/aji.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gomez-Lopez N. et al. Neutrophil extracellular traps in the amniotic cavity of women with intra-amniotic infection: a new mechanism of host defense. Reprod. Sci.24, 1139–1153 (2017). [DOI] [PMC free article] [PubMed]
- 252.Martinez-Varea A, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J. Perinat. Med. 2017;45:523–538. doi: 10.1515/jpm-2016-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gomez-Lopez N. et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am. J. Reprod. Immunol.79, e12827 (2018). [DOI] [PMC free article] [PubMed]
- 254.Gomez-Lopez N. et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am. J. Reprod. Immunol.82, e13171 (2019). [DOI] [PMC free article] [PubMed]
- 255.Galaz J, et al. Cellular immune responses in amniotic fluid of women with preterm prelabor rupture of membranes. J. Perinat. Med. 2020;48:222–233. doi: 10.1515/jpm-2019-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Galaz J, et al. Cellular immune responses in amniotic fluid of women with preterm clinical chorioamnionitis. Inflamm. Res. 2020;69:203–216. doi: 10.1007/s00011-019-01308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Romero R, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern Fetal Neonatal Med. 2015;28:1394–1409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Theis KR, et al. Microbial burden and inflammasome activation in amniotic fluid of patients with preterm prelabor rupture of membranes. J. Perinat. Med. 2020;48:115–131. doi: 10.1515/jpm-2019-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Odorizzi P. M. et al. In utero priming of highly functional effector T cell responses to human malaria. Sci. Transl. Med.10 (2018). [DOI] [PMC free article] [PubMed]
- 260.Sampson JE, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am. J. Obstet. Gynecol. 1997;176(1 Pt 1):77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 261.Gomez-Lopez N, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am. J. Obstet. Gynecol. 2017;217:693 e1–e16. doi: 10.1016/j.ajog.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gomez-Lopez N, et al. The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection. J. Perinat. Med. 2019;47:822–840. doi: 10.1515/jpm-2019-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Marquardt N, et al. Fetal CD103+ IL-17-producing group 3 innate lymphoid cells represent the dominant lymphocyte subset in human amniotic fluid. J. Immunol. 2016;197:3069–3075. doi: 10.4049/jimmunol.1502204. [DOI] [PubMed] [Google Scholar]