Abstract
Mitochondria are highly mobile organelles due to fission, fusion, transport, and mitophagy, and these processes are known as mitochondrial dynamics. Mitochondrial dynamics play an important role in energy production, cell division, cell differentiation, and cell death. In the past decade, numerous studies have revealed the importance of mitochondrial metabolism in immunity, and mitochondrial dynamics are essential for immune responses mediated by various cell types. In this review, we mainly discuss the role of mitochondrial dynamics in activation, differentiation, cytokine production, and the activity of related pathways in immune cells, particularly T cells, B cells, and other cells involved in the innate immune response.
Keywords: Mitochondrial dynamics, Metabolism, T cell, B cell, Innate immune cell
Subject terms: Immunological disorders, Cell biology
Introduction
Mitochondria have a double membrane comprising the mitochondrial outer membrane (MOM) and the mitochondrial inner membrane (MIM). The main function of mitochondria is to provide energy for cells by producing ATP. However, mitochondria are also involved in other essential cellular processes, such as cell development, the cell cycle, and cell death.1,2 As cells are not static, mitochondria display highly variable shapes, sizes, and positions due to fission, fusion, transport, and mitophagy, which are collectively known as mitochondrial dynamics.3 Mitochondrial fission produces small and fragmented mitochondria to facilitate mitochondrial transport, the segregation of damaged mitochondria and the distribution of mitochondria to daughter cells during cell division. Mitochondrial fusion enables the contents to be exchanged between different mitochondria and blocks the degradation of mitochondria. Mitochondrial transport is important for meeting the different energy needs of cells. Mitophagy removes dysfunctional or damaged mitochondria to control mitochondrial quality. Mitochondrial dynamics are related to a number of diseases, such as neurodegenerative diseases, neuropathies, cardiomyopathies, cancer, and inflammatory diseases.4
In recent years, several researchers have identified the potentially critical roles of mitochondrial dynamics in innate and adaptive immunity. Mitochondrial dynamics affect the differentiation, activation, and cytokine production of immune cells.5 Many immune cells alter their metabolism during different processes, and mitochondrial dynamics may be involved in these changes. For example, mitochondrial dynamics play an important role in the immune synapses (ISs) of T cells and natural killer (NK) cells.6,7 In this review, we mainly discuss the role of mitochondrial dynamics in immune cells, including T cells, B cells, macrophages, dendritic cells (DCs), and other immune cells.
Mitochondrial dynamics
Mitochondrial fusion
Mitochondrial fusion involves the fusion of the MOM and that of the MIM. Both of these processes are mediated by members of the GTPase family. The fusion of MOMs is mediated by mitofusin 1 (MFN1) and mitofusin 2 (MFN2), which undergo homotypic and heterotypic interactions in neighboring mitochondria.1,3,5,8,9 Misato (MSTO1) promotes mitochondrial fusion by interacting with the MOM.10 The fusion of MOMs is also controlled by the transcriptional and posttranscriptional mechanisms regulating MFN1 and MFN2.11 Fusion of the MIM is mediated by the optic atrophy 1 (OPA1) protein, which is expressed as different isoforms due to alternative splicing and protease cleavage. The main forms of OPA1 include L- optic atrophy 1 (OPA1) and S-OPA1, which both comprise four mRNA variants,12 and L-OPA1 is cleaved to produce S-OPA1 by two proteolytic enzymes named OMA1 and YME1L1 in the MIM (Fig. 1a).13 In addition, OPA1 dynamically regulates the structure of cristae. Cristae are important for mitochondrial activity and apoptosis and are related to cell death and cytochrome c release.14,15 Fusion of the MOM and the MIM are coordinated and occur almost simultaneously.1,3,5,8,9 Fusion allows different mitochondria to exchange their contents; this process can dilute abnormal contents in the mitochondrial network and rescue dysfunctional mitochondria from mitophagy.
Fig. 1.
The processes of mitochondrial dynamics. a Mitochondrial fusion involves the fusion of the MOM and the MIM. MOM fusion is mediated by MFN1/2 and promoted by MSTO1. MIM fusion is mediated by OPA1, which must be cleaved to convert L-OPA1 into S-OPA1 by two proteolytic enzymes named OMA1 and YME1L1. b Mitochondrial fission is mediated by DRP1, which is recruited to the MOM by several adaptor proteins (FIS1, MFF, MIEF1/MID51, and MIEF2/MID49). Then, DRP1 forms a ring-like structure around the mitochondria via self-assembly to induce mitochondrial fission. c Mitochondria are transported along microtubules and actin filaments after forming a complex with motor proteins (myosin, kinesin, and dynein). d The PINK1/parkin pathway is the main pathway that mediates mitophagy. PINK1 accumulates in abnormal mitochondria and recruits parkin to degrade MOM proteins through ubiquitination. Then, it forms autophagosomes to eliminate abnormal mitochondria
Mitochondrial fission
Mitochondrial fission is mediated by dynamin-related protein 1 (DRP1), a GTPase that resides in the cytosol, and thus specific adaptor proteins are required to anchor this protein in the MOM. These adaptor proteins include fission protein 1 (FIS1), mitochondrial fission factor (MFF), mitochondrial elongation factor 1 (MIEF1/MID51), and mitochondrial elongation factor 2 (MIEF2/MID49). DRP1 is recruited to the MOM by these adaptor proteins and forms a ring-like structure around the mitochondria by self-assembly (Fig. 1b).3,9,16 Ultimately, long mitochondria are divided into small and fragmented mitochondria. The activity of DRP1 is regulated by various posttranslational modifications, including phosphorylation,17 ubiquitylation,18 glycosylation,19 and SUMOylation.20,21 Mitochondrial fission plays a critical role in cell division by ensuring that daughter cells contain the same amounts of mitochondria as mother cells. Moreover, fission promotes the transport of mitochondria and the separation of damaged mitochondria.22
Mitochondrial transport
Mitochondria are transported along microtubules and actin filaments in the cytoplasm of cells. However, MOM proteins do not directly interact with these two cytoskeletal structures. Therefore, the motor proteins myosin, kinesin, and dynein appear to link MOM proteins to microtubules or actin by forming a complex (Fig. 1c).1,3,23 In the cytoplasm, small and fragmented mitochondria are transported more easily than elongated and fused mitochondria, suggesting that fission facilitates the transport of mitochondria.1,24 Mitochondrial transport contributes to the subcellular localization of mitochondria, which is important for ensuring the appropriate mitochondrial distribution during cell division and meeting the different energy demands of various cell types.
Mitochondrial autophagy
Mitochondrial autophagy, which is also called mitophagy, is the process by which damaged or dysfunctional mitochondria are degraded. Mitophagy is regulated by the PTEN-induced kinase 1 (PINK)/parkin (encoded by the PARK2 gene)-dependent or -independent pathways.25–27 The PINK1/parkin pathway is the main pathway that mediates mitophagy.28 First, PINK1 accumulates in the mitochondria and recruits parkin via a process that is promoted by fission and inhibited by fusion.29 This process segregates and fragments abnormal mitochondria, which are subsequently engulfed by autophagosomes. Then, parkin initiates the degradation of several MOM proteins by ubiquitination. Finally, it triggers autophagosome formation to remove dysfunctional mitochondria from the cell (Fig. 1d).1,3,8,16,25 Mitophagy is essential for maintaining mitochondrial quality and reducing the release of pro-apoptotic proteins from the mitochondria, which may lead to apoptosis.
The role of mitochondrial dynamics in T lymphocytes
As an essential component of adaptive immunity, T cells are involved in both cellular and humoral immunity. When recognizing antigens, naïve T (Tn) cells become activated and differentiate into T effector (Te) cells. Although most Te cells die after the immune response is resolved, a few become T memory (Tm) cells, which survive for a long time. When these Tm cells recognize the same antigen again, they are quickly reactivated and proliferate into Te cells. Regulatory T cells (Tregs) suppress the hyperactivity and hyperproliferation of Te cells to prevent an excessive immune response.30 Interestingly, T cells have different metabolic demands based on their different physiological functions.31 As quiescent cells, Tn cells mainly use phosphorylation (OXPHOS) and fatty acid oxidation (FAO) to meet their metabolic needs. After activation, Tn cells undergo a metabolic switch to aerobic glycolysis. Moreover, T cells activate diverse metabolic pathways to fuel their differentiation and functions during development.32 Mitochondria are at the center of metabolism; some connections may exist between mitochondria and T cells. Therefore, we mainly discuss the role of mitochondrial dynamics in T cell function here.
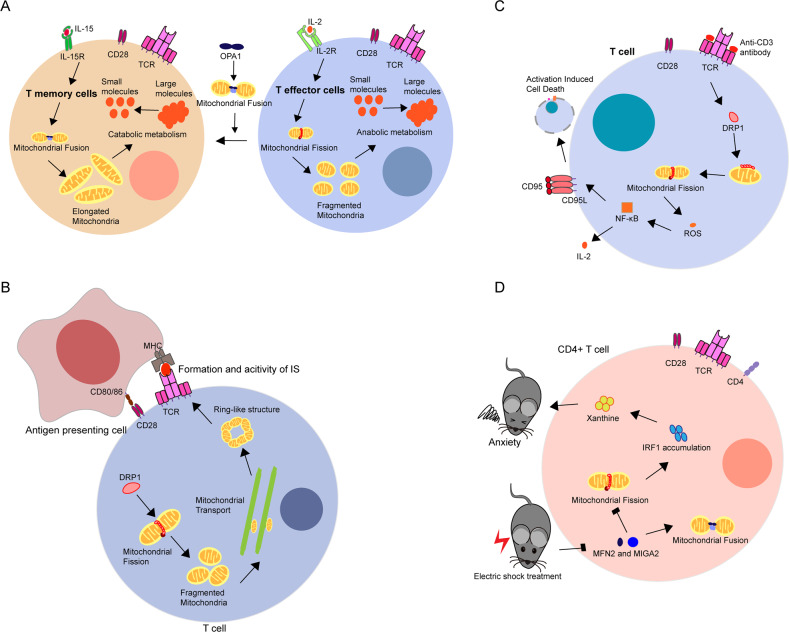
As shown in a study by Buck et al., Tm cells induced by interleukin-15 (IL-15) and Te cells induced by IL-2 engage in diverse metabolic processes that are regulated by mitochondrial dynamics.31 The mitochondria in Te cells undergo fission more frequently after the phosphorylation of DRP1 at Ser616, and thus, Te cells have small mitochondria that are dispersed throughout the cytoplasm, which results in anabolic metabolism. In contrast, Tm cells require catabolic metabolism to survive for long periods of time.33 Therefore, an increased number of fused mitochondria are observed in Tm cells. Mitochondrial fusion also affects Te cell differentiation under culture conditions and leads to a switch in the phenotype from a Tm cell to an activated Te cell. Surprisingly, in contrast to MFN1−/− or MFN2−/− T cells, OPA1−/− OT-I T cells exhibit a survival defect in the Tm culture environment, suggesting that OPA1 is important for the development of Tm cells but not for that of Te cells (Fig. 2a). Moreover, OPA1 deficiency alters and disrupts the cristae of mitochondria due to decreased OXPHOS activity. Thus, OPA1 may be related to the remodeling of mitochondrial cristae, which ultimately leads to differences in the metabolism of Tm and Te cells. Consistently, CD8+ T cells contain fewer and slightly smaller mitochondria after vaccination, which resembles a memory-like metabolic phenotype, but the mitochondria are larger than those in found in their counterparts in active infection to support clonal expansion.34 Although an association between mitochondrial dynamics and T cell fate has been reported, the detailed mechanism has not been clarified. Researchers have not elucidated the mechanism by which T cells initiate the processes of mitochondrial dynamics and whether any other elements are involved in these processes. Different Te cell subsets, such as Th1, Th17, and Th2 cells, have different metabolic demands,35 and Th1, Th2, and Th17 cells are highly glycolytic, in contrast to Treg cells, which have high lipid oxidation rates.35 Thus, these cell subsets may have some differences in mitochondrial dynamics, particularly in fission and fusion. In Tregs, the absence of FABP5 results in decreased OXPHOS activity, impaired lipid metabolism, and a loss of the cristae structure, which suppresses the activity of Tregs via cGAS-STING-dependent type I interferon (IFN) signaling.36 Inhibition of FABP5 in Tregs generated in vitro produces smaller mitochondria with larger cristae, while elongated mitochondria with tight cristae are observed in nontreated Tregs generated in vitro. Based on these findings, mitochondrial dynamics play an important role in Tregs, although the mechanism is unknown. Enhanced mitochondrial biogenesis and increased mitochondrial mass are observed in IL-15-stimulated CD8+ T cells. Mitochondrial biogenesis has also been observed in T cell receptor (TCR)-activated T cells.37 Researchers have not clearly determined the mechanisms underlying these different effects and whether mitochondrial dynamics play different roles in different subsets of T cells. However, for CD8+ T cells, the metabolite profiles of both Te and Tn cells are distinct from those of their counterparts in vitro and are related to differences in glucose utilization.38 Additional in vivo studies of mitochondrial dynamics and T cell functions are needed. Further research is required to illuminate the relationship between mitochondrial dynamics and the functions of different T cell subsets.
Fig. 2.
The essential role of mitochondrial dynamics in T cells. a Mitochondrial dynamics are involved in metabolic programming to control T cell fate. Memory T cells (Tm) undergo increased fusion to promote catabolic metabolism, while effector T cells (Te) undergo increased fission to increase anabolic metabolism. Increasing mitochondrial fusion regulated by OPA1 could convert Te cells to the Tm phenotype. b Mitochondria underwent fission and transport and formed a ring-like structure near the immune synapse (IS) during the formation and activity of the IS in T cells. c After stimulation with anti-CD3 antibodies, mitochondrial fission induced by the accumulation of DRP1 in mitochondria promoted the production of IL-2 and activation-induced cell death via the ROS-NF-κB pathway in T cells. d The reduction of MFN2 or MIGA2 was observed in electric shock (ES)-treated mice, which promoted mitochondrial fission and enhanced the synthesis of xanthine via IRF1 accumulation. These processes are significantly associated with the onset of anxiety-like behaviors in mice
Mitochondrial dynamics play critical roles in T cell differentiation and activation. According to Baixauli et al., DRP1 participates in the formation and activity of ISs via mitochondrial fission and transport.6 After undergoing fission and transport, the mitochondria form a ring-shaped structure in the central supramolecular activation cluster during formation of the ISs (Fig. 2b). DRP1 silencing in superantigen E-stimulated primary T lymphoblasts and J77 T cells results in the dispersal of the phospho-extracellular regulated protein kinase (ERK) signal, more durable phosphorylation of ERK1/2 and PLC-γ1, a longer duration of Ca2+ flux mediated by mitochondrial positioning,39 and the increased secretion of IL-2. Thus, DRP1 likely regulates TCR proximal signaling and IL-2 production by modulating the subcellular position of mitochondria. Moreover, the mitochondrial translocation observed during the formation of ISs is necessary for the activation of Th cells in a Ca2+-dependent manner.40 Consistent with these results, mitochondrial fragments and the accumulation of DRP1 at mitochondria are observed in T cells stimulated with agonistic anti-CD3 antibodies.41 The occurrence of the downstream events of CD95-dependent cytokine expression (IL-2) and activation-induced cell death is reduced when DRP1 is inhibited pharmacologically or genetically.42 As nuclear factor-κB (NF-κB) is required for the transcription of IL-2 and CD95L,43,44 the authors found that reactive oxygen species (ROS) levels are related to the transcriptional activity of NF-κB,45,46 which is controlled by DRP1 activity via S-nitrosylation at cysteine 644 (Fig. 2c).47 The morphology of the mitochondria in naïve CD4+ T cells changes from a fragmented, rounded shape to a hyperfused morphology after 9 h, and these changes are reversed within 24 h after activation using a combination of anti-CD3/anti-CD28 antibodies, which may indicate the anabolic state of rapidly dividing T cells.48 Moreover, naïve CD4+ T cells initiate mitochondrial biogenesis to induce one-carbon metabolism, which is required for their survival and antigen-specific abundance. On the other hand, primed by anti-CD3 and anti-CD28 antibodies, CD8+ naïve T cells display elongated mitochondria after early activation, while a greater number of spherical mitochondria are observed in naïve CD8+ T cells in the absence of anti-CD28 antibody.49 Mature IL-2-expressing Te cells primed by anti-CD28 antibody contain spherical mitochondria, whereas tubulated mitochondria are observed in IL-15-expressing Tm cells primed by anti-CD28 antibody, which is consistent with the mitochondrial morphology observed in Tm and Te cells mentioned above. A difference has also been observed in the morphology of cristae, which is restrained by CD28 costimulation after TCR activation, and was observed to be tighter in Tn cells and Tm cells generated in vitro and in vivo than in the Te cell counterparts. Undoubtedly, these morphological changes in mitochondria are related to T cell activation. Overall, mitochondrial dynamics are essential for T cell activation. Although mitochondrial transport affects T cell activation via ISs, researchers have not clearly determined its impact on the function of other immune cells. The additional roles of mitochondrial transport in immunity have not been clearly elucidated. Moreover, DRP1 plays critical roles in T cell proliferation and migration. DRP1 deficiency disrupts mitochondrial fission, resulting in reduced numbers of thymocytes and mature T cells during the process of T cell development and decreased migration of mature circulating T cells to secondary lymphoid organs.50 The phosphorylation of DRP1 on S616 may be involved in c-Myc activation upon TCR engagement and the subsequent phosphorylation of ERK1/2. The absence of DRP1 results in a defect in T cell migration, which has been confirmed in a solid tumor model to result in reduced T cell infiltration, and this may provide a new target for immunotherapy in tumors. As shown in the study by Zhao et al., the upregulation of MFN2 attenuates the suppressive effects of HMGB1 on the ConA-induced proliferation, IL-2 production and differentiation of CD4+ T lymphocytes,51 but the authors did not examine mitochondrial dynamics. Therefore, a study aiming to determine whether mitochondrial fusion mediates the proliferation and differentiation of T cells would be interesting.
In subjects with autoimmune diseases, mitochondrial dynamics also affect the physiological functions of T cells. HRES-1/RAB4 promotes the degradation of DRP1 and then induces the accumulation of mitochondria by inhibiting fission and mitophagy, ultimately promoting the onset of lupus in susceptible mice.52 Reciprocal changes in RAB4A and DRP1 were observed in lymphocytes from patients with systemic lupus erythematosus (SLE). These findings may facilitate the development of new therapeutic treatments for autoimmune diseases. Based on the results from these studies, mitochondrial dynamics are essential for the function of T cells, such as activation, differentiation, migration, and cytokine production, but we still know little about how mitochondrial dynamics affect the physiological functions of T cells in vivo. In recent decades, an increasing number of studies have revealed that disorders of mitochondrial dynamics are related to neurodegenerative diseases, but such disorders mainly occur in the nervous system.53 Recently, Fan et al. revealed the novel function of mitochondrial dynamics in T cells in regulating stress-induced anxiety-like behaviors.54 First, they discovered that CD4+ T cells are required for stress-induced anxiety behaviors. The differences in the biogenesis and functions of mitochondria in naïve CD4+ T cells from electric shock (ES)-treated mice implied that stress impacts mitochondrial morphology. Next, they revealed significantly reduced levels of MOM proteins that mediate mitochondrial fusion, such as MFN2 and MIGA2, in ES-treated naïve CD4+ T cells. Consistently, a disruption of mitochondrial dynamics caused by a deficiency in MFN1/2 or MIGA2 in naïve T cells promoted anxiety-like behaviors in mice (Fig. 2d). Mitochondrial dynamics are related to metabolism, but researchers have not yet determined whether mitochondrial metabolic disorder contributes to the onset of anxiety. A systemic disorder of purine metabolism was observed in mice whose CD4+ T cells contained excess numbers of fragmented mitochondria. Researchers investigated purine utilization by evaluating the metabolic flow in CD4+ T cells with 13C-labeled glucose to determine how purine metabolism was disrupted by fission. Several molecules required for the de novo synthesis of xanthine were upregulated in CD4+ T cells containing fragmented mitochondria. In addition, purine synthesis was mediated by the accumulation of interferon regulatory transcription factor 1 (IRF1), which resulted from mitochondrial fission. These results provide essential evidence linking neuropsychiatric diseases to adaptive immunity and suggest a novel therapeutic approach for neuropsychiatric diseases. However, many more questions were derived from this research. 1. Are other metabolic pathways involved in these processes? 2. What is the role of other immune cell types that contain fragmented mitochondria, and do they also affect purine metabolism? 3. Do any other important substrates regulate the function of T cells by altering mitochondrial dynamics?
The role of mitochondrial morphology in B cells
Similar to that of T cells, the life cycle of B cells includes development, differentiation, and activation, and different states have different metabolic demands.55 The early development of B cells includes the pro-B cell, pre-B cell, and immature B cell stages and occurs in the bone marrow.56 Then, immature B cells migrate to the spleen and differentiate into mature naïve B cells. Once they are activated by antigens and costimulated by T helper cells, activated B cells proliferate and differentiate into plasma cells or memory B cells in the germinal center. During these processes, the energy demands of B cells in different stages are constantly changing.55–59 B cell proliferation and activation result in increased metabolic demands, and thus, the capacities for glycolysis and oxidative phosphorylation are increased along with the mitochondrial mass in activated B cells. Mitochondria and metabolism are thought to be important for regulating the differentiation of B cells. Activated B cells likely require increased amounts of energy and utilize anabolic metabolism more frequently than quiescent naïve B cells.58 The metabolic reprogramming of B cells is similar to that of T cells. Thus, we speculate that mitochondrial dynamics may also play an essential role in B cells. The absence of ATAD3A reduces the numbers of B220+ pro-B cells, pre-B cells and immature IgM+ B cells but increases the number of early-stage B220+ prepro-B cell precursors by activating PINK1-mediated mitophagy.60 The reduction in the number of B220+ B cells is partially restored by treatment with the mitophagy inhibitor chloroquine and the deletion of PINK1. Martinez et al. reported higher ROS levels, mitochondrial mass (MM), and mitochondrial membrane potential (MMP) in WIPI2-KO naïve resting B cells than in WT cells, but the levels decreased more rapidly in WIPI2-KO cells than in WT cells. Furthermore, the phosphorylation of parkin at S65 increased in the steady state but did not increase after BCR stimulation in WIPI2-KO B cells. Based on these results, mitophagy may play an important role in regulating the differentiation and activation of B cells, but further studies are needed. In addition, Waters et al. found fewer and more elongated mitochondria with multiple nucleoids in naïve B cells, and stimulated B cells had more numerous and rounded mitochondria with a single nucleoid, suggesting that mitochondrial fission may be involved in B cell activation. Although a correlation between mitochondrial dynamics and the functions of B cells, such as activation and differentiation, has been identified, more direct evidence illuminating the role of mitochondrial dynamics in B cells is needed.
Mitochondrial dynamics regulate different functions of macrophages
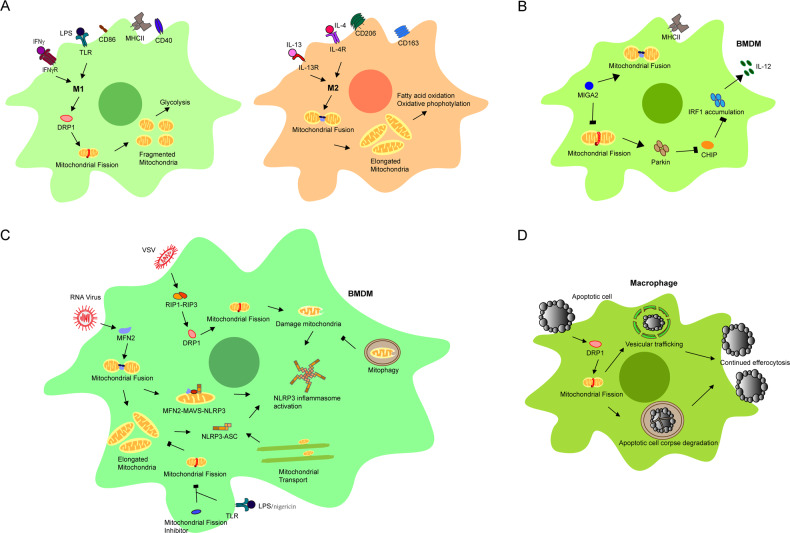
Quiescent macrophages can be polarized into two distinct subsets: M1 and M2 macrophages. M1 macrophages are induced by lipopolysaccharide (LPS) or IFN-γ and produce proinflammatory cytokines and nitric oxide (NO) during phagocytosis to defend against pathogens. M1 macrophages contain fragmented mitochondria due to the increase in mitochondrial fission induced by the activation of DRP1, which increases glycolysis. This process is also related to mitophagy, an event that follows mitochondrial fission in M1 macrophages.5,61,62 M2 macrophages are induced by IL-4 and/or IL-13 and produce IL-10 and transforming growth factor-β (TGF-β) to inhibit the inflammatory response and prevent tissue damage. Due to mitochondrial fusion, M2 macrophages have elongated mitochondria with high levels of fatty acid oxidation and oxidative phosphorylation (Fig. 3a). Consistently, M1 macrophages show more evidence of active mitophagy than M2 macrophages, which occurs in a NIX-dependent manner. Thus, mitochondrial dynamics are important for macrophage polarization, but little is known about the detailed mechanism and signaling pathways.
Fig. 3.
Different roles of mitochondrial dynamics in macrophages. a Mitochondrial dynamics are involved in the polarization of macrophages to satisfy their different metabolic demands. M1 macrophages require increased glycolysis, and M2 macrophages require increased fatty acid oxidation and oxidative phosphorylation. Therefore, fragmented mitochondria were produced in M1 macrophages by increasing mitochondrial fission mediated by DRP1, while elongated mitochondria were produced by mitochondrial fusion in M2 macrophages. b In BMDMs, MIGA2 deficiency promoted mitochondrial fission and inhibited fusion to increase the production of IL-12 via the parkin-CHIP-IRF1 signaling pathway. c Mitochondrial dynamics induced NLRP3 inflammasome formation via different signaling pathways in BMDMs. Mitochondrial fusion provided elongated mitochondria to facilitate the interaction of NLRP3, MFN2, and MAVS for the activation of the NLRP3 inflammasome after RNA virus infection. However, mitochondrial fission promoted the activation of the NLRP3 inflammasome by increasing mitochondrial damage and suppressed the activation of the NLRP3 inflammasome by decreasing the interaction of NLRP3 and ASC in different conditions. Mitochondrial transport also promoted the activation of the NLRP3 inflammasome by creating optimal sites for the interaction of NLRP3 and ASC. Mitophagy eliminated damaged mitochondria to suppress the activation of the NLRP3 inflammasome. d Mitochondrial fission induced by exposure to apoptotic cells (AC) in macrophages was essential for AC corpse degradation and vesicular trafficking, which were necessary for continued efferocytosis
In addition, the antitumor function of macrophages in the tumor microenvironment is associated with mitochondrial dynamics. FAM73b (MIGA2), a MOM protein that is required for mitochondrial fusion, has been shown to play a role in regulating antitumor immune responses.63 Gao et al. generated mice with the conditional knockout (KO) of MIGA2 in myeloid cells (MIGA2MKO). Bone marrow-derived macrophages (BMDMs) and bone marrow-derived dendritic cells (BMDCs) from MIGA2MKO mice showed higher expression of IL-12, which was consistent with the phenotype observed after the loss of MFN1/MFN2 or OPA1 expression.64 Moreover, MIGA2 KO macrophages displayed higher levels of IRF1 accumulation than WT macrophages. IRF1 is required for IL-12 production.65 Thus, the accumulation of IRF-1 leads to the hyperproduction of IL-12. Interestingly, ubiquitination of K48 in IRF1 occurs less frequently in BMDMs, indicating that mitochondrial fission suppresses the polyubiquitination and stability of IRF1. The C terminus of HSC70-interacting protein (CHIP) has been shown to mediate the degradation of IRF1 via ubiquitination.66 CHIP colocalizes with the E3 ubiquitin ligase parkin, leading to polyubiquitination that supersedes the original monoubiquitylation of CHIP. Mitochondrial fission results in an increase in the recruitment of Parkin to mitochondria and induces the degradation of monoubiquitinated CHIP colocalized with parkin. The reduction in monoubiquitinated CHIP impairs the subsequent degradation of IRF1 to further increase the production of IL-12 (Fig. 3b).64,66 Therefore, mitochondrial dynamics may contribute to the mechanism regulating cytokine production in macrophages. Yasukawa et al. reported the interaction of MFN2 with mitochondrial antiviral signaling (MAVS) and the subsequent inhibition of interferon regulatory factor 3 (IRF3) and NF-κB activation. Finally, virus-induced production of IFN-β is reduced, but this may be irrelevant to the role of MFN2 in mitochondrial fusion.67 Moreover, West et al. showed that the presence of mitochondrial DNA in the cytosol enhanced type I interferon responses mediated by the cGAS-STING-IRF3-dependent pathway after virus infection.68 Mitophagy plays a crucial role in preventing the release of mitochondrial DNA into the cytosol.69 MFN1 deficiency decreases the induction of IFN-β production via the STING-TBK1-IRF3 pathway.70 However, the specific mediators of the direct association between cytokines, particularly type I interferons, and mitochondrial dynamics in macrophages remain to be further identified.
Mitochondrial dynamics are also important for the NLRP3 inflammasome in macrophages, particularly its activation.71 After activation by a wide variety of stimuli, such as damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs), Nod-like receptor family pyrin domain-containing 3 (NLRP3) forms a multiprotein complex called the NLRP3 inflammasome, which contains adaptor protein apoptosis-associated speck-like protein (containing a caspase recruitment domain (ASC)) and procaspase-1. Activation of the NLRP3 inflammasome leads to the cleavage of procaspase-1 to yield caspase-1, which then leads to the activation of the downstream signaling pathway and initiation of the inflammatory response via the cleavage of the precursor forms of proinflammatory cytokines such as IL-1β and IL-18.72,73 MFN2, a protein that regulates mitochondrial fusion, plays an important role in the activation of the NLRP3 inflammasome in BMDMs after RNA virus infection.74 It promotes the association between NLRP3 and MAVS, which is followed by NLRP3 mitochondrial localization and inflammasome activation.75 The interaction between MFN2 and NLRP3 is also enhanced by MAVS. Moreover, the interaction between NLRP3 and MFN2 depends on the mitochondrial membrane potential [ΔΨ(m)]. The interaction of NLRP3, MFN2, and MAVS in fused mitochondria with an intact ΔΨ(m) is required to activate the NLRP3 inflammasome. In addition, mitochondrial elongation induced by pharmacological or genetic inhibition of DRP1 potentiates the assembly and activation of the NLRP3 inflammasome in BMDMs stimulated with LPS/ATP or LPS/nigericin.76 The elongation of mitochondria promotes the NLRP3-ASC association and subsequent ASC oligomerization during the assembly of the NLRP3 inflammasome, which is related to the ERK pathway but not mitochondrial damage. Thus, the mitochondria may be a platform for the activation of the NLRP3 inflammasome. However, controversy exists because Wang et al. reported the involvement of the RIP1-RIP3-DRP1 pathway in the activation of the NLRP3 inflammasome in BMDMs after RNA virus infection.77 In BMDMs, infection with VSV promotes the binding of receptor-interacting protein kinase (RIP) 1 to RIP3 to form a complex. The RIP1-RIP3 complex activates DRP1 via phosphorylation at Ser616 and translocates into mitochondria, where it induces aberrant mitochondrial fission and damages the mitochondria to activate the NLRP3 inflammasome. Although mitochondrial fission and fusion are important for the activation of the NLRP3 inflammasome, several distinct pathways likely connect mitochondrial dynamics to the NLRP3 inflammasome in response to different stimuli. Virus proteins have been shown to modulate mitochondrial dynamics in different ways,78 but researchers have not yet determined whether the differences in the aforementioned findings describing the activation of the NLRP3 inflammasome after RNA virus infection resulted from differences in the virus types. Damaged mitochondria are also involved in the activation of the NLRP3 inflammasome,79 and the elimination of damaged mitochondria by parkin attenuates the activation of the NLRP3 inflammasome in macrophages via the NF-κB-p62 pathway.80 Moreover, bone marrow-derived mesenchymal stem cells increase mitophagy and decrease mitochondrial ROS production to limit the activation of the NLRP3 inflammasome in BMDMs upon coculture.81 Consistent with the findings obtained using BMDMs, the induction of mitophagy by nucleotide-binding oligomerization domain-containing 2 (NOD2) and receptor-interacting protein kinase (RIPK) 2 is essential for the clearance of damaged mitochondria to avoid the overactivation of NLRP3 in BMDCs during influenza A virus (IAV) infection.82 Moreover, IL-10 induces mitophagy by inhibiting mammalian target of rapamycin (mTOR) to suppress the dysregulation of the activation of the NLRP3 inflammasome, and abnormal activation of the NLRP3 inflammasome in macrophages has been observed in IL-10-deficient mice and IL-10R-deficient patients with inflammatory bowel disease (IBD).83 Mitophagy is involved in activating the NLRP3 inflammasome. Mitochondrial transport is also involved in the activation of the NLRP3 inflammasome to create optimal sites for NLRP3 and ASC, which depends on dynein, a motor protein linking the microtubules and mitochondria (Fig. 3c).84 Although we identified certain connections between mitochondrial dynamics and the activation of the NLRP3 inflammasome in macrophages, many important features of the regulatory effects of mitochondrial dynamics on the NLRP3 inflammasome remain to be revealed.
Macrophages play important roles in innate immunity by phagocytosing and clearing pathogens. Phagocytic clearance of apoptotic cells (ACs), or efferocytosis, is important for macrophages in their function as phagocytes. Recognition of ACs and the subsequent phagocytosis and phagolysosomal digestion of ACs are the two main processes of efferocytosis. Macrophages exposed to ACs exhibit an increase in mitochondrial fission induced by DRP1, thereby suggesting an association between efferocytosis and mitochondrial fission.85 Likewise, pharmacological or genetic inhibition of mitochondrial fission reduces the high burden of AC efferocytosis, while the absence of MFN1 results in fragmented mitochondria and increased efferocytosis. Mitochondrial fission regulates AC corpse degradation in phagolysosomes by microtubule-associated protein 1A/1B-light chain 3 (LC3)-associated phagocytosis and calcium-dependent vesicular trafficking, which are mediated by the mitochondrial calcium uniporter to ensure efficient and continuous efferocytosis (Fig. 3d). In vivo, impaired efferocytosis was observed in the myeloid cells of mice with defective mitochondrial fission. Based on these findings, mitochondrial dynamics play a critical role in efferocytosis by macrophages. MFN1 deficiency increases efferocytosis, and the participation of LAP (LC3-associated phagocytosis)-mediated phagolysosomal AC degradation in this process suggests that mitochondrial fusion and mitophagy may be involved in this function of macrophages as well,28 but further studies are needed to confirm this hypothesis.
The different functions of mitochondrial morphology in DC subsets
As antigen-presenting cells, DCs present the epitopes of antigens that stimulate naïve T cells. They also produce chemokines and cytokines to regulate innate and adaptive immune responses. When mouse DCs are stimulated by TLR agonists, their metabolism switches from OXPHOS to aerobic glycolysis.86 This metabolic switch is required for DC maturation.86 Similar to that of lymphocytes, the maturation, activation, and migration of DCs require metabolic adaptations.87,88 CD1c+ myeloid dendritic cells (CD1c+ mDCs) and plasmacytoid dendritic cells (pDCs) are the two main subsets of circulating DCs in human peripheral blood. CD1c+ mDCs mainly induce T cell differentiation, while pDCs produce IFN-α. Upon Toll-like receptor (TLR) stimulation, increased mitochondrial fission was observed in CD1c+ mDCs overexpressing the DRP1 protein, but more frequent mitochondrial fusion was observed in pDCs overexpressing the MFN2 protein. Mitochondrial fission and subsequent mitophagy are necessary for the activation of CD1c+ mDCs via the induction of glycolysis (Fig. 4a). Another subset of DCs, CD8α+ DCs, present antigens and induce the activation of CD8+ T cells.88,89 Du et al. showed enlarged mitochondria and abnormal increases in the mitochondrial mass and membrane potential in CD8α+ DCs deficient in MST1/2.90 These changes were related to the reduced expression of OPA1 and the phosphorylation of DRP1 at serine 637, which is mediated by protein kinase A (PKA), to suppress mitochondrial fission.91,92 Moreover, MST1 interacts with PKA, but the authors did not discuss how the interaction of these proteins modulates the phosphorylation of DRP1 at serine 637. When CD8α+ DCs are treated with Mdivi-1 (mitochondrial fission inhibitor) and M1 (mitochondrial fusion promoter), the priming of CD8+ T cells is slightly increased in wild-type DCs and partially restored in MST1/2-deficient DCs. Based on this evidence, mitochondrial dynamics have diverse functions in different DC subsets; however, the mechanisms and signaling pathways mediating these differences require further investigation. Researchers have not yet clearly determined whether the unique morphology of DCs is related to different roles of mitochondrial dynamics in these cellular subtypes.
Fig. 4.
The role of mitochondrial dynamics in DCs and other immune cells. a Mitochondrial dynamics are involved in metabolic adaptations in the two main subsets of circulating DCs in human peripheral blood. In CD11c+ mDCs, mitochondrial fission mediated by DRP1 is essential for glycolysis. However, in pDCs, elongation of mitochondria mediated by MFN2 was observed, which is required for oxidative phosphorylation. b In NKT cells, activation and production of IFNγ and TNF were associated with mitochondrial fission via the RIPK-PGAM5-DRP1 pathway, which contributed to acute liver inflammation. c Upon LPS stimulation, human CD14+ monocytes showed enhanced microRNA-125b (miR-125b) activity followed by the downregulation of BIK and MTP18, which mediated mitochondrial fission and mitophagy related to rheumatoid arthritis. d Excessive mitochondrial fission in liver tumor-infiltrating NK cells was induced by hypoxia along with increased mTOR-Drp1 signaling, which reduced the antitumor activity of NK cells. In addition, in liver, NK cells outside tumors and peripheral NK cells had large, tubular mitochondria
The effects of mitochondrial dynamics on other immune cells
NKT cells produce proinflammatory and anti-inflammatory cytokines, and thus, they function as regulators of immunity to defend against tumors or infections as well as to suppress the immune response.93 DRP1 plays essential roles in the activation and induction of cytokine production in NKT cells.7 Mitochondrial phosphatase phosphoglycerate mutase 5 (PGAM5) dephosphorylates serine 637 of DRP1 and increases mitochondrial fission in NKT cells through a mechanism regulated by receptor-interacting protein kinase 3 (RIPK3). Inhibition of this process in NKT cells alleviates acute liver inflammation and tissue injury via the reduction of IFNγ and TNF production (Fig. 4b). However, it also alters the antitumor immune response of NKT cells. Together with emerging evidence of the relationship between mitochondria and inflammation,94,95 these results imply that the modulation of mitochondrial dynamics may be a new therapeutic strategy for inflammatory diseases.
Monocytes are a type of phagocyte and an important component of innate immunity. Monocytes also differentiate into DCs and macrophages.96 Upon LPS stimulation, human CD14+ monocytes exhibit reduced mitochondrial fission and an increase in mitochondrial fusion for metabolic adaptation.97 This process is mediated by the downregulation of BIK and MTP18 through an increase in the expression of microRNA-125b (miR-125b). BIK is a BH-3-only proapoptotic protein that is related to mitophagy, and MTP18 also induces mitochondrial fission and mitophagy.98 Low levels of miR-125b expression and high levels of BIK and MTP18 expression have been observed in CD14+ monocytes from patients with rheumatoid arthritis (Fig. 4c). As observed in macrophages and DCs, mitochondrial dynamics affect the inflammatory responses of CD14+ monocytes. Monocytes are heterogeneous, and further studies are needed to determine whether mitochondrial dynamics play different roles in different monocyte subsets. However, microRNAs may also regulate mitochondrial dynamics in other types of immune cells.
As mentioned above, mitochondrial dynamics are involved in the formation of T cell synapses and play a role in the formation of synapses involving NK cells. However, fewer details of this process have been revealed for NK cells than for T cells.99 Mitochondrial dynamics may also be important for NK cell activation, similar to that observed in T cells. Mitophagy plays a role in the generation of robust NK cell memory after a viral infection through a process mediated by the mitochondrial-associated proteins BNIP3 and BNIP3L, which eliminate ROS and depolarized mitochondria.100 Recently, Zheng et al. observed small, fragmented mitochondria in tumor-infiltrating NK cells, which is consistent with the upregulated expression of numerous mitochondrial fission-related genes, such as INF2, MIEF2, FIS1, and GDAP1, in these cells, while liver NK cells outside tumors and peripheral NK cells contained normal, large, tubular mitochondria.101 Excessive fission in tumor-infiltrating NK cells was induced by hypoxia along with increased mTOR-DRP1 signaling. Moreover, hypoxia in the tumor microenvironment is correlated with mitochondrial fragmentation and the loss of antitumor NK cells, which reduces the antitumor activity of NK cells (Fig. 4d). Researchers have proposed that mitochondrial dynamics are essential for the function of NK cells, including their immune synapse, antivirus, and antitumor activities, but the precise mechanism has not been clearly elucidated.
Accumulating evidence has shown the importance of mitochondrial dynamics in different immune cells, but the special functions of mitochondrial dynamics in distinct subsets of immune cells and the way in which mitochondrial morphology is regulated in different stages of activation remain unclear.
Discussion
The mitochondria play several roles in cellular metabolism. In addition to energy production, they are a metabolic hub connecting different metabolic pathways. In the last decade, the importance of mitochondria in immune cells has been discovered. In this review, we mainly discussed the roles of mitochondrial dynamics in immune cells. However, metabolism also plays critical roles in immune cells, including OXPHOS, fatty acid metabolism, and amino acid metabolism.102 In addition, mitochondrial dynamics are closely related to metabolism.61 For example, Te cells exhibit increase mitochondrial fission, resulting in anabolic metabolism, while Tm cells display increased mitochondrial fusion accompanied by catabolic metabolism.33 During different immune responses, immune cells change the shape or size of the mitochondria and metabolic state to support different functions. However, additional details regarding the interaction between mitochondrial dynamics and changes in metabolism are needed. Do these processes occur simultaneously, or does one process trigger the other? How do mitochondria monitor extracellular or intracellular cues to change their dynamics and metabolism? Although some researchers have identified posttranslational modifications of some key proteins involved in mitochondrial dynamics, such as DRP1, MFN1/2, and OPA1,11 additional studies are needed to reveal the detailed mechanisms that regulate mitochondrial dynamics in immune cells. What constitutes the sensor in mitochondria that meets the demands of different functions in immune cells? What are the roles of transcriptional regulation in mitochondrial dynamics? Notably, microRNA-125b is related to mitochondrial fission in CD14+ monocytes.97 Are microRNAs involved in the mechanism regulating mitochondrial dynamics in other immune cells? As mentioned above, mitochondrial dynamics have different functions in different immune cells, but little information is available about mitochondrial dynamics in immune cells. We expect that future studies will clarify the functions of and mechanisms regulating mitochondrial dynamics in immune cells.
Acknowledgements
This review was supported by the Excellent Young Scientist Foundation of NSFC (grant No. 31822017), the Zhejiang Provincial Natural Science Foundation of China under grant no. LR19C080001, the National Natural Science Foundation of China (grant nos. 81572651 and 81771675), and the Fundamental Research Funds for the Central Universities.
Author contributions
X.J. wrote the manuscript. J.J. and L.Y. revised the manuscript.
Competing interests
The authors declare no competing interests.
Contributor Information
Yi-Yuan Li, Email: liyiyuan@zju.edu.cn.
Jin Jin, Email: jjin4@zju.edu.cn.
References
- 1.Altieri DC. Mitochondrial dynamics and metastasis. Cell. Mol. Life Sci. 2019;76:827–835. doi: 10.1007/s00018-018-2961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohanty A, Tiwari-Pandey R, Pandey NR. Mitochondria: the indispensable players in innate immunity and guardians of the inflammatory response. J. Cell Commun. Signal. 2019;13:303–318. doi: 10.1007/s12079-019-00507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Hattab AW, Suleiman J, Almannai M, Scaglia F. Mitochondrial dynamics: Biological roles, molecular machinery, and related diseases. Mol. Genet. Metab. 2018;125:315–321. doi: 10.1016/j.ymgme.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Mishra P. Interfaces between mitochondrial dynamics and disease. Cell Calcium. 2016;60:190–198. doi: 10.1016/j.ceca.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Rambold AS, Pearce EL. Mitochondrial dynamics at the interface of immune cell metabolism and function. Trends Immunol. 2018;39:6–18. doi: 10.1016/j.it.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Baixauli F, et al. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 2011;30:1238–1250. doi: 10.1038/emboj.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang YJ, et al. Regulation of NKT cell-mediated immune responses to tumours and liver inflammation by mitochondrial PGAM5-Drp1 signalling. Nat. Commun. 2015;6:8371. doi: 10.1038/ncomms9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zemirli N, Morel E, Molino D. Mitochondrial dynamics in basal and stressful conditions. Int. J. Mol. Sci. 2018;19:564. doi: 10.3390/ijms19020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulthuis EP, Adjobo-Hermans MJW, Willems P, Koopman WJH. Mitochondrial morphofunction in mammalian cells. Antioxid. Redox Signal. 2019;30:2066–2109. doi: 10.1089/ars.2018.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gal A, et al. MSTO 1 is a cytoplasmic pro‐mitochondrial fusion protein, whose mutation induces myopathy and ataxia in humans. EMBO Mol. Med. 2017;9:967–984. doi: 10.15252/emmm.201607058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoppins S. The regulation of mitochondrial dynamics. Curr. Opin. Cell Biol. 2014;29:46–52. doi: 10.1016/j.ceb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Del Dotto V, Fogazza M, Carelli V, Rugolo M, Zanna C. Eight human OPA1 isoforms, long and short: What are they for? Biochim Biophys. Acta Bioenerg. 2018;1859:263–269. doi: 10.1016/j.bbabio.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Anand R, et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 2014;204:919–929. doi: 10.1083/jcb.201308006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frezza C, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Patten DA, et al. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 2014;33:2676–2691. doi: 10.15252/embj.201488349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan DC. Mitochondrial dynamics and its involvement in disease. Annu. Rev. Pathol. 2019;15:235–259. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 17.Cho B, et al. CDK5-dependent inhibitory phosphorylation of Drp1 during neuronal maturation. Exp. Mol. Med. 2014;46:e105. doi: 10.1038/emm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gawlowski T, et al. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J. Biol. Chem. 2012;287:30024–30034. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver D, Reddy PH. Dynamics of dynamin-related protein 1 in alzheimer’s disease and other neurodegenerative diseases. Cells. 2019;8:961. doi: 10.3390/cells8090961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simula L, Campanella M, Campello S. Targeting Drp1 and mitochondrial fission for therapeutic immune modulation. Pharmacol. Res. 2019;146:8. doi: 10.1016/j.phrs.2019.104317. [DOI] [PubMed] [Google Scholar]
- 22.Burman JL, et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J. Cell Biol. 2017;216:3231–3247. doi: 10.1083/jcb.201612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintana A, Hoth M. Mitochondrial dynamics and their impact on T cell function. Cell Calcium. 2012;52:57–63. doi: 10.1016/j.ceca.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J. Cell Sci. 2012;125:2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickles S, Vigié P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaminets A, Behl C, Dikic I. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 2016;26:6–16. doi: 10.1016/j.tcb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Lazarou M, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018;20:1013–1022. doi: 10.1038/s41556-018-0176-2. [DOI] [PubMed] [Google Scholar]
- 29.Twig G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunol. Rev. 2012;249:14–26. doi: 10.1111/j.1600-065X.2012.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buck MD, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klarquist J, et al. Clonal expansion of vaccine-elicited T cells is independent of aerobic glycolysis. Sci. Immunol. 2018;3:eaas9882. doi: 10.1126/sciimmunol.aas9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field CS, et al. Mitochondrial integrity regulated by lipid metabolism is a cell-intrinsic checkpoint for treg suppressive function. Cell Metab. 2020;31:422–437 e425. doi: 10.1016/j.cmet.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan H, et al. Integrative proteomics and phosphoproteomics profiling reveals dynamic signaling networks and bioenergetics pathways underlying T cell activation. Immunity. 2017;46:488–503. doi: 10.1016/j.immuni.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma EH, et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8(+) T cells. Immunity. 2019;51:856–870 e855. doi: 10.1016/j.immuni.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Schwindling C, Quintana A, Krause E, Hoth M. Mitochondria positioning controls local calcium influx in T cells. J. Immunol. 2010;184:184–190. doi: 10.4049/jimmunol.0902872. [DOI] [PubMed] [Google Scholar]
- 40.Quintana A, et al. T cell activation requires mitochondrial translocation to the immunological synapse. Proc. Natl Acad. Sci. USA. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth D, Krammer PH, Gulow K. Dynamin related protein 1-dependent mitochondrial fission regulates oxidative signalling in T cells. FEBS Lett. 2014;588:1749–1754. doi: 10.1016/j.febslet.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 42.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunological Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 43.Serfling E, Avots A, Neumann M. The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochimica et. Biophys. Acta. 1995;1263:181–200. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- 44.Li-Weber M, Laur O, Dern K, Krammer PH. T cell activation-induced and HIV tat-enhanced CD95(APO-1/Fas) ligand transcription involves NF-kappaB. Eur. J. Immunol. 2000;30:661–670. doi: 10.1002/1521-4141(200002)30:2<661::AID-IMMU661>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 45.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 46.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl Acad. Sci. USA. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho D-H, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Sci. (N. Y.) 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ron-Harel N, et al. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab. 2016;24:104–117. doi: 10.1016/j.cmet.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein Geltink RI, et al. Mitochondrial priming by CD28. Cell. 2017;171:385–397 e311. doi: 10.1016/j.cell.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simula L, et al. Drp1 controls effective t cell immune-surveillance by regulating t cell migration, proliferation, and cMyc-dependent metabolic reprogramming. Cell Rep. 2018;25:3059–3073 e3010. doi: 10.1016/j.celrep.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao G-j, et al. Up-regulation of mitofusin-2 protects CD4+ T cells from HMGB1-mediated immune dysfunction partly through Ca(2+)-NFAT signaling pathway. Cytokine. 2012;59:79–85. doi: 10.1016/j.cyto.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 52.Caza TN, et al. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann. Rheum. Dis. 2014;73:1888–1897. doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burté F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 2015;11:11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- 54.Fan KQ, et al. Stress-induced metabolic disorder in peripheral CD4(+) T cells leads to anxiety-like behavior. Cell. 2019;179:864–879 e819. doi: 10.1016/j.cell.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman W, Lakkis FG, Chalasani G. B cells, antibodies, and more. Clin. J. Am. Soc. Nephrology: CJASN. 2016;11:137–154. doi: 10.2215/CJN.09430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stein M, et al. A defined metabolic state in pre B cells governs B-cell development and is counterbalanced by Swiprosin-2/EFhd1. Cell Death Differ. 2017;24:1239–1252. doi: 10.1038/cdd.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melchers F. Checkpoints that control B cell development. J. Clin. Investig. 2015;125:2203–2210. doi: 10.1172/JCI78083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandoval H, Kodali S, Wang J. Regulation of B cell fate, survival, and function by mitochondria and autophagy. Mitochondrion. 2018;41:58–65. doi: 10.1016/j.mito.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boothby M, Rickert RC. Metabolic regulation of the immune humoral response. Immunity. 2017;46:743–755. doi: 10.1016/j.immuni.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin G, et al. Atad3a suppresses Pink1-dependent mitophagy to maintain homeostasis of hematopoietic progenitor cells. Nat. Immunol. 2017;19:29–40. doi: 10.1038/s41590-017-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metab. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Esteban-Martínez L, et al. Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J. 2017;36:1688–1706. doi: 10.15252/embj.201695916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, et al. Mitoguardin regulates mitochondrial fusion through mitopld and is required for neuronal homeostasis. Mol. Cell. 2016;61:111–124. doi: 10.1016/j.molcel.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 64.Gao Z, et al. Mitochondrial dynamics controls anti-tumour innate immunity by regulating CHIP-IRF1 axis stability. Nat. Commun. 2017;8:1805. doi: 10.1038/s41467-017-01919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kano S, et al. The contribution of transcription factor IRF1 to the interferon-gamma-interleukin 12 signaling axis and TH1 versus TH-17 differentiation of CD4+ T cells. Nat. Immunol. 2008;9:34–41. doi: 10.1038/ni1538. [DOI] [PubMed] [Google Scholar]
- 66.Narayan V, Pion E, Landré V, Müller P, Ball KL. Docking-dependent ubiquitination of the interferon regulatory factor-1 tumor suppressor protein by the ubiquitin ligase CHIP. J. Biol. Chem. 2011;286:607–619. doi: 10.1074/jbc.M110.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yasukawa K, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci. Signal. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- 68.West AP, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yue L, Yao H. Mitochondrial dysfunction in inflammatory responses and cellular senescence: pathogenesis and pharmacological targets for chronic lung diseases. Br. J. Pharm. 2016;173:2305–2318. doi: 10.1111/bph.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon D, Park E, Kang SJ. Stimulator of IFN genes-mediated DNA-sensing pathway is suppressed by NLRP3 agonists and regulated by mitofusin 1 and TBC1D15, mitochondrial dynamics mediators. FASEB J. 2017;31:4866–4878. doi: 10.1096/fj.201700328R. [DOI] [PubMed] [Google Scholar]
- 71.Gkikas I, Palikaras K, Tavernarakis N. The role of mitophagy in innate immunity. Front. Immun. 2018;9:1283. doi: 10.3389/fimmu.2018.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bauernfeind F, et al. Inflammasomes: current understanding and open questions. Cell Mol. Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ichinohe T, Yamazaki T, Koshiba T, Yanagi Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl Acad. Sci. USA. 2013;110:17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park S, et al. Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci. Rep. 2015;5:15489. doi: 10.1038/srep15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, et al. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat. Immunol. 2014;15:1126–1133. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- 78.Kim SJ, Ahn DG, Syed GH, Siddiqui A. The essential role of mitochondrial dynamics in antiviral immunity. Mitochondrion. 2018;41:21–27. doi: 10.1016/j.mito.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 80.Zhong Z, et al. NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li S, et al. A novel mechanism of mesenchymal stromal cell-mediated protection against sepsis: restricting inflammasome activation in macrophages by increasing mitophagy and decreasing mitochondrial ROS. Oxid. Med. Cell Longev. 2018;2018:3537609. doi: 10.1155/2018/3537609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lupfer C, et al. Receptor interacting protein kinase 2–mediated mitophagy regulates inflammasome activation during virus infection. Nat. Immunol. 2013;14:480–488. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Sci. (N. Y.) 2017;356:513–519. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Misawa T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, et al. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. 2017;171:331–345 e322. doi: 10.1016/j.cell.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 88.Basit F, Mathan T, Sancho D, de Vries IJM. Human dendritic cell subsets undergo distinct metabolic reprogramming for immune response. Front Immunol. 2018;9:2489. doi: 10.3389/fimmu.2018.02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du X, et al. Hippo/Mst signalling couples metabolic state and immune function of CD8alpha(+) dendritic cells. Nature. 2018;558:141–145. doi: 10.1038/s41586-018-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang C-R, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 92.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat. Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 94.López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 95.West AP. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology. 2017;391:54–63. doi: 10.1016/j.tox.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 96.Guilliams M, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duroux-Richard I, et al. miR-125b controls monocyte adaptation to inflammation through mitochondrial metabolism and dynamics. Blood. 2016;128:3125–3136. doi: 10.1182/blood-2016-02-697003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tondera D, et al. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J. Cell Sci. 2005;118:3049–3059. doi: 10.1242/jcs.02415. [DOI] [PubMed] [Google Scholar]
- 99.Abarca-Rojano E, et al. Re-organization of mitochondria at the NK cell immune synapse. Immunol. Lett. 2009;122:18–25. doi: 10.1016/j.imlet.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 100.O’Sullivan TE, Johnson LR, Kang HH, Sun JC. BNIP3- and BNIP3L-mediated mitophagy promotes the generation of natural killer cell memory. Immunity. 2015;43:331–342. doi: 10.1016/j.immuni.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng X, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 2019;20:1656–1667. doi: 10.1038/s41590-019-0511-1. [DOI] [PubMed] [Google Scholar]
- 102.Angajala, A., et al. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front Immunol. 9, 1605 (2018). [DOI] [PMC free article] [PubMed]