Abstract
Tissues usually super compensate during the period that follow physical exercise. Although this is widely accepted for muscle and glycogen, the compensatory effect is not usually applied to fat tissues. Notwithstanding, evidence for this has been present since the 1970s when it was first suggested that the increased lipogenic activity in response to training might be an adaptation that enables to restore an energy reserve that can be used in times of need. In this context, the present review aimed to summarize information about the effect of detraining on fat metabolism and the physiological responses associated with fat regain. A systematic search on PubMed and Scielo was performed using “training cessation,” “detraining,” “exercise detraining,” and “exercise cessation” combined with “fat tissue,” “adipose tissue,” “adipose metabolism,” and “fat metabolism,” as descriptors. From 377 results, 25 were included in this review, 12 humans and 13 rodents, resulting in a sample of 6772 humans and 613 animals. The analysis provided evidence for fat super compensation, as well as differences in humans and rodents, among different protocols and possible mechanisms for fat gain after exercise cessation. In summary, exercise cessation appears to increase the ability of the adipose tissue to store energy. However, caution should be taken, especially regarding conclusions based on investigations on humans, considering the multiple factors that could affect fat metabolism.
Keywords: Exercise detraining, adipocytes, insulin sensitivity, lipogenesis, interval training, resistance training
Introduction
It is recognized that different biological stressors can induce several positive and negative responses.1 These responses are derived from a physiological mechanism called the General Adaptation Syndrome (GAS), defined as the sum of all nonspecific systemic reactions that occur in response to stress,2 contributing to adaptation.3 In general, the stressful stimuli induce an alarm period and, if adequate conditions are provided, a compensatory effect occurs and a higher level of resilience is generated.4 Physical exercise is among the possible stressors, as it induces different responses that trigger multiple adjustments.5
These compensatory effects have been described in response to exercise in specific tissues such as bones6 and muscles.7 The same pattern appears to occur in different energetic substrates such as glycogen,8 phosphocreatine, and muscle adenosine triphosphate (ATP).9 However, this topic has been poorly discussed regarding adipose tissue. It is known that moderate-intensity continuous exercise (MICE) results in increased fat oxidation by adipose tissue.10,11 This stimulus acutely stresses fat depots, causing triglyceride breakdown and increasing free fatty acid availability into circulation to subsequent oxidation.11 Although this might lead to a reduction of adiposity and favorable changes in the lipid profile,12 it is possible that adipose tissue undergoes a compensatory effect.13–15
The compensatory effect on fat-related outcomes has been investigated in endurance athletes who showed elevated intramuscular lipid storages16 which is called the “endurance paradox.” On the other hand, these effects should also be expected on adipose tissue, especially after exercise cessation and might explain fat regain. This process might be related to the increases in tissue-specific insulin sensitivity17 or glycerol-3-phosphate esterification rate.18 Those concepts have been previously presented as hypotheses;19 however, understanding those responses is highly relevant, as it might help in reviewing the theoretical concepts for exercise prescription.
Moreover, when analyzing at a longer period, exercise cessation also leads to a detraining effect, with the reversal of many of its benefits.20–22 For example, an increase in the respiratory exchange ratio is expected after short- (<4 weeks) and long-term (>4 weeks) detraining periods, which means a shift in substrate utilization from lipids to carbohydrates.20 This detraining effect might be also evident in adipose tissue, leading to an increased fat accumulation due to changes in fat metabolism.
In this context, the main objective of this review is to summarize information about the effects of detraining on adipose tissue and physiological responses related to fat regain. The article also discusses differences between humans and rodents, different protocols, and possible mechanisms for fat gain after exercise cessation.
Methods
This systematic review searched original articles, conducted with rodents or humans, preferably randomized controlled trials (RCT), and clinical trials (CT), quasi-experimental designs, and prospective studies when humans were considered. The interventions had to include physical exercise followed by a detraining period with reevaluation in this final period.
Primary outcomes were fat cell mass, diameter, and number. Secondary outcomes were hormonal and enzymatic alterations related to adipose tissue metabolism, total body mass, lipid and glycemic profile, insulin sensibility, and transcriptional factors related to adipose super compensation.
The search was conducted independently by two of the authors (VSC and LDC) and compared against each other. If a discordance was found, a third author was consulted (FBDV). Pubmed and Scielo databases were used by applying the following descriptors: “training cessation,” “detraining,” “exercise detraining,” and “exercise cessation” combined with “fat tissue,” “adipose tissue,” “adipose metabolism,” and “fat metabolism.”
Regarding eligibility criteria, to be included, studies should (i) be conducted with humans or rodents; (ii) have both training and detraining periods; (iii) have described training and detraining characteristics, considering at least weekly training volume or its reduction (i.e., km/wk, session/wk. . .); (iv) have objectively measured any of the primary or secondary outcomes. Studies without one of the periods (training and detraining) or without direct comparison between periods were excluded.
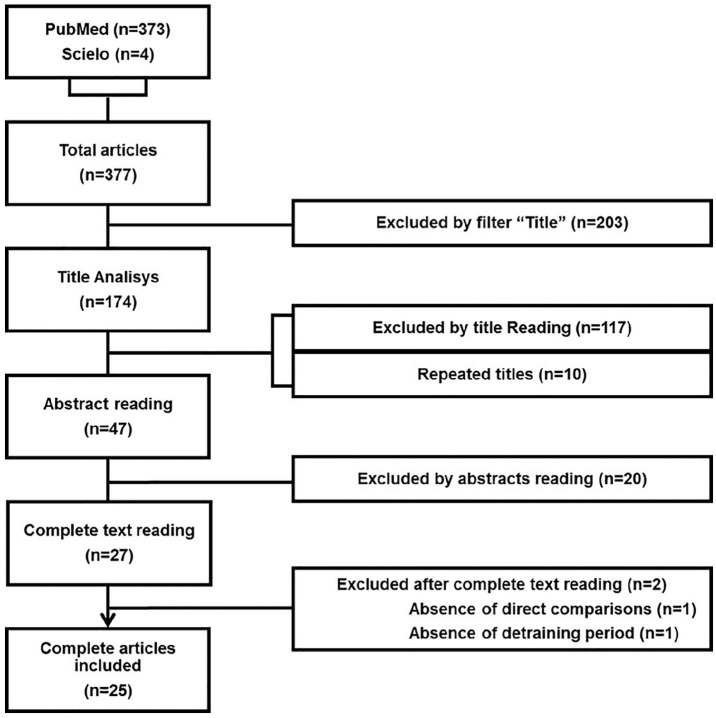
The initial search resulted in 377 articles selected for title and abstract reading, as described in Figure 1. After adequacy analysis for the minimum criteria of pre-selection, the articles were evaluated according to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA Statement). After that, 27 full articles were read and 25 of them were included in the present systematic review. The Consensus on Exercise Reporting Template23 was applied to assess the quality of description of the exercise protocols, as described in the supplementary file.
Figure 1.
Flow chart illustrating the different phases of the search and study selection.
Results
From the 25 articles inserted in the present review, 12 involved humans and 13 rodents. Together, these articles have a sample of 6772 people, of whom 6406 were from one prospective study24 and 613 rodents. The mean scores for risk of bias were 10.6 and 11.9 for studies with humans and rodents, respectively, from the 19-item CERT scale.23 In studies involving humans, the most frequent problems were related to item 5 (detailed description of how adherence to exercise is measured and reported), 6 (detailed description of motivation strategies), 11 (description of the type and number of adverse events that occur during exercise), 16a (description of how adherence or fidelity is assessed/measured), and 16b (description of the extent to which the intervention was delivered as planned). Regarding the studies in rodents, the most frequent issues were found for item 2 (detailed description of the qualifications, expertise, and/or training) and 11 (description of the type and number of adverse events that occur during exercise).
The consequences on fat-related outcomes after cessation of different types of training in humans are summarized in Table 1. Seven from 12 articles measured body/fat mass as the main outcome,24–30 while five analyzed metabolic aspects as main outcomes (i.e., insulin sensitivity, lipogenesis, etc.).31–35 Seven articles had a cohort design, analyzing previous physical activity or exercise, with no intervention,24,25,27,29,30,33,34 while the additional studies investigated responses to endurance and resistance training, mostly using running.26,28,31,32,35 The training period lasted from 1 week27 to 13 months,25 while detraining periods lasted 9 days35 to 7 years.24
Table 1.
Characteristics of studies with humans included in the systematic review (n = 12).
| Reference | Gender (n) | Training protocol (Training/Detraining periods) |
Study design Groups |
Exercise/DT-related main outcomes | Conclusion |
|---|---|---|---|---|---|
| Mujika and Padilla21 | Swimmers ♀ (17) |
High-volume Swimming ~18.8 h/week (13mo–8w) |
Total of 15 weeks high-level swimmers (6) reference group (11) |
No difference in energy intake and expenditure during the study ↑ Body mass after DT to higher values than pre ↓ Free fat mass: fat mass ratio after DT to higher values than pre |
Long-term DT period in these swimmers was associated with a substantial fat gain This increase in body fat mass appears to have been the means to restore lipid balance |
| Farias et al.26 | Overweight/Obese ♀(64) ♂(89) |
Endurance exercises 1) 1200kcal/wk at 40–55%V̇O2peak 2) 1200kcal/wk at 65–80%V̇O2peak 3) 2000kcal/wk at 65–80%V̇O2peak (8mo–2w) |
Total of 8.5 months 1) low volume/moderate intensity 2) low volume/vigorous intensity 3) high volume/vigorous intensity |
Insulin sensitivity increase 16–24 h after exercise cessation Only for groups 1 and 3 insulin sensitivity remained elevated after 15d |
Training-induced improvement in insulin action after 15 days of detraining is dependent on the intensity and weekly amount of exercise in middle-aged, overweight to obese individuals. |
| Mujika and Padilla22 | Type II Diabetes ♀(9) ♂(21) |
Running 30 min @65%V̇O2peak Resistance training 1x2x3 Resistance protocol @65%1RM (6w–6w) |
Total of 12 weeks Endurance Resistance |
Aerobic training did not induce changes after training on HbA1C and triglycerides as resistance training on weight and BMI Aerobic training showed larger effects on HbA1C after the detraining period |
Aerobic and resistance training can improve lipid profile, fasting glycemia HbA1 C in persons with DMII. Resistance training was more effective at maintaining the effects on HDL-C, LDL-C, and HbA1C after detraining |
| Rimbert et al.30 | Young adults ♀(8) ♂(6) |
Running training 40 min @ ‘somewhat hard’ to ‘hard’ on the Borg’s perceived exertion scale At least 2 days/week (13w / 9d) |
Total of 14 weeks and 2 days Sedentary control Trained Subjects |
LPL plasma activity was higher in the trained group after 60 h; Total lipemic responses were higher in the trained group after 60 h and 9 days. |
§ Increased LPL activity and blood lipaemia could indicate increased fat uptake by muscle and fat tissues. |
| Mujika and Padilla20 | Elite Taekwondo athletes ♂(16) |
Intensive regular TKD-specific training (1w/8w) |
Total of 9 weeks | Detraining impaired: Aerobic power Waist-to-hip ratio Body fat Muscle mass Blood glucose Insulin resistance index |
Insulin resistance development was positively associated with inflammatory markers, fat mass, central fat accumulation, and the decline in aerobic power |
| Slade et al.23 | College-aged men ♂(30) |
Resistance 3 days/week 10 exercises circuit to 1–2 sets 15–4 reps @75–90%1RM Endurance Treadmill running 30 minutes/day 3 days/week @70–85%HRreserve (24w / 24w) |
Total of 48 weeks Control Endurance Resistance |
No changes in body fat (%), total fat, arm, and leg fat during training. Both training groups improved resistance and aerobic endurance |
Resistance training improved lean mass and strength, which was maintained during detraining. Endurance training did not induce long-lasting changes. |
| Liao et al.27 | ♂ Obese(33) Lean(11) |
Aerobic Intervals Running treadmill 3 days/week 4sets of 4 min @80–90%HRmax by 3 min @50–65%HRmax Resistance training 3 days/week 1–4 sets 20–4 reps 40–95%1RM (12w/4w) |
Total of 16 weeks Aerobic Interval Training Non-linear resistance training Control |
↑Adiponectin levels after aerobic Intervals ↑IL-6 levels after DT for both training groups ↓ Adiponectin levels after DT in both trained ↓Fasting Insulin during training and returns during DT |
Insulin levels return to baseline after 4 weeks of DT. Aerobics induced an increase in Adiponectin; however, this variable worsened in both training groups after DT. Twelve weeks of training did not cause significant changes in serum levels of IL-6, TNF-a, and CRP, but IL-6 worsened after 4 weeks of DT for both training groups |
| Williams and Thompson24 | Swimmers (4) ♀(4) |
Swimming training > 10 h/week (5mo/5w) |
Two measures: 1. Habitual swimming training 2. DT |
↑ 1.3% in body weight ↑12.2% in Body Fat ↓7.7% in peak oxygen consumption ↓7% in RMR despite preservation of lean mass; No change in blood lipids or mood state |
5 weeks of swim DT after a competitive swim season in healthy young collegiate athletes significantly increases body weight, body fat, and waist circumference and decreases aerobic fitness and resting metabolism |
| Lo et al.28 |
Substudy 1 ♂(8) Substudy 2 ♂(10) |
Substudy 1 Reduce daily steps (-w/3w) Substudy 2 Reduce daily steps (-w/2w) |
– | Study 1 ↓Daily steps ~6000 to~1400 ↓Plasma insulin Study 2 ↓Daily steps(10.000 to1100) ↓Plasma insulin ↑Plasma triglycerides ↑Intra-abdominal fat mass ↑Total fat-free mass ↑Body mass index |
Decreased insulin sensitivity, attenuation of postprandial lipid metabolism, and physical changes suggest that calories used to maintain muscle mass with greater stepping may have been partitioned to visceral fat. |
| Almeras et al.25 | Healthy men ♂ (20) |
Running/cycling > 4 h/week (-w/4w) |
Total of 6 weeks Trained (14) Sedentary (6) |
Detraining induced fat mass gain and reduction on muscle fat oxidation capacity (21%) No alteration on insulin sensitivity |
Insulin sensitivity was not directly associated with fat mass gain and related outcomes |
| Ormsbee and Arciero29 | Healthy athletes ♂(10) ♀(6) |
Running training > 32 km/wk (-w/2w) |
– | ↑86% increase in adipose LPL activity The ratio of adipose tissue/muscle LPL (an important indicator of storage of circulating lipids in adipose tissue) increased significantly after DT. |
This decrease in muscle LPL, coupled with an increase in adipose LPL, yielded a condition favoring adipose tissue storage. |
| Mujika and Padilla20 | Runners ♂(1743) ♀(4663) |
Running (-w/7 years) |
1. Previously trained that DT 2. DT that starts training 3. Sedentary |
Inverse relationships between the changes in the amount of vigorous exercise and the changes in weight and BMI in men and older women in exercise cessation Changes in waist circumference were also inversely related to changes in the running distance in men who quit |
The initiation of vigorous exercise and its cessation decrease and increase, respectively, body weight and intra-abdominal fat, and these changes are proportional to the change in exercise dose. |
Final speed after progression; HR: Heart rate; DT: detraining; LPL: lipoprotein lipase; BM: body mass; MSG: monosodium glutamate; SAL: saline solution; §: indirect conclusions based on the study results due the absence of specific discussion on this point; OLETF: otsuka long-evans tokushima fatty; HFD: high fat diet.
The studies involving rodents are summarized in Table 2. Of the 13 studies, five were conducted using voluntary wheel running,16,26,27 while the others used treadmill running. Exercise interventions lasted from 316,28 to 16 weeks of training.36 Detraining periods lasted from 53 hours16,28 to 8 weeks.36 Regarding main outcomes, 5 from the 13 studies investigated changes in body composition,36–40 while 8 analyzed metabolic/mechanistic responses.15,17,41–46 For both humans and rodents, the large heterogeneity among protocols did not allow further comparisons.
Table 2.
Characteristics of studies with rodents included in the systematic review (n = 13).
| Reference | Specie/gender (n) | Training protocol (Training/Detraining periods) |
Study design Groups |
Exercise/DT-related main outcomes | Conclusion |
|---|---|---|---|---|---|
| Braga et al.36 | Osborne–Mendel rats ♂ (91) |
Treadmill Running 50 minutes/day, 6 days/week @20 m/minute*. (6w/2w) |
Total of 18 weeks 10 w of High fat vs control diet Control diet Sedentary Control diet Exercised High-Fat diet Sedentary High-Fat diet Exercised |
↑ Increase in food intake after 2 days of DT; ↑ Efficiency of weight gain; ↑ Lipogenic rate; ↑ Adipose LPL activity; |
DT results in a rapid state of lipid deposition by an exaggerated reversal of exercise-induced events that seem to represent a “preobese” state. It could be explained by exercise-induced enhanced insulin sensitivity. |
| Nikseresht et al.32 | Wistar rats ♂ (160) |
Continuous Swimming 45 minutes + 5%BM Intermittent Swimming 45 minutes 15s + 15%BM x 15 s rest (12w/8w) |
Total of 20 weeks MSG Sedentary MSG Continuous MSG Intermittent SAL Sedentary SAL Continuous SAL Intermittent |
Exercise-attenuated weight gain, the carcass fat, and epididymal adipose tissue weight in all groups; No differences were found in glucose oxidation and glycogen synthesis. |
Positive exercise effects were transitory with no difference between training protocols. Also, obesity induction could be explained by metabolic changes as the increased capacity of glucose transport and lipid synthesis as a result of improvement in insulin sensitivity. |
| Laye et al.37 | Wistar rats ♀ (42) |
Continuous Swimming 6 hours/day 5 days/week (10w/3w) |
Total of 13 weeks Sedentary Controls Trained Animals (Trained for 2, 4, 6, 8, or 10 weeks) Detrained Animals (DT for 1, 2, or 3 weeks) |
Smaller adipocytes showed higher insulin sensitivity; Responsive adipocytes take up glucose more rapidly; Insulin-responsiveness is related to physical fitness; Detraining did not reverse changes upon the stoppage of exercise. |
The smaller fat cells of the trained animals were significantly more insulin-responsive than the larger cells of the sedentary control animals |
| Sertie et al.14 | Fischer–Brown Norway Rats ♂ (134) |
Voluntary wheel running (3w/5–53 h) |
Total of 4 weeks Sedentary (killed at the same time as 5 h) Sedentary (killed at the same time as 10 h) Wheel Lock 5 h Wheel Lock 10 h Wheel Lock 29 h Wheel Lock 53 h |
Triacylglycerol Synthesis. . . ↑4.2-fold in SED5 than in WL5 ↑14-fold between WL5 and WL10 ↑79% in WL10 than in SED10 ↑3.5-fold in WL53 than SED5 SED Rats had a 9.4% lower body mass after 21 days than those with running wheels |
There is a rapid increase and overshoot in triacylglycerol synthesis between 5 and 10 h of reduced physical activity that was sustained at 29 and 53 h of reduced physical activity that could contribute to an increase in epididymal fat mass and adipocyte size between 29 and 53 h of no voluntary running. |
| Lehnen et al.38 | Fischer–Brown Norway Rats ♂ (60) |
Voluntary wheel running (3w/5–53 h) |
Total of 4 weeks Sedentary (killed at the same time as 5 h) Sedentary (killed at the same time as 10 h) Wheel Lock 5 h Wheel Lock 10 h Wheel Lock 29 h Wheel Lock 53 h Acute 24 h wheel access (killed after 5 or 10 h) Acute 24 h no-wheel access (killed after 5 or 10 h) |
mtGPAT1 activity in epididymal fat was suppressed at 5 h and then elevated (actually overshooting sedentary values) at 10, 29, and 53 h | More than a single day of physical activity is needed to produce the physical inactivity-induced overshoot in triacylglycerol synthesis; An increase in mtGPAT protein and mtGPAT1 activity likely contributes to the overshoot in triacylglycerol synthesis after 21 days of wheel activity, which is consistent with survival mechanisms that support the maintenance of adipose triacylglycerol stores when there is greater energy expenditure because of regular physical activity. |
| Olsen et al.33 | Harlan rats ♂ (36) |
Voluntary wheel running (6w/1w) |
Total of 7 weeks Sedentary Wheel Lock 5 h Wheel Lock 173 h |
The relative epididymal fat pad was similar in sedentary and after 173 h. Body fat % increased 2 times more after 173 h than in sedentary or 5 h Total body fat gain was higher after 173 h Impaired growth of lean body mass compared with animals that sustain wheel running or the sedentary condition Reduction of fatty acid oxidation and PGC-1a after cessation |
Fatty acid oxidation in skeletal muscle, liver, and adipocytes display strikingly different responses to a 1-wk transition from high to low daily physical activity, a period in which fat pad mass increases rapidly. Cessation of daily physical activity quickly alters the metabolic function in a manner that likely precedes the later development of chronic disease |
| Simsolo et al.34 |
Spontaneously hypertensive rats ♂ (32) Wistar Kyoto rats ♂ (32) |
Treadmill Running 60 minutes/day, 5 days/week @~ 50% to 70% maximal running speed (10w/2w) |
Total of 12 weeks SN (sedentary normotensive) SH (sedentary hypertensive), TN (trained normotensive), TH (trained hypertensive), DN1 (1w DT normotensives), DH1 (1w DT hypertensives), DN2 (2w DT normotensives) DH2 (2w DT hypertensives) |
Normotensives Bodyweight gain of ~25% No change in insulin sensitivity post-training Hypertensives Improvement of insulin sensitivity(~24%) with no decrease during DT Bodyweight increased by ~15% GLUT-4 raised during training and decreased in DT, for all groups |
GLUT4 levels do not necessarily have a major role in the development of insulin resistance associated with hypertension |
| Tsai et al.39 | C57BL/6 mice ♂ (38) |
Swimming 1.5 h/day 5 days/week (4w 2w) |
- | DT reduces food intake, resting energy metabolism, and lipolytic activity of white adipose tissue DT did not induce lipogenic activity changes |
DT reversed energy balance by reducing food intake and resting energy metabolism and impairing WAT lipolytic activity, but not lipogenic activity. |
| Bajpeyi et al.31 | OLETF rats ♂ (32) |
Voluntary wheel running (16w/5 h–1w) |
Total of 7 weeks Sedentary Wheel Lock 5 h Wheel Lock 53 h Wheel Lock 173 h |
No changes in the early days after exercise cessation in body weight, fat pad mass, food intake, serum insulin levels, PPARγ, SCD-1, cytochrome c protein, and AMPK protein ↓ Hepatic fatty acid oxidation ↑key mediators in the hepatic fatty acid synthesis |
Several hepatic lipogenesis intermediates that inhibit fatty acid oxidation were up-regulated following only 173 h of exercise cessation, whereas others were not altered. These data strongly suggest that a sudden transition to a sedentary lifestyle increases susceptibility to non-alcoholic fatty liver disease. |
| Goodpaster et al.16 | Wistar rats ♂ (30) |
Treadmill running 1 h/day 5 days/wk @50–60% of Maximal race capacity (8w/4w) |
Total of 12 weeks Trained (12w) Detrained (8w vs 4w) Sedentary (12w) |
↑Cell area in the DT group than both others ↑Malic enzyme activity in the DT group than both others ↑Fatty acid Synthase in the DT group than both others ↑Adiponectin in the DT group than both others ↑PPARy in DT group than sedentary |
Total adiposity recovery by increasing the lipogenic capacity relative to the trained group. Physical detraining may have stimulated the adipogenic process and attenuated apoptotic events which may rapidly recover the adipose mass |
| Yasari et al.40 | Wistar rats ♂ (30) |
Treadmill running 1 h/day 5 days/wk @50–60% of Maximal race capacity (8w/4w) |
Total of 12 weeks Trained (12w) Detrained (8w vs 4w) Sedentary (12w) |
Adipocytes from DT were more effective in taking up glucose compared with those from the sedentary group Adipocytes from DT were more responsive to insulin than those from sedentary animals |
During DT, the adipocytes sustained a more intense glucose transport ability in the presence of insulin, thus increasing the substrate availability for TAG production Physical training creates a favorable environment for building TAG molecules and consequently for replenishing the adipose mass at times of exercise discontinuation, which may work as an obesogenic factor. |
| Herd et al.35 | Hamsters ♂ (36) |
Voluntary running on horizontal disks (12w/6–12w) |
Total of 24 weeks Control (24w) Exercise (12w—DT 6–12) |
↑Food intake by 10–20%, and this effect persisted for about 10 days after Body fat, body cholesterol, and serum triglyceride levels reduced with exercise and returned with DT |
Increased food consumption and changes in serum insulin and glucagon may reflect compensatory adjustments to the increased energy expenditure of exercise. Discontinuation of exercise resulted in a reversal of exercise effects on body fat, body cholesterol, and serum triglyceride levels. |
| Rector et al.41 | Sprague–Dawley rats ♀ (60) |
Treadmill Running 5 days/week 60 min/day @ 26 m/min, 10% slope (8w/4w + 6w HFD) |
Total of 18 weeks Sedentary Exercise 8w and DT 4w Sedentary 4w and exercise 8w |
Exercise 8w and DT 4w accumulate more fat (~6times) in the mesenteric tissue and to a large extent in the sum of 3 intra-abdominal tissues than others during normal and HFD feeding | It is clear that previously exercise-trained rats, given a period of rest, retain a capacity to accumulate fat mass when fed an HFD. Increased lipid storage after cessation of exercise training has been attributed to factors including increased tissue sensitivity to insulin, increased lipoprotein lipase activity, and reduced resting and exercise-induced energy expenditure |
SN: sedentary normotensive; SH: sedentary hypertensive; TN: trained normotensive; TH: trained hypertensive; DN: detraining normotensives; DH: detraining hypertensives; *Final speed after progression; BM: body mass; DT: detraining; LPL: lipoprotein lipase; MSG: monosodium glutamate; SAL: saline solution; §: indirect conclusions based on the study results due the absence of specific discussion on this point; OLETF: otsuka long-evans tokushima fatty; HFD: high fat diet.
Discussion
The main objective of this review was to discuss the effects of detraining on adipose tissue and the physiological responses related to weight regain. In summary, the compensatory effect is present in most investigations as shown by changes in fat mass or body weight, as well as in fat-related outcomes such as lipid synthesis, insulin sensitivity, transcriptional factors, and others. In rodents, it seems evident that exercise causes compensatory effects. However, some caution is needed regarding the conclusions based on human studies because multifactorial aspects related to human behavior could influence the results, especially when investigations do not control for confounding factors, like diet and physical activity.
Studies involving rodents
It has been previously shown that adipose tissue shows a compensatory effect after stressful stimuli.44,45 Part of the explanation is derived from the so-called “lipostatic hypothesis,” that is, by removing part of the adipose tissue of the body, the organism will strive to recover the removed tissue, increasing its amount.47
Evidence of the compensatory effect on adipose tissue after exercise cessation is not recent; in 70’s, an “enhanced ability to esterify fatty acids in times of inactivity” was found after exercise cessation (12 weeks of running), which lead to a 60% increase in esterification in adipocytes.18 Dohm et al.48 observed an increase in fat accumulation and a higher lipogenic rate after 2 weeks of exercise cessation.48 Similar metabolic changes were found after 2 weeks of detraining in rats that performed 50 min/day of running at 20 m/min, 6 days/week for 6 weeks.42 Interestingly, these changes were independent of whether they were following a regular or high-fat diet.42 Based on this, the authors concluded that 2 weeks of detraining results in a state of rapid lipid deposition, which could represent a “pre-obesity” state.
Braga et al.36 analyzed body fat responses to detraining in rats performing continuous or intermittent exercise. The animals were divided into subgroups that received 4 milligrams (mg) of monosodium glutamate per gram (g) of body weight or a saline solution every 2 days for 14 days. The continuous exercise involved 45 minutes of swimming with individual overload equivalent to 5% of the animal’s body weight; interval training used effort to pause ratio of 1:1 for 15 seconds with an additional load equivalent to 15% of animal’s body mass, in a total duration of 45 minutes. Both groups trained 5 days a week for 12 weeks. The results revealed that detraining along with early supplementation of monosodium glutamate was associated with an increase in the carcass fat content and epididymal adipose tissue weight in both groups.
More recent studies provided further evidence for the responses of adipose tissue to detraining and its mechanisms. Evidence suggests that chronic endurance exercises may lead to a reduction in resting lipolysis, both in visceral and subcutaneous adipocytes.45 In this regard, Laye et al.37 investigated fat metabolism after 5 h and 173 h of the wheel running cessation and found a rapid increase in adipose tissue content and a deficiency in fat oxidation associated with reductions in energy expenditure and PGC-1α expression. The results suggest the occurrence of rapid metabolic changes in animals leads to increases in lipogenic processes and fat gain. Interestingly, there was an increase in the ability to oxidize fat in the skeletal muscle and liver; however, according to the authors, this was not able to prevent the increase in total fat.37 A similar study by the same group investigated the effects of physical activity interruption in hyperphagic/obese rats and reported an increased risk of hepatic steatosis by increasing hepatic fatty acid synthesis and malonyl-CoA formation due to detraining, with a significant increase in the expression of enzymes involved in fatty acid synthesis.41
In works by Goodpaster et al.16 and Lo et al.,28 rats had free access to the running wheel for 21 days and then were analyzed 5, 10, 29, or 53 hours after the exercise cessation. According to the results, 53 hours after exercise cessation, there was an increase in total and relative body mass, an increase in the diameter and the total number of fat cells and a higher concentration of blood triglycerides.44 In another study, there was an increase in the activity of mtGPAT1, a key regulator in triacylglycerol synthesis, in the detrained animals at 10, 29, and 53 hours when compared to sedentary animals.15 Moreover, there was an increase in the plasma insulin of mice after 53 hours of exercise interruption.15 The authors propose two mechanisms to explain the increase of lipid deposition and the reduction of fatty acid oxidation: i) intracellular accumulation of malonyl-CoA, a substrate for the synthesis of long-chain saturated fatty acids via fatty acid synthase; and ii) malonyl-CoA formation, which could reduce fatty acid oxidation by inhibiting carnitine palmitoyltransferase 1 (CPT-1), which is responsible for the transformation of acyl-CoA into acylcarnitine and its entry into the mitochondria.15 The increases in malonyl-CoA and acetyl CoA carboxylase in rodents submitted to endurance training was confirmed by previous studies.49 Interestingly, higher velocities were associated with a lower activity of these enzymes.49
Sertié et al.17 investigated metabolic and adipocytes changes and after exercise cessation. The study period was 12 weeks, and the rats were divided into the following: (i) control group; (ii) trained group, which performed exercise for 12 weeks; and (iii) detrained group, which exercise for 8 weeks and detrained for 4 weeks. The detrained group showed increased expression of genes associated with adiponectin synthesis (which is related to increases of newly differentiated adipocytes), increased PPARγ gene expression (favorable for adipogenesis), increased de novo lipogenesis, higher activity of fatty acid synthase and malic acid, increased weight gain, and larger adipocyte size than the control group. Based on this, the authors concluded that 4 weeks of detraining might increase “obesogenic” responses in white adipose tissue. Later, Mazzucatto et al.45 used 4 weeks of swimming for 1 h 30 min/day, 5 times a week and found that, after 2 weeks of detraining, there was an impairment in lipolytic activity in white adipose tissue, without interference in lipogenic activity, which contributed to adipose tissue remodeling.45
Sértie et al.46 submitted rats to training, detraining, and control conditions. Training consisted of five weekly sessions of 60 minutes at approximately 50–60% of the maximum running capacity for 12 weeks. The detraining group trained for 8 weeks and then had 4 weeks of detraining. According to the results, exercise induced several positive morphological and biochemical adaptations, but these were reversed during detraining. Moreover, total adipose content was increased in the detraining period, as well as its lipogenic capacity in comparison to controls.
Similarly, Yasari et al.40 conducted a study involving 8 weeks of exercise, composed by treadmill training followed by 4 weeks of detraining. Some animals also received a high-fat diet (42% fat). Interestingly, the abdominal fat content tended to be higher in detrained animals than in the other groups, suggesting the occurrence of a super compensatory effect due to the cessation of exercise. In addition, the increases in fitness and resting metabolism, obtained after training, underwent a rapid regression with detraining, reaching lower values than control.40
In an earlier study by Craig et al.,43 rats were divided into sedentary and control groups and trained and detrained groups. Training involved 10 weeks of swimming for 5/days a week for 6 h/day followed by detraining for 7, 14, and 21 days. The results showed an increase in deoxyglucose uptake, indicating an increase in insulin uptake, especially in the first 7 days of detraining. Based on these findings, the authors suggested that MICE was associated with an increased ability to respond to insulin and a consequent increase in adipose tissue.43
It has been shown that MICE-type exercises increase lipoprotein lipase activity,20,36 lipolysis rate,50 and insulin sensitivity,37,42 which might promote greater fat and glucose uptake. Detraining does not seem to immediately reverse these responses, as shown by previous studies where 2 or 3 weeks after exercise cessation, lipoprotein lipase activity,20,36 and insulin sensitivity did not return to baseline levels. In this regard, detraining for 1 and 2 weeks resulted in higher insulin sensitivity, GLUT-4 gene expression, and GLUT-4 contents in the heart, gastrocnemius muscle, and epididymal white fat tissue.38 This suggests that an increased insulin sensitivity might be a key factor in the lipogenic process.37,39
In order to better explain these processes, Sertié et al.46 compared a control group to a group of detrained rats who exercised for 8 weeks and detrained during the next 4 weeks. The results indicate higher glucose uptake and oxidation in the adipocytes of the detrained group when stimulated by insulin. The association of these results and fat super compensation might be related to the greater lipogenic capacity that would elevate glucose oxidation to provide energy for triglycerides synthesis and storage. While the improvements in the capacity of the adipocyte to uptake and oxidize glucose might be important to metabolic control during the training period, this might lead to accumulating fat after the cessation of exercise.51
Therefore, the studies in rodents show that that adipose tissues have a compensatory response after exercise cessation, as shown by fat regain and metabolic changes related to increases in adipogenesis and reductions in lipolysis. Interestingly, the exercise protocols generally involved MICE. However, it is not possible to make inferences regarding different protocols because of a lack of evidence.
Studies involving humans
In general, it has been shown that, in humans, when adipose tissue is stressed by different mechanisms, such as liposuction,13 cryolipolysis,52 or caloric restriction,51 it tends to super compensate19 in the same way other tissues and substrates do.6,8,9 The question is whether these lipogenic and adipogenic changes occur in response to exercise cessation in humans. In this regard, it has been shown that 1 day after MICE (65%VO2max for 90 min), there is an increase in diacylglycerol acyltransferase (which catalyzes the reaction in which the diacylglycerol is attached to a long-chain acyl-CoA to form the triglyceride), mitochondrial glycerol-3-phosphate acyltransferase (responsible for connecting fatty acids to glycerol), and sterol-CoA desaturase, which might induce the production of monounsaturated fatty acids in the liver.53
After 2 weeks of detraining, marathon runners experimented an increase in the activity of the lipoprotein lipase enzyme in the adipose tissue, with a decrease of this enzyme in the muscle tissue, collaborating to an increased adiposity.34 There is evidence that blood levels of adiponectin are inversely related to body fat content,32 and although there was an increase in its levels after 12 weeks of training, the levels decreased after detraining, suggesting an increase in total fat mass, which might be related to the worsening of inflammatory markers during detraining.32
When young swimmers interrupted training after a competitive season, there is a significant increase in fat mass and waist circumference along with a decrease in aerobic fitness and resting metabolic rate.29 Moreover, long-term detraining contributed to a substantial increase in body fat in swimmers, apparently by a mechanism of repair and restoration of lipid balance.25 Data given by Liao et al.27 suggest that those adverse results could be not exclusive for MICE protocols, since after detraining from specific and vigorous training, taekwondo athletes showed an increase in insulin resistance and body fat. However, it is not possible to make specific conclusions from these data, since the pre-competitive period has additional confounding factors that could interfere with these results, as rapid weight loss (frequent in combat sports) and subsequent rapid weight regain.
When recreationally active young adults performed MICE for 13 weeks and detrained for 9 days,35 there was an increase in lipoprotein lipase activity and lipemic responses in comparison to the sedentary controls, which indicates higher fat uptake by muscle and adipose tissues. Over the long term, exercise cessation in highly endurance-trained subjects induced compensatory fat gain (~ 6.5 kg), as well as a decrease in high-density lipoprotein (HDL) cholesterol and an increase in body mass index, leptin, and low-density lipoprotein (LDL) cholesterol, even with reduced caloric intake.54 A super compensatory effect was also observed because of the recurrence of rapid weight loss (lasting 1 to 3 weeks).55 In part, successive cycles of weight loss and regain seems to stimulate compensatory mechanisms in the adipose tissue and reduce the resting metabolic rate relative to body mass, probably making the organism more efficient in accumulating adipose mass.56
A previous study with obese subjects investigated the effects of a weight loss program involving caloric restriction and physical exercise and reported a large decrease in body weight, which was accompanied by a reduction in the resting metabolic rate.57 When the participants of the television program “The Biggest Loser,” which involved caloric restriction combined with physical exercise, were reassessed after 6 years, Fothergill et al.58 reported a high amount of weight regain, even though the participants maintained a reduced caloric intake and high levels of physical activity. According to the results, this might have been due to a metabolic adaptation, as can be shown by the reduction in the resting metabolic rate.
Considering insulin sensitivity, Bajpeyi et al.31 showed that overweight and obese individuals maintained an increased insulin sensitivity after detraining, which was dependent on an interaction between training intensity and volume. Low volume/moderate intensity and high volume/vigorous intensity showed longer effects (15 days), while the effects of low volume/vigorous intensity were lost earlier. While increased insulin sensitivity could indicate important improvements in health-related outcomes, the nature of the test (intravenous glucose tolerance test) did not consider tissue-specific behavior, which keeps open the question whether insulin sensitivity could increase glucose uptake (and lipogenic factors) in fat tissue or in skeletal muscle or in both. However, compensatory effects that we are suggesting might be different (or it is not exclusive to) from the insulin-related mechanism, since Rimbert et al.30 reported that insulin sensitivity was not directly associated with a fat mass gain after detraining in healthy men.
In summary, the cessation of physical exercise, especially MICE, especially those performed in an intensity that promotes high levels of fat oxidation,10 tends to induce super compensatory effects on adipose tissue by different mechanisms, including an increase in specific tissue insulin sensitivity and increased activity of lipogenic enzymes.
These evidences of a negative impact on fat metabolism and body composition add to the observed negative effects of detraining previously reported in physical function, cardiovascular system, muscle morphology, and metabolism.20–22,59 Considering that excessive fat accumulation is related to many important health problems, it is important to be alert about the risks of exercise detraining; exercise should be performed continuously in order for its benefits to be sustained.
Interestingly, this super compensatory effect might be minimized by the addition of resistance training or high-intensity interval training.19,59 A previous study involving humans analyzed the metabolic effects of a training session composed of 2 min at 90% VO2max by 2 min of recovery at 50% VO2max performed to failure (~90 min). The protocol depleted glycogen stores with no modification in skeletal muscle triacylglycerol. However, 3 hours after the exercise, the skeletal muscle triacylglycerol started to drop until the evening of the following day, while the glycogen was progressively replenished, which suggests that metabolism was shifted to a more lipolytic state to allow glycogen to restore.60 This “metabolic shift” was also confirmed in later studies that reported higher lipolysis after high-intensity activities61 and might be related to metabolic consequences of performing higher intensity exercises, such as the increase in lactate levels. In addition, recent evidence shows that lactate is associated with the browning of white adipose tissue, increasing its metabolic activity, and thermogenesis, opening new perspectives for the control of adipose tissue due to high-intensity exercises.62
Therefore, it is possible that high-intensity interval training exercises do not stress adipose tissue during efforts, avoiding its compensation and increasing adipose substrate and tissue mobilization after training.63 Interestingly, previous studies suggested that athletes involved in high-intensity activities have similar levels of body fatness than endurance athletes, disregarding the lower energy expenditure and lower training volume of their routines.64 Coincidently, these athletes are involved with activities that have a low reliance on lipolysis, such as 100, 200, 400, and 800 m. Later, evidence for low body fatness in people involved with higher intensity activities (> 9METs) was confirmed in non-athletes, even in the presence of lower energy expenditure.63,64
There is also similar evidence for resistance training, where short duration and high-intensity modalities promoted higher increases in blood lactate and maintained lipolysis elevated for up to 22 hours after its cessation, while higher volume and lower intensity protocols resulted in less pronounced metabolic responses.65 Interestingly, this kind of stimulus resulted in higher reductions in body fat than endurance training or lower-intensity resistance training, even in the absence of dietary interventions.66,67 Based on these findings, high-intensity exercises as resistance training66 and high-intensity interval training67 should be considered in weight-loss programs.
Limitations
The present study has some important limitations. The more consistent evidences, especially about mechanisms, are from studies in animals. Moreover, while studies in animals provide important information,68 the application of these results in humans is difficult,69 especially regarding the magnitude of the changes and the time they take to occur.70 Moreover, the conclusions based on human studies are limited because multifactorial aspects related to human behavior might influence the results, especially when investigations do not control for confounding factors, like diet and physical activity.
Conclusion
Exercise cessation, especially after MICE, appears to increase the ability of the adipose tissue to store energy. This can be a response to training stress on this tissue and might be a necessary, and expected, response to preserve fat storages. However, caution should be taken, especially with the conclusions based on investigations involving humans, considering the multiple factors that could affect fat metabolism. Data regarding the mechanism of this phenomenon are inconclusive and controversial; while some data suggest that increased insulin sensitivity after exercise cessation might be the main factor for fat accumulation, others failed to show this relationship. Further studies should investigate the effects of exercise cessation on fat metabolism after different types of exercises, including high-intensity interval training and high-intensity resistance training, in order to propose alternatives for more efficient and sustainable with loss.
Supplemental Material
Supplemental material, PRISMA-checklist for Effects of exercise cessation on adipose tissue physiological markers related to fat regain: A systematic review by Fabrício Boscolo Del Vecchio, Victor Silveira Coswig, Leo Dutra Cabistany, Rafael Bueno Orcy and Paulo Gentil in SAGE Open Medicine
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was not sought for the present study because this is a review article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PG receives a research grant from CNPq (304435/2018-0)
ORCID iD: Paulo Gentil  https://orcid.org/0000-0003-2459-4977
https://orcid.org/0000-0003-2459-4977
Supplemental material: Supplemental material for this article is available online.
References
- 1. Sanchis-Gomar F, Garcia-Gimenez JL, Perez-Quilis C, et al. Physical exercise as an epigenetic modulator: eustress, the “positive stress” as an effector of gene expression. J Strength Cond Res 2012; 26(12): 3469–3472. [DOI] [PubMed] [Google Scholar]
- 2. Selye H. A syndrome produced by diverse nocuous agents. Nature 1936; 138: 32. [DOI] [PubMed] [Google Scholar]
- 3. Battistuzzi PGFCM . [Hans Selye, the father of stress]. Ned Tijdschr Tandheelkd 2011; 18(10): 471. [PubMed] [Google Scholar]
- 4. Viru A. Early contributions of Russian stress and exercise physiologists. J Appl Physiol 2002; 92(4): 1378–1382. [DOI] [PubMed] [Google Scholar]
- 5. Schneider KL, Spring B, Pagoto SL. Exercise and energy intake in overweight, sedentary individuals. Eat Behav 2009; 10(1): 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gunter K, Baxter-Jones ADG, Mirwald RL, et al. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res 2008; 23: 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flann KL, Lastayo PC, McClain DA, et al. Muscle damage and muscle remodeling: no pain no gain? J Exp Biol 2011; 214: 674–679. [DOI] [PubMed] [Google Scholar]
- 8. Acheson KJ, Schutz Y, Bessard T, et al. Glycoprotein storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am J Clin Nutr 1988; 48: 240–247. [DOI] [PubMed] [Google Scholar]
- 9. Hargreaves M, McKenna MJ, Jenkins DG, et al. Muscle metabolites and performance during high-intensity, intermittent exercise. J Appl Physiol 1998; 84: 1687–1691. [DOI] [PubMed] [Google Scholar]
- 10. Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sport Exerc 2002; 34(1): 92–97. [DOI] [PubMed] [Google Scholar]
- 11. Skovgaard C, Brandt N, Pilegaard H, et al. Combined speed endurance and endurance exercise amplify the exercise-induced PGC-1α and PDK4 mRNA response in trained human muscle. Physiol Rep 2016; 4(14): e12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sene-Fiorese M, Duarte FO, Scarmagnani FRR, et al. Efficiency of intermittent exercise on adiposity and fatty liver in rats fed with high-fat diet. Obesity 2008; 16(10): 2217–2222. [DOI] [PubMed] [Google Scholar]
- 13. Benatti F, Solis M, Artioli G, et al. Liposuction induces a compensatory increase of visceral fat which is effectively counteracted by physical activity: a randomized trial. J Clin Endocrinol Metab 2012; 97(7): 2388–2395. [DOI] [PubMed] [Google Scholar]
- 14. Sertie RAL, Curi R, Oliveira AC, et al. The mechanisms involved in the increased adiposity induced by interruption of regular physical exercise practice. Life Sci 2019; 222: 103–111. [DOI] [PubMed] [Google Scholar]
- 15. Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. J Physiol 2005; 565(Pt 3): 911–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodpaster BH, He J, Watkins S, et al. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001; 86: 5755–5761. [DOI] [PubMed] [Google Scholar]
- 17. Sertie RAL, Andreotti S, Proença ARG, et al. Cessation of physical exercise changes metabolism and modifies the adipocyte cellularity of the periepididymal white adipose tissue in rats. J Appl Physiol 2013; 115: 394–402. [DOI] [PubMed] [Google Scholar]
- 18. Askew EW, Huston RL, Dohm GL. Effect of physical training on esterification of glycerol-3-phosphate by homogenates of liver, skeletal muscle, heart, and adipose tissue of rats. Metabolism 1973; 22(3): 473–480. [DOI] [PubMed] [Google Scholar]
- 19. Coswig VS, Cabistany LD, Del Vecchio FB. Hypotheses for fat tissue supercompensation after exercise cessation. Hypothesis 2016; 14(1): 1–10. [Google Scholar]
- 20. Mujika I, Padilla S. Detraining: loss of training-induced physiological and performance adaptations Part I. Short term insufficient training stimulus. Sport Med 2000; 30(2): 79–87. [DOI] [PubMed] [Google Scholar]
- 21. Mujika I, Padilla S. Muscular characteristics of detraining in humans. Med Sci Sports Exerc 2001; 33(8): 1297–1303. [DOI] [PubMed] [Google Scholar]
- 22. Mujika I, Padilla S. Cardiorespiratory and metabolic characteristics of detraining in humans. Med Sci Sports Exerc 2001; 33(3): 413–421. [DOI] [PubMed] [Google Scholar]
- 23. Slade SCC, Dionne CEE, Underwood M, et al. Consensus on exercise reporting template (CERT): explanation and elaboration statement. Br J Sports Med 2016; 50(23): 1428–1437. [DOI] [PubMed] [Google Scholar]
- 24. Williams PT, Thompson PD. Dose-dependent effects of training and detraining on weight in 6406 runners during 7.4 years. Obesity 2006; 14: 1945–1984. [DOI] [PubMed] [Google Scholar]
- 25. Almeras N, Lemieux S, Bouchard C, et al. Fat gain in female swimmers. Physiol Behav 1997; 61: 811–817. [DOI] [PubMed] [Google Scholar]
- 26. Farias TY, Santos-Lozano A, Urra PS, et al. Efectos del entrenamiento y el desentrenamiento físico sobre la hemoglobina glucosilada, la glucemia y el perfil lipídico en diabéticos tipo II. Nutr Hosp 2015; 32: 1729–1734.26545543 [Google Scholar]
- 27. Liao YH, Sung YC, Chou CC, et al. Eight-week training cessation suppresses physiological stress but rapidly impairs health metabolic profiles and aerobic capacity in elite taekwondo athletes. Plos One. Epub ahead of print 27 July 2016. DOI: 10.1371/journal.pone.0160167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lo MS, Lin LLC, Yao WJ, et al. Training and detraining effects of the resistance vsendurance program on body composition, body size, and physical performance in young men. J Strength Cond Res 2011; 25: 2246–2254. [DOI] [PubMed] [Google Scholar]
- 29. Ormsbee MJ, Arciero PJ. Detraining increases body fat and weight and decreases V̇o 2peak and metabolic rate. J Strength Cond Res 2012; 26: 2087–2095. [DOI] [PubMed] [Google Scholar]
- 30. Rimbert V, Vidal H, Duché P, et al. Rapid down-regulation of mitochondrial fat metabolism in human muscle after training cessation is dissociated from changes in insulin sensitivity. FEBS Lett 2009; 583: 2927–2933. [DOI] [PubMed] [Google Scholar]
- 31. Bajpeyi S, Tanner CJ, Slentz CA, et al. Effect of exercise intensity and volume on persistence of insulin sensitivity during training cessation. J Appl Physiol 2009; 106: 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nikseresht M, Sadeghifard N, Agha-Alinejad H, et al. Inflammatory markers and adipocytokine responses to exercise training and detraining in men who are obese. J Strength Cond Res 2014; 28: 3399–3410. [DOI] [PubMed] [Google Scholar]
- 33. Olsen RH, Krogh-Madsen R, Thomsen C, et al. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 2008; 299: 1261–1263. [DOI] [PubMed] [Google Scholar]
- 34. Simsolo RB, Ong JM, Kern PA. The regulation of adipose tissue and muscle lipoprotein lipase in runners by detraining. J Clin Invest 1993; 92: 2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Herd SL, Hardman AE, Boobis LH, et al. The effect of 13 weeks of running training followed by 9 d of detraining on postprandial lipaemia. Br J Nutr 1998; 80: 57–66. [DOI] [PubMed] [Google Scholar]
- 36. Braga L, Mello M, Manchado F, et al. Exercício contínuo e intermitente: efeitos do treinamento e do destreinamento sobre o peso corporal e o metabolismo muscular de ratos obesos. Rev Port Ciências Do Desporto 2006; 6(2). http://www.scielo.mec.pt/scielo.php?script=sci_arttext&pid=S1645-05232006000200004 [Google Scholar]
- 37. Laye MJ, Rector RS, Borengasser SJ, et al. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol 2009; 106: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lehnen AM, Leguisamo NM, Pinto GH, et al. The beneficial effects of exercise in rodents are preserved after detraining: a phenomenon unrelated to GLUT4 expression. Cardiovasc Diabetol 2010; 9: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsai AC, Bach J, Borer KT. Somatic, endocrine, and serum lipid changes during detraining in adult hamsters. Am J Clin Nutr 1981; 34: 373–376. [DOI] [PubMed] [Google Scholar]
- 40. Yasari S, Dufresne E, Prud’homme D, et al. Effect of the detraining status on high-fat diet induced fat accumulation in the adipose tissue and liver in female rats. Physiol Behav 2007; 91: 281–289. [DOI] [PubMed] [Google Scholar]
- 41. Rector SR, Thyfault JP, Laye MJ, et al. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 2008; 586(Pt 17): 4241–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Applegate EA, Upton DE, Stern JS. Exercise and detraining: effect on food intake, adiposity and lipogenesis in Osborne-Mendel rats made obese by a high fat diet. J Nutr 1984; 114: 447–459. [DOI] [PubMed] [Google Scholar]
- 43. Craig BW, Martin G, Betts J, et al. The influence of training-detraining upon the heart, muscle and adipose tissue of female rats. Mech Ageing Dev 1991; 57: 49–61. [DOI] [PubMed] [Google Scholar]
- 44. Kump DS, Laye MJ, Booth FW. Increased mitochondrial glycerol-3-phosphate acyltransferase protein and enzyme activity in rat epididymal fat upon cessation of wheel running. Am J Physiol Endocrinol Metab 2006; 290(3): E480–E489. [DOI] [PubMed] [Google Scholar]
- 45. Mazzucatto F, Higa TS, Fonseca-Alaniz MH, et al. Reversal of metabolic adaptations induced by physical training after two weeks of physical detraining. Int J Clin Exp Med 2014; 7: 2000–2008. [PMC free article] [PubMed] [Google Scholar]
- 46. Sertie RA, Andreotti S, Proenca AR, et al. Fat gain with physical detraining is correlated with increased glucose transport and oxidation in periepididymal white adipose tissue in rats. Braz J Med Biol Res. Epub ahead of print 26 May 2015. Doi: 10.1590/1414-431x20154356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehav Rev 2001; 25(1): 15–28. [DOI] [PubMed] [Google Scholar]
- 48. Dohm GL, Barakat HA, Tapscott EB, et al. Changes in body fat and lipogenic enzyme activities in rats after termination of exercise training. Proc Soc Exp Biol Med 1977; 155(2): 157–159. [DOI] [PubMed] [Google Scholar]
- 49. Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl- CoA carboxylase. J Appl Physiol 1997; 83: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 50. Despres JP, Bouchard C, Savard R, et al. Effects of exercise-training and detraining on fat cell lipolysis in men and women. Eur J Appl Physiol Occup Physiol 1984; 53(1): 25–30. [DOI] [PubMed] [Google Scholar]
- 51. Avenell A, Brown TJ, McGee MA, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J Hum Nutr Diet 2004; 17(4): 293–316. [DOI] [PubMed] [Google Scholar]
- 52. Seaman SA, Tannan SC, Cao Y, et al. Paradoxical adipose hyperplasia and cellular effects after cryolipolysis: a case report. Aesthetic Surg J 2015; 36: sjv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 2007; 117: 1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petibois C, Cassaigne A, Gin H, et al. Lipid profile disorders induced by long-term cessation of physical activity in previously highly endurance-trained subjects. J Clin Endocrinol Metab 2004; 89: 3377–3384. [DOI] [PubMed] [Google Scholar]
- 55. Saarni SE, Rissanen A, Sarna S, et al. Weight cycling of athletes and subsequent weight gain in middleage. Int J Obes 2006;30: 1639–1644. [DOI] [PubMed] [Google Scholar]
- 56. Strychar I, Lavoie M-E, Messier L, et al. Anthropometric, metabolic, psychosocial, and dietary characteristics of overweight/obese postmenopausal women with a history of weight cycling: a MONET (montreal Ottawa new emerging team) study. J Am Diet Assoc 2009; 109(4): 718–724. [DOI] [PubMed] [Google Scholar]
- 57. Byrne NM, Wood RE, Schutz Y, et al. Does metabolic compensation explain the majority of less-than-expected weight loss in obese adults during a short-term severe diet and exercise intervention? Int J Obes 2005; 36(11): 1472–1478. [DOI] [PubMed] [Google Scholar]
- 58. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “the biggest loser” competition. Obesity 2016; 24(8): 1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coswig VS, Barbalho M, Raiol R, et al. Effects of high vs moderate-intensity intermittent training on functionality, resting heart rate and blood pressure of elderly women. J Transl Med 2020; 18(1): 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol 1998; 275(2): E332–E337. [DOI] [PubMed] [Google Scholar]
- 61. Whyte LJ, Ferguson C, Wilson J, et al. Effects of single bout of very high-intensity exercise on metabolic health biomarkers in overweight/obese sedentary men. Metabolism 2012; 62(2): 212–219. [DOI] [PubMed] [Google Scholar]
- 62. Carriere A, Jeanson Y, Berger-Muller S, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 2014; 63: 3253–3265. [DOI] [PubMed] [Google Scholar]
- 63. Viana RB, Naves JPA, Coswig VS, et al. Is interval training the magic bullet for fat loss? A systematic review and meta-analysis comparing moderate-intensity continuous training with high-intensity interval training (HIIT). Br J Sports Med 2019; 53: bjsports-2018-099928. [DOI] [PubMed] [Google Scholar]
- 64. Malina RM, Mueller WH, Bouchard C, et al. Fatness and fat patterning among athletes at the montreal Olympic games, 1976. Med Sci Sports Exerc 1982; 14(6): 445–452. [DOI] [PubMed] [Google Scholar]
- 65. Paoli A, Moro T, Marcolin G, et al. High-intensity interval resistance training (HIRT) influences resting energy expenditure and respiratory ratio in non-dieting individuals. J Transl Med 2012; 10(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paoli A, Pacelli QF, Moro T, et al. Effects of high-intensity circuit training, low-intensity circuit training and endurance training on blood pressure and lipoproteins in middle-aged overweight men. Lipids Heal Dis 2013;12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Batacan RB, Duncan MJ, Dalbo VJ, et al. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med 2017; 51(6): 494–503. [DOI] [PubMed] [Google Scholar]
- 68. Bakhle YS, Blakemore C, Peatfield T. Missing evidence that animal research benefits humans. Br Med J 2004; 328: 1017–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans . JAMA 2006; 296: 1731–1112. [DOI] [PubMed] [Google Scholar]
- 70. Andreollo NA, Santos EF, Araujo MR, et al. Rat’s age versus human’s age: what is the relationship. Arq Bras Cir Dig 2012; 25(1): 49–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PRISMA-checklist for Effects of exercise cessation on adipose tissue physiological markers related to fat regain: A systematic review by Fabrício Boscolo Del Vecchio, Victor Silveira Coswig, Leo Dutra Cabistany, Rafael Bueno Orcy and Paulo Gentil in SAGE Open Medicine