Abstract
Pulmonary arterial hypertension has been reported with a prevalence of 7.9% in patients with anti-synthetase syndrome; however, anti-synthetase syndrome associated with pulmonary veno-occlusive disease (PVOD) has never before been described in the literature. We present a novel case of anti-synthetase syndrome-associated PVOD in a patient who presented with hypoxic respiratory failure associated with right heart failure and was diagnosed with anti-synthetase syndrome based on his autoimmune serology and pre-capillary pulmonary hypertension on right heart catheterization. He was initiated on pulmonary arterial hypertension therapy, but with escalating dose of parenteral epoprostenol, experienced acute clinical worsening with chest imaging concerning for PVOD that was confirmed on autopsy. Anti-synthetase syndrome can be associated with PVOD, and it should be suspected in patients who have evidence of pre-capillary pulmonary hypertension and who deteriorate with the initiation of pulmonary hypertension-specific therapy.
Keywords: pulmonary veno-occlusive disease, pulmonary arterial hypertension, anti-synthetase syndrome
Introduction
Pulmonary arterial hypertension (PAH) is a progressive pulmonary vascular disease, defined by elevation of mean pulmonary arterial pressure (mPAP) ≥20 mmHg, pulmonary wedge pressure (PAOP) ≤15 mmHg and pulmonary vascular resistance (PVR) > 3 WU.1,2 Pulmonary veno-occlusive disease (PVOD), a rare form of pulmonary arterial hypertension with overt features of venous/capillaries involvement, represents an entity whereby pathological fibrosis and intimal thickening of the pulmonary venules lead to their obliteration.3 The clinical presentations of idiopathic PAH and PVOD are quite similar; yet, the clinical course in response to therapy is aggressive in PVOD with a mortality of 72%.4 Anti-synthetase syndrome is a heterogeneous connective tissue disease, characterized by an association with interstitial lung disease (ILD) and/or myositis in the presence of anti-aminoacyl-tRNA-synthetase antibodies.5 Although the prevalence of PH in anti-synthetase syndrome is 7.9%, there are no reports of anti-synthetase syndrome associated with PVOD in the literature. We present the first case of anti-synthetase syndrome associated with PVOD.
Case
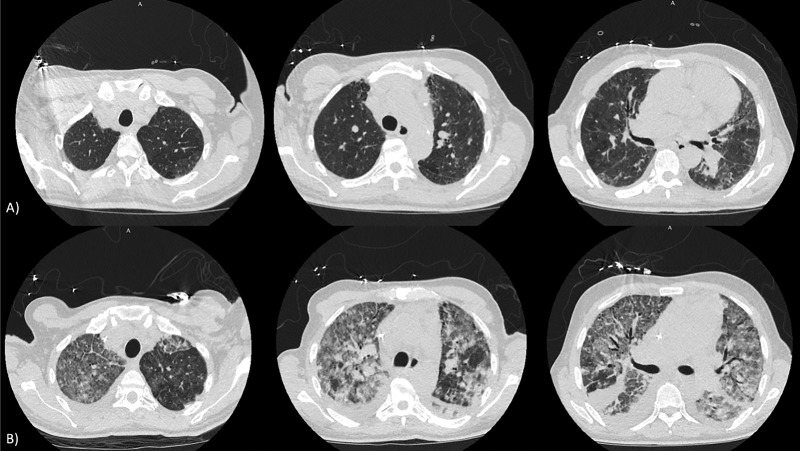
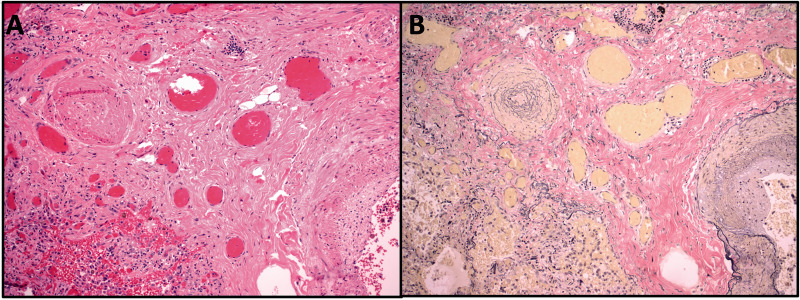
A 55-year-old man with history of interstitial lung disease of unknown etiology presented with acute worsening of his chronic shortness of breath and worsening lower extremity edema. The patient had no pulmonary function tests. Computed tomography (CT) scan of the chest showed evidence of mild bilateral lower lobe fibrosis associated with ground glass opacities without honeycombing (Fig. 1a). Autoimmune serology revealed a positive anti-nuclear antibody (1:160) and anti-Jo antibody (226 AU/mL) which in addition to the physical exam finding of mechanics hands confirmed a diagnosis of anti-synthetase syndrome. His echocardiogram revealed dilated RV with depressed function and a D-shaped septum. He underwent a right heart catheterization that showed pre-capillary pulmonary hypertension with high-risk hemodynamic findings: right atrial pressure of 10 mmHg, pulmonary artery pressure of 100/45 mmHg with mean pulmonary arterial pressure of 65 mmHg, pulmonary capillary artery occlusion pressure of 8 mmHg, pulmonary vascular resistance (PVR) of 21 WU, central venous oxygen saturation of 55% and cardiac output and cardiac index of 2.7 L/min and 1.6 L/min/m2, respectively. Despite the presence of ILD, the elevated pulmonary pressures were out of proportion to the degree of underlying parenchymal lung disease, as such, given his low cardiac output state, the patient was started on inotropic therapy with Milrinone, and pulmonary vasodilatory therapy with Macitentan 10 mg once daily, Sildenafil 20 mg three times daily, and inhaled epoprostenol. Patient initially had improvement in hypoxemia and symptoms, with PaO2 of 95 mmHg while on 40% FiO2. He was later started on low-dose parenteral epoprostenol at 2 ng/kg/min with gradual up titration of the dose, in addition to the previously initiated vasodilatory therapy. Once the epoprostenol dose was escalated to 8 ng/kg/min, the patient experienced acutely worsening hemodynamics and hypoxia with PaO2 of 53 mmHg while on 100% FiO2, and a repeat CT Chest showed new diffuse ground glass opacities with smooth septal thickening, bilateral pleural effusions, and lymphadenopathy concerning for PVOD (Fig. 1b). He was evaluated and deemed not a candidate for lung transplant. Parenteral epoprostenol dose was lowered and eventually stopped. However, in the setting of clinical deterioration and worsening hypoxemia, the patient opted for comfort measures and later passed. On autopsy, bilateral lungs revealed vascular myxoid fibrosis, venous intimal hyperplasia, dilated capillaries, and intimal thickening of the arterioles without plexiform lesions in a background of pulmonary parenchyma with mature fibrosis consistent with a nonspecific interstitial pneumonia pattern (Fig. 2). These findings confirmed underlying diagnosis of PVOD on a background of interstitial lung disease.
Fig. 1.
Axial chest CT images on admission (a), and after initiation of parenteral epoprostenol therapy showing extensive ground glass opacities, smooth septal thickening, and bilateral pleural effusions (b).
Fig. 2.
Hematoxylin and eosin (a) and elastin (b) stains showing an occluded pulmonary vein (at left) with reduplication of the elastic lamina surrounded by dilated capillaries. The edge of an arteriole is shown (bottom right) with focal fibrotic disruption of the internal elastic lamina.
Discussion
The current classification system divides PH into five main groups according to their pathophysiology, clinical features, and treatment options. Group 1 PH consists of PAH and its various subtypes such as idiopathic, heritable, and drug-induced PAH as well as those associated with connective tissue disease, HIV infection, portal hypertension, congenital heart disease, and schistosomiasis. In the most recent classification of pulmonary hypertension, PVOD and pulmonary capillary haemangiomatosis (PCH) are considered to represent varied expressions of the same disease and are now classified as the newest PAH subtype: PAH with overt features of venous/capillaries (PVOD/PCH) involvement.2 PVOD/PCH can be further sub-classified to include associated forms such as idiopathic, heritable form which has been well documented to predominantly involve biallelic mutations in EIF2AK4 and associated with a younger age of onset of symptoms,6,7 drug and toxins-induced that have been associated with organic solvents as well as high-dose chemotherapy with alkylating agents,8 and lastly autoimmune (scleroderma) and systemic causes (sarcoidosis) of PVOD which are not well recognized mostly due to their exceedingly rare presentations9 (Table 1).
Table 1.
Classification of etiologies of PVOD/PCH.
| Sub-classifications within WHO Group |
|---|
| • Idiopathic |
| • Heritable |
| • EIF2AK4 mutation |
| • Other mutations |
| • Drug/toxins/radiation induced |
| • Organic solvents |
| • Chemotherapy (alkylating agents) |
| • Autoimmune |
| • HIV infection |
The pathologic hallmark of PVOD is the extensive and diffuse occlusion of pulmonary venules and septal veins by fibrous tissue associated with thickening of the intima, as well as possible arterial involvement.10 These characteristics differentiate it from the arterialization of pulmonary veins that can be seen in post-capillary etiologies of PH.11 Furthermore, the post-capillary changes are frequently associated with capillary angioproliferation, which in some cases are found to be focally distributed.12 It is also interesting to note that in diseases such as scleroderma, PVOD-like changes have been noted,9 although no such evidence has yet been presented in individuals with ILD related to anti-synthetase syndrome.
Although the clinical presentation and hemodynamic findings of PVOD tend to be similar to PAH, the clinical course of PVOD is more aggressive, typically either refractory to PAH-specific therapy or deterioration with the initiation of therapy. It is difficult to clinically distinguish patients with PVOD from PAH prior to start of therapy as was the case in our patient. Treatment, at best, remains an extrapolation of PAH therapy as a bridge to lung transplantation.13
In anti-synthetase syndrome, the molecular pathway leading to autoantibody production against synthetases and subsequent organ pathology is presently unknown.14 ILD has been reported in 90% of patients with anti-synthetase syndrome, and the specific Anti-Jo1 synthetase antibody is prevalent in up to 70% of such patients.15 The signs of this disease have high clinical heterogeneity in which ILD can predominate as a sole manifestation of anti-synthetase syndrome without apparent myositis, fever, mechanic hands, or arthritis at a given time.14 It is important to suspect PH in such patients when symptoms and pulmonary vascular pressures appear “out of proportion” to connective tissue involvement. The prevalence of PVOD in anti-synthetase syndrome-associated PH remains unknown and may be under recognized.
Future investigations should be geared towards identifying possible biochemical markers of PVOD for more accurate diagnosis. Currently, screening efforts remain at best a high clinical suspicion. As we wait for diagnostic advancements, autoimmune associated PVOD should be suspected in patients with PH “out of proportion” to ILD that worsen with the initiation of PH therapy.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Wilkins MR, Aman J, Harbaum L, et al. Recent advances in pulmonary arterial hypertension. F1000Res 2018; 7: >F1000 Faculty Rev-1128. [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed) 2016; 69: 177. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb BW, Jr, Loyd JE, Ely EW, et al. Pulmonary veno-occlusive disease: a case series and new observations. Chest 2000; 118: 1671–1679. [PubMed] [Google Scholar]
- 5.Hervier B, Meyer A, Dieval C, et al. Pulmonary hypertension in antisynthetase syndrome: prevalence, aetiology and survival. Eur Respir J 2013; 42: 1271–1282. [DOI] [PubMed] [Google Scholar]
- 6.Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014; 46: 65–69. [DOI] [PubMed] [Google Scholar]
- 7.Montani D, Girerd B, Jaïs X, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. [DOI] [PubMed] [Google Scholar]
- 8.Ranchoux B, Gunther S, Quarck R, et al. Chemotherapy-induced pulmonary hypertension: role of alkylating agents. Am J Pathol 2015; 185: 356–371. [DOI] [PubMed] [Google Scholar]
- 9.Montani D, Lau EM, Dorfmuller P, et al. Pulmonary veno-occlusive disease. Eur Respir J 2016; 47: 1518–1534. [DOI] [PubMed] [Google Scholar]
- 10.Nossent EJ, Antigny F, Montani D, et al. Pulmonary vascular remodeling patterns and expression of general control nonderepressible 2 (GCN2) in pulmonary veno-occlusive disease. J Heart Lung Transplant 2018; 37: 647–655. [DOI] [PubMed] [Google Scholar]
- 11.Wagenvoort CA, Wagenvoort N. The pathology of pulmonary veno-occlusive disease. Virchows Arch A Pathol Anat Histol 1974; 364: 69–79. [DOI] [PubMed] [Google Scholar]
- 12.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53(1): 1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wille KM, Sharma NS, Kulkarni T, et al. Characteristics of patients with pulmonary venoocclusive disease awaiting transplantation. Ann Am Thorac Soc 2014; 11: 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witt LJ, Curran JJ, Strek ME. The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med 2016; 23: 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee S, Prayson R, Farver C. Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 2013; 80: 655–666. [DOI] [PubMed] [Google Scholar]