Abstract
Purpose of review:
Summarize recent evidence on the identification and management of rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Recent findings:
Clinical and sub-clinical ILD are frequent extra-articular manifestations of RA. Better means of identifying and treating RA-ILD are needed to improve the prognosis, with a median survival of only 3–7 years after diagnosis. Several serum biomarkers are currently being evaluated for their ability to detect RA-ILD. Thorough evaluation and multidisciplinary discussion remains the gold standard for establishing the diagnosis of RA-ILD. Management is challenging with most RA disease-modifying anti-rheumatic drugs (DMARDs) linked to pneumonitis. Methotrexate is typically avoided in clinically significant ILD, although alternative therapies including leflunomide and biologic DMARDs also carry risks in RA-ILD. Anti-fibrotics appear to slow the progression of ILD, and a large phase II trial exclusively in RA-ILD is underway. Additionally, smoking cessation, pulmonary rehabilitation, oxygen therapy, managing comorbidities, and lung transplantation evaluation are vital to improving patient outcomes in RA-ILD.
Summary:
With little high-quality evidence to guide the management of RA-ILD, multidisciplinary teams with expertise in RA-ILD are highly valuable for diagnosing and treating RA-ILD. Clinical and translational research in RA-ILD is needed to fill the many evidence gaps.
Keywords: rheumatoid arthritis, interstitial lung disease, pulmonary fibrosis
Introduction
Interstitial lung disease (ILD) is an extra-articular manifestation of rheumatoid arthritis (RA) first reported by Ellman and Ball in 1948 (1). In this review, we summarize recent evidence on the identification and management of RA-ILD.
Epidemiology and outcomes of RA-ILD
Between 5–10% of patients with RA will develop clinically significant ILD (2, 3), and another 20–30% may have subclinical involvement (4). Risk factors for RA-ILD include male sex, older age, tobacco use, higher RA disease activity, extra-articular disease features (e.g. subcutaneous nodules), and seropositivity for RA autoantibodies (rheumatoid factor [RF] and anti-citrullinated protein antibodies [ACPAs]) (2, 3, 5–7). While the median survival has been reported to be <3 years (2), two recent observational studies found the median survival to be 7 years after diagnosis (8, 9). In addition to its impact on survival, RA-ILD places a tremendous burden on healthcare systems with mean total 5-year healthcare costs exceeding $170,000 per patient (8).
Two of the most important prognostic factors in RA-ILD are the pattern of ILD and ILD severity. The most common patterns of RA-ILD are usual interstitial pneumonia (UIP), characterized radiographically by honeycombing and traction bronchiectasis, and non-specific interstitial pneumonia (NSIP), characterized radiographically by diffuse ground glass opacities and the absence of honeycombing (2, 10, 11). A meta-analysis of 10 cohort studies including 1,256 patients with RA-ILD estimated a 1.6-fold higher risk of death for those with a UIP pattern compared to other patterns (12). While radiographic appearance is clearly important, several studies have found that pulmonary physiology (e.g. forced vital capacity [FVC]) is more prognostic than ILD pattern. Severity of ILD by pulmonary physiology and high-resolution computed tomography (HRCT) is strongly associated with progression (physiologic and radiographic) and mortality in RA-ILD (13–15).
Identifying RA-ILD
Because the initial manifestation may be inflammatory arthritis (85–90% of cases) or ILD in patients who develop RA-ILD (3, 5), both rheumatologists and pulmonologists have roles in its detection and evaluation (Figure 1).
Figure 1. Approach to the identification of rheumatoid arthritis-associated interstitial lung disease.
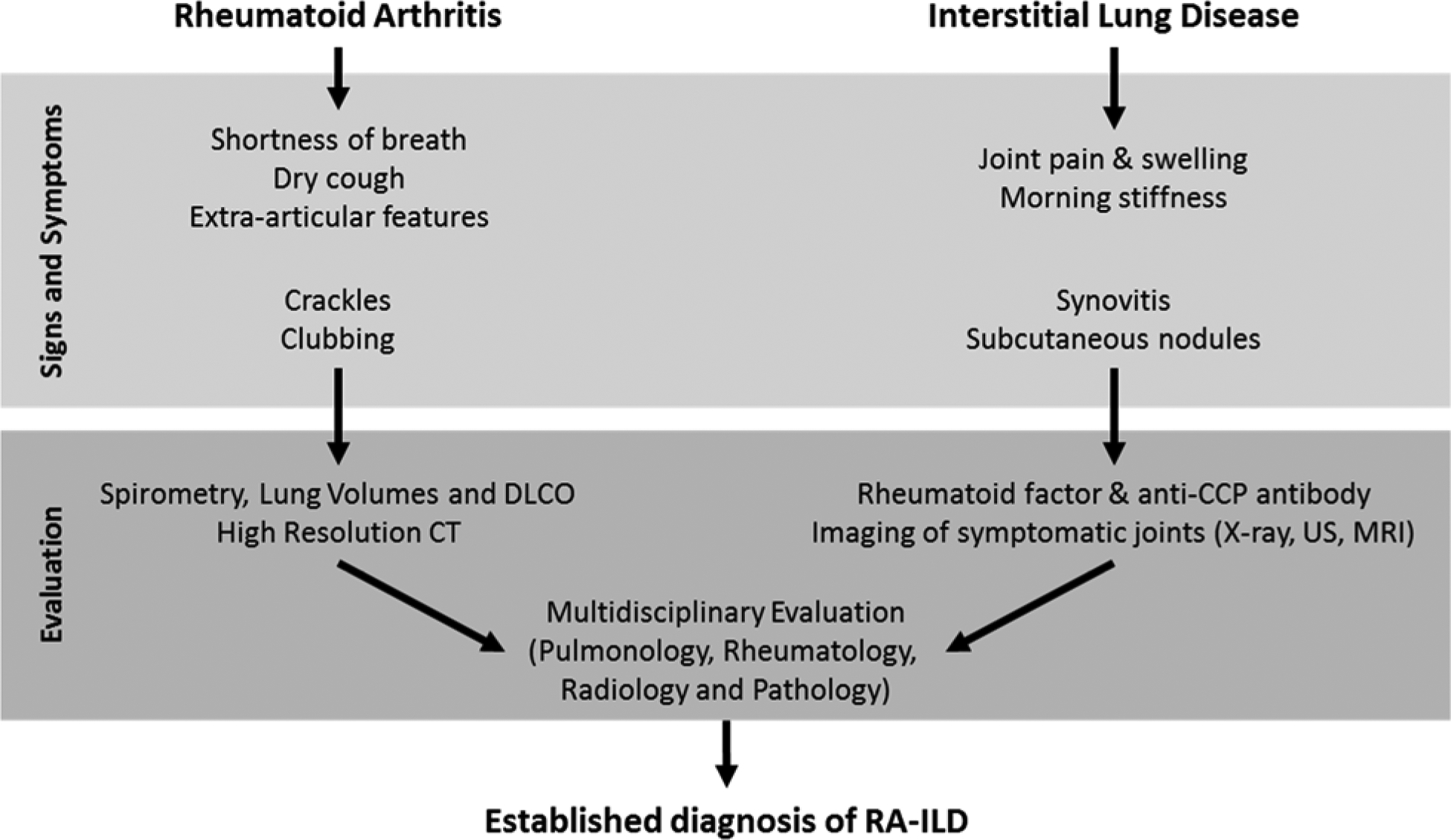
The initial presentation of rheumatoid arthritis (RA) or interstitial lung disease (ILD) should prompt evaluation for other signs and symptoms attributable to RA-ILD. Testing for pulmonary and articular manifestations followed by multidisciplinary discussion can establish the diagnosis of RA-ILD.
Abbreviations: CT = computed tomography, DLCO = diffusing capacity for carbon monoxide, MRI = magnetic resonance imaging, US = ultrasound
Identifying ILD in RA
The high prevalence of sub-clinical ILD on HRCT in patients with RA demonstrates that screening approaches relying on clinical signs and symptoms will be poorly sensitive for detecting ILD (4, 16). To improve on the sensitivity of clinical findings, an algorithm to detect Velcro rales in recorded breath sounds from an electronic stethoscope was developed. In 137 RA patients who underwent HRCT, electronic breath sounds had a sensitivity of 93.2% and specificity of 76.9% for detecting ILD and outperformed clinical symptoms, exam findings, chest x-ray, and PFTs (17). Validation in regular clinical settings is needed.
There is substantial interest in identifying serum biomarkers for RA-ILD since early identification may aid in preventing irreversible damage resulting from delays in diagnosis. In a large, international, case-control study, a MUC5B promoter variant (rs35705950) was associated with 3-fold higher odds of RA-ILD compared to RA alone (18). Our group performed a multi-center cross-sectional study that found the presence of anti-malondialdehyde-acetaldehyde (MAA) antibodies to be associated with 2-fold higher odds of ILD in RA (19). Other biomarkers that have been previously examined include matrix metalloproteinase (MMP)-7, surfactant protein D, pulmonary and activation-regulated chemokine, interferon-γ-inducible protein 10, anti-citrullinated heat shock protein 90, antibodies to cross-reactive peptidyl-arginine 3/4, and anti-citrullinated alpha enolase antibodies (20–24). To date, there has not been validation of most of these biomarkers or integration into clinical care.
Identifying RA in ILD.
When ILD is the initial manifestation, providers must differentiate RA as the underlying etiology from other connective tissue diseases (CTD) and idiopathic interstitial lung diseases. In addition to history and exam focused on articular symptoms, testing for RA autoantibodies (RF and ACPAs) should be completed. While ACPAs are highly specific (>95%) for RA in most settings (25), they may also occur in the setting of chronic lung diseases even in the absence of RA (26). Individuals with ACPAs but without inflammatory arthritis appear to be at high risk for developing RA later (27). Many of the biomarkers which show promise for identifying ILD in RA may not be useful for differentiating RA-ILD from other ILD. A recent study of two independent RA-ILD cohorts demonstrated overlap in serum pro-inflammatory cytokines and MMPs in RA-ILD and IPF (28).
Establishing the diagnosis and treatment team in RA-ILD
The gold standard for diagnosing RA-ILD is a multidisciplinary discussion of history, clinical exam, blood testing, HRCT, PFTs, and when performed, lung biopsy. While most multidisciplinary discussion of newly diagnosed ILD includes pulmonologists, radiologists, and pathologists, the inclusion of rheumatologists improves the detection of CTD-ILD (29). Given the correlation between HRCT and pathology findings as well as the morbidity accompanying surgical lung biopsy, biopsy is not typically pursued unless there is uncertainty in the diagnosis. Transbronchial cryobiopsies are a novel, less invasive method to acquire tissue to establish the diagnosis of ILD, though standardization of the procedure and delineation of its role in the diagnostic evaluation are still being determined (30). After establishing the diagnosis of RA-ILD, a multidisciplinary team including support staff (e.g. nurses, pharmacists, and respiratory therapists) is crucial for ongoing management, and referral to specialized centers with CTD-ILD programs should be considered, when available.
Overview of the management of RA-ILD
The authors approach to managing RA-ILD is shown in Figure 2. We begin by assessing disease severity, risk factors, and patient preferences. Supportive interventions are implemented for all patients. In patients with clinically significant or progressive ILD (based on clinical symptoms, PFTs, HRCT), we modify use of RA disease-modifying anti-rheumatic drugs (DMARDs). If RA-ILD progresses, we consider alternative immunomodulatory therapy and anti-fibrotics.
Figure 2. Approach to the management of rheumatoid arthritis-associated interstitial lung disease.
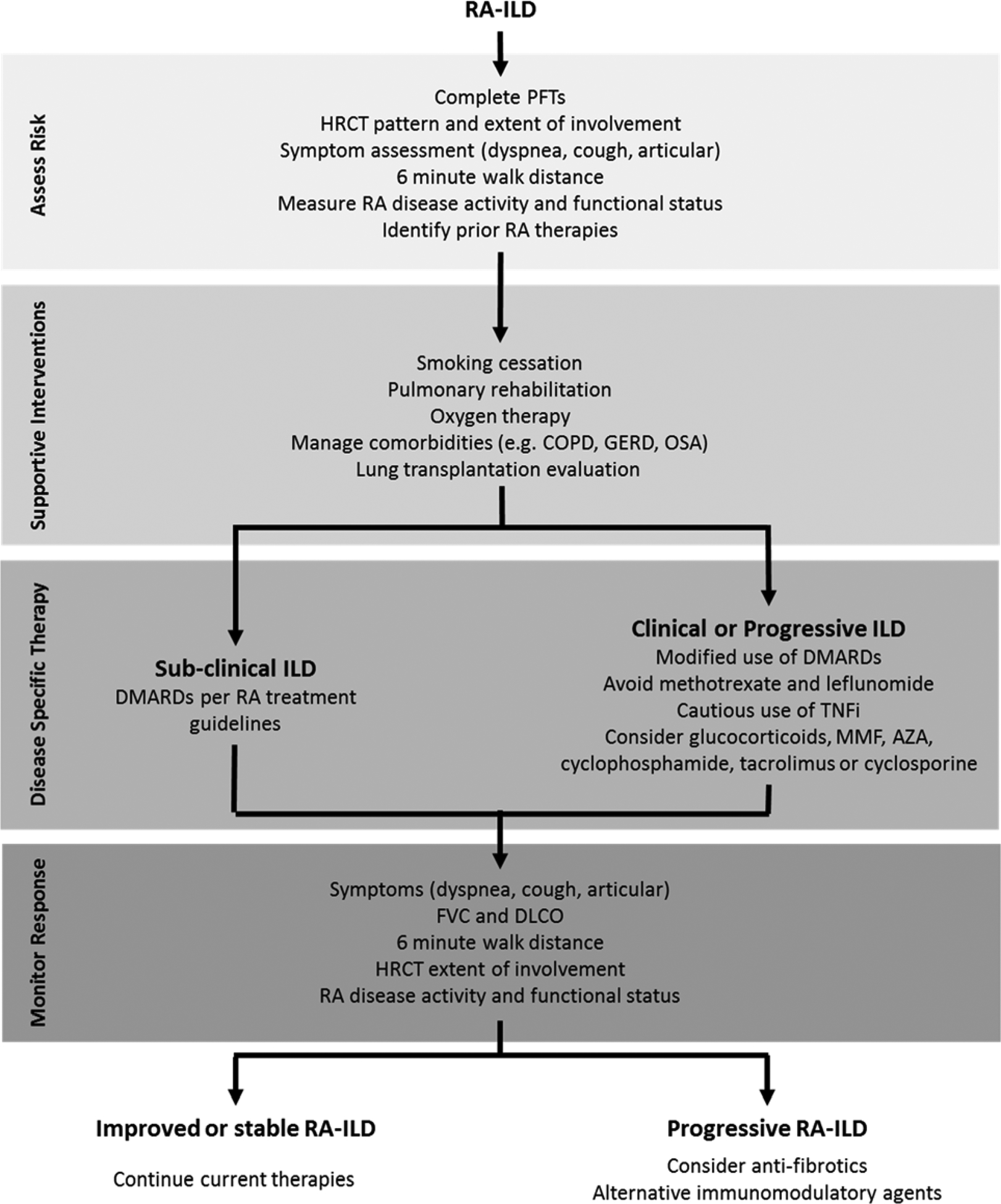
Management of rheumatoid arthritis-interstitial lung disease (RA-ILD) begins by assessing severity and risk for progression. All patients should receive non-pharmacologic therapies. Those with clinically significant RA-ILD may have their RA disease-modifying therapies adjusted and consideration given to other immunomodulatory therapies and glucocorticoids. If progression occurs despite these therapies, anti-fibrotics and alternative immunomodulatory therapies should be considered.
Abbreviations: AZA = azathioprine, COPD = chronic obstructive pulmonary disease, DLCO = diffusing capacity for carbon monoxide, DMARD = disease-modifying anti-rheumatic drug, FVC = forced vital capacity, GERD = gastroesophageal reflux disease, HRCT = high-resolution computed tomography, MMF = mycophenolate mofetil, OSA = obstructive sleep apnea, PFT = pulmonary function tests, TNFi = tumor necrosis factor inhibitor
Non-pharmacologic therapies
Smoking tobacco is the strongest environmental risk factor for both RA and ILD, and counseling on smoking cessation is of paramount importance. Ambulatory oxygen therapy is routinely prescribed for patients with a PaO2 ≤55 mm Hg or SpO2≤88%. Despite widespread use, oxygen therapy has questionable benefit in regards to dyspnea, exercise tolerance, and mortality (31–33). Even though patients with CTD-ILD appear to benefit less than patients with other forms of ILD (34), pulmonary rehabilitation meaningfully improves exercise capacity, reduces dyspnea, and improves quality of life (35). Lung transplantation evaluation should be considered in all patients with progressive ILD. Patients with RA-ILD that undergo lung transplantation have a similar risk of rejection and mortality as patients with other ILD (36, 37).
Selecting pharmacologic therapies
The outcomes in RA have dramatically improved with aggressive treatment strategies and an expanding armamentarium of DMARDs. Complicating the choice of DMARDs in RA-ILD is that most DMARDs have been linked to drug induced pneumonitis (38). We review the evidence for pharmacologic therapies in RA-ILD (all off-label), focusing on ILD outcomes given the existence of guidelines for the management of articular disease in RA (39, 40).
Glucocorticoids.
Glucocorticoids are typically part of the initial treatment regimen for clinically significant RA-ILD, based on experience in CTD-ILD rather than data demonstrating efficacy in RA-ILD (41). NSIP and organizing pneumonia ILD patterns are more responsive to glucocorticoids than UIP (41, 42), though data specifically in RA-ILD are lacking. Glucocorticoids have several dose and duration dependent long-term side effects including infection and osteoporosis (43, 44). Therefore, they are best suited for the initial management or treating acute exacerbations while transitioning to other therapies with more favorable long-term safety profiles.
Methotrexate.
Pneumonitis occurs in only 0.3 to 0.4% of patients with RA treated with methotrexate (45, 46), and methotrexate is not a risk factor for RA-ILD. In fact, results from prospective early RA inception cohorts showed trends towards lower odds of developing ILD in patients with RA treated with methotrexate (odds ratio 0.54, 95% confidence interval [CI] 0.28–1.06) (7). Because pre-existing lung disease is a risk factor for methotrexate pneumonitis (47), the difficulty in distinguishing methotrexate pneumonitis from exacerbations or progression of ILD, and that a lack of pulmonary reserve may predispose to increased mortality if pneumonitis were to occur, many providers discontinue or avoid methotrexate in RA-ILD. While prone to confounding and selection bias, the limited studies evaluating methotrexate use in RA-ILD have not observed worse outcomes with its use (48, 49). We typically avoid the use of methotrexate in clinically significant and/or progressive RA-ILD and engage patients in shared decision making prior to use of methotrexate in RA-ILD. The safety of methotrexate in RA-ILD is a critically important question.
Other conventional synthetic DMARDs (csDMARDs).
Avoidance of methotrexate in those with or at risk for RA-ILD is partially responsible for a higher rate of ILD observed with leflunomide treatment (50). However, pneumonitis is well known to also occur with leflunomide use. Pre-existing ILD and prior methotrexate pneumonitis are risk factors for death in leflunomide pneumonitis (51), suggesting it should not be the standard alternative to methotrexate in these situations. Pneumonitis has also been reported with sulfasalazine (52). There is a paucity of data on the safety of hydroxychloroquine in RA-ILD.
Tumor necrosis factor inhibitors (TNFi).
While several cases of new-onset ILD or exacerbations of ILD have been reported after TNFi use (53, 54), comparative studies are conflicting. In retrospective cohort studies in the British Society for Rheumatology Biologics Register, TNFi were not associated with a higher risk of death compared to csDMARDs in RA-ILD (55), but there were trends towards better survival with rituximab (hazard ratio 0.53, 95% CI 0.26–1.10) compared to TNFi (56). Analyses of large U.S. administrative claims databases have not found significant differences in respiratory events between patients with RA-ILD using TNFi compared to tocilizumab, rituximab, and abatacept (49, 57). However, there were numerically fewer respiratory events among initiators of abatacept compared to TNFi (57). In addition to confounding and selection bias, misclassification of RA-ILD is problematic in these observational studies as demonstrated by extensive testing of the accuracy of administrative algorithms for RA-ILD (58).
Other biologic DMARDs and janus kinase (JAK) inhibitor.
Beyond the aforementioned studies comparing to TNFi (49, 56, 57), there is only limited, uncontrolled data on these agents in RA-ILD. Small, uncontrolled studies generally have shown the majority of RA-ILD subjects treated with rituximab, tocilizumab, or abatacept to remain stable or improved by PFTs (59–62). A small case series did not find exacerbations of ILD with tofacitinib treatment (63), and in the SKG mouse model, tofacitinib effectively treats ILD (64). Biologic DMARDs appear to have a role in other CTD-ILD (65, 66), and several studies are ongoing in CTD-ILD. A double-blind RCT comparing rituximab to cyclophosphamide in CTD-ILD (RA-ILD excluded) is ongoing (67). Phase II RCTs of abatacept in RA-ILD (NCT03084419) and myositis-ILD (NCT03215927) are recruiting.
Other immunomodulatory therapies.
The role of other immunomodulatory therapies such as mycophenolate mofetil (MMF), cyclophosphamide, azathioprine, cyclosporine, and tacrolimus in RA-ILD remains unclear. In a retrospective analysis of 125 CTD-ILD treated with MMF (n=18 RA-ILD), MMF was associated with improvement in lung function in those with a NSIP pattern and stability in those with a UIP pattern (68). Both cyclophosphamide and MMF have demonstrated efficacy in SSc-ILD in double-blind RCTs (69, 70). Azathioprine is often used as an alternative to methotrexate in RA-ILD. A single center retrospective cohort study of CTD-ILD (n=97, 24% RA-ILD) found that patients treated with azathioprine had similar clinical events and longitudinal PFTs compared to MMF (71). There are case reports/series of RA-ILD improving with cyclosporine and tacrolimus (72–75). While these other immunomodulatory therapies (e.g. MMF, azathioprine) may be effective for ILD, providers must consider their potential for greater toxicities and more modest effects on articular disease (76–78).
Anti-fibrotics.
Currently two anti-fibrotics are FDA approved for the management of IPF, nintedanib and pirfenidone. They both are actively being studied in CTD-ILD. The INBUILD study was an international, double-blind RCT comparing nintedanib to placebo in patients with progressive, fibrotic lung disease (13% RA-ILD) (79). In this study, patients treated with nintedanib had a slower rate of FVC decline over 52 weeks, but there were no significant differences in subjective symptoms or clinical events. Diarrhea was the major side effect of nintedanib, occurring in 67% of treated subjects compared to 24% on placebo. A RA-ILD specific double-blind phase II RCT comparing pirfenidone to placebo is currently enrolling (TRAIL1, NCT02808871) and will illustrate the effects of anti-fibrotics on articular disease in addition to ILD (80). In a mouse model of RA-ILD, nintedanib was effective for both lung and articular manifestations (81). However, in the SENSCIS trial, a large, double-blind RCT comparing nintedanib to placebo in SSc-ILD, less decline in FVC was seen with nintedanib (resulting in FDA approval for SSc-ILD), but it was not effective for the non-ILD manifestation of skin fibrosis (82).
Managing comorbidities in RA-ILD
Chronic obstructive pulmonary disease (COPD).
Even among non-smokers, COPD frequently accompanies RA-ILD. In a multi-center retrospective study, 27% of non-smokers with RA-ILD had emphysema on CT (83). In these individuals, emphysema was independently associated with a UIP pattern and poorer survival. The high prevalence and poor outcomes of concomitant COPD in RA-ILD warrants diligent adherence to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations for COPD management (84).
Gastroesophageal reflux disease (GERD).
GERD is common in RA-ILD, with approximately 50% of patients with RA-ILD having a diagnosis of GERD (8). The relationship between GERD and ILD is debated. A recent meta-analysis of 18 case-control studies of GERD and IPF found the existing association to be confounded by smoking (85). Pharmacologic (e.g. proton pump inhibitors, H2 blockers) and non-pharmacologic (weight loss, dietary modification, elevating head of bed) treatments are frequently prescribed in ILD and conditionally recommended in IPF management guidelines (42). Equally as contentious is whether proton pump inhibitors increase the risk of pneumonia (86). Therefore, providers must balance ill-defined risks and benefits of antacid use in RA-ILD.
Monitoring treatment response
Monitoring treatment response in RA-ILD includes both assessment of articular and respiratory disease activity and severity. The American College of Rheumatology (ACR) recently convened a working group to provide recommendations on preferred RA disease activity and functional status measures (87, 88). The five preferred RA disease activity measures were the Disease Activity Score in 28-joints, Clinical Disease Activity Index, Simplified Disease Activity Index, Routine Assessment of Patient Index Data 3, and Patient Activity Scale-II (87). The three preferred functional status measures were the PROMIS physical function 10-item short form, Health Assessment Questionnaire-II, and Multidimensional Health Assessment Questionnaire (88).
The Outcomes Measures in Rheumatology (OMERACT) CTD-ILD working group performed a large Delphi process to identify important domains and outcomes measures for multicenter RCTs in CTD-ILD. The identified core domains and measures (in parentheses) were dyspnea (Medical Research Council dyspnea scale and Dyspnea-12), cough (Leicester cough questionnaire), health-related quality of life (Short Form 36 and patient global assessment), lung imaging (overall extent of ILD on HRCT), lung physiology (FVC and DLCO), and survival (89). These were selected based on relevance to multicenter RCTs, so these should serve as a guide, rather than a mandate, on measures to follow in routine care.
Conclusions
ILD frequently complicates RA, dramatically impacts patients’ lives, and places a great financial burden on patients and healthcare systems. Multidisciplinary diagnosis and management is critical to optimizing patient outcomes, especially given the paucity of data to guide treatment decisions. Non-pharmacologic therapies should be universally implemented. The optimal DMARDs and other immunodulatory therapies as well as the role for anti-fibrotics are not well established. International working groups and multi-center RCTs are needed and in place to begin to address the many evidence gaps in RA-ILD management (90).
Key Points.
Interstitial lung disease is an extra-articular manifestation of rheumatoid arthritis that leads to poor patient outcomes and substantial healthcare costs
Multidisciplinary discussion of the clinical findings, blood tests, high-resolution computed tomography images, and pulmonary function tests is considered the best approach to diagnose RA-ILD
Optimal disease-modifying anti-rheumatic drugs and other immunomodulatory therapies in RA-ILD are not known and most have been associated with cases of pneumonitis
Anti-fibrotics may have an adjunct role in managing progressive RA-ILD
Quality evidence is lacking for most diagnostic and management considerations in RA-ILD, illustrating the need for clinical and translational research in RA-ILD
Financial support and sponsorship:
BRE is supported by the National Institute of General Medical Sciences, U54 GM115458, which funds the Great Plains IDeA-CTR Network and by the Rheumatology Research Foundation through a Scientist Development Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of interest: None
Disclosures: BRE none. DH none.
References and Recommended Reading
* recent article of special interest
** recent article of outstanding interest
- 1.Ellman P, Ball RE. Rheumatoid disease with joint and pulmonary manifestations. Br Med J. 1948;2(4583):816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology. 2010;49(8):1483–9. [DOI] [PubMed] [Google Scholar]
- 4.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):528–35. [DOI] [PubMed] [Google Scholar]
- 5.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics—a large multicentre UK study. Rheumatology. 2014;53(9):1676–82. [DOI] [PubMed] [Google Scholar]
- 6.Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, et al. Rheumatoid Arthritis Disease Activity Predicting Incident Clinically Apparent Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Prospective Cohort Study. Arthritis Rheumatol. 2019;71(9):1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This cohort studied demonstrated that RA disease activity is an independent risk factor for incident ILD, with an increase in ILD risk of 35% for every unit increase in the 28-joint Disease Activity Score.
- 7.Kiely P, Busby AD, Nikiphorou E, Sullivan K, Walsh DA, Creamer P, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open. 2019;9(5):e028466. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Using two multicenter prospective RA inception cohorts, Kiely et al demonstrated that methotrexate is not a risk factor for incident RA-ILD and may actually delay the onset of ILD.
- 8.Raimundo K, Solomon JJ, Olson AL, Kong AM, Cole AL, Fischer A, et al. Rheumatoid Arthritis-Interstitial Lung Disease in the United States: Prevalence, Incidence, and Healthcare Costs and Mortality. J Rheumatol. 2019;46(4):360–9. [DOI] [PubMed] [Google Scholar]; *This large commercial insurance database estimated the incidence, prevalence, mortality, and healthcare costs for RA-ILD in the U.S.
- 9.Hyldgaard C, Ellingsen T, Hilberg O, Bendstrup E. Rheumatoid Arthritis-Associated Interstitial Lung Disease: Clinical Characteristics and Predictors of Mortality. Respiration. 2019;98(5):455–60. [DOI] [PubMed] [Google Scholar]; *This cohort study from Denmark with well characterized RA-ILD demonstrated that survival in RA-ILD is longer than previously estimated, with a median survival of 7 years.
- 10.Nurmi HM, Purokivi MK, Karkkainen MS, Kettunen HP, Selander TA, Kaarteenaho RL. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm Med. 2016;16(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HK, Kim DS, Yoo B, Seo JB, Rho JY, Colby TV, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127(6):2019–27. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Varghese J, England BR, Solomon JJ, Michaud K, Mikuls TR, et al. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: A systematic literature review and meta-analysis. Semin Arthritis Rheum. 2019;49(3):358–65. [DOI] [PubMed] [Google Scholar]; *This systematic literature review and meta-analysis of 10 cohort studies found UIP pattern to be associated with a 60% increased risk of death compared to other patterns. Results were heterogeneous and other indicators of ILD severity, namely pulmonary physiology, are important predictors of mortality in RA-ILD.
- 13.Solomon JJ, Chung JH, Cosgrove GP, Demoruelle MK, Fernandez-Perez ER, Fischer A, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2016;47(2):588–96. [DOI] [PubMed] [Google Scholar]
- 14.Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Progressive Decline of Lung Function in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2017;69(3):542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob J, Hirani N, van Moorsel CHM, Rajagopalan S, Murchison JT, van Es HW, et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J. 2019;53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salaffi F, Carotti M, Di Carlo M, Tardella M, Giovagnoni A. High-resolution computed tomography of the lung in patients with rheumatoid arthritis: Prevalence of interstitial lung disease involvement and determinants of abnormalities. Medicine (Baltimore). 2019;98(38):e17088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manfredi A, Cassone G, Cerri S, Venerito V, Fedele AL, Trevisani M, et al. Diagnostic accuracy of a velcro sound detector (VECTOR) for interstitial lung disease in rheumatoid arthritis patients: the InSPIRAtE validation study (INterStitial pneumonia in rheumatoid ArThritis with an electronic device). BMC Pulm Med. 2019;19(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, et al. MUC5B Promoter Variant and Rheumatoid Arthritis with Interstitial Lung Disease. N Engl J Med. 2018;379(23):2209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This international case-control study with discovery and multiple validation populations found that the MUC5B promoter variant rs35705959 was associated with 3-fold higher odds of ILD in RA. This association was even stronger for a UIP pattern ILD and was not observed in RA alone compared to individuals without RA.
- 19.England BR, Duryee MJ, Roul P, Mahajan TD, Singh N, Poole JA, et al. Malondialdehyde-Acetaldehyde Adducts and Antibody Responses in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2019;71(9):1483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]; *In a multi-center cross-sectional study, IgA and IgM anti-MAA antibodies were associated with 2-fold higher odds of ILD in U.S. Veterans with RA. In evaluation of lung tissues, MAA was enriched in the lungs in RA-ILD compared to other ILD, COPD, and normal subjects.
- 20.Chen J, Doyle TJ, Liu Y, Aggarwal R, Wang X, Shi Y, et al. Biomarkers of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 2015;67(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC, et al. Detection of Rheumatoid Arthritis-Interstitial Lung Disease Is Enhanced by Serum Biomarkers. Am J Respir Crit Care Med. 2015;191(12):1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harlow L, Gochuico BR, Rosas IO, Doyle TJ, Osorio JC, Travers TS, et al. Anti-citrullinated heat shock protein 90 antibodies identified in bronchoalveolar lavage fluid are a marker of lung-specific immune responses. Clin Immunol. 2014;155(1):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giles JT, Darrah E, Danoff S, Johnson C, Andrade F, Rosen A, et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS One. 2014;9(6):e98794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alunno A, Bistoni O, Pratesi F, La Paglia GMC, Puxeddu I, Migliorini P, et al. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology (Oxford). 2018;57(5):850–5. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146(11):797–808. [DOI] [PubMed] [Google Scholar]
- 26.Quirke AM, Perry E, Cartwright A, Kelly C, De Soyza A, Eggleton P, et al. Bronchiectasis is a Model for Chronic Bacterial Infection Inducing Autoimmunity in Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(9):2335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer A, Solomon JJ, du Bois RM, Deane KD, Olson AL, Fernandez-Perez ER, et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med. 2012;106(7):1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kass DJ, Nouraie M, Glassberg MK, Ramreddy N, Fernandez K, Harlow L, et al. Comparative Profiling of Serum Protein Biomarkers in Rheumatoid Arthritis-associated Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis. Arthritis Rheumatol. [Epub ahead of print] 2019. [DOI] [PubMed] [Google Scholar]; *Using two independent cohorts of RA-ILD, RA alone, and IPF, this cross sectional study of 45 protein biomarkers demonstrated biomarker signatures that differentiated RA-ILD from RA alone. Notably, there was substantial overlap between RA-ILD and IPF biomarker signatures, particularly in the cohort of U.S. Veterans.
- 29.Levi Y, Israeli-Shani L, Kuchuk M, Epstein Shochet G, Koslow M, Shitrit D. Rheumatological Assessment Is Important for Interstitial Lung Disease Diagnosis. J Rheumatol. 2018;45(11):1509–14. [DOI] [PubMed] [Google Scholar]
- 30.Hetzel J, Maldonado F, Ravaglia C, Wells AU, Colby TV, Tomassetti S, et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration. 2018;95(3):188–200. [DOI] [PubMed] [Google Scholar]
- 31.Bell EC, Cox NS, Goh N, Glaspole I, Westall GP, Watson A, et al. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev. 2017;26(143). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edvardsen A, Jarosch I, Grongstad A, Wiegand L, Gloeckl R, Kenn K, et al. A randomized cross-over trial on the direct effects of oxygen supplementation therapy using different devices on cycle endurance in hypoxemic patients with Interstitial Lung Disease. PLoS One. 2018;13(12):e0209069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visca D, Mori L, Tsipouri V, Fleming S, Firouzi A, Bonini M, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med. 2018;6(10):759–70. [DOI] [PubMed] [Google Scholar]
- 34.Dowman LM, McDonald CF, Hill CJ, Lee AL, Barker K, Boote C, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax. 2017;72(7):610–9. [DOI] [PubMed] [Google Scholar]
- 35.Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2014(10):CD006322. [DOI] [PubMed] [Google Scholar]
- 36.Courtwright AM, El-Chemaly S, Dellaripa PF, Goldberg HJ. Survival and outcomes after lung transplantation for non-scleroderma connective tissue-related interstitial lung disease. J Heart Lung Transplant. 2017;36(7):763–9. [DOI] [PubMed] [Google Scholar]
- 37.Yazdani A, Singer LG, Strand V, Gelber AC, Williams L, Mittoo S. Survival and quality of life in rheumatoid arthritis-associated interstitial lung disease after lung transplantation. J Heart Lung Transplant. 2014;33(5):514–20. [DOI] [PubMed] [Google Scholar]
- 38.Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum. 2014;43(5):613–26. [DOI] [PubMed] [Google Scholar]
- 39.Singh JA, Saag KG, Bridges SL Jr., Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26. [DOI] [PubMed] [Google Scholar]
- 40.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77. [DOI] [PubMed] [Google Scholar]
- 41.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63 Suppl 5:v1–58. [DOI] [PubMed] [Google Scholar]
- 42.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):e3–19. [DOI] [PubMed] [Google Scholar]
- 43.Youssef J, Novosad SA, Winthrop KL. Infection Risk and Safety of Corticosteroid Use. Rheum Dis Clin North Am. 2016;42(1):157–76, ix-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whittier X, Saag KG. Glucocorticoid-induced Osteoporosis. Rheum Dis Clin North Am. 2016;42(1):177–89, x. [DOI] [PubMed] [Google Scholar]
- 45.Conway R, Low C, Coughlan RJ, O’Donnell MJ, Carey JJ. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66(4):803–12. [DOI] [PubMed] [Google Scholar]
- 46.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saravanan V, Kelly CA. Reducing the risk of methotrexate pneumonitis in rheumatoid arthritis. Rheumatology (Oxford). 2004;43(2):143–7. [DOI] [PubMed] [Google Scholar]
- 48.Rojas-Serrano J, Herrera-Bringas D, Perez-Roman DI, Perez-Dorame R, Mateos-Toledo H, Mejia M. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): methotrexate and the severity of lung disease are associated to prognosis. Clin Rheumatol. 2017;36(7):1493–500. [DOI] [PubMed] [Google Scholar]
- 49.Curtis JR, Sarsour K, Napalkov P, Costa LA, Schulman KL. Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor alpha agents, a retrospective cohort study. Arthritis Res Ther. 2015;17:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suissa S, Hudson M, Ernst P. Leflunomide use and the risk of interstitial lung disease in rheumatoid arthritis. Arthritis Rheum. 2006;54(5):1435–9. [DOI] [PubMed] [Google Scholar]
- 51.Chikura B, Lane S, Dawson JK. Clinical expression of leflunomide-induced pneumonitis. Rheumatology (Oxford). 2009;48(9):1065–8. [DOI] [PubMed] [Google Scholar]
- 52.Parry SD, Barbatzas C, Peel ET, Barton JR. Sulphasalazine and lung toxicity. Eur Respir J. 2002;19(4):756–64. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, et al. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41(2):256–64. [DOI] [PubMed] [Google Scholar]
- 54.Nakashita T, Ando K, Kaneko N, Takahashi K, Motojima S. Potential risk of TNF inhibitors on the progression of interstitial lung disease in patients with rheumatoid arthritis. BMJ Open. 2014;4(8):e005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon WG, Hyrich KL, Watson KD, Lunt M, Consortium BCC, Symmons DP, et al. Influence of anti-TNF therapy on mortality in patients with rheumatoid arthritis-associated interstitial lung disease: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2010;69(6):1086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Druce KL, Iqbal K, Watson KD, Symmons DPM, Hyrich KL, Kelly C. Mortality in patients with interstitial lung disease treated with rituximab or TNFi as a first biologic. RMD Open. 2017;3(1):e000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang EH, Jin Y, Desai RJ, Liu J, Sparks JA, Kim SC. Risk of exacerbation of pulmonary comorbidities in patients with rheumatoid arthritis after initiation of abatacept versus TNF inhibitors: A cohort study. Semin Arthritis Rheum. 2019. [DOI] [PubMed] [Google Scholar]
- 58.England BR, Roul P, Mahajan TD, Singh N, Yu F, Sayles H, et al. Performance of Administrative Algorithms to Identify Interstitial Lung Disease in Rheumatoid Arthritis. Arthritis Care Res (Hoboken). [Epub ahead of print] 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matteson EL, Bongartz T, Ryu JH, Crowson CS, Hartman TE, Dellaripa PF. Open-label, pilot study of the safety and clinical effects of rituximab in patients with rheumatoid arthritis-associated interstitial pneumonia. Open Journal of Rheumatology and Autoimmune Diseases. 2012;2(03):53. [Google Scholar]
- 60.Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, et al. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology (Oxford). 2017;56(8):1348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manfredi A, Cassone G, Furini F, Gremese E, Venerito V, Atzeni F, et al. Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: a multicenter retrospective study. Intern Med J. [Epub ahead of print] 2019. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez-Diaz C, Loricera J, Castaneda S, Lopez-Mejias R, Ojeda-Garcia C, Olive A, et al. Abatacept in patients with rheumatoid arthritis and interstitial lung disease: A national multicenter study of 63 patients. Semin Arthritis Rheum. 2018;48(1):22–7. [DOI] [PubMed] [Google Scholar]
- 63.Saldarriaga-Rivera LM, López-Villegas VJ. Janus kinase inhibitors as a therapeutic option in rheumatoid arthritis and associated interstitial lung disease. Report of four cases. Revista Colombiana de Reumatología (English Edition). 2019;26(2):137–9. [Google Scholar]
- 64.Sendo S, Saegusa J, Yamada H, Nishimura K, Morinobu A. Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates interstitial lung disease in SKG mice. Arthritis Res Ther. 2019;21(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford). 2018;57(12):2106–13. [DOI] [PubMed] [Google Scholar]
- 66.Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387(10038):2630–40. [DOI] [PubMed] [Google Scholar]
- 67.Saunders P, Tsipouri V, Keir GJ, Ashby D, Flather MD, Parfrey H, et al. Rituximab versus cyclophosphamide for the treatment of connective tissue disease-associated interstitial lung disease (RECITAL): study protocol for a randomised controlled trial. Trials. 2017;18(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer A, Brown KK, Du Bois RM, Frankel SK, Cosgrove GP, Fernandez-Perez ER, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40(5):640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. [DOI] [PubMed] [Google Scholar]
- 70.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oldham JM, Lee C, Valenzi E, Witt LJ, Adegunsoye A, Hsu S, et al. Azathioprine response in patients with fibrotic connective tissue disease-associated interstitial lung disease. Respir Med. 2016;121:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang HK, Park W, Ryu DS. Successful treatment of progressive rheumatoid interstitial lung disease with cyclosporine: a case report. J Korean Med Sci. 2002;17(2):270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogawa D, Hashimoto H, Wada J, Ueno A, Yamasaki Y, Yamamura M, et al. Successful use of cyclosporin A for the treatment of acute interstitial pneumonitis associated with rheumatoid arthritis. Rheumatology (Oxford). 2000;39(12):1422–4. [DOI] [PubMed] [Google Scholar]
- 74.Ochi S, Kubota T, Sugihara T, Ogawa J, Komano Y, Nonomura Y, et al. A case report of rheumatoid arthritis complicated with rapidly progressive interstitial pneumonia, multiple bullae and pneumomediastinum, which was successfully treated with tacrolimus. Nihon Rinsho Meneki Gakkai Kaishi. 2008;31(1):62–7. [DOI] [PubMed] [Google Scholar]
- 75.Yamano Y, Taniguchi H, Kondoh Y, Ando M, Kataoka K, Furukawa T, et al. Multidimensional improvement in connective tissue disease-associated interstitial lung disease: Two courses of pulse dose methylprednisolone followed by low-dose prednisone and tacrolimus. Respirology. 2018;23(11):1041–8. [DOI] [PubMed] [Google Scholar]
- 76.Jeurissen ME, Boerbooms AM, van de Putte LB, Doesburg WH, Mulder J, Rasker JJ, et al. Methotrexate versus azathioprine in the treatment of rheumatoid arthritis. A forty-eight-week randomized, double-blind trial. Arthritis Rheum. 1991;34(8):961–72. [DOI] [PubMed] [Google Scholar]
- 77.Schiff M, Beaulieu A, Scott DL, Rashford M. Mycophenolate mofetil in the treatment of adults with advanced rheumatoid arthritis: three 24-week, randomized, double-blind, placebo- or ciclosporin-controlled trials. Clin Drug Investig. 2010;30(9):613–24. [DOI] [PubMed] [Google Scholar]
- 78.Willkens RF, Urowitz MB, Stablein DM, McKendry RJ Jr., Berger RG, Box JH, et al. Comparison of azathioprine, methotrexate, and the combination of both in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis Rheum. 1992;35(8):849–56. [DOI] [PubMed] [Google Scholar]
- 79.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019;381(18):1718–27. [DOI] [PubMed] [Google Scholar]; **This double-blind phase III RCT compared nintedanib to placebo in progressive fibrotic lung disease, finding a slower decline of lung function in subjects receiving nintedanib. CTD-ILD was not excluded from this study, with RA-ILD composing 13% of the study sample.
- 80.Solomon JJ, Danoff SK, Goldberg HJ, Woodhead F, Kolb M, Chambers DC, et al. The Design and Rationale of the Trail1 Trial: A Randomized Double-Blind Phase 2 Clinical Trial of Pirfenidone in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Adv Ther. 2019;36(11):3279–87. [DOI] [PubMed] [Google Scholar]
- 81.Redente EF, Aguilar MA, Black BP, Edelman BL, Bahadur AN, Humphries SM, et al. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2018;314(6):L998–L1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N Engl J Med. 2019;380(26):2518–28. [DOI] [PubMed] [Google Scholar]
- 83.Jacob J, Song JW, Yoon HY, Cross G, Barnett J, Woo WL, et al. Prevalence and Effects of Emphysema in Never-Smokers with Rheumatoid Arthritis Interstitial Lung Disease. EBioMedicine. 2018;28:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2020 Report. 2019.
- 85.Bedard Methot D, Leblanc E, Lacasse Y. Meta-analysis of Gastroesophageal Reflux Disease and Idiopathic Pulmonary Fibrosis. Chest. 2019;155(1):33–43. [DOI] [PubMed] [Google Scholar]
- 86.Wang CH, Li CH, Hsieh R, Fan CY, Hsu TC, Chang WC, et al. Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf. 2019;18(3):163–72. [DOI] [PubMed] [Google Scholar]
- 87.England BR, Tiong BK, Bergman MJ, Curtis JR, Kazi S, Mikuls TR, et al. 2019 Update of the American College of Rheumatology Recommended Rheumatoid Arthritis Disease Activity Measures. Arthritis Care Res (Hoboken). 2019;71(12):1540–55. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Through a systematic literature review and modified Delphi process, this working group provided updated recommendations on American College of Rheumatology endorsed RA disease activity measures.
- 88.Barber CEH, Zell J, Yazdany J, Davis AM, Cappelli L, Ehrlich-Jones L, et al. 2019 American College of Rheumatology Recommended Patient-Reported Functional Status Assessment Measures in Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2019;71(12):1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Through a systematic literature review and modified Delphi process, this working group provided initial recommendations on American College of Rheumatology endorsed RA functional status measures.
- 89.Saketkoo LA, Mittoo S, Huscher D, Khanna D, Dellaripa PF, Distler O, et al. Connective tissue disease related interstitial lung diseases and idiopathic pulmonary fibrosis: provisional core sets of domains and instruments for use in clinical trials. Thorax. 2014;69(5):428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fischer A, Strek ME, Cottin V, Dellaripa PF, Bernstein EJ, Brown KK, et al. Proceedings of the American College of Rheumatology/Association of Physicians of Great Britain and Ireland Connective Tissue Disease-Associated Interstitial Lung Disease Summit: A Multidisciplinary Approach to Address Challenges and Opportunities. Arthritis Rheumatol. 2019;71(2):182–95. [DOI] [PubMed] [Google Scholar]; **The American College of Rheumatology and the Association of Physicians of Great Britain and Ireland convened a summit on CTD-ILD with a multidisciplinary panel of experts to identify unmet needs and future research directions.


