Abstract
Purpose of review:
Chronic inflammation is a major component of HIV infection, the effects of which can be devastating in the central nervous system (CNS). Protecting the brain is therefore critical as efforts proceed to cure HIV infection by reactivating latent viral reservoirs and driving immune responses. We review the clinical presentation and pathology findings of inflammatory processes in the CNS in patients managed with ART and the drivers of these processes.
Recent findings:
Chronic inflammation is associated with increased mortality and morbidity and HIV infection increases the risk for chronic diseases, especially cognitive impairment. Latent viral reservoirs, including microglia and tissue macrophages, contribute to inflammation in the CNS. Inflammation is generated and maintained through residual viral replication, dysregulation of infected cells, continuously produced viral proteins and positive feedback loops of chronic inflammation. Novel therapeutics and lifestyle changes may help to protect the CNS from immune mediated damage.
Summary:
As therapies are developed to cure HIV, it is important to protect the CNS from additional immune-mediated damage. Adjunctive therapies to restore glial function, reduce neuro- and systemic inflammation, and inhibit expression of viral proteins are needed.
Keywords: chronic inflammation, HIV, cure, HIV-associated neurocognitive disorders
Introduction
Six years ago, President Obama announced the HIV Cure initiative with the aim of HIV eradication or development of a functional cure, to eliminate the need for long term antiretroviral therapy (ART) (1). Potential curative approaches include activation of the viral latent reservoir (2), transfusion of viral specific or viral resistant T cells (3, 4), gene editing excision of proviral DNA (5, 6*, 7), and immune checkpoint inhibition (8, 9). The majority of these strategies involve activating the latent viral reservoir, which includes T cells (10), tissue resident macrophages (11**, 12), microglia (13), and astrocytes (14) with latency-reversing agents (LRAs). Re-activated virus can then trigger proinflammatory processes involving immune antiviral responses as well as non-specific inflammation. Such inflammatory processes can also lead to unwanted collateral tissue damage and result in immune-mediated complications. Therefore, it is critical to consider the unintended immunological consequences of these curative approaches.
During a chronic inflammatory process immune responses are involved both in tissue destruction and in tissue repair (reviewed in (15*)). This results in a positive feedback loop of immune responses and damage. Chronic inflammation is a major contributor to the pathophysiology of neoplastic, neurodegenerative, autoimmune, and cardiovascular disease; conditions that persons living with HIV (PLWH) are at an increased risk for (16*). Having HIV increases the risk of developing a chronic illness by up to 80%, the risk being greatest for cognitive impairment and dementia (16). HIV-associated neurocognitive disorders (HAND) (17) are tightly associated with chronic inflammation (18–20) .
Because of the potential immunological consequences of curative approaches on the CNS, a detailed understanding of the contribution and mechanisms of inflammation-mediated damage to CNS during HIV infection becomes ever more important. This review highlights the most recent advances in our understanding of how HIV infection impacts the brain, focusing on the presentation and immunopathology of patients with CNS inflammation, the role of microglia in chronic inflammation, and the viral factors that contribute to inflammation. Additionally, the importance of protecting the brain while moving towards a functional cure will be discussed.
Clinical presentation of PLWH with chronic CNS inflammation
Even after therapeutic viral suppression has been achieved, the incidence of comorbid disorders including neurologic and psychiatric disorders, accelerated vascular disease, and frailty is very high, and it is increasingly apparent that much of this is linked to or exacerbated by chronic CNS inflammation (21).
HIV-associated neurocognitive disorders
Of all the conditions linked to CNS inflammation (Figure 1), the best studied is HAND (reviewed in (22)). HAND can be identified in 30–50% of individuals (17, 23–25) and encompasses impairments in memory, attention, processing and motor skills that can range from mild or asymptomatic to severely debilitating. The most common neurological manifestations in the era of ART are mild neurocognitive deficits (see Table 1). Importantly, once established, the deficits appear to persist even with successful ART. For example, HIV+ virally suppressed women showed deficits in verbal learning, verbal memory, executive function, attention/working memory, and fluency that persisted over four years of follow-up (26).
Figure 1. The immunopathology of HIV CNS disease.
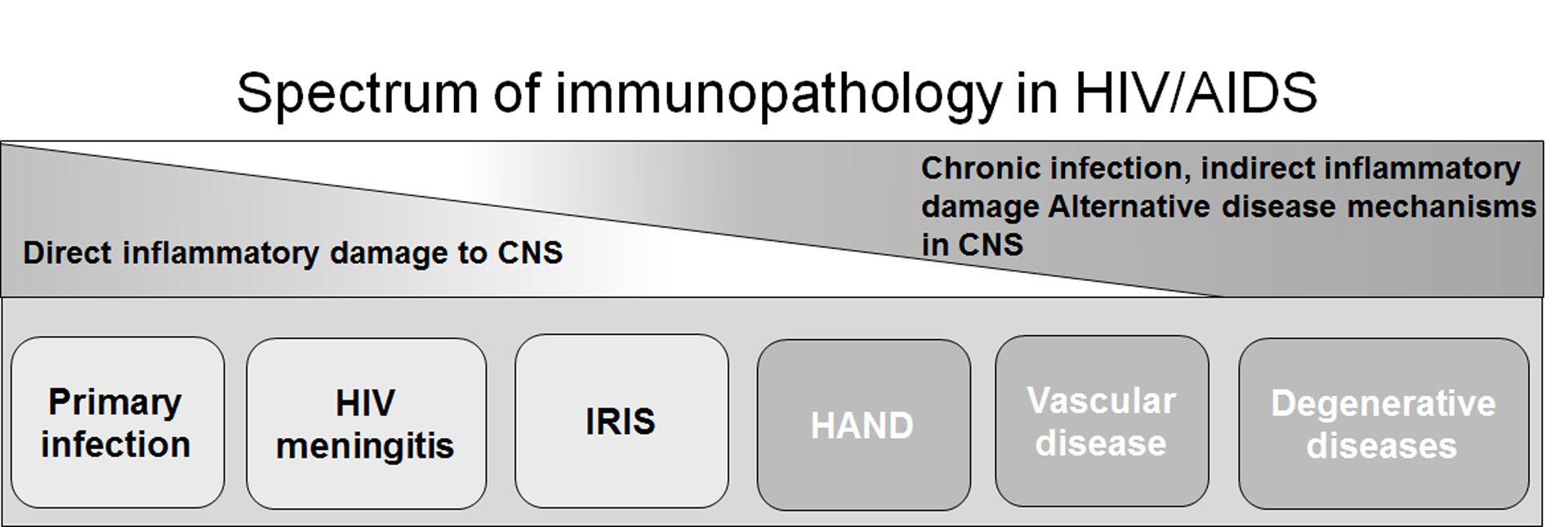
The immune response during HIV infection can damage the CNS through direct and indirect mechanisms. Shortly after HIV infection, the virus enters the CNS and infects resident cells, inducing immune responses within this compartment that result in edema and bystander tissue damage. HIV infection is also associated with a lymphocytic meningitis, often occurring within weeks or months of initial infection (132). Encephalitis, primarily driven by CD8+ T cells can also occur in the absence of opportunistic infections (OIs) and is typically due to immune reconstitution inflammatory syndrome (IRIS). IRIS phenomena unrelated to OIs are relatively rare, but is the best direct example of a harmful effect of inflammation on neurological function (133). The immune system can also damage the CNS through indirect mechanisms. The best studied neurological complication associated with chronic inflammation is HAND. However, chronic inflammation within the context of HIV infection also contributes to the development of cardiovascular disease, a major contributor to CNS pathology in the era of ART, and neurodegenerative diseases.
Table 1.
HAND in the era of ART: Key Clinical Features.
|
Although present in all patients across the spectrum of HIV infection, including the pediatric population (27**), HAND is more prevalent in older patients and in those with longer disease duration (28–30). This is particularly important as the age of PLWH is increasing. Indeed, approximately half of the PLWH in the United States are now over the age of 50 (31), suggesting a large proportion are at risk for HAND. Aging is also associated with enhanced immune activation that paradoxically results in a lack of appropriate immune response and is associated with mortality (32**). This inflammation may render older PLWH more susceptible to infections and loss of immune mediated clearance of cellular debris. Some have described this interaction of chronic CNS inflammation and aging as “inflammaging” (33, 34).
Other disease mechanisms
PLWH have substantial increased risks of cardiovascular disease (CVD) including acute myocardial infarction (35, 36), ischemic stroke (35), and heart failure (37) . CVD is strongly associated with inflammation and multiple mechanisms have been posited for how inflammation and virally driven processes accelerate atherosclerosis (38, 39). CVD risk factors including carotid intima-media thickness, central obesity and diabetes are important risk factors for the development of HAND (29, 40–42). As the cognitive profile of vascular cognitive impairment significantly overlaps with that of HAND (41), it remains to be seen whether CVD in PLWH leads to vascular cognitive impairment or accelerates more typical HAND, or some complex mix of the two. Concomitant drug use may add to accelerated CVD and drive neurological dysfunction (43). Further, given the known associations between CVD and Alzheimer disease (AD) it is also possible that HIV accelerates amyloid and tau protein deposition leading to AD and neurodegenerative processes.
CNS pathology during HIV infection and HAND
HIV infected cells enter the CNS shortly after systemic infection (44). In the CNS the virus infects tissue macrophages (11, 12), microglia (13) and astrocytes (14). Imaging studies have revealed ongoing CNS tissue loss in persons with HIV despite effective ART (45). In a recent longitudinal imaging study of 155 PLWH on ART, significant brain volume decreases, subcortical brain atrophy, ventricular expansion and white matter abnormalities were present despite undetectable viral loads (45). Brain atrophy is associated with neuroinflammation in HIV infection and HAND (19, 46–48) and recent studies utilizing magnetic resonance imaging coupled with metabolite spectroscopy have confirmed persistent neuroinflammation in individuals with HAND (49, 50). CSF biomarkers studies have also confirmed persistent inflammation and neural injury in virologically suppressed individuals (46, 51–61). Likewise, viral proteins, including the HIV transactivator of transcription (Tat) protein (62*, 63) and Nef (64), known to drive inflammation, have been found in the CSF of PLWH.
Histomorphological changes associated with HIV infection demonstrate evidence of ongoing immune processes and neuronal damage in the absence of viral replication. These include activation of infected and non-infected microglia and astrocytes (65), CD68+ cells in the white matter and vascular disease processes including infarcts, thickening of vessel walls, hemorrhage and atherosclerosis (66) as well as decreases in dendritic and synaptic complexity (67). Post-mortem studies have also revealed an increased accumulation of protein aggregates, especially Aβ, in individuals with HIV (68). This may reflect vascular damage as well as enhanced protein aggregate formation driven by Tat (69) . Biopsies from patients with IRIS, the most fulminant and recognizable form of CNS inflammation in PLWH, demonstrated CD4+ and CD8+ T cells, including IL-17+ cells, and the viral protein Tat, in the parenchyma despite the absence of replicating virus (63, 70). Indeed, patients have persistent CD4+ and CD8+ T cell activation in both the periphery and in the CSF despite having undetectable viral loads (71). In particular, effector memory T cells are elevated (72) and expression of markers of T cell exhaustion are increased (73).
Collectively, clinical and pathology findings indicate that the immune responses in the CNS observed in people with HIV includes non-specific chronic inflammatory processes that are present even during viral suppression. As chronic inflammation is a key driver in many common diseases (15), and is tightly linked with neurodegenerative diseases (74, 75), it is important to evaluate what drives the neuroinflammation observed in PLWH and how the latent reservoir contributes to these processes.
Drivers of CNS inflammation during HIV infection
Residual viral replication from the latent reservoirs despite ART, and subsequent anti-viral immune responses, may account for some of the persistent immune activation noted in the CNS (76**, 77). However, because peripheral and CSF viral loads are often undetectable, factors other than viral replication likely also drive inflammation. These factors include skewing of immune trafficking, dysregulation of gene expression from infected cells, and the persistent expression of viral proteins from latently infected cells. Once initiated, positive feedback loops of immune-mediated tissue damage and repair processes result in self-sustained chronic inflammation. Key cells in these processes are brain macrophages and microglia.
CNS viral reservoirs mediate chronic inflammation
Microglia and tissue resident macrophages are the principal cells infected with HIV in the CNS (13) and recent work has demonstrated that these cells are an important reservoir of latent virus (11, 12, 78, 79, 80**, 81**). In an SIV model, infectious virus was produced from brain macrophages after the removal of ART (11). This suggests that the viral reservoir in the CNS might re-seed the periphery (11, 81). To underline this potential scenario, almost half of virologically suppressed individuals with over eight years on ART had HIV infected cells in the CSF, although very few participants had detectable viral replication (76). Additionally, there appeared to be enrichment of virally infected cells within the CSF as compared to the periphery which was correlated with neurocognitive impairments (76). However, this study could not discern if infected cells were trafficking into the CNS at increased rates or if uninfected cells were becoming infected from virus present in the brain.
Microglia are long-lived cells that are responsible for initiating immune responses, recruiting peripheral immune cells into the CNS, and maintaining CNS homeostasis (82). Microglia are likely the key cell for initiating and sustaining positive feedback loops of chronic inflammation in the CNS. Microglia undergo functional, morphologic and phenotypic shifts when activated and in vivo PET imaging has demonstrated microglial activation during HIV infection (83, 84). Once activated, gene expression changes result in increased proliferation and alterations in cellular communication, notably the synthesis and release of proinflammatory cytokines, chemokines and effector molecules (85, 86). The release of these molecules, such as matrix metalloproteinases and reactive oxygen species, directly damage neurons resulting in loss of neuron function and neurotoxicity (87, 88). This, in turn, drives microglial activation. Additionally, the virally driven secretion of chemokines, such as CCL2 (53, 77, 89, 90), contribute to the propagation of chronic inflammation. CCL2 activates microglia, modulates their migration and encourages self-proliferation (91) as well as recruits peripheral macrophages and T cells into the CNS. These cells drive further immune-mediated damage, microglial activation, and immune cell recruitment. Viral infection early in life, as occurs in the pediatric population, may also generate an increase in proinflammatory brain resident T cells (92). These cells tend to cluster in regions of microglial activation and have also been shown to increase with age (93), promoting long-lasting proinflammatory CNS environments. The contribution of these cells during HIV infection is an area of current interest.
Astrocytes are the most common cell type in the brain (94). Although relatively few astrocytes are thought to become infected, and this infection is restricted and non-productive (14, 95) those that are may drive neuroinflammation by inducing apoptosis in uninfected astrocytes, secreting proinflammatory cytokines, altering synaptic integrity (96) and causing disruptions to the blood brain barrier (BBB) by impairing gap junctions (97, 98). In addition to the immune-mediated damage caused by activated glia, these same cells do not function in their normal role of synapse maintenance, glutamate uptake, BBB regulation, phagocytosis of dead cells and removal of protein aggregates such as Aβ (99–101), processes all critical for CNS health. Both the proinflammatory processes and the loss of brain homeostatic mechanisms contribute to CNS immunopathology. Therefore, not only eliminating HIV from the microglial and astrocyte populations, but restoring glial function is important to consider as cure strategies are implemented.
Viral proteins drive inflammation
Viral proteins produced during ART also drive inflammation. Even during ART pressure, provirus is capable of producing proteins, including Tat (62, 63) and Nef (64), which are secreted from infected cells. Tat is readily endocytosed by cells where it modulates gene expression and cellular function (102). Tat is directly neurotoxic and is potently neuroinflammatory, driving astrocyte, microglial and T cell activation as well as inducing secretion of inflammatory cytokines in both animal and in vitro models, and activating inflammasomes (63, 103–109). For example, Tat drives the production of CCL2, which recruits peripheral macrophages and T cells into the CNS and also activates these cells through chromatin remodeling. Not only does this facilitate further infection by HIV but it also causes aberrant secretion of cytokines such as granzyme B and interleukin (IL)-17 (63) which have been shown to be directly neurotoxic and to modulate the BBB (110, 111). Nef has also been shown to increase BBB permeability and drive neuroinflammation (112*, 113). Specifically, IL-1 dependent vascular changes and immune cell infiltration were noted in both the lung and the gut of animals expressing Nef in the hippocampus. These findings are of particular importance as they demonstrate that viral driven changes in the CNS can induce systemic immune responses that further damage the brain.
Protecting the brain while moving towards a cure
Neuroprotection and reducing neuronal inflammation will become even more important as cure initiatives move forward. Several LRAs are being developed to activate the viral reservoir prior to immune-mediated clearance and some consideration is being given to the use of Tat as an LRA during cure strategies (114). As discussed in this review, Tat is not only directly neurotoxic, but also drives substantial neuroinflammatory processes. Further, trials have already begun exploring the use of checkpoint inhibitor therapy in patients with HIV and have shown that some individuals have transient increased antiviral T cell responses (8). In an HIV+ patient treated with PD1 blockade for cancer, there was an increase in activation of antiviral T cells (115*). Lymphocytes transferred from elite controllers into patients with HIV also increased recipient CD8+ T cell activation with enhanced production of perforin and granzyme B (116). Importantly, antigen specific HIV responses in the brain can induce gliosis (117) and further contribute to neuronal injury. Therefore, strategies to monitor and protect the brain while activating HIV immune responses are needed.
Previous efforts at neuroprotection have been fraught with challenges. Anti-inflammatory strategies have been tested in over 20 clinical trials for HAND with few successes (22). None of these have entered mainstream therapy. However, recent work has demonstrated that protecting the CNS during HIV infection is possible. Intranasal delivered insulin reversed neuronal injury in an animal model of HAND (118), and is now being tested in PLWH. This benefit may be from a reduction in microglial activation and dampening of chronic inflammation (119). Other animal studies have shown that inhibiting JAK/STAT signaling reduces inflammation and reverses cognitive deficits (120). Further, pharmacologic inhibition of glutamate synthesis reduced the overproduction of glutamate from microglial cells and restored cognitive function in treated animals (121). Examples of targeting inflammation from outside the HIV field may also provide lessons for HIV. Immune-based therapies which lower inflammation, including IL-1β inhibition with the monoclonal antibody canakinumab, may reduce CVD in individuals without HIV (122). A recent pilot study in 10 PLWH on ART with viral suppression suggested that a single dose of canakinumab reduced plasma inflammatory markers and arterial inflammation. This finding suggests that attenuating inflammation may modulate atherosclerosis pathogenesis in HIV infection (123).
While these exciting developments suggest promising future therapeutics, some of the greatest benefits to patients are lifestyle changes. Moderate exercise improves learning and memory, which may be due to strengthening cardiovascular function, reducing inflammation and enhancing neurogenesis (124–128). Additionally, improving sleep, nutrition and social health have been linked to better cognition in PLWH (129), as has eliminating the use of drugs, alcohol and smoking (130). Importantly, these interventions are inexpensive and rapidly implementable (131).
Conclusion
Chronic inflammation is associated with mortality and significant morbidity and is a major complication in PLWH. While efforts are underway to cure HIV, a more nuanced consideration of the “unintended consequences” of further immune activation in the CNS are needed because the CNS is especially vulnerable to inflammatory processes. Residual viral replication, infected cell dysfunction, particularly long-lived microglia, and continuous expression of viral proteins all contribute to sustained chronic inflammation in PLWH. Importantly, activation of the latent viral reservoir, as part of cure initiatives, may well lead to further CNS damaging proinflammatory processes. Future research efforts should be focused on mitigating virally driven inflammation and preventing chronic inflammation.
Key Points.
Tissue macrophages and microglia are functional viral reservoirs and contribute to chronic inflammation during HIV infection.
Inflammation is a key mediator of CNS damage and is driven by residual viral replication, expression of viral proteins despite viral suppression, immune cell dysfunction and positive pro-inflammatory feedback loops.
HIV cure strategies include activating viral reservoirs and triggering immune responses. Protecting the brain during these processes will be critical for their success.
Financial Support
Supported by NIMH 5P30MH075673-13 (PI McArthur JC) and 5P30AI094189-08 (PI Chaisson R.)
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Remarks by the President on World AIDS Day. 2013.
- 2.Kim Y, Anderson JL, Lewin SR. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe. 2018;23(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Sun J, Li Z, Qin L, Liu G, Li K, et al. T Cell Therapy Targeted on HLA-A02 Restricted HIV Antigen Epitopes: An Open Label Cellular Therapy Trial Using CD8+ T Cell. Front Immunol. 2019;10:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung JA, Patel S, Clohosey ML, Roesch L, Tripic T, Kuruc JD, et al. HIV-Specific, Ex Vivo Expanded T Cell Therapy: Feasibility, Safety, and Efficacy in ART-Suppressed HIV-Infected Individuals. Mol Ther. 2018;26(10):2496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan NT, Dampier W, Chung CH, Allen AG, Atkins A, Pirrone V, et al. Novel gRNA design pipeline to develop broad-spectrum CRISPR/Cas9 gRNAs for safe targeting of the HIV-1 quasispecies in patients. Sci Rep. 2019;9(1):17088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.*.Xu L, Wang J, Liu Y, Xie L, Su B, Mou D, et al. CRISPR-Edited Stem Cells in a Patient with HIV and Acute Lymphocytic Leukemia. N Engl J Med. 2019;381(13):1240–7. [DOI] [PubMed] [Google Scholar]; Proof of concept study demonstrating crispr-cas9 gene editing of CCR5 in stem cells prior to transplantation into an HIV receipent.
- 7.Wang G, Zhao N, Berkhout B, Das AT. A Combinatorial CRISPR-Cas9 Attack on HIV-1 DNA Extinguishes All Infectious Provirus in Infected T Cell Cultures. Cell Rep. 2016;17(11):2819–26. [DOI] [PubMed] [Google Scholar]
- 8.Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J Infect Dis. 2017;215(11):1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colston E, Grasela D, Gardiner D, Bucy RP, Vakkalagadda B, Korman AJ, et al. An open-label, multiple ascending dose study of the anti-CTLA-4 antibody ipilimumab in viremic HIV patients. PLoS One. 2018;13(6):e0198158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–8. [DOI] [PubMed] [Google Scholar]
- 11.**.Abreu C, Shirk EN, Queen SE, Beck SE, Mangus LM, Pate KAM, et al. Brain macrophages harbor latent, infectious simian immunodeficiency virus. AIDS. 2019;33 Suppl 2:S181–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Quantification of viral reactivation and production of infectious virus from macrophages in brain after ART removal. These data demonstrate that perivascular macrophages in brain are a reservoirs for SIV during ART.
- 12.Arainga M, Edagwa B, Mosley RL, Poluektova LY, Gorantla S, Gendelman HE. A mature macrophage is a principal HIV-1 cellular reservoir in humanized mice after treatment with long acting antiretroviral therapy. Retrovirology. 2017;14(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallet C, De Rovere M, Van Assche J, Daouad F, De Wit S, Gautier V, et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front Cell Infect Microbiol. 2019;9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li GH, Henderson L, Nath A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr HIV Res. 2016;14(5):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.*.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review on chronic inflammation.
- 16.*.Yang HY, Beymer MR, Suen SC. Chronic Disease Onset Among People Living with HIV and AIDS in a Large Private Insurance Claims Dataset. Sci Rep. 2019;9(1):18514. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analysis of insurance claims from a large dataset, approximately 24,000 PLWH, show HIV is associated with increased risks for developing chronic illnesses as compared to HIV negative counterparts. This association remained despite controlling for demographics, behavioral risk factors and comorbidities.
- 17.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burlacu R, Umlauf A, Marcotte TD, Soontornniyomkij B, Diaconu CC, Bulacu-Talnariu A, et al. Plasma CXCL10 correlates with HAND in HIV-infected women. J Neurovirol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portilla I, Reus S, Leon R, van-der Hofstadt C, Sanchez J, Lopez N, et al. Neurocognitive Impairment in Well-Controlled HIV-Infected Patients: A Cross-Sectional Study. AIDS Res Hum Retroviruses. 2019;35(7):634–41. [DOI] [PubMed] [Google Scholar]
- 20.Palella FJ Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. [DOI] [PubMed] [Google Scholar]
- 21.Smit M, Cassidy R, Cozzi-Lepri A, Quiros-Roldan E, Girardi E, Mammone A, et al. Projections of non-communicable disease and health care costs among HIV-positive persons in Italy and the U.S.A.: A modelling study. PLoS One. 2017;12(10):e0186638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86(4):334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–21. [DOI] [PubMed] [Google Scholar]
- 26.Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, et al. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology. 2017;89(15):1594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.**.Boivin MJ, Chernoff M, Fairlie L, Laughton B, Zimmer B, Joyce C, et al. African Multi-Site 2-Year Neuropsychological Study of School-Age Children Perinatally Infected, Exposed, and Unexposed to Human Immunodeficiency Virus. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent longitudinal study involving over 600 children between the ages of 5–11 years demonstrated that HIV positive children who are well controlled with ART based on virology assessment, show evidence for neurocognitive impairment. These data demonstrate that HAND is not restricted to the aging HIV population.
- 28.Goodkin K, Miller EN, Cox C, Reynolds S, Becker JT, Martin E, et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV. 2017;4(9):e411–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73(16):1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS. 2004;18 Suppl 1:S79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC. HIV Surveillance Report, 2017; vol. 29 2018. [Google Scholar]
- 32.**.Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med. 2019;25(3):487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]; A longitudinal immunoprofiling study demonstrates alterations in the immune system that may explain immune dysfunction in aging individuals.
- 33.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90. [DOI] [PubMed] [Google Scholar]
- 34.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17(4):118–23. [PubMed] [Google Scholar]
- 35.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV. Circulation. 2018;138(11):1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2(5):536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurozumi A, Nakano K, Yamagata K, Okada Y, Nakayamada S, Tanaka Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone. 2019;124:53–61. [DOI] [PubMed] [Google Scholar]
- 39.Fukuyo S, Yamaoka K, Sonomoto K, Oshita K, Okada Y, Saito K, et al. IL-6-accelerated calcification by induction of ROR2 in human adipose tissue-derived mesenchymal stem cells is STAT3 dependent. Rheumatology (Oxford). 2014;53(7):1282–90. [DOI] [PubMed] [Google Scholar]
- 40.Hiransuthikul A, Chutinet A, Sakulrak S, Samajarn J, Vongsayan P, Kijpaisalratana N, et al. Short Communication: Carotid Intima-Media Thickness Is Not Associated with Neurocognitive Impairment Among People Older than 50 Years With and Without HIV Infection from Thailand. AIDS Res Hum Retroviruses. 2019;35(11–12):1170–3. [DOI] [PubMed] [Google Scholar]
- 41.Fabbiani M, Ciccarelli N, Tana M, Farina S, Baldonero E, Di Cristo V, et al. Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Med. 2013;14(3):136–44. [DOI] [PubMed] [Google Scholar]
- 42.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78(7):485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai H, Moore R, Celentano DD, Gerstenblith G, Treisman G, Keruly JC, et al. HIV Infection Itself May Not Be Associated With Subclinical Coronary Artery Disease Among African Americans Without Cardiovascular Symptoms. J Am Heart Assoc. 2016;5(3):e002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV-1 target cells in the CNS. J Neurovirol. 2015;21(3):276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nir TM, Jahanshad N, Ching CRK, Cohen RA, Harezlak J, Schifitto G, et al. Progressive brain atrophy in chronically infected and treated HIV+ individuals. J Neurovirol. 2019;25(3):342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eden A, Marcotte TD, Heaton RK, Nilsson S, Zetterberg H, Fuchs D, et al. Increased Intrathecal Immune Activation in Virally Suppressed HIV-1 Infected Patients with Neurocognitive Impairment. PLoS One. 2016;11(6):e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon IH, Chettimada S, Misra V, Lorenz DR, Gorelick RJ, Gelman BB, et al. White Matter Abnormalities Linked to Interferon, Stress Response, and Energy Metabolism Gene Expression Changes in Older HIV-Positive Patients on Antiretroviral Therapy. Mol Neurobiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hulgan T, Kallianpur AR, Guo Y, Barnholtz-Sloan JS, Gittleman H, Brown TT, et al. Peripheral Blood Mitochondrial DNA Copy Number Obtained From Genome-Wide Genotype Data Is Associated With Neurocognitive Impairment in Persons With Chronic HIV Infection. J Acquir Immune Defic Syndr. 2019;80(4):e95–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alakkas A, Ellis RJ, Watson CW, Umlauf A, Heaton RK, Letendre S, et al. White matter damage, neuroinflammation, and neuronal integrity in HAND. J Neurovirol. 2019;25(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cysique LA, Juge L, Gates T, Tobia M, Moffat K, Brew BJ, et al. Covertly active and progressing neurochemical abnormalities in suppressed HIV infection. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, et al. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. Biomed Res Int. 2015;2015:506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60(3):234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Monocyte chemoattractant protein-1 correlates with subcortical brain injury in HIV infection. Neurology. 2006;66(8):1255–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17(1):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528–41. [DOI] [PubMed] [Google Scholar]
- 56.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196(12):1779–83. [DOI] [PubMed] [Google Scholar]
- 57.Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cysique LA, Brew BJ, Halman M, Catalan J, Sacktor N, Price RW, et al. Undetectable cerebrospinal fluid HIV RNA and beta-2 microglobulin do not indicate inactive AIDS dementia complex in highly active antiretroviral therapy-treated patients. J Acquir Immune Defic Syndr. 2005;39(4):426–9. [DOI] [PubMed] [Google Scholar]
- 59.Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47(2):168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guha D, Mukerji SS, Chettimada S, Misra V, Lorenz DR, Morgello S, et al. Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS. 2019;33(4):615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yilmaz A, Blennow K, Hagberg L, Nilsson S, Price RW, Schouten J, et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn. 2017;17(8):761–70. [DOI] [PubMed] [Google Scholar]
- 62.*.Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS. 2019;33 Suppl 2:S145–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study confirms the ongoing expression of Tat in the CNS of individuals on ART.
- 63.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110(33):13588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell TD, Khan M, Huang MB, Bond VC, Powell MD. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn Dis. 2008;18(2 Suppl 2):S2-14-9. [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179(4):1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamers SL, Rose R, Maidji E, Agsalda-Garcia M, Nolan DJ, Fogel GB, et al. HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J Virol. 2016;90(20):8968–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80(15):1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012;26(18):2327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hategan A, Bianchet MA, Steiner J, Karnaukhova E, Masliah E, Fields A, et al. HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol. 2017;24(4):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rushing EJ, Liappis A, Smirniotopoulos JD, Smith AB, Henry JM, Man YG, et al. Immune reconstitution inflammatory syndrome of the brain: case illustrations of a challenging entity. J Neuropathol Exp Neurol. 2008;67(8):819–27. [DOI] [PubMed] [Google Scholar]
- 71.Sinclair E, Ronquillo R, Lollo N, Deeks SG, Hunt P, Yiannoutsos CT, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47(5):544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okulicz JF, Le TD, Agan BK, Camargo JF, Landrum ML, Wright E, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med. 2015;175(1):88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antonelli LR, Mahnke Y, Hodge JN, Porter BO, Barber DL, DerSimonian R, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010;116(19):3818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21(2):404–12. [DOI] [PubMed] [Google Scholar]
- 76.**.Spudich S, Robertson KR, Bosch RJ, Gandhi RT, Cyktor JC, Mar H, et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J Clin Invest. 2019;129(8):3339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]; HIV infected cells were detected in the CSF of nearly 50% of patients on ART and that detectable HIV DNA was associated with cognitive impairment. These data suggest that the CNS may be a sanctuary site for the virus and that residual viral replication may contribute to the development of HAND.
- 77.Muratori C, Mangino G, Affabris E, Federico M. Astrocytes contacting HIV-1-infected macrophages increase the release of CCL2 in response to the HIV-1-dependent enhancement of membrane-associated TNFalpha in macrophages. Glia. 2010;58(16):1893–904. [DOI] [PubMed] [Google Scholar]
- 78.Abreu C, Shirk EN, Queen SE, Mankowski JL, Gama L, Clements JE. A Quantitative Approach to SIV Functional Latency in Brain Macrophages. J Neuroimmune Pharmacol. 2019;14(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, et al. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat Med. 2017;23(5):638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.**.Ganor Y, Real F, Sennepin A, Dutertre CA, Prevedel L, Xu L, et al. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol. 2019;4(4):633–44. [DOI] [PubMed] [Google Scholar]; First demonstration that tissue macrophages are a competent viral reservoir in human disease despite long term ART.
- 81.**.Abreu CM, Veenhuis RT, Avalos CR, Graham S, Queen SE, Shirk EN, et al. Infectious Virus Persists in CD4(+) T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques. J Virol. 2019;93(15). [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study to confirm that monocytes and macrophages are functional viral reservoirs in the SIV macaque model.
- 82.Colonna M, Butovsky O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol. 2017;35:441–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hammoud DA, Sinharay S, Shah S, Schreiber-Stainthorp W, Maric D, Muthusamy S, et al. Neuroinflammatory Changes in Relation to Cerebrospinal Fluid Viral Load in Simian Immunodeficiency Virus Encephalitis. mBio. 2019;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology. 2016;86(15):1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trzeciak A, Lerman YV, Kim TH, Kim MR, Mai N, Halterman MW, et al. Long-Term Microgliosis Driven by Acute Systemic Inflammation. J Immunol. 2019;203(11):2979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ginsberg SD, Alldred MJ, Gunnam SM, Schiroli C, Lee SH, Morgello S, et al. Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann Neurol. 2018;83(2):406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cantres-Rosario YM, Ortiz-Rodriguez SC, Santos-Figueroa AG, Plaud M, Negron K, Cotto B, et al. HIV Infection Induces Extracellular Cathepsin B Uptake and Damage to Neurons. Sci Rep. 2019;9(1):8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bozzelli PL, Yin T, Avdoshina V, Mocchetti I, Conant KE, Maguire-Zeiss KA. HIV-1 Tat promotes astrocytic release of CCL2 through MMP/PAR-1 signaling. Glia. 2019;67(9):1719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95(6):3117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.D’Aversa TG, Yu KO, Berman JW. Expression of chemokines by human fetal microglia after treatment with the human immunodeficiency virus type 1 protein Tat. J Neurovirol. 2004;10(2):86–97. [DOI] [PubMed] [Google Scholar]
- 91.Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinbach K, Vincenti I, Egervari K, Kreutzfeldt M, van der Meer F, Page N, et al. Brain-resident memory T cells generated early in life predispose to autoimmune disease in mice. Sci Transl Med. 2019;11(498). [DOI] [PubMed] [Google Scholar]
- 93.Ayasoufi K, Namen S, Goddery E, Tritz Z, Fain CE, Yokanovich L, et al. Rapid activation of brain resident memory T cells following neurological insults. The Journal of Immunology. 2019;202(1 Supplement):56.20–56.20.30510068 [Google Scholar]
- 94.Almad A, Maragakis NJ. A stocked toolbox for understanding the role of astrocytes in disease. Nat Rev Neurol. 2018;14(6):351–62. [DOI] [PubMed] [Google Scholar]
- 95.Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12(2):146–52. [DOI] [PubMed] [Google Scholar]
- 96.Hu G, Niu F, Liao K, Periyasamy P, Sil S, Liu J, et al. HIV-1 Tat-Induced Astrocytic Extracellular Vesicle miR-7 Impairs Synaptic Architecture. J Neuroimmune Pharmacol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eugenin EA, Berman JW. Cytochrome C dysregulation induced by HIV infection of astrocytes results in bystander apoptosis of uninfected astrocytes by an IP3 and calcium-dependent mechanism. J Neurochem. 2013;127(5):644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011;31(26):9456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bairwa D, Kumar V, Vyas S, Das BK, Srivastava AK, Pandey RM, et al. Case control study: magnetic resonance spectroscopy of brain in HIV infected patients. BMC Neurol. 2016;16:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28(11):1579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, et al. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57(4):671–5. [DOI] [PubMed] [Google Scholar]
- 102.Spector C, Mele AR, Wigdahl B, Nonnemacher MR. Genetic variation and function of the HIV-1 Tat protein. Med Microbiol Immunol. 2019;208(2):131–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dong H, Ye X, Zhong L, Xu J, Qiu J, Wang J, et al. Role of FOXO3 Activated by HIV-1 Tat in HIV-Associated Neurocognitive Disorder Neuronal Apoptosis. Front Neurosci. 2019;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dickens AM, Yoo SW, Chin AC, Xu J, Johnson TP, Trout AL, et al. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci Rep. 2017;7(1):7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, et al. NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci. 2008;28(47):12190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Periyasamy P, Thangaraj A, Bendi VS, Buch S. HIV-1 Tat-mediated microglial inflammation involves a novel miRNA-34a-NLRC5-NFkappaB signaling axis. Brain Behav Immun. 2019;80:227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Periyasamy P, Thangaraj A, Guo ML, Hu G, Callen S, Buch S. Epigenetic Promoter DNA Methylation of miR-124 Promotes HIV-1 Tat-Mediated Microglial Activation via MECP2-STAT3 Axis. J Neurosci. 2018;38(23):5367–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J Neurosci. 2017;37(13):3599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aksenova M, Sybrandt J, Cui B, Sikirzhytski V, Ji H, Odhiambo D, et al. Inhibition of the Dead Box RNA Helicase 3 Prevents HIV-1 Tat and Cocaine-Induced Neurotoxicity by Targeting Microglia Activation. J Neuroimmune Pharmacol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee PR, Johnson TP, Gnanapavan S, Giovannoni G, Wang T, Steiner JP, et al. Protease-activated receptor-1 activation by granzyme B causes neurotoxicity that is augmented by interleukin-1beta. J Neuroinflammation. 2017;14(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Setiadi AF, Abbas AR, Jeet S, Wong K, Bischof A, Peng I, et al. IL-17A is associated with the breakdown of the blood-brain barrier in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2019;332:147–54. [DOI] [PubMed] [Google Scholar]
- 112.*.Rivera J, Isidro RA, Loucil-Alicea RY, Cruz ML, Appleyard CB, Isidro AA, et al. Infusion of HIV-1 Nef-expressing astrocytes into the rat hippocampus induces enteropathy and interstitial pneumonitis and increases blood-brain-barrier permeability. PLoS One. 2019;14(11):e0225760. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that viral protein expression in the CNS can trigger systemic inflammation through an IL-1 dependent mechanism.
- 113.Liu X, Kumar A. Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes. Sci Rep. 2015;5:9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khoury G, Mackenzie C, Ayadi L, Lewin SR, Branlant C, Purcell DFJ. Tat IRES modulator of tat mRNA (TIM-TAM): a conserved RNA structure that controls Tat expression and acts as a switch for HIV productive and latent infection. Nucleic Acids Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.*.Blanch-Lombarte O, Galvez C, Revollo B, Jimenez-Moyano E, Llibre JM, Manzano JL, et al. Enhancement of Antiviral CD8(+) T-Cell Responses and Complete Remission of Metastatic Melanoma in an HIV-1-Infected Subject Treated with Pembrolizumab. J Clin Med. 2019;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]; A case report characterizing anti-viral immune responses in a patient with HIV and cancer treated with checkpoint inhibitor therapy.
- 116.Migueles SA, Chairez C, Lin S, Gavil NV, Rosenthal DM, Pooran M, et al. Adoptive lymphocyte transfer to an HIV-infected progressor from an elite controller. JCI Insight. 2019;4(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prasad S, Hu S, Sheng WS, Chauhan P, Lokensgard JR. Recall Responses from Brain-Resident Memory CD8(+) T Cells (bTRM) Induce Reactive Gliosis. iScience. 2019;20:512–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim BH, Kelschenbach J, Borjabad A, Hadas E, He H, Potash MJ, et al. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS. 2019;33(6):973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mamik MK, Asahchop EL, Chan WF, Zhu Y, Branton WG, McKenzie BA, et al. Insulin Treatment Prevents Neuroinflammation and Neuronal Injury with Restored Neurobehavioral Function in Models of HIV/AIDS Neurodegeneration. J Neurosci. 2016;36(41):10683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gavegnano C, Haile WB, Hurwitz S, Tao S, Jiang Y, Schinazi RF, et al. Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro. J Neuroinflammation. 2019;16(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nedelcovych MT, Kim BH, Zhu X, Lovell LE, Manning AA, Kelschenbach J, et al. Glutamine Antagonist JHU083 Normalizes Aberrant Glutamate Production and Cognitive Deficits in the EcoHIV Murine Model of HIV-Associated Neurocognitive Disorders. J Neuroimmune Pharmacol. 2019;14(3):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–42. [DOI] [PubMed] [Google Scholar]
- 123.Hsue PY, Li D, Ma Y, Ishai A, Manion M, Nahrendorf M, et al. IL-1beta Inhibition Reduces Atherosclerotic Inflammation in HIV Infection. J Am Coll Cardiol. 2018;72(22):2809–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fazeli PL, Marquine MJ, Dufour C, Henry BL, Montoya J, Gouaux B, et al. Physical Activity is Associated with Better Neurocognitive and Everyday Functioning Among Older Adults with HIV Disease. AIDS Behav. 2015;19(8):1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dufour CA, Marquine MJ, Fazeli PL, Henry BL, Ellis RJ, Grant I, et al. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J Neurovirol. 2013;19(5):410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martinez-Gomez D, Lavie CJ, Hamer M, Cabanas-Sanchez V, Garcia-Esquinas E, Pareja-Galeano H, et al. Physical activity without weight loss reduces the development of cardiovascular disease risk factors - a prospective cohort study of more than one hundred thousand adults. Prog Cardiovasc Dis. 2019. [DOI] [PubMed] [Google Scholar]
- 127.Paolucci EM, Loukov D, Bowdish DME, Heisz JJ. Exercise reduces depression and inflammation but intensity matters. Biol Psychol. 2018;133:79–84. [DOI] [PubMed] [Google Scholar]
- 128.Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, et al. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol. 2013;19(5):418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cody SL, Vance DE. The neurobiology of HIV and its impact on cognitive reserve: A review of cognitive interventions for an aging population. Neurobiol Dis. 2016;92(Pt B):144–56. [DOI] [PubMed] [Google Scholar]
- 130.Monnig MA, Kahler CW, Lee H, Pantalone DW, Mayer KH, Cohen RA, et al. Effects of smoking and alcohol use on neurocognitive functioning in heavy drinking, HIV-positive men who have sex with men. AIDS Care. 2016;28(3):300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stonerock GL, Blumenthal JA. Role of Counseling to Promote Adherence in Healthy Lifestyle Medicine: Strategies to Improve Exercise Adherence and Enhance Physical Activity. Prog Cardiovasc Dis. 2017;59(5):455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McArthur JC, Cohen BA, Farzedegan H, Cornblath DR, Selnes OA, Ostrow D, et al. Cerebrospinal fluid abnormalities in homosexual men with and without neuropsychiatric findings. Ann Neurol. 1988;23 Suppl:S34–7. [DOI] [PubMed] [Google Scholar]
- 133.Venkataramana A, Pardo CA, McArthur JC, Kerr DA, Irani DN, Griffin JW, et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology. 2006;67(3):383–8. [DOI] [PubMed] [Google Scholar]


