Abstract
Whole-body positron emission tomography/computed tomography (PET/CT) and brain magnetic resonance imaging (MRI) are commonly used to stage patients with palpable lymph node metastases from melanoma, but their role in patients with satellite and/or in-transit metastasis (S&ITM) is unclear. The aim of this study was to establish the diagnostic value of PET/CT and brain MRI in these patients, and to assess their influence on subsequent management decisions. In this prospective study, 25 melanoma patients with a first presentation of S&ITM who had no clinical evidence of palpable nodal or distant metastasis underwent whole-body 18F-FDG PET/CT and brain MRI after a tentative pre-scan treatment plan had been made. Sensitivity and specificity of imaging were determined by pathological confirmation, clinical outcome and repeat PET/CT and MRI at 6 months. PET/CT led to a modification of the initial treatment plan in four patients (16%). All four were upstaged (AJCC stage eighth edition). PET/CT was false-positive in one patient, who had a Schwannoma in his trapezius muscle. A thyroid carcinoma was an incidental finding in another patient. The sensitivity of PET/CT was 58% and specificity 83%. In 6 months following the baseline PET/CT, further sites of in-transit or systemic disease were identified in 10 patients (40%). Brain MRI did not alter the treatment plan or change the disease stage in any patient. Whole-body PET/CT improved staging in melanoma patients with S&ITM and changed the originally-contemplated treatment plan in 16%. MRI of the brain appeared not to be useful.
Keywords: fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography, in-transit, melanoma, MRI, satellites, staging
Introduction
In cancer patients, the stage of their disease assists in the determination of an appropriate treatment plan, as well as providing prognostic information. Also, accurate knowledge of disease stage facilitates the exchange of information about patients and enables comparison of the outcome following different diagnostic and therapeutic strategies.
Because of the nature of their metabolism, melanoma cells require glucose to thrive. Melanoma cells have high glutamine receptor activity and high levels of intracellular hexokinase. For this reason, melanoma has high avidity for the glucose analogue 18F-fluorodeoxyglucose (FDG) that is used for positron emission tomography/computed tomography (PET/CT) [1]. This makes PET/CT an ideal staging modality for the disease, except for the detection of brain metastases. magnetic resonance imaging (MRI) has proved to be better for this purpose [2,3]. The value of whole-body PET/CT for the detection of metastatic melanoma has been well demonstrated in patients with distant disease and palpable lymph node metastases [1,4,5]. Unfortunately, PET/CT performance has proved disappointing in patients with a positive sentinel node because metastases are usually too small to be identified at this stage, and false-positive findings are frequent [6,7]. The value of PET/CT in patients with satellite and/or in-transit metastasis (S&ITM) has not been well studied.
The purposes of the current study were first to determine the implications of staging PET/CT and brain MRI for subsequent management of patients with S&ITM at the time of initial melanoma diagnosis or as a first recurrence, and secondly to establish the diagnostic accuracy of PET/CT and MRI in this population.
Materials and methods
Patients
The study was conducted with institutional ethics committee approval (HREC/13/RPAH/586). Between May 2014 and May 2015, 25 melanoma patients with a first presentation of S&ITM were prospectively enrolled. Patients were eligible if they had a first presentation of pathologically confirmed S&ITM without clinical evidence of metastases elsewhere, were at least 18 years of age and able to give informed consent, had an Eastern Cooperative Oncology Group performance status of 0, 1 or 2, and a life expectancy of at least 6 months. Patients were not eligible if they had a true local recurrence (in contact with a skin graft or scar of the primary melanoma excision) or if they were pregnant.
Microsatellites, satellites and in-transit metastases are thought to represent intralymphatic or angiotrophic tumor spread. Satellites were defined as clinically evident cutaneous and/or subcutaneous metastases within 2 cm of the primary melanoma and ITMs were defined as located between 2 cm from the primary melanoma and the first regional lymph node [8]. For microsatellites, the AJCC-UICC seventh edition definition was in use during the study period (‘any discontinuous nest of metastatic cells more than 0.05 mm in diameter that are clearly separated by normal dermis from the main invasive component of melanoma by a distance of at least 0.3 mm’) [9].
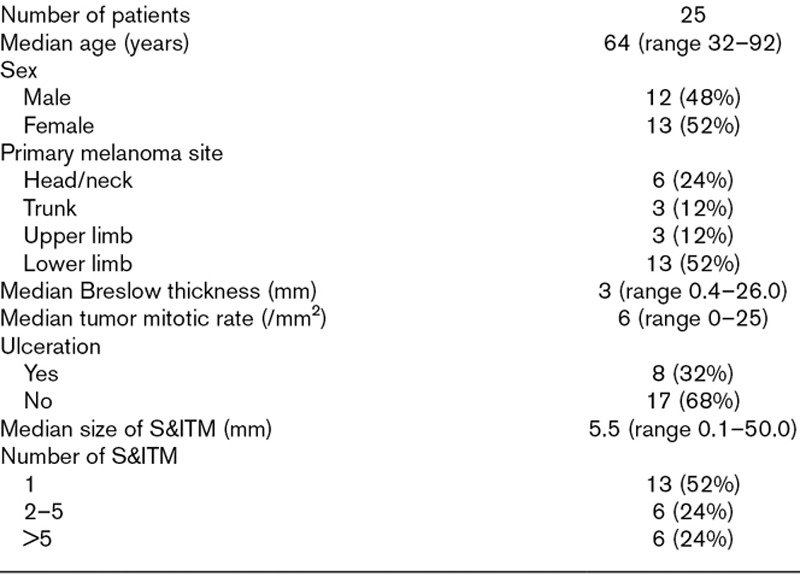
Patient and tumor characteristics are presented in Table 1. Twelve patients had newly diagnosed melanomas and simultaneous S&ITM (cTNM stage III) and 13 developed S&ITM after a preceding primary melanoma diagnosis (rcTNM stage III). Eleven of the 25 patients had satellite metastases. Eight of these 11 patients had microsatellites identified histologically in the primary tumor excision specimen. Three patients had satellite metastases still present at time of enrolment. Fourteen patients had ITMs, of whom two had undergone partial or complete excision of their lesion(s).
Table 1.
Patient and tumor characteristics
Before PET/CT and brain MRI results were obtained, a tentative treatment plan was drawn up and documented, on the assumption that no distant metastases would be demonstrated. The final treatment was compared with the pre-scan management plan. Patients were monitored for recurrence during the subsequent 6 months and at the end of this period physical examination, PET/CT and brain MRI were repeated.
18F-fluorodeoxyglucose positron emission tomography/computed tomography
A Phillips Ingenuity TOF 64 slice PET/CT scanner (Philips, Amsterdam, the Netherlands) was used for whole-body PET/CT imaging. Patients were fasted for at least 6 hours prior to intravenous administration of 3.5 mg/kg of FDG. PET images were obtained approximately 60 minutes after radioisotope injection with six- to eight-bed positions and an acquisition time of three minutes per stop. Low-dose CT was performed using a 64 slice multi-detector CT from vertex to at least proximal thighs for attenuation correction and anatomical localization. PET/CT examinations were interpreted by a nuclear physician (L.E.). Lesions were scored as negative, equivocal – probably negative, equivocal – probably positive or positive for melanoma metastasis. Anatomical location, number of lesions and maximum standardized uptake values were recorded.
Magnetic resonance imaging
MRI of the brain was acquired with a dedicated head coil in a Siemens Avanto 1.5 Tesla scanner (Siemens, Erlangen, Germany). Sequences employed included sagittal T1, FLAIR axial, axial T2, T1 axial diffusion-weighted sequence and post-intravenous gadolinium VIBE axial T1 weighted imaging. Each patient was injected with 10 cc of IV gadolinium (Gadovist 1.0; Bayer AG, Leverkusen, Germany) and the number, location and size of each metastasis were documented.
Reference standard and statistical analysis
The primary outcome measure was the percentage of patients in whom PET/CT and brain MRI led to a change in disease management. We deemed a change of treatment in at least 10% of the patients clinically relevant. Secondary outcome measures were change of AJCC-UICC stage and performance parameters of the imaging. Pathological confirmation of suspicious/positive lesions with FDG uptake on PET/CT was pursued. If pathological confirmation was not possible, clinical outcome and imaging after 6 months were used as gold standards.
The sensitivity and specificity of PET/CT and brain MRI were determined by patient-based analysis. A true-positive finding surpassed a false-negative finding in the same patient. Scans were classified as true-positive if metastatic melanoma was suggested and confirmed, and as false-positive, if the suspected metastatic melanoma was confirmed to be something else. A lesion with FDG uptake unequivocally reported and confirmed to be a non-melanoma malignancy was classified as an incidental finding, not false-positive. Scans that were considered to be negative were classified as true-negative if the patient did not develop a recurrence during the 6 months following the baseline imaging. Scans were considered false-negative if the baseline scan failed to reveal the initial S&ITM that was still present or if evidence of any further metastasis was established during the 6 months of follow-up.
Disease progression was defined as detection of melanoma metastasis after baseline imaging and treatment, by the patient, the physician or through further imaging and preferably with subsequent pathological confirmation.
Results
The baseline PET/CT showed the known tumor sites in eight of the 15 patients (53%) in whom the S&ITM was still present at the time of the imaging, but no further S&ITM were visualized. In the other seven patients, the known S&ITM was not visible on the baseline PET/CT. Additional metastases were identified in four of the 25 patients in the study (16%). These were regional lymph node metastases in two patients, distant metastases in one patient, and simultaneous regional and distant metastases in the remaining patient (Fig. 1). PET/CT was false-positive in one of the 25 patients (4%), who had a Schwannoma in the right trapezius muscle. A thyroid carcinoma was an incidental finding in another patient.
Fig. 1.
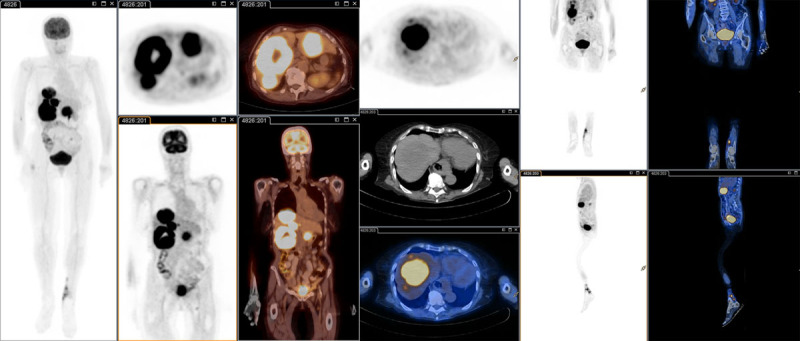
Whole-body FDG PET/CT patient 2 (Table 2). Marked FDG avidity in the liver corresponding to numerous hypodense lesions throughout both lobes. Focal FDG avidity in the left os ilium, corresponding to a partially sclerotic lesion in the low dose CT scan. Mildly hypermetabolic 12 mm left inguinal lymph node. Extensive, contiguous, FDG avid soft tissue nodular thickening in the left leg medially. CT, computed tomography; FDG, 18F-fluorodeoxyglucose PET, positron emission tomography.
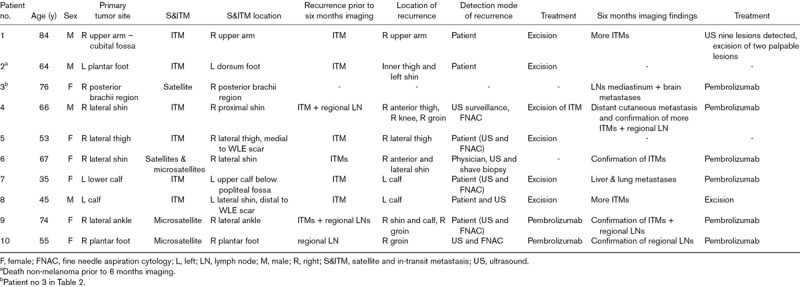
The primary aim of the study was to determine the implications of imaging for staging and subsequent management. PET/CT led to a change in the AJCC-UICC stage (eighth edition) of four of the 25 patients (16%). In all four patients (16%), PET/CT led to modification of the pre-imaging treatment plan (Table 2).
Table 2.
Characteristics of patients with a change in melanoma treatment plan

Following the baseline PET/CT and treatment, further sites of disease became evident within 6 months in 10 of the 25 patients (40%) (Table 3). In eight of these 10 patients, this was additional in-transit disease. Recurrences were detected prior to the 6-month PET/CT in nine of the 10 patients (90%). These recurrences were detected by the patient, by the physician or through ultrasound follow-up. The PET/CT after 6 months revealed even more metastases in five of these nine patients (patients 1, 3, 4, 7 and 8).
Table 3.
Disease progression and PET/CT and brain MRI findings at 6 months
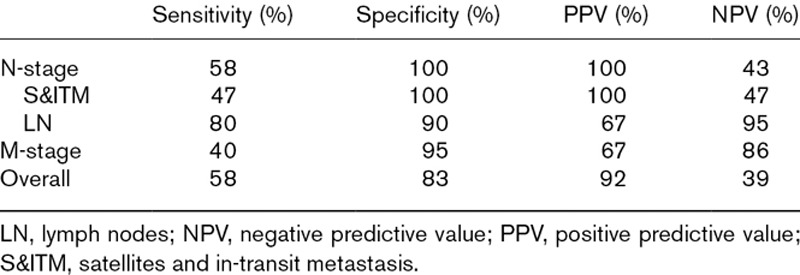
Including the S&ITM that were present initially but not apparent on the PET/CT, the baseline scans of 12 patients were false-negative. In four of these patients, the baseline scan was also true-positive. Because a true-positive finding trumped a false-negative finding in the same patient, eight scans were ultimately classified as false-negative. The performance of PET/CT in various categories is presented in Tables 4 and 5.
Table 4.
Contingency tables according to stage of disease and overall
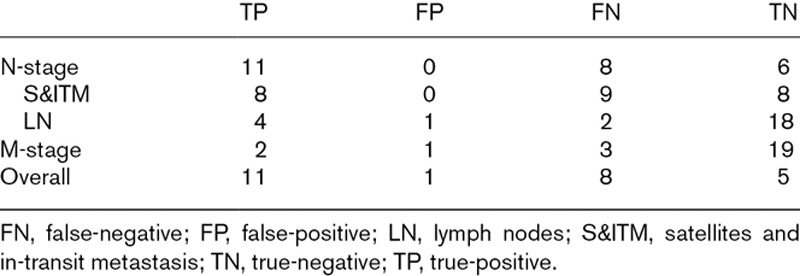
Table 5.
Performance of PET/CT
Baseline brain MRI was normal in all patients and did not result in change of stage or treatment plan in any of them. The follow-up MRI at 6 months was not performed in three patients, because two had died and a third suffered a panic attack during the imaging. The brain MRI at 6 months revealed a previously unknown right superior frontal lobe metastasis in one patient, so in this case, the baseline scan was classified as false-negative.
All melanoma metastases found at baseline as well as during the 6 months of follow-up were treated, unless the patient was unfit for any treatment or the patient and physician decided upon close monitoring until further progress of disease. Two patients died during the 6 months observation period: one from distant metastatic disease and the other from an unrelated cause.
Discussion
As expected, the results of imaging melanoma patients who at first presentation with S&ITM, which were generally small, were less likely to reveal other metastatic disease than in patients with more bulky lesions. The 58% sensitivity of PET/CT found in this study is substantially less than the 87% previously reported in patients with palpable lymph node metastases [1]. It is likely that this is because the non-visualized metastases on staging PET/CT that became clinically evident later were below the spatial resolution of PET detection (approximately 3 mm), or due to rapidly progressive new metastases in the following 6 months. Despite this, upstaging and a change in the pre-scan management plan in 16% of patients imply a meaningful impact. The importance of changes in disease management has been affirmed in the recently published Cochrane review on staging imaging in melanoma [10]. In the present study, MRI of the brain never revealed a metastasis at baseline and its usefulness in this setting therefore appears dubious.
To our knowledge, this study is the first to report the impact of whole-body PET/CT and brain MRI staging on patients with a first presentation with S&ITM. Relevant aspects are its prospective design, the strict inclusion criteria for patients with S&ITM and the use of one PET/CT scanner for all patients. There are several possible limitations to discuss. We hypothesized that PET/CT and brain MRI should be able to detect metastases up to 6 months before they become evident otherwise. This time frame is, of course, arbitrary and the choice of a shorter period would have resulted in a lower false-negative rate.
We limited our study to patients with a first diagnosis of S&ITM. If we had included patients with recurrent in-transit metastases it is likely the sensitivity of imaging would have been greater, as the disease would be likely to be more advanced.
As the AJCC eighth edition cancer staging system provides a new staging classification, which includes a separate schema for patients who recur after their initial melanoma presentation (rTNM), our 25 patients may represent a heterogeneous group. However, to our knowledge this recurrent staging classification is not yet embedded in melanoma research, hence no conclusions can be drawn concerning the differences in sensitivity of imaging for the two scenarios. In terms of survival, Read et al. [11] reported an overall 5-year survival from time of ITM presentation of 47% in patients with ITMs as a first site of recurrence versus 43% in patients presenting with ITMs at primary diagnosis, suggesting the latter group has a somewhat worse prognosis.
We hypothesized that known S&ITM would be identified depending on their size, with a higher pick-up rate for larger S&ITM. Interestingly, only one of the seven patients in whom S&ITM was not detected on the baseline scan had lesions that were less than 3 mm in diameter. In the other six patients, lesions from 3 to 7 mm (on histopathology) were missed on PET/CT. This suggests that cutaneous and subcutaneous metastases up to 7 mm in diameter might be difficult to detect on PET/CT.
Five previous studies have reported on change in treatment plan after primary staging with PET/CT. In a prospective study of 32 patients with stage III–IV melanoma being considered for surgical treatment, Bronstein et al. [12] found that the treatment plan in 12% of patients changed after treatment decisions based on PET/CT were compared with initial treatment decisions (based on conventional imaging with CT and MRI). Aukema et al. reported a change in treatment plan in 26 of 70 patients (37%) with palpable lymph node metastases [1]. In a retrospective cohort of 30 sentinel lymph node-positive patients, Constantinidou noted no change in treatment plans [6]. In a retrospective cohort, Arrangoiz et al. [13] reported a change in treatment plan in 18% of patients with T4 melanoma and no clinical evidence of metastatic disease. Their further analysis showed that 11% of the changes were due to identification of regional metastases and 7% were due to identification of distant metastases. Also, 45% of their patients had satellitosis, but no information on change in treatment in this subgroup was provided. In a retrospective study of 149 patients with stage IB–II melanoma, Barsky et al. [14] found that PET/CT never resulted in a change in treatment plan.
Two meta-analyses have addressed the value of PET/CT in primary staging of melanoma patients with AJCC-UICC (seventh edition) stages I–IV disease [4,5]. In the most recent of the two, Schröer-Günther et al. analyzed the diagnostic reliability of PET and PET/CT in prospective studies with a patient-based analysis published up to 2010 [5]. Only studies with a clinical follow-up of >6 months were included. For PET/CT the sensitivity of all included studies ranged between 17 and 85% and the specificity ranged from 74 to 96%.
Studies evaluating the diagnostic accuracy of PET/CT specifically in stage III melanoma patients are scarce. Aukema et al. found a sensitivity of 87% and a specificity of 98% in the detection of distant disease in patients with palpable lymph node disease. Accuracy was 98%, positive predictive value (PPV) 96% and negative predictive value (NPV) 91% [1].
In conclusion, the current study indicates that staging patients with S&ITM using PET/CT has a meaningful impact on patient management. There was upstaging of disease and a change in treatment as a result of whole-body PET/CT in 16% of the patients. The overall sensitivity was 58%, specificity 83%, PPV 92% and NPV 39%. MRI of the brain did not detect any metastases at baseline and might be omitted, particularly since modern-day PET/CT devices can provide a low radiation dose CT scan for screening the brain. Ten patients (40%) developed further metastasis within 6 months after the initial staging imaging and treatment. This suggests that an appropriate interval for repeat imaging in this high-risk cohort may be 3 months rather than 6 months. A future study assessing this interval would be worthwhile.
Acknowledgements
We gratefully acknowledge the support and assistance of colleagues at the Mater Hospital, St Vincent’s Hospital and Melanoma Institute Australia. Funding support from the National Health and Medical Research Council, Cancer Institute New South Wales, Melanoma Institute Australia and The Melanoma Foundation of the University of Sydney is also gratefully acknowledged.
This project received a grant from the Friends of the Mater Foundation, North Sydney NSW. L.H.J.H. received a research grant from the Groningen Melanoma and Sarcoma Foundation and was funded by het Vreedefonds and Melanoma Institute Australia.
The Friends of the Mater Foundation provided a grant of AUD 4500 to cover the costs of this research project.
Conflicts of interest
R.P.M.S. has received honoraria for advisory board participation from Novartis and MSD. J.F.T. has received honoraria for advisory board participation from MSD Australia, BMS Australia, Provectus Inc and GlaxoSmithKline, and travel support form Provectus Inc and GlaxoSmithKline. For the remaining authors, there are no conflicts of interest.
References
- 1.Aukema TS, Valdés Olmos RA, Wouters MW, Klop WM, Kroon BB, Vogel WV, Nieweg OE. Utility of preoperative 18F-FDG PET/CT and brain MRI in melanoma patients with palpable lymph node metastases. Ann Surg Oncol. 2010; 17:2773–2778 [DOI] [PubMed] [Google Scholar]
- 2.Rohren EM, Provenzale JM, Barboriak DP, Coleman RE. Screening for cerebral metastases with FDG PET in patients undergoing whole-body staging of non-central nervous system malignancy. Radiology. 2003; 226:181–187 [DOI] [PubMed] [Google Scholar]
- 3.Fogarty GB, Tartaguia C. The utility of magnetic resonance imaging in the detection of brain metastases in the staging of cutaneous melanoma. Clin Oncol (R Coll Radiol). 2006; 18:360–362 [DOI] [PubMed] [Google Scholar]
- 4.Xing Y, Bronstein Y, Ross MI, Askew RL, Lee JE, Gershenwald JE, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011; 103:129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroër-Günther M. F-18-fluoro-2-deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: a systematic review. Syst Rev. 2012; 1:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantinidou A, Hofman M, O’Doherty M, Acland KM, Healy C, Harries M. Routine positron emission tomography and positron emission tomography/computed tomography in melanoma staging with positive sentinel node biopsy is of limited benefit. Melanoma Res. 2008; 18:56–60 [DOI] [PubMed] [Google Scholar]
- 7.Holtkamp LHJ, Read RL, Emmett L, Thompson JF, Nieweg OE. Futility of imaging to stage melanoma patients with a positive sentinel lymph node. Melanoma Res. 2017; 27:457–462 [DOI] [PubMed] [Google Scholar]
- 8.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27:6199–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC. Melanoma of the skin. American Joint Committee on Cancer Staging Manual. 20107th ed, New York: Springer; 325 [Google Scholar]
- 10.Dinnes J, Ferrante di Ruffano L, Takwoingi Y, Cheung ST, Nathan P, Matin RN, et al. Ultrasound, CT, MRI or PET-CT for staging and re-staging of adults with cutaneous melanoma. Cochrane Database Syst Rev. 2019; 7:CD012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Read RL, Haydu L, Saw RP, Quinn MJ, Shannon K, Spillane AJ, et al. In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol. 2015; 22:475–481 [DOI] [PubMed] [Google Scholar]
- 12.Bronstein Y, Ng CS, Rohren E, Ross MI, Lee JE, Cormier J, et al. PET/CT in the management of patients with stage IIIC and IV metastatic melanoma considered candidates for surgery: evaluation of the additive value after conventional imaging. AJR Am J Roentgenol. 2012; 198:902–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrangoiz R, Papavasiliou P, Stransky CA, Yu JQ, Tianyu L, Sigurdson ER, et al. Preoperative FDG-PET/CT is an important tool in the management of patients with thick (T4) melanoma. Dermatol Res Pract. 2012; 2012:614349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barsky M, Cherkassky L, Vezeridis M, Miner TJ. The role of preoperative positron emission tomography/computed tomography (PET/CT) in patients with high-risk melanoma. J Surg Oncol. 2014; 109:726–729 [DOI] [PubMed] [Google Scholar]


