Abstract
Background
As the pandemic of coronavirus disease 2019 (COVID-19) continues, prognostic markers are now being identified. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are easily accessible values that have been known to correlate with inflammation and prognosis in several conditions. We used the available data to identify the association of NLR and PLR with the severity of COVID-19.
Methods
A literature search using EMBASE, MEDLINE, and Google Scholar for studies reporting the use of NLR and PLR in COVID-19 published until April 28, 2020, was performed. Random effects meta-analysis was done to estimate standard mean difference (SMD) of NLR and PLR values with 95% confidence interval (CI) between severe and non-severe COVID-19 cases.
Results
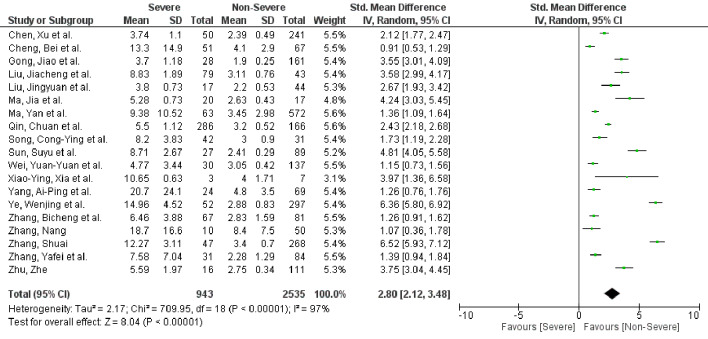
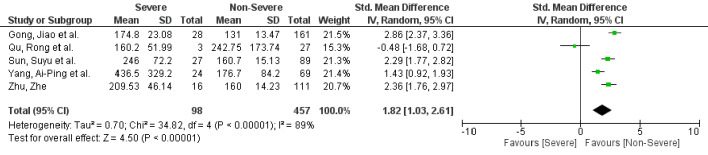
A total of 20 studies with 3,508 patients were included. Nineteen studies reported NLR values, while five studies reported PLR values between severe and non-severe COVID-19 patients. Higher levels of NLR (SMD: 2.80, 95% CI: 2.12 - 3.48, P < 0.00001) and PLR (SMD: 1.82, 95% CI: 1.03 - 2.61, P < 0.00001)) were seen in patients with severe disease compared to non-severe disease.
Conclusions
NLR and PLR can be used as independent prognostic markers of disease severity in COVID-19.
Keywords: COVID-19, NLR, PLR, Prognostic markers
Introduction
The world is currently going through an unprecedented pandemic of the coronavirus disease 2019 (COVID-19) that has now affected millions of people [1]. Compared to seasonal influenza, COVID-19 is more contagious, has a longer incubation period, and is associated with higher hospitalization and mortality rates [2-4]. The clinical presentation varies from no symptoms to acute respiratory failure, shock, and multi-organ system dysfunction [2]. Those with older ages, male gender, obesity, and chronic comorbidities such as cardiovascular disease, diabetes, chronic respiratory disease, and cancer were more likely to have worse outcomes [2, 5, 6]. Patients with COVID-19 present with multiple hematological abnormalities, of which lymphopenia and thrombocytopenia were prominent. Acute phase reactants like C-reactive protein, lactate dehydrogenase, ferritin, and D-dimer have also been well correlated with disease severity and progression [7].
Inflammation plays a major role in the pathophysiology of COVID-19. Both the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) indirectly reflect a patient’s inflammatory state. The NLR is calculated as the absolute neutrophil count divided by the absolute lymphocyte count, while the PLR is calculated by platelet count divided by absolute lymphocyte count. In the recent years, NLR and PLR have been validated as prognostic markers in various disorders such as cardiac conditions, solid tumors, sepsis, pneumonia, and acute respiratory distress syndrome (ARDS) [8-13]. Few studies have evaluated the role of NLR and PLR in patients with severe and non-severe COVID-19. We performed this meta-analysis to identify the association of NLR and PLR in relation to the severity of COVID-19.
Materials and Methods
We performed a literature search using EMBASE, MEDLINE, and Google Scholar for studies reporting the use of NLR and PLR in COVID-19 published until April 28, 2020. We used the medical subject headings (MESH) terms: “neutrophil-to-lymphocyte ratio,” “platelet-to-lymphocyte ratio,” “NLR”, “PLR,” “COVID-19,” “novel coronavirus”, and “SARS-CoV-2.” Inclusion criteria were: 1) Descriptive studies comparing between severe versus non-severe, or survivor versus deaths in COVID-19 patients; 2) Non-pregnant adult patients; 3) Studies reporting NLR or PLR values. Eligible studies were reviewed, and data including study design, sample size, baseline characteristics, NLR, and PLR values were obtained. Definitions of COVID-19 disease severity were based on individual studies.
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. We used the Cochrane Review Manager version 5.3 for our analysis. Mean and standard deviation were extrapolated from median and interquartile range (IQR) using the method outlined by Hozo et al [14]. For each outcome, standard mean difference and 95% confidence interval (CI) were calculated using the random effects model utilizing the inverse variance method. A P value of 0.05 or less was assigned as the measure of statistical significance. Study heterogeneity was assessed by calculating I2 statistics; heterogeneity was considered significant if I2 > 50%.
Results
The initial search yielded a total of 403 studies, of which 20 studies were included in the final analysis (Fig. 1) [15-34]. All studies were conducted in China. A total of 3,508 patients, with 946 in severe COVID-19 group and 2,561 in non-severe, were included. Clinical demographics are outlined in Table 1 [15-34]. The criteria for severe and non-severe disease varied between each study, but most studies considered respiratory distress and care in intensive units as severe disease. Study specific definitions are listed here (Supplementary Material 1, www.jocmr.org).
Figure 1.
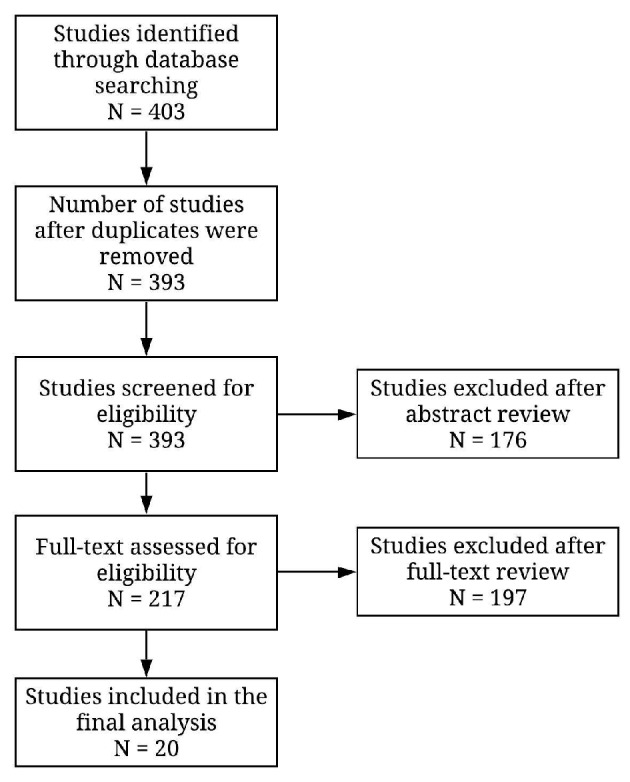
PRISMA diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1. Clinical Characteristics of Included Studies.
| Study | Baseline characteristics | Number of patients | Study design | Age, mean/median | Female, n (%) | Comorbidities |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COPD | Hypertension | Cardiovascular | Cerebrovascular | Chronic liver disease | Diabetes mellitus | Cancer | Chronic kidney disease | ||||||
| Qin et al, 2020 [15] | Non-severe | 166 | Retrospective review | 53 | 86 (51.8) | 3 (1.8) | 30 (18.1) | 3 (1.8) | 3 (1.8) | 3 (1.8) | 22 (13.3) | 4 (2.4) | 4 (2.4) |
| Severe | 286 | 53 | 131 (45.8) | 9 (3.1) | 105 (36.7) | 24 (8.4) | 8 (2.8) | 3 (1.0) | 53 (18.5) | 10 (3.5) | 6 (2.1) | ||
| Liu et al, 2020 [16] | Non-severe | 44 | Prospective review | 41 | 23 (52.3) | 2 (4.5) | 6 (13.6) | 0 (0) | - | - | 2 (4.5) | - | - |
| Severe | 17 | 56 | 7 (41.2) | 3 (17.6) | 6 (35.3) | 1 (5.9) | - | - | 3 (17.6) | - | - | ||
| Yang et al, 2020 [17] | Non-severe | 69 | Retrospective review | 42.1 | 31 (44.9) | - | 7 (10.1) | 4 (5.8) | - | 9 (13.0) | 8 (11.6) | 2 (2.9) | |
| Severe | 24 | 57.9 | 6 (25) | - | 16 (66.8) | 9 (37.5) | - | 4 (16.7) | 13 (54.2) | 8 (33.3) | |||
| Xia et al, 2020 [18] | Non-severe | 7 | Case series | 54 | 2 (28.6) | - | 1 (14) | - | - | 1 (14) | - | - | - |
| Severe | 3 | 61.6 | 2 (66.7) | - | - | - | - | - | - | - | - | ||
| Zhang et al, 2020 [19] | Non-severe | 81 | Retrospective review | 59.3 | 42 (52.8) | - | - | - | - | - | - | - | - |
| Severe | 67 | 60.8 | 29 (43.3) | - | - | - | - | - | - | - | - | ||
| Qu et al, 2020 [20] | Non-severe | 27 | Case series | 49.4 | - | - | - | - | - | - | - | - | - |
| Severe | 3 | 60 | - | - | - | - | - | - | - | - | - | ||
| Ye et al, 2020 [21] | Survivor | 297 | Retrospective review | 60 | 160 (53.9) | - | 73 (24.6) | - | - | - | 41 (13.8) | - | - |
| Non-survivor | 52 | 69 | 16 (30.8) | - | 30 (57.7) | - | - | - | 16 (30.8) | - | - | ||
| Zhang et al, 2020 [22] | Non-severe | 84 | Retrospective review | 44 | 55 (65.5) | - | - | - | - | - | - | - | - |
| Severe | 31 | 64.6 | 11 (35.5) | - | - | - | - | - | - | - | - | ||
| Sun et al, 2020 [23] | Non-severe | 89 | Retrospective review | 47 | 47 (52.8) | - | - | - | - | - | - | - | - |
| Severe | 27 | 62 | 9 (33.3) | - | - | - | - | - | - | - | - | ||
| Song et al, 2020 [24] | Non-severe | 31 | Retrospective review | 48 | 30 (71.4) | 1 (3.2) | 4 (12.9) | 1 (3.2) | - | 3 (9.7) | 2 (6.5) | - | - |
| Severe | 42 | 55.5 | 12 (28.6) | 2 (4.8) | 22 (52.4) | 4 (9.5) | - | 1 (2.4) | 4 (9.5) | - | - | ||
| Gong et al, 2020 [25] | Non-severe | 161 | Retrospective review | 45 | 89 (55.3) | - | - | - | - | - | - | - | - |
| Severe | 28 | 63.5 | 12 (42.9) | - | - | - | - | - | - | - | - | ||
| Ma et al, 2020 [26] | Non-severe | 17 | Retrospective review | 61 | 7 (41.1) | - | - | - | - | - | - | 17 (100) | - |
| Severe | 20 | 65.5 | 10 (50) | - | - | - | - | - | - | 20 (100) | - | ||
| Wei et al, 2020 [27] | Non-severe | 137 | Did not mention | 40.83 | 62 (45.3) | 2 (1.5) | - | 17 (12.4) | 2 (1.5) | - | 4 (2.9) | - | - |
| Severe | 30 | 49.03 | 10 (33.3) | 2 (6.7) | - | 7 (23.3) | 0 | - | 7 (23.3) | - | - | ||
| Zhang et al, 2020 [28] | Recovered | 50 | Retrospective review | 62.6 | 14 (28) | 4 (8) | 18 (36) | 11 (22) | 4 (8) | 5 (10) | - | - | |
| Death | 10 | 70.6 | 3 (30) | 2 (20) | 4 (40) | 3 (30) | 2 (20) | 4 (40) | - | - | |||
| Zhu et al, 2020 [29] | Non-severe | 111 | Retrospective review | 50 | 38 (34.2) | 4 (3.6) | 23 (20.7) | 4 (3.6) | 5 (4.5) | 10 (9) | 4 (3.6) | - | |
| Severe | 16 | 57.5 | 7 (43.8) | 2 (12.5) | 8 (50) | 2 (12.5) | 2 (12.5) | 0 | 1 (6.25) | - | |||
| Zhang et al, 2020 [30] | Survivors | 268 | Case-control studies | 56 | 131 (48.9) | 3 (1.12) | 64 (23.9) | 30 (11.2) | 7 (2.6) | 7 (2.6) | 34 (12.7) | 8 (3) | 2 (0.8) |
| Non-survivors | 47 | 66 | 9 (19.1) | 0 | 14 (29.8) | 5 (10.6) | 0 | 2 (4.3) | 7 (14.9) | 4 (8.5) | 0 | ||
| Liu et al, 2020 [31] | Non-severe | 43 | Retrospective review | 55 | 17 (39.5) | 0 | 13 (30.2) | 0 | 4 (9.3) | 1 (2.3) | 2 (4.7) | 0 | - |
| Severe | 79 | 65 | 33 (41.8) | 2 (2.5) | 37 (46.8) | 2 (2.5) | 6 (7.6) | 2 (2.5) | 13 (16.5) | 1 (1.3) | - | ||
| Ma et al, 2020 [32] | Non-severe | 572 | Retrospective review | 44 | 273 (47.7) | - | 90 (15.7) | - | - | - | 29 (5.1) | - | - |
| Severe | 63 | 53.3 | 34 (54) | - | 15 (24) | - | - | - | 6 (10) | - | - | ||
| Cheng et al, 2020 [33] | Survivors | 67 | Retrospective review | 70.6 | 45 (67.2) | 7 (10.4) | 39 (58.2) | 11 (16.4) | 1 (1.5) | 11 (16.4) | 2 (3) | 3 (4.5) | |
| Non-survivors | 51 | 73.1 | 20 (39.2) | 6 (11.8) | 25 (49) | 12 (23.5) | 0 | 16 (31.4) | 0 | 3 (5.9) | |||
| Chen et al, 2020 [34] | Non-severe | 241 | Retrospective review | 42.1 | 123 | 5 (2.1) | 20 (8.3) | 7 (3) | 5 (2.1) | 13 (5.4) | 15 (6.2) | 2 (0.8) | 1 (0.4) |
| Severe | 50 | 60.5 | 23 (46) | 5 (10) | 19 (38) | 5 (10) | 3 (6) | 2 (4) | 7 (14) | 0 | 1 (2) | ||
COPD: chronic obstructive pulmonary disease.
A total of 19 studies with 3,478 patients reported NLR values [15-19, 21-34]. Fifteen studies reported NLR between severe and non-severe disease, while four studies reported NLR based on survival. PLR was identified in five studies [17, 20, 23, 25, 29]. Patients with severe COVID-19 disease had higher NLR values (standard mean difference (SMD): 2.80, 95% CI: 2.12 - 3.48, P < 0.00001) when compared to patients with non-severe disease (Fig. 2). In the subgroup analysis, NLR values were higher in non-survivors when compared to survivors (SMD: 3.72, 95% CI: 0.53 - 6.90, P = 0.02) (Supplementary Material 2, www.jocmr.org). Similarly, PLR was elevated in patients with severe disease compared to non-severe disease (SMD: 1.82, 95% CI: 1.03 - 2.61, P < 0.00001) (Fig. 3). Significant heterogeneity was noted in the study results (I2 = 97% for NLR and I2 = 89% for PLR).
Figure 2.
NLR in severe versus non-severe COVID-19 patients. NLR: neutrophil-to-lymphocyte ratio; COVID-19: coronavirus disease 2019; SD: standard deviation; CI: confidence interval.
Figure 3.
PLR in severe versus non-severe COVID-19 patients. PLR: platelet-to-lymphocyte ratio; COVID-19: coronavirus disease 2019; SD: standard deviation; CI: confidence interval.
Discussion
Patients with severe COVID-19 disease had higher NLR and PLR values compared to non-severe disease. The present study shows that levels of NLR and PLR correlate with COVID-19 disease severity.
Patients with severe COVID-19 disease present with increased leukocytosis, neutrophilia, lymphopenia, and thrombocytopenia than those with non-severe disease [7]. These patients were more likely to develop ARDS and require intensive care unit (ICU) level of care [35-37]. NLR and PLR are easily obtained from a serum complete blood count with a differential profile. They serve as a function of relative neutrophilia, thrombocytosis, and lymphopenia. The different mechanisms of lymphopenia in COVID-19 patients have been linked to the virus’s ability to infect T cells through the angiotensin-converting enzyme 2 (ACE2) receptors and cluster of differentiation (CD)147-spike proteins [38, 39]. The final results were decreased levels of CD3+, CD4+, CD8+ T lymphocytes, and increased regulatory T cells. The rise of proinflammatory cytokines with T cell lymphopenia predisposes severe COVID-19 patients to cytokine storm, thus resulting in more lymphocytic apoptosis and multi-organ failure. Overall, the decreased levels of CD4+ and CD8+ T lymphocytes correlated with disease severity, which can lead to increase NLR or PLR [2, 4, 15, 16].
In cases of other viral and bacterial pneumonia, NLR was more sensitive than individual levels of neutrophils and lymphocytes [40]. Similarly, PLR correlated well with mortality and disease severity in bacterial pneumonia [12, 41]. In the study by Liu et al, NLR was found to be the most prognostic among multiple variables in determining the severity of illness. Furthermore, when compared to other risk assessment tools such as CURB-65 and multilobular infiltration, hypo-lymphocytosis, bacterial coinfection, smoking history, hypertension, and age (MuLBSTA), NLR had a higher sensitivity and specificity [16]. While many inflammatory markers like C-reactive protein, erythrocyte sedimentation rate, lactate dehydrogenase, ferritin, and procalcitonin are frequently measured in COVID-19 patients, NLR and PLR can be easily calculated using the differential count and are cost-effective especially for many third world countries. A previous meta-analysis composed of 828 patients and six studies concluded that a high NLR and low lymphocyte-to-C-reactive protein ratio indicated poor prognosis [42]. The previous study used mean or median values of neutrophils and lymphocytes from individual studies to calculate NLR. For this meta-analysis, we have chosen to exclude studies that did not have the calculated NLR values. Nevertheless, the results of this study were consistent with the previous meta-analysis and individual studies. So far, this is the first meta-analysis to evaluate the prognostic significance of PLR in COVID-19.
There are some limitations to the study. First of all, most of the studies are retrospective reviews, and all included studies were conducted in China. Second, heterogeneity exists between the included patient populations, with some studies not elucidating on underlying comorbidities. Third, it was unclear when in the disease course, the NLR and PLR values were measured. Depending on the severity of COVID-19, disease values of NLR and PLR will likely change.
Conclusions
This study establishes NLR and PLR as independent prognostic markers to differentiate severe versus non-severe disease in COVID-19 patients. Early recognition of the severe cases allows for early triaging and timely initiation of management. These markers are cost-effective and easily accessible in all laboratories. Future studies should compare the trends of NLR and PLR with disease progression.
Supplementary Material
Study Specific Definitions of Non-Severe and Severe Disease Classifications
Subgroup analysis showing NLR in severe versus non-severe disease and non-survivor versus survivor in COVID-19 patients.
Acknowledgments
None to declare.
Financial Disclosure
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
ASC and AR equally contributed to the paper in conceptualization, data collection and drafting of manuscript. AR performed the statistical analysis.
Data Availability
The studies supporting this meta-analysis are from previously reported studies and datasets, which have been cited in the references section. The processed data are available from the corresponding author upon request.
References
- 1. Johns Hopkins University & Medicine. COVID-19 map. Baltimore, MD: Johns Hopkins University; 2020. https://coronavirus.jhu.edu/map.html.
- 2.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M. et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perdersen SF, Ho Y-C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020 doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, Stachel A. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 8.Angkananard T, Anothaisintawee T, McEvoy M, Attia J, Thakkinstian A. Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:2703518. doi: 10.1155/2018/2703518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, Wang T. et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9(6):e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38(3):641–647. doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Shen Y, Huang X, Zhang W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: interaction effect with disease severity-a retrospective study. BMJ Open. 2019;9(1):e022896. doi: 10.1136/bmjopen-2018-022896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Ju M, Chen C, Yang D, Hou D, Tang X, Zhu X. et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in acute respiratory distress syndrome patients: a retrospective study. J Thorac Dis. 2018;10(1):273–282. doi: 10.21037/jtd.2017.12.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C. et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.2139/ssrn.3541136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Liu Y, Xiang P. et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. 2020 doi: 10.1101/2020.02.10.20021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia XY, Wu J, Liu HL, Xia H, Jia B, Huang WX. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID-19. J Clin Virol. 2020;127:104360. doi: 10.1016/j.jcv.2020.104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Zhou X, Zhu C. et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.12.20035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu R, Ling Y, Zhang YH, Wei LY, Chen X, Li XM, Liu XY. et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020 doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye W, Chen G, Li X. et al. Dynamic changes of D-Dimer and Neutrophil-Lymphocyte Count Ratio as prognostic biomarkers in COVID-19. BMC Respiratory Research. 2020 doi: 10.21203/rs.3.rs-21984/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020 doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 23.Sun S, Cai X, Wang H, He G, Lin Y, Lu B, Chen C. et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song C-Y, Xu J, He J-Q, Lu Y-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv. 2020 doi: 10.1101/2020.03.05.20031906. [DOI] [Google Scholar]
- 25.Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, Cao J. et al. A tool to early predict severe corona virus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020 doi: 10.1101/2020.03.17.20037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: A single center's retrospective study. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei YY, Wang RR, Zhang DW, Tu YH, Chen CS, Ji S, Li CX. et al. Risk factors for severe COVID-19: Evidence from 167 hospitalized patients in Anhui, China. J Infect. 2020;81(1):e89–e92. doi: 10.1016/j.jinf.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang N, Xu X, Zhou LY, Chen G, Li Y, Yin H, Sun Z. Clinical characteristics and chest CT imaging features of critically ill COVID-19 patients. Eur Radiol. 2020 doi: 10.1007/s00330-020-06955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, Gao G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, Guo M, Duan L. et al. Short term outcomes and risk factors for mortality in patients with COVID-19 in Wuhan, China: a retrospective study. 2020. Available at SSRN: https://ssrn.com/abstract=3551390.
- 31.Liu J, Tu C, Zhu M. et al. Exploring the law of development and prognostic factors of common and severe COVID-19: a retrospective case-control study in 122 patients with complete course of disease. 2020. Available at SSRN: https://ssrn.com/abstract=3555209.
- 32.Ma Y, Shi N, Fan Y. et al. Predictive value of the neutrophil-to-lymphocyte ratio (NLR) for diagnosis and worse clinical course of the COVID-19: findings from ten provinces in China. 2020. Available at SSRN: https://ssrn.com/abstract=3569838.
- 33.Cheng B, Gui T-M, Huang L. et al. doi: 10.1080/03007995.2020.1825365. Clinical features predicting mortality risk in older patients with COVID-19. 2020. Available at SSRN: https://ssrn.com/abstract=3569846. [DOI] [PubMed]
- 34.Chen X, Zheng F, Qing Y. et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv. 2020 doi: 10.1101/2020.03.03.20030353. [DOI] [Google Scholar]
- 35.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K, Chen W, Zhou Y-S. et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [Google Scholar]
- 40.Han Q, Wen X, Wang L, Han X, Shen Y, Cao J, Peng Q. et al. Role of hematological parameters in the diagnosis of influenza virus infection in patients with respiratory tract infection symptoms. J Clin Lab Anal. 2020;34(5):e23191. doi: 10.1002/jcla.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JH, Song S, Yoon SY, Lim CS, Song JW, Kim HS. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as diagnostic markers for pneumonia severity. Br J Biomed Sci. 2016;73(3):140–142. doi: 10.1080/09674845.2016.1209898. [DOI] [PubMed] [Google Scholar]
- 42.Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Specific Definitions of Non-Severe and Severe Disease Classifications
Subgroup analysis showing NLR in severe versus non-severe disease and non-survivor versus survivor in COVID-19 patients.
Data Availability Statement
The studies supporting this meta-analysis are from previously reported studies and datasets, which have been cited in the references section. The processed data are available from the corresponding author upon request.