Abstract
BACKGROUND & AIMS:
Data on the differences in ethnicity and race among patients with primary biliary cholangitis (PBC) awaiting liver transplantation (LT) are limited. We evaluated liver transplant waitlist trends and outcomes based on ethnicity and race in patients with PBC in the United States.
METHODS:
Using the United Network for Organ Sharing (UNOS) registry, we collected data on patients with PBC on the liver transplant waitlist, and performed analysis with a focus on ethnicity and race-based variations clinical manifestations, waitlist mortality and LT rates from 2000 to 2014. Outcomes were adjusted for demographics, complications of portal hypertension, and Model for End-stage Liver Disease score at time of waitlist registration.
RESULTS:
Although the number of white PBC waitlist registrants and additions decreased from 2000 to 2014, there were no significant changes in the number of Hispanic PBC waitlist registrants and additions each year. The proportion of Hispanic patients with PBC on the liver transplant waitlist increased from 10.7% in 2000 to 19.3% in 2014. Hispanics had the highest percentage of waitlist deaths (20.8%) of any ethnicity or race evaluated. After adjusting for demographic and clinical characteristics, Hispanic patients with PBC had the lowest overall rate for undergoing LT (adjusted hazard ratio, 0.71; 95% CI, 0. 60–0.83; P < .001) and a significantly higher risk of death while on the waitlist, compared to whites (adjusted hazard ratio, 1.41; 95% CI,1.15–1.74; P <.001). Furthermore, Hispanic patients with PBC had the highest proportion of waitlist removals due to clinical deterioration.
CONCLUSIONS:
In an analysis of data from UNOS registry focusing on outcomes, we observed differences in rates of LT and liver transplant waitlist mortality of Hispanic patients compared with white patients with PBC. Further studies are needed to improve our understanding of ethnicity and race-based differences in progression of PBC.
Keywords: OPTN, UNOS, PBC, MELD
Primary biliary cholangitis (PBC), previously referred to as primary biliary cirrhosis, is a rare, cholestatic liver disorder characterized by portal inflammation and immune-mediated destruction of intrahepatic bile ducts.1–3 The hallmark of PBC is the presence of highly specific antimitochondrial antibodies and T-alloreactive cells.4 Classically, PBC is predominantly seen in white women in the fifth and sixth decade of life with a female male prevalence ratio of 10:1.3 The global distribution of PBC is widely varied with a geographic predilection to Northern Europe and North America.5,6 In the United States, the reported prevalence of PBC is 289–402 cases per million people.7,8 Typically, PBC-related histologic damage progresses gradually and most patients outlive their liver disease, whereas a smaller proportion develops cirrhosis and needs liver transplantation (LT).7
Although epidemiologic data are lacking in the nonwhite PBC population, a US multicenter study estimated that Hispanics and African-Americans accounted for 7.9% and 3.9%, respectively, of the US PBC population.9 Demographic characteristics in nonwhite patients with PBC including mean age, gender distribution, and serologic features are comparable with their white counterparts.9 However, the rate of disease progression, clinical manifestations, response to treatment, and outcomes are vastly different. Hispanics are more likely to demonstrate evidence of advanced liver disease at the time of clinical presentation including complications related to portal hypertension.9 In addition, the mainstay treatment for PBC, ursodeoxycholic acid (UDCA), has shown to be less effective in Hispanics and those with a younger age at presentation.10 A relatively weaker response to UCDA is a predictor of progression to advanced liver disease leading to LT or death.7,11,12
Several prognostic models, including the Mayo Model, GLOBE score, and the UK-PBC risk score, have been proposed to help guide the optimal timing for LT in patients with PBC.13 Although LT for PBC has been associated with favorable post-transplant survival outcomes, the number of liver transplant surgeries performed for PBC has declined by approximately 5 cases per year.14,15 In addition to the decline in the rate of liver transplant surgeries for PBC, recent evidence has demonstrated that PBC patients awaiting LT experience higher waitlist mortality compared with waitlisted patients with other etiologies of end-stage liver disease.16,17 Using the Organ Procurement Transplant Network/ United Network for Organ Sharing (OPTN/UNOS) registry, we analyzed ethnicity/race-based temporal trends and outcomes in PBC patients waitlisted for LT.
Methods
Data Source
Our study used data from the OPTN/UNOS registry. The OPTN/UNOS registry includes national data on all liver transplant waitlist registrants and recipients in the United States.
Study Population
We performed a retrospective study analyzing adult (18 years of age or older) PBC liver transplant waitlist registrants cohort from 2000 to 2014. The etiology of chronic liver disease leading to waitlist registration and LT was determined based on primary diagnosis code in OPTN/UNOS registry. PBC waitlist registrants and liver transplant recipients were extracted and analyzed. Our primary objective was to evaluate trends in liver transplant waitlist registrations and outcomes between PBC ethnicity/race-based cohorts. The OPTN/UNOS database categorizes ethnicity/race as (1) white, (2) Hispanic, (3) African-American, and (4) other (including Asian and other ethnic/racial backgrounds). Previous reports have validated the use of ethnicity/race in the OPTN/UNOS database.18 Because of the small sample size and in concordance with previous methodology, temporal trends among PBC liver transplant waitlist registrants were reported as absolute numbers rather than incidence rates.15 Waitlist additions were defined as new or initial waitlist registrations. Overall waitlist registrants were defined as pre-existing registrants awaiting LT and new/initial waitlist additions. We studied the following waitlist outcomes among PBC waitlist registrants: liver transplant surgeries, and waitlist mortality or removal because of death ± clinical deterioration or “too sick” to transplant. Clinical and demographic information at the time of waitlist registration including mean age, gender, mean albumin (mg/dL), mean total bilirubin (mg/dL), mean creatinine, mean international normalized ratio for prothrombin time, presence of diabetes mellitus, hepatocellular carcinoma (HCC) status, mean laboratory Model for End-Stage Liver Disease (MELD) score (excluding cases of HCC) at listing, and portal hypertension-related complications (ascites, hepatic encephalopathy, and spontaneous bacterial peritonitis) were collected. However, the prevalence of other complications associated with portal hypertension including esophageal varices, hepatorenal syndrome, and hepatopulmonary syndrome were not available within the OPTN/UNOS registry. In addition, socioeconomic data, such as insurance type/status and education level, data on living donor liver transplants, mean laboratory MELD score at transplant, and waitlist death, were also collected.
Statistical Analysis
Clinical and demographic characteristics of the study cohort were presented as frequencies and proportions for categorical variables and mean for continuous variables. Continuous variables were compared using either the Student t test or Mann Whitney U test for paired comparisons and analysis of variance or Kruskal-Wallis test for more than 2 group comparisons depending on whether continuous variables were parametric or nonparametric. Cox regression models were constructed to evaluate the association of ethnicity/race with the rate of LT and risk for waitlist mortality from date of waitlist registration until date of waitlist removal including death. Waitlist mortality was modeled with LT as a competing risk.19 Adjustments in our regression models were made for age, gender, diabetes mellitus, body mass index, MELD score at listing, portal hypertension complications, and geographic OPTN/UNOS region of listing to assess waitlist outcomes among white, Hispanic, African-American, and other PBC waitlist registrants. Patients with missing data on waitlist survival follow-up and outcomes were excluded from our regression analysis. All statistical analyses were performed using the SAS statistical package version 9.4 (Cary, NC). Statistical significance was met with a P value <.05.
Results
From 2000 to 2014 there were 156,624 adult patients listed for liver transplant in the OPTN/UNOS national registry. Of those, 5472 (3.5%) patients were waitlisted for a primary diagnosis of PBC. During this time the absolute number of PBC waitlist registrants declined by an average of 24.5 (standard deviation [SD] ± 24.7) registrants per year (P < .001). Similarly, the absolute number of PBC waitlist additions (new waitlist registrations) decreased by 6.8 (SD ± 25.8) registrants per year as depicted in Supplementary Figure 1 .
Differences in Baseline Characteristics by Ethnicity/Race
The clinical characteristics among the ethnic/racial cohorts are demonstrated in Table 1. Overall, PBC wait-list registrants had a mean age of 55.6 (SD ± 9.1) with a higher distribution of females (86.2%) and a mean MELD score at listing of 16.4 (SD ± 8.6). More than half of PBC waitlist registrants presented with hepatic encephalopathy (77.2%) and ascites (55.1%), whereas only 1.3% of registrants had concomitant HCC. Of the 5472 PBC waitlist registrants, whites represented 76.4% (n = 4179), Hispanics 14.5% (n = 793), African-Americans 5.5% (n = 299), and other 3.6% (n = 199). Compared with white registrants, Hispanic and African-American registrants were significantly younger in age; had a higher proportion of females; were noted to have a higher mean MELD score at listing; and were more likely to develop complications related to portal hypertension at the time of listing for LT, including ascites, spontaneous bacterial peritonitis, and hepatic encephalopathy. There was a significant disparity in the distribution of diabetes mellitus in the whites (12.4%), Hispanics (15.9), and African-Americans (22.1%) (P < .001) (Table 1). Compared with whites, African-Americans and Hispanics were less likely to have private insurance and Hispanics more likely to have Medicaid insurance (Supplementary Table 1). Hispanics had the highest percentage without a high school degree or general equivalency diploma and the lowest percentage with a college or post-graduate education.
Table 1.
Comparison of Baseline Demographic and Characteristic Data Among PBC Waitlist Registrants by Ethnicity/Race,OPTN/UNOS 2000–2014
| All patients n = 5472 |
White n = 4179 |
Hispanic n = 793 |
African-American n = 299 |
Other n = 201 |
P value |
|
|---|---|---|---|---|---|---|
| Age (mean, SD) | 55.6 (9.1) | 56.2 (9.2) | 53.4 (9.5) | 52.59 (9.6) | 54.62 (9.2) | <.001 |
| Gender, n (%) | <.001 | |||||
| Female | 4714 (86.2) | 3559 (85.2) | 724 (91.3) | 262 (87.6) | 169 (84.1) | |
| Male | 758 (13.8) | 620 (14.8) | 69 (8.7) | 37 (12.4) | 32 (15.9) | |
| BMI (mean, SD) | 26.7 (5.4) | 26.8 (5.4) | 26.1 (5.0) | 27.8 (5.8) | 24.0 (4.7) | .126 |
| Albumin (mean, SD) | 3.00 (0.6) | 3.00 (0.6) | 2.9 (0.6) | 2.77 (0.7) | 2.92 (0.6) | <.001 |
| Total bilirubin (mean, SD) | 6.5 (5.6) | 5.88 (7.0) | 7.2 (8.7) | 10.12 (9.6) | 8.94 (10.0) | <.001 |
| Creatinine (mean, SD) | 1.1 (0.8) | 1.06 (0.7) | 1.0 (0.8) | 1.32 (1.3) | 1.06 (0.8) | <.001 |
| INR (mean, SD) | 1.40 (0.5) | 1.35 (0.4) | 1.40 (0.5) | 1.58 (0.8) | 1.41 (0.5) | <.001 |
| Diabetes, n (%) | 748 (13.7) | 520 (12.4) | 126 (15.9) | 66 (22.1) | 36 (17.9) | <.001 |
| HCC, n (%) | 71 (1.3) | 53 (1.2) | 12(1.5) | 3(1.0) | 3(1.5) | .902 |
| Laboratory MELD score at listing (mean, SD) | 16.4 (8.6) | 15.9 (8.8) | 16.8 (7.1) | 20.2 (8.0) | 16.1 (8.2) | <.001 |
| Portal hypertension complications, n (%) | ||||||
| Ascites | 3014 (55.1) | 2251 (53.9) | 465 (58.6) | 185 (61.9) | 113 (56.2) | .007 |
| SBP | 200 (3.6) | 140 (3.4) | 49 (6.2) | 5(1.7) | 6 | .002 |
| HE | 4227 (77.2) | 1164 (27.9) | 233 (29.4) | 107 (35.8) | 57 (28.4) | .030 |
| Living donor transplant, n (%) | 270 (9.9) | 233 (11.0) | 30 (8.3) | 7 (3.7) | 0 | <.001 |
| Liver transplant, n (%) | 2726 (49.8) | 2093 (50.1) | 353 (44.5) | 186 (62.2) | 94 (46.8) | <.001 |
| MELD score at transplant (mean, SD)a | 22.3 (9.3) | 21.3 (9.1) | 25.8 (10.3) | 24.9 (9.1) | 25.4 (10.4) | <.001 |
| Waitlist time (d) to transplant (median, SD) | 176 (554) | 179 (586) | 180 (472) | 98 (456) | 170 (597) | .004 |
| Waitlist removal because of death, n (%) | 994 (18.2) | 752 (18.0) | 165 (20.8) | 39 (13.0) | 38 (18.9) | <.001 |
| Waitlist time (d) to death (median, SD) | 465 (915) | 473 (1073) | 308 (892) | 613 (909) | 237 (710) | .001 |
| MELD score at death (mean, SD)b | 25.30 (11.6) | 24.8 (11.5) | 28.1 (12.5) | 30.5 (11.0) | 29.1 (10.8) | <.001 |
| Waitlist removal because of clinical deterioration, n (%) | 430 (7.8) | 313 (7.5) | 80 (10.0) | 21 (7.0) | 16 (7.9) | <.001 |
BMI, body mass index; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; INR, international normalized ratio for prothrombin time; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; OPTN/UNOS, Organ Procurement Transplant Network/United Network for Organ Sharing; PBC, primary biliary cholangitis; SBP, spontaneous bacterial peritonitis; SD, standard deviation.
Trends in Primary Biliary Cholangitis Waitlist Registrants and Waitlist Additions
During our 15-year study period, the white proportion of PBC registrants demonstrated an overall 14.5% decline from 84.8% in 2000 to 72.5% in 2014. The absolute number of white PBC waitlist registrants declined 41.8% from 906 candidates in 2000 to 527 candidates in 2014 with an average declining rate of 27.1 (SD ± 20.9) registrants per year (P < .001). Likewise, the number of white PBC waitlist additions decreased by 8.4 (SD ± 24.8) registrants per year (P < .001) as shown in Figure 1. Although there were no significant changes in the gender proportion among white PBC registrants, the absolute number of white female PBC registrants declined by 24.3 (SD ± 17.7) registrants annually with an overall decline of 43.4%. Similarly, the absolute number of white male PBC registrants declined by 2.7 (SD ± 8.6) registrants per year (Supplementary Figure 2). The declining trend in the number of white PBC registrants contributed to a demographic shift toward an increasing representation of Hispanic PBC registrants. Although the number of white PBC registrants and additions declined during the study period, there were no significant changes in the annual number of Hispanic PBC waitlist registrants or additions (Figure 1). Subsequently, this led to the Hispanic proportion of PBC registrants increasing 80.4% overall from 10.7% in 2000 to 19.3% in 2014 shown in Figure 1C. The annual number of African-American PBC waitlist additions (18.1 waitlist additions per year; SD ± 4.7) remained stable during this study period.
Figure 1.
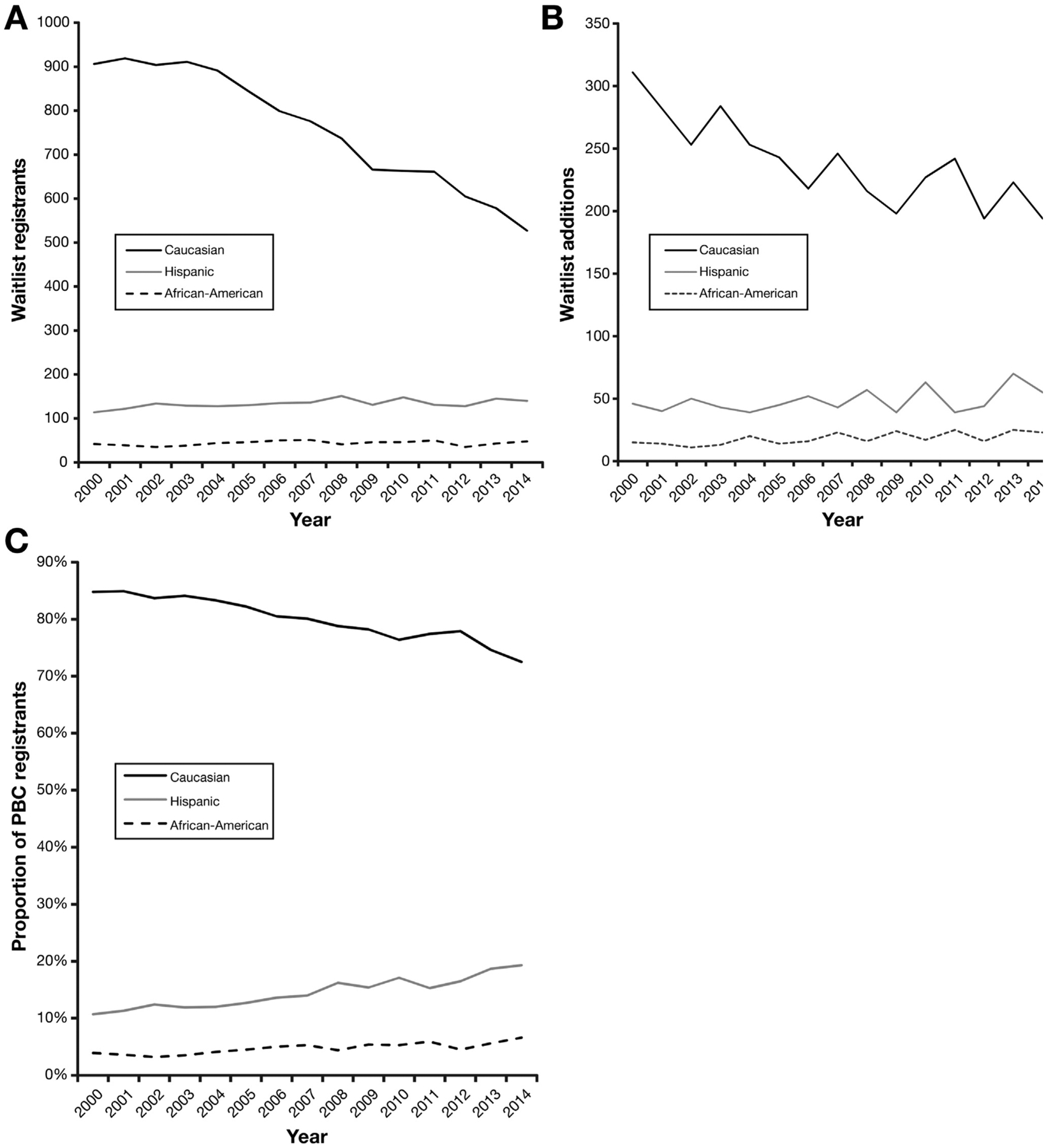
PBC liver transplant waitlist registrants and additions by ethnicity/race within the United States from 2000 to 2014. (A) Annual number of PBC liver transplant waitlist registrants by ethnicity/race. (B) Annual number of PBC liver transplant waitlist additions by ethnicity/race. (C) Annual trend in proportion of PBC liver transplant waitlist registrants by ethnicity/race.
Liver Transplantation Trends
From 2000 to 2014 there were 87,084 liver transplant recipients in the OPTN/UNOS registry. Of those, 2726 (3.1%) liver transplants were indicated for PBC. Nearly half (49.2%) of all PBC registrants during our study period underwent LT. Although the annual number of overall liver transplants for non-PBC indications increased by an average of 133 (SD ± 203.9) cases per year, the number of PBC-related liver transplants declined by 3.7 (SD ± 18.9) cases per year (Figure 2A). However, this annual decline in liver transplants was reflective of the annual reduction in the absolute number in PBC waitlist registrants. The annual percentage of PBC registrants undergoing LT was 18.9% (SD ± 1.4%) and remained relatively constant throughout the years (Figure 2B).
Figure 2.
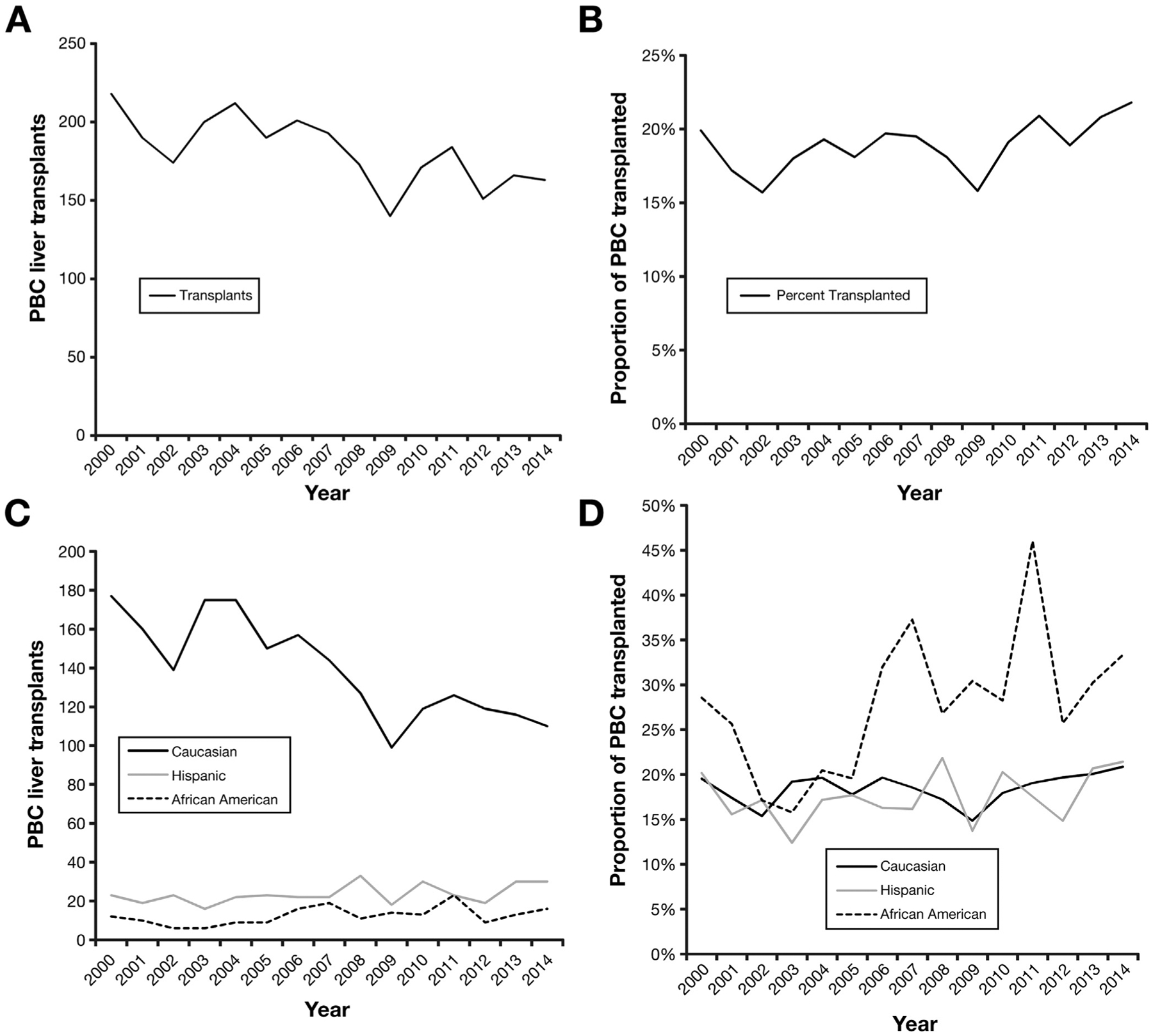
Annual trends in PBC-related liver transplants performed within the United States from 2000 to 2014. (A) Number of PBC-related liver transplants performed. (B) Proportion of PBC waitlist registrants undergoing liver transplantation. (C) Number of PBC-related liver transplants performed by ethnicity/race. (D) Proportion of PBC waitlist registrants undergoing liver transplantation by ethnicity/race.
Liver Transplantation by Ethnicity/Race
Among the ethnic/racial cohorts, whites had the highest absolute number of liver transplants (n = 2093). Overall, African-American (62.2%) had the highest percentage of PBC waitlist registrants undergoing LT, whereas Hispanics (44.5%) had the lowest percentage (P < .001) as seen in Table 1. The number of liver transplants performed for white PBC waitlist registrants declined from 177 in 2000 to 110 in 2014 (Figure 2C). The number of liver transplants performed for white PBC waitlist registrants declined by 4.8 (SD ± 7.9) cases annually. Because of the declining annual number of white PBC waitlist registrants, the annual percentage of white PBC registrants undergoing LT did not demonstrate any significant change with an annual average of 18.5% (SD ± 1.7%) of white PBC registrants undergoing LT (Figure 2D). The annual number of liver transplants performed and the annual percentage of PBC waitlist registrants undergoing LT among Hispanic and African-American PBC waitlist registrants did not demonstrate any notable trends. Moreover, after adjusting for demographics, complications of portal hypertension, MELD score at LT, and OPTN/UNOS region of listing, Hispanic PBC registrants still had the lowest overall rate of LT (adjusted hazard ratio, 0.71; 95% confidence interval, 0.60–0.83; P < .001) as demonstrated in Table 2. Mean MELD score at transplant was also higher among Hispanic PBC liver transplant recipients (25.8; SD ± 10.3) compared with all other ethnicities.
Table 2.
Multivariate Cox Regression Analysis Assessing Predictors for Overall Rate of Undergoing LT From Waitlist Registration Among PBC Waitlist Registrants, OPTN/UNOS 2000–2014
| Multivariate HRa |
95% CI |
P value |
|
|---|---|---|---|
| Initial age | 1.00 | 1.00–1.01 | .12 |
| Gender | |||
| Female | Reference | ||
| Male | 1.15 | 1.00–1.32 | .06 |
| Ethnicity | |||
| White | Reference | ||
| Black | 1.03 | 0.85–1.26 | .75 |
| Hispanic | 0.71 | 0.60–0.83 | <.001 |
| Asian | 0.76 | 0.63–1.08 | .13 |
| Other | 0.68 | 0.44–1.05 | .08 |
| Body mass index | 0.99 | 0.98–0.99 | .02 |
| Diabetes | 0.91 | 0.78–1.06 | .22 |
| Initial MELD score | 1.15 | 1.14–1.16 | <.001 |
| Ascites | 1.35 | 1.18–1.52 | .01 |
| Hepatic encephalopathy | |||
| None | Reference | ||
| Moderate | 1.13 | 1.01 −1.26 | .03 |
| Severe | 1.18 | 0.99–1.56 | .23 |
| Portal venous thrombosis | 0.90 | 0.68–1.19 | .45 |
| Spontaneous bacterial | 0.99 | 0.77–1.27 | .93 |
| peritonitis |
CI, confidence interval; HR, hazard ratio; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; OPTN/UNOS, Organ Procurement Transplant Network/United Network for Organ Sharing; PBC, primary biliary cholangitis.
Additionally adjusted for OPTN/UNOS region of listing.
From 2000 to 2014, a total of 34,311 registrants died awaiting LT in the United States. Of those deaths, PBC waitlist registrants accounted for 2.8% (n = 994) of all waitlist deaths. Within the PBC cohort, 18.2% (SD ± 8.2%) of PBC registrants died awaiting LT. The average annual number of PBC waitlist deaths was 66.3 (SD ± 6.4). This correlated to 6.9% (SD ± 1.1%) of PBC waitlist registrants dying each year. Trends in the annual number of PBC waitlist deaths are depicted in Supplementary Figure 3A.
Waitlist Mortality by Ethnicity/Race
Hispanic PBC waitlist registrants had the highest percentage (n = 165; 20.8%) of waitlist deaths compared with other ethnicity/race-based PBC waitlist registrant subcohorts. Trends in annual number of waitlist deaths among PBC registrants by ethnicity/race are shown in Supplementary Figure 3B. In addition, a higher percentage of Hispanic PBC waitlist registrants (8.3% ± 2.3%) died each year than white PBC waitlist registrants (6.7% ± 1.3%). Mean MELD score at death was highest among African-American PBC waitlist registrants and lowest among white PBC registrants seen in Table 1.
In our competing risks analysis taking into account rate of LT, cumulative PBC waitlist mortality was significantly higher (P < .001) among Hispanics compared with whites (Figure 3). Although there was no significant difference (P > .05) in the 90-day and 1-year waitlist mortality rate among the ethnic/racial cohorts, Hispanic PBC registrants had a 41% higher overall risk of waitlist mortality compared with whites (adjusted hazard ratio, 1.41; 95% confidence interval, 1.15–1.74; P < .001) after adjusting for demographics, portal hypertension complications, MELD score at listing, and OPTN/UNOS region of listing as demonstrated in Table 3. When incorporating registrant insurance status and education level into this model, Hispanic registrants still had a higher overall risk for waitlist mortality than white registrants (adjusted hazard ratio, 1.39; 95% confidence interval, 1.11–1.78; P < .001). Similarly, because of the small percentage of PBC waitlist registrants with concomitant HCC (1.3%) and MELD exceptions (6.8%), waitlist outcomes were also not impacted.
Figure 3.
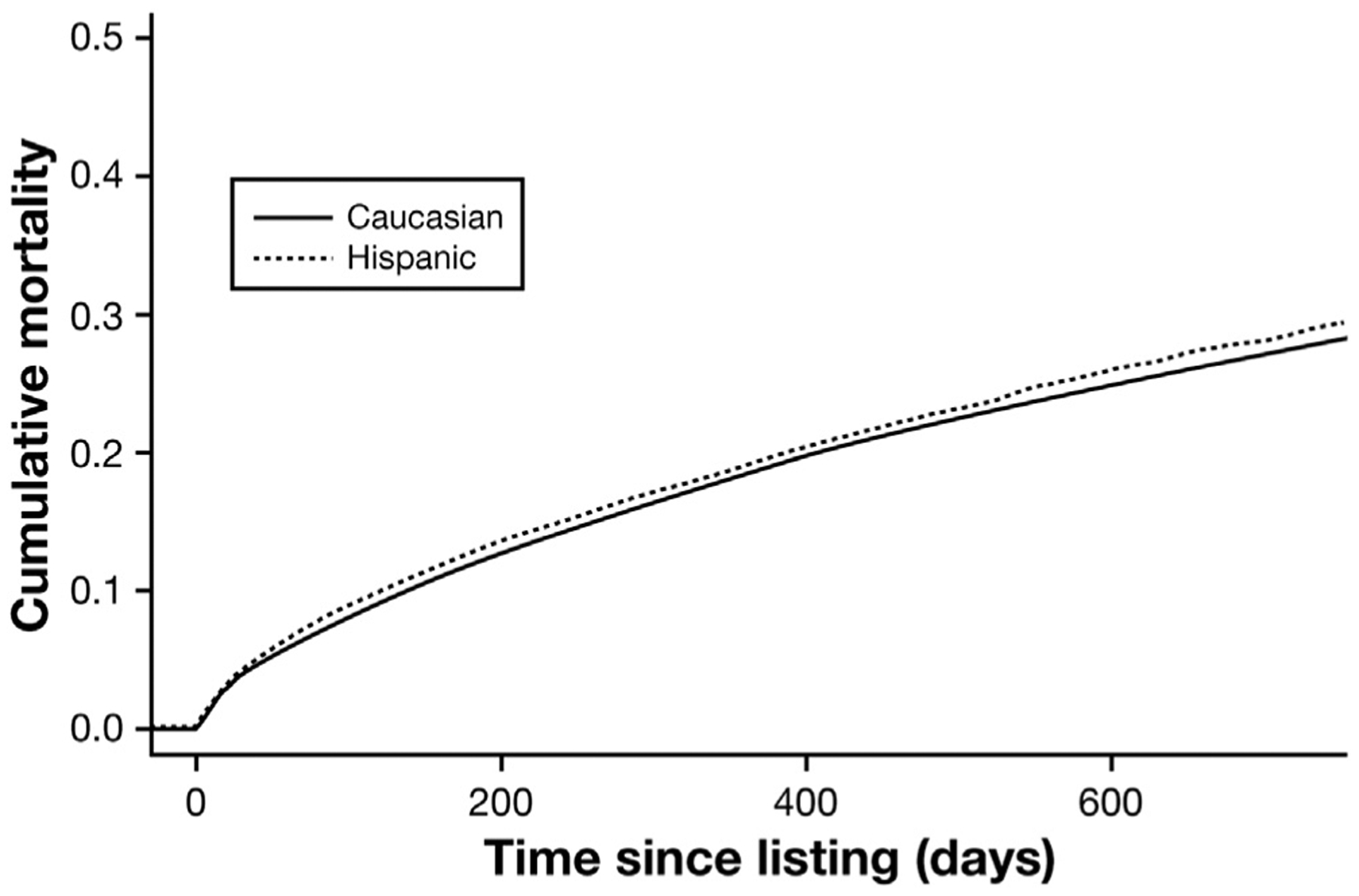
Cumulative incidence curve assessing risk of waitlist mortality among Hispanic and white PBC registrants (P < .001).
Table 3.
Multivariate Cox Regression Analysis Assessing Predictors for Overall Risk of Waitlist Mortality From Waitlist Registration Among PBC Waitlist Registrants, OPTN/UNOS 2000–2014
| Multivariate HRa | 95% CI |
P
value |
|
|---|---|---|---|
| Initial age | 1.04 | 1.03–1.04 | .04 |
| Gender | |||
| Female | Reference | ||
| Male | 0.74 | 0.58–0.95 | .02 |
| Ethnicity | |||
| White | Reference | ||
| Black | 0.69 | 0.46–1.04 | .07 |
| Hispanic | 1.41 | 1.15–1.74 | <.001 |
| Asian | 0.62 | 0.32–1.20 | .16 |
| Other | 1.49 | 0.84–2.63 | .17 |
| Body mass index | 1.00 | 0.99–1.02 | .73 |
| Diabetes | 1.08 | 0.87–1.35 | .49 |
| Initial MELD score | 1.02 | 1.01–1.04 | <.001 |
| Ascites | 1.10 | 1.01–1.18 | .22 |
| Hepatic encephalopathy | |||
| None | Reference | ||
| Moderate | 1.09 | 0.93–1.29 | .30 |
| Severe | 1.49 | 1.05–2.42 | .04 |
| Portal venous thrombosis | 0.82 | .32 | |
| Spontaneous bacterial | 1.13 | 0.77–1.02 | .54 |
| peritonitis |
CI, confidence interval; HR, hazard ratio; MELD, Model for End-Stage Liver Disease; OPTN/UNOS, Organ Procurement Transplant Network/United Network for Organ Sharing; PBC, primary biliary cholangitis.
Additionally adjusted for OPTN/UNOS Region of listing.
Discussion
The prevalence of PBC varies considerably in different geographic areas.20 Because PBC is relatively uncommon, few large cohort studies have evaluated epidemiologic data within the United States.2,9 Our study used a large cohort sampled from national transplant registry and we are able to make several important observations. First, we noted a shift in the ethnic/racial demographic representation of PBC patients listed for LT in the United States. There was a notable decline in white PBC waitlist registrants, largely contributing to a steady decline in PBC patients awaiting LT. The overall number of Hispanic PBC waitlist registrants remained stable and may reflect a steady rise in the US Hispanic population, which now constitutes more than 17% of the US population.21 The cumulative effect of this decline in whites and stability in Hispanics led to a significant rise in the proportion of Hispanics on the PBC waitlist for LT. We also demonstrated that nonwhites, African-Americans, and Hispanics demonstrated higher waitlist mortality compared with whites. Moreover, Hispanics were also shown to be disadvantaged with the lowest rate for undergoing LT.
A large US multicenter study noted a higher severity of liver disease at clinical presentation among nonwhites than whites with PBC, which could not be explained by demographic or serologic features alone.9 Hispanics are disproportionately affected by both nonalcoholic fatty liver disease and type 2 diabetes mellitus as compared with non-Hispanic black persons.22 In this current study, we noted a higher proportion of diabetes mellitus in Hispanics and also in African-Americans, which could potentially reflect the additive detrimental effect associated with increased prevalence of nonalcoholic steatohepatitis in these population with PBC.23,24 The finding of higher prevalence of diabetes mellitus in African-Americans with PBC is unexplained and needs further investigation in the future.
We were unable to assess if white patients with PBC had better access to care and thus earlier referral. Before the Affordable Care Act went into effect, severe ethnical/racial disparities were present, and 14.8% of whites,25.8% of African-Americans, and 40.5% of Hispanics were uninsured.25 African-Americans and Hispanics with PBC were less likely to have private insurance, and Hispanics less likely to have at least a high school or equivalent level of education.26 Education level and health insurance status suggestive of lower socioeconomic status have shown to negatively impact referral rates for LT and represents a major barrier in linkage to care and access to LT.
We were unable to analyze if the decline in PBC patients awaiting LT was possibly contributed by the universal use of the UDCA treatment.27–29 Evidence suggests that UDCA delays histologic progression of PBC and decreases the risk of development of esophageal varices; moreover, survival of UDCA-treated patients is better than that of untreated patients.30 In a cross-sectional study, patients of Hispanic ethnicity with PBC had an increased prevalence of overlap syndrome, reduced response to UDCA treatment, and more frequent complications of portal hypertension than non-Hispanic patients.10 In addition, genetic susceptibility for early onset PBC has been reported,31 and coexisting impact of metabolic syndrome.32 Approximately 30% of patients with PBC may have metabolic syndrome, which is consequently correlated with a higher risk of cardiovascular events.33
Finally, our analysis revealed that patients with PBC are less likely to receive MELD exceptions for HCC or other liver-related complications. Complications of portal hypertension can occur even without cirrhosis in PBC patients, placing them at disadvantage with the MELD score, criteria for listing. Therefore, Purgatory MELD for PBC is created as a higher proportion of PBC patients succumb to complications of chronic liver disease or are too sick for LT before they reach the mean MELD for LT in their respective OPTN/UNOS region.
This current study also has several limitations. Ethnicity and race are self-reported data on UNOS transplant collection forms and are subject to response bias. UNOS does not categorize Hispanic patients beyond Hispanic or Latino ethnicity. Improving collection and classification methods can help better understand these disparities. We were unable to determine the details of UDCA therapy in PBC waitlist registrants. In addition, follow-up time began at the date of waitlist registrations and therefore the natural progression of PBC in our population and ethnic/racial cohorts could not be ascertained because of lead-time bias. Our study population is not a representation of the total PBC prevalence in the United States. Because of insufficient data after June 2013, we were unable evaluate the impact of Regional Share 35 on outcomes. Finally, prognostic models, histologic staging, and data on PBC variants, such as overlap syndromes, were not available in the OPTN/UNOS data further limiting our analysis.
Despite these limitations, we have made several important observations. Most importantly, using a large US population registry, we were able to show a declining trend in white PBC waitlist registrants and additions, and growing Hispanic PBC registrants with poor waitlist outcomes, including high waitlist mortality and lower rate of undergoing LT compounded by higher severity of disease at presentation, and lower socioeconomic status. With recent data suggesting PBC waitlist mortality to be higher than other etiologies for chronic liver disease,17 focused studies and efforts to better understand the at-risk PBC subcohorts, such as Hispanics and African-Americans who may be disadvantaged for LT, may help improve overall outcomes in the future.
Supplementary Material
Acknowledgments
The authors acknowledge the United Network for Organ Sharing, a nonprofit organization that administrates the Organ Procurement and Transplantation Network, for providing them with a custom database from which the data were collected. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the United Network for Organ Sharing/Organ Procurement and Transplantation Network or the US Government.
Abbreviations used in this paper:
- HCC
hepatocellular carcinoma
- LT
liver transplantation
- MELD
Model for End-Stag-e Liver Disease
- OPTN/UNOS
Organ Procurement Transplant Network/United Network for Organ Sharing
- PBC
primary biliary cholangitis
- SD
standard deviation
- UDCA
ursodeoxycholic acid
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2017.12.017.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Beuers U, Gershwin ME, Gish RG, et al. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. Am J Gastroenterol 2015;110:1536–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershwin ME, Selmi C, Worman HJ, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology 2005; 42:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005;353:1261–1273. [DOI] [PubMed] [Google Scholar]
- 4.Selmi C, Bowlus CL, Gershwin ME, et al. Primary biliary cirrhosis. Lancet 2011;377:1600–1609. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf JV, Mitchison HC, Palmer JM, et al. Natural history of early primary biliary cirrhosis. Lancet 1996;348:1399–13402. [DOI] [PubMed] [Google Scholar]
- 6.Prince MI, Chetwynd A, Diggle P, et al. The geographical distribution of primary biliary cirrhosis in a well-defined cohort. Hepatology 2001;34:1083–1088. [DOI] [PubMed] [Google Scholar]
- 7.Kim WR, Lindor KD, Locke GR 3rd, et al. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology 2000;119:1631–1616. [DOI] [PubMed] [Google Scholar]
- 8.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol 2012;56:1181–1188. [DOI] [PubMed] [Google Scholar]
- 9.Peters MG, BisceglieAM Di, Kowdley KV, et al. Differences between caucasian, African American, and Hispanic patients with primary biliary cirrhosis in the United States. Hepatology 2007;46:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy C, Naik J, Giordano C, et al. Hispanics with primary biliary cirrhosis are more likely to have features of autoimmune hepatitis and reduced response to ursodeoxycholic acid than non-Hispanics. Clin Gastroenterol Hepatol 2014;12:1398–1405. [DOI] [PubMed] [Google Scholar]
- 11.Momah N, Silveira MG, Jorgensen R, et al. Optimizing biochemical markers as endpoints for clinical trials in primary biliary cirrhosis. Liver Int 2012;32:790–795. [DOI] [PubMed] [Google Scholar]
- 12.Lammers WJ, Kowdley KV, van Buuren HR. Predicting outcome in primary biliary cirrhosis. Ann Hepatol 2014;13:316–326. [PubMed] [Google Scholar]
- 13.Chen S, Duan W, You H, et al. A brief review on prognostic models of primary biliary cholangitis. Hepatol Int 2017; 11:412–418. [DOI] [PubMed] [Google Scholar]
- 14.Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59:1144–1165. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Belanger A, Doucette JT, et al. Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol 2007; 5:1313–1315. [DOI] [PubMed] [Google Scholar]
- 16.Genda T, Ichida T, Sakisaka S, et al. Waiting list mortality of patients with primary biliary cirrhosis in the Japanese transplant allocation system. J Gastroenterol 2014;49:324–331. [DOI] [PubMed] [Google Scholar]
- 17.Singal AK, Fang X, Kaif M, et al. Primary biliary cirrhosis has high wait-list mortality among patients listed for liver transplantation. Transpl Int 2017;30:454–462. [DOI] [PubMed] [Google Scholar]
- 18.Mathur AK, Sonnenday CJ, Merion RM. Race and ethnicity in access to and outcomes of liver transplantation: a critical literature review. Am J Transplant 2009;9:2662–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology 2006;43:345–351. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths L, Dyson JK, Jones DE. The new epidemiology of primary biliary cirrhosis. Semin Liver Dis 2014;34:318–328. [DOI] [PubMed] [Google Scholar]
- 21.United States Census Bureau. 2014. US Population Estimates. 2017 (updated February 9, 2016). Available at: https://www.census.gov/newsroom/facts-for-features/2015/cb15-ff18.html.Accessed April 23, 2017. [Google Scholar]
- 22.Lazo M, Bilal U, Perez-Escamilla R. Epidemiology of NAFLD and type 2 diabetes: health disparities among persons of Hispanic origin. Curr Diab Rep 2015;15:116. [DOI] [PubMed] [Google Scholar]
- 23.Kalia HS, Gaglio PJ. The prevalence and pathobiology of nonalcoholic fatty liver disease in patients of different races or ethnicities. Clin Liver Dis 2016;20:215–224. [DOI] [PubMed] [Google Scholar]
- 24.Gill C, Vatcheva KP, Pan JJ, et al. Frequency of nonalcoholic fatty liver disease and subclinical atherosclerosis among young Mexican Americans. Am J Cardiol 2017;119:1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchmueller TC, Levinson ZM, Levy HG, et al. Effect of the Affordable Care Act on racial and ethnic disparities in health insurance coverage. Am J Public Health 2016;106:1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemmer N Ethnic disparities in liver transplantation. Gastroenterol Hepatol 2011;7:302–307. [PMC free article] [PubMed] [Google Scholar]
- 27.Corpechot C, Carrat F, Bahr A, et al. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology 2005;128:297–303. [DOI] [PubMed] [Google Scholar]
- 28.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 2006;130:715–720. [DOI] [PubMed] [Google Scholar]
- 29.ter Borg PC, Schalm SW, Hansen BE, et al. Prognosis of ursodeoxycholic acid-treated patients with primary biliary cirrhosis. Results of a 10-yr cohort study involving 297 patients. Am J Gastroenterol 2006;101:2044–2050. [DOI] [PubMed] [Google Scholar]
- 30.Lee YM, Kaplan MM. The natural history of PBC: has it changed? Semin Liver Dis 2005;25:321–326. [DOI] [PubMed] [Google Scholar]
- 31.Almasio PL, Licata A, Maida M, et al. Clinical course and genetic susceptibility of primary biliary cirrhosis: analysis of a prospective cohort. Hepat Mon 2016;16:e31681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassallo P, Driver SL, Stone NJ. Metabolic syndrome: an evolving clinical construct. Prog Cardiovasc Dis 2016; 59:172–177. [DOI] [PubMed] [Google Scholar]
- 33.Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 2015; 149:1804–1812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


