Abstract
The glymphatic concept along with the discovery of meningeal lymphatic vessels have in recent years highlighted that fluid is directionally transported within the central nervous system. Imaging studies, as well as manipulations of fluid transport, point to a key role of the glymphatic-lymphatic system in clearance of amyloid-β and other proteins. As such, the glymphatic-lymphatic system represents a new target in combating neurodegenerative diseases. Not unexpectedly, introduction of a new plumbing system in the brain has stirred controversies. This review will highlight what we know about the brain’s fluid transport systems, where experimental data are lacking, and what is still debated.
Keywords: glymphatic system, cerebrospinal fluid, aquaporin-4, brain clearance, perivascular spaces, meningeal lymphatics, amyloid-beta
The interconnected glymphatic-lymphatic fluid transport system
The recent discoveries of the brain’s glymphatic system (see Glossary) and the meningeal lymphatic vessels have stirred considerable debate [1–3]. An obvious question is why the existence of a brain-wide fluid transport system and the presence of lymphatic vessels in the meningeal layers remained under the radar until now? Before addressing this question, we will here discuss several of the most controversial points. The debates reflect, in part, the fact that critical sets of data have yet to be collected. Other points of controversy are based on debatable assumptions and misunderstandings of the fluid dynamics within the brain. In particular, it is important to stress that the analysis of tracer distribution in histological sections is prone to artifacts. Cardiac arrest or stroke abruptly initiate a pathological influx of CSF [4, 5]. As a result, the microscopic localization of CSF tracers in post-mortem tissue will inevitably reflect, in part, CSF movements that occurred after death. We will here outline recent developments and add our points to the ongoing debate.
The glymphatic system consists of peri-arterial CSF inflow running in the same direction as blood flow, propelled by the pulsatility of the arterial wall [6, 7]. From here, CSF mixes with interstitial fluid in a process facilitated by aquaporin-4 (AQP4) water channels densely present at the vascular astrocytic endfeet [8]. Influx of fluid across the blood-brain barrier (BBB) or extrachoroidal sources of CSF may also contribute to glymphatic flow [9–12]. The mixture of CSF and interstitial fluid leaves the brain via the peri-venous space and along cranial and spinal nerves. This fluid is eventually transported out of the CNS by traditional lymphatic vessels located in meninges and in the soft tissue surrounding the skull.
Meningeal lymphatic vessels were first described in 2015, and represent a major efflux route for CSF [2, 3]. The meningeal lymphatic vessels are especially well-developed around the venous sinuses and at the base of the skull [13, 14]. Drainage by the meningeal and cervical lymphatic vessels is relatively fast, since tracers injected into brain or CSF accumulate in the cervical lymph nodes within minutes [15]. Our current model of the glymphatic-lymphatic fluid transport is depicted in Fig. 1. The model is based on analyses of how tracers distribute after they are delivery into either cisterna magna or directly into brain. The model is supported by data collected using multiple complementary methodologies, including optical and magnetic resonance imaging, and dispersion of radiolabeled tracers [16, 17]. However, it is important to note that several of the processes that guide the highly polarized fluid transport system still need to be defined. In particular, the mechanisms by which AQP4 supports transport of extracellular tracers and the interlinkage of interstitial fluid clearance with meningeal and cervical lymphatic vessels are poorly understood.
Fig. 1. Schematic model of the glymphatic-lymphatic system and its regulation by the sleep-wake cycle.
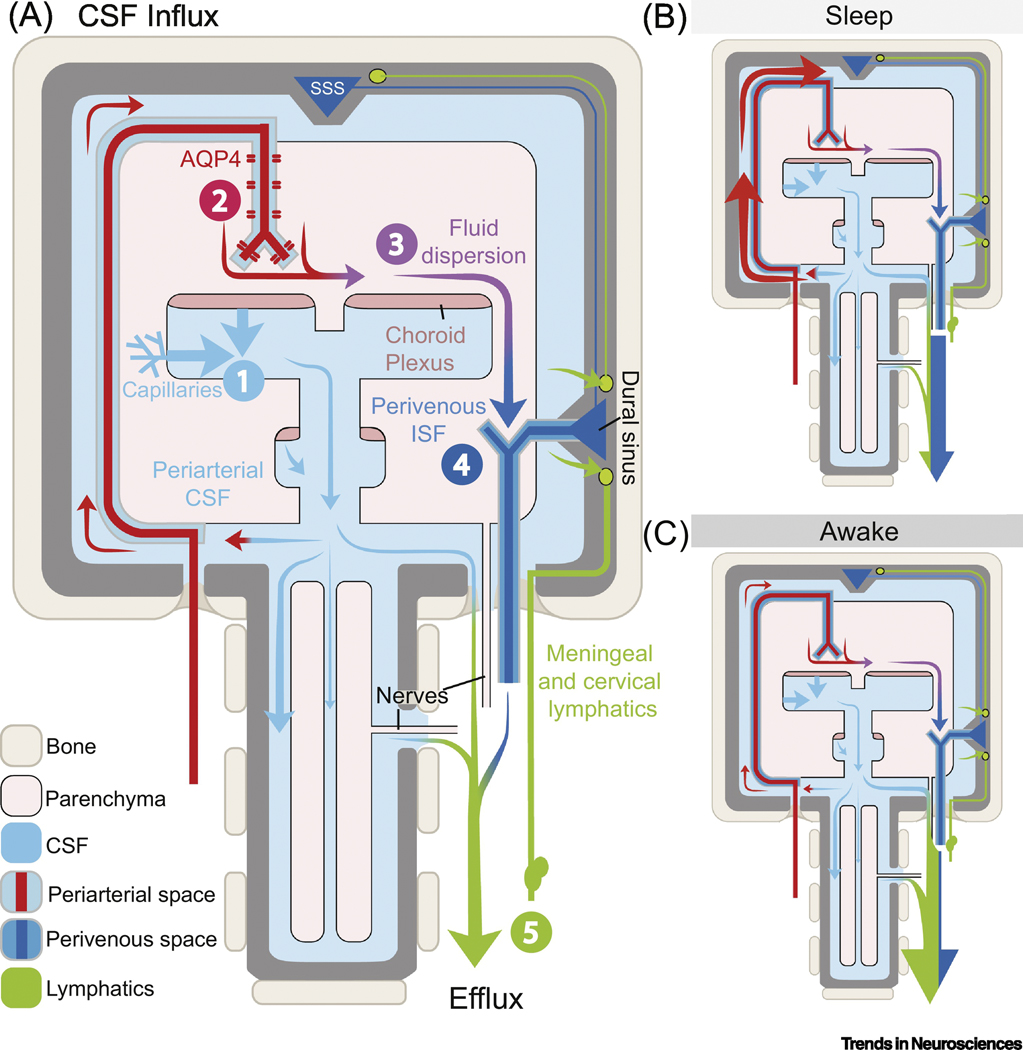
(A) The fluid transport pathway is divided into 5 distinct segments: (1) cerebrospinal fluid (CSF) is produced by the choroid plexus and likely by extrachoroidal sources (capillary influx and metabolic water production); (2) arterial wall pulsatility drives CSF deep into brain along perivascular spaces; (3) CSF enters the brain parenchyma supported by AQP4 water channels and disperses within the neuropil; (4) interstitial fluid (ISF) mixes with CSF and accumulates in the peri-venous space and drains from here out of the brain via (5) meningeal and cervical lymphatic vessels, as well as along cranial and spinal nerves. The two models on the right, map the dominant paths of CSF flow during different arousal states: during sleep (B), CSF enters the brain via glymphatic transport, but during wakefulness (C) it is mostly excluded and shunted out via lymphatic vessels [4, 43]. The exact fraction of CSF entering brain during sleep is not known, but MRI studies suggest that ~20% of contrast agents injected into cisterna magna are taken up by the brain in anesthetized rats [62]. Recent evidence collected in awake rats using contrast-enhanced MRI shows that the glymphatic system is also under circadian control [63]. According to an older, but debated literature, CSF can exit via arachnoid granulations not included in this model [64, 65]. AQP4: Aquaporin-4 channels; CSF: cerebrospinal fluid; ISF: interstitial fluid; SSS: superior sagittal sinus.
We will here discuss a number of issues that have sparked debate: (1) the loss of the fluid-filled perivascular space in histological sections, (2) the direction of flow along the periarterial space, (3) role of AQP4 water channels, (4) parenchymal convective flow, (5) intracranial pressure (ICP) changes induced by cisternal tracer injections, and (6) diurnal changes in amyloid-β and tau concentration.
Perivascular spaces are largely absent in histological sections
In vivo imaging studies in mice have recently documented that pial arteries and penetrating arterioles are surrounded by large peri-arterial spaces [18–20]. In fact, the cross-sectional area of the perivascular space often exceeds that of the pial arteries. The existence of large peri-arterial spaces was not acknowledged prior to high resolution in vivo imaging, since these spaces are absent in fixed tissue. What happens during fixation? Two-photon imaging shows that the peri-arterial spaces are lost when the animal dies. As the perivascular space shrinks and eventually disappears, CSF tracers are displaced into the surrounding smooth muscle layer and the basal lamina [7] (Fig. 2). These observations directly parallel those in relation to the fluid-filled interstitium in peripheral human tissues. The discovery of the latter was made based on routine endoscopy, which showed that fluorescein fills large fluid-filled spaces - the interstitium - but is displaced into surrounding collagen fibers when examining the same tissue ex vivo after biopsy [21]. The important broader implication of this finding is that tracer distribution in histological sections reflects displacement of the tracer after death. Accordingly, postmortem tissue should not be used to generate models of brain fluid transport. An example of such is the intramural periarterial drainage (IPAD) model. The IPAD concept argues that the accumulation of tracers in the smooth muscle cell layer and in the basement membrane in histological sections suggests that these layers can act as an efflux pathway for interstitial fluid. Two in vivo studies have provided supporting evidence for the IPAD model based on in vivo delivery of tracers next to penetrating arterioles [22, 23]. Unfortunately, one of the studies infused a large a volume of fluid (3μl) while the other used laser-induced opening of the walls of the arterioles. We will argue that the perivascular spaces are the only available space that can quickly accommodate inflow of large volumes of fluid, and that creating a lesion of the vascular wall cannot be used to study physiological fluid transport, as it impacts pressure gradients along the fluid system, regardless of its specifics. These caveats are especially notable in the aforementioned studies because no information was provided with regard to possible pathological effects of the invasive procedures. Nevertheless, it will be interesting to use particle tracking to establish whether chronic abnormalities, such as vascular amyloidosis or arteriosclerosis disturb the normally orderly CSF influx pattern documented under physiological conditions [7, 24].
Fig. 2. Diagram illustrating postmortem relocation of periarterial cerebrospinal fluid (CSF) tracers.
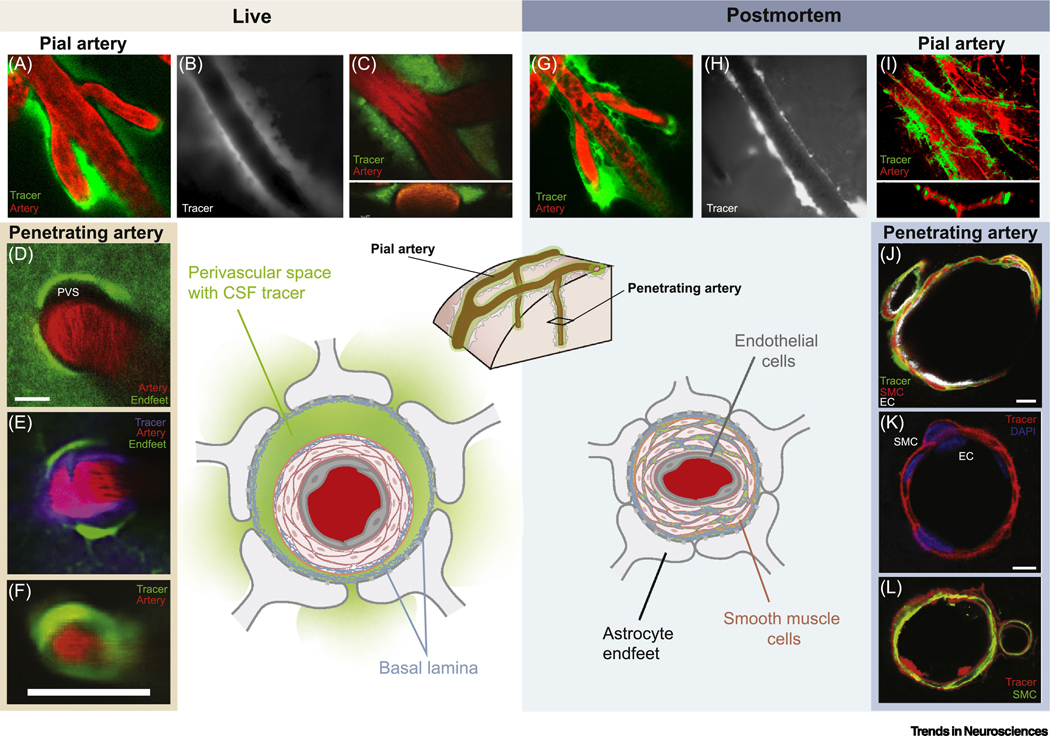
In the live brain (left), the pulsatility of pial arteries drives CSF tracers along large surface perivascular spaces (A–C; B from [66], A, C from [7]). The tracers are confined to the perivascular spaces on each side of the vessel and do not enter the compact smooth muscle cell layer (C inset, orthogonal projection). CSF tracers enter the neuropil by leaving the perivascular space (PVS) of penetrating arteries via gaps between the vascular endfeet of astrocytes (D–F; panels D, E, F are from [19], [20], and [18], respectively). Postmortem (right), the arteries collapse, and the fluid filled PVS largely disappears (G–I; compare to A–C. G, I from [7]; H from [66]), and tracers relocate into the smooth muscle cell (SMC) layer, basal lamina, and endothelial cell (EC) basement membrane (J–L; panels J, K and L are from [1], [67], and [68], respectively). Please refer to original publications for further details.
Another important concern relates to the geometry and size of the perivascular space. Most mathematical models assume that the perivascular space is shaped like the narrow layer of basement membrane depicted in histological sections, rather than the large fluid-filled perivascular space present in vivo [7](Fig. 2). The about 10-fold larger size of the perivascular space in live animals offers considerable less hydraulic resistance to CSF flow than previously assumed, possibly explaining why several models have predicted that arterial wall pulsatility cannot drive convective flow [25, 26]. Another twist to this discussion is that the perivascular space is not shaped like the circular annulus incorporated in most mathematical models. In vivo, the perivascular spaces are eccentric, and recent calculations show that the hydraulic resistance in an eccentric annular tunnel can be several-fold lower than a concentric annular tunnel [27]. Thus, the perivascular spaces appear to adapt their configuration to reduce hydraulic resistance. An obvious question is, however, whether the loss of the large fluid-filled perivascular space in histological sections invalidates the many prior studies of the glymphatic system based on ex vivo analysis of tracer distribution? We will argue that ex vivo tracer analysis is valid as long as its purpose is to quantify the magnitude of tracer influx between different groups of animals. This argument is substantiated by direct comparisons of in vivo and ex vivo tracer influx quantification in the same animal, showing a remarkably high correlation [28, 29]. That said, due to the significant artifacts from postmortem processing, it is important to bear in mind that little can be gleaned from histology with regard to the exact pathways via which tracer transport occurs [30].
Role of the aquaporin-4 (AQP4) water channel
The physiological role of aquaporins is to facilitate water transport across the plasma membrane. In brain and spinal cord, AQP4 is primarily expressed by astrocytic endfeet covering the cerebral vasculature (Fig. 2). The highly polarized expression on endfeet (rather than the soma) and the fact that brain endothelial cells are practically devoid of water channels suggests that AQP4 facilitates CSF inflow into the neuropil [31]. The term “glymphatic” was originally based on the observation that both CSF influx and ISF efflux were reduced in mice lacking glial AQP4 water channels compared with wildtype littermates [1]. A 2017 report questioned the importance of AQP4 in glymphatic transport by reporting that deletion of AQP4 did not affect CSF tracer distribution, nor did it affect dispersion of tracers injected into striatum [32]. These conclusions were cross-examined in a follow up publication with data from 5 independent groups. These authors reported that deletion of AQP4 consistently reduced inflow of CSF tracers in 4 independently generated AQP4 knockout mice lines, including the one used in the contesting study [8]. The data from [32] can be best explained by technical differences, such as the use of invasive procedures which are known to suppress glymphatic function [8], a non-optimal anesthetic regimen [29], and inclusion of mice of different ages (2–6 months). Given that the activity of the brain’s plumbing system declines rapidly in aging [33], it is imperative to compare mice within a tight age range to eliminate potential age-related confounding.
Convective flow in the interstitial space?
Helen Cserr’s group originally showed that tracers of different molecular weights were cleared from brain at the same rate despite a 5-fold difference in their diffusion coefficient [34]. Later, the existence of convective flow in the perivascular space was documented based on particle- [7, 24] and front-tracking [5, 28, 35]. These studies all mapped the dispersion of CSF tracers administered by cisterna magna injection, which is relatively non-invasive. Yet, it remains controversial whether convective flow occurs within the neuropil. Studying parenchymal tracer transport in the live brain is complex, since invasive manipulations, like direct injections into the neuropil through an open cranial window or laser-induced puncture of the vessel wall, disrupt the fine pressure gradients that drive glymphatic transport [8]. However, an innovative MRI study documented 2-fold differences in regional solute speed within the brain, and concluded that convective flow co-exists with diffusion [36]. This conclusion is in line with a recent review that made the argument that convection and diffusion cooperate in solute clearance, even if they do not take place in the same compartments: as solutes, like amyloid-β, diffuse into the perivascular space they are rapidly transported away, which in turn maintain a concentration gradient that supports the continuous diffusion of the solutes towards the perivascular spaces [37]. We conclude that convection and diffusion most likely co-exist in the brain but that the exact contributions of these two processes likely differs depending on the experimental condition, arousal state, body position, region of interest, rate and depth of respiration and more. Thus, it might be more fruitful to focus on net transport, which is more relevant for waste clearance, rather than determining which transport mechanism predominates.
Is ICP increase during tracer injection responsible for the observed CSF influx?
A common critique of glymphatic-lymphatic studies is that injection of tracers into cisterna magna is linked to artificial increases in ICP. In other words, it is alluded that brain tracer influx is artificial, and induced by nonnatural increases in ICP. This critique is based on some misunderstandings. First, physiological changes in ICP during sneezing, Valsalva maneuvers, changes in body position or agitation (10–40 mmHg) far exceed the changes in ICP that have been recorded during the injection of CSF tracers (1–3 mmHg). These physiological pressure changes are likely contributing to physiological CSF transport by transiently accelerating CSF outflow. During injections in cisterna magna there is little doubt that the local tracer injection propels its distribution in the cistern. Most rodent protocols employ a 5–10 min tracer injection protocol. During this time, the CSF tracer will typically distribute in the cisterns at the base of the brain. For the rest of the experiment, or when the tracers actually move into the brain, the pump is turned off. As shown in Fig. 3, parenchymal tracer influx first happens after ICP has returned to baseline. Thus, ICP elevations do not contribute to the parenchymal tracer distribution using standard glymphatic protocols. The same point is appreciable in experiments testing different anesthetics where all animals receive the same injection protocol, but vastly different amounts of tracers enter the brain[29]. Additional lines of evidence support the notion that tracer injections do not directly affect CSF influx. For example, in two recent studies, mean CSF flow speed in the perivascular space was comparable despite a near six-fold difference in infusion speeds [7, 24]. The much slower infusion speed did not increase ICP [24]. Moreover, the flow speed of microspheres did not change between periods when the infusion pump was on or off (Supp. Fig 1 in [7]). This is consistent for injections into compartments with high compliance and low outflow resistance such as the cisterna magna. However, this is not true in the case of intraparenchymal injections where the total volume and rate of the injection would have a considerable effect on tracer distribution within the neuropil. Neuroimaging studies have shown that several pumps contribute to physiological CSF transport, including the cardiac cycle, respiration and slow vasomotion all drive the movement of fluid within the human brain [38].
Fig. 3. Intracranial pressure (ICP) is highly dynamic in live rodents.
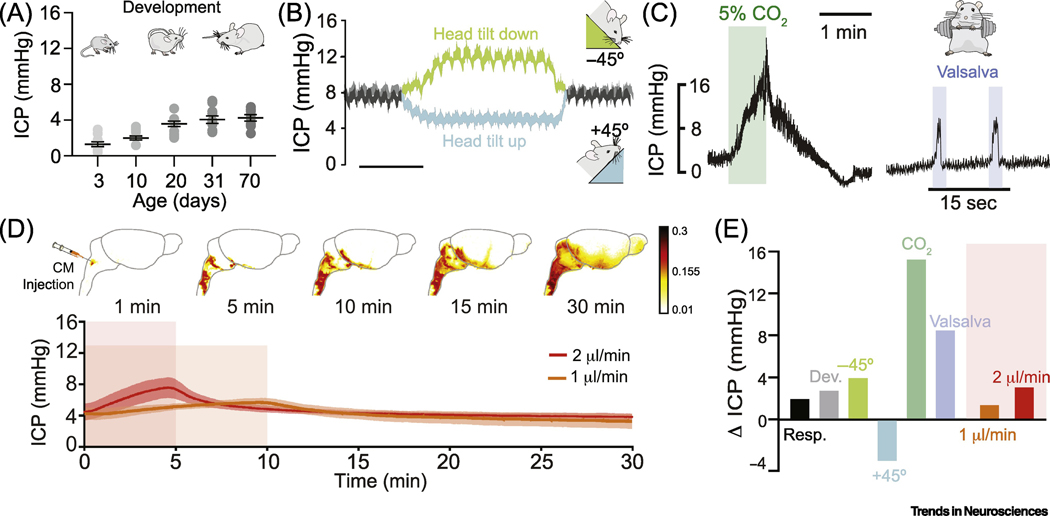
ICP pulsates in synchrony with both the cardiac and the respiratory cycle (Δ2 mmHg)[69]. (A) ICP undergoes a dramatic 3-fold increase during development, increasing in mice from 1.33±0.87 mmHg at postnatal day 3 (P3) to 4.11±0.83 mmHg at P70. Data adapted from Fig. 2 in[70]. (B) ICP is highly dependent on postural changes in rats anesthetized with isoflurane. A −45° head tilt down can elevate ICP by 4 mmHg while a +45° head tilt up can likewise decrease it 4 mmHg (time scale: 7.8 and 10 min, respectively). Adapted from Fig. 2b in[69]. (C, left panel) Respiratory acidosis or hypercapnia (high pCO2) causes cerebral blood vessels to dilate, increasing intracranial volume and in turn ICP. Hypercapnia induced by inhaling 5% CO2 in air causes a ~15 mmHg increase in baseline ICP. Right panel, chest compressions or Valsalva maneuvers increase intrathoracic pressure and reduce cerebral venous outflow elevating ICP ~9 mmHg. Adapted from Fig. 1d in [71]. (D) Top: dynamic-contrast enhanced MRI after gadobutrol intracisternal injection (12 μl at 1.5μl/min) shown as an enhancement ratio[5]. Bottom: ICP during infusion of 10 μl of tracer into cisterna magna at 1 μl/min or 2 μl/min. Intracisternal injection increases ICP by 1.4–3 mmHg and returns to baseline within 5 min after the end of the infusion. N= 5–6 mice per group. CSF influx takes place up to 20 min after the end of the ICP increase. (E) Summary of mean variations in ICP (mmHg) in response to different manipulations or state changes: respiration (Resp), development (Dev), postural changes as in panel (b), hypercapnia and Valsalva maneuvers as in panel (c), and tracer infusions as in (d). Changes in ICP caused by tracer infusion are relatively modest, and the overall comparison suggests that commonplace physiological changes for example in body posture or arterial blood gases are stronger modifiers of the glymphatic/lymphatic fluid transport than tracer injection.
Diurnal changes in amyloid-β: production vs. clearance
An elegant line of work has shown that the concentration of amyloid-β and tau in the interstitial space and CSF follow a diurnal pattern: amyloid-β and tau concentrations are highest during wakefulness and lowest during sleep in both compartments [39, 40]. According to one report, a single night of sleep deprivation, resulted in a significant increase in amyloid-β burden [41]. This pattern of regulation of protein concentrations may reflect that glymphatic clearance is turned on during sleep and largely absent during wakefulness [42]. In fact, exogenously delivered amyloid-β is cleared two-fold faster in naturally sleeping vs. awake mice [43]. However, it has also been argued that production rather than clearance may be responsible for the diurnal fluctuations in amyloid-β and tau. An important counterargument, at least in the context of animal studies, is that the diurnal changes in protein concentrations were quantified in transgenic mice overexpressing amyloid-β under the hamster prion protein (PrP) cosmid vector and tau under the control of the mouse prion protein promoter [44, 45][46]. These promoters are constitutive and not under arousal or circadian regulation. Thus, the most parsimonious synthesis of these findings is that the concentration of amyloid-β and tau in the CSF and ISF reflect the combined effects of diurnal- and state-dependent variations in CSF production rate, ISF volume, ISF turnover rate and glymphatic flow. Thus, removal rather than production is most likely responsible for the diurnal control of amyloid-β and tau.
Concluding remarks:
Interest in brain fluid transport systems has been on rapid rise in recent years. Perhaps it’s worth stepping back and asking – why should we care about this particular aspect of brain physiology? Critiques would state that many details of the glymphatic-lymphatic systems were described decades ago. For example, the Cserr group documented the existence of convective clearance almost 40 years ago [34] and the Grady group showed 10 years later that CSF is rapidly transported along perivascular spaces [47]. Even more remarkable is the hand drawn illustration of meningeal lymphatic vessels portrayed by the Italian anatomist Paolo Mascagni (1755–1815) [48]. The best explanation for the current wave of interest is probably that the glymphatic-lymphatic concepts have articulated a function of the transport system by demonstrating its role in amyloid-β clearance [1]. The glymphatic-lymphatic system clears key proteins involved in neurodegeneration, and conversely, inhibition of glymphatic-lymphatic transport accelerates protein accumulation and cognitive decline in mouse models of Alzheimer’s disease, traumatic brain injury, and Parkinson’s disease [13, 49–51]. Prior to these reports, for most scientists, brain fluid transport was simply not perceived as particularly relevant. The renewed interest in brain fluid transport also coincides with the failure of anti-amyloid-β clinical trials: the Alzheimer’s field is actively searching for alternative approaches to suppress protein aggregation [52]. Targeting the brain’s waste removal system is an attractive alternative, because hard-to-get-rid-of proteins are removed in bulk without the requirement for specific transporters. Focusing on a clearance pathway that is non-selective makes it attractive, as emerging evidence points to amyloid-β as initiating, but not directly triggering, neuronal loss and cognitive decline in Alzheimer’s disease [53]. Rather, emerging evidence points to cognitive decline coinciding with tau accumulation and microglial cell activation [54].
Another discovery, and arguably an equally important one, is that glymphatic clearance is primarily active during sleep. Thus, sleep is required for clearance of waste products that build up in the awake brain, perhaps explaining the revitalization we feel after a good night’s sleep [53]. As such, the glymphatic clearance model fulfills many of the criteria for the “need to sleep” model originally proposed by Borbely [55]. Conversely, lack of sleep is linked to a multitude of short- and long-term negative effects that are also compatible with reduced glymphatic clearance [42, 56]. Thus, the universal biological need for sleep across multiple species may at least in part reflect the need for glymphatic clearance.
The notion that glymphatic function is highly dependent on brain state also has immediate relevance for intrathecal delivery of drugs. Currently, intrathecal drugs are administered to awake patients, yet a substantial literature shows that CSF is largely shunted out of the CNS in the awake state [4]. Sleep-promoting states have been shown to improve brain-wide drug distribution, and future pharmacokinetics studies should take the state of brain activity into account [28, 57].
Finally, the glymphatic-lymphatic concept represents a unique opportunity for the neuroimaging field to develop new tools that can assess the function of non-neuronal cells. As discussed, glymphatic-lymphatic fluid transport requires the coordinated effort of glial cells and the cerebral vasculature. Pioneering studies have already documented the existence of the glymphatic system and meningeal lymphatic vessels in human brain [58, 59]. We are not aware of another neuroimaging modality that is capable of assessing the functional state of non-neuronal cell types on a macroscopic scale. Several novel non-invasive neuroimaging approaches are under development [38, 60] and can be combined with functional imaging approaches that map neuronal activity. A recent study measured the dynamics of CSF flow simultaneously with the blood-oxygen-leveldependent (BOLD) signal detected by functional magnetic resonance imaging (fMRI). The authors concluded that large waves of CSF inflow strongly correlated with changes in BOLD signals [61]. Since the BOLD signal reflects neural activity, emerging approaches in neuroimaging now enable simultaneous imaging of the largescale functional activity of neurons and fluid transport by non-neuronal cells.
Outstanding Questions Box.
Do additional astrocytic ion channels, apart from AQP4, and/or gap junctions play a role in facilitating CSFISF exchange during sleep?
Is there a circadian or state-dependent regulation in CSF/ISF production and/or turnover rate?
What are the relative contributions of meningeal lymphatics, cranial foramina, and arachnoid granulations to CSF-ISF clearance? How do changes in brain state affect CSF-ISF clearance?
Could high spatial resolution imaging and mathematical models advance our understanding of the CNS interstitial space connectome? Are there organized pathways that promote directional flow of interstitial fluid towards extracranial clearance sites? If so, are there certain brain regions that have slower clearance rates, and could this explain susceptibility to protein deposits?
Does CSF influx directly drive ISF outflow or are there additional fluid sources that aid clearance (e.g. BBB secretion)?
Is long term dysregulation of clearance pathways, or their reversal, responsible for the abnormal deposition of amyloid-β along arteries in Alzheimer’s disease and cerebral amyloid angiopathy? Alternatively, does the accumulation of amyloid-β along arteries reflect the fact that mural cells are highly phagocytotic and thereby prone to accumulation of protein aggregates?
Can ISF clearance routes be further elucidated using emerging brain-wide imaging technologies? Specifically, can non-invasive magnetic resonance imaging of fluid movements serve as a platform for assessment of glymphatic function in the clinic? In the future, will a “glymphogram” be able to predict the risk for developing proteinopathies? If so, will such a tool be useful in stratifying patients during clinical trial design?
Highlights.
Several global models of brain fluid transport have been proposed. When assessing these models, it is imperative that they are based on observations in live animals. Cerebrospinal fluid (CSF) tracer distribution in histological sections mostly reflects non-physiological events triggered after death.
The literature suggests that diffusion and convection flow both contribute to clearance of CNS solutes. Experiments that are aimed at defining the relative contribution of diffusion versus convection are difficult to interpreted, because minor changes in physiological variables, such as body posture or respiratory rate can significantly affect both pathways. Invasive procedures, such as tracer injection will primarily suppress convective flow.
The glymphatic system drives CSF into the brain along periarteral spaces and interstitial fluid out along perivenous spaces. Aquaporin-4 (AQP4) water channels densely expressed at the vascular endfeet of astrocytes facilitate glymphatic transport, based on all the studies on the topic with one exception.
Glymphatic-lymphatic efflux of amyloid-β contributes to diurnal variations in amyloid-β concentration in murine Alzheimer disease models, and represents a potential therapeutic target for Alzheimer’s disease.
Acknowledgments
We thank Dan Xue for assistance with the illustrations. This work is supported by the National Institute of Neurological Disorders and Stroke and the National Institute on Aging (U.S. National Institutes of Health: R01NS100366 and RF1AG057575), the U.S. Army Research Office (grant no. MURI W911NF1910280 to M.N.), Fondation Leducq Transatlantic Networks of Excellence Program, Novo Nordisk and Lundbeck Foundations, and the EU Horizon 2020 research and innovation program (grant no. 666881; SVDs@target). The views and conclusions contained in this review are solely those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the National Institutes of Health, Army Research Office, or the U.S. government. The U.S. government is authorized to reproduce and distribute reprints for government purposes notwithstanding any copyright notation herein.
Glossary
- Cerebrospinal fluid (CSF)
a clear, colorless fluid that is found in the ventricular system and the subarachnoid space surrounding the brain. CSF is produced at the choroid plexus but there is still debate about possible extrachoroidal sources. CSF ion concentrations are fairly similar to blood plasma and it plays a role in providing buoyancy and mechanical protection to the CNS. CSF has also been shown to act as a sink for metabolic waste produced in the neuropil
- Glymphatic (Glial-lymphatic)
Astrocyte-mediated transport of CSF and ISF that clears metabolic waste from the interstitial space of the brain parenchyma primarily during non-REM sleep and states of high slow wave activity. This process serves a pseudo-lymphatic function in the CNS
- Aquaporin-4 (AQP4)
An aquaporin water channel isoform primarily expressed by astrocytes in the CNS. AQP4 is expressed throughout the astrocytic soma but is highly polarized to the perivascular endfeet that line the brain vasculature. AQP4 reduces the hydraulic resistance of the plasma membrane allowing water to easily move into and out of the cell during ion fluxes
- Meningeal lymphatics
Lymphatic vessels found in the dura mater following the venous sinuses and at the base of the skull. These vessels have been shown to drain CSF solute to extracranial deep cervical lymph nodes and play a vital role in antigenic presentation in the CNS
- Perivascular spaces
Popularly known as Virchow-Robin spaces, although the exact anatomical boundaries that shape them and where along the cerebral vasculature they are found, is still a matter of debate. These are spaces surrounding blood vessels in the mammalian brain that function as flow pathways for CSF and interstitial fluid
- CSF influx
Entry of subarachnoidal CSF into the brain along perivascular spaces surrounding cerebral arteries and arterioles. This process is facilitated by sleep and anesthetics that exhibit high slow delta wave activity (e.g. ketamine-xylazine). During sleep-like states, arterial pulsations drive CSF bulk flow towards the brain in the direction of blood flow. This process has been shown to be highly dependent on the arousal state and AQP-4 expression
- Interstitial fluid (ISF) efflux
Outflow of ISF from the brain parenchyma towards CSF or extracranial clearance sites. This directional clearance is thought to take place at perivenous spaces and white matter tracts. This process has been shown by several independent groups to be mediated by a bulk flow mechanism and is highly dependent on arousal state and AQP-4 expression
Footnotes
Conflicts of interest
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iliff JJ et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4 (147), 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louveau A et al. (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523 (7560), 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspelund A et al. (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212 (7), 991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Q et al. (2019) Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol 137 (1), 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mestre H et al. (2020) Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 367 (6483). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliff JJ et al. (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33 (46), 18190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mestre H et al. (2018) Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9 (1), 4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mestre H et al. (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klarica M et al. (2019) The Movement of Cerebrospinal Fluid and Its Relationship with Substances Behavior in Cerebrospinal and Interstitial Fluid. Neuroscience 414, 28–48. [DOI] [PubMed] [Google Scholar]
- 10.Oreskovic D et al. (2016) Cerebrospinal fluid secretion by the choroid plexus? Physiol Rev 96 (4), 1661–2. [DOI] [PubMed] [Google Scholar]
- 11.Cserr HF (1988) Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann N Y Acad Sci 529, 9–20. [DOI] [PubMed] [Google Scholar]
- 12.Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45 (4), 545–52. [DOI] [PubMed] [Google Scholar]
- 13.Da Mesquita S et al. (2018) Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560 (7717), 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn JH et al. (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572 (7767), 62–66. [DOI] [PubMed] [Google Scholar]
- 15.Plog BA et al. (2015) Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 35 (2), 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louveau A et al. (2017) Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127 (9), 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen MK et al. (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17 (11), 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achariyar TM et al. (2016) Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener 11 (1), 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rangroo Thrane V et al. (2013) Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep 3, 2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundgaard I et al. (2015) Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat Commun 6, 6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benias PC et al. (2018) Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci Rep 8 (1), 4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Veluw SJ et al. (2019) Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbel-Ornath M et al. (2013) Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer’s disease mouse models. Acta Neuropathol 126 (3), 353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedussi B et al. (2018) Paravascular spaces at the brain surface: Low resistance pathways for cerebrospinal fluid flow. J Cereb Blood Flow Metab 38 (4), 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asgari M et al. (2016) Glymphatic solute transport does not require bulk flow. Sci Rep 6, 38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey J and Sarntinoranont M (2018) Pulsatile flow drivers in brain parenchyma and perivascular spaces: a resistance network model study. Fluids Barriers CNS 15 (1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tithof J et al. (2019) Hydraulic resistance of periarterial spaces in the brain. Fluids Barriers CNS 16 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plog BA et al. (2018) Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight in press (expected publication 18–11-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hablitz LM et al. (2019) Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 5 (2), eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albargothy NJ et al. (2018) Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 136 (1), 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeppenfeld DM et al. (2017) Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol 74 (1), 91–99. [DOI] [PubMed] [Google Scholar]
- 32.Smith AJ et al. (2017) Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kress BT et al. (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76, 845–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cserr HF et al. (1981) Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol 240 (4), F319–28. [DOI] [PubMed] [Google Scholar]
- 35.Munk AS et al. (2019) PDGF-B Is Required for Development of the Glymphatic System. Cell Rep 26 (11), 2955–2969 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koundal S et al. (2020) Optimal Mass Transport with Lagrangian Workflow Reveals Advective and Diffusion Driven Solute Transport in the Glymphatic System. Sci Rep 10 (1), 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas JH (2019) Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J R Soc Interface 16 (159), 20190572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiviniemi V et al. (2016) Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 36 (6), 1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang JE et al. (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326 (5955), 1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holth J et al. (2019) The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science, eaav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shokri-Kojori E et al. (2018) beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A 115 (17), 4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauglund NL et al. (2020) Meningeal Lymphangiogenesis and Enhanced Glymphatic Activity in Mice with Chronically Implanted EEG Electrodes. J Neurosci 40 (11), 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie L et al. (2013) Sleep drives metabolite clearance from the adult brain. Science 342 (6156), 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsiao K et al. (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274 (5284), 99–102. [DOI] [PubMed] [Google Scholar]
- 45.Hsiao KK et al. (1995) Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron 15 (5), 1203–18. [DOI] [PubMed] [Google Scholar]
- 46.Yoshiyama Y et al. (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53 (3), 337–51. [DOI] [PubMed] [Google Scholar]
- 47.Rennels ML et al. (1990) Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol 52, 431–9. [PubMed] [Google Scholar]
- 48.Sandrone S et al. (2019) A (delayed) history of the brain lymphatic system. Nat Med 25 (4), 538–540. [DOI] [PubMed] [Google Scholar]
- 49.Xu Z et al. (2015) Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iliff JJ et al. (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34 (49), 16180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou W et al. (2019) Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated alpha-synuclein. Transl Neurodegener 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knopman DS (2018) Sifting through a failed Alzheimer trial: What biomarkers tell us about what happened. Neurology 90 (10), 447–448. [DOI] [PubMed] [Google Scholar]
- 53.Brier MR et al. (2016) Tau and Abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med 8 (338), 338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terada T et al. (2019) In vivo direct relation of tau pathology with neuroinflammation in early Alzheimer’s disease. J Neurol 266 (9), 2186–2196. [DOI] [PubMed] [Google Scholar]
- 55.Borbely AA (1982) A two process model of sleep regulation. Hum Neurobiol 1 (3), 195–204. [PubMed] [Google Scholar]
- 56.Tononi G and Cirelli C (2014) Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81 (1), 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lilius TO et al. (2019) Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J Control Release 304, 29–38. [DOI] [PubMed] [Google Scholar]
- 58.Ringstad G et al. (2017) Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140 (10), 2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Absinta M et al. (2017) Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison IF et al. (2018) Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fultz NE et al. (2019) Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366 (6465), 628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H et al. (2018) Quantitative Gd-DOTA uptake from cerebrospinal fluid into rat brain using 3D VFASPGR at 9.4T. Magn Reson Med 79 (3), 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai X et al. (2020) Imaging the effect of the circadian light-dark cycle on the glymphatic system in awake rats. Proc Natl Acad Sci U S A 117 (1), 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koh L et al. (2005) Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyajima M and Arai H (2015) Evaluation of the Production and Absorption of Cerebrospinal Fluid. Neurol Med Chir (Tokyo) 55 (8), 647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bakker EN et al. (2016) Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell Mol Neurobiol 36 (2), 181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pizzo ME and Thorne RG (2017) The Extracellular and Perivascular Spaces of the Brain. In Edema Brain (Badaut J and Plesnila N eds), pp. 105–127, Academic Press. [Google Scholar]
- 68.Lam MA et al. (2017) The ultrastructure of spinal cord perivascular spaces: Implications for the circulation of cerebrospinal fluid. Sci Rep 7 (1), 12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guild SJ et al. (2015) Recording of intracranial pressure in conscious rats via telemetry. J Appl Physiol (1985) 119 (5), 576–81. [DOI] [PubMed] [Google Scholar]
- 70.Moazen M et al. (2016) Intracranial pressure changes during mouse development. J Biomech 49 (1), 123–126. [DOI] [PubMed] [Google Scholar]
- 71.Papadopoulos MC et al. (2004) Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J 18 (11), 1291–3. [DOI] [PubMed] [Google Scholar]


