Abstract
The copper-catalyzed hydroboration of benzylidenecyclopropanes, conveniently accessed in one step from readily available benzaldehydes, is reported. Under otherwise identical reaction conditions, two distinct phosphine ligands grant access to different products by either suppressing or promoting cyclopropane opening via β-carbon elimination. Computational studies provide insight into how the rigidity and steric environment of these different bis-phosphine ligands influence the relative activation energies of β-carbon elimination versus protodecupration from the key benzylcopper intermediate. The method tolerates a wide variety of heterocycles prevalent in clinical and pre-clinical drug development, giving access to valuable synthetic intermediates. The versatility of the tertiary cyclopropylboronic ester products is demonstrated through several derivatization reactions.
Keywords: Copper catalysis, hydroborations, benzylidenecyclopropanes, cyclopropylboronic esters, β-carbon elimination, heterocycles
Graphical Abstract
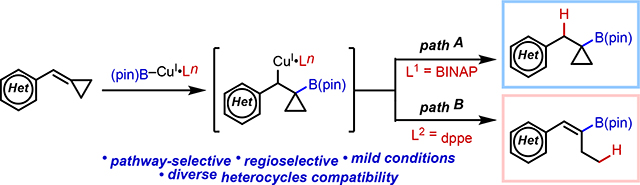
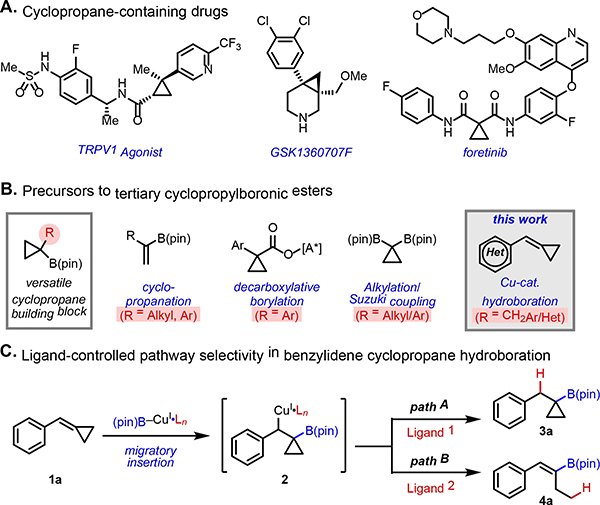
Conformationally constrained small carbocyclic ring systems are among the most important motifs in modern organic chemistry and drug discovery.[1] Cyclopropane substructures are of special importance as they can imbue a molecule with unique properties, including increased potency, metabolic stability, and brain permeability as well as attenuated pKa and lipophilicity.[2] Indeed, cyclopropyl groups are found in a wide range of secondary metabolites, insecticides, pharmaceutical products, for example a TRPV1 agonist,[3] GSK1360707F,[4] and foretinib[5] (Figure 1A). Accessing cyclopropane motifs with diverse substitution patterns is often a challenging aspect of the synthesis of such compounds.[6]
Figure 1.
Overview of Proposed Approach to Preparation of Cyclopropylboronic Esters.
Tertiary cyclopropylboronic esters have emerged as a versatile family of building blocks that can be further elaborated to prepare multi-substituted cyclopropane motifs. As a consequence, chemists have developed many important synthetic methods for their preparation (Figure 1B). For example, traditional cyclopropanation of vinyl boronates has been used to afford desired tertiary cyclopropylboronic esters.[7] More recently, the Baran and Aggarwal groups independently demonstrated that decarboxylative borylation of cyclopropyl carboxylic acids produces the corresponding cyclopropylboronic esters.[8] In addition, Morken and coworkers published a method for deborylative alkylation of gem-bis(boryl)cyclopropanes which gives tertiary cyclopropylboronic esters as products.[9] Recently, Harris and Pfizer coworkers demonstrated selective Suzuki–Miyaura coupling using these gem-bis(boryl)cyclopropane precursors to afford aryl cyclopropylboronic esters.[10] This impressive progress notwithstanding, existing methods also have some limitations in terms of restricted scope, harsh reaction conditions, cost of reagents, and requirement of pre-functionalized precursors.
For ongoing programs in drug discovery at Pfizer, we sought a convenient and reliable method to access tertiary cyclopropylboronates bearing diverse -CH2Ar/Het substitution at the α-position. In this context, we were attracted to copper–boryl chemistry, which has emerged as a powerful reactivity paradigm for hydroboration and borylative difunctionalization of alkenes. [11, 12] Indeed, the synthesis of secondary cyclopropylboronic esters from cyclopropenes[13] or allylic electrophiles[14] using copper–boryl catalysis has been previously documented. Generally speaking, this class of reactions often proceeds under mild conditions and exhibits broad functional group tolerance, making it well suited for our purposes. In particular, we envisioned highly strained benzylidenecyclopropanes (BCPs) 1 would be ideal tertiary cyclopropylboronic esters precursors upon Cu-catalyzed hydroboration. The utility of the alkylidene/benzylidenecyclopropane substrate class stems largely from the relief of strain energy, which provides a thermodynamic driving force for engaging a wide array of reaction partners in complexity-generating transformations.[15]
In considering the integration of BCP substrates into copper–boryl catalysis, we envisioned two potential competitive reaction pathways (Figure 1C) from the common intermediate 2 which is generated by migratory insertion of copper–boryl complex to BCP 1.[15] Direct protodecupration of 2 would generate cyclopropylboronic esters 3 (path A). On the other hand, alkenylboronates 4 could also be generated if β-carbon elimination of 2 is preferred over protodecupration (path B). We envisioned that by tuning the ligand sphere around the metal we could control the pathway selectivity of this process and enable straightforward access to these coveted building blocks. At the outset, we took inspiration from the work of Shi, who described a method for 1,2-aminoboration of BCPs using BINAP as ligand.[12f]
We began our study by preparing several representative BCPs (1a–1c, Table 1). All BCP substrates were derived from the corresponding aldehydes through Wittig reactions with commercially available and inexpensive starting materials (see SI for details).[16] The heterocycles were chosen based on their prevalence in bioactive compounds and their different electronic properties, which we thought might play a role in stabilizing intermediate 2 thereby impacting the innate tendency to undergo ring opening (Figure 1C).[17,18] We explored various mono- and bis-phosphine ligands with different electronic and steric properties under conditions similar to those developed for the copper-catalyzed hydroboration of styrenes.[19] For ease of visualization, the results in Table 1 are color-coded to highlight outcomes where the 1H NMR yield for one of the two isomers was higher than 70% (3 in blue and 4 in red). Entries 1–5 show results obtained with mono-phosphine ligands arranged from most electron-donating (entry 1) to most electron-withdrawing (entry 5). Although results varied from substrate to substrate, we observed a clear correlation between the electron-donating character of the ligand and the propensity for β-carbon elimination, which leads to formation of alkenylboronates 4. Notably, use of tris(pentafluorophenyl)phosphine (entry 5), a ligand not commonly used in copper–boryl catalysis, provided cyclopropylboronic esters 3 exclusively, albeit in variable yields, showing that β-carbon elimination could be fully suppressed with ligands that deplete electron density at the metal center.
Table 1.
Ligand optimization with representative substrates
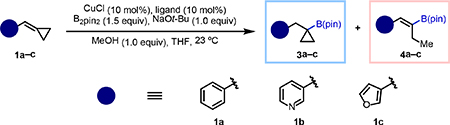 | |||||
|---|---|---|---|---|---|
| entrya | ligand | βnb | product ratio (3 : 4) in % yieldc | ||
| 1 | P(t-Bu)3·HBF4 | --- | 8 : 82 | 22 : 62 | 62 : 20 |
| 2 | PCy3 | --- | 7 : 78 | 41 : 30 | 55 : 18 |
| 3 | PPh3 | --- | 53 : 27 | 65 : 6 | 68 : 0 |
| 4 | (p-CF3-C6H4)3P | --- | 42 : 23 | 67 : 10 | 41 : 0 |
| 5 | (C6F5)3P | --- | 57 : 0 | 84 : 0 | 18 : 0 |
| 6 | dppm | 72° | 70 : 21 | 93 : 6 | 55 : 0 |
| 7 | dppbz | 83° | 0 : 88 | 2 : 72 | 21 : 66 |
| 8 | dppe | 85° | 0 : 93 | 6 : 76 | 16 : 70 |
| 9 | rac-BINAP | 92° | 79 : 0 | 83 : 6 | 91 : 0 |
| 10 | xantphos | 111° | 71 : 0 | 34 : 0 | 41 : 0 |
Reaction conditions: 1 (0.2 mmol), CuCl (10 mol%), ligand (10 mol%), B2pin2 (0.3 mmol), NaOt-Bu (0.2 mmol), MeOH (0.2 mmol) in THF (0.5 mL) at room temperature.
Natural bite angle (βn)
Yield determined by 1H NMR.
We then turned our attention to bis-phosphine ligands containing two –PPh2 arms with varied natural bite angles (βn) (entries 6–10).[20] Using dppm (entry 6, βn = 72°), all three substrates preferentially formed cyclopropylboronic esters 3. With dppbz as the ligand (entry 7, βn = 83°), most substrates gave 4 preferentially. In similar fashion, dppe (entry 8), which is known to bind copper with a larger bite-angle than dppm and dppbz (βn = 85°), favored formation of alkenylboronates 4. Larger bite angle ligands (entries 9 and 10) such as rac-BINAP (βn = 92°) and xantphos (βn = 111°) switched the selectivity to favor product 3. The lack of a clear trend between bite angle and selectivity points to a complex interplay of steric and conformational factors (vide infra). With the insights gained from this study, we opted to use rac-BINAP and dppe as the ligands for selective formation of products 3 and 4, respectively.
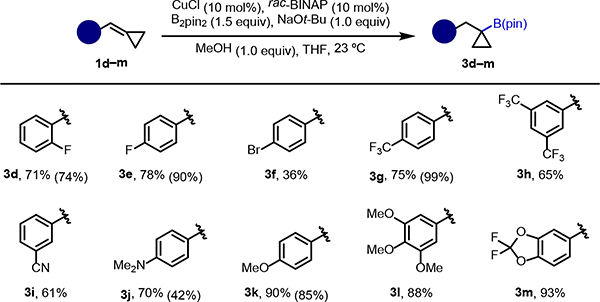
We turned our attention to exploring the scope with respect to arene substituents (Scheme 1). The protocol to prepare cyclopropylboronic esters 3 was first examined and found to tolerate a number of electron-withdrawing groups relevant to medicinal chemistry. For example, BCPs containing fluoride at the ortho and para positions gave boronic esters 3d and 3e in good yields. A substrate with a bromide at the para position gave the desired cyclopropylboronic ester 3f in 36%. As demonstrated by the synthesis of 3g and 3h, substrates containing trifluoromethyl groups can also be utilized in the reaction. Additionally, the cyano group was well-tolerated, as shown by the synthesis of product 3i. BCPs bearing electron-donating groups also worked well under the optimized reaction conditions. With certain electron-poor substrates 1d, 1e, and 1g, we found that tris(pentafluorophenyl)phosphine offered moderately higher yields (yield with this ligand in parentheses). Substrates with dimethylamine and methoxy substituents at the para position gave products 3j and 3k, respectively. A particularly electron-rich BCP bearing three methoxy groups gave the desired product 3l in 88%. Moreover, cyclopropylboronic ester 3m, containing the difluoro benzodioxole moiety, could be formed in excellent yield.
Scheme 1. Hydroboration of BCPs with substituted arenes.
aReaction conditions: 1 (0.2 mmol), CuCl (10 mol%), rac-BINAP (10 mol%), B2pin2 (0.3 mmol), NaOt-Bu (0.2 mmol), MeOH (0.2 mmol) in THF (0.5 mL) at room temperature. Percentages refer to the isolated yields. bThe values in parentheses correspond to NMR yields with (C6F5)3P as ligand in place of rac-BINAP
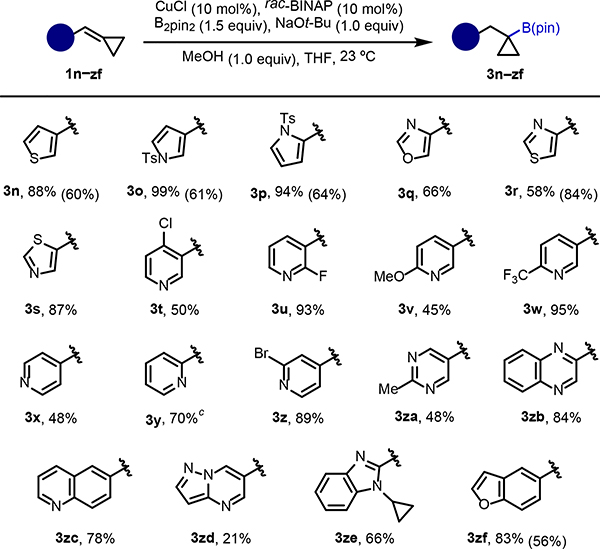
We then shifted our focus to more challenging BCPs containing heterocycles prevalent in biologically active molecules (Scheme 2). Many heterocyclic motifs are known to present problems in catalysis, as they can bind strongly to the metal center and inhibit progress. The transformation proved to be tolerant of diverse 5-membered heterocycles, including thiophene, Ts-protected pyrroles, oxazole, and thiazole to give the corresponding products 3n–3s in good to excellent yields. 6-membered heterocycles, including various substituted pyridines, performed well in the reaction, as exemplified by formation of boronic esters 3t–3z. It is worth mentioning that pyridine is the most common aromatic nitrogen-containing heterocycle in drug molecules. Synthetic methods that allow access to building blocks resembling 3t–3z are highly sought-after. We also explored other heterocycles of interest, namely pyrimidine, quinoxaline, quinoline, pyrazolo pyrimidine, benzimidazole, and benzofuran, which gave products 3za–3zf in synthetically useful to good yields.[21]
Scheme 2. Hydroboration of BCPs with heterocycles.
aReaction conditions: 1 (0.2 mmol), CuCl (10 mol%), rac-BINAP (10 mol%), B2pin2 (0.3 mmol), NaOt-Bu (0.2 mmol), MeOH (0.2 mmol) in THF (0.5 mL) at room temperature. Percentages refer to the isolated yields. b The values in parentheses correspond to NMR yields with (C6F5)3P as ligand in place of rac-BINAP. cYield determined by 1H NMR. The product was isolated as the corresponding BF3K salt in 51% yield over two steps.
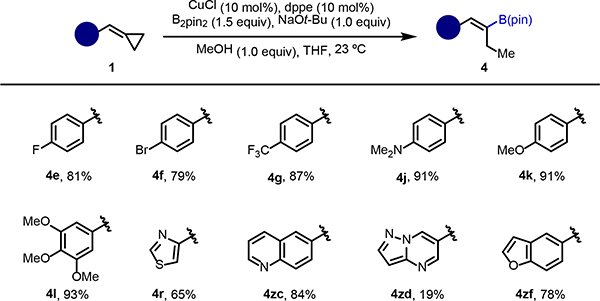
Next, we explored the feasibility of β-carbon elimination on a number of substrates containing various substituents and heterocyclic motifs (Scheme 3). The system developed utilizing dppe as the ligand served to give alkenylboronates in good to excellent yields.
Scheme 3. Synthesis of alkenyl boronates via β-C elimination.
aReaction conditions: 1 (0.2 mmol), CuCl (10 mol%), dppe (10 mol%), B2pin2 (0.3 mmol), NaOt-Bu (0.2 mmol), MeOH (0.2 mmol) in THF (0.5 mL) at room temperature. Percentages refer to the isolated yields.
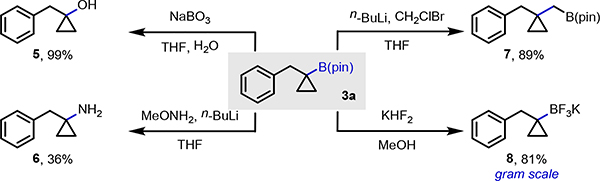
Having established access to various tertiary cyclopropylboronic esters, we set out to demonstrate their synthetic utility through derivatization reactions (Scheme 4). We chose to focus our efforts on derivatizations of the tertiary cyclopropylboronic esters, as vinyl-boronates are widely known to be versatile building blocks. Compound 3a was chosen as the model substrate for select C–X and C–C bond forming transformations. Exposure of 3a to sodium perborate gave tertiary alcohol 5 in 99%.[22] A modified amination protocol developed by Morken furnished primary amine 6 in modest yield.[23] A Matteson–Aggarwal homologation delivered boronic ester 7;[24] and lastly, the gram-scale conversion of 3a to tertiary trifluoroborate 8 occurred in 81%.[25]
Scheme 4.
Select derivatization reactions with tertiary boronic ester 3a
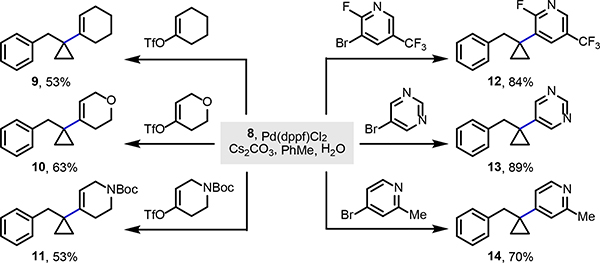
Furthermore, as illustrated in Scheme 5, facile access to trifluoroborate 8 enabled several Suzuki–Miyaura couplings. Under conditions developed by Harris and coworkers, use of alkenyl triflate electrophiles gave alkenyl cyclopropanes 9–11 in good yields.[26] Likewise, use of heteroaryl bromides efficiently delivered products 12–14 containing nitrogen-based heterocycles and substituents (e.g. fluorine, trifluoromethyl, and methyl) critical in drug development.[27]
Scheme 5.
Enabling Suzuki–Miyaura couplings with tertiary trifluoroborate 8
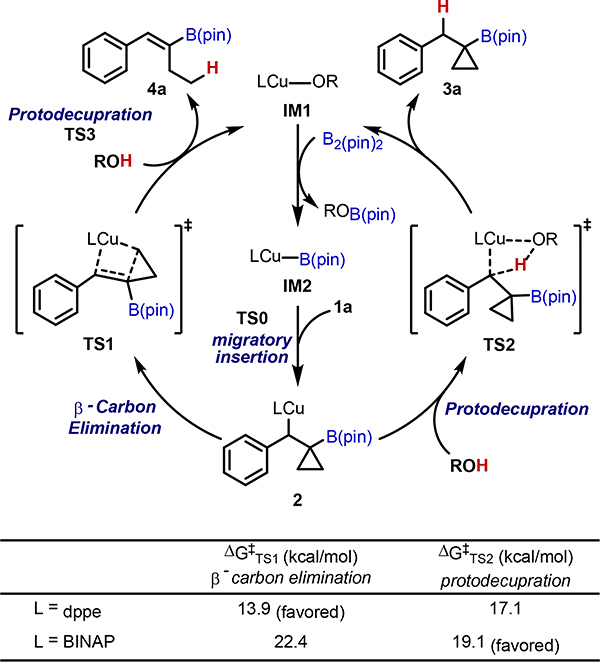
Given the wide discrepancy between outcomes obtained with seemingly related bis-phosphine ligands under otherwise identical reaction conditions, we pursued further understanding of the ligand effects with computational studies. We performed DFT calculations at the M06/6–311+G(d,p)–SDD/SMD(THF)//M06L/6–31G(d)–LANL2DZ level of theory to investigate the reaction energy profiles leading to the alkenylboronate and cyclopropylboronic ester products with dppe and BINAP ligands (see Figure S1). The calculations suggest that the pathway selectivity is determined by the activation energy difference between β-carbon elimination (TS1) and protodecupration (TS2) from the benzylic copper intermediate 2 formed via the irreversible migratory insertion of the BCP 1a (Scheme 6).[28] Consistent with the experimental observations, with the dppe ligand the β-carbon elimination is favored over protodecupration by 3.2 kcal/mol, leading to the formation of the alkenylboronate product 4a. The use of BINAP ligand completely reverses the pathway selectivity. β-Carbon elimination from the benzylic copper complex with BINAP as ligand requires a much higher barrier (ΔG‡ = 22.4 kcal/mol) than that with dppe as ligand (ΔG‡ = 13.9 kcal/mol). On the other hand, ligand effects have a much smaller impact on the barrier of protodecupration (TS2). Therefore, the reaction using BINAP as ligand forms the cyclopropylboronic ester 3a via favorable protodecupration.
Scheme 6.
Activation energies of the selectivity-determining steps.
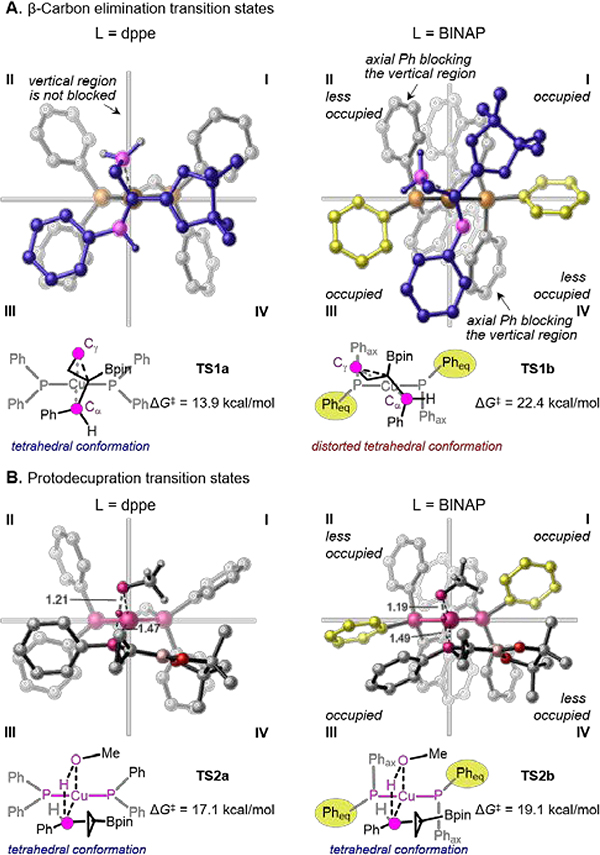
The origin of ligand effects on the β-carbon elimination barrier can be visualized in the quadrant diagrams in Figure 2A. The β-carbon elimination TS with the Cu(I) center prefers a tetrahedral geometry that places the benzylic (Cα) and γ-carbons (Cγ) within the vertical region perpendicular to the P–Cu–P plane. With dppe, this vertical region is not occupied by the –PPh2 arms. Thus, no unfavorable steric repulsions are observed in TS1a. On the other hand, the more rigid BINAP is confined to a C2-symmetric conformation, in which the vertical region is blocked by the pseudo-axial phenyl groups (Phax) of the –PPh2 arms. The benzylic and γ-carbons in TS1b are significantly distorted to be placed in the less occupied diagonal regions in quadrants II and IV to avoid repulsions with the BINAP ligand. Consequently, the distorted tetrahedral TS1b is energetically disfavored. An isomer of TS1b that places Cα and Cγ in the more occupied I and III quadrants was also located and requires an even higher activation energy (26.7 kcal/mol). By contrast, the ligand steric effects have a smaller impact on the geometry and energy of the protodecupration transition states, which are less sterically congested (TS2, Figure 2B). With either dppe or BINAP as ligand, the protodecupration transition state has an undistorted tetrahedral geometry with comparable activation energies.
Figure 2.
Origin of ligand effects on β-carbon elimination and protodecupration barriers.
In summary, we have developed conditions for the hydroboration of benzylidenecyclopropanes that lead to formation of two distinct products, namely cyclopropylboronic esters and alkenylboronates. Both products represent highly versatile building blocks that enable access to diverse derivatives based on downstream manipulation of boronic ester functionality. This work should be of particular interest to the pharmaceutical industry and represents an example of an emerging concept in catalysis, whereby pathway selectivity can be tuned through ligand space.[29] Computational analysis reveals the origins of ligand effects affecting a key β-carbon elimination step, providing a conceptual framework for strategically employing this family of strained alkenes in a broader range of catalytic transformations.
Supplementary Material
ACKNOWLEDGMENT
This work was financially supported by the National Institutes of Health (5R35GM125052-02; 1R35GM128779), The Scripps Research Institute, and Pfizer, Inc. We gratefully acknowledge the Kwanjeong Educational Foundation (Graduate Fellowship to T.K.) and the NSF for a Graduate Research Fellowship (NSF/DGE-1346837, J.D.). Calculations were performed at the Center for Research Computing at the University of Pittsburgh and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the NSF.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
Detailed experimental and computational procedures, compound characterization, Cartesian coordinates of the calculated structures. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1) (a).de Meijere A; Kozhushkov SI; Schill H Three-Membered-Ring-Based Molecular Architectures. Chem. Rev 2006, 106, 4926–4996. [DOI] [PubMed] [Google Scholar]; (b) Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; (c) Chen DY-K; Pouwer RH; Richard J-A Recent Advances in the Total Synthesis of Cyclopropane-Containing Natural Products. Chem. Soc. Rev 2012, 41, 4631–4642. [DOI] [PubMed] [Google Scholar]; (d) Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859. [DOI] [PubMed] [Google Scholar]
- (2) (a).Gagnon A; Duplessis M; Fader L Arylcyclopropanes: Properties, Synthesis and Use in Medicinal Chemistry. Org. Prep. Proced. Int 2010, 42, 1–69. [Google Scholar]; (b) Talele TT The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem 2016, 59, 8712–8756. [DOI] [PubMed] [Google Scholar]; (c) Derosa J; O’Duill ML; Holcomb M; Boulous MN; Patman RL; Wang F; Tran-Dube M; McAlpine I; Engle KM Copper-Catalyzed Chan−Lam Cyclopropylation of Phenols and Azaheterocycles. J. Org. Chem 2018, 83, 3417–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Butcher KJ; Denton SM; Field SE; Gillmore AT; Harbottle GW; Howard RM; Laity DA; Ngono CJ; Pibworth BA Convergent Asymmetric Synthesis of Two Complex TRPV1 Antagonists. Org. Process Res. Dev 2011, 15, 1192–1200. [Google Scholar]
- (4).Micheli F; Cavanni P; Andreotti D; Arban R; Benedetti R; Bertani B; Bettati M; Bettelini L; Bonanomi G; Braggio S; Carletti R; Checchia A; Corsi M; Fazzolari E; Fontana S; Marchioro C; Merlo-Pich E; Negri M; Oliosi B; Ratti E; Read KD; Roscic M; Sartori I; Spada S; Tedesco G; Tarsi L; Terreni S; Visentini F; Zocchi A; Zonzini L; Di Fabio R 6-(3,4-Dichlorophenyl)-1-[(methyloxy)methyl]-3-azabicyclo[4.1.0]heptane: A New Potent and Selective Triple Reuptake Inhibitor. J. Med. Chem 2010, 53, 4989–5001. [DOI] [PubMed] [Google Scholar]
- (5).Zillhardt M; Park S-M; Romero IL; Sawada K; Montag A; Krausz T; Yamada SD; Peter ME; Lengyel E Foretinib (GSK1363089), an Orally Available Multikinase Inhibitor of c-Met and VEGFR-2, Blocks Proliferation, Induces Anoikis, and Impairs Ovarian Cancer Metastasis. Clin. Cancer Res 2011, 17, 4042–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ebner C; Carreira EM Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev 2017, 117, 11651–11679. [DOI] [PubMed] [Google Scholar]
- (7) (a).Hussain MM; Li H; Hussain N; Ureña M; Carroll PJ; Walsh PJ Applications of 1-Alkenyl-1,1-Heterobimetallics in the Stereoselective Synthesis of Cyclopropylboronate Esters, Trisubstituted Cyclopropanols and 2,3-Disubstituted Cyclobutanones. J. Am. Chem. Soc 2009, 131, 6516–6524. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Phelan JP; Lang SB; Compton JS; Kelly CB; Dykstra R; Gutierrez O; Molander GA Redox-Neutral Photocatalytic Cyclopropanation via Radical/Polar Crossover. J. Am. Chem. Soc 2018, 140, 8037–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8)(a).Li C; Wang J; Barton LM; Yu S; Tian M; Peters DS; Kumar M; Yu AW; Johnson KA; Chatterjee AK; Yan M; Baran PS Decarboxylative Borylation. Science 2017, 356, eaam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fawcett A; Pradeilles J; Wang Y; Mutsuga T; Myers EL; Aggarwal VK Photoinduced Decarboxylative Borylation of Carboxylic Acids. Science 2017, 357, 283–286. [DOI] [PubMed] [Google Scholar]
- (9).Hong K; Liu X; Morken JP Simple Access to Elusive α-Boryl Carbanions and Their Alkylation: An Umpolung Construction for Organic Synthesis. J. Am. Chem. Soc 2014, 136, 10581–10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Harris MR; Wisniewska HM; Jiao W; Wang X; Bradow JN A Modular Approach to the Synthesis of gem-Disubstituted Cyclopropanes. Org. Lett 2018, 20, 2867–2871. [DOI] [PubMed] [Google Scholar]
- (11)(a).For early reports, see:Ito H; Yamanaka H; Tateiwa J; Hosomi A Boration of an α,β-enone Using a Diboron Promoted by a Copper(I)–Phosphine Mixture Catalyst. Tetrahedron Lett. 2000, 41, 6821–6825. [Google Scholar]; (b) Takahashi K; Tatsuo I; Norio M Addition and Coupling Reactions of Bis(pinacolato)diboron Mediated by CuCl in the Presence of Potassium Acetate. Chem. Lett 2000, 29, 982–983. [Google Scholar]
- (12)(a).For early reports, see:Mun S; Lee J-E; Yun J Copper-Catalyzed β-Boration of α,β-Unsaturated Carbonyl Compounds: Rate Acceleration by Alcohol Additives. Org. Lett 2006, 8, 4887–4889. [DOI] [PubMed] [Google Scholar]; (b) Lee Y; Hoveyda AH Efficient Boron−Copper Additions to Aryl-Substituted Alkenes Promoted by NHC−Based Catalysts. Enantioselective Cu-Catalyzed Hydroboration Reactions. J. Am. Chem. Soc 2009, 131, 3160–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee Y; Jang H; Hoveyda AH Vicinal Diboronates in High Enantiomeric Purity through Tandem Site-Selective NHC−Cu-Catalyzed Boron−Copper Additions to Terminal Alkynes. J. Am. Chem. Soc 2009, 131, 18234–18235. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Corberán R; Mszar NW; Hoveyda AH NHC-Cu-Catalyzed Enantioselective Hydroboration of Acyclic and Exocyclic 1,1-Disubstituted Aryl Alkenes. Angew. Chem. Int. Ed 2011, 50, 7079–7082. [DOI] [PubMed] [Google Scholar]; (e) Matsuda N; Hirano K; Satoh T; Miura M Regioselective and Stereospecific Copper-Catalyzed Aminoboration of Styrenes with Bis(pinacolato)diboron and O-Benzoyl-N,N-dialkylhydroxylamines. J. Am. Chem. Soc 2013, 135, 4934–4937. [DOI] [PubMed] [Google Scholar]; (f) Jiang H-C; Tang X-Y; Shi M Copper-Catalyzed Regio- and Enantioselective Aminoboration of Alkylidenecyclopropanes: The Synthesis of Cyclopropane-Containing β-Aminoalkylboranes. Chem. Commun 2016, 52, 5273–5276. [DOI] [PubMed] [Google Scholar]; (g) Kerchner H; Montgomery J Synthesis of Secondary and Tertiary Alkylboranes via Formal Hydroboration of Terminal and 1,1-Disubstituted Alkenes. Org. Lett 2016, 18, 5760–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Han JT; Han WJ; Kim N; Yun J Asymmetric Synthesis of Borylalkanes via Copper-Catalyzed Enantioselective Hydroallylation. J. Am. Chem. Soc 2016, 138, 15146–15149. [DOI] [PubMed] [Google Scholar]; (i) Jang WJ; Song SM; Moon JH; Lee JY; Yun J Copper-Catalyzed Enantioselective Hydroboration of Unactivated 1,1-Disubstituted Alkenes. J. Am. Chem. Soc 2017, 139, 13660–13663. [DOI] [PubMed] [Google Scholar]; (j) Semba K; Ohtagaki Y; Nakao Y Arylboration of 1-Arylalkenes by Cooperative Nickel/Copper Catalysis. Org. Lett 2016, 18, 3956–3959. [DOI] [PubMed] [Google Scholar]; (k) Bergmann AM; Dorn SK; Smith KB; Logan KM; Brown MK Catalyst-Controlled 1,2- and 1,1-Arylboration of α-Alkyl Alkenyl Arenes. Angew. Chem. Int. Ed 2019, 58, 1719–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13) (a).Parra A; Amenos L; Guisan-Ceinos M; Ruano JLG; Tortosa M Copper-Catalyzed Diastereo- and Enantioselective Desymmetrization of Cyclopropenes: Synthesis of Cyclopropylboronates. J. Am. Chem. Soc 2014, 136, 15833–15836. [DOI] [PubMed] [Google Scholar]; (b) Tian B; Liu Q; Tong X; Tian P; Lin G-Q Copper(I)-Catalyzed Enantioselective Hydroboration of Cyclopropenes: Facile Synthesis of Optically Active Cyclopropylboronates. Org. Chem. Front 2014, 1, 1116–1122. For reports of cyclopropene hydroboration catalyzed by other metals, see: [Google Scholar]; (c) Rubina M; Rubin M; Gevorgyan V Catalytic Enantioselective Hydroboration of Cyclopropenes. J. Am. Chem. Soc 2003, 125, 7198–7199 [DOI] [PubMed] [Google Scholar]; (d) Edwards A; Rubina M; Rubin M Directed Rh(I)-Catalyzed Asymmetric Hydroboration of Prochiral 1-Arylcycloprop-2-Ene-1-Carboxylic Acid Derivatives. Chem. Eur. J 2018, 24, 1394–1403. [DOI] [PubMed] [Google Scholar]
- (14)(a).Ito H; Kosaka Y; Nonoyama K; Sasaki Y; Sawamura M Synthesis of Optically Active Boron−Silicon Bifunctional Cyclopropane Derivatives through Enantioselective Copper(I)-Catalyzed Reaction of Allylic Carbonates with a Diboron Derivative. Angew. Chem. Int. Ed 2008, 47, 7424–7427. [DOI] [PubMed] [Google Scholar]; (b) Amenos L; Trulli L; Novoa L; Parra A; Tortosa M Stereospecific Synthesis of α-Hydroxy-Cyclopropylboronates from Allylic Epoxides. Angew. Chem. Int. Ed 2019, 58, 3188–3192. [DOI] [PubMed] [Google Scholar]
- (15) (a).Bra di A; Cicchi S; Cordero FM; Goti A Progress in the Synthesis and Transformations of Alkylidenecyclopropanes and Alkylidenecyclobutanes. Chem. Rev 2014, 114, 7317–7420. [DOI] [PubMed] [Google Scholar]; (b) Pellissier H Recent Developments in the Synthesis and Reactivity of Methylene- and Alkylidenecyclopropane Derivatives. Tetrahedron 2014, 70, 4991–5031. [Google Scholar]
- (16) (a).Masarwa A; Marek I Selectivity in Metal-Catalyzed Carbon–Carbon Bond Cleavage of Alkylidenecyclopropanes. Chem. Eur. J 2010, 16, 9712–9721. [DOI] [PubMed] [Google Scholar]; (b) Yu L-Z; Chen K; Zhu Z-Z; Shi M Recent Advances in the Chemical Transformations of Functionalized Alkylidenecyclopropanes (FACPs). Chem. Commun 2017, 53, 5935–5945. [DOI] [PubMed] [Google Scholar]
- (17). Qualitatively, the degree of aromaticity of the aryl-substituent appears to impact the inherent reactivity for ring-opening. More aromatic substituents, like phenyl in BCP 1a, appear to make the substrate more prone to rearrangement via β-carbon elimination leading to alkenylboronates 4a. The origin of this effect may be the decreased Brønsted basicity of the C–Cu bond in these types of substrates, compared to those with less aromatic substituents. A thiazole-containing substrate was found to be resistant to ring opening with all of the ligands in Table 1 (see SI).
- (18). Both the ring-opening and non-ring-opening reactions were found to proceed in the presence of radical inhibitors. This observation ruled out the possibility of radical-based processes in these reactions (see SI)
- (19).Wen Y; Xie J; Deng C; Li C Selective Synthesis of Alkylboronates by Copper(I)-Catalyzed Borylation of Allyl or Vinyl Arenes. J. Org. Chem. 2015, 80, 4142–4147. [DOI] [PubMed] [Google Scholar]
- (20) (a).Casey CP; Whiteker GT The Natural Bite Angle of Chelating Diphosphines. Isr. J. Chem 1990, 30, 299–304. [Google Scholar]; (b) Dierkes P; van Leeuwen PWNM The Bite Angle Makes the Difference: A Practical Ligand Parameter for Diphosphine Ligands. J. Chem. Soc., Dalton Trans, 1999, 1519–1530. [Google Scholar]; (c) van der Veen LA; Keeven PH Schoemaker GC; Reek JNH; Kamer PCJ; van Leeuwen PWNM; Lutz M; Spek AL Origin of the Bite Angle Effect on Rhodium Diphosphine Catalyzed Hydroformyation. Organometallics 2000, 19, 872–883. [Google Scholar]; (d) van Leeuwen PWNM; Kamer PCJ; Reek JNH; Dierkes P Ligand Bite Angle Effects in Metal-catalyzed C-C Bond Formation. Chem. Rev 2000, 100, 2741–2770. [DOI] [PubMed] [Google Scholar]
- (21). We tested an aliphatic substrate, and in this case observed <10% yield and poor regioselectivity.
- (22).Farthing CN; Marsden SP Chiral Vinyl Dioxazaborocines in Synthesis: Asymmetric Cuprate Additions to β-Boronyl Acrylates and Vinyl Sulfones. Tetrahedron Lett. 2000, 41, 4235–4238. [Google Scholar]
- (23).Mlynarski SN; Karns AS; Morken JP Direct Stereospecific Amination of Alkyl and Aryl Pinacol Boronates. J. Am. Chem. Soc 2012, 134, 16449–16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24) (a).Matteson DS α-Halo Boronic Esters: Intermediates for Stereodirected Synthesis. Chem. Rev 1989, 89, 1535–1551. [Google Scholar]; (b) Leonori D; Aggarwal VK Lithiation–Borylation Methodology and Its Application in Synthesis. Acc. Chem. Res 2014, 47, 3174–3183. [DOI] [PubMed] [Google Scholar]; (c) Fujioka Y; Amii H Boron-Substituted Difluorocyclopropanes: New Building Blocks of gem-Difluorocyclopropanes. Org. Lett 2008, 10, 769–772. [DOI] [PubMed] [Google Scholar]
- (25).Vedejs E; Chapman RW; Fields SC; Lin S; Schrimpf MR Conversion of Arylboronic Acids into Potassium Aryltrifluoroborates: Convenient Precursors of Arylboron Difluoride Lewis Acids. J. Org. Chem 1995, 60, 3020–3027. [Google Scholar]
- (26).Harris MR; Li Q; Lian Y; Xiao J; Londregan AT Construction of 1-Heteroaryl-3-azabicyclo[3.1.0]hexanes by sp3–sp2 Suzuki–Miyaura and Chan–Evans–Lam Coupling Reactions of Tertiary Trifluoroborates. Org. Lett 2017, 19, 2450–2453. [DOI] [PubMed] [Google Scholar]
- (27).Fang G-H; Yan Z-J; Deng M-Z Palladium-Catalyzed Cross-Coupling of Stereospecific Potassium Cyclopropyl Trifluoroborates with Bromides. Org. Lett 2004, 6, 357–360. [DOI] [PubMed] [Google Scholar]
- (28).Dang L; Zhao H; Lin Z; Marder TB DFT Studies of Alkene Insertions into Cu–B Bonds in Copper(I) Boryl Complexes. Organometallics 2007, 26, 2824–2832. [Google Scholar]
- (29) (a).Mahatthananchai J; Dumas AM; Bode JW Catalytic Selective Synthesis. Angew. Chem. Int. Ed 2012, 51, 10954–10990. [DOI] [PubMed] [Google Scholar]; (b) Lee Y-C; Kumar K; Waldmann H Ligand-Directed Divergent Synthesis of Carbo- and Heterocyclic Ring Systems. Angew. Chem. Int. Ed 2018, 57, 5212–5226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.