Abstract
Background
Little is known regarding optimal tacrolimus (TAC) trough levels after 1 year post-transplant in stable kidney transplant recipients (KTRs) who have not experienced renal or cardiovascular outcomes. This study aimed to investigate the effect of 1-year post-transplant TAC trough levels on long-term renal and cardiovascular outcomes and opportunistic infections in stable KTRs.
Methods
KTRs receiving TAC with mycophenolate-based immunosuppression who did not experience renal or cardiovascular outcomes within 1 year post-transplant were enrolled from a multicenter observational cohort study. Renal outcome was defined as a composite of biopsy-proven acute rejection, interstitial fibrosis and tubular atrophy, and death-censored graft loss. Cardiovascular outcome was defined as a composite of de novo cardiomegaly, left ventricular hypertrophy, and cardiovascular events. Opportunistic infections were defined as the occurrence of BK virus or cytomegalovirus infections.
Results
A total of 603 eligible KTRs were divided into the low-level TAC (LL-TAC) and high-level TAC (HL-TAC) groups based on a median TAC level of 5.9 ng/mL (range 1.3–14.3) at 1 year post-transplant. The HL-TAC group had significantly higher TAC trough levels at 2, 3, 4, and 5 years compared with the levels of the LL-TAC group. During the mean follow-up of 63.7 ± 13.0 months, there were 121 renal outcomes and 224 cardiovascular outcomes. In multivariate Cox regression analysis, LL-TAC and HL-TAC were not independent risk factors for renal and cardiovascular outcomes, respectively. No significant differences in the development of opportunistic infections and de novo donor-specific anti-human leukocyte antigen antibodies and renal allograft function were observed between the two groups.
Conclusions
TAC trough levels after 1 year post-transplant remained at a similar level until the fifth year after kidney transplantation and were not directly associated with long-term outcomes in stable Korean KTRs who did not experience renal or cardiovascular outcomes. Therefore, in Asian KTRs with a stable clinical course, TAC trough levels higher than approximately 6 ng/mL might not be required after a year of kidney transplantation.
Introduction
Underdosing of tacrolimus (TAC) in kidney transplant recipients (KTRs) can lead to biopsy-proven acute rejection (BPAR) and immunologic sensitization; however, overdosing of TAC can result in calcineurin inhibitor (CNI) toxicity and opportunistic infections including BK virus and cytomegalovirus (CMV) infections, which have detrimental effects on renal allograft outcomes [1–5]. Furthermore, CNI exposure can increase the risk of new-onset diabetes mellitus, hypertension, and lipid dysregulation, which are considered as potential risk factors for cardiovascular disease [6]. Therefore, the maintenance of optimal TAC trough levels is crucial to improve transplant outcomes.
Optimal TAC trough levels may be different according to the post-transplant period. Previous studies have reported an association between TAC trough levels within 1 year post-transplant and kidney transplantation outcomes [7–20]. The Kidney Disease: Improving Global Outcomes guidelines suggest that 5–15 ng/mL of TAC trough levels should be maintained during the first 2–4 months post-transplant and then reduced in stable KTRs to minimize toxicity, with a low quality of evidence [21]. However, little is known regarding optimal TAC trough levels after 1 year post-transplant in stable KTRs who have not experienced renal or cardiovascular outcomes. Furthermore, since ethnicity can affect tacrolimus pharmacokinetics [22], it is crucial to determine the optimal TAC trough levels in Asian KTRs.
This study aimed to investigate the effect of 1-year post-transplant TAC trough levels on renal and cardiovascular outcomes in stable Korean KTRs who did not experience renal or cardiovascular outcomes within 1 year post-transplant.
Materials and methods
Participants
De novo KTRs were enrolled from the Korean Cohort Study for Outcome in Patients with Kidney Transplantation (KNOW-KT) between 2012 and 2016 and followed up until 2019. Out of 1,080 KTRs, we included 707 KTRs receiving TAC with mycophenolate-based immunosuppression at 1 year. Overall, 101 KTRs who experienced renal or cardiovascular outcomes within 1 year post-transplant (renal = 94, cardiovascular = 33, both = 26), 1 patient with TAC trough levels > 20 ng/mL, and 2 patients with insufficient information were excluded. As a result, 603 KTRs were included in this study. The Institutional Review Committee of each participating center approved the KNOW-KT study protocol [Chonbuk National University Hospital; Gachon University Gil Medical Center; Keimyung University Dongsan Hospital; Korea University Anam Hospital; Kyungpook National University Hospital; Samsung Medical Center, Seoul; Seoul National University Hospital; Yonsei University, Severance Hospital (in alphabetical order)] [23]. All patients provided their written informed consent before participating in the study. All clinical investigations were conducted in accordance with the guidelines of the 2008 Declaration of Helsinki.
Variables
TAC trough levels and TAC dosages were recorded at 1 year and annually thereafter. TAC trough levels and TAC dosages were determined by the physicians’ clinical judgment. Eligible KTRs were divided into the low-level TAC (LL-TAC) and high-level TAC (HL-TAC) groups based on a median TAC level of 5.9 ng/mL (range 1.3–14.3) at 1 year post-transplant.
Possible confounders for renal composite endpoints included TAC trough level, TAC dosage, age, sex, body mass index (BMI), number of human leukocyte antigen (HLA) mismatches, type of transplant donor, re-transplantation, and desensitization. Possible confounders for cardiovascular composite endpoints included TAC trough levels, TAC dosage, age, sex, BMI, primary kidney disease, dialysis duration, smoking, total cholesterol, high-density lipoprotein (HDL), and systolic blood pressure (BP).
Information on de novo donor-specific anti-HLA antibodies (DSAs) was obtained at baseline, 1 year, and 5 years post-transplant and when clinically indicated.
Outcomes
Renal outcome was defined as a composite of BPAR, interstitial fibrosis and tubular atrophy (IFTA), and death-censored graft loss after 1 year post-transplant. IFTA grade I, II, and III were included. Cardiovascular outcome was defined as a composite of de novo cardiomegaly on chest X-ray, left ventricular hypertrophy on electrocardiogram, and cardiovascular events after 1 year post-transplant. Chest X-ray and electrocardiogram were performed at baseline and annual visits. Cardiomegaly on chest X-ray was defined as cardiothoracic ratio > 50%. Cardiovascular events were defined as myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass grafting, or stroke. The development of de novo DSAs and estimated glomerular filtration rates (eGFRs) were compared between the two groups.
Opportunistic infections were defined as the occurrence of BK virus or CMV infections. BK virus infection was defined as an occurrence of BK viremia (≥ 104 copies/mL) or diagnosed biopsy-proven BK virus nephropathy. CMV infection was defined as a presence of significant positive CMV polymerase chain reaction or diagnosed CMV disease.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation for normally distributed data and as the median with range when the values were not normally distributed. Differences between the groups were assessed by independent sample t-tests and chi-squared tests as appropriate. The Cox regression model was used to analyze the factors associated with the development of renal and cardiovascular outcomes and opportunistic infections. The cumulative incidences of renal and cardiovascular outcomes were analyzed according to TAC trough levels using the Kaplan-Meier method. Statistical analysis was performed using the SAS system for Windows, version 9.2 (SAS Institute Inc., Cary, NC). P values < 0.05 were considered statistically significant.
Results
Baseline characteristics
Table 1 shows the baseline characteristics according to a median TAC level of 5.9 ng/mL at 1 year post-transplant. The mean TAC trough levels were 7.8 ± 1.6 and 4.6 ± 1.0 ng/mL in KTRs with HL-TAC and LL-TAC, respectively. In comparison with patients with HL-TAC, those with LL-TAC were significantly younger (46.6 ± 11.2 vs. 44.7 ± 11.4 years, P = 0.041) and received less anti-thymocyte globulin as induction therapy (14.7 vs. 6.6%, P = 0.001). There were no significant differences in TAC dose, mycophenolic acid dose, the use of steroid, recipient sex, donor age, donor sex, BMI, primary kidney disease, dialysis duration, type of transplant donor, re-transplantation, number of HLA mismatches, panel reactive antibody positivity, desensitization, smoking, lipid profile, BP, the use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and statins, DSAs at 1 year, and eGFR at 1 year.
Table 1. Baseline characteristics.
| Variables | TAC > 5.9 ng/mL (n = 299) | TAC ≤ 5.9 ng/mL (n = 304) | P value |
|---|---|---|---|
| TAC trough levels at 1 year, ng/mL | 7.8 ± 1.6 | 4.6 ± 1.0 | < 0.001 |
| TAC dose at 1 year, mg | 3.9 ± 2.5 | 3.5 ± 1.9 | 0.065 |
| MMF dose at 1 year, mg | 942.5 ± 284.4 (n = 181) | 935.4 ± 318.4 (n = 204) | 0.819 |
| EC-MPS dose at 1 year, mg | 659.5 ± 247.2 (n = 118) | 677.8 ± 220.0 (n = 100) | 0.567 |
| Use of steroid at 1 year, n (%) | |||
| Yes | 270 (90.3) | 273 (89.8) | 0.838 |
| Missing | 29 (9.7) | 31 (10.2) | |
| Recipient age, years | 46.6 ± 11.2 | 44.7 ± 11.4 | 0.041 |
| Recipient sex, male, n (%) | 194 (64.9) | 184 (60.5) | 0.269 |
| Donor age, years | 44.1 ± 11.9 | 43.8 ± 12.1 | 0.748 |
| Donor sex, male, n (%) | 159 (53.2) | 151 (49. 7) | 0.389 |
| Recipient BMI, kg/m2 | 22.8 ± 3.5 | 22.7 ± 3.5 | 0.512 |
| Primary kidney disease, n (%) | |||
| Diabetes | 65 (21.7) | 65 (21.4) | 0.819 |
| Non-diabetes | 196 (65.6) | 195 (64.1) | |
| Unknown | 38 (12.7) | 44 (14.5) | |
| Dialysis duration, months | 25.1 ± 40.8 | 27.4 ± 46.3 | 0.523 |
| Type of donor, n (%) | |||
| Living | 246 (82.3) | 241 (79.3) | 0.350 |
| Deceased | 53 (17.7) | 63 (20.7) | |
| Re-transplantation, n (%) | 21 (7.0) | 19 (6.3) | 0.703 |
| Number of HLA mismatches | |||
| Total | 3.4 ± 1.6 | 3.3 ± 1.6 | 0.706 |
| DR | 1.1 ± 0.7 | 1.1 ± 0.7 | 0.513 |
| PRA positivity (> 0%), n (%) | 93 (31.1) | 111 (36.6) | 0.152 |
| Desensitization, n (%) | 85 (28.6) | 66 (21.8) | 0.054 |
| Induction therapy, n (%) | |||
| IL-2RB | 255 (85.3) | 284 (93.4) | 0.001 |
| ATG | 44 (14.7) | 20 (6.6) | |
| Smoking at KT | |||
| Never | 147 (49.2) | 168 (55.3) | 0.295 |
| Current or former | 150 (50.2) | 134 (44.1) | |
| Missing | 2 (0.7) | 2 (0.7) | |
| Total cholesterol at 1 year, mg/dL | 177.1 ± 34.68 | 177.7 ± 34.7 | 0.818 |
| LDL at 1 year, mg/dL | 98.2 ± 28.0 | 98.5 ± 28.6 | 0.922 |
| HDL at 1 year, mg/dL | 56.3 ± 17.1 | 59.1 ± 17.7 | 0.052 |
| SBP at 1 year, mmHg | 124.7 ± 11.9 | 123.5 ± 13.0 | 0.244 |
| DBP at 1 year, mmHg | 78.4 ± 10.4 | 77.7 ± 10.5 | 0.425 |
| Use of drugs at 1 year, n (%) | |||
| ACE inhibitors/ARBs | 42 (14.1) | 34 (11.2) | 0.300 |
| Statins | 132 (44.2) | 141 (46.5) | 0.556 |
| DSAs at 1 year, n (%) | 13 (4.3) | 17 (5.6) | 0.067 |
Values are shown as the mean ± standard deviation (range) or number (%).
Abbreviations: ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; ATG; anti-thymocyte globulin; BMI, body mass index; DBP, diastolic blood pressure; DSA, donor-specific anti-human leukocyte antigen antibodies; EC-MPS, enteric-coated mycophenolate sodium; HDL, high-density lipoprotein; HLA, human leukocyte antigen; IL-2RB, interleukin-2 receptor blocker; KT, kidney transplantation; LDL, low-density lipoprotein; MMF, mycophenolate mofetil; PRA, panel reactive antibody; SBP, systolic blood pressure; TAC, tacrolimus.
TAC trough levels and renal outcomes
Patients with HL-TAC at 1 year had significantly higher TAC trough levels at 2 years (6.4 ± 5.2 vs. 5.2 ± 1.8 ng/mL, P < 0.001), 3 years (6.0 ± 1.9 vs. 5.2 ± 1.8 ng/mL, P < 0.001), 4 years (6.1 ± 2.1 vs. 5.1 ± 1.7 ng/mL, P < 0.001), and 5 years (5.9 ± 5.3 vs. 5.3 ± 2.1 ng/mL, P < 0.001) compared with the levels of patients with LL-TAC (Fig 1).
Fig 1. TAC trough levels at 2, 3, 4, and 5 years post-transplant.
Patients with HL-TAC at 1 year had significantly higher TAC trough levels at 2 years (6.4 ± 5.2 vs. 5.2 ± 1.8 ng/mL, P < 0.001), 3 years (6.0 ± 1.9 vs. 5.2 ± 1.8 ng/mL, P < 0.001), 4 years (6.1 ± 2.1 vs. 5.1 ± 1.7 ng/mL, P < 0.001), and 5 years (5.9 ± 5.3 vs. 5.3 ± 2.1 ng/mL, P < 0.001) compared with the levels of patients with LL-TAC. *P < 0.001. Abbreviations: LL, low-level; HL, high-level; TAC, tacrolimus.
During the mean follow-up of 63.7 ± 13.0 months, 121 renal outcomes occurred after 1 year post-transplant. Detailed information on renal outcomes is presented in S1 Table. Univariate and multivariate Cox regression analysis demonstrated that TAC trough levels, TAC dose, age, sex, BMI, number of HLA mismatches, re-transplantation, and desensitization were not significantly associated with renal composite endpoints (Table 2).
Table 2. Univariate and multivariate Cox regression analysis for renal composite endpoints.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| TAC ≤ 5.9 vs. TAC > 5.9, ng/mL | 0.83 (0.58–1.18) | 0.298 | 0.85 (0.59–1.23) | 0.389 |
| TAC dose, mg | 1.06 (0.99–1.13) | 0.079 | 1.06 (0.99–1.13) | 0.079 |
| Age, years | 1.00 (0.98–1.01) | 0.525 | 0.99 (0.97–1.01) | 0.373 |
| Men vs. women | 1.28 (0.87–1.87) | 0.207 | 1.25 (0.84–1.85) | 0.272 |
| BMI, kg/m2 | 1.02 (0.97–1.07) | 0.521 | 1.02 (0.97–1.08) | 0.489 |
| Number of HLA mismatches | ||||
| Total | 1.04 (0.93–1.17) | 0.475 | 1.10 (0.90–1.33) | 0.354 |
| DR | 1.02 (0.79–1.32) | 0.889 | 0.87 (0.56–1.36) | 0.534 |
| Deceased vs. living donor | 1.19 (0.77–1.82) | 0.437 | 1.27 (0.80–2.01) | 0.311 |
| Re-transplantation | 1.01 (0.49–2.06) | 0.988 | 0.97 (0.47–2.02) | 0.938 |
| Desensitization | 1.11 (0.74–1.67) | 0.608 | 1.21 (0.79–1.86) | 0.391 |
Abbreviations: BMI, body mass index; CI, confidence interval; HLA, human leukocyte antigen; HR, hazards ratio; TAC, tacrolimus.
Multivariate Cox regression analysis for BPRA or IFTA are shown in S2 Table. TAC trough levels were not significantly associated with both BPRA and IFTA.
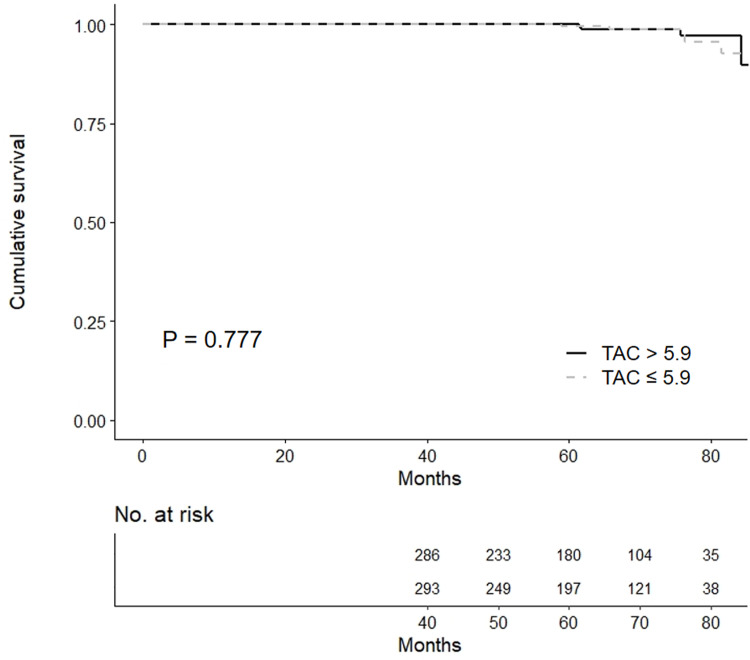
Kaplan-Meier analysis showed that there was no significant difference in the development of renal outcomes between the HL-TAC and LL-TAC groups (Fig 2A).
Fig 2.
Cumulative incidence of (A) renal composite endpoints and (B) cardiovascular composite endpoints according to TAC trough levels. No significant differences in the development of renal and cardiovascular outcomes between the HL-TAC and LL-TAC groups were observed in the Kaplan-Meier plot. Abbreviations: LL, low-level; HL, high-level; TAC, tacrolimus.
TAC trough levels of 5.0–5.9, ≤ 5, and ≤ 4 ng/mL compared with > 5.9 ng/mL were not associated with renal composite endpoints even after adjusting for TAC dose, age, sex, BMI, number of HLA mismatches, type of transplant donor, re-transplantation, and desensitization (Table 3). Subgroup analysis including deceased donor and deceased donor with donor age ≥ 60 revealed that renal outcome was not associated with LL-TAC compared with HL-TAC.
Table 3. Univariate and multivariate Cox regression analysis for renal composite endpoints in subgroups.
| Subdivided TAC trough levels | HR (95% CI) | P value | |
|---|---|---|---|
| 5.0 < TAC ≤ 5.9 vs. TAC > 5.9, ng/mL | Univariate | 1.09 (0.67–1.76) | 0.734 |
| Multivariate* | 1.13 (0.69–1.85) | 0.617 | |
| TAC ≤ 5 vs. TAC > 5.9, ng/mL | Univariate | 0.78 (0.51–1.18) | 0.231 |
| Multivariate* | 0.74 (0.49–1.13) | 0.166 | |
| TAC ≤ 4 vs. TAC > 5.9, ng/mL | Univariate | 1.01 (0.60–1.70) | 0.970 |
| Multivariate* | 1.01 (0.59–1.71) | 0.986 | |
| Subgroups: TAC ≤ 5.9 vs. TAC > 5.9, ng/mL | |||
| Deceased donor | Univariate | 0.75 (0.35–1.60) | 0.458 |
| Deceased donor & donor age ≥ 60 years | Univariate | 0.62 (0.10–3.75) | 0.606 |
Abbreviations: CI, confidence interval; HR, hazards ratio; TAC, tacrolimus.
*Adjusted for TAC dose, age, sex, BMI, number of HLA mismatches, type of transplant donor, re-transplantation, desensitization, and induction therapy.
TAC trough levels and cardiovascular outcomes
A total of 224 cardiovascular outcomes were observed during the follow-up. Detailed information on cardiovascular outcomes is presented in S1 Table. Univariate and multivariate Cox regression analysis demonstrated that TAC trough levels, TAC dose, age, sex, BMI, primary kidney disease, smoking, serum total cholesterol, HDL, and systolic BP were not significantly associated with cardiovascular composite endpoints (Table 4). Multivariate Cox regression analysis showed that dialysis duration was an independent predictor of the development of cardiovascular outcomes (HR = 1.01, 95% CI 1.00–1.01, P < 0.001).
Table 4. Univariate and multivariate Cox regression analysis for cardiovascular composite endpoints.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| TAC > 5.9 vs. TAC ≤ 5.9, ng/mL | 0.80 (0.62–1.04) | 0.102 | 0.76 (0.58–1.01) | 0.055 |
| TAC dose, mg | 0.99 (0.93–1.05) | 0.642 | 0.99 (0.93–1.05) | 0.658 |
| Age, years | 1.01 (1.00–1.02) | 0.171 | 1.01 (0.99–1.02) | 0.270 |
| Men vs. women | 1.46 (1.10–1.94) | 0.010 | 1.31 (0.90–1.90) | 0.161 |
| BMI, kg/m2 | 1.02 (0.98–1.05) | 0.420 | 0.98 (0.94–1.03) | 0.475 |
| Primary kidney disease | ||||
| Diabetes | 1.15 (0.83–1.59) | 0.397 | 1.08 (0.76–1.53) | 0.656 |
| Non-diabetes | Reference | Reference | ||
| Unknown | 1.35 (0.94–1.93) | 0.107 | 1.61 (0.80–1.69) | 0.437 |
| Dialysis duration, months | 1.01 (1.00–1.01) | < 0.001 | 1.01 (1.00–1.01) | < 0.001 |
| Smoking at KT | ||||
| Never | Reference | Reference | ||
| Current or former | 1.28 (0.98–1.66) | 0.071 | 1.07 (0.77–1.48) | 0.694 |
| Missing | 2.34 (0.58–9.48) | 0.235 | 1.79 (0.24–13.61) | 0.573 |
| Total cholesterol at 1 year, mg/dL | 1.00 (1.00–1.01) | 0.314 | 1.00 (1.00–1.01) | 0.103 |
| HDL at 1 year, mg/dL | 1.00 (0.99–1.00) | 0.360 | 1.00 (0.99–1.01) | 0.530 |
| SBP at 1 year, mmHg | 1.01 (1.00–1.02) | 0.086 | 1.01 (1.00–1.02) | 0.119 |
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; HR, hazards ratio; KT, kidney transplantation; SBP, systolic blood pressure; TAC, tacrolimus.
No significant difference in the development of cardiovascular outcomes between the HL-TAC and LL-TAC groups was observed in the Kaplan-Meier plot (Fig 2B).
TAC trough levels and opportunistic infections
During the follow-up, 5 BK virus infections and 60 CMV infections occurred after 1 year post-transplant. Univariate and multivariate Cox regression analysis demonstrated that TAC trough levels, age, sex, BMI, number of HLA mismatches, re-transplantation, and desensitization were not significantly associated with opportunistic infections (Table 5). TAC dose was an independent risk factor for the development of opportunistic infections.
Table 5. Univariate and multivariate Cox regression analysis for opportunistic infections.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| TAC ≤ 5.9 vs. TAC > 5.9, ng/mL | 0.89 (0.54–1.44) | 0.622 | 1.00 (0.60–1.64) | 0.986 |
| TAC dose, mg | 1.11 (1.03–1.20) | 0.009 | 1.11 (1.02–1.21) | 0.013 |
| Age, years | 0.99 (0.97–1.02) | 0.568 | 0.99 (0.97–1.02) | 0.568 |
| Men vs. women | 1.09 (0.65–1.81) | 0.746 | 1.19 (0.69–2.04) | 0.529 |
| BMI, kg/m2 | 0.99 (0.92–1.06) | 0.683 | 0.98 (0.90–1.06) | 0.534 |
| Number of HLA mismatches | ||||
| Total | 1.17 (0.99–1.37) | 0.059 | 1.17 (0.89–1.54) | 0.252 |
| DR | 1.30 (0.90–1.86) | 0.159 | 0.94 (0.50–1.75) | 0.841 |
| Deceased vs. living donor | 0.75 (0.38–1.46) | 0.391 | 0.92 (0.45–1.88) | 0.817 |
| Re-transplantation | 0.21 (0.03–1.51) | 0.121 | 0.18 (0.03–1.33) | 0.094 |
| Desensitization | 1.50 (0.89–2.53) | 0.126 | 1.55 (0.89–2.71) | 0.123 |
Abbreviations: BMI, body mass index; CI, confidence interval; HLA, human leukocyte antigen; HR, hazards ratio; TAC, tacrolimus.
TAC trough levels, de novo DSAs, and renal allograft function
No significant difference in the development of de novo DSAs between the HL-TAC and LL-TAC groups was observed in the Kaplan-Meier plot (Fig 3).
Fig 3. Development of de novo DSAs after 1 year post-transplant according to TAC trough levels.
No significant difference in de novo DSA development between the HL-TAC and LL-TAC groups was observed in the Kaplan-Meier plot. Abbreviations: DSA, donor-specific anti-HLA antibody; LL, low-level; HL, high-level; TAC, tacrolimus.
There were no significant differences in eGFRs at 2 years (67.0 ± 18.1 vs. 65.5 ± 18.8 mL/min/1.73 m2, P = 0.648), 3 years (67.8 ± 19.4 vs. 67.1 ± 20.2 mL/min/1.73 m2, P = 0.662), 4 years (66.9 ± 21.4 vs. 65.2 ± 20.8 mL/min/1.73 m2, P = 0.417), and 5 years (68.3 ± 21.3 vs. 64.9 ± 24.4 mL/min/1.73 m2, P = 0.273) between the HL-TAC and LL-TAC groups (Fig 4).
Fig 4. eGFRs at 2, 3, 4, and 5 years post-transplant.
There were no significant differences in eGFRs at 2 years (67.0 ± 18.1 vs. 65.5 ± 18.8 mL/min/1.73 m2, P = 0.648), 3 years (67.8 ± 19.4 vs. 67.1 ± 20.2 mL/min/1.73 m2, P = 0.662), 4 years (66.9 ± 21.4 vs. 65.2 ± 20.8 mL/min/1.73 m2, P = 0.417), and 5 years (68.3 ± 21.3 vs. 64.9 ± 24.4 mL/min/1.73 m2, P = 0.273) between the HL-TAC and LL-TAC groups. Abbreviations: eGFR, estimated glomerular filtration rate; LL, low-level; HL, high-level; TAC, tacrolimus.
Discussion
In this multicenter cohort study, TAC trough levels after 1 year post-transplant remained at a similar level until the fifth year after kidney transplantation. No significant association was found between 1-year post-transplant TAC trough levels and long-term renal and cardiovascular outcomes and opportunistic infections in stable Korean KTRs who did not experience renal and cardiovascular outcomes within 1 year post-transplant. Furthermore, TAC trough levels of > 5.9 ng/mL compared with ≤ 5, 5.0–5.9, and ≤ 5.9 ng/mL did not show any benefits in terms of outcomes as well as the development of de novo DSAs and renal allograft function. Subgroup analysis including deceased donor or deceased donor with donor age ≥ 60 also showed consistent results.
There are limited data on stable KTRs, especially in the Asian population, who have not experienced any outcomes including rejection in the first year following kidney transplantation. In this study, TAC trough levels higher than approximately 6 ng/mL did not exhibit any beneficial effect on reducing renal outcomes including the development of de novo DSAs in stable KTRs. Although an existing large-scale study reported TAC trough levels > 5 ng/mL after 1 year post-transplant as optimal for preventing the development of de novo DSAs, the study differed considerably from this study in that it included all patients who experienced BPAR within a year after kidney transplantation [19]. As BPAR itself can affect transplant outcomes and the target range of TAC trough levels, this study excluded those with clinical events within 1 year. Furthermore, TAC trough levels < 5 ng/mL in patients with low HLA alloimmune response did not increase the risk of de novo DSA development, indicating that not all KTRs may have a standard TAC trough level target of above 5 ng/mL [19]. Therefore, the TAC trough level target should be adjusted based on previous BPAR history as well as the individual’s alloimmune risk.
CNIs have been reported to cause dyslipidemia and vascular calcification dose-dependently, and these conditions may increase cardiovascular outcomes [6]. However, the reduction of cardiovascular outcomes by optimal TAC trough levels has not been investigated. Contrary to our expectations, high TAC trough levels did not increase the risk of cardiovascular outcomes in this study. Instead, pretransplant dialysis duration was an independent risk factor for cardiovascular outcomes even after adjusting for Framingham risk factors. The result of the present study is consistent with that of a previous study, which reported pretransplant dialysis duration as an independent predictor of patient death resulting from cardiovascular diseases [24]. The pathogenesis may be attributed to the rapid progression of cardiovascular changes including vascular calcification and left ventricular hypertrophy [25], increased inflammation [26], and changes in concentrations of advanced glycosylation end products [27].
This study has some limitations. First, this study was not a randomized controlled study but an observational study. The results of our study are not completely unaffected by the physician’s strategies and clinical circumstances of KTRs. Second, as the TAC trough level involved a single drug concentration, there may be a lack of representation. However, KTRs with HL-TAC at 1 year post-transplant also had significantly higher TAC trough levels until 5 years post-transplant. The sustained higher or lower TAC trough levels for the following 5 years may be explained by individual genetic predispositions, as reported in previous studies about the association between cytochromeP450(CYP)3A5 gene polymorphisms, the dose requirement of TAC, and TAC trough levels [28–30]. CYP3A5 is the major enzyme responsible for the metabolisms TAC. The CYP3A5 genetic polymorphisms can affect the pharmacokinetics of TAC and contribute to the interindividual variability of TAC trough levels. Previous studies reported that CYP3A5 genotype is associated with dose-adjusted level of TAC and complications among Korean KTRs [30, 31]. Although no information on CYP3A5 gene polymorphism has been obtained from the current study, further studies regarding the genotype‐guided TAC dosage control will ultimately be necessary to achieve individual optimized TAC trough levels and achieve the optimal transplant outcomes. Third, there are no data on the presence of proteinuria over time, which may have yielded more useful information for the evaluation of recurrent or newly occurring glomerulonephritis after kidney transplantation which are a frequent cause of allograft loss at 10 years [32]. Despite these limitations, this study could provide Asian clinicians with some guidance about TAC trough levels after 1 year post-transplant because it included an Asian population, in contrast to prior studies that included Caucasian, African American, and Hispanic populations. Furthermore, the results of this study are clinically significant, demonstrating that stable KTRs who have never experienced renal or cardiovascular outcomes make up the largest number of patients in clinical practice. In addition, the mean follow-up duration was considerably long.
In conclusion, TAC trough levels after 1 year post-transplant were not directly associated with long-term outcomes in stable Korean KTRs who did not experience renal or cardiovascular outcomes. Therefore, in Asian KTRs who have not experienced any outcomes in the first year, TAC trough levels higher than approximately 6 ng/mL might not be required after a year of kidney transplantation.
Supporting information
(DOCX)
(DOCX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (No. 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, and 2019E320100) to CA and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, which is funded by the Ministry of Health & Welfare, Republic of Korea (No. HI13C1232) to CDK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004;66(1):329–37. 10.1111/j.1523-1755.2004.00735.x . [DOI] [PubMed] [Google Scholar]
- 2.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46(6):840–6. 10.1086/528718 . [DOI] [PubMed] [Google Scholar]
- 3.Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87(5):621–30. 10.1097/TP.0b013e318197c17d . [DOI] [PubMed] [Google Scholar]
- 4.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–99. 10.1111/j.1600-6143.2011.03840.x . [DOI] [PubMed] [Google Scholar]
- 5.Park WY, Kang SS, Jin K, Park SB, Choe M, Han S. Long-term prognosis of BK virus-associated nephropathy in kidney transplant recipients. Kidney Res Clin Pract. 2018;37(2):167–73. Epub 2018/07/05. 10.23876/j.krcp.2018.37.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jardine AG. Assessing the relative risk of cardiovascular disease among renal transplant patients receiving tacrolimus or cyclosporine. Transpl Int. 2005;18(4):379–84. 10.1111/j.1432-2277.2005.00080.x . [DOI] [PubMed] [Google Scholar]
- 7.van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation. 2008;86(8):1043–51. 10.1097/TP.0b013e318186f98a . [DOI] [PubMed] [Google Scholar]
- 8.Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562–75. 10.1056/NEJMoa067411 . [DOI] [PubMed] [Google Scholar]
- 9.Bouamar R, Shuker N, Hesselink DA, Weimar W, Ekberg H, Kaplan B, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials(dagger). Am J Transplant. 2013;13(5):1253–61. 10.1111/ajt.12191 . [DOI] [PubMed] [Google Scholar]
- 10.Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant. 2001;16(9):1905–9. 10.1093/ndt/16.9.1905 . [DOI] [PubMed] [Google Scholar]
- 11.O'Seaghdha CM, McQuillan R, Moran AM, Lavin P, Dorman A, O'Kelly P, et al. Higher tacrolimus trough levels on days 2–5 post-renal transplant are associated with reduced rates of acute rejection. Clin Transplant. 2009;23(4):462–8. 10.1111/j.1399-0012.2009.01021.x . [DOI] [PubMed] [Google Scholar]
- 12.Israni AK, Riad SM, Leduc R, Oetting WS, Guan W, Schladt D, et al. Tacrolimus trough levels after month 3 as a predictor of acute rejection following kidney transplantation: a lesson learned from DeKAF Genomics. Transpl Int. 2013;26(10):982–9. 10.1111/tri.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaynor JJ, Ciancio G, Guerra G, Sageshima J, Roth D, Goldstein MJ, et al. Lower tacrolimus trough levels are associated with subsequently higher acute rejection risk during the first 12 months after kidney transplantation. Transpl Int. 2016;29(2):216–26. 10.1111/tri.12699 . [DOI] [PubMed] [Google Scholar]
- 14.Arreola-Guerra JM, Serrano M, Morales-Buenrostro LE, Vilatoba M, Alberu J. Tacrolimus Trough Levels as a Risk Factor for Acute Rejection in Renal Transplant Patients. Ann Transplant. 2016;21:105–14. 10.12659/aot.895104 . [DOI] [PubMed] [Google Scholar]
- 15.Rehman S, Wen X, Casey MJ, Santos AH, Andreoni K. Effect of different tacrolimus levels on early outcomes after kidney transplantation. Ann Transplant. 2014;19:68–75. 10.12659/AOT.889858 . [DOI] [PubMed] [Google Scholar]
- 16.Richards KR, Hager D, Muth B, Astor BC, Kaufman D, Djamali A. Tacrolimus trough level at discharge predicts acute rejection in moderately sensitized renal transplant recipients. Transplantation. 2014;97(10):986–91. 10.1097/TP.0000000000000149 . [DOI] [PubMed] [Google Scholar]
- 17.Davis S, Gralla J, Klem P, Tong S, Wedermyer G, Freed B, et al. Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor-specific antibodies in the first year of kidney transplantation. Am J Transplant. 2018;18(4):907–15. 10.1111/ajt.14504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung HY, Cho SY, Choi JY, Cho JH, Park SH, Kim YL, et al. Comparison of Transplant Outcomes for Low-level and Standard-level Tacrolimus at Different Time Points after Kidney Transplantation. J Korean Med Sci. 2019;34(12):e103 10.3346/jkms.2019.34.e103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiebe C, Rush DN, Nevins TE, Birk PE, Blydt-Hansen T, Gibson IW, et al. Class II Eplet Mismatch Modulates Tacrolimus Trough Levels Required to Prevent Donor-Specific Antibody Development. J Am Soc Nephrol. 2017;28(11):3353–62. 10.1681/ASN.2017030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatault P, Kamar N, Buchler M, Colosio C, Bertrand D, Durrbach A, et al. Reduction of Extended-Release Tacrolimus Dose in Low-Immunological-Risk Kidney Transplant Recipients Increases Risk of Rejection and Appearance of Donor-Specific Antibodies: A Randomized Study. Am J Transplant. 2017;17(5):1370–9. 10.1111/ajt.14109 . [DOI] [PubMed] [Google Scholar]
- 21.Eckardt KU, Kasiske BL. Kidney disease: improving global outcomes. Nat Rev Nephrol. 2009;5(11):650–7. 10.1038/nrneph.2009.153 . [DOI] [PubMed] [Google Scholar]
- 22.Trofe-Clark J, Brennan DC, West-Thielke P, Milone MC, Lim MA, Neubauer R, et al. Results of ASERTAA, a Randomized Prospective Crossover Pharmacogenetic Study of Immediate-Release Versus Extended-Release Tacrolimus in African American Kidney Transplant Recipients. Am J Kidney Dis. 2018;71(3):315–26. 10.1053/j.ajkd.2017.07.018 . [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Lee J, Huh KH, Park JB, Cho JH, Lee S, et al. KNOW-KT (KoreaN cohort study for outcome in patients with kidney transplantation: a 9-year longitudinal cohort study): study rationale and methodology. BMC Nephrol. 2014;15:77 10.1186/1471-2369-15-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helantera I, Salmela K, Kyllonen L, Koskinen P, Gronhagen-Riska C, Finne P. Pretransplant dialysis duration and risk of death after kidney transplantation in the current era. Transplantation. 2014;98(4):458–64. 10.1097/TP.0000000000000085 . [DOI] [PubMed] [Google Scholar]
- 25.Himmelfarb J, Ikizler TA. Hemodialysis. N Engl J Med. 2010;363(19):1833–45. 10.1056/NEJMra0902710 . [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648–58. 10.1046/j.1523-1755.1999.00273.x . [DOI] [PubMed] [Google Scholar]
- 27.Degenhardt TP, Grass L, Reddy S, Thorpe SR, Diamandis EP, Baynes JW. Technical note. The serum concentration of the advanced glycation end-product N epsilon-(carboxymethyl)lysine is increased in uremia. Kidney Int. 1997;52(4):1064–7. 10.1038/ki.1997.429 . [DOI] [PubMed] [Google Scholar]
- 28.Thishya K, Vattam KK, Naushad SM, Raju SB, Kutala VK. Artificial neural network model for predicting the bioavailability of tacrolimus in patients with renal transplantation. PLoS One. 2018;13(4):e0191921 Epub 2018/04/06. 10.1371/journal.pone.0191921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anutrakulchai S, Pongskul C, Kritmetapak K, Limwattananon C, Vannaprasaht S. Therapeutic concentration achievement and allograft survival comparing usage of conventional tacrolimus doses and CYP3A5 genotype-guided doses in renal transplantation patients. Br J Clin Pharmacol. 2019;85(9):1964–73. Epub 2019/05/12. 10.1111/bcp.13980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim IW, Noh H, Ji E, Han N, Hong SH, Ha J, et al. Identification of factors affecting tacrolimus level and 5-year clinical outcome in kidney transplant patients. Basic Clin Pharmacol Toxicol. 2012;111(4):217–23. Epub 2012/04/04. 10.1111/j.1742-7843.2012.00892.x . [DOI] [PubMed] [Google Scholar]
- 31.Cho JH, Yoon YD, Park JY, Song EJ, Choi JY, Yoon SH, et al. Impact of cytochrome P450 3A and ATP-binding cassette subfamily B member 1 polymorphisms on tacrolimus dose-adjusted trough concentrations among Korean renal transplant recipients. Transplant Proc. 2012;44(1):109–14. Epub 2012/02/09. 10.1016/j.transproceed.2011.11.004 . [DOI] [PubMed] [Google Scholar]
- 32.Floege J, Regele H, Gesualdo L, Group E-EIW. The ERA-EDTA Database on Recurrent Glomerulonephritis following renal transplantation. Nephrol Dial Transplant. 2014;29(1):15–21. Epub 2013/08/30. 10.1093/ndt/gft299 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.