Abstract
Ionizing radiation has historically been used to treat cancer by killing tumour cells, in particular by inducing DNA damage. This view of radiotherapy (RT) as a simple cytotoxic agent has dramatically changed in recent years, and it is now widely accepted that RT can deeply reshape the tumour environment by modulating the immune response. Such evidence gives a strong rationale for the use of immunomodulators to boost the therapeutic value of RT, introducing the era of ‘immunoradiotherapy’. The increasing amount of preclinical and clinical data concerning the combination of RT with immunomodulators, in particular with immune checkpoint inhibitors such as anti‐PD‐1/PD‐L1 and anti‐CTLA4, reflects the interest of the scientific and medical community concerning immunoradiotherapy. The expectations are enormous since the rationale for performing such combinations is strong, with the possibility to use a local treatment such as RT to amplify a systemic antitumour response, as illustrated by the case of the abscopal effect. Nevertheless, several points remain to be addressed such as the need to find biomarkers to identify patients who will benefit from immunoradiotherapy, the identification of the best sequences/schedules for combination with immunomodulators and mechanisms to overcome resistance. Additionally, the effects of immunoradiotherapy on healthy tissues and related toxicity remain largely unexplored. To answer these critical questions and make immunoradiotherapy keep its promising qualities, large efforts are needed from both the pharmaceutical industry and academic/governmental research. Moreover, because of the work of both these entities, the arsenal of available immunomodulators is quickly expanding, thus opening the field to increasing combinations with RT. We thus forecast that the field of immunoradiotherapy will further expand in the coming years, and it needs to be supported by appropriate investment plans.
Keywords: abscopal effect, immune modulation, immunotherapy, radiotherapy, toxicity
This review briefly summarizes the results obtained so far using the combination of immunomodulators with radiotherapy (immunoradiotherapy), both preclinically and clinically, and discusses the perspectives and challenges, including the still limited exploration of the effects of immunoradiotherapy on healthy tissues and related toxicity.

Abbreviations
- cGAS
cyclic GMP‐AMP synthase
- CTLA4
cytotoxic T‐lymphocyte‐associated protein 4
- DAMPs
damage‐associated molecular patterns
- DCs
dendritic cells
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- HMGB1
high‐mobility group box 1
- ICD
immunogenic cell death
- ICI
immune checkpoint inhibitors
- IFNβ
interferon beta
- IR
irradiation
- IRF3
interferon regulatory factor 3
- LAG‐3
lymphocyte‐activation gene 3
- MDSC
myeloid‐derived suppressor cells
- PD‐1
programmed cell death protein 1
- PD‐L1
programmed death‐ligand 1
- RT
radiotherapy
- SBRT
stereotactic body radiation therapy
- STING
stimulator of interferon genes
- TAM
tumour‐associated macrophages
- TGF‐β
transforming growth factor beta
- TIM‐3
T‐cell immunoglobulin and mucin‐domain containing‐3
- Treg
regulatory T cells
1. Introduction
The efficacy of radiotherapy (RT) has largely improved in recent decades, mainly due to improved treatment planning, imaging and novel irradiation (IR) techniques. On the other hand, the promise to pharmacologically improve the therapeutic index of RT, that is, by combining it with drugs such as DNA repair inhibitors, pro‐apoptotic agents or antiangiogenic agents has been broken by unexpected and deceiving results (Chargari et al., 2016). This scenario has largely changed in recent years, and new hopes relying on a combination of RT with drugs have transpired since the ‘immunotherapy revolution’ in oncology has begun. Indeed, a ‘paradigm shift’ has been proposed when a large bulk of experimental data provided enough evidence that some of the effects of ionizing radiation contribute to antitumour immunity (Formenti and Demaria, 2013). Currently, it is widely accepted that RT does not act merely as a cytotoxic agent, but it can deeply reshape the tumour environment in many ways and in particular by modulating the immune response (Frey et al., 2017), giving a strong rationale to use immunomodulators to boost the therapeutic value of RT (immunoradiotherapy).
Herein, we briefly summarize the results obtained so far using immunoradiotherapy, both preclinically and clinically, and we discuss the perspectives and challenges, including the still limited exploration of the effect of immunoradiotherapy on healthy tissues.
2. Rationale for immunoradiotherapy combinations
2.1. Immunostimulation
Historically, the main rationale for the use of ionizing radiation to treat cancer initially came from empirical clinical observations and has been attributed to its cytotoxic activity, in particular by inducing DNA damage eventually leading to tumour cell killing. Consequently, most efforts in classical radiobiology have been spent trying to improve tumour cell killing by IR and/or to reduce the damage to healthy cells, that is, to improve the differential cytotoxic effect of RT (Bhattacharya and Asaithamby, 2017). Even though some effects of RT on the immune system have been known since the late 1970s (Stone et al., 1979), the view of cancer as a mostly cell‐autonomous process together with preclinical research on RT performed using in vitro models and in vivo models in immunodeficient mice did not allow researchers to fully appreciate the contribution of the immune response to the therapeutic effect. When the landscape of oncology research shifted towards a view of cancer as disease strongly affected by the tumour stroma, radiation biology started to accumulate evidence of the involvement of the immune system in mediating its therapeutic efficacy, especially in the last decade.
Ionizing radiation has been recognized as one of the anticancer agents able to induce ‘immunogenic cell death’ (ICD), a type of cell death that promotes a T‐cell‐mediated immune response against antigens derived from dying cells (reviewed in Wennerberg et al., 2017). Ionizing radiation has the potential to activate the key molecular steps (e.g., the translocation of the ER protein calreticulin to the cell surface, the release of the nuclear protein HMGB1 and the release of adenosine triphosphate) described as ‘damage‐associated molecular patterns’ (DAMPs) and that are required to achieve ICD. This process enhances the uptake of tumour‐derived antigens, including neo‐antigens due to immunogenic mutations driven by RT, by antigen‐presenting cells such as dendritic cells (DCs) and macrophages. This action is accompanied by the release of pro‐inflammatory cytokines, such as CXCL10 and CXCL16, which, together with the RT‐promoted process of vascular normalization (Klug et al., 2013; Mondini et al., 2015), can enhance the infiltration of activated CD8+ T cells (Matsumura et al., 2008). It has also recently been reported that newly recruited T cells contribute to RT efficacy and that tumour‐reprogrammed tissue‐resident T cells, which can survive to clinically used RT doses, can mediate tumour control (Arina et al., 2019).
Another central element contributing to immunostimulation by RT is the induction of the interferon (IFN) cascade through the activation of the STING DNA‐sensing pathway. After exposure to IR, DNA can shuttle to the cytoplasm and be sensed by cGAS, which triggers the nuclear translocation of STING. Through a signalling cascade that leads to the activation of IRF3, STING leads to the production of type I interferon (Galluzzi et al., 2018), fostering the maturation of DCs and their antigen presentation to T cells, contributing to the amplification of an antitumour adaptive immune response. It interesting to note that the secretion of type I IFN appears to be dependent on the irradiation dose, where 8 Gy fractions induce a strong upregulation of IFNβ levels, while higher doses failed to achieve this effect.
In addition, RT has been shown to enhance the expression of major histocompatibility complex‐I molecules favouring antigen presentation (Reits et al., 2006). IR doses in the range of those most used in clinical settings (2 Gy) have also been shown to reprogram the phenotype of tumour‐associated macrophages (TAMs) towards pro‐immunogenic behaviour (Klug et al., 2013).
All these observations demonstrate that RT can trigger both the adaptive and innate immune responses towards antitumour activity, thus enhancing immunotherapy.
2.2. Abscopal effect
The occurrence of tumour responses at sites distant from the irradiated volume has been known for more than 60 years and is termed the abscopal effect (Brix et al., 2017). The abscopal effect is a rare event, and the number of cases described in the literature is extremely low. Indeed, only 47 cases were reported from 1960 to 2018 in patients treated with RT alone. With the advent of the immunoradiotherapy era, this number is quickly growing, with the same number of cases (47) described in 6 years (2012–2018) in patients treated with immunomodulators combined with RT compared to 50 years for RT‐only treated patients (Dagoglu et al., 2019). This finding likely results from the induction of a systemic immune response trigger by the combined immunostimulatory effect of RT with immunotherapy. Although investigated in preclinical settings, the precise mechanisms underlying abscopal responses remain unclear and relatively unexplored in clinical settings (Rodriguez‐Ruiz et al., 2019), even if the first clues stem from dedicated clinical trials (Formenti et al., 2018; Golden et al., 2015), in which immunoradiotherapy resulted in 18–27% of abscopal responses.
One of the most ambitious goals of immunoradiotherapy is to make the occurrence of the abscopal response systematic. In addition, the abscopal effect is a direct witness of the systemic synergy of immunoradiotherapy that can also be exploited at the locally advanced tumour stage to prevent metastasis and increase control, as illustrated by the recent approval of immune checkpoints inhibitors (ICI) adjuvant to chemoradiation in locally advanced lung cancer. A large amount of investigations is still needed to understand why abscopal effect remains a relatively rare phenomenon, with its clinical benefit restricted to a proportion of patients. One explanation is that the immunosuppressive mechanisms amplified by RT could limit a widespread systemic immune response.
2.3. Immunosuppression
As the balance between stimulating and suppressive signals finely tunes the activity of the immune system, it is not surprising that together with immunostimulatory responses, RT can also trigger immunosuppression. For example, RT has been shown to upregulate the expression of the immune checkpoint PD‐L1 in both preclinical (Deng et al., 2014) and clinical settings (Twyman‐Saint Victor et al., 2015), thus limiting the activation of tumour T cells. RT can also enhance the release of immunosuppressive cytokines such as transforming growth factor beta (TGF‐β) in the tumour environment. TGF‐β can repress the proliferation, activation and effector function of T cells and can also impact the maturation and function of tumour natural killer cells and macrophages; on the other hand, TGF‐β promotes Treg differentiation (Dahmani and Delisle, 2018). Tregs can contribute to immunosuppression after RT, as their infiltration is increased in irradiated tumours (Mondini et al., 2019; Muroyama et al., 2017). RT also triggers an influx of myeloid cells through the upregulation of the secretion of chemokines such as CCL2 by tumours, which can contribute to the generation of an immunosuppressive environment (Kalbasi et al., 2017; Mondini et al., 2019).
The interplay between these populations and inhibitory signals can thus impair or limit an effective antitumour immune response after irradiation.
2.4. Combination of radiotherapy with immunotherapeutics
Given the essential role of lymphocytes in the response to RT, the combination of RT with immune checkpoint inhibitors (ICI) can unleash the potential of the T‐cell compartment. Associations of anti‐CTLA4 with RT were among the first immunoradiotherapy combinations tested, which gave promising results such as the induction of systemic immune responses. As PD‐L1 expression is increased after RT, the combination of RT with anti‐PD‐1/PD‐L1 has a strong rationale, and it has been widely tested in preclinical settings and then translated into clinical trials (Shevtsov et al., 2019).
Even if the combination of RT and anti‐CTLA4 or PD‐1/PD‐L1 can ameliorate the efficacy of RT, several mechanisms of resistance to therapies can be developed by the tumours. Thus, many additional immunomodulators are being tested in combination with RT (either alone or together with anti‐CTLA4 and anti‐PD‐1/PD‐L1) such as ICI including anti‐TIM‐3 or anti‐LAG‐3. Other strategies for novel immunoradiotherapies include the administration of costimulatory molecules as agonists of OX‐40 or CD‐40. The modulation of the tumour environment also has a strong rationale, such as targeting the TGF‐β pathway or chemo‐attractive axes such as that of CCL2/CCR2. Other new treatment approaches are based on the use of agents aimed at increasing antigen presentation as agonists of Toll‐like receptors or at boosting the RT‐induced interferon response using modulators of the cGAS/STING pathway.
The arsenal of available immunomodulators is rapidly expanding, paving the way for novel potent immunoradiotherapies but also unravelling several new challenges, such as identifying the optimal combination of molecules, the sequence of administration and the handling of potential associated toxicities.
3. Immunoradiotherapy and normal tissue toxicity
The enthusiasm for immunotherapy and radiation therapy combinations also raises the question of normal tissue toxicity and the safety of these treatments (Deutsch et al., 2019). For radiation therapy alone, normal tissue injuries are the main limiting factor of the dose that could be delivered to the target. For organs at risk, tolerance doses and volumes are defined according to clinical treatment guidelines and are based on several parameters including the fractionation of the dose and the organization of the organ at risk. Moreover, concerning immunotherapy alone, previous clinical reports have described acute and long‐term toxicities to the skin, colon, liver and lungs (Michot et al., 2016). For example, lung cancer will increasingly be treated using stereotactic body radiation therapy (SBRT), and several clinical trials plan to combine SBRT and ICI (Lin et al., 2019). Moreover, it was reported that patients treated with concomitant ICI and SBRT can develop radiation pneumonitis even if it remains unclear whether the pneumonitis is due to SBRT or to an enhancing effect of the combination with ICI (Delaunay et al., 2017; Louvel et al., 2018). Preclinical experiments using small animals are very useful in translational cancer research and the clinical implementation of novel treatments. The influence of dose fractionation has not yet been satisfactorily investigated in depth. Preclinical models are now available to model lung SBRT in rodents, and the method to deliver the dose (in terms of fraction) associated with immunotherapy could be used to obtain crucial information about putative acute and long‐term toxicity (Lavigne et al., 2019). Moreover, follow‐up clinical data are not yet available about the effects of SBRT/immunotherapy combination, and prospective data from a large series of patients regarding safety are a key issue for the future.
Radiation‐induced normal tissue injuries are characterized by a very complex dynamic process involving a large number of molecular and cellular factors. The immune system is known to play a pivotal role not only in the onset of cancer development, but also in radiation‐induced normal tissue injury. There is a close association between inflammation and injury, and innate immune cells, including neutrophils, monocytes and macrophages, are the first line of defence against infection and release highly toxic chemicals to kill pathogens. Most of these molecules act as DAMPs. However, similar mediators are also released from immune cells for the resolution of injury and the proper wound healing process. The outcome (toxicity or not) is dependent on many parameters, including the phenotype and molecular footprints of these cells, their relative spatial localization and the dynamics of their recruitment/elimination. It is crucial to obtain more information about the effect of immunotherapy/RT combinations on the temporal and spatial effects on immune cells, which are involved in normal tissue toxicity. For example, it was shown that TAMs express PD‐1 and that immunotherapy could also act through a direct effect on macrophages (Gordon et al., 2017). However, macrophages play a crucial role not only in the development of inflammation but also in its resolution as well as in tissue regeneration. Details are still lacking about what happens to macrophage subpopulations in normal tissues exposed to immunoradiotherapy and the consequences of their putative toxic effects (Meziani et al., 2018). From a more general point of view, this issue raises the question about the relative contribution and biologic significance of PD‐1/PD‐L1 expression by cells in normal tissue and how such expression could be involved in response to immunoradiotherapy combination regimens. Pertinent preclinical models combined with state‐of‐the‐art methods of molecular biology such as ‘single‐cell RNA‐seq’ could help answer several concerns and notably to exactly identify the effects of RT/immunotherapy combinations on both pro‐regenerating cell types and cell types involved in the toxic effects.
Experiments to obtain information on the toxicity of normal tissues will be very important for translation into clinical applications. In general, these concerns are not priorities compared to research projects aimed at investigating the antitumour effects of combination treatments. However, the recent history of combinations of targeted therapies with radiotherapy (such anti‐antigenic factors) has taught us that this aspect should not be overlooked.
4. Immunoradiotherapy in the clinical tracks
Immunotherapies using anti‐PD‐L1 and/or anti‐CTLA4 have recently become new standards of care in several cancer types. Given the strong abovementioned rationale for combining ICIs with radiotherapy, there are a rapidly increasing number of immunoradiotherapy clinical trials (Fig. 1; Cushman et al., 2018; Kang et al., 2016). The first clinical advantage of triggering a systemic (out of the radiation field, ‘abscopal’), immune response was presented in a melanoma patient progressing on ipilimumab (anti‐CTLA4, Postow et al., 2012). A secondary analysis of a phase I study assessing the anti‐PD‐1 agent pembrolizumab in patients with advanced non‐small‐cell lung cancer (NSCLC) then showed that patients who received radiotherapy before (median: 9 months) the anti‐PD1 treatment had increased progression‐free survival (PFS) and overall survival (OS) compared with patients who did not receive prior radiotherapy (Shaverdian et al., 2017). One of the largest randomized clinical trials (CA184‐043 study) on advanced castration‐resistant prostate cancer receiving palliative (single fraction of 8 Gy) radiotherapy on a bony lesion compared the anti‐CTLA4 agent ipilimumab versus placebo. The trial was negative for OS, but it should be noted that ipilimumab alone (without irradiation) did not become a standard in such patients (Kwon et al., 2014). The recent PACIFIC trial assessing the anti‐PD‐L1 agent durvalumab as consolidation after thoracic chemoradiotherapy in NSCLC patients demonstrated an improved OS compared to placebo (Antonia et al., 2018). In a multicentre, randomized phase 2 study (PEMBRO‐RT) of 92 patients with advanced NSCLC, better outcomes were observed when SBRT (3 times 8 Gy) was administered 7 days before the anti‐PD1 agent pembrolizumab compared to the nonirradiated group (Theelen et al., 2019). Since then, several prospective trials have assessed the addition of a PD‐1/PD‐L1 to concurrent chemoradiotherapy (e.g., NCT03728556, NCT03519971, NCT03745222: RATIONALE001) or to SBRT (e.g., NCT03774732: NIRVANA‐Lung) in NSCLC patients.
Fig. 1.
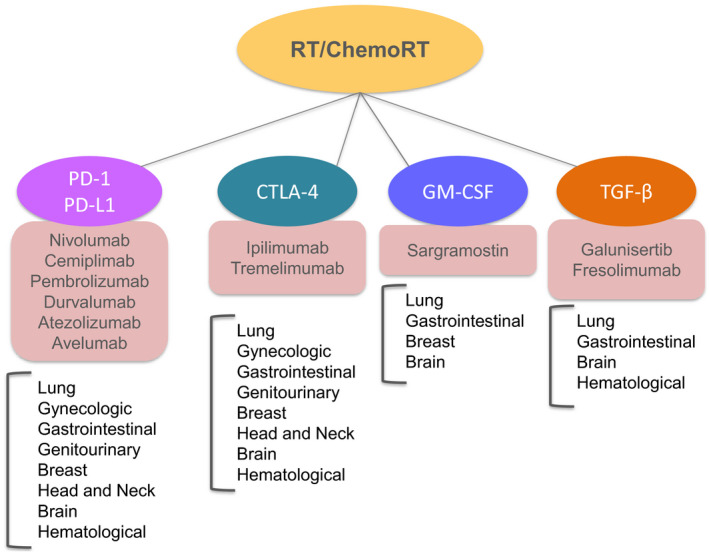
Immunomodulators used in ongoing/past clinical trials, in combination with radiotherapy/chemoradiotherapy. For each immunotherapy category, main drug names and the tumour sites investigated are listed.
The results from the first clinical trials, though encouraging, have shown that most patients do not respond to immunoradiotherapy combinations. Indeed, several limitations should be highlighted. (a) The best surrogate immune biomarker for radioimmunotherapy remains to be confirmed. In an unplanned post hoc subgroup analysis based on PD‐L1 expression levels on tumour cells at initial biopsy, a benefit in OS was not proven in the PD‐L1‐negative patients of the PACIFIC trial (Antonia et al., 2018). In contrast, positive results were largely influenced by the PD‐L1‐negative subgroup in the PEMBRO‐RT trial (Theelen et al., 2019). Further potential predictive and prognostic immune assays are being studied at the cellular (tumour microenvironment composition), genomic (mutational/neoantigen load) and peripheral (blood and microbiota) levels (Levy et al., 2017). Radiomics approaches, using artificial intelligence algorithms to analyse radiographic characteristics, have been recently proposed as noninvasive biomarkers for response to immunotherapy and may be useful to improve patient stratification and for predicting clinical outcomes of patients treated with immunotherapy (Sun et al., 2018; Trebeschi et al., 2019). (b) The best volume of irradiation in patients receiving ICI may be different from that in patients without receiving immunostimulatory agents, in particular concerning the need to perform elective lymph node irradiation (Luke et al., 2018). Irradiation in great vessels and draining lymph nodes (main location of T‐cell cross‐priming by DCs) could affect immune cell functions and migration (Deutsch et al., 2019). Modern techniques such as volumetric modulated arc therapy (VMAT) induce larger volumes of healthy tissues receiving low doses of ionizing radiation that could affect circulating lymphocytes and decrease the adaptive immune response (Tang et al., 2014). (c) The immune response may depend on the timing, the number of irradiated sites and the employed dose fractionation. The irradiation of many lesions within multiple tissue beds could increase the repertoire of released antigens and activate immune signals from various tumour microenvironments (Brooks and Chang, 2019). For instance, in the CA184‐043 trial, a single dose fraction of 8 Gy to the bone did not lead to strong immune stimulation (Kwon et al., 2014), while multiple fractions (at least 3 sessions) of ‘moderate’ dose level to the lung (cf. PEMBRO‐RT study) have been more efficient in stimulating the immune response (Theelen et al., 2019). (d) Negative effects (radiation‐induced toxicity and immune‐related events) could also be increased through the synergistic stimulation of both local and systemic immunities. Ideally, the pretreatment determination of individuals’ normal tissue and/or tumour sensitivity will help identify patients more likely to achieve the best therapeutic index ratio (Chargari et al., 2016). (e) Abscopal response, as it has not been sufficiently well documented. Stratification of patients and monitoring of out‐of‐field responses are, however, now incorporated in current trials (e.g. NCT03426657 and NCT03386357 for head and neck cancers).
5. Conclusions
The large and increasing amount of preclinical and clinical data concerning the combination of immunomodulators with radiotherapy reflects the great interest of the scientific and medical community concerning immunoradiotherapy. The expectations are enormous since the rationale for performing such combinations is strong, and the first positive results are coming from clinical settings. Nevertheless, several points remain to be addressed or better elucidated (Fig. 2) such as (a) what are the best sequences/schedules to follow when combining RT with immunomodulators; (b) how to select the best types of immunotherapy; (c) what are the effects of immunotherapies on healthy tissues; (d) which biomarkers are useful for selecting the best candidates for immunoradiotherapies; (e) how to overcome resistance and increase the number of responsive patients; and (f) how to increase the (still very limited) number of systemic antitumour responses leading to an effective abscopal effect.
Fig. 2.
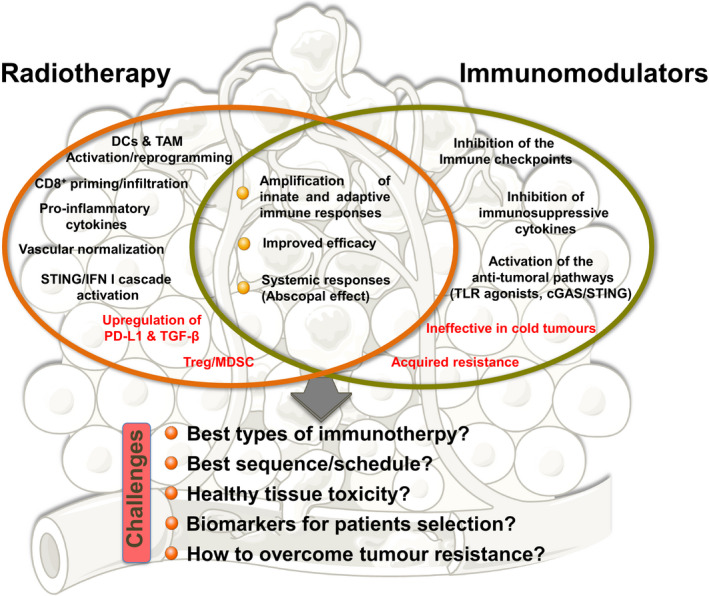
Strengths, weaknesses and challenges of radiotherapy and immunotherapy combinations.
To answer these critical questions and make immunoradiotherapy keep its promising qualities, great efforts are needed from both the pharmaceutical industry and academic/governmental research. Moreover, because of the work of both these entities, the arsenal of available immunomodulators is quickly expanding, thus providing the field with increasing combinations with RT. We thus forecast that the field of immunoradiotherapy will further expand in the coming years, and it needs to be supported by appropriate investment plans.
Conflict of interest
ED, MM and LM declare grants from Roche Genentech, Servier, AstraZeneca, Merck Serono, Bristol‐Myers Squibb, Boehringer Ingelheim, Eli Lilly and MSD, outside the submitted work. ED declares personal fees from Roche Genentech, AstraZeneca, MSD, AMGEN, Accuray and Boehringer Ingelheim outside the submitted work. ED declares shared patents with NH‐Theraguix and Clevexel.
Acknowledgements
The authors received financial support from INSERM, SIRIC SOCRATE, Fondation ARC pour la recherche sur le cancer, Agence Nationale de la Recherche (ANR) and Institut National du Cancer (INCa 2018‐1‐PL BIO‐06‐1 and 2014‐1‐PL BIO‐03).
Contributor Information
Michele Mondini, Email: michele.mondini@gustaveroussy.fr.
Eric Deutsch, Email: eric.deutsch@gustaveroussy.fr.
References
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M et al (2018) Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379, 2342–2350. [DOI] [PubMed] [Google Scholar]
- Arina A, Beckett M, Fernandez C, Zheng W, Pitroda S, Chmura SJ, Luke JJ, Forde M, Hou Y, Burnette B et al (2019) Tumor‐reprogrammed resident T cells resist radiation to control tumors. Nat Commun 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S and Asaithamby A (2017) Repurposing DNA repair factors to eradicate tumor cells upon radiotherapy. Transl Cancer Res 6, S822–S839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix N, Tiefenthaller A, Anders H, Belka C and Lauber K (2017) Abscopal, immunological effects of radiotherapy: narrowing the gap between clinical and preclinical experiences. Immunol Rev 280, 249–279. [DOI] [PubMed] [Google Scholar]
- Brooks ED and Chang JY (2019) Time to abandon single‐site irradiation for inducing abscopal effects. Nat Rev Clin Oncol 16, 123–135. [DOI] [PubMed] [Google Scholar]
- Chargari C, Magne N, Guy J‐B, Rancoule C, Levy A, Goodman KA and Deutsch E (2016) Optimize and refine therapeutic index in radiation therapy: overview of a century. Cancer Treat Rev 45, 58–67. [DOI] [PubMed] [Google Scholar]
- Cushman TR, Caetano MS, Welsh JW and Verma V (2018) Overview of ongoing clinical trials investigating combined radiotherapy and immunotherapy. Immunotherapy 10, 851–850. [DOI] [PubMed] [Google Scholar]
- Dagoglu N, Karaman S, Caglar HB and Oral EN (2019) Abscopal effect of radiotherapy in the immunotherapy era. Systematic review of reported cases. Cureus 11, e4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmani A and Delisle J‐S (2018) TGF‐β in T cell biology: implications for cancer immunotherapy. Cancers (Basel) 10, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro‐Sibilot D, Michot J‐M, Raimbourg J, Girard N, Guisier F et al (2017) Immune‐checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 50, pii: 1700050. [DOI] [PubMed] [Google Scholar]
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR and Fu Y‐X (2014) Irradiation and anti‐PD‐L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124, 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch E, Chargari C, Galluzzi L and Kroemer G (2019) Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol 20, e452–e463. [DOI] [PubMed] [Google Scholar]
- Formenti SC and Demaria S (2013) Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 105, 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formenti SC, Rudqvist N‐P, Golden E, Cooper B, Wennerberg E, Lhuillier C, Vanpouille‐Box C, Friedman K, de Andrade LF, Wucherpfennig KW et al (2018) Radiotherapy induces responses of lung cancer to CTLA‐4 blockade. Nat Med 24, 1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B, Rückert M, Deloch L, Rühle PF, Derer A, Fietkau R and Gaipl US (2017) Immunomodulation by ionizing radiation‐impact for design of radio‐immunotherapies and for treatment of inflammatory diseases. Immunol Rev 280, 231–248. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vanpouille‐Box C, Bakhoum SF, and Demaria S (2018) SnapShot: CGAS‐STING signalling. Cell 173, 276–276.e1. [DOI] [PubMed] [Google Scholar]
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton‐Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J et al (2015) Local radiotherapy and granulocyte‐macrophage colony‐stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof‐of‐principle trial. Lancet Oncol 16, 795–803. [DOI] [PubMed] [Google Scholar]
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D et al (2017) PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, Ben‐Josef E and Beatty GL (2017) Tumor‐derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res 23, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Demaria S and Formenti S (2016) Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 4, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L et al (2013) Low‐dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602. [DOI] [PubMed] [Google Scholar]
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, Krainer M, Houede N, Santos R, Mahammedi H et al (2014) Ipilimumab versus placebo after radiotherapy in patients with metastatic castration‐resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184‐ 043): a multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol 15, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne J, Suissa A, Verger N, Dos Santos M, Benadjaoud M, Mille‐Hamard L, Momken I, Soysouvanh F, Buard V, Guipaud O et al (2019) Lung stereotactic arc therapy in mice: development of radiation pneumopathy and influence of HIF‐1α endothelial deletion. Int J Radiat Oncol Biol Phys 104, 279–290. [DOI] [PubMed] [Google Scholar]
- Levy A, Nigro G, Sansonetti PJ and Deutsch E (2017) Candidate immune biomarkers for radioimmunotherapy. Biochim Biophys Acta Rev Cancer 1868, 58–68. [DOI] [PubMed] [Google Scholar]
- Lin AJ, Roach M, Bradley J and Robinson C (2019) Combining stereotactic body radiation therapy with immunotherapy: current data and future directions. Transl Lung Cancer Res 8, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvel G, Bahleda R, Ammari S, Le Péchoux C, Levy A, Massard C, Le Pavec J, Champiat S and Deutsch E (2018) Immunotherapy and pulmonary toxicities: can concomitant immune‐checkpoint inhibitors with radiotherapy increase the risk of radiation pneumonitis? Eur Respir J 51, 1701737. [DOI] [PubMed] [Google Scholar]
- Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, Al‐Hallaq HA, Arina A, Khodarev NN, Janisch L et al (2018) Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 36, 1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML et al (2008) Radiation‐induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 181, 3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziani L, Deutsch E and Mondini M (2018) Macrophages in radiation injury: a new therapeutic target. Oncoimmunology 7, e1494488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel‐Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A et al (2016) Immune‐related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54, 139–148. [DOI] [PubMed] [Google Scholar]
- Mondini M, Loyher P‐L, Hamon P, Gerbé de Thoré M, Laviron M, Berthelot K, Clémenson C, Salomon BL, Combadière C, Deutsch E et al (2019) CCR2‐dependent recruitment of Tregs and monocytes following radiotherapy is associated with TNFα‐mediated resistance. Cancer Immunol Res 7, 376–387. [DOI] [PubMed] [Google Scholar]
- Mondini M, Nizard M, Tran T, Mauge L, Loi M, Clémenson C, Dugue D, Maroun P, Louvet E, Adam J et al (2015) Synergy of radiotherapy and a cancer vaccine for the treatment of HPV‐associated head and neck cancer. Mol Cancer Ther 14, 1336–1345. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Nirschl TR, Kochel CM, Lopez‐Bujanda Z, Theodros D, Mao W, Carrera‐Haro MA, Ghasemzadeh A, Marciscano AE, Velarde E et al (2017) Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol Res 5, 992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E et al (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J et al (2006) Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203, 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Ruiz ME, Rodriguez I, Leaman O, López‐Campos F, Montero A, Conde AJ, Aristu JJ, Lara P, Calvo FM and Melero I (2019) Immune mechanisms mediating abscopal effects in radioimmunotherapy. Pharmacol Ther 196, 195–203. [DOI] [PubMed] [Google Scholar]
- Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P (2017) Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non‐small‐cell lung cancer: a secondary analysis of the KEYNOTE‐001 phase 1 trial. Lancet Oncol 18, 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsov M, Sato H, Multhoff G and Shibata A (2019) Novel approaches to improve the efficacy of immuno‐radiotherapy. Front Oncol 9, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone HB, Peters LJ and Milas L (1979) Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst 63, 1229–1235. [PubMed] [Google Scholar]
- Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S et al (2018) A radiomics approach to assess tumour‐infiltrating CD8 cells and response to anti‐PD‐1 or anti‐PD‐L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol 19, 1180–1191. [DOI] [PubMed] [Google Scholar]
- Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, Hong DS, Komaki R and Welsh JW (2014) Lymphopenia association with gross tumor volume and lung V5 and its effects on non‐small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 89, 1084–1091. [DOI] [PubMed] [Google Scholar]
- Theelen WSME, Peulen HMU, Lalezari F, van der Noort V , de Vries JF , Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer A‐LN, de Langen AJ et al (2019) Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non‐small cell lung cancer: results of the PEMBRO‐RT phase 2 randomized clinical trial. JAMA Oncol 5, 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebeschi S, Drago SG, Birkbak NJ, Kurilova I, Cǎlin AM, Pizzi AD, Lalezari F, Lambregts DMJ, Rohaan M, Parmar C et al (2019) Predicting response to cancer immunotherapy using non‐invasive radiomic biomarkers. Ann Oncol 30, 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman‐Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM et al (2015) Radiation and dual checkpoint blockade activate non‐redundant immune mechanisms in cancer. Nature 520, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg E, Vanpouille‐Box C, Bornstein S, Yamazaki T, Demaria S and Galluzzi L (2017) Immune recognition of irradiated cancer cells. Immunol Rev 280, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]


