Abstract
Radiotherapy remains a mainstay of cancer treatment, being used in roughly 50% of patients. The precision with which the radiation dose can be delivered is rapidly improving. This precision allows the more accurate targeting of radiation dose to the tumor and reduces the amount of surrounding normal tissue exposed. Although this often reduces the unwanted side effects of radiotherapy, we still need to further improve patients’ quality of life and to escalate radiation doses to tumors when necessary. High‐precision radiotherapy forces one to choose which organ or functional organ substructures should be spared. To be able to make such choices, we urgently need to better understand the molecular and physiological mechanisms of normal tissue responses to radiotherapy. Currently, oversimplified approaches using constraints on mean doses, and irradiated volumes of normal tissues are used to plan treatments with minimized risk of radiation side effects. In this review, we discuss the responses of three different normal tissues to radiotherapy: the salivary glands, cardiopulmonary system, and brain. We show that although they may share very similar local cellular processes, they respond very differently through organ‐specific, nonlocal mechanisms. We also discuss how a better knowledge of these mechanisms can be used to treat or to prevent the effects of radiotherapy on normal tissue and to optimize radiotherapy delivery.
Keywords: brain, cardiopulmonary system, dose distribution, normal tissue effects, salivary gland
Here, we discuss the complexity of radiotherapy‐induced side effects and approaches for prevention or treatment. Radiation damage develops at a cellular level, progresses to intercellular interactions resulting in intra‐ and interorgan functional responses. These need different approaches for prevention or treatment, for instance intracellular signaling modification, sparing of specific stem cell‐containing areas or contributing organs, and stem cell therapies.
Abbreviations
- ACE
angiotensin‐converting enzyme
- ATM
ataxia telangiectasia mutated
- BBB
blood–brain barrier ; CSF1R, colony‐stimulating factor‐1 receptor ;
- CREB
cAMP response element‐binding
- ECM
extracellular matrix
- ESC
embryonic stem cells
- FDA
food and drug administration ;
- FGF
fibroblast growth factor
- GDNF
glial cell line‐derived neurotrophic factor
- HNC
head and neck cancer
- IMRT
intensity‐modulated radiotherapy
- MSC
mesenchymal stem cells
- NOS3
nitric oxide synthase 3
- OPC
oligodendrocyte progenitor cells
- PSC
pluripotent stem cells
- RAAS
renin–angiotensin–aldosterone system
- ROS/RNS
reactive oxygen and nitrogen species
- SASP
senescence‐associated secretory phenotype
- SGZ
subgranular zone
- SSPC
salivary gland stem/progenitor cells
- SVZ
subventricular zone
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- VMAT
volumetric modulated arc therapy
1. Introduction
The number of new cancer cases per year is estimated to rise to 22.2 million by the year 2030 worldwide [1], and about 12 million patients will receive radiotherapy as part of their treatment [2,3]. Although radiotherapy is well tolerated by most patients, some experience radiation‐induced side effects, the severity and frequency of which can be reduced by modern, more precise therapies, such as particle therapy and advanced image‐guided technologies. This improved precision can be used to minimize the radiation dose to normal tissue thereby reducing side effects, but can also be used for escalation of dose to poorly responding tumors without increasing the risk of side effects.
Currently, oversimplified approaches using constraints on mean doses and irradiated volumes of normal tissues receiving a specified dose are used to plan treatments with minimized risk of radiation side effects. Initially, consensus publications such as the Emami paper were the main sources for constraints [4]. More recently, these have been updated by systematic literature reviews, such as the one performed in the Quantec effort [5].
Modern radiotherapy techniques, such as intensity‐modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) (Box 1), reduce the amount of normal tissue that receives a high dose of radiation but at the cost of large volumes of tissue receiving a low dose. In contrast, particle therapy (Box 1) allows to concentrate a high dose of radiation to the tumor while limiting the integral dose to normal tissues. As such, modern radiotherapy technologies offer greater precision but their optimal use requires radiation oncologists to have a better understanding of how these therapies affect normal tissue.
BOX 1.
Radiotherapy techniques
From the 90s until today, radiotherapy has undergone a strong technological development aimed at improving precision of radiation dose delivery to the tumor while minimizing the dose to the normal tissue. By the end of the 90s, 3‐dimensional conformal radiotherapy (3DCRT) was introduced. In this technique, 3D imaging data prior to treatment are used to design a limited number of radiation beams with a fixed shape and uniform dose distribution matching the shape of the tumor volume. During the first decade of this century, this technique was enhanced into IMRT, which allows variations of dose within each beam, thus providing a new dimension of optimization. Typically IMRT also uses more beams than 3DCRT allowing a more conformal dose distribution. In the past decade, this was further developed into techniques irradiating while rotating the irradiator around the patient in VMAT. This technique results in arcs rather than discrete beams. Though technically all of these modalities use anatomical and more recently also functional information obtained before the start of treatment, utility of imaging obtained during the treatment was recognized. The use of such imaging is termed image‐guided radiotherapy. In parallel to the development of these photon‐based irradiation techniques, particle therapy, which is based on the use of ions, such as protons in proton therapy or carbon ions, can offer opportunities to further reduce the radiation dose to the normal tissue. In contrast to photons, particle therapy aims to achieve radiation dose deposition concentrated predominantly at a precise depth by exploiting the intrinsic physical properties of ions. This allows additional sparing of the normal tissue.
The risk of side effects on normal tissue depends on the radiation dose and the volume of normal tissue irradiated [6]. Recently, it has been shown that volume effects can be region‐dependent [7] and can even involve interactions between different organs [8,9]. Recent technologies might, for the first time, allow treatments to be optimized by taking into account such intra‐organ variations in sensitivity and interorgan interactions. In this review, we show that such optimization requires knowledge of the tissue and organ‐level mechanisms that are responsible for such regional variations and organ interactions. To this end, we address different aspects of the mechanisms of radiation‐induced normal tissue effects in general and more specifically of the salivary glands, cardiopulmonary system, and brain. These three organs exhibit similar local cellular responses but nevertheless differ strongly in their response to radiotherapy due to fundamental differences in tissue organization and in the consequences of tissue damage for their function. We discuss these consequences, focusing on their implications for the prevention and treatment of radiation‐induced side effects.
2. Radiation‐induced side effects
Radiation activates a damage repair cascade in normal tissues. This cascade initiates with the DNA damage response that includes apoptosis, mitotic cell death, and cellular senescence [10], and is followed by a perpetual cytokine cascade, which induces inflammation and excessive extracellular matrix (ECM) and collagen deposition, processes that are largely modulated by reactive oxygen and nitrogen species (ROS/RNS) imbalance and tissue hypoxia [11]. The side effects of radiotherapy in normal tissue can be divided into early (or acute) and late responses, depending mostly on tissue turnover time and their modulation by processes that mimic a wound healing response [11]. Early (or acute) side effects occur during, immediately after, or soon after (within weeks of) radiotherapy treatment [11,12]. Early side effects are often reversible when the dose is limited and tissue turnover is high, such as in the oral mucosa [13] and gut, or partly reversible, such as in lungs (pneumonitis) [14], skin [15], and brain (memory loss and fatigue [16]).
Late normal tissue side effects are defined by their occurrence several months to years after radiotherapy [11,12]. Late side effects are in general chronic and often progressive, leading to a reduction in patients’ quality of life following treatment. These are, therefore, often employed to determine radiation dose limits [11]. In contrast to early side effects, the time to response of late‐responding tissues depends on the dose and is modulated by processes such as cellular senescence, chronic inflammation, hypoxia, and fibrosis [11]. All of these responses subsequently inhibit the regenerative potential of the tissue. Importantly, fibrosis is involved in the pathogenesis of side effects in most tissues, such as heart [17], lung [14], and liver [18].
Although different normal tissues may share very similar localized cellular processes, they may respond very differently owing to organ‐specific, nonlocal mechanisms, such as loss of peripheral tissue secondary to loss of stem cells located in specific regions and functional dependence between organs. In the following sections, we describe such responses for three different tissues, the salivary gland, lung, and brain. We also illustrate how these responses can offer unique opportunities for therapeutic and preventative strategies.
3. Salivary glands
Most head and neck cancer (HNC) patients are treated with radiotherapy alone, or in combination with chemotherapy and/or surgery. This often results in the unavoidable co‐irradiation of the peripherally positioned salivary glands. Forty percent of HNC patients receiving IMRT will experience moderate or severe xerostomia (‘dry mouth syndrome’), resulting from hyposalivation, leading to alterations in speech and taste, difficulties with mastication and deglutition, and an increased risk of developing oral infections and dental caries [19, 20, 21]. These sequelae severely hamper the quality of life of affected patients.
3.1. Cellular and tissue responses over time
Salivary glands contain saliva‐producing mucous and serous acinar cells, myoepithelial cells, duct cells, cholinergic and adrenergic nerve fibers, blood vessels, and supporting stromal tissue [22,23], which can all be affected by irradiation. Interestingly, although salivary gland parenchymal cells are mostly postmitotic with a cell turnover time of 60–120 days, their response to radiation is acute as observed both in rodents [24] and in humans [25] and is followed by a later response, which is induced by different mechanisms [26,27] (Fig. 1). The early response cannot be due to mitotic failure and has been attributed to several abnormalities in murine and rhesus monkey: the apoptosis of acinar cells [28,29], the membrane damage‐induced dysfunction of acinar cells [24,26], the impairment of microvessels [30], and reduced parasympathetic signaling [31]. A major characteristic of late radiation‐damaged salivary glands is the accumulation of chronic inflammation and fibrosis and consequent tissue dysfunction and atrophy [11,24] (Fig. 1). This coincides with a lack of regenerative potential of salivary gland stem/progenitor cells (SSPCs) [32]. Indeed, the radiation‐surviving SSPCs in and outside of the radiation field have been shown to determine the regenerative capacity of the gland post‐treatment [32,33]. Interestingly, senescent cells accumulate in the murine salivary gland ducts [34], where SSPCs seem to reside [32]. These senescent cells develop a unique secretory phenotype, called senescence‐associated secretory phenotype (SASP). The SASP includes several proinflammatory and profibrotic growth factors [10] and is associated with reduced tissue regenerative capacities, inflammatory processes, and fibrosis [35]. In rodent models, acini have been shown to have some regeneration capacity even 1 year after treatment, as large acinar cell clusters have been found in irradiated salivary gland tissues (Fig. 1) [36,34]. This is probably due to acinar cell proliferation. However, the resulting clusters do not seem to be functional [36]. While there is relative consensus in the field regarding the late effects of radiation on salivary glands, there is less clarity about the early effects, which depend on the preclinical model used. The salivary glands of different rodent species and strains respond quite differently to irradiation. FVB mice [38] and Wistar rats [26, 27, 28] have a clear early response [38], whereas C57BL/6 mice have a relatively mild early response but a strong late response [39]. Additionally, different radiation dose tolerance and responses depend on whether the radiation field is localized or includes the whole head [40].
Fig. 1.
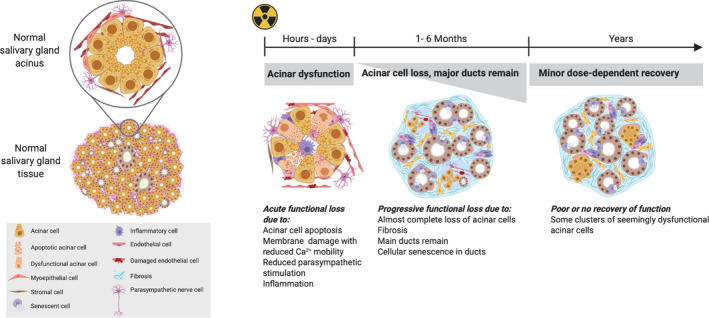
A schematic of the cellular and tissue responses of the salivary gland to radiotherapy over time. (Left panel) The left panel depicts a model of a salivary gland and shows the structure of the acinus, which when enlarged features the different cell types it is composed of. (Right panel) The early and late responses of salivary gland tissue to radiotherapy. The early response (which occurs within hours or days) is completely different mechanistically to the late response. The early response is too rapid to be explained by mitotic failure or related cell death and seems to be due to failure of vasculature function, parasympathetic nerve function, acinar cell signal transduction, and possibly inflammation and acinar cell apoptosis (which is limited, depending on the experimental model used). The later effects (which occur > 30 days after radiotherapy) result from acinar cell loss, which coincides with chronic inflammation and fibrosis. Depending on the radiotherapy dose used, some morphological recovery might follow, as shown by the appearance of acinar cell clusters.
3.2. Therapeutic approaches
Our increased level of understanding of radiation‐induced damage has led to a multitude of therapeutic strategies to ameliorate salivary gland radiation damage. These have recently been reviewed by Jensen et al. [41], here, we focus on some that are related to the above‐described mechanisms.
Many radical scavengers have been tested in a variety of models. ROS/RNS scavengers aim to reduce the effective radiation dose to the tissue, thereby potentially sparing normal tissue cells but not the tumor. One such scavenger is amifostine, which in rat salivary glands shows amelioration of function loss depending on the irradiated region in rat salivary glands [42] and improved protection when it is injected in a retrograde manner in the secretory ducts [43], where SSPCs seem to be located [32]. However, amifostine clinical trials have been inconclusive, owing to limited statistical power or a lack of proper control arms [41]. In addition, amifostine has serious side effects at the moment of administration, such as nausea, and probably cannot be administered in the effective dose range used in animal experiments [44]. An alternative with less side effects could be tempol, which has been shown to protect the murine salivary gland and not the tumor [45]. Other strategies have been developed to optimize the salivary gland’s regenerative potential after radiotherapy. Most of these treatments stimulate the proliferation of the remaining SSPCs, limiting their use to (parts of) the tissue that have received a relatively low dose [46]. These approaches encompass both pharmacological agents that stimulate the parasympathetic response of the gland, such as pilocarpine, and growth factors that stimulate proliferation [46]. The saliva secretion inducing sialagogue, Pilocarpine, has been relatively well studied, both as a treatment before and after radiotherapy. Animal and human studies show that this drug produces some improvement in salivary flow, which seems again to be limited by the maximum dose received [47,48] and which probably relies on improving the proliferation of the remaining radiation‐surviving cells [49]. Similarly, growth factors, such as insulin growth factor 1 [50], keratinocyte growth factor [51], as well as cytokine producing mesenchymal stem cells (MSCs), have been shown to improve salivary gland function after relatively low‐dose irradiation by stimulating the proliferation of radiation‐surviving cells. MSCs derived from different sources, such as bone marrow or adipose tissue, have been associated with the regeneration of radiation‐damaged normal tissues [52] including salivary glands [53,54]. A recent phase I/II clinical trial has shown the clinical feasibility and a marginal effect of such a therapy in the treatment of postradiotherapy hyposalivation [55]. However, since MSCs do not transdifferentiate into salivary gland cells but rather stimulate remaining surviving cells to proliferate, their action depends on the number of surviving SSPCs and their effect might be limited to the lower radiation dose regions [46]. Interestingly, MSCs have been shown to remodel radiation‐induced fibrosis [52]. Therefore, sequential or combined treatment with senolytics, drugs that kill senescent cells, and MSCs might improve the radiation‐damaged salivary gland environment for transplantation. The dependence of such therapies on the viability of remaining stem cells demands new research to enhance the number of surviving SSPCs.
Stem cell‐based therapy may provide a means to reduce radiation‐induced hyposalivation in patients after radiotherapy treatment [32]. Recently, we have shown the potential of stem cell therapy to ameliorate radiation‐induced hyposalivation in mice using expanded murine and human autologous adult SSPCs [56, 57, 58]. Interestingly, the positive effect of human SSPCs was partially due to the remodeling of the tissue [58]. SSPCs, however, cannot be obtained from patients with late radiation toxicity. A solution for this would be to use episomal reprogrammed somatic cells, such as blood mononuclear cells [59], that can generate pluripotent stem cells (PSCs), which are able to differentiate into every cell type in the body [60]. Similarly, embryonic stem cells (ESCs) have recently been shown to be able to differentiate into salivary gland cells [61], opening up novel avenues for regenerative medicine. A stem cell‐based approach to treating the side effects of radiotherapy on normal glands might be most effective when combined with the remodeling of the radiation‐damaged salivary gland environment.
3.3. Preventing radiation‐induced damage of salivary glands
Knowing where SSPCs are localized could help to prevent radiation‐induced salivary gland dysfunction. We have shown that the stem cells of the rodent and human parotid salivary gland localize to a specific region where the main excretory ducts are [7]. Sparing this region from radiation during radiotherapy for HNC is currently being evaluated in a double‐blind randomized clinical trial [62], with promising preliminary results. However, the complete sparing of the area that contains the highest proportion of SSPCs proved to be difficult due to the close vicinity of the tumor. This and the finding that these stem/progenitor cells might be very sensitive to low doses of irradiation [63] warrant the use of very accurate radiotherapy technologies, such as proton therapy (Box 1). The above‐described approaches have all been developed after obtaining a deeper knowledge of the response of the salivary gland to irradiation. Expanding this knowledge might, in the future, result in patient‐specific approaches that can prevent or ameliorate radiotherapy‐induced hyposalivation.
4. Cardiopulmonary system
Treatment of thoracic cancers with radiotherapy can cause side effects. Traditionally, early pulmonary and late cardiac damage has received the most attention [64]. The clinical sequelae of radiation lung injury usually start with the acute onset of radiation pneumonitis at 2–6 months after radiotherapy with symptoms that range from cough, fever, and dyspnea to even death from respiratory failure. Radiation‐induced lung fibrosis often develops subclinically from several months to years after radiotherapy. In rodents, symptoms of toxicity manifest as increased breathing frequency [65, 66, 67]. Several inflammatory responses initiated by radiation and radiation‐induced damage contribute to radiation pneumonitis [68,69]. Acute alveolar and interstitial inflammation leads to the loss of type I epithelial cells and endothelial cells, while inducing the proliferation of type II epithelial cells. These events initiate a cascade of inflammatory cytokines, which plays an important role in radiation pneumonitis [70]. This can be aggravated by combined treatment with chemotherapeutic agents [70]. Furthermore, beginning at 4 weeks after irradiation, an increase in ECM collagen deposition can be observed in the lungs of mice [71] (Fig. 2, Box 2).
Fig. 2.
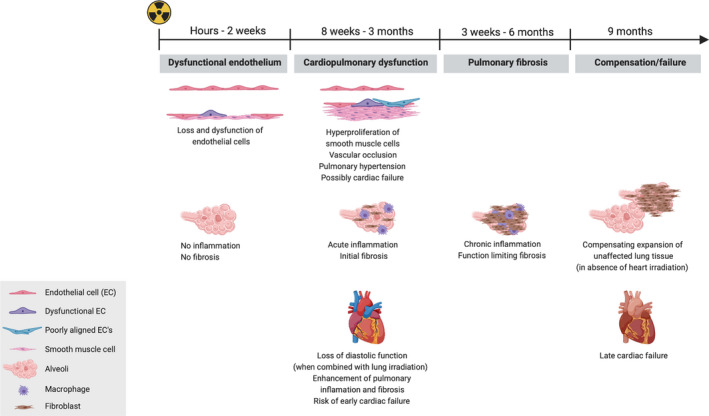
A schematic of the side effects of radiotherapy on the cardiopulmonary system over time. Shown are the cellular, tissue, and organ responses over time in the tissues of the lung and heart. The loss and dysfunction of lung vascular ECs are the first visible forms of damage in the irradiated lung. This is followed by acute inflammation and, depending on the dose, the first signs of fibrosis. These local processes are aggravated by loss of diastolic function if the heart is irradiated concomitantly. In the subsequent months, lung damage progresses and features chronic inflammation and function‐limiting fibrosis. The resulting dyspnea might resolve at later time points by compensatory inflation of nonirradiated parts of the lung. The adequacy of this compensatory response, however, depends on the irradiated lung volume. In addition, cardiac irradiation might also lead to later onset cardiac failure.
BOX 1.
Glossary
Pneumonitis: inflammation of lung tissue.
Radiation pneumonitis: pneumonitis caused by radiation.
Episomal reprogramming: system that reprograms somatic cells into induced PSCs, which are able to differentiate into almost any type of cell in the body.
The risk and severity of lung radiation side effects depend on the dose and volume of the tissue involved [65,72, 73, 74]. Therefore, technical advances that enable treatment optimization are currently used to reduce dose and volume of normal tissue receiving radiation.
4.1. Cellular and tissue responses
In the 1990s, Travis et al. [75] hypothesized and confirmed that the consequences of irradiating the lung might vary depending on which part of the lung was irradiated. Their studies in mice found that responses to the irradiation of basal subvolumes of the lung were consistently more severe than those observed after the irradiation of apical subvolumes. Based on these results, they hypothesized that this variation originated from a nonuniform distribution of alveolar tissue over the lung [76]. The latter hypothesis was indeed confirmed in rat studies, in which irradiation of the lateral parts of the lungs resulted in more severe respiratory dysfunction than did irradiation of the medially located parts of the lung that also contain the major airways [66]. However, interestingly in the same model, it was also observed that part of the regional variation in response could be explained by variations in dose in the heart [9,77]. Although also in patients a regional variation in the risk of radiation pneumonitis was observed, the data cannot distinguish between a role of alveolar distribution and involvement of the heart [78].
Since the heart and lungs are linked by the cardiopulmonary circulation, it was hypothesized that damage in these two organs after radiotherapy is mediated by changes in the cardiopulmonary circulation. Indeed, in rats it was found that heart irradiation reduced left ventricle diastolic function, leading to signs of congestion in the lungs that closely resembled the classical signs of radiation pneumonitis, such as shortness of breath, inflammation, and fibrosis [8]. Interestingly, also in rats it was shown that lung irradiation damages the endothelial cell (EC) layer of the pulmonary microvasculature within the first 2 weeks after irradiation, which precedes parenchymal damage [79]. These damages occur both in irradiated and spared lung tissue [79]. It was suggested that irradiation induces EC loss and that this leads to a contraction of the vasculature, which subsequentially results in an increase in pulmonary pressure in the unirradiated part of the lung. This increased pressure induces the sheering of ECs, much like that observed in pulmonary hypertension models [80,81]. EC injury might initiate or mediate structural changes in pulmonary vasculature, as described for pulmonary hypertension [82,83]. Indeed, pronounced vascular remodeling, including muscularization, adventitia thickening, and neointima formation throughout the lungs, was observed to result in increased pulmonary vascular resistance, leading to pulmonary hypertension [8,79]. The pathological features of the pulmonary vasculature were highly specific for pulmonary arterial hypertension. Pulmonary arterial hypertension can also impair left ventricle function, further aggravating the symptoms of cardiopulmonary dysfunction [8]. These effects depend on the irradiated lung volume, possibly pointing to a critical role for the amount of irradiated vasculature in the etiology of cardiopulmonary dysfunction [79]. As a consequence, limiting the irradiated volume may be more effective in preventing cardiopulmonary dysfunction than reducing the radiation dose within irradiated volumes.
Interestingly, cardiac irradiation has also been shown in a rat model to cause early interstitial and perivascular fibrosis of the heart in combination with loss of diastolic function [8]. Loss of diastolic function, and its associated congestion in the pulmonary circulation, increases the risk of cardiopulmonary dysfunction. When combined with pulmonary hypertension, loss of diastolic function increases the risk of cardiac failure [8].
In the ongoing CLARIFY study, the occurrence and impact of these and potentially other disturbances of cardiopulmonary physiology on patients are currently studied in a large prospective cohort study in lung and esophageal cancer patients using pre‐ and post‐treatment echocardiography, cardiac magnetic resonance imaging, and blood biomarkers [84].
These findings indicate that in the development of radiation damage, the heart and lung must be considered as an integrated system. This broader view of integrated responses of organs and organ systems provides us with novel opportunities to optimize radiotherapy treatment, as well as treatment of toxicity. Firstly, the functional interaction that exists between the heart and lung calls for the combined dose distribution in both organs to be optimized for the underlying systemic changes. Secondly, the process underlying the interaction between heart and lung provides novel targets for interventions to prevent toxicity.
4.2. Therapeutic approaches
Multiple approaches have been explored to reduce pulmonary toxicity but very few have been used in the clinic. Nevertheless, the mechanisms described above could inform the development of novel preventive or treatment measures.
For the nononcological patient, the prolonged activation of the renin–angiotensin–aldosterone system (RAAS) plays an important role in the progression of cardiac failure [85]. Consequently, inhibiting various components of the RAAS is a cornerstone of current treatments for heart failure [85,86]. The inhibition of angiotensin‐converting enzyme (ACE) to prevent the conversion of angiotensin I into angiotensin II and the activation of downstream mechanisms has been the most common approach used to achieve this.
Cardiac irradiation causes loss of diastolic function with detrimental consequences for the lung, as observed in a rat model [8,87]. Given this, could ACE inhibition be a promising strategy by which to reduce or prevent the failure of the cardiopulmonary system after thoracic radiotherapy? This does indeed appear to be the case. In a rat model, the ACE inhibitor, captopril, was observed to reduce dyspnea after whole‐thorax irradiation [88,89]. In experiments where radiation doses in heart and lung were varied in a controlled manner, this effect was shown to be likely achieved by reducing interstitial and perivascular fibrosis in the heart, leading to the preservation of diastolic function [87]. These results suggest that the RAAS is indeed involved in the development of radiation‐induced loss of diastolic function. Moreover, this finding suggests that treatments for conditions that lead to cardiac failure in nononcological patients might be potentially useful for preventing radiotherapy‐induced cardiopulmonary complications.
4.3. Preventing radiation‐induced damage in the cardiopulmonary system
As indicated, research on the regional responses of the lung was inspired by the idea that such regional responses might offer opportunities to reduce, or even prevent, normal tissue toxicity by avoiding the most important regions of the lung [75]. Indeed, this idea led to clinical studies in which risk estimation improved, the use of lung doses was replaced by a hybrid model that incorporated the local function of the lung and that optimized treatment strategies by avoiding vital lung tissue. This was achieved by moving the radiation dose to parts of the lung with a lower contribution to function [90].
However, as described in the previous section, recent animal work illustrates that cardiac and pulmonary toxicity can no longer be seen as separate entities and that both organs need to be spared. Unfortunately, the ability of photon‐based treatment technology to achieve this is limited due to dose deposited beyond the tumor volume. Interestingly, the peaking of the dose distribution of particles near the end of their penetration depth and the lack of dose beyond is expected to offer unprecedented opportunities to achieve this [91,92].
5. Brain
Radiotherapy‐induced neurocognitive dysfunction represents the major side effect of cranial radiotherapy in adult and pediatric cancer survivors, affecting school performance, employment, and independent living [93]. The brain is mostly formed by postmitotic neurons and glial cells. Glial cells primarily consist of the following cell types: astrocytes, which support neuronal function;oligodendrocytes, which are responsible for coating axons with myelin; and microglia, which are the resident macrophages of the brain. The brain also harbors a limited number of neural stem and progenitor cells in two restricted regions of adult neurogenesis. Importantly, the brain is isolated from the rest of the body's bloodstream by the blood–brain barrier (BBB), which is formed of highly selective junctions between ECs. Although it is difficult to dissect the contribution of each cell type and their interaction to the pathogenesis of the neurocognitive dysfunction that occurs as a late response (> 4 months) after radiotherapy, these cell types have been shown to be all somehow affected by radiation (Fig. 3).
Fig. 3.
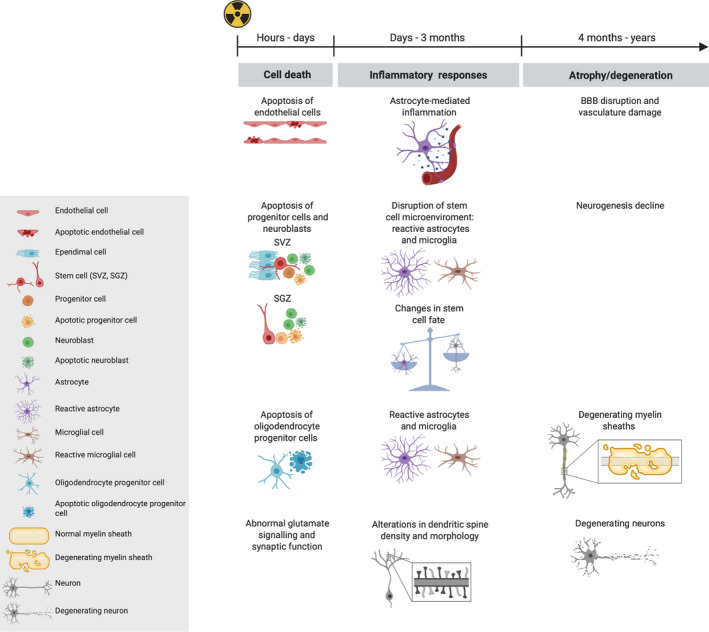
A schematic of the cellular and tissue responses of the brain to radiotherapy over time. Radiation causes multiple effects in the brain, including vascular damage, neurogenesis decline, white matter damage, and neuronal damage. Within hours after irradiation, cell death, largely via apoptosis, occurs in ECs, progenitor cells, and neuroblasts of the SVZ and SGZ, and in OPCs. Neurons exhibit abnormal glutamate signaling and synaptic function relatively early after irradiation, alongside alterations in dendritic spines and morphology. These early responses are followed by an inflammatory response that is characterized by the release of cytokines, and the reactivity of astrocytes and microglial cells. This inflammatory response can contribute to both early and late effects that affect different cell types and their interactions.
5.1. Cellular and tissue responses over time
Endothelial cells form the inner layer of blood vessel walls and, together with mural cells and astrocytes, ensure cerebral blood flow and BBB integrity [94]. Within hours after irradiation, in rodent models, ECs apoptose in a p53‐independent manner via the acid sphingomyelinase pathway, leading to the early disruption of BBB permeability [95,96]. This is followed, at 1–3 months after irradiation, by the irreversible disruption of the BBB, which is linked to persistent inflammation modulated by tumor necrosis factor (TNF)‐alpha and vascular endothelial growth factor (VEGF) overexpression in the astrocytes surrounding the ECs [97]. This delayed response is often restricted to white matter regions and precedes the development of radiation‐induced necrosis.
As in the salivary gland, the adult brain contains slowly proliferating stem and progenitor cells. Neurogenesis in the adult mammalian brain primarily occurs in two regions, the subventricular zone (SVZ) adjacent to the lateral ventricles and the subgranular zone (SGZ) in the dentate gyrus of the hippocampus. Within hours after irradiation, ataxia‐telangiectasia mutated (ATM) and p53‐dependent apoptosis of neural progenitor cells is observed in the SVZ [98,99] and in the SGZ region of rodent models [100]. In the longer term (> 2 months postirradiation), a change in cell fate differentiation from a neuronal to a glial fate is also observed [100]. This delayed response seems to be due to changes in the stem cell niche microenvironment, which include elevated levels of activated microglia and a disrupted vasculature that affects the regenerative potential of the remaining stem cells [100].
Glial cells include oligodendrocytes, astrocytes, and microglia. Oligodendrocyte progenitor cells (OPCs) in rodent models, similar to the progenitor cells of the SVZ and SGZ regions, have been shown to apoptose hours after irradiation. This in the long‐term (> 4 months postirradiation) results in the irreversible depletion of mature myelinating oligodendrocytes, leading to white matter damage [101,102]. This effect seems to be partially mediated by the upregulation of VEGF by reactive astrocytes in proximity to white matter regions [103]. Astrocytes and microglia in rodent models have indeed been shown to similarly respond to radiation with an initial (within hours) overexpression of TNF‐alpha and other cytokines, leading over time to chronic neuroinflammation characterized by reactive astrogliosis and subsequent microglial activation lasting for years after irradiation [104,105].
Despite being the largest cell population in the brain, a limited number of studies have focused on the direct impact of radiation on postmitotic neurons. Extensive changes in mouse neuronal dendritic and spine morphology have been reported after doses of 1 to 10 Gy, starting as early as 10 days after irradiation and progressively worsening thereafter [106]. Among the different types of dendritic spines, early filopodia membranous protrusions were most sensitive to radiation, thus possibly hindering their development into mature dendritic spines. These morphological changes were accompanied by a decrease in the presynaptic marker synaptophysin, followed by an upregulation of the synaptic protein PSD‐95. Abnormal glutamate signaling (as early as 1 h after irradiation with 10 Gy) has been proposed as being a contributing factor to radiation‐induced synaptic changes that lead to excitotoxicity by excessive synaptic stimulation [107]. However, how radiation directly impacts the dendritic spines remain to be fully elucidated and other intermediated responses are likely mediating this effect. Additionally, whether all of the above responses faithfully recapitulate what is occurring in the human brain after radiotherapy treatment still remains unanswered.
5.2. Therapeutic approaches
Many therapeutic strategies have been explored preclinically in the quest to ameliorate the radiotherapy‐induced neurocognitive sequelae. For example, in rats, the PPAR‐gamma agonist, pioglitazone, and the ACE inhibitor, ramipril, have been shown to indirectly improve neurocognitive dysfunction, although without directly improving the already damaged brain vasculature [108, 109, 110]. In patients with brain tumors, pioglitazone and ramipril have been, or are currently being, tested in phase I and II clinical trials [111] (ClinicalTrials.gov Identifier: NCT03475186).
With the goal of improving tissue regeneration, stem cell transplantation therapies have also been explored in the brain. Preclinical studies using either human embryonic or neural stem cells in rodent models have demonstrated the ability of these cells to integrate in the brain and to differentiate into neurons and glial cells, leading to improved neurocognitive function [112,113]. The underlying mechanisms of this benefit are not fully established and seem to not be limited to the replacement of the lost or damaged cells but rather to include the microvesicle‐mediated release of trophic factors (such as glial cell line‐derived neurotrophic factor, GDNF, and basic fibroblast growth factor, FGF) by the transplanted stem cells themselves [114]. Intranasally delivered human MSCs have also been used and have recently been shown to effectively improve neurocognitive function after irradiation in mice, conferring protection against several responses, including radiation‐induced persistent cAMP response element‐binding (CREB) activation, inflammation, oxidative stress, and neuronal loss [115].
Pharmacological interventions using food and drug administration‐approved psychiatric medications have also been explored as possible ways to reduce the long‐lasting impact that radiation has on adult neurogenesis. For example, in mice lithium [116] has been shown to reduce apoptosis of neural stem and progenitor cells in the SGZ, and in rodent models melatonin has been shown to increase the engraftment of neural stem cells in the SVZ [117,118]. Running‐based exercise has also been shown to improve neurogenesis in mice after irradiation [119]. The underlying mechanisms of how these pharmacological and physical interventions increase neurogenesis, however, remain to be fully elucidated and yet to be confirmed in patients.
A promising preclinical strategy to overcome radiation‐induced white matter damage is the transplantation of human embryonic stem cell‐derived OPCs into the corpus callosum and cerebellum of irradiated rats [120]. This was shown to lead to an improvement in neurocognitive and motor functions. However, the clinical translation of such an approach remains challenging. In mice, the depletion of microglia using PLX5622, a dietary inhibitor of colony‐stimulating factor‐1 receptor (CSF1R), was shown to prevent radiation‐induced neurocognitive dysfunction [121]. Other interventions to reduce glial cell reactivity have focused on reducing inflammation using nonsteroidal anti‐inflammatory drugs or selective inhibitors of proinflammatory cytokines.
In terms of neuronal damage, the administration of memantine, which blocks the glutamate receptor NMDAR, in mice just before irradiation prevented some of the radiation‐induced synaptic alterations [107]. Clinically memantine has been shown to improved neurocognitive function over time in patients receiving whole‐brain radiation therapy [122].
5.3. Preventing radiation‐induced damage in the brain
One strategy to prevent radiation‐induced side effects is to physically minimize the dose and volume of irradiated normal brain tissue or to minimize the exposure of specific brain structures to irradiation. This can be achieved using modern radiotherapy technologies, such as particle therapy. Our current knowledge of the potential role of different neuroanatomical structures in the pathogenesis of radiotherapy‐induced neurocognitive decline is largely limited to the hippocampus and its memory function [93]. However, other brain structures are likely to contribute to the complex neurocognitive sequelae that occur after radiotherapy, especially in pediatric patients, in whom a large proportion of tumors are located in the posterior fossa [123]. Future preclinical and clinical efforts should focus on discerning the contribution of different brain structures to radiation‐induced neurocognitive dysfunction in order to make optimal use of increasingly advanced radiotherapy technologies.
Another area that is little understood is the contribution of genetic variation to neurocognitive outcome. This topic has been recently reviewed in Ref. [124]. Strikingly, and possibly due to methodological issues, very little research has focused on trying to identify genes that are specifically associated with the development of radiotherapy‐induced neurocognitive dysfunction, with only one study showing a potential role for nitric oxide synthase 3 (NOS3) 894Thomozygosity [125]. Future research on the contribution of germline mutations, as well as the role of tumor genetic variation, is urgently needed to identify those patients who are most at risk of developing neurocognitive impairment and to offer them personalized preventive or therapeutic options.
6. Concluding remarks
In this review, we have discussed how recent developments in understanding how radiotherapy causes toxicity in normal tissue can be understood by describing three normal tissues at risk of such toxicity. The increasing availability of high‐precision radiotherapy is changing the way that we look at its effects on normal tissue. Changes to dose distribution and our increasing knowledge of the local and nonlocal effects of radiotherapy on normal tissue warrant the use of a different approach to prevent and/or to treat these effects. One of the commonalities of the three tissues described in this review is the occurrence of (micro‐) vascular effects. These effects have been described in many tissues but have received relatively little attention when compared to inflammatory processes and fibrosis. Since vascular effects might result in volume‐dependent within‐tissue and between‐tissue responses, the incorporation of such mechanisms in dose distribution planning would improve treatment outcomes. Such a strategy, however, requires quantitative information on the associated responses in patients that can only be obtained in translational clinical studies, such as our ongoing study into cardiopulmonary toxicity (acronym CLARIFY) [84]. Another effect, possibly one that is not independent of the vascular effect, is the stem cell region‐dependent regenerative potential of some normal tissues. Here also, altering dose distributions and sparing region that contain stem cells (as described in the salivary gland and brain) might also result in the further optimization of radiotherapy. Sparing or reducing the dose that somatic stem/progenitor cells are exposed to could result in the recovery of even late‐responding tissues. Their subsequent stimulation, using cytokines or MSCs, could further improve the regenerative potential of, and prevent excess damage to, normal tissue. In addition, regenerative therapies, based on the replacement of the damaged tissue‐specific stem/progenitor cells, might provide a means by which to further optimize the regenerative potential of the irradiated tissue. Recently, the development of in vitro tissue resembling models, such as organoids and organs‐on‐chip, derived from human tissue‐specific adult stem cells and from ESCs/PSCs and containing different cell types, including stem/progenitor cells and specialized differentiated functional cells [126], open up endless possibilities for modeling radiation‐induced side effects.
A deeper knowledge of the mechanisms that underlie normal tissue damage might also help to develop better preventive and therapeutic strategies. We need to progress from understanding local molecular/cellular events toward having a better understanding of tissue and organ interactions; this progress does not occur automatically and needs to be supported by subsequent translational research using animal models or tissue resembling models [63,127]. Typically, studies of the importance of different structures, in particular for the adult and pediatric brain, are needed to define (functional) structures that need to be spared or that can tolerate a somehow larger dose. Moreover, these structures are very likely to be interacting with each other, hence increasing the complexity of such studies. The most optimal animal models should also be used to address specific research questions. Although mice are more available and amenable to genetic manipulation, they might be too small to achieve accurate irradiation fields and are also characterized by significant differences in responses between strains. Rats or even larger experimental animals might be needed to design preclinical studies to test the optimal use of modern radiotherapy technologies. Genetic clinical studies to identify those patients that are most at risk of developing late side effects (although not reviewed here) are certainly of importance and, when possible, should be validated in combination with animal studies considering the above‐described principles.
The here‐described therapeutic and preventative strategies warrant further translation; however, many have yet to reach the clinic. The progress of an idea from the laboratory to the clinic and back to the laboratory to address further questions requires a well‐connected multidisciplinary team, which regretfully is often lacking within one institute. Alongside this, it seems that findings from well‐controlled experiments in animal models optimized for specific targets are very difficult to mimic in clinical studies that involve a diverse group of patients, who are often suffering from underlying diseases. Obtaining insight into the potential relevance of preclinical ideas using small clinical proof‐of‐concept studies, such as the MRI‐HART study [128], which precedes the much larger ongoing CLARIFY study [84], is essential for optimizing the design of clinical studies and for maximizing the probability that preclinical findings will reach clinical practice. However, such translational paths require long‐term commitments from both the laboratory and the clinic. Offering opportunities or work settings that allow a better understanding of each other’s fields, for instance, by spending internships in the laboratory or in the clinic, may help to achieve this.
Although the translation of preclinical research remains a challenge, several of the above‐described research discoveries are slowly entering the clinic. Stem cell‐sparing trials, such as the one described in Ref. [62], are currently being performed and some stem cell therapies are close to or in phase I/II clinical trials. However, the future improvement of combined biology and modern radiotherapy technologies depends on a constant, intense effort based on interdisciplinary and international collaborations between all the fields involved in (radiation) oncology.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
LB, RPC, and PVL equally contributed to the writing of the manuscript and design of the figures.
Acknowledgements
LB received funding by ZonMw (Off‐Road grant 451001001), Dutch Cancer Society (KWF) (Young Investigator grant 11148 and Unique High‐Risk grant 12487). RPC received funding from KWF (Project grants 10650, 12092 and 12458), EU (Grant agreement number: 755523–MEDIRAD), ZonMw (Grant 11.600.1023, 40‐43600‐98‐14003). PvL received funding from KWF (Project grants 11349 and 12134). Figures were created with BioRender.com.
Lara Barazzuol, Rob P. Coppes and Peter van Luijk contributed equally
References
- 1. Bray F, Jemal A, Grey N, Ferlay J & Forman D (2012) Global cancer transitions according to the Human Development Index (2008–2030): a population‐based study. Lancet Oncol 13, 790–801. [DOI] [PubMed] [Google Scholar]
- 2. Thariat J, Hannoun‐Levi JM, Sun Myint A, Vuong T, Gerard JP. Past, present, and future of radiotherapy for the benefit of patients. Nature Reviews Clinical Oncology. 2013;10 1:52–60. 10.1038/nrclinonc.2012.203. [DOI] [PubMed] [Google Scholar]
- 3. Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nature Reviews Drug Discovery. 2013;12 7:526–542. 10.1038/nrd4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider JE, Shank B, Solin LJ & Wesson M (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21, 109–122. [DOI] [PubMed] [Google Scholar]
- 5. Marks LB, Ten Haken RK & Martel MK (2010) Guest Editor’s introduction to QUANTEC: a users guide. Sci Rep 12(6), 1–11. [DOI] [PubMed] [Google Scholar]
- 6. Dörr W & van der Kogel AJ (2018) Normal tissue tolerance and the effect of dose inhomogeneities In Basic Clinical Radiobiology (Joiner MC. & Van Der Kogel AJ, eds), pp. 182–187. CRC Press, Boca Raton, FL. [Google Scholar]
- 7. Van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, Baanstra M, Van Der Laan HP, Kierkels RGJ, Van Der Schaaf A et al (2015) Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med 7, 305ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghobadi G, Van Der Veen S, Bartelds B, De Boer RA, Dickinson MG, De Jong JR, Faber H, Niemantsverdriet M, Brandenburg S, Berger RMF et al (2012) Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys 84, e639–e646 [DOI] [PubMed] [Google Scholar]
- 9. Van Luijk P, Novakova‐Jiresova A, Faber H, Schippers JM, Kampinga HH, Meertens H & Coppes RP (2005) Radiation damage to the heart enhances early radiation‐induced lung function loss. Cancer Res 65, 6509–6511. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen HQ, To NH, Zadigue P, Kerbrat S, De La Taille A, Le Gouvello S & Belkacemi Y (2018) Ionizing radiation‐induced cellular senescence promotes tissue fibrosis after radiotherapy. A review. Crit Rev Oncol Hematol 129, 13–26. [DOI] [PubMed] [Google Scholar]
- 11. Bentzen SM (2006) Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 6, 702–713. [DOI] [PubMed] [Google Scholar]
- 12. De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM & Hegi‐Johnson F (2019) Radiotherapy toxicity. Nat Rev Dis Primers 5, 13. [DOI] [PubMed] [Google Scholar]
- 13. Gruber S, Dorr W. Tissue reactions to ionizing radiation‐Oral mucosa. Mutation Research ‐ Reviews in Mutation Research. 2016;770: 292–298. 10.1016/j.mrrev.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 14. McDonald S, Rubin P, Phillips TL & Marks LB (1995) Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int J Radiat Oncol Biol Phys 31, 1187–1203. [DOI] [PubMed] [Google Scholar]
- 15. Hamilton CS, Denham JW, O’Brien M, Ostwald P, Kron T, Wright S & Dörr W (1996) Underprediction of human skin erythema at low doses per fraction by the linear quadratic model. Radiother Oncol 40, 23–30. [DOI] [PubMed] [Google Scholar]
- 16. Tofilon PJ & Fike JR (2000) The radioresponse of the central nervous system: a dynamic process. Radiat Res 153, 357–370. [DOI] [PubMed] [Google Scholar]
- 17. Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S & Moslehi J (2014) Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non‐invasive imaging for detection of cardiovascular disease. Eur Heart J 35, 612–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guha C & Kavanagh BD (2011) Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol 21, 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vergeer MR, Doornaert PAH, Rietveld DHF, Leemans CR, Slotman BJ & Langendijk JA (2009) Intensity‐modulated radiotherapy reduces radiation‐induced morbidity and improves health‐related quality of life: results of a nonrandomized prospective study using a standardized follow‐up program. Int J Radiat Oncol Biol Phys 74, 1–8. [DOI] [PubMed] [Google Scholar]
- 20. Vissink A, Jansma J, Spijkervet FKL, Burlage FR & Coppes RP (2003) Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14, 199–212. [DOI] [PubMed] [Google Scholar]
- 21. Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, Elting LS, Langendijk JA, Coppes RP & Reyland ME (2010) Clinical management of salivary gland hypofunction and xerostomia in head‐and‐neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys 78, 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pavlov I (1906) The scientific investigation of the psychical faculties or processes in the higher animals. Science 24, 613–619. [DOI] [PubMed] [Google Scholar]
- 23. Proctor GB and Carpenter GH (2007) Regulation of salivary gland function by autonomic nerves. Auton Neurosci 133, 3–18. [DOI] [PubMed] [Google Scholar]
- 24. Konings AWT, Coppes RP & Vissink A (2005) On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys 62, 1187–1194. [DOI] [PubMed] [Google Scholar]
- 25. Burlage FR, Coppes RP, Meertens H, Stokman MA & Vissink A (2001) Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol 61, 271–274. [DOI] [PubMed] [Google Scholar]
- 26. Coppes RP, Meter A, Latumalea SP, Roffel AF & Kampinga HH (2005) Defects in muscarinic receptor‐coupled signal transduction in isolated parotid gland cells after in vivo irradiation: evidence for a non‐DNA target of radiation. Br J Cancer 92, 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coppes RP, Zeilstra LJW, Kampinga HH & Konings AWT (2001) Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br J Cancer 85, 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paardekooper GMRM, Cammelli S, Zeilstra LJW, Coppes RP & Konings AWT (1998) Radiation‐induced apoptosis in relation to acute impairment of rat salivary gland function. Int J Radiat Biol 73, 641–648. [DOI] [PubMed] [Google Scholar]
- 29. Stephens LC, Schultheiss TE, Price RE, Ang KK & Peters LJ (1991) Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer 67, 1539–1543. [DOI] [PubMed] [Google Scholar]
- 30. Cotrim AP, Sowers A, Mitchell JB & Baum BJ (2007) Prevention of irradiation‐induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther 15, 2101–2106. [DOI] [PubMed] [Google Scholar]
- 31. Knox SM, Lombaert IMA, Haddox CL, Abrams SR, Cotrim A, Wilson AJ & Hoffman MP (2013). Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun 4, 1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pringle S, Van Os R & Coppes RP (2013) Concise review: adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 31, 613–619. [DOI] [PubMed] [Google Scholar]
- 33. Braam PM, Roesink JM, Moerland MA, Raaijmakers CPJ, Schipper M & Terhaard CHJ (2005) Long‐term parotid gland function after radiotherapy. Int J Radiat Oncol Biol Phys 62, 659–664. [DOI] [PubMed] [Google Scholar]
- 34. Marmary Y, Adar R, Gaska S, Wygoda A, Maly A, Cohen J, Eliashar R, Mizrachi L, Orfaig‐Geva C, Baum BJ et al (2016) Radiation‐induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer Res 76, 1170–1180. [DOI] [PubMed] [Google Scholar]
- 35. Hernandez‐Segura A, Nehme J & Demaria M (2018) Hallmarks of cellular senescence. Trends Cell Biol 28, 436–453. [DOI] [PubMed] [Google Scholar]
- 36. Coppes RP, Vissink A & Konings AWT (2002) Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother Oncol 63, 321–328. [DOI] [PubMed] [Google Scholar]
- 37. Shubin AD, Sharipol A, Felong TJ, Weng PL, Schutrum BE, Joe DS, Aure MH, Benoit DSW & Ovitt CE (2020) Stress or injury induces cellular plasticity in salivary gland acinar cells. Cell Tissue Res 380, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wong WY, Allie S & Limesand KH (2019) PKCζ and JNK signaling regulate radiation‐induced compensatory proliferation in parotid salivary glands. PLoS One 14, e0219572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nanduri LSY, Maimets M, Pringle SA, Van Der Zwaag M, Van Os RP & Coppes RP (2011) Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol 99, 367–372. [DOI] [PubMed] [Google Scholar]
- 40. Konings AWT, Vissink A & Coppes RP (2002) Comments on: extended‐term effects of head and neck irradiation in a rodent (multiple letters). Eur J Cancer 38, 851–852. [DOI] [PubMed] [Google Scholar]
- 41. Jensen SB, Vissink A, Limesand KH & Reyland ME (2019) Salivary gland hypofunction and xerostomia in head and neck radiation patients. J Natl Cancer Inst Monogr 2019, lgz016. [DOI] [PubMed] [Google Scholar]
- 42. Konings AWT, Faber H, Vissink A & Coppes RP (2005) Radioprotective effect of amifostine on parotid gland functioning is region dependent. Int J Radiat Oncol Biol Phys 63, 1584–1591. [DOI] [PubMed] [Google Scholar]
- 43. Varghese JJ, Schmale IL, Mickelsen D, Hansen ME, Newlands SD, Benoit DSW, Korshunov VA & Ovitt CE (2018) Localized delivery of amifostine enhances salivary gland radioprotection. J Dental Res 97, 1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gu J, Zhu S, Li X, Wu H, Li Y & Hua F (2014) Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta‐analysis based on randomized controlled trials. PLoS One 9, e95968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cotrim AP, Hyodo F, Matsumoto KI, Sowers AL, Cook JA, Baum BJ, Krishna MC & Mitchell JB (2007) Differential radiation protection of salivary glands versus tumor by tempol with accompanying tissue assessment of tempol by magnetic resonance imaging. Clin Cancer Res 13, 4928–4933. [DOI] [PubMed] [Google Scholar]
- 46. Coppes RP & van de Water TA (2015) Future prevention and treatment of radiation‐induced hyposalivation Guy C, In Dry Mouth, pp. 195–212. Berlin, Heidelberg, Springer. [Google Scholar]
- 47. Burlage FR, Roesink JM, Faber H, Vissink A, Langendijk JA, Kampinga HH & Coppes RP (2008) Optimum dose range for the amelioration of long term radiation‐induced hyposalivation using prophylactic pilocarpine treatment. Radiother Oncol 86, 347–353. [DOI] [PubMed] [Google Scholar]
- 48. Burlage FR, Roesink JM, Kampinga HH, Coppes RP, Terhaard C, Langendijk JA, van Luijk P, Stokman MA & Vissink A (2008) Protection of salivary function by concomitant pilocarpine during radiotherapy: a double‐blind, randomized, placebo‐controlled study. Int J Radiat Oncol Biol Phys 70, 14–22. [DOI] [PubMed] [Google Scholar]
- 49. Burlage FR, Faber H, Kampinga HH, Langendijk JA, Vissink A & Coppes RP (2009) Enhanced proliferation of acinar and progenitor cells by prophylactic pilocarpine treatment underlies the observed amelioration of radiation injury to parotid glands. Radiother Oncol 90, 253–256. [DOI] [PubMed] [Google Scholar]
- 50. Mitchell GC, Fillinger JL, Sittadjody S, Avila JL, Burd R & Limesand KH (2010) IGF1 activates cell cycle arrest following irradiation by reducing binding of ΔNp63 to the p21 promoter. Cell Death Dis 1, e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lombaert IMA, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G & Coppes RP (2008) Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 26, 2595–2601. [DOI] [PubMed] [Google Scholar]
- 52. Benderitter M, Caviggioli F, Chapel A, Coppes RP, Guha C, Klinger M, Malard O, Stewart F, Tamarat R, van Luijk P et al (2014) Stem cell therapies for the treatment of radiation‐induced normal tissue side effects. Antioxid Redox Signal 21, 338–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. An HY, Shin HS, Choi JS, Kim HJ, Lim JY & Kim YM (2015) Adipose mesenchymal stem cell secretome modulated in hypoxia for remodeling of radiation‐induced salivary gland damage. PLoS One 10, e0141862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lombaert IMA, Wierenga PK, Kok T, Kampinga HH, DeHaan G & Coppes RP (2006) Mobilization of bone marrow stem cells by granulocyte colony‐stimulating factor ameliorates radiation‐induced damage to salivary glands. Clin Cancer Res 12, 1804–1812. [DOI] [PubMed] [Google Scholar]
- 55. Grønhøj C, Jensen DH, Vester‐Glowinski P, Jensen SB, Bardow A, Oliveri RS, Fog LM, Specht L, Thomsen C, Darkner S et al (2018) Safety and efficacy of mesenchymal stem cells for radiation‐induced xerostomia: a randomized, placebo‐controlled phase 1/2 trial (MESRIX). Int J Radiat Oncol Biol Phys 101, 581–592. [DOI] [PubMed] [Google Scholar]
- 56. Maimets M, Rocchi C, Bron R, Pringle S, Kuipers J, Giepmans BNG, Vries RGJ, Clevers H, De Haan G, Van Os R et al (2016) Long‐term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep 6, 150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nanduri LSY, Baanstra M, Faber H, Rocchi C, Zwart E, De Haan G, Van Os R & Coppes RP (2014) Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep 3, 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pringle S, Maimets M, Van Der Zwaag M, Stokman MA, Van Gosliga D, Zwart E, Witjes MJH, De Haan G, Van Os R & Coppes RP (2016) Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34, 640–652. [DOI] [PubMed] [Google Scholar]
- 59. Wen W, Zhang JP, Xu J, Su RJ, Neises A, Ji GZ, Yuan W, Cheng T & Zhang XB (2016) Enhanced generation of integration‐free iPSCs from human adult peripheral blood mononuclear cells with an optimal combination of episomal vectors. Stem Cell Rep 6, 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu SM & Hochedlinger K (2011) Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 13, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tanaka J, Ogawa M, Hojo H, Kawashima Y, Mabuchi Y, Hata K, Nakamura S, Yasuhara R, Takamatsu K, Irié T et al (2018) Generation of orthotopically functional salivary gland from embryonic stem cells. Nature Commun 9, 4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Steenbakkers RJHM (2013) Parotid‐gland Stem‐cell Sparing Intensity‐modulated Radiotherapy (SCS‐IMRT) . Clinicaltrials.Gov. https://clinicaltrials.gov/ct2/show/NCT01955239.
- 63. Nagle PW, Hosper NA, Barazzuol L, Jellema AL, Baanstra M, Van Goethem MJ, Brandenburg S, Giesen U, Langendijk JA, Van Luijk P et al (2018) Lack of DNA damage response at low radiation doses in adult stem cells contributes to organ dysfunction. Clin Cancer Res 24, 6583–6593. [DOI] [PubMed] [Google Scholar]
- 64. Simone CB (2017) Thoracic radiation normal tissue injury. Semin Radiat Oncol 27, 370–377. [DOI] [PubMed] [Google Scholar]
- 65. Liao ZX, Travis EL & Tucker SL (1995) Damage and morbidity from pneumonitis after irradiation of partial volumes of mouse lung. Int J Radiat Oncol Biol Phys 32, 1359–1370. [DOI] [PubMed] [Google Scholar]
- 66. Novakova‐Jiresova A, Van Luijk P, Van Goor H, Kampinga HH & Coppes RP (2005) Pulmonary radiation injury: identification of risk factors associated with regional hypersensitivity. Cancer Res 65, 3568–3576. [DOI] [PubMed] [Google Scholar]
- 67. Vujaskovic Z, Down JD, Van T'Veld AA, Mooyaart EL, Meertens H, Piers DA, Szabo BG & Konings AWT(1998) Radiological and functional assessment of radiation‐induced lung injury in the rat. Exp Lung Res 24, 137–148. [DOI] [PubMed] [Google Scholar]
- 68. Johnston CJ, Williams JP, Elder A, Hernady E & Finkelstein JN (2004) Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res 30, 369–382. [DOI] [PubMed] [Google Scholar]
- 69. Marks LB, Yu X, Vujaskovic Z, Small W, Folz R & Anscher MS (2003) Radiation‐induced lung injury. Semin Radiat Oncol 13, 333–345. [DOI] [PubMed] [Google Scholar]
- 70. Rübe CE, Wilfert F, Uthe D, König J, Liu L, Schuck A, Willich N, Remberger K & Rübe C (2004) Increased expression of pro‐inflammatory cytokines as a cause of lung toxicity after combined treatment with gemcitabine and thoracic irradiation. Radiother Oncol 72, 231–241. [DOI] [PubMed] [Google Scholar]
- 71. Rubin P, Johnston CJ, Williams JP, McDonald S & Finkelstein JN (1995) A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 33, 99–109. [DOI] [PubMed] [Google Scholar]
- 72. Bentzen SM, Skoczylas JZ & Bernier J (2000) Quantitative clinical radiobiology of early and late lung reactions. Int J Radiat Biol 76, 453–462. [DOI] [PubMed] [Google Scholar]
- 73. Coppes RP, Muijs CT, Faber H, Gross S, Schippers JM, Brandenburg S, Langendijk JA & Van Luijk P (2011) Volume‐dependent expression of in‐field and out‐of‐field effects in the proton‐irradiated rat lung. Int J Radiat Oncol Biol Phys 81, 262–269. [DOI] [PubMed] [Google Scholar]
- 74. Marks LB, Bentzen SM, Deasy JO, Kong F‐M (Spring), Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD et al (2010) Radiation dose‐volume effects in the lung. J Pineal Res 63, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Travis EL, Liao ZX & Tucker SL (1997) Spatial heterogeneity of the volume effect for radiation pneumonitis in mouse lung. Int J Radiat Oncol Biol Phys 38, 1045–1054. [DOI] [PubMed] [Google Scholar]
- 76. Tucker SL, Liao ZX & Travis EL (1997) Estimation of the spatial distribution of target cells for radiation pneumonitis in mouse lung. Int J Radiat Oncol Biol Phys 38, 1055–1066. [DOI] [PubMed] [Google Scholar]
- 77. van Luijk P, Faber H, Meertens H, Schippers JM, Langendijk JA, Brandenburg S, Kampinga HH & Coppes RP (2007) The impact of heart irradiation on dose‐volume effects in the rat lung. Int J Radiat Oncol Biol Phys 69, 552–559. [DOI] [PubMed] [Google Scholar]
- 78. Vinogradskiy Y, Tucker SL, Liao Z & Martel MK (2012) Investigation of the relationship between gross tumor volume location and pneumonitis rates using a large clinical database of non‐small‐cell lung cancer patients. Int J Radiat Oncol Biol Phys 82, 1650–1658. [DOI] [PubMed] [Google Scholar]
- 79. Ghobadi G, Bartelds B, Van Der Veen SJ, Dickinson MG, Brandenburg S, Berger RMF, Langendijk JA, Coppes RP & Van Luijk P (2012) Lung irradiation induces pulmonary vascular remodelling resembling pulmonary arterial hypertension. Thorax 67, 334–341. [DOI] [PubMed] [Google Scholar]
- 80. Macario DK, Entersz I, Paul Abboud J & Nackman GB (2008) Inhibition of apoptosis prevents shear‐induced detachment of endothelial cells. J Surg Res 147, 282–289. [DOI] [PubMed] [Google Scholar]
- 81. Ueno H, Kanellakis P, Agrotis A & Bobik A (2000) Blood flow regulates the development of vascular hypertrophy, smooth muscle cell proliferation, and endothelial cell nitric oxide synthase in hypertension. Hypertension 36, 89–96. [DOI] [PubMed] [Google Scholar]
- 82. Chan SY & Loscalzo J (2008) Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 44, 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jurasz P, Courtman D, Babaie S & Stewart DJ (2010) Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Therap 126, 1–8. [DOI] [PubMed] [Google Scholar]
- 84. Muijs CT (2019) Cardiopulmonary toxicity of thoracic radiotherapy. Clinicaltrials.Gov. 10.31525/ct1-nct03978377 [DOI]
- 85. Lang CC & Struthers AD (2013) Targeting the renin‐angiotensin‐aldosterone system in heart failure. Nat Rev Cardiol 10, 125–134. [DOI] [PubMed] [Google Scholar]
- 86. Mann DL & Bristow MR (2005) Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111, 2837–2849. [DOI] [PubMed] [Google Scholar]
- 87. Van Der Veen SJ, Ghobadi G, De Boer RA, Faber H, Cannon MV, Nagle PW, Brandenburg S, Langendijk JA, Van Luijk P & Coppes RP (2015) ACE inhibition attenuates radiation‐induced cardiopulmonary damage. Radiother Oncol 114, 96–103. [DOI] [PubMed] [Google Scholar]
- 88. Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER & Medhora M (2009) Renin‐angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys 75, 1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ward WF, Molteni A, Ts'ao C‐H, Kim YT & Hinz JM (1992) Radiation pneumotoxicity in rats: modification by inhibitors of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys 22, 623–625 [DOI] [PubMed] [Google Scholar]
- 90. Kocak Z, Borst GR, Zeng J, Zhou S, Hollis DR, Zhang J, Evans ES, Folz RJ, Wong T, Kahn D et al (2007) Prospective assessment of dosimetric/physiologic‐based models for predicting radiation pneumonitis. Int J Radiat Oncol Biol Phys 67, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Loeffler JS, Smith AR & Suit HD (1997) The potential role of proton beams in radiation oncology. Semin Oncol 24, 686–695. [PubMed] [Google Scholar]
- 92. Wilson RR(1946) Radiological use of fast protons. Radiology 47, 487–491. [DOI] [PubMed] [Google Scholar]
- 93. Makale MT, McDonald CR, Hattangadi‐Gluth JA & Kesari S (2016) Mechanisms of radiotherapy‐associated cognitive disability in patients with brain tumours. Nat Rev Neurol 13, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sweeney MD, Kisler K, Montagne A, Toga AW & Zlokovic BV (2018) The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21, 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li YQ, Chen P, Haimovitz‐Friedman A, Reilly RM & Wong CS (2003) Endothelial apoptosis initiates acute blood‐brain barrier disruption after ionizing radiation. Cancer Res 63, 5950–5956. [PubMed] [Google Scholar]
- 96. Peña LA, Fuks Z & Kolesnick RN (2000) Radiation‐induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 60, 321–327. [PubMed] [Google Scholar]
- 97. Nordal RA, Nagy A, Pintilie M & Wong CS (2004) Hypoxia and hypoxia‐inducible factor‐1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res 10, 3342–3353. [DOI] [PubMed] [Google Scholar]
- 98. Barazzuol L, Hopkins SR, Ju L & Jeggo PA (2019) Distinct response of adult neural stem cells to low versus high dose ionising radiation. DNA Repair 76, 70–75. [DOI] [PubMed] [Google Scholar]
- 99. Barazzuol L, Ju L & Jeggo PA (2017) A coordinated DNA damage response promotes adult quiescent neural stem cell activation. PLoS Biol 15, e2001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Monje ML, Mizumatsu S, Fike JR & Palmer TD (2002) Irradiation induces neural precursor‐cell dysfunction. Nat Med 8, 955–962. [DOI] [PubMed] [Google Scholar]
- 101. Chari DM, Gilson JM, Franklin RJM & Blakemore WF (2006) Oligodendrocyte progenitor cell (OPC) transplantation is unlikely to offer a means of preventing X‐irradiation induced damage in the CNS. Exp Neurol 198, 145–153. [DOI] [PubMed] [Google Scholar]
- 102. Panagiotakos G, Alshamy G, Chan B, Abrams R, Greenberg E, Saxena A, Bradbury M, Edgar M, Gutin P & Tabar V (2007) Long‐term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One 2, e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tsao MN, Li YQ, Lu G, Xu Y & Wong CS (1999) Upregulation of vascular endothelial growth factor is associated with radiation‐induced blood‐spinal cord barrier breakdown. J Neuropathol Exp Neurol 58, 1051–1060. [DOI] [PubMed] [Google Scholar]
- 104. Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH & Han IO (2006) Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis 21, 457–467. 6. [DOI] [PubMed] [Google Scholar]
- 105. Kyrkanides S, Olschowka JA, Williams JP, Hansen JT & O’Banion MK (1999) TNFα and IL‐1β mediate intercellular adhesion molecule‐1 induction via microglia‐astrocyte interaction in CNS radiation injury. J Neuroimmunol 95, 95–106. [DOI] [PubMed] [Google Scholar]
- 106. Parihar VK & Limoli CL (2013) Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci USA 110, 12822–12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Duman JG, Dinh J, Zhou W, Cham H, Mavratsas VC, Pavešković M, Mulherkar S, McGovern SL, Tolias KF & Grosshans DR (2018) Memantine prevents acute radiation‐induced toxicities at hippocampal excitatory synapses. Neuro Oncol 20, 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jenrow KA, Brown SL, Liu J, Kolozsvary A, Lapanowski K & Kim JH (2010) Ramipril mitigates radiation‐induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee TC, Greene‐Schloesser D, Payne V, Diz DI, Hsu F‐C, Kooshki M, Mustafa R, Riddle DR, Zhao W, Chan MD et al (2012) Chronic administration of the angiotensin‐converting enzyme inhibitor, ramipril, prevents fractionated whole‐brain irradiation‐induced perirhinal cortex‐dependent cognitive impairment. Radiat Res 178, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhao W, Payne V, Tommasi E, Diz DI, Hsu FC & Robbins ME (2007) Administration of the peroxisomal proliferator‐activated receptor γ agonist pioglitazone during fractionated brain irradiation prevents radiation‐induced cognitive impairment. Int J Radiat Oncol Biol Phys 67, 6–9. [DOI] [PubMed] [Google Scholar]
- 111. Cramer CK, Alphonse‐Sullivan N, Isom S, Metheny‐Barlow LJ, Cummings TL, Page BR, Brown DR, Blackstock AW, Peiffer AM, Strowd RE et al (2019) Safety of pioglitazone during and after radiation therapy in patients with brain tumors: a phase I clinical trial. J Cancer Res Clin Oncol 145, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, Fike JR & Limoli CL (2009) Rescue of radiation‐induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci 106, 19150–19155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, Rosi S & Limoli CL (2011) Human neural stem cell transplantation ameliorates radiation‐induced cognitive dysfunction. Cancer Res 71, 4834–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Baulch JE, Acharya MM, Allen BD, Ru N, Chmielewski NN, Martirosian V, Giedzinski E, Syage A, Park AL, Benke SN et al (2016) Cranial grafting of stem cell‐derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc Natl Acad Sci USA 113, 4836–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Soria B, Martin‐Montalvo A, Aguilera Y, Mellado‐Damas N, López‐Beas J, Herrera‐Herrera I, López E, Barcia JA, Alvarez‐Dolado M, Hmadcha A et al (2019) Human mesenchymal stem cells prevent neurological complications of radiotherapy. Front Cell Neurosci 13, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO, Boone B, Shinohara ET & Hallahan DE (2006) Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res 66, 11179–11186. [DOI] [PubMed] [Google Scholar]
- 117. Mendivil‐Perez M, Soto‐Mercado V, Guerra‐Librero A, Fernandez‐Gil BI, Florido J, Shen YQ, Tejada MA, Capilla‐Gonzalez V, Rusanova I, Garcia‐Verdugo JM et al (2017) Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J Pineal Res 63. [DOI] [PubMed] [Google Scholar]
- 118. Naseri S, Moghahi SMHN, Mokhtari T, Roghani M, Shirazi AR, Malek F & Rastegar T (2017) Radio‐protective effects of melatonin on ubventricular zone in irradiated rat: decrease in apoptosis and upregulation of nestin. J Mol Neurosci 63, 198–205. [DOI] [PubMed] [Google Scholar]
- 119. Wong‐Goodrich SJE, Pfau ML, Flores CT, Fraser JA, Williams CL & Jones LW (2010) Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole‐brain irradiation. Cancer Res 70, 9329–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D et al (2015) Human embryonic stem cell‐derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell 16, 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, Le MT, Kawashita T, Giedzinski E, Parihar VK et al (2016). Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D et al (2013) Memantine for the prevention of cognitive dysfunction in patients receiving whole‐brain radiotherapy: a randomized, double‐blind, placebo‐controlled trial. Neuro Oncol 15, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ajithkumar T, Price S, Horan G, Burke A & Jefferies S (2017) Prevention of radiotherapy‐induced neurocognitive dysfunction in survivors of paediatric brain tumours: the potential role of modern imaging and radiotherapy techniques. Lancet Oncol 18, e91–e100. [DOI] [PubMed] [Google Scholar]
- 124. Wefel JS, Noll KR & Scheurer ME(2016). Neurocognitive functioning and genetic variation in patients with primary brain tumours. Lancet Oncol 17, e97–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Krajinovic M, Robaey P, Chiasson S, Lemieux‐Blanchard E, Rouillard M, Primeau M, Garcia Bournissen F & Moghrabi A (2005) Polymorphisms of genes controlling homocysteine levels and IQ score following the treatment for childhood ALL. Pharmacogenomics 6, 293–302. [DOI] [PubMed] [Google Scholar]
- 126. Bartfeld S & Clevers H (2017) Stem cell‐derived organoids and their application for medical research and patient treatment. J Mol Med 95, 729–738. [DOI] [PubMed] [Google Scholar]
- 127. Nagle PW, Plukker JTM, Muijs CT, van Luijk P & Coppes RP (2018) Patient‐derived tumor organoids for prediction of cancer treatment response. Semin Cancer Biol 53, 258–264. [DOI] [PubMed] [Google Scholar]
- 128. Widder J (2015) Evaluation of Radiation Induced Pulmonary Hypertension Using MRI in Stage III NSCLC Patients Treated With Chemoradiotherapy. A Pilot Study (MRI‐HART). Clinicaltrials.Gov. https://clinicaltrials.gov/ct2/show/NCT02377934


