Abstract
Particle therapy using protons or heavier ions is currently the most advanced form of radiotherapy and offers new opportunities for improving cancer care and research. Ions deposit the dose with a sharp maximum – the Bragg peak – and normal tissue receives a much lower dose than what is delivered by X‐ray therapy. Particle therapy has also biological advantages due to the high linear energy transfer of the charged particles around the Bragg peak. The introduction of particle therapy has been slow in Europe, but within the last decade, more than 20 clinical facilities have opened and facilitated access to this frontline therapy. In this review article, the basic concepts of particle therapy are reviewed along with a presentation of the current clinical indications, the European clinical research, and the established networks.
Keywords: cancer, ion beam therapy, particle therapy, proton therapy, radiation, radiotherapy
Particle therapy using protons, or heavier ions, is the most advanced form of radiotherapy today and offers new opportunities for improving cancer care and research. Within the last decade, more than 20 new clinical facilities have opened in Europe, facilitating access to this frontline therapy. This review presents the physics, biology, and clinical aspects of particle therapy.
Abbreviations
- CT
computer tomography
- EORTC
European Organization for Research and Treatment of Cancer
- EPTN
European Particle Therapy Network
- ESTRO
European Society for Radiotherapy and Oncology
- LET
linear energy transfer
- MeV·u−1
energy range per nucleon
- MR
magnetic resonance imaging
- NTCP
normal tissue complication probability
- PET
positron emission tomography
- PTCOG
Particle Therapy Co‐Operative Group
- RBE
relative biological effectiveness
1. Introduction
Particle therapy denotes the clinical use of ion beams (protons or heavier ions) to treat patients with malignant or nonmalignant tumors. Particle therapy is currently the most advanced form of radiotherapy. Despite a somewhat higher cost compared to megavoltage X‐rays, ion beam therapy holds great potential for significant advances in cancer care and research. Within the last decade, more than 20 clinical facilities have opened in European countries, which has enabled easier access to this frontline therapy also for European citizens.
The application of accelerated charged particles in radiotherapy was originally proposed in 1946 by Robert Rathnub Wilson, a student of Ernest Orlando Lawrence at the University of California in Berkeley (CA, USA) (Wilson, 1946). The physical advantages of particle therapy are due to the favorable depth–dose distribution compared to conventional X‐rays (Durante and Paganetti, 2016). While photon dose decreases exponentially as a function of the depth, swift ions deposit initially little energy (plateau), but their energy loss per unit track increases with depth, reaching a sharp maximum, called the Bragg peak, when they are close to their range in tissue (Fig. 1). Therefore, the dose to the organs at risk can be reduced with ion beam without compromising the dose to the tumor. In photon radiotherapy, it is necessary to combine many different beam angles, whereas only a few beams are necessary in ion beam therapy (Durante and Paganetti, 2016).
Fig. 1.
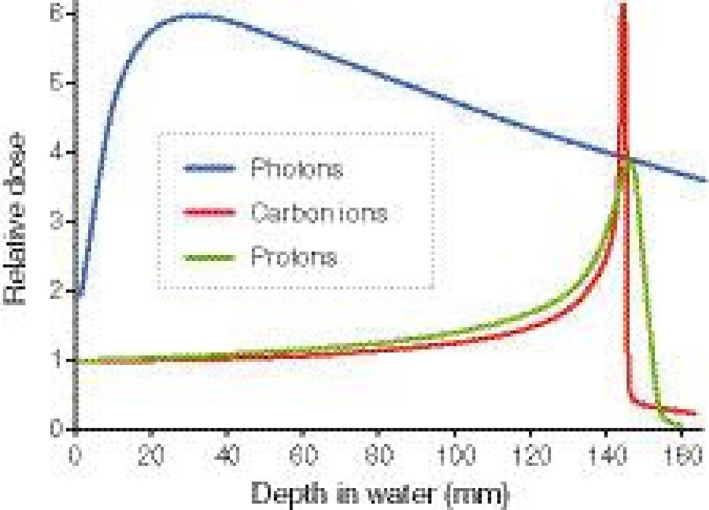
Physical and biological advantages of particle therapy (protons and carbon ions) as compared to megavoltage X‐rays (photons). The depth–dose curves of charged particles are defined by a plateau phase and the Bragg peak, situated in a specific depth depending on the energy of the beam.
In addition, particle therapy has several biological advantages compared to conventional photon radiotherapy (Durante and Loeffler, 2010). The advantages are due to the high linear energy transfer (LET) of the charged particles around the Bragg peak: The dense ionization patterns induce clustered DNA lesions that are difficult to repair and trigger different signaling pathways. The effectiveness of high‐LET radiation in tumor control was the main rationale for using fast neutrons in radiotherapy (Specht et al., 2015), but in neutron therapy, the LET is high in both normal and tumor tissues, and the depth–dose distribution is similar to photons. On the other hand, in particle therapy the LET can be low in the entrance (plateau) and high in the Bragg peak, and the high‐LET radiobiological properties are therefore confined to the tumor region (Fig. 1). Because LET ∝z 2, where z is the atomic number of the ion, heavy ions have higher LET than light ions, but for z ≥ 10, the LET is too high already in the normal tissue, thus leading to unacceptable toxicities such as those observed in neutron therapy. For these very reasons, particle therapy is limited to light ions, and currently, only protons and 12C‐ions are used in the clinics.
Protons (z = 1) have always low LET, and therefore, their advantage is limited to the sparing of the normal tissue due to the Bragg peak. However, it has been recently observed that the increased LET of slow protons at the end of their range can lead to unexpected toxicities, such as brain necrosis (Haas‐Kogan et al., 2018). Carbon ions (z = 6) are a good compromise because they have low LET in the entrance channel and high LET in the tumor region (Durante and Debus, 2018). For this reason, carbon ion therapy is currently ongoing in nine centers in Asia and four in Europe (Weber et al., 2017). However, carbon ion therapy is more expensive than proton therapy, because large synchrotrons are needed to accelerate heavy ions compared to compact cyclotrons used in proton therapy (Durante and Flanz, 2019).
The aim of the current paper is to introduce the basic concepts of particle therapy, including the biological properties and the technical and physical aspects of various delivery techniques, along with a review of the contemporary clinical use of protons in Europe. Finally, the European networks for experimental and clinical research will be described.
2. Ion beam delivery techniques for clinical use
The energies required in proton beam therapy range from about 60 to 250 MeV to cover penetration depth from a few centimeters (e.g., for ocular tumors) to more than 25 cm (e.g., for pelvic tumors). For heavier particles such as carbon ions, the respective energy range per nucleon (MeV·u−1) is between about around 120 and 430 MeV·u−1 (Stock et al., 2018). Most European particle therapy facilities that focus on proton therapy use cyclotrons for beam production, while the four European particle therapy facilities that offer both protons and carbon ions are synchrotron‐based. A cyclotron is operated at a static magnetic field; hence, the radius of the beam cycling in the magnetic field increases with increasing particle beam energy. The beam is extracted from the cyclotron when it reaches the maximum energy and is reduced passively by inserting range shifters in the beam line to reduce penetration depth. In a synchrotron, the particle beam is cycling through a ring with a fixed radius at variable magnetic field strengths to compensate for the particle energy. The beam is incrementally accelerated and can be extracted from the ring at any energy. This allows to actively select beam energies instead of degrading a high energy by inserting passive elements.
Irrespective of accelerator type, after beam extraction the narrow particle beam (‘pencil beam’) is transported through vacuum pipes into the treatment room. In order to shape the narrow beam to an irregular tumor, two main delivery techniques are in clinical use, that is, the passive scattering and pencil beam scanning technique. In the traditionally used passive scattering technique, the pencil beam is widened by mechanical elements that scatter the beam; field shaping according to the 2D projection of the tumor is achieved by individual collimators. The depth–dose modulation of the Bragg peak to cover the tumor extent in longitudinal direction is also realized via passive elements. All newly planned and recently established particle therapy facilities apply or aim for pencil beam scanning, where the narrow PB is deflected by magnetic fields in vertical and horizontal directions. The position in depth of the Bragg peak is adjusted via active energy variations in case of synchrotrons, or by reducing the maximum particle energy with range shifter in case of cyclotrons. As the scanning technique does not need mechanical elements for beam broadening or beam shaping, which cause unwanted secondary particles (mostly neutrons), it results in a lower integral dose to healthy tissue (Schneider et al., 2016). Two European particle therapy facilities, the Paul Scherrer Institute in Villigen, Switzerland, for proton therapy (spot scanning) and the Helmholtzzentrum für Schwerionenforschung in Darmstadt, Germany, for carbon ion therapy (raster scanning), pioneered scanned beam delivery and thus paved the way for its global clinical utilization.
In order to detect, assess, delineate, and track the tumor volume with the highest possible precision, imaging plays a vital role in particle therapy. Irrespective of beam delivery technique, treatment planning is commonly based on multimodality imaging (CT, MR, PET). The pencil beam scanning technique is linked with computerized treatment plan optimization, where the particle fluence pattern to be delivered is calculated from desired treatment aims, that is, the intended target dose and the acceptable organ at risk exposure (Oelfke and Bortfeld, 2003). Treatment planning for the traditional passive scattering technique is performed in a manual trial and error optimization process.
Influenced and motivated by the standards of image‐guided photon beam therapy, the integration of advanced X‐ray imaging (e.g., cone‐beam computed tomography) in the treatment room has just started in particle therapy (Bolsi et al., 2018). In‐room imaging enables to account for time variable anatomic variations during the course of therapy. Moreover, frequent and repetitive imaging is the prerequisite for adaptive radiotherapy, where patient‐specific variations that were detected via imaging methods trigger treatment modifications (van de Water et al., 2018). Treating moving targets with particle therapy is challenging and certainly needs an image‐guided approach (Knopf et al., 2010). In the context of motion mitigation techniques like gating or tumor tracking, the time structure of the beam produced by either a synchrotron or a cyclotron is important as well.
3. Clinical experience and current indications
It took only 6 years after the publication by Robert R. Wilson in 1946 before John Lawrence and Cornelius A. Tobias at Berkeley Radiation Laboratory applied protons clinically for the first time when a 340‐MeV proton beam was used to irradiate the pituitary gland in a patient with acromegaly. Since then, more than 190 000 patients have been treated with protons and 28 000 patients have received carbon ions (www.ptcog.ch). Eighty proton therapy centers are currently active worldwide; about 30% of those centers are in Europe. The number of European proton therapy clinics is rapidly increasing: While in 2017 there were 15 operational facilities, by the end of 2020 there will be 31 proton therapy facilities in clinical operation (Fig. 2). Carbon ion therapy is currently ongoing in nine centers in Asia and four in Europe.
Fig. 2.
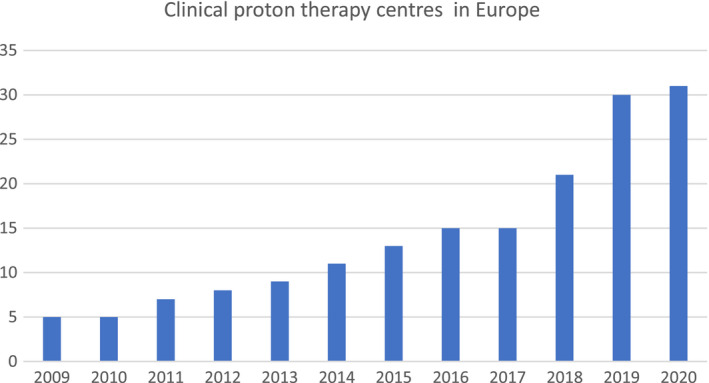
Graph showing the number of clinical proton facilities in Europe 2009–2020. Source: www.ptcog.ch.
The clinical rationale for using proton therapy is primarily based on the advantageous physical dose distribution. Protons can be used to increase the target dose, as to optimize local tumor control probability, and/or to decrease the likelihood of radiation‐induced toxicity by delivering less dose to organs at risk in the direct vicinity of the tumor volume (Fig. 3). Skull base tumors (i.e., chordoma or chondrosarcoma), which are radioresistant tumors, are good examples where tumor control is increased by a factor of approximately three to four when using protons as compared to historical photon series (Romero et al., 1993; Weber et al., 2016). Reduction of radiation‐induced toxicity is seen, for example, in medulloblastoma in children, wherein the radiation dose delivered outside the target volume in the patient's body is reduced by 4.5–6.0 times with proton therapy as compared to photon therapy, resulting in a substantial risk of radiation‐induced secondary malignancies (Sakthivel et al., 2019).
Fig. 3.
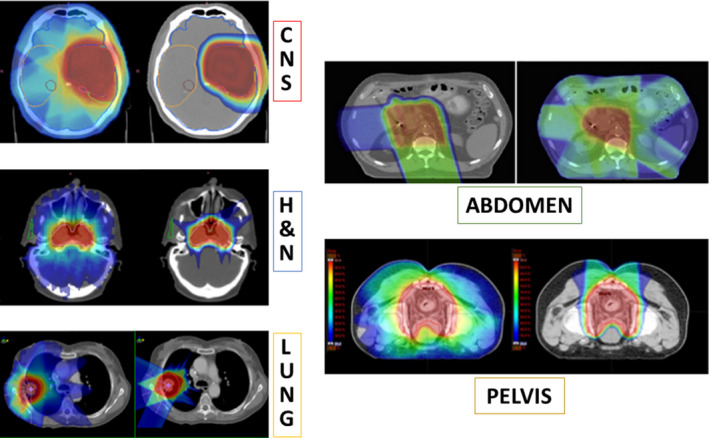
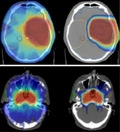
Comparison of treatment plans with X‐rays (left) and protons (right) for different tumor sites. Figure reproduced from Durante et al. (2019), reproduced with permission of Elsevier.
Proton therapy is currently considered standard therapy only in a limited number of cancer types. A current list of core indications is detailed in Table 1. The list may vary from country to country. In Europe, such indications are generally negotiated on a national level. Most European countries accept proton therapy as a standard for pediatric indications. For all other indications, some countries reimburse proton therapy according to a binding list (e.g., France, Italy, Poland, Switzerland). Other countries (e.g., Sweden, Denmark, the Czech Republic) do not have a fixed list of approved indications; here, the administration of proton therapy is based on decisions from multidisciplinary tumor boards. One country (the Netherlands) applies the model‐based approach (Widder et al., 2016) for selected novel indications (see the next section). In many countries, including Germany and the United States, cancers are treated with proton therapy providing that the patient's healthcare package reimburses this treatment. In the United States, a substantial number of patients however have been refused proton therapy (Odei et al., 2017; Shen et al., 2017).
Table 1.
Core indications for proton therapy.
| Ocular tumors |
| Uveal, iris, and conjunctival melanoma; hemangioma |
| Skull base tumors |
| Primary skull base tumors |
| Secondary infiltration from intracranial tumors |
| Head and neck tumors |
| Nasopharyngeal carcinoma, paranasal sinus carcinoma, adenoid cystic carcinoma, parotid carcinoma, soft tissue sarcomas |
| Nonmoving extracranial (paraspinal, retroperitoneal, sacral) tumors |
| Chondrosarcoma, chordoma, osteosarcoma, soft tissue sarcoma |
| Intracranial and spinal tumors |
| Low‐grade glioma, ependymoma, medulloblastoma, meningioma, chordoma, chondrosarcoma, neuroblastoma, low‐grade glioma NOS |
| Tumors of pediatric patients |
| The indications listed above and other pediatric tumors, for example, rhabdomyosarcoma, primitive neuroectodermal tumors, atypical teratoid/rhabdoid tumors, and Ewing sarcoma |
The clinical rationale for using carbon ion therapy is related to the depth–dose characteristics, a sharper penumbra, and the biological effectiveness, which is greater than that of protons. Carbon ions are used only in clinical protocols, and for certain types of, for example, radioresistant cancer.
4. Randomized clinical trials and new evidence‐based methodologies
Although particle therapy has been clinically introduced already for decades for several indications, limited data exist on treatment outcome based on systemically collected prospective studies, such as randomized controlled trials or observational cohort studies. Since particle therapy is more expensive than conventional photon radiotherapy, it is of utmost importance that robust data are generated to justify its use (Grau et al., 2018); evidence from well‐designed prospective trials is needed to demonstrate that protons delivered to selected cancer patients have a meaningful clinical benefit and should be routinely administered to a broader range of patients (Langendijk et al., 2018a; Weber et al., 2019, 2020).
There are currently a number of prospective phase III randomized trials ongoing or in the late planning phase in Europe. Most advanced are the plans for comparing protons with photons (IMRT) for head and neck cancers. In the UK, a randomized trial (TORPEdO) in oropharyngeal cancer patients has been launched (Price et al., 2020). In Denmark, the Danish Head and Neck Cancer Group (DAHANCA) is preparing a trial in oropharyngeal and laryngeal cancers, where patients who have a dosimetric benefit of protons will be randomized to either modality. EORTC is considering a trial, which in addition to the randomized arms allows patients to be enrolled using a model‐based approach without randomization. Other European randomized trials are under way in breast cancer, esophageal cancer, and prostate and lung cancers. These trials will form a much‐needed balance to a large series of US trials already recruiting.
Next to the randomized controlled trial, there is a need for alternative evidence‐based methodologies. In the Netherlands, the so‐called model‐based approach has been introduced not only to select which patients likely benefit most from proton therapy (model‐based selection), but also to define optimization criteria for radiotherapy treatment planning based on dose parameters included in normal tissue complication probability (NTCP) models (model‐based optimization) and model‐based clinical validation (Christianen et al., 2016; Langendijk et al., 2018a, 2013, 2018a, 2013; Rwigema et al., 2019; Widder et al., 2016). Prospective data registries are the backbone of the model‐based approach and are essential to continuously develop, externally validate, and update NTCP models for photons and protons. However, the model‐based approach is only applicable when protons are used to decrease the dose to critical anatomical structures aiming at reduction of radiation‐induced side effects, while the target dose remains biologically equivalent as compared to photons (Langendijk et al., 2013). Consequently, the model‐based approach cannot be used to validate the added value of carbon ions, assuming a relative biological effectiveness (RBE) that is different from photons, and/or if particles are used for target dose escalation. Moreover, the model‐based approach assumes that high‐quality NTCP models are available, which is not always the case for all indications. Therefore, in these circumstances, randomized controlled trials remain the gold standard.
Other novel trial methods are currently being suggested, many of which utilize information from rigorously collected prospective clinical data. One of these, the so‐called cohort multiple randomized controlled trial, will be described in the next section.
5. Prospective clinical databases
Prospective real‐life data registries may provide important information on the outcome of patients treated with particle therapy, both in terms of efficacy (e.g., local control and survival) and in terms of side effects and patient‐rated outcome measures. As such, prospective registrations offer a unique opportunity to find out which factors in the care process lead to the best results for patients. Identifying variation between centers and sharing positive results and care processes of best practices may give major incentives to improve the quality of all particle therapy centers or to follow implementation of guidelines (Beck et al., 2019; Gietelink et al., 2016).
The European Society for Radiotherapy and Oncology (ESTRO) is setting up a prospective data registration program for patients treated with particle therapy in the ParticleCare project (Weber et al., 2019). ParticleCare is part of E2‐RADIatE (www.estro.org/Science/E2RADIATE), which stands for the EORTC‐ESTRO RADiotherapy InfrAstrucTure for Europe, a collaboration between the European Organization for Research and Treatment of Cancer (EORTC) and the European Society for Radiotherapy. The overarching aim of ParticleCare is to establish a uniform prospective data registry on a European level for the most common tumor types treated with particle therapy, such as for cancers of the central nervous system, head and neck, breast, lung, esophagus, and prostate (Langendijk et al., 2018b).
Prospective data registries are an essential component of the so‐called cohort multiple randomized controlled trials (Lambin et al., 2015; Langendijk et al., 2018a). The principle is that a large cohort of patients is monitored prospectively for various parameters (Relton et al., 2010). Compared to a classical randomized trial, the main difference is that the study population can be randomly assigned to one of several new interventions (e.g., protons instead of photons) when they meet specific conditions. In this way, several novel approaches can be tested in the same trial. The trial design is particularly appropriate when testing expensive interventions like protons, and in situations where patients' preference to accept a new intervention is high (Lambin et al., 2015).
6. Collaborative networks
Collaboration between European particle therapy centers for generation of scientific and clinical evidence is of critical importance (Weber et al., 2019).
European particle therapy centers are active members of the global Particle Therapy Co‐Operative Group (PTCOG; www.ptcog.ch). In addition, there are currently three active European networks working in complementary fields of particle therapy: the European Network for Light Ion Hadron Therapy (ENLIGHT; https://enlight.web.cern.ch), the Infrastructure in Proton International Research (INSPIRE; www.protonsinspire.eu), and the European Particle Therapy Network (EPTN; www.estro.org/Science/EPTN). In addition, research collaboration is facilitated through the International Biophysics Collaboration (www.gsi.de/bio‐coll) and an EU collaboration exists to support particle therapy in southeastern Europe (www.seeiist.eu). The EU also sponsored a previous collaboration, the Union of Light Ion Centres in Europe (ULICE; www.cordis.europa.eu/project/rcn/92176/reporting/en). The ULICE project ended in 2014, leaving a substantial contribution of reports and white papers in the public domain (Potter et al., 2018).
The ENLIGHT network has coordinated European efforts in ion beam research since 2002. The network has its main focus on science dissemination within basic and translational research issues (Dosanjh et al., 2018a).
INSPIRE was funded by EU FP7 for the period of 2018–2021. A broad consortium led by the University of Manchester works together to develop a new infrastructure, bringing research activities in clinical proton therapy centers, associated academic establishments, and industry across Europe together. The aim is to enable researchers from across Europe, in both the public and private sectors, to access this infrastructure and conduct research. Training will be provided for the next generation of researchers in this field where there is an internationally recognized skill shortage. INSPIRE will also facilitate knowledge exchange and allow best research practice to be shared across and between centers throughout Europe and develop an innovation pipeline allowing research to be translated into clinical practice and industrial products (www.inspireprotons.eu).
The EPTN was established in 2015 in response to the increase in the number of clinical particle therapy centers in Europe (Grau et al., 2018; Weber et al., 2019). The primary aim of EPTN is to enable cooperation among particle therapy centers and to integrate particle therapy in the radiation oncology community (Grau et al., 2018). Therefore, EPTN is an official task force of the European Society for Radiotherapy and Oncology (ESTRO). The seven working groups have already produced a series of recommendations and whitepapers, and more activities are expected in the coming years (Bolsi et al., 2018; Dosanjh et al., 2018b; Eekers et al., 2018; Grau et al., 2018; Lambrecht et al., 2018; Langendijk et al., 2018b; Weber et al., 2019, 2018, 2019, 2018).
7. Conclusions and future directions
Particle therapy holds great promise to improve the therapeutic outcome of cancer patients treated with this modality. There is an urgency to produce high‐quality clinical evidence. With the opening of a substantial number of particle centers in Europe, there is now a great opportunity to collaborate across institutions and countries to secure evidence‐based implementation of particle therapy. In Europe, collaboration is catalyzed by several networks and organizations, including INSPIRE, EPTN, ESTRO, and EORTC. There is now a platform that enables European particle centers to collaborate in prospective data collection as well as prospective clinical trials. With the combined efforts of all these dedicated working groups and institutions, there is no doubt that European particle therapy is ready to meet the challenge and generate the much‐needed clinical evidence for particle therapy.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
All authors contributed to the manuscript and approved the final version.
References
- Beck N, van Brakel TJ, Smit HJM, van Klaveren D, Wouters M and Schreurs WH (2019) Pneumonectomy for lung cancer treatment in the Netherlands: between‐hospital variation and outcomes. World J Surg 44, 285–294. [DOI] [PubMed] [Google Scholar]
- Bolsi A, Peroni M, Amelio D, Dasu A, Stock M, Toma‐Dasu I, Nystrom PW and Hoffmann A (2018) Practice patterns of image guided particle therapy in Europe: a 2016 survey of the European Particle Therapy Network (EPTN). Radiother Oncol 128, 4–8. [DOI] [PubMed] [Google Scholar]
- Christianen ME, van der Schaaf A, van der Laan HP, Verdonck‐de Leeuw IM, Doornaert P, Chouvalova O, Steenbakkers RJ, Leemans CR, Oosting SF, van der Laan BF et al (2016) Swallowing sparing intensity modulated radiotherapy (SW‐IMRT) in head and neck cancer: clinical validation according to the model‐based approach. Radiother Oncol 118, 298–303. [DOI] [PubMed] [Google Scholar]
- Dosanjh M, Amaldi U, Mayer R, Poetter R and Network E (2018a) ENLIGHT: European network for light ion hadron therapy. Radiother Oncol 128, 76–82. [DOI] [PubMed] [Google Scholar]
- Dosanjh M, Jones B, Pawelke J, Pruschy M and Sorensen BS (2018b) Overview of research and therapy facilities for radiobiological experimental work in particle therapy. Report from the European Particle Therapy Network radiobiology group. Radiother Oncol 128, 14–18. [DOI] [PubMed] [Google Scholar]
- Durante M and Debus J (2018) Heavy charged particles: does improved precision and higher biological effectiveness translate to better outcome in patients? Semin Radiat Oncol 28, 160–167.29735192 [Google Scholar]
- Durante M and Flanz J (2019) Charged particle beams to cure cancer: strengths and challenges. Semin Oncol 46, 219–225. [DOI] [PubMed] [Google Scholar]
- Durante M, Golubev A, Park W‐Y and Trautmann C (2019) Applied nuclear physics at the new high‐energy particle accelerator facilities. Phys Rep 800, 1–37. [Google Scholar]
- Durante M and Loeffler JS (2010) Charged particles in radiation oncology. Nat Rev Clin Oncol 7, 37–43. [DOI] [PubMed] [Google Scholar]
- Durante M and Paganetti H (2016) Nuclear physics in particle therapy: a review. Rep Prog Phys 79, 096702. [DOI] [PubMed] [Google Scholar]
- Eekers DBP, in't Ven L, Roelofs E, Postma A, Alapetite C, Burnet NG, Calugaru V, Compter I, Coremans IEM, Høyer M et al (2018) The EPTN consensus‐based atlas for CT‐ and MR‐based contouring in neuro‐oncology. Radiother Oncol 128, 37–43. [DOI] [PubMed] [Google Scholar]
- Gietelink L, Wouters MW, Bemelman WA, Dekker JW, Tollenaar RA, Tanis PJ and Dutch Surgical Colorectal Cancer Audit Group (2016) Reduced 30‐day mortality after laparoscopic colorectal cancer surgery: a population based study from the Dutch Surgical Colorectal Audit (DSCA). Ann Surg 264, 135–140. [DOI] [PubMed] [Google Scholar]
- Grau C, Baumann M and Weber DC (2018) Optimizing clinical research and generating prospective high‐quality data in particle therapy in Europe: introducing the European Particle Therapy Network (EPTN). Radiother Oncol 128, 1–3. [DOI] [PubMed] [Google Scholar]
- Haas‐Kogan D, Indelicato D, Paganetti H, Esiashvili N, Mahajan A, Yock T, Flampouri S, MacDonald S, Fouladi M, Stephen K et al (2018) National Cancer Institute workshop on proton therapy for children: considerations regarding brainstem injury. Int J Radiat Oncol Biol Phys 101, 152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf A, Bert C, Heath E, Nill S, Kraus K, Richter D, Hug E, Pedroni E, Safai S, Albertini F et al (2010) Special report: workshop on 4D‐treatment planning in actively scanned particle therapy–recommendations, technical challenges, and future research directions. Med Phys 37, 4608–4614. [DOI] [PubMed] [Google Scholar]
- Lambin P, Zindler J, Vanneste B, van de Voorde L, Jacobs M, Eekers D, Peerlings J, Reymen B, Larue RT, Deist TM et al (2015) Modern clinical research: how rapid learning health care and cohort multiple randomised clinical trials complement traditional evidence based medicine. Acta Oncol 54, 1289–1300. [DOI] [PubMed] [Google Scholar]
- Lambrecht M, Eekers DBP, Alapetite C, Burnet NG, Calugaru V, Coremans IEM, Fossati P, Hoyer M, Langendijk JA, Mendez Romero A et al (2018) Radiation dose constraints for organs at risk in neuro‐oncology; the European Particle Therapy Network consensus. Radiother Oncol 128, 26–36. [DOI] [PubMed] [Google Scholar]
- Langendijk JA, Boersma LJ, Rasch CRN, van Vulpen M, Reitsma JB, van der Schaaf A and Schuit E (2018a) Clinical trial strategies to compare protons with photons. Semin Radiat Oncol 28, 79–87. [DOI] [PubMed] [Google Scholar]
- Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M and Verheij M (2013) Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model‐based approach. Radiother Oncol 107, 267–273. [DOI] [PubMed] [Google Scholar]
- Langendijk JA, Orecchia R, Haustermans K, Zips D, Balosso J, Lacombe D, Lievens Y, Weber DC, Grau C and Troost EGC (2018b) Prospective data registration and clinical trials for particle therapy in Europe. Radiother Oncol 128, 9–13. [DOI] [PubMed] [Google Scholar]
- Odei B, Frandsen JE, Boothe D, Ermoian RP and Poppe MM (2017) Patterns of care in proton radiation therapy for pediatric central nervous system malignancies. Int J Radiat Oncol Biol Phys 97, 60–63. [DOI] [PubMed] [Google Scholar]
- Oelfke U and Bortfeld T (2003) Optimization of physical dose distributions with hadron beams: comparing photon IMRT with IMPT. Technol Cancer Res Treat 2, 401–412. [DOI] [PubMed] [Google Scholar]
- Potter R, Balosso J, Baumann M, Bert C, Davies J, Enghardt W, Fossati P, Harris S, Jones B, Kramer M et al (2018) Union of light ion therapy centers in Europe (ULICE EC FP7) – objectives and achievements of joint research activities. Radiother Oncol 128, 83–100. [DOI] [PubMed] [Google Scholar]
- Price J, Hall E, West C and Thomson D (2020) TORPEdO – a phase III trial of intensity‐modulated proton beam therapy versus intensity‐modulated radiotherapy for multi‐toxicity reduction in oropharyngeal cancer. Clin Oncol (R Coll Radiol) 32, 84–88. [DOI] [PubMed] [Google Scholar]
- Relton C, Torgerson D, O'Cathain A and Nicholl J (2010) Rethinking pragmatic randomised controlled trials: introducing the “cohort multiple randomised controlled trial” design. BMJ 340, c1066. [DOI] [PubMed] [Google Scholar]
- Romero J, Cardenes H, la Torre A, Valcarcel F, Magallon R, Regueiro C and Aragon G (1993) Chordoma: results of radiation therapy in eighteen patients. Radiother Oncol 29, 27–32. [DOI] [PubMed] [Google Scholar]
- Rwigema JM, Langendijk JA, Paul van der Laan H, Lukens JN, Swisher‐McClure SD and Lin A (2019) A model‐based approach to predict short‐term toxicity benefits with proton therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys 104, 553–562. [DOI] [PubMed] [Google Scholar]
- Sakthivel V, Ganesh KM, McKenzie C, Boopathy R and Selvaraj J (2019) Second malignant neoplasm risk after craniospinal irradiation in X‐ray‐based techniques compared to proton therapy. Australas Phys Eng Sci Med 42, 201–209. [DOI] [PubMed] [Google Scholar]
- Schneider U, Halg RA, Baiocco G and Lomax T (2016) Neutrons in proton pencil beam scanning: parameterization of energy, quality factors and RBE. Phys Med Biol 61, 6231–6242. [DOI] [PubMed] [Google Scholar]
- Shen CJ, Hu C, Ladra MM, Narang AK, Pollack CE and Terezakis SA (2017) Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer 123, 4048–4056. [DOI] [PubMed] [Google Scholar]
- Specht HM, Neff T, Reuschel W, Wagner FM, Kampfer S, Wilkens JJ, Petry W and Combs SE (2015) Paving the road for modern particle therapy – what can we learn from the experience gained with fast neutron therapy in Munich? Front Oncol 5, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock M, Georg D, Ableitinger A, Zechner A, Utz A, Mumot M, Kragl G, Hopfgartner J, Gora J, Bohlen T et al (2018) The technological basis for adaptive ion beam therapy at MedAustron: status and outlook. Z Med Phys 28, 196–210. [DOI] [PubMed] [Google Scholar]
- van de Water S, Albertini F, Weber DC, Heijmen BJM, Hoogeman MS and Lomax AJ (2018) Anatomical robust optimization to account for nasal cavity filling variation during intensity‐modulated proton therapy: a comparison with conventional and adaptive planning strategies. Phys Med Biol 63, 025020. [DOI] [PubMed] [Google Scholar]
- Weber DC, Abrunhosa‐Branquinho A, Bolsi A, Kacperek A, Dendale R, Geismar D, Bachtiary B, Hall A, Heufelder J, Herfarth K et al (2017) Profile of European proton and carbon ion therapy centers assessed by the EORTC facility questionnaire. Radiother Oncol 124, 185–189. [DOI] [PubMed] [Google Scholar]
- Weber DC, Grau C, Lim PS, Georg D and Lievens Y (2019) Bringing Europe together in building clinical evidence for proton therapy – the EPTN‐ESTRO‐EORTC endeavor. Acta Oncol 58, 1340–1342. [DOI] [PubMed] [Google Scholar]
- Weber DC, Habrand JL, Hoppe BS, Hill Kayser C, Laack NN, Langendijk JA, MacDonald SM, McGovern SL, Pater L, Perentesis JP et al (2018) Proton therapy for pediatric malignancies: Fact, figures and costs. A joint consensus statement from the pediatric subcommittee of PTCOG, PROS and EPTN. Radiother Oncol 128, 44–55. [DOI] [PubMed] [Google Scholar]
- Weber DC, Lim PS, Tran S, Walser M, Bolsi A, Kliebsch U, Beer J, Bachtiary B, Lomax T and Pica A (2020) Proton therapy for brain tumours in the area of evidence‐based medicine. Br J Radiol 93, 20190237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DC, Malyapa R, Albertini F, Bolsi A, Kliebsch U, Walser M, Pica A, Combescure C, Lomax AJ and Schneider R (2016) Long term outcomes of patients with skull‐base low‐grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol 120, 169–174. [DOI] [PubMed] [Google Scholar]
- Widder J, van der Schaaf A, Lambin P, Marijnen CA, Pignol JP, Rasch CR, Slotman BJ, Verheij M and Langendijk JA (2016) The quest for evidence for proton therapy: model‐based approach and precision medicine. Int J Radiat Oncol Biol Phys 95, 30–36. [DOI] [PubMed] [Google Scholar]
- Wilson RR (1946) Radiological use of fast protons. Radiology 47, 487–491. [DOI] [PubMed] [Google Scholar]


