Summary
Background
Kawasaki disease is an acute febrile systemic childhood vasculitis, which is suspected to be triggered by respiratory viral infections. We aimed to examine whether the ongoing COVID-19 epidemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is associated with an increase in the incidence of Kawasaki disease.
Methods
We did a quasi-experimental interrupted time series analysis over the past 15 years in a tertiary paediatric centre in the Paris region, a French epicentre of the COVID-19 outbreak. The main outcome was the number of Kawasaki disease cases over time, estimated by quasi-Poisson regression. In the same centre, we recorded the number of hospital admissions from the emergency department (2005–2020) and the results of nasopharyngeal multiplex PCR to identify respiratory pathogens (2017–2020). These data were compared with daily hospital admissions due to confirmed COVID-19 in the same region, recorded by Public Health France.
Findings
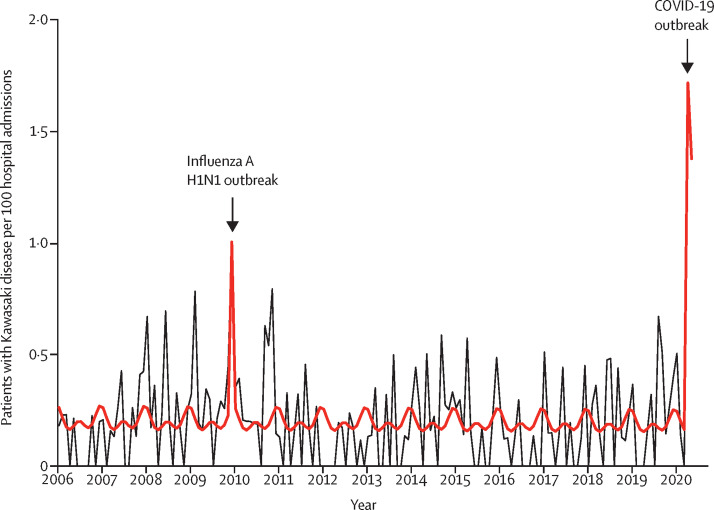
Between Dec 1, 2005, and May 20, 2020, we included 230 patients with Kawasaki disease. The median number of Kawasaki disease hospitalisations estimated by the quasi-Poisson model was 1·2 per month (IQR 1·1–1·3). In April, 2020, we identified a rapid increase of Kawasaki disease that was related to SARS-CoV-2 (six cases per month; 497% increase [95% CI 72–1082]; p=0·0011), starting 2 weeks after the peak of the COVID-19 epidemic. SARS-CoV-2 was the only virus circulating intensely during this period, and was found in eight (80%) of ten patients with Kawasaki disease since April 15 (SARS-CoV-2-positive PCR or serology). A second peak of hospital admissions due to Kawasaki disease was observed in December, 2009 (six cases per month; 365% increase ([31–719]; p=0.0053), concomitant with the influenza A H1N1 pandemic.
Interpretation
Our study further suggests that viral respiratory infections, including SAR-CoV-2, could be triggers for Kawasaki disease and indicates the potential timing of an increase in incidence of the disease in COVID-19 epidemics. Health-care providers should be prepared to manage an influx of patients with severe Kawasaki disease, particularly in countries where the peak of COVID-19 has recently been reached.
Funding
French National Research Agency.
Introduction
The rapid spread of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global pandemic.1 The paediatric population appears to be much less affected than adults are, with less than 3% of reported cases in those younger than 20 years.1 Available data suggested that compared with adult patients, paediatric patients have milder symptoms and a better prognosis.2, 3
By April, 2020, physicians from several countries had suggested that the numbers of children and adolescents with multisystem inflammatory symptoms, including Kawasaki disease and features of shock with intensive care unit admissions, were increasing.4 This suggestion led to reports of an increased incidence of paediatric shock and myocarditis by several health authorities in Europe and North America.5, 6 A link between COVID-19 and Kawasaki disease has been suggested by scattered case reports7, 8, 9 and an observational cohort study reported an outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic.10 Acute heart failure in multisystem inflammatory syndrome in the context of SARS-CoV-2 has also been reported in a multicentre cohort.11 Whether or not these various manifestations should be considered as the clinical spectrum of a novel disease or as an association of various suspected post-infectious diseases triggered by SARS-CoV-2 is unknown.
Kawasaki disease is an acute febrile systemic childhood vasculitis that affects medium and small-sized blood vessels with a coronary artery tropism.12, 13 The disease is one of the leading causes of acquired heart disease in industrialised countries.12, 13 No specific test exists to confirm the diagnosis. The diagnosis of Kawasaki disease is based on internationally accepted clinical criteria,14, 15 combining a fever lasting 5 days or more with at least four of the following clinical signs: bilateral conjunctival injection, changes affecting the lips and oral cavity (inflammation of the oral and pharyngeal mucosa), polymorphic exanthema, changes in the peripheral extremities or perineal area, and cervical lymphadenopathy of 15 mm or larger. Patients who meet the case definition on the basis of principal clinical findings are said to have complete Kawasaki disease. Patients who do not have sufficient principal clinical findings might be diagnosed with incomplete Kawasaki disease.
Research in context.
Evidence before this study
The rapid spread of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global pandemic. Physicians from several countries have suggested that the numbers of children with multisystem inflammatory symptoms, including Kawasaki disease, were increasing. This suggestion led several health authorities in Europe and North America to issue alerts to medical professionals. We searched PubMed articles published until May 31, 2020, using the terms “Kawasaki disease” AND “coronavirus” OR “COVID-19” OR “virus involvement” OR “SARS-CoV-2”. We identified scattered case reports and an observational cohort study from Italy, indicating a potential link between COVID-19 and Kawasaki disease. Previous studies have suggested that viral infections could be the trigger of Kawasaki disease. Thus, epidemiological studies are needed to enlighten the potential link between SARS-CoV-2 and the emergence of Kawasaki disease.
Added value of this study
We did a time-series analysis that was based on a retrospective review of Kawasaki disease cases in a French tertiary paediatric centre over the past 15 years. The centre is located in the Paris region, an epicentre of the country's COVID-19 outbreak. We found an increase in the incidence of Kawasaki disease during the COVID-19 pandemic. During this period, which included the national lockdown, the circulation of all other respiratory viruses had an unprecedented drop. Our observations further suggest that an association exists between SARS-CoV-2 infection and Kawasaki disease. Interestingly, a second significant peak of Kawasaki disease was observed in December, 2009, during the influenza A H1N1 pandemic. To our knowledge, this is the first study to use a time series analysis over a 15-year period to assess incidence of Kawasaki disease and concurrent circulating viruses, including SARS-CoV-2.
Implications of all the available evidence
The ongoing SARS-CoV-2 pandemic has been followed by a rapid emergence of Kawasaki disease, suggesting that children can develop severe forms of COVID-19. This increased incidence is similar to the peak of Kawasaki disease that occurred after the 2009 influenza A H1N1 pandemic, providing evidence of the role of viral infections in triggering Kawasaki disease. Physicians should prepare to manage an increase in the incidence of Kawasaki disease, depending on the magnitude of their local COVID-19 outbreak.
The cause of Kawasaki disease is unknown.16 Regions of the extratropical northern hemisphere have two seasonal peaks in winter and spring, and some outbreaks have been observed in Japan, without any clear cause known.17 A correlation between this bimodal seasonality and viruses such as influenza and respiratory syncytial virus in winter and respiratory enteroviruses in summer has been suggested.18, 19, 20, 21 This correlation has led to the speculation that viral infections could be the trigger of Kawasaki disease. To date, whether SARS-CoV-2 can lead to Kawasaki disease is unknown.
We aimed to examine whether the outbreak of COVID-19 is associated with an increase of Kawasaki disease in a tertiary hospital in the French epicentre of the COVID-19 outbreak.
Methods
Study design
We did a quasi-experimental, interrupted time-series analysis based on a retrospective review of data of all patients with Kawasaki disease admitted over the past 15 years to a tertiary centre, Robert Debré University Hospital, in Paris, France. This centre is a paediatric hospital that mainly receives patients from the north and north-eastern part of Paris and the Paris region, which has been one of the epicentres of the COVID-19 epidemic in France. Robert Debré Hospital provides paediatric care support and retrieval to a population of approximately 2 million inhabitants. The referring hospitals and the reasons for referral to Robert Debré Hospital remained unchanged during the observation period and during the COVID-19 epidemic.
Data collection
From Dec 1, 2005, to May 20, 2020, we recorded the number of patients diagnosed with Kawasaki disease using the validated diagnostic criteria15 of the American Heart Association. Patients for whom another diagnosis was confirmed during the follow-up were excluded. For each patient, demographic and clinical data were prospectively recorded. All collected data were anonymous. Furthermore, we recorded the number of hospital admissions of paediatric patients (aged <18 years) from the emergency department in the same centre in the study period. The results of the respiratory pathogens identified by multiplex nasopharyngeal PCR from Sept 1, 2017, to May 20, 2020, were also recorded. These time series were compared with daily paediatric hospital admissions due to confirmed COVID-19 disease in the same region, recorded by Public Health France. This study followed national ethical guidelines and was approved by French data protection authorities (Commission Nationale de l'Information et des Libertés 2014908 and 1980120).
Outcomes
The main outcome was the number of monthly Kawasaki disease cases over the study period estimated by our model. Secondary outcomes were the number of hospital admissions following paediatric emergency department visits, and the proportion of positive nasopharyngeal multiplex PCRs in our centre over the past 3 years. Our centre used multiplex PCR (FilmArray Respiratory Panel 2 plus [Biomérieux, Marcy l'Etoile, France) to test for respiratory pathogens in nasopharyngeal swabs from patients. The tests were for the following respiratory pathogens: adenovirus, coronavirus 229E, coronavirus HKU, coronavirus NL63, coronavirus OC43, metapneumovirus, rhinovirus or enterovirus, influenza A, influenza B, parainfluenza (types 1–4), respiratory syncytial virus, Bordetella parapertussis, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae. Furthermore, all Kawasaki disease cases diagnosed since April 1, 2020, were tested with SARS-CoV-2 nasopharyngeal PCR (Xpert® Xpress SARS-CoV-2 [Cepheid, Maurens-Scopont, France]). We also analysed the monthly Kawasaki disease rate per 100 hospital admissions following paediatric emergency department visits over the study period.
Analysis
The outcomes were analysed using quasi-Poisson regression, accounting for seasonality, secular trends, and overdispersion of data.22, 23, 24 Seasonality was taken into account by including harmonic terms (sines and cosines) with 12-month and 6-month periods.22 To provide optimal precision to the model, the chosen time unit was 1 month.24 Because a delay between the onset of respiratory viral infection and Kawasaki disease is expected on the basis of previous studies on seasonality and Kawasaki disease, we hypothesised that the COVID-19 outbreak would have an effect on Kawasaki disease cases 1 month (ie, one time unit) after the peak number of hospital admissions in the region. Thus, the COVID-19 impact assessment involved a dummy variable in the model estimating the immediate post-outbreak change in April, 2020. The only previous major respiratory virus pandemic identified over the study period was the influenza A H1N1 pandemic in 2009. Thus, another dummy variable was included in the model to allow for the immediate effect of influenza A H1N1 on Kawasaki occurrence in December, 2009. The validity of the quasi-Poisson regression model was assessed using visual inspection of the correlograms (autocorrelation and partial autocorrelation functions) and analysis of the residuals. All statistical tests were two-sided, and we considered a result as significant when the p-value was less than 0·05. All statistical analysis was done in R (version 3.6.1).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication
Results
Between Dec 1, 2005, and May 20, 2020, 230 patients were admitted to hospital with incomplete or complete Kawasaki disease (134 [58%] boys, 96 [42%] girls, aged 1 month to 15·5 years). The median number of Kawasaki disease hospitalisations estimated by the quasi-Poisson model was 1·2 per month (IQR 1·1–1·3).
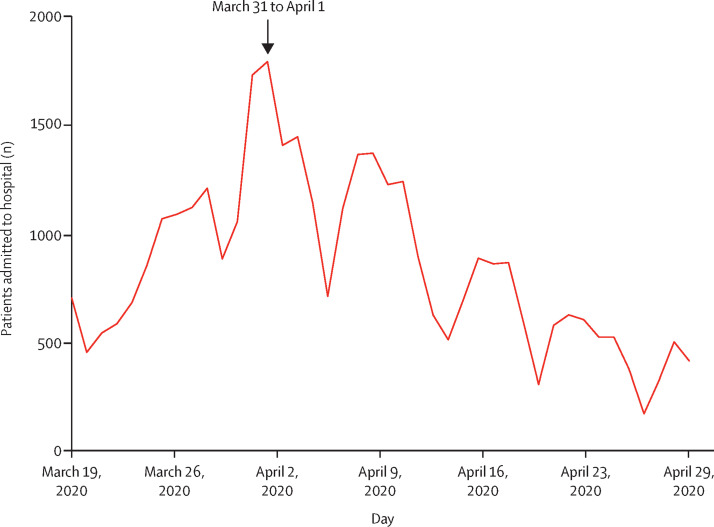
In April, 2020, we observed a significant increase in the number of patients with Kawasaki disease admitted to our centre (six patients per month; 497% increase [95% CI 72–1082]; p=0.0011). Similar results were obtained when analysing Kawasaki disease rate per 100 hospitalisations over time (figure 1 ; appendix p 3) with an occurrence of a spike between April 15 and May 15, 2020. Thus, the increase of Kawasaki disease hospitalisations started 2 weeks after the first peak of the COVID-19 epidemic in the Paris region, which occurred around March 31 to April 1, 2020 (figure 2 ).
Figure 1.
Kawasaki disease rate per 100 hospital admissions, 2006 to 2020
230 patients had Kawasaki disease during this period. The total number of hospital admission following paediatric emergency department visits was 110 824. The black line depicts the observed data. The bold red line depicts the model estimates based on the quasi-Poisson regression model. The influenza A H1N1 outbreak occurred November to December, 2009, and the COVID-19 outbreak March to April, 2020.
Figure 2.
COVID-19 hospital admissions during the outbreak in the Paris region, France (n=35 732)
Data are from Public Health France. The figure depicts the daily number of new admissions to hospital for confirmed COVID-19 for all ages in the Paris region, France.
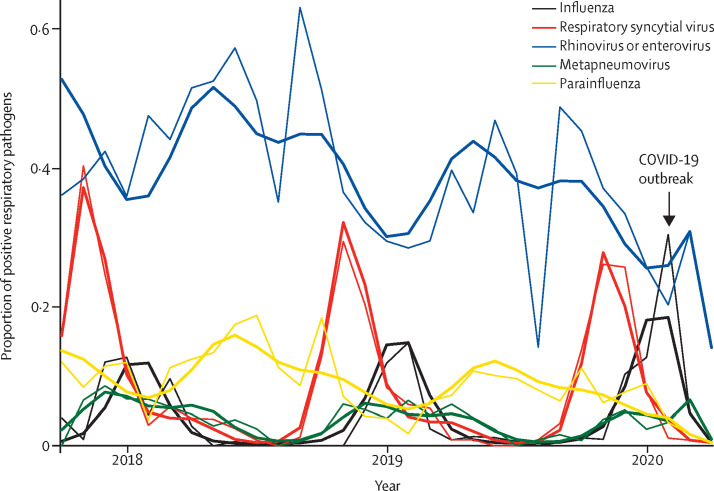
The increase in the rate of Kawasaki disease observed in April, 2020, was not related to an increase in overall hospital admissions, which dropped since March, 2020 (360 hospital admissions per month; 31% decrease [95% CI −42 to −18]; appendix p 2), probably because of the requirement for people to stay home during national lockdown. Furthermore, this increase coincided with an unprecedented decrease in the proportion of other respiratory viruses circulating in the Paris region population since March, 2020 (figure 3 ), probably because of the reduction of social contacts after the lockdown. SARS-CoV-2 was circulating intensely during this period (figure 2), reinforcing the link between this virus and the increase in the rate of Kawasaki disease.
Figure 3.
Rate of respiratory pathogens in our centre, 2017 to 2020
The thin lines depict observed data (n=4662). The bold lines show the model estimates from the final quasi-Poisson regression models.
The characteristics of patients with Kawasaki disease during the SARS-CoV-2 epidemic are shown in table 1 . Among the ten children presenting with Kawasaki disease from April 15 to May 20, 2020, eight (80%) had a positive nasopharyngeal SARS-CoV-2 PCR or positive SARS-CoV-2 serology (table 1). One (10%) patient had a prolonged exposure with an individual with confirmed COVID-19 but had negative SARS-CoV-2 PCR and serological tests. Among these patients, five (50%) had complete Kawasaki disease and five (50%) patients had fever with only three other Kawasaki disease criteria (incomplete Kawasaki disease). The age of patients with Kawasaki disease ranged from 18 months to 15·8 years. Six (60%) children had cardiac abnormalities, including one major coronary aneurysm (Z score=12) and five with myocarditis. Six (60%) patients required intensive care and five (50%) had inotrope treatment. None required mechanical ventilation, and no fatal outcome was observed.
Table 1.
Characteristics of patients with Kawasaki disease who presented during the SARS-CoV-2 epidemic, April 15 to May 20, 2020
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Date of admission | April 17, 2020 | April 17, 2020 | April 21, 2020 | April 24, 2020 | April 26, 2020 | April 28, 2020 | May 4, 2020 | May 10, 2020 | May 12, 2020 | May 16, 2020 |
| Age (years) | 12·0 | 1·8 | 11·5 | 1·5 | 15·5 | 13·5 | 9·8 | 14·5 | 15·8 | 6·3 |
| Sex | Female | Female | Male | Male | Female | Female | Female | Female | Male | Male |
| SARS-CoV-2 nasopharyngeal PCR | Positive | NA | Positive | Negative | Positive | Positive | Negative | Positive | Negative | Negative |
| SARS-CoV-2 serology | NA | NA | IgG positive | Negative | IgG positive | NA | IgG positive | NA | IgG positive | IgG positive |
| Contact with suspected or confirmed case | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Type of Kawasaki disease | Complete | Complete | Complete | Incomplete | Incomplete | Complete | Incomplete | Complete | Incomplete | Incomplete |
| Kobayashi score1 | 8 | 1 | 4 | 0 | 4 | 8 | 7 | 6 | 8 | 6 |
| Kawasaki disease shock syndrome2 | No | No | No | No | No | Yes | Yes | Yes | Yes | No |
| Cardiac involvement | Myocarditis | No | No | Coronary dilatation (Z=12) | No | Myocarditis | Myocarditis | Myocarditis, pericarditis | Myocarditis | No |
| First-line treatment | IVIg | IVIg | None | IVIg | IVIg | IVIg | IVIg | IVIg | IVIg | IVIg |
| Unsuccessful first-line treatment | Yes | No | No | Yes | No | Yes | Yes | No | Yes | Yes |
| Second-line treatment | IVIg plus methyl-prednisolone | No | No | IVIg plus methyl-prednisolone | No | Tocilizumab | IVIg plus methyl-prednisolone | No | IVIg plus methyl-prednisolone | IVIg plus methyl-prednisolone |
| Admission to PICU | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Inotropes treatment | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Lengths of hospital stay (days) | 14 | 5 | 4 | 27 | 5 | 18 | 19 | 13 | 9 | 17 |
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. IVIg=intravenous immunoglobulin. PICU=paediatric intensive care unit. NA=not available.
A second peak of Kawasaki disease hospitalisations was detected by the model in December, 2009, (six cases per month; 365% increase [95% CI 31–719]; p=0·0053), concomitant with the H1N1 outbreak,25 peaking in November to December, 2009, in France (figure 1; appendix p 3). The increase in the number of Kawasaki disease hospitalisations occurred approximately 1–3 weeks after the peak of the H1N1 epidemic in the Paris region.
A comparison between patients with Kawasaki disease who were hospitalised in our centre during the SARS-CoV-2 epidemic, during the H1N1 epidemics, and outside of these major epidemics is shown in table 2 . Furthermore, we compared these patient groups with paediatric patients from another study10 who were hospitalised with a Kawasaki-like disease during the SARS-CoV-2 epidemic in Bergamo, Italy (table 2). The characteristics of patients with Kawasaki disease who were hospitalised during the SARS-CoV-2 epidemic in our centre were similar to those of patients who were hospitalised during the SARS-CoV-2 epidemic in Bergamo, with a high proportion of patients with severe Kawasaki disease with cardiac involvement and the need for a second line of treatment. By contrast, the characteristics of patients with Kawasaki disease diagnosed during SARS-COV-2 epidemics appeared to be different from those diagnosed during the H1N1 epidemics (median age 11·8 vs 2·1 years; p=0.034; median C-reactive protein concentration 23·6 mg/dL vs 8·4 mg/dL; p=0·042; median lymphocytes count 1042 × 109 per L vs 3410 × 109 per L; p=0·028).
Table 2.
Comparison between patients with Kawasaki disease presenting during the SARS-CoV-2 epidemic in the Paris region, France, and in Bergamo, Italy, during the influenza A H1N1 epidemics and outside of major viral epidemics
| SARS-CoV-2 epidemic in Robert Debré Hospital, France* | SARS-CoV-2 epidemic in Bergamo, Italy10 | Influenza A H1N1 epidemic in Robert Debré Hospital, France | Kawasaki disease outside of major viral epidemics in Robert Debré Hospital, France | ||
|---|---|---|---|---|---|
| Time of presentation | April–May, 2020† | March–April, 2020 | December, 2009 | 2005–2020 | |
| Patients | 10 | 10 | 6 | 214 | |
| Incidence per month | 6 | 10 | 6 | 1 | |
| Rate of Kawasaki disease per 100 hospital admissions | 1·5 | NA | 1·0 | 0·2 | |
| Sex | |||||
| Female | 4/10 (40%) | 3/10 (30%) | 5/6 (83%) | 87/214 (41%) | |
| Male | 6/10 (60%) | 7/10 (70%) | 1/6 (17%) | 127/214 (59%) | |
| Median age, years | 11·8 (7·4–14·3) | 7·2 (5·5–8·1) | 2·1 (1·7–3·8) | 2·1 (1·1–3·7) | |
| Complete Kawasaki disease | 6/10 (60%) | 5/10 (50%) | 4/6 (67%) | 138/214 (64%) | |
| C-reactive protein concentration, mg/dL | 23·6 (13·2–30·9) | 24·1 (13·0–29·5) | 8·4 (5·1–13·3) | 14·4 (9·9–19·8) | |
| Lymphocytes count, × 109 per L | 1042 (650–1150) | 832 (543–960) | 3410 (2010–4590) | 3044 (1855–4770) | |
| Platelet count, × 109 per L | 274 (192–715) | 130 (120–142) | 613 (454–715) | 379 (285–484) | |
| Sodium concentration, mEq/L | 130 (129–135) | 131·5 (129–133) | 137·5 (136–139) | 135 (134–137) | |
| Aspartate aminotransferase concentration, U/L | 35 (35–53) | 57 (35–112) | 30 (28–37) | 35 (28–57) | |
| Alanine aminotransferase concentration, U/L | 33 (27–38) | 55 (34–79) | 20 (15–28) | 40 (19–97) | |
| Kobayashi score ≥51 | 7/10 (70%) | 7/10 (70%) | 0 | 39/153 (25%) | |
| Kawasaki disease shock syndrome29 | 4/10 (40%) | 5/10 (50%) | 0/6 (0%) | NA | |
| Abnormal echocardiography | 6/10 (60%) | 6/10 (60%) | 3/6 (50%) | 51/214 (24%) | |
| Need for additional treatment to first dose of IVIg | 6/10 (60%) | 8/10 (80%) | 1/6 (17%) | 44/203 (22%) | |
| Admission to PICU | 6/10 (60%) | NA | 0 | 14/160 (9%) | |
| Inotropes treatment | 5/10 (50%) | 2/10 (20%) | NA | NA | |
Data are n, n (%), or median (IQR). SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. IVIg=intravenous immunoglobulin. PICU=paediatric intensive care unit. NA=not available.
The total number of paediatric patients with COVID-19 who were referred to our hospital was 39.
Data after May 20, 2020 were not available.
Discussion
We found a link between an emergence of Kawasaki disease and the ongoing COVID-19 pandemic. Several findings support this association. First, we observed that the unexpected high emergence of Kawasaki disease occurred 2 weeks after the peak of the COVID-19 epidemic, in line with the post-infection mechanism of Kawasaki disease, as previously reported.15 Second, following the nationwide lockdown in March, 2020, SARS-CoV-2 was the only respiratory virus with intense circulation in the weeks before the emergence of Kawasaki disease, as indicated by our analysis of the results from respiratory multiplex PCR showing a decrease in the proportion of other respiratory viruses in the hospital population at the time. Third, almost all patients with Kawasaki disease in April, 2020, had a positive nasopharyngeal SARS-CoV-2 PCR, exposure to an individual with confirmed COVID-19, or positive serology.
The only other peak in Kawasaki disease observed during the 15-year observation period occurred during the context of the H1N1 pandemic, suggesting that SARS-CoV-2 is not the only virus capable of triggering such an emergence of Kawasaki disease. These findings are in line with previous observations reporting associations between seasonality and outbreaks of Kawasaki disease, especially in Japan, where nationwide epidemics were observed in 1979, 1982, and 1986.12, 17, 26 Seasonal patterns of Kawasaki disease have also been observed in many other countries, including in Europe and North America. This finding has led to the speculation that viral infections might underline Kawasaki disease pathogenesis. However, large outbreaks of Kawasaki disease seem to be a rare event. The surges in the incidence of Kawasaki disease following two different major epidemic outbreaks observed in our study provide a unique, quasi-experimental condition, further implicating the crucial role of viral infections in the trigger of Kawasaki disease in susceptible patients.
In both observed epidemic peaks the increase in the incidence of Kawasaki disease was sudden. Further studies will be necessary to understand the immunological reasons leading to this severe and sudden increase in the context of SARS-CoV-2 and H1N1 epidemics when compared with seasonal viral infections. The sudden exposure to new antigens might trigger a particular uncontrolled immune response in susceptible children. By contrast, frequent seasonal viruses are encountered by children with trained immune systems, after exposures to similar antigens during precedent seasons, and therefore only a minority of children develop Kawasaki disease. Although our study has shown an emergence of Kawasaki disease associated with COVID-19, even in the context of the pandemic, this strong inflammatory disease is very rare.
The strength of our study was that it was done over a long time period (15 years) in a stable multi-ethnic population, living in a defined area, with stable medical orientation to our tertiary centre. Doing so allowed us to observe the rate of Kawasaki disease over time, enabling the detection of peaks in cases. Our study also had some limitations. Although the sample size was sufficient to reach statistical significance, our study is limited by its single-centre nature and a small number of patients. However, we did our analysis in a tertiary centre that was located in an epicentre of the COVID-19 outbreak to increase the sensitivity of assessing the association between SARS-CoV-2 and Kawasaki disease. This approach might have led to an overestimation of the increased risk for Kawasaki disease. Our study design allowed us to provide a proof of concept of the link between the COVID-19 pandemic and Kawasaki disease. Larger population-based studies are required to investigate the exact additional risk of Kawasaki disease associated with SARS-CoV-2 infections.
The clinical spectrum of multisystem inflammatory diseases in children observed during the COVID-19 pandemic is believed to include features of Kawasaki disease (complete or incomplete), toxic shock syndrome, and myocarditis.4, 11, 27 Paediatric and scientific societies in several countries are gathering information to provide a detailed clinical description30 and suggest new classifications—eg, paediatric multisystem inflammatory syndrome temporally associated with COVID-19 in Europe and multisystem inflammatory syndrome in children in the USA.29, 30 To reduce bias due to heterogeneous clinical presentations, we decided to focus only on patients with clinical presentation of Kawasaki disease according to established criteria.27 Further studies will be necessary to understand whether these patients should be classified as having SARS-CoV-2 infection with a Kawasaki disease phenotype or SARS-CoV-2-triggered Kawasaki disease.
Our observations could have practical implications for clinicians and health-care providers. Indeed, the clinical characteristics of Kawasaki disease patients hospitalised during the COVID-19 epidemic in our centre in France resembled those of patients hospitalised in an epicentre of COVID-19 in Italy,10 with a high proportion of patients with severe Kawasaki disease with cardiac involvement (abnormal echocardiography, coronary aneurysm, or myocarditis), intensive care unit admissions, inotrope requirement, and the need for additional treatments after first intravenous immunoglobulin treatment. By contrast, the clinical characteristics of Kawasaki disease occurring during the only other observed Kawasaki disease peak during an H1N1 epidemic resembled those of typical Kawasaki disease cohorts.15, 31, 32 These observations suggest that in the context of SARS-CoV-2 circulation a higher proportion of patients with Kawasaki disease might present a severe phenotype. Furthermore, these observations indicate that in such a pandemic, health-care systems should be prepared to manage an increased influx of patients with Kawasaki disease and potential severe cardiac involvement, particularly in countries where the peak of COVID-19 has just been reached. Our study provides evidence of a rapid emergence of Kawasaki disease in children related to SARS-CoV-2, starting approximately 2 weeks after the peak of the COVID-19 epidemic. Health-care providers and health authorities need to be prepared to manage a potential increased influx of children with severe Kawasaki disease.
Acknowledgments
Acknowledgments
This study was funded by the French National Research Agency (ANR-16-CE17-0009).
Contributors
UM and NO designed the study. NO, MP, PM, CB, AB, SB, KD, MCh, LM, FLB, MCa, JG, JP, RC, LT, AF, IM, and UM analysed and interpreted the data and drafted the article. NO and MP performed the statistical analysis. SB and PM did the microbiological analysis. All authors revised and approved the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Parri N, Lenge M, Buonsenso D. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020 doi: 10.1056/NEJMc2007617. published online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tagarro A, Epalza C, Santos M. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1346. published online April 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loomba RS, Raskin A, Gudausky TM, Kirkpatrick E. Role of the Egami Score in Predicting Intravenous Immunoglobulin Resistance in Kawasaki Disease Among Different Ethnicities. Am J Ther. 2016;23:e1293–e1299. doi: 10.1097/MJT.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 7.Jones VG, Mills M, Suarez D. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 8.Rivera-Figueroa EI, Santos R, Simpson S, Garg P. Incomplete Kawasaki disease in a child with Covid-19. Indian Pediatr. 2020 doi: 10.1007/s13312-020-1900-0. S097475591600179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder AR, Wilson KM, Ralston SL. COVID-19 and Kawasaki disease: finding the signal in the noise. Hosp Pediatr. 2020 doi: 10.1542/hpeds.2020-000356. published online May 13. [DOI] [PubMed] [Google Scholar]
- 10.Verdoni L, Mazza A, Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belhadjer Z, Méot M, Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. published online May 17. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Yashiro M, Uehara R, Oki I, Watanabe M, Yanagawa H. Epidemiologic features of Kawasaki disease in Japan: results from the nationwide survey in 2005–2006. J Epidemiol. 2008;18:167–172. doi: 10.2188/jea.JE2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003;112:495–501. doi: 10.1542/peds.112.3.495. [DOI] [PubMed] [Google Scholar]
- 14.Ozen S, Ruperto N, Dillon MJ. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936–941. doi: 10.1136/ard.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCrindle BW, Rowley AH, Newburger JW. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 16.Newburger JW, Takahashi M, Burns JC. Kawasaki Disease. J Am Coll Cardiol. 2016;67:1738–1749. doi: 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 17.Burns JC, Herzog L, Fabri O. Seasonality of Kawasaki disease: a global perspective. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns JC, Cayan DR, Tong G. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–225. doi: 10.1097/01.ede.0000152901.06689.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GB, Park S, Kwon BS, Han JW, Park YW, Hong YM. Evaluation of the temporal association between Kawasaki disease and viral infections in South Korea. Korean Circ J. 2014;44:250–254. doi: 10.4070/kcj.2014.44.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan-Villegas A, Chang ML, Ramilo O, Mejías A. Concomitant respiratory viral infections in children with Kawasaki disease. Pediatr Infect Dis J. 2010;29:770–772. doi: 10.1097/INF.0b013e3181dba70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnier JL, Anderson MS, Heizer HR, Jone PN, Glodé MP, Dominguez SR. Concurrent respiratory viruses and Kawasaki disease. Pediatrics. 2015;136:e609–e614. doi: 10.1542/peds.2015-0950. [DOI] [PubMed] [Google Scholar]
- 22.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350 doi: 10.1136/bmj.h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 25.Dawood FS, Iuliano AD, Reed C. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12:687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Yashiro M, Uehara R. Epidemiologic features of Kawasaki disease in Japan: results of the 2009–2010 nationwide survey. J Epidemiol. 2012;22:216–221. doi: 10.2188/jea.JE20110126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanegaye JT, Wilder MS, Molkara D. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittaker E, Bamford A, Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndome temporally associated with SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.10369. published online June 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaushik S, Aydin SI, Derespina KR. Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York City. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.045. published online June 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chbeir D, Gaschignard J, Bonnefoy R. Kawasaki disease: abnormal initial echocardiogram is associated with resistance to IV Ig and development of coronary artery lesions. Pediatr Rheumatol Online J. 2018;16:48. doi: 10.1186/s12969-018-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piram M, Darce Bello M, Tellier S. Defining the risk of first intravenous immunoglobulin unresponsiveness in non-Asian patients with Kawasaki disease. Sci Rep. 2020;10 doi: 10.1038/s41598-020-59972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited References
- 28.Belot A, Antona D, Renolleau S. SARS-Cov-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.