CONTRIBUTED BY, J. van Duin, S. van den Wor
FAMILY LEVIVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Leviviridae |
| Genus | Levivirus |
| Genus | Allolevivirus |
VIRION PROPERTIES
MORPHOLOGY
Virions are spherical and exhibit icosahedral symmetry (T=3) with a diameter of about 26 nm. There is no envelope (Fig. 1 ).
Figure 1.

(Left) Schematic representation of a levivirus: the RNA inside the virion is highly structured. (Upper right) Escherichia coli bacterium with Enterobacteria phage MS2 (MS2) particles attached its F-pili (Courtesy A.B. Jacobson). The inset is a -pilus with phage-enlargement. (Courtesy R.I. Koning and H.K. Koerten). (Lower right) Reconstruction of images obtained from cryo-electron microscopy of infectious MS2. View from outside (left) and inside (right). (Courtesy R.I. Koning and H.K. Koerten).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr varies from 3.6-4.2 × 106 depending on the genus. The S20w value is 80-84S and buoyant density in CsCl is 1.46 g/cm3. Infectivity is ether-, chloroform-, and low-pH-resistant, but is sensitive to RNase and detergents. Inactivation by UV light and chemicals is comparable to that of other icosahedral viruses containing ssRNA.
NUCLEIC ACID
Virions contain one molecule of positive sense ssRNA of 3466-4276 nt: size and gene arrangement vary with genus. RNA makes up 39% of the virion weight. The 5′ nucleotide carries a triphosphate, while at the 3′ terminus a non-templated A residue is added by the replicase (Figs. 2 , 3 ).
Figure 2.

General genetic map of a representative levivirus; Enterobacteria phage MS2 (MS2) and an allolevivirus; Enterobacteria phage Q (Q). The maturation protein is also called A-protein. The lysis gene overlaps the replicase gene in a +1 frameshift. Arrows indicate repression of replicase translation by capsid protein binding to an RNA hairpin structure (the operator) present at the start of the gene. The UGA nonsense codon (nt 1742) is occasionally (∼6%) misread as tryptophan to produce the read-through protein.
Figure 3.

Genetic map of Acinetobacter phage AP205 (AP205). Note the location of the tentative lysis gene at the 5′-terminus. AP205 is unusually long for a levivirus. This map corrects the one previously published (Klovins et al, 2002). A: A-protein CP: capsid protein R: replicase L: lysis.
PROTEINS
The capsid contains 180 copies of the CP (14 kDa) arranged in an icosahedron. The structure of the protein shell of several ssRNA phages has been solved by X-ray crystallography, and shows 60 quasi-symmetric AB- and 30 symmetric CC′-dimers. The A and C subunits are situated around the 3-fold axes, and the B subunits around the 5-fold axes of the icosahedron. The CP has no structural similarity to those of eukaryote icosahedral RNA viruses. The X-ray structure of the capsid in a complex with the 19 nt operator shows interaction of the dimers with this hairpin. Each virion contains one copy of the A-protein (35-61 kDa), which is required for maturation of the virion and for pilus attachment (Fig. 1). Alloleviviruses also contain several copies of the read-through protein in their capsid. Virions lacking the A-protein are RNase-sensitive.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Members of the family Leviviridae that propagate in E. coli infect by adsorption to the sides of F(ertility) pili (Non-coliphages such as Pseudomonas phage PP7 (PP7) and Acinetobacter phage AP205 (AP205) bind to other pili). This event leads to cleavage of the A-protein and release of the RNA from the virion into the bacterium. The infecting RNA encodes a replicase, which assembles with three host proteins (ribosomal protein S1 and translation elongation factors EF-Tu and EF-Ts) to form the active RNA polymerase. A fourth protein, called Host Factor, not associated with the polymerase complex but acting directly on the RNA, is needed for synthesis of the minus strand. Members of the two genera use different Host Factors. Plus-strand synthesis requires, besides the virus-coded replicase, only EF-Tu and EF-Ts as cofactors. Late in infection coat-protein dimers act as translational repressors of the replicase gene by binding to an RNA hairpin, the operator, that contains the start site of this gene. This protein-RNA complex is considered to also be the nucleation site for encapsidation. Virions assemble in the cytoplasm around phage RNA. It is unknown at which point the A-protein (and read-through protein) is assembled in the virion but it is assumed to be an early step since the A-protein can not be incorporated into preformed virions lacking the protein. Infection usually results in cell lysis releasing thousands of phages per cell. The lysis protein short-circuits the membrane potential and somehow activates the bacterial autolysins leading to degradation of the peptidoglycan network.
ANTIGENIC PROPERTIES
Members of the family Leviviridae are highly antigenic.
BIOLOGICAL PROPERTIES
Members of the Leviviridae family occur worldwide and are abundantly present in sewage, waste water, animal and human faeces. In Asia a particular geographic distribution has been noticed with respect to the four levivirus species. It has also been proposed that the various species have a preference for particular hosts e.g. Members of Enterobacteria phage Qβ are found predominantly in human waste. The evidence is not conclusive. RNA bacteriophages are harmless for humans. Members of the family Leviviridae not only infect enterobacteria but also species of the genera Caulobacter, Pseudomonas, and Acinetobacter and probably many other Gram-negative bacteria, provided they express the appropriate pili on their surface. RNA coliphages are often used as indicators for the presence of enteroviruses in waste and surface water. There is renewed interest in phage therapy to combat bacterial infections.
GENUS LEVIVIRUS
Type Species Enterobacteria phage MS2
DISTINGUISHING FEATURES
Leviviruses contain the short version of the genome and have a separate gene for cell lysis, which partly overlaps the replicase coding region in the +1 reading frame (Fig. 2). Overlap with the CP gene is variable. Genome size ranges from 3466 for GA (Enterobacteria phage BZ13) to 3577 for fr (Enterobacteria phage MS2) (Fig. 2). Leviviruses and alloleviviruses use different Host Factors for their polymerase holoenzyme. The levivirus Host Factor has been isolated but has not been genetically identified. Generally, the replicases from leviviruses poorly replicate allolevivirus RNA and vice versa.
Recently, the sequence of RNA of AP205, an RNA phage growing on Acinetobacter tentatively identified its lysis gene in the unusual location of the 5′-end. The absence of a read-through protein was taken as criterion to classify AP205 as a levivirus (Fig. 3).
GENOME ORGANIZATION AND REPLICATION
Figure 2 shows the map of the levivirus genome. Lysis and replicase synthesis are dependent on translation of the CP gene: early CP nonsense mutants are deficient in replicase and lysis protein synthesis. Translational starts at the lysis gene were shown to be reinitiations by ribosomes that had completed CP-gene reading but had not yet detached themselves from the message. A small fraction of these ribosomes manages to back up to the lysis start. Part of the replicase ribosome binding site is base-paired to an upstream sequence located in the coat coding region. A ribosome translating the CP cistron disrupts this interaction, thereby exposing the replicase start site (when not blocked by a CP dimer, which is the case late in infection). The CP gene is freely accessible to ribosomes.
Maturation or A-protein is translated from an RNA folding intermediate which has an accessible ribosome-binding site. This intermediate exists for a short time on nascent strands. Full-length RNA reaches an equilibrium folding in which the start site of the A-protein gene is inaccessible. It is believed that the purpose of these control mechanisms is to facilitate the switch from translation of the viral RNA to its replication. One of the binding sites of the replicase holoenzyme is the start of the CP gene. Binding of the enzyme to this site squeezes out ribosomes from CP, lysis and replicase genes. At this stage the A-protein gene is folded in its ribosome-inaccessible state and replication can proceed without interference from translation.
The polymerase of GA has been purified, that of MS2 may be unstable. Except for the Host Factor the polymerases of leviviruses and alloleviviruses contain the same subunits.
ANTIGENIC PROPERTIES
Antigenic specificity is distinct from that of members of the genus Allolevivirus.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
A major difference between members of the species Enterobacteria phage MS2 and Enterobacteria phage BZ13 (formerly called subgroups I and II) is a ∼60 nt deletion in the 3′-UTR of members of Enterobacteria phage BZ13, comprising three small RNA hairpins (Fig. 4 ). There is also a 35 nt deletion in the replicase gene of members of Enterobacteria phage BZ13 producing a shorter hairpin stem. Furthermore, the percentage of aa or nt sequence identity is dramatically lower between the two species than between strains within a species. Species can also be distinguished by serological means and by species-specific antisense DNA probes.
Figure 4.

Comparison of the RNA folding in the 3′UTR of Enterobacteria phage MS2 (MS2) and Enterobacteria phage GA (GA). GA lacks the three stem-loops U4, U5 and U6. In MS2 stem-loops V1 and V2 are part of the A-protein binding site. The other part of the protein's binding site is located around nt 400.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Enterobacteria phage MS2 | ||
| Enterobacteria phage f2 | (f2) | |
| Enterobacteria phage fr | [X15031] | (fr) |
| Enterobacteria phage JP501 | [AF227251] | (JP501) |
| Enterobacteria phage M12 | [AF195778] | (M12) |
| Enterobacteria phage MS2 | [GB-PH:MS2CG] | (MS2) |
| Enterobacteria phage R17 | (R17) | |
| Enterobacteria phage BZ13 | ||
| Enterobacteria phage BZ13 | (BZ13) | |
| Enterobacteria phage GA | [NC_001426] | (GA) |
| Enterobacteria phage JP34 | [J04343] | (JP34) |
| Enterobacteria phage KU1 | [AF227250] | (KU1) |
| Enterobacteria phage TH1 | (TH1) | |
TENTATIVE SPECIES IN THE GENUS
GENUS ALLOLEVIVIRUS
Type Species Enterobacteria phage Q
DISTINGUISHING FEATURES
Alloleviviruses contain the longer version of the genome (Fig. 2). The extra RNA encodes a C-terminal extension of the CP arising by occasional suppression of the CP gene termination codon. The read-through protein, is present at ∼12 copies per virion, together with the A-protein, is necessary for infection. Its precise role is not known. There is no separate lysis gene. Cell lysis is a secondary function of the A-protein. Genome length varies between 4217 nt for Q (Enterobacteria phage Q) and 4276 nt for SP (Enterobacteria phage F1) (Fig. 2).
GENOME ORGANIZATION AND REPLICATION
Genome organization is shown Figure 2. The RNA polymerase of Q has been purified and the enzyme can amplify Q RNA in vitro. The Host Factor has been purified and genetically characterized. It is the product of the hfq gene. In the uninfected cell the protein functions in the transition to stationary phase. In particular, it stimulates translation of the mRNA encoding the a38 protein involved in transcription of stationary phase genes. Hfq is a sequence non-specific ssRNA binding protein with some preference for A-residues. It is heat resistant and acts as a pentamer. The protein helps the polymerase to get access to the 3′-end of the plus strand, which exists in a base-paired and therefore inactive state. In Fig. 5 the secondary structure of the 3′UTR of Q RNA is shown; the 3′-terminal 6 nt are taken up in long-distance interaction with ld IX.
Figure 5.

RNA secondary structure for Enterobacteria phage Q (Qp) RNA from nt 2966 to the 3′-end (nt 4217) marked as AOH. The UAA stop codon (nt 4119) of the replicase gene is boxed. Replicase Domain 2 (RD2) containing 1062 nt has been replaced by a dotted circle. Breaking two or three basepairs in the central pseudoknot (ldX) or ldVIII abolishes replication. However, breaking the pairs in ld IX, which buries the 3′ terminal nucleotides, stimulates replication. Production of minus strand is also inhibited by deletion of stem-loops U1, V1, V2 or U2. (R1 and R2 were not tested).
Although the polymerases are specific for their own RNA, the interaction with RNA involves host-encoded subunits (EF-Tu, S1 and Hfq) that have no sequence specificity. An important contribution to template activity is provided by the higher order structure of Q RNA (Fig. 5). For instance, destroying 2 out of the 8 base pairs that make up the central pseudoknot in Q RNA, here indicated as ld X, lowers replication 100-fold. The higher order structures of the RNAs of phages PP7 (tentative) and SP (Enterobacteria phage F1) are shown in Fig. 6 .
Figure 6.

RNA secondary structure in the 3′UTR of Pseudomonas phage PP7 (PP7) and Enterobacteria phage SP (SP). The folding of PP7 RNA is much more like that of SP RNA than that of either MS2 or GA (Fig. 4). Compared to MS2 the stem-loops U3, U4, U5, U6 and one of the two V-loops are missing. The boxed sequence in the loop of hairpin U1 is conserved in all viruses of the family Leviviridae. The sequence is part of the central pseudoknot in Q. The pseudoknot is believed to exist also in the other phages.
The switch from translation to replication is as in leviviruses and was first formulated for Q. Control of the maturation protein is slightly different. The time window for producing the A-protein is not set by the lifetime of a folding intermediate, as for MS2, but by the time it takes the polymerase to move from position ∼60, the start of the A-protein gene, to position ∼470 where the complement to the Shine-Dalgarno sequence of the A-protein gene is located. Once this complement is synthesized pairing between the two regions blocks further translation.
ANTIGENIC PROPERTIES
Antigenic specificity is distinct from that of members of the genus Levivirus.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The major difference between Enterobacteria phage Q and Enterobacteria phage F1 (formerly called subgroups III and IV respectively) is a ∼90-nt deletion in the maturation-protein gene of Q, corresponding to a bifurcated hairpin. There is also the extra stem-loop (V1) in the 3′UTR of members of Enterobacteria phage Q that is lacking in members of Enterobacteria phage F1. Species can also be differentiated by serological criteria and by species-specific antisense DNA probes. Finally, the percentage of aa or nt sequence identity is dramatically lower between the two species than between strains within a species.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Enterobacteria phage F1 | ||
| Enterobacteria phage F1 | (F1) | |
| Enterobacteria phage ID2 | (ID2) | |
| Enterobacteria phage NL95 | [AF059243] | (NL95) |
| Enterobacteria phage SP | [NC_004301] | (SP) |
| Enterobacteria phage TW28 | (TW28) | |
| Enterobacteria phage Qβ | ||
| Enterobacteria phage Qβ | [AY099114] | (Qβ) |
| Enterobacteria phage M11 | [NC_004304] | (M11) |
| Enterobacteria phage MX1 | [NC_001890] | (MX1) |
| Enterobacteria phage ST | (ST) | |
| Enterobacteria phage TW18 | (TW18) | |
| Enterobacteria phage VK | (VK) | |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
| Caulobacter phage ϕCb12r | (ϕCb12r) |
| Caulobacter phage ϕCb2 | (ϕCb2) |
| Caulobacter phage ϕCb23r | (ϕCb23r) |
| Caulobacter phage ϕCb4 | (ϕCb4) |
| Caulobacter phage ϕCb5 | (ϕCb5) |
| Caulobacter phage ϕCb8r | (ϕCb8r) |
| Caulobacter phage ϕCb9 | (ϕCb9) |
| Caulobacter phage ϕCP18 | (ϕCP18) |
| Caulobacter phage ϕCP2 | (ϕCP2) |
| Caulobacter phage ϕCr14 | (ϕCr14) |
| Caulobacter phage ϕCr28 | (ϕCr28) |
| Enterobacteria phage β | β |
| Enterobacteria phage τ | (τ) |
| Enterobacteria phage α15 | (α 15) |
| Enterobacteria phage μ2 | (μ 2) |
| Enterobacteria phage B6 | (B6) |
| Enterobacteria phage B7 | (B7) |
| Enterobacteria phage C-1 | (C-1) |
| Enterobacteria phage C2 | (C2) |
| Enterobacteria phage fcan | (fcan) |
| Enterobacteria phage Folac | (Folac) |
| Enterobacteria phage Iα | (Ia) |
| Enterobacteria phage M | (M) |
| Enterobacteria phage pilHα | (pilHa) |
| Enterobacteria phage R23 | (R23) |
| Enterobacteria phage R34 | (R34) |
| Enterobacteria phage ZG/1 | (ZG/1) |
| Enterobacteria phage ZIK/1 | (ZIK/1) |
| Enterobacteria phage ZJ/1 | (ZJ/1) |
| Enterobacteria phage ZL/3 | (ZL/3) |
| Enterobacteria phage ZS/3 | (ZS/3) |
| (other enterobacteriophages, with many plasmid specificities, have been reported). | |
| Pseudomonas phage 7s | (7s) |
| Pseudomonas phage PRR1 | (PRR1) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
A tentative phylogenetic tree of the family Leviviridae is given in Figure 7 . Relationships have been based first on deeply rooted features such as the genetic map and second on similarity in RNA folding, in particular the one present at the 3′UTR which is conserved in its outline. As a result there is a fundamental split between leviviruses and alloleviviruses because they have different maps. The two non-coli leviviruses AP205 and PP7 have been placed closer to the ancestor than the coli leviviruses because they have the same folding of their 3′UTR as the alloleviviruses (Fig. 6). As a result MS2 and GA are closer to the non-coliphages than to coliphage Q. PP7 is placed closer to MS2 than AP205 because AP205 has its lysis gene in a different position. PP7 has it in the same position as MS2, fr and GA.
Figure 7.

Proposed phylogenetic tree for the family Leviviridae. Distances are arbitrary. The ancestor only has the three basic genes. Lysis is effected by the A-protein as it still is today in Q. Presumably, fitness of the ancestor was restricted by the double function of the A-protein (Bollback and Huelsenbeck, 2001). The leviviruses solved the problem by evolving a separate lysis protein either encoded on a vacant region of the genome (AP205) or resulting from a ribosomal restart following translation termination at the end of the capsid gene (other leviviruses). Once restrictions on the A-protein were relaxed the gene could evolve in various directions to better fulfill its remaining function: virion maturation and infection. Two features of leviviruses can be explained by this scenario: first, lysis genes have variable startpoints (even between MS2 and fr or between GA and KU1) and secondly, of the three “old” genes, the A-protein gene shows the lowest sequence conservation. The alloleviviruses solved the dual-function problem by transfering part of the maturation and infection function to a new protein, read-through, which arose by an insertion between coat and replicase genes. Presumably, this allowed the A-protein to improve its lysis function. Such a scenario would provide a different reason why also in the alloleviviruses the A-protein is least conserved of the “old” genes. Signification of the abbreviations of virus names are to be found in “List of Species in the genus”.
Figure 6.

Virions of an isolate of Barley yellow mosaic virus, stained with 1% PTA, pH 7.0. The bar represents 200 nm (from D. Lesemann).
In this scheme, the ancestor contains only the three basic genes and the A-protein has the double function of lysis and maturation (infection). We assume that its 3′-UTR is folded in the simple way of PP7 (AP205) and Q (SP) (Fig. 6).
The subdivision of each genus in two species is based on criteria explained above. Based on the sequence it is possible to make subtle distinctions between strains within a species. For example, MS2, R17, f2, M12 and JP501 are extremely close (∼95% identity) whereas fr is much further away (∼80% identity), has some features of members of Enterobacteria phage BZ13, but is still clearly a member of Enterobacteria phage MS2.
SIMILARITY WITH OTHER TAXA
Not reported.
DERIVATION OF NAMES
Levi: from Latin levis, “light”.
Allo: from Greek allov, “other”.
REFERENCES
- Ackermann H.W., Dubow M.S., editors. Vol. 2. CRC Press; Boca Raton, Florida: 1987. (Viruses of Prokaryotes). [Google Scholar]
- Bollback J.P., Huelsenbeck J.P. Phylogeny, genome evolution and host specificity of single-stranded RNA bacteriophage (Family Leviviridae) J. Mol. Evol. 2001;52:117–128. doi: 10.1007/s002390010140. [DOI] [PubMed] [Google Scholar]
- Brown D., Gold L. RNA replication by Qß replicase: A working model. Proc. Natl. Acad. Sci. USA. 1996;93:11558–11562. doi: 10.1073/pnas.93.21.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convery M.A., Rowsell S., Stonehouse N.J., Ellington A.D., Hirao I., Murray J.B., Peabody D.S., Phillips S.E.V., Stockley P.G. Crystal structure of an RNA aptamer-protein complex at 2.8 Å resolution. Nature Struct. Biol. 1998;5:133–139. doi: 10.1038/nsb0298-133. [DOI] [PubMed] [Google Scholar]
- Fiers W. Structure and function of RNA bacteriophages. In: Fraenkel-Conrat H., Wagner R.R., editors. Vol. 13. Plenum Press; New York: 1979. pp. 69–204. (Comprehensive Virology). [Google Scholar]
- Furuse K. Distribution of the coliphages in the environment. In: Goyal S.M., Gerber C.P., Bitton G., editors. Phage Ecology. John Wiley and Sons; New York: 1987. pp. 87–124. [Google Scholar]
- Havelaar A.H., IAWPRC Study group on health related water microbiology Bacteriophages as model viruses in water quality control. Wat. Res. 1991;25:529–545. [Google Scholar]
- Jacobson A.B., Arora R., Zuker M., Priano C., Liu C.H., Mills D.R. Structural plasticity in RNA and its role in the regulation of translation in Qß. J. Mol. Biol. 1998;275:589–600. doi: 10.1006/jmbi.1997.1472. [DOI] [PubMed] [Google Scholar]
- Klovins J., Overbeek G.P., van den Worm S.H.E., Ackermann H.-W., van Duin J. Nucleotide sequence of a ssRNA phage from Acinetobacter: kinship to coliphages. J. Gen. Virol. 2002;83:1523–1533. doi: 10.1099/0022-1317-83-6-1523. [DOI] [PubMed] [Google Scholar]
- Miranda G., Schuppli D., Barrera I., Hausherr C., Sogo J.M., Weber H. Recognition of Qß plusstrand RNA as a template by Qß replicase: role of RNA interactions mediated by ribosomal protein S1 and Host Factor. J. Mol. Biol. 1997;267:1089–1103. doi: 10.1006/jmbi.1997.0939. [DOI] [PubMed] [Google Scholar]
- Rohde N., Daum H., Biebricher K. The mutant distribution of an RNA species replicated by Qß replicase. J. Mol. Biol. 1995;294:754–762. doi: 10.1006/jmbi.1995.0334. [DOI] [PubMed] [Google Scholar]
- Schuppli D., Georgijevic J., Weber H. Synergism of mutations in Qß RNA affecting host factor dependence of Qß replicase. J. Mol. Biol. 2000;295:149–154. doi: 10.1006/jmbi.1999.3373. [DOI] [PubMed] [Google Scholar]
- Sledjeski D., Whitman C., Zhang A. Hfq is necessary for regulation by the untranslated RNA DsrA. J. Bacteriol. 2001;183:1997–2005. doi: 10.1128/JB.183.6.1997-2005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin J. Single stranded RNA bacteriophages. In: Calendar R., editor. The Bacteriophages. Plenum Press; New York: 1988. pp. 117–167. [Google Scholar]
- Valegård K., Liljas L., Fridborg K., Unge T. The three-dimensional structure of the bacterial virus MS2. Nature. 1990;345:36–41. doi: 10.1038/345036a0. [DOI] [PubMed] [Google Scholar]
- Valegård K., Murray J.B., Stockley P.G., Stonehouse N.J., Liljas L. Crystal structure of an RNA bacteriophage coat protein-operator complex. Nature. 1994;371:623–626. doi: 10.1038/371623a0. [DOI] [PubMed] [Google Scholar]
- Zinder N.D., editor. RNA phages. Cold Spring Harbor Laboratory Press. Monograph Series; Cold Spring Harbor, New York: 1975. [Google Scholar]
CONTRIBUTED BY, K.W. Buck, R. Esteban, B.I. Hillman
FAMILY NARNAVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Narnaviridae |
| Genus | Narnavirus |
| Genus | Mitovirus |
Viruses in the family Narnaviridae consist of a single molecule of non-encapsidated positive-strand RNA of 2.3-2.9 kb, which encodes a single protein of 80-104 kDa with amino acid sequence motifs characteristic of RdRps.
GENUS NARNAVIRUS
Type Species Saccharomyces 20S RNA narnavirus
VIRION PROPERTIES
MORPHOLOGY
No true virions are found associated with members of this genus. The genomes, however, are associated with their RdRps forming ribonucleoprotein complexes in 1:1 stoichiometry. Genetic and biochemical evidence show that they are cytoplasmically-located.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The ribonucleoprotein complex sediments through a sucrose gradient with a sedimentation coefficient ∼20S. These complexes are quite stable at pH 9.0 and have in vitro RNA polymerase activity that synthesizes mainly 20S RNA, and a minor amount of complementary strands.
NUCLEIC ACID
The Saccharomyces 20S RNA narnavirus (ScNV-20S) genome is a linear ssRNA of 2.5 kb in size with a high G+C content (∼60%). There is no poly(A) tail at the 3′-end and it is not known whether the 5′-end is capped. It is present in a high copy number under stress conditions, such as growth under nitrogen starvation, reaching up to 100,000 copies/cell.
PROTEINS
No structural proteins have been described for members of this family. ScNV-20S has coding capacity for a protein of 91 kDa (p91), with sequences conserved among RdRps. The conserved sequences are more similar to those of replicases of ssRNA enterobacteria phages than polymerases of members of the family Totiviridae in the same host. This protein is quite basic (estimated pI of 11) and has ssRNA binding activity. Protein p91 is essential for replication and responsible for the in vitro RdRp activity that synthesizes 20S RNA. P91 does not undergo proteolytic processing after translation. Studies using antibodies against this protein show that it is expressed in yeast cells grown exponentially or under induction conditions.
LIPIDS
No lipids have been described associated to ScNV-20S.
CARBOHYDRATES
None reported.
GENOMIC ORGANIZATION AND REPLICATION
ScNV-20S has only one ORF that encodes p91, and there are no ORFs with coding capacity larger than 100 aa in the complementary strand. The ORF for p91 spans almost the entire sequence of 20S RNA, with a short untranslated leader sequence at the 5′-end (12 nt) and an UTR at the 3′-end of 12 nt. Two replication models for 20S RNA have been proposed based on the similarity of p91 to the replicases of RNA enterobacteria phages and the replication intermediates obtained in the in vitro RNA polymerase reaction. One model is similar to the replication cycle of ssRNA enterobacteria phages such as Qβ; that is, ScNV-20S is copied into the complementary strands and these copies serve as templates for 20S RNA synthesis. Annealing of 20S RNA and its complementary strand gives a double-stranded form of ScNV-20S. This dsRNA called W can be easily isolated from all ScNV-20S-containing yeast strains. The other model hypothesizes that W dsRNA is the replicative form of ScNV-20S. At present, available data support the first model. Recently, a reverse genetics system for ScNV-20S has been established. Like native viruses, viruses generated from cDNA vectors can be transmitted to daughter cells indefinitely without the vector or any selection.
Figure 1.

Genomic organization of Saccharomyces 20S RNA narnavirus (ScNV-20S) and Saccharomyces 23S RNA narnavirus (ScNV-23S) and the proteins encoded on them (p91 and p104, respectively). Sequence motifs (A to D) conserved in RdRp are boxed and shaded. Motifs 1, 2 and 3 are present only in p91 and p104.
BIOLOGICAL PROPERTIES
ScNV-20S infects more than 90% of laboratory strains of the baker's yeast Saccharomyces cerevisiae. Some strains isolated from the brewery industry also have been found to carry ScNV-20S. There is no phenotype associated with the presence of this RNA. Like other viruses of fungi, there is no extracellular stage in the ScNV-20S life cycle. Transmission takes place through mating or cytoplasmic mixing. These viruses are very stable. Known curing procedures that eliminate members of the family Totiviridae in the same host, such us growth at high temperature, or with cycloheximide, acridine orange, or guanidine HCl, do not eliminate ScNV-20S.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Narnaviruses generally replicate stably within the cell as the cells grow. Virus strains of the same species are expected to segregate relative to each other as the cells grow, whereas those of different species should be stably co-maintained. Viruses of the same species should be similarly affected by host chromosomal mutations. Viruses that can recombine or exchange segments with each other to give viable progeny should be considered the same species. Although these biological criteria are the prime determinants of species, sequence criteria also are used. Less than 50% sequence identity at the protein level generally reflects a species difference. None of the above criteria is absolute, but narnaviruses described so far leave little doubt about species demarcation. For example, ScNV-20S and ScNV-23S are only 30% identical in the 439 aa region of highest similarity. More important, they are stably compatible with each other in the same yeast strain.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS MITOVIRUS
Type Species Cryphonectria mitovirus 1
VIRION PROPERTIES
No virions have been reported for members of this genus.
NUCLEIC ACID
The virus genome consists of a single molecule of RNA of 2.3-2.7 kb. Double-stranded RNAs in this size range can be isolated from mitochondria of infected isolates. Single-stranded RNA of the same size, and corresponding to the coding strand of the dsRNA, is present in infected tissue in greater molar amount than the dsRNA. The 5′ and 3′ sequences can be folded into stable stem-loop structures. For some mitoviruses, the 5′ and 3′ sequences are complementary. The coding strand has 62-73% A+U residues, but no poly(A) tail is associated with the 3′-end.
PROTEINS
No structural proteins are known to be associated with the virus ssRNA or dsRNA.
ANTIGENIC PROPERTIES
None reported.
GENOME ORGANIZATION AND REPLICATION
The putative coding strand is predicted to be translatable only in mitochondria, not in cytoplasm. When mitochondrial codon usage is invoked (UGA coding for tryptophan), the deduced translation product is a protein of 80-97 kDa, containing RdRp motifs. RdRp activity and an 80 kDa RdRp protein have been detected in mitochondria from an infected Ophiostoma novo-ulmi isolate. No large polypeptide is predicted from the complementary strand of any mitovirus.
BIOLOGICAL PROPERTIES
Mitoviruses have been found in isolates of the chestnut blight fungus, Cryphonectria parasitica, Dutch elm disease fungi, Ophiostoma novo-ulmi and O. ulmi, and Sclerotinia homoeocarpa, the cause of dollar spot of turf grass. Fungal isolates may contain one or several mitoviruses. Some, but not all, member viruses reduce virulence of the fungus (i.e., cause “hypovirulence”). Mitoviruses are localized in mitochondria. They can be transmitted to uninfected strains by hyphal fusion (anastomosis). The transmission rate through asexual spores (conidia) is virus-specific and varies from 10-100%. In C. parasitica, transmission through sexual spores (ascospores) occurs at 20-50% when the infected parent is the female in matings, but does not occur when the infected parent is male in matings. In O. novo-ulmi, viruses are usually excluded from ascospores, even when both parents are infected. Identical mitoviruses have been found in O. novo-ulmi and O. ulmi, and a strain of Ophiostoma mitovirus 3a has been reported in Sclerotinia homoeocarpa, suggesting that both interspecies and intergenus virus transmission occurs in nature.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Species demarcation criteria have not been precisely defined. However, amino acid sequence identities of putative RdRp proteins between the different mitovirus species so far defined are less than 40%. Amino acid sequence identities of putative RdRp proteins between strains of the same mitovirus species are greater than 90%.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Cryphonectria mitovirus 1 | ||
| Cryphonectria mitovirus 1 | [L31849] | (CMV1) |
| Ophiostoma mitovirus 3a | ||
| Ophiostoma mitovirus 3a | [AJ004930; AY172454] | (OMV3a) |
| Ophiostoma mitovirus 4 | ||
| Ophiostoma mitovirus 4 | [AJ132754] | (OMV4) |
| Ophiostoma mitovirus 5 | ||
| Ophiostoma mitovirus 5 | [AJ132755] | (OMV5) |
| Ophiostoma mitovirus 6 | ||
| Ophiostoma mitovirus 6 | [AJ132756] | (OMV6) |
TENTATIVE SPECIES IN THE GENUS
| Gremmeniella mitovirus S1 | [AF534641] | (GMVS1) |
| Ophiostoma mitovirus 1a | (OMV1a) | |
| Ophiostoma mitovirus 1b | (OMV1b) | |
| Ophiostoma mitovirus 2 | (OMV2) | |
| Ophiostoma mitovirus 3b | (OMV3b) |
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
| Rhizoctonia virus M2 | [U51331] | (RVM2) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
In a neighbor-joining phylogenetic tree based on aa sequences of the putative RdRp proteins, the mitovirus and narnavirus genera are clearly distinguished, but nevertheless form a significant cluster (Fig. 2 ). The putative RdRp protein of the unassigned virus, Rhizoctonia virus M2 (RVM2), clusters with those of the mitoviruses (Fig. 2). However, since only a small proportion of RVM2 copurifies with mitochondria with most being found in the cytoplasm, RVM2 does not use the mitochondrial code, and there is evidence for a DNA copy in the host genome, this suggests significant differences from the mitoviruses.
Figure 2.

Phylogenetic tree based on aa sequences of motifs A to E (Hong et al. 1998) of the putative RdRp proteins of members of the family Narnaviridae, other families of RNA viruses of fungi and related viruses in other host taxa, and the family Leviviridae of RNA bacteriophages. Sequence alignments and the neighbor-joining tree were made using the Clustal X program. Bootstrap numbers (1000 replicates) are shown on the nodes. Abbreviations and sequence acquisition numbers [ ] are: AbVL1, Agaricus bisporus virus L1 [X94361]; AhV, Atkinsonella hypoxylon virus [L39126]; BaYMV, Barley yellow mosaic virus [D01091]; BcV3, Beet cryptic virus 3 [S63913]; CHV1, Cryphonectria hypovirus 1 [M57938]; CMV1, Cryphonectria mitovirus 1 [L31849]; FusoV1, Fusarium solani virus 1 [D55668]; GlV, Giardia lamblia virus [L13218]; GMVS1, Gremmeniella mitovirus S1 [AF534641]; Hv145SV, Helminthosporium victoriae 145S virus [AF297176]; Hv190SV, Helminthosporium victoriae 190S virus [U41345]; LRV1, Leishmania RNA virus 1-1 [M92355]; MBV, Mushroom bacilliform virus [U07551]; MS2, Enterobacteria phage MS2 [GB-PHMS2CG]; OMV3a, Ophiostoma mitovirus 3a [AJ004930]; OMV4, Ophiostoma mitovirus 4 [AJ132754]; OMV5, Ophiostoma mitovirus 5 [AJ132755]; OMV6, Ophiostoma mitovirus 6 [AJ132756]; PcV, Penicillium chrysogenum virus [AF296439]; PLRV, Potato leafroll virus [X14600]; Qbeta, Enterobacteria phage Qβ [AY099114]; RVM2, Rhizoctonia virus M2 [U51331]; ScV-L-A, Saccharomyces cerevisiae virus L-A [J04692]; ScV-L-BC, Saccharomyces cerevisiae virus L-BC [U01060]; ScNV-20S, Saccharomyces 20S RNA narnavirus [M63893]; ScNV-23S, Saccharomyces 23S RNA narnavirus [M86595]; TEV, Tobacco etch virus [M15239]; TvV, Trichomonas vaginalis virus [U08999]; UmVH1, Ustilago maydis virus H1 [NC_003823].
SIMILARITY WITH OTHER TAXA
The putative RdRp proteins of narnaviruses and mitoviruses are distantly related to those of bacteriophages in the family Leviviridae (Fig. 2). Furthermore, the 3′-end secondary structures of members of the genus Narnavirus resemble those of coliphages in the family Leviviridae. In a neighbor-joining phylogenetic tree of families of fungus viruses and related viruses in other taxa, based on aa sequences of the putative RdRp proteins, the families Narnaviridae and Leviviridae form a cluster with 69.2% bootstrap support (Fig. 2).
DERIVATION OF NAMES
Mito: sigla from mitochondrial.
Narna: sigla from naked RNA virus.
REFERENCES
- Buck K.W., Brasier C.M. Viruses of the Dutch elm disease fungi. In: Tavantzis S.M., editor. DsRNA genetic elements: concepts and applications in agriculture, forestry and medicine. CRC Press; Boca Raton, Florida: 2002. pp. 165–190. [Google Scholar]
- Cole T.E., Müller B., Hong Y., Brasier C.M., Buck K.W. Complexity of virus-like double-stranded RNA elements in a diseased isolate of the Dutch elm disease fungus, Ophiostoma novo-ulmi. J. Phytopathol. 1998;146:593–598. [Google Scholar]
- Cole T.E., Hong Y., Brasier C.M., Buck K.W. Detection of an RNA-dependent RNA polymerase in mitochondria from a mitovirtus-infected isolate of the Dutch elm disease fungus, Ophiostoma novo-ulmi. Virology. 2000;268:239–243. doi: 10.1006/viro.1999.0097. [DOI] [PubMed] [Google Scholar]
- Esteban R., Fujimura, T. Launching the yeast 23S RNA narnavirus shows 5’ and 3’ cis-acting signals for replication. Proc. Natl. Acad. Sci. USA. 2003;100:2568–2573. doi: 10.1073/pnas.0530167100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban L.M., Fujimura T., García-Cuéllar M.P., Esteban R. Association of yeast viral 23S RNA with its putative RNA-dependent RNA polymerase. J. Biol. Chem. 1994;269:29771–29777. [PubMed] [Google Scholar]
- García-Cuéllar M.P., Esteban R., Fujimura T. RNA-dependent RNA polymerase activity associated with the yeast viral p91/20S RNA ribonucleoprotein complex. RNA. 1997;3:27–36. [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Cole T.E., Brasier C.M., Buck K.W. Evolutionary relationships among putative RNA-dependent RNA polymerases encoded by a mitochondrial virus-like RNA in the Dutch elm disease fungus, Ophiostoma novo-ulmi, by other viruses and virus-like RNAs and by the Arabidopsis mitochondrial genome. Virology. 1998;246:158–169. doi: 10.1006/viro.1998.9178. [DOI] [PubMed] [Google Scholar]
- Hong Y., Dover S.L., Cole T.E., Brasier C.M., Buck K.W. Multiple mitochondrial viruses in an isolate of the Dutch elm disease fungus Ophiostoma novo-ulmi. Virology. 1999;258:118–127. doi: 10.1006/viro.1999.9691. [DOI] [PubMed] [Google Scholar]
- Lakshman D.K., Jian J., Tavantzis S.M. A double-stranded RNA element from a hypovirulent strain of Rhizoctonia solani occurs in DNA forms and is genetically related to the pentafunctional AROM protein of the shikimate pathway. Proc. Natl. Acad. Sci. USA. 1998;95:6425–6429. doi: 10.1073/pnas.95.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polashock J.J., Hillman B.I. A small mitochondrial double-stranded (ds) RNA element associated with a hypovirulent strain of the chestnut blight fungus and ancestrally related to yeast cytoplasmic T and W dsRNAs. Proc. Natl. Acad. Sci. USA. 1994;91:8680–8684. doi: 10.1073/pnas.91.18.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polashock J.J., Anagnostakis S.L., Milgroom M.G., Hillman B.I. Isolation and characterization of a virus-resistant mutant of Cryphonectria parasitica. Current Genetics. 1994;26:528–534. doi: 10.1007/BF00309945. [DOI] [PubMed] [Google Scholar]
- Polashock J.J., Bedker P.J., Hillman B.I. A mitochondrial dsRNA of Cryphonectria parasitica: Ascospore inheritance and mitochondrial recombination. Mol. Gen. Genet. 1997;256:566–571. doi: 10.1007/s004380050602. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cousiño N., Esteban L.M., Esteban R. Molecular cloning and characterization of W double-stranded RNA, a linear molecule present in Saccharomyces cerevisiae: identification of its single-stranded RNA form as 20S RNA. J. Biol. Chem. 1991;266:12772–12778. [PubMed] [Google Scholar]
- Rodriguez-Cousiño N., Solórzano A., Fujimura T., Esteban R. Yeast positive-strand virus-like RNA replicons: 20S and 23S RNA terminal nucleotide sequences and 3’-end secondary structures resemble those of RNA coliphages. J. Biol. Chem. 1998;273:20363–20371. doi: 10.1074/jbc.273.32.20363. [DOI] [PubMed] [Google Scholar]
- Solórzano A., Rodríguez-Cousiño N., Esteban R., Fujimura T. Persistent yeast single-stranded RNA viruses exist in vivo as genomic RNA:RNA polymerase complexes in 1:1 stoichiometry. J. Biol. Chem. 2000;275:26428–26435. doi: 10.1074/jbc.M002281200. [DOI] [PubMed] [Google Scholar]
- Tavantzis S.M. Lakshman, Liu C. Double-stranded RNA elements modulating virulence in Rhizoctonia solani. In: Tavantzis S.M., editor. DsRNA genetic elements: concepts and applications in agriculture, forestry and medicine. CRC Press; Boca Raton, Florida: 2002. pp. 191–211. [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nuc. Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner W.R., Matsumoto Y., Wickner R.B. Is 20S RNA naked? Mol. Cell. Biol. 1991;11:2905–2908. doi: 10.1128/mcb.11.5.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONTRIBUTED BY, G. Stanway, F. Brown, P. Christian, T. Hovi, T. Hyypiä, A.M.Q. King, N.J. Knowles, S.M. Lemon, P.D. Minor, M.A. Pallansch, A.C. Palmenberg, T. Skern
FAMILY PICORNAVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Picornaviridae |
| Genus | Enterovirus |
| Genus | Rhinovirus |
| Genus | Cardiovirus |
| Genus | Aphthovirus |
| Genus | Hepatovirus |
| Genus | Parechovirus |
| Genus | Erbovirus |
| Genus | Kobuvirus |
| Genus | Teschovirus |
VIRION PROPERTIES
MORPHOLOGY
Virions consist of a capsid, with no envelope, surrounding a core of ssRNA. Hydrated native particles are 30 nm in diameter, but vary from 22-30 nm in electron micrographs due to drying and flattening during preparation. Electron micrographs reveal no projections, the virion appearing as an almost featureless sphere (Fig. 1 ). The capsid is composed of 60 identical units (protomers), each consisting of three surface proteins, 1B, 1C and 1D, of 24-41 kDa, and, in most picornaviruses, an internal protein, 1A of 5.5-13.5 kDa. Total protomer is 80-97 kDa. Proteins 1A, 1B, 1C and 1D are also commonly named VP4, VP2, VP3, and VP1, respectively. Proteins 1B, 1C and 1D each possess a core structure comprising an eight-stranded -sandwich (“-barrel”). The -barrels pack together in the capsid with T=l, pseudo T=3, icosahedral symmetry. (These structural features are shared by certain plant viruses that exhibit T=3, or pseudo T=3, symmetry, e.g. Sobemovirus and Comoviridae, respectively). Genera differ in the external loops that interconnect the strands. These loops account for differences in surface relief of each genus (Fig. 1) and in thickness of the capsid wall. Assembly occurs via pentameric intermediates (pentamer=five protomers). Proteins within each pentamer are held together by an internal network formed from the N-termini of the three major CPs, the C-termini lying on the outer capsid surface. Empty capsids, which are produced by some picornaviruses, are very similar to virions, except that 1A and 1B are normally replaced by the uncleaved precursor, 1AB.
Figure 1.

(Top) Pictures of picornavirus structures; Poliovirus type 1 (PV-1) (Left), Mengo virus (Center) and Foot-and-mouth disease virus serotype O (FMDV-O) (Right), PDB entries: 2PLV, 2MEV and 1FOD respectively. The bar represents 10 nm. (Images courtesy of J.Y. Sgro, with permission). (Bottom left) Diagram of a picornavirus particle. The surface shows proteins VP1, VP2 and VP3. The fourth capsid protein, VP4, is located about the internal surface of the pentameric apex of the icosahedron. (Right) Negative contrast electron micrograph of Poliovirus (PV) particles. The bar represents 100 nm. (Courtesy of Ann C Palmenberg).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is 8 × 106 − 9 × 106, S20w is 140-165S (empty particle S20w is 70-80S). Buoyant density in CsCl is 1.33-1.45 g/cm3, depending on the genus. Some species are unstable below pH 7; many are less stable at low ionic strength than at high ionic strength. Virions are insensitive to ether, chloroform, or non-ionic detergents. Viruses are inactivated by light when grown with, or in the presence of photodynamic dyes such as neutral red or proflavin. Thermal stability varies with viruses as does stabilization by divalent cations.
NUCLEIC ACID
Virions contain one molecule of positive sense, ssRNA, 7-8.8 kb in size, and possessing a single long ORF. A poly(A) tail, heterogeneous in length, is located after the 3′-terminal heteropolymeric sequence. A small protein, VPg (∼2.4 kDa), is linked covalently to the 5′-terminus. The NTRs at both termini contain regions of secondary structure which are essential to genome function. The very long 5′-NTR (0.5-1.5 kb) includes a 5′-terminal domain involved in replication (e.g. the poliovirus “clover-leaf”) and an IRES of 400-450 nt upstream of the translational start site; most picornaviral IRES elements can be assigned to one of two types, according to their secondary structure. Between the 5′-terminal domain and the IRES there may be one, or more, pseudoknots and/or a poly(C) tract (Fig. 2). The 3′-NTR, which may also contain a pseudoknot, ranges from 40 to 165 nt in length. The overall sequence identity between the genomes of viruses of different genera is typically less than 40%.
Figure 2.

Genome structure and gene organization of members of the family Picornaviridae. Each of the 9 genera is represented, as are species where there is a significant difference within a genus. Circles within the 5′-NTR indicate poly(C) tracts that are present in some members. The 1A gene products of many members are myristylated at the amino terminal glycine. The 5-’NTR is followed by a long ORF encoding the polyprotein, that is in turn followed by the 3′-NTR and a poly(A) tail. The eventual cleavage products of the polyprotein are indicated by vertical lines and different shading. The nomenclature of the polypeptides follows an L:4:3:4 scheme corresponding to the genes (numbers) encoded by the L, P1, P2, P3 regions. The P1 region encodes the structural proteins 1A, 1B, 1C and 1D, also referred to as VP4, VP2, VP3 and VP1, respectively. VP0 (1AB) is the intermediate precursor for VP4 and VP2 and in parechoviruses and kobuviruses it remains uncleaved. In all viruses 3C is a protease, in enteroviruses and rhinoviruses 2A is a protease, while in all viruses 3D is considered to be a component of the RNA replicase. Only Foot-and-mouth disease virus encodes 3 VPg proteins that map in tandem.
PROTEINS
In addition to the major CPs, 1A, 1B, 1C and 1D, and 3B (Vpg), described above, small amounts of 1AB (VP0) are commonly seen in lieu of one or more copies of 1A and 1B. Protein 1A is small in hepatoviruses, and 1AB is uncleaved in parechoviruses and kobuviruses. Traces of other proteins, including the viral RdRp, 3Dpol, may also be present in purified virus preparations.
LIPIDS
Some picornaviruses carry a sphingosine-like molecule (“pocket factor”) in a cavity (“pocket”) located inside 1D. Protein 1A, where present, has a molecule of myristic acid covalently attached to the amino terminal glycine.
CARBOHYDRATES
None of the viral proteins are glycosylated.
GENOME ORGANIZATION AND REPLICATION
The virion RNA is infectious and serves as both the genome and the viral mRNA. Gene maps are shown in Figure 2 . Initiation of protein synthesis is stimulated by the IRES. Translation of the single ORF produces the polyprotein precursor 240-250 kDa) to the structural proteins (derived from the P1 region of the genome) and the nonstructural proteins (from the P2 and P3 regions). In some viruses P1 is preceded by a leader protein (L). The polyprotein is cleaved to functional proteins by specific proteases contained within it. Intermediates are denoted by letter combinations (e.g. 3CD, the uncleaved precursor of 3C and 3D). The viral proteases are as follows: Protease 3Cpro, a serine-like cysteine protease encoded by all picornaviruses, performs most of the cleavages. In most genera, 2A is also associated with proteolytic activity; the 2Apro of cardioviruses and aphthoviruses acts only in cis. The leader protein of aphthoviruses has proteolytic activity (Lpro). Some intermediates are stable and serve functions distinct from those of their cleavage products (e.g. cleavage of poliovirus P1 by 3CDpro, not by 3Cpro). The cleavage of 1AB, which accompanies RNA encapsidation, is thought to be autocatalytic.
Replication of viral RNA occurs in complexes associated with cytoplasmic membranes. These complexes contain proteins derived from the whole of the 2BC-P3 region of the polyprotein, including the polymerase (3Dpol, an RNA chain-elongating enzyme), and 2C (an ATPase containing a nucleotide binding sequence motif). The poliovirus 3Cpro component has been shown to be required for binding to the 5′-terminal RNA cloverleaf. Many compounds that specifically inhibit replication have been described. Mutants resistant to, or dependent on drugs have been reported. Genetic recombination, complementation, and phenotypic mixing occur. Defective particles, carrying deletions in the CPs or L, have been produced experimentally but have not been observed in natural virus populations.
ANTIGENIC PROPERTIES
Serotypes are classified by cross-protection, neutralization of infectivity, complement-fixation, specific ELISA using a capture format or immunodiffusion. Some serotypes can be identified by hemagglutination. Antigenic sites, defined by mutations that confer resistance to neutralization by monoclonal antibodies, typically number 3 or 4 per protomer. Neutralization by antibody follows first-order inactivation kinetics.
BIOLOGICAL PROPERTIES
Most picornaviruses are specific for one, or a very few host species (exceptions are Foot-and-mouth disease virus (FMDV) and Encephalomyocarditis virus (EMCV)). Members of most species can be grown in cell culture. Resistant host cells (e.g., mouse cells in the case of the primate-specific polioviruses) can often be infected (for a single round) by transfection with naked, infectious RNA. Transmission is horizontal, mainly by fecal-oral, fomite or airborne routes. Transmission by arthropod vectors is not known, although EMCV has been isolated from mosquitoes and ticks.
Infection is generally cytolytic, but persistent infections are common with some species and reported with others. Poliovirus infected cells undergo extensive vacuolation as membranes are reorganized into viral replication complexes. Infection may be accompanied by rapid inhibition of cap-dependent translation of cellular mRNAs (2Apro of poliovirus and Lpro of aphthovirus are each powerful inhibitors), mRNA synthesis, and the cellular secretary pathway (poliovirus 2B and 3A have been implicated).
SPECIES DEMARCATION CRITERIA IN THE FAMILY
A picornavirus species is a polythetic class of phylogenetically related serotypes or strains which would normally be expected to share (i) a limited range of hosts and cellular receptors, (ii) a significant degree of compatibility in proteolytic processing, replication, encapsidation and genetic recombination, and (iii) essentially identical genome maps.
GENUS ENTEROVIRUS
Type Species Poliovirus
DISTINGUISHING FEATURES
VIRION PROPERTIES
MORPHOLOGY
CPs 1B, 1C and 1D of the human enteroviruses are among the largest in the family (VP1-3 chain lengths, 238-302 aa), and this is reflected in the typically long inter–strand loops, the larger than average thickness of the capsid wall (46 Å), and a surface relief that is strongly pronounced compared to most other picornaviruses. Encircling a raised area at the 5-fold axis is a 25 Å deep groove, or “canyon”, into which the cellular receptor for poliovirus binds. The binding site for the pocket factor lies beneath the floor of this canyon within the 1D -barrel. Virions can be converted by a variety of treatments (gentle heating, binding to receptor, or some neutralizing antibodies) to altered (A′) particles of 135S which lack VP4 and possess altered antigenicity.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Typically virions are stable at acid pH. Buoyant density in CsCl is 1.30-1.34 g/cm3. Empty capsids are often observed in virus preparations. Sometimes a small proportion (about 1% of the population) of heavy particles (density: 1.43 g/cm3) is observed.
NUCLEIC ACID
The genome contains a type-1 IRES and no poly(C) tract. Sequence identities for different enteroviruses, or between enteroviruses and rhinoviruses are more than 50% over the genome as a whole.
GENOME ORGANIZATION AND REPLICATION
Genomes encode a single VPg and no L protein. Protease 2Apro, which is related to the family of small bacterial serine proteases, cleaves the polyprotein at its own N-terminus. Certain hydrophobic molecules that bind to the capsid in competition with pocket factor exert a powerful antiviral action by interfering with receptor binding and/or uncoating.
ANTIGENIC PROPERTIES
Native virions are antigenically serotype-specific (designated “N” or “D” for poliovirus), whereas ‘A’ particles exhibit group specificity (designated “H” or “C” for poliovirus).
BIOLOGICAL PROPERTIES
Viruses multiply primarily in the gastrointestinal tract, but they can also multiply in other tissues, e.g., respiratory mucosa, nerve, muscle, etc. Infection may frequently be asymptomatic. Clinical manifestations include mild meningitis, encephalitis, myelitis, myocarditis and conjunctivitis. Cap-dependent translation of host mRNA is inhibited by 2Apro, which cleaves the host eukaryotic initiation factor 4G (eIF-4G). Many different cell surface molecules, many of them uncharacterized, serve as viral receptors.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a species of the genus Enterovirus:
-
•
share greater than 70% aa identity in P1,
-
•
share greater than 70% aa identity in the non-structural proteins 2C + 3CD,
-
•
share a limited range of host cell receptors,
-
•
share a limited natural host range,
-
•
have a genome base composition (G+C) which varies by no more than 2.5%,
-
•
share a significant degree of compatibility in proteolytic processing, replication, encapsidation and genetic recombination.
LIST OF SPECIES IN THE GENUS
Swine vesicular disease virus is a porcine variant of Human coxsackievirus B5 (CV-B5). Certain viruses initially reported as novel echoviruses were later shown to have been misidentified. Thus E-8 is the same serotype as E-1, E-10 is now Reovirus 1, E-28 is now Human rhinovirus 1A, E-22 is now Human parechovirus 1, E-23 is now Human parechovirus 2. Similarly CV-A23 is the same serotype as E-9, and CV-A15 is the same serotype as CV-A11 and CV-A18. Porcine enteroviruses belonging to CPE group I have been moved to the genus Teschovirus and renamed Porcine teschovirus 1-10.
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Bovine enterovirus | ||
| Bovine enterovirus 1 | [D00214] | (BEV-1) |
| Bovine enterovirus 2 | [X79369] | (BEV-2) |
| Human enterovirus A | ||
| Human coxsackievirus A2 | [L28146, X87585] | (CV-A2)* |
| Human coxsackievirus A3 | [X87586] | (CV-A3) |
| Human coxsackievirus A4 | [AF081295] | (CV-A4) |
| Human coxsackievirus A5 | [X87588] | (CV-A5) |
| Human coxsackievirus A6 | [AF081297] | (CV-A6) |
| Human coxsackievirus A7 | [X87589] | (CV-A7) |
| Human coxsackievirus A8 | [X87590] | (CV-A8) |
| Human coxsackievirus A10 | [X87591] | (CV-A10) |
| Human coxsackievirus A12 | [X87593] | (CV-A12) |
| Human coxsackievirus A14 | [X87595] | (CV-A14) |
| Human coxsackievirus A16 | [U05876] | (CV-A16) |
| Human enterovirus 71 | [U22521] | (EV-71) |
| Human enterovirus 76 | (EV-76) | |
| Human enterovirus B | ||
| Human coxsackievirus B1 | [M16560] | (CV-B1) |
| Human coxsackievirus B2 | [AF081485] | (CV-B2) |
| Human coxsackievirus B3 | [M88483] | (CV-B3) |
| Human coxsackievirus B4 | [X05690] | (CV-B4) |
| Human coxsackievirus B5 (including Swine vesicular disease virus) | [X67706, D00435] | (CV-B5) |
| Human coxsackievirus B6 | [AF039205] | (CV-B6) |
| Human coxsackievirus A9 | [D00627] | (CV-A9) |
| Human echovirus 1 | [X89531, AF029859] | (E-1) |
| Human echovirus 2 | [X89532, AY302545] | (E-2) |
| Human echovirus 3 | [X89533, AY302553] | (E-3) |
| Human echovirus 4 | [X89534, AY302557] | (E-4) |
| Human echovirus 5 | [X89535, AF083069] | (E-5) |
| Human echovirus 6 | [U16283, AY302558] | (E-6) |
| Human echovirus 7 | [X89538, AY036579, AY036578] | (E-7) |
| Human echovirus 9 | [X84981, X92886] | (E-9) |
| Human echovirus 11 | [X80059] | (E-11) |
| Human echovirus 12 | [X79047] | (E-12) |
| Human echovirus 13 | [X89542, AY302539] | (E-13) |
| Human echovirus 14 | [X89543, AY302540] | (E-14) |
| Human echovirus 15 | [X89544, AY302541] | (E-15) |
| Human echovirus 16 | [X89545, AY302542] | (E-16) |
| Human echovirus 17 | [X89546, AY302543] | (E-17) |
| Human echovirus 18 | [X89547, AF317694] | (E-18) |
| Human echovirus 19 | [X89548, AY302544] | (E-19) |
| Human echovirus 20 | [X89549, AY302546] | (E-20) |
| Human echovirus 21 | [X89550, AY302547] | (E-21) |
| Human echovirus 24 | [X89551, AY302548] | (E-24) |
| Human echovirus 25 | [X90722, X89552, AY302549] | (E-25) |
| Human echovirus 26 | [X89553, AY302550] | (E-26) |
| Human echovirus 27 | [X89554, AY302551] | (E-27) |
| Human echovirus 29 | [X89555, AY302552] | (E-29) |
| Human echovirus 30 | [X89556, AF102711] | (E-30) |
| Human echovirus 31 | [X89557, AY302554] | (E-31) |
| Human echovirus 32 | [X89558, AY302555] | (E-32) |
| Human echovirus 33 | [X89559, AY302556] | (E-33) |
| Human enterovirus 69 | [X87605, AY302560] | (EV-69) |
| Human enterovirus 73 | [AF241359] | (EV-73) |
| Human enterovirus 74 | [AY208118] | (EV-74) |
| Human enterovirus 75 | [AF152280] | (EV-75) |
| Human enterovirus 77 | [AY208119] | (EV-77) |
| Human enterovirus 78 | [AY208120] | (EV-78) |
| Human enterovirus C | ||
| Human coxsackievirus A1 | [X87584, AF499035] | (CV-A1) |
| Human coxsackievirus A11 | [X87592, AF499636, AF499638] | (CV-A11) |
| Human coxsackievirus A13 | [X87594, AF499637, AF499640] | (CV-A13) |
| Human coxsackievirus A17 | [X87597, AF499039] | (CV-A17) |
| Human coxsackievirus A19 | [X87599, AF499641] | (CV-A19) |
| Human coxsackievirus A20 | [X87600, AF499642] | (CV-A20) |
| Human coxsackievirus A21 | [D00538] | (CV-A21) |
| Human coxsackievirus A22 | [X87603, AF499643] | (CV-A22) |
| Human coxsackievirus A24 | [D90457] | (CV-A24) |
| Human enterovirus D | ||
| Human enterovirus 68 | [X87604] | (EV-68) |
| Human enterovirus 70 | [D00820] | (EV-70) |
| Poliovirus | ||
| Human poliovirus 1 | [J02281] | (PV-1) |
| Human poliovirus 2 | [M12197] | (PV-2) |
| Human poliovirus 3 | [K01392] | (PV-3) |
| Porcine enterovirus A | ||
| Porcine enterovirus 8 | [AF406813] | (PEV-8) |
| Porcine enterovirus B | ||
| Porcine enterovirus 9 | [Y14459] | (PEV-9) |
| Porcine enterovirus 10 | [AF363455] | (PEV-10) |
| Simian enterovirus A | ||
| Simian enterovirus A1 | (SEV-A1) | |
| Simian enterovirus A2-plaque virus** | [AF201894] | (SEV-A2) |
| Simian enterovirus SV4** | [AF326759] | (SEV-SV4) |
| Simian enterovirus SV28** | [AF326757] | (SEV-SV28) |
| Simian enterovirus SA4** | (SEV-SA4) | |
Note: The alternative abbreviations, CAV-2, etc, are widely used.
the 4 isolates are closely related and probably constitute a single serotype.
TENTATIVE SPECIES IN THE GENUS
| Simian enterovirus A13 | [AF326750] | (A13) |
| Simian enterovirus N125 | [AF414372] | (N125) |
| Simian enterovirus N203 | [AF414373] | (N203) |
| Simian enterovirus SA5 | [AF326751] | (SA5) |
| Simian enterovirus SV16 | [AY064715; AF326752; AY064709] | (SV16) |
| Simian enterovirus SV18 | [AY064716; AF326753; AY064710] | (SV18) |
| Simian enterovirus SV19 | [AF326754] | (SV19) |
| Simian enterovirus SV2 | [AY064708] | (SV2) |
| Simian enterovirus SV26 | [AF326756] | (SV26) |
| Simian enterovirus SV35 | [AF326758] | (SV35) |
| Simian enterovirus SV42 | [AY064717; AF326760; AY064711] | (SV42) |
| Simian enterovirus SV43 | [AF326761] | (SV43) |
| Simian enterovirus SV44 | [AY064718; AF326762; AY064712] | (SV44) |
| Simian enterovirus SV45 | [AY064719; AF326763; AY064713] | (SV45) |
| Simian enterovirus SV47 | (SV47) | |
| Simian enterovirus SV49 | [AY064720; AF326765; AY064714] | (SV49) |
| Simian enterovirus SV6 | [AF326766] | (SV6) |
GENUS RHINOVIRUS
Type Species Human rhinovirus A
DISTINGUISHING FEATURES
VIRION PROPERTIES
MORPHOLOGY
Rhinoviruses share with human enteroviruses the same, comparatively uneven, surface, with its characteristic canyon around the 5-fold axis (attachment site for the intercellular adhesion molecule-1 (ICAM-1) receptor), thick-walled capsid, and pocket factor-binding cavity.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Typically virions are unstable below pH 5-6. They can also be distinguished from enteroviruses by their porosity to CsCl, which gives rise to a buoyant density in the range 1.38-1.42 g/cm3.
NUCLEIC ACID
The 5′-NTR of 650 nt is shorter than that of enteroviruses, owing to a deletion of approximately 100 nt between the IRES and the translation start site. The IRES is of type 1 and there is no poly(C) tract. Nucleotide sequence identity over the entire genome for different species of the genus Rhinovirus, or between enteroviruses and rhinoviruses is more than 50%, although it may be greater or less than this for particular genomic regions.
PROTEINS
Virion proteins are very similar in size to those of human enteroviruses.
GENOME ORGANIZATION AND REPLICATION
These are similar to human enteroviruses. Antiviral, pocket-binding drugs, analogous to those used against enteroviruses, have been described.
ANTIGENIC PROPERTIES
Antigenic properties, including the N-D conversion, are as for human enteroviruses.
BIOLOGICAL PROPERTIES
Human rhinoviruses can be divided into major and minor receptor group viruses. Eighty-nine serotypes (major group) use ICAM-l as receptor, 10 serotypes (minor group) bind members of the low-density lipoprotein receptor (LDLR) family. Clinical manifestations include the common cold and other upper and lower respiratory tract illnesses of humans. Cap-dependent translation of host mRNA is inhibited by 2Apro, which cleaves the host eIF-4G.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a species in the genus Rhinovirus share:
-
•
greater than 70% aa identity in P1,
-
•
greater than 70% aa identity in 2C + 3CD,
-
•
similar susceptibility of receptor attachment to inhibition by pocket-binding antiviral agents (“inhibitor group” A or B).
LIST OF SPECIES IN THE GENUS
Human rhinovirus 87 is now considered to be the same serotype as Human enterovirus 68.
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Human rhinovirus A | ||
| Human rhinovirus 1† | [D00239] | (HRV-1) |
| Human rhinovirus 2 | [X02316] | (HRV-2) |
| Human rhinovirus 7 | [Z47564] | (HRV-7) |
| Human rhinovirus 8 | [AF343594] | (HRV-8) |
| Human rhinovirus 9 | [AF343605] | (HRV-9) |
| Human rhinovirus 10 | [AF343609] | (HRV-10) |
| Human rhinovirus 11 | [Z47565] | (HRV-11) |
| Human rhinovirus 12 | [AY016405] | (HRV-12) |
| Human rhinovirus 13 | [AF343599] | (HRV-13) |
| Human rhinovirus 15 | [AF343630] | (HRV-15) |
| Human rhinovirus 16 | [L24917] | (HRV-16) |
| Human rhinovirus 18 | [AY016407] | (HRV-18) |
| Human rhinovirus 19 | [AF343632] | (HRV-19) |
| Human rhinovirus 20 | [AF343644] | (HRV-20) |
| Human rhinovirus 21 | [Z47566] | (HRV-21) |
| Human rhinovirus 22 | [AF343628] | (HRV-22) |
| Human rhinovirus 23 | [AF343597] | (HRV-23) |
| Human rhinovirus 24 | [AF343619] | (HRV-24) |
| Human rhinovirus 25 | [AF343617] | (HRV-25) |
| Human rhinovirus 28 | [AY016406] | (HRV-28) |
| Human rhinovirus 29 | [Z47567] | (HRV-29) |
| Human rhinovirus 30 | [AF343596] | (HRV-30) |
| Human rhinovirus 31 | [AF343583] | (HRV-31) |
| Human rhinovirus 32 | [AF343584] | (HRV-32) |
| Human rhinovirus 33 | [AF343625] | (HRV-33) |
| Human rhinovirus 34 | [AF343634] | (HRV-34) |
| Human rhinovirus 36 | [Z49123] | (HRV-36) |
| Human rhinovirus 38 | [AF343614] | (HRV-38) |
| Human rhinovirus 39 | [AF343637] | (HRV-39) |
| Human rhinovirus 40 | [AF343641] | (HRV-40) |
| Human rhinovirus 41 | [AF343600] | (HRV-41) |
| Human rhinovirus 43 | [AY040232] | (HRV-43) |
| Human rhinovirus 44 | [AF343616] | (HRV-44) |
| Human rhinovirus 45 | [AY016409] | (HRV-45) |
| Human rhinovirus 46 | [AY040235] | (HRV-46) |
| Human rhinovirus 47 | [AF343607] | (HRV-47) |
| Human rhinovirus 49 | [Z47568] | (HRV-49) |
| Human rhinovirus 50 | [Z47569] | (HRV-50) |
| Human rhinovirus 51 | [AF343585] | (HRV-51) |
| Human rhinovirus 53 | [AF343592] | (HRV-53) |
| Human rhinovirus 54 | [AF343612] | (HRV-54) |
| Human rhinovirus 55 | [AF343621] | (HRV-55) |
| Human rhinovirus 56 | [AF343610] | (HRV-56) |
| Human rhinovirus 57 | [AF343622] | (HRV-57) |
| Human rhinovirus 58 | [Z47570] | (HRV-58) |
| Human rhinovirus 59 | [AF343611] | (HRV-59) |
| Human rhinovirus 60 | [AF343627] | (HRV-60) |
| Human rhinovirus 61 | [AF343601] | (HRV-61) |
| Human rhinovirus 62 | [Z47571] | (HRV-62) |
| Human rhinovirus 63 | [AF343636] | (HRV-63) |
| Human rhinovirus 64 | [AF343629] | (HRV-64) |
| Human rhinovirus 65 | [Z47572] | (HRV-65) |
| Human rhinovirus 66 | [AF343640] | (HRV-66) |
| Human rhinovirus 67 | [AF343603] | (HRV-67) |
| Human rhinovirus 68 | [AF343591] | (HRV-68) |
| Human rhinovirus 71 | [AF343587] | (HRV-71) |
| Human rhinovirus 73 | [AF343602] | (HRV-73) |
| Human rhinovirus 74 | [AF343631] | (HRV-74) |
| Human rhinovirus 75 | [AF343639] | (HRV-75) |
| Human rhinovirus 76 | [AF343624] | (HRV-76) |
| Human rhinovirus 77 | [AF343608] | (HRV-77) |
| Human rhinovirus 78 | [AY016408] | (HRV-78) |
| Human rhinovirus 80 | [AF343593] | (HRV-80) |
| Human rhinovirus 81 | [AF343606] | (HRV-81) |
| Human rhinovirus 82 | [AY040233] | (HRV-82) |
| Human rhinovirus 85 | [AF343642] | (HRV-85) |
| Human rhinovirus 88 | [AF343590] | (HRV-88) |
| Human rhinovirus 89 | [M16248] | (HRV-89) |
| Human rhinovirus 90 | [AF343620] | (HRV-90) |
| Human rhinovirus 94 | [AF343638] | (HRV-94) |
| Human rhinovirus 95 | [AF343595] | (HRV-95) |
| Human rhinovirus 96 | [AF343604] | (HRV-96) |
| Human rhinovirus 98 | [AF343613] | (HRV-98) |
| Human rhinovirus 100 | [AF343643] | (HRV-100) |
| Human rhinovirus Hanks | [AY040234] | (HRV-Hanks) |
| Human rhinovirus B | ||
| Human rhinovirus 3 | [U60874] | (HRV-3) |
| Human rhinovirus 4 | [AF343655] | (HRV-4) |
| Human rhinovirus 5 | [AF343651] | (HRV-5) |
| Human rhinovirus 6 | [AY016402] | (HRV-6) |
| Human rhinovirus 14 | [K02121, K01087, L05355] | (HRV-14) |
| Human rhinovirus 17 | [AF343645] | (HRV-17) |
| Human rhinovirus 26 | [AF343653] | (HRV-26) |
| Human rhinovirus 27 | [AF343654] | (HRV-27) |
| Human rhinovirus 35 | [AY040241] | (HRV-35) |
| Human rhinovirus 37 | [AY016401] | (HRV-37) |
| Human rhinovirus 42 | [AY016404] | (HRV-42) |
| Human rhinovirus 48 | [AY016400] | (HRV-48) |
| Human rhinovirus 52 | [AY016398] | (HRV-52) |
| Human rhinovirus 69 | [AY016399] | (HRV-69) |
| Human rhinovirus 70 | [AF343646] | (HRV-70) |
| Human rhinovirus 72 | [Z47574] | (HRV-72) |
| Human rhinovirus 79 | [AF343649] | (HRV-79) |
| Human rhinovirus 83 | [AF343647] | (HRV-83) |
| Human rhinovirus 84 | [AY040240] | (HRV-84) |
| Human rhinovirus 86 | [AF343648] | (HRV-86) |
| Human rhinovirus 91 | [AY040237] | (HRV-91) |
| Human rhinovirus 92 | [AY040238] | (HRV-92) |
| Human rhinovirus 93 | [AY040239] | (HRV-93) |
| Human rhinovirus 97 | [AY040242] | (HRV-97) |
| Human rhinovirus 99 | [AF343652] | (HRV-93) |
HRV-1 is divided into two antigenic subtypes referred to as HRV-1A and HRV-1B.
TENTATIVE SPECIES IN THE GENUS
| Bovine rhinovirus 1 | (BRV-1) |
| Bovine rhinovirus 2 | (BRV-2) |
| Bovine rhinovirus 3 | (BRV-3) |
GENUS CARDIOVIRUS
Type Species Encephalomyocarditis virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
MORPHOLOGY
Empty capsids are seen only rarely, if ever. When compared by mean wall thickness, surface unevenness, and chain length of the major proteins, the cardiovirus capsid is intermediate between the enteroviruses and aphthoviruses. In place of a continuous, circular, canyon, seen in enteroviruses, is a five-fold repeated pit. There is no pocket factor.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion buoyant density in CsCl is 1.33-1.34 g/cm3. Virions are moderately stable to acidic pH.
NUCLEIC ACID
EMCV has a poly(C) tract of variable length (usually 80-250 nt) about 150 nt from the 5′-terminus of the viral RNA, while Theilovirus isolates lack this feature. All EMCV members have two pseudoknots 5′ to their poly(C) tracts. The IRES is of type 2. The nt sequence identity over the entire genome for different species of the genus Cardiovirus is more than 50% (e.g. TMEV has 54% nt sequence identity to EMCV).
GENOME ORGANIZATION AND REPLICATION
The viral genome encodes a leader (L) protein which lacks proteolytic activity, unlike the L of aphthoviruses; thus L is cleaved from P1 by the virus encoded protease 3C. The 1D/2A junction is also cleaved by 3Cpro, rather than by 2A. The 2A protein causes cleavage, or polypeptide chain interruption, between P1-2A and downstream sequences at an essential sequence, –NPG/P–.
ANTIGENIC PROPERTIES
Four independent antigenic sites have been described. There is no evidence of an N-D conversion, nor of ‘A’ particles.
BIOLOGICAL PROPERTIES
Encephalomyocarditis viruses have been isolated from over 30 host species including mammals, birds and insects. Clinical manifestations include encephalitis and myocarditis in mice and many other animals. TMEV can be divided into two biological subgroups which both infect mice; one causes an acute and fatal polioencephalomyelitis and the other causes a chronic persistent demyelinating infection of the white matter. Vilyuisk human encephalomyelitis virus (VHEV) is thought to be the cause of a degenerative neurological disease in man which has been reported in the Vilyuy valley in Siberia. Cardiovirus infection does not cause cleavage of the host eIF-4G. The cellular receptor used by EMCV to attach to murine vascular endothelial cells has been identified as VCAM-1. However, in human cell lines an as yet unidentified sialoglycoprotein(s) has been found. EMCV binds to human erythrocytes via glycophorin A.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a species of the genus Cardiovirus:
-
•
share greater than 70% aa identity in P1,
-
•
share greater than 70% aa identity in 2C + 3CD,
-
•
share a natural host range,
-
•
share a common genome organization.
LIST OF SPECIES IN THE GENUS
Mengovirus, Columbia SK virus and Maus Elberfeld virus are strains of EMCV, based on serological cross-reaction and sequence identity. The rat encephalomyelitis virus MHG appears to be a strain of TMEV; however, the serological relationship of a genetically divergent Theiler-like virus (TLV) of rats to TMEV is not presently clear.
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Encephalomyocarditis virus | ||
| Columbia SK virus | ||
| Encephalomyocarditis virus* | [M81861] | (EMCV) |
| Maus Elberfeld virus | ||
| Mengovirus | [L22089] | |
| Theilovirus | ||
| Theiler's murine encephalomyelitis virus | [M20562] | (TMEV) |
| Theiler-like virus of rats | [AB090161] | (TLV) |
| Vilyuisk Human encephalomyelitis virus | [M80888, M94868] | (VHEV) |
The significance of the reported serological cross-reaction between Cricket paralysis virus, a member of the family Dicistroviridae, and EMCV is not presently understood.
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS APHTHOVIRUS
Type Species Foot-and-mouth disease virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
MORPHOLOGY
The capsid of FMDV is thin-walled (mean thickness ∼33 Å), and has an unusually smooth surface. A long (17-23 aa), mobile loop, the G-H loop, projects from the surface of 1D. There is a pore at the 5-fold axis, where part of the underlying 1C is exposed. Some serotypes of FMDV accumulate empty capsids.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions are acid labile; FMDV particles are unstable below pH 6.8; Equine rhinitis A virus (ERAV) particles are unstable below pH 5.5. The buoyant density in CsCl is 1.43-1.45g/cm3. Virions of FMDV sediment at 146S, empty capsids at 75S.
NUCLEIC ACID
There is a poly(C) tract close to the 5′-terminus of the genome. In FMDV it is located about 360 nt from the end, and varies in length from 100 to more than 400 nt. Current data suggest that the poly(C) tract in ERAV is shorter (∼40 nt) and closer to the 5′-end. In the RNA of members of both species there is a series of pseudoknots on the 3′-side of the poly(C); the total 5′-NTR is thus extremely long (1.1-1.5 kb). ERAV and FMDV differ by approximately 50% in nt sequence across the entire genome.
PROTEINS
The major CPs of FMDV have the shortest chain lengths of any picornavirus (208-220 aa); those of ERAV are only slightly longer. At the tip of the 1D G-H loop of FMDV is the conserved integrin recognition motif, RGD.
GENOME ORGANIZATION AND REPLICATION
Translation starts at two alternative in-frame initiation sites, resulting in two forms of the L protein (Lab and Lb). L is a papain-like cysteine protease which cleaves itself from the virus polyprotein. The 2A polypeptide is very short (chain length = 18 aa in FMDV), and is involved in NPGP-dependent polypeptide chain interruption at its C-terminus as in cardioviruses. The genome of FMDV encodes 3 species of VPg while that of ERAV encodes only one.
ANTIGENIC PROPERTIES
Five independent antigenic sites have been reported in FMDV type O, two of which have determinants in the G-H loop of 1D. There is no evidence of N-D conversion, nor ‘A’ particles.
BIOLOGICAL PROPERTIES
FMDV infects mainly cloven-hooved animals, but has been isolated from at least 70 species of mammals. Clinical manifestations of FMDV infections include foot-and-mouth disease (vesicular lesions), sometimes with associated acute fatal myocarditis in young animals; of ERAV, upper respiratory tract infections of horses. Both species may produce persistent upper respiratory tract infections. FMDV infects cells by binding to integral membrane proteins of the integrin family through its 1D G-H loop; heparan sulfate proteoglycans may also serve as receptors. Cap-dependent translation of host mRNA is inhibited by Lpro, which cleaves the host eIF-4G.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a species of the genus Aphthovirus:
-
•
share greater than 50% aa identity in P1,
-
•
share greater than 70% aa identity in 2C + 3CD,
-
•
share a natural host range,
-
•
have a genome base composition which varies by no more than 1%,
-
•
share a common genome organization.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Equine rhinitis A virus | ||
| Equine rhinitis A virus (Equine rhinovirus 1) | [L43052, X96870] | (ERAV) |
| Foot-and-mouth disease virus | ||
| Foot-and-mouth disease virus – A | [L11360, M10975] | (FMDV-A) |
| Foot-and-mouth disease virus – Asia 1 | [U01207] | (FMDV-Asia1) |
| Foot-and-mouth disease virus – C | [X00130, J02191] | (FMDV-C) |
| Foot-and-mouth disease virus – O | [M35873, X00871] | (FMDV-O) |
| Foot-and-mouth disease virus – SAT 1 | [Z98203] | (FMDV-SAT1) |
| Foot-and-mouth disease virus – SAT 2 | [AJ251473] | (FMDV-SAT2) |
| Foot-and-mouth disease virus – SAT 3 | [M28719] | (FMDV-SAT3) |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS HEPATOVIRUS
Type Species Hepatitis A virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Viruses are very stable, resistant to acid pH and elevated temperatures (60C for 10 min). Buoyant density in CsCl is 1.32-1.34 g/cm3.
NUCLEIC ACID
There is little similarity between the genome sequences of hepatoviruses and those of other picornaviruses, although the IRES is distantly related to the type 2 IRES. The 5′-NTR contains a 5′-terminal hairpin, two putative pseudoknots, and a short (∼40 nt) pyrimidine-rich (i.e. not pure polyC) tract upstream of the IRES. Nucleotide sequence identity between different Hepatitis A virus (HAV) strains is generally greater than 80%. Avian encephalomyelitis virus (AEV) RNA contains the shortest of all picornavirus 5′-NTRs, at 494 nt.
PROTEINS
In contrast to those of other picornaviruses, protein lA of hepatoviruses is extremely small, does not appear to be myristylated at its N-terminus, and may not be a component of the mature virus particle. Immature HAV may contain uncleaved 1D2A (PX) precursor protein.
GENOME ORGANIZATION AND REPLICATION
The polyprotein contains only a single protease (3Cpro). There is no clearly defined L protein, and 2A has no proteolytic activity. The primary cleavage of the polyprotein occurs at the 2A/2B junction, and is catalyzed by 3Cpro. The 1D/2A cleavage may be directed by an unknown cellular protease, or the VP1 protein may be subject to C-terminal trimming as in cardioviruses. Replication in cell culture occurs slowly, with little CPE, and with low yields of virus compared to most other picornaviruses. The IRES differs from those of other picornaviruses in that its activity is dependent on intact eIF-4G. The 2A protein of hHepatitis A virus (which is unique among picornaviruses) is distinct from that in the tentative species Avian encephalomyelitis-like virus (which is distantly related to the 2A of parechoviruses and kobuviruses).
ANTIGENIC PROPERTIES
Hepatitis A viruses are strongly conserved in their antigenic properties. Most antibodies are directed against a single, conformationally defined immunodominant antigenic site that is comprised of aa residues of the VP3 and VP1 proteins on the surface of the virion.
BIOLOGICAL PROPERTIES
HAV infects epithelial cells of the small intestine and hepatocytes of primates. Virus is predominantly replicated within the liver, excreted via the bile and present in feces in high titer. Viral shedding is maximal shortly before the onset of clinical signs of hepatitis, which probably represents immunopathologically mediated liver injury. Clinical manifestations are fever, jaundice, light stools, abdominal pain, and occasionally diarrhea. Hepatoviruses generally establish persistent infection when inoculated on to any of a wide range of primate cells in vitro, but persistent infection does not occur in vivo, and the viruses are not associated with chronic hepatitis. HAVs can be divided into two distinct biotypes that are phylogenetically distinct and have different preferred hosts (all species of primates: humans, chimpanzees, owl monkeys and marmosets, for one biotype, vs. green monkeys and cynomolgus monkeys for the other). These two biotypes share cross-reacting antigens, but have biotype-specific epitopes that can be distinguished by monoclonal antibodies. AEV causes encephalomyelitis in young chickens, pheasants, quail and turkeys. It can be transmitted both vertically and by the fecal-oral route; field strains are enterotropic.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a species of the genus Hepatovirus have:
-
•
greater than 70% aa identity in P1,
-
•
greater than 70% aa identity in 2C + 3CD,
-
•
greater than 75% nt sequence identity over the genome as a whole,
-
•
cross-protective antigens,
-
•
a defined tissue tropism and host range,
-
•
a similar genome base composition which varies by no more than 1%,
-
•
a common genome organization.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
| Avian encephalomyelitis-like virus | [AJ225173] | (AEV) |
GENUS PARECHOVIRUS
Type Species Human parechovirus
DISTINGUISHING FEATURES
VIRION PROPERTIES
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions are acid stable. The buoyant density in CsCl is 1.36g/cm3.
NUCLEIC ACID
The 5′-NTR is 710 (Human parechovirus) − 730 (Ljungan virus) nt and contains a typical type 2 IRES. The ORF is 2180/2250 codons and the 3′-NTR 87 and 111 nt in Human parechovirus and Ljungan virus, respectively.
PROTEINS
Predicted protein sequences of parechoviruses are highly divergent, no protein having a greater than 30% level of identity when compared with corresponding proteins of any other picornavirus. In contrast to most other picornaviruses, protein 1AB of parechoviruses appears not to be cleaved, and its N-terminus, also unusually, is not myristylated. The mature capsid therefore appears to comprise only three proteins, 1AB, 1C and 1D.
GENOME ORGANIZATION AND REPLICATION
The polyprotein contains only a single protease (3Cpro). The 2A protein is believed to lack protease activity and is related distantly to a family of cellular proteins involved in the control of cell proliferation, as well as to that of Kobuvirus and AEV. Ljungan virus isolates additionally contain sequences resembling the aphthovirus 2A at this locus.
BIOLOGICAL PROPERTIES
Human parechoviruses replicate in the respiratory and gastrointestinal tract. Infection is particularly prevalent in young children but it is probably often asymptomatic. In addition to respiratory infections and diarrhea, infections of the central nervous system have occasionally been reported. The cytopathology may be unusual in including changes in granularity and chromatin distribution in the nucleus, when viewed in the electron microscope. Isolates of ljungan viruses appear to infect predominantly rodents.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a species of the genus Parechovirus:
-
•
share greater than 70% aa identity in P1,
-
•
share greater than 70% aa identity in 2C + 3CD,
-
•
share a natural host range,
-
•
share a common genome organization,
-
•
have a similar genome base composition which varies by no more than 1%.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Human parechovirus | ||
| Human parechovirus 1 | [L02971] | (HPeV-1) |
| (Human echovirus 22) | ||
| Human parechovirus 2 | [AF055846, AJ005695] | (HPeV-2) |
| (Human echovirus 23) | ||
| Human parechovirus 3 | [AB084913] | (HPeV-3) |
| Ljungan virus | ||
| Ljungan virus* | [AF327920, AF327921, AF327922, AF538689] | (LV) |
The American isolate M1146 is relatively divergent from the Swedish isolates 87–012, 174F and 145SL, but it is not known if they are distinct serotypes.
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS ERBOVIRUS
Type Species Equine rhinitis B virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Equine rhinitis B virus (ERBV) has a buoyant density in CsCl of 1.41-1.45 g/cm3. The virus is unstable below pH 5.
NUCLEIC ACID
ERBV possesses an unusually long 3′-NTR of 167 nt. The IRES is of type 2, and a poly(C) tract is thought to be present. No pseudoknots have been identified.
PROTEINS
The CPs have between 25% and 47% aa sequence identity to those of ERAV, FMDV and EMCV, though protein modelling studies indicate that they more closely resemble those of EMCV.
GENOME ORGANIZATION AND REPLICATION
No evidence for alternative sites of initiation of protein synthesis is available. The L protein appears to be a protease, but has only 23% and 18% aa sequence identity to the L proteins of FMDV and ERAV, respectively. The 2B and 3C proteins have exceptionally large chain lengths (283 and 251 aa). The 2A protein has a chain length of 18 aa, ending in NPGP, and there is only one VPg.
BIOLOGICAL PROPERTIES
ERBV causes upper respiratory tract disease in horses, with a viremia and fecal shedding. Infections may be persistent.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS KOBUVIRUS
Type Species Aichi virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Unlike other picornaviruses, kobuviruses exhibit icosahedral surface structure under the electron microscope. Virions are stable at pH 3.5.
NUCLEIC ACID
The genome of Aichi virus (AiV) has a high G+C base composition (59%), and a very long 3′-NTR (240 nt). There is a 5′-proximal stem-loop involved in RNA replication and encapsidation.
PROTEINS
Protein 1AB appears not to be cleaved.
GENOME ORGANIZATION AND REPLICATION
There is a leader polypeptide of unknown function, and distinctive length (170 aa rather than 67 aa or 217 aa in EMCV and FMDV, respectively). The 2A protein is distantly related to that of parechoviruses.
BIOLOGICAL PROPERTIES
AiV grows in cell cultures (BSC-1, Vero). AiV is thought to be a cause of human gastroenteritis associated with eating shellfish.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a species of the genus Kobuvirus:
-
•
share greater than 70% aa identity in P1,
-
•
share greater than 70% aa identity in 2C + 3CD,
-
•
share a common genome organization.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS TESCHOVIRUS
Type Species Porcine teschovirus
DISTINGUISHING FEATURES
VIRION PROPERTIES
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions are stable at acid pH. Buoyant density in CsCl is 1.33 g/cm3. Empty capsids are often observed in virus preparations.
NUCLEIC ACID
Teschoviruses have an IRES which is unlike that of other picornaviruses in being shorter (290 nt) and functional in the absence of eIF-4G. In both these properties the IRES resembles that of Hepatitis C virus (family Flaviviridae) and sequence similarity has also been observed.
PROTEINS
Genomes encode a single VPg and a leader (L) protein. The 2A polypeptide is very short and ends in NPGP, indicative of an aphthovirus 2A-like molecule.
BIOLOGICAL PROPERTIES
Clinical manifestations may include a polioencephalomyelitis (“Teschen disease”), which may vary in severity.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Porcine teschovirus | ||
| Porcine teschovirus 1 | [AF231769, AB038528] | (PTV-1) |
| (Porcine enterovirus 1) | ||
| Porcine teschovirus 2 | [AF296087] | (PTV-2) |
| (Porcine enterovirus 2) | ||
| Porcine teschovirus 3 | [AF296088] | (PTV-3) |
| (Porcine enterovirus 3) | ||
| Porcine teschovirus 4 | [AF296089] | (PTV-4) |
| (Porcine enterovirus 4) | ||
| Porcine teschovirus 5 | [AF296090] | (PTV-5) |
| (Porcine enterovirus 5) | ||
| Porcine teschovirus 6 | [AF296091] | (PTV-6) |
| (Porcine enterovirus 6) | ||
| Porcine teschovirus 7 | [AF296092] | (PTV-7) |
| (Porcine enterovirus 7) | ||
| Porcine teschovirus 8 | [AF296093] | (PTV-8) |
| (Porcine enterovirus 11) | ||
| Porcine teschovirus 9 | [AF296094] | (PTV-9) |
| (Porcine enterovirus 12) | ||
| Porcine teschovirus 10 | [AF296119, AF296095] | (PTV-10) |
| (Porcine enterovirus 13) | ||
| Porcine teschovirus 11 | [AF296096] | (PTV-11) |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
| Acid-stable equine picornaviruses | (EqPV) |
| Avian entero-like virus 2 | (AELV-2) |
| Avian entero-like virus 3 | (AELV-3) |
| Avian entero-like virus 4 | (AELV-4) |
| Avian nephritis virus 3* | (ANV-3) |
| Barramundi virus-1† | (BaV) |
| Cockatoo entero-like virus | (CELV) |
| Duck hepatitis virus 1 | (DHV-1) |
| Duck hepatitis virus 3 | (DHV-3) |
| Guineafowl transmissible enteritis virus | (GTEV) |
| Harbour seals picorna-like virus | (SPLV) |
| Sea-bass virus-1† | (SBV) |
| Sikhote-Alyn virus | (SAV) |
| Smelt virus-1† | (SmV-1) |
| Smelt virus-2† | (SmV-2) |
| Syr-Daria Valley fever virus | (SDFV) |
| Turbot virus-1 | (TuV-1) |
| Turkey entero-like virus | (TELV) |
| Turkey hepatitis virus | (THV) |
| Turkey pseudo enterovirus 1 | (TPEV-1) |
| Turkey pseudo enterovirus 2 | (TPEV-2) |
Avian nephritis virus 1 (ANV-1) and Avian nephritis virus 2 (ANV-2) have been shown to be members of the family Astroviridae. Taura syndrome virus (TSV) of marine penaeid shrimp has been shown to be a member of the family Dicistroviridae.
PHYLOGNETIC RELATIONSHIPS WITHIN THE FAMILY
Figure 3.

Phylogenetic trees showing the relationships between the species and genera of the family Picornaviridae. (Top) Protein P1 and (Bottom) Proteins 2C+3CD. The Neighbor-joining trees were produced and bootstrapped (1000 replicates) using CLUSTALX and an aa weight matrix (BLOSUM). The trees were drawn using TreeView v1.5.2. Only bootstrap values of >90% are indicated.
SIMILARITY WITH OTHER TAXA
A “picornavirus-like superfamily” has previously been proposed to include the families Picornaviridae, Sequiviridae, Comoviridae and Potyviridae, which share the following features:
(i) Genome: This consists of one, or two (in the case of the bipartite plant viruses), molecules (segments) of ssRNA of positive sense. Each segment acts as the exclusive mRNA for the genes it carries (i.e. there are no sgRNAs), contains a single ORF, and is linked at its 5′-end to a tyrosine or serine residue of a genome-linked protein (VPg) via an O-phosphate ester bond.
(ii) Gene expression: The single polyprotein encoded by each genome segment is processed proteolytically to functional proteins by proteases, and these proteases are exclusively virus encoded.
(iii) Genes: All members of the “superfamily” encode, in addition to VPg, a 2C-like protein having a nucleotide binding sequence motif, a 3Cpro-like protease, and a 3Dpol-like polymerase.
(iv) Gene maps: The gene order, 2C-VPg-3Cpro-3Dpol, is common to all members of the “superfamily”, the 3Dpol gene always being located at the 3′-terminus of the relevant ORF. Similarly, where CP gene(s) are present in a single ORF, they are always 5′-proximal.
In addition to the families already mentioned above, there are two groups of insect viruses, those in the unassigned genus Iflavirus and those comprising the Dicistroviridae, that show many of the above characteristics and clear affinities with the putative “picornavirus-like superfamily”. At a structural level the “superfamily” is polythetic and contains both icosahedral and rod-shaped viruses. Where the crystal structure is known for icosahedral representatives, of members of the Picornaviridae and Comoviridae, for example, they exhibit the same, pseudo T=3, arrangement of three major protein subunits, which themselves share the same, -barrel tertiary structure. This structure is also shared with members of the Dicistroviridae.
Despite these notable affinities, the “picornavirus-like superfamily” has no formal taxonomic status.
DERIVATION OF NAMES
Aphtho: from Greek aphthae, “vesicles in the mouth” English: aphtha, “thrush” French:
fièvre aphteuse.
Cardio: from Greek kardia, “heart”.
Entero: from Greek enteron, “intestine”.
Erbo: sigla for equine rhinitis B virus.
Hepato: from Greek hepatos, “liver”.
Kobu: from Japanese kobu, “knuckle” (reference to surface structure of virus particle).
Parecho: from par(a)echo (echo, the former name of the type species, a sigla for “enteric
cytopathic human orphan”).
Picorna: from the prefix “pico” (=′micro-micro′) and RNA.
Rhino: from Greek rhis, rhinos, “nose”.
Tescho: from Teschen disease.
REFERENCES
- Agol V.I. Picornavirus genome: an overview. In: Semler B.L., Wimmer E., editors. Molecular biology of picornaviruses. ASM Press; Washington DC: 2002. pp. 127–148. [Google Scholar]
- Blomqvist S., Savolainen C., Raman L., Roivainen M., Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J. Clin. Miocrobiol. 2002;40:4218–4422. doi: 10.1128/JCM.40.11.4218-4223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnek B.W. Avian encephalomyelitis. In: McFerran J.B., McNulty M.S., editors. Vol. 4. Elsevier; Amsterdam: 1993. pp. 469–478. (Virus infections of vertebrates, virus infections of birds). [Google Scholar]
- Calnek B.W. Duck virus hepatitis. In: McFerran J.B., McNulty M.S., editors. Vol. 4. Elsevier; Amsterdam: 1993. pp. 485–495. (Virus infections of vertebrates, virus infections of birds). [Google Scholar]
- Hamparian V.V., Colonno R.J., Dick E.C., Gwaltney J.M., Hughes J.H., Jordan W.S., Kapikian A.Z., Mogabgab W.J., Mores A., Phillips C.A., Rueckert R.R., Scheble J.H., Stott E.J., Tyrrell D.A.J. A collaborative report: rhinoviruses – extension of the numbering system from 89 to 100. Virology. 1987;159:191–192. doi: 10.1016/0042-6822(87)90367-9. [DOI] [PubMed] [Google Scholar]
- Huang J.A., Ficorilli N., Hartley C.A., Wilcox R.S., Weiss M., Studdert M.J. Equine rhinitis B virus: a new serotype. J. Gen. Virol. 2001;82:2641–2645. doi: 10.1099/0022-1317-82-11-2641. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Hovi T., Knowles N.J., Stanway G. Classification of enteroviruses based on molecular and biological properties. J. Gen. Virol. 1997;78:1–11. doi: 10.1099/0022-1317-78-1-1. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Horsnell C., Maaronen M., Khan M., Kalkinnen N., Auvinen P., Kinnuren L., Stanway G. A novel picornavirus group identified by sequence analysis. Proc. Natl. Acad. Sci. USA. 1992;89:8847–8851. doi: 10.1073/pnas.89.18.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada T. Avian nephritis virus infection. In: McFerran J.B., McNulty M.S., editors. Vol. 4. Elsevier; Amsterdam: 1993. pp. 479–483. (Virus infections of vertebrates, virus infections of birds). [Google Scholar]
- McFerran J.B. Other avian enterovirus infections. In: McFerran J.B., McNulty M.S., editors. Vol. 4. Elsevier; Amsterdam: 1993. pp. 497–503. (Virus infections of vertebrates, virus infections of birds). [Google Scholar]
- Oberste M.S., Maher K., Kilpatrick D.R., Pallansch M.A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M.S., Maher K., Pallansch M.A. Molecular phylogeny and proposed classification of the simian picornaviruses. J. Virol. 2002;76:1244–1251. doi: 10.1128/JVI.76.3.1244-1251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert R.R., Wimmer E. Systematic nomenclature of picornavirus proteins. J. Virol. 1984;50:957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen C., Blomqvist S., Mulders M.N., Hovi T. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 2002;83:333–340. doi: 10.1099/0022-1317-83-2-333. [DOI] [PubMed] [Google Scholar]
- Stanway G., Hovi T., Knowles N.J., Hyypiä, T. Molecular and biological basis of picornavirus taxonomy. In: Semler B.L., Wimmer E., editors. Molecular Biology of Picornaviruses. ASM Press; Washington DC: 2002. pp. 17–24. [Google Scholar]
- Wutz G., Auer H., Nowotny N., Grosse B., Skern T., Kuechler E. Equine rhinovirus serotypes 1 and 2: relationship to each other and to aphthoviruses and cardioviruses. J. Gen. Virol. 1996;77:1719–1730. doi: 10.1099/0022-1317-77-8-1719. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Sakae K., Tsuzuki H., Suzuki Y., Ishikawa N., Takeda N., Miyamura T., Yamazaki S. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J. Virol. 1998;72:8408–8412. doi: 10.1128/jvi.72.10.8408-8412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell R., Dauber M., Krumbholz A., Henke A., Birch-Hirschfeld E., Stelzner A., Prager D., Wurm R. Porcine teschoviruses comprise at least eleven distinct serotypes: molecular and evolutionary aspects. J. Virol. 2001;75:1620–1631. doi: 10.1128/JVI.75.4.1620-1631.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONTRIBUTED BY, P. Christian, E. Carstens, L. Domier, J. Johnson, K. Johnson, N. Nakashima, P. Scotti, F. van der Wilk
GENUS IFLAVIRUS
Type Species Infectious flacherie virus
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

Negative contrast electron micrograph of isometric particles of an isolate of Infectious flacherie virus. The bar represents 100nm (Courtesy of H. Bando).
Virions are roughly spherical with a particle diameter of approximately 30 nm and no envelope.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions have a buoyant density of between 1.33 g/ml and 1.38 g/ml.
NUCLEIC ACID
Particles contain a single molecule of linear, positive sense, ssRNA of between ∼8,800-9,700 nt in size. The 3′-end of the viral RNA is polyadenylated and in Infectious flacherie virus (IFV) there is a protein, VPg, covalently linked to the 5′-end of the genome. The 5′-UTR is quite small and ranges from 156 nt (IFV) to 473 nt (Perina Nuda virus – PnV). The 3′-UTR ranges from 45 nt (PnV) to 239 nt (IFV).
PROTEINS
Mature virions contain three major structural proteins with size generally between 28-35 kDa. In IFV and PnV a fourth smaller structural protein (4-12 kDa) protein has also been reported which may be analogous to the VP4 of picornaviruses and dicistroviruses. There are no reports of other minor structural proteins in the capsid. The structural proteins have been termed VP2, VP3, and VP1 (N-C) to reflect the sequence and deduced structural homology with the equivalent proteins in picornaviruses and dicistroviruses.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Figure 2.

Genome structure of Infectious flacherie virus (IFV). The genome encodes a single polyprotein that is processed to produce the three major structural proteins (VP2, VP3 and VP1) and the non-structural proteins. The structural proteins are encoded at the 5′-end of the polyprotein and the non-structural proteins at the 3′-end. The polyprotein is preceded by a leader sequence (L) of unknown function that is removed from VP2 before capsid assembly. The VP4 is in analogous to the VP4 present in some dicistroviruses and in the case of IFV is present as a minor structural component of the capsid. The approximate positions of the helicase (Hel), protease (Pro) and replicase (RdRp) domains in the non-structural protein are shown.
The genome consists of a ssRNA with a 5′-UTR of 156-473 nt followed by a single ORF of 8,400-9,500 nt and a 3′-UTR of 45-239 nt. The genome is arranged with the structural proteins at the 5′-end of the genome and the non-structural proteins at the 3′-end. The CPs are preceded by a leader sequence of greater than 140 aa which has no known function. Sequence analysis reveals the presence of a small protein between VP2 and VP3 that is analogous to the VP4 of dicistroviruses. In IFV this protein appears to be present as a minor CP, however, its structural role is yet to be elucidated.
The mechanism of virus entry into cells is unknown. In vitro translation studies with genomic RNA of IFV have shown that the viral polyprotein is processed to form an array of smaller polypeptides including the CPs. There is no evidence to indicate that translation of the polyprotein is mediated by an IRES. Mechanisms of polyprotein processing and the effects on host cell macromolecular synthesis during infection have not been well studied as suitable cell culture systems have not been available.
ANTIGENIC PROPERTIES
No reported antigenic relationships between species.
BIOLOGICAL PROPERTIES
All members appear to have restricted host ranges. IFV is known only from the lepidopteran species Bombyx mori and Glyphodes pyloalis and is not know to replicate in any cultured cell line. Sacbrood virus (SBV) is a common infection of larvae of the honeybee, Apis mellifera. No other hosts or permissive cell lines are known. PnV is known only from the lepidopteran Perina nuda. Unlike the other two viruses in the genus, PnV replicates in a homologous cell line established from Perina nuda.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The list of species demarcation criteria is:
-
•
Natural host range: species can be differentiated on the basis of their natural host range
-
•
Sequence identity between the CPs of isolates and strains of a species is above 90%.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Figure 3.

Unrooted phenogram derived from the RdRp domain of the viral non-structural proteins showing the relationships of representative picornaviruses, sequiviruses, dicistroviruses and the three members of the genus Iflavirus (IFV, PnV and SBV). Taxa used (with sequence accession numbers shown in brackets [ ]) were Cowpea severe mosaic virus (CPSMV) [M83830], Drosophila C virus (DCV) [AF014388], Encephalomyocarditis virus (EMCV) [M81861], Foot-and-mouth disease virus (FMDV) [X00871], Hepatitis A virus (HAV) [M14707], Poliovirus (PV) [J02281], Parsnip yellow fleck virus (PYFV) [D14066], Plautia stali intestine virus (PSIV), Rhopalosiphum padi virus (RhPV) [AF022937], and Rice tungro spherical virus (RTSV) [M95497]. A bootstrap analysis was performed and values obtained are shown next to the branching points. Branch lengths are proportional to distance.
The current members of the genus are related only distantly to each other. Phenetic analyses of the CP coding regions show that the VP2 and VP3 of the iflaviruses share between 17-22% and 18-24% aa identity in pairwise comparisons. These levels of variation are below those seen between members of the genus Cripavirus in the Dicistroviridae and even below the levels of variation seen between genera within the Picornaviridae.
SIMILARITY WITH OTHER TAXA
Members of the genus Iflavirus have similarities to other viruses with positive sense ssRNA genomes within the picornavirus “superfamily” (Comoviridae, Dicistroviridae, Picornaviridae, Potyviridae and Sequiviridae). For instance the gene order of the nonstructural proteins is the same for all groups within this assemblage i.e. Hel-Pro-Rep.
However, in many respects the iflaviruses superficially appear to be entomogenous picornaviruses. The genome organization (with the structural proteins located at the 5′-end of the genome), three CPs, a single ORF and no sgRNAs, all point to strong affinities with the picornaviruses. However, there are some important differences between the iflaviruses and the picornaviruses. First, the position of VP4 in the CPs is very different from the picornaviruses and suggests an affinity with the dicistroviruses. Second the very small size of the 5′-UTR – which is much smaller than that of either the picornaviruses or the dicistroviruses – may well indicate the lack of an IRES-like element in this region which would make this group unique amongst the animal-infecting members of the “picornavirus-like superfamily”.
In addition to the known members of this genus and the Dicistroviridae there are a large number of viruses of insects/invertebrates with icosahedral/spherical particles, 3 major CPs and ssRNA genomes. While many have been described as either picornaviruses or picorna-like viruses many remain relatively uncharacterized. In the case of Acyrthosiphon pisum virus (APV) [AF14514], the complete genome has been sequenced. This virus has a structural and genomic organization, which are quite different from any other. Among the remaining 20 or so picorna-like viruses of insects there are undoubtedly a number of viruses that will eventually be aligned with members of the genus Iflavirus but which are currently classified as unassigned viruses.
DERIVATION OF NAMES
Ifla: a sigla from the type virus of the genus Infectious flacherie virus
REFERENCES
- Christian P.D., Scotti P.D. The picorna-like viruses of insects. In: Miller L.K., Ball L.A., editors. The Insect Viruses. Plenum Publishing Company; New York: 1998. pp. 301–336. [Google Scholar]
- Ghosh R., Ball B.V., Willcocks M.M., Carter M.J. The nucleotide sequence of sacbrood virus of the honey bee: an insect picorna-like virus. J. Gen. Virol. 1999;80:1541–1549. doi: 10.1099/0022-1317-80-6-1541. [DOI] [PubMed] [Google Scholar]
- Isawa H., Asano S., Sahara K., Iizuka T., Bando H. Analysis of the genetic information of an insect picorna-like virus, infectious flacherie of silkworm: evidence for evolutionary relationships among insect, mammalian and plant picorna(-like) viruses. Arch. Virol. 1998;143:127–143. doi: 10.1007/s007050050273. [DOI] [PubMed] [Google Scholar]
- Wu C.-Y., Lo C.-F., Huang C.-J., Yu H.-T., Wang C.-H. The complete genome sequence of Perina nuda picorna-like virus, an insect-infecting RNA virus with a genome organization similar to that of the mammalian picornaviruses. Virology. 2002;294:312–323. doi: 10.1006/viro.2001.1344. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, P. Christian, E. Carstens, L. Domier, J. Johnson, K. Johnson, N. Nakashima, P. Scotti, F. van der Wilk
FAMILY DICISTROVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Dicistroviridae |
| Genus | Cripavirus |
Since only one genus is currently recognized, the family description corresponds to the genus description.
GENUS CRIPAVIRUS
Type Species Cricket paralysis virus
VIRION PROPERTIES
MORPHOLOGY
Virions are roughly spherical with a particle diameter of approximately 30 nm and no envelope (Fig. 1 ). The virions exhibit icosahedral, pseudo T=3 symmetry and are composed of 60 protomers, each comprised of a single molecule of each of VP2, VP3 and VP1 (Fig. 1). A smaller protein, VP4, is also present in the virions of some members and is located on the internal surface of the 5-fold axis below VP1.
Figure 1.

(Left) Rendering of a particle of an isolate of Cricket paralysis virus at 2.4Å resolution (Courtesy of Reddy et al., 2001). (Center) Diagram showing the packing of surface proteins of Cricket paralysis virus (CrPV). (Right) Negative contrast electron micrograph of isometric particles of CrPV. The bar represents 100nm (Courtesy of C. Reinganum).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions have a buoyant density in CsCl of between 1.34 and 1.39 g/cm3 and sedimentation coefficients of between 153 and 167S. For those viruses where physicochemical stability has been assessed like Cricket paralysis virus (CrPV) the virions are stable at pH 3.0.
NUCLEIC ACID
Particles contain a single molecule of linear, positive sense, ssRNA of approximately 9,000-10,000 nt in size. The 3′-end of the viral RNA is polyadenylated and in most species there is a protein, VPg, covalently linked to the 5′-end of the genome. The 500-800 nt 5′-UTR and the untranslated intergenic region (IGR) between the two ORFS can both initiate translation as IRES. In the case of the IGR the predicted secondary structure is found to be highly conserved across all members of the family and to have a characteristic series of stem-loop structures and a pseudo-knot immediately upstream of the initiation codon. Structural conservation in the putative 5′-IRES is much less conserved between viruses than the IGR IRES and there are no clear structural homologies between the 5′- and IGR IRES.
PROTEINS
Mature virions contain three major structural proteins with size generally between 28-37 kDa. In the case of Taura syndrome virus (TSV) one of the structural proteins, VP3, is much larger than this with a deduced size (from nt sequence analysis) of 56 kDa. In some species a fourth smaller structural protein (4.5-9 kDa) protein has also been reported. In most species a minor structural component – larger than the major CPs – has also been reported and is presumed to be the precursor of one of the major structural proteins (VP3) and the minor structural
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genome consists of ssRNA with a 5′-UTR of 500-800 nt followed by two ORFs of around 5,500 nt and 2,600 nt separated by an untranslated region of approximately 190 nt known as the IGR (Fig. 2 ). The CPs have been shown by direct sequence analysis to be encoded by the ORF proximal to the 3′-end. The 5′ ORF encodes protein(s) with sequence motifs related to the helicase, protease and replicase domains of other positive sense RNA viruses of plants and animals e.g. picornaviruses, comoviruses, sequiviruses and iflaviruses.
Figure 2.

Genome structure of Cricket paralysis virus (CrPV). The approximate positions of the helicase (Hel), protease (Pro) and replicase (RdRp) domains in the non-structural protein encoded by the 5′ ORF are shown. The structural proteins are encoded by ORF 2 and are expressed as a polyprotein. This is processed to produce the three major structural proteins (VP2, VP3 and VP1). VP4 is presumed to be cleaved from a precursor comprising VP4-VP3 and is a minor structural component of the virion. VP4 is not produced by all members of the family.
The mechanism of virus entry into cells in unknown. Initiation of protein synthesis coincides with shutdown or down-regulation of host cell protein synthesis. Large precursor are produced in infected cells which are then cleaved to produce an array of smaller polypeptides.
In the case of CrPV the structural proteins are observed to be synthesized in supramolar excess relative to the non-structural proteins. No sgRNA is produced during infection and translation of ORF 2 is presumed to be initiated by an IRES-like element. Studies have shown that the 5′-UTR and IGR of several members can direct initiation of translation in in vitro translation systems and in the case of CrPV, both regions function as IRES elements and direct translation from bi-cistronic messages transfected into cultured cells. Initiation of translation from the IGR does not require the presence of a methionine residue and in all known cases is initiated from alanine or glutamine codons.
Virions are assembled in the cytoplasm of infected cells and tend to form large paracrystalline arrays with most species.
ANTIGENIC PROPERTIES
All species are serologically distinct. However, there is some serological relatedness between CrPV and Drosophila C virus (DCV) which show a reaction of partial identity in double diffusion in agar with some sera raised against CrPV.
BIOLOGICAL PROPERTIES
All member viruses have been isolated from invertebrate species. CrPV has been isolated from species of Orthoptera, Hymenoptera, Lepidoptera, Hemiptera and Diptera. DCV has only been isolated from dipteran species while Himetobi P virus (HiPV), Plautia stali intestine virus (PSIV), Rhopalosiphon padi virus (RhPV) and Triatoma virus (TrV) have only come from hemipteran species. Acute bee paralysis virus (ABPV) and Black queen cell virus (BQCV) are known only from honeybees (Apis mellifera) and TSV only from penaeid shrimps. No vectors are known to be involved in transmission.
CrPV is known to have a wide host range and to be widely distributed in nature. DCV is commonly associated with Drosophila species both in nature and in laboratory cultures. Infections with most viruses are usually not associated with a noticeable disease state although they commonly lead to reduced life expectancy of infected individuals. Under some circumstances CrPV shows increased tropism for neural cells and can lead to an obvious paralytic disease state.
CrPV and DCV replicate readily in several established Drosophila cell lines. CrPV has also been found to replicate in a number of other established insect cell lines. There are no cell lines known that are able to support replication of the other members.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The list of species demarcation criteria is:
-
•
Natural host range: species can be differentiated on the basis of their natural host range and their relative ability to replicate in a range of cultured insect cells.
-
•
Serology: all species are serologically distinct.
-
•
Sequence identity between the CPs of isolates and strains of a species is above 90%.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Aphid lethal paralysis virus | ||
| Aphid lethal paralysis virus | [AF536531] | (ALPV) |
| Black queen cell virus | ||
| Black queen cell virus | [AF183905] | (BQCV) |
| Cricket paralysis virus | ||
| Cricket paralysis virus | [AF218039] | (CrPV) |
| Drosophila C virus | ||
| Drosophila C virus | [AF014388] | (DCV) |
| Himetobi P virus | ||
| Himetobi P virus | [AB017037] | (HiPV) |
| Plautia stali intestine virus | ||
| Plautia stali intestine virus | [AB006531] | (PSIV) |
| Rhopalosiphum padi virus | ||
| Rhopalosiphum padi virus | [AF022937] | (RhPV) |
| Triatoma virus | ||
| Triatoma virus | [AF178440] | (TrV) |
TENTATIVE SPECIES IN THE GENUS
None reported
LIST OF UNASSIGNED SPECIES IN THE FAMILY
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Figure 3.
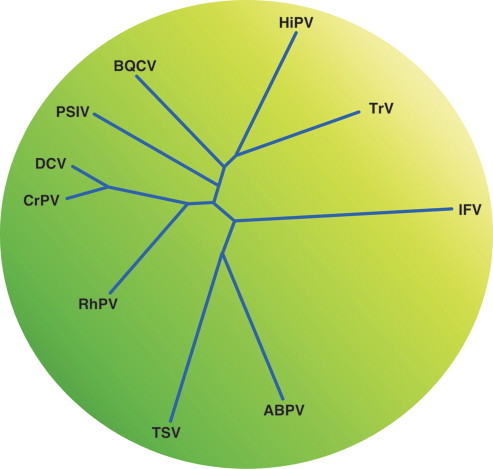
Phenogram showing the relationships among members of the family Dicistroviridae, constructed from the aa similarity of VP2 using the neighbor-joining algorithm of the MEGA software. The sequence from Infectious flacherie virus (IFV) [AB000906] was used as an outgroup for the analysis. The abbreviations refer to the virus names above. Branch lengths are drawn to scale.
SIMILARITY WITH OTHER TAXA
Members of the Dicistroviridae have similarities to other viruses with positive sense ssRNA genomes within the “picornavirus-like superfamily” (Comoviridae, Iflavirus, Picornaviridae, Potyviridae and Sequiviridae). For instance the gene order of the non-structural proteins is the same for all groups within this assemblage i.e. Hel-Pro-RdRp. Like other isometric members of this “superfamily” for which the structure is known, CrPV has a pseudo T=3 symmetry. However, the members of the family Dicistroviridae can be distinguished from the members of the taxa Iflavirus, Picornaviridae and Sequiviridae by the organization of the genome i.e. having the structural proteins at the 3‘-end of the genome rather than the 5′-end and by the presence of the IGR, and they can be separated from members of the family Comoviridae by having only a single rather than two genomic segments.
There are a large number of RNA-containing viruses of approximately 30 nm in diameter that have been described from insects for which the taxonomic status is not known. Many of these have characteristics that are superficially similar to members of the family Dicistroviridae and have been described in the literature as either picornaviruses or picorna-like viruses. While many of these viruses remain relatively uncharacterized, for Acyrthosiphon pisum virus (APV) [AF14514], the complete genome has been sequenced.
This virus has a structural and organization which is quite different from either the members of the family Dicistroviridae or of the recently established unassigned genus, Iflavirus. Among the remaining 20 or so picorna-like viruses of insects there are undoubtedly a number of viruses that will eventually be members of the family Dicistroviridae but they are currently classified as unassigned viruses.
Figure 4.

Unrooted phenogram showing the relationships of members of the family Dicistroviridae to representatives of the families Picornaviridae, Comoviridae, Sequiviridae and the unassigned genus Iflavirus. The phenogram was constructed from an aa similarity matrix of the replicase (RdRp) region of the non-structural proteins using the neighbor-joining method. A bootstrap analysis was performed and the percentage values are indicated at the branching points. Viruses other than dicistroviruses included in the analysis, abbreviation ( ) and accession numbers [ ] are; Cowpea severe mosaic virus (CPSMV) [M83830], Encephalomyocarditis virus (EMCV) [M81861], Foot-and-mouth disease virus (FMDV) [X00871], Hepatitis A virus (HAV) [M14707], Infectious flacherie virus (IFV) [AB000906], Parsnip yellow fleck virus (PYFV) [D14066], Perina nuda virus (PnV) [AF323747], Poliovirus (PV) [J02281], Rice tungro spherical virus (RTSV) [M95497], Sacbrood virus (SBV) [AF469603]. Branch lengths are drawn to scale.
DERIVATION OF NAMES
Cripa: sigla from the name of the type member of the genus, Cricket paralysis virus
Dicistro: sigla from the characteristic di-cistronic arrangement of the genome.
REFERENCES
- Christian P.D., Scotti P.D. The picorna-like viruses of insects. In: Miller L.K., Ball A., editors. The insect viruses. Plenum Press; New York: 1998. pp. 301–336. [Google Scholar]
- Domier L.L., McCoppin N.K., D'Arcy C.J. Sequence requirements for translation initiation of Rhopalosiphum padi virus ORF2. Virology. 2000;268:264–271. doi: 10.1006/viro.2000.0189. [DOI] [PubMed] [Google Scholar]
- Kanamori Y., Nakashima N. A tertiary structure model of the internal ribosome entry site (IRES) for methionine-independent initiation of translation. RNA. 2001;7:266–274. doi: 10.1017/s1355838201001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljas L., Tate J., Christian P., Johnson J.E. Evolutionary and taxonomic implications of conserved structural; motifs between picornaviruses and insect picorna-like viruses. Arch. Virol. 2002;147:59–84. doi: 10.1007/s705-002-8303-1. [DOI] [PubMed] [Google Scholar]
- Mari J., Poulos B.T., Lightner D.V., Bonami J.-R. Shrimp Taura syndrome virus: genomic characterization and similarity with members of the genus Cricket paralysis-like viruses. J. Gen. Virol. 2002;, 83:915–926. doi: 10.1099/0022-1317-83-4-915. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Nakashima N. Translation initiation at the CUU codon is mediated by the internal ribosome entry site of an insect picorna-like virus in vitro. J. Virol. 1999;73:1219–1226. doi: 10.1128/jvi.73.2.1219-1226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J., Nakashima N. Methionine-independent initiation of translation in the capsid protein of an insect RNA virus. Proc. Nat. Acad. Sci. USA. 2000;97:1512–1515. doi: 10.1073/pnas.010426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate, J., Liljas, L., Scotti P., Christian P., Lin T., Johnson J.E. The crystal structure of cricket paralysis virus: the first view of a new virus family. Nature Struct. Biol. 1999;6:765–774. doi: 10.1038/11543. [DOI] [PubMed] [Google Scholar]
- Wilson J.E., Powell M.J., Hoover S.E., Sarnow P. Naturally occurring dicistronic Cricket Paralysis Virus RNA is regulated by two internal ribosome entry sites. Mol. Cell. Biol. 2000;20:4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONTRIBUTED BY, A.I. Culley, A.S. Lang, C.A. Suttle
FAMILY MARNAVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Marnaviridae |
| Genus | Marnavirus |
Since only one genus is currently recognized, the family description corresponds to the genus description.
GENUS MARNAVIRUS
Type Species Heterosigma akashiwo RNA virus
VIRION PROPERTIES
MORPHOLOGY
At present, the only characterized representative of the family Marnaviridae is Heterosigma akashiwo RNA virus (HaRNAV). Based on electron micrographs, HaRNAV virions are approximately 25 nm in diameter, polyhedral in shape, do not appear to have an envelope, and have no discernable projections (Fig. 1 ).
Figure 1.

(Left) Diagrammatic representation of the possible structure of Heterosigma akashiwo RNA virus (HaRNAV) particles. (Right) Electron micrograph of HaRNAV particles stained with phosphotungstic acid. The bar represents 50 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
HaRNAV is not sensitive to chloroform.
NUCLEIC ACID
HaRNAV has a 8.6-kb ssRNA genome containing a single ORF. The genome has a poly(A) tail at the 3′-terminus. The 5′- and 3′-UTRs are 483 and 361 nt long, respectively, accounting for a total of 9.8% of the genome. Computer predictions (mfold 3.0) of secondary structure of the 5′-UTR and a notable pyrimidine-rich stretch of sequence upstream of the predicted start codon, suggest the presence of an IRES, a feature observed in many picorna-like viruses. The genome sequence has 2 large pseudo repeats in the 5′- and 3′-UTRs. A 136 nt sequence in the 5′-UTR shares 123 exact bases with a 137 nt sequence in the 3′-end. These repeated sequences may have some function in replication or translation.
PROTEINS
The major structural proteins of HaRNAV are characterized in Table 1 . The 33 and 29 kDa protein sequence revealed similarities to the VP3 proteins from the Dicistroviridae and Picornaviridae and the VP1 proteins from the Dicstroviridae, respectively. The lack of a recognizable pattern at the protein cleavage site suggests there may be more than one protease involved in polyprotein processing.
Table 1.
Characteristics of major HaRNAV structural proteins
| Protein (kDa)a | Position of N-teminus in polyproteinb | Putative sequence at cleavage siteb |
|---|---|---|
| 39 | 1990 | PTST-SEIV |
| 33 | 2318 | FVST-SEII |
| 29 | 2060 | LFGY-SRPP |
| 26 | 1776 | EKLL-TETL |
| 24 | 1810 | RPGE-VDGD |
Based on SDS-PAGE
Based on N-terminal sequencing and genome sequence analysis
LIPIDS
Undetermined.
CARBOHYDRATES
Undetermined.
GENOME ORGANIZATION AND REPLICATION
The map of protein domains within the predicted HaRNAV polyprotein sequence is shown in Figure 2 . Domains were identified on the basis of similarities with conserved domains for picorna-like helicases, RdRps, and CPs. HaRNAV encodes an amino acid sequence that matches the chymotrypsin-related serine protease catalytic domain. A VPglike protein, characteristic of most picorna-like viruses, has not been identified.
Figure 2.

Representation of the genome organization of Heterosigma akashiwo RNA virus (HaRNAV). The location of conserved picorna-like protein domains are indicated within the polyprotein box: Hel, helicase; Pro, protease; RdRp, RNA-dependent RNA polymerase; VP3 and VP1, structural proteins. The locations of Ntermini found by sequencing the HaRNAV structural proteins are shown by black lines in the box.
ANTIGENIC PROPERTIES
Undetermined.
BIOLOGICAL PROPERTIES
The host range of HaRNAV is restricted to specific strains of Heterosigma akashiwo. Of 15 host strains isolated from the Northwest Pacific, Western Atlantic Ocean, and Japanese coastal waters, five were permissive to HaRNAV infection. HaRNAV replication appears to be cytolytic. Cytopathic effects begin approximately 48 hrs after infection. Ultrastructural changes include swelling of the endoplasmic reticulum, vacuolation and disintegration of the cytoplasm, and the appearance of fibrous material in vacuolated areas. Particles of HaRNAV are distributed in the cytoplasm in crystalline arrays or as individuals.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Heterosigma akashiwo RNA virus | |
| Heterosigma akashiwo RNA virus | (HaRNAV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
SIMILARITY WITH OTHER TAXA
There is strong evidence placing the family Marnaviridae in the “picorna-like superfamily”. The HaRNAV genome is composed of one molecule of ssRNA of positive sense that exhibits the 2C-3Cpro-3Dpol gene order and particles are icosahedral with a diameter ∼25 nm, a size and structure consistent with picorna-like viruses. However, the structure of the viral genome and the patterns of sequence relationships of HaRNAV proteins to other known viral picorna-like proteins clearly show that it does not belong in any of the currently established picorna-like families. The HaRNAV genome structure is mostly like the potyviruses (e.g., Tobacco etch virus), in that the non-structural protein domains are located at the N-terminus and the structural proteins are at the C-terminus in a single large polyprotein encoded on a monopartite genome. However, potyvirus capsids are filamentous and phylogenetic analyses demonstrated no significant homology with this family (Fig. 3 ). Moreover, a phylogenetic analysis of picorna-like RdRps does not place the HaRNAV sequence within any established family of picorna-like viruses (Fig. 3). It is not surprising that HaRNAV represents a new family as it is the first picorna-like virus that has been described that infects a protist.
Figure 3.

Phylogenetic analysis of picorna-like RdRp domain protein sequences. CLUSTAL_X alignments were done with residues 1362-1619 of the HaRNAV polyprotein that represent the conserved regions I-VIII (defined in Koonin and Dolja (1993)) and the corresponding regions from the other viruses included. The tree is based on maximum likelihood distances generated with TREE-PUZZLE. The sequence from the Carnation mottle virus (CarMV) was used as an outgroup. Support values based on 10,000 puzzling steps are shown above the branches. Bootstrap values (based on 1,000 replicates) for branches that are supported by >50% by neighbor-joining analysis are labeled below the branches (a dash indicates there was no corresponding branch in the neighbor-joining tree). The maximum likelihood scale bar is shown.
DERIVATION OF NAMES
Marna is a sigla derived from mare: Latin, “sea”, and RNA
REFERENCES
- Allison R.F., Johnson R.E., Dougherty W.G. The nucleotide sequence of the coding region of Tobacco etch virus genomic RNA: Evidence for the synthesis of a single polyprotein. Virology. 1986;154:9–20. doi: 10.1016/0042-6822(86)90425-3. [DOI] [PubMed] [Google Scholar]
- Andino R., Rieckhof G.E., Achacoso P.L., Baltimore D.A. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5’-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E.V., Dolja V.V. Evolution and taxonomy of positive-strand RNA viruses: Implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Lang A.S., Culley A.I., Suttle C.A. Nucleotide sequence and characterization of HaRNAV: a marine virus related to picorna-like viruses infecting the photosynthetic alga Heterosigma akashiwo. Virology. 2003;310:359–371. doi: 10.1016/j.virol.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Liljas L., Tate J., Lin T., Christian P., Johnson J.E. Evolutionary and taxonomic implications of conserved structural motifs between picornaviruses and insect picorna-like viruses. Arch. Virol. 2002;147:59–84. doi: 10.1007/s705-002-8303-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Salas E., Ramos R., Lafuente E., Lopex de Quinto S. Functional interactions in internal translation initiation directed by viral and cellular IRES elements. J. Gen. Virol. 2001;82:973–984. doi: 10.1099/0022-1317-82-5-973. [DOI] [PubMed] [Google Scholar]
- Mathews D.H., Sabina J., Zuker M., Turner D.H. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- Pestova T.V., Hellen C.U.T., Wimmer E. Translation of Poliovirus RNA: Role of essential cisacting oligopyrimidine element within the 5’ nontranslated region and involvement of a cellular 57-kilodalton protein. J. Virol. 1991;65:6194–6204. doi: 10.1128/jvi.65.11.6194-6204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.A., Strimmer K., Vingron M., Von Haeseler A. Tree-puzzle: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- Tai V., Lawrence J.E., Lang A.S., Chan A.M., Culley A.I., Suttle C.A. Characterization of HaRNAV, a single-stranded RNA virus causing lysis of Heterosigma akashiwo (Raphidophyceae) J. Phycol. 2003;39:343–352. [Google Scholar]
CONTRIBUTED BY, O. Le Gall, T. Iwanami, A.V. Karasev, T. Jones, K. Lehto, H. Sanfaçon, J. Wellink, T. Wetzel, N. Yoshikawa
FAMILY SEQUIVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Sequiviridae |
| Genus | Sequivirus |
| Genus | Waikavirus |
VIRION PROPERTIES
MORPHOLOGY
Particles are icosahedral, about 25-30 nm in diameter (Fig. 1 ).
Figure 1.

(Left) Putative diagram representation of the capsid structure of sequiviruses. (Right) Negative contrast electron micrograph of particles of an isolate of Parsnip yellow fleck virus, stained in 1% uranyl acetate. The bar represents 100 nm. (Courtesy I.M. Roberts).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
For a given virus, two classes of virions are distinguished according to their buoyant densities: the main virion component contains RNA and sediments at 150-190S, and some preparation also contain empty shells that sediment at about 60S.
NUCLEIC ACID
The genome is a single positive-sense ssRNA, encoding a polyprotein. Infectivity is protease-sensitive and a 5′-linked VPg molecule is probably present.
PROTEINS
Virions contain three major CP of about 32-34, 22-26 and 22-24 kDa. Virion and non-structural proteins arise by proteolytic cleavage of polyproteins.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Genetic information encoded by the RNA genome is organized as a single ORF (Fig. 2 ). The genomic organizations are essentially similar between each genus, and similar to that of other “picorna-like” viruses of plants and animals, with domains characteristic of proteins with NTP binding, proteinase and RdRp. The domain encoding the structural proteins is located upstream of this “replication block”, as in picornavirus genomes but unlike in those of other plant “picorna-like” viruses with monopartite genomes.
Figure 2.

Genome organizations characteristic of Parsnip yellow fleck virus (PYFV; Sequivirus) and Rice tungro spherical virus (RTSV; Waikavirus). The boxes represent the polyproteins. The vertical solid lines show where cleavages are known to occur in the polyproteins and the dashed lines show where cleavages are presumed to occur. The approximate positions of NTP-binding (Hel), proteinase (Pro) and polymerase (Pol) are shown. Other putative proteins include the movement protein (MP). Ovals represent the putative VPg, and An the 3′ poly-A in RTSV.
ANTIGENIC PROPERTIES
Polyclonal sera contain antibodies to all virion proteins.
BIOLOGICAL PROPERTIES
Natural host ranges are usually restricted. Transmission is in the semi-persistent mannerby aphids, or by leafhoppers. Co-infection with a “helper” waikavirus seems to be required for aphid transmission of sequiviruses. RTSV also serves as a helper virus for leafhopper transmission of Rice tungro bacilliform virus, a member of the family Caulimoviridae. The viruses are graft-transmissible. Sequiviruses, but not waikaviruses, are also mechanically transmitted in standard laboratory conditions.
GENUS SEQUIVIRUS
Type Species Parsnip yellow fleck virus
DISTINGUISHING FEATURES
The main virion component sediments around 150S, contains about 40% RNA and has a correspondingly high equilibrium density in cesium salts (1.49-1.52 g/cm3). Some preparations also contain less dense particles (about 60S) that contain no RNA. Virions of PYFV contain three major CP of about 32, 26 and 23 kDa.
The RNA is about 10 kb. PYFV RNA is not polyadenylated and has no small ORF near the 3′-end. There are about 400 aa upstream of the structural proteins in the large polyprotein. Aphid transmission of PYFV depends on simultaneous or prior access to plants infected by a helper virus, Anthriscus yellows virus (genus Waikavirus).
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains one major ORF that encodes a polyprotein of about 3,000 to 3,500 aa. The structural proteins are in the N-terminal half of the polyprotein but are separated from the N-terminus by polypeptide(s) of about 40-60 kDa. Sequences downstream of the structural proteins contain domains characteristic of proteins with NTP binding, protease and RdRp activities.
BIOLOGICAL PROPERTIES
The natural host range includes several species in several families. Transmission is in the semi-persistent manner by aphids. However, it is dependent on the presence of a helper virus in the genus Waikavirus.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of the species Parsnip yellow fleck virus and the species Dandelion yellow mosaic virus are distinguished because :
-
•
they do not cross-react with heterologous antibodies; PYFV can be divided into two serotypes (formerly regarded as separate species) that differ by serological differentiation index of 4 to 5,
-
•
their principal hosts belong to different families; PYFV infects umbelliferous plants (although the serotypes differ in host range) and Dandelion yellow mosaqic virus infects plants in the Compositae,
-
•
they are transmitted by different species of vector aphid; PYFV is transmitted by Cavariella spp. and DaYMV is transmitted by Aulacorthum solani and some Myzus spp.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Dandelion yellow mosaic virus | ||
| Dandelion yellow mosaic virus | (DaYMV) | |
| Parsnip yellow fleck virus | ||
| Celery yellow net virus | (CeYNV) | |
| Parsnip yellow fleck virus | [NC_003628] | (PYFV) |
TENTATIVE SPECIES IN THE GENUS
| Lettuce mottle virus | (LeMoV) |
GENUS WAIKAVIRUS
Type Species Rice tungro spherical virus
DISTINGUISHING FEATURES
The main virion component sediments at 180-190S, contains about 40% RNA and has a correspondingly high equilibrium density in cesium salts (1.5 g/cm3). Virions contain three major CPs of about 33-34, 22-24 and 22-25 kDa. Particles of some waikaviruses are thought to contain other proteins that may be derived from one of the three major proteins.
The RNA is about 12 kb and has a poly(A) tail. Genomes of Rice tungro spherical virus (RTSV) and Maize chlorotic dwarf virus (MCDV) RNA contain a small ORF near the 3′-end and have about 600 to 700 aa upstream of the structural proteins in the large polyproteins. Transmission by aphids or leafhoppers is thought to depend on a self-encoded helper protein. The helper protein of some species can assist insect transmission of other viruses, e.g. sequiviruses, when they are present in co-infection.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains one major ORF that encodes a polyprotein. A smaller ORF is present downstream of the region encoding the polyprotein. The structural proteins are in the N-terminal half of the polyprotein but are separated from the N-terminus by polypeptide(s) of about 40-60 kDa. Sequences downstream of the structural proteins contain domains characteristic of proteins with NTP-binding, protease and RdRp activities.
BIOLOGICAL PROPERTIES
Natural host ranges are restricted to few species within few families. Waikaviruses are not sap-transmitted. Field transmission is in the semi-persistent manner by aphids or leafhoppers. A virus-encoded helper protein is probably needed. Some waikaviruses are helper viruses for the insect transmission of other viruses: Parsnip yellow fleck virus (Sequivirus) in the case of Anthriscus yellows virus and Rice tungro bacilliform virus (Caulimoviridae) in the case of Rice tungro spherical virus (this association being responsible for the very damaging rice tungro disease).
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Isolates belong to distinct species if:
-
•
gene products differ in aa sequence; from limited comparisons values of <70% homology over the entire polyprotein and <80% between NTP-binding domains or proteinase domains or polymerase domains would suggest distinct species (the extent is not possible to define with certainty as too few sequences are at hand),
-
•
they differ serologically; at most, there is a very weak cross-reaction between RTSV and MCDV in immunoblots,
-
•
they differ in host range; RTSV infects rice and some other graminaceous hosts, MCDV infects maize and some other graminaceous hosts, but not rice and AYV infects umbelliferous (dicotyledonous) hosts,
-
•
they are transmitted by different vector species; MCDV and RTSV are transmitted by leafhoppers (Graminella spp. and Nephotettix spp. respectively) and AYV is transmitted by aphids.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Figure 3.

Unrooted dendrogram derived from the alignment of the sequences of the proteinase-polymerase domains of virus species belonging to the families Comoviridae and Sequiviridae, and the unassigned genera Cheravirus and Sadwavirus. Branches supported by bootstrap values lower than 75% were merged. The horizontal scale is proportional to the level of divergence while the vertical scale is arbitrary.
SIMILARITY WITH OTHER TAXA
The amino acid sequences in the conserved NTP-binding and RNA polymerase domains of the polyproteins resemble those in the polyproteins encoded by RNA of viruses in the unassigned genera Cheravirus and Sadwavirus, and in the families Comoviridae, Picornaviridae and Dicistroviridae. The number and sizes of the CPs resemble those of viruses in the family Picornaviridae although the protein(s) upstream of the CPs is larger than the L protein of aphthoviruses. The properties of the particles and the genomes of these viruses have sometimes prompted their description as ‘plant picornaviruses’.
DERIVATION OF NAMES
Sequi: sigla from latin sequi, to follow, accompany, attend (in reference to the dependent aphid transmission of PYFV).
Waika: from Japanese, describing the symptoms induced in rice by infection with RTSV alone (i.e. in the absence of Rice tungro bacilliform virus, RTBV).
REFERENCES
- Bos L., Huijberts N., Huttinga H., Maat D.Z. Further characterization of dandelion yellow mosaic virus from lettuce and dandelion. Neth. J. Pl. Path. 1983;89:207–222. [Google Scholar]
- Elnagar S., Murant A.F. The role of the helper virus, anthriscus yellows, in the transmission of parsnip yellow fleck virus by the aphid Cavariella aegopodii. Ann. Appl. Biol. 1976;84:169–181. [Google Scholar]
- Ge X., Gordon D.T., Gingery R.E. Occurrence of a small RNA in maize chlorotic dwarf virus-like particles. Phytopathology. 1989;79:1195. [Google Scholar]
- Gingery R.E. Maize chlorotic dwarf and related viruses. In: Koenig R., editor. Vol. 3. Plenum Press; New York: 1988. pp. 259–272. (The plant viruses; polyhedral virions with monopartite RNA). [Google Scholar]
- Hemida S.K., Murant A.F., Duncan G.H. Purification and some particle properties of anthriscus yellows virus, a phloem-limited, semi-persistent, aphid-borne virus. Ann. Appl. Biol. 1989;114:71–86. [Google Scholar]
- Hunt R.E., Nault L.R., Gingery R.E. Evidence for infectivity of maize chlorotic dwarf virus and for a helper component in its leafhopper transmission. Phytopathology. 1988;78:499–504. [Google Scholar]
- Murant A.F. Parsnip yellow fleck virus, type member of a proposed new plant virus group, and a possible second member, dandelion yellow mosaic virus. In: Koenig R., editor. Vol. 3. Plenum Press; New York: 1988. pp. 273–288. (The plant viruses; polyhedral virions wit h monopartite RNA). [Google Scholar]
- Reddick B.B., Habera L.F., Law M.D. Nucleotide sequence and taxonomy of maize chlorotic dwarf virus within the Sequiviridae. J. Gen. Virol. 1997;78:1165–1174. doi: 10.1099/0022-1317-78-5-1165. [DOI] [PubMed] [Google Scholar]
- Shen P., Kaniewska M.B., Smith C., Beachy R.N. Nucleotide sequence and genomic organization of rice tungro spherical virus. Virology. 1993;193:621–630. doi: 10.1006/viro.1993.1170. [DOI] [PubMed] [Google Scholar]
- Thole V., Hull R. Characterization of a protein from rice tungro spherical virus with serine proteinase-like activity. J. Gen. Virol. 2002;83:3179–3186. doi: 10.1099/0022-1317-83-12-3179. [DOI] [PubMed] [Google Scholar]
- Turnbull-Ross A.D., Mayo M.A., Reavy B., Murant A.F. Sequence analysis of the parsnip yellow fleck virus polyprotein: evidence of affinities with picornaviruses. J. Gen. Virol. 1993;74:555–561. doi: 10.1099/0022-1317-74-4-555. [DOI] [PubMed] [Google Scholar]
- Zhang S., Jones M.C., Barker P., Davies J.W., Hull R. Molecular cloning and sequencing of coat protein-encoding cDNA of rice tungro spherical virus – a plant picornavirus. Virus Genes. 1993;7:121–132. doi: 10.1007/BF01702392. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, O. Le Gall, T. Iwanami, A.V. Karasev, T. Jones, K. Lehto, H. Sanfaçon, J. Wellink, T. Wetzel, N. Yoshikawa
GENUS SADWAVIRUS
Type Species Satsuma dwarf virus
VIRION PROPERTIES
MORPHOLOGY
Particles are icosahedral, about 25-30 nm in diameter (Fig. 1 ).
Figure 1.

Negative contrast electron micrograph of particles of isolates of Satsuma dwarf virus. The bar represents 100 nm. (Photograph T. Iwanami).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Three types of virions are found, differing in their buoyant densities. Top (T) particles, found in small amounts in purified virus preparations, are empty and 50-60S. Middle (M) and bottom (B) particles contain genomic RNA. Their buoyant densities in CsCl are about 1.43 and 1.46 g/cm3 respectively. Strawberry latent ringspot virus (SLRSV) has only B particles that contain either one RNA with Mr of ∼ 2.6 × 106 or two RNAs of 1.6 × 106, while Satsuma dwarf virus (SDV) has M and B particles that contain either of the different RNA molecules.
NUCLEIC ACID
The genome consists of two species of linear positive-sense ssRNA. Both RNAs are polyadenylated at their 3′-end and encode a single polyprotein that is processed to yield the mature proteins. Both RNAs are necessary for systemic infection. RNA-1 is about 7,000 nt in length, and RNA-2 is 4,600-5,400 nt long. RNA-2 of SLRSV, with 3,800 nt, is somewhat shorter. SLRSV RNAs have a VPg attached at the 5′-end of their genome. Some isolates of Strawberry latent ringspot virus are associated with a large ss satellite RNA that encodes a protein.
PROTEINS
Sadwaviruses have two CP subunits (Large subunit: 40-45 kDa and Small subunit: 21-29 kDa). Virion and non-structural proteins arise by proteolytic cleavage of polyproteins.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Genetic information encoded by the RNA genome is organized as a single ORF for each RNA molecule (Fig. 2 ). The general genetic organization is similar to that of other “picorna-like” viruses of plants and animals, with domains characteristic of proteins with NTP-binding, proteinase and RdRp. As in the family Comoviridae, the polyprotein encoded by RNA-1 contains the domains for proteins likely to be involved in the replication of the virus genome, while RNA-2 encodes the CPs and the putative MP. Extensive sequence identity between RNA-1 and RNA-2 are found in the 5′ and 3′ UTR as well as in the 5′-end of the putative coding region.
Figure 2.

Genome organization characteristic of Satsuma dwarf virus (SDV). The boxes represent the polyproteins. The vertical solid lines show where cleavages are known to occur in the polyproteins and the dashed lines show where cleavages are presumed to occur. The approximate positions of NTP-binding (Hel), proteinase (Pro) and polymerase (Pol) are shown. Other putative proteins include the MP. Arrows show the portions of the RNAs that are similar in both genomic RNAs. Ovals represent the putative VPg, and An the 3′ poly-A.
ANTIGENIC PROPERTIES
Purified virions are moderate to good immunogens.
BIOLOGICAL PROPERTIES
SLRSV is transmitted by nematodes of several species within the genus Xiphinema, while SDV has no known vector and Strawberry mottle virus (SMoV) is transmitted in a semipersistent manner by Chaetosiphon spp. and other aphids. In several hosts, SLRSV is transmitted through seed at a relatively high rate.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•
Type of biological vector
-
•
Host range
-
•
Absence of serological cross-reaction
-
•
Absence of cross-protection
-
•Sequence similarity:
-
○less than 75% aa sequence identity in the Large CP within a species.
-
○less than 75% aa sequence identity in the proteinase-polymerase region.
-
○
Citrus mosaic virus (CiMV), Natsudaidai dwarf virus (NDV) and Navel orange infectious mottling virus (NIMV) are considered to be distantly related strains of Satsuma dwarf virus because:
-
•
Their host ranges are similar to that of “type” SDV.
-
•
They are serologically related although they form three different serogroups.
-
•
SDV protects Satsuma orange against infection (except in the case of NDV).
-
•
The aa sequence identities in the Large CP sequence is 81-85% between groups.
-
•
The aa sequence identity between NIMV and SDV is 82% in the proteinase-polymerase region.
Natural isolates of SMoV have more than 90% sequence identity with each other in the large CP aa sequence and more than 94% in the RdRp region.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| RNA-1 Accession# | RNA-2 Accession # | ||
| Satsuma dwarf virus | |||
| Citrus mosaic virus | [AB032751 (part.)] | (CiMV) | |
| Natsudaidai dwarf virus | [AB032750] | (NDV) | |
| Navel orange infectious mottling virus | [AB022887 (part.)] | [AB000282 (part.)] | (NIMV) |
| Satsuma dwarf virus | [NC_003785] | [NC_003786] | (SDV) |
| Strawberry latent ringspot virus | |||
| Rhubarb virus 5 | (RhuV5) | ||
| Strawberry latent ringspot virus | [X77466] | (SLRSV) | |
| Strawberry mottle virus | |||
| Strawberry mottle virus | [NC_003445] | [NC_003446] | (SMoV) |
TENTATIVE SPECIES IN THE GENUS
| Lucerne Australian symptomless virus | (LASV) |
| Rubus Chinese seed-borne virus | (RCSV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Not available.
SIMILARITY WITH OTHER TAXA
The aa sequences in the conserved NTP-binding and RNA polymerase domains of the polyproteins resemble those in the polyproteins encoded by RNA of viruses in the unassigned genera Cheravirus, in the families Sequiviridae, Comoviridae, Picornaviridae and Dicistroviridae. Like fabaviruses and comoviruses (family Comoviridae), sadwaviruses have 2 CPs of significantly different sizes. Sadwaviruses were previously considered as atypical but tentative members of the genus Nepovirus (family Comoviridae), but were distinguished on the basis of their genomic organization, in particular the number of CP species, as well as sequence homologies and, for some of them, natural transmission by insects.
DERIVATION OF NAMES
Sadwa: from Satsuma dwarf virus, the type member.
REFERENCES
- Barbara D.J., Ashby S.C., McNamara D.G. Host range, purification and some properties of Rubus Chinese seed-borne virus. Ann. Appl. Biol. 1985;107:45–55. [Google Scholar]
- Bellardi M.G., Bertaccini A. Parsley seeds infected by strawberry latent ringspot virus (SLRV) Phytopath. Medit. 1991;30:198–199. [Google Scholar]
- Everett K.R., Milne K.S., Forster R.L. Nucleotide sequence of the coat protein genes of strawberry latent ringspot virus: lack of homology to the nepoviruses and comoviruses. J. Gen. Virol. 1994;75:1821–1825. doi: 10.1099/0022-1317-75-7-1821. [DOI] [PubMed] [Google Scholar]
- Hellen C.U.T., Yuanyi L., Cooper J.I. Synthesis and proteolytic processing of arabis mosaic nepovirus, cherry leaf roll nepovirus, and strawberry latent ringspot nepovirus proteins in reticulocyte lysate. Arch. Virol. 1991;120:19–31. doi: 10.1007/BF01310946. [DOI] [PubMed] [Google Scholar]
- Iwanami T., Kondo Y., Karasev A.V. Nucleotide sequences and taxonomy of satsuma dwarf virus. J. Gen. Virol. 1999;80:793–797. doi: 10.1099/0022-1317-80-3-793. [DOI] [PubMed] [Google Scholar]
- Iwanami T., Kondo Y., Kobayashi M., Han S.S., Karasev A.V. Sequence diversity and interrelationships among isolates of satsuma dwarf-related viruses. Arch. Virol. 2001;146:807–813. doi: 10.1007/s007050170149. [DOI] [PubMed] [Google Scholar]
- Karasev A.V., Han S.S., Iwanami T. Satsuma dwarf and related viruses belong to a new lineage of plant picorna-like viruses. Virus Genes. 2001;23:45–52. doi: 10.1023/a:1011131328951. [DOI] [PubMed] [Google Scholar]
- Kreiah S., Strunk G., Cooper J.I. Sequence analysis and location of the capsid proteins within RNA 2 of strawberry latent ringspot virus. J. Gen. Virol. 1994;75:2527–2532. doi: 10.1099/0022-1317-75-9-2527. [DOI] [PubMed] [Google Scholar]
- Mayo M.A., Barker H., Harrison B.D. Polyadenylate in the RNA of five nepoviruses. J. Gen. Virol. 1979;43:603–610. [Google Scholar]
- Mayo M.A., Barker H., Harrison B.D. Specificity and properties of the genome-linked proteins of nepoviruses. J. Gen. Virol. 1982;59:149–162. [Google Scholar]
- Mayo M.A., Barker H., Robinson D.J. Satellite RNA in particles of strawberry latent ringspot virus. J. Gen. Virol. 1982;63:417–423. [Google Scholar]
- Mayo M.A., Murant A.F., Harrison B.D., Goold R.A. Two protein and two RNA species in particles of strawberry latent ringspot virus. J. Gen. Virol. 1974;24:29–37. doi: 10.1099/0022-1317-24-1-29. [DOI] [PubMed] [Google Scholar]
- Remah A., Jones A.T., Mitchell M.J. Purification and properties of lucerne Australian symptomless virus, a new virus infecting lucerne in Australia. Ann. Appl. Biol. 1986;109:307–315. [Google Scholar]
- Roberts I.M., Harrison B.D. Inclusion bodies and tubular structures in Chenopodium amaranticolor plants infected with strawberry latent ringspot virus. J. Gen. Virol. 1970;7:47–54. doi: 10.1099/0022-1317-7-1-47. [DOI] [PubMed] [Google Scholar]
- Thompson J.R., Leone G., Lindner J.L., Jelkmann W., Schoen C.D. Characterization and complete nucleotide sequence of Strawberry mottle virus: a tentative member of a new family of bipartite plant picorna-like viruses. J. Gen. Virol. 2002;83:229–239. doi: 10.1099/0022-1317-83-1-229. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, O. Le Gall, T. Iwanami, A.V. Karasev, T. Jones, K. Lehto, H. Sanfaçon, J. Wellink, T. Wetzel, N. Yoshikawa
GENUS CHERAVIRUS
Type Species Cherry rasp leaf virus
VIRION PROPERTIES
MORPHOLOGY
Particles are icosahedral, about 25-30 nm in diameter (Fig. 1 ).
Figure 1.

Negative contrast electron micrograph of particles of an isolate of Cherry rasp leaf virus, stained in 2% ammonium molybdate, pH 5. The bar represents 100 nm. (Photograph A.T. Jones).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Three types of virions are found in purified Cherry rasp leaf virus (CRLV) preparations that differ in their buoyant densities. Top (T) particles, found in small amounts in purified virus preparations, are empty and sediment at 56S. Middle (M) and bottom (B) particles contain genomic RNA and sediment at 96S and 120S respectively for CRLV. Apple latent spherical virus (ALSV) has two components (M and B) with densities in CsCl of 1.41 and 1.45 g/cm3, respectively. M and B particles probably contain two molecules of RNA-2 and a single molecule of RNA-1, respectively.
NUCLEIC ACID
The genome consists of two species of linear positive-sense ssRNA. RNA-1 is about 7,000 nt in length, and RNA-2 is about 3,300 nt long. Both RNAs are polyadenylated at their 3′-end and encode a single polyprotein that is processed to yield the mature proteins (Fig. 2 ). Proteinase treatment abolishes the infectivity of purified RNA, which suggests the presence of a VPg.
Figure 2.

Genome organization characteristic of Apple latent spherical virus (ALSV). The boxes represent the polyproteins. The vertical solid lines show where cleavages are known to occur in the polyproteins and the dashed lines show where cleavages are presumed to occur. The approximate positions of NTP-binding (Hel), proteinase (Pro) and polymerase (Pol) are shown. Other putative proteins include the movement protein (MP). Ovals represent the putative VPg, and An the 3′ poly-A.
PROTEINS
Cheraviruses have three CP subunits of similar sizes (24, 22 and 20 kDa for CRLV, and 25, 24 and 20 kDa for ALSV). In some cases, these proteins are not fully or reproducibly resolved from each other by electrophoresis. Virion and non-structural proteins arise by proteolytic cleavage of polyproteins.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Genetic information encoded by the RNA genome is organized as a single ORF for each RNA molecule (Fig. 2). The general genetic organization is similar to that of other picorna-like viruses of plants and animals, with domains characteristic of proteins with NTP-binding, proteinase and RdRp. As in the family Comoviridae, the polyprotein encoded by RNA-1 contains the domains for proteins likely to be involved in the replication of the virus genome, while RNA-2 encodes the CPs and the putative movement protein.
ANTIGENIC PROPERTIES
Purified virions are moderate immunogens.
BIOLOGICAL PROPERTIES
The host range is broad or narrow, depending on viruses, and includes weed plants found in the vicinity of infected crops. Symptoms are usually mild or absent. CRLV is transmitted by nematodes (Xiphinema americanum) in the field, and is readily seed-transmitted. No information is available for other cheraviruses.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•
Type of biological vector
-
•
Host range
-
•
Absence of serological cross-reaction
-
•
Absence of cross-protection
-
•Sequence similarity:
-
○less than 75% aa sequence identity in the CPs within a species.
-
○less than 75% aa sequence identity in the proteinase-polymerase region.
-
○
Members of CRLV and ALSV were distinguished because:
-
•
The host ranges differ, although some hosts including apple are common.
-
•
Purified ALSV particles did not react with polyclonal antibodies against CRLV and AVBV in either gel diffusion tests or immunoblot analysis.
-
•
The aa sequence identities between three CPs, Vp25, Vp20, and Vp24, are 54%, 59%, and 65%, respectively.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
| Arracacha virus B | (AVB) |
| Artichoke vein banding virus | (AVBV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Not available.
SIMILARITY WITH OTHER TAXA
The aa sequences in the conserved NTP-binding and RdRp domains of the polyproteins resemble those in the polyproteins encoded by RNA of viruses in the unassigned genus Sadwavirus, in the families Sequiviridae, Comoviridae, Picornaviridae and Dicistroviridae. The number and sizes of the CPs resemble those of viruses in the families Picornaviridae and Sequiviridae.
Like sadwaviruses, cheraviruses were previously considered as atypical but tentative members of the genus Nepovirus (family Comoviridae), but were distinguished on the basis of their genomic organization, in particular the number of CP species, as well as sequence homologies and, for some of them, natural transmission by insects.
DERIVATION OF NAMES
Chera: from Cherry rasp leaf virus, the type member. CRLV was preferred as a type member to ALSV despite the lack of a complete sequence because more biological data were available.
REFERENCES
- Brown D.J.F., Halbrendt J.M., Jones A.T., Vrain T.C., Robbins R.T. Transmission of three North American nepoviruses by populations of four distinct species of the Xiphinema americanum group. Phytopathology. 1994;84:646–649. [Google Scholar]
- Hansen A.J., Nyland G., McElroy F.D., Stace-Smith R. Origin, cause, host range and spread of cherry rasp leaf disease in North America. Phytopathology. 1974;64:721–727. [Google Scholar]
- James D., Upton C. Nucleotide sequence analysis of RNA-2 of a flat apple isolate of Cherry rasp leaf virus with regions showing greater identity to animal picornaviruses than to related plant viruses. Arch. Virol. 2002;147:1631–1641. doi: 10.1007/s00705-002-0833-3. [DOI] [PubMed] [Google Scholar]
- Jones A.T., Mayo M.A., Henderson S.J. Biological and biochemical properties of an isolate of cherry rasp leaf virus from red raspberry. Ann. Appl. Biol. 1985;106:101–110. [Google Scholar]
- Li C., Yoshikawa N., Takahashi T., Ito T., Yoshida K., Koganezawa H. Nucleotide sequence and genome organization of apple latent spherical virus: a new virus classified into the family Comoviridae. J. Gen. Virol. 2000;81:541–547. doi: 10.1099/0022-1317-81-2-541. [DOI] [PubMed] [Google Scholar]
- Parish C.L. A relationship between flat apple disease and cherry rasp leaf disease. Phytopathology. 1977;67:982–984. [Google Scholar]
- Stace-Smith, R. and Hansen, A.J. (1976). Cherry rasp leaf virus. CMI/AAB Description of Plant Viruses, n°159.
CONTRIBUTED BY, O. Le Gall, T. Iwanami, A.V. Karasev, T. Jones, K. Lehto, H. Sanfaçon, J. Wellink, T. Wetzel, N. Yoshikawa
FAMILY COMOVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Comoviridae |
| Genus | Comovirus |
| Genus | Fabavirus |
| Genus | Nepovirus |
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

(Top left) Molecular rendering of the Cowpea mosaic virus (CPMV) particle (Lin et al., 1999, with permission). (Top central) Diagrammatic representation of a T=1 lattice. A= Small capsid protein, B= C-terminal & C= N-terminal domains of the Large capsid protein. (Top right) Molecular rendering of the Red clover mosaic virus (RCMV) particle (Lin et al., 2000, with permission). (Center) Diagram of the three types of comovirus particles. (Bottom) Negative contrast electron micrograph of particles of CPMV. The bar represents 100 nm.
Virions are non-enveloped 28-30 nm in diameter and exhibit icosahedral symmetry (T=1, pseudo T=3). They contain two types of positive sense ssRNA molecules, RNA-1 and RNA-2. Virus preparations contain three types of components differing in their buoyant properties: T (“Top”, empty particles), M (“Middle”, particles usually containing a single molecule of RNA-2) and B (“Bottom”, particles containing a single molecule of RNA-1 or, in some nepoviruses, two molecules of RNA-2).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions are heat-stable (thermal inactivation is usually above 60C), and most are insensitive to organic solvents. Particles sediment as three components, T, M and B, with S20w values of 49-63S, 84-128S and 111-134S, respectively, (values vary within each genus). Mr of particles are 3.2-3.8 × 106 (T), 4.6-5.8 × 106 (M) and 6.0-6.2 × 106 (B). Buoyant densities in CsCl are 1.28-1.30 (T), 1.41-1.48 (M) and 1.44-1.53 (B) g/cm3 (density values refer only to comoviruses and nepoviruses).
NUCLEIC ACID
The genome consists of two species of linear positive-sense ssRNA. Both RNAs are necessary for systemic infection. Sizes of RNAs differ among genera; nepovirus RNA-1 (7.2-8.4 kb) and RNA-2 (3.9-7.2 kb) are larger than fabavirus and comovirus RNA-1 (5.9-7.2 kb) and RNA-2 (3.5-4.5 kb). For the genera Comovirus and Nepovirus the genomic RNAs contain a 3′-terminal poly(A) tract of variable length, and a polypeptide, designated VPg (2 – 4 kDa), covalently bound at the 5′-end (this has not been confirmed for fabaviruses).
Table 1.
Sizes of the genomes of viruses in the family Comoviridae, in nucleotides.
| Genus (virus) | RNA-1 | RNA-2 |
|---|---|---|
| Comovirus | 5,900 – 7,200 | 3,300 – 3,800 |
| Cowpea mosaic virus (CPMV) | (5,889) | (3,810) |
| Fabavirus | 5,900 – 6,300 | 3,100 – 4,500 |
| Broad bean wilt virus 2 (BBWV2) | (5,951) | (3,607) |
| Nepovirus | 7,200 – 8,400 | 3,700 – 7,300 |
| Grapevine fanleaf virus (GFLV) | (7,342) | (3,774) |
| Beet ringspot virus (BRSV) | (7,356) | (4,662) |
| Tomato ringspot virus (ToRSV) | (8,214) | (7,273) |
PROTEINS
Comoviruses and fabaviruses have two CPs of 40-45 kDa and 21-27 kDa; nepoviruses normally have a single CP of 52-60 kDa. Virions have 60 copies of each CP per particle. For three comoviruses (CPMV, BPMV and RCMV) and one nepovirus (TRSV) the atomic structure has been solved and found to be very similar (pseudo T=3) to that of viruses belonging to the family Picornaviridae. Each capsid subunit is made of three beta-barrels that are present in two CPs (Comovirus) or a single CP (Nepovirus).
LIPIDS
None reported.
CARBOHYDRATES
None reported. No carbohydrate in comoviruses (a report that the CPs contain about 1.9% carbohydrates covalently linked has recently been shown to be mistaken).
Figure 2.

Capsid architecture of a picornavirus (top; 3 beta-barrels in 3 CPs), a comovirus (middle; 3 beta-barrels in 2 CPs) and a nepovirus (bottom; 3 beta-barrels in 1 CP).
GENOME ORGANIZATION AND REPLICATION
Unfractionated RNA is highly infective but neither RNA species alone can infect plants systemically. Cytoplasm of infected cells contains conspicuous inclusions consisting primarily of membranous elements and electron-dense material that are sites of virus genome replication. The following information only refers to como- and nepoviruses (fabaviruses have not been studied): RNA-1 can replicate in protoplasts but in the absence of RNA-2 (encoding the CPs) no virus particles are produced. RNA-1 carries all the information for RNA replication, including the polymerase. Both RNA species are translated into polyproteins that are cleaved by a viral proteinase (encoded by RNA-1) to give several intermediate and final processing products. Virions assemble and accumulate in the cytoplasm, often in crystalline or paracrystalline arrays. They are also found within infection-specific tubules, which contain the viral MP and cross cell walls, and which have been implicated in cell-to-cell transport.
ANTIGENIC PROPERTIES
Virus preparations are good immunogens. Species belonging to the same genus are serologically interrelated (especially comoviruses), but often distantly.
BIOLOGICAL PROPERTIES
Comoviruses have narrow host ranges; nepoviruses and fabaviruses have wide host ranges. Symptoms vary widely within each genus. Viruses in the family Comoviridae all have biological vectors, comoviruses are transmitted by beetles (especially members of the family Chrysomelidae), fabaviruses are transmitted by aphids and some nepoviruses are transmitted by nematodes. All are readily transmissible experimentally by mechanical inoculation. Seed and/or pollen transmission is very common among nepoviruses, but is rare for comoviruses and fabaviruses.
GENUS COMOVIRUS
Type Species Cowpea mosaic virus
DISTINGUISHING FEATURES
The comovirus genome is made of two genomic ssRNAs with a 5′-bound polypeptide (VPg) and a 3′ poly-A (see section on Comoviridae). The comovirus capsid is made of two types of polypeptides (Large subunit: 40-45 kDa and Small subunit: 21-27 kDa).
RNA-2 is translated into two largely overlapping polyproteins that are processed into three domains. P2A is involved in RNA-2 replication. P2B is the MP, with a typical “LPL” motif. The CP domains are encoded at the C-terminus of the polyprotein. RNA-1 is translated into a single polyprotein that is processed into five domains. P1A limits the processing of the RNA-1-encoded polyprotein in cis and assists the processing of the RNA-2-encoded polyprotein. P1B has sequence motifs characteristic of an NTP-binding helicase, P1C is the VPg, P1D is the proteinase and P1E has sequence motifs characteristic of an RdRp. P1A and P1B are involved in inducing the cytopathic structure. The 5′ and 3′ NTRs of RNA-1 and RNA-2 are homologous.
Figure 3.
Genome organization characteristic of Cowpea mosaic virus (CPMV). The ORFs are boxed, and functions of the proteins are indicated. MP, movement protein; CPL and CPS, large and small capsid proteins; Hel, helicase; Pro, proteinase; Pol, polymerase. Proteolytic cleavage sites are indicated on the polyproteins. All intermediate and final cleavage products have been detected in infected cells. The ovals at the 5′-end of the RNAs represents the VPg, and An at the 3′-end the poly-A.
BIOLOGICAL PROPERTIES
Comoviruses have narrow host ranges, 11 of the 15 species being restricted to a few species of the family Leguminosae. Mosaic and mottle symptoms are characteristic, but usually not ringspots. Transmission in nature is exclusively by beetles, especially members of the family Chrysomelidae. Beetles retain their ability to transmit virus for days or weeks.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Large CP aa sequence less than 75% homologous,
-
•
Polymerase aa sequence less than 75% homologous,
-
•
No pseudo-recombination between components possible,
-
•
Differences in antigenic reactions.
Using polyclonal rabbit antisera in agar gel diffusion tests, isolates of Cowpea mosaic virus and Cowpea severe mosaic virus differ by four two-fold steps. Their CPs have less than 54% identity and 61% homology between the aligned respective nt and aa sequences. Their Pol proteins have less than 55% identity and 62% homology between the aligned respective nt and aa sequences. No component reassortment has been demonstrated between these viruses.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| RNA-1 Acc. # | RNA-2 Acc. # | ||
| Andean potato mottle virus | |||
| Andean potato mottle virus | [L19239] | (APMoV) | |
| Bean pod mottle virus | |||
| Bean pod mottle virus – KentuckyG7 | [NC_003496] | [NC_003495] | (BPMV-KenG7) |
| Bean pod mottle virus – K-Hopkins1 | [AF394608] | [AF394609] | (BPMV-KHop) |
| Bean pod mottle virus – K-Hancock1 | [AF394606] | [AF394607] | (BPMV-KHan) |
| Bean rugose mosaic virus | |||
| Bean rugose mosaic virus | [AF263548] | (BRMV) | |
| Broad bean stain virus | |||
| Broad bean stain virus | (BBSV) | ||
| Broad bean true mosaic virus | |||
| Broad bean true mosaic virus | (BBTMV) | ||
| Echtes Ackerbohnemosaik virus | (EABV) | ||
| Vicia virus 1 | (VV1) | ||
| Cowpea mosaic virus | |||
| Cowpea mosaic virus | [NC_003549] | [NC_003550] | (CPMV) |
| Cowpea yellow mosaic virus | (CPYMV) | ||
| Cowpea severe mosaic virus | |||
| Arkansas cowpea mosaic virus | (CPSMV-Ark) | ||
| Cowpea mosaic virus – severe | (CPSMV-Svr) | ||
| Cowpea severe mosaic virus – DG | [NC_003545] | [NC_003544] | (CPSMV-DG) |
| Trinidad cowpea mosaic virus | (CPSMV-Tri) | ||
| Glycine mosaic virus | |||
| Glycine mosaic virus | (GMV) | ||
| Pea green mottle virus | |||
| Pea green mottle virus | (PGMV) | ||
| Pea mild mosaic virus | |||
| Pea mild mosaic virus | (PMiMV) | ||
| Quail pea mosaic virus | |||
| Bean curly dwarf mosaic virus | (BCDMV) | ||
| Quail pea mosaic virus | (QPMV) | ||
| Radish mosaic virus | |||
| Radish enation mosaic virus | (RaEMV) | ||
| Radish mosaic virus | (RaMV) | ||
| Red clover mottle virus | |||
| Red clover mottle virus – S | [NC_003741] | [NC_003738] | (RCMV-S) |
| Squash mosaic virus | |||
| Cucurbit ring mosaic virus | (CuRMV) | ||
| Muskmelon mosaic virus | (MuMV) | ||
| Squash mosaic virus – Arizona | [AF059533] | (SqMV-Ari) | |
| Squash mosaic virus – Kimble | [AF059532] | (SqMV-Kim) | |
| Squash mosaic virus – Y | [NC_003799] | [NC_003800] | (SqMV-Y) |
| Ullucus virus C | |||
| Ullucus virus C | (UVC) | ||
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS FABAVIRUS
Type Species Broad bean wilt virus 1
DISTINGUISHING FEATURES
Capsids contain two polypeptide species (Large and Small). road bean wilt virus 2 (BBWV-2) RNA-2 is translated into a polyprotein that is cleaved into the two CPs and a 52 kDa protein that is possibly involved in movement (Fig. 4 ). Fabaviruses have wide host ranges among dicotyledonous plants and some families of monocotyledonous plants. Symptoms are ringspots, mottling, mosaic, distortion, wilting and apical necrosis. In nature, fabaviruses are transmitted by aphids in a non-persistent manner. In other respects, fabaviruses are similar to comoviruses.
Figure 4.

Genome organization characteristic of Broad bean wilt virus 2 (BBWV-2). The ORFs are boxed, and the putative functions of the proteins are indicated. MP, movement protein; CPL and CPS, large and small capsid proteins; Hel, helicase; Pro, proteinase; Pol, polymerase. Proteolytic cleavage sites are indicated on the polyproteins. The ovals at the 5’-end of the RNAs represents the putative VPg, question marks some uncertainty regarding the presence of a cleavage site in the polyprotein, and An at the 3’-end the poly-A.
RNA-2 is translated into a polyprotein that is processed into three domains. The occurrence of two overlapping reading frames as in comoviruses is not known. P2B is the MP, with a typical “LPL” motif. The CP domains are encoded at the C-terminus of the polyprotein. RNA-1 is translated into a single polyprotein. A cleavage site between putative P1A and P1B domains has not been investigated. P1B has sequence motifs characteristic of a NTP-binding helicase, P1C is the (putative) VPg, P1D is the proteinase and P1E has sequence motifs characteristic of an RdRp. The 5’ and 3’ non-translated regions (NTRs) are homologous between RNA-1 and RNA-2.
BIOLOGICAL PROPERTIES
Fabaviruses have wide host ranges among dicotyledonous plants and some families of monocotyledonous plants. Symptoms are ringspots, mottling, mosaic, distortion, wilting and apical necrosis. In nature, fabaviruses are transmitted by aphids in a non-persistent manner.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
No pseudo-recombination between components possible,
-
•
Differences in antigenic reactions.
-
•
Sequence identity lower than 75% in the CP and polymerase coding regions.
Using polyclonal rabbit antisera in agar gel diffusion tests Broad bean wilt virus 1 (BBWV-1) and Lamium mild mosaic virus (LMMV) differ by eight twofold steps. No reassortment has been demonstrated between these viruses.
On the other hand, Patchouli mild mosaic virus is now believed to be a strain of BBWV-2 because these two viruses differ only by 3-21% in sequence (21% in the Large CP).
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| RNA-1 Acc. # | RNA-2 Acc. # | ||
| Broad bean wilt virus 1 | |||
| Broad bean wilt virus 1 | [AF225955] | (BBWV-1) | |
| Broad bean wilt virus 2 | |||
| Broad bean wilt virus | [E31398] | (BBWV) | |
| Broad bean wilt virus 2 | [AF144234] | [E31397] | (BBWV-2) |
| Broad bean wilt virus 2 – B935 | [AF149425] | (BBWV2-B935) | |
| Broad bean wilt virus 2 – Chinese | [AJ132844] | (BBWV2-Chi) | |
| Broad bean wilt virus 2 – IA | [AB051386] | [AB032403] | (BBWV2-IA) |
| Broad bean wilt virus 2 – IP | [AB023484] | [AB018698] | (BBWV2-IP) |
| Broad bean wilt virus 2 – Korean | [AF104335] | (BBWV2-Kor) | |
| Broad bean wilt virus 2 – MB7 | [AB013615] | [AB013616] | (BBWV2-MB7) |
| Broad bean wilt virus 2 – ME | [NC_003003] | [NC_003004] | (BBWV2-ME) |
| Broad bean wilt virus 2 – P158 | [AF228423] | (BBWV2-P158) | |
| Broad bean wilt virus 2 – PV131 | [U65985] | (BBWV2-PV131) | |
| Nasturtium ringspot virus | (NaRSV) | ||
| Parsley virus 3 | (PaV-3) | ||
| Patchouli mild mosaic virus ‡ | [NC_003975] | [NC_003974] | (PatMMV) |
| Petunia ringspot virus | (PeRSV) | ||
| Plantago II virus | (PlIIV) | ||
| Lamium mild mosaic virus | |||
| Lamium mild mosaic virus | (LMMV) | ||
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS NEPOVIRUS
Type Species Tobacco ringspot virus
DISTINGUISHING FEATURES
The capsid of nepoviruses contains a single polypeptide species. Genome organization and expression are similar to those of comoviruses, except that RNA-2 specifies a single primary translation product of 105-207 kDa (Fig. 5 ). Definitive nepoviruses can be divided in three subgroups. Subgroup A has an RNA-2 with Mr 1.3-1.5 × 106, present in both M and B components. Subgroup B has an RNA-2 with Mr 1.4-1.6 × 106, present only in M component. Subgroup C has an RNA-2 with Mr 1.9-2.2 × 106, present in M component particles that are sometimes barely separable from those of B component.
Figure 5.

Genome organization characteristic of Grapevine fanleaf virus (GFLV) (subgroup A; top), BRSV; Beet ringspot virus (subgroup B; middle) and ToRSV; Tomato ringspot virus (subgroup C; bottom). The ORFs are boxed, and functions of the proteins released by proteolysis are indicated. MP, movement protein; CP, capsid protein; Hel, helicase; Pro, proteinase; Pol, polymerase. Proteolytic cleavage sites are indicated on the polyproteins. The ovals at the 5′-end of the RNAs represents the VPg, and An at the 3′-end the poly-A. Braces show the portions of the RNAs that are highly homologous or identical in both RNAs in a subgroup. Diamonds indicate typical sequence motifs.
The nepovirus capsid is typically made of 60 subunits of a 52-60 kDa polypeptide. Additional linear or circular satellite RNAs, which sometimes modulate symptoms, are found associated with several nepoviruses of all three subgroups. They are either linear (1100-1800 nt) with a VPg, a poly-A and encoding a 36-48 kDa polypeptide, or circular (300-460 nt) and apparently non-coding, are present in some natural isolates but are not necessary for virus accumulation.
GENOME ORGANIZATION AND REPLICATION
RNA-2 is translated into a single polyprotein that is processed into three domains. In Grapevine fanleaf virus (GFLV), P2A is involved in RNA-2 replication. P2B is the MP, with a typical “LPL” motif. P2C is the single CP, with two conserved motifs. In Tomato ringspot virus (ToRSV) (subgroup C), a third cleavage site occurs within the 5′-most domain.
RNA-1 is translated into a single polyprotein that is processed into five domains. The function of P1A is unknown, P1B has sequence motifs characteristic of an NTP-binding helicase, P1C is the VPg, P1D is the proteinase and P1E has sequence motifs characteristic of an RdRp. In ToRSV (subgroup C), a fifth cleavage site is present in the 5′-terminal region of the polyprotein. The 5′ and 3′ NTRs are homologous but different between RNA-1 and RNA-2 in subgroup A. The 5′-NTRs of RNA-1 and RNA-2 are also homologous in subgroup B, while the 3′-NTRs are identical. Both NTRs are identical or nearly identical in subgroup C, and include in part the coding region of the polyproteins in ToRSV, but not in Blackcurrant reversion virus (BRV).
BIOLOGICAL PROPERTIES
Nepoviruses are widely distributed in temperate regions. Natural host ranges vary from wide to restricted to a single plant species, depending on the virus. Ringspot symptoms are characteristic, but mottling and spotting are equally frequent. Twelve species are acquired and transmitted persistently by longidorid nematodes (Xiphinema, Longidorus or Paralongidorus spp), three are transmitted by pollen, one (BRV) is transmitted by mites (Blackcurrant reversion virus, BRV) and the others have no known biological vector. Seed and/or pollen transmission is very common. In herbaceous plants, the symptoms induced by nepoviruses are often transient, with newly emerging leaves appearing symptomless a few weeks after infection (the so-called “recovery” phenomenon) due to inefficient plant defense inhibition by the virus.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
CP aa sequence less than 75% homologous,
-
•
Polymerase aa sequence less than 75% homologous,
-
•
No pseudo-recombination between components possible,
-
•
Differences in antigenic reactions,
-
•
Different vector species.
Using polyclonal rabbit antisera in agar gel diffusion tests, Grapevine chrome mosaic virus (GCMV) and Grapevine fanleaf virus (GFLV) differ by more than 10 two-fold dilution steps. Their CP sequences have less than 25% identity and 32% homology between the aligned nt and aa sequences, respectively. Their RdRp sequences have less than 40% identity and 51% homology between the aligned nt and aa sequences, respectively. No reassortment has been demonstrated between these viruses. GFLV is vectored by Xiphinema index nematodes while the vector of GCMV is not known (but is not Xiphinema index).
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
Three clusters of species in the genus, designated as Subgroups A, B and C, are based on length and packaging of RNA-2, sequence similarities and serological relationships.
| Subgroup A | |||
| RNA-1 Acc. # | RNA-2 Acc. # | ||
| Arabis mosaic virus | |||
| Arabis mosaic virus – NW | [AY303786] | [AY017339] | (ArMV-NW) |
| Arabis mosaic virus – P2 | [X81814, X81815] | (ArMV-P2) | |
| Raspberry yellow dwarf virus | (RYDV) | ||
| Rhubarb mosaic virus | (RhuMV) | ||
| Arracacha virus A | |||
| Arracacha virus A | (AVA) | ||
| Artichoke Aegean ringspot virus | |||
| Artichoke Aegean ringspot virus | (AARSV) | ||
| Cassava American latent virus | |||
| Cassava American latent virus | (CsALV) | ||
| Grapevine fanleaf virus | |||
| Grapevine fanleaf virus – F13 | [NC_003615] | [NC_003623] | (GFLV-F13) |
| Grapevine fanleaf virus – NW | [AY017338] | (GLFV-NW) | |
| Grapevine infectious degeneration virus | (GIDV) | ||
| Potato black ringspot virus | |||
| Potato black ringspot virus | (PBRSV) | ||
| Tobacco ringspot virus – potato calico | (TobRSV-PC) | ||
| Raspberry ringspot virus | |||
| Raspberry leaf curl virus | (RpLCV) | ||
| Raspberry ringspot virus | [S46011] | (RpRSV) | |
| Raspberry ringspot virus – Cherry | [AY303787] | [AY303788] | (RpRSV-Che) |
| Raspberry ringspot virus – Grapevine | [AY310444] | [AY310445] | (RpRSV-Gra) |
| Redcurrant ringspot virus | (RcRSV) | ||
| Tobacco ringspot virus | |||
| Tobacco ringspot virus | [U50869] | (TRSV) | |
| Tobacco ringspot virus n°1 | (TRSV-1) | ||
| Subgroup B | |||
| RNA-1 Acc. # | RNA-2 Acc. # | ||
| Artichoke Italian latent virus | |||
| Artichoke Italian latent virus | [X87254 (part.)] | (AILV) | |
| Beet ringspot virus | |||
| Beet ringspot virus | [NC_003693] | [NC_003694] | (BRSV) |
| Tomato black ring virus – Scottish | (TBRV-S) | ||
| Cocoa necrosis virus | |||
| Cocoa necrosis virus | (CoNV) | ||
| Cocoa swollen shoot virus – S | (CSSV-S) | ||
| Crimson clover latent virus | |||
| Crimson clover latent virus | (CCLV) | ||
| Cycas necrotic stunt virus | |||
| Cycas necrotic stunt virus | [NC_003791] | [NC_003792] | (CNSV) |
| Grapevine chrome mosaic virus | |||
| Grapevine chrome mosaic virus | [NC_003622] | [NC_003621] | (GCMV) |
| Mulberry ringspot virus | |||
| Mulberry ringspot virus | (MRSV) | ||
| Olive latent ringspot virus | |||
| Olive latent ringspot virus | [AJ277435] | (OLRSV) | |
| Tomato black ring virus | |||
| Bean ringspot virus | (BRSV) | ||
| Lettuce ringspot virus | (LRSV) | ||
| Potato bouquet virus | (PBV) | ||
| Tomato black ring virus – ED | [X80831] | (TBRV-ED) | |
| Tomato black ring virus – MJ | [NC_004439] | [NC_004440] | (TBRV-MJ) |
| Subgroup C | |||
| RNA-1 Acc. # | RNA-2 Acc. # | ||
| Apricot latent ringspot virus | |||
| Apricot latent ringspot virus | [AJ278875 (part.)] | (ALRSV) | |
| Artichoke yellow ringspot virus | |||
| Artichoke yellow ringspot virus | (AYRSV) | ||
| Blackcurrant reversion virus | |||
| Blackcurrant reversion virus | [NC_003509] | [NC_003502] | (BRV) |
| Blueberry leaf mottle virus | |||
| Blueberry leaf mottle virus | [U20622 (part.)] | [U20621 (part.)] | (BLMoV) |
| Cassava green mottle virus | |||
| Cassava green mottle virus | (CsGMV) | ||
| Cherry leaf roll virus | |||
| Cherry leaf roll virus | [Z34265 (part.)] | [U24694 (part.)] | (CLRV) |
| Elm mosaic virus | (ElMV) | ||
| Golden elderberry virus | (GEBV) | ||
| Walnut black line virus | (WBLV) | ||
| Chicory yellow mottle virus | |||
| Chicory yellow mottle virus | (ChYMV) | ||
| Parsley carrot leaf virus | (PaCLV) | ||
| Grapevine Bulgarian latent virus | |||
| Grapevine Bulgarian latent virus | (GBLV) | ||
| Grapevine Tunisian ringspot virus | |||
| Grapevine Tunisian ringspot virus | (GTRSV) | ||
| Hibiscus latent ringspot virus | |||
| Hibiscus latent ringspot virus | (HLRSV) | ||
| Lucerne Australian latent virus | |||
| Lucerne Australian latent virus | (LALV) | ||
| Lucerne latent virus | (LLV) | ||
| Myrobalan latent ringspot virus | |||
| Myrobalan latent ringspot virus | (MLRSV) | ||
| Peach rosette mosaic virus | |||
| Grape decline virus | (GrDV) | ||
| Grapevine degeneration virus | (GraDV) | ||
| Peach rosette mosaic virus | [AF016626] | (PRMV) | |
| Potato virus U | |||
| Potato virus U | (PVU) | ||
| Tomato ringspot virus | |||
| Grape yellow vein virus | (GraYVV) | ||
| Nicotiana virus 13 | (NV13) | ||
| Peach yellow bud mosaic virus | (PYBMV) | ||
| Tobacco ringspot virus n°2 | (TbRSV-2) | ||
| Tomato ringspot virus | [NC_003840] | [NC_003839] | (ToRSV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Figure 6.
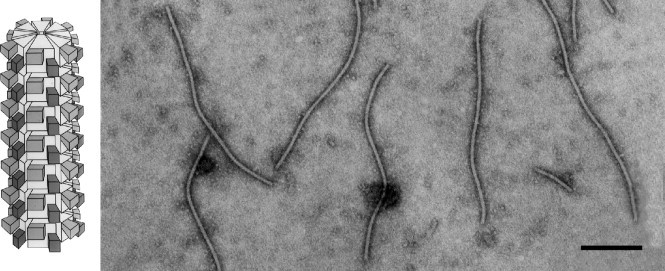
Unrooted dendrogram derived from the alignment of the sequences of the proteinase-polymerase domains of virus species belonging to the families Comoviridae and Sequiviridae, and the unassigned genera Cheravirus and Sadwavirus. Branches supported by bootstrap values lower than 75% were merged. The horizontal scale is proportional to the level of divergence while the vertical scale is arbitrary.
The dendrograms derived from multiple sequence alignments from various regions of the genome agree with each other to indicate that the genera Comovirus and Fabavirus are more closely related to each other than they are from the genus Nepovirus. This is supported by genetic organization data. Within the genus Nepovirus, subgroup B forms a distinct entity according to the dendrograms. Some viruses considered previously as tentative members of the genus Nepovirus are now considered to form two unassigned genera, Cheravirus and Sadwavirus.
SIMILARITY WITH OTHER TAXA
Several features of the family Comoviridae are similar to those of the families and genera Sequiviridae, Cheravirus, Sadwavirus, Picornaviridae, Dicistroviridae, Caliciviridae and Potyviridae e.g. genome organization, VPg at 5′-end and poly(A) tract at 3′-end of genomes, post-translational processing of polyproteins and sequence similarities among nonstructural proteins (Fig. 6). Moreover all these virus families except the family Potyviridae have very similar capsid morphologies.
DERIVATION OF NAMES
Como: sigla from Cowpea mosaic
Faba: Latin Faba, bean; also Vicia faba, broad bean
Nepo: sigla from Nematode-transmitted, polyhedral particles (to distinguish them from the tobraviruses, also nematode-transmitted but with elongated particles).
REFERENCES
- Altmann F., Lomonossoff G.P. Glycosylation of the capsid proteins of cowpea mosaic virus: a reinvestigation shows the absence of sugar residues. J. Gen. Virol. 2000;81:1111–1114. doi: 10.1099/0022-1317-81-4-1111. [DOI] [PubMed] [Google Scholar]
- Carette J.E., Stuiver M., Van Lent J., Wellink J., Van Kammen A. Cowpea mosaic virus infection induces a massive proliferation of endoplasmic reticulum but not Golgi membranes and is dependent on de novo membrane synthesis. J. Virol. 2000;714:6556–6563. doi: 10.1128/jvi.74.14.6556-6563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier K., Xiang Y., Sanfacon H. Genomic organization of RNA2 of Tomato ringspot virus: processing at a third cleavage site in the N-terminal region of the polyprotein in vitro. J. Gen. Virol. 2001;82:1785–1790. doi: 10.1099/0022-1317-82-7-1785. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V., Johnson J.E. The structure of tobacco ringspot virus: a link in the evolution of icosahedral capsids in the picornavirus superfamily. Structure. 1998;6:157–171. doi: 10.1016/s0969-2126(98)00018-5. [DOI] [PubMed] [Google Scholar]
- Francki R.I.B., Milne R.G., Hatta T., editors. II. CRC Press; Boca Raton, Florida: 1985. Comovirus group; pp. 1–22. (Atlas of Plant Viruses). [Google Scholar]
- Gergerich R.C., Scott H.A. Comoviruses: transmission epidemiology and control. In: Harrison B.D., Murant A.F., editors. The Plant Viruses. 5th ed. Plenum Press; New York: 1996. pp. 77–98. [Google Scholar]
- Goldbach R., Wellink J. Comoviruses: molecular biology and replication. In: Harrison B.D., Murant A.F., editors. The Plant Viruses. 5th ed. Plenum Press; New York: 1996. pp. 35–76. [Google Scholar]
- Harrison B.D., Murant A.F. Nepoviruses: ecology and control. In: Harrison B.D., Murant A.F., editors. The Plant Viruses. 5th ed. Plenum Press; New York: 1996. pp. 211–228. [Google Scholar]
- Le Gall O., Candresse T., Dunez J. A multiple alignment of the capsid protein sequences of nepoviruses and comoviruses suggests a common structure. Arch. Virol. 1995;140:2041–2053. doi: 10.1007/BF01322691. [DOI] [PubMed] [Google Scholar]
- Lin T., Chen Z., Usha R., Stauffacher C.V., Dai J.B., Schmidt T., Johnson J.E. The refined crystal structure of cowpea mosaic virus at 2.8 A resolution. Virology. 1999;265:20–34. doi: 10.1006/viro.1999.0038. [DOI] [PubMed] [Google Scholar]
- Lisa V., Boccardo G. Fabaviruses: broad bean wilt and allied viruses. In: Harrison B.D., Murant A.F., editors. The Plant Viruses. 5th ed. Plenum Press; New York: 1996. pp. 229–250. [Google Scholar]
- Mayo M.A., Robinson D.J. Nepoviruses: molecular biology and replication. In: Harrison B.D., Murant A.F., editors. The Plant Viruses. 5th ed. Plenum Press; New York: 1996. pp. 139–186. [Google Scholar]
- Murant A.F., Jones A.T., Martelli G.P., Stace-Smith R. Nepoviruses: general properties, diseases and viru identification. In: Harrison B.D., Murant A.F., editors. The Plant Viruses. 5th ed. Plenum Press; New York: 1996. pp. 99–138. [Google Scholar]
- Pouwels J., Carette J.E., Van Lent J., Wellink J. Cowpea mosaic virus: effects on host cell processes. Mol. Plant Pathol. 2002;3:411–418. doi: 10.1046/j.1364-3703.2002.00135.x. [DOI] [PubMed] [Google Scholar]
- Rochon D., Sanfaçon H. Nepoviruses. In: Maloy O.C., Murray T.D., editors. Encyclopedia of Plant Pathology. Academic Press; San Diego: 2001. pp. 704–708. [Google Scholar]
- Ritzenthaler C., Laporte C., Gaire F., Dunoyer P., Schmitt C., Duval S., Piequet A., Loudes A.M., Rohfritsch O., Stuss Garaud C., Pfeiffer P. Grapevine fanleaf virus replication occurs on endoplasmic reticulum-derived membranes. J. Virol. 2002;76:8808–8819. doi: 10.1128/JVI.76.17.8808-8819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde R.A., Fulton J.P. Comoviruses: identification and diseases caused. In: Harrison B.D., Murant A.F., editors. The Plant Viruses. 5th ed. Plenum Press; New York: 1996. pp. 17–34. [Google Scholar]
CONTRIBUTED BY, P.H. Berger, M.J. Adams, O.W. Barnett, A.A. Brunt, J. Hammond, J.H. Hill, R.L. Jordan, S. Kashiwazaki, E. Rybicki, N. Spence, D.C. Stenger, S.T. Ohki, I. Uyeda, A. van Zaayen, J. Valkonen, H.J. Vetten
FAMILY POTYVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Potyviridae |
| Genus | Potyvirus |
| Genus | Ipomovirus |
| Genus | Macluravirus |
| Genus | Rymovirus |
| Genus | Tritimovirus |
| Genus | Bymovirus |
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments with no envelope and are 11-15 nm in diameter, with a helical pitch of about 3.4 nm (Fig. 1 ). Particle lengths of members of some of the six genera differ. Members of the genera Potyvirus, Ipomovirus, Macluravirus, Rymovirus, Tritimovirus and the unassigned viruses are monopartite with particle modal lengths of 650-900 nm; members of the genus Bymovirus are bipartite with particles of two modal lengths of 250-300 and 500-600 nm.
Figure 1.

(Left) Schematic diagram of a potyvirus particle. The N-terminal ∼30 aa (large rectangle) and C-terminal ∼19 aa (small rectangle) of the CP molecules are exposed on the surface of the intact virus particle (from Shukla and Ward, 1989). (Right) Negative contrast electron micrograph of particles of an isolate of Plum pox virus, stained with 1% PTA, pH 6.0. The bar represents 200 nm (Courtesy of I.M. Roberts).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions of viruses in the genera Potyvirus and Rymovirus have densities in CsCl of about 1.31 g/cm3 and S20w of 137-160S. Those of viruses of the genus Bymovirus have a density in CsCl of about 1.29 g/cm3.
NUCLEIC ACID
Viruses in all genera except Bymovirus have a single molecule of positive sense, ssRNA, 9.3-10.8 kb in size. Virions are infectious. A VPg of about 24 kDais covalently linked to the 5′-terminal nt. A polyadenylate tract (20 to 160 adenosines) is present at the 3′ terminus. The complete nt sequence is known for over 30 potyviruses, one ipomovirus, one rymovirus, two tritimovirus, and four bymoviruses. Bymoviruses have two positive sense, ssRNA molecules; RNA-1 is 7.3-7.6 kb in size and RNA-2 is 3.5-3.7 kb in size. Both RNAs have 3′ terminal polyadenylate tracts and probably a VPg at the 5′-termini.
PROTEINS
The genome-derived polyprotein is cleaved into several proteins, some of which form inclusion bodies in the cell. Virions contain one type of CP of 28.5-47 kDa. N- and C-terminal residues are positioned on the exterior of the virion. Mild trypsin treatment removes N- and C-terminal segments, leaving a trypsin-resistant core of about 24 kDa. Plant proteases may degrade the CP in vivo, as happens in vitro during purification using some procedures or from certain hosts. All potyvirus CPs display significant aa sequence identity in the trypsin-resistant core, but little identity in their N and C-terminal segments.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Genetic information encoded by the RNA genome is organized as a single ORF. Genetic maps for members of the genus Potyvirus, and BaYMV (genus Bymovirus) are presented in genera descriptions. For potyviruses, the genome is expressed initially as a polyprotein, which then undergoes co- and post-translational proteolytic processing by three viral-encoded proteinases to form individual gene products. Genomic RNA replicates via the production of a full-length negative sense RNA.
ANTIGENIC PROPERTIES
The viral proteins are moderately immunogenic; there are serological relationships among members. An epitope of the CP in the conserved internal trypsin-resistant core has been identified that is similar in most members of the family.
BIOLOGICAL PROPERTIES
INCLUSION BODY FORMATION
All members of the family Potyviridae form cytoplasmic cylindrical inclusion (CI) bodies during infection. The CI is an array of a 70 kDa viral protein that possesses ATPase and helicase activities. Some potyviruses induce nuclear inclusion bodies that are co-crystals of two viral-encoded proteins – NIa and NIb – present in equimolar amounts. The small nuclear inclusion (NIa) protein (49 kDa) is a polyprotein consisting of the VPg and proteinase. The large nuclear inclusion (NIb) protein has aa motifs of RdRps. NIa and NIb are also found in the cytoplasm. Amorphous inclusion bodies are also evident in the cytoplasm during certain potyvirus infections and represent aggregations of the protein HC-Pro and perhaps other non-structural proteins. HC-Pro has a helper component activity and a proteolytic activity associated with it. Bymoviruses do not encode a protein similar in length to the helper component, but a 28 kDa protein from RNA-2 of BaYMV has aa domains with sequence similarities to the potyvirus protein HC-Pro.
HOST RANGE
Some members have a narrow host range, most members infect an intermediate number of plants, and a few members infect species in up to 30 families. Transmission to most hosts is readily accomplished by mechanical inoculation. Many viruses are widely distributed. Distribution is aided by seed transmission in some cases.
TRANSMISSION
Potyviruses are vectored by a variety of organisms. Members of the genera Potyvirus and Macluravirus have aphid vectors that transmit in a non-persistent, non-circulative manner. A helper component and a particular CP aa triplet (i.e., DAG for some potyviruses) are required for aphid transmission. Rymoviruses and tritimoviruses are transmitted by eriophyid mites, in a semi-persistent manner. Bymoviruses are transmitted persistently by a fungus vector. Ipomoviruses appear to be transmitted by whiteflies.
GENUS POTYVIRUS
Type Species Potato virus Y
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments, 680-900 nm long and 11-13 nm wide, with helical symmetry and a pitch of about 3.4 nm. Particles of some viruses are longer in the presence of divalent cations than in the presence of EDTA.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion S20w is 137-160S; density in CsCl is 1.31 g/cm3; E0.1% 1 cm, 260 nm = 2.4-2.7.
NUCLEIC ACID
Virions contain a single molecule of linear, positive sense ssRNA, about 9.7 kb in size; virions contain 5% RNA by weight. RNA molecules have poly (A) tracts at their 3′-ends. A 24 kDa VPg is covalently linked at or near the 5′ terminus (Fig. 2 ).
Figure 2.

Genomic map of a member of the genus Potyvirus, using a strain of Tobacco etch virus as an example. The RNA genome is represented by a thin line and an open box which represents translated segments of the ssRNA. Functions associated with these products are shown. VPg, genome-linked viral protein covalently attached to the 5′ terminal nt (represented by the oval at the 5′-end); P1-Pro, a protein with a proteolytic activity responsible for cleavage at typically Tyr/Phe-Ser (O); HC-Pro, a protein with aphid transmission helper component activity and proteolytic activity responsible for cleavage at typically Gly-Gly (?); Pro, serine-like proteolytic activity responsible for cleavage at Gln/Glu-(Ser/Gly/Ala) (T). Some of these proteins of particular viruses of the family Potyviridae aggregate to form inclusion bodies during infection. The protein involved and the particular type of inclusion body is shown above the genetic map; AI, amorphous inclusion; CI, cylindrical-shaped inclusion body found in the cytoplasm; NIa and NIb, small and large nuclear inclusion proteins, respectively, which aggregate in the nucleus to form a nuclear inclusion body.
PROTEINS
Virions contain a single CP, 30 to 47 kDa in size. The CP of most isolates of the type species, PVY, contains 267 aa.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Potyvirus genomes are ∼9.7 kb in size (Fig. 2).
ANTIGENIC PROPERTIES
Virions are moderately immunogenic; there are serological relationships among many members. One monoclonal antibody reacts with most aphid-transmitted potyviruses. The CP aa sequence identity among aphid-transmitted viruses is 40-70%. Some viruses are serologically related to viruses in the genera Rymovirus and Bymovirus.
BIOLOGICAL PROPERTIES
Many individual viruses have a narrow host range, but a few infect plant species in up to 30 host families. The viruses are transmitted by aphids in a non-persistent manner and are transmissible experimentally by mechanical inoculation. Some isolates are inefficiently transmitted by aphids and others are not transmissible by aphids at all. This is apparently due to mutations within the helper component and/or CP cistrons. Some viruses are seed-transmitted.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•Genome sequence relatedness.
- –CP aa sequence identity less than ca. 80%,
- –nt sequence identity of less than 85% over whole genome,
- –different polyprotein cleavage sites.
-
•Natural host range.
- –host range may be related to species but usually not helpful in identifying species; may delineate strains.
-
•Pathogenicity and cytopathology.
- –different inclusion body morphology,
- –lack of cross protection,
- –seed transmissibility, or lack thereof,
- –some aspects of host reaction may be useful (e.g., different responses in key host species, and particular genetic interactions).
-
•Mode of transmission.
- –different primary vectors, but vector species not use in identification to virus species.
-
•Antigenic properties.
- –serological differences.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Alpinia mosaic virus | ||
| Alpinia mosaic virus | (AlpMV) | |
| Alstroemeria mosaic virus | ||
| Alstroemeria mosaic virus | (AlMV) | |
| Alstroemeria streak virus | ||
| Amaranthus leaf mottle virus | ||
| Amaranthus leaf mottle virus | (AmLMV) | |
| Apium virus Y‡ | ||
| Apium virus Y | (ApVY) | |
| Parsley virus Y | ||
| Araujia mosaic virus | ||
| Araujia mosaic virus | (ArjMV) | |
| Artichoke latent virus | ||
| Artichoke latent virus | (ArLV) | |
| Asparagus virus 1 | ||
| Asparagus virus 1 | (AV-1) | |
| Banana bract mosaic virus | ||
| Banana bract mosaic virus | (BBrMV) | |
| Bean common mosaic necrosis virus | ||
| Bean common mosaic necrosis virus | [AY138897, AY282577, U19287] | (BCMNV) |
| Bean common mosaic virus serotype A | ||
| Bean common mosaic virus | ||
| Azuki bean mosaic virus | ||
| Bean common mosaic virus | [AY112735, U34972] | (BCMV) |
| Blackeye cowpea mosaic virus | [AJ312437-8] | |
| Dendrobium mosaic virus | ||
| Guar green sterile virus | ||
| Peanut chlorotic ring mottle virus | ||
| Peanut mild mottle virus | ||
| Peanut stripe virus | [U05771-2] | |
| Bean yellow mosaic virus | ||
| Bean yellow mosaic virus | [AY192568, D83749, U47033] | (BYMV) |
| Crocus tomasinianus virus | ||
| Pea mosaic virus | ||
| White lupin mosaic virus | ||
| Beet mosaic virus | ||
| Beet mosaic virus | [AY206394] | (BtMV) |
| Dioscorea alata ring mottle virus | ||
| Bidens mottle virus | ||
| Bidens mottle virus | (BiMoV) | |
| Calanthe mild mosaic virus | ||
| Calanthe mild mosaic virus | (CalMMV) | |
| Carnation vein mottle virus | ||
| Carnation vein mottle virus | (CVMoV) | |
| Carrot thin leaf virus | ||
| Carrot thin leaf virus | (CTLV) | |
| Carrot virus Y | ||
| Carrot virus Y | (CtVY) | |
| Celery mosaic virus | ||
| Celery mosaic virus | (CeMV) | |
| Ceratobium mosaic virus | ||
| Ceratobium mosaic virus | (CerMV) | |
| Chilli veinal mottle virus | ||
| Chilli veinal mottle virus | [AJ237843] | (ChiVMV) |
| Indian pepper mottle virus | ||
| Pepper vein banding mosaic virus | ||
| Clitoria virus Y | ||
| Clitoria virus Y | (ClVY) | |
| Clover yellow vein virus | ||
| Clover yellow vein virus | [AB011819] | (ClYVV) |
| Pea necrosis virus | ||
| Statice virus Y | ||
| Cocksfoot streak virus | ||
| Cocksfoot streak virus | [AF499738] | (CSV) |
| Colombian datura virus | ||
| Colombian datura virus | (CDV) | |
| Petunia flower mottle virus ‡ | ||
| Commelina mosaic virus | ||
| Commelina mosaic virus | (ComMV) | |
| Cowpea aphid-borne mosaic virus | ||
| Cowpea aphid-borne mosaic virus | [AF348210] | (CABMV) |
| Sesame mosaic virus | ||
| South African passiflora virus | ||
| Cowpea green vein banding virus | ||
| Cowpea green vein banding virus | (CGVBV) | |
| Cypripedium virus Y | ||
| Cypripedium virus Y | (CypVY)) | |
| Dasheen mosaic virus | ||
| Dasheen mosaic virus | [AJ298033] | (DsMV) |
| Datura shoestring virus | ||
| Datura shoestring virus | (DSSV) | |
| Diuris virus Y | ||
| Diuris virus Y | (DiVY) | |
| Endive necrotic mosaic virus | ||
| Endive necrotic mosaic virus | (ENMV) | |
| Freesia mosaic virus | ||
| Freesia mosaic virus | (FreMV) | |
| Gloriosa stripe mosaic virus | ||
| Gloriosa stripe mosaic virus | (GSMV) | |
| Groundnut eyespot virus | ||
| Groundnut eyespot virus | (GEV) | |
| Guinea grass mosaic virus | ||
| Guinea grass mosaic virus | (GGMV) | |
| Helenium virus Y | ||
| Helenium virus Y | (HVY) | |
| Henbane mosaic virus | ||
| Henbane mosaic virus | (HMV) | |
| Hibbertia virus Y‡ | ||
| Hibbertia virus Y | (HiVY) | |
| Hippeastrum mosaic virus | ||
| Hippeastrum mosaic virus | (HiMV) | |
| Hyacinth mosaic virus | ||
| Hyacinth mosaic virus | (HyaMV) | |
| Iris fulva mosaic virus | ||
| Iris fulva mosaic virus | (IFMV) | |
| Iris mild mosaic virus | ||
| Iris mild mosaic virus | (IMMV) | |
| Iris severe mosaic virus | ||
| Bearded iris mosaic virus | ||
| Iris severe mosaic virus | (ISMV) | |
| Japanese yam mosaic virus | ||
| Japanese yam mosaic virus | [AB016500, AB027007] | (JYMV) |
| Johnsongrass mosaic virus | ||
| Johnsongrass mosaic virus | [Z26920] | (JGMV) |
| Kalanchoë mosaic virus | ||
| Kalanchoë mosaic virus | (KMV) | |
| Konjac mosaic virus | ||
| Konjac mosaic virus | (KoMV) | |
| Leek yellow stripe virus | ||
| Garlic mosaic virus | ||
| Garlic virus | ||
| Garlic virus 2 | ||
| Leek yellow stripe virus | [AJ307057] | (LYSV) |
| Lettuce mosaic virus | ||
| Lettuce mosaic virus | [AJ278854, AJ306288, X97704-5] | (LMV) |
| Lily mottle virus | ||
| Lily mild mottle virus | ||
| Lily mottle virus | [AJ564636] | (LMoV) |
| Tulip band breaking virus | ||
| Lycoris mild mottle virus | ||
| Lycoris mild mottle virus | (LyMMoV) | |
| Maize dwarf mosaic virus | ||
| Maize dwarf mosaic virus | [AJ001691] | (MDMV) |
| Moroccan watermelon mosaic virus | ||
| Moroccan watermelon mosaic virus | (MWMV) | |
| Narcissus degeneration virus | ||
| Narcissus degeneration virus | (NDV) | |
| Narcissus late season yellows virus | ||
| Jonquil mild mosaic virus | ||
| Narcissus late season yellows virus | (NLSYV) | |
| Narcissus yellow stripe virus | ||
| Narcissus yellow stripe virus | (NYSV) | |
| Nerine yellow stripe virus | ||
| Nerine yellow stripe virus | (NeYSV) | |
| Nothoscordum mosaic virus | ||
| Nothoscordum mosaic virus | (NoMV) | |
| Onion yellow dwarf virus | ||
| Onion yellow dwarf virus | [AJ510223] | (OYDV) |
| Ornithogalum mosaic virus | ||
| Ornithogalum mosaic virus | (OrMV) | |
| Pterostylis virus Y | ||
| Ornithogalum virus 2 | ||
| Ornithogalum virus 2 | (OrV2) | |
| Ornithogalum virus 3 | ||
| Ornithogalum virus 3 | (OrV3) | |
| Papaya leaf distortion mosaic virus | ||
| Papaya leaf distortion mosaic virus | [AB088221] | (PLDMV) |
| Papaya ringspot virus | ||
| Papaya ringspot virus | [S46722, X97251, AY010722, AY027810, AY162218, AY231130] | (PRSV) |
| Watermelon mosaic virus 1 | ||
| Parsnip mosaic virus | ||
| Parsnip mosaic virus | (ParMV) | |
| Passion fruit woodiness virus | ||
| Passion fruit woodiness virus | (PWV) | |
| Pea seed-borne mosaic virus | ||
| Pea seed-borne mosaic virus | [AJ252242, D10930, X89997] | (PSbMV) |
| Peanut mottle virus | ||
| Peanut mottle virus | [AF023848] | (PeMoV) |
| Pepper mottle virus | ||
| Pepper mottle virus | [AF501591, M96425] | (PepMoV) |
| Pepper severe mosaic virus | ||
| Pepper severe mosaic virus | (PepSMV) | |
| Pepper veinal mottle virus | ||
| Pepper veinal mottle virus | (PVMV) | |
| Pepper yellow mosaic virus | ||
| Pepper yellow mosaic virus | (PepYMV) | |
| Peru tomato mosaic virus | ||
| Peru tomato mosaic virus | (PTV) | |
| Pleione virus Y | ||
| Pleione virus Y | (PlVY) | |
| Plum pox virus | ||
| Plum pox virus | [AF401295-6, AJ243957, AY184478, D13751, M92280, X16415, X81083, Y09851] | (PPV) |
| Pokeweed mosaic virus | ||
| Pokeweed mosaic virus | (PkMV) | |
| Potato virus A | ||
| Potato virus A | [AF543212, AF543709, AJ131400-2, AJ296311] | (PVA) |
| Tamarillo mosaic virus | [AJ131403] | |
| Potato virus V | ||
| Potato virus V | [AJ243766] | (PVV) |
| Potato virus Y | ||
| Potato virus Y | [AF237963, AF463399, AF522296, AJ439544-5, AY166866-7, D00441, M95491, U09509, X12456, X97895] | (PVY) |
| Sunflower chlorotic mottle virus | ||
| Rhopalanthe virus Y | ||
| Rhopalanthe virus Y | (RhVY) | |
| Sarcochilus virus Y | ||
| Sarcochilus virus Y | (SaVY) | |
| Scallion mosaic virus | ||
| Scallion mosaic virus | [AJ316084] | (ScMV) |
| Shallot yellow stripe virus | ||
| Shallot yellow stripe virus | (SYSV) | |
| Welsh onion yellow stripe virus | ||
| Sorghum mosaic virus | ||
| Sorghum mosaic virus | [AJ310197-8, U57358] | (SrMV) |
| Soybean mosaic virus | ||
| Soybean mosaic virus | [AB100442-3, AF241739, AJ310200, AJ312439, AJ507388, AJ628750, AY216010, AY216987, AY294044-5, D00507, S42280] | (SMV) |
| Sugarcane mosaic virus | ||
| Sugarcane mosaic virus | [AF494510, AJ278405, AJ297628, AJ310102-5, AY042184, AY149118] | (SCMV) |
| Sunflower mosaic virus | ||
| Sunflower mosaic virus | (SuMV) | |
| Sweet potato feathery mottle virus | ||
| Sweet potato chlorotic leafspot virus | ||
| Sweet potato feathery mottle virus | [D86371] | (SPFMV) |
| Sweet potato internal cork virus | ||
| Sweet potato russet crack virus | ||
| Sweet potato virus A | ||
| Sweet potato latent virus | ||
| Sweet potato latent virus | (SPLV) | |
| Sweet potato mild speckling virus‡ | ||
| Sweet potato mild speckling virus | (SPMSV) | |
| Sweet potato virus G‡ | ||
| Sweet potato virus G | (SPVG) | |
| Telfairia mosaic virus | ||
| Telfairia mosaic virus | (TeMV) | |
| Tobacco etch virus | ||
| Tobacco etch virus | [L38714, M11458, M15239] | (TEV) |
| Tobacco vein banding mosaic virus | ||
| Tobacco vein banding mosaic virus | (TVBMV) | |
| Tobacco vein mottling virus | ||
| Tobacco vein mottling virus | [U38621, X04083] | (TVMV) |
| Tropaeolum mosaic virus | ||
| Nasturtium mosaic virus | ||
| Tropaeolum mosaic virus | (TrMV) | |
| Tuberose mild mosaic virus | ||
| Tuberose mild mosaic virus | (TuMMV) | |
| Tulip breaking virus | ||
| Tulip breaking virus | (TBV) | |
| Tulip mosaic virus‡ | ||
| Tulip mosaic virus | (TulMV) | |
| Turnip mosaic virus | ||
| Tulip chlorotic blotch virus | ||
| Tulip top breaking virus | ||
| Turnip mosaic virus | [AB093596-627, AF169561, AF394601-2, AY090660, AY227024, D10927, D83184] | (TuMV) |
| Watermelon leaf mottle virus‡ | ||
| Watermelon leaf mottle virus | (WLMV) | |
| Watermelon mosaic virus | ||
| Vanilla necrosis virus | ||
| Watermelon mosaic virus | (WMV) | |
| Watermelon mosaic virus 2 | ||
| Wild potato mosaic virus | ||
| Wild potato mosaic virus | [AJ437279] | (WPMV) |
| Wisteria vein mosaic virus | ||
| Wisteria vein mosaic virus | (WVMV) | |
| Yam mild mosaic virus‡ | ||
| Dioscorea alata virus | ||
| Yam mild mosaic virus | (YMMV) | |
| Yam mosaic virus | ||
| Dioscorea green banding virus | ||
| Yam mosaic virus | [U42596] | (YMV) |
| Zantedeschia mosaic virus‡ | ||
| Japanese hornwort mosaic virus | ||
| Zantedeschia mosaic virus | (ZaMV) | |
| Zea mosaic virus | ||
| Iranian johnsongrass mosaic virus | ||
| Zea mosaic virus | (ZeMV) | |
| Zucchini yellow fleck virus | ||
| Zucchini yellow fleck virus | (ZYFV) | |
| Zucchini yellow mosaic virus | ||
| Zucchini yellow mosaic virus | [AF014811, AF127929, AJ307036, AJ316228-9, AJ515911, AY278998-9, AY279000, L29569, L31350] | (ZYMV) |
TENTATIVE SPECIES IN THE GENUS
Aphid-borne (*aphid transmission not confirmed; + denotes plant species with a report of a potyvirus infection)
| Alstroemeria flower banding virus | (AlFBV) |
| Amazon lily mosaic virus | (ALiMV) |
| Aneilema mosaic virus | (AneMV) |
| Anthoxanthum mosaic virus* | (AntMV) |
| Aquilegia necrotic ringspot virus* | (AqNRSV) |
| Arracacha virus Y | (AVY) |
| Asystasia gangetica mottle virus* | (AGMoV) |
| Bidens mosaic virus | (BiMV) |
| Bramble yellow mosaic virus | (BrmYMV) |
| Bryonia mottle virus | (BryMoV) |
| Canary reed mosaic virus | (CRMV) |
| Canavalia maritima mosaic virus | (CnMMV) |
| Carrot mosaic virus | (CtMV) |
| Cassia yellow spot virus | (CasYSV) |
| Celery yellow mosaic virus | (CeYMV) |
| Chickpea bushy dwarf virus | (CpBDV) |
| Chickpea filiform virus | (CpFV) |
| Chrysanthemum spot virus | (ChSV) |
| Clitoria yellow mosaic virus | (CtYMV) |
| Cowpea rugose mosaic virus | (CPRMV) |
| Crinum mosaic virus* | (CriMV) |
| Croatian clover virus+ | (CroCV) |
| Cypripedium chlorotic streak virus* | (CypCSV) |
| Daphne virus Y | (DVY) |
| Datura virus 437 | (DV-437) |
| Datura distortion mosaic virus | (DDMV) |
| Datura mosaic virus* | (DTMV) |
| Datura necrosis virus | (DNV) |
| Desmodium mosaic virus | (DesMV) |
| Dioscorea dumentorum virus | (DDV) |
| Dioscorea trifida virus+ | (DTV) |
| Dipladenia mosaic virus | (DipMV) |
| Dock mottling mosaic virus | (DMMV) |
| Eggplant green mosaic virus | (EGMV) |
| Eggplant severe mottle virus | (ESMoV) |
| Euphorbia ringspot virus | (EuRSV) |
| Fig leaf chlorosis virus | (FigLCV) |
| Guar symptomless virus* | (GSLV) |
| Habenaria mosaic virus | (HaMV) |
| Holcus streak virus* | (HSV) |
| Hungarian datura innoxia virus* | (HDIV) |
| Isachne mosaic virus* | (IsaMV) |
| Kennedya virus Y | (KVY) |
| Malva vein clearing virus | (MVCV) |
| Marigold mottle virus | (MaMoV) |
| Melilotus mosaic virus | (MeMV) |
| Melon vein-banding mosaic virus | (MVBMV) |
| Melothria mottle virus | (MeMoV) |
| Mungbean mosaic virus* | (MbMV) |
| Mungbean mottle virus | (MMoV) |
| Palm mosaic virus* | (PalMV) |
| Passion fruit mottle virus | (PFMoV) |
| Passion fruit ringspot virus | (PFRSV) |
| Patchouli mottle virus | (PatMoV) |
| Peanut chlorotic blotch virus | (PeClBlV) |
| Peanut green mottle virus | (PeGMoV) |
| Peanut top paralysis virus | (PeTPV) |
| Pecteilis mosaic virus | (PcMV) |
| Pepper mild mosaic virus | (PMMV) |
| Perilla mottle virus | (PerMoV |
| Plantain virus 7 | (PlV-7) |
| Pleioblastus mosaic virus | (PleMV) |
| Poplar decline virus* | (PopDV) |
| Primula mosaic virus | (PrMV) |
| Primula mottle virus | (PrMoV) |
| Radish vein clearing virus | (RaVCV) |
| Ranunculus mottle virus | (RanMoV) |
| Rembrandt tulip breaking virus | (ReTBV) |
| Rudbeckia mosaic virus | (RuMV) |
| Sri Lankan passion fruit mottle virus | (SLPMoV) |
| Sweet potato vein mosaic virus | (SPVMV) |
| Sweet potato mild speckling virus | (SPMSV) |
| Sword bean distortion mosaic virus | (SBDMV) |
| Taro feathery mottle virus | (TFMoV) |
| Teasel mosaic virus | (TeaMV) |
| Tobacco wilt virus | (TWV) |
| Tongan vanilla virus | (TVV) |
| Tradescantia mosaic virus | (TraMV) |
| Trichosanthes mottle virus | (TrMoV) |
| Tropaeolum virus 1 | (TV-1) |
| Tropaeolum virus 2 | (TV-2) |
| Ullucus mosaic virus | (UMV) |
| Vallota mosaic virus | (ValMV) |
| Vanilla mosaic virus | (VanMV) |
| White bryony virus | (WBV) |
| Zoysia mosaic virus | (ZoMV) |
GENUS IPOMOVIRUS
Type Species Sweet potato mild mottle virus
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments 800-950 nm long.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion S20w is 155S for Sweet potato mild mottle virus (SPMMV).
NUCLEIC ACID
Virions contain a positive sense ssRNA, ∼10.8 kb in size, with a 3′-poly(A) terminus.
PROTEINS
The viral CP of is a single peptide of 270-275 aa (37.7 kDa).
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The SPMMV genome consists of 10818 nt excluding the 3′-terminal poly(A) tail. Sequence analysis reveals an ORF of 3456 aa (Fig. 3 ). The structure and organization of the SPMMV genome appear to be similar to those of other members of the family Potyviridae except the bymoviruses. Almost all known potyvirus motifs are present in the polyprotein of SPMMV. However, motifs in the putative HC-Pro and CP of SPMMV are incomplete or missing, which may account for its vector relations. Comparative sequence analyses show only limited similarities between the nine mature proteins and those of other species in other genera of the family Potyviridae.
Figure 3.

Genomic map of an isolate of Sweet potato mild mottle virus. The RNA genome is represented by thin lines and an open box which represent translated segments of the ssRNA. Conventions are as for the potyvirus genome organization map (Fig. 2). Activities of most gene products are postulated by analogy with genus Potyvirus.
ANTIGENIC PROPERTIES
Moderately immunogenic. No serological relationships with other members of the family Potyviridae have been found.
BIOLOGICAL PROPERTIES
INCLUSION BODY FORMATION
Characteristic cytoplasmic cylindrical (“pinwheel”) inclusions are present in infected cells.
HOST RANGE
The natural host range of SPMMV is wide, with more than nine families susceptible.
TRANSMISSION
SPMMV is transmitted by the whitefly Bemisia tabaci in a non-persistent manner and is transmissible experimentally by mechanical inoculation and by grafting.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•Genome sequence relatedness.
- –CP aa sequence identity less than ca. 80%,
- –nt sequence identity of less than 85% over whole genome,
- –different polyprotein cleavage sites.
-
•Natural host range.
- –host range may be related to species but usually not helpful in identifying species. May delineate strains.
-
•Pathogenicity and cytopathology.
- –different inclusion body morphology,
- –lack of cross protection,
- –seed transmissibility, or lack thereof,
- –some aspects of host reaction may be useful (e.g., resistance genes, different responses in key host species).
-
•Antigenic properties.
- –serological differences.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Cassava brown streak virus | ||
| Cassava brown streak virus | (CBSV) | |
| Cucumber vein yellowing virus | ||
| Cucumber vein yellowing virus | (CVYV) | |
| Sweet potato mild mottle virus | ||
| Sweet potato mild mottle virus | [Z73124] | (SPMMV) |
TENTATIVE SPECIES IN THE GENUS
| Sweet potato yellow dwarf virus | (SPYDV) |
GENUS MACLURAVIRUS
Type Species Maclura mosaic virus
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments mostly 650-675 nm × 13-16 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion S20w is 155-158S; density in CsCl is 1.31-1.33 g/cm3.
NUCLEIC ACID
Virions contain one molecule of linear positive sense, ssRNA. RNA is ∼8.0 kb.
PROTEINS
Macluraviruses have a single CP species of 33-34 kDa.
LIPIDS
None reported.
CARBOHYDRATES
None reported
GENOME ORGANIZATION AND REPLICATION
The aa sequences of macluravirus CPs show limited (14-23%) identity with CP sequences of some aphid-transmitted potyviruses. Macluraviruses show significant aa sequence identity in portions of the replicase protein with viruses in other genera of the family Potyviridae. Characteristic cytoplasmic cylindrical (“pinwheel”) inclusions are present in infected cells. The macluraviruses seem to have a genome organization and replication strategy typical of viruses in the family Potyviridae.
ANTIGENIC PROPERTIES
Moderately immunogenic. No serological relationships to members of the genus Potyvirus have been found except for a weak reactions between Maclura mosaic virus (MacMV) and Narcissus latent virus (NLV) and between MacMV and Bean yellow mosaic virus (BYMV).
BIOLOGICAL PROPERTIES
HOST RANGE
Both MacMV and NLV have a narrow host range, infecting species in up to 9 host families. MacMV has only been reported from the former Yugoslavia while NLV is likely to occur wherever narcissus, gladiolus and bulbous iris are grown.
TRANSMISSION
The viruses are transmitted by aphids in a non-persistent manner and experimentally by mechanical inoculation.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•Genome sequence relatedness.
- –CP aa sequence identity less than ca. 80%,
- –nt sequence identity less than 85% over whole genome,
- –different polyprotein cleavage sites.
-
•Natural host range.
- –host range may be correlated with species but usually not helpful in identifying species; may delineate strains.
-
•Pathogenicity and cytopathology.
- –different inclusion body morphology,
- –lack of cross protection,
- –seed transmissibility, or lack thereof,
- –some aspects of host reaction may be useful (e.g., different responses in key host species, and particular genetic interactions).
-
•Mode of transmission.
- –different primary vectors, but vector species are not of use in demarcating virus species.
-
•Antigenic properties.
- –serological differences.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Cardamom mosaic virus | |
| Cardamom mosaic virus | (CdMV) |
| Indian cardamom mosaic virus | |
| Maclura mosaic virus | |
| Maclura mosaic virus | (MacMV) |
| Narcissus latent virus | |
| Narcissus latent virus | (NLV) |
TENTATIVE SPECIES IN THE GENUS
| Chinese yam necrotic mosaic virus | (CYNMV) |
GENUS RYMOVIRUS
Type Species Ryegrass mosaic virus
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments 690-720 × 11-15 nm in size.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion density in CsCl is 1.325 g/cm3 (for Ryegrass mosaic virus, RGMV). Virion S20w is 165-166S for most members.
NUCLEIC ACID
Virions contain a single molecule of linear positive sense ssRNA with a 3′-poly(A) terminus. Virion RNA is about 9-10 kb in size.
PROTEINS
Rymoviruses have one type of CP, of 29.2 kDa for RGMV.
LIPIDS
None reported
CARBOHYDRATES
None reported
GENOME ORGANIZATION AND REPLICATION
The rymoviruses presumably have a genome organization (Fig. 4 ) and replication strategy typical of viruses in the family Potyviridae.
Figure 4.

Genomic map of an isolate of Ryegrass mosaic virus. The RNA genome is represented by thin lines and an open box which represent translated segments of the ssRNA. Conventions are as for the potyvirus genome organization map (Fig. 2). Activities of most gene products are postulated by analogy with genus Potyvirus.
ANTIGENIC PROPERTIES
Particles of most rymoviruses are moderately immunogenic. No serological relationships have been found among member viruses.
BIOLOGICAL PROPERTIES
HOST RANGE
Most rymoviruses have limited but widespread host ranges within the family Graminae but some have relatively narrow host ranges.
TRANSMISSION
Transmission by eriophyid mites and mechanical transmission have been reported for most members.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•Genome sequence relatedness.
- –CP aa sequence identity less than ca. 80%,
- –nt sequence identity less than 85% over whole genome,
- –different polyprotein cleavage sites.
-
•Natural host range.
- –host range may be related to species but usually not helpful in identifying species; may delineate strains.
-
•Pathogenicity and cytopathology.
- –different inclusion body morphology,
- –lack of cross protection,
- –seed transmissibility, or lack thereof,
- –some aspects of host reaction may be useful (e.g., different responses in key host species, and particular genetic interactions).
-
•Mode of transmission.
- –different primary vectors, but vector species not of use in demarcating virus species.
-
•Antigenic properties.
- –serological differences.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported
GENUS TRITIMOVIRUS
Type Species Wheat streak mosaic virus
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments 690-700 nm long.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion S20w is 166S for Wheat streak mosaic virus (WSMV).
NUCLEIC ACID
Virions contain a positive sense ssRNA, ∼9.4-9.6 kb in size, with a 3′-poly(A) terminus.
PROTEINS
The viral CP is a single peptide of ∼349 aa for WSMV and 320 aa for Brome streak mosaic virus (BrSMV). The Mr estimated by electrophoresis is ∼42 kDa.
LIPIDS
None reported
CARBOHYDRATES
None reported
GENOME ORGANIZATION AND REPLICATION
The WSMV genome consists of 9384 nt excluding the 3′-terminal poly(A) tail. Sequence analysis reveals an ORF of 3035 aa (Fig. 5 ). The structure and organization of the WSMV genome is similar to those of other members of the family Potyviridae except the bymoviruses. Most known potyvirus motifs are present in the polyprotein of WSMV. However, motifs in the putative helper-component and CP of BrSMV are incomplete or missing, which may account for different vector relations of the tritimoviruses. The WSMV CP sequence shows limited (22-25%) identity with CP sequences of some aphid-transmitted potyviruses. WSMV shows significant aa sequence identity with aphid-transmitted potyviruses in the cylindrical inclusion protein and portions of the nuclear inclusion proteins. WSMV RNA has been translated in vitro into several large proteins immunoprecipitable with WSMV CP antiserum, suggesting that WSMV uses a proteolytic processing strategy to express functional proteins such as the CP. Antiserum to TEV 58 kDa nuclear inclusion protein also reacts with in vitro translation products of WSMV. An in vitro translation product is precipitated with antiserum to HC-Pro helper component of an isolate of Tobacco vein mottling virus. Comparative sequence analyses show similarities with other members of the family Potyviridae, but these are limited to the nine mature proteins. WSMV has CP molecules of 42, 36 and 32 kDa; the two smaller proteins are parts of the 42 kDa protein.
Figure 5.

Genomic map of an isolate of Wheat steak mosaic virus. The RNA genome is represented by thin lines and an open box which represent translated segments of the ssRNA. Conventions are as for the potyvirus genome organization map (Fig. 2). Activities of most gene products are postulated by analogy with genus Potyvirus.
ANTIGENIC PROPERTIES
Moderately immunogenic. The WSMV and ONMV are serologically related to each other, but not to the other members of the family Potyviridae.
BIOLOGICAL PROPERTIES
CYTOLOGY
Characteristic cytoplasmic cylindrical (“pinwheel”) inclusions are present in infected cells.
HOST RANGE
WSMV has a wide host range within the Graminae. BrSMV and ONMV have a narrow host range, also restricted to the Graminae.
TRANSMISSION
WSMV and BrSMV are transmitted by eriophyid mites in a semi-persistent manner. All definitive tritimoviruses are transmissible experimentally by mechanical inoculation.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•Genome sequence relatedness.
- –CP aa sequence identity less than ca. 80%,
- –nt sequence identity less than 85% over whole genome,
- –different polyprotein cleavage sites.
-
•Natural host range.
- –host range may be related to species but usually not helpful in identifying species. May delineate strains.
-
•Pathogenicity and cytopathology.
- –different inclusion body morphology,
- –lack of cross protection,
- –seed transmissibility, or lack thereof,
- –some aspects of host reaction may be useful (e.g., resistance genes, different responses in key host species).
-
•Mode of transmission.
- –different primary vectors, but vector species not of use in demarcating virus species.
-
•Antigenic properties.
- –serological differences.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported
TENTATIVE SPECIES IN THE GENUS
None reported
GENUS BYMOVIRUS
Type Species Barley yellow mosaic virus
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments of two modal lengths, 250-300 and 500-600 nm; both are 13 nm in width (Fig. 6 ).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion buoyant density in CsCl is 1.28-1.30 g/cm3.
NUCLEIC ACID
Virions contain two molecules of linear positive sense, ssRNA. RNA-1 is 7.5-8.0 kb and RNA-2 is 3.5-4.0 kb; RNA makes up 5% by weight of particles. Both RNA molecules have 3′-terminal poly(A) tracts. There is little base sequence homology between the two RNAs except in the 5′ NTR. The CP gene is located in the 3′-proximal region of RNA-1
PROTEINS
Virions have a single CP of 28.5-33 kDa. The CP of Barley yellow mosaic virus (BaYMV) has 297 aa.
LIPIDS
None reported
CARBOHYDRATES
None reported
GENOME ORGANIZATION AND REPLICATION
The two RNA molecules appear to be translated initially into precursor polypeptides from which functional proteins are derived by proteolytic processing (Fig. 4).
Figure 7.

Genomic map of the bymovirus bipartite genome, using, as an example, an isolate of Barley yellow mosaic virus. Conventions are as for potyvirus genome organization map (Fig. 2). Function of most gene products are postulated by analogy with genus Potyvirus. P1 corresponds to the C-terminal protease of HC-Pro
ANTIGENIC PROPERTIES
The viral proteins are moderately immunogenic; serological relationships exist among members except Barley mild mosaic virus (BaMMV). The CP aa sequence identity among members is 35-74%.
BIOLOGICAL PROPERTIES
CYTOLOGY
There are characteristic pinwheel-like inclusions and membranous network structures are formed in the cytoplasm of infected plant cells. No nuclear inclusions are found.
HOST RANGE
The host range of member viruses is narrow, restricted to the host family Graminae.
TRANSMISSION
These viruses are transmitted by Polymyxa graminis in a persistent manner, surviving in resting spores as long as these remain viable; transmissible experimentally by mechanical inoculation.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•Genome sequence relatedness.
- –CP aa sequence identity less than ca. 80%,
- –nt sequence identity less than 85% over whole genome,
- –different polyprotein cleavage sites.
-
•Natural host range.
- –host range may be related to species but usually not helpful in identifying species.
-
•Mode of transmission.
- –transmitted by Polymyxa graminis.
-
•Antigenic properties.
- –serological differences.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Barley mild mosaic virus | ||
| Barley mild mosaic virus | [AF536942, AJ242725, L49381, D83408-9, X75933, X82625, X84802, X90904, Y10973-4,] | (BaMMV) |
| Barley yellow mosaic virus | ||
| Barley yellow mosaic virus | [AF536958, AJ132268-9, AJ515479-85, D01091-2, D01099, X69757] | (BaYMV) |
| Oat mosaic virus | ||
| Oat mosaic virus | [AJ306718-9] | (OMV) |
| Rice necrosis mosaic virus | ||
| Rice necrosis mosaic virus | (RNMV) | |
| Wheat spindle streak mosaic virus | ||
| Wheat spindle streak mosaic virus | (WSSMV) | |
| Wheat yellow mosaic virus | ||
| Wheat yellow mosaic virus | [AF041041, AF067124, AJ131981-2, AJ239039, AJ242490, D86634-5] | (WYMV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED SPECIES IN THE FAMILY
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
Three viruses have been assigned to the family Potyviridae on the basis of similarity to recognized members of the family. TomMMoV, SpMoV and SCSMV have CP aa sequence similarity to other members of the family Potyviridae, but are sufficiently different from other members of the family to suggest that they may represent new genera. TomMMoV is most closely related to members of the genus Ipomovirus, but differs from other members of this genus on the basis of vector relationships; ipomoviruses are transmitted by the whitefly Bemisia tabaci while TomMMoV is transmitted in a non-circulative manner by aphids. No vectors have been identified for SpMoV or SCSMV.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Figure 8.

Phylogenetic tree for the family Potyviridae using aa sequence relationships of the CP. Inference based on the Fitch-Margoliash (1967) least squares method. The sequences were aligned using PILEUP (Devereux et al., 1984). Branch lengths are proportional to sequence distances. The dendrogram was bootstrapped 1000 times (percent scores shown at nodes; bootstrap values for genus Potyvirus not shown), and the tree is not rooted. Many branches are condensed and may contain multiple viruses, e.g., BCMV, for example, includes BCMNV, CABMV, DMV, PWV, SAPV, SMV, WMV and ZYMV, AzMV, BlCMV, DeMV, and PStV. Signification of abbreviations are in the “List of Species”.
SIMILARITY WITH OTHER TAXA
Viruses in the family Potyviridae have similarity to members of the families Comoviridae, Picornaviridae, and Hypoviridae. Genomes of member viruses of these taxa are positive sense ssRNAs, except those of the family Hypoviridae, which have dsRNA genomes. Most have a VPg at their 5′-termini and a poly(A) tract at their 3′-termini. Their genomes are expressed initially as high molecular weight polyprotein precursors which are processed by virus-encoded proteases. Gene products involved in replication are conserved in gene order and gene sequence.
DERIVATION OF NAMES
Bymo: sigla from Barley yellow mosaic. Ipomo: sigla from Ipomea and mosaic. Maclura: sigla from host genus name “Maclura”. Poty: sigla from Potato virus Y. Rymo: siglafrom Ryegrass mosaic. Tritimo: sigla from Triticum and mosaic.
REFERENCES
- Berger P.H., Wyatt S.D., Shiel P.J., Silbernagel M.J., Druffel K., Mink G.I. Phylogenetic analysis of the Potyviridae with emphasis on legume-infecting potyviruses. Arch. Virol. 1997;142:1979–1999. doi: 10.1007/s007050050216. [DOI] [PubMed] [Google Scholar]
- Badge J., Robinson D.J., Brunt A.A., Foster G.D. 3’-terminal sequences of the RNA genomes of narcissus latent and Maclura mosaic viruses suggest that they represent a new genus of the Potyviridae. J. Gen. Virol. 1997;78:253–257. doi: 10.1099/0022-1317-78-1-253. [DOI] [PubMed] [Google Scholar]
- Barnett O.W., editor. Potyvirus taxonomy. Springer-Verlag; Vienna: 1992. [Google Scholar]
- Colinet D., Kummert J., Lepoivre P. The nucleotide sequence and genome organization of the whitefly transmitted sweet potato mild mottle virus: A close relationship with members of the family Potyviridae. Virus Res. 1998;53:187–196. doi: 10.1016/s0168-1702(97)00148-2. [DOI] [PubMed] [Google Scholar]
- Dougherty W.G., Carrington J.C. Expression and function of potyviral gene products. Ann. Rev. Phytopathol. 1988;26:123–143. [Google Scholar]
- Dougherty W.G., Parks T.D. Post-translational processing of the tobacco etch virus 49-kDa small nuclear inclusion polyprotein: identification of an internal cleavage site and delimitation of VPg and proteinase domains. Virology. 1991;183:449–456. doi: 10.1016/0042-6822(91)90974-g. [DOI] [PubMed] [Google Scholar]
- Edwardson J.R., Christie R.G., editors. I-IV. Univ Florida; Agric Exp Stn. Mono 16, Gainesville Florida: 1991. (The potyvirus group). [Google Scholar]
- Götz R., Huth W., Lesemann D.-E., Maiss E. Molecular and serological relationships of Spartina mottle virus (SpMV) strains from Spartina spec. and from Cynodon dactylon to other members of the Potyviridae. Arch. Virol. 2002;147:379–391. doi: 10.1007/s705-002-8326-x. [DOI] [PubMed] [Google Scholar]
- Götz R., Maiss E. The complete nucleotide sequence and genome organization of the mite-transmitted brome streak mosaic rymovirus in comparison with those of potyviruses. J. Gen. Virol. 1995;76:2035–2042. doi: 10.1099/0022-1317-76-8-2035. [DOI] [PubMed] [Google Scholar]
- Hall J.S., Adams B., Parsons T.J., French R., Lane L.C., Jensen S.G. Molecular cloning, sequencing, and phylogenetic relationships of a new potyvirus: sugarcane streak mosaic virus, and a reevaluation of the classification of the Potyviridae. Mol. Phylogenet. Evol. 1998;10:323–332. doi: 10.1006/mpev.1998.0535. [DOI] [PubMed] [Google Scholar]
- Jordan R., Hammond J. Comparison and differentiation of potyvirus isolates and identification of strain-, virus-, subgroup-specific and potyvirus group-common epitopes using monoclonal antibodies. J. Gen. Virol. 1991;72:25–36. doi: 10.1099/0022-1317-72-1-25. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki S., Minobe Y., Hibino H. Nucleotide sequence of barley yellow mosaic virus RNA 2. J. Gen. Virol. 1991;72:995–999. doi: 10.1099/0022-1317-72-4-995. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki S., Minobe Y., Omura T., Hibino H. Nucleotide sequence of barley yellow mosaic virus RNA 1: a close evolutionary relationship with potyviruses. J. Gen. Virol. 1990;71:2781–2790. doi: 10.1099/0022-1317-71-12-2781. [DOI] [PubMed] [Google Scholar]
- Monger W.A., Spence N.J., Foster G.D. Molecular evidence that the aphid-transmitted Tomato mild mottle virus belongs to the Potyviridae family but not the Potyvirus genus. Arch. Virol. 2002;146:2345–2441. doi: 10.1007/s007050170013. [DOI] [PubMed] [Google Scholar]
- Revers F., Le Gall O., Candresse T., Maule A.J. New advances in understanding the molecular biology of plant/potyvirus interactions. MPMI. 1999;12:367–376. [Google Scholar]
- Salm S.N., Rey M.E.C., Rybicki E.P. Phylogenetic justification for splitting the Rymovirus genus of the taxonomic family Potyviridae. Arch. Virol. 1996;141:2237–2242. doi: 10.1007/BF01718229. [DOI] [PubMed] [Google Scholar]
- Shukla D.D., Ward C.W., Brunt A.A., editors. The Potyviridae. CAB International; Wallingford, UK: 1994. [Google Scholar]
- Stenger D.C., Hall J.S., Choi I.R., French R. Phylogenetic relationships within the family Potyviridae: Wheat streak mosaic virus and brome streak mosaic virus are not members of the genus Rymovirus. Phytopathology. 1998;88:782–787. doi: 10.1094/PHYTO.1998.88.8.782. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, M.K. Koopmans, K.Y. Green, T. Ando, I.N. Clarke, M.K. Estes, D.O. Matson, S. Nakata, J.D. Neill, A.W. Smith, M.J. Studdert, H.-J. Thiel
FAMILY CALICIVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Caliciviridae |
| Genus | Lagovirus |
| Genus | Norovirus |
| Genus | Sapovirus |
| Genus | Vesivirus |
VIRION PROPERTIES
MORPHOLOGY
Virions are nonenveloped with icosahedral symmetry. They are 27-40 nm in diameter by negative stain electron microscopy and 35-40 nm by electron cryo-microscopy. The capsid is composed of 90 dimers of the major structural protein arranged on a T=3 icosahedral lattice. A characteristic feature of the capsid architecture is the 32 cup-shaped depressions at each of the icosahedral 5-fold and 3-fold axes. In some negative stain virus preparations, the cup-shaped depressions appear distinct and well-defined, while in others, these depressions are less prominent (Fig. 1 ).
Figure 1.

(Top left) Cryo-image reconstruction of recombinant Norwalk virus (NV)-like particles (rNV VLPs). (Top central) Cryo-image reconstruction of Primate calicivirus. A set of icosahedral 5- and 3-fold axes is marked. (Courtesy of Prasad, B.V.V.). (Top right) Central cross-section of rNV VLPs. (Bottom left) Electronic rendering of Norwalk virus (Prasad et al.,1999). (Bottom center) Diagram representing a T=3 icosahedral structure. (Bottom right) Negative stain electron micrographs of Bovine calicivirus particles (Courtesy of S. McNulty). The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is ∼15 × 106. Virion buoyant density is 1.33-1.41 g/cm3 in CsCl and 1.29 g/cm3 in glycerol-potassium tartrate gradients. Virion S20w is 170-187S. Physicochemical properties have not been fully established for all members of the family. Rabbit hemorrhagic disease virus (RHDV) in the genus Lagovirus has been reported as stable over a wide range of pH values (4.-10.5). The genus Norovirus has been shown in studies with one of its members (Norwalk virus, NV) to be acid, ether, and relatively heat stable. For strains examined in the genus Vesivirus, inactivation occurs at pH 3-5, thermal inactivation is accelerated in high concentrations of Mg++ ions, and virions are insensitive to treatment with ether, chloroform, or mild detergents.
NUCLEIC ACID
The genome consists of a linear, positive sense, ssRNA molecule of 7.4-8.3 kb. A protein (VPg, 10-15 kDa) is covalently attached to the 5′-end of the genomic RNA and the 3′-end is polyadenylated. SgRNA (2.2-2.4 kb) is synthesized intracellularly and is VPg-linked in RHDV and Feline calicivirus (FCV). The FCV sgRNA can be packaged into viral particles with lower density than the particles with the full-length genome. The gene order for RHDV and FCV was determined by in vitro translation studies and cleavage mapping, respectively, as 5′-p16-p23-p37(helicase)-p30-VPg-protease-polymerase-VP60 (major CP)-VP10-3′ (RHDV), and 5′-p5.6-p32-p39 (helicase)-p30-p13 (VPg)-p76 (Pro-Pol)-VP62 (major CP)-VP8.5 (minor CP).
PROTEINS
Virions are constructed predominantly from one major species of CP (58-60 kDa). A second minor structural protein (8.5-23 kDa) has been found in association with FCV, NV, and RHDV virions. Nonstructural proteins have homology with those of the family Picornaviridae replicative enzymes and include 2C helicase, 3C cysteine protease, and 3D RdRp domains. The calicivirus VPg (10-15 kDa) is covalently linked to the viral RNA and maps to the region of the calicivirus genome analogous to the 3B region of picornaviruses, but has no apparent amino acid homology with those of picornaviruses. Mapping studies are in progress to establish precursor and product relationships of the calicivirus nonstructural proteins.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The positive strand genomic RNA is organized into either two or three major ORFs. The nonstructural proteins are encoded in the 5′-end of the genome and the structural proteins in the 3′-end. Replication occurs in the cytoplasm and two major positive-sense RNA species are found in infected cells. The genome-sized positive-sense RNA serves as the template for translation of a large polyprotein that undergoes cleavage by a virus-encoded protease to form the mature nonstructural proteins. A subgenomic-sized positive strand RNA, co-terminal with the 3′-end of the genome, is the template for translation of the major viral CP as well as the 3′-terminal ORF product. A dsRNA corresponding in size to full-length genomic RNA has been identified in FCV and San Miguel sea lion virus (SMSV)-infected cells, indicating that replication occurs via a negative strand intermediate (Fig. 2 ).
Figure 2.

Genome organizations of viruses of the family Caliciviridae. The genomic organization and ORF usage are shown for representative species (with strain indication shown in brackets) in the following genera: Lagovirus: Rabbit hemorrhagic disease virus (RHDV) [Ra/LV/RHDV/GH/1988/GE]; Norovirus: Norwalk virus (NV) [Hu/NV/Nor/1968/US]; Sapovirus: Sapporo virus (SV) [Hu/SV/Man/1993/UK]; and Vesivirus: Feline calicivirus (FCV) [Fe/VV/FCV/F9/1958/US. Viruses in two genera (Lagovirus and Sapovirus) contain a large ORF1 in which the nonstructural polyprotein gene is continuous and in frame with the CP coding sequence. Some strains in the genus Sapovirus encode a third predicted ORF that overlaps ORF1 (not shown). Viruses in the other two genera (Norovirus and Vesivirus) encode the major structural CP in a separate reading frame (ORF2). The RNA helicase (HEL), protease (PRO), and polymerase (POL) regions of the genome are indicated. The linkage of VPg to the RNA of viruses in the genera Norovirus and Sapovirus has not been confirmed. The designated VPg region of the genomes of representative viruses from the genera Norovirus, Sapovirus, and Vesivirus is shown by homology with the mapped VPg of RHDV. The shaded region of the ORF2 of the representative member of the genus Vesivirus illustrates the leader sequence (approximately 125 aa in length) of the precursor CP. Studies of FCV and RHDV have identified two major positive sense RNA molecules in infected cells. One RNA molecule corresponds in size to the full-length genome and the other, a subgenomic-sized RNA, is co-terminal with the 3′-end of the genome. The sgRNA is the template for translation of the major viral CP and the 3′-terminal ORF product that has been identified as a minor structural protein in FCV.
ANTIGENIC PROPERTIES
Cross-challenge studies in the natural host and experiments with monoclonal antibodies indicate that RHDV and European brown hare syndrome virus (EBHSV) are antigenically distinct. Antigenic types have been defined by cross-challenge studies, immune electron microscopy or solid phase immune electron microscopy for noncultivatable strains in the genera Norovirus and Sapovirus. Numerous serotypes have been established by neutralization for Vesicular exanthema of swine virus (VESV) and SMSV strains. One serotype has been described for FCV strains, but considerable antigenic variation within this serotype has been reported. Recombinant virus-like particles (rVLPs) have been generated by expression of the major calicivirus structural CP in baculovirus and plant expression systems. These VLPs are highly immunogenic and similar in antigenicity to native virions.
BIOLOGICAL PROPERTIES
Caliciviruses infect a broad range of animals that includes hares, rabbits, pigs, cats, pinnipeds, mice, cattle, reptiles, skunks, cetaceans, chimpanzees, and humans. Although individual calicivirus species generally exhibit a natural host restriction, the VESV species of the genus Vesivirus is an exception, showing a broad host range. For example, VESV-like viruses have been isolated from several marine animal species (including fish), birds, reptiles, and land mammals. The geographic distribution of each calicivirus species usually reflects the host distribution.
Transmission is via contaminated food, water, fomites, and on occasion via aerosolization of fecal material, vomitus or respiratory secretions. In general, no vectors appear to be involved in transmission; however, mechanical arthropod vector transmission of RHDV has been described.
Caliciviruses are associated with a number of disease syndromes. RHDV is associated with a generalized viremic infection in which there is massive liver necrosis that triggers a disseminated intravascular coagulation and rapid death in rabbits greater than three months of age. A nonvirulent RHDV strain has been described. EBHSV is similar to RHDV but appears to be less virulent. Human caliciviruses in the genera Norovirus and Sapovirus induce a generally self-limited gastroenteritis with symptoms that may include nausea, diarrhea, vomiting, abdominal cramping, fever, and malaise. VESV produces clinical signs in swine that are sometimes indistinguishable from foot-and-mouth disease, including vesicles in the mouth, tongue, lips, snout and feet between the digits. In addition, the virus may cause encephalitis, myocarditis, fever, diarrhea, abortion and failure of infected animals to thrive. SMSV is similar to VESV, although there have been limited studies of natural infection in the marine host. Primate calicivirus causes mucosal vesiculation and persistent infection. FCV is associated in cats with conjunctivitis, rhinitis, pneumonia, mucosal vesiculation, diarrhea, urinary tract infection, and paresis and can produce a persistent infection with virus latent in the tonsils.
GENUS LAGOVIRUS
Type Species Rabbit hemorrhagic disease virus
DISTINGUISHING FEATURES
The strains in this genus form a distinct phylogenetic clade within the family. The genome is organized into two major ORFs. ORF1 encodes the nonstructural polyprotein, with the major structural CP gene (VP60) in frame with the nonstructural polyprotein coding sequence. ORF2 overlaps ORF1 by 17 nt in the RHDV genome and 5 nt in the EBHSV genome. The ORF2 encodes a small protein (VP10) of unknown function that has been identified as a minor structural component in the RHDV virion. These viruses have characteristically been associated with infection in rabbits and hares (lagomorphs), and can cause epidemics with high mortality in these animals.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a Lagovirus species share:
-
•
a major phylogenetic branch within the genus,
-
•
a common genome layout,
-
•
greater than 80% aa identity in the CP,
-
•
a natural host range,
-
•
cross-protection antigens.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| European brown hare syndrome virus | ||
| EBHSV-BS89 | [X98002] | (Ha/LV/EBHSV/BS89/1989/IT) |
| EBHSV-FRG | [U09199] | (Ha/LV/EBHSV/FRG/1989/GE) |
| EBHSV-GD | [Z69620] | (Ha/LV/EBHSV/GD/1989/FR) |
| EBHSV-UK91 | [U65372] | (Ha/LV/EBHSV/UK91/1991/UK) |
| Rabbit hemorrhagic disease virus | ||
| Rabbit calicivirus | [X96868] | (Ra/LV/RHDV/RCV/1995/IT) |
| RHDV-AST89 | [Z49271] | (Ra/LV/RHDV/AST89/1989/SP) |
| RHDV-BS89 | [X87607] | (Ra/LV/RHDV/BS89/1989/IT) |
| RHDV-FRG | [M67473] | (Ra/LV/RHDV/GH/1988/GE) |
| RHDV-SD | [Z29514] | (Ra/LV/RHDV/SD/1989/FR) |
| RHDV-V351 | [U54983] | (Ra/LV/RHDV/V351/1987/CK) |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS NOROVIRUS
Type Species Norwalk virus
DISTINGUISHING FEATURES
The strains in this genus form a distinct phylogenetic clade within the family. The genome is organized into three major ORFs. ORF1 encodes the nonstructural polyprotein. ORF2 encodes the major structural CP and overlaps by 14 nt with ORF1 in the Norwalk and Southampton virus strains and by 17 nt in the Lordsdale virus strain, resulting in a −2 frameshift of ORF2 in all three viruses. ORF3 overlaps by one nt with ORF2 in a −1 frameshift and encodes a small virion-associated protein. Members of the genus often (but not always) have a less-defined surface structure when observed by negative stain electron microscopy, leading to the now historic descriptive term “small round structured viruses” for the viruses in this genus associated with epidemic gastroenteritis in humans.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable. Additional characterization of the viruses in this genus will be required in order to delineate species criteria.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Norwalk virus | ||
| Desert Shield virus | [U04469] | (Hu/NV/DSV395/1990/SR) |
| Hawaii virus | [U07611] | (Hu/NV/HV/1971/US) |
| Lordsdale virus | [X86557] | (Hu/NV/LD/1993/UK) |
| Mexico virus | [U22498] | (Hu/NV/MX/1989/MX) |
| Norwalk virus | [M87661] | (Hu/NV/NV/1968/US) |
| Snow Mountain virus | [L23831] | (Hu/NV/SMV/1976/US) |
| Southampton virus | [L07418] | (Hu/NV/SHV/1991/UK) |
TENTATIVE SPECIES IN THE GENUS
GENUS VESIVIRUS
Type Species Sapporo virus
DISTINGUISHING FEATURES
The strains in this genus form a distinct phylogenetic clade within the family. The full-length genomic sequence is available for the Manchester virus, and for a porcine enteric calicivirus (PEC strain Cowden). The Manchester virus genome is organized into three major ORFs. ORF1 encodes the non-structural polyprotein, with the major structural CP gene in frame with the nonstructural polyprotein coding sequence. ORF2 overlaps ORF1 in a −1 frameshift and encodes a predicted small protein of unknown function. ORF3 begins 11 nt downstream from the predicted start codon of the CP in a +1 frameshift and encodes a predicted protein of ∼160 aa. However, in certain strains of this genus, and in the PEC Cowden strain, ORF3 is absent. The viruses in this genus have characteristically been associated with sporadic outbreaks and cases of gastroenteritis in humans and diarrhea in pigs, and often (but not always) have distinct calicivirus cup-like morphology when observed by negative stain electron microscopy.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable. Additional characterization of the viruses in this genus will be required in order to delineate species criteria.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Sapporo virus | ||
| Sapporo virus | [U65427] | (Hu/SV/SV/1982/JA) |
| Sapporo virus Houston/86 | [U95643] | (Hu/SV/Hou/1986/US) |
| Sapporo virus Houston/90 | [U95644] | (Hu/SV/Hou 27/1990/US) |
| Sapporo virus London 29845 | [U95645] | (Hu/SV/Lon 29845/1992/UK) |
| Sapporo virus Manchester virus | [X86560] | (Hu/SV/Man/1993/UK) |
| Sapporo virus Parkville virus | [U73124] | (Hu/SV/Park/1994/US) |
TENTATIVE SPECIES IN THE GENUS
GENUS VESIVIRUS
Type Species Vesicular exanthema of swine virus
DISTINGUISHING FEATURES
The species in this genus form a distinct phylogenetic clade within the family. The genome is organized into three major ORFs. ORF1 encodes the nonstructural polyprotein.
ORF2 encodes the major structural CP that is translated as a larger precursor protein before cleavage into the mature CP, a feature that appears unique to this genus. ORF1 and ORF2 of the viruses in this genus are separated by either 2 nt (GC for FCV strains) or 5 nt (CCACT/C for SMSV and VESV strains). A third ORF (ORF3) encodes a small, basic protein of unknown function and overlaps by one nt with ORF2 in a −1 frameshift. The ORF3 product has been detected in FCV-infected cells. Most members of this genus can be readily propagated in cell culture, which contrasts with viruses in the other three genera, none of which has been cultured in conventional cell culture systems. FCV grows most efficiently in cells of feline origin; in vivo, the primary site of replication is the upper respiratory tract. VESV isolates grow in a number of cell lines and infect a broad range of hosts, with vesicles of the skin a prevalent disease symptom with the possible exception of the canine caliciviruses. The RNA genomes of VESV and FCV are infectious, as is synthetic RNA derived from a full-length cDNA clone of the FCV genome.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Members of a Vesivirus species share:
-
•
a major phylogenetic branch within the genus,
-
•
a common genome layout,
-
•
greater than 60% aa identity in the CP.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Feline calicivirus | ||
| Feline calicivirus CFI/68 | [U13992] | (Fe/VV/FCV/CFI/1968/US) |
| Feline calicivirus F9 | [M86379] | (Fe/VV/FCV/F9/1958/US) |
| Vesicular exanthema of swine virus | ||
| Bovine calicivirus | [U18741] | (Bo/VV/VESV/Bos-1/1981/US) |
| Cetacean calicivirus | [U52091] | (Ce/VV/VESV/Tur-1/1977/US) |
| Primate calicivirus | [U52086] | (Pr/VV/VESV/Pan-1/1979/US) |
| Reptile calicivirus | [U52092] | (Re/VV/VESV/Cro-1/1978/US) |
| San Miguel sea lion virus, serotype 1 | [M87481] | (Pi/VV/VESV/SMSV-1/1972/US) |
| San Miguel sea lion virus, serotype 4 | [M87482] | (Pi/VV/VESV/SMSV-4/1973/US) |
| San Miguel sea lion virus, serotype 17 | [U52005] | (Pi/VV/VESV/SMSV-17/1991/US) |
| Skunk calicivirus | [U14667] | (Pi/VV/VESV/SCV/1992/US) |
| Vesicular exanthema of swine virus-A48 | [U76874] | (Sw/VV/VESV/A48/1948/US) |
TENTATIVE SPECIES IN THE GENUS
| Mink calicivirus | [AF338407] | (Mi/VV/MCV 20/1980/US) |
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
| Amyelosis chronic stunt virus (insects) | (ACSV) |
| Bovine enteric calicivirus strain NB | (BEC-NB) |
| Canine calicivirus | (CaCV) |
| Fowl calicivirus | (FCV) |
| Walrus calicivirus | (WCV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Figure 3.

Phylogenetic relationships within the family Caliciviridae and comparison with the family Picornaviridae (Courtesy of E. van Strien and H. Vennema). Full-length capsid aa sequences were used for the phylogenetic analysis and included representative strains from each genus in the family Caliciviridae. Clustal W analysis was used to create a multiple alignment for the aa sequences, which were verified by alignment of known motifs in the region (e.g., PPG/N). The PHYLIP v3.5c package was used for the phylogenetic analyses of the aligned nt sequences. The multiple alignment data were bootstrapped (N = 1000) and submitted for the distance method. The unrooted phylogenetic tree is presented. Scale bar represents units for expected number of substitutions per site. The following numbers represent the phylogenetic distances, in units of expected number of substitutions per site, among the calicivirus genera (representing distinct clades) as measured by the distances between the first branchpoints of: Norovirus and Sapovirus, 1.59; Norovirus and Lagovirus, 1.48; Norovirus and Vesivirus, 1.46; Sapovirus and Lagovirus, 1.21; Sapovirus and Vesivirus, 0.84; and Lagovirus and Vesivirus, 1.07. A fifth branch is constituted by the Bovine enteric calicivirus strain NB, which has not yet been assigned. The BEC-NB is at present the sole representative of this potential new genus. Genbank accession numbers for the strains in this analysis were: AB032758, AF053720, AF091736, AF182760, AF195847, AJ011099, AY082890, AY228235, L07418, M67473, M86379, M87481, M87482, M87661, NC_004541, U04469, U07611, U13992, U22498, U52005, U65427, U76874, U95644, U95645, X86557, Z69620.
SIMILARITY WITH OTHER TAXA
Caliciviruses have some properties similar to the viruses of the families Picornaviridae, Potyviridae and Comoviridae relative to the presence of a VPg at the 5′-end and a poly(A) tract at the 3′-end of the positive sense ssRNA genome. The putative viral replicase of caliciviruses shares sequence homology with that of picornaviruses.
DERIVATION OF NAMES
Calici: from Latin calix, “cup” or “goblet”, from cup-shaped depressions on the virion
surface observed by electron microscopy.
Lago: from Lagomorpha, the mammalian host order for the prototype strain rabbit
hemorrhagic disease virus.
Noro: modified from the type species name, Norwalk virus
Sapo: modified from the type species name, Sapporo virus
Vesi: from the type species name, vesicular exanthema of swine virus.
REFERENCES
- Berke T., Golding B., Jiang X., Cubitt D.W., Wolfaardt M., Smith A.W., Matson D.O. Phylogenetic analysis of the caliciviruses. J. Med. Virol. 1997;52:419–424. doi: 10.1002/(sici)1096-9071(199708)52:4<419::aid-jmv13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Carter M.J., Milton I.D., Meanger J., Bennett M., Gaskell R.M., Turner P.C. The complete nucleotide sequence of a feline calicivirus. Virology. 1992;190:443–448. doi: 10.1016/0042-6822(92)91231-i. [DOI] [PubMed] [Google Scholar]
- Green K.Y., Chancock R.M., Kapikian A.Z. Human caliciviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 841–874. [Google Scholar]
- Guo M., Chang K.O., Hardy M.E., Zhang Q., Parwani A.V., Saif L.J. Molecular characterization of a porcine enteric calicivirus genetically related to Sapporo-like human caliciviruses. J. Virol. 1999;73:9531–9625. doi: 10.1128/jvi.73.11.9625-9631.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Evermann J.F., Saif L.J. Detection and molecular characterization of cultivable caliciviruses from clinically normal mink and enteric caliciviruses associated with diarrhea in mink. Arch Virol. 2001;146:479–493. doi: 10.1007/s007050170157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang M., Wang K., Estes M.K. Sequence and genome organization of Norwalk virus. Virology. 1993;195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- Lambden P.R., Caul E.O., Ashley C.R., Clarke I.N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993;259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- le Gall G., Huguet S., Vende P., Vautherot J.-F., Rasschaert D. European brown hare syndrome virus: molecular cloning and sequencing of the genome. J. Gen. Virol. 1996;77:1693–1697. doi: 10.1099/0022-1317-77-8-1693. [DOI] [PubMed] [Google Scholar]
- Liu B.L., Clarke I.N., Caul E.O., Lambden P.R. Human enteric caliciviruses have a unique genome structure and are distinct from the Norwalk-like viruses. Arch. Virol. 1995;140:1345–1356. doi: 10.1007/BF01322662. [DOI] [PubMed] [Google Scholar]
- Liu B.L., Lambden P.R., Gunther H., Otto P., Elschner M., Clarke I.N. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 1999;73:819–825. doi: 10.1128/jvi.73.1.819-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers G., Wirblich C., Thiel H.-J. Rabbit hemorrhagic disease virus-molecular cloning and nucleotide sequence of a calicivirus genome. Virology. 1991;184:664–676. doi: 10.1016/0042-6822(91)90436-f. [DOI] [PubMed] [Google Scholar]
- Neill J.D., Meyer R.F., Seal B.S. Genetic relatedness of the caliciviruses: San Miguel sea lion and vesicular exanthema of swine viruses constitute a single genotype within the Caliciviridae. J. Virol. 1995;69:4484–4488. doi: 10.1128/jvi.69.7.4484-4488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill J.D. The subgenomic RNA of feline calicivirus is packaged into viral particles during infection. Virus Res. 2002;87:89–93. doi: 10.1016/s0168-1702(02)00086-2. [DOI] [PubMed] [Google Scholar]
- Noel J.S., Liu B.L., Humphrey C.D., Rodriguez E.M., Lambden P.R., Clarke I.N., Dwyer D.M., Ando T., Glass R.I., Monroe S.S. Parkville virus: a novel genetic variant of human calicivirus in the Sapporo virus clade, associated with an outbreak of gastroenteritis in adults. J. Med. Virol. 1997;52:173–178. [PubMed] [Google Scholar]
- Numata K., Hardy M.E., Nakata S., Chiba S., Estes M.K. Molecular characterization of morphologically typical human calicivirus Sapporo. Arch. Virol. 1997;142:1537–1552. doi: 10.1007/s007050050178. [DOI] [PubMed] [Google Scholar]
- Prasad B.V., Hardy M.E., Dokland T., Bella J., Rossmann M.G., Estes M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- Wirblich C., Meyers G., Ohlinger V.F., Capucci L., Eskens U., Haas B., Thiel H.-J. European brown hare syndrome virus: relationship to rabbit hemorrhagic disease virus and other caliciviruses. J. Virol. 1994;68:5164–5173. doi: 10.1128/jvi.68.8.5164-5173.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONTRIBUTED BY, S.U. Emerson, D. Anderson, A. Arankalle, X.-J. Meng, M. Purdy, G.G. Schlauder, S.A. Tsarev
GENUS HEPEVIRUS
Type Species Hepatitis E virus
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

Negative contrast electron micrograph of virions of an isolate of Hepatitis E virus, in the bile fluid from a monkey challenged with the Mexico strain of human Hepatitis E virus. The bar represents 100 nm. (From Ticehurst et al., 1992, with permission).
Virions (27-34 nm) are icosahedral and non-enveloped.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion buoyant density is 1.35 g/cm3 in CsCl and 1.29 g/cm3 in glycerol potassium tartrate gradients. Virion S20w is 183S.
NUCLEIC ACID
The genome is a positive sense ssRNA of approximately 7.2 kb, with a 5′ m7G cap and a 3′-poly(A) tail.
PROTEINS
Virions are constructed from a major CP (72 kDa) which may be proteolytically processed: its size in the virion is unknown. A small immunoreactive protein (1.5 kDa) of unknown function has been identified. Non-structural proteins have limited similarity with the “alpha-like supergroup” of viruses and include domains consistent with a Mtr, RNA helicase, papain-like cysteine protease and RdRp. RdRp and Mtr/guanlytransferase activities have been demonstrated for ORF1 recombinant proteins.
LIPIDS
None reported.
CARBOHYDRATES
Evidence for glycosylation of the major CP in mammalian expression studies has been reported, but the biological significance is unknown.
GENOME ORGANIZATION AND REPLICATION
The RNA genome of Hepatitis E virus (HEV) is organized into three ORFs, with the non-structural proteins encoded toward the 5′-end of the genome and the structural protein(s) toward the 3′-end. Capped genomic RNA is infectious for rhesus monkeys and chimpanzees. The 5′-NTR is only ∼26 nt long. The 3′-NTR contains a cis-reactive element. ORF1 encodes the non-structural polyprotein. ORF2 encodes the major CP. A third ORF overlaps ORF1 and ORF2 and encodes a small phosphoprotein (123 aa) of unknown function (Fig. 2 ). ORF2 and ORF3 are thought to be translated from subgenomic messenger RNAs.
Figure 2.

Genome organization of Hepatitis E virus (HEV) (human strain Burma, M73218). The putative MTR, Pro, “X”, Hel, and RdRp domains are indicated.
ANTIGENIC PROPERTIES
A single serotype has been described, with extensive cross-reactivity among circulating human and swine strains. Antibodies cross-reactive with ORF2 epitopes of human strains have been identified in numerous species of rodents and other mammals but the putative viruses have not been characterized. A distantly related virus that infects, and causes hepatitis in, chickens also cross-reacts serologically.
BIOLOGICAL PROPERTIES
HEV is associated in humans with outbreaks and sporadic cases of enterically transmitted acute hepatitis. The virus is considered endemic in tropical and subtropical countries of Asia and Africa, as well as in Mexico, but antibody prevalence studies suggest global distribution of this virus, perhaps in a non-pathogenic form. Antibody prevalence studies have found evidence for HEV or a related agent in animals and there is speculation that the virus may be zoonotic. HEV strains have been identified in swine and are closely related antigenically and genetically to human HEV genotype 3 and 4 strains. Interspecies transmission of genotype 3 HEV between swine and primates has been experimentally demonstrated but direct evidence for natural interspecies transmission has not been obtained.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
| Avian hepatitis E virus | [AY043166] | (AHEV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Figure 4.

Unrooted phylogenetic tree depicting the relationship of nt sequences over a 287 nt fragment of ORF1. Isolates represented are Burma (B1, B2), China/Taiwan (C1, C2, C3, C4, Cs15, Ct1), Pakistan, (P1), India (I1, I2), Mexico (M1), United States (US1, US2), Greece (Gr1, Gr2), Spain (Sp1, Sp2), Italy (It1), Argentina (Ar1, Ar2), Austria (Au1), swine US (swUS1), swine New Zealand (swNZ1). The Arabic number adjacent to the shaded circles indicates genotypic designations. The dashed lines and circles indicate the putative positions of additional isolates from China/Taiwan (Cx), Nigeria (Nix) and swine sewage from Spain (swSp?) based on overlap of ORF 2 sequence. (From Schlauder and Mushahwar, 2001, with permission.)
SIMILARITY WITH OTHER TAXA
HEV is similar to members of the family Caliciviridae in its structural morphology, as assessed by electron microscopy, and in its genome organization. HEV shows highest, but limited, aa similarity in its replicative enzymes with Rubella virus and alphaviruses of the family Togaviridae and with plant furoviruses. The HEV capping enzyme has properties very similar to those of viruses within the “alphavirus-like supergroup”.
Figure 3.

Phylogenetic relationships of Hepatitis E virus (HEV) with members of the families Picornaviridae, Caliciviridae, and Togaviridae. The helicase (Hel) and polymerase (Pol) regions of the genome were analyzed (Courtesy of Berke, T. and Matson, D.O.).
A. Partial gene sequences (200 aa) from the proposed helicase region were used for the phylogenetic analysis and included representative strains from each family. Clustal W v1.7 was used to create a multiple alignment for the aa sequences, which was verified by alignment of known motifs in the region (e.g. GxGKS/T). The nt sequences were added and aligned by hand using the corresponding aa sequences as template resulting in a consensus length of 608 nt. A phylogenetic tree was constructed from the nt sequence alignment using the maximum likelihood algorithm in the program DNAML from the PHYLIP 3.52c package within UNIX environment. For the algorithm, the global rearrangement option was invoked and the order of sequence input was randomized ten times. Other menu options were left as default. The resultant tree is unrooted and the phylogenetic distances are in the unit of expected number of substitutions per site. Branch points of the resulting tree had a confidence level of P<0.01 (P<0.05*). Genbank accession numbers for the strains in this analysis were M87661, X86557, U52086, U13992, Z69620, M67473, X86560, J02281, K02121, M22458, X00429, K02990, M15240, J02363, M73218, M80581, M74506, AF011921.
B. Partial gene sequences (200 aa) from the proposed polymerase region were used for the phylogenetic analysis and included representative strains from each family. Clustal W v1.7 was used to create a multiple alignment for the aa sequences, which was verified by alignment of known motifs in the region (e.g. SGxxxTxxxMT/S, GDD). The nt sequences were added and aligned by hand using the corresponding aa sequences as template resulting in a consensus length of 590 nt. A phylogenetic tree was constructed as described above and Genbank accession numbers for the strains in this analysis were identical to those above.
DERIVATION OF NAMES
Hepevirus is derived from the sigla of Hepatitis E virus
REFERENCES
- Agrawal S., Gupta D., Panda S.K. The 3’ end of Hepatitis E virus (HEV) genome binds specifically to the viral RNA-dependent RNA polymerase (RdRp) Virology. 2001;282:87–101. doi: 10.1006/viro.2000.0819. [DOI] [PubMed] [Google Scholar]
- Balayan M.S., Andjaparidze A.G., Savinskaya S.S., Ketiladze E.S., Braginsky D.M., Savinov A.P., Poleschuk V.F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- Berke T., Golding B., Jiang X., Cubitt D.W., Wolfaardt M., Smith A.W., Matson D.O. Phylogenetic analysis of the caliciviruses. J. Med. Virol. 1997;52:419–424. doi: 10.1002/(sici)1096-9071(199708)52:4<419::aid-jmv13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Emerson S.U., Zhang M., Meng X.-J., Nguyen H., St. Claire M., Govindarajan S., Huang Y.K., Purcell R.H. Recombinant Hepatitis E virus genomes infectious for primates: importance of capping and discovery of a cis-reactive element. Proc. Natl. Acad. Sci. USA. 2001;98:15270–15275. doi: 10.1073/pnas.251555098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.5c. Distributed by the author. Department of Genetics, University of Washington; Seattle: 1993. [Google Scholar]
- Koonin E.V., Dolja V.V. Evolution and taxonomy of positive-strand RNA viruses: Implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Koonin E.V., Gorbalenya A.E., Purdy M.A., Rozanov M.N., Reyes G.R., Bradley D.W. Computer-assisted assignment of functional domains in the non-structural polyprotein of hepatitis E virus: Delineation of an additional group of positive-strand RNA plant and animal viruses. Proc. Natl. Acad. Sci. USA. 1992;89:8259–8263. doi: 10.1073/pnas.89.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magden J., Takeda N., Li T., Auvinen P., Ahola T., Miyamura T., Merits A., Kaariainen L. Virus-specific mRNA capping enzyme encoded by Hepatitis E virus. J. Virol. 2001;75:6249–6255. doi: 10.1128/JVI.75.14.6249-6255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.-J., Halbur P.G., Shapiro M.S., Govindarajan S., Bruna J.D., Mushahwar I.K., Purcell R.H., Emerson S.U. Genetic and experimental evidence for cross-species infection by swine Hepatitis E virus. J. Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.-J. Novel strains of Hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J. Hepatology. 2000;33:842–845. doi: 10.1016/s0168-8278(00)80319-0. [DOI] [PubMed] [Google Scholar]
- Purcell R.H., Emerson S.U. Hepatitis E virus. In: Knipe D., Howley P., editors. Fields Virology. Lippincott, Willliams and Wilkins; Philadelphia: 2001. pp. 3051–3061. [Google Scholar]
- Schlauder G.G., Mushahwar I.K. Genetic heterogeneity of Hepatitis E virus. J. Med. Virol. 2001;65:282–292. doi: 10.1002/jmv.2031. [DOI] [PubMed] [Google Scholar]
- Tam A.W., Smith M.W., Guerra M.E., Huang C.C., Bradley D.W., Fry K.E., Reyes G.R. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nuc. Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticehurst J., Rhodes L.L., Jr., Krawczynski K., Asher L.V., Engler W.F., Mensing T.L., Caudill J.D., Sjogren M.H., Hoke C.H., LeDuc J.W., Bradley D.W., Binn L.N. Infection of owl monkeys (Aotus trivigatus) and cynomologous monkeys (Macaca fasicularis) with Hepatitis E virus from Mexico. J. Infect. Dis. 1992;165:835–845. doi: 10.1093/infdis/165.5.835. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, S.S. Monroe, M.J. Carter, J. Herrmann, D.K. Mitchel, A. Sanchez-Fauquier
FAMILY ASTROVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Astroviridae |
| Genus | Avastrovirus |
| Genus | Mamastrovirus |
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

Negative contrast electron micrograph of virions of Human astrovirus (HAstV), from a stoolspecimen. The bar represents 100 nm (Courtesy of Dr. C. Humphrey).
Virions shed in feces samples are 28-30 nm in diameter, spherical in shape and non-enveloped. A distinctive five- or six-pointed star is discernible on the surface of about 10% of virions. Virions derived from cell culture are up to 41 nm in diameter, with well -defined surface spikes.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is about 8 × 106, S20w is about 160S. Virion buoyant density in CsCl is 1.36-1.39 gm/cm3. Virions are resistant to pH 3, 50°C for 1 hr, 60°C for 5 min, chloroform, lipid solvents and non-ionic, anionic, and zwitterionic detergents.
NUCLEIC ACID
Virions contain one molecule of infectious, positive sense, ssRNA, 6.4-7.4 kb in size. A poly(A) tract is located after the 3-terminal heteropolymeric sequence. The structure of the 5′-end of the genome in not known. A small protein with similarity to picornavirus VPg has been identified by sequence comparisons, but its presence on viral RNA has not been experimentally verified. The lengths of the non-translated regions at both ends of the genome vary by genus.
PROTEINS
Virion protein composition remains unclear; however, all isolates have at least two, usually three, major proteins of between 24 and 39 kDa.
LIPIDS
Virions do not contain a lipid envelope.
CARBOHYDRATES
None of the viral proteins is glycosylated.
GENOME ORGANIZATION AND REPLICATION
The virion RNA is infectious and serves as a messenger RNA for the non-structural polyproteins, p1A and p1AB. A polyadenylated, sgRNA (∼2.8 kb) is detected in the cytoplasm of infected cells. Viral RNA replication is resistant to actinomycin D. A precursor of CP of 86-90 kDa has been detected in the cytoplasm of infected cells. Virions cultivated in a trypsin-free environment are composed of a single 79 kDa protein (VP79). N-terminal microsequencing indicates that VP79 results from the cleavage of the N-terminal 70 aa of the full-length ORF2 product. When VP79-containing particles are exposed to trypsin, capsid subunits of 26, 29 and 34 kDa are detected, and infectivity is enhanced.
Figure 2.

Genome organization and replication strategy of Human astrovirus − 1 (HAstV-1). ORF 2 isexpressed from a sgRNA detected in the cytoplasm of infected cells.
ANTIGENIC PROPERTIES
Eight serotypes of Human astrovirus have been defined by immune electron microscopy and neutralization tests and been confirmed by sequence comparisons. They share at least one common epitope recognized by monoclonal antibody. Neutralization epitopes have been mapped to the VP26 and VP29 proteins of Human astrovirus 1 (HAstV-1) and HAstV-2. Two distinct serotypes of bovine astrovirus have been defined by neutralization.
BIOLOGICAL PROPERTIES
Astroviruses appear to have very limited host ranges. They have been detected in stool samples from humans, cats, cattle, deer, dogs, ducks, mice, pigs, sheep, mink, turkeys, and chickens. Transmission is by the fecal-oral route and no intermediate vectors have been described. Astrovirus pathogenesis appears to vary by genus.
GENUS AVASTROVIRUS
Type Species Turkey astrovirus
DISTINGUISHING FEATURES
Members of the genus Avastrovirus infect avian species.
BIOLOGICAL PROPERTIES
Infection with Avastrovirus species often involves extra-intestinal manifestations (e.g. damage to liver, kidney, or the immune system). Duck astrovirus (DastV) causes an often fatal hepatitis in ducklings. Astroviruses infecting turkeys (TAstV) and chickens (ANV) affect multiple organs, including the kidney and thymus.
Duck astrovirus grows in embryonated hen's eggs following blind passage in the amniotic sac. Few infected embryos die in less than 7 days. Infected embryos appear stunted and have greenish, necrotic livers in which astrovirus particles have been identified.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Species are defined on the basis of host of origin. Serotypes are defined on the basis of twenty-fold, or greater, two-way cross-neutralization titers. Serotypes assigned to the species are given consecutive numbers.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS MAMASTROVIRUS
Type Species Human astrovirus
DISTINGUISHING FEATURES
Members of the genus Mamastrovirus infect mammalian species.
BIOLOGICAL PROPERTIES
The predominant feature of infection with mamastroviruses is gastroenteritis. In humans, astrovirus has been detected in duodenal biopsies in epithelial cells located in the lower part of villi. In experimentally infected sheep, astrovirus was found in the small intestine in the apical two-thirds of villi. In calves, astrovirus infection was localized to specialized M cells overlying the Peyer's patches. Human astroviruses are distributed worldwide and have been associated with 2-8% of acute, non-bacterial gastroenteritis in children. Astroviruses have also been associated with gastroenteritis outbreaks and with gastroenteritis in immunocompromised children and adults.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Species are defined on the basis of host of origin. Serotypes are defined on the basis of twenty-fold, or greater, two-way cross-neutralization titers. Serotypes assigned to the species are given consecutive numbers.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Bovine astrovirus | ||
| Bovine astrovirus 1 | (BAstV-1) | |
| Bovine astrovirus 2 | (BAstV-2) | |
| Feline astrovirus | ||
| Feline astrovirus 1 | (FAstV-1) | |
| Human astrovirus | ||
| Human astrovirus 1 | [Z25771; L13745] | (HAstV-1) |
| Human astrovirus 2 | [L12745] | (HAstV-2) |
| Human astrovirus 3 | [AF141381] | (HAstV-3) |
| Human astrovirus 4 | [Z33883] | (HAstV-4) |
| Human astrovirus 5 | [U15136] | (HAstV-5) |
| Human astrovirus 6 | [Z46658] | (HAstV-6) |
| Human astrovirus 7 | [Y08632] | (HAstV-7) |
| Human astrovirus 8 | [AF260508] | (HAstV-8) |
| Mink astrovirus‡ | ||
| Mink astrovirus 1 | [AY179509] | (MastV-1) |
| Ovine astrovirus | ||
| Ovine astrovirus 1 | [Y15937] | (OAstV-1) |
| Porcine astrovirus | ||
| Porcine astrovirus 1 | [Y15938] | (PAstV-1) |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
Figure 3.

Phylogenetic relationships between the species and serotypes of the family Astroviridae. Thepredicted aa sequences of the entire capsid polyprotein were aligned using CLUSTALX (v1.83) and the ntsequences were aligned on the basis of the protein alignments using DAMBE (v4.1.19). The phylogenetic treewas generated using the maximum likelihood algorithm as implemented in the DAMBE program. Sequences usedfor the comparison are indicated in the list of species.
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
SIMILARITY WITH OTHER TAXA
None reported.
DERIVATION OF NAMES
Astro: from Greek astron, “star”, representing the star-like surface structure on virions.
Av: from Latin avis, “bird”, representing avian host species
Mam: from Latin mamma, “breast”, representing mammalian host species
REFERENCES
- Aroonprasert D., Fagerland J.A., Kelso N.E., Zheng S., Woode G.N. Cultivation and partial characterization of bovine astrovirus. Vet. Microbiol. 1989;19:113–125. doi: 10.1016/0378-1135(89)90077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D.M., Qiu S. Proteolytic processing of the astrovirus capsid. J. Virol. 2000;74:1810–1814. doi: 10.1128/jvi.74.4.1810-1814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behling-Kelly E., Schultz-Cherry S., Koci M., Kelley L., Larsen D., Brown C. Localization of astrovirus in experimentally infected turkeys as determined by in situ hybridization. Vet. Pathol. 2002;39:595–598. doi: 10.1354/vp.39-5-595. [DOI] [PubMed] [Google Scholar]
- Englund L., Chriel M., Dietz H.H., Hedlund K.O. Astrovirus epidemiologically linked to pre-weaning diarrhoea in mink. Vet. Microbiol. 2002;85:1–11. doi: 10.1016/s0378-1135(01)00472-2. [DOI] [PubMed] [Google Scholar]
- Geigenmueller U., Ginzton N.H., Matsui S.M. Construction of a genome-length cDNA clone for human astrovirus serotype 1 and synthesis of infectious RNA transcripts. J. Virol. 1997;71:1713–1717. doi: 10.1128/jvi.71.2.1713-1717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenmueller U., Chew T., Ginzton N., Matsui S.M. Processing of nonstructural protein 1a of human astrovirus. J. Virol. 2002;76:2003–2008. doi: 10.1128/JVI.76.4.2003-2008.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A.J., Gray E.W., Snodgrass D.R. Purification and characterization of ovine astrovirus. J. Gen. Virol. 1981;53:47–55. doi: 10.1099/0022-1317-53-1-47. [DOI] [PubMed] [Google Scholar]
- Jiang B., Monroe S.S., Koonin E.V., Stine S.E., Glass R.I. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc. Natl. Acad. Sci. USA. 1993;90:10539–10543. doi: 10.1073/pnas.90.22.10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen C.M., Jonassen T.T., Sveen T.M., Grinde B. Complete genomic sequences of astroviruses from sheep and turkey: comparison with related viruses. Virus Res. 2003;91:195–201. doi: 10.1016/s0168-1702(02)00269-1. [DOI] [PubMed] [Google Scholar]
- Kiang D., Matsui S.M. Proteolytic processing of a human astrovirus nonstructural protein. J. Gen. Virol. 2002;83:25–34. doi: 10.1099/0022-1317-83-1-25. [DOI] [PubMed] [Google Scholar]
- Koci M.D., Schultz-Cherry S. Avian astroviruses. Avian Pathol. 2002;31:213–227. doi: 10.1080/03079450220136521. [DOI] [PubMed] [Google Scholar]
- Lee T.W., Kurtz J.B. Prevalence of human astrovirus serotypes in the Oxford region 1976–92, with evidence for two new serotypes. Epidemiol. Infect. 1994;112:187–193. doi: 10.1017/s0950268800057551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinke B., Bloys A.J., Brown T.D., Willcocks M.M., Carter M.J., Brierley I. The human astrovirus RNA-dependent RNA polymerase coding region is expressed by ribosomal frameshifting. J. Virol. 1994;68:5588–5595. doi: 10.1128/jvi.68.9.5588-5595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E., Fernandez-Luna T., Lopez S., Mendez-Toss M., Arias C.F. Proteolytic processing of a serotype 8 Human Astrovirus ORF2 polyprotein. J. Virol. 2002;76:7996–8002. doi: 10.1128/JVI.76.16.7996-8002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S.S., Stine S.E., Gorelkin L., Herrmann J.E., Blacklow N.R., Glass R.I. Temporal synthesis of proteins and RNAs during human astrovirus infection of cultured cells. J. Virol. 1991;65:641–648. doi: 10.1128/jvi.65.2.641-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J.S., Lee T.W., Kurtz J.B., Glass R.I., Monroe S.S. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J. Clin. Microbiol. 1995;33:797–801. doi: 10.1128/jcm.33.4.797-801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco C., Carrascosa J.L., Pedregosa A.M., Humphrey C.D., Sanchez-Fauquier A. Ultrastructure of human astrovirus serotype 2. J. Gen. Virol. 1995;76:2075–2080. doi: 10.1099/0022-1317-76-8-2075. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fauquier A., Carrascosa A.L., Carrascosa J.L., Otero A., Glass R.I., Lopez J.A., San Martin C., Melero J.A. Characterization of a human astrovirus serotype 2 structural protein (VP26) that contains an epitope involved in virus neutralization. Virology. 1994;201:312–320. doi: 10.1006/viro.1994.1296. [DOI] [PubMed] [Google Scholar]
- Willcocks M.M., Carter M.J., Laidler F.R., Madeley C.R. Growth and characterisation of human faecal astrovirus in a continuous cell line. Arch. Virol. 1990;113:73–81. doi: 10.1007/BF01318354. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, A. Schneemann, L.A. Ball, C. Delsert, J.E. Johnson, T. Nishizawa
FAMILY NODAVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Nodaviridae |
| Genus | Alphanodavirus |
| Genus | Betanodavirus |
GENUS ALPHANODAVIRUS
Type Species Nodamura virus
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

(Left) Image reconstruction of a particle of Flock House virus (FHV). (Center) Schematic representation of a T=3 icosahedral lattice. (Right) Cryo-electron micrograph of particles of FHV; the bar represents 50 nm. (Courtesy of N. Olson and T. Baker).
Virions are non-enveloped, roughly spherical in shape, 32-33 nm in diameter and have icosahedral symmetry (T=3). No distinct surface structure is seen by electron microscopy of negatively stained preparations. Empty shells are seldom seen in virus preparations.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is about 9 × 106; S20w is 135-145S. Virion buoyant density in CsCl is 1.30-1.34 g/cm3 (varies with species). Infectivity of aqueous suspensions is stable to extraction with chloroform. Infectivity of Nodamura virus (NoV), Black beetle virus (BBV), or Flock House virus (FHV) is stable at room temperature in 1% sodium dodecyl sulfate but Boolarra virus (BoV) is inactivated. Virions are stable at acid pH. The RNA content of the virion is about 16%.
NUCLEIC ACID
The genome consists of two molecules of positive sense ssRNA: RNA1 (Mr 1.1 × 106, 3.1 kb) and RNA2 (Mr 0.48 × 106, 1.4 kb). Both molecules are required for infectivity, and both are encapsidated in the same virus particle. Both RNA molecules are capped at their 5′-ends with cap zero structures and lack poly(A) tails at their 3′-ends. RNA 3′-ends cannot be chemically derivatized even after treatment with denaturing solvents, indicating that the expected 3′-terminal-OH groups are unreactive.
PROTEINS
The capsid consists of 180 protein subunits (protomers) arranged on a T=3 surface lattice. Each protomer is composed of a single CP (protein α) or the two products of its cleavage (proteins β? and γ). Mass spectrometry of the FHV CP indicates that the initiating methionine is removed. Thus, for FHV, the capsid proteins are: protein α: (44 kDa), aa 2-407; protein β: (39 kDa), aa 2-363; protein γ: (4 kDa), aa 364-407. Morphogenesis involves the formation of a non-infectious provirion which acquires infectivity by autocatalytic cleavage of protein α?to form proteins β and γ. Maturation cleavage is often incomplete and virions typically contain residual uncleaved protein α.
LIPIDS
None.
CARBOHYDRATES
None.
GENOME ORGANIZATION AND REPLICATION
Alphanodaviruses replicate in the cytoplasm of infected cells (Fig.2 ). RNA synthesis is resistant to actinomycin D. Infected cells contain three ssRNAs: RNA1 (Mr 1.1 × 106; 3.1 kb); RNA2 (Mr 0.48 × 106; 1.4 kb) and a sgRNA3 (Mr 1.10.13 × 106; 0.39 kb), whose nt sequence corresponds to the 3′-end of RNA1 (387 nt in the case of FHV). RNA3 is not packaged into virions; its 3′-end is chemically unreactive like those of RNAs 1 and 2. RNA1 encodes protein A (112 kDa), which is the catalytic subunit of the viral RdRp. RNA2 encodes protein α, the CP precursor (44 kDa). Depending on virus species, RNA3 encodes one or two small proteins (proteins B1 and B2, 11 kDa). B1 is encoded in the same ORF as protein A. Protein B2 is encoded in an overlapping ORF. BoV RNA3 does not encode protein B1 but all known alphanodavirus RNA3 molecules encode protein B2. Protein B2 of FHV has been shown to function as a suppressor of RNA silencing in cultured Drosophila melanogaster cells (Schneider's line 2) and tobacco plants (Nicotiana benthamiana). The function of protein B1 is unknown. Cells transfected with isolated RNA1 synthesize RNA1 and overproduce RNA3, but do not make RNA2. RNA2 replication strongly inhibits synthesis of RNA3 and the translation of RNA2 suppresses the translation of RNA1.
Figure 2.

Alphanodavirus (Flock House virus; FHV) genome organization and strategy of replication. (Adapted from L.A. Ball and K.L. Johnson).
ANTIGENIC PROPERTIES
NoV, BBV, FHV and BoV are cross-reactive by double-diffusion immunoprecipitation tests, but all four members represent different serotypes (neutralization titer of each antiserum less than 0.5% in heterotypic crosses).
BIOLOGICAL PROPERTIES
HOST RANGE
All species of alphanodaviruses were isolated in nature from insects, although serological data suggest that NoV also naturally infects pigs and perhaps herons. NoV seems to be unique among the nodaviruses in its ability to infect both vertebrates and invertebrates. It is also very unusual in being able to kill both insect and mammalian hosts. The other alphanodaviruses do not show strict specificity for particular insect hosts.
In the laboratory, most alphanodaviruses can be propagated in larvae of the common wax moth, Galleria mellonella, where they cause paralysis and death. NoV, isolated from mosquitoes, also grows in suckling mice but not in cultured cells of Drosophila melanogaster. FHV, BBV, and BoV grow well in cultured Drosophila melanogaster cells and form plaques on monolayers of these cells. Defective-interfering particles are readily formed unless the viruses are passaged at low multiplicity of infection. Persistent infections, with subsequent resistance to superinfection, occur readily in cultured Drosophila melanogaster cells. NoV multiplies poorly in most cultured cells but can be propagated by transfecting insect or vertebrate cell cultures with virion RNA at temperatures below about 34°C.
TRANSMISSION
NoV is transmissible to suckling mice by Aedes aegypti mosquitoes. It causes paralysis and death when injected into suckling mice, but no disease in adult animals. In their insect hosts, alphanodaviruses typically cause stunting, paralysis, and death.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The following criteria can be applied to the demarcation of species within the Alphanodavirus genus:
-
•
Biological properties (host range, vectors, mode of transmission). Since the natural host ranges of the nodaviruses have generally not been examined in detail but may in some cases be broad, virus isolation from a new host is not, in itself, evidence of a new nodavirus species.
-
•
Antigenic properties. Antisera raised against different isolates or strains of a single nodavirus species should exhibit high levels of cross-reactivity in Western blot and/or neutralization analyses. Lower levels of cross-reactivity in these assays using antisera against all previously recognized nodavirus species can provide evidence of a novel nodavirus.
-
•Virion physical/physicochemical characteristics.
-
○Virion electrophoretic mobility. Intact virus particles migrate with characteristic electrophoretic mobilities in non-denaturing agarose gel, so virion mobility should be compared with those of other nodavirus species.
-
○Sedimentation coefficient, buoyant density. Virion sedimentation coefficient and buoyant density should be compared with those of other nodavirus species.
-
○
-
•
Structural protein characteristics. The electrophoretic mobilities in SDS-PAGE of the CP precursor or its cleavage products should be compared with those of other nodavirus species.
-
•Genome molecular characteristics.
-
○RNA electrophoretic mobilities. In the absence of sequence information, the electrophoretic mobilities of the viral genomic RNAs should be compared with those of other nodaviruses.
-
○RNA hybridization properties. In the absence of differences in RNA electrophoretic mobilities, the molecular hybridization properties of the viral genomic RNAs should be compared with those of other nodaviruses.
-
○Genome sequence characteristics. The nt sequence of the two genomic RNAs should be compared with those of other nodaviruses. Because the nodavirus genome is segmented, reassortment can occur and the two genome segments may have distinct evolutionary lineages.
-
○
Application of these criteria. In practice, while the five criteria above may be suggestive of a new species, definitive demarcation is based on the nt sequence of the viral CP gene. The two closest recognized species are BBV and FHV, whose RNA2 sequences show 80% identity at the nt level and 87% identity at the aa sequence level. Their RNA1 sequences, however, are 99% identical.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Black beetle virus | ||
| Black beetle virus | (BBV) | |
| Boolarra virus | ||
| Boolarra virus | (BoV) | |
| Flock House virus | ||
| Flock House virus | (FHV) | |
| Nodamura virus | ||
| Nodamura virus | (NoV) | |
| Pariacoto virus | ||
| Pariacoto virus | (PaV) | |
TENTATIVE SPECIES IN THE GENUS
| Gypsy moth virus | (GMV) |
| Lymantria ninayi virus Greenwood | (LNV) |
| Manawatu virus | (MwV) |
| New Zealand virus | (NZV) |
| Drosophila line 1 virus | (DLV) |
GENUS BETANODAVIRUS
Type Species Striped jack nervous necrosis virus
VIRION PROPERTIES
MORPHOLOGY
Virions are non-enveloped, spherical in shape, and have icosahedral symmetry (T=3). Distinct surface protrusions are observed by electron microscopy of negatively stained preparations (Fig. 3 ). Image reconstruction of virus-like particles of Malabaricus grouper nervous necrosis virus (MGNNV) indicates that the CP of betanodaviruses has a two domain structure compared to the single domain structure of the CP of alphanodaviruses. The average diameter of the particle is 37 nm. In contrast with most alphanodaviruses, empty particles have been seen by electron microscopy of some preparations of betanodaviruses.
Figure 3.

(Left) Image reconstruction of virus-like particles of Malabaricus grouper nervous necrosis virus (MGNNV) generated in Spodoptera frugiperda cells from a recombinant baculovirus expressing the MGNNV coat protein gene. (Center) Schematic representation of a T=3 icosahedral lattice. (Right) Cryo-electron micrograph of virus-like particles MGNNV; the bar represents 40 nm. (Courtesy of L. Tang and J.E. Johnson).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion buoyant density in CsCl of Striped jack nervous necrosis virus (SJNNV) has not been reported but that of Dicentrarchus labrax encephalitis virus (DlEV) is about 1.31-1.36 g/cm3. Virions of DlEV are stable between pH 2-9 and resistant to heating at 56°C for 30 min. Infectivity is resistant to extraction of virions with chloroform.
NUCLEIC ACID
The genome consists of two molecules of positive sense ssRNA: RNA1 (Mr 1.01 × 106) and RNA2 (Mr 0.49 × 106). Both RNA molecules lack poly(A) tails at their 3′-ends.
PROTEINS
Betanodavirus capsids contain 180 copies of a single structural protein of 42 kDa. In contrast to alphanodaviruses, maturation cleavage of this protein is not observed. The appearance of a protein doublet on denaturing polyacrylamide gels is an artefact of incomplete reduction of a disulfide bond.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The betanodaviruses replicate in the cytoplasm. Infected cells contain three ssRNAs: RNA1 (Mr 1.01 × 106; 3.1 kb); RNA2 (Mr 0.49 × 106; 1.4 kb) and a sgRNA3 (Mr about 0.13 × 106; 0.4 kb) derived from RNA1. RNA3 is not packaged into virions. RNA1 encodes protein 1a (110 kDa), theRdRp. RNA2 encodes protein 2a (42 kDa), the CP. The protein(s) encoded by RNA3 have not yet been identified.
ANTIGENIC PROPERTIES
Betanodaviruses are cross-reactive by immunoblot analysis using polyclonal antisera but differential reactivity is observed with monoclonal antibodies.
BIOLOGICAL PROPERTIES
HOST RANGE
Nature: All species of the betanodaviruses were isolated from juvenile marine fish, in which they cause a vacuolating encephalopathy and retinopathy associated with behavioral abnormalities and high mortalities. These diseases have been detected particularly in commercial fish hatcheries, where they cause significant problems for the marine aquaculture industry.
Laboratory: Betanodaviruses replicate in cultured cells from striped snakehead fish (SNN-1) and sea bass larvae (SBL). A low level of virus replication was observed in mammalian (COS-1 and HeLa) cells at 28°C.
TRANSMISSION
Antibodies to SJNNV were found in 65% of plasma samples collected from wild and domestic brood stocks of striped jack, suggesting that the virus is very prevalent. Viral antigens were detected in eggs, larvae, and ovaries of hatchery-reared and wild spawner fish, suggesting both horizontal and vertical modes of transmission of the virus.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The species demarcation criteria applied above for the alphanodaviruses also apply to betanodaviruses.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Barfin flounder nervous necrosis virus | ||
| Barfin flounder nervous necrosis virus | RNA2[D38635] | (BFNNV) |
| Redspotted grouper nervous necrosis virus | ||
| Redspotted grouper nervous necrosis virus | RNA2[D38636] | (RGNNV) |
| Striped jack nervous necrosis virus | ||
| Striped jack nervous necrosis virus | (SJNNV) | |
| Tiger puffer nervous necrosis virus | ||
| Tiger puffer nervous necrosis virus | RNA2[D38637] | (TPNNV) |
TENTATIVE SPECIES IN THE GENUS
| Atlantic cod nervous necrosis virus | RNA2[AF445800] | (ACNNV) |
| Atlantic halibut nodavirus | RNA2[AJ245641] | (AHNV) |
| Dicentrarchus labrax encephalitis virus | RNA2[U39876, Y08700, AJ277803-10, AF175509-20] | (DlEV) |
| Dragon grouper nervous necrosis virus | RNA2[AF245004] | (DGNNV) |
| Greasy grouper nervous necrosis virus | (GGNNV) | |
| Grouper nervous necrosis virus | (GNNV) | |
| Halibut nervous necrosis virus | (HNNV) | |
| Japanese flounder nervous necrosis virus | RNA2[D38527] | (JFNNV) |
| Lates calcarifer encephalitis virus | (LcEV) | |
| Malabaricus grouper nervous necrosis virus | RNA2[AF245003] | (MGNNV) |
| Seabass nervous necrosis virus | (SBNNV) | |
| Umbrina cirrosa nodavirus | (UCNV) |
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
None reported
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Within the alphanodaviruses, CP sequences are 44-87% identical to one another at the aa level, whereas within the betanodaviruses, CP aa sequence identities are 80% or greater. The phylogenetic relationship between betanodavirus species and tentative species has not yet been rigorously defined. However, the sequences of RNA2 of RGNNV, GGNNV, MGNNV and DGNNV are >95% identical at both the nt and aa level suggesting that future reclassification of some tentative species as strains of current betanodavirus species may be possible. The CP aa sequences of the alphanodaviruses are only about 10% identical to those of the betanodaviruses, insufficient to indicate common ancestry.
SIMILARITY WITH OTHER TAXA
The omegatetraviruses such as Nudaurelia capensis ω?virus (NωV) and Helicoverpa armigera stunt virus (HaSV) contain bipartite ssRNA genomes, but their RNAs are about twice the size of nodavirus RNAs and they have no 3′-terminal blockage. Tetraviruses also have larger capsids with T=4 icosahedral symmetry.
DERIVATION OF NAMES
Noda is from Nodamura, the name of a village (now a city; Nodashi) in the vicinity of the site where NoV was isolated in Japan. Other nodaviruses are similarly named after the place of isolation or after the host name from which they were isolated.
REFERENCES
- Ball L.A., Johnson K.L. Nodaviruses of insects. In: Miller L.K., Ball L.A., editors. The Insect Viruses. Plenum Publishing Company; New York: 1998. pp. 225–267. [Google Scholar]
- Ball L.A., Amann J.M., Garrett B.K. Replication of nodamura virus after transfection of viral RNA into mammalian cells in culture. J. Virol. 1992;66:2326–2334. doi: 10.1128/jvi.66.4.2326-2334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comps M., Pepin J.F., Bonami J.R. Purification and characterization of two fish encephalitis viruses (FEV) infecting Lates calcarifer and Dicentrarchus labrax. Aquaculture. 1994;123:1–10. [Google Scholar]
- Dearing S.C., Scotti P.D., Wigley P.J., Dhana S.D. A small RNA virus isolated from the grass grub, Costelytra zealandica (Coleoptera: Scarabaeidae) N. Z. J. Zool. 1980;7:267–269. [Google Scholar]
- Delsert C., Morin N., Comps M. Fish nodavirus lytic cycle and semipermissive expression in mammalian and fish cell cultures. J. Virol. 1997;71:5673–5677. doi: 10.1128/jvi.71.7.5673-5677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs G.N., Rodger H.D., Peric Z. Cell culture isolation of piscine neuropathy nodavirus from juvenile sea bass, Dicentrarchus labrax. J. Gen. Virol. 1996;77:2067–2071. doi: 10.1099/0022-1317-77-9-2067. [DOI] [PubMed] [Google Scholar]
- Garzon S., Charpentier G. Nodaviridae. In: Adams J.R., Bonami J.R., editors. Atlas of Invertebrate Viruses. CRC Press; Boca Raton. Florida: 1992. pp. 351–370. [Google Scholar]
- Hendry D.A. Nodaviridae of Invertebrates. In: Kurstak E., editor. Viruses of Invertebrates. Marcel Dekker; New York: 1991. pp. 227–276. [Google Scholar]
- Johnson J.E., Reddy V. Structural studies of noda and tetraviruses. In: Miller L.K., Ball L.A., editors. The Insect Viruses. Plenum Publishing Company; New York: 1998. pp. 171–223. [Google Scholar]
- Krondiris J.V., Sideris D.C. Intramolecular disulfide bonding is essential for betanodavirus coat protein conformation. J. Gen. Virol. 2002;83:2211–2214. doi: 10.1099/0022-1317-83-9-2211. [DOI] [PubMed] [Google Scholar]
- Li H., Li W.X., Ding S.W. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- Mori K.I., Nakai T., Muroga K., Arimoto M., Musiake K., Firusawa I. Properties of a new virus belonging to the Nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology. 1992;187:368–385. doi: 10.1016/0042-6822(92)90329-n. [DOI] [PubMed] [Google Scholar]
- Munday B.L., Nakai T., Nguyen H.D. Antigenic relationship of the picorna-like virus of larval barramundi, Lates calcarifer Bloch to the nodavirus of larval striped jack, Pseudocaranx dentex (Bloch and Schneider) Aust. Vet. J. 1994;71:384–385. doi: 10.1111/j.1751-0813.1994.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Nishizawa T., Mori K., Furuhashi M., Nakai T., Furusawa I., Muroga K. Comparison of the coat protein genes of five fish nodaviruses, the causative agents of viral nervous necrosis in marine fish. J. Gen. Virol. 1995;76:1563–1569. doi: 10.1099/0022-1317-76-7-1563. [DOI] [PubMed] [Google Scholar]
- Nishizawa T., Furuhashi M., Nagai T., Nakai T., Muroga K. Genomic classification of fish nodaviruses by molecular phylogenetic analysis of the coat protein gene. Appl. Environ. Microbiol. 1997;63:1633–1636. doi: 10.1128/aem.63.4.1633-1636.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa T., Takano R., Muroga K. Mapping a neutralizing epitope on the coat protein of striped jack nervous necrosis virus (SJNNV) J. Gen. Virol. 1999;80:3023–3028. doi: 10.1099/0022-1317-80-11-3023. [DOI] [PubMed] [Google Scholar]
- Reinganum C., Bashirrudin J.B., Cross G.F. Boolarra virus: a member of the Nodaviridae isolated from Oncopera intricoides (Lepidoptera: Hapealidae) Intervirology. 1985;24:10–17. doi: 10.1159/000149613. [DOI] [PubMed] [Google Scholar]
- Schneemann A., Reddy V., Johnson J.E. The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv. Virus Res. 1998;50:381–446. doi: 10.1016/s0065-3527(08)60812-x. [DOI] [PubMed] [Google Scholar]
- Schneemann A., Zhong W., Gallagher T.M., Rueckert R.R. Maturation cleavage required for infectivity of a nodavirus. J. Virol. 1992;66:6728–6734. doi: 10.1128/jvi.66.11.6728-6734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti P.D., Fredericksen S. Manawatu virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae) Arch. Virol. 1987;97:85–92. doi: 10.1007/BF01310736. [DOI] [PubMed] [Google Scholar]
- Tang L., Lin C.S., Krishna N.K., Yeager M., Schneemann A., Johnson J.E. Virus-like particles of a fish nodavirus display a capsid subunit domain organization different from insect nodaviruses. J. Virol. 2002;76:6370–6375. doi: 10.1128/JVI.76.12.6370-6375.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONTRIBUTED BY, T.N. Hanzlik, K.H.J. Gordon, A.E. Gorbalenya, D.A. Hendry, F.M. Pringle, V.K. Ward, J.-L. Zeddam
FAMILY TETRAVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Tetraviridae |
| Genus | Betatetravirus |
| Genus | Omegatetravirus |
GENUS BETATETRAVIRUS
Type Species Nudaurelia capensis fivirus
VIRION PROPERTIES
MORPHOLOGY
Virions are non-enveloped, roughly spherical, about 40 nm in diameter and exhibit T=4 icosahedral shell quasi-symmetry. Distinct capsomers have been resolved by cryo-electron microscopy and image reconstruction (Fig. 1 ). The genome consists of ssRNA. Viruses in the genus Betatetravirus have monopartite genomes, whereas those in the genus Omegatetravirus have bipartite genomes.
Figure 1.

(Left) Schematic representation of a T=4 icosahedral lattice. (Center and Right) Cryo-electron image reconstruction of a particle of Nudaurelia capensis β virus (NβV) on the symmetry axis 3 and 5; the bar represents 20 nm. (Courtesy of H.R. Cheng, N. Olson and T. Baker).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is about 18 × 106. Virion S20w is 194-217S. Virion buoyant density in CsCl is
usually 1.28-1.30 g/cm3 but occasionally as high as 1.33 g/cm3 (varies with species). Virions are stable over a broad range of pH and their infectivity can resist desiccation and protease treatment.
NUCLEIC ACID
Virions of the type species, Nudaurelia capensis β virus, contain a single, positive sense, ssRNA segment of about 6.5 kb (Mr 1.8 × 106) which represents about 10% of the particle mass. This genomic RNA is not polyadenylated at its 3′-end, nor blocked like nodaviral RNAs, but terminates instead with a distinctive tRNA-like structure. A subgenomic message for the CPs, which is derived from the 3′-end of the genomic RNA, can also be encapsidated in some species.
PROTEINS
Capsids consist of 240 protein subunits (protomers) arranged on a T=4 surface lattice. Each protomer consists of the two cleavage products (β, 58.4 kDa and ? γ, 8 kDa), of a single CP precursor (a, 66.4 kDa). Minor amounts of the uncleaved precursor may be found in virions.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The viruses replicate in the cytoplasm. The 6,625 nt genomic RNA of Nudaurelia capensis β virus (NβV), contains two ORFs that overlap for 1517 nt: that nearer the 5′-end contains 1821 codons and encodes the multidomain RNA replicase (204 kDa), whereas that nearer the 3′-end contains 612 codons and encodes a precursor (66.4 kDa) to the two Cps (Fig. 2 ). The RNA replicase includes three highly conserved enzymatic domains, Mtr, RNA Hel and RdRp that are yet to be characterized experimentally.
Figure 2.

Genome organization of betatetraviruses. The locations of the methyltransferase (Mtr), RNA helicase (Hel) and RdRp motifs within the replicase ORFs are indicated. The amino-terminal portion of the TaV and EeV CP precursor removed by processing is boxed. The 5′-ends of the TaV/EeV genomic and sgRNAs have not been characterized and are indicated by “?”.
The genomic RNA of the recently sequenced Thosea asigna virus (TaV) is 5,715 nt. Like that of the closely related Euprosterna elaeasa virus (EeV)(5,698 nt), it carries a shorter ORF (1257 codons) encoding the RdRp-containing replicase, resulting in a much shorter overlap between the replicase and CP ORFs. The TaV CP ORF is longer than that of NβV, yielding a putative precursor of 757 aa in length that is processed by an additional step to remove an amino terminal portion of 17 kDa (Fig. 2).
During RNA replication of NβV and TaV, a sgRNA, which represents the 3′ 2.5 kb of the genome is synthesized, and this serves as the mRNA for the CP precursor (Fig. 2). In some betatetravirus species, including both NβV and TaV, the sgRNA can be encapsidated in virus particles, which complicates the distinction between the monopartite genome organization of the betatetraviruses and the bipartite genome organization of the omegatetraviruses.
ANTIGENIC PROPERTIES
Most of the members of the group are serologically interrelated but distinguishable. The majority of the isolates were identified on the basis of their serological reaction with antiserum raised against NβV.
BIOLOGICAL PROPERTIES
HOST RANGE
Nature: All virus species were isolated from Lepidoptera species (moths and butterflies), principally from Saturniid, Limacodid and Noctuid moths and no replication in other animals has been detected. In larvae, virus replication is restricted predominantly to the cells of the midgut.
Laboratory: With the exception of Providence virus (PrV), no infections by members of the Betatetravirus genus have been achieved in cultured cells, even when genomic RNA was transfected directly into cells.
TRANSMISSION
Oral transmission of NβV to Antherea eucalypti (the emperor gum moth) has been demonstrated experimentally. Oral transmission is implied by the midgut site of viral replication and by reports of some tetraviruses being used as sprayed insecticides in Malaysia (e.g. DtV; Darna trima virus). At high host densities, horizontal spread appears to be the major route of infection. Suggestive evidence exists for vertical transmission, which could be responsible for the observed persistence of tetraviruses within insect populations.
CYTOPATHIC EFFECTS
The viruses replicate primarily in the cytoplasm of gut cells of several Lepidoptera species. Crystalline arrays of virus particles are often seen within cytoplasmic vesicles. Different isolates vary considerably in pathogenicity; symptoms can vary from inapparent to acutely lethal infections.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The following criteria can be applied to the demarcation of species within the betatetravirus genus:
-
•
Biological properties (host range, vectors, mode of transmission). Since the natural host-ranges of individual recognized tetravirus species appear to be narrow, virus isolation from a new host can provide suggestive evidence of a new tetravirus species.
-
•
Antigenic properties. Antisera raised against different isolates or strains of a single tetravirus species should exhibit high levels of cross-reactivity in Western blot and/or neutralization analyses. Lower levels of cross-reactivity in these assays using antisera against previously recognized tetraviruses can provide evidence of a new tetravirus species.
-
•
Virion physical/physicochemical characteristics. In the absence of more definitive criteria, significant (>5%) differences in virion sedimentation coefficient or buoyant density from those of all previously recognized tetravirus species can provide evidence of a new virus species.
-
•
Structural protein characteristics. The electrophoretic mobilities in SDS-PAGE of the CP precursor or its cleavage products should be compared with those of other tetravirus species.
-
•Genome molecular characteristics.
-
○RNA electrophoretic mobilities. In the absence of sequence information, the electrophoretic mobilities of the viral genomic RNAs should be compared with those of other tetraviruses.
-
○RNA hybridization properties. In the absence of differences in RNA electrophoretic mobilities, the molecular hybridization properties of the viral genomic RNAs should be compared with those of other tetraviruses.
-
○
-
•
Genome sequence characteristics. The nt sequences of the genomic RNA(s) should be compared with those of other tetraviruses.
Application of these criteria. In practice, while criteria 1 − 5 above may be suggestive of a new species, definitive demarcation is based on the nt sequence of the viral CP gene.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Antheraea eucalypti virus | ||
| Antheraea eucalypti virus | (AeV) | |
| Darna trima virus | ||
| Darna trima virus | (DtV) | |
| Dasychira pudibunda virus | ||
| Dasychira pudibunda virus | (DpV) | |
| (Calliteara pudibunda virus) | (CpV) | |
| Euprosterna elaeasa virus | ||
| Euprosterna elaeasa virus | (EeV) | |
| Nudaurelia capensis β virus | ||
| Nudaurelia capensis β virus | [AF102884] | (NβV) |
| Antheraea eucalypti virus | (AeV) | |
| Philosamia cynthia x ricini virus | ||
| Philosamia cynthia x ricini virus | (PxV) | |
| Providence virus | ||
| Providence virus | (PrV) | |
| Pseudoplusia includens virus | ||
| Pseudoplusia includens virus | (PiV) | |
| Thosea asigna virus | ||
| Thosea asigna virus | [AF82930, AF062037] | (TaV) |
| (Setothosea asigna virus) | (SaV) | |
| Trichoplusia ni virus | ||
| Trichoplusia ni virus | (TnV) | |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS OMEGATETRAVIRUS
Type Species Nudaurelia capensis m virus
VIRION PROPERTIES
MORPHOLOGY
Virions are non-enveloped, roughly spherical, about 40 nm in diameter and exhibit T=4 icosahedral shell quasi-symmetry. Distinct capsomers have been resolved by cryo-electron microscopy and image reconstruction (Fig. 3 ). The genome consists of ssRNA. Viruses in the genus Omegatetravirus have bipartite genomes, whereas those in the genus Betatetravirus have monopartite genomes.
Figure 3.

(Left) Schematic representation of a T=4 icosahedral lattice. (Center and Right) Cryo-electron image reconstruction of a particle of Nudaurelia capensis β virus (NβV)(Center) and of Nudaurelia capensis ? virus (N ? V)(Right). The bar represents 25 nm. (From Johnson et al. (1994)).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is about 16 × 106. Virion S20w 194-217S. Virion buoyant density in CsCl is
1.28-1.30 g/cm3 (varies with species). Virions of Helicoverpa armigera stunt virus (HaSV) are stable between pH 3.0-11.0, and at temperatures up to 55°C.
NUCLEIC ACID
Virions of the type species, Nudaurelia capensis to virus, contain two positive-sense, ssRNA segments of 5.3 kb (RNA1, Mr 1.75 × 106) and 2.45 kb (RNA2, Mr 0.8 × 106). These genomic RNAs are capped at their 5′-ends but their 3′-ends are not polyadenylated, nor blocked like nodaviral RNAs, but instead terminate with a distinctive tRNA-like structure. It is likely that omegatetraviruses encapsidate both genomic RNAs within a single particle, in which case the RNAs would represent about 10% of the particle mass.
PROTEINS
Capsids consist of 240 protein subunits (protomers) arranged on a T=4 surface lattice. Each protomer consists of the two cleavage products (β, 62 kDa and ?, 7.8 kDa) of a single CP precursor (a, 69.8 kDa). Overall, the CPs of Nudaurelia capensis ? irus (N?V) and NβV share less than 20% aa sequence homology, indicating that the beta and omega genera of the tetraviruses have substantially diverged.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Omegatetraviruses replicate in the cytoplasm. The viral genome consists of two unique molecules of RNA, probably encapsidated in the same virus particle (Fig. 4 ). A single ORF of 1704 codons on Helicoverpa armigera stunt virus (HaSV) RNA1 (Mr 1.75 × 106; 5.3 kb) encodes the RNA replicase with a domain organization similar to that encoded by the betatetravirus NβV. RNA2 (Mr 0.8 ×106; 2.45 kb) encodes the CP precursor (70 kDa). A 157-codon ORF precedes and partially overlaps that for the CP; it encodes a non-structural protein (p17) of unknown function. Separate dsRNAs that correspond to the two genome segments are observed.
Figure 4.

Genome organization of omegatetraviruses. The locations of the Mtr, RNA Hel and RdRp motifs within the replicase ORF are indicated.
ANTIGENIC PROPERTIES
The two recognized omegatetraviruses (N?V and HaSV) display no serological relationship although their CPs show a high degree of aa sequence homology.
BIOLOGICAL PROPERTIES
HOST RANGE
Nature: All species were isolated from Lepidoptera species, principally from Saturniid, Limacodid and Noctuid moths.
Laboratory: No infections by members of the genus Omegatetravirus have yet been achieved in cultured cells, but infectious HaSV particles were produced by plant protoplasts transfected with plasmids carrying full-length cDNAs that corresponded to the viral genome segments.
TRANSMISSION
As with the betatetraviruses, oral transmission is implied by the midgut site of viral replication. At high host densities, horizontal spread appears to be the major route of infection, but evidence exists for vertical transmission which might be responsible for the observed persistence of tetraviruses within insect populations.
CYTOPATHIC EFFECTS
The viruses replicate primarily in the cytoplasm of midgut cells of the larvae of several Lepidoptera species. Crystalline arrays of virus particles are often seen within cytoplasmic vesicles. There is a considerable range of pathogenicity with different isolates, and symptoms can vary from inapparent to acutely lethal infections.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The species demarcation criteria of betatetraviruses also apply to omegatetraviruses. Also, because the genome of omegatetraviruses is segmented, reassortment is possible and the two genome segments may have different evolutionary lineages. However, no chimeric omegatetraviruses have yet been detected.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
| Acherontia atropas virus | (AaV) |
| Agraulis vanillae virus | (AvV) |
| Callimorpha quadripuntata virus | (CqV) |
| Eucocytis meeki virus | (EmV) |
| Euploea corea virus | (EcV) |
| Hyalophora cecropia virus | (HcV) |
| Hypocritae jacobeae virus | (HjV) |
| Lymantria ninayi virus | (LnV) |
| Saturnia pavonia virus | (SpV) |
| Setora nitens virus | (SnV) |
| Nudaurelia capensis ɛ virusa | (NɛV) |
NɛV resembles the tetraviruses in appearance but is serologically unrelated to any known species.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Capsid proteins of all sequenced tetraviruses form a compact group (Fig. 5 ). Two major clusters are evident in the tetravirus capsid phylogram, with one comprising NβV, TaV and EeV, and the other HaSV, N?V and PrV. It is striking that in each of these clusters, both CP processing strategies (i.e. that employed by NβV, HaSV and N?V and that identified for TaV, EeV, and PrV are present.
Figure 5.

Unrooted phenogram showing the relationships amongst CPs of members of the family Tetraviridae. The phenogram was constructed from an aa similarity matrix of CP alignments using the neighbor-joining method. Branch lengths are drawn to scale. Viruses included in the analysis, abbreviation ( ) and accession numbers [ ] are: Nudaurelia capensis ? virus (N?V) [S43937], Helicoverpa armigera stunt virus (HaSV) [L37299], Nudaurelia capensis β virus (N|V) [AF102884], Providence virus (PrV) [AF548354], Thosea asigna virus (TaV) [AF062037] and Euprosterna elaeasa virus (EeV) (AF461742). The nodavirus Flock House virus (FHV) [X15959] is included as an outgroup.
In contrast to the CPs, the replicases of the tetraviruses for which genomic sequences have been determined fall into at least two distinct phylogenetic groups that do not reflect the taxonomic demarcation. The first of these groups includes the betatetravirus NβV and the omegatetravirus HaSV. (Although no complete sequence has been published for the replicase of N?V, unpublished data show it to be very closely related to that of HaSV). Replicases of these viruses include three conserved domains, Mtr, Hel and RdRp, and cluster together with the similarly organized replicases of a dozen other ssRNA+ virus families (Fig. 6 ; see also below). The second group includes the betatetraviruses TaV and EeV. The replicases of these viruses are also multi-domain proteins with RdRp being the only domain with provisionally assigned function. The phylogenetic neighborhood of the TaV, EeV RdRps includes diverse viruses that are discussed below but not those of the NβV/HaSV group (Fig. 7 ). That there appears to be a third distinct RdRp lineage within the tetraviruses is indicated by available (unpublished) data on PrV, whose RdRp clusters with viruses other than those that form either of the above two groups.
Figure 6.
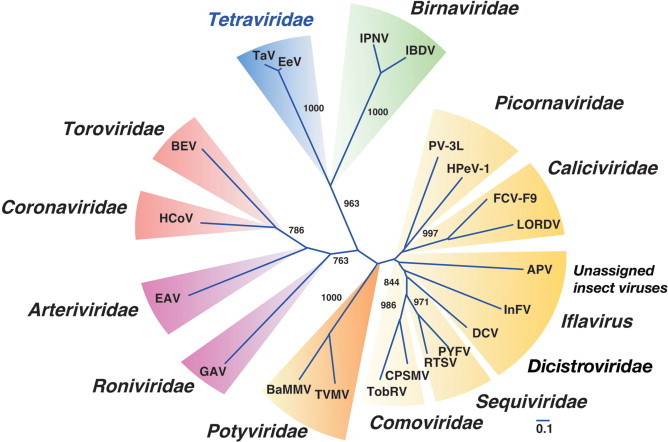
Unrooted phenogram showing the relationships of the RdRps of the tetraviruses NβV and HaSV to representatives of other virus families in the “alphavirus-like supercluster”. From the alignment, an unrooted neighbor-joining tree was inferred by the ClustalX1.82 program. Columns containing gaps were removed from the alignment and the Kimura correction for multiple substitutions was on. Branch lengths are drawn to scale. A bootstrap analysis was performed and the values of all bifurcations with support in > 700 out of 1000 bootstraps are indicated at the branching points. Viruses included in the analysis, abbreviation ( ) and accession numbers [ ] are: Bromoviridae: Alfalfa mosaic virus (AMV-LE) [L00163, K02792, K02730], Olive latent virus 2 (OLV-2) [X94346, X94327, X76933, X77115], Brome mosaic virus (BMV) [V00099, J02042, J02043, K02706, K02707, X01678, X02380, M25172]; Closteroviridae: Beet yellows virus (BYV) [X73476], Grapevine leafroll-associated virus 3 (GLAV-3) [U82937], Little cherry virus (LCV) [Y10237]; Tetraviridae: Helicoverpa armigera stunt virus (HaSV) [U18246], Nudaurelia capensis beta virus (NβV) [AF102884]; Togaviridae: Ross River virus strain NB5092 (RRV-N) [M20162], Salmon pancreas disease virus (SPDV) [AJ316244], Rubella virus strain Therien (RUBV-T) [M15240, X05259, X72393, L78917]; Hepevirus: Hepatitis E virus strain Pakistan (HeV-PA) [AF185822], Avian hepatitis E virus (AHEV) [AY043166]; Idaeovirus: Raspberry bushy dwarf virus (RBDV) [S51557, S55890, D01052]; Pomovirus: Beet virus Q (BVQ) [AJ223596 to AJ223598]; Tobamovirus: Tobacco mosaic virus strain Ob (TMV-Ob) [L11665]; Tobravirus: Tobacco rattle virus (TRV) [AF034622].
Figure 7.

Unrooted phenogram showing the relationships of the RdRps of the tetraviruses TaV and EeV to other virus families and viruses in the “picornavirus-like supercluster”. The RdRps of TaV, EeV and the birnaviruses were converted into the canonical form by relocating the motif C sequence (18-20 aa) downstream of the motif B, as in canonical polymerase motifs. These sequences were aligned with those of polymerases from representative viruses in the Picornaviridae, Dicistroviridae, Sequiviridae, Comoviridae, Caliciviridae, Potyviridae, Coronaviridae, Roniviridae, Arteriviridae, the genus Iflavirus and unclassified insect viruses. Using an extended, gap-free version of the alignment containing 332 informative characters, an unrooted neighbor-joining tree was inferred by the ClustalX1.81 program. All bifurcations with support in > 700 out of 1000 bootstraps are indicated. Different groups of viruses are highlighted. Virus families and groups, viruses included in the analysis, abbreviations ( ) and the NCBI protein (unless other specified) IDs [ ] are as follows: Picornaviridae: Human poliovirus type 3 Leon strain (PV-3L) [130503] and Human parechovirus 1 (HpeV-1) [6174922]; Iflavirus: Infectious flacherie virus (InFV) [3025415]; unclassified insect virus Acyrthosiphon pisum virus (APV) [7520835]; Dicistroviridae: Drosophila C virus (DCV) [2388673]; Sequiviridae: Rice tungro spherical virus (RTSV) [9627951] and Parsnip yellow fleck virus (PYFV) [464431]; Comoviridae: Cowpea severe mosaic virus (CPSMV) [549316] and Tobacco ringspot virus (TobRV) [1255221]; Caliciviridae: Feline calicivirus F9 (FCV-F9) [130538] and Lordsdale virus (LORDV) [1709710]; Potyviridae: Tobacco vein mottling virus (TVMV) [8247947] and Barley mild mosaic virus (BaMMV) [1905770]; Coronaviridae: Human coronavirus 229E (HCoV) [12175747] and Berne torovirus (BEV) [94017]; Arteriviridae: Equine arteritis virus (EAV) [14583262]; Roniviridae: Gill-associated virus (GAV) [9082018]; Tetraviridae: Thosea asigna virus (TaV) [AF82930; nt sequence] and Euprosterna elaeasa virus (EeV) [AF461742; nt sequence]; Birnaviridae: Infectious pancreatic necrosis virus (IPNV) [133634] and Infectious bursal disease virus (IBDV) [1296811]. Coronaviridae, Arteriviridae and Roniviridae belong to the order Nidovirales.
SIMILARITY WITH OTHER TAXA
The tetravirus CPs form a monophyletic group with the jelly-roll fold β subunits being distantly related to the CPs of nodaviruses having the T=3 capsids. It has been speculated that the tetravirus capsid might have evolved from a nodavirus-like ancestor through a process that included insertion of an immunoglobulin-like protein domain coding sequence (either acquired or evolved through sequence duplication) within the CP gene.
In contrast, comparative analysis of currently available non-structural protein sequences split tetraviruses into at least two distinct lineages, prototyped by NβV/HaSV and TaV/EeV respectively, within two different virus superclusters. The replicases of NβV and HaSV resemble those of the “alphavirus-like” supercluster, having the distinct Mtr-Hel-RdRp domain organization and through phylogenetic clustering of these three domains. The replicases of TaV and EeV lack both Mtr and Hel domains. Furthermore, their RdRp domain has a unique C-A-B motif arrangement in the palm subdomain of the active site that differs from the canonical A-B-C arrangement found in the other tetraviruses, all “alphavirus-like” viruses and indeed almost all known template-dependent polynucleotide polymerases (viral and cellular) carrying the palm sub-domain. Interestingly, the same C-A-B permutation of the motif arrangement is also found in replicases of all dsRNA birnaviruses. This motif rearrangement is a result of migration of ∼22 aa residues encompassing motif C between two internal positions, separated by ∼110 aa, in a conserved region of ∼400 aa. The permuted TaV, EeV and birnavirus enzymes form a minor, deeply separated cluster in the RdRp tree that also includes viruses of the “picornavirus-like supercluster” and order Nidovirales. Thus, TaV/EeV and birnaviruses may represent their own virus supercluster. The RdRp of PrV clusters with viruses of other families and therefore appears to belong to yet another lineage.
These complex and incongruent relationships of CP and replicase proteins imply that viruses currently classified as tetraviruses on the basis of their CP and other properties form a polyphyletic group. It is likely that immediate ancestors of TaV/EeV and PrV have independently acquired CP genes from ancestral tetraviruses resembling NβV and HaSV, respectively. Future revision of tetravirus taxonomy will need to address these complexities.
DERIVATION OF NAMES
Nudaurelia capensis is the emperor pine moth. Tetra: from Greek ‘tettares’ meaning four, as T = 4.
REFERENCES
- Agrawal D.K., Johnson J.E. Sequence and analysis of the capsid protein of Nudaurelia capensis w virus, an insect virus with T = 4 icosahedral symmetry. Virology. 1992;190:806–814. doi: 10.1016/0042-6822(92)90918-f. [DOI] [PubMed] [Google Scholar]
- Agrawal D.K., Johnson J.E. Assembly of the T = 4 Nudaurelia capensisco virus capsid protein, post-translational cleavage, and specific encapsidation of its mRNA in a baculovirus expression system. Virology. 1995;207:89–97. doi: 10.1006/viro.1995.1054. [DOI] [PubMed] [Google Scholar]
- Brooks E.M., Gordon K.H.J., Dorrian S.J., Hines E.R., Hanzlik T.N. Infection of its lepidopteran host by the Helicoverpa armigera stunt virus (Tetraviridae) J. Invertebrate Pathol. 2002;80:97–111. doi: 10.1016/s0022-2011(02)00103-9. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E., Pringle F.M., Zeddam J.-L., Luke B.T., Cameron C.E., Kalmakoff J., Hanzlik T.N., Gordon K.H.J., Ward V.K. The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J. Mol. Biol. 2002;324:47–62. doi: 10.1016/S0022-2836(02)01033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K.H.J., Hanzlik T.N. Tetraviruses. In: Miller L.K., Ball L.A., editors. The Insect Viruses. Plenum Publishing Company; New York: 1998. pp. 269–299. [Google Scholar]
- Gordon K.H.J., Johnson K.N., Hanzlik T.N. The larger genomic RNA of Helicoverpa armigera stunt tetravirus encodes the viral RNA polymerase and has a novel 3′-terminal tRNA-like structure. Virology. 1995;208:84–98. doi: 10.1006/viro.1995.1132. [DOI] [PubMed] [Google Scholar]
- Gordon K.H.J., Williams M.R., Hendry D.A., Hanzlik T.N. Sequence of the genomic RNA o Nudaurelia ß virus (Tetraviridae) defines a novel virus genome organization. Virology. 1999;258:42–53. doi: 10.1006/viro.1999.9677. [DOI] [PubMed] [Google Scholar]
- Gordon K.H.J., Williams M.R., Baker J.S., Gibson J., Bawden A.L., Millgate A., Larkin P.J., Hanzlik T.N. Replication-independent assembly of an insect virus (Tetraviridae) in plant cells. Virology. 2001;288:36–50. doi: 10.1006/viro.2001.1049. [DOI] [PubMed] [Google Scholar]
- Hanzlik T.N., Dorrian S.J., Gordon K.H.J., Christian P.D. A novel small RNA virus isolated fro the cotton bollworm, Helicoverpa armigera. J. Gen. Virol. 1993;74:1105–1110. doi: 10.1099/0022-1317-74-9-1805. [DOI] [PubMed] [Google Scholar]
- Hanzlik T.N., Johnson K.N., Gordon K.H.J. Sequence of RNA2 of the Helicoverpa armigera stun virus (Tetraviridae) and bacterial expression of its genes. J. Gen. Virol. 1995;76:799–811. doi: 10.1099/0022-1317-76-4-799. [DOI] [PubMed] [Google Scholar]
- Hanzlik T.N., Gordon K.H.J. The Tetraviridae. Adv. Virus Res. 1997;48:101–168. [PubMed] [Google Scholar]
- Johnson J.E., Munshi S., Liljas L., Agrawal D., Olson N.H., Reddy V., Fisher A., McKinney B., Schmidt T., Baker T.S. Comparative studies of T = 3 and T = 4 icosahedral RNA insect viruses, Arch. Virol. 1994;9(suppl.):497–512. doi: 10.1007/978-3-7091-9326-6_48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N.F. The Nudaurelia ß family of insect viruses. In: Kurstak E., editor. Viruses of Invertebrates. Marcel Dekker; New York: 1991. pp. 277–285. [Google Scholar]
- Munshi S., Liljas L., Cavarelli J., Bomu W., McKinney B., Reddy V., Johnson J.E. The 2.8 Å tructure of a T = 4 animal virus and its implications for membrane translocation of RNA. J. Mol. Biol. 1996;261:1–10. doi: 10.1006/jmbi.1996.0437. [DOI] [PubMed] [Google Scholar]
- Olson N.H., Baker T.S., Johnson J.E., Hendry D.A. The three-dimensional structure of frozen-hydrated Nudaurelia capensis ß virus, a T = 4 insect virus. J. Structural Biol. 1990;105:111–122. doi: 10.1016/1047-8477(90)90105-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle F.M., Gordon K.H.J., Hanzlik T.N., Kalmakoff J., Scotti P.D., Ward V.K. A novel capsid expression strategy for Thosea asigna virus, a member of the Tetraviridae. J. Gen. Virol. 1999;80:1855–1863. doi: 10.1099/0022-1317-80-7-1855. [DOI] [PubMed] [Google Scholar]
- Pringle F.M., Johnson K.N., Goodman C.L., McIntosh A.H., Ball L.A. Providence virus: a new member of the Tetraviridae that infects cultured insect cells. Virology. 2003;306:359–370. doi: 10.1016/s0042-6822(02)00052-1. [DOI] [PubMed] [Google Scholar]
- Reinganum C. Tetraviridae. In: Adams J.R., Bonami J.R., editors. Atlas of Invertebrate Viruses. CRC Press; Boca. Raton, Florida: 1991. pp. 553–592. [Google Scholar]
CONTRIBUTED BY, R. Hull, D. Fargette
GENUS SOBEMOVIRUS
Type Species Southern bean mosaic virus
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

(Left) Electronic rendering of particles of Southern bean mosaic virus (SBMV) (T=3); (Center)Diagrammatic representation of a T=3 structure capsid; (Right) Negative contrast electron micrograph of Riceyellow mottle virus (RYMV) particles stained in uranyl acetate. The bar represents 100 nm.
Virions are about 30 nm in diameter. They have a single tightly packed capsid layer with 180 subunits of about 26-34 kDa assembled on a T = 3 icosahedral lattice. Sobemoviruses are stabilized by divalent cations, pH-dependent protein-protein interactions and salt bridges between protein and RNA. Upon alkali treatment in the presence of divalent chelators, the capsid swells and become sensitive to enzymes and denaturants.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The virion Mr is about 6.6 × 106; S20w is about 115S; density is about 1.36 g/cm3 in CsCl (but virus forms two or more bands in Cs2SO4); particles swell reversibly in EDTA and higher pH with concomitant changes in capsid conformation and partial loss of stability.
NUCLEIC ACID
Particles contain a single molecule of positive sense ssRNA, ∼ 4.0-4.5 kb in size. A sgRNA molecule, co-terminal with the 3′-end of the genomic RNA, with an Mr of 0.3-0.4 × 106, is synthesized in the virus infected cell. Both genomic and subgenomic RNAs have a viral protein (VPg) covalently bound to their 5′-end. The 3′ terminus is non-polyadenylated and does not contain a tRNA-like structure. Several sobemoviruses encapsidate a circular viroid-like satellite RNA (220-390 nt).
PROTEINS
The CP subunits are chemically identical but structurally not equivalent. Three types of CP subunits termed A, B, C are related by quasi three-fold axes of symmetry and are involved in different inter-subunit contacts. The CP has two distinct domains. The N-terminal R (random) domain, partially ordered, is localized to the interior of the particle. The S domain which forms the surface of the particle displays a canonical ß-barrel motif. The arrangement of the N-terminal part (in particular the ßA arm) of the sub-unit plays a crucial role in determining the capsid size.
The CP of RYMV is required for cell-to-cell movement as well as for long-distance movement. The protein P1, coded by ORF1, has been associated with cell-to-cell movement for SBMV and RYMV and also for suppression of RNA silencing in RYMV-infected plants. Sequence similarities, suggest that the ORF2 protein has replication functions. No function has been attributed to the protein encoded by ORF3.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Figure 2.

Genomic organization for representatives of four species of sobemoviruses. The lines represent theviral RNA genomes, the boxes indicate the ORFs and the numbers correspond to the nt numbers for the beginningand the ending of the ORFs. The vertical lines indicate the position of serine and cysteine protease motifs andthe horizontal lines indicate a polymerase conserved domain of luteoviruses. The ORF frameshift signal: UUUAAAC is positioned by a striped bar and the presence of a second ATG in ORF2b of CoMV is indicated atnt 1740.
The genome of sobemoviruses harbors four ORFs. Based on organizational differences in the central part of the genome (encoding the viral polyprotein), the sobemoviruses are subdivided into Southern cowpea mosaic virus (SCPMV)-like and Cocksfoot mottle virus (CoMV)-like types. The polyprotein of SCPMV is encoded by a large continuous ORF2. The genome of SCPMV also contains an internal coding region, ORF3, situated in the −1 reading frame within ORF2. Similar organization has been reported for LTSV, RGMoV, SBMV and SeMV. In contrast, CoMV lacks the continuous ORF2 and the nested coding region of the SCPMV. Instead, CoMV has two overlapping ORFs, ORF2a and ORF2b. ORF2b is expressed as a fusion protein through a −1 ribosome frameshift mechanism. A similar genomic organization is characteristic of SCMoV and of all strains of RYMV.
Translation initiation of sobemovirus ORF1 and ORF2 proteins occurred via a leaky ribosomal scanning mechanism. ORF1 encodes a small protein involved in virus movement and in suppressing gene silencing. ORF2 codes for a polyprotein having the putative serine-protease, VPg and RdRp domains. The polyprotein is cleaved by the N-terminal serine protease. The CP gene (ORF4) is encoded by a sgRNA at the 3′-end of the genome. The coat protein is required for cell-to-cell and long-distance movement.
ANTIGENIC PROPERTIES
Viral proteins and virions are efficient immunogens. A single precipitin line is formed in gel diffusion tests. There are serological relationships between SBMV, SCPMV and SeMV. SCMoV and LTSV virions are serologically distantly related. Several serotypes with different geographical origins have been identified in some species.
BIOLOGICAL PROPERTIES
HOST RANGE
Sobemoviruses infect both monocotyledonous and dicotyledonous plants, but the natural host range of each virus species is relatively narrow. Disease symptoms are mainly mosaics and mottles. Systemic infections are caused in most natural hosts with most cell types being infected.
TRANSMISSION
Seed transmission occurs in several host plants for some sobemoviruses (SBMV, Southern cowpea mosaic virus; SCPMV). The viruses are transmitted by beetles or, for Velvet tobacco mottle virus (VTMoV), a myrid. All sobemoviruses are readily transmitted mechanically.
GEOGRAPHICAL DISTRIBUTION
Most members have limited distribution but some species are found worldwide.
CYTOPATHIC EFFECTS
Virions are found in both the cytoplasm and nuclei, and late in infection occur as large crystalline aggregates in the cytoplasm and the vacuoles. Infected cells show extensive cytoplasmic vacuolation. Some members invade phloem and xylem cells (SBMV, RYMV), and virions are also found in pit membranes of primary cell walls (RYMV).
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Different host ranges in certain plant species.
-
•
Antigenic differences, and
-
•
Values of 40% or more sequence difference as assessed by hybridization tests or by comparisons of sequence data.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Blueberry shoestring virus | ||
| Blueberry shoestring virus | [NC003138] | (BSSV) |
| Cocksfoot mottle virus | ||
| Cocksfoot mottle virus | [Z48630, NC002618, L40905] | (CoMV) |
| Lucerne transient streak virus | ||
| Lucerne transient streak virus | [NC001696] | (LTSV) |
| Rice yellow mottle virus | ||
| Rice yellow mottle virus – Cote d’Ivoire | [NC001575] | (RYMV-CI) |
| Rice yellow mottle virus – Nigeria | [U23142] | (RYMV-Ni) |
| Ryegrass mottle virus | ||
| Ryegrass mottle virus | [NC003747] | (RGMoV) |
| Sesbania mosaic virus | ||
| Sesbania mosaic virus | [NC802568] | (SeMV) |
| Solanum nodiflorum mottle virus | ||
| Solanum nodiflorum mottle virus | (SNMoV) | |
| Southern bean mosaic virus | ||
| Southern bean mosaic virus | [L34672] | (SBMV) |
| Southern bean mosaic virus – B/Ark | [NC004060] | (SBMV-BAr) |
| Southern cowpea mosaic virus | ||
| Southern cowpea mosaic virus (previously cowpea strain of SBMV) | [NC001625] | (SCPMV) |
| Sowbane mosaic virus | ||
| Sowbane mosaic virus | (SoMV) | |
| Subterranean clover mottle virus | [NC004346] | |
| Subterranean clover mottle virus | (SCMoV) | |
| Turnip rosette virus | ||
| Turnip rosette virus | [NC004553] | (TRoV) |
| Velvet tobacco mottle virus | ||
| Velvet tobacco mottle virus | (VTMoV) | |
TENTATIVE SPECIES IN THE GENUS
| Cocksfoot mild mosaic virus | (CMMV) |
| Cynosurus mottle virus | (CnMoV) |
| Ginger chlorotic fleck virus | (GCFV) |
| Rottboellia mottle virus | (RoMoV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Figure 3.

Phylogenetic tree reconstructed by maximum parsimony of representatives of the nine species ofsobemoviruses. It includes Cocksfoot mottle virus (CoMV; NC 002618, L40905), Lucerne transient streak virus(LTSV; NC 001696), Rice yellow mottle virus (RYMV; NC 001575, AJ608219), Ryegrass mottle virus (RGMoV;NC 003747), Sesbania mosaic virus (SeMV; NC 002568), Southern bean mosaic virus (SBMV; NC 004060,L34672), Southern cowpea mosaic virus (SCPMV; NC 001625), Subterranean clover mottle virus (SCMoV; NC004346) and Turnip rosette virus (TRoV; NC 004553). The sequences of the Potato leaf roll virus(Luteoviridae)(PLRV??; AF453394) and of the Mushroom bacilliform virus (Barnaviridae)(MBV??; NC001633)were used as out-groups. The numbers at each node indicate the percentage of bootstrap support after 1000 replicates.
From their genomic organization, sobemoviruses can be divided into two distinct subgroups: those that express the RdRp from a single in-frame polyprotein, and those that express it via a −1 translational frameshifting mechanism. However, the sobemoviruses with either of the two genomic organizations do not cluster into two discrete sub-groups in phylogenetic analyses.
SIMILARITY WITH OTHER TAXA
The 5′-terminal half of the sobemovirus genome resembles that of the poleroviruses and enamoviruses with the successive functional domains of a serine protease-like, a VPg and an RdRp. Moreover, polymerase sequences of the sobemoviruses are phylogenetically related to those of the poleroviruses and enamoviruses. By contrast, the CP of the sobemoviruses – encoded by the 3′terminal half of the genome – shows sequences and structural similarities with the CP of members of the genus Necrovirus of the family Tombusviridae. A member of the species Mushroom bacilliform virus, the unique species of the family Barnaviridae, has a genomic organization similar to that of the sobemoviruses and showed sequence identities with them, both in the polymerase and in the CP genes. Genomic organization of animal viruses of the family Astroviridae also shows similarities to that of sobemoviruses, but the sequence identities are more remote.
DERIVATION OF NAMES
Sobemo: sigla derived from the name of type species southern bean mosaic.
REFERENCES
- Bonneau C., Brugidou C., Chen L., Beachy R.N., Fauquet C.M. Expression of the Rice yellow mottle virus P1 protein in vitro and in vivo and its involvement in virus spread. Virology. 1998;244:79–96. doi: 10.1006/viro.1998.9100. [DOI] [PubMed] [Google Scholar]
- Dwyer G.I., Njeru R., Williamson S., Fosu-Nyarko J., Hopkins R., Jones R.A.C., Waterhouse P.M., Jones M.G.K. The complete nucleotide sequence of Subterranean clover mottle virus. Arch. Virol. 2003;148:2237–2247. doi: 10.1007/s00705-003-0144-3. [DOI] [PubMed] [Google Scholar]
- Fargette D., Pinel A., Abubakar Z., Traoré O., Brugidou C., Fatogoma S., Hébrard E., Choisy M., Yacouba S., Fauquet C., Konaté, G. Inferring the evolutionary history of Rice yellow mottle virus from genomic, phylogenetic and phylogeographic studies. J. Virol. 2004;7:3252–3261. doi: 10.1128/JVI.78.7.3252-3261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker D.L., Sivahumaran K. Mapping and expression of Southern bean mosaic virus genomic and subgenomic RNAs. Virology. 1997;234:317–327. doi: 10.1006/viro.1997.8667. [DOI] [PubMed] [Google Scholar]
- Jones A.T., Mayo M.A. Satellite nature of the viroid-like RNA-2 of Solanum nodiflorum mottle virus and the ability of other plant viruses to support the replication of viroid-like RNA molecules. J. Gen. Virol. 1984;65:1713–1721. [Google Scholar]
- Konate G., Sarra S., Traore R. Rice yellow mottle virus is seed-borne but not seed transmitted in rice seeds. Eur. J. Plant Pathol. 2001;107:361–364. [Google Scholar]
- Lee L., Anderson E. Nucleotide sequence of a resistance breaking mutant of southern bean mosaic virus. Arch. Virol. 1998;143:2189–2201. doi: 10.1007/s007050050451. [DOI] [PubMed] [Google Scholar]
- Lokesh G.L., Gopinath K., Satheshkumar P.S., Savithri H.S. Complete nucleotide sequence of Sesbania mosaic virus: a new virus of the genus Sobemovirus. Arch. Virol. 2001;146:209–223. doi: 10.1007/s007050170170. [DOI] [PubMed] [Google Scholar]
- Mäkinen K., Tamm T., Næss V., Truve E., Puurand U., Munthe T., Saarma M. Characterization of Cocksfoot mottle virus genomic RNA and sequence comparisons with related viruses. J. Gen. Virol. 1995;76:2817–2825. doi: 10.1099/0022-1317-76-11-2817. [DOI] [PubMed] [Google Scholar]
- Ngon A., Yassi, M., Ritzenthaler C., Brugidou C., Fauquet C.M., Beachy R.N. Nucleotide sequence and genome characterization of Rice yellow mottle virus RNA. J. Gen. Virol. 1994;75:249–257. doi: 10.1099/0022-1317-75-2-249. [DOI] [PubMed] [Google Scholar]
- Opalka N., Brugidou C., Bonneau C., Nicole M., Beachy R., Yeager M., Fauquet C.M. Movement of rice yellow mottle virus between xylem cells through pit membranes. Proc. Natl. Acad. Sci. USA. 1998;95:3323–3328. doi: 10.1073/pnas.95.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman Y., Hull R. Nucleotide sequence of the bean strain of Southern bean mosaic virus. Virology. 1995;206:287–297. doi: 10.1016/S0042-6822(95)80044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C., Liljas L., Opalka N., Brugidou C., Yeager M., Beachy R., Fauquet C., Johnson J., Lin T. 3D domain swapping modulates the stability of members of an icosahedral virus group. Structure. 2000;8:1095–1103. doi: 10.1016/s0969-2126(00)00508-6. [DOI] [PubMed] [Google Scholar]
- Sara S., Peters D. Rice yellow mottle virus is transmitted by cows, donkeys, and grass rats in irrigated rice crops. Plant Disease. 2003;87:804–808. doi: 10.1094/PDIS.2003.87.7.804. [DOI] [PubMed] [Google Scholar]
- Satheshkumar P., Lokesh G., Savithri H. Polyprotein processing: cis and trans proteolytic activities of Sesbania mosaic virus serine protease. Virology. 2004;318:429–438. doi: 10.1016/j.virol.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Sivakumaran K., Hacker D. The 105-k-Da polyprotein of Southern bean mosaic virus is translated by scanning ribosomes. Virology. 1998;246:34–44. doi: 10.1006/viro.1998.9183. [DOI] [PubMed] [Google Scholar]
- Tars K., Zeltins A., Liljas L. The three-dimensional structure of cocksfoot mottle virus at 2.7 A resolution. Virology. 2003;310:287–297. doi: 10.1016/s0042-6822(03)00148-x. [DOI] [PubMed] [Google Scholar]
- Tamm T., Truve E. Sobemoviruses. J. Virol. 2000;74:6231–6241. doi: 10.1128/jvi.74.14.6231-6241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O., Pinto M., Baulcombe D. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA. 1999;96:14147–14152. doi: 10.1073/pnas.96.24.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Rinehart C.A., Kaesberg P. Sequence and organization of Southern bean mosaic virus genomic RNA. Virology. 1987;161:73–80. doi: 10.1016/0042-6822(87)90172-3. [DOI] [PubMed] [Google Scholar]
- Zhang F.Y., Toriyama S., Takahashi M. Complete nucleotide sequence of Ryegrass mottle virus: a new species of the genus Sobemovirus. J. Gen. Plant Pathol. 2001;67:63–68. doi: 10.1007/PL00012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONTRIBUTED BY, C.J. D'Arcy, L.L. Domier
FAMILY LUTEOVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Luteoviridae |
| Genus | Luteovirus |
| Genus | Polerovirus |
| Genus | Enamovirus |
VIRION PROPERTIES
MORPHOLOGY
Virions are 25 to 30 nm in diameter, hexagonal in outline and have no envelope (Fig. 1 ). They exhibit icosahedral symmetry (T=3). Particles are composed of two proteins and a core of genomic ssRNA. A small protein (VPg) has been reported to be covalently linked to the 5′-end of the genomic RNA of two poleroviruses and the one enamovirus.
Figure 1.

(Left) Diagram of the proposed structure of luteovirus particles. (Center) Negative contrast electron micrograph of particles of Barley yellow dwarf virus-PAV (BYDV-PAV) and (Right) Pea enation mosaic virus-1 (PEMV-1), isolated by means of sucrose density gradient centrifugation and stained with uranyl acetate. The bars represent 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
For luteoviruses and poleroviruses, virion Mr is about 6 × 106; buoyant density in CsCl is 1.40 g/cm3; S20w is 106-127S. For enamoviruses (B component), the Mr is about 5.6 × 106; buoyant density in CsCl is 1.42 g/cm3; S20w is 107-122S. Virions are moderately stable and are insensitive to treatment with chloroform or non-ionic detergents, but are disrupted by prolonged treatment with high concentrations of salts. Luteovirus and polerovirus particles are insensitive to freezing.
NUCLEIC ACID
Virions contain a single molecule of infectious, linear, positive-sense ssRNA. The genome size is fairly uniform: 5,677 nt for BYDV-PAV, 5,697 nt for BYDV-PAS, 5,964 nt for Bean leafroll virus(BLRV), 5,708-5,853 nt for Soybean dwarf virus (SbDV), 5,882 nt for Potato leafroll virus (PLRV), 5,776 nt for Beet chlorosis virus (BChV), 5,722 nt for Beet mild yellowing virus (BMYV), 5,641 nt for Beet western yellows virus (BWYV), 5,600 nt for Cereal yellow dwarf virus (CYDV-RPV) (partial sequence), 5,669 nt for Cucurbit aphid borne yellows virus (CABYV), 5,899 nt for Sugarcane yellow leaf virus (ScYLV) and 5,705 nt for (PEMV-1). The RNAs do not have a 3′-terminal poly(A) tract. A VPg is linked to the genome RNA of the poleroviruses PLRV and BYDV-RPV and the enamovirus PEMV-1.
PROTEINS
The five or six proteins encoded by genome RNA are between 4 and 84 kDa (Table 1 ). The CP gene has been assigned to ORF3, which is followed in frame by ORF5, and the polerovirus VPg has been assigned to ORF1.
Table 1.
Proteins of the different ORFs with sizes (kDa) and possible function(s).
| ORF | Luteovirus | Polerovirus | Enamovirus | Function of product |
|---|---|---|---|---|
| 0 | NA | 28-30 | 34 | possible membrane-linked replication factor |
| 1 | 39–42 | 66–72 | 84 | helicase motifs in luteoviruses; protease and VPg in poleroviruses |
| 2 | 60–62 | 65–72 | 67 | probable RdRp |
| 3 | 22 | 22–23 | 21 | CP gene |
| 4 | 16–21 | 17–21 | NA | probable MP |
| 5 | 43–59 | 50–56 | 29 | possible aphid transmission or virus particle stability factor |
| 6 | 4–7 | 7–9 | NA | unknown |
Virion structural proteins are CP and a “readthrough” protein, which is a fusion of the products of the CP gene and the contiguous ORF5. The readthrough protein may be associated with aphid transmission or virus particle stability. The product of ORF4 has been shown to be required for long distance movement of some luteoviruses and poleroviruses. ORF4 is absent from enamovirus RNA. The region containing ORF6 in the luteoviruses, but not the ORF6 translation product, acts as a translational enhancer of the expression of BYDV-PAV RNA.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomes contain 5 or 6 ORFs (Fig. 2 ). The genera can be distinguished on the basis of the arrangements and sizes of the ORFs. The ORFs encoding the replication-related proteins (1 and 2) of the luteoviruses are not homologous to the corresponding ORFs of the poleroviruses. The products of ORFs 1 and 2 of the luteoviruses are most similar to those of viruses in the family Tombusviridae, while the products of ORFs 1 and 2 of the poleroviruses and enamoviruses are related to those of sobemoviruses. Also, ORF0 is present in the genomes of poleroviruses and enamoviruses, ORF4 is present in the genomes of luteoviruses and poleroviruses, and ORF6 is present in the genomes of some luteoviruses and poleroviruses. ORF0 overlaps ORF1 (poleroviruses and enamoviruses), which overlaps ORF2. ORF4 is contained completely within ORF3 (luteoviruses and poleroviruses). Finally, ORF5 is positioned directly downstream of, and contiguous with, ORF3.
Figure 2.

Diagram of the genome organization and map of the translation products typical of viruses in each genus of the family Luteoviridae. Solid lines represent RNA; boxes represent ORFs; thinner boxes represent translation products; grey circles represent VPgs.
The differences among luteoviruses, poleroviruses and enamoviruses are principally in the 5′-end of the genome. ORFs 0, 1 and 2 are translated from the genomic RNA. ORF2 is translated by frameshift from ORF1 and thus shares an amino terminus with the product of ORF1. ORFs 3, 4 (in luteoviruses and poleroviruses) and 5 are expressed from a sgRNA. ORF5 is probably translated via a readthrough following translation of ORF3. Luteoviruses produce two additional sgRNAs, the larger of which contains ORF6. Some poleroviruses produce a second sgRNA.
There are no data on post-translational modification. Particles of some strains of CYDV-RPV contain 322 nt satellite RNAs and virions of some isolates that consist of PEMV-1 together with the umbravirus Pea enation mosaic virus-2 (PEMV-2) contain 717 nt satellite RNAs in addition to genomic RNAs.
ANTIGENIC PROPERTIES
Luteovirus and polerovirus particles are strongly immunogenic. Species within a genus are more closely related serologically than are species in different genera. Serological relationships may be detected when comparing disrupted virus particles that are not detectable when intact virions are tested. Virions produced in plants infected with PEMV-1 together with PEMV-2 (Umbravirus) are moderately antigenic. In gel diffusion assays, aphid-transmissible isolates display an antigenic determinant that is absent from aphid-non-transmissible isolates. No serological relationships have been reported between enamoviruses and either luteoviruses or poleroviruses.
BIOLOGICAL PROPERTIES
HOST RANGE
Several members of the family Luteoviridae have host ranges largely restricted to one plant family. For example, BYDV and CYDV infect many grasses, BLRV infects mainly legumes, and Carrot red leaf virus infects mainly plants in the family Umbelliferae. Other members of the family Luteoviridae infect plants in several or many different families. For example, BWYV infects more than 150 species of plants in over 20 families.
GEOGRAPHIC DISTRIBUTION
Members of the family Luteoviridae have been reported from arctic, temperate, subtropical, and tropical regions. Some of the viruses are found worldwide, such as BYDV, BWYV and PLRV. Others have more restricted distributions, such as Tobacco necrotic dwarf virus, which has been reported only from Japan, and Groundnut rosette assistor virus, which has been reported from south Saharan countries in Africa.
TRANSMISSION
Transmission is in a circulative, non-propagative manner by specific aphid vectors. Virus is acquired by phloem feeding, enters the hemocoel of the aphid via the hindgut (e.g., BYDV-PAV) or posterior midgut (e.g., PLRV), circulates in hemolymph and enters the accessory salivary gland. Inoculation probably results from transport of virus into the salivary duct and introduction of saliva into the plant during feeding. PEMV-1 is readily transmitted mechanically, a property dependent on its multiplication in cells co-infected with PEMV-2 (Umbravirus), but aphid transmissibility can be lost after several mechanical passages.
CYTOPATHOLOGY
Luteovirus and polerovirus particles are largely confined to phloem cells; PEMV-1, with PEMV-2, is found in phloem and mesophyll tissue. Virus particles are found in both the nuclei and cytoplasm of infected cells. Luteoviruses and poleroviruses often cause phloem necrosis that spreads from inoculated sieve elements and causes symptoms by inhibiting translocation, slowing plant growth and inducing the loss of chlorophyll. The genome of PEMV-1 is capable of autonomous replication in protoplasts, but is dependent on PEMV-2 to support systemic invasion, which induces mosaic and enation symptoms.
LIST OF SPECIES DEMARCATION CRITERIA IN THE FAMILY
Criteria used to demarcate species of the family Luteoviridae include:
-
•
Differences in breadth and specificity of host range;
-
•
Failure of cross protection in either one-way or two-way relationships;
-
•
Differences in serological specificity with discriminatory polyclonal or monoclonal antibodies;
-
•
Differences in aa sequences of any gene product of greater than 10%.
The nt sequences of BLRV and SbDV (genus Luteovirus) lack ORF0, like those of luteoviruses, and the predicted aa sequences of their replication proteins are similar to those of the luteoviruses. However, BLRV and SbDV structural proteins are more closely related to those of the poleroviruses. The genome of the polerovirus ScYLV contains an ORF0. ScYLV ORFs 1 and 2 are most closely related to those of the polerovirses, ORFs 3 and 4 are most closely related to those of the luteoviruses and ORF5 is most closely related to the read-through protein gene of the enamovirus. These viruses may represent hybrids of sequences from these three genera.
GENUS LUTEOVIRUS
Type Species Barley yellow dwarf virus – PAV
DISTINGUISHING FEATURES
Virion buoyant density in CsCl is 1.39-1.40 g/cm3; S20w is 106-118S. Genome sizes are 5273 nt (BYDV-MAV) (partial sequence), 5,677 nt (BYDV-PAV), and 5,697 nt (BYDV-PAS). The genome RNA does not have a VPg. Genome properties are the key features. There is no ORF0 and frameshift from ORF1 into ORF2 occurs at the termination codon of ORF1. The translation products of ORF1 and ORF2 form replication-related proteins, which are most similar to those of viruses in the family Tombusviridae. The length of the non-coding sequence between ORF2 and ORF3 is about 100 nt. There is no evidence for the presence of a genome-linked protein and translation is by a cap-independent mechanism. ORF4 is present and contained within ORF3.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
See above “List of Species Demarcation Criteria in the Family”.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Barley yellow dwarf virus-MAV | ||
| Barley yellow dwarf virus-MAV | [D01213] | (BYDV-MAV) |
| Barley yellow dwarf virus-PAS‡ | ||
| Barley yellow dwarf virus-PAS | [AF218798, U29604] | (BYDV-PAS) |
| Barley yellow dwarf virus-PAV | ||
| Barley yellow dwarf virus-PAV | [D01214, L25299, D85783, AF235167] | (BYDV-PAV) |
| (Barley yellow dwarf virus-RGV( | ||
| (Rice giallume virus) | ||
| Bean leafroll virus | ||
| Bean leafroll virus | [AF441393] | (BLRV) |
| (Legume yellows virus) | ||
| (Michigan alfalfa virus) | ||
| (Pea leafroll virus) | ||
| Soybean dwarf virus | ||
| Soybean dwarf virus | [L24049] | (SbDV) |
| (Subterranean clover red leaf virus) | ||
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS POLEROVIRUS
Type Species Potato leafroll virus
DISTINGUISHING FEATURES
Particles are thought to have 180 subunits arranged in a T=3 icosahedron. Virion buoyant density in CsCl is 1.39-1.42; S20w is 115-127S. Genome sizes range from 5,641 nt for BWYV to 5,882 nt for PLRV. Poleroviruses and enamoviruses are distinguished from luteoviruses by genome features. The polerovirus genome has a VPg linked to the 5′-end of the genome RNA. The presence of a VPg has been confirmed for PLRV and CYDV-RPV. Poleroviruses possess an ORF0 and a non-coding region between ORF2 and ORF3 of about 200 nt. The translation products of ORF1 and ORF2 form replication-related proteins, which are most similar to those of sobemoviruses. Frameshift from ORF1 into ORF2 occurs upstream of the termination of ORF1. Polerovirus genomes differ from those of enamoviruses in that ORF4 is present within ORF3 and ORF5 is about 1400 nt.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
See above “List of Species Demarcation Criteria in the Family”.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Beet chlorosis virus | ||
| Beet chlorosis virus | [NC_002766] | (BChV) |
| Beet mild yellowing virus | ||
| Beet mild yellowing virus | [X83110] | (BMYV) |
| Beet western yellows virus | ||
| Beet western yellows virus | [X13062, X13063] | (BWYV) |
| (Malva yellows virus) | ||
| (Turnip mild yellows virus) | ||
| Cereal yellow dwarf virus-RPS | ||
| Cereal yellow dwarf virus-RPS | [AF235168] | (CYDV-RPS) |
| Cereal yellow dwarf virus-RPV | ||
| Cereal yellow dwarf virus-RPV | [Y07496] | (CYDV-RPV) |
| Cucurbit aphid-borne yellows virus | ||
| Cucurbit aphid-borne yellows virus | [X76931] | (CABYV) |
| Potato leafroll virus | ||
| Potato leafroll virus | [X14600, X74789, D13954, D00734, D13953, D00733, D00530, X14600] | (PLRV) |
| (Solanum yellows virus) | ||
| (Tomato yellow top virus) | ||
| Sugarcane yellow leaf virus | ||
| Sugarcane yellow leaf virus | [AF157029, AJ249447, AY236971] | (ScYLV) |
| Turnip yellows virus‡ | ||
| Turnip yellows virus | [AF168608, AF168606] | (TuYV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS ENAMOVIRUS
Type Species Pea enation mosaic virus-1
DISTINGUISHING FEATURES
PEMV-1 occurs as part of a complex with PEMV-2 (Umbravirus). Unlike other members of the family Luteoviridae, PEMV-1 is readily transmitted mechanically, a property dependent on its multiplication in cells co-infected with PEMV-2, but aphid transmissibility can be lost after several mechanical passages. Virions are found in mesophyll tissue as well as in vascular tissue. PEMV-1 will multiply when inoculated to isolated leaf protoplasts, but there is no evidence that it can spread in plants.
Enamovirus particles (B component) are 25-28 nm in diameter. A 180 subunit arrangement in a T=3 icosahedron has been proposed. The virions have Mr of about 5.6 × 106, buoyant densities in CsCl of 1.42 g/cm3 and S20w of 107-122S.
Genome size is 5,706 nt (PEMV-1). A VPg is associated with virion RNA of PEMV-1. The PEMV-1 genome contains an ORF0, but does not contain an ORF4 (present in luteoviruses and poleroviruses). The non-coding intergenic region between ORF2 and ORF3 is about 200 nt in length. The translation products of ORF1 and ORF2 form replication-related proteins, which are most similar to those of sobemoviruses. Frameshift from ORF1 into ORF2 occurs upstream of the termination of ORF1. The PEMV-1 genome contains an ORF5 of about 730 nt.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Pea enation mosaic virus-1 | ||
| Pea enation mosaic virus-1 | [L04573] | (PEMV-1) |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED SPECIES IN THE FAMILY
| Barley yellow dwarf virus-GPV | ||
| Barley yellow dwarf virus-GPV | [L10356] | (BYDV-GPV) |
| Barley yellow dwarf virus-RMV | ||
| Barley yellow dwarf virus-RMV | [Z14123, L12757-9] | (BYDV-RMV) |
| Barley yellow dwarf virus-SGV | ||
| Barley yellow dwarf virus-SGV | [U06865] | (BYDV-SGV) |
| Carrot red leaf virus | ||
| Carrot red leaf virus | (CtRLV) | |
| Chickpea stunt disease associated virus | ||
| Chickpea stunt disease associated virus | [Y11530] | (CpSDaV) |
| Groundnut rosette assistor virus | ||
| Groundnut rosette assistor virus | [Z68894] | (GRAV) |
| Indonesian soybean dwarf virus | ||
| Indonesian soybean dwarf virus | (ISDV) | |
| Strawberry mild yellow edge associated virus | ||
| Strawberry mild yellow edge associated virus | (SMYEaV) | |
| Sweet potato leaf speckling virus | ||
| Sweet potato leaf speckling virus | (SPLSV) | |
| Tobacco necrotic dwarf virus | ||
| Tobacco necrotic dwarf virus | (TNDV) | |
| Tobacco vein distorting virus‡ | ||
| Tobacco vein distorting virus | [AJ459320, AJ457176, AF402621] | (TVDV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
The three genera within the family Luteoviridae share very similar structural protein genes (ORFs 3 and 5) whose products show varying levels of serological relatedness. Phylogenetic analysis of the predicted aa sequences of the polymerases (ORF2) most clearly separate the members of the family Luteoviridae into the three genera (Fig. 3 ). ORFs 1 and 2 of the luteoviruses are most closely related to the polymerase genes of viruses in the family Tombusviridae, while ORFs 1 and 2 of the poleroviruses and enamoviruses are related to those of viruses in the genus Sobemovirus. This is manifest in differences in gene number and placement and the presence or absence of a VPg. BLRV, SbDV and ScYLV, unassigned species, group with different genera depending on which sequences are analyzed. The CP sequences of BYDV-GPV, BYDV-RMV, CpSDaV, GRAV and SPLSV group with the poleroviruses; BYDV-SGV groups with the luteoviruses.
Figure 3.

Phylogenetic analyses of the (Top) Capsid Protein, and (Bottom) Polymerase sequences of representatives of species in the family Luteoviridae. Amino acid sequences were aligned with CLUSTALX and trees constructed with PAUP. Bootstraps values above 50% are indicated.
SIMILARITY WITH OTHER TAXA
Viruses in the family Luteoviridae have replication-related proteins which are sufficiently similar to those in other genera to suggest evolutionary relationships. The putative luteovirus polymerases resemble those of members of the family Tombusviridae. In contrast, polymerases of poleroviruses and enamoviruses resemble those of viruses in the genus Sobemovirus. These polymerase types are thought to be very distant in evolutionary terms and it has been suggested that the origin of these genomes was recombination between ancestral genomes containing the CP genes characteristic of the family Luteoviridae and genomes containing either of the two polymerase types. The CP sequences of PLRV and Rice yellow mottle virus, a sobemovirus, share 33% similarity, which has been used to predict the structure of PLRV and other members of the family Luteoviridae.
DERIVATION OF NAMES
Enamo: sigla from Pea enation mosaic virus
Luteo: from Latin luteus, “yellow”
Polero: sigla from Potato leaf roll virus
REFERENCES
- Ashoub A., Rohde W., Prufer D. In planta transcription of a second subgenomic RNA increases the complexity of the subgroup 2 luteovirus genome. Nuc. Acids Res. 1998;26:420–426. doi: 10.1093/nar/26.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencharki B., Mutterer J., El Yamani M., Ziegler-Graff V., Zaoui D., Jonard G. Severity of infection of Moroccan barley yellow dwarf virus PAV isolates correlates with variability in their coat protein sequences. Ann. Appl. Biol. 1999;134:89–99. [Google Scholar]
- Brault V., van Den Heuvel J.F.J.M., Verbeek M., Ziegler-Graff V., Reutenauer A., Herrbach E., Garaud J.-C., Guilley H., Richards K., Jonard G. Aphid transmission of beet western yellows luteovirus requires the minor capsid read-through protein P74. EMBO J. 1995;14:650–659. doi: 10.1002/j.1460-2075.1995.tb07043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demler S.A., De Zoeten G.A. The nucleotide sequence and luteovirus-like nature of RNA 1 of an aphid non-transmissible strain of pea enation mosaic virus. J. Gen. Virol. 1991;72:1819–1834. doi: 10.1099/0022-1317-72-8-1819. [DOI] [PubMed] [Google Scholar]
- Demler S.A., De Zoeten G.A., Adam G., Harris K.F. Pea enation mosaic enamovirus: properties and aphid transmission. In: Harrison B.D., Murant A.F., editors. The Plant Viruses 5: polyhedral virions and bipartite RNA genomes. Plenum; New York and London: 1995. pp. 303–344. [Google Scholar]
- Domier L.L., McCoppin N.K., Larsen R.C., D'Arcy C.J. Nucleotide sequence shows that Bean leafroll virus has a Luteovirus-like genome organization. J. Gen. Virol. 2002;83:1791–1798. doi: 10.1099/0022-1317-83-7-1791. [DOI] [PubMed] [Google Scholar]
- Fuentes S., Mayo M.A., Jolly C.A., Nakano M., Querci M., Salazar L.F. A novel luteovirus from sweet potato, sweet potato leaf speckling virus. Ann. Appl. Biol. 1996;128:491–504. [Google Scholar]
- Hauser S., Stevens M., Beuve M., Lemaire O. Biological properties and molecular characterization of Beet chlorosis virus (BChV) Arch. Virol. 2002;147:745–762. doi: 10.1007/s007050200023. [DOI] [PubMed] [Google Scholar]
- Hauser S., Stevens M., Mougel C., Smith H.G., Fritsch C., Herrbach E., Lemaire O. Biological, serological, and molecular variability suggest three distinct polerovirus species infecting beet or rape. Phytopathology. 2000;90:460–466. doi: 10.1094/PHYTO.2000.90.5.460. [DOI] [PubMed] [Google Scholar]
- Martin R.R., D'Arcy C.J. Relationships among luteoviruses based on nucleic acid hybridization and serological studies. Intervirology. 1990;31:23–30. doi: 10.1159/000150130. [DOI] [PubMed] [Google Scholar]
- Mayo M.A., Ziegler-Graff V. Molecular biology of luteoviruses. Adv. Virus Res. 1996;46:413–460. doi: 10.1016/s0065-3527(08)60077-9. [DOI] [PubMed] [Google Scholar]
- Miller W.A., Rasochova L. Barley yellow dwarf viruses. Ann. Rev. Phytopath. 1997;35:167–190. doi: 10.1146/annurev.phyto.35.1.167. [DOI] [PubMed] [Google Scholar]
- Mo X.H., Qin X.Y., Wu J., Yang C., Wu J.Y., Duan Y.Q., Li T.F., Chen H.R. Complete nucleotide sequence and genome organization of a Chinese isolate of tobacco bushy top virus. Arch. Virol. 2003;148:389–397. doi: 10.1007/s00705-002-0919-y. [DOI] [PubMed] [Google Scholar]
- Moonan F., Molina J., Mirkov T.E. Sugarcane yellow leaf virus: An emerging virus that has evolved by recombination between luteoviral and poleroviral ancestors. Virology. 2000;269:156–171. doi: 10.1006/viro.1999.0162. [DOI] [PubMed] [Google Scholar]
- Naidu R.A., Mayo M.A., Reddy S.V., Jolly C.A., Torrance L. Diversity among the coat proteins of luteoviruses associated with chickpea stunt disease in India. Ann. Appl. Biol. 1997;130:37–47. [Google Scholar]
- Sadowy E., Maasen A., Juszczuk M., David C., Zagorski-Ostoja W., Gronenborn B., Hulanicka M.D. The ORF0 product of Potato leafroll virus is indispensable for virus accumulation. J. Gen. Virol. 2001;82:1529–1532. doi: 10.1099/0022-1317-82-6-1529. [DOI] [PubMed] [Google Scholar]
- Terauchi H., Kanematsu S., Honda K., Mikoshiba Y., Ishiguro K., Hidaka S. Comparison of complete nucleotide sequences of genomic RNAs of four Soybean dwarf virus strains that differ in their vector specificity and symptom production. Arch. Virol. 2001;146:1885–1898. doi: 10.1007/s007050170040. [DOI] [PubMed] [Google Scholar]
- Terradot L., Souchet M., Tran V., Giblot Ducray-Bourdin D. Analysis of a three-dimensional structure of Potato leafroll virus coat protein obtained by homology modeling. Virology. 2001;286:72–82. doi: 10.1006/viro.2001.0900. [DOI] [PubMed] [Google Scholar]
- van Der Wilk F., Verbeek M., Dullemans A.M., van Den Heuvel J.F.J.M. The genome-linked protein of potato leafroll virus is located downstream of the putative protease domain of the ORF1 product. Virology. 1997;234:300–303. doi: 10.1006/viro.1997.8654. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, M.E. Taliansky, D.J. Robinson, P.M. Waterhouse, A.F. Murant, G.A. de Zoeten, B.W. Falk, M.J. Gibbs
GENUS UMBRAVIRUS
Type Species Carrot mottle virus
VIRION PROPERTIES
MORPHOLOGY
Umbraviruses do not form conventional virus particles, and the four genomes whose complete sequences are known lack plausible ORFs for capsid CPs. Umbraviruses rely on the CP of a helper virus, characteristically from a virus in the family Luteoviridae, for encapsidation and for transmission by the vector of the helper virus. However, in single infections by umbraviruses, the infectivity in buffer extracts of leaves is surprisingly stable, though very sensitive to treatment with organic solvents, suggesting that the infective RNA is protected in lipid-containing structures. In plants infected with Carrot mottle virus (CMoV), enveloped structures ∼52 nm in diameter (Fig. 1 ) occur in the vacuoles of infected cells and in partially purified preparations. These structures may be involved in virus replication and/or serve to protect the RNA. Similar structures occur in plants infected with the bean yellow vein-banding strain of Pea enation mosaic virus-2 (BYVBV), Groundnut rosette virus (GRV) and Lettuce speckles mottle virus (LSMV), but no information is available for other umbraviruses.
Figure 1.

(Left) Section of palisade mesophyll cell from a leaf of Nicotiana clevelandii systemically infected with Carrot mottle virus (CMoV), showing enveloped structures (E) ∼52 nm in diameter in the cell vacuole (V) in association with the tonoplast (T). The bar represents 250 nm. (Right) Enveloped structures ∼52 nm in diameter in a partially purified preparation from CMoV-infected N. clevelandii, stained with 2% uranyl acetate. The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Infectivity in leaf extracts is stable for several hours at room temperature or several days at 5°C, but is abolished by treatment with organic solvents. Partially purified preparations of CMoV consist predominantly of cell membranes but contain infective components which, because they have a sedimentation coefficient of ∼270S and a buoyant density of ∼1.15 g/cm3 in CsCl, are probably the 52 nm-diameter enveloped structures observed in these preparations. An infective fraction from GRV-infected tissue contained complexes with a buoyant density of 1.34-1.45 g/cm3 consisting of filamentous ribonucleoprotein particles, composed of the ORF3 protein and virus RNA, embedded in a matrix. The relationship between these two kinds of structure is unclear.
NUCLEIC ACID
Nucleic acid preparations made by extracting leaves with phenol are often much more infective than buffer extracts. The infective agent in these preparations is a ssRNA, but the preparations also contain abundant dsRNA. The genome consists of one linear segment of positive-sense ssRNA. Nucleotide sequences have been determined for five umbraviruses: Carrot mottle mimic virus, (CMoMV) (4,201 nt), GRV (4,019 nt), Pea enation mosaic virus (PEMV-2) (4,253 nt), Tobacco bushy top virus (TBTV) (4,152 nt) and Tobacco mottle virus (TMoV) (incomplete). These genomes are probably not polyadenylated at their 3′-ends; there is no information about the structures at their 5′-ends.
PROTEINS
No structural proteins are reported. The nucleotide sequences lack plausible ORFs for CPs but possess ORFs for four potential non-structural protein products.
LIPIDS
Although no conventional virus particles are formed, the sensitivity to organic solvents, and low buoyant density, of the infective components in partially purified preparations of CMoV suggests that this infectivity is associated with lipid, probably of plant origin. The infective components probably correspond to the enveloped structures seen in sections of infected leaves.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Figure 2 shows the genome organization of GRV; those of other umbraviruses are very similar. For each RNA, there is at the 5′-end a very short non-coding region preceding ORF1, which encodes a putative product of 31-37 kDa. ORF2, which slightly overlaps the end of ORF1, could encode a product of 63-65 kDa but lacks an AUG initiation codon near its 5′-end. However, immediately before the stop codon of ORF1 there is a 7-nt sequence that is associated with frameshifting in several plant and animal viruses, and it seems probable that ORF1 and ORF2 are translated as a single polypeptide of 94-98 kDa by a mechanism involving a -1 frameshift. The predicted product contains, in the ORF2 region, sequence motifs characteristic of viral RdRp. A short untranslated region separates ORF2 from ORF3 and ORF4, which overlap each other almost completely in different reading frames and each yield a putative product of 26-29 kDa. The ORF4 product contains sequences characteristic of plant virus MPs. The ORF3 product of different umbraviruses studied have up to 50% similarity to each other but no significant similarity to any other viral or non-viral proteins; their function is to protect viral RNA and enable its transport through the phloem.
Figure 2.

Diagram showing the genomic organization of Groundnut rosette virus (GRV). The continuous horizontal line represents the genome RNA, and the numbered blocks the correspondingly numbered ORFs. The lower part of the diagram shows the predicted translation products, with their size. The potential product of ORF1 has not been shown to exist. The single product produced from ORFs 1 and 2, probably as a result of a -1 frameshift event (FS), is thought to be a polymerase because it contains, in the ORF2 region, sequences characteristic of viral RdRp. The ORF3 product functions to protect viral RNA and enable its transport through the phloem. The ORF4 product, marked MP, has a cell-to-cell movement function.
Umbravirus-infected leaf tissue contains abundant dsRNA that is not itself infective but that becomes so when heat-denatured. Two dsRNA species are common to all umbraviruses: dsRNA-1 (∼4.2-4.8 kbp) and dsRNA-2 (∼1.1-1.5 kbp). cDNA copies of dsRNA-1 hybridize with dsRNA-2 and these molecules are thought to represent doublestranded forms of, respectively, genomic and subgenomic ssRNA species. ORFs 3 and 4 are probably expressed from sgRNA. There is evidence for the presence in GRV-infected plants of two less-than-full-length RNA species of very similar size, close to that expected for such sgRNAs, and corresponding to that of dsRNA-2. The dsRNA-2 of CMoMV has been shown to include the sequences of ORFs 3 and 4, and the 3’ UTR.
Some umbraviruses possess one or more additional dsRNA species, associated in at least one instance (GRV) with the presence of a satellite RNA. PEMV-2 too has a satellite RNA, and each of these satellites can be supported by the helper virus of the other.
ANTIGENIC PROPERTIES
None reported.
BIOLOGICAL PROPERTIES
HOST RANGE
Individual umbraviruses are confined in nature to one or a few host plant species. Their experimental host ranges are broader but still restricted. The symptoms induced in infected plants are usually mottles or mosaics. Symptoms of GRV are greatly influenced by the associated satellite RNA.
TRANSMISSION
Umbraviruses are transmissible, sometimes with difficulty, by mechanical inoculation. However, in nature each is dependent on a specific helper virus for transmission in a persistent (circulative, non-propagative) manner by aphids. All helper viruses that have been characterized are members of the family Luteoviridae. The mechanism of this dependence is encapsidation of the dependent virus RNA in the CP of the helper. In GRV, the satellite RNA plays an essential role in mediating this luteovirus-dependent aphid transmission. There is no evidence for multiplication of umbraviruses in the insect vector. Seed transmission has not been reported.
GEOGRAPHICAL DISTRIBUTION
CMoV and/or CMoMV, and PEMV-2, apparently occur worldwide wherever their crop hosts are grown; other umbraviruses have a restricted distribution. Several umbraviruses, notably GRV, occur only in Africa.
PATHOGENICITY
Although all umbraviruses depend on a helper virus for transmission by vector insects, several of them are as important or more important than their helpers in the causation of disease symptoms. The umbravirus of greatest economic importance is GRV, which causes the most devastating virus disease of groundnut (peanut) in Africa. However, in this case it is a GRV-dependent satellite RNA that is the actual cause of the symptoms. In most instances umbraviruses have not been shown to contribute functions essential for the biological success of their associated helper viruses. However, a notable exception is PEMV-2, which is essential for the systemic spread of PEMV-1 in plants, and even allows it, unlike typical members of the family Luteoviridae, to spread out of the phloem into mesophyll tissue and thereby to become transmissible by manual inoculation. Another member of the Luteoviridae, Beet western yellows virus, has been reported to show limited manual transmissibility when in the presence of Lettuce speckles mottle virus, a member of the genus Umbravirus.
CYTOPATHIC EFFECTS
Umbraviruses, even in the absence of their helper viruses, exhibit rapid systemic spread in plants. They infect cells throughout the leaf, though presumably the aphid transmissible particles are restricted to the same tissues (in most instances the phloem) as the luteoviruses that provide their CP. In mesophyll cells infected with CMoV there is extensive development of cell wall outgrowths sheathing elongated plasmodesmatal tubules.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Natural host range,
-
•
dsRNA band pattern (bearing in mind that some bands may represent satellite RNA species),
-
•
Nucleotide sequence identity less than 55%, and
-
•
Little or no hybridization with cDNA probes representing most parts of the genome.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Carrot mottle mimic virus | ||
| Carrot mottle mimic virus | [U57305] | (CMoMV) |
| Carrot mottle virus | ||
| Carrot mottle virus | (CMoV) | |
| Groundnut rosette virus | ||
| Groundnut rosette virus | [Z69910; satellite RNA: Z29702-Z29711] | (GRV) |
| Lettuce speckles mottle virus | ||
| Lettuce speckles mottle virus | (LSMV) | |
| Pea enation mosaic virus-2 | ||
| Bean yellow vein-banding virus | (BYVBV) | |
| Pea enation mosaic virus-2 | [U03563; satellite RNA: U03564] | (PEMV-2) |
| Tobacco bushy top virus | ||
| Tobacco bushy top virus | [AF431890] | (TBTV) |
| Tobacco mottle virus | ||
| Tobacco mottle virus | [AY007231] | (TMoV) |
TENTATIVE SPECIES IN THE GENUS
| Sunflower crinkle virus | (SuCV) |
| (Sunflower rugose mosaic virus) | |
| Sunflower yellow blotch virus | (SuYBV) |
| (Sunflower yellow ringspot virus) | |
| Tobacco yellow vein virus | (TYVV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
SIMILARITY WITH OTHER TAXA
Amino acid sequence comparisons show that the putative RdRp encoded by the genomic RNA molecules of CMoMV, GRV, PEMV-2 and TBTV belong to the so-called supergroup 2 of RNA polymerases, as do those of viruses in the genera Carmovirus, Dianthovirus, Luteovirus, Machlomovirus, Necrovirus, and Tombusvirus (Fig. 3 ). Since these enzymes are the only universally conserved proteins of positive-strand RNA viruses, the genus Umbravirus might be considered to be in or close to the family Tombusviridae.
Figure 3.

Phylogenetic relationships of the RdRps of umbraviruses and some other plant viruses. Amino acid sequences were aligned using the “Clustal” method and the phylogenetic tree was constructed with MEGA software using the following parameters: P-distance; Neighbor-joining; pairwise deletion; 2000 bootstraps.
DERIVATION OF NAMES
Umbra: From Latin, a shadow. In English, a shadow, an uninvited guest that comes with an invited one.
REFERENCES
- Adams A.N., Hull R. Tobacco yellow vein, a virus dependent on assistor viruses for its transmission by aphids. Ann. Appl. Biol. 1972;71:135–140. [Google Scholar]
- Demler S.A., de Zoeten G.A., Adam G., Harris K.F. Pea enation mosaic enamovirus: properties and aphid transmission. In: Harrison B.D., Murant A.F., editors. Vol. 5. Plenum Press; New York: 1996. pp. 303–344. (The Plant Viruses polyhedral virions and bipartite RNA genomes). [Google Scholar]
- Demler S.A., Rucker D.G., de Zoeten G.A., Ziegler A., Robinson D.J., Murant A.F. The satellite RNAs associated with the groundnut rosette disease complex and pea enation mosaic virus: sequence similarities and ability of each other's helper virus to support their replication. J. Gen. Virol. 1996;77:2847–2855. doi: 10.1099/0022-1317-77-11-2847. [DOI] [PubMed] [Google Scholar]
- Falk B.W., Duffus J.E., Morris T.J. Transmission, host range, and serological properties of the viruses that cause lettuce speckles disease. Phytopathology. 1979;69:612–617. [Google Scholar]
- Falk B.W., Morris T.J., Duffus J.E. Unstable infectivity and sedimentable ds-RNA associated with lettuce speckles mottle virus. Virology. 1979;96:239–248. doi: 10.1016/0042-6822(79)90187-9. [DOI] [PubMed] [Google Scholar]
- Gibbs M.J., Cooper J.I., Waterhouse P.M. The genome organization and affinities of an Australian isolate of carrot mottle umbravirus. Virology. 1996;224:310–313. doi: 10.1006/viro.1996.0533. [DOI] [PubMed] [Google Scholar]
- Gibbs M.J., Ziegler A., Robinson D.J., Waterhouse P.M., Cooper J.I. Carrot mottle mimic virus (CMoMV): a second umbravirus associated with carrot motley dwarf disease recognised by nucleic acid hybridisation. Mol. Plant Path. On-Line. 1996 http://www.bspp.org.uk/mppol/1996/1111gibbs. [Google Scholar]
- Mo X.H., Gin X.Y., Wu J., Yang C., Wu J.Y., Duan Y.G., Li T.F., Chen H.R. Complete nucleotide sequence and genome organization of a Chinese isolate of tobacco bushy top virus. Arch. Virol. 2003;148:389–397. doi: 10.1007/s00705-002-0919-y. [DOI] [PubMed] [Google Scholar]
- Murant A.F. Dependence of groundnut rosette virus on its satellite RNA as well as on groundnut rosette assistor luteovirus for transmission by Aphis craccivora. J. Gen. Virol. 1990;71:2163–2166. doi: 10.1099/0022-1317-71-9-2163. [DOI] [PubMed] [Google Scholar]
- Murant A.F. Complexes of transmission-dependent and helper viruses. In: Matthews R.E.F., editor. Diagnosis of Plant Virus Diseases. CRC Press; Boca Raton: 1993. pp. 333–357. [Google Scholar]
- Murant A.F., Goold R.A., Roberts I.M., Cathro J. Carrot mottle – a persistent aphid-borne virus with unusual properties and particles. J. Gen. Virol. 1969;4:329–341. [Google Scholar]
- Murant A.F., Rajeshwari R., Robinson D.J., Raschké, J.H. A satellite RNA of groundnut rosette virus that is largely responsible for symptoms of groundnut rosette disease. J. Gen. Virol. 1988;69:1479–1486. [Google Scholar]
- Murant A.F., Roberts I.M., Goold R.A. Cytopathological changes and extractable infectivity in Nicotiana clevelandii leaves infected with carrot mottle virus. J. Gen. Virol. 1973;21:269–283. [Google Scholar]
- Reddy D.V.R., Murant A.F., Raschké J.H., Mayo M.A., Ansa O.A. Properties and partial purification of infective material from plants containing groundnut rosette virus. Ann. Appl. Biol. 1985;107:65–78. [Google Scholar]
- Ryabov E.V., Robinson D.J., Taliansky M.E. Umbravirus-encoded proteins both stabilize heterologous viral RNA and mediate its systemic movement in some plant species. Virology. 2001;288:391–400. doi: 10.1006/viro.2001.1078. [DOI] [PubMed] [Google Scholar]
- Smith K.M. The transmission of a plant virus complex by aphides. Parasitology. 1946;37:131–134. doi: 10.1017/s0031182000013275. [DOI] [PubMed] [Google Scholar]
- Taliansky M.E., Robinson D.J., Murant A.F. Complete nucleotide sequence and organization of the RNA genome of groundnut rosette umbravirus. J. Gen. Virol. 1996;77:2335–2345. doi: 10.1099/0022-1317-77-9-2335. [DOI] [PubMed] [Google Scholar]
- Taliansky M.E., Roberts I.M., Kalinina N.O., Ryabov E.V., Raj S.K., Robinson D.J., Oparka K.J. An umbraviral protein, involved in long-distance RNA movement, binds viral RNA and forms unique, protective ribonucleoprotein complexes. J. Virol. 2003;77:3031–3040. doi: 10.1128/JVI.77.5.3031-3040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuri J.M., Bock K.R., Woods R.D. Distribution, host range and some properties of a virus disease of sunflower in Kenya. Trop. Pest Manag. 1987;33:202–207. [Google Scholar]
CONTRIBUTED BY, S.A. Lommel, G.P. Martelli, L. Rubino, M. Russo
FAMILY TOMBUSVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Tombusviridae |
| Genus | Dianthovirus |
| Genus | Tombusvirus |
| Genus | Aureusvirus |
| Genus | Avenavirus |
| Genus | Carmovirus |
| Genus | Necrovirus |
| Genus | Panicovirus |
| Genus | Machlomovirus |
VIRION PROPERTIES
MORPHOLOGY
Capsids exhibit a T=3 icosahedral symmetry and are composed of 180 protein subunits (Fig. 1 ). Capsids are formed with CP having one of two distinct phylogenetic origins. The virions from the genera Aureusvirus, Avenavirus, Carmovirus, Dianthovirus, and Tombusvirus, have a rounded outline, a granular surface, and a diameter of about 32-35 nm. Each subunit folds into three distinct structural domains: R, the N-terminal internal domain interacting with RNA; S, the shell domain constituting the capsid backbone; and P, the protruding C-terminal domain, which gives the virus its granular appearance. P domains are clustered in pairs to form 90 projections. These dimeric contacts are important in the assembly and stabilization of the capsid structure. The R domain, which contains many positively charged residues, binds RNA. The S domain forms a barrel structure made up of p-strands. Two Ca2+ binding sites stabilize contacts between adjacent S domains. The capsids of viruses in the genera Machlomovirus, Necrovirus, and Panicovirus are composed of CPs that lack the protruding domain. Consequently, the surfaces of the virions have a smooth appearance. They range in diameter between 30-32 nm, and the shell domain is related to the CPs of sobemoviruses.
Figure 1.

(Top row, left) Computer reconstruction of a Tomato bushy stunt virus (TBSV) particle based on X-ray crystallography at 2.9Å resolution (J.Y. Sgro, Univ. Wisconsin-Madison; Olson et al., 1983). (Top row center) Diagrammatic representation of T=3 TBSV particles (from Hopper et al., 1984, with permission). (Top row, right) Negative contrast electron micrograph of TBSV particles. (Bottom row, left) Computer reconstruction of a Tobacco necrosis virus A (TNV-A) particle, based on X-ray crystallography at 2.25Å resolution (from Oda et al., 2000; Reddy V. et al., 2001). (Bottom row center) Schematic representation of the T=3 structure of TNV particles. (Bottom row, right) Negative contrast electron micrograph of TNV particles. The bars represent 50 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as one component with an S20w of 118-140S, have a buoyant density ranging from 1.34-1.36 g/cm3 in CsCl, and a virion Mr of 8.2-8.9 × 106. Virions are stable at acidic pH, but expand above pH 7 and in the presence of EDTA. Lowering pH or adding Ca++ recompacts the particles. Virions are resistant to elevated temperatures (thermal inactivation usually occurs above 80°C) and are insensitive to organic solvents and non-ionic detergents.
NUCLEIC ACID
With the exception of those of the genus Dianthovirus, virions contain a single molecule of positive sense, linear ssRNA, that constitutes about 17% of the particle weight, and have a size ranging from 3.7 to 4.8 kb, depending on the genus. Dianthovirus virions contain two genomic RNAs. The large RNA1 is ∼3.9 kb and the smaller RNA2 is 1.5 kb. The 3′-ends are not polyadenylated. The 5′-termini are probably not protected however the presence of a cap was demonstrated for Carnation mottle virus (CarMV), Red clover necrotic mosaic virus (RCNMV) and Maize chlorotic mottle virus (MCMV). Addition of a cap analogue to in vitro RNA transcripts enhances infectivity little or not at all. With the possible exception of MCMV, all species express the CP from a sgRNA. DI RNAs occur in some genera. In addition some members have satellite RNAs or satellite viruses associated with them.
PROTEINS
All capsids are composed of 180 copies of a single CP type. Capsids are composed of one of two phylogenetically distinct groups of CP. Those CPs form one phylogenetically conserved group, containing a protruding domain, possess a single major CP of 37-48 kDa. In those genera that have a CP from the second phylogenetically distinct group, lacking a protruding domain, the CPs range in size from 25-29 kDa.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Even though variability exists in the number and location of genes within members of the family, a number of organizational features are highly conserved (Fig. 2 ). The unifying feature of the family is that each member species possesses a highly conserved polymerase that is interrupted by an in-frame termination codon that is periodically suppressed to express catalytic quantities of the core polymerase containing the canonical “GDD” motif. Dianthoviruses utilize a -1 ribosomal frameshifting mechanism to accomplish the same result. The polymerase is further characterized by possessing no obvious helicase motif. In this description, as well as those for each genus, the polymerase is labeled as a single ORF with the read through portion labeled ORF1-RT or ORF1-FS. With the exception of MCMV, the polymerase is the first 5′-proximal ORF encountered when translated from the genomic RNA.
Figure 2.

Genome organization of the type species for each genus in the family Tombusviridae. Boxes represent known and predicted ORFs. Similarly shaded boxes represent proteins with extensive sequence conservation. The yellow boxes represent ORFs encoding the phylogenetically conserved polymerase. Red hatched boxes represent CP encoding ORFs. Right-hatched boxes identify CPs lacking a protruding domain that are related to those of the genus Sobemovirus while those which are left-hatched represent tombusvirus-like CPs that contain protruding domains. Dark blue boxes represent viral MPs that have a high degree of sequence conservation at the carboxyl-terminus. Light blue boxes identify MPs (Dianthovirus ORF3, Tombusvirus ORF4, Aureusvirus ORF3) involved in movement with no sequence similarity, and shaded boxes identify other unrelated ORFs whose proteins encode an accessory function. RT: translational readthrough of termination codon. -1FS: -1 ribosomal frameshifting event. CP: capsid protein.
Genomes of members of the genera Dianthovirus and Avenavirus encode 3 ORFs while all others encode 5 ORFs. The genera Machlomovirus and Panicovirus have additional terminator readthrough and ribosomal frameshifting events to extend putative MP and accessory ORFs. Products of the 5′-proximal ORFs 1 and 1RT or 1FS are expressed by translation directly from the genomic RNA, whereas translation products of the internal and 3′-proximal ORFs 2, 3 and 4, are expressed from sgRNAs. dsRNAs corresponding in size to virus-related RNAs (genomic and subgenomic) are present in infected tissues. For all genera, the CP ORF is either internal or 3’-proximal and requires the synthesis of a sgRNA for expression in vivo.
Non-structural proteins include the phylogenetically conserved polymerase proteins of 22-50 kDa and its 82-112 kDa readthrough product. Viruses in the family utilize at least three phylogenetically distinct MPs. The avenaviruses, carmoviruses, machlomoviruses, necroviruses, and panicoviruses encode a 7-9 kDa MP that in all these genera, excluding Avenavirus, is associated with another small ORF encoding an 8-9 kDa polypeptide. Genomes of the genera Tombusvirus and Aureusvirus encode a 22-27 kDa MP and that of the genus Dianthovirus utilizes the third type of MP of around 35 kDa. The genera Tombusvirus and Aureusvirus encode a 14-19 kDa accessory protein whose function is associated with the suppression of virus-induced gene silencing. The genera Panicovirus and Machlomovirus also have several additional accessory ORFs whose functions have not been determined.
Replication occurs in the cytoplasm, possibly in membranous vesicles that may be associated with endoplasmic reticulum, or modified organelles such as peroxisomes, mitochondria and, more rarely, chloroplasts. Virions are assembled in the cytoplasm and occasionally in mitochondria and nuclei. Virions accumulate, sometimes in crystalline form, in the cytoplasm and in vacuoles.
ANTIGENIC PROPERTIES
Virions are efficient immunogens. Antisera yield single precipitin lines in immunodiffusion tests. Depending on the genus, serological cross-reactivity among species ranges from nil to near-homologous titers. Many serologically related strains have been identified in several species.
BIOLOGICAL PROPERTIES
HOST RANGE
The natural host range of individual virus species is relatively narrow. Members can either infect monocotyledonous or dicotyledonous plants, but no species can infect both. The experimental host range is wide. Infection is often limited to the root system, but when hosts are invaded systemically, viruses enter all tissues. Many members induce a necrosis symptom in the foliar parts of the plant. Diseases are characterized by mottling, crinkling, necrosis, and deformation of foliage. Some virus species infections are symptomless in their natural hosts.
TRANSMISSION
All species are readily transmitted by mechanical inoculation and through plant material used for propagation. Some may be transmitted by contact and through seeds. Viruses are often found in natural environments, i.e. surface waters and soils from which they can be acquired without assistance of vectors. Transmission by the chytrid fungi in the genus Olpidium and beetles has also been reported for members of several genera. Most, if not all, members can be transmitted through the soil either dependent on, or independent of, a biological vector.
GEOGRAPHICAL DISTRIBUTION
Geographical distribution of particular species varies from wide to restricted. The majority of the species occur in temperate regions. Legume-infecting carmoviruses and one tentative member of the genus Dianthovirus have been recorded from tropical areas.
CYTOPATHIC EFFECTS
Distinctive cytopathological features occur in association with exceedingly high accumulations of virus particles in cells and “multivesicular bodies”, i.e. cytoplasmic membranous inclusions originated from profoundly modified mitochondria and/or peroxisomes.
LIST OF GENUS DEMARCATION CRITERIA IN THE FAMILY
The list of criteria demarcating genera in the family are:
-
•
Structural criteria: T=3 icosahedral virions 28-35 nm in diameter that are insensitive to organic solvents, composed of 180 copies of a single subunit.
-
•
Genomic criteria: genome composed of positive polarity ssRNA on one or two segments with the total genome size being less that 5.5 kb.
-
•
Polymerase criteria: gene interrupted by either a termination codon or a -1 ribosomal frameshifting element that is periodically readthrough. Polymerase with at least 25% aa sequence identity. Polymerase located at or near the 5’-end of the genomic RNA. Polymerase lacking obvious helicase motifs.
-
•
Capsid protein criteria: one of two phylogenetic origins; a CP with a protruding domain having 25% aa sequence identity with other CPs in the family having a protruding domain, a CP lacking a protruding domain with 20% or higher sequence identity with the sobemovirus CP. CP expressed from a sgRNA in vivo.
-
•
Transmission criteria: virions that are mechanically transmissible. Soil transmission either with or without the aid of a biological vector.
GENUS DIANTHOVIRUS
Type Species Carnation ringspot virus
DISTINGUISHING FEATURES
Virions sediment in sucrose gradients as a single band of S20w 126-135S. The genome is in two genomic RNAs of 3.9 and 1.5 kb. The first ORF1 encoding the polymerase is interrupted by a ribosomal frameshifting event yielding a pre-frameshift 27 kDa protein and a 88 kDa frameshift polypeptide. The CP possessing a protruding domain, is encoded by the 3’-proximal ORF on RNA1 and is expressed from a 1.5 kb sgRNA in vivo. The ORF for the phylogenetically distinct 34-35 kDa MP is in the monocistronic RNA2. Species are transmitted through the soil without the aid of a biological vector.
VIRION PROPERTIES
MORPHOLOGY
Virions are not enveloped. Capsids are 32-35 nm in diameter and have a T=3 icosahedral symmetry (Fig. 3 ). The isometric nucleocapsids have an obvious regular surface structure giving a granular appearance in the electron microscope. The surface capsomer arrangement is not obvious. Capsids are composed of 180 protein subunits, but the detailed structure is not known. However, based on similarity of CP sequence, it is predicted that the capsid is similar in structure to those of species in the genera Carmovirus and Tombusvirus. Each subunit is predicted to fold into three distinct structural domains: R, the N-terminal internal domain interacting with RNA; S, the shell domain constituting the capsid backbone; and P, the protruding C-terminal domain. P domains are clustered in pairs to form 90 projections.
Figure 3.

(Left) Cryo-reconstruction image at 10Å resolution of a particle of Red clover necrotic mosaic virus (RCNMV) (from Baker, Sherman and Lommel, with permission). (Right) Negative contrast electron micrograph of RCNMV particles (from S. A. Lommel, with permission). The bar represents 50 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as one component in sucrose with S20w of 126-135S. The buoyant density in CsCl is 1.363-1.366 g/cm3, and the virion Mr is 8.6 × 106. Particles exhibit an A260/A280 ratio of 1.67 and a thermal inactivation point between 80-90°C. A longevity in vitro of around 10 weeks has been reported for most species. Virions are insensitive to ether, chloroform and non-ionic detergents. Virions are stable at pH 6 and lower; alkaline conditions (pH 7-8) induce particle swelling. Virions are stabilized by divalent cations.
NUCLEIC ACID
Virions contain two molecules of infectious linear positive sense ssRNA. Genomic RNA1 is 3,876-3,940 nt and RNA2 is between 1,412-1,449 nt in size. The genomic RNAs are not capped however there is a report that the 5′-end of each RNA is capped with m7GpppA. The RNAs do not contain a 3′-terminal poly(A) tract or a tRNA-like structure. It is assumed that the virion packages only one copy of each genomic RNA. There is no evidence that the sgRNAs are packaged into virions. Three virus-specific dsRNA species are found in infected cells. The largest and smallest dsRNAs correspond to the genomic RNA1 and RNA2, respectively. The intermediate sized dsRNA corresponds to a sgRNA of 1.5 kb representing the 3’ portion of genomic RNA1.
PROTEINS
Capsids are composed of 180 copies of a single CP species of 339 aa (37-38 kDa).
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Only the 5′-terminal 13 nt and 3′-terminal 27 nt are identical in RNA1 and RNA2 (Fig. 4 ). The 3’ 27 nt are predicted to form a stem-loop structure. RNA1 contains two ORFs. ORF1 is capable of encoding a 27 kDa protein. A -1 ribosomal frameshift event at the canonical shifty heptanucleotide allows translation to continue into ORF1-FS in about 5% of the times the RNA is translated to yield an 88 kDa protein. Both the 27 kDa and 88 kDa proteins are observed in vivo and are made by translation of virion RNA in vitro. The ORF1 and ORF1-FS encoded proteins form the viral polymerase. ORF2 encodes the 37-38 kDa CP. This ORF is expressed in vivo from the 1.5 kb sgRNA. ORF3 on RNA2 encodes the 34-35 kDa MP. For the sgRNA to be expressed, the loop of a stem-loop in RNA2 must base pair within the RNA1 sgRNA promoter.
Figure 4.

Genome organization and replication strategy of Carnation ringspot virus (CRSV). Boxes represent known ORFs with the sizes of the respective proteins (or readthrough products) indicated within. Yellow ORFs on RNA1 indicate polymerase proteins that have a high degree of sequence conservation within the family Tombusviridae. Left-hatched red box on RNA1 identifies the CP that is highly conserved among other genera within the family Tombusviridae that have a protruding domain. The blue box on RNA2 identifies the ORF that encodes the MP. This protein exhibits a small region of sequence conservation with MPs in the family Bromoviridae. The line under RNA1 depicts the 1.5 kb CP sgRNA. -1 FS = site of -1 ribosomal frameshifting.
ANTIGENIC PROPERTIES
Virus particles are moderately to highly immunogenic. Various serologically distinct strains have been identified. Antisera yield a single precipitin line in agar gel-diffusion assays. Monoclonal antibodies have been identified that cross-react between species.
BIOLOGICAL PROPERTIES
HOST RANGE
In nature, dianthoviruses have moderately broad natural host ranges restricted to dicotyledonous plants. In the laboratory, the experimental host range is much broader, and includes a wide range of herbaceous species in the families Solanaceae, Leguminosae, Cucurbitaceae, and Compositae. Species infect a larger number of plants locally (non-systemically).
TRANSMISSION
The viruses are readily transmitted by mechanical inoculation; they are not known to be seed-transmitted. The viruses are not transmitted by insects, nematodes, or soil-inhabiting fungi. However, viruses are readily transmitted through the soil without the aid of a biological vector.
GEOGRAPHIC DISTRIBUTION
Dianthoviruses, with the possible exception of FNSV, which appears to be tropical in range, are widespread throughout the temperate regions of the world.
CYTOPATHIC EFFECTS
None reported.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The list of species demarcation criteria in the genus is:
-
•
Extent of serological relationship as determined by immunodiffusion and/or ELISA,
-
•Extent of sequence identity between relevant gene products,
-
○Less than 79% aa sequence identity of the CP,
-
○Less than 54% aa sequence identity of the polymerase,
-
○
-
•
Ability to form pseudorecombinants with the two RNA components,
-
•
Transmission through the soil,
-
•
Natural host range,
-
•
Artificial host range reactions.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
| Furcraea necrotic streak virus | (FNSV) | |
| Rice virus X | [AB033715] | (RVX) |
| Sesame necrotic mosaic virus | (SNMV) |
GENUS TOMBUSVIRUS
Type Species Tomato bushy stunt virus
DISTINGUISHING FEATURES
The genome is approximately 4.8 kb and contains four ORFs. The CP ORF is located internally on the genomic RNA and is expressed in vivo from a 2.1 kb sgRNA. ORFs 3 and 4 are 3’ proximally located and ORF4 is contained within ORF3 in a different reading frame. Both ORFs are expressed from a second 0.9 kb sgRNA. The genome organization and expression strategy are identical to that of PoLV (genus Aureusvirus). The tombusvirus ORF3 is significantly smaller and ORF4 significantly larger than that in PoLV. All members elicit formation of multivesicular inclusion bodies. Diseases caused by tombusviruses prevail in temperate climates. All species are soil-borne, but only one, Cucumber necrosis virus (CNV), has a recognized fungus vector (Olpidium bornovanus).
VIRION PROPERTIES
MORPHOLOGY
Capsids are 32-35 nm in diameter and have a T=3 icosahedral symmetry (Fig. 5 ). The isometric nucleocapsids have a regular surface structure giving a granular appearance under the electron microscope. The surface capsomer arrangement is not obvious. Capsids comprise 32 capsomers composed of 180 protein subunits. Each subunit folds into three distinct structural domains: R, the N-terminal internal domain interacting with RNA; S, the shell domain constituting the capsid backbone; and P, the protruding C-terminal domain. P domains are clustered in pairs to form 90 projections. These dimeric contacts are important in the assembly and stabilization of the virion structure. The R domain, which contains many positively charged residues, binds RNA. The S domain forms a barrel structure made up of (β-strands. Two Ca++ binding sites stabilize contacts between S domains.
Figure 5.

(Left) Computer reconstruction of a Tomato bushy stunt virus (TBSV) particle based on X-ray crystallography at 2.9Å resolution (Courtesy J.Y. Sgro, Univ. Wisconsin-Madison; Olson et al., 1983). (Center) Diagrammatic representation of a T=3 TBSV particles (from Hopper et al., 1984, with permission). (Right) Negative contrast electron micrograph of TBSV particles. The bar represents 50 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The virus sediments as one component with an S20w of 132-140S, has a buoyant density of 1.34-1.36 g/cm3 in CsCl, and a virion Mr of 8.9 × 106. The virion isoelectric point is pH 4.1. Particles exhibit an A260/A280 ratio of 1.64 and a thermal inactivation point of 80-90°C. Longevity in vitro of 130-150 days has been reported. Virions have a dilution end point in excess of 10-6. Virions are insensitive to ether, chloroform and non-ionic detergents. Virions are stabilized by divalent cations.
NUCLEIC ACID
Nucleic acid represents 17% of the virion, and consists of one molecule of linear positive-sense ssRNA. Total genome length averages around 4.8 kb. The 5′-end of the genome lacks a cap structure and the 3’-terminus has neither a poly(A) tract nor a terminal tRNAlike structure. A 3′-proximal segment is involved in facilitating cap-independent translation. In addition to genomic RNA, virions of some species harbor and package DI and/or satellite RNAs. SgRNAs may also be packaged into virions at various levels. SgRNAs are generated by premature termination during genome minus strand synthesis, followed by sgRNA production using the truncated minus strand RNA as template. Three virus-specific dsRNA species are found in infected cells. The size of largest virus specific dsRNA corresponds to the genomic RNA. The second largest, 2.1 kbp, and the smallest, 0.9 kbp, dsRNAs correspond to sgRNAs 1 and 2, respectively.
PROTEINS
Virions contain 83% protein. One species of structural protein found in virions. The CP is about 41 kDa and is not glycosylated nor phosphorylated.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains four ORFs (Fig. 6 ). ORF1 encodes a 32-36 kDa protein. Readthrough of the ORF1 amber termination codon allows the expression of a 92-95 kDa protein (ORF1-RT). Both the 32-36 and 92-95 kDa proteins are produced by translation of virion RNA. The ORF1 and ORF1-RT-encoded proteins are the viral polymerase. The internal ORF2 encodes the CP, which is expressed from the 2.1 kb sgRNA1. Two nested ORFs (ORF3 and ORF4) located at the 3’ terminus of the genome, encode 22 (p22) and 19 (p19) kDa proteins, respectively. ORF3 and ORF4 initiation codons are in a sub-optimal and optimal translational context, respectively. Ribosome scanning occurs to allow for translation of ORF4. p22 has a role in symptom induction and is required for cell-to-cell movement, interacting with a host homeodomain leucine-zipper protein. p19 is a suppressor of post-transcriptional gene silencing. It has a role in the systemic spread of the virus, and is involved in the development of necrotic host response to infection.
Figure 6.
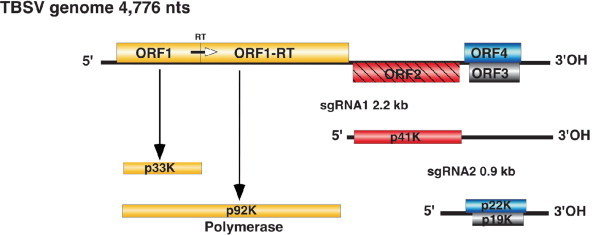
Genome organization and replication strategy of Tomato bushy stunt virus (TBSV). Boxes represent known and predicted ORFs with the sizes of the respective proteins (or readthrough products) indicated within. Shaded ORFs indicate polymerase proteins that have a high degree of sequence conservation within the family Tombusviridae. Left-hatched box identifies the CP that is highly conserved among other genera within the family Tombusviridae that have a protruding domain. The grey boxes identify ORFs whose proteins are unique to the genus. Lines underneath depict the two sgRNAs that are synthesized in infected cells and allow for the expression of ORF2 and ORFs 3 and 4 from sgRNAs 1 and 2, respectively. RT = Amber termination codon that is read through.
Necrotic response may be determined by the interaction of p19 with the protein encoded by ORF1, which, in turn, is mediated by DI RNAs.
Genome replication is carried out by the virus-coded RdRp, probably in conjunction with host factors. The replication process begins with the synthesis of a minus-strand RNA from a plus-strand template. The minus-strand is then used as template for the synthesis of the progeny genomes. Replication requires the presence of several cis-acting elements in the 5’ and 3’ UTRs and in internal positions. DI RNAs are generated during replication of some species following multiple and progressive deletions of genomic RNA templates. Polymerase proteins of CymRSV and CIRV are expressed in cells of the yeast Saccharomyces cerevisiae and the sequences involved in targeting and anchoring to peroxisomal and mitochondrial membranes were identified. In addition, yeast cells expressing CIRV polymerase replicate molecules of DI RNA following the same basic mechanisms as in plant cells.
ANTIGENIC PROPERTIES
Most species are serologically interrelated, though to a variable extent. Species with serologically related virions are: Artichoke mottled crinkle virus (AMCV), Pelargonium leaf curl virus (PLCV), and Petunia asteroid mosaic virus (PAMV), which are closely related; Morrocan pepper virus (MPV) which is distantly related; Eggplant mottled crinkle virus (EMCV), Carnation Italian ringspot virus (CIRV), Lato River virus (LRV) and Neckar River virus (NRV) which are very distantly related. Species with serologically unrelated virions are Cymbidium ringspot virus (CymRSV) and Cucumber necrosis virus (CNV).
BIOLOGICAL PROPERTIES
HOST RANGE
Most species have a narrow natural host range. However, most also have a wide experimental host range. Even though the host range of an individual species is restricted in nature, tombusviruses are present in a wide range of both monocotyledonous and dicotyledonous plants. Viruses tend to remain localized, forming a necrosis in artificially infected hosts.
TRANSMISSION
Members are easily transmitted mechanically in the field and experimentally. Most, if not all, species are soil-borne without the aid of a biological vector. They appear to be directly transmitted through the soil. CNV is transmitted by Olpidium bornovanus. Some species may be transmitted through the seed at a very low level.
GEOGRAPHICAL DISTRIBUTION
TBSV strains are present throughout North and South America, Europe, and the Mediterranean. Other tombusviruses are present wherever the primary hosts exist.
CYTOPATHIC EFFECTS
Virions are found in all parts of the host plant cells including cytoplasm, nuclei, nucleoli, mitochondria, and cell vacuoles. Virus crystals are present in the cytoplasm of infected cells. Multivesicular bodies (MVBs), i.e. cytopathic structures made up of a main body surrounded by spherical to ovoid vesicles 80-150 nm in diameter, are consistently present in infected cells. MVBs originate from the proliferation of the limiting membrane of peroxisomes (e.g. CymRSV) or mitochondria (CIRV), their formation being determined by an ORF1 sequence as short as c. 600 nt. MVBs do not contain virions which, however, are present within seemingly intact mitochondria in cells infected by EMCV, CymRSV, GALV, MPV, NRV, TBSV, and, occasionally, PAMV.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The list of species demarcation criteria in the genus is:
-
•
Extent of serological relationship as determined by immunodiffusion usually not below 3, and/or ELISA,
-
•Extent of sequence identity between relevant gene products,
-
○Less than 87% aa sequence identity of the CP,
-
○Less than 96% aa sequence identity of the polymerase,
-
○
-
•
Size of the CP,
-
•
Differential cytopathological features; organelles from which multivesicular bodies arise,
-
•
Natural host range,
-
•
Artificial host range reactions.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Artichoke mottled crinkle virus | ||
| Artichoke mottled crinkle virus | [X62493] | (AMCV) |
| Carnation Italian ringspot virus | ||
| Carnation Italian ringspot virus | [X85215] | (CIRV) |
| Cucumber Bulgarian latent virus | ||
| Cucumber Bulgarian latent virus | [AY163842] | (CBLV) |
| Cucumber necrosis virus | ||
| Cucumber necrosis virus | [M25270] | (CNV) |
| Cymbidium ringspot virus | ||
| Cymbidium ringspot virus | [X15511] | (CymRSV) |
| Eggplant mottled crinkle virus | ||
| Eggplant mottled crinkle virus | (EMCV) | |
| Grapevine Algerian latent virus | ||
| Grapevine Algerian latent virus | [AF540885] | (GALV) |
| Lato river virus | ||
| Lato river virus | (LRV) | |
| Moroccan pepper virus | ||
| Moroccan pepper virus | [AF540886] | (MPV) |
| Neckar river virus | ||
| Neckar river virus | (NRV) | |
| Pear latent virus | ||
| Pear latent virus | [AY100482] | (PeLV) |
| Pelargonium leaf curl virus | ||
| Pelargonium leaf curl virus | [AF290026] | (PLCV) |
| Petunia asteroid mosaic virus | ||
| Petunia asteroid mosaic virus | (PAMV) | |
| Sikte waterborne virus | ||
| Sikte waterborne virus | (SWBV) | |
| Tomato bushy stunt virus | ||
| Tomato bushy stunt virus | [M21958, U80935, AJ249740] | (TBSV) |
TENTATIVE SPECIES IN THE GENUS
| Maize necrotic streak virus | [AF266518] | (MNeSV) |
GENUS AUREUSVIRUS
Type Species Pothos latent virus
DISTINGUISHING FEATURES
The virion is a 30 nm icosahedron that packages the 4.4 kb genomic RNA. The RNA contains four ORFs. The CP ORF is located internally in the genomic RNA and is expressed in vivo from a 2 kb sgRNA. ORF3 and ORF4 are 3′-proximal and ORF4 is contained within ORF3, in a different reading frame. Both ORFs are expressed from a second 0.8 kb sgRNA. The genome organization and expression strategy are identical to those of viruses in genus Tombusvirus. While conserved, the polymerases of the two genera are no more closely related than either is with even the least conserved genus in the family Tombusviridae. The aureusvirus ORF3 is significantly larger and ORF4 significantly smaller than those in the genus Tombusvirus. Transmission is through the soil without (PoLV) or with (CLSV) the aid of a biological vector.
VIRION PROPERTIES
MORPHOLOGY
Virions are isometric with a rounded outline, a knobby surface and a diameter of c. 30 nm (Fig. 7 ). Based on comparative aa sequence alignments, the CP subunits of Pothos latent virus (PoLV) appear to be made up of three structural domains, i.e. the N-terminal internal domain, the shell domain and the C-terminal protruding domain.
Figure 7.

(Left) Diagrammatic representation of a T=3 TBSV particles (from Hopper et al., 1984, with permission). (Right) Negative contrast electron micrograph of Pothos latent virus (PoLV) particles (G. P. Martelli, with permission). The bar represents 50 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Preparations of purified virus sediment as a single component in sucrose density gradients, and to equilibrium in solutions of CsCl and Cs2SO4. Buoyant density in CsCl and Cs2SO4 is 1.34-1.36 and 1.37 g/cm3, respectively. The thermal inactivation point is above 80°C. Virus particles resist organic solvents but are readily disrupted by SDS.
NUCLEIC ACID
Virions contain a single molecule of linear, uncapped, non-polyadenylated, positive sense ssRNA of 4,415 nt, constituting 17% of the particle weight. Virions can contain two sgRNAs 2.0 and 0.8 kb in size. Three dsRNA species corresponding to the full-size genomic RNA and the two sgRNAs can be recovered from infected plants. Satellite or DI RNAs do not occur naturally, nor does PoLV genomic RNA support the replication of tombusvirus satellite or DI RNAs.
PROTEINS
Capsids possess 180 copies of a single CP species of 40-41 kDa.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The viral genome contains four ORFs (Fig. 8 ). ORF1 encodes a 25 kDa protein. The readthrough of its amber stop codon results in translation into ORF1-RT yielding an 84 kDa protein possessing the conserved motifs of RdRps. ORF2 encodes the 40 kDa CP. ORF3 and 4 are nested in different reading frames. The 27 kDa product of ORF3 is the MP and the 14 kDa product of ORF4 is responsible for symptom severity. CP is important in regulating the synthesis of the 14 kDa protein, the excess production of which is lethal to infected plants. In vitro translation of genome-length RNA transcribed from an infectious full-length cDNA clone, yields only one 25 kDa protein. Translation of the 2.0 kb and 0.8 kb sgRNAs gives rise to the 40 kDa CP and the 27 kDa and 14 kDa proteins, respectively. Replication may occur in the cytoplasm, possibly in association with nucleus-derived vesicles and vesiculated bodies, i.e. globose aggregates of vesicular elements surrounded by a unit membrane. The strategy of replication includes readthrough and sgRNA production. Virus particles assemble and accumulate in the cytoplasm.
Figure 8.

Genome organization and replication strategy of Pothos latent virus (PoLV). Boxes represent known and predicted ORFs with the sizes of the respective proteins (or readthrough products) indicated within. Yellow ORFs indicate polymerase proteins that have a high degree of sequence conservation within the family Tombusviridae. Left-hatched red box identifies the CP that is highly conserved among other genera within the family Tombusviridae that share a protruding domain. The grey boxes identify ORFs whose proteins are unique to the genus. Lines underneath depict the genomic RNA and two sgRNAs that are synthesized in infected cells and allow for the expression of ORF2 and ORFs 3 and 4 from sgRNAs 1 and 2, respectively. RT = termination codon that is read through.
ANTIGENIC PROPERTIES
Virions are efficient immunogens. Polyclonal antisera yield a single precipitin line in immunodiffusion tests and uniformly decorate virus particles. Distant relationships were found with members of the genera Tombusvirus and Carmovirus.
BIOLOGICAL PROPERTIES
HOST RANGE
Pothos (Scindapsus aureus) and pigeonpea (Cajanus cajan) are the only known natural hosts of PoLV, and cucumber (Cucumis sativus) is the natural host of Cucumber leaf spot virus (CLSV). The experimental host range is moderately wide. Localized infections are induced in most hosts, except for Nicotiana benthamiana and N. clevelandii, which are systemically invaded.
TRANSMISSION
PoLV is readily transmitted by mechanical inoculation. Natural transmission occurs through the soil or the circulating solution in hydroponics, apparently without the intervention of a vector. CLSV is transmitted by the soil-inhabiting fungus Olpidium bornovanus and, to a low rate (c. 1%), through seeds.
GEOGRAPHICAL DISTRIBUTION
Reported from several European countries, Jordan, and India.
CYTOPATHIC EFFECTS
PoLV is very invasive in systemically infected plants and is found in parenchyma and conducting tissues. Virus particles often form intracellular crystalline aggregates. Distinctive cytopathological features are the extensive vesiculation of the nuclear envelope and the single-membrane vesiculated bodies in the cytoplasm. Cytopathology of CLSV infections is characterized by occasional peripheral vesiculation of mitochondria and by the presence of membranous cytoplasmic vesicles with fibrillar content.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The list of species demarcation criteria in the genus is:
-
•
Serological specificity (known species are serologically unrelated),
-
•Extent of sequence identity between relevant gene products;
-
○Less than 45% aa sequence identity of the CP,
-
○Less than 90% aa sequence identity of the polymerase,
-
○
-
•
Differential cytopathological features,
-
•
Transmission by a fungal vector,
-
•
Natural host range,
-
•
Artificial host range reactions.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS AVENAVIRUS
Type Species Oat chlorotic stunt virus
DISTINGUISHING FEATURES
This monotypic genus is distinguished from other genera in the family Tombusviridae because the CP is significantly larger than other members with a protruding domain. The genome organization is intermediate between those of the genera Carmovirus and Tombusvirus. The MP of Oat chlorotic stunt virus (OCSV) is related to the MPs of viruses in the genera Carmovirus, Machlomovirus and Necrovirus, but it lacks the second MP ORF associated with these viruses.
VIRION PROPERTIES
MORPHOLOGY
Particles are isometric and approximately 35 nm in diameter. Based on sequence similarity with the CPs of members of the family Tombusviridae that contain a protruding domain, it is assumed that the particle has T=3 icosahedral symmetry and are composed of 180 protein subunits.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
No information exists on physicochemical and physical properties.
NUCLEIC ACID
The genome is composed of a single positive-sense ssRNA molecule of 4,114 nt. It is not known if the 5′-terminus is capped. The 3′-end does not possess a poly(A) tail. A single sgRNA of 1,772 nt is expressed in infected tissues and is encapsidated at low concentrations within the virions.
PROTEINS
The capsid is probably composed of 180 copies of the single 48 kDa CP.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains 3 ORFs (Fig. 9 ). The genome organization is intermediate between those of the genera Carmovirus and Tombusvirus. ORF1 encodes a 23 kDa protein. Readthrough of the ORF1 amber termination codon allows the expression of a 84 kDa protein. ORF2 encodes the 48 kDa CP. ORF3 is within ORF2, in a different reading frame. This ORF encodes an 8 kDa polypeptide. A single sgRNA is formed to allow for the expression of the 3′-proximal CP ORF as well as ORF3. The ORF1 product and its amber terminator readthrough product are thought to form the viral polymerase. ORF2 encodes the virus CP. The ORF3 gene product is believed to encode the MP.
Figure 9.

Genome organization of Oat chlorotic stunt virus (OCSV). Boxes represent known and predicted ORFs with the sizes of the respective proteins (or readthrough products) indicated within. Yellow ORFs indicate polymerase proteins that have a high degree of sequence conservation within the family Tombusviridae. Left-hatched red box identifies the CP that is highly conserved among other genera within the family Tombusviridae that share a protruding domain. The blue box identifies the putative cell-to-cell MP that exhibits sequence conservation with similar proteins in the genera Carmovirus, Machlomovirus, Necrovirus, and Panicovirus. A single 1.8 kb sgRNA is expressed in vivo for the expression of the ORF2 CP and ORF. RT = termination codon that is read through.
ANTIGENIC PROPERTIES
The virus is a moderate immunogen. Antibodies do not cross-react with other unrelated icosahedral viruses of oats or with representative members of the genera Carmovirus and Machlomovirus.
BIOLOGICAL PROPERTIES
HOST RANGE
The virus has only been identified and studied in oats (Avena sativa).
TRANSMISSION
The virus is easily mechanically transmitted from oat plant to oat plant. Infection patterns in winter oat fields are consistent with the virus being soil-borne, and possibly transmitted by zoosporic fungi.
GEOGRAPHICAL DISTRIBUTION
This virus has only been reported in the United Kingdom.
CYTOPATHIC EFFECTS
None reported.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Oat chlorotic stunt virus | ||
| Oat chlorotic stunt virus | [X89864] | (OCSV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS CARMOVIRUS
Type Species Carnation mottle virus
DISTINGUISHING FEATURES
Virion Mr is 8.2 × 106 and S20w is 118-130S. Some viruses sediment as two entities in Cs2SO4 gradients. The genomic RNA is 4.0 kb in size and contains four ORFs. Translation of the genome yields a 28 kDa polypeptide encoded by ORF1 and an 88 kDa polypeptide (ORF1RT) originating from readthrough of the amber terminator of ORF1. ORFs 2 and 3 code for two small polypeptides of 7-8 kDa and 8-9 kDa, respectively, depending on the virus. CP contains a protruding domain and is encoded by ORF4, which is 3’ co-terminal. The ORFs 2, 3 and 4 polypeptides are translated from two sgRNAs with sizes of ∼1.7 and 1.5 kb, respectively. Viral species are not serologically related. Multivesicular bodies are formed only by some viruses. Most species are found in temperate regions. Those infecting legumes are reported from tropical areas. Several species are soil-borne. Melon necrotic spot virus (MNSV) is transmitted by Olpidium bornovanus.
VIRION PROPERTIES
MORPHOLOGY
Figure 10.

(Left) Computer reconstruction of a Carnation mottle virus (CarMV) particle based on X-ray crystallography at 3.2Å resolution. (from Morgunova et al., 1994, with permission). (Center) Diagrammatic representation of a carmovirus particle (from Hopper et al., 1984, with permission). (Right) Negative contrast electron micrograph of CarMV particles (from Morgunova et al., 1994, with permission).. (Right) Negative contrast electron micrograph of CarMV particles. The bar represents 50 nm.
Virions are 32-35 nm in diameter and have a T=3 icosahedral symmetry (Fig. 15). The isometric nucleocapsids have an obvious regular surface structure giving them a granular appearance in the electron microscope. Surface capsomer arrangement not obvious, there are 32 capsomers per nucleocapsid. Virions are composed of 180 protein subunits. Each subunit is folded into three distinct structural domains: R, the N-terminal internal domain interacting with RNA; S, the shell domain constituting the capsid backbone; and P, the protruding C-terminal domain. P domains are clustered in pairs to form 90 projections. These dimeric contacts are important in the assembly and stabilization of the virion structure. The R domain, which contains many positively charged residues, binds RNA. The S domain forms a barrel structure made up of β-strands. Two Ca++ binding sites stabilize contacts between S domains. X-ray crystallography analysis indicates large similarities to the TBSV structure except that CarMV lacks a β-annulus and thus may assemble by a different mechanism to that proposed for the tombusviruses.
Figure 15.
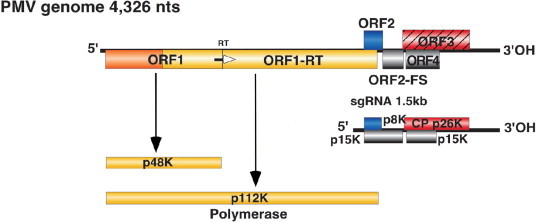
Genome organization and replication strategy of Panicum mosaic virus (PMV). Boxes represent known and predicted ORFs with the sizes of the respective proteins (or readthrough products) indicated within, or beside. Yellow shaded ORFs indicate polymerase proteins that have a high degree of sequence conservation within the family Tombusviridae. The red right-hatched box identifies the CP that is highly conserved among other genera within the family Tombusviridae that lack a protruding domain. The black box identifies the putative cell-to-cell movement protein that exhibits sequence conservation with similar proteins in the genera Avenavirus, Carmovirus, Machlomovirus, and Necrovirus. The gray boxes identify ORFs not having significant sequence similarity with a known viral protein. The 1.5 kb sgRNA is illustrated as a line below the genomic RNA. RT = amber termination codon that can be read through.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as one component with an S20w of 118-130S. The buoyant density of virions is 1.33-1.36 g/cm3 in CsCl, and the Mr is 8.2 × 106. Carnation mottle virus (CarMV) has an isoelectric point of pH 5.2. Particles exhibit an A260/A280 ratio of 1.48-1.66 and a thermal inactivation point of 95°C. Longevity in vitro of 395 days has been reported. Virions have dilution end points often in excess of 10-6. Virions are insensitive to ether, chloroform and non-ionic detergents, and are stabilized by divalent cations.
NUCLEIC ACID
Nucleic acid comprises 14% of the virion. Virions contain one molecule of linear positive-sense ssRNA. Total genome length varies between 3,879 and 4,450 nt. For CarMV, the 5′-end of the genome is probably capped with a m7GpppG or A whereas Turnip crinkle virus (TCV) genomic RNA appears not to be capped. The 3’-terminus lacks either a poly(A) tract or a terminal tRNA-like structure. Generally only the genomic RNA is encapsidated. Some species also harbor and package DI and/or satellite RNAs. SgRNAs may also be packaged into virions at a very low level. Three virus-specific dsRNA species are found in infected cells. The size of the largest virus-specific dsRNA corresponds to that of the genomic RNA. The second largest 1.5-1.9 kbp and the smallest 1.1-1.6 kbp dsRNA correspond to sgRNAs1 and 2 respectively. Complete nucleotide sequences are available for most of the species. Partial sequences are available for Pelargonium flower break virus (PFBV) and Elderberry latent virus (ELV).
PROTEINS
Virions contain 86% protein. The capsids are composed of 180 copies of a single structural CP ranging in size from 36.4 to 40.6 kDa. The CPs are not glycosylated or phosphorylated.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains four ORFs (Fig. 11). ORF1 can encode a 28 kDa protein. Readthrough of the ORF1 amber termination codon (ORF1-RT) yields an 86 kDa protein. Both the 27 and 86 kDa proteins are made in vivo and by translation of virion RNA in vitro. The proteins encoded by ORF1 and ORF1-RT make the viral polymerase. ORF2 encodes the 7 kDa MP. ORF3 encodes the 9 kDa polypeptide that also has been implicated in facilitating movement of the infection throughout the plant. Both ORFs 2 and 3 are thought to be expressed in vivo from the larger 1.7 kb sgRNA1 synthesized in infected cells.
Figure 11.

Phylogenetic (NEIGHBOR) analysis of carlaviruses using the aa sequences of the products of three ORFs: (Top left) Polymerase, (Top right) Triple gene block 1 (TGBp1), (Bottom) Capsid protein. Sequences were aligned using GCG PILEUP and genetic distances estimated by PROTDIST (Dayhoff PAM method). Trees were displayed in TreeView. Bootstrap values based on 100 replicates are shown where >60%. Apple stem pitting virus sequences (ASPV), genus Foveavirus is included for comparison. The abbreviations of the other viruses are indicated in the list of species and all the available Genbank accessions were used.
Figure 11.

Genome organization and replication strategy of Carnation mottle virus (CarMV). Boxes represent known and predicted ORFs with the sizes of the respective proteins (or readthrough products) indicated within or to the right or beside. Yellow ORFs indicate polymerase proteins that have a high degree of sequence conservation within the family Tombusviridae. Left-hatched red box identifies the CP that is conserved among other genera in the family Tombusviridae that have a protruding domain. The dark blue box identifies one of the two proteins involved in cell-to-cell movement that exhibits carboxyl-terminal sequence conservation with like proteins in the genera Avenavirus, Machlomovirus, Necrovirus, and Panicovirus. An ORF for a 9kDa protein (p9K), is also involved in movement, shares no sequence similarity with RNAs of other viruses in the family Tombusviridae. Lines underneath the gene map depict the genomic RNA and the two sgRNAs that are synthesized in infected cells and allow for the expression of ORFs 2 and 3, and ORF4 from sgRNAs 1 and 2, respectively. RT = amber termination codon that is read through.
The ORF2 initiation codon is in a sub-optimal translational context and the ORF3 initiation codon is in an optimal translational context. Ribosome scanning allows translation of ORF3 from the 1.7 kb sgRNA1. ORF4 encodes the 38 kDa CP and is expressed in vivo from the 1.5 kb sgRNA2. For TCV, the CP has also been shown to be a suppressor of virus-induced gene silencing.
ANTIGENIC PROPERTIES
Virions are efficient immunogens. Polyclonal antisera yield a single precipitin line in immunodiffusion tests. Virus species are not serologically related.
BIOLOGICAL PROPERTIES
HOST RANGE
Most species have a narrow natural host range. However, most also have a wide experimental host range. Even though the host range of an individual species is restricted in nature, species infect a wide range of both monocotyledonous and dicotyledonous plants. Viruses tend to remain localized, forming a necrosis in artificially infected hosts.
TRANSMISSION
Species are easily mechanically transmitted experimentally and in nature. CarMV has spread worldwide by the dispersal of infected carnation cuttings. Some species may be transmitted through seed at a low level. Several viruses are soil-borne, but only MNSV is transmitted by Olpidium bornovanus. Cowpea mottle virus (CPMoV), Bean mild mosaic virus (BMMV), Blackgram mottle virus (BmoV) and TCV are transmitted by beetles (Coleoptera).
GEOGRAPHICAL DISTRIBUTION
Probably distributed worldwide. Most species are found in temperate regions of the world. Those infecting legumes are reported from tropical areas.
CYTOPATHIC EFFECTS
In systemically infected plants virus particles are found in parenchyma and conducting tissues, sometimes forming intracellular crystalline aggregates. Membranous vesicles are produced from the endoplasmic reticulum. Multivesicular bodies are formed only in cells infected by some viruses.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The list of species demarcation criteria in the genus is:
-
•
Extent of serological relationship as determined by immunodiffusion and/or ELISA,
-
•Extent of sequence identity between relevant gene products,
-
○Less than 41% aa sequence identity of the CP,
-
○Less than 52% aa sequence identity of the polymerase,
-
○
-
•
Cytopathological features. Presence or absence of multivesicular bodies,
-
•
Transmission by a fungal vector,
-
•
Natural host range,
-
•
Artificial host range reactions.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Ahlum waterborne virus | ||
| Ahlum waterborne virus | (AWBV) | |
| Bean mild mosaic virus | ||
| Bean mild mosaic virus | (BMMV) | |
| Cardamine chlorotic fleck virus | ||
| Cardamine chlorotic fleck virus | [L16015] | (CCFV) |
| Carnation mottle virus | ||
| Carnation mottle virus | [X02986] | (CarMV) |
| Cowpea mottle virus | ||
| Cowpea mottle virus | [U20976, U07227] | (CPMoV) |
| Cucumber soil-borne virus | ||
| Cucumber soil-borne virus | (CuSBV) | |
| Galinsoga mosaic virus | ||
| Galinsoga mosaic virus | [Y13463, NC001818] | (GaMV) |
| Hibiscus chlorotic ringspot virus | ||
| Hibiscus chlorotic ringspot virus | [X86448] | (HCRSV) |
| Japanese iris necrotic ring virus | ||
| Japanese iris necrotic ring virus | [D86123] | (JINRV) |
| Melon necrotic spot virus | ||
| Melon necrotic spot virus | [M29671] | (MNSV) |
| Pelargonium flower break virus | ||
| Pelargonium flower break virus | [AJ003153, Z28395] | (PFBV) |
| Saguaro cactus virus | ||
| Saguaro cactus virus | [U72332] | (SgCV) |
| Turnip crinkle virus | ||
| Turnip crinkle virus | [M22445] | (TCV) |
| Weddel waterborne virus | ||
| Weddel waterborne virus | (WWBV) | |
TENTATIVE SPECIES IN THE GENUS
| Blackgram mottle virus | (BMoV) | |
| Elderberry latent virus | [AY038066] | (ElLDV) |
| Glycine mottle virus | (GMoV) | |
| Narcissus tip necrosis virus | (NTNV) | |
| Pea stem necrosis virus | (PSNV) | |
| Plantain virus 6 | (PlV-6) | |
| Squash necrosis virus | (SqNV) | |
| Tephrosia symptomless virus | (TeSV) |
GENUS NECROVIRUS
Type Species Tobacco necrosis virus A
DISTINGUISHING FEATURES
Virions sediment as a single component with an S20w of 118S. The genomic RNA is about 3.8 kb in size and contains four ORFs. A fifth potential smaller ORF is predicted within the 3’-leader of the type species TNV-A. The genome organization and expression strategy are similar to members of the genus Carmovirus. Necroviruses have a small CP that forms smooth virions and is phylogenetically related to the sobemovirus CP. This feature distinguishes the necroviruses from carmoviruses, which have a larger CP with a protruding domain
VIRION PROPERTIES
MORPHOLOGY
Virions are approximately 28 nm in diameter and exhibit a T=3 icosahedral symmetry. The shell has a smooth appearance (Fig. 12 ).
Figure 12.

(Left) Computer reconstruction of a Tobacco necrosis virus A (TNV-A) particle based on X-ray crystallography at 2.25Å resolution (from Oda et al., 2000; Reddy V. et al., (2001). (Center) Diagrammatic representation of a necrovirus particle. (Right) Negative contrast electron micrograph of TNV-A virions. The bar represents 50 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The virus sediments as one component with S20w of 118S, and has a buoyant density of 1.40 g/cm3 in CsCl. The particle has a Mr of 7.6 × 106. The thermal inactivation point of TNV is between 85 and 95°C. Virion isoelectric point is pH 4.5. Virions are insensitive to ether, chloroform and non-ionic detergents.
NUCLEIC ACID
Virions contain one molecule of infectious linear positive sense ssRNA. The type species RNA is 3684 nt. The 5′-end of the RNA does not have a covalently linked virion protein and is uncapped, possessing a ppA… terminus. The RNA does not contain a 3′-terminal poly(A) tract. The virion packages exclusively the genomic RNA. Three virus specific dsRNA species are found in infected cells. The size of the largest virus specific dsRNA corresponds to that of the genomic RNA. The second largest 1.6 kbp and the smallest 1.3 kbp dsRNAs correspond to sgRNA1 and 2 respectively. Infectious RNA transcripts have been synthesized from a full-length cDNA clone of the Olive latent virus 1 (OLV-1) and TNV-D genomes.
PROTEINS
The capsid is composed of 180 copies of a single CP species. This protein has 268-275 aa and a size of 29-30 kDa.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains four ORFs (Fig. 13 ). However, TNV-A also contains a small 3’ proximal ORF5. ORF1 is capable of encoding a 22-23 kDa polypeptide. Readthrough of the ORF1 amber termination codon allows translation to continue into ORF1-RT for the expression of an 82 kDa polypeptide. The 82 kDa protein is predicted to be the RdRp. ORF2 can encode a 7-8 kDa polypeptide implicated in cell-to-cell movement. ORF3 encodes a 6-7 kDa polypeptide that also may be involved in movement. ORF4 encodes the 29-30 kDa CP. ORF5, present only in the TNV-A, encodes a 6.7 kDa protein. Two sgRNAs of 1.6 and 1.3 kb are synthesized in infected cells. The smaller sgRNA is the translational template for CP and the larger is the translational template for the ORF2 and possibly ORF3 products. The function of the ORF5 product is not known. The 8K protein encoded by the OLV-1 genome was detected in close proximity to plasmodesmata or within plasmodesma channels and accumulated in the cytoplasm of infected cells as bundles of thin filaments.
Figure 13.

Genome organization and replication strategy of Tobacco necrosis virus A (TNV-A). Boxes represent known and predicted ORFs with the sizes of the respective proteins (or readthrough products) indicated within. Shaded ORFs indicate polymerase proteins that have a high degree of sequence conservation within the family Tombusviridae. Right-hatched red box identifies the CP that is highly conserved among other genera within the family Tombusviridae that lack a protruding domain. The blue box identifies one of the two proteins involved in cell-to-cell movement that exhibits sequence conservation with a like protein in the genera Avenavirus, Carmovirus, Machlomovirus, and Panicovirus. The grey boxes identify ORFs whose proteins share no sequence similarity with other viruses in the family Tombusviridae. Lines underneath the gene map depict the two sgRNAs that are synthesized in infected cells; these allow for the expression of ORFs 2 and 3 and ORF4 from sgRNA1 and 2, respectively. RT = amber termination codon that can be read through.
ANTIGENIC PROPERTIES
Particles of necroviruses are moderately immunogenic. Species can be distinguished serologically. Antisera yield a single precipitin line in agar gel-diffusion assays.
BIOLOGICAL PROPERTIES
HOST RANGE
Necroviruses have wide host ranges that include monocotyledonous and dicotyledonous plants. In nature, infections are typically restricted to roots. Experimental inoculations usually cause necrotic lesions on the inoculated leaves, but rarely result in systemic infection.
TRANSMISSION
Virions are readily transmitted by mechanical inoculation. Member viruses are soil-borne. Some (TNV-A, TNV-D, Beet black scorch virus; BBSV) are naturally transmitted by the chytrid fungus Olpidium brassicae, while others (OLV-1) are transmitted through the soil without the apparent intervention of a vector.
GEOGRAPHICAL DISTRIBUTION
TNV-A and TNV-D are ubiquitous, OLV-1 was reported from several Mediterranean countries, Leek white stripe virus (LWSV) from France, and BBSV from China.
CYTOPATHIC EFFECTS
Virus particles occur, often in prominent crystalline arrays, in infected cells in all tissue types, including vessels. Clumps of electron-dense amorphous material resembling accumulations of excess coat protein are present in the cytoplasm of cells infected by TNV or OLV-1. Membranous vesicles with fibrillar material, lining the tonoplast, or derived from the endoplasmic reticulum and accumulating in the cytoplasm are elicited by LWSV or OLV-1.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
-
•
Extent of serological relationship as determined by immunodiffusion and/or ELISA,
-
•Extent of sequence identity between relevant gene products,
-
○Less than 62% aa sequence identity of the CP,
-
○Less than 76% aa sequence identity of the polymerase,
-
○
-
•
Transmission by a fungal vector,
-
•
Natural host range,
-
•
Artificial host range reactions.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Beet black scorch virus | ||
| Beet black scorch virus | [NC004452] | (BBSV) |
| Chenopodium necrosis virus | ||
| Chenopodium necrosis virus | (ChNV) | |
| Leek white stripe virus | ||
| Leek white stripe virus | [X94560] | (LWSV) |
| Olive latent virus 1 | ||
| Olive latent virus 1 | [X85989] | (OLV-1) |
| Tobacco necrosis virus A | ||
| Tobacco necrosis virus A | [M33002] | (TNV-A) |
| Tobacco necrosis virus D | ||
| Tobacco necrosis virus D | [D00942, U62546] | (TNV-D) |
TENTATIVE SPECIES IN THE GENUS
| Carnation yellow stripe virus | (CYSV) |
| Lisianthus necrosis virus | (LNV) |
GENUS PANICOVIRUS
Type Species Panicum mosaic virus
DISTINGUISHING FEATURES
Virions sediment at S20w 109S. The genomic RNA is 4.3 kb and contains four ORFs. The polymerase is larger than those encoded by members of the family Tombusviridae. Like that of the machlomoviruses, the panicovirus polymerase has an amino terminal extension fused to the rest of the polymerase that is phylogenetically conserved among the family Tombusviridae. The virus produces only a single 1.5 kb sgRNA that is a template for the expression of ORFs 2 through 5. A second smaller sgRNA that could be used for the expression of the CP ORF4 and ORF5 has not been identified. The overall size and organization of the genome is similar to that of the genus Machlomovirus. However, viruses in the genus Panicovirus lack the additional 5’-proximally located ORF encoding a 32 kDa protein of unknown function. The virus is restricted to monocotyledonous hosts.
VIRION PROPERTIES
MORPHOLOGY
Virions are approximately 30 nm in diameter and exhibit icosahedral symmetry (Fig. 14 ). Detailed capsid structure is not known. Based on CP sequence similarity, it is predicted that the capsid is structurally similar to the T=3 capsid of Southern bean mosaic virus (genus Sobemovirus).
Figure 14.

(Left) Diagrammatic representation of a particle of Panicum mosaic virus (PMV). (Right) Negative contrast electron micrograph of PMV particles (K. B. Scholthof, with permission).. The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as one sedimenting component with an S20w of 109S, and have a buoyant density of 1.365 g/cm3 in CsCl, and a virion Mr of 6.1 × 106. Virus stored in desiccated tissue retained infectivity after twelve years. The thermal inactivation point of the type strain is 85°C, and 60°C for the St. Augustine grass decline virus strain. Virions are stable at pH 6 and lower. Virions are insensitive to ether, chloroform and non-ionic detergents. Virions are stabilized by divalent cations.
NUCLEIC ACID
Virions contain a single molecule of infectious linear positive sense ssRNA. The RNA is 4326 nt in length. The 5′-end of the RNA appears not to be capped. The RNA does not contain a 3′-terminal poly(A) tract. A 1475 nt sgRNA is also produced in vivo that appears not to be packaged into the virions.
PROTEINS
The virion is probably composed of 180 copies of a single 26 kDa CP.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains five ORFs (Fig. 15 ). ORF1 encodes a 48 kDa protein. Readthrough of the ORF1 amber termination codon allows the production of a 112 kDa protein. Both the 48 kDa and 112 kDa polypeptides are produced by translation of virion RNA in vitro. ORF2 encodes an 8 kDa protein that is produced by in vitro translation of a transcript representing the subgenomic RNA. ORF 3 is expressed from a noncanonical start codon (GUG) and encodes a 6.6 kDa protein. ORF 4 encodes the 26 kDa CP. ORF5 is nested within ORF4 in a different reading frame and encodes a 15 kDa polypeptide. This ORF is likely expressed by leaky ribosome scanning. From a single subgenomic RNA, ORFs 2-5 are expressed, most likely through a combination of translational strategies including leaky scanning and internal ribosome entry (IRES). ORFs 2, 3, and 5 likely encode proteins involved in virus movement.
ANTIGENIC PROPERTIES
PMV particles are highly immunogenic. Antisera yield a single precipitin line in agar gel-diffusion assays. There are several serological strains of the type strain as well as the serologically distinct St. Augustine grass decline virus strain.
BIOLOGICAL PROPERTIES
HOST RANGE
In nature, PMV is restricted to grass species in the Paniceae tribe of the Poaceae. It is known to cause diseases of note in switch grass (Panicum virgatum), St. Augustinegrass (Stenotaphrum secundatum), and centipede grass (Eremochloa ophiuroides). In the laboratory, a number of additional species in the Graminae can be symptomless hosts. Zea mays is used as a propagation host for the type strain. St. Augustinegrass must be used as a propagation host for the St. Augustinegrass decline virus strain.
TRANSMISSION
The virus is readily transmitted by mechanical inoculation. The virus is typically transmitted by the transport and replanting of infected sod. There is one report that the St. Augustinegrass decline virus strain was seed-transmitted through Setaria italica.
GEOGRAPHIC DISTRIBUTION
The virus has been reported in the USA and Mexico. The type strain is widely distributed in a number of turf grasses throughout the central United States whereas the St. Augustinegrass decline virus strain is widely distributed throughout the southern US.
PATHOGENICITY, ASSOCIATION WITH DISEASE
The virus typically forms a systemic mosaic. More severe symptoms, including chlorotic mottling, stunting and seed yield reduction, occur in forage grasses when PMV is in a mixed infection with Panicum mosaic satellite virus. The St. Augustinegrass decline virus strain can cause a severe disease on St. Augustinegrass with symptoms being more severe in the hot summer months.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The list of species demarcation criteria in the genus is:
-
•
Extent of serological relationship as determined by immunodiffusion and/or ELISA,
-
•
Extent of sequence identity between relevant gene products,
-
•
Soil transmission with the aid of a biological vector,
-
•
Natural host range,
-
•
Artificial host range reactions.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Panicum mosaic virus | ||
| Panicum mosaic virus | [U55002] | (PMV) |
TENTATIVE SPECIES IN THE GENUS
| Molinia streak virus | (MoSV) |
GENUS MACHLOMOVIRUS
Type Species Maize chlorotic mottle virus
DISTINGUISHING FEATURES
Virions sediment at an S20w of 109S. The genomic RNA is about 4.4 kb and contains four ORFs. The polymerase is larger than those encoded by other species in the family Tombusviridae. Like the panicoviruses, the machlomovirus polymerase has an amino terminal extension fused to the rest of the polymerase that exhibits sequence conservation with the family Tombusviridae. It is not clear how the 3’ proximally located CP ORF is expressed in vivo. There are conflicting reports of either a 1.1 kb or a 1.47 kb sgRNA being made in vivo. Apparently another 0.34 kb sgRNA is made in vivo that would not act as a mRNA for any viral ORF. The overall size and organization of the genome is quite similar to that of the genus Panicovirus. However, genomes of machlomoviruses encode an additional 5’ proximally located ORF encoding a 32 kDa protein of unknown function. The virus is restricted to monocotyledonous hosts.
VIRION PROPERTIES
MORPHOLOGY
Virions are approximately 30 nm in diameter and exhibit icosahedral symmetry (Fig. 16 ). Detailed structure of virions is not known. Based on CP sequence similarity, it is predicted that the capsid is structurally similar to the T=3 capsids of Southern bean mosaic virus (genus Sobemovirus).
Figure 16.

(Left) Diagrammatic representation of a particle of Maize chlorotic mottle virus (MCMV). (Right) Negative contrast electron micrograph of MCMV virions (S.A. Lommel, with permission).. The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Mr of virions is 6.1 × 106; S20w is 109S; buoyant density in CsCl is 1.365 g/cm3. Virions are insensitive to ether, chloroform and non-ionic detergents. Virions are stable in vitro for up to 33 days and the thermal inactivation point of virions is between 80-85°C. Virions are stable at pH 6 and lower. Virions are stabilized by divalent cations.
NUCLEIC ACID
Virions contain a single molecule of infectious linear positive-sense ssRNA. The RNA is 4437 nt in length. The 5′-end of the RNA has been reported to be capped with m7GpppA, however the absence of a cap analog from full length transcripts does not reduce infectivity. The RNA does not contain a 3′-terminal poly(A) tract. Either a 1470 or an 1100 nt sgRNA is also packaged into virions at a very low frequency.
PROTEINS
Capsids are composed of 180 copies of a single CP of 238 aa (25.1 kDa).
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains four ORFs (Fig. 17 ). ORF1 is capable of encoding a 32 kDa protein. ORF2 can encode a 50 kDa protein. Readthrough of the ORF2 amber termination codon allows for translation to continue into ORF2-RT, yielding a 111 kDa protein. A 111 kDa protein is produced by translation of virion RNA in vitro. ORF3 encodes a 9 kDa protein whose carboxyl terminus is like those of proteins encoded by similarly located small ORFs in the genomes of the genera Avenavirus, Carmovirus, Necrovirus, and Panicovirus. Assuming readthrough of the ORF3 opal termination codon, a 33 kDa protein would be produced. ORF4 encodes the 25.1 kDa CP. It is not clear how the internal ORFs are expressed in vivo. A major sgRNA is synthesized in vivo with a size of either 1.1 kb or 1.47 kb. If the sgRNA is 1.1 kb it serves as the translational template for the CP ORF. However, a 1.1 kb sgRNA does not explain the expression of the small internally located ORF. Conversely, if the sgRNA is 1.47 kb it does explain the expression of the two internal ORFs but does not explain how CP would be expressed. A second 0.34 kb sgRNA is made in vivo which does not act as a mRNA for any viral ORF. The functions of proteins encoded by ORF1 and ORF3 and the ORF3 readthrough products are not known. The ORF2-encoded protein and its readthrough product are thought to be the viral polymerase.
Figure 17.

Genome organization and replication strategy of Maize chlorotic mottle virus (MCMV). Boxes represent known and predicted ORFs with the sizes of the respective proteins (or readthrough products) indicated within. Shaded ORFs indicate polymerase proteins that have a high degree of sequence conservation within the family Tombusviridae. Right-hatched red box identifies the CP that is highly conserved among those genera within the family Tombusviridae that lack a protruding domain. The blue box identifies the putative cell-to-cell MP that exhibits sequence conservation with similar proteins in the genera Avenavirus, Carmovirus, Necrovirus, and Panicovirus. The gray boxes identify ORFs not having significant sequence similarity with a known viral protein. The 1.47 kb, the 1.1 kb CP and the 0.34 kb sgRNAs illustrated as lines below the genomic RNA. RT = termination codon that is read through.
ANTIGENIC PROPERTIES
MCMV particles are moderately to highly immunogenic. Serological variants have been identified. Antisera yield a single precipitin line in agar gel-diffusion assays
BIOLOGICAL PROPERTIES
HOST RANGE
In nature, the virus systemically infects varieties of maize (Zea mays). In the laboratory, the virus is restricted to members of the family Graminae.
TRANSMISSION
The virus is readily transmitted by mechanical inoculation. The virus is also seed-transmitted. Kansas and Nebraska isolates can be transmitted by six species of chrysomelid beetles in the laboratory. A Hawaiian isolate is transmitted by thrips.
GEOGRAPHIC DISTRIBUTION
The virus has been reported in Argentina, Mexico, Peru, and the United States. Within the United States, the virus is restricted to the Republican River valley of Kansas and Nebraska, and to Kauai, Hawaii.
PATHOGENICITY, ASSOCIATION WITH DISEASE
(MCMV causes a mild mosaic on maize in nature. When plants are also infected with one of several Graminae-specific potyviruses, a severe necrotic disease results, termed corn lethal necrosis.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Maize chlorotic mottle virus | ||
| Maize chlorotic mottle virus | [X14736] | (MCMV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
None reported.
SIMILARITY WITH OTHER TAXA
The polymerases of members of the family Tombusviridae are related to the polymerase utilized by Barley yellow dwarf virus-PAV and similar species in the genus Luteovirus (Fig 18 ). The CPs of viruses in the genera Machlomovirus, Necrovirus, and Panicovirus are similar in sequence, and presumably in structure, to those of sobemoviruses (Fig 18). The dianthovirus MP has limited sequence similarity over a limited region with MPs of viruses in the family Bromoviridae.
Figure 18.

Phylogenetic analysis of CP (left) and polymerase (right) proteins of genera of the family Tombusviridae. The two different morphological types of virions, smooth versus those with a protruding domain are outlined with colored boxes. Protein sequences were aligned with CLUSTAL W and phylogenetic trees constructed with the SEQBOOT, PROTPARS, and CONSENSE programs of the PHYLIP package. SBMV CP and polymerase proteins were used as out-groups.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
DERIVATION OF NAMES
Aureus: from the specific epithet of Scindapsus aureus (pothos), natural host of the virus.
Avena: from Avena, the generic name for oats.
Carmo: sigla from carnation mottle.
Diantho: from Dianthus, the generic name of carnation.
Machlomo: sigla from maize chlorotic mottle.
Necro: from Greek nekros, “dead body”.
Panico: sigla from panicum mosaic.
Tombus: sigla from tomato bushy stunt.
REFERENCES
- Booonham N., Henry CM., Wood R.R. The characterization of a subgenomic RNA and in vitro translation products of oat chlorotic stunt virus. Virus Genes. 1998;16:141–145. doi: 10.1023/a:1007985404933. [DOI] [PubMed] [Google Scholar]
- Campbell R.N., Tim S.T., Lecoq H. Virus transmission of host-specific strains of Olpidium bornovanus and Olpidium brassicae. Euro. J. Plant Pathol. 1995;101:273–282. [Google Scholar]
- Cao Y., Cai Z., Ding Q., Li D., Han C., Yu J., Liu Y. The complete nucleotide sequence of beet black scorch virus (BBSV), a new member of the genus necrovirus. Arch. Virol. 2002;147:2431–2435. doi: 10.1007/s00705-002-0896-1. [DOI] [PubMed] [Google Scholar]
- Ciuffreda P., Rubino L., Russo M. Molecular cloning and complete nucleotide sequence of galinsoga mosaic virus genomic RNA. Arch. Virol. 1998;143:173–180. doi: 10.1007/s007050050277. [DOI] [PubMed] [Google Scholar]
- Coutts R.H.A., Rigden J.E., Slabas A.R., Lomonossoff G.P., Wise, PJ. The complete nucleotide sequence of tobacco necrosis virus strain D. J. Gen. Virol. 1991;72:1521–1529. doi: 10.1099/0022-1317-72-7-1521. [DOI] [PubMed] [Google Scholar]
- Giesman-Cookmeyer D., Kim K.H., Lommel S.A. Dianthoviruses. In: Singh R.P.,, Singh U.S.,, Kohomoto K.,, editors. Vol. 3. Pergamon Press; Oxford: 1995. pp. 157–176. (Pathogenesis and Host Specificity in Plant Diseases; histopathological, biochemical, genetic and molecular basis). [Google Scholar]
- Grieco F., Savino V., Martelli G.P. Nucleotide sequence of a citrus isolate of olive latent virus 1. Arch. Virol. 1996;141:825–838. doi: 10.1007/BF01718158. [DOI] [PubMed] [Google Scholar]
- Hamilton R.I., Tremaine J.H. Dianthoviruses: Properties, Molecular Biology, Ecology, and Control. In: B.D. Harrison, Murant A.F., editors. Vol. 5. Plenum Press; New York: 1996. pp. 251–282. (The Plant Viruses). [Google Scholar]
- Kumar P.L., Jones A.T., Sreenivasulu P., Fenton B., Reddy V.R. Characterization of a virus from pigeonpea with affinities to species of the genus Aureusvirus, family Tombusviridae. Plant Dis. 2001;85:208–215. doi: 10.1094/PDIS.2001.85.2.208. [DOI] [PubMed] [Google Scholar]
- Lesnaw J.A., Reichmann M.E. The structure of tobacco necrosis virus I. The protein subunit and the nature of the nucleic acid. Virology. 1969;39:729–737. doi: 10.1016/0042-6822(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Lot H., Rubino L., Delecolle B., Jaquemond M., Turturo C., Russo M. Characterization, nucleotide sequence and genome organization of leek stripe virus, a putative new species of the genus Necrovirus. Arch. Virol. 1996;141:2375–2386. doi: 10.1007/BF01718638. [DOI] [PubMed] [Google Scholar]
- Miller J.S., Damude H., Robbins M.A., Reade R.D., Rochon D.M. Genome structure of cucumber leaf spot virus: sequence analysis suggests it belongs to a distinct species within the Tombusviridae. Virus Res. 1997;52:51–60. doi: 10.1016/s0168-1702(97)00105-6. [DOI] [PubMed] [Google Scholar]
- Molnar A., Havelda Z., Dalmay T., Szutorisz H., Burgyan J. Complete nucleotide sequence of tobacco necrosis virus strain DH and genes required for RNA replication and virus movement. J. Gen. Virol. 1997;78:1235–1239. doi: 10.1099/0022-1317-78-6-1235. [DOI] [PubMed] [Google Scholar]
- Morgunova E.Y., Dauter Z., Fry E., Stuart D.I., Stel'maschchuk V.Y., Mikhailov A.M., Wilson K.S., Vainshtein B.K. The atomic structure of carnation mottle virus capsid protein. FEBS Letters. 1994;338:267–271. doi: 10.1016/0014-5793(94)80281-5. [DOI] [PubMed] [Google Scholar]
- Oda Y., Saeki K., Takahashi Y., Maeda T., Naitow H., Tsukihara T., Fukuyama K. Crystal structure of tobacco necrosis virus at 2.25Å resolution. J. Mol. Biol. 2000;300:153–179. doi: 10.1006/jmbi.2000.3831. [DOI] [PubMed] [Google Scholar]
- Olson A.J., Bricogne G., Harrison S.C. Structure of tomato bushy stunt virus. IV. The virus particle at 2.9 Å resolution. J. Mol. Biol. 1983;171:61–93. doi: 10.1016/s0022-2836(83)80314-3. [DOI] [PubMed] [Google Scholar]
- Pantaleo V., Rubino L., Russo M. Replication of Carnation Italian ringspot virus defective interfering RNA in Saccharomyces cerevisiae. J. Virol. 2003;77:2116–2123. doi: 10.1128/JVI.77.3.2116-2123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino L., Russo M. Molecular analysis of the pothos latent virus genome. J. Gen. Virol. 1997;78:1219–1226. doi: 10.1099/0022-1317-78-6-1219. [DOI] [PubMed] [Google Scholar]
- Rubino L., Di Franco A., Russo M. Expression of a plant virus non-structural protein in Saccharomyces cerevisiae causes membrane proliferation and altered mitochondrial morphology. J. Gen. Virol. 2000;81:279–286. doi: 10.1099/0022-1317-81-1-279. [DOI] [PubMed] [Google Scholar]
- Russo M., Burgyan J., Martelli G.P. Molecular biology of Tombusviridae. Adv. Virus Res. 1994;44:381–428. doi: 10.1016/s0065-3527(08)60334-6. [DOI] [PubMed] [Google Scholar]
- Sabanadzovic S., Boscia D., Saldarelli P., Martelli G.P., Lafortezza R., Koenig R. Characterization of a pothos (Scindapsus aureus) virus with unusual properties. Eur. J. Plant Pathol. 1995;101:171–182. [Google Scholar]
- Scheets K. Maize chlorotic mottle machlomovirus expresses its coat protein from a 1.47 kb subgenomic RNA and makes a 0.34 kb subgenomic RNA. Virology. 2000;267:90–101. doi: 10.1006/viro.1999.0107. [DOI] [PubMed] [Google Scholar]
- Takemoto Y., Kanehira T., Shinohara M., Yamashita S., Hibi T. The nucleotide sequence and genome organization of Japanese iris necrotic ringspot virus, a new species in the genus carmovirus. Arch. Virol. 2000;145:651–657. doi: 10.1007/s007050050054. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, W.J.M. Spaan, D. Cavanagh, R.J. de Groot, L. Enjuanes, A.E. Gorbalenya, E.J. Snijder, P.J. Walker
ORDER NIDOVIRALES
TAXONOMIC STRUCTURE OF THE ORDER
| Order | Nidovirales |
| Family | Coronaviridae |
| Genus | Coronavirus |
| Genus | Torovirus |
| Family | Arteriviridae |
| Genus | Arterivirus |
| Family | Roniviridae |
| Genus | Okavirus |
VIRION PROPERTIES
GENERAL
The order comprises three families of viruses. Currently recognized unique molecular markers that distinguish this order from all other RNA viruses are part of replicase:
-
1.
An ORF1a-encoded cysteine or serine protease with chymotrypsin-like fold and the substrate specificity resembling that of picornavirus 3C proteases (3C-like or main protease) that is flanked by two hydrophobic trans-membrane domains (M-3CL-M);
-
2.
An ORF1b-encoded putative multinuclear Zn-finger-like domain associated with nucleoside triphosphate (NTP)-binding/5’-to-3’-helicase domain (Zn-HEL);
-
3.
An ORF1b-encoded putative poly(U)-specific endoribonuclease (NendoU);
-
4.
The replicase gene constellation separated by a ribosomal frameshifting signal (FS): M-3CL-M_FS_RdRp_Zn-HEL_NendoU.
The characteristics which are common for the members of the order Nidovirales and not listed above are:
-
•
Linear, non-segmented, positive-sense, ssRNA genomes.
-
•
The general genome organization 5’-UTR-replicase gene-structural protein genes-UTR-3’.
-
•
A 3’ co-terminal nested set of two or more sg mRNAs.
-
•
The genomic RNA functions as the mRNA for the replicase gene.
-
•
Only the 5’-proximal one or two ORFs of mRNAs are translationally active.
-
•
Presence of a virion envelope.
-
•
Genome and sg mRNAs contain a 3’-poly[A] tail.
-
•
Two large overlapping open reading frames (ORF1a and ORF1b) encoding replicase subunits that are derived from the translation products of ORF1a and ORF1a plus ORF1b (ORF1ab), the latter generated by ribosomal frameshifting.
MORPHOLOGY
The members of the order Nidovirales are enveloped viruses with an architecture that shows various similarities and differences, depending on whether the external appearance or the nucleocapsid of the virions is studied (Fig. 1 ). The two genera of the family Coronaviridae (Coronavirus and Torovirus) and members of the family Roniviridae show large projections protruding from the envelope (peplomers) that are formed, at least in coronaviruses, by trimers of the spike protein. These oligomeric structures provide them with the characteristic “crown” observed by electron microscopy that inspired the name of the coronavirus family. Coronaviruses have an internal core shell that protects a nucleocapsid having helical symmetry. Vitrified coronavirus particles have a diameter of 145 nm (including the extended peplomers) and an internal core shell of 65 nm. The torovirus nucleocapsid shows an unusual morphology resembling a toroid, which inspired their name. Toroviruses have a virion size similar to coronaviruses, while Roniviruses (for rod-shaped nidoviruses) are bacilliform in shape and have a 150-200 nm long nucleocapsid with helical symmetry and a diameter of 20-30 nm. Also ronivirus envelopes are studded with prominent peplomers projecting approximately 11 nm from the surface. Arterivirus virions are spherical and significantly smaller than other nidoviruses with a complete particle of 50-70 nm in diameter, a nucleocapsid of 25-35 nm in diameter. Arteriviruses probably have an icosahedral core shell that contains the genome. No spikes are obvious on the arterivirus surface, but a surface pattern of relatively small and indistinct projections has been observed.
Figure 1.

Schematic structure of particles of members of the order Nidovirales. MEM, lipid membrane; CS, core shell; NC, nucleocapsid; N, nucleocapsid protein; S, spike protein; M, membrane protein; E, envelope protein; HE, hemagglutinin-esterase.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The coronavirus virion Mr is 400 × 106, the buoyant density in sucrose is 1.15 to 1.20 g/ml, the density in CsCl is 1.23-1.24 g/ml, and viron S20W is 300 to 500S. Toroviruses have bouyant densities of 1.14-1.18 g/ml in sucrose. Arterivirus virion buoyant density is about 1.13-1.17 g/ml in sucrose and 1.17-1.20 g/ml in CsCl. Virion S20W is 200 to 300S. Ronivirus virion buoyant density in sucrose is 1.18-1.20 g/ml. Nidovirus virions are sensitive to heat, lipid solvents, non-ionic detergents, formaldehyde, oxidizing agents and UV irradiation.
NUCLEIC ACID
The nidoviruses genome is an infectious, single-stranded linear, positive-sense RNA molecule, which is polyadenylated and, at least for arteri- and coronaviruses, carries a 5’ cap. The size of the genomes of members of the order Nidovirales are 27.6 to 31 kb (Coronavirus), 25 to 30 kb (Torovirus), ∼28 kb (Okavirus) and 12.7 to 15.7 kb (Arterivirus). The coronavirus genome is the largest known non-segmented viral RNA genome. Complete genome sequences have been determined for several coronaviruses (Mouse hepatitis virus (MHV), Transmissible gastroenteritis virus (TGEV), Infectious bronchitis virus (IBV), Bovine coronavirus (BCoV), Porcine epidemic diarrhea virus (PEDV), Severe acute respiratory syndrome coronavirus (SARS-CoV), Human coronavirus OC43 (HCoV-OC43) and Human coronavirus 229E (HCoV-229E), the ronivirus Gill-associated virus (GAV), and all known arteriviruses (Equine arteritis virus (EAV), Lactate dehydrogenase-elevating virus (LDV), Porcine reproductive and respiratory syndrome virus (PRRSV), Simian hemorrhagic fever virus (SHFV)). A sequence of the (estimated) 3′-proximal half of the genome is available for the Equine torovirus (EToV).
PROTEINS
No virion protein common to all nidoviruses has so far been recognized. The virion proteins typical for the various nidovirus families are summarized in Table 2 .
Table 2.
Nidovirus virion-associated proteins (kDa)
| Protein | Coronavirusa | Torovirusa | Arterivirusa | Okavirusa | |
|---|---|---|---|---|---|
| Spike glycoprotein | S | 180–220 | 200 | – | – |
| Major surface glycoprotein | GP5 | – | – | 30–45 | – |
| Minor surface glycoprotein | GP2 | – | – | 25 | – |
| GP3 | 36–42 | – | |||
| GP4 | – | – | 15–28 | – | |
| Large spike glycoprotein | Gp116 | – | – | – | 110–135 |
| Small spike glycoprotein | Gp64 | – | – | – | 60–65 |
| Membrane protein | M | 23–35 | 27 | 16 | |
| Nucleocapsid protein | N | 50–60 | 19 | 12 | 20–22 |
| Small envelope protein | E | 9–12 | – | 9 | – |
| Hemagglutinin-esterase protein | HE | 65 | 65 | – | – |
apparent molecular weight estimated by SDS-PAGE electrophoresis (kDa)
The envelope of coronaviruses contains three or four proteins. The spike proteins (S) of coronaviruses and toroviruses have a highly exposed globular domain and a stem portion containing heptad repeats, indicative of a coiled-coil structure. The membrane (M) proteins of coronaviruses and toroviruses are different in sequence but alike in size, structure and function. The M proteins have a similar triple- or quadruple-spanning membrane topology. In addition, coronaviruses have a small structural protein (E) within the envelope (around 20 copies per virion). Toroviruses seem to lack a homolog for the E protein. Some coronaviruses (MHV, HCoV-OC43, BCV) and toroviruses contain an additional membrane protein with hemagglutinin-esterase activity (HE).
The structural proteins of arteriviruses are apparently unrelated to those of the other members of the family Coronaviridae. There are six envelope proteins that have been identified in EAV and PRRSV virions and may be common to all arteriviruses: a 16-20 kDa non-glycosylated membrane protein (M) is thought to transverse the membrane three times and thus structurally resembles the M protein of corona- and toroviruses. The heterogeneously N-glycosylated, putative triple-spanning major glycoprotein (GP5 for EAV, LDV, and PRRSV) of variable size forms a disulfide-linked heterodimer with the M protein. Recently, a trimeric complex consisting of the three remaining viral glycoproteins (GP2, GP3, and GP4), which are all a minor virion components, was described for EAV. The final structural protein of arteriviruses is a small, non-glycosylated, hydrophobic protein designated E (for envelope).
Ronivirus structural proteins have been studied only for Yellow head virus (YHV). YHV virions contain three major structural proteins (110-135 kDa, 63-67 kDa and 20-22 kDa). The 110-135 kDa protein (gp116) and the 63-67 kDa protein (gp64) are glycosylated and appear to be envelope proteins that form the prominent peplomers on the virion surface. Mature gp116 and gp64 are generated by post-translational processing of a precursor glycopolyprotein. Gp116 and gp64 are not linked by intramolecular disulfide bonds but each is anchored in the virion by C-terminal hydrophobic transmembrane domains. The 20-22 kDa protein (p20) is associated with nucleocapsids and appears to function as the nucleoprotein.
LIPIDS
Nidoviruses have lipid envelopes. The S protein (MHV, BCV) and the E protein (MHV) of coronaviruses are acylated (palmitic acid).
CARBOHYDRATES
Coronavirus S and HE proteins contain N-linked glycans, the S protein being heavily glycosylated (about 20-35 glycans). The M protein of coronaviruses contains a small number of either N- or O-linked glycans, depending on the species. These side chains are located near the amino-terminus, but the M protein of TGEV also has a potential glycosylation site in the carboxy-terminus. At present there is no evidence to suggest that the E protein is glycosylated or phosphorylated.
Torovirus S protein has 18 potential N-glycosylation sites. Also their HE protein (Bovine torovirus (BToV)) is N-glycosylated and binds 9-O-acetylated receptors, but their M protein is not glycosylated.
In the arteriviruses the GP2, GP3, GP4 and GP5 contain N-linked glycans. GP5 of EAV, LDV, and PRRSV are modified by heterogeneous N-acetyl lactosamine addition. The M and E proteins are not glycosylated.
GENOME ORGANIZATION AND REPLICATION
Despite the differences in genetic complexity and gene composition, the genome organization of corona-, toro, roni-, and arteriviruses are remarkably similar (Fig. 2 ). Two thirds of each genome contain two large ORFs, designated ORF1a and ORF1b. Downstream of ORF1b there are three (Okavirus) to ten (BCV, MHV, SHFV), or 12 (SARS-CoV) ORFs that encode the structural proteins and, at least for coronaviruses, a number of non-structural “accessory” proteins.
Figure 2.

Schematic representation of the genome structure of members of the order Nidovirales (from top to bottom: MHV, BEV, EAV, GAV) and the 3’ genome organization can differ between members of each genus. ORFs are represented by boxes. Untranslated sequences are indicated by solid lines. The ribosomal frameshift sites in ORF1 are aligned and represented b y arrows. The proteins encoded by the ORFs are indicated. The 5’ leader sequences are depicted by a small black box. Poly(A) tails are indicated by An. S, spike protein; M, membrane protein; N, nucleocapsid protein, HE, hemagglutinin-esterase protein; I, internal ORF. PolyA, is indicated by An.
Nidovirus replication proceeds through the synthesis of the full-length negative antigenome in the cytoplasm of infected cells. It is catalyzed by a poorly characterized membrane-bound replicative complex. The products of the ORF1a and ORF1ab are sufficient to maintain genomic and sgRNA synthesis in arteriviruses and sgRNA synthesis in coronaviruses. These ORFs encode a variety of (putative) enzymes (see section on replicase) that may be part of the replicative complex per se or control its composition and functioning.
The genome is expressed by diverse mechanisms including replication, transcription, translation and co- and post-translational regulation. The virion RNA functions as the mRNA (mRNA1) for the two 5’-most ORFs (1a and 1b) encoding replicase components. The translation of ORF1a yields the pp1a polyprotein. In approximately 20-30% of cases, ribosomes slip at the overlap between the ORF1a and ORF1b regions, containing a specific seven-nucleotide “slippery” sequence and a downstream pseudoknot structure (ribosomal frameshifting signal), to continue translation into ORF1b, which is in the -1 frame relative to ORF1a. Translation of ORF1ab yields the pp1ab polyprotein. The pp1a and pp1ab polyproteins have not been described in infected cells; they are believed to be co- and post-translationally processed by several virus-encoded proteases to more than a dozen mature and intermediate replicase subunits. There is no nidovirus-wide nomenclature of replicase subunits; they may be listed in the order in which they are encoded in polyproteins, or according to their molecular size or function. In arteriviruses and coronaviruses, from one to three (and possibly four) papain-like proteases (PLpro) control processing of the N-terminal part of pp1a/pp1ab polyproteins. A chymotrypsin-like protease (known as 3CLpro or ‘main’ protease – Mpro) is responsible for processing of the rest part of pp1a/pp1ab polyproteins at 8-11 conserved cleavage sites.
All ORFs downstream of ORF1b are expressed from a 3’-coterminal nested set of specialized sgRNAs whose number varies between 2 and 8. Except for the smallest mRNA, all of the mRNAs are structurally polycistronic. As a rule, only the 5’-most one or two ORFs of each mRNA are translated while downstream ORF(s) remain translationally silent.
The sg mRNAs of corona- and arteriviruses carry a 5’ leader sequence of 55 to 92 and 170 to 210 nt, respectively, which is derived from the 5’-end of the viral genome. The sgRNA synthesis thus involves a mechanism of discontinuous RNA synthesis, which is currently presumed to occur during minus strand synthesis. The torovirus mRNAs lack a common 5’ leader sequence, with the exception of mRNA-2. The ronivirus sg mRNAs also lack a common 5’ leader sequence. Both coronaviruses and arteriviruses contain conserved AU-rich sequences at the fusion sites of leader and mRNA bodies. These sequences are most commonly termed transcription-regulating sequences (TRS), but were previously also referred to as intergenic sequences (IS), subgenomic promoters, or leader-to-body junction sites. Cells infected by arteriviruses or coronaviruses contain negative-stranded sgRNAs that correspond to each sg mRNA. The combined biochemical and reverse genetics data obtained for coronaviruses and arteriviruses strongly suggests that these subgenomic minus strands serve as template for sg mRNA synthesis. In agreement with this notion, replicative intermediates (RI)/replicative forms (RF) with sizes corresponding to the different sg mRNAs have been shown to be present and actively involved in RNA synthesis.
Several models have been proposed to explain the wealth of experimental data and, in particular, how the common leader sequence is ‘fused’ to different mRNA bodies. These models are not necessary mutually exclusive, as components of each model may operate at different stages of the viral replication cycle. The model compatible with most of the experimental data is discontinuous extension of nascent negative strand RNA. This model proposes that during negative strand RNA synthesis the discontinuous transcription step occurs, generating negative-stranded sgRNAs, which then serve as templates for the (uninterrupted) synthesis of sg mRNAs. In this model, the body TRSs on the genomic RNA serve as termination or pausing signals for negative strand synthesis, and the nascent negative-stranded sgRNA then jumps to the leader TRS sequence at the 5′-end of the genomic RNA where, following a base pairing interaction, minus strand synthesis is resumed to complete the negative-stranded sgRNA. The alternative model, the leader-primed transcription model, proposes that the genomic RNA is first transcribed into a genome-length, negative-stranded RNA, which, in turn, becomes the template for subsequent synthesis of the entire repertoire of sgRNAs. The leader of nascent sgRNAs is transcribed from the 3′-end of the negative-strand RNA and dissociates from the template to subsequently associate with the template RNA at one of the (body) TRSs to prime the synthesis of the particular sg mRNA. In this mechanism, the discontinuous transcription step takes place during positive strand RNA synthesis.
The replicase gene
A large variety of virus proteins known as non-structural proteins are not incorporated into virions. The largest non-structural protein is the essential replicase, which is encoded by the slightly overlapping ORFs 1a and 1b (gene 1) and accounts for approximately two-thirds of the genome size. The replicase gene encodes two proteins, one of which is encoded by ORF1a (pp1a), whereas the other (pp1ab) is the fused product of ORF1a and ORF1b translation and is expressed through -1 ribosomal frameshifting. Neither of these giant proteins (from the approximately 2000-aa pp1a of arteriviruses to the >7000-aa pp1ab of coronaviruses) has been observed in infected cells, and they appear to be co- and post-translationally processed at conserved junctions by viral proteases, yielding dozens of mature and intermediate replicase products. Replicases of all nidoviruses, irrespective of their more than two-fold size differences, have a common backbone of conserved domains. Sequence alignments and phylogenetic analysis imply that this replicase conservation is likely due to continuous evolution from a common nidovirus ancestor. A number of activities and functions have been provisionally assigned to many of the conserved replicase subunits. These assignments largely derived from bioinformatics analyses, although an increasing number of these predictions is being verified experimentally. Replicase subunits conserved among nidoviruses include (from the N-terminus to C-terminus): a chymotrypsin-like protease with a substrate specificity resembling that of picornavirus 3C proteases (3C-like or main protease) that is flanked by two hydrophobic trans-membrane domains (M-3CL-M), a large RdRp, a protein including a putative multinuclear Zn-finger-like domain and a nucleoside triphosphate (NTP)-binding/5’-to-3’-helicase domain (Zn-HEL), a putative poly(U)-specific endoribonuclease (NendoU).
Unfortunately, no complete genome sequence is available for any torovirus. For the best characterized torovirus (EToV), a large part of ORF1a between the known 5’- and 3’-terminal sequences is yet to be determined. The N-terminal half of the ORF1a protein is quite variable in other nidoviruses. This variability is largely responsible for the size differences in coronaviruses, or arterivirus genomes. A comparison between the coronavirus and arterivirus N-terminal ORF1a protein sequences does not yield any significant similarities beyond the presence of one or more papain-like proteases.
ANTIGENIC PROPERTIES
Serological interfamily cross-reactivity has not been demonstrated. Coronaviruses have four structural proteins: (i) the spike (S) protein forms trimers and is the major inducer of virus-neutralizing antibodies which are elicited by several domains located in the amino terminal half of the molecule; (ii) the membrane (M) protein has three or four transmembrane domains, with either the amino-terminus alone or both the amino-terminus and the carboxy-terminus being exposed at the virus surface. Most of the antibodies elicited by the M protein are directed to the carboxy-terminus. In general, polyvalent or monovalent antibodies to the amino-terminus weakly neutralize virus infectivity, but in the presence of complement they reduce infectivity around 100-fold; (iii) nucleoprotein (N) is a dominant antigen during virus infection; a N peptide is presented on the surface of infected cells and induces protective T cell responses; (iv) the envelope (E) protein is also exposed at the surface of virus and virus-infected cells. In the case of MHV, E protein-specific antiserum neutralizes viral infectivity in the presence of complement
The four toroviruses described (EToV, BToV, Porcine torovirus, Human torovirus) are serologically related. Toroviruses have four structural proteins: (i) the spike (S) protein of 180 kDa that forms large 17-20-nm spikes and induces virus-neutralizing and hemagglutination-inhibiting antibodies, (ii) the 26-kDa triple-spanning integral membrane protein (M), (iii) a 65 kDa class I membrane protein (HE) exhibiting acetylesterase activity; this protein in BToV virions forms short surface projections of 6 nm on average that act as a prominent antigen during infection, and (iv) the 19-kDa nucleocapsid protein.
Antibodies against the known arteriviruses infecting different species (EAV, LDV, PRRSV, SHFV) do not cross-react. Arteriviruses have six structural proteins: (i) a major glycoprotein (GP5) which, due to heterogeneous glycosylation, has a size between 30 and 42 kDa and is the main determinant of virus-neutralization; (ii) a trimer of GP2, GP3, and GP4, which are all a minor virion components and of which (at least) GP4 can play a role in humoral immunity; (ii) (iii) an unglycosylated transmembrane (M) protein; (iv) a 12 kDa nucleocapsid (N) protein involved in the induction of protection; and (v) a small nonglycosylated hydrophobic protein E.
BIOLOGICAL PROPERTIES
Coronaviruses infect many mammals, including humans. The main targets are epithelial cells, and consequently respiratory and gastrointestinal organ disorders result. Biological vectors are not known. Respiratory, fecal-oral and mechanical transmission are common. Swine and domestic fowl may become persistently infected with TGEV and IBV, respectively, and shed virus from the enteric tract. Hepatitis and neurological (MHV), heart and eye (RbCoV) infections have also been described.
Toroviruses infect ungulates: horses (EToV, Berne virus), bovines (BToV, Breda virus) and swine (PToV). Humans (HToV) and probably carnivores (mustellids) are also hosts for toroviruses. The transmission is probably by the fecal-oral route.
Arteriviruses infect horses (EAV), mice (LDV), monkeys (SHFV) and swine (PRRSV). EAV causes inflammation of small arteries and EAV infection can lead to extremely variable clinical signs. A fatal outcome of the disease has been reported in both natural and experimental infections, but most natural infections are either mild or subclinical. Primary host cells for all arteriviruses are macrophages. Persistent infections are frequently established. Spread is in general horizontal (respiratory, biting), by venereal routes and semen (EAV and PRRSV). Males may become persistently infected and shed virus from their reproductive tract. In pregnant animals arteriviruses (PRRSV and EAV) can cause abortions or in utero fetal death (PRRSV).
Roniviruses are the only known invertebrate nidoviruses and have been detected only in crustaceans. The black tiger prawn (Penaeus monodon) appears to be the natural host of GAV but other prawn species are susceptible to experimental infection. Infections may be chronic or acute and transmission can occur horizontally and vertically. During acute infections, mortalitiy is usually high and virus occurs in most tissues of ectodermal and mesodermal origin, and particularly in the ‘Oka’ or lymphoid organ. Necrotic cells display intensely basophilic cytoplasmic inclusions. The geographic range of infection presently appears to be restricted to Asia and Australia where the prevalence of sub-clinical chronic infections in P. monodon is commonly high. There are no known prophylactic or curative treatments.
PHYLOGENETIC RELATIONSHIPS WITHIN THE ORDER
SIMILARITY WITH OTHER TAXA
Homologs of several (putative) enzymes encoded by viruses of the order Nidovirales have been found in non-nidoviruses. The proteolytic enzymes and RdRps cluster together with homologs of viruses of the “Picornavirus-like” supergroup, double-stranded RNA Birnaviridae family members and a subset of members of the family Tetraviridae (Fig. 3 ).
Figure 3.

Unrooted phenogram showing the relationships of the RdRps of the Nidovirales lineages with virus of the families of the “Picornavirus-like” supergroup, Tetraviridae and Birnaviridae. The most conserved part of RdRps from representative viruses in the Picornaviridae, Dicistroviridae, Sequiviridae, Comoviridae, Caliciviridae, Potyviridae, Coronaviridae, Roniviridae, Arteriviridae, Birnaviridae, Tetraviridae and unclassified insect viruses was aligned. The RdRps of Thosea asigna virus (TaV), Euprosterna elaeasa virus (EeV) and the birnaviruses were converted into the canonical ABC motif form before the analysis. Using an extended, gap-free version of the alignment containing 332 informative characters, an unrooted neighbor-joining tree was inferred by the ClustalX1.81 program. All bifurcations with support in > 700 out of 1000 bootstraps are indicated. Different groups of viruses are highlighted. Note that the relative positions of members of the families Arteriviridae and Roniviridae within the Nidovirales are not resolved in this tree.
Virus families and groups, viruses included in the analysis, abbreviations ( ) and the NCBI protein (unless other specified) IDs [ ] are as follows: Picornaviridae: Human poliovirus type 3 Leon strain (PV-3L) [130503] and parechovirus 1 (HpeV-1) [6174922]; Iflavirus: Infectious flacherie virus (InFV) [3025415] and Unclassified insect viruses: Acyrthosiphon pisum virus (APV) [7520835]; Dicistroviridae: Drosophila C virus (DCV) [2388673]; Sequiviridae: Rice tungro spherical virus (RTSV) [9627951] and Parsnip yellow fleck virus (PYFV) [464431]; Comoviridae: Cowpea severe mosaic virus (CPSMV) [549316] and Tobacco ringspot virus (TRSV) [1255221]; Caliciviridae: Feline calicivirus F9 (FCV-F9) [130538] and Lordsdale virus (LORDV) [1709710]; Potyviridae: Tobacco vein mottling virus (TVMV) [8247947] and Barley mild mosaic virus (BaMMV) [1905770]; Coronaviridae: Human coronavirus 229E (HCoV) [12175747] and Equine torovirus (EToV) [94017]; Arteriviridae: Equine arteritis virus (EAV) [14583262]; Roniviridae: Gill-associated virus (GAV) [9082018]; Tetraviridae: Thosea asigna virus (TaV) [AF82930; nt sequence] and Euprosterna elaeasa virus (EeV) [AF461742; nt sequence]; Birnaviridae: Infectious pancreatic necrosis virus (IPNV) [133634] and Infectious bursal disease virus (IBDV) [1296811]. (Tree was modified from Gorbalenya et al., (2002), with permission).
The HEL enzyme has a counterpart in viruses of the “Alphavirus-like” supergroup. The organization of the replicase ORFs, including the 3CLpro_FS_RdRp constellation, is also conserved in the family Astroviridae and some Sobemoviruses and related viruses. Some parallels in the genome organization and expression strategy are evident between members of the order Nidovirales and the family Closteroviridae.
DERIVATION OF NAMES
Arteri, from equine arteritis, the disease caused by the reference virus.
Corona, derived from Latin corona, meaning crown, representing the appearance of surface projections.
Nido, from Latin nidus, meaning nest, representing the nested set of mRNAs.
Roni refers to rod-shaped nidoviruses and is derived from the morphology of the virus.
Toro, from Latin torus, the lowest convex moulding in the base of a column, refers to the nucleocapsid morphology.
REFERENCES
- Cavanagh D., Brian D.A., Briton P., Enjuanes L., Horzinek M.C., Lai M.M.C., Laude H., Plagemann P.G. W., Siddell S., Spaan W., Talbot P.J. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–635. [PubMed] [Google Scholar]
- De Vries A.A.F., Horzinek M.C., Rottier P.J.M., De Groot R.J. The genome organization of the Nidovirales: similarities and differences between arteri-, toro-, and coronaviruses. Sem. Virol. 1997;8:33–47. doi: 10.1006/smvy.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Siddell S.G., Spaan W.J., editors. Coronaviruses and Arteriviruses. Plenum Press; New York: 1998. [Google Scholar]
- González J.M., Gomez-Puertas P., Cavanagh D., Gorbalenya A.E., Enjuanes L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch. Virol. 2003;148:2207–2235. doi: 10.1007/s00705-003-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E. Big nidovirus genome: when count and order of domains matter. Adv. Exp. Med. Biol. 2001;494:1–17. [PubMed] [Google Scholar]
- Gorbalenya A.E., Pringle F.M., Zeddam J.-L., Luke B.T., Cameron C.E., Kalmakoff J., Hanzlik T.N., Gordon K.H.J., Ward V.K. The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J. Mol. Biol. 2002;324:47–62. doi: 10.1016/S0022-2836(02)01033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C., Cavanagh D. The molecular biology of coronaviruses. Adv. Vir. Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S.G., Fraenkel-Conrat H., Wagner R.R., editors. The Viruses. Plenum Press; New York: 1995. “The Coronaviridae”. [Google Scholar]
- Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L.M., Guan Y., Rozanov M., Spaan W.J.M., Gorbalenya A.E. Unique and conserved features of genome and proteome of SARS-Coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Horzinek M.C. The molecular biology of toroviruses. In: Siddell S.G., editor. The Coronaviridae. Plenum Press; New York: 1995. pp. 219–238. [Google Scholar]
- Snijder E.J., Meulenberg J.J.M. The molecular biology of arteriviruses. J. Gen. Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., Spaan W.J.M. The coronavirus-like superfamily. In: Siddell S.G., editor. The Coronaviridae. Plenum press; New York: 1995. pp. 239–252. [Google Scholar]
- Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- Ziebuhr J., Bayer S., Cowley J.A., Gorbalenya A.E. The 3C-like proteinase of an invertebrate nidovirus links coronavirus and potyvirus homologs. J. Virol. 2003;77:1415–1426. doi: 10.1128/JVI.77.2.1415-1426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
CORONAVIRIDAE
CONTRIBUTED BY, W.J.M. Spaan, D. Brian, D. Cavanagh, R.J. de Groot, L. Enjuanes, A.E. Gorbalenya, K.V. Holmes, P. Masters, P. Rottier, F. Taguchi, P. Talbot
FAMILY CORONAVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Order | Nidovirales |
| Family | Coronaviridae |
| Genus | Coronavirus |
| Genus | Torovirus |
GENUS CORONAVIRUS
Type Specie Infectious bronchitis virus
VIRION PROPERTIES
MORPHOLOGY
Virions are enveloped and spherical in shape; those of members of the genus Coronavirus being commonly 120-160 nm in diameter, with an internal, possibly icosahedral, core shell of around 65 nm, and a helical nucleocapsid comprising the nucleocapsid protein (N) and RNA genome (Fig. 1 ). Coronaviruses have large surface projections formed by glycoproteins (peplomers; trimers of the spike protein) with a globular and a stem portion. The peplomers (trimers of the spike protein) are about 20 nm in length. In some coronaviruses such as Bovine coronavirus (BCoV) and some strains of Mouse hepatitis virus (MHV), a second layer of peplomers formed by the hemagglutinin esterase (HE) glycoprotein is also observed. A gap separating the internal core from the envelope has been observed in coronaviruses using cryoelectron microscopy. The core can be released after treatment with detergents. Disruption of these cores releases N-protein-containing helical nucleocapsids.
Figure 1.

Structure of coronavirus virions. (Top left) Schematic diagram of virus structure; (Bottom left) Diagram of virion surface. (Top right) Electron micrograph of virus particles of Transmissible gastroenteritis virus (TGEV) stained with uranyl acetate (top right) or sodium phosphotungstate (insert bottom left) showing the surface of the virus particles. The peplomers are better defined using sodium phosphotungstate. (insert bottom right) Cryo-electron microscopic visualization of unstained TGEV in vitreous ice. The particles contain an internal structure inside the viral envelope and well extended peplomers. MEM, lipid membrane; S, spike protein; M, large membrane protein, E, small envelope protein; HE, hemagglutinin-esterase; N, nucleocapsid protein; CS, core-shell; NC, nucleocapsid. The bars represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr has been estimated at 400 × 106 for coronaviruses. Virion buoyant density in sucrose is 1.15-1.20 g/ml; density in CsCl is 1.23-1.24 g/ml. Virion S20,w is 300-500. Virions are inactivated by heat, lipid solvents, non-ionic detergents, formaldehyde, oxidizing agents and UV irradiation. After incubating 24 hrs at 37°C an at least 10-fold decrease in virus infectivity has been observed with certain strains in tissue culture. Magnesium ions (1M) reduce the extent of heat inactivation in MHV. Some viruses in both genera are stable at pH 3.0.
NUCLEIC ACID
Virions contain a single molecule of linear, positive-sense, ssRNA which functions as an mRNA and is infectious. The genomic RNA is the largest viral RNA genome known ranging from 27.6 to 31 kb in size. The cap structure at the 5′-end of genome is followed by an UTR of 200-400 nt that includes a so-called leader sequence of 65-98 nt at the very 5′-end. At the 3′ end of the genome is another UTR of 200-500 nt followed by a poly(A) tail. RNA secondary structures, including a bulged stem-loop, a pseudoknot, or both (depending on the coronavirus group), are found in the 3′ untranslated regions of genomic and sgRNA. There may be from 6 to 14 ORFs of different sizes between 5′- and 3′-UTRs. The two 5′-most and overlapping ORFs comprise the replicase gene and each other ORF is a separate gene. The genes are arranged in the order 5′-replicase-(HE)-S-E-M-N-3′, with a variable number of other genes dispersed downstream of the replicase in different coronaviruses. The products of these genes (known as non-structural, ns) are believed not to be part of virions and they are largely non-essential, at least in tissue culture. A genome structure of a coronavirus is shown in Figure 2 .
Figure 2.

Representation of the genome of MHV as a coronavirus genome example. ORFs are represented by boxes. The proteins encoded by the ORFs are indicated; ORF1a encodes pp1a and, together with ORF1b, pp1ab polyproteins. The 5′ leader sequence is depicted by a small black box; poly(A) tail is indicated by An; M, membrane protein; N, nucleocapsid protein; S, spike protein; HE, hemagglutinin-esterase glycoprotein; I, internal ORF; no designated boxes, nonstructural proteins; the leader is indicated by a small black box; The arrow between ORF 1a and 1b represents the ribosomal frameshifting site.
The complete sequences of several coronavirus genomes have been determined and are accessible from international databases: BCoV, Human coronavirus strains 229E and OC43 (HCoV-229E and HCoV-OC43, respectively), IBV, MHV, Porcine epidemic diarrhea virus (PEDV), Severe acute respiratory syndrome coronavirus (SARS-CoV), TGEV.
PROTEINS
Virions contain a large surface glycoprotein (or spike, S), an integral membrane protein (M) that has three or four transmembrane segments, a small membrane protein (E) and a nucleocapsid protein (N) (Table 1 ). The ratios of S:E:M:N proteins vary in different reports. For TGEV, these ratios have been estimated to be 20:1:300:140, respectively. The S protein is large, ranging from 1160 to 1452 aa, and in some coronaviruses is cleaved into S1 and S2 subunits. The S protein is responsible for attachment to cells, hemagglutination, membrane fusion, and induction of neutralizing antibodies. Immunization with S alone can induce protection from challenge with some coronaviruses (IBV, MHV, TGEV). The S protein has a carboxy-terminal half with a coiled-coil structure and belongs to the class I virus fusion proteins. The M protein contains 221 to 260 aa and can induce a-interferon. Envelopes of most coronaviruses in group 2 (see below) also contain a hemagglutinin-esterase glycoprotein (HE) that forms short surface projections. This apparently nonessential protein has a receptor binding domain for 9-O-acetylated neuraminic acid, hemagglutination activity, as well as receptor destroying activities (neuraminate-O-acetylesterase). The E protein (76 to 109 aa), together with the M protein, plays an essential role in coronavirus particle assembly. No E protein (gene) has been identified in toroviruses. The N protein (377 to 455 aa) is a highly basic phosphoprotein that modulates viral RNA synthesis, binds to the viral RNA and forms a helical nucleocapsid.
Table 1.
Virion-associated proteins of coronaviruses.
| Protein | kDa | |
|---|---|---|
| Spike glycoprotein | S | 180 – 220 |
| Membrane protein | M | 23 – 35 |
| Nucleocapsid protein | N | 50 – 60 |
| Small-envelope protein | E | 9 – 12 |
| Hemagglutinin-esterase protein | HE | 65 |
a, apparent molecular mass estimated by electrophoresis (kDa)
A large variety of virus proteins known as non-structural proteins are not incorporated into virions. The largest non-structural protein is the essential replicase encoded by slightly overlapping ORF1a and ORF1b (gene 1), which accounts for two-thirds of the genome (from 18 to 22 kb). The replicase gene is predicted to encode two proteins of approximately 450 kDa (encoded by ORF1a) and 740-800 kDa (a fused product of ORF1a and ORF1b expressed through −1 frameshifting). Neither of these giant proteins has been observed in infected cells, and they appear to be co- and post-translationaly processed at the conserved junctions by viral proteases to form 15 to 16 mature replicase products and an unknown number of intermediates. A number of activities and functions has been provisionally assigned to most of the conserved replicase subunits by bioinformatics methods and some of these predictions were verified experimentally. Thus, replicase of viruses of Coronavirus genus includes (from the N-terminus to C-terminus): putative adenosine diphosphate-ribose 1”-phosphatase (ADRP), Zn-ribbon-dependent papain-like cysteine proteinase, a chymotrypsin-like cysteine protease with the substrate specificity resembling that of picornavirus 3C proteases (3C-like or main protease) that is flanked by two hydrophobic trans-membrane domains, a small cysteine-rich protein previously known as the growth-factor-related protein, a large RdRp, a protein including putative multinuclear Zn-finger-like domain and nucleoside triphosphate (NTP)-binding/5′-to-3′-helicase domain (HEL), putative Zn-finger-containing 3′-to-5′ exonuclease, putative poly(U)-specific endoribonuclease, and S-adenosylmethionine-dependent ribose 2′-O-methyltransferase (MTR). Coronaviruses also differ in respect to a small number of proteins and domains, which are derived from the N-terminus of the replicase. They include a distant copy of a Zn-ribbon-dependent papain-like cysteine proteinase and SARS-CoV unique domain (SUD) located in the largest replicase subunit nsp3 (also known as p195/p210).
The other non-structural proteins that are encoded downstream of the replicase gene vary in type and location among coronaviruses. The locations of the genes encoding these non-structural proteins are indicated in Figure 3 . These non-structural proteins are generally not essential for virus replication in tissue culture or in vivo, although (provisional) functional assignment was made only for two proteins including HE and putative cyclic phosphodiesterase (CPD).
Figure 3.

Genome organization in coronaviruses. ORFs are represented by boxes and indicated by numbers in, below or above the box except for the SARS CoV ORF 1a and 1b (see below). The names below or on top of the bars indicate the protein encoded by the corresponding ORF. The structural protein genes are marked by various symbols while the proteins of the nonstructuralgenesare not designated. The 5′ leader sequence is depicted by a small black box. Protein acronyms are as in figure 2. Δ, deleted. nsp, non-structural protein. The arrow between ORF 1a and 1b represents the ribosomal frameshifting site. HCoV-OC43 does not have the two non-structural proteins encoded by gene 4. The translation products of ORFs MHV 5a, BCoV 4a,b, FCoV and CCoV 3b have not been detected. ORFs TGEV 3b, FCoV and CCoV 3c, and HCV229E 3a,b are homologous. The 3a ORFs of TGEV, FCoV and CCoV are homologous. ORFs TGEV 7 and the 7a of FCoV and CCoV are homologous. Genetic structure of SARS-CoV: the numbers above ORF 1a and 1b indicate the putative proteolytic cleavage products; the red arrowheads depict the papain-like cysteine protease cleavage sites and the blue arrowheads represent the 3C-like cysteine protease cleavage sites.
LIPIDS
Virions have lipid-containing envelopes derived from the host cell. The S protein (BCoV, MHV) and E protein (MHV) of coronaviruses are acylated.
CARBOHYDRATES
The S and HE proteins contain N-linked glycans, the S protein being extensively glycosylated. The M protein of coronaviruses contains a small number of either N- or O-linked glycans, depending on the virus or strain.
GENOME ORGANIZATION AND REPLICATION
Coronavirus replication proceeds through the synthesis of the full-length negative antigenome in the cytoplasm of infected cells. It is catalyzed by a poorly characterized membrane-bound replicative complex. The products of the ORF1a and ORF1ab are sufficient to maintain the coronavirus RNA synthesis. These ORFs encode a variety of (putative) enzymes (see section on proteins) that may be part of the replicative complex per se or control its composition and functioning.
During the synthesis of positive- and negative-strand RNAs MHV undergoes recombination at very high frequency. A lower recombination frequency has been described for IBV and TGEV. Interspecies recombination limited to viruses of the same group, e.g. between Feline coronavirus (FCoV) and Canine coronavirus (CCoV), has also been documented.
The genome is expressed by different mechanisms that includes replication, transcription, translation and co- and post-translation regulation. The virion RNA functions as the mRNA1 for the two 5′-most ORF1a and ORF1b encoding replicase components. The translation of ORF1a yields the pp1a polyprotein. In approximately 20% of cases, ribosomes slip at the overlap between the ORF1a and ORF1b regions, containing a specific seven-nucleotide “slippery” sequence and a pseudoknot structure (ribosomal frameshifting signal), to continue translation into ORF1b, which is in the −1 frame relative to ORF1a. Translation of ORF1ab yields the pp1ab polyprotein. The pp1a and pp1ab polyproteins have not been described in infected cells; they are believed to be co- and post-translationally processed by two or three virus encoded proteases to 15 or 16 mature replicase subunits and an unknown number of functional intermediates. There is no universally accepted nomenclature of replicase subunits; they may be listed in the order of encoding in polyproteins, or according to the molecular size or function. For SARS-CoV a nomenclature is proposed as indicated in Figure 3. One or two papain-like proteases (PLpro) control processing of the N-terminal part of pp1a/pp1ab polyproteins at two or three conserved cleavage sites. A chymotrypsin-like protease (3CLpro or Mpro) is responsible for processing of the rest part of pp1a/pp1ab polyproteins at 11 conserved cleavage sites.
All ORFs downstream of ORF1b are expressed from a 3′-coterminal nested set of specialized sgRNAs whose number varies between 5 and 7 among coronaviruses (Fig. 4 ). Subgenomic mRNAs were originally designated with numbers from 2 to 7, in order of decreasing size. Some later discovered mRNAs have been given a hyphenated name, e.g. mRNA2-1, to show that they are variants of previously recognized mRNAs. Except for the smallest mRNA, all of the mRNAs are structurally polycistronic. As a rule, only the 5′-most ORF of each mRNA is translated while downstream ORF(s) remain translationally silent. However, there are exceptions which, in addition to the expression of ORFs 1a and 1b from the same RNA detailed above, include 2nd and 3rd ORFs of some mRNAs, e.g. mRNA5 of MHV, mRNA3 of IBV and mRNA encoding nucleocapsid of BCoV. These ORFs may be translated by internal, as well as 5′-terminal, initiation to produce from two to three proteins from a single sg mRNA.
Figure 4.

Structural relationship between mRNAs and the genomic RNA of coronaviruses. Thick lines represent the translated sequences. Thinner lines, untranslated sequences. The names below the boxes indicate the proteins encoded by the corresponding genes. Acronyms are explained in the legend to Figure 2.
Coronavirus mRNAs have another unique structural feature: their 5′-ends have a leader sequence of approximately 65 to 98 nt, which is identical to the 5′-end of the genomic RNA. At the mRNA start sites on the viral genomic RNA, there is a short stretch of sequence that is an imperfect repeat of the 3′-end of the leader RNA. This sequence constitutes part of the signal for sgRNA transcription.
The synthesis of coronavirus sgRNAs occurs in the cytoplasm at negative-stranded template(s) through a complex mechanism for which essential details remain a matter of debate. Both genome-size and negative-strand sgRNAs, which correspond in number of species and size to those of the virus-specific mRNAs, have been detected in infected cells. The 5′-end of the negative-strand RNA contains short stretches of oligo(U). The negative-strand sgRNAs appear to be complementary copies of the positive-strand sgRNAs. An almost perfect repeat of a sequence stretch known as the consensus or core sequence, which is UCUAAAC in MHV or a related sequence for other coronaviruses, was found immediately upstream of gene (ORFs) in the coronavirus genome. These sequences represent crucial signals for the synthesis of sgRNAs and they are also called transcription regulating sequences (TRS) (previously designated as intergenic sequences, IGSs). The 5′-most TRS is at the 3′-border of the leader sequence (leader TRS) that is common for all mRNAs including genomic RNA. Several models have been proposed to explain a wealth of experimental data and, particularly, how the common leader sequence is fused to different bodies in the variety of mRNAs. These models are not mutually exclusive, as components of each model may operate at different stages of the viral replication cycle. The model compatible with most of the experimental data is discontinuous transcription during negative-strand RNA synthesis. This model proposes that the discontinuous transcription step occurs during negative-strand RNA synthesis, generating negative-strand sgRNAs, which then serve as templates for uninterrupted synthesis of sg mRNAs. In this model, the body TRSs on the genomic RNA serve as termination or pausing signals for negative-strand synthesis, and the nascent negative-strand sgRNA then jumps to the leader TRS sequence at the 5′-end of the genomic RNA to complete the synthesis of the negative copy of sg mRNA. In the alternative model, the leader-primed transcription model proposes that the virion genomic RNA is first transcribed into a genomic-length, negative-strand RNA, which, in turn, becomes the template for subsequent synthesis of the entire repertoire of sg mRNAs. The leader of nascent sg mRNA is transcribed from the 3′-end of the negative-strand RNA and dissociates from the template to subsequently associate with the template RNA at one of the (body) TRSs to prime the synthesis of the particular sg mRNA. In this mechanism, the discontinuous transcription step takes place during positive-strand RNA synthesis. The composition of the machinery responsible for the synthesis of sgRNAs (transcriptase) is unknown, although many replicase subunits described above must be involved in transcription.
The packaging signal for MHV RNA, as determined using defective minigenomes, is localized near the 3′-end of gene 1. This packaging signal forms a stem loop and is sufficient for the packaging of DI RNA or heterologous RNAs into virions. In contrast, the packaging signal of TGEV and IBV has been localized within the first 650 nt of gene 1.
The assembly of virus particles probably starts with the formation of an RNP that interacts with components of the core shell. Virions mature in the cytoplasm by budding through the endoplasmic reticulum and other pre-Golgi membranes. The interaction between the M and E proteins appears to be a key event for virus particle assembly. The S and HE proteins are not necessary for virus particle formation though the S protein is essential for infectivity.
Coronavirus reverse genetics has been facilitated by targeted recombination, the construction of infectious cDNA clones using different strategies: as bacterial artificial chromosomes, using poxviruses as cloning vectors, and as independent fragments that are assembled in vitro.
ANTIGENIC PROPERTIES
Strong humoral immune responses are elicited by the structural proteins S, M, N, and, when present, HE. The S and HE proteins are the predominant antigens involved in virus neutralization. Reduction of infectivity with anti-M antibodies also has been shown, but generally in the presence of complement. Protection against coronavirus infections (IBV, MHV, TGEV) is provided by S protein that has been affinity-purified or expressed by recombinant adenovirus. N- and M-specific antibodies also give some protection in vivo. The most efficient induction of virus neutralizing antibodies has been achieved with a combination of S and N proteins. The globular portion of the S protein contains many dominant antigenic sites targeted by the humoral immune response and also by cytotoxic T lymphocytes. Other important antibody epitopes are also found in the stem portion, at least for MHV. Both the amino- and carboxy-termini of the M protein elicit strong immune responses. While neutralizing antibodies can prevent disease if present prior to infection, cytotoxic T cell responses are important in virus clearance. Hypervariable domains in the S1 portion of the S protein facilitate the selection of virus escape mutants that evade both humoral and cellular immune responses. N protein also elicits a protective cellular immune response.
The immune system is known to play a major role in the pathogenesis of some coronavirus-induced diseases, such as the experimental induction of demyelination by MHV, and the antibody-dependent enhancement of disease (feline infectious peritonitis) caused by FCoV.
BIOLOGICAL PROPERTIES
Coronaviruses infect birds and many mammals, including humans. The respiratory tract, gastrointestinal tractand neurological tissues are the most frequent targets of coronaviruses, but other organs including liver, kidney, heart, and eye can also be affected. Epithelial cells are the main target of coronaviruses, plus, with some coronavirus species, widely distributed cells such as macrophages. Coronaviruses have a wider host range in vivo than would be expected from in vitro studies. In experimental infections BCoV caused enteric disease in turkeys. FCoV and CCoV replicated in experimentally infected pigs, clinical disease being caused by virulent FCoV. Turkey coronavirus has been demonstrated to replicate in chickens asymptomatically. Type II FCoV has arisen by natural recombination of type I FCoV with CCoV. The cause of severe acute respiratory syndrome (SARS) in man is a coronavirus that is believed to have jumped from another animal species. BCoV and some isolates of HCoV-OC43 have >99% aa identity in their S and HE proteins, raising the possibility of a shared host range in vivo. Leader-switching between a defective RNA of BCoV and HCoV-OC43 virus has been demonstrated, and the BCoV defective RNA could be replicated by the replicative machinery of HCoV-OC43 and other members of coronavirus Group 2, suggesting a potential for recombination between members of Group 2. Biological vectors are not known. Respiratory, fecal-oral and mechanical transmission are common.
Although coronaviruses may bind cells through ubiquitous acetylated forms of glycoproteins and lipids, a more specific binding between the virus and a cellular receptor is required for the establishment of viral infection. Coronaviruses are divided into three groups called 1, 2 and 3 (see below). MHV in Group 2 (see below), uses as receptors CEACAM1 glycoproteins in the immunoglobulin superfamily. SARS-CoV uses angiotensin converting enzyme 2 (ACE2) as a receptor. Members of the group 1 (see below) coronaviruses (including TGEV, FCoV and HCoV-229E) use aminopeptidase N (APN or CD13) as a receptor for cell entry. Sialic acid (N-acetyl-9-O-acetylneuraminic acid)-containing glycoproteins are probably a component of the cell surface molecules required for BCoV and HCoV-OC43 infectivity, and oc2,3-linked sialic acid in the case of the Group 3 coronavirus IBV. Nevertheless, binding to CEACAM1 and APN receptors does not explain the differences in coronavirus tropism. In addition to the binding to the aforementioned receptors, a second factor mapping in the S protein (possibly, a second receptor binding site) has been implicated in coronavirus tropism.
Infections of man and animals by coronaviruses seem to be ubiquitous, as evidence of infection has been obtained in every country where serological or virological studies have been done.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Practically, species in the Coronavirus genus have been differentiated using four parameters: (i) the position and variety of the non-essential non-structural proteins in the 3′-part of genome, (ii) antigenic properties, (iii) the processing of the S protein into two halves (S1 and S2), and (iv) the host range. No rigorous guidelines have been developed to apply the above criteria for defining new coronavirus species or differentiating between existing species. A brief posteriori analysis of the coronavirus species diversity in respect to these criteria is summarized below.
The number, type and location of the non-essential genes (encoding non-structural proteins) significantly vary. Some of these genes are common for a set of closely related viruses, e.g. CPD and HE genes in MHV, BCoV and HCoV-O43, and others may be specific for a virus species, e.g. all unique ORFs in SARS-CoV. Importantly, different isolates of the same coronavirus may also differ in respect to certain 3′-located ORFs, e.g. ORF8a and ORF8b are fused in a single ORF in some human vs. animal isolates of SARS-CoV. Serological characterization allows placing a virus within an antigenic cluster and nucleic (amino) acid similarity within a genetic cluster or group; the characterized coronaviruses form at least 3 genetic clusters or groups (see below). All coronaviruses fall in one of two divisions depending whether the S protein is processed into S1 and S2 subunits or not. Those with uncleaved S protein include Group 1 coronaviruses (CCoV, FCoV, HCoV229E, PEDV and TGEV) and Group 2, SARS-CoV, and those with the cleaved S protein include species of Groups 2 (BCoV, MHV and HCoV-OC43) and 3 (IBV). Coronaviruses infect a wide range of mammals and distantly related coronaviruses may infect the same host, e.g. HCoV-229E, HCoV-O43 and SARS-CoV, from different groups of coronaviruses, all infect humans. The group 3 consists exclusively of avian coronaviruses. Future revision of the taxonomy of the family Coronaviridae may propose a quantitative measure of genome similarity or other criterion to discriminate coronavirus species rigorously.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Group 1 species | ||
| Canine coronavirus | ||
| Canine coronavirus | [DL3096] | (CCoV) |
| Feline coronavirus | ||
| Feline coronavirus | (FCoV) | |
| Feline infectious peritonitis virus | (FIPV) | |
| Human coronavirus 229E | ||
| Human coronavirus 229E | [X69721] | (HCoV-229E) |
| Porcine epidemic diarrhea virus | ||
| Porcine epidemic diarrhea virus | [Z35758] | (PEDV) |
| Transmissible gastroenteritis virus | ||
| Transmissible gastroenteritis virus | [Z24675, Z34093, D00118, X06371] | (TGEV) |
| Porcine respiratory coronavirus | (PRCoV) | |
| Group 2 species | ||
| Bovine coronavirus | ||
| Bovine coronavirus | (BCoV) | |
| Human coronavirus OC43 | ||
| Human coronavirus OC43 | (HCoV-OC43) | |
| Human enteric coronavirus‡ | ||
| Human enteric coronavirus | (HECoV) | |
| Murine hepatitis virus | ||
| Murine hepatitis virus | [AF029248] | (MHV) |
| Porcine hemagglutinating encephalomyelitis virus | ||
| Porcine hemagglutinating encephalomyelitis virus | (HEV) | |
| Puffinosis coronavirus‡ | ||
| Puffinosis coronavirus | (PCoV) | |
| Rat coronavirus | ||
| Rat coronavirus | (RtCoV) | |
| Sialodacryoadenitis virus, | (SDAV) | |
| Severe acute respiratory syndrome coronavirus | ||
| Severe acute respiratory syndrome coronavirus | (SARS-CoV) | |
| Group 3 species | ||
| Infectious bronchitis virus | ||
| Infectious bronchitis virus | [M95169] | (IBV) |
| Pheasant coronavirus‡ | ||
| Pheasant coronavirus | (PhCoV) | |
| Turkey coronavirus | ||
| Turkey coronavirus | (TCoV) | |
TENTATIVE SPECIES IN THE GENUS
| Rabbit coronavirus | (RbCoV) |
GENUS TOROVIRUS
Type Species Equine torovirus
DISTINGUISHING FEATURES
RNAs and ORFs are summarized in Figure 6 .
Figure 6.

Genome organization structure in toroviruses. ORFs are represented by boxes. The proteins encoded by the ORFs are indicated. ORF1a encodes pp1a and, together with ORF1b, pp1ab polyproteins. Poly(A) tails are indicated by An; M, membrane protein; N, nucleocapsid protein; S, spike protein; HE, hemagglutinin-esterase glycoprotein.
The nucleocapsid has a tubular appearance and virions are disc-, kidney- or rod-shaped (Fig. 5 ). There are 4 structural proteins: N, M, S and HE. The later protein is dispensable for replication in vitro; in Berne virus (BEV) most of the HE gene has been deleted and only the 3′ 426 nt are present (Table 2 ). The toroviral and coronaviral HE proteins share 30% sequence identity and display a similar degree of sequence relatedness with subunit 1 of the HE fusion protein of Influenza C virus. Although the nature of the presumed gene acquisition is uncertain, toroviruses and coronaviruses appear to have acquired their HE gene through independent heterologous RNA recombination events. Toroviruses seem to combine discontinuous and non-discontinuous transcription to produce their set of sgRNAs. In the case of EToV, sgRNAs 3 through 5 lack a leader; mRNA-2, however, carries a short 15-18 nt extension at its 5′-end, which is derived from the very 5′-end of the genome.
Figure 5.

Torovirus particle structure. (Top) Schematic representation of the architecture of a particle of Equine torovirus (EToV). The localization of the structural proteins and genome are indicated. S, spike protein; M, large membrane protein; HE, hemagglutinin-esterase; N, nucleocapsid protein. (Bottom left) Negative contrast electron micrograph of EToV Berne strain particles. (Bottom right) Different forms of EToV particles in ultrathin sections of EoTV-infected equine dermis cells. The bar represents 100 nm.
Table 2.
Virus-associated proteins of toroviruses.
| Protein | kDa | |
|---|---|---|
| Spike glycoprotein | S | 200 |
| Membrane protein | M | 27 |
| Nucleocapsid protein | N | 19 |
| Small-envelope protein | E | nk |
| Hemagglutinin-esterase protein | HE | 65 |
aapparent molecular weight estimated by electrophoresis (kDa)
nk, presence not known.
VIRION PROPERTIES
MORPHOLOGY
Toroviruses are pleomorphic and measure 120 to 140 nm at their largest diameter. Spherical, oval elongated and kidney-shaped particles are observed. The two most conspicuous features of toroviruses are the double fringe of small and large spikes on the envelope, the latter of which resemble the peplomers of coronaviruses, and the tubular nucleocapsid of helical symmetry, which appears to determine the shape of the virion. Toroviruses are enveloped viruses; an isometric core shell has not been identified, in contrast to coronaviruses.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Buoyant densities of 1.16, 1.18, and 1.14 g/cm3 in sucrose were determined for EToV, BToV serotype 2 and HToV, respectively.
NUCLEIC ACID
The EToV genome is 28 kb in length, capped and polyadenylated. It contains six ORFs. The 5′-UTR includes 820 nt and 3′-UTR encompasses 200 nt, excluding the poly(A) tail. The characteristics of EToV genome RNA and ORFs are summarized in Figure 6. The full genome sequence has not yet been reported for any torovirus.
PROTEINS
In EToV strain Berne, four structural proteins have been identified: a 180 kDa spike (S) protein of 1581 aa (which is post-translationaly cleaved into two subunits, S1 and S2), a 26 kDa integral membrane (M) protein of 233 aa, a HE of 65 kDa and a 19 kDa N protein of 160 aa. The S protein has an N-terminal signal sequence, a putative C-terminal transmembrane anchor, two putative heptad-repeat domains, and a possible cleavage site for a “trypsin-like” protease. The M protein is unglycosylated, accounts for about 13% of the virion protein mass and does not contain an N-terminal signal sequence. The HE is a class I membrane protein displaying 30% sequence identity with the HE of coronaviruses and influenza C viruses. The N protein is the most abundant structural protein of the EToV particle, accounting for about 80% of its protein mass. It is a phosphorylated protein with RNA-binding properties.
LIPIDS
Virions contain lipid bilayer envelopes.
CARBOHYDRATES
The torovirus S and HE proteins are N-glycosylated, carrying up to 24 and 11 potential N-glycosylation sites, respectively. The M protein is not glycosylated.
GENOME ORGANIZATION AND REPLICATION
The first two ORFs 1a and 1b from the 5′-end are translated from genomic RNA, to yield two large polyproteins, pp1a and pp1ab. These are believed to be proteolytically cleaved to yield the mature proteins from which the viral replicase/transcriptase is composed. The ORF1a initiation codon is located at nt position 821-823 in the genome. The four remaining ORFs 2, 3, 4, and 5 have been identified as structural genes (Fig. 6), and are expressed by the generation of a 3′-coterminal nested set of 4 sg mRNAs (Figure 7 ).
Figure 7.

Structural relationship between sgRNAs and the genomic RNA of toroviruses. Thick lines represent the translated sequences, thinner lines, untranslated sequences. The names below the boxes indicate the proteins encoded by the corresponding genes. The 5′ leader sequence is depicted by a small black box; poly(A) tail is indicated by An; M, membrane protein; N, nucleocapsid protein; S, spike protein; HE, hemagglutinin-esterase glycoprotein; the leader is indicated by a small black box.
Sequence analysis of EToV and BToV has shown that the genes for M, HE and N are preceded by a conserved AU-rich sequence (5′AC-N2-3-UCUUUAGA3′), which is also present at the very 5′-end of the genome. Although this sequence resembles the transcription-regulating sequences (TRSs) in coronaviruses, there is no evidence for fusion of a common leader to mRNAs 3, 4 and 5. These mRNAs are produced via a non-discontinuous transcription mechanism with transcription-initiation occurring at the AC-dinucleotide preceeding the UCUUUAGA core sequence. This appears to be an important difference between toroviruses and coronaviruses. In terms of transcription, however, the consequences of this dissimilarity between toro- and coronaviruses may be limited: whereas in coronaviruses the TRSs may act as a site for homology-assisted RNA recombination during subgenomic negative strand synthesis to add an anti-leader, the conserved intergenic sequences in toroviruses may act as a termination signal. Subgenomic mRNA synthesis would then occur via direct binding of the polymerase to the torovirus “core promoters” at the 3′-ends of the subgenomic negative strand templates. It is of note, that one subgenomic EToV mRNA, mRNA2 apparently is produced via discontinuous transcription during which a 15-18 nt “leader” derived from the 5′-end of the genome is added. Hence, toroviruses seem to use a combination of discontinuous and non-discontinuous RNA synthesis to produce their set of sgRNAs.
Evidence for two independent non-homologous recombination events during torovirus evolution have been obtained. The first putative recombination involves ORF 4, the HE gene. The second putative recombination site involves the C-terminus of the EToV ORF 1a, which contains 31 to 36% identical aa residues compared with the N-terminal 190 aa of the 30-32 kDa non-structural 2A protein (CPD) of coronavirus.
In addition to the products of ORFs 2, 3, 4, and 5, which are assumed to be synthesized by monocistronic translation of a nested set of structurally polycistronic mRNAs, the 3′ part of the EToV genome may encode one more protein in ORF5, which completely overlaps 264 nt with N gene and potentially encodes a hydrophobic 10 kDa protein. Although no such protein has been observed in virions or EToV-infected cells, it is interesting to note that a similar situation, a small hydrophobic protein expressed from an ORF that completely overlaps with the N protein gene, has been reported for the BCV.
The composition of a 1 kb DI genome in a replication competent virus suggests that the minimal sequences required for EToV RNA replication (and probably also for packaging) are located in two small domains present at the termini of the genomic RNA. This suggests a difference with members of the genus coronavirus.
Extensive N-glycosylation and proteolytic cleavage of the precursor are part of the posttranslational processing of the torovirus S protein. The EToV M protein accumulates in intracellular membranes and is thought to play a role in budding through intracellular membranes.
ANTIGENIC PROPERTIES
The S protein is recognized by neutralizing and hemagglutination-inhibiting monoclonal antibodies.
BIOLOGICAL PROPERTIES
The BToV has been identified as a pathogen causing gastroenteritis in calves and possibly pneumonia in older cattle. BToV infections are usually limited to the gut, although the respiratory system may be sporadically involved. No disease has been associated to EToV. Serological evidence indicates that it infects ungulates (horses, cattle, sheep, goats, pigs), rats, rabbits, and some species of feral mice. Torovirus-like particles have been detected by electron microscopy in humans, dogs and cats. The torovirus-like particles found in humans cross-reacts antigenically with BToV and sequence similarity. No antibody to toroviruses has been found in the sera of cats. Infections by BToV are also quite common in dairy cattle. The presence of maternal antibodies in calves does not prevent infection, but may modify the outcome of the disease. Infections by BToV appear to be ubiquitous, as evidence of infection has been obtained in every country where serological or virological studies have been done: Western Europe, North America, India, South Africa, and New Zealand. Torovirus infects the epithelial cells lining the small and large intestine, with progression from areas of the midje junum down to the ileum and colon. Within the small intestine, cells of the upper third of the crypt and the epithelium overlying the Peyer's patches, including M cells, are also infected. Chronic torovirus infections may also occur.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
So far, only limited molecular genetic information is available for toroviruses. Comparative sequence analysis of the structural protein genes of a set torovirus field variants identified three distinct genotypes, displaying 20 to 40% divergence. These are exemplified by BToV Breda strain, PToV Markelo strain and EToV Berne strain. Human torovirus, for which only the HE gene has been characterized, may represent a fourth genotype.
The bovine and porcine toroviruses apparently display host species preference. In phylogenetic analyses, all PToV variants cluster, while the extant European BToVs mostly resemble the New World BToV variant Breda, identified 19 years ago. However, there is evidence for recurring intergenotypic recombination. All newly characterized European BToV variants seem to have arisen from a genetic exchange, during which the 3′-end of the HE gene, the N gene, and the 3′ -UTR of a Breda virus-like parent had been swapped for those of PToV. Moreover, some PToV and BToV variants carried chimeric HE genes, which apparently resulted from recombination events involving hitherto unknown toroviruses. From these observations, the existence of two additional torovirus genotypes can be inferred.
Sequencing of C-terminus of the N gene and the 3′-UTR have shown > 93% identity between HToV, BToV and EToV. Nevertheless, small but consistent sequence differences were noted among five HToV isolated and EToV. The published HToV HE sequence is most related to that of BToV variant Breda (83%), and more distantly related to those of the European BToV strains (∼73%) and PToV strain (∼56%).
BToV, PToV and HToV cause gastroenteritis and the BToV sporadically infects the respiratory system, in contrast to EToV that has remained as a virus in search of a disease.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Bovine torovirus | ||
| Bovine torovirus | [Y10866] | (BToV) |
| Breda virus | (BRV) | |
| Equine torovirus | ||
| Berne virus | (BEV) | |
| Equine torovirus | (EToV) | |
| Human torovirus | ||
| Human torovirus | (HToV) | |
| Porcine torovirus | ||
| Porcine torovirus | (PToV) | |
TENTATIVE SPECIES IN THE GENUS
Not reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
None reported.
DISTINGUISHING FEATURES BETWEEN CORONAVIRUSES AND TOROVIRUSES
Coronavirus mRNAs contain a 5′ leader sequence, which is acquired via discontinuous RNA transcription, most likely during (-)strand RNA synthesis. In contrast, the torovirus mRNAs seem to be produced mainly via a non-discontinuous transcription mechanism: subgenomic mRNAs 3 through 5 of EToV strain Berne do not possess a leader. EToV mRNA2, however, contains a short 15-18 nt sequence at its 5′end, which apparently is derived from the 5′-end of the genome. Coronaviruses have a loosely wound helical nucleocapsid protected by a core shell while the toroviruses have a more rigid tubular nucleocapsid. All torovirus field strains studied so far express an HE protein; of the coronaviruses only those belonging to group 2 encode an HE protein. The N protein is much larger in coronaviruses than in toroviruses. The M protein is glycosylated only in coronaviruses. Toroviruses do not have a counterpart to the E protein and direct the synthesis of fewer subgenomic mRNAs than any coronavirus. A putative CPD was mapped to the very C-terminus of pp1a in a torovirus but it is encoded immediately downstream of ORF1b in some coronaviruses. Upon comparison of every pair of homologous protein, toroviruses and coronaviruses do not interleaved and form separate groups.
Table 3.
Features of coronaviruses and toroviruses.
| Feature | Coronavirus | Torovirus |
|---|---|---|
| Enveloped virions | + | + |
| Core shell | + | – |
| Nucleocapsid architecture | helical | tubular |
| Prominent spikes | + | + |
| Coiled-coil structure in spikes | + | + |
| M protein with three membrane-spanning sequences | + | + |
| Intracellular budding | + | + |
| Linear positive-sense ssRNA genome with poly (A) tail | + | + |
| Genome size (kb) | 27–31 | ∼25 |
| The genome organization includes 5′-UTR-replicase gene-structural protein genes-UTR 3′ | + | + |
| An −1 ribosomal frameshifting in the replicase gene | + | + |
| Transcriptome includes 3′ co-terminal nested set of ≥ 4 sgRNAs | + | + |
| Only 5′-most one or two ORFs of mRNAs are translationally active | + | + |
| 5′ leader sequence in mRNAs | + | +/− |
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
On the basis of antigenic cross-reactivity, coronaviruses were classified into three groups, known as groups 1, 2 and 3. This classification was corroborated by phylogenetic analysis of virion proteins as well as replicase proteins that do not contribute to the virion antigenicity. Subsequent expansion of the coronavirus groups with new species was made using a phylogenetic criterion rather than results of serological characterization. Consequently, Group 1 coronaviruses include three distinct antigenic clusters, one formed by TGEV, CCoV and FCoV, and two others formed by HCoV229E and PEDV, respectively. The phylogenetic position of the recently identified SARS-CoV was subject of several studies all of which found it not to be closely related to any of previously established coronaviruses. This prompted placing SARS-CoV in a separate 4th group. However, in an analysis dealing with the most conserved pp1b part of replicase and involving a torovirus outgroup, SARS-CoV confidently clusters together with group 2 species as the most distant member. This clustering was also observed in studies of several other proteins including pp1a replicase domains and virion S, M, and N components.
Most closely related coronaviruses (members of the same antigenic group) share also a common pattern of the accessory non-structural genes in the 3′-end of genome that distinguish them from other coronaviruses. Such unique ORFs, encoding CPD and HE, were described for closely related MHV, BCoV and HCoV-O43 of group 2. They are absent in SARS-CoV, which has 5 (4) unique ORFs. Likewise, in diverse group 1, different patterns of accessory genes have been described for distant viruses. These observations argue that the 3′-located accessory ORFs may be reliable markers for antigenic clusters rather than for the evolutionary groups. In contrast, group-specific genetic markers were recently identified in the N-terminal cleavage products nsp1 and nsp3 of pp1a/pp1ab replicase. According to this criterion, SARS-CoV belongs to the coronavirus group 2.
Figure 8.

Phylogenetic relationship of the large-surface glycoprotein of coronaviruses and toroviruses. Amino acid sequences were aligned using the “clustal” method and phylogenetic trees were constructed using the neighborhood-joining method. The analyses were done using the MegAlign module of the Lasergene software suite (DNASTAR). The phylogenies are rooted assuming a biological clock (Siddell, 1995).
The intra-group diversity in coronaviruses is comparable to that found between viruses of different genera in other RNA virus families, e.g. Picornaviridae. A proposal was put forward to accept the evolutionary coronavirus groups as the basis for the three new genera in the family Coronaviridae.
Immunological evidence has shown that equine and bovine torovirus are antigenically related to each other, and to torovirus-like particles found in human fecal specimens but not to other animal viruses, including coronaviruses. The available sequences also show that all known toroviruses are closely related, although the actual phylogenetic diversity of toroviruses remains unknown. In a broad comparative sequence analysis aimed at a revision of the taxonomy of the family Coronaviridae, toroviruses proved to be well separated from coronaviruses with which they share some characteristics not found in two other families of the order Nidovirales. Among these unique shared characteristics are distantly related S and M proteins. If coronavirus groups are to be elevated to the genera ranks, a special distant torovirus-coronavirus relationship could be recognized through assigning the subfamily ranks to the coronaviruses and toroviruses within the family Coronaviridae. Future revision of the Coronaviridae family taxonomy must address this issue.
SIMILARITY WITH OTHER TAXA
The family Coronaviridae together with the Arteriviridae and the Roniviridae families form the order Nidovirales. The viruses of these families share the genome organization, expression strategy and have a similarly organized replicase gene. Non-nidovirus homologs of several (putative) enzymes encoded by viruses of the family Coronaviridae have been found in other RNA viruses. The proteolytic enzymes and RdRps cluster together with homologs of viruses of “picorna-like” supergroup. The ADRP and Hel enzymes have counterparts in viruses of the “alphavirus-like” supergroup. Mtr has homologs in alphaviruses, flaviviruses, and mononegavirales. CPD has homologs among some rotaviruses. HE has homologs in the Influenza C viruses. Also some parallels in the genome organization and expression strategy are evident between members of the Coronaviridae and Closteroviridae families.
DERIVATION OF NAMES
Corona, from the Latin corona for “crown”, representing the appearance of surface projections in negatively-stained electron micrographs of members of the Coronavirus genus.
Toro, from the Latin torus, “lowest convex moulding in the base of a column”, representing the toroviruses nucleocapsid shape.
REFERENCES
- Cavanagh D., Brian D.A., Briton P., Enjuanes L., Horzinek M.C., Lai M.M.C., Laude H., Plagemann P.G. W., Siddell S., Spaan W., Talbot P.J. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;145:629–635. [PubMed] [Google Scholar]
- Cavanagh D., Mawditt K., Welchman D., de Britton P.B., Gough R.E. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathology. 2002;31:81–93. doi: 10.1080/03079450120106651. [DOI] [PubMed] [Google Scholar]
- Cornelissen L.A.H.M., Wierda C.M.H., Van Der Meer F.J., Herrewegh A.P.M., Horzinek M.C., Egberonk H.F., Groot R.J. Hemagglutinin-esterase, a novel structural protein of torovirus. J. Virol. 1997;71:5277–5286. doi: 10.1128/jvi.71.7.5277-5286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J.M., Gomez-Puertas P., Cavanagh D., Gorbalenya A.E., Enjuanes L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch. Virol. 2003;148:2207–2235. doi: 10.1007/s00705-003-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E. Big nidovirus genome: when count and order of domains matter. Adv. Exp. Med. Biol. 2001;494:1–17. [PubMed] [Google Scholar]
- De Vries A.A.F., Horzinek M.C., Rottier P.J.M., De Groot R.J. The genome organization of the Nidovirales: similarities and differences between arteri-, toro-, and coronaviruses. Sem. Virol. 1997;8:33–47. doi: 10.1006/smvy.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escors D., Capiscol M.C., Enjuanes L. Location of transmissible gastroenteritis coronavirus encapsidation signal (*P) J. Virol. 2003;77:7890–7902. doi: 10.1128/JVI.77.14.7890-7902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Siddell S.G., Spaan W.J., editors. Coronaviruses and Arteriviruses. Plenum Press; New York: 1998. [Google Scholar]
- Ismail M.M., Tang Y., Saif Y.M. Pathogenicity of turkey coronavirus in turkeys and chickens. Avian Diseases. 2003;47:515–522. doi: 10.1637/5917. [DOI] [PubMed] [Google Scholar]
- Lai M.M. C., Cavanagh D. The molecular biology of coronaviruses. Adv. Vir. Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C., Holmes K.V. Coronaviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. Lippincott, Williams & Wilkins; Philadelphia, Pa: 2001. pp. 1163–1185. [Google Scholar]
- Sawicki S.G., Sawicki D.L. Coronaviruses use discontinuous extension for synthesis of subgenome-length negative strands. Adv. Exp. Biol. Med. 1995;380:499–506. doi: 10.1007/978-1-4615-1899-0_79. [DOI] [PubMed] [Google Scholar]
- Sethna P.B., Hung S.-L., Brian D.A. Coronavirus subgenomic minus-strand RNAs and the potential for mRNA replicons. Proc. Natl. Acad. Sci. USA. 1989;86:5626–5630. doi: 10.1073/pnas.86.14.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S.G., Fraenkel-Conrat H., Wagner R.R., editors. The Coronaviridae. Series: The Viruses. Plenum Press; New York: 1995. [Google Scholar]
- Smits S.L., Lavazza A., Matiz K., Horzinek M.C., Koopmans M.P., de Groot R.J. Phylogenetic and evolutionary relationships among torovirus field variants: evidence for multiple intertypic recombination events. J. Virol. 2003;77:9567–9577. doi: 10.1128/JVI.77.17.9567-9577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Horzinek M.C. The molecular biology of toroviruses. In: Siddell S.G., editor. The Coronaviridae. Plenum Press; New York: 1995. pp. 219–238. [Google Scholar]
- Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L.M., Guan Y., Rozanov M., Spaan W.J.M., Gorbalenya A.E. Unique and conserved features of genome and proteome of SARS-Coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet A.L., Smits S.L., Rottier P.J., de Groot R.J. Discontinuous and non-discontinuous subgenomic RNA transcription in a nidovirus. EMBO J. 2002;21:6571–6580. doi: 10.1093/emboj/cdf635. [DOI] [PMC free article] [PubMed] [Google Scholar]
ARTERIVIRIDAE
CONTRIBUTED BY, E.J. Snijder, M.A. Brinton, K.S. Faaberg, E.K. Godeny, A.E. Gorbalenya, N.J. MacLachlan, W.L. Mengeling, P.G.W. Plagemann
FAMILY ARTERIVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Order | Nidovirales |
| Family | Arteriviridae |
| Genus | Arterivirus |
Since only one genus is currently recognized, the family description corresponds to the genus description
GENUS ARTERIVIRUS
Type Species Equine arteritis virus
VIRION PROPERTIES
MORPHOLOGY
Arteriviruses are spherical particles with a diameter of 45 to 60 nm. Virions consist of an isometric nucleocapsid of about 25 to 35 nm in diameter, surrounded by a lipid envelope (Fig. 1 ). No spikes are obvious on the virion surface, but a surface pattern of relatively small and indistinct projections has been observed.
Figure 1.

Structure of arterivirus virions. (Upper right) Electron micrograph of negatively stained particles of Porcine reproductive and respiratory syndrome virus (PRRSV). Bar is 50 nm. (Lower right) Electron micrograph of Equine arteritis virus (EAV) particles, budding from smooth intracellular membranes (BHK-21 cells). The bar represents 50 nm. (Left) Schematic representation of an arterivirus particle. Seven virion-associated proteins have been identified in EAV and PRRSV virions. In addition to the nucleocapsid protein(N), there are two major (GP5 and M) and four minor (GP2, GP3, GP4, E) envelope proteins. By reverse genetics (EAV), each of these proteins was shown to be required for the production of infectious progeny. The proteins encoded by ORFs 2a, 3 and 4 have not been identified as structural components of Lactate dehydrogenase-elevating virus (LDV). The virion proteins of Simian hemorrhagic fever virus (SHFV) remain largely uncharacterized. The major virion glycoprotein of arteriviruses (GP5 in EAV, PRRSV, and LDV) forms a disulfide-linked heterodimer with M that is essential for virus infectivity. GP2, GP3, and GP4 were recently described to form heterotrimers in the virus particle (EAV).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The buoyant density of arteriviruses has been estimated to be 1.13 to 1.17 g/cm3 in-sucrose. Reported sedimentation coefficients for arteriviruses range from 214S to 230S. Virion stability is affected by temperature and pH. Virions are stable when stored at −70°C. The half-life of arteriviruses decreases progressively with increasing temperature. Virions are relatively stable between pH 6.0 and 7.5, but are inactivated at higher or lower pHs. Arteriviruses are inactivated by lipid solvents, such as ether, butanol, and chloroform and are extremely sensitive to detergents. For example, a brief incubation with a nonionic detergent such as 0.01% NP40 or Triton X-100, efficiently disrupts the viral envelope (Lactate dehydrogenase-elevating virus, LDV; Simian hemorrhagic fever virus; SHFV).
NUCLEIC ACID
Virions contain a single molecule of linear, positive-sense, ssRNA that ranges in length from 12.7 to 15.7 kb. The genome RNA has a 5′ type I cap structure (SHFV) and a 3′-terminal poly(A) tract. Full-length sequences are available in the GenBank database for all currently known arteriviruses. Arterivirus genomes contain 5′- and 3′-UTR of 156-224 and 59-117 nt, respectively. The 5′ 156-211 nt are used as a common 5′ “leader sequence” on viral sgRNAs (see below). For Equine arteritis virus (EAV), Porcine reproductive and respiratory syndrome virus (PRRSV), and LDV, the genome contains 9 functional genes, whereas the single reported SHFV sequence contains 12 ORFs, due to a postulated 3-gene duplication. The genes are arranged in the order 5′-replicase-E/GP2-GP3-GP4-GP5-M-N-3′ (EAV) (Fig. 2A ). The virion RNA functions as the mRNA for replicase gene translation.
Figure 2.

Overview of arterivirus genome organization and replicase polyproteins. (A) General genome organization. ORFs are represented by boxes. The proteins encoded by the EAV ORFs are indicated. The 5′ leader sequence is depicted by a small black box; 3′ poly(A) tails are not shown. The arrow between ORF1a and ORF1b represents the ribosomal frameshift site. The grey boxes represent regions where PRRSV, LDV, and SHFV contain major insertions compared to Equine arteritis virus (EAV). (B) Overview of proteolytic processing and domain organization of the EAV replicase polyproteins, pp1a and pp1ab, with differences in PRRSV and LDV indicated. Polyprotein cleavage sites are depicted with arrowheads matching the color of the proteinase involved. Abbreviations: PCP, papain-like cysteine proteinase; CP, nsp2 cysteine proteinase; SP, nsp4 chymotrypsin-like serine proteinase; h, hydrophobic domain; RdRp, RNA-dependent RNA polymerase; ZF, zinc finger; HEL, NTPase/helicase; EN, putative endoribonuclease.
NON-STRUCTURAL PROTEINS
Arteriviruses encode two large non-structural polyproteins, the ORF1a-encoded pp1a (187-260 kDa) and the ORF1ab-encoded pp1ab (345-421 kDa), whose synthesis involves ribosomal frameshifting. The EAV replicase polyproteins are cleaved into 12 mature nonstructural proteins by three ORF1a-encoded proteinases (PCP, CP, and SP; Fig. 2B), whereas PRRSV and LDV produce an additional cleavage product due to the fact that their nsp1 equivalent contains an additional internal autoproteinase that cleaves the nsp1 region into nsp1oc and nsp1(3. In EAV, this second papain-like proteinase (PCP β in the nsp1 region has become inactivated, although its remnants were detected in the EAV nsp1 aequivalent. A third papain-like cysteine protease (CP) with some unique properties is located in nsp2. A chymotrypsin-like serine protease (SP), related to the members of the 3C-like cysteine proteinase family, is located in nsp4 and constitutes the main proteinase of arteriviruses. EAV nsp4 is the first arterivirus protein for which a crystal structure was determined. The nsp4 SP is responsible for 8 proteolytic cleavages that occur in the C-terminal half of the ORF1a-encoded polyprotein (5 sites) and in the ORF1b-encoded part of pp1ab (3 sites).
Table 1.
Non-structural proteins of Equine arteritis virus (EAV)a
| Protein | sizeb | mode of expressionc | (putative) function(s) |
|---|---|---|---|
| nsp1d | 260 | TI + nsp1 PCP | proteinase (PCP), mononuclear zinc finger, role in sgRNA synthesis |
| nsp2 | 571 | TI + nsp1 PCP + nsp2 CP | proteinase (CP), integral membrane protein, replication complex formation |
| nsp3 | 233 | TI + nsp2 CP + nsp4 SP | integral membrane protein, replication complex formation |
| nsp4 | 204 | TI + nsp4 SP | main proteinase (SP) |
| nsp5 | 162 | TI + nsp4 SP | integral membrane protein |
| nsp6 | 22 | TI + nsp4 SP | ? |
| nsp7 | 225 | TI + nsp4 SP | ? |
| nsp8e | 50 | TI + nsp4 SP + TT | ? |
| nsp9 | 693 | TI + RFS + nsp4 SP | RdRp |
| nsp10 | 467 | TI + RFS + nsp4 SP | RNA helicase/NTPase, polynuclear zinc finger, role in sgRNA synthesis |
| nsp11 | 219 | TI + RFS + nsp4 SP | nidovirus-specific endoribonuclease |
| nsp12 | 119 | TI + RFS + nsp4 SP + TT | ? |
Based on the currently known replicase processing scheme of EAV
in aa
TI, translation initiation; RFS, ORF1a/ORF1b ribosomal frameshifting; TT, translation termination; PCP, nsp1 cysteine proteinase; CP, nsp2 cysteine proteinase; SP, nsp4 serine proteinase
Nsp1 of LDV and PRRSV is cleaved internally by an additional papain-like proteinase to yield nsp1α and nsp1β
Due to ribosomal frameshifting, nsp8 is identical to the N-terminal 50 aa of nsp9
In EAV, nsp1 was found to be fully dispensable for genome replication, but essential for the synthesis of sgRNAs. Nsp2 and nsp3 (and also nsp5) contain hydrophobic domains and have been shown to interact, a step that is essential for the formation of ER-derived double membrane vesicles that were shown to carry the viral RNA replication complex. The core replication complex is formed by nsp9, the putative RdRp, and nsp10, which was shown to possess NTPase and RNA helicase activities. In addition, nsp10 contains an N-terminal putative zinc finger region, which is also conserved in other nidoviruses. This region was implicated to have a function specific to sgRNA synthesis (EAV), but also appears to be essential for genome replication. By comparative sequence analysis, an nsp11 domain that is conserved in all nidoviruses was recently predicted to be an RNA endoribonuclease on the basis of a distant relationship with XendoU, a poly(U)-specific endoribonuclease of cellular origin. Functions or putative functions for the other arterivirus non-structural proteins remain to be identified.
VIRION-ASSOCIATED PROTEINS
The arterivirus nucleocapsid is composed of a single basic nucleocapsid protein (N) that is 12-15 kDa in size. In all arteriviruses, this protein is encoded by the 3′-proximal ORF. For the two best studied arteriviruses, EAV and PRRSV, six envelope proteins (encoded by ORFs 2a to 6) have now been identified in virus particles, an unusually large number for a positive strand RNA virus. It can be assumed that, given the right reagents, the same number of virion-associated proteins may also be found in LDV and SHFV particles. So far, three envelope proteins were identified in LDV and an attempt to detect the E protein was unsuccessful. By reverse genetics (EAV), each of the seven virion-associated proteins was shown to be required for the production of infectious progeny. The arterivirus envelope contains a heterodimer and a heterotrimer, which are probably both essential for virus infectivity. The heterodimer is formed between the integral membrane protein M and the major virion glycoprotein GP5 (Fig. 1), both of which are probably triple-spanning. The two proteins are linked by a single disulfide bond between conserved cysteine residues in their respective ectodomains. The recently described heterotrimer (EAV) is composed of the remaining three glycoproteins, GP2, GP3, and GP4. Homodimers of the GP2 protein have also been described (EAV). A soluble, non-virion-associated form of the ORF3 glycoprotein has been reported to be released from infected cells (LDV and PRRSV). SHFV encodes three additional 3′ ORFs, which may be duplications of ORFs 2 to 4 (Fig. 2).
Table 2.
Virion-associated proteins of arterivirusesa
| Protein | sizeb | ORF | mRNA | (putative) function(s) |
|---|---|---|---|---|
| Ed | 67–70 | 2a/2bc | 2d | small integral envelope protein |
| GP2 | 227–249 | 2b/2ac | 2d | minor glycoprotein, part of GP2/GP3/GP4 heterotrimer |
| GP3 | 163–265 | 3 | 3 | minor glycoprotein, part of GP2/GP3/GP4 heterotrimer |
| GP4 | 152–183 | 4 | 4 | minor glycoprotein, part of GP2/GP3/GP4 heterotrimer |
| GP5 | 199–255 | 5 | 5 | major glycoprotein, carries main determinants for neutralization, part of GP5/M heterodimer |
| M | 162–173 | 6 | 6 | integral (triple-spanning) membrane protein, part of GP5/M heterodimer |
| N | 110–128 | 7 | 7 | nucleocapsid protein, partially localizes to the nucleus of infected cells |
Based on the genome organization of EAV, PRRSV and LDV
in aa
in PRRSV
sgRNA2 is assumed to be functionally bicistronic
LIPIDS
Virions have lipid-containing envelopes derived from the host cell. Budding occurs from smooth intracellular membranes, probably including those of the endoplasmic reticulum and the Golgi complex.
CARBOHYDRATES
The four glycoproteins of arteriviruses all contain one or more putative N-linked glycosylation signals. Glycosylation of GP5 (EAV, LDV, PRRSV) occurs by the addition of variable numbers of lactosamine repeats.
GENOME ORGANIZATION AND REPLICATION
About three-quarters of the arterivirus genome is occupied by the replicase gene (ORF1a and ORF1b), which is expressed directly from the genomic RNA. The very short region in which ORF1a and ORF1b overlap contains a specific “slippery” sequence. Together with a downstream pseudoknot structure, this sequence directs a −1 ribosomal frameshift which is required for the translation of ORF1b and results in the synthesis of an ORF1ab polyprotein (pp1ab), in addition to the ORF1a polyprotein (pp1a) that results from normal translation termination.
The arterivirus replicase polyproteins are co- and post-translationally processed by three or four viral proteinases (Fig. 2), which reside within the ORF1a polyprotein. Replicase subunits containing hydrophobic domains (see above) are assumed to target the replication/transcription complex to modified intracellular (double) membranes, which are probably derived from the endoplasmic reticulum. Four host cell proteins have been identified that bind to a cis-acting region required for plus-strand RNA synthesis from the minus-strand template.
The cytoplasmic, membrane-associated replication/transcription complex of arteriviruses engages in RNA-dependent RNA synthesis (Fig. 3 ), leading to the synthesis of genomic and subgenomic plus- and minus-stranded RNAs. Subgenomic mRNAs are used to express the virion-associated proteins, which are encoded by overlapping ORFs in the 3′-proximal quarter of the genome. Subgenomic mRNAs contain both 5′ and 3′ co-terminal sgRNAs (“nested set” structure). Whereas the coding region of the sgRNA (the mRNA “body”) is colinear with a 3′-proximal portion of the genome, the 5′-proximal 156-211 nt of the sgRNAs (“leader”) are identical to the 5′-end of the genome. In addition to a full-length minus strand, infected cells also contain a nested set of minus strand sgRNAs, which are the complements of the sgRNAs and are believed to function as templates for their synthesis. The production of the minus strands sgRNA, which involves the “fusion” of sequences that are noncontiguous in the genome, is currently thought to occur by discontinuous extension of minus strand synthesis. According to this model, transcription-regulating sequences (TRSs) would direct attenuation of minus strand synthesis, a step that would be followed by translocation of the nascent strand to the leader region in the 5′-end of the genomic template. Guided by a base pairing interaction, minus strand synthesis would resume to add the leader complement to the nascent minus strand sgRNA.
Figure 3.
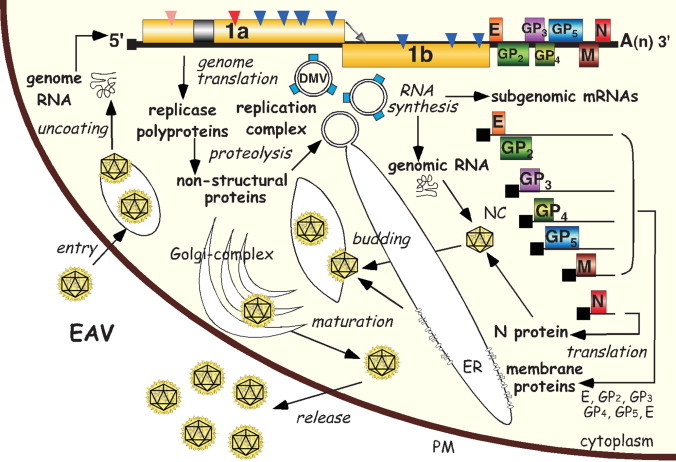
Overview of the life cycle of the arterivirus prototype (EAV). The genome organization, including replicase cleavage sites (arrowheads; see also Fig. 2), is shown at the top of the figure. Abbreviations: ER, endoplasmic reticulum; PM, plasma membrane; DMV, double membrane vesicle; NC, nucleocapsid.
Arteriviruses produce 6 (EAV, LDV, PRRSV) to 8 (SHFV) major sgRNA species. In addition, the production of multiple, alternative sgRNAs (from alternative TRSs) has been reported for various arteriviruses. Despite their polycistronic structure, it is assumed that – as a rule – only the 5′ proximal ORF of each sgRNA is translated. However, one (EAV, PRRSV and LDV) or two (SHFV) sgRNAs are thought to be functionally bicistronic.
PRRSV has been reported to enter cells via a low pH-dependent endocytic pathway and recent studies have identified sialoadhesin, a macrophage-restricted surface molecule, as a receptor for PRRSV. Arteriviruses replicate in the cytoplasm of infected cells, although a fraction of nsp1 and the N protein localize to the nucleus. Nucleocapids bud into the lumen of smooth intracellular membranes of the exocytic pathway, probably including those of the Golgi complex. Virions are released from infected cells via exocytosis.
Thus far, infectious molecular clones (full-length cDNA clones) have been constructed for EAV and both European and North American prototype strains of PRRSV. Using these tools, knowledge on the details of various aspects of arterivirus molecular biology and immunology has been expanded considerably. Among the topics studied by reverse genetics are nonstructural protein processing and function, sgRNA synthesis, virus assembly, virus immunogenicity, and the development of arterivirus-based vector systems.
ANTIGENIC PROPERTIES
GP5-specific neutralizing antibodies have been described for EAV, LDV and PRRSV. Also monoclonal antibodies against the GP5 equivalent of SHFV can neutralize virus infectivity. The neutralization domain has been mapped to various epitopes/regions in the ectodomain of this protein. Monoclonal antibodies specific for the GP4 can also be neutralizing (PRRSV) and a neutralizing epitope in PRRSV GP4 was mapped to the region between aa 39 and 79. No antigenic cross reactivity between different arterivirus members has been found. LDV- and PRRSV-specific T-cell responses have been described.
BIOLOGICAL PROPERTIES
Infections with arteriviruses can cause acute or persistent asymptomatic infections or respiratory disease (EAV and PRRSV), in utero fetal death (PRRSV) and abortion (EAV and PRRSV), fatal age-dependent poliomyelitis (LDV) or fatal hemorrhagic fever (SHFV).
The host range of arteriviruses is restricted. EAV infects horses and donkeys, LDV infects mice, PRRSV infects swine, and SHFV infects some species of African (patas monkeys, African green monkeys and baboons) and Asian (macaque) monkeys. Macrophages are the primary target cells for all arteriviruses in their respective hosts. Laboratory mutants of LDV can also replicate in ventral motor neurons in some strains of inbred mice. In tissue culture, LDV replicates only in primary mouse macrophages, SHFV and PRRSV replicate in primary macrophages (rhesus and porcine alveolar lung macrophages, respectively) as well as in the MA-104 established cell line (an African green monkey kidney cell line), and EAV replicates in primary equine macrophages and kidney cells as well as in BHK-21, RK-13, Vero and MA-104 cells.
Arteriviruses are spread horizontally and vertically. Horizontal transmission can occur via the respiratory route (EAV and PRRSV), via the sexual route in semen (EAV and PRRSV) and via infected blood or body fluids (LDV, PRRSV, and SHFV). Congenital infection is common for PRRSV. Arterivirus replication is characterized by the formation of double-membrane vesicles (see above) in infected cells. One-step growth experiments have shown that the replication cycle of arteriviruses (in cell culture) is relatively short, maximum progeny virus titers being released by 10 to 15 hrs post infection. The maximum titers obtained in cell culture are 106-107 TCID50/ml for PRRSV, but may exceed 108 PFU/ml for EAV and SHFV. The infection of macrophages and cell lines is highly cytocidal, resulting in rounding of the cells and detachment from the culture plate surface. PRRSV, SHFV, and EAV are titrated by endpoint dilution or plaque assays. However, LDV is titrated in mice, because the percentage of susceptible cells in primary mouse macrophage cultures is too low to detect their destruction. Apoptosis has been observed in PRRSV-infected porcine alveolar macrophages, MA-104 cells, and testicular germ cells. Expression of PRRSV GP5 from a Vaccinia virus recombinant also induced apoptosis.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The members of the genus Arterivirus form a distinct phylogenic group. Their genomes are polycistronic positive-stranded RNA molecules ranging in size from 12 to 16 kb, which is considerably smaller than the genome sizes of other members of the order Nidovirales (corona-, toro- and ronivirus genomes are all larger than 25 kb). The genome encodes two large 5′ ORFs, ORFs 1a and 1b, that are expressed from the genomic RNA. ORF1b is only expressed after a −1 frameshift has occurred. The 3′ ORFs encode virion-associated proteins and are expressed from a set of 3′- and 5′-co-terminal sgRNAs. The arterivirus nucleocapsid is isometric and is composed of the genome RNA and a single nucleocapsid protein N. Virions contain 2 major and 4 minor envelope proteins, 4 of which are glycoproteins. The virion surface projections are relatively small and indistinct, and none of the virion glycoproteins contain a coiled-coil structure. With the exception of the triple-spanning character of the membrane protein M, there are no obvious similarities between the virion-associated proteins of Arteriviridae and those of other members of the order Nidovirales. Members of the four species in the genus Arterivirus (EAV, LDV, PRRSV, and SHFV) each constitute a phylogenetic branch within the genus (Fig. 4 ), with each species represented by a cluster of strains. LDV and PRRSV are most closely related to each other. There are two ‘subspecies’ of PRRSV, a European (type I) and an American (type II). Members of the four virus species are antigenically distinct. The viruses of each species have a restricted host range but infects different hosts. The variability in the length of the N-terminal region of ORF1a among arteriviruses suggests that this region may contain species-specific functions.
Figure 4.

Unrooted phylogenetic analysis of the full-length ORF1b replicase polyprotein of the members of the family Arteriviridae. The tree was generated using the neighbor-joining algorithm implemented in the ClustalX program (with the Kimura correction on and using an alignment from which all columns containing gaps had been deleted). One thousand bootstrap replicates were done. Branch lengths indicate number of substitutions per residue.
(A.E. Gorbalenya; unpublished data).
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Equine arteritis virus | ||
| Equine arteritis virus – Bucyrus (North American) | [X53459] | (EAV-Buc) |
| Equine arteritis virus – CW (European) | [AY349167] | (EAV-CW) |
| Lactate dehydrogenase-elevating virus | ||
| Lactate dehydrogenase-elevating virus – C | [L13298] | (LDV-C) |
| Lactate dehydrogenase-elevating virus – P | [U15146] | (LDV-P) |
| Porcine reproductive and respiratory syndrome virus | ||
| Porcine reproductive and respiratory syndrome virus - | [M96262] | (PRRSV-L) |
| Lelystad (type I (European) prototype) | ||
| Porcine reproductive and respiratory syndrome virus - | [U87392] | (PRRSV-VR) |
| VR-2332 (type II (North American) prototype) | ||
| Simian hemorrhagic fever virus | ||
| Simian hemorrhagic fever virus – LVR42-0 | [AF180391] | (SHFV-LVR) |
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
SIMILARITY WITH OTHER TAXA
The family Arteriviridae together with the family Coronaviridae and the family Roniviridae form the order Nidovirales. These viruses have important common features at the level of genome organization, genome expression strategy, and phylogeny and internal organization of their large replicase gene. Despite these overall similarities, arterivirus genomes are substantially smaller, and the size, structure, and composition of their virions do not resemble those of other members of the order Nidovirales. Various non-structural proteins contain arterivirus- and/or nidovirus-specific domains or signatures, including an SDD signature (instead of the canonical GDD) in the RdRp (nsp9), a complex, N-terminal (putative) zinc binding domain in the helicase (nsp10), and a nidovirus-specific putative endonuclease domain in nsp11. Non-nidovirus homologs of several (putative) enzymes encoded by viruses of the family Arteriviridae have been found in other RNA viruses. The main proteinase and RdRp cluster together with homologs of viruses in the “picornavirus-like” and “sobemovirus-like” supergroups. The helicase has counterparts in viruses of the “alphavirus-like” supergroup. Also, some parallels are evident between the genome organization and expression strategy of members of the family Arteriviridae (and other members of the order Nidovirales) and those of the plant family Closteroviridae.
DERIVATION OF NAMES
Arteri: from equine arteritis, the disease caused by the reference virus.
REFERENCES
- Barrette-Ng I.H., Ng K.K., Mark B.L., van Aken D., Cherney M.M., Garen C., Kolodenko Y., Gorbalenya A.E., Snijder E.J., James M.N. Structure of arterivirus nsp4: The smallest chymotrypsin-like proteinase with an alpha/beta C-terminal extension and alternate conformations of the oxyanion hole. J. Biol. Chem. 2002;277:39960–39966. doi: 10.1074/jbc.M206978200. [DOI] [PubMed] [Google Scholar]
- Chen Z., Li K., Plagemann P.G. Neuropathogenicity and sensitivity to antibody neutralization of lactate dehydrogenase-elevating virus are determined by polylactosaminoglycan chains on the primary envelope glycoprotein. Virology. 2000;266:88–98. doi: 10.1006/viro.1999.0050. [DOI] [PubMed] [Google Scholar]
- Godeny E.K., de Vries A.A.F., Wang X.C., Smith S.L., de Groot R.J. Identification of the leader-body junctions for the viral subgenomic mRNAs and organization of the simian hemorrhagic fever virus genome: Evidence for gene duplication during arterivirus evolution. J. Virol. 1998;72:862–867. doi: 10.1128/jvi.72.1.862-867.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E. Big nidovirus genome: when count and order of domains matter. Adv. Exp. Med. Biol. 2001;494:1–17. [PubMed] [Google Scholar]
- Hwang Y.-K., Brinton M.A. A 68-nucleotide sequence within the 3’ non-coding region of simian hemorrhagic fever virus negative-strand RNA binds to four MA104 cell proteins. J. Virol. 1998;72:4341–4351. doi: 10.1128/jvi.72.5.4341-4351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutz L.C., Ackermann M.R. Porcine reproductive and respiratory syndrome virus enters cells through a low pH dependent endocytic pathway. Virus Res. 1996;42:137–147. doi: 10.1016/0168-1702(96)01313-5. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J.M., Petersen-den Besten A., de Kluyver E.P., Moormann R.J.M., Schaaper W.M.M., Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995;206:155–163. doi: 10.1016/S0042-6822(95)80030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenkamp R., van Tol H., Rozier B.C.D., van der Meer Y., Spaan W.J.M., Snijder E.J. The arterivirus replicase is the only viral protein required for genome replication and subgenomic mRNA transcription. J. Gen. Virol. 2000;81:2491–2496. doi: 10.1099/0022-1317-81-10-2491. [DOI] [PubMed] [Google Scholar]
- Pasternak A.O., van den Born E., Spaan W.J.M., Snijder E.J. Sequence requirements for RNA strand transfer during nidovirus discontinuous subgenomic RNA synthesis. EMBO J. 2001;20:7220–7228. doi: 10.1093/emboj/20.24.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K.W., van der Meer Y., Roos N., Snijder E.J. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J. Virol. 1999;73:2016–2026. doi: 10.1128/jvi.73.3.2016-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L., Guan Y., Rozanov M., Spaan W.J.M., Gorbalenya A.E. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Dobbe J.C., Spaan W.J.M. Heterodimerization of the two major envelope proteins is essential for arterivirus infectivity. J. Virol. 2003;77:97–104. doi: 10.1128/JVI.77.1.97-104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Meulenberg J.J.M. Arteriviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott, Williams, and Wilkins; Philadelphia: 2001. pp. 1205–1220. [Google Scholar]
- Tijms M.A., van Dinten L.C., Gorbalenya A.E., Snijder E.J. A zinc finger-containing papain-like protease couples subgenomic mRNA synthesis to genome translation in a positive-stranded RNA virus Proc. Nat. Acad. Sci. USA. 2001;98:1889–1894. doi: 10.1073/pnas.041390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheijden N., Delputte P.L., Favoreel H.W., Vandekerckhove J., Van Damme J., van Woensel P.A., Nauwynck H.J. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 2003;77:8207–8215. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa R., de Vries A.A.F., Rottier P.J.M. Formation of disulfide-linked complexes between the three minor envelope glycoproteins (GP2b, GP3, and GP4) of equine arteritis virus. J. Virol. 2003;77:6216–6226. doi: 10.1128/JVI.77.11.6216-6226.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
RONIVIRIDAE
CONTRIBUTED BY, P.J. Walker, J.R. Bonami, V. Boonsaeng, P.S. Chang, J.A. Cowley, L. Enjuanes, T.W. Flegel, D.V. Lightner, P.C. Loh, E.J. Snijder, K. Tang
FAMILY RONIVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Order | Nidovirales |
| Family | Roniviridae |
| Genus | Okavirus |
Since only one genus is currently recognized, the family description corresponds to the genus description.
GENUS OKAVIRUS
Type Species Gill-associated virus
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

(Top left) Schematic illustration of an okavirus virion. (Top right) Transmission electron micrograph of negative-stained particles of Gill-associated virus (GAV). (Bottom left). Transmission electron micrograph of partially disrupted Yellow head virus (YHV) virion displaying the internal nucleocapsid and a ring-like structure which appears to be a disrupted virion in cross-section. (Bottom right) Transmission electron micrograph of cytoplasmic unenveloped nucleocapsids in a thin section of GAV-infected lymphoid organ cells. The bars represent 100 nm.
(Courtesy of K. Spann, P. Loh, J. Cowley and R.J McCulloch and reproduced with permission).
Virions are enveloped and bacilliform in shape, with rounded ends and dimensions of 150-200 nm × 40-60 nm. Envelopes are studded with prominent peplomers projecting approximately 11 nm from the surface. Nucleocapsids have helical symmetry with diameter of 20-30 nm, apparently consisting of coiled structures with a periodicity of 5-7 nm. Long filamentous nucleocapsid precursors (approximately 15 × 80-450 nm) occur in the cytoplasm of infected cells and appear to acquire envelopes by budding into vesicles at the endoplasmic reticulum, generating intracytoplasmic paracrystalline arrays of enveloped virions.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion buoyant density in sucrose is 1.18-1.20 g/ml. Yellow head virus is inactivated by heating at 60°C for 15-30 min but has been reported to survive in seawater at 25-28°C for at least 4 days. Virions are sensitive to calcium hypochlorite and SDS but sensitivity to other treatments is not known.
NUCLEIC ACID
Virions contain a single segment of linear, positive-sense ssRNA. The genome of Gillassociated virus comprises 26,235 nt. A 68 nt 5′-UTR is followed by 5 long ORFs (5′-ORF1a-ORF1b-ORF2-ORF3-ORF4-3′), a 129 nt UTR and a 3′-poly[A] tail. ORF4 is significantly truncated in Yellow head virus (YHV) and other known genotypes and it may not always be expressed. Untranslated regions upstream of ORF2 (93 nt), ORF3 (57 nt) and ORF4 (256 nt) each contain a core of relatively conserved sequence. The genome structure of GAV is shown in Figure 2 . The complete genome sequence of GAV and partial sequences of YHV are available.
Figure 2.

Schematic representation of the okavirus genome which comprises 5 long ORFs (ORF1a, ORF1b, ORF2, ORF3, ORF4). ORF 1a and ORF1b overlap at a ribosomal frame-shift site and contain the conserved sequence motifs (3CLP, Pol, MIB, Hel, M1 and M3). ORF2 encodes the nucleoprotein (p20). ORF3 encodes the virion transmembrane glycoproteins gp116 and gp64. The products of ORF4 have not yet been identified. Two sub-genomic mRNAs initiate at conserved sequences in the intergenic regions upstream of ORF2 and ORF3.
PROTEINS
Virion structural proteins have been identified only for YHV. YHV virions contain three major structural proteins (110-135 kDa, 63-67 kDa and 20-22 kDa). The 110-135 kDa protein (gp116) and the 63-67 kDa protein (gp64) are glycosylated and appear to be envelope proteins that form the prominent peplomers on the virion surface. Mature gp116 and gp64 are generated by post-translational processing of a precursor polyglycoprotein. Gp116 and gp64 are not linked by intramolecular disulfide bonds but each is anchored in the virion by C-terminal hydrophobic transmembrane domains. The 20-22 kDa protein (p20) is associated with nucleocapsids and appears to function as the nucleoprotein.
Table 1.
Virus-associated proteins of okaviruses
| Protein | Okavirusa | ||
|---|---|---|---|
| Large spike glycoprotein | S1 | gp116 | 110–135 |
| Small spike glycoprotein | S2 | gp64 | 60–65 |
| Nucleocapsid protein | N | p20 | 20–22 |
Apparent sizes by electrophoresis (kDa)
LIPIDS
Viruses have tri-laminar envelopes derived from the host cell.
CARBOHYDRATES
Gp116 and gp64 are extensively glycosylated. N-linked glycosylation sites are present in both gp116 (7-8 sites) and gp64 (4 sites) and the size estimate for each protein is consistent with glycosylation at these sites. Multiple O-linked glycosylation sites are also present but it is not known if they are utilized.
GENOME ORGANIZATION AND REPLICATION
The genome comprises a large replicase gene (ORF1a/ORF1b) followed by the nucleoprotein gene (ORF2), a glycoprotein gene (ORF3), a small gene or pseudogene of unknown function (ORF4), and a 3′-poly[A] tail. At the overlap between ORF1a and ORF1b there is a pseudoknot structure and a slippery sequence (AAAUUUU) that allow expression of ORF1b through a −1 ribosomal frame-shift. The pseudoknot structure and slippery sequence are not of the ‘H-type’ that occurs commonly in other nidoviruses but resemble the complex pseudoknot at the gag/pol junction of retroviruses. ORF1a contains a 3C-like protease flanked by hydrophobic domains that shares common sequence motifs with coronavirus homologues and appears to be involved in autolytic processing of the polyprotein pp1a. ORF1b contains multiple conserved sequence motifs including helicase and metal-ion binding domains, and a polymerase domain with the ‘SDD’ active site motif that is characteristic of nidoviruses.
Okaviruses are unique amongst members of the Nidovirales in that the nucleoprotein gene (ORF2) is located upstream of the glycoprotein gene (ORF3). ORF3 encodes a polyprotein that is processed by proteolysis at two predicted signal cleavage sites to generate virion envelope glycoproteins gp116 and gp64. The N-terminal fragment of the ORF3 polyprotein is predicted to be a triple-membrane-spanning glycoprotein of similar size to the M proteins of other nidoviruses. However, this product does not appear to be a major structural protein and has not yet been detected in infected cells. The 3′-terminal ORF4 varies significantly in size in different viruses. The 83 aa GAV ORF4 product is poorly characterized but has been detected in infected cells.
Okavirus transcription occurs via a nested set of 3′-coterminal polyadenylated mRNAs. Two sgRNAs are transcribed from highly conserved sequences in intergenic regions preceding ORF2 and ORF3. As for most torovirus sgRNAs, but unlike coronaviruses or arteriviruses, okavirus sgRNAs lack a common 5′-leader sequence acquired from the genomic RNA and initiate by templated transcription at common 5′-AC…sites. A less conserved but similar sequence also occurs in the long intergenic region preceding ORF4.
ANTIGENIC PROPERTIES
Not known.
BIOLOGICAL PROPERTIES
Okaviruses are the only known invertebrate nidoviruses and have been detected only in crustaceans. The black tiger prawn (Penaeus monodon) appears to be the natural host of GAV but other prawn species are susceptible to experimental infection. Infections may be chronic or acute and transmission can occur horizontally and vertically. During acute infections, mortalities are usually high and virus occurs in most tissues of ectodermal and mesodermal origin, and particularly in the ‘Oka’ or lymphoid organ. Necrotic cells display intensely basophilic cytoplasmic inclusions. The known geographic range of infection presently appears to be restricted to Asia and Australia where the prevalence of sub-clinical chronic infections in P. monodon is commonly high. There are no known prophylactic or curative treatments.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
Genotype 3
Genotype 4
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Phylogenetic analysis of sequences in the ORF1b gene has clustered the viruses into four distinct genotypes: GAV, YHV, genotype 3 and genotype 4. GAV has been detected in Australia, Vietnam and Thailand; YHV has been detected in Thailand and Taiwan; genotype 3 has been detected in Thailand, Vietnam and Malaysia; genotype 4 has been detected only in India.
Figure 3.

Unrooted phylogenetic tree of okaviruses based on a 781 nt region of ORF1b. The sequences were aligned using Clustal W, phylogenetic inference was determined by the neighbor-joining method and the tree was constructed using NJ-plot and Treeview software. Bootstrap analysis was conducted on 1000 replicates. Branch lengths are proportional to phylogenetic distance. The bar represents a sequence divergence of 1.0%.
SIMILARITY WITH OTHER TAXA
Various structural and genetic similarities with other viruses in the order Nidovirales. Virion morphology distantly resembles that of plant rhabdoviruses. The pseudoknot structure in the ORF1a/1b overlap resembles the gag/pol pseudoknots of some retroviruses. The structure and substrate specificity of the ORF1a protease bridges the gap between the chymotrypsin-like cysteine proteases of coronaviruses and plant potyviruses.
DERIVATION OF NANES
Roni is a sigla derived from rod-shaped nidovirus referring to the unique virion morphology of viruses in the family.
Oka refers to the ‘Oka’ or lymphoid organ in which the viruses are commonly detected and in which pathology occurs during acute infections. Lymphoid organs are anatomical structures common to penaeid shrimp.
REFERENCES
- Boonyaratpalin S., Supamattaya K., Kasornchandra J., Direkbusaracom S., Aekpanithanpong U., Chantanachookin C. Non-occluded baculo-like virus, the causative agent of yellow head disease in the black tiger shrimp (Penaeus monodon) Gyobyo Kenkyu. 1993;28:103–109. [Google Scholar]
- Chantanachookin C., Boonyaratpalin S., Kasornchandra J., Direkbusarakom S., Ekpanithanpong U., Supamataya K., Siurairatana S., Flegel T.W. Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by “yellow-head” disease. Dis. Aquat. Org. 1993;17:145–157. [Google Scholar]
- Cowley J.A., Dimmock C.M., Spann K.M., Walker P.J. Gill-associated virus of Penaeus monodon shrimp: an invertebrate virus with ORF1a and ORF1b genes related to arteri- and coronaviruses. J. Gen. Virol. 2000;81:1473–1484. doi: 10.1099/0022-1317-81-6-1473. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Dimmock C.M., Walker P.J. Gill-associated nidovirus of Penaeus monodon prawns transcribes 3′-coterminal subgenomic RNAs that do not possess 5′-leader sequences. J. Gen. Virol. 2001;83:927–935. doi: 10.1099/0022-1317-83-4-927. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Dimmock C.M., Wongteerasupaya C., Boonsaeng V., Panyim S., Walker P.J. Yellow head virus from Thailand and gill-associated virus from Australia are closely related but distinct prawn viruses. Dis. Aquat. Org. 1999;36:153–157. doi: 10.3354/dao036153. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Hall M.R., Cadogan L.C., Spann K.M., Walker P.J. Vertical transmission of covert gill-associated virus (GAV) infections in Penaeus monodon. Dis. Aquat. Org. 2002;50:95–104. doi: 10.3354/dao050095. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Walker P.J. The complete genome sequence of gill-associated virus of Penaeus monodon prawns indicates a gene organisation unique among nidoviruses. Arch. Virol. 2002;147:1977–1987. doi: 10.1007/s00705-002-0847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitrapakdee S., Unajak S., Sittidilokratna N., Hodgson R.A.J., Cowley J.A., Walker P.J., Panyim S., Boonsaeng V. Identification and analysis of gp116 and gp64 structural glycoproteins of yellow head nidovirus of Penaeus monodon shrimp. J. Gen Virol. 2003;84:863–873. doi: 10.1099/vir.0.18811-0. [DOI] [PubMed] [Google Scholar]
- Loh P.C., Tapay L.M., Lu Y., Nadala E.C.B. Viral pathogens of penaeid shrimp. Adv. Virus Res. 1997;48:263–312. doi: 10.1016/S0065-3527(08)60290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadala E.C.B., Tapay L.M., Loh P.C. Yellow-head virus: a rhabdovirus-like pathogen of penaeid shrimp. Dis. Aquat. Org. 1997;31:141–146. [Google Scholar]
- Sittidilokratna N., Hodgson R.A.J., Cowley, J.A., Jitrapakdee, S., Boonsaeng V., Panyim S., Walker P.J. Complete ORF1b-gene sequence indicates yellow head virus is an invertebrate nidovirus. Dis. Aquat. Org. 2002;50:87–93. doi: 10.3354/dao050087. [DOI] [PubMed] [Google Scholar]
- Spann K.M., Cowley J.A., Walker P.J., Lester R.J.G. Gill-associated virus (GAV), a yellow head-like virus from Penaeus monodon cultured in Australia. Dis. Aquat. Org. 1997;31:169–179. [Google Scholar]
- Spann K.M., McCulloch R.J., Cowley, J.A., East, I.J., Walker P.J. Detection of gill-associated virus (GAV) by in situ hybridization during acute and chronic infections of Penaeus monodon and P. esculentus. Dis. Aquat. Org. 2003;56:1–10. doi: 10.3354/dao056001. [DOI] [PubMed] [Google Scholar]
- Spann K.M., Vickers J.E., Lester R.J.G. Lymphoid organ virus of Penaeus monodon from Australia. Dis. Aquat. Org. 1995;23:127–134. [Google Scholar]
- Walker P.J., Cowley J.A., Spann K.M., Hodgson R.A.J., Hall M.R., Withychumnarnkul B. Yellow head complex viruses: transmission cycles and topographical distribution in the Asia-Pacific region. In: Browdy C.L., Jory D.E., editors. The New Wave: Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture 2001. The World Aquaculture Society; Baton Rouge, LA: 2001. pp. 227–237. [Google Scholar]
- Wang Y.C., Chang P.S. Yellow head virus infection in the giant tiger prawn Penaeus monodon cultured in Taiwan. Fish Pathol. 2000;35:1–10. [Google Scholar]
- Wongteerasupaya C., Sriurairatana S., Vickers J.E., Akrajamorn A., Boonsaeng V., Panyim S., Tassanakajon A., Withyachumnarnjul B., Flegel T.W. Yellow-head virus of Penaeus monodon is an RNA virus. Dis. Aquat. Org. 1995;22:45–50. [Google Scholar]
- Ziebuhr J., Bayer S., Cowley J.A., Gorbalenya A.E. The 3C-like proteinase of an invertebrate nidovirus links coronavirus and potyvirus homologs. J. Virol. 2003;77:1415–1426. doi: 10.1128/JVI.77.2.1415-1426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
FLAVIVIRIDAE
CONTRIBUTED BY, H.-J. Thiel, M.S. Collett, E.A. Gould, F.X. Heinz, M. Houghton, G. Meyers, R.H. Purcell, C.M. Rice
FAMILY FLAVIVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Flaviviridae |
| Genus | Flavivirus |
| Genus | Pestivirus |
| Genus | Hepacivirus |
VIRION PROPERTIES
MORPHOLOGY
Virions are 40-60 nm in diameter, spherical in shape and contain a lipid envelope. The capsid is composed of a single capsid protein (C) and the envelope contains two or three virus-encoded membrane proteins. The behavior of hepaciviruses during filtration and their susceptibility to chemical and physical treatments suggest that its overall structural properties are similar to those of flaviviruses and pestiviruses. Specific descriptions of the three individual genera are given in the corresponding sections.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The virion Mr, buoyant density, sedimentation coefficient and other physicochemical properties differ among the members of the three genera and are described separately in the corresponding sections.
NUCLEIC ACID
The genome RNA of all three genera is a positive sense ssRNA of approximately 11, 12.3, and 9.6 kb for flavi-, pesti-, and hepaciviruses, respectively. All members of the family lack a 3‘-terminal poly(A) tract. Flaviviruses contain a 5‘-terminal type I cap structure, while pestiviruses and hepaciviruses do not.
PROTEINS
The virions of all members of the family have a single, small basic C and two (Flavivirus and Hepacivirus) or three (Pestivirus) membrane-associated proteins. The non-structural proteins contain sequence motifs characteristic of a serine proteinase, RNA helicase, and RdRp that are encoded at similar locations along the genome in all three genera. Further details of specific functional properties are given in the corresponding sections of the individual genera.
LIPIDS
Lipids present in virions are derived from host cell membranes and make up 17% of the total virion weight in the case of flaviviruses. The lipid content of pesti- and hepaciviruses has not been determined.
CARBOHYDRATES
Virions contain carbohydrates in the form of glycolipids and glycoproteins.
GENOME ORGANIZATION AND REPLICATION
The genome RNA of all three genera has a similar organization and is the only viral mRNA found in infected cells. It contains a single long ORF flanked by 5‘- and 3‘-terminal NCRs that form specific secondary structures required for genome replication and translation. Translation-initiation is cap-dependent in the case of flaviviruses, whereas IRES have been demonstrated for pestiviruses and hepaciviruses. Viral proteins are synthesized as part of a polyprotein of more than 3000 aa that is co- and post-translationally cleaved by viral and cellular proteinases. The structural proteins are contained in the N-terminal portion of this polyprotein and the non-structural proteins in the remainder. The latter include a serine proteinase, an RNA helicase, and an RdRp.
RNA synthesis occurs in the cytoplasm in association with the endoplasmic reticulum via synthesis of a full-length negative-strand intermediate. Virion assembly and envelopment are thought to take place at intracellular membranes. Viral particles are transported in cytoplasmic vesicles through the secretory pathway before they are released by exocytosis, as shown for members of the genus Flavivirus and assumed for the pestiviruses and hepaciviruses.
ANTIGENIC PROPERTIES
The three genera are antigenically unrelated, but serological cross-reactivities exist among members within the genera Flavivirus and Pestivirus. Hepaciviruses have so far not been amenable to antigenic analysis.
BIOLOGICAL PROPERTIES
The biological properties of the three genera exhibit different characteristics and are described in the corresponding sections.
GENUS FLAVIVIRUS
Type Species Yellow fever virus
DISTINGUISHING FEATURES
The 5′-end of the genome possesses a type I cap (m-7GpppAmp) not seen in the other genera. Most flaviviruses are transmitted to vertebrate hosts by arthropod vectors, mosquitos or ticks, in which they actively replicate. Some flaviviruses are zoonotic agents transmitted between rodents or bats without known arthropod vectors.
VIRION PROPERTIES
MORPHOLOGY
Virions are 50 nm in diameter and spherical in shape (Fig. 1 ). Two virus forms can be distinguished. Mature virions contain two virus encoded membrane-associated proteins, E and M. Intracellular, immature virions contain the precursor prM instead of M, which is proteolytically cleaved in the course of maturation. The atomic structure of the major envelope protein E from Tick-borne encephalitis virus (TBEV) and Dengue virus (DENV) has been determined by X-ray crystallography. It is a dimeric, rod-shaped molecule that is oriented parallel to the membrane and does not form spike-like projections in its neutral pH conformation. Image reconstructions from cryo-electron micrographs have shown that the virion envelope has icosahedral symmetry.
Figure 1.

(Left) Schematic of immature and mature virion. (Center and right) Three-dimensional cryo-electron microscopic reconstructions of immature and mature particles of an isolate of Dengue virus
(courtesy of R.J. Kuhn).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr has not been precisely determined but can be estimated from the virus composition to be ∼6 × 107. Mature virions sediment at ∼200S and have a buoyant density of ∼1.19 g/cm3 in sucrose. Viruses are stable at slightly alkaline pH 8.0 but are readily inactivated at acidic pH, temperatures above 40°C, organic solvents, detergents, ultraviolet light, and gamma-irradiation.
NUCLEIC ACID
The virion RNA of flaviviruses is a positive-sense infectious ssRNA of ∼11 kb. The 5′-end of the genome possesses a type I cap (m-7GpppAmp) followed by the conserved dinucleotide AG. The 3′-ends lack a terminal poly(A) tract and terminate with the conserved dinucleotide CU.
PROTEINS
Virions contain three structural proteins: C (11 kDa), E (50 kDa), the major envelope protein; and either prM (26 kDa), in immature virions, or M (8 kDa), in mature virions. The E protein is the viral hemagglutinin and is believed to mediate both receptor binding and acid pH-dependent fusion activity after uptake by receptor-mediated endocytosis. Seven nonstructural proteins are synthesized in infected cells: NS1 (46 kDa), NS2A (22 kDa), NS2B (14 kDa), NS3 (70 kDa), NS4A (16 kDa), NS4B (27 kDa) and NS5 (103 kDa). NS3 is a multi-functional protein. The N-terminal one-third of the protein forms the viral serine proteinase complex together with NS2B that is involved in processing the polyprotein. The C-terminal portion of NS3 contains an RNA helicase domain involved in RNA replication, as well as an RNA triphosphatase activity that is probably involved in formation of the 5′-terminal cap structure of the viral RNA. NS5 is the largest and most highly conserved flavivirus protein. NS5 is the flavivirus RdRp and also possesses motifs suggesting that it encodes the methyltransferase activity necessary for methylation of the 5′-cap structure.
LIPIDS
Virions contain about 17% lipid by weight; lipids are derived from host cell membranes.
CARBOHYDRATES
Virions contain about 9% carbohydrate by weight (glycolipids, glycoproteins); their composition and structure are dependent on the host cell (vertebrate or arthropod). N-glycosylation sites are present in the proteins prM (1 to 3 sites), E (0 to 2 sites), and NS1 (1 to 3 sites).
GENOME ORGANIZATION AND REPLICATION
The genome RNA represents the only viral messenger RNA in flavivirus-infected cells. It consists of a single long ORF of more than 10,000 nt that codes for all structural and nonstructural proteins and is flanked by short NCRs at the 5′- and 3′-terminal ends (Fig. 2 ).
Figure 2.
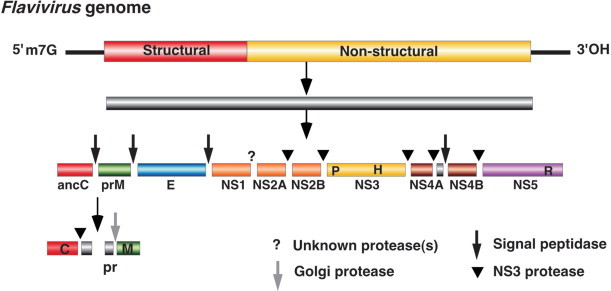
Flavivirus genome organization (not to scale) and polyprotein processing. At the top is the viral genome with the structural and non-structural protein coding regions and the 5′- and 3′-NCRs indicated. Boxes below the genome indicate viral proteins generated by the proteolytic processing cascade. AncC: anchored CP (with C-terminal hydrophobic domain). The viral structural proteins are shown in black. P, H, and R symbols indicate the localization of the NS3 proteinase, the NS3 RNA helicase, and the NS5 RdRp, respectively.
While nt sequences are divergent, the predicted secondary structures within the 5′- and 3′-NCRs are conserved among different flaviviruses. The NCRs contain stretches of conserved RNA sequences that are distinct in mosquito- and tick-borne flaviviruses. The length of the 3′-NCR of Tick-borne encephalitis virus can vary significantly, from 450 to almost 800 nt, and in some cases may contain an internal poly(A) tract. RNA synthesis appears to occur on the membranes of the perinuclear endoplasmic reticulum. After translation of the incoming genomic RNA, RNA replication begins with synthesis of complementary negative strands, which are then used as templates to produce additional genome-length positive-stranded molecules. These are synthesized by a semi-conservative mechanism involving replicative intermediates (containing double-stranded regions as well as nascent single-stranded molecules) and replicative forms (duplex RNA molecules). Negative strand synthesis in flavivirus-infected cells continues throughout the replication cycle. Translation usually starts at the first AUG of the ORF but may also occur at a second in-frame AUG located 12 to 14 codons downstream in mosquito-borne flaviviruses. The polyprotein is processed by cellular proteinases and the viral NS2B-NS3 serine proteinase to give rise to the mature structural and nonstructural proteins. Protein topology with respect to the ER and cytoplasm is determined by internal signal and stop-transfer sequences. Proliferation and hypertrophy of intracellular membranes is a characteristic feature of flavivirus-infected cells. The replication complex sediments with membranous fractions of extracts from infected cells. Virus particles can first be observed in the rough endoplasmic reticulum, which is believed to be the site of virus assembly. These immature virions are then transported through the membrane systems of the host secretory pathway to the cell surface where exocytosis occurs. Shortly before virion release, the prM protein is cleaved by furin or a furin-like cellular proteinase to generate mature virions. Flavivirus-infected cells also release a noninfectious subviral particle that has a lower sedimentation coefficient than whole virus (70S vs. 200S) and exhibits hemagglutination activity (slowly sedimenting hemagglutinin; SHA).
ANTIGENIC PROPERTIES
All flaviviruses are serologically related, which can be demonstrated by binding assays such as ELISA and by hemagglutination-inhibition using polyclonal and monoclonal antibodies. Neutralization assays are more discriminating and have been used to define several serocomplexes of more closely related flaviviruses (see List of Species in the Genus). The envelope protein E is the major target for neutralizing antibodies and induces protective immunity. The E protein also induces flavivirus cross-reactive non-neutralizing antibodies. Antigenic sites involved in neutralization have been mapped to each of the three structural domains of the E protein. Antibodies to prM can also mediate immunity, probably by neutralizing viruses with partially uncleaved prM.
BIOLOGICAL PROPERTIES
HOST RANGE
Flaviviruses can infect a variety of vertebrate species and in many cases arthropods. Some viruses have a limited vertebrate host range (e.g., only primates), others can infect and replicate in a wide variety of species (mammals, birds, etc.). Arthropods are usually infected when they feed on a vertebrate host during viremia, but non-viremic transmission has also been described for tick-borne flaviviruses.
TRANSMISSION
Most flaviviruses are arthropod-borne viruses that are maintained in nature by transmission from hematophagous arthropod vectors to vertebrate hosts. About 50% of known flaviviruses are mosquito-borne, 28% are tick-borne, and the rest are zoonotic agents transmitted between rodents or bats without known arthropod vectors. In some instances, the transmission cycle has not yet been identified. In the arthropod vectors, the viruses may also be passed on trans-ovarially (mosquitoes, ticks) and trans-stadially (ticks).
GEOGRAPHICAL DISTRIBUTION
Flaviviruses have a world-wide distribution but individual species are restricted to specific endemic or epidemic areas (e.g., Yellow fever virus in tropical and subtropical regions of Africa and South America; Dengue virus in tropical areas of Asia, Oceania, Africa, Australia, and the Americas; Japanese encephalitis virus in South-East Asia; Tick-borne encephalitis virus in Europe and Northern Asia).
PATHOGENICITY
More than 50% of known flaviviruses have been associated with human disease, including the most important human pathogens: Yellow fever virus, Dengue virus, Japanese encephalitis virus, West Nile virus and Tick-borne encephalitis virus. Flavivirus-induced diseases may be associated with symptoms of the central nervous system (e.g., meningitis, encephalitis), fever, arthralgia, rash, and hemorrhagic fever. Several flaviviruses are pathogenic for domestic or wild animals (turkey, pig, horse, sheep, dog, grouse, muskrat) and cause economically important diseases.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Species demarcation criteria in the genus include:
-
•
Nucleotide and deduced amino acid sequence data,
-
•
Antigenic characteristics,
-
•
Geographic association,
-
•
Vector association,
-
•
Host association,
-
•
Disease association,
-
•
Ecological characteristics.
Other defined members of individual species, which do not constitute a species on their own, are shown below the species. Those viruses for which insufficient information is available are listed as ‘tentative’ within the group of viruses to which they are most closely related by sequence analysis.
LIST OF SPECIES IN THE GENUS
Virus species in the Genus can be grouped serologically and in terms of their vector preferences as shown in the list provided.
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| ||
| Mammalian tick-borne virus group | ||
| Gadgets Gully virus | ||
| Gadgets Gully virus | [AF013374] | (GGYV) |
| Kyasanur Forest disease virus | ||
| Kyasanur Forest disease virus | [X74111] | (KFDV) |
| Langat virus | ||
| Langat virus | [M73835] | (LGTV) |
| Louping ill virus | ||
| Louping ill virus | [Y07863] | (LIV) |
| British subtype | [D12937] | (LIV-Brit) |
| Irish subtype | [X86784] | (LIV-Ir) |
| Spanish subtype | [X77470] | (LIV-Span) |
| Turkish subtype | [X69125] | (LIV-Turk) |
| Omsk hemorrhagic fever virus | ||
| Omsk hemorrhagic fever virus | [X66694] | (OHFV) |
| Powassan virus | ||
| Powassan virus | [L06436] | (POWV) |
| Royal Farm virus | ||
| Karshi virus | [AF013381] | (KSIV) |
| Royal Farm virus | [AF013398] | (RFV) |
| Tick-borne encephalitis virus | ||
| Tick-borne encephalitis virus | (TBEV) | |
| European subtype | [M27157, M33668] | (TBEV-Eu) |
| Far Eastern subtype | [X07755] | (TBEV-FE) |
| Siberian subtype | [L40361] | (TBEV-Sib) |
| Seabird tick-borne virus group | ||
| Kadam virus | ||
| Kadam virus | [AF013380] | (KADV) |
| Meaban virus | ||
| Meaban virus | [AF013386] | (MEAV) |
| Saumarez Reef virus | ||
| Saumarez Reef virus | [X80589] | (SREV) |
| Tyuleniy virus | ||
| Tyuleniy virus | [X80588] | (TYUV) |
| ||
| Aroa virus group | ||
| Aroa virus | ||
| Aroa virus | [AF013362] | (AROAV) |
| Bussuquara virus | [AF013366] | (BSQV) |
| Iguape virus | [AF013375] | (IGUV) |
| Naranjal virus | [AF013390] | (NJLV) |
| Dengue virus group | ||
| Dengue virus | ||
| Dengue virus 1 | [23027] | (DENV-1) |
| Dengue virus 2 | [M19197] | (DENV-2) |
| Dengue virus 3 | [A34774] | (DENV-3) |
| Dengue virus 4 | [M14931] | (DENV-4) |
| Kedougou virus | ||
| Kedougou virus | [AF013382] | (KEDV) |
| Japanese encephalitis virus group | ||
| Cacipacore virus | ||
| Cacipacore virus | [AF013367] | (CPCV) |
| Japanese encephalitis virus | ||
| Japanese encephalitis virus | [M18370] | (JEV) |
| Koutango virus | ||
| Koutango virus | [AF013384] | (KOUV) |
| Murray Valley encephalitis virus | ||
| Alfuy virus | [AF013360] | (ALFV) |
| Murray Valley encephalitis virus | [X03467] | (MVEV) |
| St. Louis encephalitis virus | ||
| St. Louis encephalitis virus | [M1661] | (SLEV) |
| Usutu virus | ||
| Usutu virus | [AF013412] | (USUV) |
| West Nile virus | ||
| Kunjin virus | [D00246] | (KUNV) |
| West Nile virus | [M12294] | (WNV) |
| Yaounde virus | ||
| Yaounde virus | [AF013413] | (YAOV) |
| Kokobera virus group | ||
| Kokobera virus | ||
| Kokobera virus | [AF013383] | (KOKV) |
| Stratford virus | [AF013407] | (STRV) |
| Ntaya virus group | ||
| Bagaza virus | ||
| Bagaza virus | [AF013363] | (BAGV) |
| Ilheus virus | ||
| Ilheus virus | [AF013376] | (ILHV) |
| Rocio virus | [AF013397] | (ROCV) |
| Israel turkey meningoencephalomyelitis virus | ||
| Israel turkey meningoencephalomyelitis virus | [AF013377] | (ITV) |
| Ntaya virus | ||
| Ntaya virus | [AF013392] | (NTAV) |
| Tembusu virus | ||
| Tembusu virus | [AF013408] | (TMUV) |
| Spondweni virus group | ||
| Zika virus | ||
| Spondweni virus | [AF013406] | (SPOV) |
| Zika virus | [AF013415] | (ZIKV) |
| Yellow fever virus group | ||
| Banzi virus | ||
| Banzi virus | [L40951] | (BANV) |
| Bouboui virus | ||
| Bouboui virus | [AF013364] | (BOUV) |
| Edge Hill virus | ||
| Edge Hill virus | [AF013372] | (EHV) |
| Jugra virus | ||
| Jugra virus | [AF013378] | (JUGV) |
| Saboya virus | ||
| Potiskum virus | [AF013395] | (POTV) |
| Saboya virus | [AF013400] | (SABV) |
| Sepik virus | ||
| Sepik virus | [AF013404] | (SEPV) |
| Uganda S virus | ||
| Uganda S virus | (UGSV) | |
| Wesselsbron virus | ||
| Wesselsbron virus | (WESSV) | |
| Yellow fever virus | ||
| Yellow fever virus | [X03700] | (YFV) |
| ||
| Entebbe bat virus group | ||
| Entebbe bat virus | ||
| Entebbe bat virus | [AF013373] | (ENTV) |
| Sokoluk virus | [AF013405] | (SOKV) |
| Yokose virus | ||
| Yokose virus | [AF013414] | (YOKV) |
| Modoc virus group | ||
| Apoi virus | ||
| Apoi virus | [AF013361] | (APOIV) |
| Cowbone Ridge virus | ||
| Cowbone Ridge virus | [AF013370] | (CRV) |
| Jutiapa virus | ||
| Jutiapa virus | [AF013379] | (JUTV) |
| Modoc virus | ||
| Modoc virus | [AF013387] | (MODV) |
| Sal Vieja virus | ||
| Sal Vieja virus | [AF013401] | (SVV) |
| San Perlita virus | ||
| San Perlita virus | [AF013402] | (SPV) |
| Rio Bravo virus group | ||
| Bukalasa bat virus | ||
| Bukalasa bat virus | [AF013365] | (BBV) |
| Carey Island virus | ||
| Carey Island virus | [AF013368] | (CIV) |
| Dakar bat virus | ||
| Dakar bat virus | [AF013371] | (DBV) |
| Montana myotis leukoencephalitis virus | ||
| Montana myotis leukoencephalitis virus | [AF013388] | (MMLV) |
| Phnom Penh bat virus | ||
| Batu Cave virus | [AF013369] | (BCV) |
| Phnom Penh bat virus | [AF013394] | (PPBV) |
| Rio Bravo virus | ||
| Rio Bravo virus | [AF013396] | (RBV) |
TENTATIVE SPECIES IN THE GENUS
| Cell fusing agent virus | [M91671] | (CFAV) |
| Tamana bat virus | (TABV) |
GENUS PESTIVIRUS
Type Species Bovine viral diarrhea virus 1
DISTINGUISHING FEATURES
Relative to the other genera, pestiviruses encode two unique gene products, namely Npro and Erns. The first protein of the ORF, nonstructural protein Npro, which possesses an autoproteolytic activity and is responsible for its release from the nascent polyprotein, is not essential for virus replication in cell culture. One of the three viral envelope glycoproteins, Erns, possesses an intrinsic RNAse activity. Two biotypes of pestiviruses, cytopathogenic (cp) and non-cytopathogenic (noncp) viruses, are distinguished by their ability to cause cytopathic effects in cell culture.
VIRION PROPERTIES
MORPHOLOGY
Virions are 40-60 nm in diameter and spherical in shape (Fig. 3 ). The virion envelope has 10-12 nm ring-like subunits on its surface. Structure and symmetry of the core have not been characterized.
Figure 3.

Negative contrast electron micrograph of particles of an isolate of Bovine viral diarrhea virus 1. The bar represents 100 nm.
(From M. König, with permission).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr has not been determined precisely, but can be estimated from the virus composition to be ∼6 × 107. Buoyant density in sucrose is 1.10-1.15 g/cm3; S20W is 140-150S. Virion infectivity is stable over a relatively broad pH range, but unstable at temperatures above 40°C. Organic solvents and detergents rapidly inactivate the viruses.
NUCLEIC ACID
The virion RNA is a positive sense, infectious molecule of ssRNA ∼12.3 kb in size. The 5′-NCR contains an IRES and is ∼370-385 nt in length. The 3′-NCR with ∼185-273 nt in length is complex and contains a region with variable sequences and a highly conserved terminal region. Genomic RNA contains a single ORF spanning the viral genome. For some cp pestivirus strains, a small and variable segment of host cell or viral nucleic acid is integrated into particular regions (often within NS2 or at the junction between NS2 and NS3) of the viral genome, sometimes accompanied by viral gene duplications or deletions. Other cp pestiviruses contain only viral gene duplications involving all or part of the Npro and NS3 protein-coding regions, resulting in genomic RNA of up to ∼16.5 kb. In all cases, the single large ORF is maintained. Finally, cp viruses may also arise by deletion of large portions of their genomes. Such defective genomes may be rescued by intact helper viruses.
PROTEINS
Virions are composed of 4 structural proteins: a basic nucleocapsid core protein, C (14 kDa), and 3 envelope glycoproteins, Erns (gp44/48), E1 (gp33) and E2 (gp55). All three glycoproteins exist as intermolecular disulfide-linked complexes: Erns homodimers, E1-E2 heterodimers, and E2 homodimers. Erns possesses an intrinsic RNase activity. Pestiviruses encode 7-8 non-structural (NS) proteins among which Npro (23 kDa), p7 (7 kDa) and NS2 (40 kDa) are not necessary for RNA replication. Npro is a proteinase that autocatalytically releases itself from the nascent polyprotein. Nonstructural protein p7 is presumed to have a role in virus maturation. NS2-3 (120 kDa) is a multifunctional protein. The N-terminal 40% (NS2) is hydrophobic and contains a zinc finger motif suggesting divalent metal ion binding. The C-terminal 60% (NS3, 80 kDa) acts as both a serine proteinase involved in polyprotein processing and an RNA helicase/NTPase likely involved in RNA replication. NS2-3 is found after infection with all pestiviruses. In cells infected with cp pestiviruses, large amounts of NS3 can be detected. For some noncp BDV and CSFV strains, a minor part of NS2-3 is cleaved into NS3 and NS2. The NS4A (7 kDa) protein acts as a cofactor to the NS3 proteinase activity. The role of NS4B (33 kDa) is unknown. NS5A (58 kDa) represents a phosphorylated protein and presumably also plays a yet to be identified role in RNA replication. NS5B (75 kDa) possesses RdRp activity.
LIPIDS
The viruses are enveloped, but no reports have described the lipid composition.
CARBOHYDRATES
All virus envelope glycoproteins contain N-linked glycans.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains a single large ORF encoding a polyprotein of ∼3,900 aa that is preceded by a 5′-NCR of ∼370-385 nt and followed by a 3′-NCR of ∼185-273 nt. The gene order is 5′-Npro-C-Erns-E1-E2-p7-NS2-3(NS2-NS3)-NS4A-NS4B-NS5A-NS5B-3′ (Fig. 4 ).
Figure 4.

Pestivirus genome organization (not to scale) and polyprotein processing. The RNA is usually ∼12.3 kb in size (depending on the virus). The 5′-NCR is ∼370-385 nt, the ORF ∼11.7 kb and the 3′-NCR is 185-273 nt. P′, P″, H, and R symbols indicate the localization of the Npro proteinase, the NS3 proteinase, the NS3 RNA helicase, and the NS5B RdRp, respectively. The viral non-structural proteins are indicated as NS. The proteinases (where known) and proteolytic steps involved in the generation of individual proteins are indicated. In noncp BVD viruses, NS2-3 is not cleaved. In cp BVD viruses, NS3 is produced in addition to NS2-3. If NS3 production is a result of NS3 gene duplication, NS2 is not necessarily produced.
Pestivirus replication is probably initiated by receptor-mediated endocytosis involving one or more cell surface molecules and viral glycoproteins Erns and E2. After endocytosis and uncoating, the genome RNA serves as mRNA; there are no subgenomic mRNA molecules. Translation initiation occurs by a cap-independent internal initiation mechanism involving an IRES within the 5′-NCR of the RNA. Polyprotein processing occurs co- and post-translationally by both cellular and viral proteinases. Nonstructural protein Npro, the first protein of the ORF, autoproteolytically removes itself from the nascent polyprotein by cleavage at the Npro/C site. Downstream cleavages that produce structural proteins Erns, E1 and E2 as well as p7 are probably mediated by cellular signal peptidase(s). Glycoprotein translocation to the endoplasmic reticulum probably occurs by an internal signal sequence, perhaps within the C protein. Cleavage between E2 and p7 is not complete, leading to two intracellular forms of E2 with different C-termini. Depending on the particular pestivirus and its biotype, NS2-3 remains intact or is found together with its N- and C-terminal products NS2 and NS3. The generation of NS3 in cp pestiviruses is in most cases due to RNA recombination. Most cp pestiviruses have gene insertions, deletions, duplications or rearrangements that result in (enhanced) NS3 production. The NS3/NS2-3 serine proteinase activity is responsible for all processing events downstream of NS3. NS4A facilitates cleavages by NS3 of sites 4B/5A and 5A/5B.
RNA replication occurs most likely in association with intracytoplasmic membranes, presumably in a replication complex composed of viral RNA and viral nonstructural proteins. Nonstructural proteins NS3, 4A, 4B, 5A and 5B are necessary for RNA replication; only NS5A can be provided in trans. Replicative forms of pestiviral RNA have been detected. The ratio of positive-to-negative sense RNA in cells 12 hr post-infection is about 10. RNA synthesis is resistant to actinomycin D. Virus maturation is poorly understood. However, viral proteins are not found on the cell surface, suggesting that viruses mature in intracellular vesicles and are released by exocytosis. Considerable amounts of infectious virus remain cell-associated. Host cell RNA and protein synthesis continue throughout infection.
ANTIGENIC PROPERTIES
Pestiviruses are antigenically related, and cross-reactive epitopes are present on all pestiviruses. Separate antigenic determinants defined by monoclonal antibodies (Mabs) have also been identified. Antigenic variation is particularly pronounced among BVDV and BDV isolates. The N-terminal portion of E2 contains an antigenically hypervariable region. Mab binding patterns are generally consistent with the genetic relatedness of viruses.
Infected animals mount potent antibody responses to two structural glycoproteins (Erns, E2) and to the NS2-3/NS3 protein, while antibody responses are weak or nonexistent to other virus-encoded polypeptides. Erns and E2 are able to induce protection independently. Mabs reactive with these proteins can neutralize virus infectivity. Antibodies to NS2-3/NS3 are not able to neutralize virus infectivity.
BIOLOGICAL PROPERTIES
HOST RANGE
Pestiviruses infect pigs and ruminants, including cattle, sheep, goats and wild ruminants. There are no invertebrate hosts.
TRANSMISSION
Transmission occurs by direct and indirect contact (e.g., nasal or urine secretion, contaminated food, etc.). Transplacental and congenital transmission occur in all host species.
PATHOGENICITY
Pestivirus infections may be subclinical or produce a range of clinical conditions including acute diarrhea, acute hemorrhagic syndrome, acute fatal disease, and a wasting disease. Diplacental infection can result in fetal death, congenital abnormalities, or lifelong persistent infection. Fatal mucosal disease can occur in cattle persistently infected with noncp viruses when a cp virus is generated by mutation or introduced by superinfection. Pestivirus infections of livestock are economically important worldwide.
EXPERIMENTAL HOSTS
Experimental infection models have not been established for bovine or ovine pestiviruses outside their natural mammalian hosts. However, CSFV can be adapted to propagate in rabbits.
CELL CULTURE
Cells derived from natural host species (bovine, porcine, ovine) support virus replication. Most virus isolates are noncp and can establish persistent infections in cell culture. Infectious noncp BVDV is often present in bovine serum products used for cell culture. Cp pestiviruses induce extensive cytopathology and form virus plaques under appropriate conditions. Death of cp pestivirus infected cells is due to apoptosis. It is not known whether apoptosis is induced by cp viruses or actively blocked by noncp viruses.
HEMAGGLUTINATION
No hemagglutinating activity has been found associated with pestiviruses.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Species demarcation criteria in the genus includes:
-
•
Nucleotide sequence relatedness,
-
•
Serological relatedness,
-
•
Host of origin.
Pestivirus species demarcation considers several parameters and their relationship to the type viruses of the currently recognized species (BVDV-1-NADL; BVDV-2-890; BDV-BD31; and CSFV-A187). Nucleotide sequence relatedness is an important criterion for pestivirus species demarcation. For example, the 5′-NCR sequences among the four currently recognized species are over 15% divergent. In most cases, the degree of homology within the 5′-NCR will allow pestivirus species demarcation. However, in some cases the nt sequence relatedness may be ambiguous and must be complemented with additional comparative analyses. Convalescent animal sera generated against members of a given pestivirus species (e.g., Bovine viral diarrhea virus 1) generally show a several-fold higher neutralization titer against viruses of the same same species than against viruses from the other species. Finally, differences in host of origin and disease can assist in species identification.
For example, Bovine viral diarrhea virus 1 and Classical swine fever virus are considered different species because their members differ from each other by: (i) at least 25% at the sequence level (complete genomes), (ii) at least 10-fold difference in neutralization titer in cross-neutralization tests with polyclonal immune sera, and (iii) host range, in that under natural conditions CSFV infects only pigs while BVDV-1 infects ruminants as well as pigs.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Border disease virus | ||
| Border disease virus – BD31 | [U70263] | (BDV-BD31) |
| Border disease virus – X818 | [AF037405] | (BDV-X818) |
| Bovine viral diarrhea virus 1 | ||
| Bovine viral diarrhea virus 1-CP7 | [U63479] | (BVDV-1-CP7) |
| Bovine viral diarrhea virus 1-NADL | [M31182] | (BVDV-1-NADL) |
| Bovine viral diarrhea virus 1-Osloss | [M96687] | (BVDV-1-O) |
| Bovine viral diarrhea virus 1-SD1 | [M96751] | (BVDV-1-SD1) |
| Bovine viral diarrhea virus 2 | ||
| Bovine viral diarrhea virus 2-C413 | [AF002227] | (BVDV-2-C413) |
| Bovine viral diarrhea virus 2-NewYork’93 | [AF502399] | (BVDV-2-NY93) |
| Bovine viral diarrhea virus 2-strain 890 | [U18059] | (BVDV-2-890) |
| Classical swine fever virus | ||
| (Hog cholera virus) | ||
| Classical swine fever virus – Alfort/187 | [X87939] | (CSFV-A187) |
| Classical swine fever virus – Alfort-Tübingen | [J04358] | (CSFV-ATub) |
| Classical swine fever virus – Brescia | [M31768] | (CSFV-Bre) |
| Classical swine fever virus – C | [Z46258] | (CSFV-C) |
TENTATIVE SPECIES IN THE GENUS
| Pestivirus of giraffe | [AF144617] |
GENUS HEPACIVIRUS
Type Species Hepatitis C virus
DISTINGUISHING FEATURES
Hepaciviruses are transmitted between humans, principally via exposure to contaminated blood or blood products. There is no known invertebrate vector. Hepaciviruses differ from the other Flaviviridae genera by their inability to be propagated efficiently in cell culture. In the hepacivirus precursor protein, the NS2-3 junction is autocatalytically cleaved by a Zn-dependent NS2-3 proteinase activity.
VIRION PROPERTIES
MORPHOLOGY
Virions are ∼50 nm in diameter, as determined by filtration and electron microscopy. They are spherical in shape and contain a lipid envelope, as determined by electron microscopy and inactivation by chloroform. The viral core is spherical and ∼30 nm in diameter. Detailed structural properties have not been determined.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr has not been determined. Buoyant density in sucrose is ∼1.06 g/cm3 when the virus is recovered from the serum of an acute infection and ∼1.15-1.18 g/cm3 when recovered from the serum of a chronically infected patient. The lower density results from physical association of the virion with serum very-low-density lipoproteins. The higher density results from the binding of serum antibodies to the virion. A buoyant density in sucrose of 1.12 g/cm3 has been measured for Hepatitis C virus (HCV) recovered from cell culture. The S20w is equal to or greater than 150S. The virus is stable in buffer at pH 8.0-8.7. Virions are sensitive to heat, organic solvents and detergents.
NUCLEIC ACID
Virions contain a single positive-sense, infectious ssRNA (Fig. 5 ). The genome length is ∼9.6 kb. The 5′-NCR contains an IRES and is 341 nt in length. The 3′-NCR contains a sequence-variable region of ∼50 nt, a polypyrimidine-rich region averaging ∼100 nt (but variable in length) and a highly conserved terminal region (98 nt).
Figure 5.

Hepacivirus genome organization (not to scale) and polyprotein processing. The RNA is ∼9.6 kb in size. The 5′-NCR is 341 nt, the 3′-NCR is ∼250 nt, and the ORF is ∼9 kb. The proteinases involved in cleavage of the polyprotein are indicated. The structural proteins are shown in dark. The locations of the proteinases, helicase and RdRp are indicated by P′, P″, H and R, respectively.
PROTEINS
The virion consists of at least 3 proteins: the nucleocapsid core protein C (p19), and two envelope proteins, E1 (gp31) and E2 (gp70). One or more additional proteins, resulting from ribosomal frame-shifting within the C gene, have been reported although it is not known if these are part of the virion. An additional protein, p7 (believed to have properties of an ion channel protein), is incompletely cleaved from a precursor of E2 to yield E2-p7 and p7, but it is not known whether these are virion structural components. The two envelope proteins form heterodimers (probably non-covalently linked) in virions. The recognized nonstructural proteins include NS2, (21 kDa protein that, before cleavage, is part of a Zn-dependent proteinase that bridges NS2 and NS3 and mediates autocatalytic cleavage of the NS2/NS3 junction), NS3 (70 kDa protein with additional serine proteinase, helicase and NTPase acitivities; the NS3 proteinase cleaves the remaining junctions between nonstructural proteins), NS4A (6 kDa cofactor essential for NS3 serine proteinase activity), NS4B (27 kDa protein that induces a membranous replication complex at the endoplasmic reticulum), NS5A (a serine phosphoprotein of unknown function that exists in 56 and 58 kDa forms, depending on the degree of phosphorylation) and NS5B (68 kDa protein with RdRp activity).
LIPIDS
Lipids have not been demonstrated directly. However, based on observed removal of the viral envelope and loss of infectivity following exposure to solvents or detergents, the presence of lipids is inferred.
CARBOHYDRATES
Carbohydrates have not been demonstrated directly but the presence of glycosylation sites in the predicted coding sequences of the E1 and E2 genes, and the demonstration of carbohydrate associated with the products of these two genes expressed as recombinant proteins is consistent with the presence of carbohydrates in virions. When expressed in transfected cells, the E1 and E2 gene products normally remain tightly anchored within the lumen of the endoplasmic reticulum and contain high-mannose chains lacking complex carbohydrate.
GENOME ORGANIZATION AND REPLICATION
The genome contains a single large ORF encoding a polyprotein of ∼3000 aa (Fig. 5). The gene order is 5′-C-E1-E2-p7-NS2-NS3-NS4A-NS4B-NS5A-NS5B-3′. All 3 structural proteins (C, E1, E2), are encoded within the amino-terminal portion of the large ORF. Immediately downstream is the small protein, p7. The non-structural proteins are encoded in the 3′ portion of the ORF. Replication is poorly understood but is believed to occur in association with intracytoplasmic membranes. Replicative forms of viral RNA have been detected in liver tissue. The genomic RNA is translated into a polyprotein that is rapidly processed both co- and post-translationally. Translation initiation occurs via an IRES within the 5′-NCR, which also contains several closely spaced AUGs. Translocation of the structural glycoproteins to the endoplasmic reticulum probably occurs via an internal signal sequence. Cleavage of the structural proteins is effected by host cell signal peptidases, and signal peptide peptidase. Viral proteinases cleave all non-structural protein junctions. Virus assembly is believed to occur by budding into vesicles from the endoplasmic reticulum.
ANTIGENIC PROPERTIES
Virus-specific antibodies to recombinant-expressed structural proteins (C, E1 and E2) and non-structural proteins (principally NS3, NS4 and NS5) have been detected in individuals infected with HCV. Both linear and conformational epitopes are believed to be involved in the humoral immune response of the host to infection. Significant genetic heterogeneity throughout the genome is reflected in some serologic heterogeneity in the humoral immune response, especially to the product of the NS4 gene. The most extensive heterogeneity of HCV is found in the N-terminal 27 aa of E2 (hypervariable region 1; HVR-1). There is some evidence that HVR-1 is a neutralization epitope of HCV and that neutralization-escape variants of HVR-1 are positively selected by the humoral immune response of the host. Other neutralization epitopes may exist but have not been defined. Cell-mediated immune responses to all HCV proteins have been detected; it is believed that such responses are associated with amelioration or resolution of infection. Because there is no efficient or standardized cell culture system for the propagation of HCV, it has not been possible to carry out in vitro virus neutralization assays.
BIOLOGICAL PROPERTIES
HOST RANGE
Humans are the natural host and apparent reservoir of HCV. The virus can be transmitted experimentally to chimpanzees. No other natural host has been identified.
TRANSMISSION
Hepatitis C virus is transmitted almost exclusively by parenteral exposure to blood, blood products and objects contaminated with blood. Effective screening of blood donors and implementation of inactivation procedures have virtually eliminated the transmission of HCV via blood and blood products, but other routes of exposure, principally via blood-contaminated syringes, are now the most important recognized risk factors in developed countries. Sexual and perinatal transmission have been reported but are relatively uncommon.
GEOGRAPHICAL DISTRIBUTION
HCV has a worldwide distribution. Antibody-based epidemiological studies suggest that ∼0.1-2% of the populations of developed countries may be infected with HCV, but antibody prevalence as high as 20% has been detected in some developing countries. The high prevalence of antibody to HCV is thought to be the result of using contaminated needles and syringes in such countries. Overall, it has been estimated that about 3% of the world population has been infected with HCV, resulting in ∼170 million chronically infected individuals.
PATHOGENICITY
Infections range from subclinical to clinical acute and chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. Persistence of the virus occurs in ∼80% of HCV infections. Of these, ∼20% progress to chronic active hepatitis and cirrhosis, usually over the course of many years. Persistent HCV infection has been epidemiologically linked to primary liver cancer, cryptogenic cirrhosis, and some forms of auto-immune hepatitis. Extra-hepatic manifestations of HCV infection include mixed cryoglobulinemia with associated membranoproliferative glomerulonephritis and, possibly, porphyria cutanea tarda, Sjögren's-like syndromes and other autoimmune conditions.
CELL TROPISM
HCV has been reported to replicate in several cell lines derived from hepatocytes and lymphocytes, but virus growth has not been sufficient for practical application of these systems. In vivo, HCV replicates in hepatocytes and possibly in lymphocytes.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Hepatitis C virus can be classified into 6 genetic groups, based upon the genome-wide heterogeneity of isolates recovered throughout the world. These have been called HCV clades 1-6; clades differ from each other by ∼25-35% at the nt level. Genotypes 7-11 have been described, but more extensive genetic analysis has placed genotypes 7, 8, 9 and 11 within clade 6 and genotype 10 within clade 3. The 6 clades have been further subdivided into over 100 subtypes. These differ from each other by ∼15-25% at the nt level. Although the clades are more or less distinct, discrimination of subtypes is less clear, owing to overlap in the degree of heterogeneity. Because serotyping of HCV isolates is not possible at present, and because major genotypes do not have any other taxonomic characteristics except, in some cases, geographic distribution, the 6 genetic groups of HCV currently comprise one species.
LIST OF SPECIES IN THE GENUS
A number of clades are recognized for Hepatitis C virus. Examples are listed below.
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Hepatitis C virus | ||
| HCV clade 1 | ||
| HCV genotype 1a | [M62321] | (HCV-1) |
| HCV genotype 1b | [D90208] | (HCV-J) |
| HCV clade 2 | ||
| HCV genotype 2a | [D00944] | (HCV-J6) |
| HCV genotype 2b | [D01221] | (HCV-J8) |
| HCV clade 3 | ||
| HCV genotype 3a | [D17763] | (HCV-NZL1) |
| HCV genotype 10 | [D63821] | (HCV-JK049) |
| HCV clade 4 | ||
| HCV genotype 4a | [Y11604] | (HCV-ED43) |
| HCV clade 5 | ||
| HCV genotype 5a | [Y13184] | (HCV-EVH1480) |
| HCV clade 6 | ||
| HCV genotype 6a | [Y12083] | (HCV-EUHK2) |
| HCV genotype 11 | [D63822] | (HCV-JK046) |
TENTATIVE SPECIES IN THE GENUS
Phylogenetically, GBV-B is related to but distinct from the hepatitis C viruses. Like the latter, it causes hepatitis and replicates in the liver. However, it only infects tamarins and owl monkeys, not humans or chimpanzees. Only one isolate of GBV-B has been identified to date, in contrast to the several hundred HCV isolates. While clearly related to HCV at the level of both nucleotide sequence and genetic organization, there is more sequence divergence evident between GBV-B and HCV (28% aa identity between the encoded polyproteins) than within the HCV species itself (> 60% aa identity). GBV-B causes self-limiting hepatitis in tamarins and owl monkeys, whereas HCV typically causes chronic hepatitis in man and chimpanzees.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
Two distinct groups of viruses have been assigned tentatively to the family Flaviviridae, based upon their genomic organization and genetic similarity to recognized members of the family.
DISTINGUISHING FEATURES
GBV-A and GBV-A-like agents are a group of related viruses that have been identified in at least 6 species of New World monkeys. They do not cause hepatitis in the unique host species of each virus nor in other susceptible species. Their organ site of replication has not been identified and, although the viruses are transmissible via blood, their natural route of transmission is unknown. These viruses share an overall genomic organization and distant genetic similarity with hepaciviruses, but differ in that they appear to lack a complete nucleocapsid protein gene and the organization of their 3′-NCR is less complex than that of the hepaciviruses.
DISTINGUISHING FEATURES
GBV-C is a genetically heterogeneous virus of human and chimpanzee origin. It is transmitted via blood and blood products and possibly sexually, but other routes of transmission may exist. Although originally described as a hepatitis virus, it rarely, if ever, causes hepatitis, and its pathogenicity and organ site of replication remain controversial. Lymphocytes may be its primary site of replication. Although distinct, GBV-C is most closely related to the GBV-A group of viruses, both in genomic organization and genetic relatedness.
Figure 6.

Phylogenetic relationship of the helicase region of members of the family Flaviviridae. Partial gene sequences (∼280 aa) from the proposed helicase region were used for the phylogenetic analysis and included representative strains from each genus. CLUSTALX v1.81 was used to create a multiple alignment for the aa sequences which was verified by alignment of the known motifs in the region (e.g., GxGKS/T). An unrooted phylogenetic tree was constructed from the sequence alignment using the distance method, Neighbor-joining from the PAUP 4b10 package within a Macintosh environment. The virus names corresponding to the abbreviations can be found in the “List of Species” in each genus and the Genbank accession numbers are AF037405, U18059, M31182, J04358, M87512, M29095, U22303, U22304, NC 002348, AF144617, M62321, D17763, D00944, D10988, X61596, M18370, M12294, NC_002031. Bootstrap replicates of 1000 trees was also examined using PAUP and the percentages are drawn at the branch points of the tree.
(Courtesy of L. McMullan).
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
SIMILARITY WITH OTHER TAXA
None reported.
DERIVATION OF NAMES
Flavi: from Latin flavus, ”yellow”.
Hepaci: from Greek hepar, hepatos, ”liver”.
Pesti: from Latin pestis, ”plague”.
REFERENCES
- Avalos-Ramirez R., Orlich M., Thiel H.-J., Becher P. Evidence for the presence of two novel pestivirus species. Virology. 2001;286:456–465. doi: 10.1006/viro.2001.1001. [DOI] [PubMed] [Google Scholar]
- Gould E.A., de Lamballerie X., Zanotto P.M., Holmes E.C. Evolution, epidemiology and dispersal of flaviviruses revealed by molecular phylogenies. Adv. Virus Res. 2001;57:71–103. doi: 10.1016/s0065-3527(01)57001-3. [DOI] [PubMed] [Google Scholar]
- Kuhn R.J., Zhang W., Rossmann M.G., Pletnev S.V., Corver J., Lenches E., Jones C.T., Mukhopadhyay S., Chipman P.R., Strauss E.G., Baker T.S., Strauss J.H. Structure of Dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno G., Chang G.-J.J., Tsuchiya K.R., Karabatsos N., Cropp C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998;72:73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach B.D., Rice C.M. Flaviviridae: The viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 991–1041. [Google Scholar]
- Major M.E., Rehermann B., Feinstone S.M. Hepatitis C viruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott Williams and Wilkins; Philadephia: 2001. pp. 1127–1161. [Google Scholar]
- Meyers G., Thiel H.-J. Molecular characterization of pestiviruses. Adv. Virus Res. 1996;47:53–118. doi: 10.1016/s0065-3527(08)60734-4. [DOI] [PubMed] [Google Scholar]
- Modis Y., Ogata S., Clements D., Harrison S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath T.P., Heinz F.X. Flaviviruses. In: Fields B.N., Knipe B.M., Howley P.M., editors. Fields Virology. Lippincott-Raven; Philadelphia: 1996. pp. 961–1034. [Google Scholar]
- Rey F.A., Heinz F.X., Mandl C., Kunz C., Harrison S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- Robertson B. Viral hepatitis and primates: historical and molecular analysis of human and nonhuman primate hepatitis A, B, and the GB-related viruses. J. Viral Hepatitis. 2001;8:233–242. doi: 10.1046/j.1365-2893.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- Simmonds P. The origin and evolution of hepatitis viruses in humans. J. Gen. Virol. 2001;82:693–712. doi: 10.1099/0022-1317-82-4-693. [DOI] [PubMed] [Google Scholar]
TOGAVIRIDAE
CONTRIBUTED BY, S.C Weaver, T. K Frey, H.V. Huang, R.M. Kinney, CM. Rice, J.T. Roehrig, R.E. Shope, E.G. Strauss
FAMILY TOGAVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Togaviridae |
| Genus | Alphavirus |
| Genus | Rubivirus |
VIRION PROPERTIES
MORPHOLOGY
Virions are 70 nm in diameter, spherical, with a lipid envelope containing heterodimeric glycoprotein spikes composed of two virus glycoproteins. For alphaviruses, the heterodimers are organized in a T=4 icosahedral lattice consisting of 80 trimers (Fig. 1 ). The envelope is tightly organized around an icosahedral nucleocapsid that is 40 nm in diameter. The nucleocapsid is composed of 240 copies of the CP, organized in a T=4 icosahedral symmetry, and the genomic RNA. Some virions such as those of Aura virus also encapsidate the 26S sgRNA. The Rubella virus (RUBV) core is reported to have T=3 symmetry, and is composed of genomic RNA and multiple copies of the CP in the form of homodimers. For alphaviruses, the one-to-one relation between glycoprotein heterodimers and nucleocapsid proteins is believed to be important in virus assembly. The E1 envelope glycoprotein, which as been solved structurally by crystallography, is the fusion protein for entry into the cytoplasm from acidic endosomes. The E2 envelope glycoprotein predominates in the viral spikes that extend outward from the envelope, and forms the petals of the spike that cover the underlying E1 protein fusion peptide at neutral pH. The three dimensional structure of RUBV virions has not been determined. RUBV virions are 60-70 nm in diameter and pleomorphic in nature, indicating that the capsid-glycoprotein interaction is not as tight as it is in virions of alphaviruses.
Figure 1.

(Top panel) On the left a diagrammatic representation of Sindbis virus (SINV) particle. The knobs on the surface represent the external portions of the E1+E2 heterodimers. The heterodimers associate to form trimers. The 240 heterodimers and 240 copies of SINV capsid (C) proteins are arranged in an icosahedral lattice with a T=4 symmetry (from Harrison, 1990). On the upper right; thin section of pelletted particles of Semliki forest virus (SFV) (Courtesy of B.V.V. Prasad). On the lower right, negative contrast electron micrograph of particles of SFV (Courtesy of C.H. von Bonsdorff). The bars represent 100 nm. (Bottom panel) Structure of Sindbis virus particle. Left: Surface shaded view as determined by cryo-electronmicroscopy and image reconstruction. The view is looking down the icosahedral 3-fold axis. The image is calculated to 20Å resolution. Center: Surface view of SINV particle showing the organization of the E1 glycoprotein on the surface of the virus particle. The C-alpha backbone of E1 is shown in white. The view is identical to that shown in the left panel. Right: The image represents the nucleocapsid core showing the pentameric and hexameric capsomeres. 240 copies of the nucleocapsid protein together with the genome RNA form a T=4 icosahedron.
(Courtesy of R. Kuhn).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is about 52 × 106. Alphaviruses have a buoyant density in sucrose of 1.22 g/cm3 and an S20w of 280S. RUBV has a buoyant density of 1.18-1.19 g/cm3 and a similar S value. Alphaviruses are stable between pH 7 and 8, but are rapidly inactivated by very acidic pH. Virions have a half-life at 37°C of about 7 hr in culture medium. Most alphaviruses are rapidly inactivated at 58°C with a half-life measured in minutes. RUBV virions are more heat labile than alphaviruses, with a half-life at 37°C of 1-2 hrs and a half-life at 58°C of 5-20 min. Generally, togaviruses are sensitive to organic solvents and detergents which solubilize their lipoprotein envelopes. Sensitivity to irradiation is directly proportional to the size of the viral genome.
NUCLEIC ACID
The genome consists of a linear, positive sense, ssRNA molecule 9.7-11.8 kb in size. The viral RNA is capped (7-methylguanosine) at the 5′-terminus and polyadenylated at the 3′-terminus.
PROTEINS
The structural proteins of togaviruses include a basic CP (30-33 kDa) and two envelope glycoproteins, E1 and E2 (45-58 kDa). Some alphaviruses may contain a third envelope protein, E3 (10 kDa). The four non-structural proteins, which are present in infected cells but not found in virions, are called nsP1-nsP4. Their functions are described below.
LIPIDS
Lipids comprise about 30% of the dry weight of virions. They are derived from the host-cell membrane from which budding occurs: the plasma membrane for alphaviruses, both intracellular membranes and the plasma membrane for RUBV. Their composition depends upon the cells in which the virus was grown. Phospholipids (including phosphatidyl ethanolamine, phosphatidyl choline, phosphatidyl serine, and sphingomyelin) and cholesterol are present in a molar ratio of about 2:1 for alphaviruses, 4:1 for RUBV, presumably because the latter matures primarily at intracellular membranes.
CARBOHYDRATES
Both high mannose and complex N-linked glycans are found on the envelope glycoproteins. In addition, RUBV E2 protein contains O-linked glycans.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA serves as the mRNA for the non-structural proteins of the virus (Fig. 2 ). In alphaviruses, the polyprotein precursor is cleaved by a viral-encoded protease in nsP2 to produce four final products, nsP1, nsP2, nsP3 and nsP4 (Fig. 3 ). In eight of ten alphaviruses sequenced, there is a termination codon (UGA) between nsP3 and nsP4 genes which is read-through with moderate efficiency (5-20%), whereas in the two other alphaviruses this codon has been replaced by a codon for arginine (CGA). Polyproteins containing nsP2 are enzymes and function primarily in trans to produce the cleaved non-structural proteins. In RUBV the polyprotein precursor is cleaved into two products, P150 and P90. The protease mediating this cleavage is located near the C-terminus of P150, and can function either in cis or in trans.
Figure 2.

Togavirus genomic coding strategies. Shown are comparative schematic representations of the alphavirus and rubivirus genomic RNAs with untranslated regions represented as solid black lines and ORFs as open boxes (NS-ORF = non-structural protein ORF; S-ORF = structural protein ORF). Within each ORF, the coding sequences for the proteins processed from the translation product of the ORF are delineated. The asterisk between nsP3 and nsP4 in the alphavirus NS-ORF indicates the stop codon present in some alphaviruses that must be translationally read through to produce a precursor containing nsP4. Additionally, within the NS-ORFs, the locations of motifs associated with the following activities are indicated: (Mtr) methyl transferase, (Pro) protease, (Hel) helicase, (X) unknown function, and (Rep) replicase. The sequences encompassed by the sgRNA are also shown.
(Courtesy of T. K. Frey).
Figure 3.

Genome organization, translation, transcription and replication strategies of Sindbis virus (SINV). The regions of the 11.7 kb genomic RNA and 26S sgRNA (dark lines) that code respectively for the non-structural (nsP) and structural proteins (colored boxes) are shown. Replication and transcription are indicated by thick arrows. The grey line is the replicative intermediate that is also the template for the sgRNA. E3 is a structural protein in some alphaviruses (not present in Rubella virus (RUBV). Initiation codons are indicated by (*), termination codons by (†) and ($)(the latter is readthrough to produce P1234, hence nsP4 is cleaved off). Dark triangles represent nsP2 protease activity.
(From Strauss and Strauss, 1994).
The non-structural proteins, as individual entities and as polyproteins, are required to replicate viral RNA and probably act in association with cellular proteins. The alphavirus nsP1 protein is thought to be involved in capping of viral RNAs and in initiation of negative-strand RNA synthesis. The nsP2 functions as a protease to process the non-structural proteins and is believed to be a helicase required for RNA replication. Protein nsP4 is believed to be the viral RNA polymerase. Protein nsP3 is also required for RNA replication; P123 (the uncleaved precursor of nsP1, nsP2 and nsP3) and nsP4 form the replicase complex for minus strand synthesis, whereas efficient plus-strand synthesis requires cleavage of P123. In RUBV, P150 contains a MTR motif of unknown function that is also present in nsP3 of alphaviruses. RUBV P90 contains both helicase and replicase motifs. These motifs are in a different order than in the alphavirus genome. The difference in processing and order of non-structural protein motifs between alphaviruses and RUBV suggests that evolution of these two genera was more complicated than simple divergence from a common ancestor. In RNA replication, a negative-strand copy is produced that is used as template in the synthesis of both genome-sized RNA as well as a subgenomic 26S mRNA that corresponds to the 3′ third of the viral genome and encodes the viral structural proteins.
The mRNA is capped and polyadenylated. It is translated as a polyprotein, which is processed in alphaviruses by a combination of an autoprotease activity present in the CP and cellular organelle-bound proteases, to produce the viral structural proteins. The RUBV CP lacks autoprotease activity, and all of the cleavages of this precursor are mediated by a cellular signal endopeptidase.
Cis-acting regulatory elements in the 5′- and 3′-non-translated regions of the genomic RNA are required to produce alphavirus minus strands and to copy the minus strand into plus strands. There are believed to be other cis-acting regulatory elements within the viral RNA as well. For alphaviruses, the promoter for the production of the 26S sgRNA is a stretch of 24 nt that span the start point of the sgRNA. This minimal 24 nt sequence element is upreg-ulated by upstream sequences. The RUBV subgenomic promoter is 50 nt upstream from the sgRNA start site. RUBV also contains cis-acting untranslated sequences preceding each ORF that are believed to form stem-loops and to regulate translation and RNA replication. RUBV and alphaviruses share homology in the cis-acting elements at the 5′-end of the genome and subgenomic promoter region.
The non-structural proteins function in the cytoplasm of infected cells in association with the surface of membranes, and attachment appears to be mediated by nsP1 palmitoylation. Some alphavirus nsP2 is translocated into the nucleus. The CP assembles with the viral RNA to form the viral nucleocapsids in the cytosol. Glycoproteins inserted into the endoplasmic reticulum during translation are translocated via the Golgi apparatus to the plasma membrane for alphaviruses; for RUBV they are also found at intracellular membranes. Assembled nucleocapsids bud through these membranes and acquire a lipid envelope containing the two integral membrane glycoproteins. For RUBV, the glycoproteins are retained in the Golgi apparatus, the preferred site of budding. Unlike in alphaviruses, rubellavirus capsids are not pre-assembled in the cytosol and only form during the budding process. Late in infection, the rubellavirus glycoproteins also accumulate at the plasma membrane, and budding also occurs at this site.
ANTIGENIC PROPERTIES
Member viruses of the genus Alphavirus were originally defined on the basis of serological cross-reactions. Thus, all alphaviruses are antigenically related to each other. They share a minimum aa sequence identity of about 40% in the more divergent structural proteins and about 60% in the non-structural proteins. Alphaviruses can be grouped into 8 antigenic complexes based on serologic cross-reactivity: the eastern, Venezuelan and western equine encephalitis, Trocara, Middelburg, Ndumu, Semliki Forest and Barmah Forest complexes. The non-arthropod-borne alphaviruses, Salmon pancretic disease and southern elephant seal viruses, are also antigenically distinct from the remaining members of the genus. RUBV is serologically distinct from alphaviruses and no structural protein aa sequence homology can be detected between RUBV and the alphaviruses.
BIOLOGICAL PROPERTIES
Most alphaviruses are transmitted biologically between vertebrates by mosquitoes or other hematophagous arthropods. However, salmon pancreatic disease virus is not known to have an arthropod vector. Alphaviruses have a wide host range and nearly worldwide distribution. The infection of cells of vertebrate origin is generally cytolytic and involves the shutdown of hostcell macromolecular synthesis. In mosquito cells, most alphaviruses usually establish a non-cytolytic infection in which the cells survive and become persistently infected. Cytopathology has been described in the midguts of mosquitoes infected with Eastern equine encephalitis virus (EEEV) and Western equine encephalitis virus (WEEV). In contrast, humans are the only known host for RUBV, which is spread via the respiratory route. RUBV replicates in a number of mammalian cell culture lines, including lines from humans, monkeys, rabbits and hamsters. The virus is not cytopathic in most of these lines and has a propensity to initiate persistent infections.
GENUS ALPHAVIRUS
Type Species Sindbis virus
DISTINGUISHING FEATURES
Genomes are 11-12 kb in size, exclusive of the 3′-terminal poly(A) tract: Sindbis virus (SINV), 11,703 nt; O'nyong-nyong virus (ONNV), 11,835 nt; Ross River virus (RRV), 11,851 nt; VEEV, 11,444 nt; Semliki Forest virus (SFV) 11,442 nt; RNA S20w about 49S. The order of the genes for the non-structural proteins in the genomic RNA is nsP1, nsP2, nsP3, nsP4 (Fig. 2). These are made as polyprotein precursors and processed by the nsP2 protease (Fig. 3). The gene order in the 26S mRNA is CP-E3-E2-6K-E1. The derived polyprotein is processed by an auto-proteolytic activity in the CP, by cellular signal peptidase, and by an enzyme thought to be a component of the Golgi apparatus (Fig. 3). Glycoprotein E2 is produced as a precursor, PE2 (otherwise called p62), that is cleaved during virus maturation. For some viruses the N-terminal cleavage product of PE2, referred to as E3 (∼10 kDa), remains associated with the virion. Carbohydrates comprise about 14% of the mass of the envelope glycoproteins and about 5% of the mass of the alphavirus virion.
Alphaviruses possess the ability to replicate in and be transmitted horizontally by mosquitoes. Some viruses have a preferred mosquito vector; however, as a group these viruses use a wide range of mosquitoes. Fort Morgan virus (FMV) is transmitted by arthropods of the family Cimicidae (Order Hemiptera) associated with birds. Most alphaviruses can infect a wide range of vertebrates. Many alphaviruses have different species of birds as their primary vertebrate reservoir host, but most are able to replicate in mammals as well. A number of alphaviruses have mammals as their primary vertebrate reservoir host. Some of these, such as RRV, replicate poorly in birds. Alphavirus isolations from reptiles and amphibians have also been reported. Southern elephant seal virus replicates in several mammalian cell lines, and salmon pancreatic disease virus has only been propagated in salmonid cell lines. As a group, the alphaviruses are found on all continents and on many islands. However, most viruses have a more limited distribution. Sindbis virus (SINV), the type species virus, has been isolated from many regions of Europe, Africa, Asia, the Philippines and Australia. WEEV is distributed discontinuously from Canada to Argentina. At the other extreme, ONNV has been isolated only from East Africa where it caused epidemics in the years 1959-60 and 1996-97. Many Old World alphaviruses cause serious, but not life threatening illnesses that are characterized by fever, rash and a painful arthralgia. RRV, Mayaro virus (MAYV), and the Ockelbo subtype of SINV cause epidemic polyarthritis in humans with symptoms (in a minority of cases) that may persist for months, or years. The New World alphaviruses, EEEV, VEEV and WEEV, regularly cause fatal encephalitis in humans, although the fraction of infections that leads to clinical disease is small. EEEV, WEEV, and VEEV also cause encephalitis in horses, and EEEV causes encephalitis in pheasants, emus, pigs and other domestic animals. Highlands J virus (HJV) is generally not believed to be pathogenic for humans or horses, but is recognized as an important pathogen of turkeys, pheasants, chukar partridges, ducks, emus, and whooping cranes.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not available.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Aura virus | ||
| Aura virus | [S78478] | (AURAV) |
| Barmah Forest virus | ||
| Barmah Forest virus | [U73745] | (BFV) |
| Bebaru virus | ||
| Bebaru virus | [U94595] | (BEBV) |
| Cabassou virus | ||
| Cabassou virus | [AF075259, U94611] | (CABV) |
| Chikungunya virus | ||
| Chikungunya virus | [L37661, U94597] | (CHIKV) |
| Eastern equine encephalitis virus | ||
| Eastern equine encephalitis virus | [D01034] | (EEEV) |
| Everglades virus | ||
| Everglades virus | [AF075251, U94608] | (EVEV) |
| Fort Morgan virus | ||
| Buggy Creek virus | [U94607, U60403, U60395] | |
| Fort Morgan virus | [U60399, U60404] | (FMV) |
| Getah virus | ||
| Getah virus | [U94568] | (GETV) |
| Highlands J virus | ||
| Highlands J virus | [J02206, U60401, U94609] | (HJV) |
| Mayaro virus | ||
| Mayaro virus | [U94602] | (MAYV) |
| Middelburg virus | ||
| Middelburg virus | [J02246, U94599] | (MIDV) |
| Mosso das Pedras virus‡ | ||
| Mosso das Pedras virus (78V3531) | [AF075257] | (MDPV) |
| Mucambo virus | ||
| Mucambo virus | [AF075253, U94615] | (MUCV) |
| Ndumu virus | ||
| Ndumu virus | [U94600] | (NDUV) |
| O'nyong-nyong virus | ||
| O'nyong-nyong virus | [M33999] | (ONNV) |
| Pixuna virus | ||
| Pixuna virus | [AF075256, U94613] | (PIXV) |
| Rio Negro virus | ||
| Rio Negro virus (strain Ag80-663) | [AF075258, U94610] | (RNV) |
| Ross River virus | ||
| Ross River virus | [M20162] | (RRV) |
| Sagiyama virus | [U94601] | |
| Salmon pancreas disease virus | ||
| Salmon pancreas disease virus | [AJ012631] | (SPDV) |
| Sleeping disease virus | [AJ238578] | |
| Semliki Forest virus | ||
| Semliki Forest virus | [X04129] | (SFV) |
| Sindbis virus | ||
| Babanki virus | [U94604, U60394, U60400] | |
| Kyzylagach virus | [U94605, U60396, U60402] | |
| Ockelbo virus | [M69205] | |
| Sindbis virus | [V00073] | (SINV) |
| Southern elephant seal virus‡ | ||
| Southern elephant seal virus | [AF315122] | (SESV) |
| Tonate virus‡ | ||
| Tonate virus | [AF075254] | (TONV) |
| Trocara virus | ||
| Trocara virus | [AF252265] | (TROV) |
| Una virus | ||
| Una virus | [U94603] | (UNAV) |
| Venezuelan equine encephalitis virus | ||
| Venezuelan equine encephalitis virus | [X04368] | (VEEV) |
| Western equine encephalitis virus | ||
| Western equine encephalitis virus | [J03854, U01065, AF109297] | (WEEV) |
| Whataroa virus | ||
| Whataroa virus | [U94606, U60398, U60408] | (WHAV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS RUBIVIRUS
Type Species Rubella virus
DISTINGUISHING FEATURES
The genome is 9,757 nt in size exclusive of the 3′-terminal poly(A) tract (RNA S20W of 40S). The order of the genes in the nonstructural protein ORF is NH2-P150-P90-COOH. The non-structural protein precursor is cleaved by a papain-like cysteine protease located near the C-terminus of P150. The virion has a CP (34 kDa) and two envelope glycoproteins (E1, 59 kDa; E2, 44-50 kDa), but no equivalent of E3 or the 6K protein of the alphaviruses. The order of the proteins in the structural polyprotein precursor is NH2-CP-E2-E1-COOH. The two cleavages that separate these three structural proteins are effected by signal peptidase (the E2 signal sequence remains attached to CP). Carbohydrates make up 10% of the mass of E1 and 30-40% of E2. E2 is heterogeneous in size due to differential processing of glycans (N- and O-linked). RUBV is transmitted by aerosol. The illness, rubella or German measles, is generally benign in nature, but complications such as arthritis, thrombocytopenia purpura, and encephalitis can occur. Intrauterine transmission is the most serious consequence of rubella as viral infection of the fetus during the first trimester of pregnancy leads to a constellation of serious birth defects known as congenital rubella syndrome (CRS). In addition, CRS patients suffer a variety of autoimmune and psychiatric disorders in later life, including a fatal neurodegenerative disease known as progressive rubella panencephalitis (which has also been described in post-natally infected individuals). CRS infants shed virus for up to six months. RUBV is endemic worldwide, although it is controlled by vaccination in most developed countries. Only one serotype has been described, although two genotypes have been detected.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not appropriate.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
| Triniti virus | (TRIV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Amino acid sequence homology between RUBV and the alphaviruses has been detected only within the nonstructural proteins (see Fig. 2). Nucleotide and aa sequence homology has been demonstrated among all alphaviruses sequenced, and phylogenetic relationships have been estimated using the nsP1, nsP4 and E1 genes. The 8 antigenic complexes (see above) are supported as distinct monophyletic groups by these analyses (Fig. 4 ).
Figure 4.

Phylogenetic tree of all alphavirus species except Southern elephant seal virus (the homologous sequence region is not available), and selected subtypes and variants, generated from partial E1 envelope glycoprotein gene sequences using the neighbor joining program with the F84 distance formula. Rubella virus cannot be included in this analysis because there is no detectable primary sequence homology in comparisons with alphavirus structural protein sequences. Bootstrap values for 100 replicates are indicated.
SIMILARITY WITH OTHER TAXA
Togavirus non-structural proteins (alphavirus nsP1, nsP2, and nsP4 and the methyl transferase, helicase and replicase regions of RUBV) share some sequence homology with the non-structural proteins of Hepatitis E virus (Hepevirus) and several groups of plant viruses, including tobamoviruses, bromoviruses and tobraviruses, suggesting a common origin for the replicases of these viruses. Differences in genome organization and segmentation presumably reflect extensive recombination and modular evolution.
DERIVATION OF NAMES
Alpha: from Greek letter α.
Rubi: from Latin rubeus “reddish”.
Toga: from Latin toga “cloak”.
REFERENCES
- Calisher C.H., Karabatsos N. Arbovirus serogroups: Definition and geographic distribution. In: Monath T.P., editor. I. CRC Press; Boca Raton, Florida: 1988. pp. 19–57. (The Arboviruses: Epidemiology and Ecology). [Google Scholar]
- Chantler J., Wolinsky J.S., Tingle A. Rubella virus. In: Knipe D.M., Howley P.M., editors. Fields’ Virology. 4th ed. Lippincott, Williams and Wilkins; Philadelphia: 2001. pp. 963–990. [Google Scholar]
- Cheng R.H., Kuhn R.J., Olson N.H., Rossmann M.G., Choi H.K., Smith T.J., Baker T.S. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell. 1995;80:621–630. doi: 10.1016/0092-8674(95)90516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.E. Alphaviruses. In: Knipe D.M., Howley P.M., editors. Fields’ Virology. 4th ed. Lippincott, Williams and Wilkins; Philadelphia: 2001. pp. 917–962. [Google Scholar]
- Hobman T.C., Lemon H.F., Jewell K. Characterization of an endoplasmic reticulum retention signal in the rubella virus E1 protein. J. Virol. 1997;71:7670–7680. doi: 10.1128/jvi.71.10.7670-7680.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsos N. International Catalog of Arboviruses Including Certain Other Viruses of Vertebrates. American Society of Tropical Medicine and Hygiene; San Antonio: 1985. [Google Scholar]
- La Linn M., Gardner J., Warrilow D., Darnell G.A., McMahon C.R., Field I., Hyatt A.D., Slade R.W., Suhrbier A. Arbovirus of Marine Mammals: a New Alphavirus Isolated from the Elephant Seal Louse, Lepidophthirus macrorhini. J. Virol. 2001;75:4103–4109. doi: 10.1128/JVI.75.9.4103-4109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescar J., Roussel A., Wien M.W., Navaza J., Fuller S.D., Wengler G., Rey F.A. The fusion glycoprotein shell of Semliki Forest virus: an icosahedral assembly primed for fusogenic activation at endosomal pH. Cell. 2001;105:137–148. doi: 10.1016/s0092-8674(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Monath T.P., editor. The Arboviruses: Epidemiology and Ecology. CRC Press; Boca Raton, Florida: 1988. [Google Scholar]
- Pletnev S.V., Zhang W., Mukhopadhyay S., Fisher B.R., Hernandez R., Brown D.T., Baker T.S., Rossmann M.G, Kuhn R.J. Locations of carbohydrate sites on alphavirus glycoproteins show that E1 forms an icosahedral scaffold. Cell. 2001;105:127–136. doi: 10.1016/s0092-8674(01)00302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A.M., Brault A.C., Shirako Y., Strauss E.G., Kang W., Strauss J.H., Weaver S.C. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001;75:10118–10131. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugachev K.V., Abernathy E.S., Frey T.K. Genomic sequence of the RA27/3 vaccine strain of rubella virus. Arch. Virol. 1997;142:1165–1180. doi: 10.1007/s007050050150. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M.J. Togaviridae: The viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott, Williams and Wilkins; Philadelphia: 2001. pp. 895–916. [Google Scholar]
- Strauss J.H., Strauss E.G. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villoing S., Bearzotti M., Chilmonczyk S., Castric J., Bremont M. Rainbow trout sleeping disease virus is an atypical alphavirus. J. Virol. 2000;74:173–183. doi: 10.1128/jvi.74.1.173-183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C, Kang W., Shirako Y., Rumenapf T., Strauss E.G., Strauss J.H. Recombinational history and molecular evolution of western equine encephalomyelitis complex alphaviruses. J. Virol. 1997;71:613–623. doi: 10.1128/jvi.71.1.613-623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Mukhopadhyay S., Pletnev S.V., Baker T.S., Kuhn R.J., Rossmann M.G. Placement of the structural proteins in Sindbis virus. J. Virol. 2002;76:11645–11658. doi: 10.1128/JVI.76.22.11645-11658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
TOBAMOVIRUS
CONTRIBUTED BY, D.J. Lewandowski
GENUS TOBAMOVIRUS
Type Species Tobacco mosaic virus
VIRION PROPERTIES
MORPHOLOGY
Virions are elongated rigid cylinders, approximately 18 nm in diameter with a central hollow cavity and helical symmetry (pitch 2.3 nm) (Fig. 1 ). The predominant virion has a length of 300-310 nm, and contains the genomic RNA. Shorter virions produced by the encapsidation of sgRNA are a minor component of the virion population, although at least two species produce an abundant short virion 32-34 nm in length.
Figure 1.

(Left) Model of particle of Tobacco mosaic virus (TMV). Also shown is the RNA as it is thought to participate in the assembly process. (Right) Negative contrast electron micrograph of TMV particle stained with uranyl acetate. The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is 40 × 106. Buoyant density in CsCl is 1.325 g/cm3. S20w is 194S. Virions are very stable.
NUCLEIC ACID
The genome consists of one molecule of linear positive sense ssRNA 6.3-6.6 kb in size. An m7GpppGp cap structure is found at the 5′-terminus of the genomic RNA, followed by an approximately 70 nt long 5′-untranslated sequence, containing many AAC repeats and few or no G residues. The 0.2-0.4 kb 3′-untranslated region contains sequences that can be folded into pseudoknots followed by 3′-terminal sequences that can be folded into a tRNA-like, amino acid-accepting structure. SgRNAs also contain a 5′-terminal cap and 3′-tRNA-like structure. The origin of assembly for encapsidation is located within the ORF for the MP in most species, but within the ORF for the CP in at least two species: Cucumber green mottle mosaic virus and Sunn hemp mosaic virus.
PROTEINS
Virions contain a single structural protein (17-18 kDa). Two nonstructural proteins are expressed directly from the genomic RNA: a 124-132 kDa protein terminated by an amber stop codon and a 181-189 kDa protein produced by readthrough of this stop codon, both of which are required for efficient replication. A third nonstructural protein (28-31 kDa) is required for cell-to-cell and long-distance movement. The MP is associated with plasmodesmata and has single-stranded nucleic acid binding activity in vitro. The CP is not required for cell-to-cell movement, but has a role in vascular tissue dependent virus accumulation. The replication proteins have also been implicated in virus movement. The MP and CP are expressed from individual 3′-co-terminal sgRNAs. The MP is expressed early during infection, whereas the CP is expressed later, and at higher levels. The MP and CP are not required for replication in single cells. The N-terminal one third of the 124-132 kDa protein has similarity with methyltransferase / guanylyl transferases whereas the C-terminal one third of the 124-132 kDa protein has similarity with RNA helicases (including an NTP-binding motif). The readthrough domain of the 181-189 kDa protein has motifs common to RdRps.
LIPIDS
Virions contain no lipids.
CARBOHYDRATES
Virions contain no carbohydrates.
GENOME ORGANIZATION AND REPLICATION
The single genomic RNA encodes at least 4 proteins. The 124-132 kDa and 181-189 kDa replication proteins are translated directly from the genomic RNA. The 124-132 kDa replication protein contains the Mtr and Hel domains. The 181-189 kDa replication protein that also contains the polymerase domain is synthesized by occasional readthrough of the leaky termination codon of the 124-132 kDa ORF. The 181-189 kDa replication protein is the only protein required for replication in single cells, although the 124-132 kDa replication protein is also required for efficient replication. The next ORFs encode the 28-31 kDa MP and 17-18 kDa CP in 5′ to 3′ order. MP and CP are translated from their respective 3′ co-terminal sgRNAs, both of which contain a 5′-cap (Fig. 2 ). In some species, the MP ORF overlaps both of the 181-189 kDa protein and CP ORFs, and in others does not overlap either ORF or overlaps one of the ORFs. Replication is cytoplasmic where the positive-sense genomic RNA is copied into a negative-sense RNA, which is used as template to produce positive sense genomic and sgRNAs.
Figure 2.

Genome organization of Tobacco mosaic virus (TMV). Conserved replicase domains are indicated as shaded boxes. Genomic RNA is capped and is template for expression of the 126 and 183 kDa proteins. The 3′ distal movement and CP ORFs are expressed from individual 3′ co-terminal sgRNAs. CP = coat protein; MP = movement protein.
ANTIGENIC PROPERTIES
The virions act as strong immunogens. Different species can be identified by intragel crossabsorption immunodiffusion tests using polyclonal antisera or by ELISA using monoclonal antibodies. Antigenic distances between individual species expressed as serological differentiation indices are correlated with the degree of sequence difference in their CPs.
BIOLOGICAL PROPERTIES
Most species have moderate to wide host ranges under experimental conditions, although in nature host ranges are usually quite narrow. Transmission occurs without the help of vectors by contact between plants and sometimes by seed, although this occurs in the absence of infection of the embryo. Geographic distribution is worldwide. The viruses are found in all parts of host plants. Virions often form large crystalline arrays visible by light microscopy.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Tobamoviruses have historically been designated as strains of TMV, but many of these are now defined as separate species based on nucleotide sequence data.
The criteria demarcating species in the genus are:
-
•
Sequence similarity; less than 10% overall nt sequence difference is considered to characterize strains of the same species, although most of the sequenced species have considerably less than 90% sequence identity,
-
•
Host range; however many of these viruses have wider and more overlapping host ranges in experimental rather than natural situations,
-
•
Antigenic relationships between the CPs.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Cucumber fruit mottle mosaic virus | ||
| Cucumber fruit mottle mosaic virus | [NC_002633] | (CFMMV) |
| Cucumber green mottle mosaic virus | ||
| Cucumber green mottle mosaic virus-SH | [NC_001801] | (CGMMV-SH) |
| Cucumber green mottle mosaic virus-W | [AB015146] | (CGMMV-W) |
| Frangipani mosaic virus | ||
| Frangipani mosaic virus | [AF165884] | (FrMV) |
| Hibiscus latent Fort Pierce virus | ||
| Hibiscus latent Fort Pierce virus | [AY250831] | (HLFPV) |
| Hibiscus latent Singapore virus | ||
| Hibiscus latent Singapore virus | [AF395898, AF395899] | (HLSV) |
| Kyuri green mottle mosaic virus | ||
| Kyuri green mottle mosaic-C1 | [NC_003610] | (KGMMV-C1) |
| Kyuri green mottle mosaic-Yodo | [AB015145] | (KGMMV-Y) |
| Obuda pepper virus | ||
| Obuda pepper virus | [NC_003852, L11665] | (ObPV) |
| Odontoglossum ringspot virus | ||
| Odontoglossum ringspot virus | [U89894, S83257, U34586] | (ORSV) |
| Paprika mild mottle virus | ||
| Paprika mild mottle virus-J | [NC_004106] | (PaMMV-J) |
| Pepper mild mottle virus | ||
| Pepper mild mottle virus-S | [NC_003630] | (PMMoV-S) |
| Pepper mild mottle virus-Ia | [AJ308228] | (PMMoV-Ia) |
| Ribgrass mosaic virus | ||
| Ribgrass mosaic virus | [U69271] | (RMV) |
| Sammons's Opuntia virus | ||
| Sammons's Opuntia virus | (SOV) | |
| Sunn-hemp mosaic virus | ||
| Sunn-hemp mosaic virus | [U47034, J02413] | (SHMV) |
| Tobacco latent virus | ||
| Tobacco latent virus | [AY137775] | (TLV) |
| Tobacco mild green mosaic virus | ||
| Tobacco mild green mosaic virus-Japan | [AB078435] | (TMGMV-J) |
| Tobacco mild green mosaic virus-U2 | [NC_001556] | (TMGMV-U2) |
| Tobacco mosaic virus | ||
| Tobacco mosaic virus | [NC_001367, AF395127, AF165190, AJ011933, X68110] | (TMV) |
| Tobacco mosaic virus-Rakkyo | [D63809] | (TMV-Rakkyo) |
| Tomato mosaic virus | ||
| Tomato mosaic virus | [X02144, NC_002692, AJ132845, AJ243571, AJ417701, Z92909] | (ToMV) |
| Turnip vein-clearing virus | ||
| Turnip vein clearing virus | [NC_001873] | (TVCV) |
| Turnip vein clearing virus-cr | [Z29370] | (TVCV-cr) |
| Ullucus mild mottle virus | ||
| Ullucus mild mottle virus | (UMMV) | |
| Wasabi mottle virus | ||
| Wasabi mottle virus | [NC_003355] | (WMoV) |
| Youcai mosaic virus | ||
| Youcai mosaic virus | [NC_004422] | (YoMV) |
| Youcai mosaic virus-Cg | [D38444] | (YoMV-Cg) |
| Zucchini green mottle mosaic virus | ||
| Zucchini green mottle mosaic virus | [NC_003878] | (ZGMMV) |
TENTATIVE SPECIES IN THE GENUS
| Maracuja mosaic virus | (MarMV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Fully sequenced tobamovirus genomes share 43-83% identity. However, tobamoviruses grouped based on host range share a much higher degree of sequence similarity. Nucleotide sequence of viruses within the groups of tobamoviruses that infect cruciferous (CFMMV, CGMMV, KGMMV, ZGMMV), brassicaceous (RMV, TVCV, WMoV, YoMV) or solanaceous plants (ObPV, PaMMV, PMMoV, TMGMV, TMV, ToMV) share 60-83%, 81-82%, and 56-79% identity, respectively. ORSV, an orchid infecting tobamovirus, shares 56-58% and 55-56% nt sequence identity with the groups infecting solanaceous plants and cucurbitaceous, respectively. SHMV, a legume infecting tobamovirus, shares 44-47% identity with other tobamoviruses.
Relationships between deduced aa sequences of each of the ORFs are similar to the relationship based on nt sequence: aa sequence identity is higher within groups based on host range, than between groups (Fig. 3 ). The CP, MP and large replication protein of tobamoviruses that infect brassicas share 84-92%, 81-87% and 97% identity, respectively, but share only 30-49%, 21-36% and 42-68% identity, respectively, with other tobamoviruses. The CP, MP and large replication protein of tobamoviruses that infect cucurbits share 44-81%, 57-88% and 63-93% identity within the group, and 30-43%, 20-42% and 40-45% identity outside the group, respectively. Within the group of cucurbit-infecting tobamoviruses, CGMMV is the most distinct, sharing 44-46%, 57-61% and 63-64% identity for the CP, MP and the large replication protein, respectively. SHMV CP and large replication protein share 41-43% and 41-42% identity, respectively, with the cucurbit-infecting tobamoviruses, although the SHMV MP shares only 18-22% identity with other tobamoviruses. FrMV CP and MP share only 33-39% and 20-33% identity with other tobamovirus species. The CP and MP of tobamoviruses that infect malavaceous hosts (HLFPV, HLSV) are 73% and 70% identical, respectively, but share 36-47% and 19-28% identity to the remainder of the genus. Although the ORSV CP and MP are more similar to tobamoviruses that infect solanaceous hosts (63-72% and 50-62% identity, respectively), the large replication protein is more similar to tobamoviruses that infect brassicas (67-68% identity).
Figure 3.

Dendrograms based on the deduced aa sequences of the 181-189 kDa replication protein (left), the MP (center) and the CP (right) of selected strains of tobamovirus species. The corresponding proteins from an isolate of Tobacco rattle virus, the type species of the Tobravirus genus, were used as the outgroup. Sequences were aligned using CLUSTALW, and the trees were constructed by a distance method using the PHYLIP programs PROTDIST and NEIGHBOR. The scale represents a distance value of 0.1.
SIMILARITY WITH OTHER TAXA
The 124-132 kDa and 181-189 kDa non-structural proteins contain conserved motifs common to the RdRp of many viruses, and are most similar to the corresponding proteins of Tobacco rattle virus. The 28-31 kDa MP is the prototypical plant virus MP and resembles MPs of a wide variety of plant viruses, including Tobacco rattle virus. The 17-18 kDa structural protein share similarities with those of other plant viruses that form rodshaped virions.
DERIVATION OF NAMES
Tobamo: sigla from tobacco mosaic virus.
REFERENCES
- Buck K. Replication of tobacco mosaic virus RNA. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:613–627. doi: 10.1098/rstb.1999.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V. Tobacco mosaic virus: a pioneer of cell-to-cell movement. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:637–643. doi: 10.1098/rstb.1999.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W.O., Lehto K.M. Regulation of tobamovirus gene expression. Adv. Virus Res. 1990;38:307–342. doi: 10.1016/S0065-3527(08)60865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deom C.M., Lapidot M., Beachy R.N. Plant virus movement proteins. Cell. 1992;69:221–224. doi: 10.1016/0092-8674(92)90403-y. [DOI] [PubMed] [Google Scholar]
- Gibbs A.J. Tobamovirus group. CMI/AAB Descriptions of Plant Viruses. 1977;(No 184) [Google Scholar]
- Gibbs A. Origin and evolution of tobamoviruses. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:593–602. doi: 10.1098/rstb.1999.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A. The tobacco mosaic virus particle: structure and assembly. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:531–535. doi: 10.1098/rstb.1999.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartey R.T., Voss T.C., Melcher U. Tobamovirus evolution: gene overlaps, recombination, and taxonomic implications. Mol. Biol. Evol. 1996;13:1327–1338. doi: 10.1093/oxfordjournals.molbev.a025579. [DOI] [PubMed] [Google Scholar]
- Nelson R.S., van Bel A.J.E. The mystery of virus trafficking into, through and out of vascular tissue. In: Luttge U., editor. Vol. 59. Springer-Verlag; Berlin: 1998. pp. 476–553. (Progress in Botany). [Google Scholar]
- Okada Y. Historical overview of research on the tobacco mosaic virus genome: genome organization, infectivity and gene manipulation. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:569–582. doi: 10.1098/rstb.1999.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof K.-B., Shaw J.G., Zaitlin M., editors. Tobacco mosaic virus: One hundred years of contributions to virology. APS Press; St. Paul, Minnesota: 1999. [Google Scholar]
- Van Regenmortel M.H.V., Fraenkel-Conrat H., editors. The Plant Viruses. The Rod-Shaped Plant Viruses. Plenum Press; New York: 1986. [Google Scholar]
- Wang H., Stubbs G. Structure determination of cucumber green mottle mosaic virus by X-ray fiber diffraction. Significance for the evolution of tobamoviruses. J. Mol. Biol. 1994;239:371–384. doi: 10.1006/jmbi.1994.1379. [DOI] [PubMed] [Google Scholar]
TOBRAVIRUS
CONTRIBUTED BY, D.J. Robinson
GENUS TOBRAVIRUS
Type Species Tobacco rattle virus
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

(Left) Diagram of a virion of Tobacco rattle virus (TRV), in section. (Right) Negative contrast electron micrograph of particles of TRV. The bar represents 100 nm.
Virions are tubular particles with no envelope. They are of two predominant lengths, (L) 180-215 nm and (S) ranging from 46 to 115 nm, depending on the isolate. Many strains produce in addition small amounts of shorter particles. The particle diameter is 21.3-23.1 nm by electron microscopy or 20.5-22.5 nm by X-ray diffraction, and there is a central canal 4-5 nm in diameter. Virions have helical symmetry with a pitch of 2.5 nm; the number of subunits per turn has been variously estimated as 25 or 32.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is 48-50 × 106 (L particles) and 11-29 × 106 (S Particles). Buoyant density in CsCl is 1.306-1.324 g/cm3. S20,W is 286-306S (L particles) and 155-245S (S particles). Virions are stable over a wide range of pH and ionic conditions and are resistant to many organic solvents, but are sensitive to treatment with EDTA.
NUCLEIC ACID
The genome consists of two molecules of linear positive sense ssRNA; RNA-1 is about 6.8 kb and RNA-2 ranges from 1.8 kb to about 4.5 kb in size (varying in different isolates). The 5′-terminus is capped with the structure m7G5′ppp5′Ap. There is no genome-linked protein or poly(A) tract.
PROTEINS
Virions contain a single structural protein of 22-24 kDa. RNA-1 codes for four non-structural proteins: a 134-141 kDa protein terminated by an opal stop codon and a 194-201 kDa protein produced by read through of this stop codon, both of which are probably involved in RNA replication; a 29-30 kDa protein (P1a) involved in intercellular transport of the virus; and a 12-16 kDa protein (P1b), which in Pea early-browning virus (PEBV) is involved in transmission of the virus in pea seeds. In addition to the virion structural protein, RNA-2 codes for two non-structural proteins, P2b and P2c. The size of P2b ranges from 27 to 40 kDa in different isolates, and that of P2c from 18 to 33 kDa. P2b is absolutely required for transmission by nematodes, whereas mutation of the P2c gene affects nematode transmission in some strains but not in others. The genes for P2b and P2c are missing from some laboratory strains that have been maintained by mechanical transmission. RNA-2 of some tobravirus isolates contains an additional small ORF between the CP and P2b genes, which codes for a potential 9 kDa protein.
LIPIDS
Virions contain no lipids.
CARBOHYDRATES
Virions contain no carbohydrates.
GENOME ORGANIZATION AND REPLICATION
RNA-1 is capable of independent replication and systemic spread in plants. The 134-141 kDa and 194-201 kDa replication proteins are translated directly from it, whereas P1a and P1b are translated from sgRNA species 1a and 1b, respectively. RNA-2 does not itself have messenger activity; the CP is translated from sgRNA-2a. The means by which the other RNA-2 encoded proteins are expressed is unknown (Fig. 2 ). There is sequence homology between RNA-1 and RNA-2 at both ends, but the extent of the homology varies between strains. In some strains, the homologous region at the 3′-end is large enough to include some or all of the P1a and P1b genes of RNA-1, but it is not known if these genes are expressed from RNA-2. Accumulation of virus particles is sensitive to cycloheximide but not to chloramphenicol, suggesting that cytoplasmic ribosomes are involved in viral protein synthesis. Virions accumulate in the cytoplasm. L particles of Pepper ringspot virus (PepRSV) become radially arranged around mitochondria, which are often distorted, and in cells infected with some Tobacco rattle virus isolates, ‘X-bodies’ largely composed of abnormal mitochondria and containing small aggregates of virus particles may be produced.
Figure 2.

Genome organization and strategy of expression of Tobacco rattle virus (TRV). The means by which P2b and P2c are expressed is unknown.
ANTIGENIC PROPERTIES
Tobravirus particles are moderately immunogenic. There is little or no serological relationship between members of the genus, and considerable antigenic heterogeneity among different isolates of the same virus.
BIOLOGICAL PROPERTIES
The host ranges are wide, including members of more than 50 monocotyledonous and dicotyledonous plant families. The natural vectors are nematodes in the genera Trichodorus and Paratrichodorus (Trichodoridae), different species being specific for particular virus strains. Adults and juveniles can transmit, but virus is probably not retained through the moult. Ingested virus particles become attached to the esophageal wall of the nematodes, and are thought to be released by salivary gland secretions and introduced into susceptible root cells during exploratory feeding probes. Virus can be retained for many months by non-feeding nematodes. There is no evidence for multiplication of virus in the vector and it is probably not transmitted through nematode eggs. The viruses are transmitted through seed of many host species. Tobacco rattle virus (TRV) occurs in Europe (including Russia), Japan, New Zealand and North America; PEBV occurs in Europe and North Africa, and PepRSV occurs in South America. TRV causes diseases in a wide variety of crop plants as well as weeds and other wild plants, including spraing (corky ringspot) and stem mottle in potato, rattle in tobacco, streaky mottle in narcissus and tulip, ringspot in aster, notched leaf in gladiolus, malaria in hyacinth and yellow blotch in sugar beet. PEBV is the cause of diseases in several legumes, including broad bean yellow band, distorting mosaic of bean and pea early-browning. PepRSV causes diseases in artichoke, pepper and tomato.
Most tissues of systemically invaded plants can become infected, but in many species virus remains localized at the initial infection site in the roots. In some virus-host combinations, notably TRV in some potato cultivars, limited systemic invasion occurs, and virus may not be passed on to all the vegetative progeny of infected mother plants.
Normal particle-producing isolates (called M-type) are readily transmitted by inoculation with sap and by nematodes. Other isolates (called NM-type) have only RNA-1, do not produce particles, are transmitted with difficulty by inoculation with sap, and are probably not transmitted by nematodes. NM-type isolates are obtained from M-type isolates by using inocula containing only L particles, and are also found in naturally infected plants. They often cause more necrosis in plants than do their parent M-type cultures.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Nucleotide sequences of RNA-1 show <75% identity.
-
•
Interspecific pseudo-recombinant isolates cannot be made.
-
•
Host ranges differ in specific hosts (e.g. legumes).
-
•
RNA-2 sequences and serological relationships are of limited value.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Pea early-browning virus | ||
| Pea early-browning virus – E116 | [RNA-2: AJ006500] | (PEBV-E116) |
| Pea early-browning virus – SP5 | [RNA-1: X14006; RNA-2: X51828] | (PEBV-SP5) |
| Pea early-browning virus – TpA56 | [RNA-2: X78455] | (PEBV-TpA56) |
| Pepper ringspot virus | ||
| Pepper ringspot virus | [RNA-1: L23972; RNA-2: X03241] | (PepRSV) |
| Tobacco rattle virus | ||
| Tobacco rattle virus-ON | [RNA-2: Z97357] | (TRV-ON) |
| Tobacco rattle virus-ORY | [RNA-1: AF034622; RNA-2: AF034621] | (TRV-ORY) |
| Tobacco rattle virus-PaY4 | [RNA-2: AJ250488] | (TRV-PaY4) |
| Tobacco rattle virus-PLB | [RNA-2: J04347] | (TRV-PLB) |
| Tobacco rattle virus-PpK20 | [RNA-1: AF314165; RNA-2: Z36974] | (TRV-PpK20) |
| Tobacco rattle virus-PSG | [RNA-2: X03686] | (TRV-PSG) |
| Tobacco rattle virus-Rostock | [RNA-2: AJ272198] | (TRV-Rostock) |
| Tobacco rattle virus-SP | [RNA-2: AJ007293] | (TRV-SP) |
| Tobacco rattle virus-SYM | [RNA-1: D00155] | (TRV-SYM) |
| Tobacco rattle virus-TCM | [RNA-2: X03955] | (TRV-TCM) |
| Tobacco rattle virus-TpO1 | [RNA-2: AJ009833] | (TRV-TpO1) |
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Nucleotide sequences of RNA-1 of PEBV, PepRSV and TRV share only 40-45% identity, whereas those of three strains of TRV are more than 99% identical. However, relationships assessed using RNA-2 nt sequences or CP aa sequences are more complex. In particular, the CPs of several TRV strains are similar to those of typical PEBV strains, although at the extremities of RNA-2 they have nt sequences typical of TRV. Such strains are believed to have arisen through recombination.
Figure 3.

Dendrogram based on the coat protein amino acid sequences of representative tobravirus strains and a representative of the type species of the other genera of plant viruses with rod-shaped particles. Sequences were aligned using CLUSTAL, and the tree was constructed by a distance method using the PHYLIP programs PROTDIST and NEIGHBOR-NJ. The scale represents a distance value of 0.1.
SIMILARITY WITH OTHER TAXA
The 134-141 kDa and 194-201 kDa non-structural proteins contain conserved sequence motifs common to RdRp of many viruses, and are most closely related to the analogous proteins of Tobacco mosaic virus. The 29-30 kDa P1a protein also shares sequence similarities with the analogous 30 kDa protein of Tobacco mosaic virus and, to a lesser extent, with non-structural proteins of some other plant viruses. The structural proteins share sequence motifs with those of other plant viruses with rod-shaped particles. Hypochoeris mosaic virus, previously listed as a tentative species in the genus Furovirus, is now believed to have been an isolate of TRV.
DERIVATION OF NAMES
Tobra : sigla from tobacco rattle virus.
REFERENCES
- Harrison B.D., Robinson D.J. The tobraviruses. Adv. Virus Res. 1978;23:25–77. doi: 10.1016/s0065-3527(08)60097-4. [DOI] [PubMed] [Google Scholar]
- Harrison B.D., Robinson D.J. Tobraviruses. In: Kurstak E., editor. Handbook of plant virus infections and comparative diagnosis. Elsevier/North-Holland; Amsterdam: 1981. pp. 515–540. [Google Scholar]
- Harrison B.D., Robinson D.J. Tobraviruses. In: van Regenmortel M.H.V., Fraenkel-Conrat H., editors. Vol. 2. Plenum Press; New York: 1986. pp. 339–369. (The plant viruses). [Google Scholar]
- MacFarlane S.A. Molecular biology of the tobraviruses. J. Gen. Virol. 1999;80:2799–2807. doi: 10.1099/0022-1317-80-11-2799. [DOI] [PubMed] [Google Scholar]
- Uhde K., Koenig R., Lesemann D.-E. An onion isolate of tobacco rattle virus: reactivity with an antiserum to Hypochoeris mosaic virus, a putative furovirus, and molecular analysis of its RNA 2. Arch. Virol. 1998;143:1041–1053. doi: 10.1007/s007050050354. [DOI] [PubMed] [Google Scholar]
HORDEIVIRUS
CONTRIBUTED BY, J.N. Bragg, A.G. Solovyev, S. Yu Morozov, J.G. Atabekov, A.O. Jackson
GENUS HORDEIVIRUS
Type Species Barley stripe mosaic virus
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

Electron micrograph of purified Barley stripe mosaic virus (BSMV) particles stained with 2% uranyl acetate. The particles are approximately 20 nm wide and have a length that varies depending on the size of the encapsidated RNA. The field was selected to represent monomers, but often a range of heterodisperse end-to-end aggregates up to 1000 nm in length predominate in purified preparations. The particles in the top left, bottom center, and upper left side of the micrograph are end-to-end aggregates that occur during purification. The bar represents 150 nm.
Virions are non-enveloped, elongated and rigid, about 20 × 110-150 nm in size; they are helically symmetrical with a pitch of 2.5 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
BSMV virions occur as heterodisperse sedimenting species with an S20w of about 182-193S; other species have an S20w of about 165-200S, depending on the virus. The BSMV isoelectric point is pH 4.5. Anionic detergents, added to purification buffers, increase virus yield by preventing particle aggregation. Thermal inactivation of infectivity occurs at 63-70°C. Virions are stable and their survival in sap ranges from a few days to several weeks.
NUCLEIC ACID
Virions normally contain three positive sense ssRNAs. The RNAs are designated α, β, and γ, and their respective sizes are 3.8, 3.2, and 2.8 kb (BSMV-ND18 strain), 3.7, 3.1, and 2.6 kb (Lychnis ringspot virus, LRSV), and 3.9, 3.6, and 3.2 kb (Poa semilatent virus, PSLV). The sizes of the α and β RNAs are similar between different strains of BSMV, whilst RNAy varies in size. The ND18 RNAy is 2.8 kb, that of the type strain is 3.2 kb. This difference is due to a 266 nt duplication near the 5′-end of the RNA that produces a γa protein of 87 kDa in the type strain compared to a 74 kDa γa ND18 protein. The Argentine mild strain contains mixtures of RNAγ species of 3.2, 2.8, and 2.6 kb. The 3.2 kb molecule contains a duplication similar to that of the type strain and the 2.6 kb RNA encodes a defective polymerase. No extensive hybridization can be detected between RNAs of BSMV, LRSV, and PSLV. Each RNA has m7GpppGUA at its 5′-end, and a highly conserved 238 nt (BSMV), 148 nt (LRSV), or 330 nt (PSLV) tRNA-like structure at the 3′-end. In the case of BSMV, this structure can be charged with tyrosine. In the BSMV and LRSV genomes, a poly(A) sequence that is variable in length separates the coding region from the tRNA-like structure; however, this sequence is not present in the PSLV genome. A close sequence similarity between the first 70 nt of RNAα and RNAγ of the CV17 strain of BSMV suggests that a natural recombination event has occurred between RNAα and RNAγ of this strain. A similar recombination appears to have occurred between the 5′-untranslated leaders of RNAα and RNAβ of LRSV These results plus sequence duplications in RNAγ provide persuasive evidence that RNA recombination has had a substantial role in the evolution of hordeiviruses.
PROTEINS
The virion capsid is constructed from subunits of a single protein. The CP of all species is 22 kDa in size, yet the proteins differ in electrophoretic mobility.
LIPIDS
None present.
CARBOHYDRATES
The BSMV virion CP has been reported to be glycosylated, based on results of a staining procedure. However, glycosylation sites are not present in the deduced protein sequence and independent experiments failed to substantiate the report.
GENOME ORGANIZATION AND REPLICATION
All three BSMV genomic RNAs are required for systemic infection of plants, but RNAs α and γ alone can infect protoplasts. The 5′- and 3′-NCR of each BSMV RNA are required for replication. The hordeivirus genome encodes seven proteins as illustrated for BSMV in Figure 2 . RNAα is monocistronic and encodes the αa protein (130 kDa in BSMV, 129 kDa in LRSV, and 131 kDa in PSLV) that functions as the helicase subunit of the viral replicase. The αa protein has two conserved sequence domains, an amino-terminal Mtr and a carboxy-terminal NTPase/Hel. The 5′-terminal RNAβ ORFs (βa) of all three viruses encode a 22 kDa CP. The BSMV CP, which is dispensable for systemic movement of the virus, is more closely related to the PSLV CP (55.2% identity) than to the LRSV CP (41.5% identity). An intergenic region separates a “triple gene block” (TGB) that encodes three non-structural proteins, βb (TGB1), βc (TGB3) and βd (TGB2), in which the βd protein overlaps the other two genes. In BSMV, The βb protein is expressed from a 2,450 nt sgRNA, and the βc and βd proteins are expressed from a second bicistronic 960 nt sgRNA with βc being expressed via a leaky scanning mechanism. In BSMV, a minor 23 kDa translational readthrough extension of the βd protein, designated βd', is present in plants. However, genetic experiments have not identified a function for βd', so it appears to be dispensable for infection in all local lesion and systemic hosts tested. The BSMV sgRNAβ1 and sgRNAβ2 promoters reside between positions −29 to −2 and −32 to −17 relative to the transcription initiation sites, respectively, and the nt sequences preceding the transcription initiation sites of these sgRNAs are conserved in LRSV and PSLV. The βb protein (58 kDa in BSMV, 50 kDa in LRSV, and 63 kDa in PSLV) contains a conserved NTPase/Hel domain. The BSMV βb protein binds RNA, NTPs, and exhibits ATPase and helicase activity in vitro. The βc (17 kDa in BSMV, and 18 kDa in LRSV and PSLV) and βd (14 kDa in BSMV and LRSV, and 18 kDa in PSLV) proteins are hydrophobic and membrane-associated. Each of the BSMV TGB proteins (βb, βc, and βd) is required for virus cell-to-cell movement in plants. RNAγ is bicistronic and encodes the γa polymerase subunit of the viral replicase (74 kDa in the BSMV-ND18 strain, 71 kDa in LRSV, and 84 kDa in PSLV), and the cysteine-rich γb protein (17 kDa in BSMV, 16 kDa in LRSV, and 20 kDa in PSLV).
Figure 2.
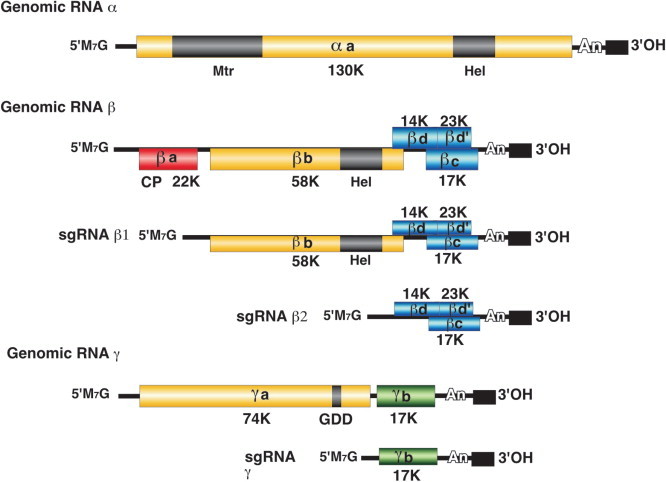
Genome organization of Barley stripe mosaic virus (BSMV). The filled circle, open rectangles and solid rectangles represent the 5′-cap structure, the ORFs, and the 3′-terminal tRNA-like structure, respectively. The 3′-proximal ORFs on each RNA terminate with an UAA that initiates the short poly (A) tract that directly precedes the 238 nt tRNA-like terminus. RNAα encodes a single protein, αa, with an amino-terminal Mtr domain (Mtr) and a carboxy-terminal helicase domain (Hel). This protein is referred to as the helicase subunit of the replicase (RdRp). RNAβ encodes five proteins: βa, the CP is translated from the genomic RNA; βb, a 58 kDa protein that contains a helicase domain (Hel). βb is translated from sgRNA β1, whose promoter resides between positions −29 to −2 relative to the transcription start site; βc, a 17 kDa protein that is separated from the βb ORF by 173 nt; βd, a 14 kDa protein which overlaps the βb and the βc ORFs; and βd', a 23 kDa translational extension product of unknown function. The βd, βd', and βc proteins are translated from sgRNA β2. The sgRNA β2 promoter is located between nt −32 to −17 relative to its transcription start site. RNAγ encodes two proteins. The γa protein contains the GDD domain and is the polymerase subunit of the replicase. The cysteine-rich, 17 kDa γb protein has RNA binding ability, and is translated from a sgRNAγ, Note: what is this? whose promoter lies between positions −21 to +2 relative to the transcription start site.
The γa protein is variable in size because of the approximately 250 nt RNAγ repeated sequence present in different strains of BSMV. The BSMV γb protein is expressed from a 737 nt sgRNA and is a pathogenicity determinant that is involved in regulating expression of genes encoded by RNAβ. The sgRNAγ promoter is between nt −21 to +2 relative to its transcription start site, and this sequence has similarity to sequences upstream of the γb proteins in PSLV and LRSV. The BSMV γb protein has both RNA binding and zinc binding ability, participates in homologous interactions, and may act as a suppressor of post-transcriptional gene silencing. Translation of a functional αa protein is required for replication of RNAα in cis, whilst replication of RNAβ is dependent on the presence of the βa and βb intergenic region, and RNAy requires approximately 600 nt of the 5′-terminal region. The TGB proteins on RNA β (b, c, d) are required for cell-to-cell and systemic movement in plants, but the CP and βd' are dispensable. The γb protein is also dispensable in some genetic backgrounds. A mutation in the 5′-leader sequence of the γa ORF interfered with systemic infection of Nicotiana benthamiana, suggesting that modulation of γa expression can affect movement. Full-length dsRNAs corresponding to all viral genomic ssRNAs can be isolated from infected plants. Virus particles accumulate predominantly in the cytoplasm and also in nuclei. Infected barley plants develop pronounced enlargements of the plasmodesmata that contain the βb protein, and prominent peripheral vesicles appear in proplastids and chloroplasts. These vesicles may be the sites of replication because antibodies raised against poly(I):poly(C) have detected dsRNA in proplastids from infected barley root tips.
ANTIGENIC PROPERTIES
Hordeivirus particles are efficient immunogens. Member species are distantly related serologically with BSMV being more closely related to PSLV than to LRSV, which is in agreement with sequence analyses.
BIOLOGICAL PROPERTIES
HOST RANGE
The native hosts of three viruses (ALBV, BSMV, PSLV) are grasses (family Gramineae); strains of LRSV occur naturally in dicotyledonous plants of the families Caryophyllaceae and Labiatae. Various strains of these viruses elicit local lesions in Chenopodium species and are able to establish systemic infections in a common host, Nicotiana benthamiana.
TRANSMISSION
BSMV and LRSV are efficiently seed-transmitted, and are transmitted less efficiently by pollen. Field spread from primary infection foci occurs efficiently by direct leaf contact. There are no known vectors for any members of the family.
GEOGRAPHIC DISTRIBUTION
ALBV has been reported only from Great Britain; BSMV occurs world-wide wherever barley is grown; LRSV (mentha strain) has been isolated in Hungary, and the type strain which is highly seed-transmissible in the family Caryophyllaceae, was initially discovered in California from seed of Lychnis divaricata introduced from Europe. PSLV has been recovered from Poa palustris isolated from two locations in Western Canada.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not available.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Anthoxanthum latent blanching virus | ||
| Anthoxanthum latent blanching virus | (ALBV) | |
| Barley stripe mosaic virus | ||
| Barley stripe mosaic virus | [U35768, X03854, X52774] | (BSMV) |
| Lychnis ringspot virus | ||
| Lychnis ringspot virus | [Z46630, Z46351, Z46353] | (LRSV) |
| Poa semilatent virus | ||
| Poa semilatent virus | [Z46352, M81486, M81487] | (PSLV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONS WITHIN THE GENUS
Not available.
SIMILARITY WITH OTHER TAXA
The hordeivirus replicase sequences are most similar to the pecluvirus, Peanut clump virus (PCV; 65-66 % identity in the RdRp domain), the furovirus Soil borne wheat mosaic virus (SBWMV; 63-67 %), and to the tobravirus, Tobacco rattle virus (TRV; 52-54 %). Besides the hordeiviruses, the TGB MPs are found in allexiviruses, benyviruses, carlaviruses, foveaviruses, furoviruses, pecluviruses, pomoviruses, and potexviruses. The hordeivirus TGB proteins are most similar to PCV proteins, and to those of Potato mop-top virus and Beet soil-borne virus. However, SBWMV and TRV lack the TGB, and encode single MPs unrelated to the TGB proteins. Moreover, in the other TGB-containing viruses, the replicase sequences are only distantly related to those of hordeiviruses. Apparently, reassortment events during evolution have resulted in different phylogenetic trees of hordeivirus TGB MPs and replication-associated proteins. The hordeivirus γb proteins show significant similarity to the respective proteins of SBWMV and PCV, and have a marginal similarity to the cysteine-rich proteins of tobraviruses. The hordeivirus CP sequences are most closely related to CP of PCV. Taking into account the high degree of sequence similarity of the encoded proteins and some common features of genome organization, the hordeiviruses are most closely related to PCV.
DERIVATION OF NAMES
hordei from hordeus, latin name of the primary host of the type species virus of the genus.
REFERENCES
- Beczner L., Hamilton R.I., Rochon D.M. Properties of the mentha strain of lychnis ringspot virus. Intervirology. 1992;33:49–56. doi: 10.1159/000150230. [DOI] [PubMed] [Google Scholar]
- Carroll T.W. Hordeiviruses: biology and pathology. In: van Regenmortel M.H.V., Fraenkel-Conrat H., editors. Vol. 2. Plenum Press; New York: 1986. pp. 373–395. (The Plant Viruses, The Rod-shaped Plant Viruses). [Google Scholar]
- Donald R.G.K., Jackson A.O. The barley stripe mosaic virus yb protein encodes a multifunctional cysteine-rich protein that affects pathogenesis. Plant Cell. 1994;6:1593–1606. doi: 10.1105/tpc.6.11.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R.G.K., Jackson A.O. RNA-binding activities of barley stripe mosaic virus ?b fusion proteins. J. Gen. Virol. 1996;77:879–888. doi: 10.1099/0022-1317-77-5-879. [DOI] [PubMed] [Google Scholar]
- Donald R.G.K., Lawrence D.M., Jackson A.O. The BSMV 58 kDa ßb protein is a multifunctional RNA binding protein. J. Virol. 1997;71:1538–1546. doi: 10.1128/jvi.71.2.1538-1546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M.C., Petty I.T.D., Jackson A.O. RNA recombination in the genome of barley stripe mosaic virus. Virology. 1992;189:389–392. doi: 10.1016/0042-6822(92)90722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M.L., Kelley S.E., Arnold M.K., Cooper J.I. Properties of a hordeivirus from Anthoxanthum odoratum. Plant Pathol. 1989;38:209–221. [Google Scholar]
- Jackson A.O., Hunter B.G, Gustafson, GD. Hordeivirus relationships and genome organization. Ann. Rev. Phytopathol. 1989;27:95–121. [Google Scholar]
- Jackson A.O., Petty I.T.D., Jones R.W., Edwards M.C., French R. Analysis of Barley stripe mosaic virus pathogenicity. Semin. Virol. 1991;2:107–119. [Google Scholar]
- Johnson J.A., Bragg J.N., Lawrence D.M., Jackson A.O. Sequences controlling expression of Barley stripe mosaic virus subgenomic RNAs. Virology. 2003;313:66–80. doi: 10.1016/S0042-6822(03)00285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina N.O., Rakitina D.V., Soloyev A.G., Schiemann J., Morozov, S. Yu. RNA helicase activity of the plant movement proteins encoded by the first gene of the triple gene block. Virology. 2002;296:321–329. doi: 10.1006/viro.2001.1328. [DOI] [PubMed] [Google Scholar]
- Lawrence D.M., Jackson A.O. Interactions of the TGB1 protein during cell-to-cell movement of Barley stripe mosaic virus. J. Virol. 2001;75:8712–8723. doi: 10.1128/JVI.75.18.8712-8723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D.M., Jackson A.O. Requirements for cell-to-cell movement of barley stripe mosaic virus in monocot and dicot hosts. Mol. Plant Path. 2001;2:65–75. doi: 10.1046/j.1364-3703.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- Morozov, S. Yu., Solovyev A.G. Triple gene block: modular design of a multifunctional machine for plant virus movement. J. Gen. Virol. 2003;84:1351–1366. doi: 10.1099/vir.0.18922-0. [DOI] [PubMed] [Google Scholar]
- Solovyev A.G., Savenkov E., Agranovsky A.A., Morozov, S. Yu. Comparisons of the genomic cis- elements and coding regions in RNA ß components of the hordeiviruses Barley stripe mosaic virus, Lychnis ringspot virus, and Poa semilatent virus. Virology. 1996;219:9–18. doi: 10.1006/viro.1996.0217. [DOI] [PubMed] [Google Scholar]
- Savenkov E., Solovyev A.G., Morozov, S. Yu. Genome sequences of Poa semilatent and Lychnis ringspot hordeiviruses. Arch. Virol. 1997;143:1379–1393. doi: 10.1007/s007050050382. [DOI] [PubMed] [Google Scholar]
- Yelina N.E., Savenkov E.I., Solovyev A.G., Morozov, S. Yu., Valkonen J.P.T. Long-distance movement, virulence, and RNA silencing suppression controlled by a single protein in hordeivirus and potyvirus: complementary functions between virus families. J. Virol. 2002;76:12981–12991. doi: 10.1128/JVI.76.24.12981-12991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONTRIBUTED BY, L. Torrance, R. Koenig
GENUS FUROVIRUS
Type Species Soil-borne wheat mosaic virus
VIRION PROPERTIES
MORPHOLOGY
Virions are non-enveloped hollow rods, which have helical symmetry. Virions are about 20 nm in diameter, with predominant lengths of 140-160 nm and 260-300 nm. The length distribution of the Soil-borne wheat mosaic virus (SBWMV) short particles is broad, 80-160 nm, due to the presence of deletion mutants in some cultures (Fig. 1 ).
Figure 1.

Negative contrast electron micrograph of stained (ammonium molybdate pH 7.0) particles of Soilbornewheat mosaic virus (SBWMV). The bar represents 200 nm. Inset: Negative contrast electronmicrograph of particles SBWMV stained with 1% uranyl acetate. The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as two (or three) components; for SBWMV the S20w values are 220-230S (long particles) and 170-225S (short particles), and 126-177S (deletion mutants). SBWMV loses infectivity in extracts of wheat kept at 60-65°C for 10 min.
NUCLEIC ACID
Complete or almost complete nt sequences are available for all five species in the genus. The genome is bipartite, linear, positive-sense ssRNA. RNA-1 is c. 6-7 kb and RNA-2 c. 3.5-3.6 kb. The RNA molecules of SBWMV have a 5’-cap (m7GpppG) and in all of the species where the complete sequences have been determined there is a 3’-terminal tRNA-like structure with a putative anticodon for valine. The 3’-terminus of SBWMV RNA was shown experimentally to accept valine.
PROTEINS
The capsid comprises multiple copies of a single polypeptide of ∼19-20.5 kDa. The CPs of SBWMV, Chinese wheat mosaic virus (CWMV), Soil-borne cereal mosaic virus (SBCMV) and Oat golden stripe virus (OGSV) comprise 176 aa with 76-82% aa homologies, they share only 46% homology with SrCSV. The CP gene terminates in a leaky (UGA) stop codon that can be suppressed to produce a read-through protein (∼85 kDa), which is thought to be involved in natural transmission by the plasmodiophorid vector. In addition to replicase proteins the furoviruses encode a single MP (∼37 kDa) and a cysteine rich protein (∼18 kDa) of unknown function.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
Genome organization and structure is conserved between species but there are substantial differences in the nt sequences. SBWMV RNA-1 encodes a 150 kDa protein, a 209 kDa readthrough product and a 37 kDa protein (Fig. 2 ). The 150 kDa protein contains Mtr and NTP-binding Hel motifs and the readthrough protein, in addition, contains RNA polymerase motifs, indicating that these proteins are involved in replication. The 37 kDa protein is thought to be involved in virus movement as it shares partial sequence similarity to the MPs of dianthoviruses. RNA-2 encodes the CP (19 kDa), the sequence of which terminates in a UGA codon that can be suppressed to give a readthrough product of 84 kDa. A 25 kDa polypeptide is initiated from a CUG codon upstream of the CP AUG. An ORF towards the 3’-end of the RNA-2 encodes a 19 kDa protein that contains 7 conserved cysteine residues. Products corresponding to the 37 kDa protein and the cysteine-rich 19 kDa protein were not found in in vitro transcription/translation experiments, and these proteins are thought to be expressed from sgRNAs. Spontaneous deletions in the CP readthrough domain occur on successive passage by manual inoculation, and in field isolates in older infected plants.
Figure 2.

Genome organization of Soil-borne wheat mosaic virus (SBWMV). The tRNA structure motifs at the3’-ends of the RNAs are represented by a dark square, the Met, Hel and RdRp motifs of the polymerase byasterisks and the readthrough of the polymerase and coat protein ORFs by RT and an arrow.
ANTIGENIC PROPERTIES
Virions are immunogenic and the five virus species can be distinguished serologically.
BIOLOGICAL PROPERTIES
HOST RANGE
The natural host ranges of furoviruses are narrow and confined to species within the Graminae. SBWMV induces green or yellow mosaic and stunting in winter wheat (Triticum aestivum) causing up to 80% yield loss in severely infected crops. It also may infect barley and rye. SBCMV infects mainly wheat and triticale in Western and Southern Europe and mainly rye in Central and North-Eastern Europe. Both viruses are (not readily) mechanically transmissible to Chenopodium quinoa. OGSV infects oats (Avena sativa) but failed to infect wheat when plants were grown in viruliferous soil. Mechanically it can be transmitted to some Nicotiana and Chenopodium species. SrCSV infects Sorghum bicolor. Mechanically it can be transmitted to a range of species including Chenopodium quinoa, C. amaranticolor, Nicotiana clevelandii, Arachis hypogaea, Zea mays and T. aestivum.
TRANSMISSION
The viruses are soil-borne, and Polymyxa graminis has been identified as a vector for SBWMV. Virions are thought to be carried within the motile zoospores. Soil containing the resting spores remains infectious for many years.
GEOGRAPHICAL DISTRIBUTION
Furoviruses are found in temperate regions worldwide including the United States of America, Europe, China, Japan.
CYTOPATHIC EFFECTS
Virions are found scattered, or in aggregates and inclusion bodies in the cytoplasm and vacuole. Inclusion bodies can be crystalline inclusions or comprise loose clusters of virus particles in association with masses of microtubules. Amorphous inclusion bodies can be seen in tissue sections by light microscopy.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The species within the genus Furovirus are presently mainly differentiated on the basis of the nt sequences of their RNAs and the deduced aa sequences of their putative gene products. On these bases, SBWMV (Japan) may represent an additional species. RNA-1 sequences of these viruses share 58-74% identity and RNA-2 46-80% (Fig. 3 ). SBWMV, SBCMV, CWMV and OGSV can be discriminated also by reactivity with selected monoclonal and polyclonal antibodies. OGSV and SrCSV differ in host range to SBWMV, SBCMV and CWMV. Especially the latter three viruses have similar biological properties and genetic reassortants can be formed with RNA-1 and RNA-2 of SBWMV (Nebraska), SBWMV (Japan) and SBCMV. With the other viruses this possibility has not yet been checked. The relative merits of using genetic reassortment in furovirus species demarcation in the future are currently being debated in the furovirus study group.
Figure 3.

Percentage sequence identities of total RNAs of furoviruses.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Chinese wheat mosaic virus | ||
| Chinese wheat mosaic virus | [RNA1: AJ012005; RNA2: AJ012006] | (CWMV) |
| Oat golden stripe virus | ||
| Oat golden stripe virus | [RNA1: AJ132578; RNA2: J132579] | (OGSV) |
| Soil-borne cereal mosaic virus | ||
| Soil-borne cereal mosaic virus (European wheat mosaic virus) (Soil-borne rye mosaic virus) | [RNA1: AF146278-80, AJ252151; RNA2: AF146281-83, AJ252152] | (SBCMV) |
| Soil-borne wheat mosaic virus | ||
| Soil-borne wheat mosaic virus – Japan | [RNA1: AB033689; RNA2: AB033690] | (SBWMV-JP) |
| Soil-borne wheat mosaic virus – Nebraska | [RNA1: L07937; RNA2: L07938] | (SBWMV-Ne) |
| Soil-borne wheat mosaic virus – New York | [RNA1: AY016007, AF361641; RNA2: AY016008, AF361642] | (SBWMV-NY) |
| Sorghum chlorotic spot virus | ||
| Sorghum chlorotic spot virus | [RNA1: AB033691; RNA2: AB033692] | (SrCSV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
The genome organization is identical for all furoviruses. However, there are considerable differences in percent nt sequence identity between the species with OGSV and SrCSV being the most distantly related ones (Fig 3).
SIMILARITY WITH OTHER TAXA
Furoviruses have similar particle morphology to members of the genera Pecluvirus, Pomovirus, Benyvirus, Tobamovirus, Tobravirus and Hordeivirus. The CPs of these viruses show various degrees of aa sequence homologies (Fig. 4 ) and have a number of conserved residues, e.g. RF and FE in their central and C-terminal parts, respectively, which are thought to be involved in the formation of salt bridges. The aa sequences of the putative replicase proteins form a cluster with those of the pomo-, peclu-, hordei-, tobra- and tobamoviruses (Fig. 4). The furoviruses also resemble the pomoviruses and surprisingly also the tymoviruses in having valine-specific tRNA-like structures at the 3’-ends of their genomic RNAs. The furoviruses differ from the pomo-, beny-, hordei- and tobamoviruses by their bipartite genomes and from peclu-, pomo-, beny- and hordeiviruses in that they have a single movement protein gene rather a triple block of genes encoding movement proteins. SBWMV has distant serological relationships with Potato mop-top virus and Tobacco mosaic virus.
Figure 4.

Percentage identities of the deduced aa sequences of replication-associated and CPs of furovirusesand other viruses with tubular particles
DERIVATION OF NAMES
Furo: sigla from fungus-borne, rod-shaped virus.
REFERENCES
- Diao A., Chen J., Ye R., Zheng T., Yu S., Antoniw J.F., Adams M.J. Complete sequence nd genome properties of Chinese wheat mosaic virus, a new furovirus from China. J. Gen. Virol. 1999;0:1141–1145. doi: 10.1099/0022-1317-80-5-1141. [DOI] [PubMed] [Google Scholar]
- Diao A., Chen J., Gitton F., Antoniw J.F., Mullins J., Hall A.M., Adams M.J. Sequences of European wheat mosaic virus and Oat golden stripe virus and genome analysis of the genus Furovirus. Virology. 1999;261:331–339. doi: 10.1006/viro.1999.9880. [DOI] [PubMed] [Google Scholar]
- Chen J., MacFarlane S.A., Wilson T.M.A. Detection and sequence analysis of a spontaneous deletion mutant of Soil-borne wheat mosaic virus RNA2 associated with increased symptom severity. Virology. 1994;202:921–929. doi: 10.1006/viro.1994.1414. [DOI] [PubMed] [Google Scholar]
- Chen J., Torrance L., Cowan G.H., MacFarlane S.A., Stubbs G., Wilson T.M.A. Monoclonal antibodies detect a single amino acid difference between the coat proteins of Soil-borne wheat mosaic virus isolates: Implications for virus structure. Phytopathology. 1997;87:295–301. doi: 10.1094/PHYTO.1997.87.3.295. [DOI] [PubMed] [Google Scholar]
- Goodwin J.B., Dreher T.W. Transfer RNA mimicry in a new group of positive-strand RNA plant viruses, the furoviruses: differential aminoacylation between the RNA components of one genome. Virology. 1998;246:170–178. doi: 10.1006/viro.1998.9193. [DOI] [PubMed] [Google Scholar]
- Kendall T.L., Langenberg W.G., Lommel S. Molecular characterization of Sorghum chlorotic spot virus, a proposed furovirus. J. Gen. Virol. 1988;69:2335–2345. [Google Scholar]
- Koenig R., Bergstrom G.C., Gray S.M., Loss S. A New York isolate of Soil-borne wheat mosaic virus differs considerably from the Nebraska type strain in the nucleotide sequences of various coding regions but not in the deduced amino acid sequences. Arch. Virol. 2002;147:265–617. doi: 10.1007/s007050200011. [DOI] [PubMed] [Google Scholar]
- Koenig R., Huth W. Soil-borne rye mosaic and European wheat mosaic virus: two names for a furovirus with variable genome properties which is widely distributed in several cereal crops in Europe. Arch. Virol. 2000;145:689–697. doi: 10.1007/s007050050663. [DOI] [PubMed] [Google Scholar]
- Koenig R., Pleij C.W.A., Huth H. Molecular characterization of a new furovirus mainly infecting rye. Arch. Virol. 1999;144:2125–2140. doi: 10.1007/s007050050627. [DOI] [PubMed] [Google Scholar]
- Miyanishi M., Roh S.H., Yamamiya A., Ohsato S., Shirako Y. Reassortment between genetically distinct Japanese and US strains of Soil-borne wheat mosaic virus: RNA1 from a Japanese strain and RNA2 from a US strain make a pseudorecombinant virus. Arch. Virol. 2002;147:1141–1153. doi: 10.1007/s00705-002-0798-2. [DOI] [PubMed] [Google Scholar]
- Rao A.S., Brakke M.K. Relation of soil-borne wheat mosaic virus and its fungal vector. Phytopathology. 1969;59:581–587. [Google Scholar]
- Shirako Y. Non-AUG translation initiation in a plant RNA virus: a forty-amino-acid extension is added to the N terminus of soil-borne wheat mosaic virus capsid protein. J. Virol. 1998;72:1677–1682. doi: 10.1128/jvi.72.2.1677-1682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y., Wilson T.M.A. Complete nucleotide sequence and organization of the bipartite genome of Soil-borne wheat mosaic virus. Virology. 1993;195:16–32. doi: 10.1006/viro.1993.1342. [DOI] [PubMed] [Google Scholar]
- Shirako Y., Suzuki N., French R.C. Similarity and divergence among viruses in the genus Furovirus. Virology. 2000;270:201–207. doi: 10.1006/viro.2000.0251. [DOI] [PubMed] [Google Scholar]
- Ye R., Xu L., Gao Z.Z., Yang J.P., Chen J.P., Chen M.J., Adams M.J., Yu S.Q. Use of monoclonal antibodies for the serological differentiation of wheat and oat furoviruses. J. Phytopathology. 2000;148:257–262. [Google Scholar]
CONTRIBUTED BY, R. Koenig, D.-E. Lesemann
GENUS POMOVIRUS
Type Species Potato mop-top virus
VIRION PROPERTIES
MORPHOLOGY
The non-enveloped, rod-shaped particles are helically constructed with a pitch of 2.4 to 2.5 nm and an axial canal. They have predominant lengths of ∼ 65-80, 150-160 and 290-310 nm and diameters of 18-20 nm. Crude extracts of plants infected with Beet soil-borne virus (BSBV), Beet virus Q (BVQ) and Potato mop-top virus (PMTV) contain characteristic small bundles of a few side-by-side aggregated particles in addition to singly dispersed particles.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as three components with S20w of c. 125S, 170S and 230S, respectively. In sap at room temperature, most of the infectivity is lost within a few hours.
NUCLEIC ACID
Virions contain three molecules of linear positive-sense ssRNA of ∼ 6, 3-3.5 and 2.5-3 kb, respectively. The sequence has been determined for all three RNA species of BSBV, BVQ, PMTV and Broad bean necrosis virus (BBNV). The RNAs are probably capped at the 5′-end; their 3’-ends can be folded into tRNA-like structures that are preceded by a long hairpin-like structure and an upstream pseudoknot domain. The tRNA-like structures of pomoviruses like those of tymoviruses contain an anticodon for valine and are capable of high-efficiency valylation.
PROTEINS
The major capsid protein (CP) species is 20 kDa in size. It is not needed for systemic infection. The CP readthrough protein, which may be detected in some PMTV particles near one extremity by means of immunogold labeling, apparently initiates virus assembly. Sequences in the CP readthrough protein are also necessary for the transmission of PMTV by Spongospora subterranea. PMTV triple gene block protein 1 and 2 (TGBp2 and TGBp3) are membrane-associated and bind ssRNA in a sequence nonspecific manner. It has been suggested that they may form a complex with PMTV RNA that is translocated and localized to the plasmodesmata by TGBp3.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
RNA-1 of BSBV has ORF for a 149 kDa protein and a 207 kDa readthrough protein that are presumably involved in replication. The shorter ORF is terminated by an apparently suppressible UAA stop codon (Fig. 2 ). Proteins of similar sizes are encoded on RNA-1 of BVQ, BBNV and PMTV. The smaller protein contains in its N-terminal part Mtr (Mt) and in its C-terminal part Hel motifs; the motifs for RdRp are found in the C-terminal part of the readthrough protein (Fig. 2). The two proteins contain also other highly conserved domains of unknown function in their N- and C-terminal parts, but their central regions (designated as ‘variable’ in Fig. 2) are highly specific for each virus. RNAs-2 contain the CP gene, which terminates with a suppressible UAG stop codon and then continues in frame to form a CP readthrough protein gene that varies considerably in size between different pomoviruses, possibly because it readily undergoes internal deletions. PMTV RNA-2 has therefore originally been referred to as RNA-3 and vice versa. PMTV RNA-2 contains a gene for a cysteine-rich protein that is not found on the RNAs of BSBV and BVQ. A triple gene block (TGB) coding for proteins involved in viral movement is found on RNAs-3. TGBp1 also contains Hel motifs. The sequences of the C-terminal part of TGBp1, of the entire TGBp2 and of the N-terminal part of TGBp3 are highly conserved among pomoviruses. The replication mechanisms are unknown.
Figure 2.

Genome organization of Beet soil-borne virus (BSBV). Areas in the putative translation products indicate respectively the UAA and UAG stop codons that are thought to be suppressible, and solid squares indicate a 3’-terminal tRNA-like structure. Dark areas indicate conserved motifs. Hel; helicase, Mt; methyltransferase, RdRp; RNA dependent RNA polymerase, RT; readthrough.
ANTIGENIC PROPERTIES
Virions are moderately antigenic. Distant serological relationships have been found between the particles of BSBV and BVQ but not between those of the two beet viruses and PMTV. This is probably due to the fact that PMTV CP has ten extra amino acids on its immunodominant N-terminus that are missing in the two beet viruses. A conserved sequence EDSALNVAHQL is found in the CPs of PMTV, BSBV and BVQ. It contains an epitope for which the monoclonal antibody SCR 70 is specific and which is only detectable by Western blotting after disruption of the particles. Other epitopes are either exposed along the entire particle length, e.g. the immunodominant N-terminus, or are accessible only on one extremity (Fig. 1 ). PMTV and BBNV show distant serological relationships to tobamoviruses.
Figure 1.

Negative contrast electron micrograph of particles of Potato mop-top virus (PMTV). The gold-labelling shows the binding of monoclonal antibody SCR 68 to one extremity of the particles. The bar represents 100 nm. (Courtesy I. M. Roberts).
BIOLOGICAL PROPERTIES
HOST RANGE
The natural host range of pomoviruses is very narrow; only dicotyledoneous hosts have been described.
TRANSMISSION
Pomoviruses are transmitted by soil. Spongospora subterranea and Polymyxa betae have been identified as vectors for PMTV and BSBV, respectively. The viruses are also transmissible mechanically.
GEOGRAPHICAL DISTRIBUTION
Countries with temperate climate.
CYTOPATHIC EFFECTS
PMTV-infected cells contain in the cytoplasm virions aggregated in sheaves. Infections by BSBV and BVQ induce voluminous cytoplasmic inclusions which consist of hypertrophied endoplasmic reticulum, convoluted membrane accumulations, numerous small virion bundles and rarely compact virus aggregates.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Differences in host range,
-
•
Effects in infected tissue: different inclusion body morphology,
-
•
Transmission: different vector species,
-
•
Serology: virions are distantly related serologically,
-
•
Genome: different numbers of genome components (presence or absence of a gene for a cysteine-rich protein).
-
•
Sequence: less than ∼80% identical over whole sequence,
-
•
Sequence: less than ∼90% identical in CP amino acid sequence.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Beet soil-borne virus | ||
| Beet soil-borne virus – Ahlum | [RNA-1: NC_003020; RNA-2: NC_003518; RNA-3: NC_003519] | (BSBV-Ahl) |
| Beet virus Q | ||
| Beet virus Q – Wierthe | [RNA-1: NC_003510; RNA-2: NC_003511; RNA-3: NC_003512] | (BVQ-Wie) |
| Broad bean necrosis virus | ||
| Broad bean necrosis virus | [RNA-1: NC_004423; RNA-2: NC_004424; RNA-3: NC_004425] | (BBNV) |
| Potato mop-top virus | ||
| Potato mop-top virus – SW | [RNA-1: NC_003723; RNA-2*: NC_003724; RNA-3*: NC_003725] | (PMTV-SW) |
| Potato mop-top virus – T | [RNA-2*: D16193; RNA-3*: D30753] | (PMTV-T) |
RNA2 of PMTV has originally been referred to as RNA 3 and vice versa
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
The replication-associated proteins (RNA-1-encoded readthrough proteins) and the CPs of the pomoviruses described so far share less than 65% and 55% aa sequence identity, respectively (Fig. 3 ). The highest percentages of aa sequence identities are found in TGBp2 (75% for BSBV and BVQ – Fig. 3).
Figure 3.

Phylogenetic trees showing the percentages of aa sequence identities among the replication-associated proteins, the CPs and the TGBp2 of pomoviruses, of other plant viruses with rod-shaped particles and of Turnip yellow mosaic virus (as an unrelated plant virus, only for the replication-associated and CPs). The alignments were made by the GCG programs LINEUP and PILEUP and the trees were generated by the program DNAMAN (Lynnon Bio/Soft).
SIMILARITY WITH OTHER TAXA
Pomoviruses are morphologically similar to other rod-shaped viruses, i.e. furoviruses, pecluviruses, hordeiviruses, tobraviruses, tobamoviruses and benyviruses. The coat proteins of these viruses have a number of conserved residues, e.g. RF and FE in their central and C-terminal parts, respectively, which are thought to be involved in the formation of salt bridges. The derived aa sequences for the putative RNA-1-encoded proteins also suggest relationships to furoviruses, pecluviruses, tobraviruses, hordeiviruses and tobamoviruses (Fig. 3). Affinities not only to pecluviruses, benyviruses and hordeiviruses, but also to potexviruses and carlaviruses are suggested by the derived aa sequences of their triple gene block-encoded proteins (Fig. 3). The folding properties of the 5-UTRs of their genomic RNAs suggest affinities to the tymoviruses, those of the 3’-UTRs to tymoviruses, tobamoviruses and hordeiviruses. Pomoviruses differ from furoviruses, pecluviruses and tobamoviruses by having a tripartite genome and from hordeiviruses by having the proteins presumably involved in replication encoded on one rather than two RNA molecules.
DERIVATION OF NAMES
Pomo: sigla from Potato mop-top virus.
REFERENCES
- Cowan G.H., Lioliopoulou F., Ziegler A., Torrance L. Subcellular localisation, protein interactions, and RNA binding of Potato mop-top virus triple block proteins. Virology. 2002;298:106–115. doi: 10.1006/viro.2002.1435. [DOI] [PubMed] [Google Scholar]
- Cowan G.H., Torrance L., Reavy B. Detection of potato mop-top virus capsid readthrough protein in virus particles. J. Gen. Virol. 1997;78:1779–1783. doi: 10.1099/0022-1317-78-7-1779. [DOI] [PubMed] [Google Scholar]
- Goodwin J.B., Dreher T.W. Transfer RNA mimicry in a new group of positive-strand RNA plant viruses, the furoviruses: differential aminoacylation between the RNA components of one genome. Virology. 1998;246:170–178. doi: 10.1006/viro.1998.9193. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki S., Scott K.P., Reavy B., Harrison B.D. Sequence analysis and gene content of potato mop-top virus RNA 3: further evidence of heterogeneity in the genome organization of furoviruses. Virology. 1995;206:701–706. doi: 10.1016/s0042-6822(95)80092-1. [DOI] [PubMed] [Google Scholar]
- Koenig R., Beier C., Commandeur U., Lüth U., Kaufmann A., Lüddecke P. Beet soil-borne virus RNA 3 – a further example for the heterogeneity of the gene content of furovirus genomes and of triple gene block-carrying RNAs. Virology. 1996;216:202–207. doi: 10.1006/viro.1996.0047. [DOI] [PubMed] [Google Scholar]
- Koenig R., Commandeur U., Loss S., Beier C., Kaufmann A., Lesemann D.-E. Beet soil-borne virus RNA-2: similarities and dissimilarities to the coat protein gene-carrying RNAs of other furoviruses. J. Gen. Virol. 1997;78:469–477. doi: 10.1099/0022-1317-78-2-469. [DOI] [PubMed] [Google Scholar]
- Koenig R., Loss S. Beet soil-borne virus RNA 1: genetic analysis enabled by a starting sequence generated with primers to highly conserved helicase-encoding domains. J. Gen. Virol. 1997;78:3161–3165. doi: 10.1099/0022-1317-78-12-3161. [DOI] [PubMed] [Google Scholar]
- Koenig R., Pleij C.W.A., Beier C., Commandeur U. Genome properties of beet virus Q, a new furo-like virus from sugarbeet, determined from unpurified virus. J. Gen. Virol. 1998;79:2027–2036. doi: 10.1099/0022-1317-79-8-2027. [DOI] [PubMed] [Google Scholar]
- Koonin E.V., Dolja V.V. Evolution and taxonomy of positive-strand RNA viruses: Implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Lu X., Yamamoto S., Tanaka M., Hibi T., Namaba S. The genome organization of the broad bean necrosis virus (BBNV) Arch. Virol. 1998;143:1335–1348. doi: 10.1007/s007050050379. [DOI] [PubMed] [Google Scholar]
- McGeachy K.D., Barker H. Potato mop-top virus RNA can move long distance in the absence of coat protein: evidence from resistant, transgenic plants. Mol. Plant Microbe Interact. 2000;13:125–128. doi: 10.1094/MPMI.2000.13.1.125. [DOI] [PubMed] [Google Scholar]
- Pereira L.G., Torrance L., Roberts I.M., Harrison B.D. Antigenic structure of the coat protein of potato mop-top virus. Virology. 1994;203:277–285. doi: 10.1006/viro.1994.1485. [DOI] [PubMed] [Google Scholar]
- Reavy B., Arif M., Cowan G.H., Torrance L. Association of sequences in the coat protein/readthrough domain of potato mop-top virus with transmission by Spongospora subterranea. J. Gen. Virol. 1998;79:2343–2347. doi: 10.1099/0022-1317-79-10-2343. [DOI] [PubMed] [Google Scholar]
- Sandgren M., Savenkov E.I., Valkonen J.P. The readthrough region of Potato mop-top virus (PMTV) coat protein encoding RNA, the second largest RNA of PMTV genome, undergoes structural changes in naturally infected and experimentally inoculated plants. Arch. Virol. 2001;146:467–477. doi: 10.1007/s007050170156. [DOI] [PubMed] [Google Scholar]
- Savenkov E.I., Sandgren M., Valkonen J.P. Complete sequence of RNA 1 and the presence of tRNA-like structures in all RNAs of Potato mop-top virus, genus Pomovirus. J. Gen. Virol. 1999;80:2779–2784. doi: 10.1099/0022-1317-80-10-2779. [DOI] [PubMed] [Google Scholar]
- Torrance L., Mayo M.A. Proposed re-classification of furoviruses. Arch. Virol. 1997;142:435–439. [PubMed] [Google Scholar]
CONTRIBUTED BY, K.E. Richards, C.M. Deom, R.A. Naidu
GENUS PECLUVIRUS
Type Species Peanut clump virus
VIRION PROPERTIES
MORPHOLOGY
Virions are rod-shaped of about 21 nm in diameter and of two predominant lengths of190 and 245 nm (Fig. 1 ). The length distribution of the short particles is broad and insome preparations an additional class of 160 nm is recognizable. Virions have helicalsymmetry with a pitch of 2.6 nm.
Figure 1.
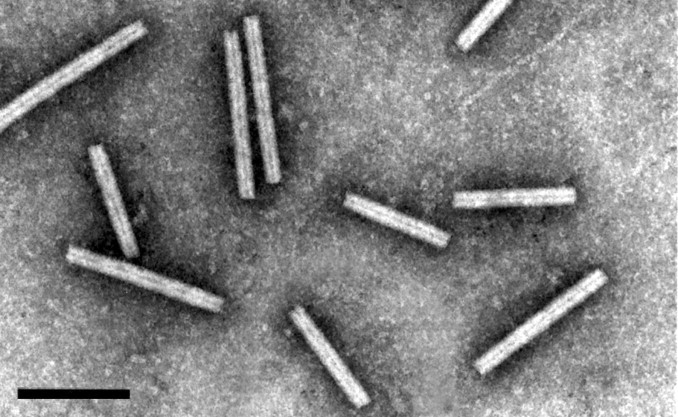
Negative contrast electron micrograph of virions of Indian peanut clump virus (IPCV) (L serotype)negatively stained with 2% phosphotungstic acid, pH 6. The bar represents 150 nm. (Courtesy, G.H. Duncan).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as two major components with S20w of 183S and 224S. Buoyant density in CsCl is 1.32 g/cm3. Virion isoelectric point is pH 6.45. Thermal inactivation of infectivity occurs at 64°C. Virions are stable in frozen leaves.
NUCLEIC ACID
The genome consists of two molecules of linear positive sense ssRNA; RNA-1 of ∼5,900 nt and RNA-2 of ∼4,500 nt. RNAs are thought to have a 5′-cap structure but this has not been confirmed. The 3′-ends of the RNAs are not polyadenylated.
PROTEINS
The virion CP subunits are 23 kDa.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
RNA-1 contains two ORFs (Fig. 2 ). The 5’ ORF encodes a 131 kDa protein, and suppression of a termination codon results in the synthesis of a readthrough protein of 191 kDa. The 3’ ORF encodes a 15 kDa protein. The proteins of 131 and 191 kDa contain NTP-binding, Hel and RNA polymerase motifs that make a putative replication complex. For Peanut clump virus (PCV), these proteins are respectively 88%, 95% and 75% similar to the products of an isolate of one serotype of Indian peanut clump virus (IPCV). The 15 kDa protein is translated from a sgRNA. It is a suppressor of posttranscriptional gene silencing and is targeted to peroxisomes or related punctate bodies during infection. RNA-2 contains 5 ORFs: the ORF near the 5′-end encodes the CP, the second ORF which, in PCV RNA-1, overlaps the first ORF by 2 nts encodes a 39 kDa protein. This protein is expressed by leaky scanning and is thought to be involved in the transmission of PCV by its fungus vector. Further downstream, separated by a 135 nt intergenic region, is a triple gene block sequence that codes for proteins of 51, 14 and 17 kDa that are thought to be involved in the movement of virus from cell to cell. The proteins are probably expressed via sgRNAs, but these have not been clearly identified. The 3’-NCR for PCV are 298 nt for RNA-1 and of 275 nt for RNA-2; the last 96 nt are identical in both RNAs. The NCRs differ in size among isolates from the different serotypes of IPCV.
Figure 2.
Genomic organization of Peanut clump virus (PCV) RNAs. ORFs are indicated by rectanglesand suppressible termination codon by an arrow (RT readthrough).
The two RNAs are required for systemic invasion of plants but RNA-1 is able to replicate in absence of RNA-2 in protoplasts. The virus is found in the cells of roots, stems and leaves of systemically infected plants.
ANTIGENIC PROPERTIES
The virus is highly immunogenic. There is a great serological variability among isolates of PCV. IPCV isolates fall into one of three very distinct serotypes; IPCV-H, IPCV-L, IPCVT. All are serologically distinct from PCV.
BIOLOGICAL PROPERTIES
HOST RANGE
The natural host first reported was Arachis hypogea (groundnut, Leguminosae). Disease symptoms are stunting – mottle – mosaic – chlorotic ringspot. PCV infects Sorghum arundinaceum, usually symptomlessly. IPCV infects a number of cereal crops and graminaceous weeds, some symptomlessly and others to induce stunting. The experimental host range is wide and includes species of Aizoaceae, Amaranthaceae, Chenopodiaceae, Cucurbitaceae, Graminae, Leguminosae, Scrophulariaceae, and Solanaceae. Nicotiana benthamiana and Phaseolus vulgaris are experimental propagation hosts, Chenopodium amaranticolor, and Chenopodium quinoa are local lesions hosts.
TRANSMISSION
The virus is transmitted naturally by a plasmodiophorid fungus (Polymyxa graminis) or by seed (in groundnuts). It is mechanically transmissible.
GEOGRAPHICAL DISTRIBUTION
PCV spreads in West Africa (Bénin, Burkina Faso, Congo, Côte d'Ivoire, Mali, Niger, Senegal and Pakistan). IPCV is widely distributed in India and Pakistan. A soil type favorable to the vector is a prerequisite for virus to cause disease.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The species are distinguished by different reactions with particular antisera (heterologous reactions are weak or undetectable). Also, PCV occurs only in Africa whereas IPCV occurs in the Indian subcontinent. However, isolates of IPCV can be readily assigned to one of three serotypes as protein preparations made from particles of each serotype barely react with heterologous antisera in immunoblotting tests. Isolates of PCV are also heterogeneous in their reactions with a panel of monoclonal antibodies. Moreover, several of the proteins encoded by genes in RNA of the different serotypes of IPCV differ in sequence from corresponding proteins of other IPCV serotypes by about as much as each differs from the corresponding protein of one isolate of PCV.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Indian peanut clump virus | ||
| Indian peanut clump virus – H | [X99149, AF447397] | (IPCV-H) |
| Indian peanut clump virus – D | [–, AF447396] | (IPCV-D) |
| Indian peanut clump virus – L | [–, AF239729] | (IPCV-L) |
| Peanut clump virus | ||
| Peanut clump virus – S | [X78602, L07269] | (PCV-S) |
| Peanut clump virus – Ni | [–, AF447398] | (PCV-Ni) |
| Peanut clump virus – N | [–, AF447399] | (PCV-N) |
| Peanut clump virus – M | [–, AF447400] | (PCV-M) |
| Peanut clump virus – B | [–, AF447401] | (PCV-B) |
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Not available.
SIMILARITY WITH OTHER TAXA
There are similarities among the putative replicase proteins of PCV and IPCV with those of Soil-borne wheat mosaic virus (SBWMV), the type species of the genus Furovirus. These proteins are more closely related to those of Barley stripe mosaic virus (BSMV; genus Hordeivirus) than to those of Beet necrotic yellow vein virus (BNYVV: genus Benyvirus, previously classified in the genus Furovirus). The CP sequences are 37% (IPCV) and 36% (PCV) identical to that of the CP of BSMV.
REFERENCES
- Bouzoubaa S. Furovirus isolation and RNA extraction. In: Foster G.D., Taylor S., editors. Vol. 81. Humana Press Inc; Totowa, NJ: 1998. pp. 107–114. (Plant Virology Protocols. From virus isolation to transgenic resistance). [Google Scholar]
- Dunoyer P., Pfeffer S., Fritsch C., Hemmer O., Voinnet O., Richards K.E. Identification, subcellular localization and some properties of a cysteine-rich suppressor of gene silencing encoded by peanut clump virus. Plant J. 2002;29:555–567. doi: 10.1046/j.0960-7412.2001.01242.x. [DOI] [PubMed] [Google Scholar]
- Herzog E., Guilley H., Fritsch C. Translation of the second gene of peanut clump virus RNA-2 occurs by leaky scanning in vitro. Virology. 1995;208:215–225. doi: 10.1006/viro.1995.1145. [DOI] [PubMed] [Google Scholar]
- Herzog E., Guilley H., Manohar S.K., Dollet M., Richards K., Fritsch C., Jonard G. Complete nucleotide sequence of peanut clump virus RNA 1 and relationships with other fungus-transmitted rodshaped viruses. J. Gen. Virol. 1994;75:3147–3155. doi: 10.1099/0022-1317-75-11-3147. [DOI] [PubMed] [Google Scholar]
- Legrève, A., Vanpee, B., Risopoulos, J., Ward, E. Maraite, H. (1996). Characterization of Polymyxa sp. associated with the transmission of indian peanut clump virus. Third symposium of the international working group on plant viruses with fungal vectors. (J.L. Sherwood and C.M. Rush, eds),157–160.
- Manohar S.K., Guilley H., Dollet M., Richards K., Jonard G. Nucleotide sequence and genetic organization of peanut clump virus RNA-2 and partial characterization of deleted forms. Virology. 1993;195:33–41. doi: 10.1006/viro.1993.1343. [DOI] [PubMed] [Google Scholar]
- Mayo M.A., Reddy D.V.R. Translation products of RNA from Indian peanut clump virus. J. Gen. Virol. 1985;66:1347–1351. [Google Scholar]
- Miller J.S., Wesley S.V., Naidu R.A., Reddy D.V.R., Mayo M.A. The nucleotide sequence of RNA-1 of Indian peanut clump furovirus. Arch. Virol. 1996;141:2301–2312. doi: 10.1007/BF01718632. [DOI] [PubMed] [Google Scholar]
- Naidu R.A., Sawyer S., Deom C.M. Molecular diversity of RNA-2 genome segments in pecluviruses causing peanut clump disease in West Africa and India. Arch. Virol. 2003;148:83–98. doi: 10.1007/s00705-002-0900-9. [DOI] [PubMed] [Google Scholar]
- Nolt B.L., Rajeshwari R., Reddy D.V.R., Bharathan N., Manohar S.K. Indian peanut clump virus isolates: host range, symptomatology, serological relationships, and some physical properties. Phytopathology. 1988;78:310–313. [Google Scholar]
- Reddy D.V.R., Rajeshwari R., Iisuka W., Lesemann D.E., Nolt B.L., Goto T. The occurrence of Indian peanut clump, a soil-borne virus disease of groundnuts (Arachis hypogaea) in India. Ann. Appl. Biol. 1983;102:305–310. [Google Scholar]
- Reddy D.V.R., Robinson D.J., Roberts I.M., Harrison B.D. Genome properties and relationships of Indian peanut clump virus. J. Gen. Virol. 1985;66:2011–2016. [Google Scholar]
- Thouvenel J.-C., Fauquet C. Further properties of peanut clump virus and studies on its natural transmission. Ann. Appl. Biol. 1981;97:99–107. [Google Scholar]
- Thouvenel J.C., Dollet M., Fauquet C. Some properties of peanut clump, a newly discovered virus. Ann. Appl. Biol. 1976;84:311–320. [Google Scholar]
- Torrance L., Mayo M.A. Proposed re-classification of furoviruses. Arch. Virol. 1997;142:435–439. [PubMed] [Google Scholar]
- Wesley S.V., Mayo M.A., Jolly C.A., Naidu R.A., Reddy D.V.R., Jana M.K., Parnaik V.K. The coat protein of Indian peanut clump virus: relationships with other furoviruses and with barley stripe mosaic virus. Arch. Virol. 1994;134:271–278. doi: 10.1007/BF01310566. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, R. Koenig, D.-E. Lesemann
GENUS BENYVIRUS
Type Species Beet necrotic yellow vein virus
VIRION PROPERTIES
MORPHOLOGY
The non-enveloped, rod-shaped particles are helically constructed with an axial canal (Fig. 1 ). They have predominant lengths of c. 85, 100, 265 and 390 nm and diameters of 20 nm. The right-handed helix with a pitch of 2.6 nm has an axial repeat of four turns, involving49 CP subunits. Each CP subunit occupies four nucleotides on the RNA.
Figure 1.

(Left) Scheme showing the accessibility to antibodies of various parts of the coat protein amino acid (aa) sequence in particles of Beet necrotic yellow vein virus (BNYVV). Encircled numbers designate different epitopes. (Center) Negative contrast electron micrograph of stained purified particles of BNYVV. (Right)From left (a) negative contrast electron micrograph of a BNYVV particle and (b, c, d) computer-filtered micrographs of BNYVV particles (Courtesy of A.C. Steven, from Virology 113, 428, (1981), with permission). The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The viruses are rather unstable in sap. At room temperature, most of the infectivity is lost within one or a few days.
NUCLEIC ACID
In naturally infected plants, virions contain four – or with some BNYVV isolates five-molecules of linear positive-sense ssRNA of c. 6.7, 4.6, 1.8, 1.4 and 1.3 kb, respectively. After mechanical transmission to test plants, RNAs 3, 4 and 5 of BNYVV may become partially deleted or may be lost entirely. The RNAs are capped at the 5′-end and – unlike the RNAs of all other plant viruses with rod-shaped particles – they are 3’-polyadenylated. The complete sequence has been determined for all five RNAs of different isolates of BNYVV and for the four RNAs of an isolate of Beet soil-borne mosaic virus (BSBMV). Partial sequences which have not yet been released have been determined for RNA-1 of Rice stripe necrosis virus (RSNV) as well as for RNAs 1 and 2 of Burdock mottle virus (BdMV).
PROTEIN
The major CP species is 21-23 kDa in size. The CP read through protein which may be detected in some BNYVV particles near one extremity by means of immunogold labeling apparently initiates virus assembly. A KTER motif in the C terminal part of the BNYVVCP read through protein is necessary for the transmission of the virus by Polymyxa betae. The three triple gene block (TBG)-encoded proteins (TGBp1, TGBp2 and TGBp3) are necessary for cell to cell movement. BNYVV TGBp1 labeled with green fluorescent protein (GFP) on its N-terminus is targeted by TGBp2 and TGBp3 to punctate bodies associated with plasmodesmata. The three proteins can be functionally substituted by the MP of Tobacco mosaic virus or the three TGB-encoded proteins of Peanut clump virus. The latter have to be supplied together, they are unable to substitute their BNYVV counterparts one by one. The N-terminal part of BNYVV TGBp1 has nucleic acid binding activity, its C-terminal part contains consensus sequence motifs characteristic of an ATP/GTP-dependent helicase.
LIPID
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
RNA-1 contains one large ORF for a putative replication-associated protein which is cleaved post-translationally. This distinguishes the benyviruses from all other viruses with rod-shaped particles, which have their replication-associated proteins encoded on two ORFs. In vitro translation of BNYVV RNA-1 may initiate at two sites: at the first AUG in the sequence at position 154 or at a downstream AUG at position 496. The resulting proteins of 237 and 220 kDa, respectively, both contain in their N-terminal part Mtr motifs, in their central part Hel and papain-like protease motifs (Prot) and in their C-terminal part RdRp motifs (Fig. 2 ). RNA-2 of BNYVV contains six ORFs, i.e. the CP gene which is terminated by a suppressible UAG stop codon, the CP read through protein gene, the TGB coding for TGBp1, TGBp2 and TGBp3 (42, 13 and 15 kDa respectively, with BNYVV) and agene coding for a 14 kDa cysteine-rich protein. The TGB ORFs and the P14 gene are expressed by means of subgenomic RNAs (Fig. 2). RNAs 1 and 2 are sufficient for replication of BNYVV in the local lesion host Chenopodium quinoa. The typical rhizomania symptoms in beet are produced only in the presence of RNA-3; RNA-4 greatly increases the transmission rate by Polymyxa betae and RNA-5 may modulate the type of symptoms formed. RNAs-3 and -4 are always present in natural BNYVV infections.
Figure 2.

Genome organization and translation strategies of Beet necrotic yellow-vein virus (BNYVV). The scheme indicates a suppressible UAG stop codon (arrow), and (An) the 3’ poly (A)-tails. Dark areas indicate conserved motifs. Hel; helicase, Mtr; methyltransferase, Pro: protease, RdRp; RNA dependent RNA polymerase, RT; read through.
ANTIGENIC PROPERTIES
Virions are moderately to strongly antigenic. BNYVV and BSBMV are very distantly related serologically. Epitope mapping has revealed portions of the aa sequence of BNYVV CP which are either exposed along the entire particle length, e.g. the immunodominant C-terminus, or are accessible only on one extremity or after disruption of the particles (Fig. 1).
BIOLOGICAL PROPERTIES
HOST RANGE
The natural host ranges of benyviruses are very narrow. Species of Chenopodium are infected experimentally, often only locally.
TRANSMISSION
In nature BNYVV and BSBMV are transmitted by Polymyxa betae and RNSV by P. graminis. The viruses are also mechanically transmissible.
GEOGRAPHICAL DISTRIBUTION
BNYVV has spread to most sugarbeet-growing areas world-wide. Different variants (A type, B type, P type) occur in different geographical areas. BSBMV is widely distributed in the USA. RSNV occurs in Africa, South and Central America. BdMV has been identified in a restricted area in Japan.
CYTOPATHIC EFFECTS
At early times post infection BNYVV has been found at the cytoplasmic surface of mitochondria. Later virions of most BNYVV isolates are scattered throughout the cytoplasm of infected cells or occur in aggregates. More or less dense masses of particles arranged in parallel or angle-layer arrays may be formed. Depending on the isolate only one or both types of aggregates occur. Membraneous accumulations of endoplasmic reticulum may also be found.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Distantly related serologically,
-
•
Less than 90% identity in their CP aa sequence
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Beet necrotic yellow vein virus | ||
| Beet necrotic yellow vein virus – C, China | [RNA-3: AJ239200; RNA-4: AJ239199; RNA-5: AJ236894-5] | (BNYVV-C) |
| Beet necrotic yellow vein virus – F2, France | [RNA-1: X05147; RNA-2: X04197; RNA-3: M36894; RNA-4: M36896] | (BNYVV-B-Eur) |
| Beet necrotic yellow vein virus – F72, France | [RNA-2: AF197547*; RNA-4: AF197546*; RNA-5: U78293*] | (BNYVV-P-Eur) |
| Beet necrotic yellow vein virus – N7, The Netherlands | [RNA-3: AF197558*; RNA-4: AF197559*] | (BNYVV-A-Eur) |
| Beet necrotic yellow vein virus – S, Japan | [RNA-1: D84410; RNA-2: D84411; RNA-3: D84412; RNA-4: D84413; RNA-5: AB018607-10*] | (BNYVV-A-Jap) |
| Beet necrotic yellow vein virus, Kazakhstan | [RNA-2: AF197556; RNA-4: AF197554*; RNA 5: AF197555*] | (BNYVV-P-Kaz) |
| Beet soil-borne mosaic virus | ||
| Beet soil-borne mosaic virus – EA | [RNA-1: NC_003506; RNA-2: NC_003503; RNA-3: NC_003507; RNA-4: NC_003508] | (BSBMV) |
• Sequences lack short stretches at the 5′ and 3′-ends
-
•
Sequences lack short stretches at the 5’ and 3’-ends
TENTATIVE SPECIES IN THE GENUS
| Burdock mottle virus | (BdMV) |
| Rice stripe necrosis virus | (RSNV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
The CP aa sequences of BNYVV and BSBMV share less than 60% homology (Fig. 3 ). The percentages of identity between various non-structural proteins of the two viruses range between 38% (cysteine-rich protein) and 84% (replication-associated protein). The CP of BdMV shares 38% of sequence identity with that of BNYVV. TGBp1 of BdMV shares c.50% identity with the corresponding proteins of BNYVV and BSBMV. A 2,239 nt fragment of RSNV RNA-1 shares about 50% sequence identity with the corresponding regions of BNYVV and BSBMV RNA-1.
Figure 3.

Phylogenetic trees showing the percentages of aa sequence identities of the replication-associated proteins and the CPs of benyviruses, of other plant viruses with rod-shaped particles, of Hepatitis virus E and Rubella virus (replication-associated proteins only) and of Turnip yellow mosaic virus as an unrelated plant virus (CP only). The alignments were made by the GCG programs LINEUP and PILEUP and the trees were generated by the program DNAMAN (Lynnon Bio/Soft).
SIMILARITIES WITH OTHER TAXA
Benyviruses are morphologically similar to other rod-shaped viruses, i.e. furo-, peclu-,pomo-, hordei-, tobra- and tobamoviruses. The CPs of these viruses have a number of conserved residues, e.g. RF and FE in their central and C-terminal parts, respectively, which are presumably involved in the formation of salt bridges. Like pomo-, peclu- and hordeiviruses, but unlike furo-, tobamo- and tobraviruses, the benyviruses have their movement function encoded on a triple gene block. Sequence identities in the first and second triple gene block-encoded proteins reveal affinities not only to pomo- and hordei-, but also to potex- and carlaviruses. The Mtr, Hel and RdRp motifs in the putative replication-associated proteins show a higher degree of similarity to those of the viruses of the family Togaviridae (Rubella virus) and of Hepatitis virus E than to those of other rod-shaped plant viruses.
DERIVATION OF NAME
Beny: siglaw from Beet necrotic yellow vein virus.
REFERENCES
- Commandeur U., Koenig R., Manteuffel R., Torrance L., Lüddecke P., Frank R. Location, size and complexity of epitopes on the coat protein of beet necrotic yellow vein virus studied by means of synthetic overlapping peptides. Virology. 1994;198:282–287. doi: 10.1006/viro.1994.1031. [DOI] [PubMed] [Google Scholar]
- Erhardt M., Morant M., Ritzenthaler C., Stussi-Garaud C., Guilley H., Richards K., Jonard G., Bouzoubaa S., Gilmer D. P42 movement protein of Beet necrotic yellow vein virus is targeted by the movement proteins P13 and P15 to punctate bodies associated with plasmodesmata. Mol. Plant Microbe Interact. 2000;13:520–528. doi: 10.1094/MPMI.2000.13.5.520. [DOI] [PubMed] [Google Scholar]
- Erhardt M., Dunoyer P., Guilley H., Richards K., Jonard G., Bouzoubaa S. Beet necrotic yellow vein virus particles localize to mitochondria during infection. Virology. 2001;286:256–262. doi: 10.1006/viro.2001.0931. [DOI] [PubMed] [Google Scholar]
- Haeberlé A.M., Stussi-Garaud C., Schmitt C., Garaud J.C., Richards K.E., Guilley H., Jonard G. Detection by immunogold labelling of P75 readthrough protein near an extremity of Beet necrotic yellow vein virus particles. Arch. Virol. 1994;134:195–203. doi: 10.1007/BF01379118. [DOI] [PubMed] [Google Scholar]
- Hehn A., Fritsch C., Richards K.E., Guilley H., Jonard G. Evidence for in vitro and in vivo autocatalytic processing of the primary translation product of Beet necrotic yellow vein virus RNA 1 by a papain-like proteinase. Arch. Virol. 1997;142:1051–1058. doi: 10.1007/s007050050141. [DOI] [PubMed] [Google Scholar]
- Hirano, S., Kondo, H., Maeda, T. and Tamada, T. (1999) Burdock mottle virus has a high genome similarity to beet necrotic yellow vein virus. Proceedings of the Fourth Symposium of the International Working Group on Plant Viruses with Fungal Vectors, Asilomar, October 5–8-1999, (J.L. Sherwood and C.M. Rush, eds.), 33–36.
- Kiguchi T., Saito M., Tamada T. Nucleotide sequence analysis of RNA-5 of five isolates of Beet necrotic yellow vein virus and the identity of a deletion mutant. J. Gen. Virol. 1996;77:575–580. doi: 10.1099/0022-1317-77-4-575. [DOI] [PubMed] [Google Scholar]
- Koenig R., Haeberlé A.M., Commandeur U. Detection and characterization of a distinct type of beet necrotic yellow vein virus RNA 5 in a sugarbeet growing area in Europe. Arch. Virol. 1997;142:1499–1504. [PubMed] [Google Scholar]
- Koenig R., Jarausch W., Li Y., Commandeur U., Burgermeister W., Gehrke M., Lüddecke P. Effect of recombinant Beet necrotic yellow vein virus with different RNA compositions on mechanically inoculated sugarbeets. J. Gen. Virol. 1991;72:2243–2246. doi: 10.1099/0022-1317-72-9-2243. [DOI] [PubMed] [Google Scholar]
- Koenig R., Lüddecke P., Haeberlé, A.M. Detection of Beet necrotic yellow vein virus strains, variants and mixed infections by examining single-strand conformation polymorphisms of immunocapture RT-PCR products. J. Gen. Virol. 1995;76:2051–2055. doi: 10.1099/0022-1317-76-8-2051. [DOI] [PubMed] [Google Scholar]
- Lauber E., Bleykasten-Grosshans C., Erhardt M., Bouzoubaa S., Jonard G., Richards K.E., Guilley H. Cell-to-cell movement of Beet necrotic yellow vein virus: I. Heterologous complementation experiments provide evidence for specific interactions among the triple gene block proteins. Mol Plant Microbe Interact. 1998;11:618–625. doi: 10.1094/MPMI.1998.11.7.618. [DOI] [PubMed] [Google Scholar]
- Lee L., Telford E.B., Batten J.S., Scholthof K.B., Rush C.M. Complete nucleotide sequence and genome organization of Beet soilborne mosaic virus, a proposed member of the genus Benyvirus. Arch. Virol. 2001;146:2443–2453. doi: 10.1007/s007050170014. [DOI] [PubMed] [Google Scholar]
- Richards K., Tamada T. Mapping functions on the multipartite genome of beet necrotic yellow vein virus. Ann. Rev. Phytopathol. 1992;30:291–313. [Google Scholar]
- Tamada T., Abe H. Evidence that beet necrotic yellow vein virus RNA-4 is essential for efficient transmission by the fungus Polymyxa betae. J. Gen. Virol. 1989;70:3391–3398. doi: 10.1099/0022-1317-72-7-1497. [DOI] [PubMed] [Google Scholar]
- Tamada T., Schmitt C., Saito M., Guilley H., Richards K., Jonard G. High resolution analysis of the readthrough domain of Beet necrotic yellow vein virus readthrough protein: a KTER motif is important for efficient transmission of the virus by Polymyxa betae. J. Gen. Virol. 1996;77:1359–1367. doi: 10.1099/0022-1317-77-7-1359. [DOI] [PubMed] [Google Scholar]
BROMOVIRIDAE
CONTRIBUTED BY, M.J. Roossinck, J. Bujarski, S.W. Ding, R. Hajimorad, K. Hanada, S. Scott, M. Tousignant
FAMILY BROMOVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Bromoviridae |
| Genus | Alfamovirus |
| Genus | Bromovirus |
| Genus | Cucumovirus |
| Genus | Ilarvirus |
| Genus | Oleavirus |
VIRION PROPERTIES
MORPHOLOGY
Virions are either spherical (Fig. 1 ), having T=3 icosahedral symmetry, and a diameter of 26-35 nm (genera Bromovirus, Cucumovirus, and Ilarvirus) or bacilliform (genera Alfamovirus, Ilarvirus, and Oleavirus) having within a species constant diameters of 18-26 nm and lengths from 30 to 85 nm. Genomic RNAs are packaged in separate virions that may also contain sgRNAs, defective RNAs or satellite RNAs.
Figure 1.

(Top left) Image reconstruction of a particle of Cowpea chlorotic mottle virus (CCMV)(genus Bromovirus), showing pentamer and hexamer clustering in a T=3 quasi-icosahedron (Lucas et al., 2001); (Top central) Schematic representation of a T=3 particle; (Top right) Negative contrast electron micrograph of particles of Cucumber mosaic virus (CMV)(genus Cucumovirus), (Courtesy of G. Kasdorf); (Bottom left) Electron density representation of a Ta particle of Alfalfa mosaic virus (AMV)(genus Alfamovirus), showing T=1 structure (Kumar et al., 1997); (Bottom Center) Schematic representation of a T=1 particle; (Bottom right) Negative contrast electron micrograph of particles of Prune dwarf virus (PDV)(genus Ilarvirus).
(Courtesy of G. Kasdorf). The bars represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The Mr of the virions varies from 3.5-6.9 × 106, depending on the nucleic acid content. Virion Mr is constant among members of the genera Bromovirus, Cucumovirus, and some Ilarvirus members, and varies among the remaining members of the family. The buoyant density of formaldehyde-fixed virions ranges from 1.35-1.37 g/cm3 in CsCl. The S20w varies from 63S to 99S. Virion integrity is dependent on RNA-protein interactions and virion RNA is susceptible to RNAse degradation in situ at neutral pH. Heat inactivationoccurs at 60°C in some genera, others have not been tested. In most cases, virions are unstable in the presence of divalent cations. Virions are generally stable in the presence of chloroform, but not in the presence of phenol. Virions are unstable in the presence of strong anionic detergents such as SDS, but can tolerate low concentrations of mild detergents such as Triton X-100.
NUCLEIC ACID
Total genome length is approximately 8 kb. Genomes consist of three linear, positive sense ssRNAs with 5′-terminal cap structures. The 3′-termini are not polyadenylated, but generally are highly conserved within a species or isolate, and form strong secondary structures. They are either tRNA-like and can be aminoacylated (genera Bromovirus and Cucumovirus) or form other structures that are not aminoacylated (genera Alfamovirus, Ilarvirus and Oleavirus) (Table 1 ).
Table 1.
Genomic RNA sizes in nucleotide number for typical members of each genus.
| Genus | species | strain | RNA-1 | RNA-2 | RNA-3 | 3′ term. DIs/sat | RNAs |
|---|---|---|---|---|---|---|---|
| Alfamovirus | AMV | 425 | 3,644 | 2,593 | 2,037 | complexa | −/− |
| Bromovirus | BMV | Russian | 3,234 | 2,865 | 2,117 | tRNAb | +/v |
| Cucumovirus | CMV | Fny | 3,357 | 3,050 | 2,216 | tRNA | +/+ |
| Ilarvirus | TSV | n.a | 3,491 | 2,926 | 2,205 | complex | −/− |
| Oleavirus | OLV-2 | n.a | 3,126 | 2,734 | 2,438 | complex | ?/? |
complex secondary structure.
aminoacylatable, with pseudoknot folding. n.a.: not applicable, only 1 strain reported
PROTEINS
A single 20-24 kDa CP is expressed from a sgRNA. The CP is generally required for systemic movement and may be required for cell to cell spread in some cases. There are at least three ORFs for non-structural proteins: the replicase (consisting of the 1a and 2a proteins, along with host factors) and the MP (3a) (Table 2 ). Cucumovirus RNA-2 encodes 2b proteins that are involved in post-transcriptional gene silencing. TSV RNA-2 also encodes an ORF for a 2b protein.
Table 2.
Virus proteins, sizes and functional activities.
| Protein | size (kDa) | mRNA | Function1 |
|---|---|---|---|
| 1a | 102.7-125.8 | RNA-1 | Mtr, helicase |
| 2a | 89.8-96.7 | RNA-2 | RdRp |
| 3a | 30.5-36.5 | RNA-3 | cell to cell movement |
| CP | 19.8-26.2 | sgRNA-42 | encapsidation, movement |
Functions of 1a and 2a are putative in most cases, by analogy to related viruses
The sgRNA for the CP derived from RNA-3 is encapsidated in all but the genus Oleavirus.
LIPIDS
There are no lipids associated with the virions.
CARBOHYDRATES
There are no carbohydrates associated with the virions.
GENOME ORGANIZATION AND REPLICATION
RNA-1, −2, and −3, can act as mRNAs. The 2b ORF is found in cucumovirus RNA-2 the genus Cucumovirus, and is expressed from an additional sgRNA that may or may not be encapsidated. The CP ORF is expressed from a sgRNA that is usually encapsidated (RNA-4). The genomic RNA-1 and −2 each encode a single large ORF, and in some genera RNA-2 also encodes a second ORF that is translated from a sgRNA. RNA-3 encodes a 5′ protein, the MP, and the CP, which is translated from a sgRNA. There is no clear evidence of proteolytic or other post-translational processing. Virus replication occurs on cytoplasmic membranes via full length minus (-) strand synthesis and subsequent plus (+) strand synthesis. The sgRNAs are synthesized from the (-) template, and may or may not be found in the virions. The CP accumulates to high levels in infected cells, whereas the nonstructural proteins accumulate to much lower levels. Virions accumulate in the cytoplasm. The life cycle of the virus takes place predominantly in the cytoplasm.
Figure 2.

Schematic genome organization for members of the family Bromoviridae.
ANTIGENIC PROPERTIES
Native virions are generally poor immunogens and require stabilization with formaldehyde prior to use as antigens. There are little or no serological relationships between the genera, and weak relationships between species of the same genus.
BIOLOGICAL PROPERTIES
The natural host range of the viruses ranges from very narrow (genus Bromovirus) to extremely broad (genus Cucumovirus). They are predominantly transmitted by insects, in a non-persistent manner, or mechanically. Vectors have not been identified for some of the members of the family. They are distributed worldwide, and several are responsible for major disease epidemics in crop plants.
GENUS ALFAMOVIRUS
Type Species Alfalfa mosaic virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
Virions are generally bacilliform, having a constant diameter of 18 nm, and varying from 30-57 nm in length, depending on the nucleic acid species encapsidated. The Mr are from 3.5-6.9 × 106. Virions are readily separated into components by sucrose density.
GENOME ORGANIZATION AND REPLICATION
Replication is activated by CP binding to the complex 3′-end structure. CP from members of the genus Ilarvirus can also activate replication.
BIOLOGICAL PROPERTIES
The host range is very broad. Viruses are transmitted by aphids in a non-persistent manner.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS BROMOVIRUS
Type Species Brome mosaic virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
MORPHOLOGY
Virions are polyhedral, and all the same size, with a diameter of 27 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions prepared below pH 6.0 have S20w of 88S, are stable to high salt and low detergent concentrations, and are nuclease- and protease-resistant. At pH 7.0 and above, virions swell to a diameter of 31 nm, S20w decreases to 78S, salt and detergent stability decreases dramatically, and protein and RNA are susceptible to hydrolytic enzymes. This swelling is accompanied by conformational changes of the capsid that are detectable by physical and serological means.
NUCLEIC ACID
RNA 3′-termini are tRNA-like, are very similar in all viruses sequenced so far, and can be aminoacylated with tyrosine.
PROTEINS
Coat protein size is 20 kDa, unlike the 24-26 kDa of other members of the family Bromoviridae. The 3a proteins and CPs of bromoviruses share sequence similarities with one another, and more distantly with cucumoviruses, but not with ilarviruses or alfamoviruses.
ANTIGENIC PROPERTIES
All members are serologically related, with large antigenic differences between species.
BIOLOGICAL PROPERTIES
The natural host range is narrow, and is limited to a few plant hosts for each species. All species are thought to be beetle-transmitted, although BMV is inefficiently transmitted by aphids in a non-persistent manner.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Host range,
-
•
Serological relationships,
-
•
Compatible replicase proteins (i.e. 1a and 2a proteins),
-
•
Nucleotide sequence similarity between species ranges from 50 to 80% depending on the gene used for comparison.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Broad bean mottle virus | ||
| Broad bean mottle virus | [RNA1: M65138, RNA2: M64713, RNA3: M60291,] | (BBMV) |
| Brome mosaic virus | ||
| Brome mosaic virus | Russian strain [RNA1: X02380, RNA2: X01678, RNA3: V00099] | (BMV) |
| Cassia yellow blotch virus | ||
| Cassia yellow blotch virus | (CYBV) | |
| Cowpea chlorotic mottle virus | ||
| Cowpea chlorotic mottle virus | [RNA1: M65139, RNA2: M28817, RNA3: M28818,] | (CCMV) |
| Melandrium yellow fleck virus | ||
| Melandrium yellow fleck virus | (MYFV) | |
| Spring beauty latent virus | ||
| Spring beauty latent virus | [RNA1: AB080598, RNA2: AB080599, RNA3: AB080600] | (SBLV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS CUCUMOVIRUS
Type Species Cucumber mosaic virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
MORPHOLOGY
Virions are icosahedral, of uniform size and sedimentaion properties. In electron micrographs they appear to have electron dense centers.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Purified virions are labile, and are especially susceptible to anionic detergents and high ionic strength buffers that disrupt the RNA-protein interactions required for particle integrity. Most strains are unstable in the presence of Mg2+, but at least one strain of CMV requires Mg2+ for stability.
NUCLEIC ACID
The 3′-termini of all RNAs within a species are highly similar and can form tRNA-like structures that are aminoacylatable with tyrosine. Within a species, the 5′-NTR of RNA-1 and RNA-2 are also very similar. At least one strain of CMV can form defective RNAs that arise by deletions in the 3a ORF of RNA-3. Subgroup II strains of CMV encapsidate the sgRNA for the 2b ORF, called RNA-4a, and an additional small RNA of about 300 nt, called RNA-5, that is co-terminal with the 3′-ends of RNAs-3 and 4. In addition, CMV and Peanut stunt virus (PSV) may harbor satellite RNAs of about 330 to 400 nt. The satellite RNAs are more common under experimental conditions than in field conditions, and may dramatically alter the symptoms of infection by the helper virus.
PROTEINS
Although the CPs are estimated to be about 24 kDa by sequence analysis, most migrate in polyacrylamide gels with a slower than predicted mobility.
GENOME ORGANIZATION AND REPLICATION
An additional ORF, the 2b ORF is found in all cucumoviruses and has been shown to be active in CMV and Tomato aspermy virus (TAV).
ANTIGENIC PROPERTIES
CMV has been divided into two subgroups, based on serology. PSV also has more than one serological group. Sequence analysis has upheld the divisions, although CMV subgroup I can be further divided into two groups by phylogenetic analyses.
BIOLOGICAL PROPERTIES
CMV has an extremely broad host range, infecting 85 distinct plant families, and up to 1000 species experimentally. The other cucumovirus species have narrower host ranges; PSV is largely limited to legumes and solanaceous hosts, and TAV predominantly infects composites and solanaceous plants. All species are transmitted by aphids in a non-persistent manner.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Serological relatedness,
-
•
Compatibility of replicase proteins (1a and 2a proteins), but these distinctions may break down in the case of naturally occurring reassortants,
-
•
Sequence similarity.
Serology and nucleotide sequence similarity is used to distinguish subgroups within a species. Subgroups generally have at least 65% sequence similarity.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Cucumber mosaic virus | ||
| Cucumber mosaic virus | [Fny strain; RNA1: D00356, RNA2: D00355, RNA3: D10538, Q strain; RNA1: X02733, RNA2: X00985, RNA3: M21463] | (CMV) |
| Peanut stunt virus | ||
| Peanut stunt virus | [ER strain; RNA1: U15728, RNA2: U15729, RNA3: U15730, J strain; RNA1: D11126, RNA2: D11127, RNA3: D00668] | (PSV) |
| (Robinia mosaic virus) | ||
| Tomato aspermy virus | ||
| Tomato aspermy virus | [RNA1: D10663, RNA2: D10044, RNA3: D01015] | (TAV) |
| (Chrysanthemum aspermy virus) | ||
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS ILARVIRUS
Type Species: Tobacco streak virus
DISTINGUISHING FEATURES
VIRION PROPERTIES
MORPHOLOGY
Virions are quasi-isometric or occasionally bacilliform, and are about 30 nm in diameter.
NUCLEIC ACID
There is a short region of sequence similarity at the 3′-ends of the RNAs.
PROTEINS
The CP is required for activation of replication, but may be substituted with CP from AMV (genus Alfamovirus).
ANTIGENIC PROPERTIES
Viruses in each subgroup are all serologically related, and there are also some serological cross-reactions between certain subgroups; however, there are no cross-reactions between subgroup 1 viruses and any other viruses.
BIOLOGICAL PROPERTIES
The viruses infect mainly woody plants.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Serology,
-
•
Host range,
-
•
Sequence similarity (Specific levels of sequence similarity have not been defined). Clusters of viruses (Subgroup 1-7) that are antigenically related are indicated in the list.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Parietaria mottle virus | ||
| Parietaria mottle virus | [RNA1: AY496068, RNA2: AY496069] | (PMoV) |
| Tobacco streak virus | ||
| Tobacco streak virus | [RNA1: U80934, RNA2: U75538, RNA3: X00435,] | (TSV) |
| Subgroup 2 | ||
| Asparagus virus 2 | ||
| Asparagus virus 2 | [RNA1: U93601, RNA1: U93602, RNA2: U93603, RNA3: X86352] | (AV-2) |
| Citrus leaf rugose virus | ||
| Citrus leaf rugose virus | [RNA1: U23715, RNA2: U17726, RNA3: U17390] | (CiLRV) |
| Citrus variegation virus | ||
| Citrus variegation virus | [RNA1: U93604, RNA2: U93605, RNA3: U17389] | (CVV) |
| Elm mottle virus | ||
| Elm mottle virus | [RNA1: U57047, RNA2: U34050, RNA3: U57048] | (EMoV) |
| Hydrangea mosaic virus | [RNA1: AF172968, RNA2: AF172967, RNA3: AF192965] | (HdMV) |
| Spinach latent virus | ||
| Spinach latent virus | [RNA1: U93192, RNA2: U93193, RNA3: U93194] | (SpLV) |
| Tulare apple mosaic virus | ||
| Tulare apple mosaic virus | [RNA1: AF226160, RNA2: AF226161, RNA3: AF226162] | (TAMV) |
| Subgroup 3 | ||
| Apple mosaic virus | ||
| Apple mosaic virus | [RNA1: AF174584, RNA2: AF174585, RNA3: U15608] | (ApMV) |
| Blueberry shock virus | ||
| Blueberry shock virus | (BlShV) | |
| Humulus japonicus latent virus | ||
| Humulus japonicus latent virus | (HJLV) | |
| Prunus necrotic ringspot virus | ||
| Prunus necrotic ringspot virus | [RNA1: AF278534, RNA2: AF278535, RNA3: L38823] | (PNRSV) |
| Subgroup 4 | ||
| Prune dwarf virus | ||
| Prune dwarf virus | [RNA1: U57648, RNA2: AF277662, RNA3: L28145] | (PDV) |
| Subgroup 5 | ||
| American plum line pattern virus | ||
| American plum line pattern virus | [RNA1: AF235033, RNA2: AF235165, RNA3: AF235166] | (APLPV) |
| Subgroup 6 | ||
| Fragaria chiloensis latent virus | ||
| Fragaria chiloensis latent virus | (FClLV) | |
| Lilac ring mottle virus | ||
| Lilac ring mottle virus | [RNA3: U17391] | (LiRMoV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS OLEAVIRUS
Type Species: Olive latent virus 2
DISTINGUISHING FEATURES
VIRION PROPERTIES
MORPHOLOGY
Virions have different shape and size, ranging from quasispherical with a diamter of 26 nm, to bacilliform with lengths of 37, 43, 38, and 55 nm, and diameters of 18 nm. Particles up to 85 nm in length occasionally are present.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
In sucrose gradients, virions sediment as 5 or 6 components.
NUCLEIC ACID
Virion RNA differs in size from that of other members of the family, encapsidating the three genomic RNAs and a sgRNA of about 2 kb, that is apparently not an mRNA. The sgRNA for the CP ORF is not encapsidated. Three additional RNAs of 200 to 550 nt are also present in virions. The 5′-termini of the genomic RNAs are capped, but not the 5′-terminus of the encapsidated sgRNA. The 3′-termini of the RNAs are similar to those of the genera Alfamovirus and Ilarvirus, but do not interact with CP to activate replication.
ANTIGENIC PROPERTIES
Virions are efficient immunogens.
BIOLOGICAL PROPERTIES
The only known host is olive (Olea europaea), which is infected asymptomatically. The virus is transmitted by inoculations, but no insect vector is known.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
LIST OF UNASSIGNED SPECIES IN THE FAMILY
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Phylogenetic relationships have not been thoroughly characterized in this family. The CP and Mps are too distant to use for phylogenetic analyses. The replicase genes reveal discrepancies between the accepted taxonomy and the probable phylogenetic relationships. However, the genera Bromovirus and Cucumovirus form a distinct clade from genera Alfamovirus and Ilarvirus, and genus Oleavirus is the most distant from all the others.
SIMILARITY WITH OTHER TAXA
The viruses are members of the “alpha-like” supergroup, sharing sequence similarity in the 1a protein domains for Mtr and Hel activities, and in the 2a protein polymerase domain with members of the genera Tobravirus, Hordeivirus, Tobamovirus, Potexvirus, Carlavirus, and Tymovirus, and animal viruses in the family Togaviridae. The 3a proteins of bromoviruses and the 35 kDa protein of the members of the genus Dianthovirus (RCNMV) form a distinct “family” of movement-associated proteins. Raspberry bushy dwarf virus (genus Idaeovirus) is similar to bromoviruses in genome organization and in the sequence of certain genes.
DERIVATION OF NAMES
Alfamo: derived from Alfalfa mosaic virus.
Bromo: derived from Brome mosaic, also, from Bromus (host of Brome mosaic virus).
Cucumo: derived from Cucumber mosaic virus.
Ilar: derived from isometric labile ringspot.
Olea: derived from the genus name of the host, olive (Olea).
REFERENCES
- Ahlquist P. Bromovirus RNA replication and transcription. Curr. Opin. Gen. Dev. 1992;2:71–76. doi: 10.1016/s0959-437x(05)80325-9. [DOI] [PubMed] [Google Scholar]
- Bernal J.J., Moriones E., García-Arenal F. Evolutionary relationships in the cucumoviruses: nucleotide sequence of tomato aspermy virus RNA-1. J. Gen. Virol. 1991;72:2191–2195. doi: 10.1099/0022-1317-72-9-2191. [DOI] [PubMed] [Google Scholar]
- Bol J.F. Alfalfa mosaic virus and ilarviruses: involvement of coat protein in multiple steps of the replication cycle. J. Gen. Virol. 1999;80:1089–1102. doi: 10.1099/0022-1317-80-5-1089. [DOI] [PubMed] [Google Scholar]
- Ding S.-W., Anderson B.J., Haase H.R., Symons R.H. New overlapping gene encoded by the cucumber mosaic virus genome. Virology. 1994;198:593–601. doi: 10.1006/viro.1994.1071. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Palukaitis P., Park Y.I. Phosphorylation of cucumber mosaic virus RNA polymerase 2a protein inhibits formation of replicase comples. EMBO J. 2002;21:2292–2300. doi: 10.1093/emboj/21.9.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Reddy V.S., Yusibov V., Chipman P.R., Hata Y., Fita I., Fukuyama K., Rossmann M.G., Loesch-Fries L.S., Baker T.S., Johnson J.E. The structure of Alfalfa mosaic virus capsid protein assembled as a T=1 icosahedral particle at 4.0-A resolution. J. Virol. 1997;71:7911–7916. doi: 10.1128/jvi.71.10.7911-7916.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R.W., Larson S.B., McPherson A. The crystallographic structure of Brome mosaic virus. J. Mol. Biol. 2001;317:95–108. doi: 10.1006/jmbi.2001.5389. [DOI] [PubMed] [Google Scholar]
- Martelli G.P., Grieco F. Oleavirus, a new genus i n t h e f a m i l y Bromoviridae. Arch. Virol. 1997;142:1933–1936. [PubMed] [Google Scholar]
- Naidu R.A., Hu C.-C., Pennington R.E., Ghabrial S.A. Differentiation of eastern and western strains of Peanut stunt cucumovirus based on satellite RNA support and nucleotide sequence homology. Phytopathology. 1995;85:502–507. [Google Scholar]
- Noueiry A.O., Ahlquist P. Brome mosaic virus RNA replication: revealing the role of the host in RNA virus replication. Ann. Rev. Phytopathol. 2003;41:77–98. doi: 10.1146/annurev.phyto.41.052002.095717. [DOI] [PubMed] [Google Scholar]
- O'Reilly D., Thomas C.J.R., Coutts R.H.A. Tomato aspermy virus has an evolutionary relationship with other tripartite RNA plant viruses. J. Gen. Virol. 1991;72:1–7. doi: 10.1099/0022-1317-72-1-1. [DOI] [PubMed] [Google Scholar]
- Palukaitis P., Roossinck M.J., Dietzgen R.G., Francki R.I.B. Cucumber mosaic virus. Adv. Virus Res. 1992;41:281–348. doi: 10.1016/s0065-3527(08)60039-1. [DOI] [PubMed] [Google Scholar]
- Roossinck M.J. Evolutionary history of cucumber mosaic virus deduced by phylogenetic analyses. J Virol. 2002;76:3382–3387. doi: 10.1128/JVI.76.7.3382-3387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Navarro J.A., Pállas V. Evolutionary relationships in the ilarviruses: nucleotide sequence of Prunus necrotic ringspot virus RNA-3. Arch. Virol. 1997;142:749–763. doi: 10.1007/s007050050116. [DOI] [PubMed] [Google Scholar]
- Scott S.W., Zimmerman M.T., Ge X. Viruses in subgroup 2 of the genus Ilarvirus share both serological relationships and characteristics at the molecular level. Arch. Virol. 2003;148:2063–2075. doi: 10.1007/s00705-003-0148-z. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Yoshida M., Yoshinuma T., Hibi T. Interaction of replicase components between Cucumber mosaic virus and Peanut stunt virus. J. Gen. Virol. 2003;84:1931–1939. doi: 10.1099/vir.0.19070-0. [DOI] [PubMed] [Google Scholar]
OURMIAVIRUS
CONTRIBUTED BY, R.G. Milne
GENUS OURMIAVIRUS
Type Species Ourmia melon virus
VIRION PROPERTIES
MORPHOLOGY
The bacilliform virions constitute a series of particles with conical ends (apparently hemi-icosahedra) and cylindrical bodies 18 nm in diameter. The bodies of the particles are composed of a series of double disks, the commonest particles having 2 disks (particle length 30 nm), a second common particle having 3 disks (particle length 37 nm) with rarer particles having 4 disks (particle length 45.5 nm) and 6 disks (particle length 62 nm). There is no envelope (Fig. 1 and 2 ).
Figure 1.

Diagram of virion surface of a member of the genus Ourmiavirus, showing arrangement of double disks and conical ends in particles of different length. Each row of 5 triangles represents a double disk.
Figure 2.

(A, B, C) Negative contrast electron micrographs (uranyl acetate) of purified particles of Ourmia melon virus (OuMV). The bar represents 100 nm. D and E, features of the two commonest particle types, enhanced by photographic superimposition.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
The Mr of virions and their sedimentation coefficients are not known. The buoyant density in CsCl of all particle sizes is 1.375 g/cm3. The particles are stable near pH 7. Thermally, they are relatively stable; infectivity survives in crude sap after heating for 10 min at 70°C but not 80°C, and is retained after at least one freeze-thaw cycle. Cation stability is not known, except that the particles survive in CsCl density gradients. The particles survive treatment with chloroform but not n-butanol, and survive treatment with 1% Triton X-100 detergent.
NUCLEIC ACID
The nucleic acid is linear positive-sense ssRNA with an estimated Mr of 1.58 × 106, divided into 3 segments with estimated Mr of 0.91, 0.35 and 0.32 × 106. It is not known if there is a 5′-terminal cap, a 5′-terminal covalently-linked polypeptide or a 3′-terminal poly(A) tract. No nucleotide sequences are available.
PROTEINS
There is one CP of 25.2 kDa. In addition, one nonstructural protein of unknown function accumulates abundantly in the cytoplasm of infected plants, forming fibers or tubules.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The CP is encoded by RNA-3. The fibrous nonstructural protein is encoded by RNA-1 or RNA-2. Particles accumulate to high levels in the cytoplasm of parenchyma cells and become associated with the fibrous protein.
ANTIGENIC PROPERTIES
The native virions and the nonstructural protein are good immunogens. They do not cross-react.
BIOLOGIC PROPERTIES
The type species can easily be mechanically transmitted to a rather wide range of dicotyledonous plants (34 species in 14 families reported), usually inducing systemic ringspots, mosaic and necrosis, with local lesions on some hosts. There is no particular tissue tropism. No vector has been identified. No experimental transmission was obtained with several aphid species, the whiteflies Trialeurodes and Bemisia or a Tetranychus mite. Experimental seed-transmission rates are 1 − 2% in Nicotiana benthamiana and N. megalosiphon. Different species in the genus are reported to occur in geographically diverse areas and on widely differing hosts, though there are experimental hosts in common.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The species demarcation criteria are:
-
•
No evident sequence homologies between the respective RNA-2s and RNA-3s (by hybridization).
-
•
CPs of distinctly different size (25 or 21 kDa).
-
•
No serological relationships between CPs (by gel-diffusion or EM decoration tests) or distant serological (in Western blots).
-
•
Natural host ranges non-overlapping (cucurbits; cherry; cassava).
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Cassava virus C | |
| Cassava virus C | (CsVC) |
| Epirus cherry virus | |
| Epirus cherry virus | (EpCV) |
| Ourmia melon virus | |
| Ourmia melon virus | (OuMV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Not available.
SIMILARITY WITH OTHER TAXA
The virion morphology, in particular the architecture of double disks and conical ends, forming particles of different lengths, is unique, differing clearly from that of other known “bacilliform” viruses of similar size. The virions are notably resistant to treatment with solvents, detergent and heat. There are 3 ssRNAs of Mr about 0.9, 0.35 and 0.32 × 106 combined with a single CP of 21-25 kDa. A fibrous nonstructural protein accumulates conspicuously in the cytoplasm. The genus may have affinities with other ssRNA positive-strand multipartite viruses such as the members of the family Bromoviridae.
DERIVATION OF NAMES
Ourmia: derived from the district of Ourmia in Northern Iran where the type species was found.
REFERENCES
- Accotto G.P., Riccioni L., Barba M., Boccardo G. Comparison of some molecular properties of Ourmia melon and Epirus cherry viruses, two representatives of a proposed new virus group. J. Plant Pathol. 1997;78:87–91. [Google Scholar]
- Aiton M.M., Roberts I.M., Harrison B.D. Two new cassava viruses from Africa. Abstracts 5th Inter. Congress Plant Pathol.; Kyoto: 1988. p. 43. 1988. [Google Scholar]
- Avgelis A., Barba M., Rumbos I. Epirus cherry virus, an unusual virus isolated from cherry with rasp-leaf symptoms in Greece. J. Phytopathol. 1989;126:51–58. [Google Scholar]
- Lisa V., Milne R.G., Accotto G.P., Boccardo G., Caciagli P., Parvizy R. Ourmia melon virus, a virus from Iran with novel properties. Ann. Appl. Biol. 1988;112:291–302. [Google Scholar]
IDAEOVIRUS
CONTRIBUTED BY, A.T. Jones
GENUS IDAEOVIRUS
Type Species Raspberry bushy dwarf virus
VIRION PROPERTIES
MORPHOLOGY
Virions are isometric, about 33 nm in diameter and are not enveloped. They appear flattened in electron micrographs of preparations negatively stained with uranyl salts (Fig. 1 ).
Figure 1.

Negative contrast electron micrograph of particles of an isolate of Raspberry bushy dwarf virus, stained with uranyl formate/sodium hydroxide. The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is about 7.5 × 106 (calculated from the S20w of 115S). The buoyant density of aldehyde-fixed particles in CsCl is 1.37 g/cm3. Particles are readily disrupted in neutral chloride salts and by SDS.
NUCLEIC ACID
Virion preparations contain three species of linear, positive sense, ssRNA of about 5.5 kb (RNA-1), 2.2 kb (RNA-2) and 1 kb (RNA-3). These RNA molecules are not polyadenylated.
PROTEINS
Virions possess one major CP species of ∼30 kDa. Sequence data indicate that there are two non-structural proteins of 188 and 39 kDa.
LIPIDS
Not known.
CARBOHYDRATES
Not known.
GENOME ORGANIZATION AND REPLICATION
The genome is bipartite. RNA-1 has a major ORF encoding a 188 kDa protein, and also in a different frame and overlapping the 188 kDa protein, a putative small ORF that encodes a 12 kDa protein that is not essential for infectivity. The 188 kDa protein contains sequence motifs characteristic of helicases and polymerases. RNA-2 has two in-frame ORFs: that in the 5′-terminal half encodes a ∼39 kDa protein which has some slight sequence similarities with proteins of other viruses that are thought to have roles in virus transport; that in the 3′-terminal half encodes the CP. RNA-2 is probably a template for the production of RNA-3 that comprises the 3′-most 946 nt of RNA-2 and is a sgRNA for CP. The 3′-terminal non-coding 18 nt of RNA-1 and RNA-2 (and hence of RNA-3) are the same and the 3′-terminal 70 nt can be arranged in similar extensively base-paired structures. Infected leaves contain dsRNA corresponding in size to double-stranded forms of RNA-1 and RNA-2. In vitro translation yields three major proteins, of ∼190, 44 and 31 kDa (CP), which are the translation products, respectively, of RNA-1, RNA-2 and RNA-3.
Figure 2.

Scale diagram of RNA species found in particles of Raspberry bushy dwarf virus (RBDV). The open boxes represent the ORFs. The positions of putative domains are indicated as ‘Mtr’, a Mtr motif, ‘Hel’, an NTP-binding motif, ‘Pol’, an RdRp motif. MP signifies the putative movement protein role of the 39 kDa protein and CP the coat protein.
ANTIGENIC PROPERTIES
Particles are moderate immunogens.
BIOLOGICAL PROPERTIES
In nature, the host range is confined to Rubus species, all but one in the subgenus Idaeobatus; the experimental host range is fairly wide. The virus occurs in all tissues of the plant, including seed and pollen, and Raspberry bushy dwarf virus (RBDV) is transmitted in association with pollen, both vertically to the seed and horizontally to the pollinated plant. This is the only known method of natural spread. Experimentally, the virus can be transmitted by mechanical inoculation. The virus occurs throughout the world wherever raspberry is grown. Infection of raspberry and blackberry is often symptomless but in some cultivars may be associated with ‘yellows disease’ and/or ‘crumbly fruit’. Confusingly, Black raspberry necrosis virus is the main cause of bushy dwarf disease in Lloyd George raspberry. However, because the additional presence of RBDV in plants contributes significantly to the intensity of the disease symptoms usually observed in the field, it can also be regarded as an integral component of the disease syndrome.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Not applicable.
SIMILARITY WITH OTHER TAXA
RBDV resembles viruses of the genus Ilarvirus, family Bromoviridae, in having easily deformable particles that are transmitted in association with pollen. RNA-2 resembles RNA-3 of viruses in family Bromoviridae in the arrangement and sizes of its encoded gene products, the generation of a 3′-terminal sgRNA and in the structured nature of the 3′-ends of the molecules. The sequence of the translation product of RBDV RNA-1 resembles, in different parts, sequences in the translation products of viruses in the family Bromoviridae and to a lesser extent the sequence of the helicase + polymerase protein (∼183 kDa) of tobamoviruses. Idaeoviruses, therefore, belong to the ”alphavirus-like” supergroup.
DERIVATION OF NAMES
Idaeo: from idaeus, specific name of raspberry, Rubus idaeus.
REFERENCES
- Barnett O.W., Murant A.F. Host range, properties and purification of raspberry bushy dwarf virus. Ann. Appl. Biol. 1970;65:435–449. [Google Scholar]
- Jones A.T., Mayo M.A., Murant A.F. Raspberry bushy dwarf idaeovirus. In: Harrison B.D., Murant A.F., editors. The Plant Viruses, Vol. 5: Polyhedral Virions and Bipartite RNA Genomes. Plenum Press; New York: 1996. pp. 283–301. [Google Scholar]
- Jones A.T., Murant A.F., Jennings D.L., Wood G.A. Association of raspberry bushy dwarf virus with raspberry yellows disease; reaction of Rubus species and cultivars, and the inheritance of resistance. Ann. Appl. Biol. 1982;100:135–147. [Google Scholar]
- Mayo M.A., Jolly C.A., Murant A.F., Raschke J.H. Nucleotide sequence of raspberry bushy dwarf virus RNA-3. J. Gen. Virol. 1991;72:469–472. doi: 10.1099/0022-1317-72-2-469. [DOI] [PubMed] [Google Scholar]
- Murant A.F. Some properties of the particles of raspberry bushy dwarf virus. Proc. Am. Phytopath. Soc. 1975;2:116–117. [Google Scholar]
- Murant A.F. Raspberry bushy dwarf. In: Converse R.H., editor. Virus Diseases of Small Fruits. USDA Agriculture Handbook N° 631; 1987. pp. 229–234. [Google Scholar]
- Murant A.F., Chambers J., Jones A.T. Spread of raspberry bushy dwarf virus by pollination, its association with crumbly fruit, and problems of control. Ann. Appl. Biol. 1974;77:271–281. [Google Scholar]
- Murant A.F., Mayo M.A., Raschke J.H. Some biochemical properties of raspberry bushy dwarf virus. Acta Hort. 1986;186:23–30. [Google Scholar]
- Natsuaki T., Mayo M.A., Jolly C.A., Murant A.F. Nucleotide sequence of raspberry bushy dwarf virus RNA-2: a bicistronic component of a bipartite genome. J. Gen. Virol. 1991;72:2183–2189. doi: 10.1099/0022-1317-72-9-2183. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Natsuaki T., Mayo M.A., Jolly C.A., Murant A.F. Nucleotide sequence of raspberry bushy dwarf virus RNA-1. J. Gen. Virol. 1992;73:3213–3218. doi: 10.1099/0022-1317-73-12-3213. [DOI] [PubMed] [Google Scholar]
Tymoviridae
CONTRIBUTED BY, T.W. Dreher, M.C. Edwards, A.J. Gibbs, A.-L. Haenni, R.W. Hammond, I. Jupin, R. Koenig, S. Sabanadzovic, N. Abou Ghanem-Sabanadzovic, G.P. Martelli
FAMILY TYMOVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Tymoviridae |
| Genus | Tymovirus |
| Genus | Marafivirus |
| Genus | Maculavirus |
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

(Left) Atomic rendering of a virion of Turnip yellow mosaic virus (TYMV) (Canady et al., 1996).(Center) Diagram of a TYMV virion with CP clusters in hexa- and pentamers. (Right) Negative contrast electron micrograph of virions and ‘empty particles’ of Belladonna mottle virus (BeMV), a representative of the genus Tymovirus. Particles of members of the genera Marafivirus and Maculavirus have the same morphology. The bar represents 100 nm.
Virions are isometric, non-enveloped, ∼ 30 nm in diameter, with a rounded contour and prominent surface structure, with clustering of CP subunits in pentamers and hexamers. The capsids of tymoviruses are made up of 20 hexameric and 12 pentameric subunits arranged in a T=3 icosahedron and the RNA appears to be at least partially ordered in an icosahedral arrangement in the center of the protein shell.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virus particles sediment as two centrifugal components: T, made up of non-infectious protein shells that contain a small amount of RNA (primarily subgenomic CP mRNA) and B, composed of intact nucleoprotein particles. Sedimentation coefficients (Sw20) of component T and B range from 42 to 55 and from 109 to 125, respectively. Buoyant densities in CsCl of components T and B are 1.26-1.28 g/cm3 and 1.40-1.46 g/cm3 respectively. Virions resist high temperatures (thermal inactivation point is 60-65°C up to above 80°C with some tymoviruses) and organic solvents, but are disrupted by SDS.
NUCLEIC ACID
Virions contain a single molecule of positive sense, ssRNA constituting 25 to 35% of the particle weight. The RNA has a very high cytidine content (from 32 to about 50%) and ranges from 6.0 to 7.5 kb in length. The complete genomic sequences have been determined for representative of seven species of the genus Tymovirus (Chayote mosaic virus; ChMV, Eggplant mosaic virus; EMV, Erysimum latent virus; ErLV, Kennedya yellow mosaic virus, KYMV, Ononis yellow mosaic virus; OYMV, Physalis mottle virus; PhyMV and Turnip yellow mosaic virus; TYMV), two of the genus Marafivirus (Maize rayado fino virus; MRFV, Oat blue dwarf virus; OBDV), one of the genus Maculavirus (Grapevine fleck virus; GFkV), and for the unassigned Poinsettia mosaic virus (PnMV). The genomes of several other viruses have been partially sequenced (see lists of species). Sequenced tymovirus genomes are capped at the 5′-terminus and have a tRNA-like structure at the 3′-end, which for TYMV and several other species, accepts valine. The genomes of GFkV, PnMV and OBDV are polyadenylated at the 3′-terminus and are thought to be capped at the 5′-end. The genome of the marafivirus MRFV also is thought to be 5′-capped, but it lacks a poly(A) tail at the 3′-terminus. Infectious RNA transcripts have been generated from cDNA clones of the tymoviruses TYMV, EMV, and OYMV.
PROTEINS
The CP of virus particles contains either a single protein species with molecular mass of 20 kDa (tymoviruses), 24.5-25 kDa (maculaviruses), or a major protein of 21-21.5 kDa and a minor protein of 24.4-25 kDa (marafiviruses OBDV and MRFV). The capsids of PnMVand a strain of the Bermuda grass etched line virus are made up of a single protein of ∼21kDa.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomes of tymoviruses contain two extensively overlapping ORFs that begin 7 nt apart, and a third ORF (for CP) that is expressed from a 3′-coterminal sgRNA (Fig. 2 ). The longest ORF encodes a ∼220 kDa replication polyprotein. The genomes of the marafiviruses, maculaviruses and PnMV possess a similar ∼220 kDa-coding ORF, and a CP ORF in the same reading frame that is either fused to the long ORF (marafiviruses and PnMV) or separated from it by two adjacent stop codons (GFkV). MRFV is the sole marafivirus with an extensive ORF near the 5′-end of the genome that entirely overlaps the replication protein ORF (Fig. 3 ). The genome of GFkV possesses two additional ORFs towards the 3′-end. Viral RNA of tymoviruses replicates in the cytoplasm in association with the double-membraned invaginations that line the periphery of the chloroplasts. Comparable vesicles occurring at the peripheries of mitochondria or chloroplasts of cells infected with maculaviruses, the tentative marafivirus Grapevine asteroid mosaic-associated virus (GAMaV), and PnMV may have the same function. Genome expression of the tymoviruses includes post-translational autocatalytic cleavage of the ∼220 kDa protein by a virus-encoded papain-like protease, and synthesis and translation of a 3′-coterminal sgRNA for CP expression.
Figure 2.

Organization and expression of the genome of Turnip yellow mosaic virus (TYMV).
Figure 3.

The two known types of genome structure in the genus Marafivirus, exemplified by Maize rayado fino virus (MRFV) and Oat blue dwarf virus (OBDV), showing the relative position of the ORFs and their expression products. Mtr, methyltransferase; Pro, papain-like protease; Hel, helicase; Pol, polymerase (RdRp); CPs, capsid proteins; p43, proline-rich protein.
ANTIGENIC PROPERTIES
Virions are moderately to highly antigenic. Monoclonal antibodies have been produced to GFkV. Tymoviruses cluster roughly into two serological groups with cross-reactivity ranging from strong to weak within each group. Cross-reactivities between group members are weak to undetectable. Serological relationships occur between some marafiviruses, but not between individual maculaviruses.
BIOLOGICAL PROPERTIES
HOST RANGE
Tymoviruses and maculaviruses infect dicotyledonous plants, whereas some marafiviruses primarily infect plants in the Graminae. Natural and experimental host ranges of individual virus species are narrow, sometimes restricted to a single type of host (e.g., GFkV, GRGV, GAMaV and Grapevine rupestris vein feathering virus (GRVFV) infect only Vitis; MRFV only Zea). Disease symptoms are bright yellow mosaic or mottling (tymoviruses and PnMV), chlorotic stripes, vein clearing, etched lines or dwarfing (marafiviruses), and flecking of the leaves (maculaviruses).
TRANSMISSION
Tymoviruses and PnMV, but not other members of the family, are readily transmissible by mechanical inoculation to leaf surfaces. They replicate to high titres and invade all main tissues of the host. Marafi- and maculaviruses are phloem-limited. Marafiviruses, except for tentative members GAMaV and GRVFV, are distinguished by being vectored in a persistent manner by leafhoppers, in which they replicate. Some tymoviruses are weakly seed-transmissible, and also are spread by beetles, which serve as low-efficiency local vectors. Maculaviruses do not have recognized vectors, and the grapevine-associated marafi- and maculaviruses are disseminated primarily through infected propagating material.
GEOGRAPHICAL DISTRIBUTION
Members of the family have been recorded from most parts of the world. Geographical distribution of individual species varies from restricted to widespread.
CYTOPATHIC EFFECTS
Most species elicit derangement of the internal structure and alteration of the shape of chloroplasts and/or mitochondria, which also show rows of peripheral vesicles derived from localized invaginations of the limiting membrane.
LIST OF GENUS DEMARCATION CRITERIA IN THE FAMILY
The criteria demarcating genera in the family are:
-
•
Biological criteria: Tymoviruses are mechanically transmissible, invade parenchyma tissues, and infect dicotyledonous plants. Marafiviruses and maculaviruses are both phloem-limited and are not mechanically transmissible to leaf surfaces. Marafiviruses infect primarily monocotyledonous plants, maculaviruses infect dicotyledonous plants.
-
•
Epidemiological criteria: Tymoviruses are transmitted by beetles, most marafiviruses by leafhoppers. Maculaviruses have no known vector.
-
•
Cytopathological criteria: Tymoviruses elicit the formation of double-membraned vesicles at the periphery of chloroplasts, maculaviruses elicit them at the periphery of mitochondria. Chloroplast vesicles induced by Poinsettia mosaic virus are single-membraned.
-
•
Physicochemical criteria: (a) Genome size: 6.0-6.7 kb (tymoviruses), 6.3-6.5 kb (marafiviruses), 7.5 kb (maculaviruses); (b) number and molecular mass of the CP: one subunit type of 20 kDa (tymoviruses), 24 kDa (maculaviruses) or 21 kDa (PnMV), or two subunit types (21 and 24 kDa) (marafiviruses).
-
•
Molecular criteria: Tymovirus genomes have three ORFs and a tRNA-like structure at the 3′-terminus. Maculavirus genomes have four ORFs, a 3′-terminal poly(A) tail. Two genome types have been reported for marafiviruses: (i) a single large ORF and a 3′-terminal poly(A) tail; (ii) two ORFs and no poly(A) tract. Tymovirus genomes possess a conserved sequence that serves as a sgRNA promoter known as the “tymobox”; the genomes from marafiviruses and PnMV contain a variant termed the“marafibox”, while no such sequence is evident in maculavirus genomes.
GENUS TYMOVIRUS
Type Species Turnip yellow mosaic virus
DISTINGUISHING FEATURES
The genomic RNA (6.0-6.7 kb in size) contains three ORFs (Fig. 2). ORF1 encodes a 206 kDa protein with the conserved sequence motifs characteristic of Mtr, papain-like proteases, Hel, and RdRp. The 3′-ends of these large ORFs are highly conserved and contain a 16 nt sequence known as the “tymobox”, which functions as subgenomic RNA promoter. ORF2 initiates 7 nt upstream of ORF1, almost entirely overlaps ORF1, and encodes a 50-80 kDa proline-rich protein that for TYMV is dispensable for replication but is required for cell-to-cell movement. ORF3 codes for the viral CP (20 kDa), which is expressed via a sgRNA. The narrow host range of tymoviruses makes host susceptibility an important property in distinguishing among species. All species induce vesicles at the periphery of chloroplasts and to a lesser degree of mitochondria, and they induce characteristic aggregates of swollen and modified chloroplasts. All main tissues of the hosts are invaded. Empty virion shells sometimes accumulate in the nuclei. Beetles of the families Chrysomelidae and Curculionidae serve as local vectors, transmitting the virus in a semi-persistent manner. All members of the genus are mechanically transmissible and a few (TYMV, EMV, DuMV) are transmitted through seeds.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Overall sequence identity of less than 80%
-
•
Capsid protein sequences less than 90% identical
-
•
Differential host range
-
•
Differences in the 3′-terminal structure
-
•
Serological specificity
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Andean potato latent virus | ||
| Andean potato latent virus | [AF035402] | (APLV) |
| Belladonna mottle virus | ||
| Belladonna mottle virus | [X54529] | (BeMV) |
| Cacao yellow mosaic virus | ||
| Cacao yellow mosaic virus | [X54354] | (CYMV) |
| Calopogonium yellow vein virus | ||
| Calopogonium yellow vein virus | [U91413] | (CalYVV) |
| Chayote mosaic virus | ||
| Chayote mosaic virus | [AF195000] | (ChMV) |
| Clitoria yellow vein virus | ||
| Clitoria yellow vein virus | [M15963] | (CYVV) |
| Desmodium yellow mottle virus | ||
| Desmodium yellow mottle virus | [AF035201] | (DYMoV) |
| Dulcamara mottle virus | ||
| Dulcamara mottle virus | [AF035634] | (DuMV) |
| Eggplant mosaic virus | ||
| Eggplant mosaic virus | [J04374] | (EMV) |
| Erysimum latent virus | ||
| Erysimum latent virus | [AF098523] | (ErLV) |
| Kennedya yellow mosaic virus | ||
| Kennedya yellow mosaic virus | [D00637] | (KYMV) |
| Melon rugose mosaic virus | ||
| Melon rugose mosaic virus | (MRMV) | |
| Okra mosaic virus | ||
| Okra mosaic virus | [AF035202] | (OkMV) |
| Ononis yellow mosaic virus | ||
| Ononis yellow mosaic virus | [J04375] | (OYMV) |
| Passion fruit yellow mosaic virus | ||
| Passion fruit yellow mosaic virus | [AF47107] | (PFYMV) |
| Peanut yellow mosaic virus | ||
| Peanut yellow mosaic virus | (PeYMV) | |
| Petunia vein banding virus | ||
| Petunia vein banding virus | [AF210709] | (PetVBV) |
| Physalis mottle virus | ||
| Physalis mottle virus | [Y16104] | (PhyMV) |
| Plantago mottle virus | ||
| Plantago mottle virus | (PlMoV) | |
| Scrophularia mottle virus | ||
| Scrophularia mottle virus (Anagyris vein yellowing virus) | (SrMV) | |
| Turnip yellow mosaic virus | ||
| Turnip yellow mosaic virus | [J04373, X16378, X07441] | (TYMV) |
| Voandzeia necrotic mosaic virus | ||
| Voandzeia necrotic mosaic virus | (VNMV) | |
| Wild cucumber mosaic virus | ||
| Wild cucumber mosaic virus | [AF035633] | (WCMV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
GENUS MARAFIVIRUS
Type Species Maize rayado fino virus
DISTINGUISHING FEATURES
The distinct feature of the marafivirus genome (6.3-6.5 kb in size) is a large ORF encoding a precursor polypeptide consisting of the replication-associated proteins and the larger (∼25 kDa) of the two forms of CP found in the virions. The ∼225 kDa replication protein domains (Fig. 3) contain the conserved signature motifs of the replication-associated proteins (Mtr, Hel, RdRp), a papain-like protease domain, and the “marafibox”, a conserved 16 nt stretch comparable to the “tymobox”, from which it differs by three residue changes. The ∼25 kDa CP is produced initially as a C-terminal fusion of the replication protein, while the ∼21 kDa CP is produced from a 3′-co-terminal sgRNA. Viruses presently classified as marafiviruses exhibit some diversity in genome design: the MRFV and OBDV genomes differ by the presence or absence of an extensive ORF overlapping the 5′ regions of the replication protein ORF, and by the presence or absence of a poly(A) tail at the 3′-terminus (MRFV, OBDV, Fig. 3). Host ranges are very narrow, making host susceptibility an important identifying criterion. Marafiviruses are strictly confined to the phloem of infected hosts and are not readily transmissible by sap inoculation, although MRFV has been transmitted by vascular puncture. Except for GAMaV and GRVFV, which have no known vector, transmission is by leafhoppers in a persistent-propagative manner, with virus replication occurring in the insect host. Species-specific virus-vector associations occur: i.e., MRFV is transmitted by Dalbulus, OBDV by Macrosteles, and BELV by Aconurella. The insect-transmitted species infect primarily plants in the Graminae, although a notable exception is the infection of flax by OBDV. None of the species is transmitted through seeds. The tentative species GAMaV induces peripheral vesiculation of chloroplasts.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Overall sequence identity of less than 80%
-
•
Capsid protein sequences less than 90% identical
-
•
Differences in the 3′-terminal structure and in the number of ORFs
-
•
Differential host range
-
•
Vector specificity
-
•
Serological specificity
-
•
Different effects on cell ultrastructure
-
•
Marafibox that is distinct in sequence from the tymobox
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
GENUS MACULAVIRUS
Type Species Grapevine fleck virus
DISTINGUISHING FEATURES
The genomic RNA of GFkV (7.5 kb in size) is the largest in the family, contains four ORFs and is 3′-polyadenylated (Fig. 4 ). ORF1 encodes a polypeptide of 215 kDa that contains the conserved signature motifs of the replication-associated proteins (Mtr, Hel, RdRp) and a papain-like protease domain, but does not seem to have a conserved sequence comparable to the “tymobox” or the “marafibox”. ORF2 codes for the 24 kDa CP. ORF3 and ORF4, which are located at the extreme 3′-end of the genome, code for proline-rich proteins of 31 and 16 kDa, respectively, which show a distant relationship with the putative MPs of tymoviruses. There are two known species in the genus (GFkV and GRGV), both of which are restricted to Vitis species, which are infected latently, with the exception of V. rupestris, which reacts to GFkV with translucent spots (flecks) on the leaves. Both species are strictly confined to the phloem of infected hosts and are not transmissible by sap inoculation. The cytopathology of GFkV infections is characterized by a severe modification of mitochondria into structures called “multivesiculate bodies”. Field spread of GFkV has been reported, but the vector is unknown. GFkV is not transmitted through seeds, thus virus dissemination is primarily through distribution of infected propagative material.
Figure 4.

Genome structure of an isolate of Grapevine fleck virus, the type species of the genus Maculavirus showing the relative position of the ORFs and their expression products. Mtr, methyltransferase; Pro, papain-like protease; Hel, helicase; Pol, polymerase (RdRp); CP, capsid protein; p31 and p16, proline-rich proteins.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Overall sequence identity of less than 80%
-
•
Capsid protein sequences identity less than 90%
-
•
Serological specificity
-
•
Lack of tymobox/marafibox sequence
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Grapevine fleck virus | ||
| Grapevine fleck virus | [AJ309022] | (GFkV) |
TENTATIVE SPECIES IN THE GENUS
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
| Poinsettia mosaic virus | ||
| Poinsettia mosaic virus | [AJ271595] | (PnMV) |
PnMV has characteristics of both the tymoviruses and marafiviruses, and some distinct characteristics. Like tymoviruses, PnMV is mechanically transmissible, infects all main tissues of the host, and amplifies to high titre. Like that of the marafivirus OBDV, the genome contains a single long ORF comprising fused replication and CP domains, a poly(A) tail at the 3′-end and a “marafibox” variant of the “tymobox”. Unlike marafiviruses, PnMV is not insect-transmitted, and possesses only a single CP. PnMV infection induces invaginations of the chloroplast membrane, but these are bounded by a single membrane, rather than the double membrane characteristic of tymoviruses. Sequence relationships (Fig. 6 ) support classification distinct from the existing genera of the family Tymoviridae.
Figure 6.

Phylogenetic tree showing the relationship between the species and genera of the family Tymoviridae based on the RdRp (Top) and CP (Bottom) sequences. The meaning of the abbreviations and the sequence accession # are indicated in the list of species in the genera descriptions. The tree was produced using CLUSTAL W. Branch lengths are proportional to sequence distances.
Figure 5.

Genome structure of Poinsettia mosaic virus (PnMV), unassigned within the family Tymoviridae, showing the single ORF encoding domains characteristic of Mtr, papain-like protease (Pro), helicase (Hel), and RdRp (Pol), and the 21-kDa CP. It is unknown whether the 5′-end of the genome is capped.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
SIMILARITY WITH OTHER TAXA
Replication-associated proteins (RdRp, Mtr, and Hel) contain signature sequences homologous to those of other taxa of the “alpha-like” supergroup of ssRNA viruses, especially those of the genera Carlavirus and Potexvirus.
DERIVATION OF NAMES
Macula: from macula, Latin for fleck.
Marafi: sigla from maize rayado fino virus,
Tymo: sigla from turnip yellow mosaic virus,
REFERENCES
- Abou Ghanem-Sabanadzovic N., Sabanadzovic S., Martelli G.P. Sequence analysis of the 3′-end of three Grapevine fleck virus-like viruses from grapevine. Virus Genes. 2003;27:11–16. doi: 10.1023/a:1025164200412. [DOI] [PubMed] [Google Scholar]
- Boscia D., Sabanadzovic S., Savino V., Kyriakopoulou P.E., Martelli G.P., Lafortezza R. A non mechanically-transmissibile virus associated with asteroid mosaic of the grapevine. Vitis. 1994;33:101–102. [Google Scholar]
- Bozarth C.S., Weiland J.J., Dreher T.W. Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology. 1992;187:124–130. doi: 10.1016/0042-6822(92)90301-5. [DOI] [PubMed] [Google Scholar]
- Bradel B.G., Preil W.X., Jeske H. Sequence analysis and genome organisation of Poinsettia mosaic virus (PnMV) reveal closer relationship to marafiviruses than to tymoviruses. Virology. 2000;271:289–297. doi: 10.1006/viro.2000.0335. [DOI] [PubMed] [Google Scholar]
- Canady M.A., Larson S.B., Day J., McPherson A. Crystal structure of turnip yellow mosaic virus. Nature Struct. Biol. 1996;3:771–781. doi: 10.1038/nsb0996-771. [DOI] [PubMed] [Google Scholar]
- Castellano M.A., Martelli G.P. Ultrastructure and nature of the vesiculated bodies associated with isometric virus-like particles in diseased grapevines. J. Ultrastruct. Res. 1984;89:56–64. [Google Scholar]
- Ding S.W., Howe J., Keese P., Mackenzie A., Meek D., Osorio-Keese M., Skotnicki M., Pattana S., Torronen M., Gibbs A. The tymobox, a sequence shared by most tymoviruses: its use in molecular studies of tymoviruses. Nucl. Acids Res. 1990;18:1181–1187. doi: 10.1093/nar/18.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher T.W., Goodwin J.B. Transfer RNA mimicry among tymoviral genomic RNAs ranges from highly efficient to vestigial. Nucl. Acids Res. 1998;26:4356–4364. doi: 10.1093/nar/26.19.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M.C., Zhang Z., Weiland J.J. Oat blue dwarf marafivirus resembles the tymoviruses in sequence, genome organization, and expression strategy. Virology. 1997;232:217–229. doi: 10.1006/viro.1997.8555. [DOI] [PubMed] [Google Scholar]
- Hammond R.W., Ramirez P. Molecular characterization of the genome of Maize rayado fino virus, the type member of the genus Marafivirus. Virology. 2001;282:338–347. doi: 10.1006/viro.2001.0859. [DOI] [PubMed] [Google Scholar]
- Hellendoorn K., Mat A.W., Gultyaev A.P., Pleij C.W.A. Secondary structure model of the coat protein gene of turnip yellow mosaic virus RNA: long C-rich, single-stranded regions. Virology. 1996;224:43–54. doi: 10.1006/viro.1996.0505. [DOI] [PubMed] [Google Scholar]
- Izadpanah K., Zhang Y.P., Daubert S., Rowhani A. Sequence of the coat protein gene of Bermuda grass etched-line virus and of the adjacent “marafibox” motif. Virus Genes. 2002;24:131–134. doi: 10.1023/a:1014516515454. [DOI] [PubMed] [Google Scholar]
- Koenig R. A loop structure in the serological classification system of tymoviruses. Virology. 1976;72:1–5. doi: 10.1016/0042-6822(76)90305-6. [DOI] [PubMed] [Google Scholar]
- Lesemann D.E. Virus group-specific and virus-specific cytological alterations induced by members of the tymovirus group. Phytopathol. Z. 1977;90:315–336. [Google Scholar]
- Martelli G.P., Sabanadzovic S., Abou Ghanem-Sabanadzovic N., Saldarelli P. Maculavirus, a new genus of plant viruses. Arch. Virol. 2002;147:1847–1853. doi: 10.1007/s007050200046. [DOI] [PubMed] [Google Scholar]
- Ranjith-Kumar C.T., Gopinath K., Jacob A.N., Srividhya V., Elango P., Savithri H.S. Genomic sequence of Physalis mottle virus and its evolutionary relationship with other tymoviruses. Arch. Virol. 1998;143:1489–1500. doi: 10.1007/s007050050392. [DOI] [PubMed] [Google Scholar]
- Sabanadzovic S., Abou Ghanem-Sabanadzovic N., Saldarelli P., Martelli G.P. Complete nucleotide sequence and genome organization of Grapevine fleck virus. J. Gen. Virol. 2001;82:2009–2015. doi: 10.1099/0022-1317-82-8-2009. [DOI] [PubMed] [Google Scholar]
- Weiland J.J., Dreher T.W. Infectious TYMV RNA from cloned cDNA. Effects in vitro and in vivo of point substitutions in the initiation codons of two extensively overlapping ORFs. Nucl. Acids Res. 1989;17:4675–4687. doi: 10.1093/nar/17.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Closteroviridae
CONTRIBUTED BY, G.P. Martelli, A.A. Agranovsky, M. Bar-Joseph, D. Boscia, T. Candresse, R.H.A. Coutts, V.V. Dolja, B.W. Falk, D. Gonsalves, J.S. Hu, W. Jelkmann, A.V. Karasev, A. Minafra, S. Namba, H.J. Vetten, C.G. Wisler, N. Yoshikawa
FAMILY CLOSTEROVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Closteroviridae |
| Genus | Closterovirus |
| Genus | Ampelovirus |
| Genus | Crinivirus |
VIRION PROPERTIES
MORPHOLOGY
Figure 1.

(Top) Negative contrast electron micrograph of virions of Citrus tristeza virus (CTV) (genusClosterovirus). Particles of all members of the genera Ampelovirus and Crinivirus have a similar morphology. The bar represents 100 nm (Courtesy of R.G. Milne). (Bottom) Beet yellows virus (BYV) particles showing adecorated extremity (arrow heads) following exposure to an antiserum to the N-terminal peptide expressed fromthe minor CP gene. The bar represents 100 nm
(Courtsey of D.E. Lesemann).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions of all genera usually sediment as a single band in sucrose or Cs2SO4 gradients. S20w range from 96 to 140, buoyant densities are 1.30-1.34 g/cm3 in CsCl and 1.30-1.34 g/cm3 in Cs2SO4. Virions of most species are degraded by CsCl and are unstable in high salt concentration. Virions resist moderately high temperatures (thermal inactivation is around 45-55°C) and organic solvents, but are sensitive to RNAse and chelation.
NUCLEIC ACID
Regardless of the genome type, monopartite or bipartite, virions contain a single molecule of linear, positive sense, ssRNA, constituting 5-6% of the particle weight. Genome size is related to particle length. In monopartite genome species (genera Closterovirus and Ampelovirus), genome sizes range from 15.5 kb (BYV) to 19.3 kb (CTV), the largest genome among positive-strand RNA plant viruses. Genome sizes of the bipartite genome criniviruses Sweet potato chlorotic stunt virus (SPCSV), Lettuce infectious yellows virus (LIYV) and Cucurbit yellow stunting disorder (CYSDV) are 17.6 kb, 15.3 kb and 16.4 kb, respectively. The 5′-end of the genome is likely to be capped. The 3′-end is not polyadenylated and does not possess a tRNA-like structure, but may have several hairpin structures. The genomic RNAs of isolates of two species of the genus Closterovirus, i.e., BYV and CTV (four isolates from California, Florida, Spain, and Israel), three species of the genus Ampelovirus, i.e. Little cherry virus 2 (LChV-2), Grapevine leafroll-associated virus 2 (GLRaV-2), Grapevine leafroll-associated virus 3 (GLRaV-3), and three viruses of the genus Crinivirus, i.e., LIYV, CYSDV, SPCSV, and Beet pseudo yellows virus (BPYV) have been completely sequenced. Partial to almost complete sequences are available for Beet yellow stunt virus (BYSV) and Carnation necrotic fleck virus (CNFV) from the genus Closterovirus; Grapevine leafroll-associated virus 1 (GLRaV-1), Grapevine leafroll-associated virus 5 (GLRaV-5), Pineapple mealybug wilt associated virus 1 (PMWaV-1), Pineapple mealybug wilt associated virus 2 (PMWaV-2), Grapevine leafroll-associated virus 4 (GLRaV-4), Grapevine leafroll-associated virus 6 (GLRaV-6), Plum bark necrosis and stem pitting associated virus (PBNSPaV) from the genus Ampelovirus; Tomato infectious chlorosis virus (TICV), Tomato chlorosis virus (ToCV), from the genus Crinivirus, and for the unassigned species in the family, Grapevine leafroll-associated virus 7 (GLRaV-7), Olive leaf yellowing associated virus (OLYaV) and Little cherry virus 1 (LChV-1).
PROTEINS
Virions of all members of the family are composed of a major CP ranging from 22 to 46 kDa, according to the individual species, but the capsids of BYV, CTV, and LIYV also incorporate the minor CP (CPm) at one end of the particle. Additional evidence suggests that HSP70h and CPm are tightly associated with the virions and that CPm is required for the assembly of virion tails. The CP of some isolates (BYV, CNFV and Lilac chlorotic leafspot virus) lack tryptophan, resulting in a high A260/A280 ratio (1.4-1.8) for the virions. The reverse is true for the CP of CTV, which contains a significant amount of tryptophan (A260/A280 =1.21-1.22). Non-structural proteins common to all members of the family are: (i) a large polypeptide (295 to 349 kDa) containing the conserved domains of papain-like protease (P-Pro), Mtr, and Hel; (ii) a 48 to 57 kDa protein with all sequence motifs of viral RdRp of the “alpha-like” supergroup of positive-strand RNA viruses; (iii) a 6 kDa hydrophobic protein with membrane-binding properties; (iv) a 59 to 70 kDa homolog of the cellular HSP70 heat-shock proteins (HSP70h) implicated in the cell-to-cell movement of the viruses; (v) a 55 to 64 kDa product. At least five different virus-coded proteins are required for BYV cell-to-cell movement, i.e. CP, CPm, three MPs, namely, the 6 kDa protein (p6), the HSP70h, and the 64 kDa protein (p64) (Fig. 2 ). The L-Pro domain of ORF1a and the p20 protein coded for by ORF7 of BYV are involved in systemic transport. The BYV HSP70h localizes near to plasmodesmata and inside plasmodesma channels and interacts with p20, providing a site for its attachment to virions. Both proteins associate with intracellular accumulations of virus particles, forming a physical complex with them. p20 is dispensable for assembly and cell-to-cell movement of BYV, but is required for long-distance transport of BYV through the phloem.
Figure 2.

Genome organization and strategy of replication characteristic of Beet yellows virus (BYV)showing the relative position of the ORFs, their expression products, and the 3′ nested set of sgRNAs. L-Pro, leader proteinase; Mtr, methyltransferase;Hel, helicase; RdRp, RNA polymerase; HSP70h, heat shock proteinhomologue; CP coat protein; CPm, minor capsid protein. The five boxes under “cell-to-cell movement” representthe five gene block conserved among closteroviruses (from Dolja, 2003).
LIPIDS
None present.
CARBOHYDRATES
None present.
GENOME ORGANIZATION AND REPLICATION
Closteroviruses have the largest genomes among positive-strand RNA plant viruses. The genome is monopartite for the members of the genera Closterovirus and Ampelovirus and bipartite for most of those in the genus Crinivirus. The genomes of viruses in all genera are characterized by the presence of unique genes coding for HSP70h proteins and for an analogue of the CP. The genome organizations, the numbers and relative positions of the ORFs vary with the genus and/or individual virus species. In species of the genus Closterovirus (BYV, CTV, BYSV, GLRaV-2), the CPm is upstream of the CP, whereas the reverse is true for members of the genus Ampelovirus (GLRaV-1, GLRaV-3, LChV-2, PMWaV-1, PMWaV-2) and Crinivirus (LIYV, SPSVV, CCSV, CYSDV, BPYV). The ORFs coding for the 6 kDa small hydrophic protein, the HSP70h, the 55-64 kDa product, the CP, and CPm, form a five-gene module that is conserved among members of the family (Fig. 2 and 3 ). The genome expression strategy is based on: (i) proteolytic processing of the polyprotein encoded by ORF1a; (ii) +1 ribosomal frameshift for the expression of the RdRp domain encoded by ORF1b; (iii) expression of the downstream ORFs via the formation of a nested set of 3′ co-terminal sgRNAs (Fig. 2 and 3). The dsRNA patterns are very complex and variable among species, reflecting the different numbers and sizes of the ORFs present in individual genomes. Replication occurs in the cytoplasm, possibly in association with endoplasmic reticulum-derived membranous vesicles and vesiculated mitochondria.
Figure 3.

Organization and expression strategy of genomes characteristic of Citrus tristeza virus. Dashedlines represent putative protein products corresponding to the respective ORFs. +1 FS the putative ribosomalframeshift. Solid lines define the two genomic regions expressed through proteolytic processing of thepolyprotein precursor(s) and through the formation of a nested set of 3′ co-terminal sgRNAs (from Karasev andHilf, 1997).
ANTIGENIC PROPERTIES
Virion proteins are moderately antigenic. Most of the species are serologically unrelated or distantly related to one another. Monoclonal antibodies have been produced to proteins of a number of members, i.e., CTV, GLRaV-1 to −6, PMWaV-2, and SPCSV. Polyclonal antisera to CTV, LChV-2, GLRaV-2, GLRaV-3, and SPCSV have been raised from fusion proteins produced in bacterial expression systems.
BIOLOGICAL PROPERTIES
HOST RANGE
The natural and experimental host ranges of individual virus species are restricted. Disease symptoms are of the yellowing type (i.e. stunting, rolling, yellowing or reddening of the leaves, small and late ripening fruits), or pitting and/or grooving of the woody cylinder. Infection is systemic, but usually limited to the phloem, which may necrotize to a varying extent
TRANSMISSION
Few species of the genus Closterovirus are transmissible by mechanical inoculation, though with difficulty, but none of those in the genera Ampelovirus and Crinivirus can be. In vegetatively propagated crops, long distance virus dissemination is primarily through infected propagating material. Transmission through seeds is very rare. According to the genus, natural vectors are aphids, whiteflies (Bemisia and Trialeurodes), pseudococcid (Pseudococcus, Planococcus; Phenacoccus, Saccharicoccus, and Dysmicoccus), and coccid (Pulvinaria, Neopulvinaria, and Parthenolecanium) mealybugs. Transmission is semi-persistent regardless of the type of vector.
GEOGRAPHICAL DISTRIBUTION
Geographical distribution varies from restricted to widespread, depending on the virus species, most of which occur in temperate or subtropical regions.
CYTOPATHIC EFFECTS
Virions are usually found in the phloem (sieve tubes, companion cells, phloem parenchyma), occasionally in the mesophyll and epidermis. Ultrastructural modifications arise by membrane proliferation, vesiculation of chloroplasts, degeneration and vesiculation of mitochondria, and formation of inclusion bodies. These are made up of aggregates of virions or membranous vesicles, or a combination of the two. Virions accumulate in conspicuous cross-banded fibrous masses or, more typically, in more or less loose bundles intermingled with single or clustered membranous vesicles. Inclusions of this type are one of the hallmarks of the family. The vesicles contain a fibrillar network and derive either from the endoplasmic reticulum, or from peripheral vesiculation of mitochondria.
GENUS CLOSTEROVIRUS
Type Species Beet yellows virus
DISTINGUISHING FEATURES
Virions are of one size (>1000 nm, usually 1250 to 2200 nm in length) and contain a single molecule of linear, positive sense, ssRNA from 15.5 to 19.3 kb in size. CTV has also smaller than full-length particles that may encapsidate subgenomic or multiple species of defective RNAs (D-RNA) containing all of the cis-acting sequences required for replication. SgRNAs may be involved in the construction of recombinant D-RNAs. The presence of D-RNA makes the dsRNA pattern of CTV isolates more complex than that of other members of the genus. The major CP subunit (22-25 kDa) coats most of the virion length and CPm coats a short segment (∼75 nm) at one end of the particle. There are three types of genome organization among the sequenced species of the genus, typified by BYV (Fig. 2), CTV (Fig. 3) and BYSV. The BYV genome contains nine ORFs flanked by 5′- and 3′-UTR of 107 and 181 nt, respectively. CTV has twelve ORFs and UTRs of 107 nt at the 5′-end and 275 nt at the 3′-end. Differences with BYV are the presence of two papain-like protease domains in ORF 1a, of an extra 5′-proximal ORF (ORF2) encoding a 33 kDa product with no similarity to any other protein in databases, and of two extra 3′-proximal ORFs (ORF9 and ORF11). The BYSV genome has ten ORFs and a 3′ UTR of 241 nt, a length intermediate between that of the BYSV and CTV UTRs. A further difference with the BYV genome is the presence of an extra ORF (ORF2) encoding a 30 kDa polypetide with no similarity to any other protein in the databases. This ORF is located downstream of ORF1b, i.e. in the same position as the unrelated CTV ORF2. Thus, the organization of BYSV genomes is intermediate between that of BYV and CTV, suggesting that these three viruses might represent three distinct stages in closterovirus evolution. In contrast to the other two genera in the family (Ampelovirus and Crinivirus), the closterovirus gene encoding the CPm is upstream of the CP gene. The dsRNA-containing cytoplasmic vesicles that accumulate in the cytoplasm of infected cells arise primarily by proliferation of the endoplasmic reticulum. The genus contains only viruses that are transmitted by aphids in a semi-persistent manner and that infect primarily dicotyledonous hosts. Several of the members are transmissible by sap inoculation, though with some difficulty.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Particle size,
-
•
Size of CP, as determined by deduced amino acid sequence data,
-
•
Serological specificity using discriminatory monoclonal or polyclonal antibodies,
-
•
Genome structure and organization (number and relative location of the ORFs),
-
•
Amino acid sequence of relevant gene products (CP, CPm, HSP70h) differing by more than 10%,
-
•
Vector species and specificity,
-
•
Magnitude and specificity of natural and experimental host range,
-
•
Cytopathological features (i.e., aspect of inclusion bodies and origin of cytoplasmic vesicles).
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| 1-Aphid-transmitted | ||
| Beet yellows virus | ||
| Beet yellows virus | [X73476] | (BYV) |
| Beet yellow stunt virus | ||
| Beet yellow stunt virus | [U51931] | (BYSV) |
| Burdock yellows virus | ||
| Burdock yellows virus | (BuYV) | |
| Carnation necrotic fleck virus | ||
| Carnation necrotic fleck virus | (CNFV) | |
| Carrot yellow leaf virus | ||
| Carrot yellow leaf virus | (CYLV) | |
| Citrus tristeza virus | ||
| Citrus tristeza virus | [U56902, U163304, AY170468, Y18420, AF001623, AF260651, AB04398] | (CTV) |
| Wheat yellow leaf virus | ||
| Wheat yellow leaf virus | (WYLV) | |
| 2-Vector unknown | ||
| Grapevine leafroll-associated virus 2 | ||
| Grapevine leafroll-associated virus 2 | [Y14131] | (GLRAV-2) |
TENTATIVE SPECIES IN THE GENUS
| Clover yellows virus | (CYV) |
| Dendrobium vein necrosis virus | (DVNV) |
| Festuca necrosis virus | (FNV) |
| Heracleum virus 6 | (HV-6) |
GENUS AMPELOVIRUS
Type Species Grapevine leafroll-associated virus 3
DISTINGUISHING FEATURES
Virions are of one size (>1000 nm, usually 1400-2200 nm in length) and contain a single molecule or linear, positive sense, single stranded RNA 16.9-17.9 kb in size. CP subunits have a high molecular mass (35-46 kDa). There are two types of genome structure in the genus typified by GLRaV-3 (Fig. 4 ) and LChV-2. The GLRaV-3 genome contains 12 ORFs with a 3′ UTR of 277 nt. The LChV-2 genome (16.9 kb), the third largest monopartite closterovirus sequenced to date, contains nine ORFs and UTRs of 76 nt at the 5′-end and 210 nt at the 3′-end, respectively. LChV-2 has the largest HSP70h (70 kDa), CP (46 kDa) and CPm (76 kDa) among ampeloviruses. With GLRaV-3 and several other sequenced members of the genus, the CPm gene is downstream of the CP gene, whereas with LChV-2, the CPm gene is five ORFs upstream of the CP cistron. GLRaV-1 shows a further peculiarity in that its CPm gene is duplicated. The dsRNA-containing vesicles that accumulate in the cytoplasm of infected cells may arise either by proliferation of the endoplasmic reticulum or from vesiculation and fragmentation of mitochondria. The genus contains viruses that infect only dicotyledonous hosts, and that are transmitted semi-persistently by coccid (Parthenolecanium, Pulvinaria, Neopulvinaria) or pseudococcid (Pseudococcus, Planococcus, Saccharicoccus, Dysmiococcus, Phenacoccus, Heliococcus) mealybugs. None of the members is transmissible by sap inoculation.
Figure 4.

Genome structure characteristic of a representative of Grapevine leafroll-associated virus 3, the typespecies of the genus Ampelovirus, showing the relative position of the ORFs and their expression products. Pro, papain-like protease; Mtr; Hel, helicase; RdRp, RNA polymerase; HSP70h, heat shock-related proteinhomologue; CP, capsid protein; CPm, minor capsid protein.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Particle size,
-
•
Size of CP, as determined by deduced aa sequence data,
-
•
Serological specificity using discriminatory monoclonal or polyclonal antibodies,
-
•
Genome structure and organization (number and relative location of the ORFs),
-
•
Amino acid sequence of relevant gene products (CP, CPm, HSP70h) differing by more than 10%,
-
•
Vector species and specificity,
-
•
Magnitude and specificity of natural and experimental host range,
-
•
Cytopathological features (i.e., aspect of inclusion bodies and origin of cytoplasmic vesicles).
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Grapevine leafroll-associated virus 1 | ||
| Grapevine leafroll-associated virus 1 | [AF195822] | (GLRaV-1) |
| Grapevine leafroll-associated virus 3 | ||
| Grapevine leafroll-associated virus 3 | [U82937] | (GLRaV-3) |
| Grapevine leafroll-associated virus 5 | ||
| Grapevine leafroll-associated virus 5 | [AF039552] | (GLRaV-5) |
| Little cherry virus 2‡ | ||
| Little cherry virus 2 | [AF 531505, AF333237, AF416335] | (LChV-2) |
| Pineapple mealybug wilt-associated virus 1 | ||
| Pineapple mealybug wilt-associated virus 1 | [AF414119] | (PMWaV-1) |
| Pineapple mealybug wilt-associated virus 2 | ||
| Pineapple mealybug wilt-associated virus 2 | [AF283103] | (PMWaV-2) |
TENTATIVE SPECIES IN THE GENUS
| 1-Mealybug-transmitted | ||
| Sugarcane mild mosaic virus | (SMMV) | |
| 2 -Vector unknown | ||
| Grapevine leafroll-associated virus 4 | [AF039553] | (GLRaV-4) |
| Grapevine leafroll-associated virus 6 | [AJ496796] | (GLRaV-6) |
| Grapevine leafroll-associated virus 8 | (GLRaV-8) | |
| Plum bark necrosis and stem pitting-associated virus | [AF195501] | (PBNSTaV) |
GENUS CRINIVIRUS
Type Species Lettuce infectious yellows virus
DISTINGUISHING FEATURES
Figure 5.

Genome organization of Lettuce infectious yellows virus (LIYV) showing the relative position of the ORFs and their expression products. P-Pro, papain-like protease; Mtr, methyltranferase; Hel, helicase; POL, RNA polymerase; HSP70h, heat shock-related protein homologue; CP capsid protein; CPm, minor capsid protein.
Virions are shorter that 1000 nm and have two modal lengths (650-850 and 700-900 nm). The genome is a linear, positive sense, ssRNA 15.3 kb (LIYV), 16.4 kb (CYSDV), and 17.6 kb (SPCSV) in size, divided into two molecules, both needed for infectivity and separately encapsidated (Fig. 4). CPs of known members range from 28 to 33 kDa, but the size of CPm can be as large as c. 80 kDa. There are three types of genome structure in the genus, typified by LIYV, SPCSV and BPYV. LIYV RNA-1 is a bicistronic molecule comprising a 5′- and 3′-UTR of 97 and 219 nt, respectively. By contrast, the SPCSV 3′-UTR is longer (208 nt). LIYV RNA-1 encodes replication-related proteins (ORF1), including RdRp, which is expressed via a putative ribosomal frameshift, and a trans-enhancer for RNA-2 accumulation (ORF2). SPCSV RNA-1 has two extra ORFs coding for an RNAse III-like protein (ORF2) and a putative small hydrophobic protein (p7) (ORF3). This protein could be homologous to a hydrophobic protein of similar size encoded by ORF1 of LIYV RNA-2. BPYV RNA-1 is a monocistronic molecule comprising only ORF1a and ORF1b. RNA-2 of all sequenced members of the genus (LIYV, CYSDV, SPCSV, BPYV) has seven ORFs. It contains the five-gene module typical of the family, which, however, differs from that of the genera Closterovirus and Ampelovirus because of the insertion of an extra small gene (ORF4) upstream of the CP gene. SPCSV and ToCV have notably large ORFs for the CPm (75-79 kDa), unlike LIYV (52 kDa). In all members of the genus the position of the CPm ORF is downstream of the CP gene. The dsRNA-containing vesicles that accumulate in the cytoplasm of infected cells may arise by proliferation of the endoplamic reticulum. None of the members can be transmitted by sap inoculation. Natural vectors are whiteflies (Bemisia, Trialeurodes) that transmit in a semi-persistent manner. PYVV and DVCV are transmitted by Trialeurodes seem to have undivided genomes and arrangements of genes similar to those of bipartite viruses. They have been assigned to this genus as tentative species because the type of vector transmission is a key biological character recognized as a major classification feature of closteroviruses.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Particle size,
-
•
Size of CP, as determined by deduced amino acid sequence data,
-
•
Serological specificity using discriminatory monoclonal or polyclonal antibodies,
-
•
Genome structure and organization (number and relative location of the ORFs),
-
•
Amino acid sequence of relevant gene products (CP, CPm, HSP70h) differing by more than 10%,
-
•
Vector species and specificity,
-
•
Magnitude and specificity of natural and experimental host range,
-
•
Cytopathological features (i.e., aspect of inclusion bodies and origin of cytoplasmic vesicles).
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Abutilon yellows virus | ||
| Abutilon yellows virus | (AbYV) | |
| Beet pseudoyellows virus | ||
| Beet pseudoyellows virus | [Y15568, U67447] | (BPYV) |
| (Cucumber chlorotic spot virus) | ||
| (Cucumber yellows virus) | [AB085612, AB085613] | |
| Cucurbit yellow stunting disorder virus | ||
| Cucurbit yellow stunting disorder virus | [AJ439690, AJ537493] | (CYSDV) |
| Lettuce chlorosis virus | ||
| Lettuce chlorosis virus | (LCV) | |
| Lettuce infectious yellows virus | ||
| Lettuce infectious yellows virus | [U15440, U15441] | (LIYV) |
| Sweet potato chlorotic stunt virus | ||
| Sweet potato chlorotic stunt virus | [AJ428554, AJ428555] | (SPCSV) |
| (Sweet potato sunken vein virus) | ||
| Tomato chlorosis virus | ||
| Tomato chlorosis virus | (ToCV) | |
| Tomato infectious chlorosis virus | ||
| Tomato infectious chlorosis virus | (TICV) | |
TENTATIVE SPECIES IN THE GENUS
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
The species that follow could not be allocated to any of the genera either because the available information is scanty (Megakepasma mosaic virus; MegMV and Alligatorweed stunting virus; AWSV), or because more molecular (Olive leaf yellowing-associated virus; OLYaV and Grapevine leafroll-associated virus 7; GLRaV-7) or biological data (Little cherry virus 1; LChV-1) are needed for unequivocal classification.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Figure 6.

Phylogenetic tree showing the relationships between the species and genera of the family Closterovoridae based on the sequence of the HSP70h gene. The neighbor-joining tree was produced and bootsrapped using CLUSTAL W. Branch lengths are proportional to sequence distances. All the abbreviations signification can be found in the “List of Soecies” in the description. GLRaV-7, LChV-1, and OLYaV are unassigned species in the family.
SIMILARITY WITH OTHER TAXA
Virions of the genera Capillovirus, Trichovirus, and Vitivirus have the same particle morphology as those of the family Closteroviridae. However, the sequence of the CP of members of this family has little homology with that of CPs of viruses in the three above genera, and major differences exist in genome size and organization, and strategy of expression. Replication-associated proteins (RdRp, Mtr and Hel) contain signature sequences homologous to those of other taxa of the “alpha-like” supergroup of ssRNA viruses, the closest affinity being with the family Bromoviridae and the genera Tobravirus, Tobamovirus, and Hordeivirus. The replication strategy, based on polyprotein processing, translational frameshifting, and multiple sgRNA generation, closely resembles that of viruses in the families Coronaviridae and Arteriviridae. However, unlike closteroviruses, the RdRp of coronaviruses and arteriviruses belong to the “picorna-like” supergroup of polymerases. Hence, the transcriptional strategy of members of the family Closteroviridae follows the mechanism of other “alpha-like” viruses, and is dissimilar from the discontinuous, leader-primed transcription of coronaviruses and arteriviruses.
DERIVATION OF NAMES
ampelo: from ampelos, Greek for grapevine, the host of the type species of the genus clostero: from Greek kloster, ‘spindle, thread’.
crini from crinis, Latin for hair, from the appearance of the very long thread-like particles.
REFERENCES
- Agranovsky A.A. Principles of molecular organization, expression and evolution of closteroviruses: over the barriers. Adv. Virus Res. 1996;47:119–158. doi: 10.1016/S0065-3527(08)60735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agranovsky A.A., Lesemann D.E., Maiss E., Hull R., Atabekov J.G. Rattlesnake structure of a filamentous plant RNA virus built of two capsid proteins. Proc. Natl. Acad. Sci. USA. 1995;92:2470–2473. doi: 10.1073/pnas.92.7.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko V.P., Karasev A.A., Agranovsky A.A., Koonin E.V., Dolja V.V. Coat protein gene duplication in a filamentous RNA virus of plants. Proc. Natl. Acad. Sci. USA. 1992;89:9156–9160. doi: 10.1073/pnas.89.19.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin R.S., Coutts R.H.A. Relationships among Trialeurodes vaporariorum-transmittedyellowing viruses from Europe and North America. J. Phytopathol. 1995;143:375–380. [Google Scholar]
- Dawson W.O. Complete sequence of the citrus tristeza virus RNA genome. Virology. 1995;208:511–520. doi: 10.1006/viro.1995.1182. [DOI] [PubMed] [Google Scholar]
- Dolja V.V. Beet yellows virus: the importance of being different. Mol. Plant Pathol. 2003;4:91–98. doi: 10.1046/j.1364-3703.2003.00154.x. [DOI] [PubMed] [Google Scholar]
- Dolja V.V., Karasev A.V., Koonin E.V. Molecular biology and evolution of closteroviruses: sophisticated build up of large RNA genomes. Annu. Rev. Phytopathol. 1994;32:261–285. [Google Scholar]
- Faoro F. Cytopathology of closteroviruses and trichoviruses infecting grapevines. In: Monette P.L., editor. Filamentous Viruses of Woody Plants. Research Signpost; Trivandrum: 1997. pp. 29–47. [Google Scholar]
- Karasev A.V. Genetic diversity and evolution of closteroviruses. Annu. Rev. Phytopathol. 2000;38:293–324. doi: 10.1146/annurev.phyto.38.1.293. [DOI] [PubMed] [Google Scholar]
- Karasev A.V., Hilf M.E. Molecular biology of the citrus tristeza virus. In: Monette P.L., editor. Filamentous Viruses o f Woody Plants. Research Signpost; Trivandrum: 1997. pp. 121–131. [Google Scholar]
- Klaassen V.A., Boeshore M.L., Koonin E.V., Tian T., Falk B.W. Genome structure and phylogenetic analysis of lettuce infectious yellows virus, a whithefly-transmitted, bipartite closterovirus. Virology. 1995;208:99–110. doi: 10.1006/viro.1995.1133. [DOI] [PubMed] [Google Scholar]
- Mawassi M., Mietkiewska E., Hilf M.E., Ashoulin L., Karasev A.V., Gafny R., Lee R.F., Garnsey S.E., Dawson W.O., Bar-Joseph M. Multiple species of defective RNAs in plants infected with citrus tristeza virus. Virology. 1995;214:264–268. doi: 10.1006/viro.1995.9930. [DOI] [PubMed] [Google Scholar]
- Medina V., Peremyslov V.V., Hagiwara Y., Dolja V.V. Subcellular localization of the Hsp70-homolog encoded by beet yellows closterovirus. Virology. 1999;260:173–181. doi: 10.1006/viro.1999.9807. [DOI] [PubMed] [Google Scholar]
- Napuli A.J., Alzhanova D.V., Doneanu C.E., Barofsky D.F., Koonin E.V., Dolja V.V. The 64-kilodaltons capsid protein homolog of Beet yellows virus is required for assembly of virion tails. J irol. 2003;77:2377–2384. doi: 10.1128/JVI.77.4.2377-2384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhnevsky A.I., Peremyslov V.V., Napuli A.J., Dolja V.V. Interaction between long-distance transport factor and Hsp70-related movement protein of Beet yellows virus. J. Virol. 2002;76:11003–11011. doi: 10.1128/JVI.76.21.11003-11011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Klaasssen V.A., Soong J., Wisler G., Duffus J.E., Falk B.W. Generation of cDNAs pecific to lettuce infectious yellows closterovirus and other whitefly-transmitted viruses by RT-PCR and egenerate oligonucleotide primers corresponding to the closterovirus gene encoding the heat shock protein 70 homolog. Phytopathology. 1996;86:1167–1173. [Google Scholar]
- Wisler G.C., Duffus J.E., Liu H.Y., Li R.H. Ecology and epidemiology of whitefly-transmitted closteroviruses. Plant Dis. 1998;82:270–280. doi: 10.1094/PDIS.1998.82.3.270. [DOI] [PubMed] [Google Scholar]
- Yeh H.H., Tian T., Rubio L., Crawford B., Falk B.W. Asynchronous accumulation of Lettuce infectious yellows virus RNAs 1 and 2 and identification of an RNA 1 trans enhancer of RNA 2 accumulation. J. Virol. 2000;74:5762–5768. doi: 10.1128/jvi.74.13.5762-5768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flexiviridae
CONTRIBUTED BY, M.J. Adams, G.P. Accotto, A.A. Agranovsky, M. Bar-Joseph, D. Boscia, A.A. Brunt, T. Candresse, R.H.A. Coutts, V.V. Dolja, B.W. Falk, G.D. Foster, D. Gonsalves, W. Jelkmann, A. Karasev, G.P. Martelli, M. Mawassi, R.G. Milne, A. Minafra, S. Namba, A. Rowhani, H.J. Vetten, V.K. Vishnichenko, G.C. Wisler, N. Yoshikawa, S.K. Zavriev
FAMILY FLEXIVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Flexiviridae |
| Genus | Potexvirus |
| Genus | Mandarivirus |
| Genus | Allexivirus |
| Genus | Carlavirus |
| Genus | Foveavirus |
| Genus | Capillovirus |
| Genus | Vitivirus |
| Genus | Trichovirus |
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments, usually 12-13 nm in diameter (range 10-15 nm) and from 470 to over 1000 nm in length, depending on the genus. They have helical symmetry with a pitch of about 3.4 nm (range 3.3-3.7 nm) and in some genera there is clearly visible crossbanding.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as single (or occasionally two very close) bands with an S20w of 92-176S, depending on the genus.
NUCLEIC ACID
Virions contain a single molecule of linear ssRNA of ∼ 5.9-9.0 kb which is 5-6% by weight of the virion. The RNA is capped (or probably capped) at the 5′-terminus and has a polyadenylated tract at the 3′-terminus. Smaller 3′-co-terminal sgRNAs are encapsidated in some, but not all, members of the genus Potexvirus, while in the genus Carlavirus some viruses have two sgRNAs of 2.1-3.3 kb and 1.3-1.6 kb, which are possibly encapsidated in shorter particles.
PROTEINS
The viral capsid of all species is composed of a single polypeptide ranging in size from 18-44 kDa. In allexiviruses a 42 kDa polypeptide was also detected as a minor component of virions.
GENOME ORGANIZATION AND REPLICATION
The number of genes is between 3 and 6 depending upon the genus (Fig. 1 ) but, in all species, the ORF1-encoded product, which follows a short 5′-UTR sequence, has homologies with putative polymerase proteins of the “alphavirus-like” supergroup of RNA viruses. This protein (150-250 kDa) contains conserved Mtr, Hel and RdRp motifs. Smaller ORFs encode the proteins involved in cell-to-cell movement, either a single MP of the ‘30K’ superfamily (Capillovirus, Trichovirus, Vitivirus, Citrus leaf blotch virus) or a ‘triple gene block’ (TGB) (remaining genera and viruses). These are usually located following (3′-proximal to) the polymerase but in capillovirus genomes the MP ORF2 is nested within the ORF1. The CP gene always follows the MP(s) and in some genera (Mandarivirus, Allexivirus, Carlavirus, Vitivirus) a final ORF encodes a protein with a zinc binding finger motif and the ability to bind nucleic acids. ORFs downstream of the polymerase are translated from 3′-terminal sgRNAs that can often be found in infected tissue. In some viruses, notably in the genera Vitivirus and Trichovirus and in Citrus leaf blotch virus, nested sets of 5′-terminal sgRNAs and their associated dsRNAs can also be detected. Replication is (or is presumed to be) cytoplasmic and the product of ORF1 is the only virus-encoded protein known to be involved.
Figure 1.

Genome organization of viruses in the family Flexiviridae. Motifs in the polymerase protein ORF1 are Methyltransferase (Mtr), Helicase (Hel), Papain-like protease (P-Pro) and RdRp (Pol). Triple gene block (TGB) proteins; capsid protein (CP) genes; movement protein of the ‘30K’ superfamily (MP); NB, nucleic acidbinding protein.
ANTIGENIC PROPERTIES
Virions are highly immunogenic in some genera (Potexvirus, Mandarivirus, Allexivirus, Carlavirus) but those of others are only moderate to poor antigens. Within (but not usually between) genera, some viruses are serologically related.
BIOLOGICAL PROPERTIES
Flexiviruses have been reported from a wide range of herbaceous and woody mono- and dicotyledonous plant species but the host range of individual members is usually limited. Natural infections by members of the genera Mandarivirus, Foveavirus, Capillovirus, Vitivirus and Trichovirus are mostly or exclusively of woody hosts. Many of the viruses have relatively mild effects on their host. All species can be transmitted by mechanical inoculation, often readily. Many of the viruses have no known invertebrate or fungus vectors; however, allexiviruses and some trichoviruses are thought to be mite-borne, most carlaviruses are transmitted naturally by aphids in the non-persistent manner and a range of vectors have been reported for different vitiviruses. Aggregates of virus particles accumulate in the cytoplasm. Many carlaviruses induce the formation of ovoid or irregularly shaped inclusions but otherwise there are usually no specific cytopathic structures.
SPECIES AND GENUS DEMARCATION CRITERIA IN THE FAMILY
Throughout the family, distinct species have less than ∼ 72% identical nt or 80% identical aa between their CP or polymerase genes. Viruses from different genera usually have less than ∼ 45% identical nt in these genes.
GENUS POTEXVIRUS
Type Species Potato virus X
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments, 470-580 nm in length and 13 nm in diameter, with helical symmetry and a pitch of 3.3-3.7 nm (Fig. 2 ). A central axial canal, ∼ 3 nm in diameter, is discernible only in best preparations. The number of protein subunits per turn of the primary helix is slightly less than 9.0. The RNA backbone is at a radial position of 3.3 nm.
Figure 2.

Negative contrast electron micrograph of particles of an isolate of Potato virus X. The bar represents 100 nm.
(Courtesy of D.-E. Lesemann).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is ∼ 3.5 × 106; S20w is 115 − 130S; buoyant density in CsCl is 1.31 g/cm3.
NUCLEIC ACID
Virions contain a single linear molecule of positive sense ssRNA of ∼ 5.9-7.0 kb which is ∼ 6% by weight of the virion. The RNA is capped at the 5′-terminus and has a polyadenylated tract at the 3′-terminus. The genomic RNA sequences are available for isolates of many species: Potato virus X (PVX, 6,435 nt), White clover mosaic virus (WCMV, 5,845 nt), Clover yellow mosaic virus (ClYMV, 7,015 nt), Papaya mosaic virus (PapMV, 6,656 nt), Bamboo mosaic virus (BaMV, 6,366 nt), Cactus virus X (CVX, 6,689 nt) Cymbidium mosaic virus (CymMV, 6,227 nt), Foxtail mosaic virus (FoMV, 6,151 nt), Pepino mosaic virus (PepMV, 6,450 nt), Plantago asiatica mosaic virus (PlAMV, 6,128 nt), Potato aucuba mosaic virus (7,059 nt) Strawberry mild yellow edge virus (SMYEV, 5,966 nt) Tulip virus X (PVX, 6,056 nt) and Narcissus mosaic virus (NMV, 6,955 nt). Many potexviruses have 5 ORFs; however, some (e.g., Cassava common mosaic virus, CsCMV; NMV and SMYEV have a sixth smaller ORF located completely within ORF5, although it has no known protein product and is of unknown function.
PROTEINS
The virus capsid consists of 1,000-1,500 protein subunits of a single 18-27 kDa polypeptide; the CP of some strains of Potato virus X is glycosylated. Partial proteolytic cleavage of the CP subunits can occur during storage of purified virus. Four nonstructural proteins are coded by the PVX genome including an RNA polymerase (166 kDa) and three proteins (25, 12 and 8 kDa) involved in cell-to-cell movement of the virus (Fig. 3 ).
Figure 3.

Potato virus X (PVX) genome structure and expression.
LIPIDS
None reported.
CARBOHYDRATES
The CP of some strains of PVX is glycosylated.
GENOME ORGANIZATION AND REPLICATION
Virions of PVX contain only genomic RNA, but other potexviruses also encapsidate the sgRNA for the CP. Genomic RNA is translated as a functionally monocistronic message; only the 5′-proximal RNA-polymerase gene is translated directly by ribosomes, producing the RNA polymerase (150-181 kDa). The 5′-UTR leader sequence of PVX RNA (ab – leader) consists of 83 nt (excluding the cap-structure) and efficiently enhances translation. In infected plants, some potexviruses produce sgRNAs including one that acts as messenger RNA for the CP (Fig. 3).
The genomic RNA of potexviruses typically has five ORFs; however, some (including CsCMV, NMV, White clover mosaic virus (WClMV) and SMYEV) have a sixth smaller ORF located completely within ORF5. ORF1, at the 5′-terminus, is the polymerase gene and ORF5, located at the 3′-terminus, is the CP gene. Between ORF1 and ORF5 is the TGB of three overlapping ORFs, the products of which (25, 12, and 8 kDa) are involved in cellto-cell movement of viral RNA. The 25 kDa protein (as well as the 166 kDa replicase) contains an NTPase-helicase domain, but is not involved in RNA replication. The 12 and 8 kDa proteins contain large blocks of uncharged aa and are membrane-bound. ORFs 2 to 5 are expressed via the production (and subsequent translation) of appropriate sgRNAs. Two or three 3′-co-terminal sgRNAs can be isolated from plants infected with potexviruses (2.1; 1.2 and 1.0 kb); the double-stranded counterparts of these sgRNAs have also been detected. The medium-size sgRNA (1.2 kb) is probably functionally bicistronic, its translation yielding the 12 and 8 kDa proteins.
ANTIGENIC PROPERTIES
Virions are highly immunogenic; some species are antigenically related, but others are serologically distinct.
BIOLOGICAL PROPERTIES
HOST RANGE
Some of the viruses are moderately pathogenic, causing mosaic or ringspot symptoms in a wide range of mono- and dicotyledonous plant species, but others alone cause little damage to infected plants. The host range of individual members is limited.
TRANSMISSION
The viruses are mechanically transmissible, but none has known invertebrate or fungal vectors. The viruses are transmitted in nature by mechanical contact.
GEOGRAPHICAL DISTRIBUTION
The viruses have world-wide distribution.
CYTOPATHIC EFFECTS
The cytoplasm of infected cells contains fibrous, banded or irregular aggregates of virus particles, and often membrane accumulations. There is no cytopathology specific to potexviruses, although some viruses induce unique structures such as the beaded sheets found in PVX-infected cells.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The list of species demarcation criteria in the genus is:
-
•
Host range: the natural host range is usually specific to different species.
-
•
Distinct species fail to cross-protect in infected plants.
-
•
Serology; species and strains of some species are also readily distinguishable in differential reactions with monoclonal antibodies.
-
•
Sequence: Distinct species have less than ∼ 72% identical nt or 80% identical aa between their CP or polymerase genes.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Alternanthera mosaic virus | ||
| Alternanthera mosaic virus | [AF080448] | (AltMV) |
| Asparagus virus 3 | ||
| Asparagus virus 3 | (AV-3) | |
| Bamboo mosaic virus | ||
| Bamboo mosaic virus | [D26017, L77962, AF018156] | (BaMV) |
| Cactus virus X | ||
| Cactus virus X | (CVX) | |
| Cassava common mosaic virus | ||
| Cassava common mosaic virus | [U23414] | (CsCMV) |
| Cassava virus X | ||
| Cassava virus X | (CsVX) | |
| Clover yellow mosaic virus | ||
| Clover yellow mosaic virus | [M63511, M63512, M63512, M63514, D00485, D29630] | (ClYMV) |
| Commelina virus X | ||
| Commelina virus X | (ComVX) | |
| Cymbidium mosaic virus | ||
| Cymbidium mosaic virus | [U62963, X62663, X62664, X62665, X62133, X81051, AF016914] | (CymMV) |
| Daphne virus X | ||
| Daphne virus X | (DVX) | |
| Foxtail mosaic virus | ||
| Foxtail mosaic virus | [AY121833, M62730] | (FoMV) |
| Hosta virus X | ||
| Hosta virus X | [AY181252] | (HVX) |
| Hydrangea ringspot virus | ||
| Hydrangea ringspot virus | [AJ270987, AJ550524] | (HdRSV) |
| Lily virus X | ||
| Lily virus X | [X15342]] | (LVX) |
| Narcissus mosaic virus | ||
| Narcissus mosaic virus | [D13747] | (NMV) |
| Nerine virus X | ||
| Nerine virus X | (NVX) | |
| Papaya mosaic virus | ||
| Papaya mosaic virus | [D13957, AY017186-88, D00240] | (PapMV) |
| Pepino mosaic virus | ||
| Pepino mosaic virus | [AF484251, AJ438767, AF340024] | (PepMV) |
| Plantago asiatica mosaic virus | ||
| Plantago asiatica mosaic virus | [Z21647] | (PlAMV) |
| Plantago severe mottle virus | ||
| Plantago severe mottle virus | (PlSMoV) | |
| Plantain virus X | ||
| Plantain virus X | (PlVX) | |
| Potato aucuba mosaic virus | ||
| Potato aucuba mosaic virus | [S73580] | (PAMV) |
| Potato virus X | ||
| Potato virus X | [M38655, M95516, U19790, X55802, X72214, X88782, X88784-88, Z29333] | (PVX) |
| Scallion virus X‡ | ||
| Scallion virus X | [AJ316085] | (ScaVX) |
| Strawberry mild yellow edge virus | ||
| Strawberry mild yellow edge virus | [D12517, D12515, D01227, D00866] | (SMYEV) |
| Tamus red mosaic virus | ||
| Tamus red mosaic virus | (TRMV) | |
| Tulip virus X | ||
| Tulip virus X | [AB066288] | (TVX) |
| White clover mosaic virus | ||
| White clover mosaic virus | [X06728, X16636] | (WClMV) |
TENTATIVE SPECIES IN THE GENUS
| Artichoke curly dwarf virus | (ACDV) |
| Barley virus B1 | (BarV-B1) |
| Boletus virus X | (BolVX) |
| Centrosema mosaic virus | (CenMV) |
| Dioscorea latent virus | (DLV) |
| Lychnis symptomless virus | (LycSLV) |
| Malva veinal necrosis virus | (MVNV) |
| Nandina mosaic virus | (NaMV) |
| Negro coffee mosaic virus | (NeCMV) |
| Parsley virus 5 | (PaV-5) |
| Parsnip virus 3 | (ParV-3) |
| Parsnip virus 5 | (ParV-5) |
| Patchouli virus X | (PatVX) |
| Rhododendron necrotic ringspot virus | (RoNRSV) |
| Rhubarb virus 1 | (RV-1) |
| Smithiantha latent virus | (SmiLV) |
| Viola mottle virus | (VMoV) |
| Zygocactus symptomless virus | (ZSLV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Figure 4.

Phylogenetic analysis of potexviruses using the aa sequences of the polymerase (Left), the CP (Center) and the TGBp1 protein (Right). Sequences were aligned using GCG PILEUP and genetic distances estimated by PROTDIST (Dayhoff PAM method). Trees were displayed in TreeView. Bootstrap values based on 100 replicates are shown where >60%. Shallot virus X (ShV-X), genus Allexivirus is included for comparison. The abbreviations of the other virus names are indicated in the list of species.
GENUS MANDARIVIRUS
Type Species Indian citrus ringspot virus
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments of 650 nm modal length, 13 nm in diameter, with clearly visible cross-banding (Fig. 5 ).
Figure 5.

Electron micrograph of particles of an isolate of Indian citrus ringspot virus, stained in 1% uranyl acetate. The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
ICRSV forms a single band in cesium sulfate density gradients. Purified preparations show maximum absorption at 260 nm with a A260/280 ratio of 1.1 (corrected for light scattering).
NUCLEIC ACID
Indian citrus ringspot virus (ICRSV) virions contain a single molecule of linear ssRNA, 7,560 nt in length, excluding the 3′-poly(A) tail.
PROTEINS
The only structural protein of Indian citrus ringspot virus (ICRSV) is the CP composed of 325 aa (34 kDa).
LIPIDS
Not reported.
CARBOHYDRATES
Not reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA of ICRSV comprises six ORFs on the positive strand, a 5′-UTR of 78 nt and a 3′-UTR of 40 nt, followed by a poly(A) tail (Fig. 6 ). No significant ORFs are in the negative strand. The putative polypeptides encoded by the different ORFs are: ORF1, 1658 aa, 187.3 kDa; ORF2, 225 aa, 25 kDa; ORF3, 109 aa, 12 kDa; ORF4, 60 aa, 6.4 kDa; ORF5, 325 aa, 34 kDa, and ORF6, 222 aa, 23 kDa.
Figure 6.

Genome organization of Indian citrus ringspot virus (ICRSV).
ORF1 encodes the viral polymerase. ORFs 2, 3 and 4 form the TGBck, as in potexvirus genomes. ORF5 encodes the CP. ORF6 encodes a putative protein of unknown function that shows limited similarity with nucleic acid-binding regulatory proteins encoded by ORF6 of allexi- and carlaviruses.
ANTIGENIC PROPERTIES
ICRSV particles are good immunogens, rabbit antisera can have titers of 1/128 and 1/2048 in gel diffusion and EM decoration, respectively.
BIOLOGICAL PROPERTIES
ICRSV causes a serious disease of citrus, especially Kinnow mandarin, in India, with bright yellow ringspots on mature leaves, followed by rapid decline of the tree. Experimentally the virus can be mechanically inoculated to leaves of Chenopodium quinoa, ∼ amaranticolor, Glycine max, Vigna unguiculata and Phaseolus vulgaris, giving local lesions, but systemic infection only in P. vulgaris. No natural vector is known, but ICRSV is transmitted by grafting and persists in the host.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Indian citrus ringspot virus | ||
| Indian citrus ringspot virus | [AF406744] | (ICRSV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Not applicable.
GENUS ALLEXIVIRUS
Type Species Shallot virus X
VIRION PROPERTIES
MORPHOLOGY
Virions are highly flexible filamentous particles, about 800 nm in length and 12 nm in diameter. They resemble potyviruses in their length, but closteroviruses in their flexibility and cross-banded substructure (Fig. 7 ).
Figure 7.

Negative staining electron micrograph of virions of an isolate of Shallot virus X. The bar represents 200 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions of Shallot virus X (ShVX) virions sediment with an S20w of about 170S in 0.1 M tris-HC1, pH 7.5 at 20°C and have abuoyant density in CsCl of 1.33 g/cm3.
NUCLEIC ACID
Virions contain a single molecule of linear ssRNA, about 9.0 kb in size, with a 3′poly(A) tract. RNA preparations contain genomic ssRNA and molecules of dsRNA, 1.5 kb in length, whose genesis and function(s) are unknown. The complete nt sequences of the genomic RNA of ShVX and Garlic virus X (GVX), and the partial sequences of the RNA of four allexiviruses have been determined.
PROPTEINS
Virions are composed of a 28-36 kDa polypeptide as a major CP. A 42 kDa polypeptide is a minor component of virions.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA contains six large ORFs and UTRs of 98 nt at the 5′ terminus, and 112 nt followed by poly (A) tail at the 3′ terminus (Fig. 9 ). The ORFs code for polypeptides of 195, 26, 11, 42, 28, and 15 kDa, respectively from 5′-end to 3′-end. The gene arrangement of other incompletely sequenced allexiviruses is similar. The 195 kDa polypeptide is probably the RdRp. In comparisons among the aa sequences of Mtr, Hel or RdRp domains, those of allexiviruses were most similar to those of potexviruses. The 26 and 11 kDa proteins are similar to the first two proteins encoded by the TGB of potexviruses and carlaviruses and are probably involved in cell-to-cell movement of the virus. There is a coding sequence for a small (7-8 kDa) TGB protein but it lacks the initiation AUG-codon. The 42 kDa polypeptide has no significant homology with any proteins known but has been shown to be expressed in plants infected with ShVX in relatively large amounts and was shown to be involved in the virion assembly. The 28 kDa polypeptide is the CP. In PAGE it migrates as an apparently 32-36 kDa protein, which could be due to its high hydrophilicity. The 15 kDa protein is similar to the 11-14 kDa proteins encoded by the 3′ ORFs of carlaviruses, has a zinc binding finger motif and an ability to bind nucleic acids. The function of this polypeptide is not known.
Figure 9.

Filamentous particles of an isolate of Carnation latent virus. The bar represents 100 nm.
(Courtesy R.G. Milne).
Figure 8.

Genome organization of Shallot virus X (ShVX).
ANTIGENIC PROPERTIES
Allexivirus particles are good immunogens. Some members of the genus are serologically interrelated. Specific antisera and monoclonal antibodies against pure virus particles as well as antisera against recombinant CPs have been used for differentiation purposes.
BIOLOGICAL PROPERTIES
HOST RANGE
Host range is extremely restricted. Some isolates from shallot, onion, garlic and sand leek have been experimentally transmitted to Chenopodium murale, in which they induced local lesions.
TRANSMISSION
Allexiviruses are thought to be mite-borne. Garlic virus C (GarV-C) and Garlic virus D (GarV-D) have been shown to be transmitted by the eriophyd mite, Aceria tulipae. All are manually transmissible by sap inoculation of healthy host plants. None could be transmitted by aphids.
GEOGRAPHICAL DISTRIBUTION
Allexiviruses have been identified in Russia, Japan, France, Germany, UK, The Netherlands, Korea, Taiwan, Thailand and Argentina.
CYTOPATHIC EFFECTS
Most induce no visible or only very mild symptoms in many species, although certain isolates can cause severe damage to crops. In infected tissue allexiviruses can induce formation of granular inclusion bodies and small bundles of flexible particles.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Members of distinct species have less than ∼ 72% identical nt or 80% identical aa between their CP or polymerase genes,
-
•
Different reactions with antisera.
LIST OF SPECIES IN THE GENUS
The available information about the identity of the members in the genus Allexivirus is still fragmentary. There are almost always found in mixed infections of vegetatively propagated species and it is difficult or impossible to isolate and/or separate the viruses.
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Garlic mite-borne filamentous virus | ||
| Garlic mite-borne filamentous virus | (GarMbFV) | |
| Garlic virus A | ||
| Garlic virus A | [AB010300, AF478197, X98991] | (GarV-A) |
| Garlic virus B | ||
| Garlic virus B | [AB010301, AF543829] | (GarV-B) |
| Garlic virus C | ||
| Garlic virus C | [AB010302, D49443] | (GarV-C) |
| Garlic virus D | ||
| Garlic virus D | [AB010303, AF519572, L38892] | (GarV-D) |
| Garlic virus E‡ | ||
| Garlic virus E | [AJ292230] | (GarV-E) |
| Garlic virus X | ||
| Garlic virus X | [AJ292229, U89243] | (GarV-X) |
| Shallot virus X | ||
| Shallot virus X | [M97264, L76292] | (ShVX) |
TENTATIVE SPECIES IN THE GENUS
| Garlic mite-borne latent virus | (GarMbLV) |
| Onion mite-borne latent virus | (OMbLV) |
| Shallot mite-borne latent virus | (ShMbLV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
A phylogenetic tree based on the coat protein aa sequence alignment shows that ShVX is closer to GarV-A, GarV-D and GarV-E.
GENUS CARLAVIRUS
Type Species Carnation latent virus
VIRION PROPERTIES
MORPHOLOGY
Virions are slightly flexuous filaments, 610-700 nm in length and 12-15 nm in diameter (Fig. 9). They have helical symmetry with a pitch of about 3.4 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is about 60 × 106, with a nucleic acid content of ∼ 6%. Virion S20,w is 147-176S, and the buoyant density in CsCl solutions is 1.3 g/cm3.
NUCLEIC ACID
Virions contain a single molecule of linear ssRNA that has a size range of 7.4-7.7 kb when estimated by agarose gel analysis, although full-length sequence analysis suggests that genome sizes are in the 8.3-8.6 kb range. Some species also have two sgRNAs of 2.1-3.3 kb and 1.3-1.6 kb, which are possibly encapsidated in shorter particles. The genomic RNAs have a 3′-poly(A) tract and a 5′-cap. They contain six ORFs. The nt sequence or partial sequence of a large number of carlavirus RNAs has been determined.
PROTEINS
Virions contain a single 31-36 kDa polypeptide.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNAs of Potato virus M (PVM)(Fig. 10 ), (PCMV, 8,530 nt) and Blueberry scorch virus (BlScV, 8,514 nt), the better characterized viruses, each contain six ORFs; PVM RNA has UTRs of 75 nt at the 5′-terminus, 70 nt followed by a poly(A) tail at the 3′-terminus and 38 and 21 nt between the three large blocks of coding sequences. Full-length genomic sequences have recently been obtained for Garlic common latent virus (GarCLV, 8,353 nt) and Aconitum latent virus (AcLV, 8,657 nt).
Figure 10.

Genome organization of Potato virus M (PVM).
ORF1 encodes a polypeptide of 223 kDa that is the viral replicase; ORFs 2, 3 and 4 form the triple gene block and encode polypeptides of 25, 12 and 7 kDa which facilitate virus movement. ORF5 encodes the 34 kDa CP and overlaps ORF5, which encodes a cysteinerich protein of 11-16 kDa. The gene arrangement of other incompletely sequenced carlaviruses is similar. The proteins encoded by the triple gene block facilitate cell-to-cell movement of virus. The 34 kDa polypeptide is the CP. The function of the 11-16 kDa polypeptide has yet to be determined, but its ability to bind nucleic acid indicates that it may facilitate aphid transmission or be involved in host gene transcription/gene silencing and/or viral RNA replication.
Only the 223 kDa replicase ORF is translated from the full length genomic RNA. With BlScV and probably other carlaviruses the 223 kDa protein is proteolytically processed by a papain-like proteinase activity, with ∼ 30-40 kDa being removed. The 3′-terminal ORFs appear to be translated from two sgRNAs that can be found in infected tissue, and, for some viruses, can be detected in purified virus preparations. The 5′-untranslated leader sequence of the genomic RNA and the sgRNA for the CP of Potato virus S (PVS) have both been shown to act as efficient enhancers of translation.
ANTIGENIC PROPERTIES
Carlavirus virions are good immunogens. Some species are serologically interrelated, but others are apparently distinct.
BIOLOGICAL PROPERTIES
HOST RANGE
Individual viruses have restricted natural host ranges, but some can infect a wide range of experimental hosts.
TRANSMISSION
Most species are transmitted naturally by aphids in the non-persistent manner; Cowpea mild mottle virus CPMMV) is transmitted by whiteflies (Bemisia tabaci). Pea streak virus, PeSV; Red clover vein mosaic virus, RCVMV and Cowpea mild mottle virus CPMMV) are seedborne in their leguminous hosts. All are mechanically transmissible; some (e.g. Carnation latent virus, CLV and PVS) are sufficiently infectious to be so transmitted this way in the field.
GEOGRAPHICAL DISTRIBUTION
The geographical distribution of many species is restricted, but those infecting vegetatively-propagated crops are usually widely distributed, presumably due to inadvertent dissemination in vegetative propagules. Most species commonly occur in temperate climates, but that transmitted by whiteflies is restricted to tropical and subtropical regions.
CYTOPATHIC EFFECTS
Virions of aphid-borne species are scattered throughout the cytoplasm or occur in membrane-associated bundle-like or plate-like aggregates. Many species also induce the formation of ovoid or irregularly shaped inclusions that appear in the light microscope as vacuolate bodies; these consist of aggregates of virus particles, mitochondria, endoplasmic reticulum and lipid globules. The particles of CPMMV, the whitefly-transmitted carlavirus, also occur in aggregates in cytoplasm; those of most, but not all, strains of CPMMV form brush-like inclusions.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Each distinct species usually has a specific natural host range. Distinct species do not cross-protect in infected common host plant species. Distinct species are readily differentiated by serological procedures; strains of individual species are often distinguishable in reactions with polyclonal antisera, but more readily so with monoclonal antibodies. Distinct species have less than ∼72% identical nt or 80% identical aa between their CP or polymerase genes.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Aphid-borne species: | ||
| American hop latent virus | ||
| American hop latent virus | (AHLV) | |
| Blueberry scorch virus | ||
| Blueberry scorch virus | [L25658] | (BlScV) |
| Cactus virus 2 | ||
| Cactus virus 2 | (CV-2) | |
| Caper latent virus | ||
| Caper latent virus | (CapLV) | |
| Carnation latent virus | ||
| Carnation latent virus | [AJ010697, X55331, X55897, U43905] | (CLV) |
| Chrysanthemum virus B | ||
| Chrysanthemum virus B | [AJ564854, S60150] | (CVB) |
| Cole latent virus | ||
| Cole latent virus | [AY340584] | (CoLV) |
| Dandelion latent virus | ||
| Dandelion latent virus | (DaLV) | |
| Elderberry symptomless virus | ||
| Elderberry symptomless virus | (ESLV) | |
| Elderberry virus A | ||
| Garlic common latent virus | ||
| Garlic common latent virus | [AB004566, AB004804-05, AF228416, AF538951, X81138, X81139] | (GarCLV) |
| Helenium virus S | ||
| Helenium virus S | [D10454, S71594] | (HVS) |
| Honeysuckle latent virus | ||
| Honeysuckle latent virus | (HnLV) | |
| Hop latent virus | ||
| Hop latent virus | [AB032469] | (HpLV) |
| Hop mosaic virus | ||
| Hop mosaic virus | [AB051109] | (HpMV) |
| Hydrangea latent virus | ||
| Hydrangea latent virus | (HdLV) | |
| Kalanchoe latent virus | ||
| Kalanchoe latent virus | [AJ293570, AJ293571, AY238136-AY238143] | (KLV) |
| Lilac mottle virus | ||
| Lilac mottle virus | (LiMoV) | |
| Lily symptomless virus | ||
| Alstroemeria carlavirus | ||
| Lily symptomless virus | [AJ564638, AJ516059, AJ131812, AF103784, AF015286, X15343, D43801, U43905] | (LSV) |
| Mulberry latent virus | ||
| Mulberry latent virus | (MLV) | |
| Muskmelon vein necrosis virus | ||
| Muskmelon vein necrosis virus | (MuVNV) | |
| Nerine latent virus | ||
| Hippeastrum latent virus | ||
| Nerine latent virus | (NeLV) | |
| Passiflora latent virus | ||
| Passiflora latent virus | [AF354652] | (PLV) |
| Pea streak virus | ||
| Alfalfa latent virus | [AY037925] | |
| Pea streak virus | (PeSV) | |
| Potato latent virus | ||
| Potato latent virus | [AY007728] | (PotLV) |
| Potato virus M | ||
| Potato virus M | [X53062, X57440, D144449, AF23877] | (PVM) |
| Potato virus S | ||
| Pepino latent virus | ||
| Potato virus S | [D00461, S45593] | (PVS) |
| Red clover vein mosaic virus | ||
| Red clover vein mosaic virus | (RCVMV) | |
| Shallot latent virus | ||
| Garlic latent virus | [AB004458, AB004565-67, AB004684-86, NC_003557] | (SLV) |
| Shallot latent virus | [AB004456-57, AB004544, AB004802-03] | (GarLV) |
| Sint-Jan's onion latent virus | ||
| Sint-Jan's onion latent virus | (SJOLV) | |
| Strawberry pseudo mild yellow edge virus | ||
| Strawberry pseudo mild yellow edge virus | (SPMYEV) | |
| Whitefly-transmitted species: | ||
| Cowpea mild mottle virus | ||
| Bean angular mosaic virus | ||
| Cowpea mild mottle virus | [AF024628-29] | (CPMMV) |
| Groundnut crinkle virus | ||
| Psophocarpus necrotic mosaic virus | ||
| Tomato pale chlorosis virus | ||
| Voandzeia mosaic virus | ||
| Unknown vector species: | ||
| Aconitum latent virus | ||
| Aconitum latent virus | [AB051848] | (AcLV) |
| Narcissus common latent virus | ||
| Narcissus common latent virus | [AJ311375, AJ311376] | (NCLV) |
| Poplar mosaic virus | ||
| Poplar mosaic virus | [X65102, D13364, X97683, X97765] | (PopMV) |
| Verbena latent virus | ||
| Verbena latent virus | [AF271218] | (VeLV) |
TENTATIVE SPECIES IN THE GENUS
| Anthriscus latent virus | (AntLV) | |
| Arracacha latent virus | (ALV) | |
| Artichoke latent virus M | (ArLVM) | |
| Artichoke latent virus S | (ArLVS) | |
| Butterbur mosaic virus | (ButMV) | |
| Caraway latent virus | (CawLV) | |
| Cardamine latent virus | (CaLV) | |
| Cassia mild mosaic virus | (CasMMV) | |
| Chicory yellow blotch virus | (ChYBV) | |
| Cynodon mosaic virus | (CynMV) | |
| Daphne virus S | [AJ535084] | (DVS) |
| Dulcamara virus A | (DuVA) | |
| Dulcamara virus B | (DuVB) | |
| Eggplant mild mottle virus (Eggplant virus) | (EMMV) | |
| Euonymus mosaic virus | (EuoMV) | |
| Fig virus S | (FVS) | |
| Fuchsia latent virus | (FLV) | |
| Garlic mosaic virus | (GarMV) | |
| Gentiana latent virus | (GenLV) | |
| Gynura latent virus (Chrysanthemum virus B) | (GyLV) | |
| Helleborus mosaic virus | (HeMV) | |
| Impatiens latent virus | (ILV) | |
| Lilac ringspot virus | (LiRSV) | |
| Plantain virus 8 | (PlV-8) | |
| Potato rough dwarf virus | [AJ250314] | (PRDV) |
| Potato virus P | (PVP) | |
| Prunus virus S | (PruVS) | |
| Southern potato latent virus | (SoPLV) | |
| White bryony mosaic virus | (WBMV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
The phylogenetic analysis is presented for the aa sequences of the polymerase, TGBp1 and the CP are presented in Figure 11 . ASPV, genus Foveavirus has been included as a nearest neighbor.
Virions of Narcissus latent virus (NLV) are filamentous and ∼650 nm long. It was previously considered to be a carlavirus. However, it differs from carlaviruses in inducing the formation of intracellular inclusions (“pinwheels”) and in having a CP of 46 kDa; it is thus now better placed in the genus Macluravirus (family Potyviridae), together with Maclura mosaic virus, to which it is serologically related. Chinese yam necrotic mosaic virus, (ChYNMV) which was also listed as a tentative carlavirus, has now been sequenced and is also placed in the genus Macluravirus (family Potyviridae).
GENUS FOVEAVIRUS
Type Species Apple stem pitting virus
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments, ∼800 to over 1000 nm in length and 12-15 nm in diameter with helical symmetry exhibiting a surface pattern with cross-banding and longitudinal lines (Fig. 12 ). Particles of some viruses such as Apple stem pitting virus (ASPV) and Cherry green ring mottle virus (CGRMV) show a tendency to end-to-end aggregation.
Figure 12.

Negative contrast electron micrograph of particles of an isolate of Apple stem pitting virus. The bar represents 100 nm.
(Courtesy of H. Koganezawa).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
ASPV virions sediment as two or three bands in sucrose density gradients but yield a single band at equilibrium in Omnipaque 350 density gradients. They resist moderately high temperatures (thermal inactivation is around 60°C) but not organic solvents, and are unstable in cesium chloride and sulfate. CGRMV virions resist organic solvents and are stable in cesium sulfate.
NUCLEIC ACID
Virions contain a single molecule of positive sense ssRNA, polyadenylated at the 3′-terminus. The genome of the tentative species CGRMV is capped at the 5′-terminus. The complete nt sequences are known for Apple stem pitting virus (ASPV, 9.3 kb), Rupestris stem pitting-associated virus (RSPaV, 8.7 kb), Cherry green ring mottle virus (CGRMV, 8.4 kb) and Cherry necrotic rusty mottle (CNRMV, 8.4 kb) and the a partial sequence of Apricot latent virus (ApLV) is available.
PROTEINS
The viral capsid of all species is composed of a single polypeptide with a size ranging from 23 kDa (SCSMaV), to 30 kDa (CGRMV), to 44 kDa (ASPV and ApLV). Non structural proteins consist of a large polypeptide (205-247 kDa) containing the Mtr, Hel, and viral RdRp signatures, a 23-25 kDa polypeptide with the NTP-binding helicase domain, and two small polypetides (12-13 kDa and 7-8 kDa) with membrane-binding functions.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomes of all fully sequenced members, except for CGRMV contain 5 ORFs (Fig. 13 ). The 5′ region initiates with a UTR of 33-72 nt, ORF1 codes for the replication-related proteins with conserved motifs of the “alphavirus-like” supergroup of positive-strand ssRNA viruses (i.e. Mtr and RdRp); ORF2, ORF3, and ORF4 constitute the TGB thought to be involved in cell-to-cell spread of the virus; ORF5 is the CP cistron. A non-coding sequence of 176-312 nt followed by a poly(A) tail terminate the genome. The 5′-end of CGRMV RNA is capped and two additional ORFs (ORF2a and ORF4a) nested in ORF2 and ORF5, respectively, which potentially encode 14 and 18 kDa polypeptides, have been reported from computer analysis only. No similarity was found with other proteins in databases. ASPV virions accumulate in the cytoplasm, where multiplication is likely to occur following a strategy comparable to that of potexviruses, based on direct expression of the 5′-proximal ORF, and expression of downstream ORFs from sgRNAs. Multiple dsRNAs are found in infected hosts.
Figure 13.

Genome organization of Apple stem pitting virus (ASPV) showing the relative position of the ORFs and their expression products. Mtr, methytransferase; Hel, helicase; Pol, polymerase; TGB, triple gene block; CP, capsid protein.
ANTIGENIC PROPERTIES
Antisera to ASPV that can be used for serological detection tests have been raised from purified virions or chimeric fusion CPs expressed in E. coli. A CGRMV particle-decorating antiserum was also produced by using fusion proteins. ASPV and ApLV are serologically related, but there is no recognized serological relationships among other virus species.
BIOLOGICAL PROPERTIES
HOST RANGE
The natural host range of individual species is restricted to a single (RSPaV) or a few hosts (ASPV, ApLV, CGRMV). ASPV infects primarily pome fruits, causing diseases of apple (topworking disease) when grafted on susceptible rootstocks, and of pear (vein yellows and necrotic spot). ApLV is the putative agent of peach asteroid spot and peach sooty ringspot diseases. RSPaV is a pathogen of grapevine. Stone fruits (sweet, sour and flowering cherries, peach, and apricot) are the natural hosts of CGRMV, ApLV, and CNRMV. Experimental host ranges are restricted.
TRANSMISSION
No vector is known for any of the viruses. ASPV, CGRMV, and CNRMV are transmitted by grafting and persist in the host propagative material. ASPV is mechanically transmissible, with some difficulty, to Nicotiana occidentalis and its subspecies obliqua. CGRMV was transmitted by sap inoculation from cherry to cherry but not to a range of herbaceous hosts.
GEOGRAPHICAL DISTRIBUTION
All species have a rather wide geographical distribution.
CYTOPATHIC EFFECTS
ASPV elicits a severe derangement of the cytology of infected cells but no specific cytopathic structures or inclusion bodies. Virus particles accumulate in bundles in the cytoplasm. Massive accumulations of virus-like particles were also observed in mesophyll cells of CGRMV-infected Kwazan flowering cherry.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Natural host range,
-
•
Mechanical transmissibility,
-
•
Serological specificity,
-
•
CP size,
-
•
Less than ∼ 72% identical nt or 80% homologous aa between CP or polymerase genes.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Figure 14.

Phylogenetic tree showing the relationships among definitive and tentative species of the genus Foveavirus based on the CP gene sequence. The tree was produced and bootstrapped using CLUSTAL W.
GENUS CAPILLOVIRUS
Type Species Apple stem grooving virus
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments, 640-700 × 12 nm, constructed from helically arranged protein subunits in a primary helix with a pitch of 3.4 nm and between 9 and 10 subunits per turn (Fig. 15 ).
Figure 15.

(Left) Schematic representation of a fragment of a particle of a capillovirus. (Right) Negative contrast electron micrograph of particles of an isolate of Apple stem grooving virus. The bar represents 100 nm.
(Courtesy of N. Yoshikawa).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
S20w of particles of Apple stem grooving virus (ASGV) is ∼112S, isoelectric point is about pH 4.3 at ionic strength 0.1 M, and electrophoretic mobility is 10.3 and 6.5 × 10-5 cm2/sec/volt, at pH 7.0 and 6.0 respectively (ionic strength 0.1 M).
NUCLEIC ACID
Virions contain linear positive sense ssRNA, 6.5-7.4 kb in size, constituting about 5%, by weight, of virions. The RNA is polyadenylated at its 3′-end. The complete nt sequences are known for genome RNA of three isolates of Apple stem grooving virus and one of Cherry virus A. Isolates of Apple stem grooving virus from different hosts show wide variations in the sequence of a 284 aa region of ORF1-encoded protein, between the polymerase and CP domains.
PROTEINS
Virions are composed of a single 24-27 kDa protein. Non-structural proteins include a 36-52 kDa protein with sequence homology with putative MPs, and a large replication-associated protein fused with the CP, with conserved Mtr, Hel and RNA polymerase motifs, expressed as a precursor with probable maturation by proteolysis.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomic RNA of all sequenced viruses has the same structural organization, and two ORFs (Fig. 16 ). ORF1 encodes a putative 240-266 kDa protein followed by a UTR of 142 nt upstream of the 3′-poly(A) tail. ORF2 is nested within ORF1 near its 3′-end, and encodes a 36-52 kDa protein. The ORF1-encoded product has homologies with putative polymerase proteins of the “alphavirus-like” supergroup of RNA viruses. Although the CP cistron is located in the C-terminal end of ORF1, and ORF2 is nested within ORF1, the strategy of expression of both CP and putative MP may be based on sgRNA production, as suggested by the analysis of dsRNA patterns from infected tissues. dsRNAs of ASGV consist of five major bands with sizes of approximately 6.5, 5.5, 4.5, 2.0 and 1.0 kbp. The 6.5 kbp species probably represents the double-stranded form of the full-length genome, and the 2.0 and the 1.0 kbp species may be the double-stranded forms of sgRNAs that code for the putative MP and the CP, respectively. Replication is likely to occur in the cytoplasm, in which virus particles accumulate in discrete bundles.
Figure 16.

Genome organization of Apple stem grooving virus (ASGV) showing the relative positions of the ORFs and their expression products. Mtr, methyltransferase; P-Pro, papain-like protease; Hel, helicase; Pol, polymerase; MP, putative movement protein; CP, capsid protein.
ANTIGENIC PROPERTIES
Virions are moderately antigenic. There are no serological relationship between species.
BIOLOGICAL PROPERTIES
HOST RANGE
ASGV is pathogenic to pome fruits and citrus and induces stock/scion incompatibility, i.e. top-working disease of apple and bud union crease syndrome of citrus. It also infects lily. CVA is found in cherry but no disease has been associated with it.
TRANSMISSION
No vectors are known. ASGV was transmitted through seed to progeny seedlings of Chenopodium quinoa, and lily. ASGV, Cherry virus A (CVA), and Nandina stem pitting virus (NSPV) are transmitted by grafting. NSPV has not been transmitted by sap inoculation, but by slashing stems with a partially purified virus preparation.
GEOGRAPHICAL DISTRIBUTION
Geographical distribution ranges from wide to restricted according to the virus. ASGV has been recorded from most areas where apples are grown, and is widespread in citrus in China, Japan, United States, Australia, and South Africa. Lilac chlorotic leafspot virus (LiCLV) occurs in England and possibly in continental Europe and the United States. NSPV is found only in the United States and CVA in Germany.
CYTOPATHIC EFFECTS
No distinct cytological alterations have been observed in infected cells. Virus particles occur in bundles in mesophyll and phloem parenchyma cells, but not in the epidermis and sieve elements.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Natural host range,
-
•
Serological specificity (all known species are serologically unrelated),
-
•
Less than ∼ 72% identical nt or 80% identical aa between the CP or polymerase genes.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
| Nandina stem pitting virus | (NSPV) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Not available.
GENUS VITIVIRUS
Type Species Grapevine virus A
VIRION PROPERTIES
MORPHOLOGY
Virions are flexuous filaments 725-825 × 12 nm in size, showing distinct cross banding, helically constructed with a pitch of 3.3-3.5 nm and about 10 subunits per turn of the helix (Fig. 17 ).
Figure 17.

Negative contrast electron micrograph of particles of an isolate of Grapevine virus A. The bar represents 100 nm.
(Courtesy of A.A. Castellano).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as a single or two very close bands in sucrose or Cs2SO4 gradients, with an S20w of ∼92S. Virions of Heracleum latent virus (HLV), are sensitive to ribonucleases. Virions of all species resist moderately high temperatures (thermal inactivation is around 60°C) and are moderately resistant to organic solvents.
NUCLEIC ACID
Virions contain a single molecule of positive sense ssRNA, ∼ 7.6 kb in size, capped at the 5′-terminus and polyadenylated at the 3′-terminus. The RNA accounts for ∼ 5% of the particle weight. The complete nt sequences are available for Grapevine virus A (GVA) and Grapevine virus B (GVB). The genomes of Grapevine virus D (GVD) and HLV have been sequenced in part. Infectious cDNA clones have been produced for GVA and GVB.
PROTEINS
The CPs are composed of a single 18-21.5 kDa polypeptide. Non-structural proteins are: (i) a 194 kDa polypeptide with conserved motifs of replication-related proteins of the “alphavirus-like” supergroup of positive-strand ssRNA viruses (i.e., Mtr, Hel and RdRp, in that order from the N- to the C-terminus); (ii) a 19-20 kDa polypeptide of unknown function with no significant sequence homology to known proteins, which, in GVB infections does not accumulate in phase with MPs (iii) a 31-36.5 kDa polypeptide that possesses the G/D motif of the “30K superfamily” MP and which is associated with cell walls and plasmodesmata (GVA, GVB) and/or with cytoplasmic viral aggregates (GVA)(iv) a 10-14 kDa polypeptide with weak homologies to proteins with RNA-binding properties.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomes of GVA, GVB and GVD contain five slightly overlapping ORFs (Fig. 18 ). The 5′ region of GVA and GVB initiates with an A/T-rich (60-68%) UTR of 47-86 nt. In addition to the 21.5 kDa CP encoded by ORF4, virus genomes express the non-structural proteins. The strategy of expression is based on sgRNA production, as suggested by the analysis of dsRNA patterns from infected tissues. The four dsRNAs have sizes of 7.6, 6.48, 5.68 and 5.1 kbp for GVA and GVD, and 7.6, 6.25, 5.03 and 1.97 kbp for GVB. Replication occurs in the cytoplasm, possibly in association with membranous vesicles. The replication strategy of GVA encompasses the production of nested sets of 5′-terminal sgRNAs 5.1, 5.5, and 6.0 kb in size and of 3′-terminal sgRNAs 1.0, 1.8, and 2.2 kb that serve for the expression of all ORFs, except for ORF5, which may be expressed via a bi- or polycistronic mRNA. The generation of these 5′- and 3′-terminal sgRNAs appears to be controlled by internal cis-acting elements.
Figure 18.

Organization and expression the genome of Grapevine virus A (GVA) showing the relative position of the ORFs, their expression products, and the nested sets of 5′- and 3′-terminal sgRNAs. Mtr, methyltransferase; Hel, helicase; Pol, polymerase; MP, putative movement protein; CP, capsid protein.
(adapted from Galiakparov et al., 2003).
ANTIGENIC PROPERTIES
Virions are moderate or poor antigens. Most species are very distantly serologically related. Monoclonal antibodies to GVA, GVB, and GVD and recombinant protein antibodies to the putative MP of GVA have been produced. The relationship between GVA, GVB and GVD is due to a few common internal antigenic determinants (cryptotopes). GVA particles carry a highly structured epitope centered in a common peptide region of the CP sequence.
BIOLOGICAL PROPERTIES
HOST RANGE
The natural host range of individual species is restricted to a single host. Infections induce either no symptoms (HLV) or severe diseases characterized by pitting and grooving of the wood (GVA, GVB, GVD). The experimental host range varies from wide (HLV) to restricted (GVA, GVB, GVC, GVD)
TRANSMISSION
All species are transmitted by mechanical inoculation, those infecting grapevines with greater difficulty. Transmission by grafting and dispersal through propagating material is common with grapevine-infecting species. GVA and GVB are transmitted in a semi-persistent manner by different species of pseudococcid mealybugs of the genera Pseudococcus and Planococcus. GVA is also transmitted by the scale insect Neopulvinaria innumerabilis. HLV is transmitted semi-persistently by aphids, in association with a helper virus.
GEOGRAPHICAL DISTRIBUTION
Geographical distribution varies from very wide (GVA, GVB, GVD) to restricted (HLV).
CYTOPATHIC EFFECTS
Infected cells are damaged to a varying extent. All species elicit the formation of vesicular evaginations of the tonoplast containing finely fibrillar material, possibly representing replicative forms of viral RNA. Virions of grapevine-infecting species are strictly phloem-limited, but in herbaceous hosts they also invadethe parenchyma. Virus particles accumulate in the cytoplasm in bundles or paracrystalline aggregates.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
The natural host range,
-
•
Serological specificity using discriminatory polyclonal and monoclonal antibodies,
-
•
Epidemiology: individual species or groups of species are transmitted by different types and species of vectors,
-
•
Differences in dsRNA pattern,
-
•
Less than ∼ 72% identical nt or 80% identical aa between the CP or polymerase genes.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
TENTATIVE SPECIES IN THE GENUS
| Grapevine virus C | (GVC) |
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Figure 19.

Phylogenetic tree based on CP sequences, showing the relationships among species of the genus Vitivirus and between these and some members of related genera. The tree was produced using the Neighbor, Seqboot, Protdist and Consense programs of the PHYLIP package
(adapted from Abou-Ghanem et al., 1997).
GENUS TRICHOVIRUS
Type Species Apple chlorotic leaf spot virus
VIRION PROPERTIES
MORPHOLOGY
Virions are very flexuous filaments, 640-760 × 10-12 nm in size, helically constructed with a pitch of 3.3-3.5 nm, and about 10 subunits per turn of the helix. Virions may show cross banding, criss-cross or rope-like features according to the negative contrast material used (Fig. 20 ).
Figure 20.

Negative contrast electron micrograph of particles of an isolate of Apple chlorotic leaf spot virus. The bar represents 100 nm.
(Courtesy of M.A. Castellano).
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virions sediment as single or as two very close bands with an S20w of ∼100S. Virions of Apple chlorotic leaf spot virus (ACLSV) are sensitive to ribonucleases. Virions of all species resist moderately high temperatures (thermal inactivation is around 55-60°C) and are moderately resistant to organic solvents.
NUCLEIC ACID
Virions contain a single molecule of linear, positive sense, ssRNA ∼ 7.5 to 8.0 kb in size, with a polyadenylated 3′-terminus, accounting for ∼ 5% of the particle weight. Indirect evidence suggests that the genome RNA of ACLSV is capped at its 5′-end. The complete nt sequences are available for four isolates of ACLSV and for Cherry mottle leaf virus (CMLV). Partial sequences are known for RNAs of Potato virus T (PVT), Grapevine berry inner necrosis virus (GINV), and Peach mosaic virus (PcMV). An infectious cDNA clone of ACLSV has been produced. ACLSV isolates show a high variability in their nt sequence with an overall homology between 76 and 82%. The CP is the most conserved protein (87-93% homology), whilst the putative MP is the most divergent (77-85% homology)
PROTEINS
Virions of all species are composed of a single 20.5-27 kDa polypeptide. Non-structural proteins of sequenced members are: (i) a protein of about 180-220 kDa containing RdRp, Hel and Mtr signature sequences typical of replication-associated proteins of the “alphavirus-like” supergroup of ssRNA viruses; (ii) a 40-50 kDa polypeptide with weak homologies to some plant virus MP.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The genomes of ACLSV, PVT, and GINV contain three slightly overlapping ORFs (Fig. 21 ). An additional ORF is present at the 3′-terminus of CMLV genome. The large 5′ ORF of ACLSV is directly expressed from genomic RNA, whereas the two smaller downstream ORFs that code, respectively, for the putative MP and CP, are expressed via sgRNAs. ACLSV-infected tissues contain six dsRNA species of approximately 7.5, 6.4, 5.4, 2.2, 1.1, and 1.0 kbp. The 7.5 kbp species represents the double-stranded form of the full-length genome, whereas the 2.2 and the 1.1 kbp species are the double-stranded forms of sgRNAs coding for the putative MP and the CP, respectively. The most abundant dsRNA species, the function of which are unknown, are 5′ co-terminal with genomic RNA, and have sizes of 6.4 and 5.4 kbp, respectively. Replication is presumed to be cytoplasmic and to involve the translation product of ORF1.
Figure 21.

Genome organization of Apple chlorotic leaf spot virus (ACLSV), showing the relative positions of the ORFs and their expression products. Mtr, methyltransferase; Hel, helicase; Pol, polymerase; MP, putative movement protein; CP, capsid protein.
ANTIGENIC PROPERTIES
Virions are moderate to poor antigens. CMLV and PcMV are serologically related with one another but with none of the other species in the genus.
BIOLOGICAL PROPERTIES
HOST RANGE
The natural host range of individual species is relatively narrow (ACLSV, PcMV), or restricted to a single host (PVT, GINV, CMLV). The experimental host range is somewhat wider, but still limited to a few herbaceous species. In the natural hosts, infections induce few or no symptoms (PVT, ACLSV in certain hosts), or mottling, rings, line patterns, and fruit injuries (i.e. pseudosharka) (ACLSV), mottling with stunting and internal necrosis of shoots and berries (GINV), mottling and severe distortion of the leaves (CMLV), mottling and deformation of leaves and fruits and color break in the petals (PcMV).
TRANSMISSION
The viruses are readily transmitted by mechanical inoculation, by grafting (ACLSV, GINV, CMLV, PcMV) and through propagating material. PVT is seed-transmitted in several hosts, including Solanum spp. GINV is transmitted by the grape erineum mite Colomerus vitis, CMLV by the scale mite Eriophyes inequalis, and PcMV by the peach bud mite Eriophyes insidiosus.
GEOGRAPHICAL DISTRIBUTION
Geographical distribution varies from wide to restricted, according to the virus species. ACLSV is ubiquitous, whereas PVT is reported only from the Andean region of South America, GINV from Japan, and CMLV and PcMV from North America.
CYTOPATHIC EFFECTS
Infected cells are damaged by ACLSV to varying extents. Virions are found in phloem and parenchyma cells of leaves and roots and accumulate in the cytoplasm, sometimes in the nucleus, in bundles or paracrystalline aggregates. No inclusion bodies are formed.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
The criteria demarcating species in the genus are:
-
•
Natural and experimental host range,
-
•
Serological specificity,
-
•
Less than ∼ 72% identical nt or 80% identical aa between the CP or polymerase genes,
-
•
Transmission by a vector,
-
•
Vector specificity.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Apple chlorotic leaf spot virus | ||
| Apple chlorotic leaf spot virus | [M58152, D14996, X99752, AJ243438] | (ACLSV) |
| Cherry mottle leaf virus | ||
| Cherry mottle leaf virus | [AF170028] | (CMLV) |
| Grapevine berry inner necrosis virus | ||
| Grapevine berry inner necrosis virus | [D88448] | (GINV) |
| Peach mosaic virus | ||
| Peach mosaic virus | (PcMV) | |
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
Figure 22.

Phylogenetic tree showing the relationships between the species in the genus Trichovirus, based on the sequence of the CP gene. The tree was produced and bootstrapped using CLUSTAL W and drawn using Treeview. The figures are protein database accession numbers.
LIST OF UNASSIGNED VIRUSES IN THE FAMILY
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession numbers, and assigned abbreviations ( ) are also listed.
SPECIES IN THE FAMILY
| Banana mild mosaic virus‡ | ||
| Banana mild mosaic virus | [AF314662] | (BanMMV) |
| Cherry green ring mottle virus | ||
| Cherry green ring mottle virus | [AF017780, AJ291761] | (CGRMV) |
| Cherry necrotic rusty mottle virus | ||
| Cherry necrotic rusty mottle virus | [AF237816] | (CNRMV) |
| Citrus leaf blotch virus‡ | ||
| Citrus leaf blotch virus | [AJ318061] | (CLBV) |
| Potato virus T | ||
| Potato virus T | [D10172] | (PVT) |
| Sugarcane striate mosaic-associated virus‡ | ||
| Sugarcane striate mosaic-associated virus | [AF315308] | (SCSMaV) |
TENTATIVE SPECIES IN THE FAMILY
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE FAMILY
Genera fall into two groups but these are different depending upon the part of the genome examined (Fig. 23 ). The polymerase analysis places the genera Potexvirus, Allexivirus and Mandarivirus in one group and the remaining genera and viruses in a second cluster. This grouping may reflect some differences in replication strategy as 5′-terminal sgRNAs are present in at least some members of the second cluster, but experimental details are lacking for some genera. In the CP analysis, the genera Capillovirus, Trichovirus and Vitivirus form one group and the remaining viruses and genera (most of which have a triple gene block) are in a distantly related cluster.
Figure 23.

Phylogenetic (Neighbor) analysis of viruses in the family Flexiviridae using the aa sequences of the Polymerase (left) and coat protein (right). Sequences were aligned using GCG PileUp and genetic distances estimated by ProtDist (Dayhoff PAM method). Trees were displayed in TreeView. Bootstrap values based on 100 replicates are shown where >60%. The abbreviations and the accession numbers of the viruses are indicated in the lists of species in each genus.
SIMILARITY WITH OTHER TAXA
The polymerase proteins are members of the “alphavirus-like” supergroup of RNA viruses and therefore are likely to have some relationship to those of other virus genera, including the tymoviruses. The TGB proteins of members of the family Flexiviridae are related to those of rod-shaped viruses in the genera Benyvirus, Hordeivirus, Pecluvirus and Pomovirus, but form a distinct subgroup.
DERIVATION OF NAMES
Allexi: sigla from All ium (the genus name for the principal host, shallot) + X
Capillo: from Latin capillus, a hair.
Carla: sigla from Car nation la tent virus.
Flexi: from flexus, Latin for bent.
Fovea: from fovea, Latin for pit, hole, type of symptom induced by the type species.
Mandari: from mandarin (Citrus reticulata), the host of the type species, Indian citrus
ringspot virus.
Potex: sigla from Pot ato virus X.
Tricho: from thrix, Greek for hair.
Viti: from Vitis, generic name of the grapevine, Vitis vinifera.
REFERENCES POTEXVIRUS
- Angell S.M., Davies C., Baulcombe D.C. Cell-to-cell movement of potato virus X is associated with a change in the size-exclusion limit of plasmodesmata in trichome cells of Nicotiana clevelandii. Virology. 1996;216:197–201. doi: 10.1006/viro.1996.0046. [DOI] [PubMed] [Google Scholar]
- Batten J.S., Yoshinari Y., Hemenway C. Potato virus X: a model system for virus replication, movement and gene expression. Mol. Plant Pathol. 2003;4:125–131. doi: 10.1046/j.1364-3703.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- Calvert L.A., Cuervo M.I., Ospino M.D., Fauquet C.M., Ramirez B.-C. Characterization of cassava common mosaic virus and a defective RNA species. J. Gen. Virol. 1996;77:525–530. doi: 10.1099/0022-1317-77-3-525. [DOI] [PubMed] [Google Scholar]
- Chen J., Zheng H.Y., Chen J.P., Adams M.J. Characterisation of a potyvirus and a potexvirus from Chinese scallion. Arch. Virol. 2002;147:683–693. doi: 10.1007/s007050200018. [DOI] [PubMed] [Google Scholar]
- Davenport W.F., Baulcombe D.C. Mutation of the GKS motif of the RNA-dependent RNA polymerase from potato virus X disables or eliminates virus replication. J. Gen. Virol. 1997;78:1247–1251. doi: 10.1099/0022-1317-78-6-1247. [DOI] [PubMed] [Google Scholar]
- Geering A.D., Thomas J.E. Characterisation of a virus from Australia that is closely related to papaya mosaic potexvirus. Arch. Virol. 1999;144:577–592. doi: 10.1007/s007050050526. [DOI] [PubMed] [Google Scholar]
- Hefferon K.L., Doyle S., AbouHaidar M.G. Immunological detection of the 8K protein of potato virus X (PVX) in cell walls of PVX-infected tobacco and transgenic potato. Arch. Virol. 1997;142:425–433. doi: 10.1007/s007050050089. [DOI] [PubMed] [Google Scholar]
- Hefferon K.L., Khalilian H., Xu H., AbouHaidar M.G. Expression of the coat protein of Potato virus X from a dicistronic mRNA in transgenic potato plants. J Gen. Virol. 1997;78:3051–3059. doi: 10.1099/0022-1317-78-11-3051. [DOI] [PubMed] [Google Scholar]
- Kalinina N.O., Fedorkin O.N., Samuilova O.V., Maiss E., Korpela T., Morozov S.Y., Atebekov J.G. Expression and biochemical analysis of the recombinant potato virus X 25K movement protein. FEBS Lett. 1996;397:75–78. doi: 10.1016/s0014-5793(96)01138-6. [DOI] [PubMed] [Google Scholar]
- Kavanagh T., Goulden M., Santa Cruz S., Chapman S., Barker I., Baulcombe D. Molecular analysis of a resistance-breaking strain of potato virus X. Virology. 1992;189:609–617. doi: 10.1016/0042-6822(92)90584-c. [DOI] [PubMed] [Google Scholar]
- Kim K.-H., Hemenway C. Mutations that alter a conserved element upstream of the potato virus X triple block and coat protein genes affect subgenomic RNA accumulation. Virology. 1997;232:187–197. doi: 10.1006/viro.1997.8565. [DOI] [PubMed] [Google Scholar]
- Lambrecht S., Jelkmann W. Infectious cDNA clone used to identify strawberry mild yellow edge-associated potexvirus as causal agent of the disease. J. Gen. Virol. 1997;78:2347–2353. doi: 10.1099/0022-1317-78-9-2347. [DOI] [PubMed] [Google Scholar]
- Lee Y.S., Lin B.Y., Hsu Y.H., Chang B.Y., Lin N.S. Subgenomic RNAs of bamboo mosaic potexvirus-V isolate are packaged into virions. J. Gen. Virol. 1998;79:1825–1832. doi: 10.1099/0022-1317-79-7-1825. [DOI] [PubMed] [Google Scholar]
- Park M.H., Ryu K.H. Molecular evidence supporting the classification of Hosta virus X as a distinct species of the genus Potexvirus. Arch. Virol. 2003;148:2039–2045. doi: 10.1007/s00705-003-0154-1. [DOI] [PubMed] [Google Scholar]
- Solovyev S.G., Novikov V.K., Merits A., Savenkov E.I., Zeleninu D.A., Tyulkina L.G., Morozov S.Y. Genome characterization and taxonomy of Plantago asiatica mosaic potexvirus. J. Gen. Virol. 1994;75:259–267. doi: 10.1099/0022-1317-75-2-259. [DOI] [PubMed] [Google Scholar]
- Tomashevskaya O.L., Solovyev A.G., Karpova O.V., Fedorkin O.N., Rodionova NP, Morozov S.Y., Atabekov J.G. Effects of sequence elements in the potato virus X RNA non-translated -leader on its tramslation enhancing activity. J. Gen. Virol. 1993;74:2717–2724. doi: 10.1099/0022-1317-74-12-2717. [DOI] [PubMed] [Google Scholar]
REFERENCES MANDARIVIRUS
- Byadgi A.S., Ahlawat Y.S., Chakraborty N.K., Varma A., Srivastava M., Milne R.G. Characterization of a filamentous virus associated with citrus ringspot in India. In: Moreno P., editor. Proc. 12th Conf. International Organization of Citrus Virologists. Riverside; California: 1993. pp. 155–162. [Google Scholar]
- Rustici G., Accotto G.P., Noris E., Masenga V., Luisoni E., Milne R.G. Indian itrus ringspot virus: a proposed new species with some affinities to potex-, carla-, fovea- and allexivirusers. Arch. Virol. 2000;145:1895–1908. doi: 10.1007/s007050070064. [DOI] [PubMed] [Google Scholar]
- Rustici G., Milne R.G., Accotto G.P. Nucleotide sequence, genome organization and phylogenetic analysis of Indian citrus ringspot virus. Arch. Virol. 2002;147:2215–2224. doi: 10.1007/s00705-002-0875-6. [DOI] [PubMed] [Google Scholar]
REFERENCES ALLEXIVIRUS
- Arshava N.V., Konareva T.N., Ryabov E.V., Zavriev S.K. The 42K protein of shallot virus X is expressed in the infected Allium plants. Mol. Biol. (Russia) 1995;29:192–198. [PubMed] [Google Scholar]
- Barg E., Lesemann D.E., Vetten H.J., Green S.K. Identification, partial characterization and distribution of viruses infecting crops in south and south-east Asian countries. Acta Horti. 1994;358:251–258. [Google Scholar]
- Chen J., Chen J., Adams MJ. Molecular characterization of a complex mixture of viruses in garlic with mosaic symptoms in China. Arch. Virol. 2001;146:1841–1853. doi: 10.1007/s007050170037. [DOI] [PubMed] [Google Scholar]
- Kanyuka K.V., Vishnichenko V.K., Levay K.E., Kondrikov D.Yu, Ryabov E.V., Zavriev S.K. Nucleotide sequence of shallot virus X RNA reveals a 5’-proximal cistron closely related to those of potexviruses and a unique arrangement of the 3’-proximal cistrons. J. Gen. Virol. 1992;73:2553–2560. doi: 10.1099/0022-1317-73-10-2553. [DOI] [PubMed] [Google Scholar]
- Ryabov E.V., Generozov E.V., Vetten H.J., Zavriev S.K. Analysis of the 3’-region of the mite born filamentous virus genome testifies its relation to the shallot virus X group. Mol. Biol. (Russia) 1996;30:103–110. [Google Scholar]
- Song S.I., Song J.T., Kim C.H., Lee J.S., Choi Y.D. Molecular characterization of the garlic virus X genome. J. Gen. Virol. 1998;79:155–159. doi: 10.1099/0022-1317-79-1-155. [DOI] [PubMed] [Google Scholar]
- Sumi S., Tsuneyoshi T., Furuntani H. Novel rod-shaped viruses isolated from garlic, possessing a unique genome organization. J. Gen. Virol. 1993;74:1879–1885. doi: 10.1099/0022-1317-74-9-1879. [DOI] [PubMed] [Google Scholar]
- Vishnichenko V.K., Konareva T.N., Zavriev S.K. A new filamentous virus in shallot. Pl. Path. 1993;42:121–126. [Google Scholar]
- Vishnichenko V.K., Stelmashchuk V.Y., Zavriev S.K. The 42K protein of the Shallot virus X participates in Formation of virus particles. Mol. Biol. (Russia) 2002;36:1080–1084. [PubMed] [Google Scholar]
- Yamashita K., Sakai J., Hanada K. Characterization of a new virus from garlic (Allium sativum L.), garlic mite-borne virus. Ann. Phytopath. Soc. Japan. 1996;62:483–489. [Google Scholar]
REFERENCES CARLAVIRUS
- Belintani P., Gaspar J.O., Targon M., Machado M.A. Evidence supporting the recognition of Cole latent virus as a distinct carlavirus. J. Phytopath. 2002;150:330–333. [Google Scholar]
- Brattey C., Badge J.L., Burns R., Foster G.D., George E., Goodfellow H.A., Mulholland V., McDonald J.G., Jeffries C.J. Potato latent virus: a new species in the genus Carlavirus. Plant Pathol. 2002;51:495–505. [Google Scholar]
- Chen J., Chen J.P., Langeveld S.A., Derks A.F.L.M., Adams M.J. Molecular characterisation of carla- and potyviruses from Narcissus in China. J. Phytopath. 2003;151:26–29. [Google Scholar]
- Chen J., Chen J., Adams M.J. Molecular characterisation of a complex mixture of viruses in garlic with mosaic symptoms in China. Arch. Virol. 2001;146:1841–1853. doi: 10.1007/s007050170037. [DOI] [PubMed] [Google Scholar]
- Cohen J., Zeidan M., Feigelson L., Maslenin L., Rosner A., Gera A. Characterization of a distinct carlavirus isolated from Verbena. Arch. Virol. 2003;148:1007–1015. doi: 10.1007/s00705-002-0973-5. [DOI] [PubMed] [Google Scholar]
- Foster G.D. The structure and expression of the genome of carlaviruses. Res. Virol. 1992;143:103–112. doi: 10.1016/s0923-2516(06)80089-0. [DOI] [PubMed] [Google Scholar]
- Foster G.D. Carlavirus isolation and RNA extraction. Plant Virology Protocols: From virus isolation to transgenic resistance. Methods Mol. Biol. 1998;81:145–151. doi: 10.1385/0-89603-385-6:145. [DOI] [PubMed] [Google Scholar]
- Fuji S., Yamamoto H., Inoue M., Yamashita K., Fukui Y., Furuya H., Naito H. Complete nucleotide sequence of the genomic RNA of Aconitum latent virus (genus Carlavirus) isolated from Delphinium sp. Arch. Virol. 2002;147:865–870. doi: 10.1007/s007050200034. [DOI] [PubMed] [Google Scholar]
- Hataya T., Uchino K., Arimoto R., Suda N., Sano T., Shikata E., Uyeda I. Molecular characterization of Hop latent virus and phylogenetic relationships among viruses closely related to carlaviruses. Arch. Virol. 2000;145:2503–2524. doi: 10.1007/s007050070005. [DOI] [PubMed] [Google Scholar]
- Hataya T., Arimoto R., Suda N., Uyeda I. Molecular characterization of Hop mosaic virus: its serological and molecular relationships to Hop latent virus. Arch. Virol. 2001;146:1935–1948. doi: 10.1007/s007050170043. [DOI] [PubMed] [Google Scholar]
- Lawrence D.M., Rozanov M.N., Hillman B.I. Autocatalytic processing of the 223-kDa protein of blueberry scorch carlavirus by a papain-like proteinase. Virology. 1995;207:127–135. doi: 10.1006/viro.1995.1058. [DOI] [PubMed] [Google Scholar]
- Monger W., Seal S., Issacs A., Foster G.D. The molecular characterisation of the cassava brown streak virus coat protein. Plant Pathol. 2001;50:527–534. [Google Scholar]
- Nicolaisen M., Nielsen Lykke S. Analysis of the triple gene block and coat protein sequences of two strains of Kalanchoe latent carlavirus. Virus Genes. 2001;22:265–270. doi: 10.1023/a:1011101904002. [DOI] [PubMed] [Google Scholar]
- Song S.I., Choi J.N., Song J.T., Ahn J.H., Lee J.S., Kim M., Cheong J.J., Choi Y.D. Complete genome sequence of garlic latent virus, a member of the carlavirus family. Mol. Cells. 2002;14:205–213. [PubMed] [Google Scholar]
- Tsuneyoshi T., Matsumi T., Deng T., Sako I., Sumi S. Differentiation of Allium carlaviruses isolated from different parts of the world based on the viral coat protein sequence. Arch. Virol. 1998;143:1093–1107. doi: 10.1007/s007050050358. [DOI] [PubMed] [Google Scholar]
REFERENCES FOVEAVIRUS
- Giunchedi L., Poggi Pollini C. Cytopathological, negative staining and serological electron microscopy of a clostero-like virus associated with pear vein yellows disease. J. Phytopatol. 1992;34:329–335. [Google Scholar]
- Jelkmann W. Nucleotide sequence of apple stem pitting virus and of the coat protein gene of a similar virus from pear associated with vein yellows disease and their relationship with potex- and carlaviruses. J. Gen. Virol. 1994;75:1535–1542. doi: 10.1099/0022-1317-75-7-1535. [DOI] [PubMed] [Google Scholar]
- Jelkmann W., Keim-Konrad R. An immunocapture-polymerase chain reaction and plate trapped ELISA for the detection of apple stem pitting virus. J. Phytopathol. 1997;145:499–504. [Google Scholar]
- Jelkmann W., Kunze L., Vetten H.J., Lesemann D.E. cDNA cloning of dsRNA associated with apple stem pitting disease and evidence of the relationship of the virus-like agents associated with apple stem pitting and pear vein yellows. Acta Hort. 1992;309:55–62. [Google Scholar]
- Martelli G.P., Jelkmann W. Foveavirus, a new plant genus. Arch. Virol. 1998;143:1245–1249. doi: 10.1007/s007050050372. [DOI] [PubMed] [Google Scholar]
- Meng B., Pang S., Forsline P.L., McPherson J.R., Gonzalves D. Nucleotide sequence and genome structure of grapevine rupestris stem pitting associated virus 1 reveal similarities to apple stem pitting virus. J. Gen. Virol. 1998;79:2059–2069. doi: 10.1099/0022-1317-79-8-2059. [DOI] [PubMed] [Google Scholar]
- Nemchinov, L., Shamloul, A.M., Zemtchik E.Z., Verdervskaya T.D., Hadidi A. Apricot latent virus: a new species in the genus Foveavirus. Arch, Virol. 2000;145:1801–1813. doi: 10.1007/s007050070057. [DOI] [PubMed] [Google Scholar]
- Rott M.E., Jelkmann W. Complete nucleotide sequence of cherry necrotic rusty mottle virus. Arch. Virol. 2001;146:395–401. doi: 10.1007/s007050170184. [DOI] [PubMed] [Google Scholar]
- Zagula K., Aref N.M., Ramsdell D.C. Purification serology, and some properties of a mechanically transmissible virus associated with green ring mottle disease in peach and cherry. Phytopathology. 1989;79:451–456. [Google Scholar]
- Zhang Y.P., Kirkpatrick B.C., Smart C.D., Uyemoto J.K. cDNA cloning and molecular characterization of sour cherry green ring mottle virus. J. Gen. Virol. 1998;79:2275–2281. doi: 10.1099/0022-1317-79-9-2275. [DOI] [PubMed] [Google Scholar]
- Zhang Y.P., Uyemoto J.K., Golino D.A., Rowhani A. Nucleotide sequence and RT-PCR detection of a virus associated with grapevine rupestris stem-pitting disease. Phytopathology. 1998;88:1231–1237. doi: 10.1094/PHYTO.1998.88.11.1231. [DOI] [PubMed] [Google Scholar]
REFERENCES CAPILLOVIRUS
- Magome H., Yoshikawa N., Takahashi T., Ito T., Miyakawa T. Molecular variability of the genome of capilloviruses from apple, Japanese pear, European pear, and citrus trees. Phytopathology. 1997;87:389–396. doi: 10.1094/PHYTO.1997.87.4.389. [DOI] [PubMed] [Google Scholar]
- Magome H., Terauchi H., Yoshikawa N., Takahashi T. Analysis of double-stranded RNA in tissues infected with apple stem grooving capillovirus. Ann. Phytopath. Soc. Japan. 1997;63:450–454. [Google Scholar]
- Ohira K., Namba S., Rozanov M., Kusumi T., Tsuchizaki T. Complete sequence of and infectious full-length cDNA clone of citrus tatter leaf capillovirus: comparative sequence analysis of capillovirus genomes. J. Gen. Virol. 1995;76:2305–2309. doi: 10.1099/0022-1317-76-9-2305. [DOI] [PubMed] [Google Scholar]
- Ohki S., Yoshikawa N., Inouye N., Inouye T. Comparative electron microscopy of Chenopodium quinoa leaves infected with apple chlorotic leaf spot, apple stem grooving, or citrus tatter leaf virus. Ann. Phytopath. Soc. Japan. 1989;55:245–249. [Google Scholar]
- Terauchi H., Magome H., Yoshikawa N., Takahashi T., Inouye N. Construction of an infectious cDNA clone of the apple stem pitting capillovirus (isolate Li-23) genome containing a cauliflower mosaic virus 35S RNA promoter. Ann. Phytopath. Soc. Japan. 1997;63:432–436. [Google Scholar]
- Yoshikawa N., Takahashi T. Properties of RNAs and proteins of apple stem grooving and apple chlorotic leaf spot viruses. J. Gen. Virol. 1988;69:241–245. [Google Scholar]
- Yoshikawa N., Takahashi T. Evidence for genomic translation of apple stem grooving capillovirus RNA. J. Gen. Virol. 1992;73:1313–1315. doi: 10.1099/0022-1317-73-5-1313. [DOI] [PubMed] [Google Scholar]
REFERENCES VITIVIRUS
- Abou-Ghanem N., Saldarelli P., Minafra A., Castellano M.A., Martelli G.P. Properties of grapevine virus D, a novel putative trichovirus. J. Plant Pathol. 1997;79:1–11. [Google Scholar]
- Bonavia M., Digiaro M., Boscia D., Boari A., Bottalico G., Savino V., Martelli G.P. Studies on “corky rugose wood” of grapevine and on the diagnosis of grapevine virus B. Vitis. 1996;35:53–58. [Google Scholar]
- Boscia D., Digiaro M., Safi M., Garau R., Zhou Z., Minafra A., Abou Ghanem-Sabanadzovic N., Bottalico G., Potere O. Production of monoclonal antibodies to Grapevine virus D and contribution to the study of its aetiologial role in grapevine diseases. Vitis. 2001;40:69–74. [Google Scholar]
- Choueiri E., Abou-Ghanem N., Boscia D. Grapevine virus A and grapevine virus D are serologically distantly related. Vitis. 1997;36:39–41. [Google Scholar]
- Dell'Orco M., Saldarelli P., Minafra A., Boscia D., Gallitelli D. Epitope mapping of Grapevine virus A capsid protein. Arch. Virol. 2002;147:627–634. doi: 10.1007/s007050200012. [DOI] [PubMed] [Google Scholar]
- Galiakparov N., Tanne E., Sela I., Gafny R. Infectious RNA transcripts from a grapevine virus A cDNA clone. Virus Genes. 1999;19:235–242. doi: 10.1023/a:1008192831883. [DOI] [PubMed] [Google Scholar]
- Galiakparov N., Tanne E., Sela I., Gafny R. Functional analysis of the grapevine virus A genome. Virology. 2003;306:42–50. doi: 10.1016/s0042-6822(02)00019-3. [DOI] [PubMed] [Google Scholar]
- Galiakparov N., Goszczynski D., Che X., Batuman O., Bar-Joseph M., Mawassi M. Two classes of subgenomic RNA of grapevine virus A produced by internal controller elements. Virology. 2003;312:434–448. doi: 10.1016/s0042-6822(03)00239-3. [DOI] [PubMed] [Google Scholar]
- Garau R., Prota V.A., Boscia D., Fiori M., Prota U. Pseudococcus affinis Mask., new vector of grapevine trichovirus A and B. Vitis. 1995;34:67–68. [Google Scholar]
- Goszczynski D. G.G.F., Pietersen G. Western blot reveals that grapevine viruses A and B are serologically related. J. Phytopathol. 1996;144:581–583. [Google Scholar]
- La Notte P., Buzkan N., Choueiri E., Minafra A., Martelli G.P. Acquisition and transmission of grapevine virus A by the mealybug Pseudococcus longispinus. J. Plant Pathol. 1997;79:79–85. [Google Scholar]
- Martelli G.P., Minafra A., Saldarelli P. Vitivirus, a new genus of plant viruses. Arch. Virol. 1997;142:1929–1932. [PubMed] [Google Scholar]
- Rubinson E., Galiakparov N., Rasian S., Sela I., Tanne E., Gafny R. Serological detection of grapevine virus A using an antiserum to a non structiural protein, the putative movement protein. Phytopathology. 1997;87:1041–1045. doi: 10.1094/PHYTO.1997.87.10.1041. [DOI] [PubMed] [Google Scholar]
- Saldarelli P., Minafra A. Immunodetection of the 20kDa protein encoded by ORF 2 of Grapevine virus B. J. Plant Pathol. 2000;82:157–158. [Google Scholar]
- Saldarelli P., Dell'Orco M., Minafra A. Infectious cDNA clones of two grapevine viruses. Arch. Virol. 2000;145:397–405. doi: 10.1007/s007050050031. [DOI] [PubMed] [Google Scholar]
- Saldarelli P., Minafra A., Castellano M.A., Martelli G.P. Immunodetection and subcellular localization of proteins encoded by ORF 3 of grapevine viruses A and B. Arch. Virol. 2000;145:1535–1542. doi: 10.1007/s007050070074. [DOI] [PubMed] [Google Scholar]
REFERENCES TRICHOVIRUS
- German S., Candresse T., Le Gall O., Lanneau M., Dunez J. Analysis of dsRNAs of apple chlorotic leaf spot virus. J. Gen. Virol. 1992;73:767–773. doi: 10.1099/0022-1317-73-4-767. [DOI] [PubMed] [Google Scholar]
- German-Retana S., Bergey B., Delbos R.P., Candresse T., Dunez J. Complete nucleotide sequence of the genome of a severe cherry isolate of apple chlorotic leafspot trichovirus (ACLSV) Arch. Virol. 1997;142:833–841. doi: 10.1007/s007050050122. [DOI] [PubMed] [Google Scholar]
- James D., Upton C. Single primer pair designs that facilitate simultaneous detection and differentiation of peach mosaic virus and cherry mottle leaf virus. J. Virol. Meth. 1999;83:103–111. doi: 10.1016/s0166-0934(99)00112-3. [DOI] [PubMed] [Google Scholar]
- James D. Isolation and partial characterization of a filamentous virus associated with peach mosaic disease. Plant Dis. 1998;82:909–913. doi: 10.1094/PDIS.1998.82.8.909. [DOI] [PubMed] [Google Scholar]
- James D., Mukerji S. Mechanical transmission, identification and characterization of a virus associated with mottle leaf in cherry. Plant Dis. 1993;77:271–275. [Google Scholar]
- James D., Jelkmann W., Upton C. Nucleotide sequence and genome organisation of cherry mottle leaf virus and its relationship to members of the Trichovirus genus. Arch. Virol. 2000;145:995–1007. doi: 10.1007/s007050050690. [DOI] [PubMed] [Google Scholar]
- Kunugi Y., Asari S., Terai Y., Shinkau A. Studies on the grapevine berry inner necrosis virus disease. 2. Transmission of Grapevine berry inner mecrosis virus by the grape erineum mite Colomerus vitis in Yamanashi. Bull. Yamanashi Fruit Tree Exp. Sta. 2000;10:57–64. [Google Scholar]
- Ohki S.T., Yoshikawa N., Inouye N., Inouye T. Comparative electron microscopy of Chenopodium quinoa leaves infected with apple chlorotic leaf spot, apple stem grooving or citrus tatter leaf virus. Ann. Phytopath. Soc. Japan. 1989;55:245–249. [Google Scholar]
- Satoh H., Yoshikawa N., Takahashi T. Construction and biolistic inoculation of an infectious cDNA clone of apple chlorotic leafspot trichovirus. Ann. Phythopathol. Soc. Japan. 1999;65:301–304. [Google Scholar]
- Yoshikawa N., Iida H., Goto S., Magome H., Takahashi T., Terai Y. Grapevine berry inner necrosis, a new trichovirus: comparative studies with several trichoviruses. Arch. Virol. 1997;142:1351–1363. doi: 10.1007/s007050050165. [DOI] [PubMed] [Google Scholar]
REFERENCES FLEXIVIRIDAE
- Gambley C.F., Thomas J.E. Molecular characterisation of Banana mild mottle virus, a new filamentous virus in Musa spp. Arch. Virol. 2001;146:1369–1379. doi: 10.1007/s007050170097. [DOI] [PubMed] [Google Scholar]
- Hataya T., Uchino K., Arimoto R., Suda N., Sano T., Shikata E., Uyeda I. Molecular characterization of Hop latent virus and phylogenetic relationships among viruses closely related to carlaviruses. Arch. Virol. 2000;145:2503–2524. doi: 10.1007/s007050070005. [DOI] [PubMed] [Google Scholar]
- Melcher U. The ‘30K’ superfamily of viral movement proteins. J. Gen. Virol. 2000;81:257–266. doi: 10.1099/0022-1317-81-1-257. [DOI] [PubMed] [Google Scholar]
- Morozov S.Y., Solovyev A.G. Triple gene block: modular design of a multifunctional machine for plant virus movement. J. Gen. Virol. 2003;84:1351–1366. doi: 10.1099/vir.0.18922-0. [DOI] [PubMed] [Google Scholar]
- Thompson N., Randles J.W. The genome organization and taxonomy of Sugarcane striate mosaic associated virus. Arch. Virol. 2001;146:1441–1451. doi: 10.1007/s007050170070. [DOI] [PubMed] [Google Scholar]
- Vives M.C., Galipienso L., Navarro L., Moreno P., Guerri J. The nucleotide sequence and genomic organization of Citrus leaf blotch virus: Candidate type species for a new virus genus. Virology. 2001;287:225–233. doi: 10.1006/viro.2001.1040. [DOI] [PubMed] [Google Scholar]
- Vives M.C., Galipienso L., Navarro L., Moreno P., Guerri J. Characterization of two kinds of subgenomic RNAs produced by citrus leaf blotch virus. Virology. 2002;295:328–336. doi: 10.1006/viro.2001.1349. [DOI] [PubMed] [Google Scholar]
- Wong S.-M., Lee K.-C, Yu H.-H., Leong W.-F. Phylogenetic analysis of triple gene block viruses based on the TGB 1 homolog gene indicate a convergent evolution. Virus Genes, 1998;16:295–302. doi: 10.1023/a:1008034807216. [DOI] [PubMed] [Google Scholar]
CONTRIBUTED BY, P.J. Wright, P.A. Revill
FAMILY BARNAVIRIDAE
TAXONOMIC STRUCTURE OF THE FAMILY
| Family | Barnaviridae |
| Genus | Barnavirus |
Since only one genus is currently recognized, the family description corresponds to the genus description.
GENUS BARNAVIRUS
Type Species Mushroom bacilliform virus
VIRION PROPERTIES
MORPHOLOGY
Virions are bacilliform, non-enveloped and lack prominent surface projections. Typically, virions are 19 × 50 nm, but range between 18-20 nm in width and 48-53 nm in length (Fig. 1 ). Optical diffraction patterns of the virions resemble those of virions of Alfalfa mosaic virus, suggesting a morphological subunit diameter of about 10 nm and a T=1 icosahedral symmetry.
Figure 1.

Negative contrast electron micrograph of particles of an isolate of Mushroom bacilliform virus. The bar represents 100 nm.
PHYSICOCHEMICAL AND PHYSICAL PROPERTIES
Virion Mr is 7.1 × 106, buoyant density in Cs2SO4 is 1.32 g/cm3. Virions are stable between pH 6 and 8 and ionic strength of 0.01 to 0.1M phosphate, and are insensitive to chloroform.
NUCLEIC ACID
Virions contain a single linear molecule of a positive sense ssRNA, 4.0 kb in size. The complete 4,009 nt sequence is available. The RNA has a linked VPg and appears to lack a poly(A) tail. RNA constitutes about 20% of virion weight.
PROTEINS
Virions contain a single major CP of 21.9 kDa. There are probably 240 molecules in each capsid.
LIPIDS
None reported.
CARBOHYDRATES
None reported.
GENOME ORGANIZATION AND REPLICATION
The RNA genome (4,009 nt) contains four major and three minor ORFs and has 5′- and 3′-UTRs of 60 nt and 250 nt, respectively. ORFs 1 to 4 encode polypeptides of 20, 73, 47, and 22 kDa, respectively. The deduced aa sequence of ORF2 contains putative serine protease motifs related to chymotrypsin. ORF3 encodes a putative RdRp and ORF4 encodes the CP. ORFs 5 to 7 encode 8, 6.5, and 6 kDa polypeptides, respectively. The polypeptides potentially encoded by ORFs 1, 5, 6 and 7 show no homology to known polypeptides (Fig. 2 ).
Figure 2.

Genome organization of Mushroom bacilliform virus (MBV).
In a cell-free system, genomic length RNA directs the synthesis of a 21 kDA and a 77 kDa polypeptide and several minor polypeptides of 18-60 kDa. The full-length genomic RNA and a sgRNA (0.9 kb) encoding ORF4 (CP) are found in infected cells. Virions accumulate singly or as aggregates in the cytoplasm.
ANTIGENIC PROPERTIES
Virions are highly immunogenic.
BIOLOGICAL PROPERTIES
The virus infects the common cultivated button mushroom (Agaricus bisporus). Bacilliform particles, which are morphologically similar to MBV, have been observed in the field mushroom A. campestris. Transmission is horizontal via mycelium and possibly basidiospores. Distribution of MBV coincides with that of the commercial cultivation of A. bisporus; the virus has been reported to occur in most major mushroom-growing countries. MBV is capable of autonomous replication, but commonly occurs as a double infection with a dsRNA virus (LaFrance isometric virus, LFIV) in mushrooms afflicted with La France disease. MBV is not required in pathogenesis involving LFIV, but it remains to be determined if it is a second, minor causal agent of LaFrance disease, the etiologic agent of an unrecognized pathology or benign. MBV RNA and LFIV dsRNA do not share extensive sequence homology.
LIST OF SPECIES DEMARCATION CRITERIA IN THE GENUS
Not applicable.
LIST OF SPECIES IN THE GENUS
Species names are in green italic script; strain names and synonyms are in black roman script; tentative species names are in blue roman script. Sequence accession, and assigned abbreviations ( ) are also listed.
SPECIES IN THE GENUS
| Mushroom bacilliform virus | ||
| Mushroom bacilliform virus | U07551 | (MBV) |
TENTATIVE SPECIES IN THE GENUS
None reported.
PHYLOGENETIC RELATIONSHIPS WITHIN THE GENUS
None reported.
SIMILARITY WITH OTHER TAXA
The aa sequences of the putative chymotrypsin-related serine protease and RdRp suggest an evolutionary relationship with some ssRNA positive-sense plant viruses, particularly poleroviruses, sobemoviruses and enamoviruses (Fig. 3 ).
Figure 3.

An unrooted neighbour-joining dendrogram showing comparison of the aa sequence of the putative Mushroom bacilliform virus RdRp with those of selected sobemoviruses and poleroviruses, and an enamovirus. The luteovirus BYDV-PAV was included as an outgroup. Amino acid sequences were aligned with Clustal X and the tree was generated with Treeview. Bootstrap values (1000 replicates) are indicated. CYDV-RPV = Cereal yellow dwarf virus-RPV; SBMV = Southern bean mosaic virus; RYMV = Rice yellow mottle virus; PEMV = Pea enation mosaic virus; BWYV = Beet western yellows virus; PLRV = Potato leafroll virus. Accession numbers are AF218798 (BYDV-PAV), U07551 (MBV), AF055887 (SBMV), L20893 (RYMV), L04573 (PEMV) X13063 (BWYV), AF020090 (CYDV-RPV), AY138970 (PLRV).
DERIVATION OF NAMES
Barna: sigla from bacilliform-shaped RNA viruses.
REFERENCES
- Buck K.W. Fungal virology: an overview. In: Buck K.W., editor. Fungal Virology. CRC Press; Boca Raton, Florida: 1986. pp. 1–84. [Google Scholar]
- Ghabrial S.A. New developments in fungal virology. Adv. Virus Res. 1994;43:303–388. doi: 10.1016/S0065-3527(08)60052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin M.M., Schlagnhaufer B., Romaine C.P. Encapsidation of the La France disease specific double-stranded RNAs in 36 nm isometric virus-like particles. Phytopathology. 1992;82:285–290. [Google Scholar]
- Moyer J.W., Smith S.H. Partial purification and antiserum production to the 19 × 50 nm mushroom virus particle. Phytopathology. 1976;66:1260–1261. [Google Scholar]
- Moyer J.W., Smith S.H. Purification and serological detection of mushroom virus-like particles. Phytopathology. 1977;67:1207–1210. [Google Scholar]
- Revill P.A., Davidson A.D., Wright P.J. The nucleotide sequence and genome organization of mushroom bacilliform virus: a single-stranded RNA virus of Agaricus bisporus (Lange) Imbach. Virology. 1994;202:904–911. doi: 10.1006/viro.1994.1412. [DOI] [PubMed] [Google Scholar]
- Revill P.A., Davidson A.D., Wright P.J. Mushroom bacilliform virus RNA: the initiation of translation at the 5’-end of the genome and identification of the VPg. Virology. 1998;249:231–237. doi: 10.1006/viro.1998.9345. [DOI] [PubMed] [Google Scholar]
- Revill P.A., Davidson A.D., Wright P.J. Identification of a subgenomic mRNA encoding the capsid protein of mushroom bacilliform virus, a single-stranded RNA mycovirus. Virology. 1999;260:273–276. doi: 10.1006/viro.1999.9839. [DOI] [PubMed] [Google Scholar]
- Romaine C.P., Schlagnhaufer B. Hybridization analysis of the single-stranded RNA bacilliform virus associated with La France disease of Agaricus bisporus. Phytopathology. 1991;81:1336–1340. [Google Scholar]
- Romaine C.P., Schlagnhaufer B. PCR analysis of the viral complex associated with La France disease of Agaricus bisporus. Appl. Environ. Microbiol. 1995;61:2322–2325. doi: 10.1128/aem.61.6.2322-2325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaine C.P., Schlagnhaufer B. PCR analysis of three RNA genetic elements in Agaricus bisporus. In: Royse D.J., editor. Mushroom biology and mushroom products. The Pennsylvania State University Press, University Park; Pennsylvania: 1996. pp. 449–458. [Google Scholar]
- Tavantzis S.M., Romaine C.P., Smith S.H. Purification and partial characterization of a bacilliform virus from Agaricus bisporus: a single-stranded RNA mycovirus. Virology. 1980;105:94–102. doi: 10.1016/0042-6822(80)90159-2. [DOI] [PubMed] [Google Scholar]
- Tavantzis S.M., Romaine C.P., Smith S.H. Mechanism of genome expression in a single-stranded RNA virus from the cultivated mushroom Agaricus bisporus. Phytopathology. 1983;106:45–50. [Google Scholar]
- van Zaayen A.M. Mushroom viruses. In: Lemke P.A., editor. Viruses and Plasmids in Fungi. Marcel Dekker; New York: 1979. pp. 239–324. [Google Scholar]


