Abstract
Background
Several risk factors in the first 1,000 days are linked with increased obesity risk in later childhood. The role of potentially modifiable eating behaviors in this association is unclear.
Objective
This study examined if the association between cumulated risk factors in the first 1,000 days and adiposity at 6 years is moderated by eating behaviors.
Design
Participants were 302 children from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort. Risk factors included maternal pre-pregnancy and paternal overweight, excessive gestational weight gain, raised fasting glucose during pregnancy, short breastfeeding duration and early introduction of solid foods. Composite risk scores reflecting the prevalence and the importance of risk factors present were computed. Adiposity outcomes were child BMI and sum of skinfolds (SSF), and candidate eating behavior moderators were portion size, eating rate, and energy intake during lunch and in an eating in the absence of hunger task.
Results
Higher composite risk score predicted higher BMI z-scores (B= 0.08, 95%CI [0.04, 0.13]) and larger SSF (0.70 [0.23, 1.18]mm), and was associated with larger self-served food portions (5.03 [0.47, 9.60] kcal), faster eating rates (0.40 [0.21, 0.59] g/min) and larger lunch intakes (7.05 [3.37, 10.74] kcal). Importantly, the association between composite risk score and adiposity was moderated by eating behaviors. The composite risk score was unrelated to SSF in children who selected smaller food portions, ate slower, and consumed less energy, but was positively associated with SSF among children who selected larger food portions, ate faster, and consumed more energy (eating behavior*risk score interactions p<0.05).
Conclusions
The association between risk factors in the first 1,000 days and adiposity at 6 years varies by eating behaviors, highlighting modifiable behavioral targets for interventions.
Keywords: risk factors; 1,000 days; eating behavior; childhood obesity; adiposity; portion size; eating rate; energy intake; eating in the absence of hunger; adiposity outcomes
1.0. Introduction
The first 1,000 days, from conception through to two years, has been recognized as an important period in the development of childhood obesity (1). Risk factors in the first 1,000 days linked with obesity risk include maternal pre-pregnancy overweight, excessive gestational weight gain, smoking in pregnancy, gestational diabetes, early introduction of solid foods, infant anti-biotic exposure, short duration of breastfeeding, maternal depression, low vitamin D status in pregnancy and high paternal BMI (1). Other known risk factors include high infant birth weight and accelerated weight gain in the first 1,000 days (2), but these are to some extent already a consequence of the features of the perinatal environment (3, 4). While the majority of these studies established independent associations between individual risk factors and obesity risk, some children are exposed to multiple risk factors that vary in severity, highlighting the need to investigate their cumulative effects. Evidence suggests that when aggregated, a higher number of risk factors linearly increased future risk of overweight/obesity in American (5, 6) and British (2, 7) populations of 3 to 10 year olds, and recently also in the Singaporean sample of 4-year-old children from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort (8). Across the studies there are large variations in the strength of the reported associations, suggesting that these associations may be contingent on additional, potentially modifiable, factors that require further investigation.
Behavioral factors have been increasingly recognized as an important predictor of variation in weight status (9) that might moderate the associations between risk factors and adiposity. Findings from 2-6 year olds show that children, particularly those with overweight (10), consume more energy when served larger food portions (11) and larger food portions predict accelerated weight gain in the first two years of life (12). Already at 4.5 years of age faster eating rates facilitate larger energy intakes at a meal, are linked with higher adiposity (13) and larger increases in BMI over time (14). Similarly, higher intake of energy-dense snacks in an eating in the absence of hunger (EAH) paradigm (15) has been linked with higher overall energy intakes (16), higher prevalence of overweight (17) and accelerated weight gain over time (18, 19). As risk factors in the first 1,000 days predict future obesity risk, it is plausible that they are also linked with eating behaviors that facilitate greater energy intakes and increased adiposity. Importantly, it is unclear if the association between such risk factors and adiposity is moderated by eating behavior, or whether these are independent.
The aim of this study was to examine whether eating behaviors at 6 years moderate the associations between cumulative risk factors in the first 1,000 days and adiposity outcomes at age 6 years among children from the GUSTO cohort. A priori selected risk factors included maternal pre-pregnancy and paternal overweight/obesity, excessive gestational weight gain, raised fasting glucose during pregnancy, short breastfeeding duration and early introduction of solid foods. The primary outcomes were a composite risk score computed from risk factors and candidate eating behaviors, which included portion size selection, eating rate, energy intake at lunch and intake of snacks in the EAH task. We hypothesized that a higher composite risk score would be linked with larger food portions, faster eating rates, higher energy intakes at lunch and during the EAH task, and would continue to predict higher BMI and skinfold thickness at age 6 years. We further hypothesized that higher composite risk scores would show the strongest positive associations with adiposity among children who exhibit eating behaviors associated with higher energy intakes.
2.0. Methods
2.1. Participants
The participants were families from the multi-ethnic GUSTO cohort, who were recruited during the first trimester antenatal ultrasound scan between June 2009 and September 2010. Full recruitment and eligibility criteria are described in detail elsewhere (20). Data on risk factors in the first 1,000 days and eating behaviors at age 6 years were available for 436 children. To ensure reliability of the eating behavior measurements we excluded children who selected less than 10 kcal (n=1) as their ideal portion, consumed less than 50 kcal at lunch (n= 67), ate for less than 3 minutes (n= 37), and those with very high eating rates exceeding 3.8 SD (n= 2). In addition, children who still rated themselves as hungry after lunch were excluded specifically from the analysis of the EAH task data (n= 37), following the original protocol (15).
Data from 329 children were available for the analysis of lunchtime eating behavior, and from 292 children for the EAH assessment. Among children with the available eating behavior measures, BMI data at 6 years were available for 302 children and skinfolds thickness measures for 292 children, and these were the final analytical samples. Detailed selection criteria and loss to follow-up are described in the participant flowchart (Supplementary Figure 1). Informed written consent was obtained from all participants and the study was approved by the National Healthcare Group Domain Specific Review Board and SingHealth Centralized Institutional Review Board.
2.2. Risk factors for overweight
Risk factors considered in this study were selected a priori as important predictors of child overweight in the Singaporean context and strong predictors of overweight risk among GUSTO sample at age 4 years (8). The six risk factors were coded as binary variables: maternal pre-pregnancy overweight/ obesity (PPO), paternal overweight/obesity at 24 months, maternal excessive gestational weight gain (eGWG), high fasting plasma glucose (FPG) during pregnancy, short breastfeeding duration and early introduction of solid foods. For simplicity, ‘overweight/obesity’ are referred to as ‘overweight’ throughout. Maternal PPO (self-reported) and paternal overweight (measured by partner or self-reported) were defined using WHO guidelines as BMI ≥25 kg/m2. GWG was calculated as the difference between the final weight before delivery and pre-pregnancy weight, and guidelines from the Institute of Medicine (21) were used to define eGWG. An oral glucose tolerance test (2h, 75g) was conducted at 26-28 weeks of gestation and FPG ≥5.1 mmol l-1 was used as a cut-off for high FPG (22). A cut-off of <4 months was chosen to define short duration of any breastfeeding and introduction of solid foods before 4m was defined as early introduction of solid foods (self-reported). Data collection procedures for these variables have been reported in detail elsewhere (8).
A composite risk score was computed for each child, which accounted for the number and the severity of risk factors present, based on a risk-prediction procedure described and validated in an earlier study (2). The binary risk factors described above were used as predictors of overweight in the GUSTO cohort at age 4 years in multivariable regressions (8). Beta coefficient of each predictor was transformed to an integer score, with higher integer values representing greater risk of overweight, as recommended earlier (2). When a risk factor was present the child was allocated the associated integer score, and if the risk factor was absent they received 0. The integer scores were summed to calculate a child’s composite risk score, which could range from 0 to 15, with higher scores representing higher risk of overweight at age 4 years. A summary of beta coefficients and the associated integer scores is presented in Supplementary Table 1.
2.3. Computerized portion selection task
All eating behavior measures were pilot tested prior to data collection. At the 6-year-old time-point children attended an eating behavior assessment in the hospital, with which they were familiar from their previous GUSTO visits. Children were requested to fast for the minimum of 3 hours before the lunchtime meal. Research coordinators received standardized instructions and record sheets for all assessments and were requested to note down any unusual events or behaviors. Immediately before the meal children participated in an age-appropriate computerized portion selection task. High-resolution images of eight different foods varying in energy density and commonly available in Singapore were presented on a computer screen and included Rice Porridge (0.7 kcal/g), Mozzarella Pizza (2.71 kcal/g), Salad (1.32 kcal/g), Fried Rice (1.86 kcal/g), Macaroni Cheese (3.71 kcal/g), Steamed Buns (2.49 kcal/g), Garlic Bread (3.48 kcal/g) and Roti Prata (2.46 kcal/g). Children were asked to imagine that they were going to consume each of the presented foods for lunch, and were subsequently asked the question ‘How much <food> would you like to eat for lunch right now?’. The foods were presented in increasing portion sizes ranging from 20 kcal to 900 kcal, in 20 kcal steps (44 images per food type), one food item at a time. Children used computer keys to increase or decrease the portion size of the food presented to select their ideal portion. The energy content of the selected image was used as an ideal portion size of each of the different foods. The portion sizes were averaged across the 8 foods to compute a mean ideal portion size (kcal).
2.4. Lunchtime meal
A video-recorded lunchtime meal was served in a test room equipped with child appropriate furniture immediately after the computer task. Vegetarian fried rice (1.86 kcal/g), which is a popular local food, was provided ad libitum in a large bowl (800g). Children self-served the food portion they wanted to consume onto a smaller plate and were told they could eat as much as they wished, and could serve themselves multiple times. Children were allotted 20 minutes for lunch with a further 10-minute extension if that was not enough time (granted for 24 children). The two main study outcomes were the self-served portion size (kcal) and energy consumed at lunch (kcal) and these were calculated from the leftovers in the serving bowl and on the child’s plate, which were recorded on standardized sheets. Both before and after lunch children rated their hunger using a 5-point picture scale ranging from ‘Very hungry’ to ‘Very full’. After lunch children also rated dish liking on a 3-point scale (Yummy, OK, Yucky).
2.5. Eating rate
Oral processing behaviors were coded using behavioral annotation software (ELAN 4.9.1, Max Planck Institute for Psycholinguistics, The Netherlands). The bites, chews and swallows were coded throughout the lunchtime meal, and the detailed procedure is described elsewhere (13). The time between every bite and every swallow was cumulated to estimate the total time food spent in mouth. To calculate the child’s average eating rate (g/min) during lunch, the total number of grams consumed was divided by the total time food spent in mouth. Coding of the videos was completed by a single researcher. For quality control, in line with best practice for video coded data (23), 10% of the videos were selected at random and coded by another researcher to test inter-rater agreement as a measure of reliability. Intra-class correlation coefficient showed excellent agreement (ICC=0.995, CI95% [0.991, 0.997]).
2.6. Eating in the absence of hunger (EAH) task
Approximately 20 minutes after lunch children took part in the EAH task following the standard ‘free access’ protocol (15). Two types of sweet (18 units of M&M, 4.83 kcal/g; 10 units of Hello Panda, 5.43kcal/g) and two types of savory (10 units of Rollercoster, 5.55 kcal/g; 2 units of Want Want, 4.83 kcal/g) snacks were placed in small bowls (310 kcal in total). To start the task, children were given crayons and coloring paper and the researcher placed the bowls with snacks on the table within a child’s reach. The researcher told the child they had to briefly leave the room, and the child was welcome to have any of the foods served in the bowls. The researcher returned after 5 minutes and removed the bowls, which marked the end of the task. The food was weighed before and after the task to calculate the energy consumed in the absence of hunger (kcal) and recorded on a standardized sheet.
2.7. Anthropometry
A child’s weight and height, and skinfold thicknesses in the triceps, biceps, suprailiac and subscapular region were collected within two weeks of completing the eating behavior assessment at the 6-year-old time-point, following the standard guidelines and using the recommended anatomical landmarks (24, 25). Age-and sex-specific BMI z-scores were calculated based on WHO 2006 references (25). Skinfold thicknesses in the four regions were summed to derive a sum of skinfolds (SSF) as an adiposity index.
3.0. Statistical analysis
Composite risk scores, eating behavior and adiposity outcomes were treated as continuous variables in the main analyses. Composite risk scores were additionally categorized into quartiles of risk to provide a measure of the relative group differences in the study outcomes. Covariates in all models were identified a priori. Mother’s education and height, and child ethnicity, sex and birth order were used as covariates due to their associations with childhood adiposity in the GUSTO cohort. Child’s reported hunger before lunch and liking of fried rice predicted energy intake at lunch, so they were used as additional covariates in the analyses focused on the serving size, eating rate and energy intake at lunch and cumulatively. Finally, as snack intake during the EAH may depend on energy intake at lunch and on a child’s fullness, child hunger after lunch and energy consumed at lunch were additional covariates in the analyses that examined the EAH task. Chi-squared tests and t-tests were conducted to compare the final sample to those excluded.
Multivariable linear regressions were used to investigate whether composite risk scores predicted child eating behaviors and adiposity outcomes. ANCOVAs were used to describe eating behaviors and adiposity outcomes across the quartiles of risk score and post-hoc polynomial contrasts were conducted to test for linear trends across the quartiles of risk score. To examine whether the associations between composite risk scores and adiposity outcomes at 6 years are moderated by eating behaviors, we included interaction terms for each eating behavior and composite risk score (‘eating behavior*composite risk score’) in the linear regression models, adjusted for covariates and the main effects. All interactions were tested individually in separate models. To test for potential issues linked with multiple comparisons, an additional Benjamini-Hochberg False Discovery Rate (FDR) correction was conducted and reported. Composite risk scores and eating behavior outcomes were mean-centered for this analysis to aid interpretation. The interaction terms were subsequently examined in more detail and presented graphically using the simple slopes procedure, in which both variables (eating behavior and risk score) were standardized. Three slopes were computed for each eating behavior, which represented the standardized mean ± 1SD. As part of the sensitivity analysis, data were re-examined without covariates in the models and with fewer covariates after removing self-reported hunger and liking of the dish. Finally, analyses were repeated using composite risk scores based on the sum of the raw Beta values without conversion to integers to account for the fact that the allocated integers do not reflect the absolute differences in Beta values. The results of all sensitivity analyses supported the findings presented here (Supplementary Table 2 and Table 3). Analyses were performed in IBM SPSS version 23.0 and PROCESS macro (27) was used to examine the simple slopes. Alpha level of 0.05 was used for the inferential tests of significance.
4.0. Results
Sample characteristics
Children lost to follow-up did not differ from the final sample in in sex, ethnicity, parity, maternal education, maternal age, birth length, birth weight, risk score, BMI z-score or SSF (based on p-value thresholds of p> 0.05, unreported). Children who completed the eating behavior assessments but were excluded from the analyses did not differ from the final sample in ethnicity, maternal education, maternal age, BMI, SSF, birth order, birth length, birth weight or composite risk score (based on p-value thresholds p> 0.05, unreported), but were more likely to be of male sex (χ2= 5.25, p= 0.022).
Table 1 provides a summary of socio-demographic characteristics, gestation duration and birth size, presented for the total sample and across the quartiles of risk scores. Children in different quartiles did not differ in sex, gestational age, birth length or any maternal characteristics. Children of Chinese ethnicity were more likely to be in the lowest quartile of risk (Q1), while a higher proportion of children of Malay or Indian ethnicity were more likely be in the highest quartile (Q4). There was a linear increase in birth weight across the quartiles. Table 2 summarizes the prevalence of individual risk factors in the sample and mean values for the behavioral and adiposity outcomes. Individually, risk factors showed similar prevalence and each were present in 35-41% of the sample, with the exception of the early introduction of solid foods which was only observed in 2% of the sample. A fifth of the sample did not present any of the risk factors examined in this study. The prevalence of individual risk factors across the composite risk score quartiles is presented in Table 3. The majority of children in the lowest quartile of risk (Q1) had no risk factors for overweight and few experienced short breastfeeding duration, while children in the higher quartiles (Q3 and Q4) generally had multiple risk factors.
Table 1. Mother-child socio-demographic and early life characteristics, presented for the total sample and across the quartiles of risk for overweight.
| N (% of sample) or Mean ± SD | ||||||
|---|---|---|---|---|---|---|
| Total | Q1 | Q2 | Q3 | Q4 | P-value | |
| CHILD | ||||||
| Sex | 0.65 | |||||
| Boys | 167 (51%) | 53 (32%) | 47 (28%) | 27 (16%) | 40 (24%) | |
| Girls | 162 (49%) | 48 (30%) | 44 (27%) | 35 (22%) | 35 (22%) | |
| Ethnicity | <0.001 | |||||
| Chinese | 188 (57%) | 74 (40%) | 59 (31%) | 29 (15%) | 26 (14%) | |
| Indian | 56 (17%) | 11 (20%) | 10 (18%) | 16 (27%) | 19 (34%) | |
| Malay | 85 (26%) | 16 (19%) | 22 (26%) | 17 (20%) | 30 (35%) | |
| GA (wks.) | 38.4 ± 1.4 | 38.4 ± 1.6 | 38.3 ± 1.4 | 38.5 ± 1.4 | 38.6 ± 1.1 | 0.62 |
| BW (kg) | 3.10 ± 0.43 | 3.00 ± 0.43 | 3.10 ± 0.37 | 3.20 ± 0.46 | 3.22 ± 0.44 | 0.005 |
| BL (cm) | 48.7 ± 2.4 | 48.4 ± 2.8 | 48.6 ± 2.1 | 49.0 ± 2.4 | 48.7 ± 2.1 | 0.39 |
| MOTHER | ||||||
| Age | 37.8 ± 5.0 | 37.9 ± 4.8 | 37.6 ± 5.0 | 38.0 ± 4.6 | 37.9 ± 5.7 | 0.97 |
| Education | 0.17 | |||||
| Primary | 13 (4%) | 4 (31%) | 1 (8%) | 4 (31%) | 4 (31%) | |
| Secondary | 208 (63%) | 56 (27%) | 62 (30%) | 37 (18%) | 53 (26%) | |
| Tertiary | 108 (33%) | 41 (38%) | 28 (26%) | 21 (19%) | 18 (17%) | |
| Parity | 0.41 | |||||
| Primiparous | 145 (44%) | 48 (33%) | 44 (30%) | 23 (16%) | 30 (21%) | |
| Multiparous | 184 (56%) | 53 (29%) | 47 (26%) | 39 (21%) | 45 (25%) | |
Note BL- Birth length; BW- birth weight; GA (wks.)- Gestational age (weeks); Chi-squared tests were used to compare quartiles in categorical outcomes and one-way ANOVAs for continuous outcomes.
Table 2. Prevalence of the individual risk factors during the first 1,000 days in the sample, and the key Behavioral and adiposity outcomes at age 6 years.
| N (% of sample) or mean ± SD | |
|---|---|
| Risk factor prevalence | |
| Pre-pregnancy overweight | 116 (35%) |
| Paternal overweight | 129 (39%) |
| High fasting plasma glucose | 124 (38%) |
| Excessive GWG | 124 (38%) |
| Early introduction to solid foods | 7 (2%) |
| Short breastfeeding duration | 135 (41%) |
| Child outcomes at 6 years | |
| BMIz | -0.06 ± 1.36 |
| SSF (mm) | 30.7 ± 14.6 |
| Ideal portion (kcal) | 291.6 ± 179.9 |
| Portion self-served (kcal) | 248.3 ± 143.3 |
| Eating rate (g/min) | 12.9 ± 6.1 |
| Intake at lunch (kcal) | 190.5 ± 117.2 |
| Intake during EAH task (kcal) | 61.0 ± 49.0 |
| Cumulative intake (kcal) | 250.0 ± 131.5 |
Note BMIz- BMI z-score; EAH- Eating in the absence of hunger; FDR- False Discovery Rate correction; GWG-Gestational weight gain; SSF- Sum of Skinfolds
Table 3. Prevalence of different risk factors across the composite risk score quartiles together with the associated composite risk score and risk factor ranges.
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| n= 101 | n= 91 | n= 62 | n= 75 | |
| Risk score range | 0-1 | 2-4 | 5-7 | 8-12 |
| Risk factors (range)1 | 0-1 | 1-2 | 1-4 | 2-5 |
| No risk factors | 62.4% | 0% | 0% | 0% |
| Short breastfeeding | 26.7% | 25.9% | 13.3% | 34.1% |
| High fasting glucose | 11.8% | 5.9% | 29.4% | 52.9% |
| Paternal overweight | 0% | 47.3% | 14.0% | 38.8% |
| Excessive GWG | 0% | 22.6% | 33.1% | 44.4% |
| Early intro. solids | 0% | 14.3% | 42.9% | 42.9% |
| Pre-pregnancy overweight | 0% | 0% | 35.3% | 64.7% |
Note GWG-Gestational weight gain; intro.-introduction to;
Number of individual risk factors observed among individuals in each quartile
Do composite risk scores predict eating behaviors and adiposity outcomes at 6 years?
Regression coefficients predicting eating behaviors and adiposity outcomes from composite risk scores are summarized in Table 4. Higher risk scores predicted higher BMI and SSF at age 6 years. Children with higher risk scores self-served larger portions of food at lunch, consumed food at faster rates and consumed more energy at lunch. Although composite risk scores did not predict energy consumed during the EAH task, children with higher risk scores consumed more energy cumulatively during lunch and the EAH task. Risk scores did not predict the ideal portion size selected in the computer-based task.
Table 4.
Regression coefficients for composite risk scores for overweight during the first 1,000 days predicting child eating behaviors1 (n=329), BMI z-scores (n=302) and sum of skinfolds (n= 292) at age 6 years. Both uncorrected and corrected p-values2 are reported.
| B | β [95% CIs] | p-value | FDR p-value | |
|---|---|---|---|---|
| Ideal portion size (kcal) | -0.06 | -3.04 [-8.86, 2.78] | 0.31 | 0.35 |
| Self-served portion (kcal) | 0.12 | 5.03 [0.47, 9.60] | 0.031 | 0.041 |
| Eating rate (g/min) | 0.23 | 0.40 [0.21, 0.59] | <0.001 | <0.001 |
| Lunch intake (kcal) | 0.21 | 7.05 [3.37, 10.74] | 0.001 | 0.002 |
| EAH (kcal) | 0.09 | 0.12 [-1.64, 1.89] | 0.89 | 0.89 |
| Cumulative intake (kcal) | 0.19 | 7.00 [2.88, 11.13] | 0.001 | 0.002 |
| BMIz | 0.21 | 0.08 [0.04, 0.13] | <0.001 | <0.001 |
| SSF (mm) | 0.17 | 0.70 [0.23, 1.18] | 0.004 | 0.006 |
Note B= standardised coefficient; β= unstandardized coefficient; BMIz- BMI z-score; EAH- Eating in the absence of hunger; FDR- False Discovery Rate correction; SSF- Sum of Skinfolds
All outcomes were adjusted for mother’s education and height, and child sex, ethnicity, and birth order; Self-served portion size, eating rate, lunch intake and cumulative intake were additionally adjusted for reported hunger before lunch and liking of the dish; EAH was additionally adjusted for hunger after lunch and energy consumed at lunch;
False Discovery Rate computed using Benjamini-Hochberg procedure.
Relative differences in eating behaviors and adiposity outcomes between children with different risk scores were examined across the risk score quartiles and summarized in Table 5. The lowest adiposity and indices of eating behaviors were observed in the first quartile (Q1) and the highest in the fourth quartile (Q4). Analysis of the group differences generally supported the regression outcomes, and showed significant group differences in BMIz, SSF, eating rates and energy intake at lunch and cumulatively during lunch and the EAH task. Polynomial contrasts across the quartiles of risk suggested that linear trends were the best fit for the data. The largest absolute differences were consistently observed between Q1 and Q4. Despite the trend for linear increase, the outcomes of children from Q1 tended to resemble those of children from Q2, and similarly, those of children from Q3 were similar to those observed in Q4. Based on this, we proposed a subjective denomination for each risk score quartile that describes the level of overweight risk, ranging from Low to Very high risk.
Table 5.
Mean values (± SE), group differences1 (with uncorrected and corrected p-values2) and linear trends across the risk score quartiles (Q1-Q4) in eating behaviors and adiposity outcomes. Each quartile has been assigned a subjective risk of overweight category.
| Q1 n= 101 |
Q2 n=91 |
Q3 n=62 |
Q4 n=75 |
ANCOVA p-value |
ANCOVA FDR p-value |
Linear trend p-value |
|
|---|---|---|---|---|---|---|---|
| Risk | Low | Moderate | High | Very high | |||
| BMIz | -0.53 ± 0.14 | 0.07 ± 0.14 | 0.11 ± 0.18 | 0.30 ± 0.16 | 0.001 | 0.004 | <0.001 |
| SSF (mm) | 26.9 ± 1.5 | 31.4 ± 1.5 | 31.0 ± 1.9 | 35.1 ± 1.1 | 0.005 | 0.010 | 0.001 |
| Ideal potion size (kcal) | 312.2 ± 18.2 | 280.7 ±18.9 | 299.7 ± 23.1 | 270.5 ± 21.3 | 0.44 | 0.44 | — |
| Self-served portion (kcal) | 232.4 ± 14.3 | 233.2 ± 14.8 | 265.4 ± 18.1 | 274.0 ± 16.6 | 0.16 | 0.21 | — |
| Eating Rate (g/min) | 11.2 ± 0.6 | 12.4 ± 0.6 | 13.6 ± 0.7 | 15.0 ± 0.7 | <0.001 | 0.001 | <0.001 |
| Lunch intake (kcal) | 168.6 ± 11.5 | 174.2 ± 12.0 | 204.6 ± 14.6 | 228.1 ± 13.5 | 0.005 | 0.01 | <0.001 |
| EAH (kcal) | 59.9 ± 5.3 | 64.2 ± 5.4 | 68.5 ± 6.5 | 55.4 ± 6.4 | 0.37 | 0.42 | — |
| Cumulative intake (kcal) | 224.7 ± 13.0 | 236.3 ± 13.4 | 272.9 ± 16.4 | 282.0 ± 15.1 | 0.016 | 0.026 | 0.002 |
Note BMIz- BMI z-score; EAH- Eating in the absence of hunger; FDR- False Discovery Rate correction; SSF- Sum of Skinfolds
ANCOVAs with polynomial post-hoc contrasts for linear trends (presented for the significant effects only), adjusted for mother’s education and height and child sex, ethnicity, birth order; Self-served portion, eating rate, lunch intake and cumulative intake were additionally adjusted for hunger before lunch and liking of the dish; EAH was additionally adjusted for hunger after lunch and energy consumed at lunch;
False Discovery Rate computed using Benjamini-Hochberg procedure.
Is the association between risk factors from the first 1,000 days and adiposity moderated by eating behaviors?
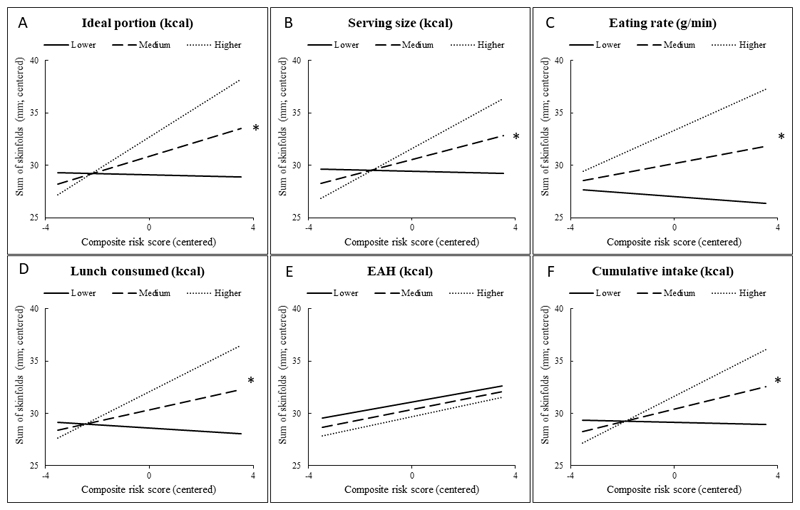
Regression coefficients for the main effects of the composite risk scores and eating behaviors on the adiposity outcomes, as well as the inferential tests for interactive effects between the two variables are reported in Table 6. The interactions between composite risk scores and eating behaviors on the adiposity outcomes have been presented graphically in Figure 1 (A-F) for BMI, and Figure 2 (A-F) for SSF. Positive associations between composite risk scores and adiposity were more pronounced among children who exhibited eating behaviors linked with higher energy intakes (i.e. selected larger food portions, ate at faster rates) and who ate more at lunch and cumulatively. Conversely, there was little relationship between risk scores and adiposity among children who ate less and who showed eating behaviors linked with lower energy intakes. This pattern was observed across all eating behaviors measured in this study, except for the energy consumed during the EAH task. Positive associations between composite risk scores and child BMI showed similar non-significant trends to those for adiposity. Ideal portion size selection was the only behavior significantly moderating the associations with BMI.
Table 6.
Standardized regression coefficients for the main effects of eating behavior and composite risk score, and the inferential test of interaction1between eating behavior and composite risk score (eating behavior*risk score) predicting child’s BMI z-score (n=302) and sum of skinfolds (n=292), with uncorrected and corrected p-values2. Composite risk score and eating behavior outcomes were centered prior to the analysis.
| BMIz | SSF | |||||
|---|---|---|---|---|---|---|
| B | p-value | FDR p-value | B | p-value | FDR p-value | |
| Ideal portion (kcal) | 0.12 | 0.036 | - | 0.12 | 0.04 | - |
| Risk score | 0.22 | <0.001 | - | 0.18 | 0.002 | - |
| Ideal portion*Risk score | — | 0.017 | 0.034 | — | <0.001 | 0.012 |
| Self-served portion (kcal) | 0.18 | 0.002 | - | 0.13 | 0.026 | - |
| Risk score | 0.19 | 0.001 | - | 0.16 | 0.007 | - |
| Self-served portion*Risk score | — | 0.088 | 0.13 | — | 0.003 | 0.014 |
| Eating rates (g/min) | 0.26 | <0.001 | - | 0.25 | <0.001 | - |
| Risk score | 0.15 | 0.011 | - | 0.11 | 0.052 | - |
| Eating rates*Risk score | — | 0.11 | 0.15 | — | 0.005 | 0.014 |
| Lunch intake (kcal) | 0.23 | <0.001 | - | 0.19 | 0.001 | - |
| Risk score | 0.17 | 0.005 | - | 0.13 | 0.023 | - |
| Lunch intake*Risk score | — | 0.15 | 0.18 | — | 0.004 | 0.014 |
| EAH (kcal) | -0.01 | 0.92 | - | -0.04 | 0.51 | - |
| Risk score | 0.18 | 0.004 | - | 0.13 | 0.036 | - |
| EAH*Risk score | — | 0.29 | 0.32 | — | 0.82 | 0.82 |
| Cumulative intake (kcal) | 0.19 | 0.001 | - | 0.15 | 0.012 | - |
| Risk score | 0.18 | 0.003 | - | 0.14 | 0.14 | - |
| Cumulative intake*Risk score | — | 0.08 | 0.13 | — | 0.006 | 0.014 |
Note BMIz- BMI z-score; EAH- Eating in the absence of hunger; FDR- False Discovery Rate correction; SSF- Sum of Skinfolds
All outcomes were adjusted for mother’s education and height, and child sex, ethnicity, birth order; Self-served portion, eating rate, lunch intake and cumulative intake were additionally adjusted for child hunger before lunch and liking of fried rice; EAH was additionally adjusted for child hunger after lunch and energy consumed at lunch.
False Discovery Rate computed using Benjamini-Hochberg procedure.
Figure 1.
(A-F). Simple slopes demonstrating the associations between risk scores and BMI z-scores at 6 years at different levels of eating behavior: mean ideal portion size (A), serving size at lunch (B), eating rate (C), intake of energy at lunch (D), eating in the absence of hunger (E) and cumulative intake of energy at lunch and during the EAH task (F). For simple slopes analysis both risk scores and eating behaviorus were centered; * FDR-corrected p< 0.05 for interaction term
Figure 2.
(A-F). Simple slopes demonstrating the associations between risk scores and sum of skinfolds (SSF) at 6 years at different levels of eating behavior: mean ideal portion size (A), serving size at lunch (B), eating rate (C), intake of energy at lunch (D), eating in the absence of hunger (E) and cumulative intake of energy at lunch and during the EAH task (F). For simple slopes analysis both risk scores and eating behaviorus were centered. * FDR-corrected p< 0.05 for interaction term
5.0. Discussion
Composite risk scores showed modest but significant associations with measures of adiposity and eating behaviors. Importantly, the association between composite risk scores and adiposity outcomes at age 6 years was not uniform across the sample, but was stronger among children who showed a greater tendency towards larger food portions, faster eating rates and who had higher energy intakes.
The results of the current study show that certain behaviors, such as selecting larger food portions, rapid eating and overall higher energy intakes, are associated with risk factors in the first 1,000 days of life, though the specific mechanisms of how this occurs of are less clear. Eating behaviors have not been previously examined in the context of risk factors from the first 1,000 days, and direct causal associations cannot be excluded. However, it is likely that these risk factors are at least in part markers of a less healthy home food environment and a reflection of parental dietary habits. Here, children in the highest quartile of risk had both parents with overweight, mothers with excessive gestational weight gain and high plasma glucose. This group of children may have been exposed to similar lifestyle and eating behaviors that contributed to their parents developing overweight and the related conditions, as parental healthy and unhealthy dietary behaviors are a good predictor of children’s diet quality (28). Parents decide what and how much food is accessible to children, they use techniques to encourage or discourage food intake, and are primary models of eating behaviors in the first years of life (29). Children exposed to larger food quantities tend to select larger food portions (30, 31), which can encourage faster eating rates and result in higher energy intakes (32). At the same time, the observed associations between risk factors and child eating behaviors could also potentially be explained by familial transmission of biological predispositions to overweight via programming of appetitive traits that promote higher energy intakes (33). Children with the highest overweight risk would be those with appetitive traits that facilitate increased energy intakes, who are exposed to environmental conditions linked with overweight, such as shorter breastfeeding duration or early introduction of solid foods, and who have opportunities to habitually express these appetitive traits and overeat.
Consistent with the studies in other populations (2, 5–7), and with the earlier findings from the GUSTO cohort (8), risk factors in the first 1,000 days predicted higher BMI and larger whole-body adiposity at 6 years. Our composite score approach to defining risk in the first 1,000 days captured variation in the strength of the associations between various risk factors and overweight, and complemented the findings of the previous studies, which were primarily focused on the risk factors prevalence. Furthermore, the current findings extend the previous research, and show that the association between early life risk factors and subsequent adiposity is not uniform across the sample, but varies depending on a child’s eating behavior. Children with lower composite risk scores tended to have lower adiposity, independently of their eating behavior. However, children with higher risk scores had the highest adiposity when they ate more and exhibited eating behaviors that facilitate higher energy intakes. These findings highlight that not every child with multiple risk factors for overweight will in fact develop overweight. We identified the most vulnerable group of children, who had multiple risk factors in the first 1,000 days and exhibited eating behaviors that promote the highest energy intakes. Future studies should aim to examine these associations in non-Asian populations.
Risk factors examined here are often referred to as “modifiable”, but the extent to which some of these risks truly can be modified may be limited. Many of risk factors stratify by sociocultural and economic status (34), making it challenging to address in intervention programs without considering the wider context. Risk factors often cluster together and many stem from maternal overweight (35, 36), suggesting that, theoretically, addressing this specific risk factor might have a cascade effect on alleviating the other risks. Yet, the lack of success in addressing the obesity crisis worldwide suggests that reducing maternal overweight might be a big, if not the biggest, challenge. Past intervention attempts at weight-loss in preparation for pregnancy and guidance prior to, and during pregnancy, showed little effectiveness and low compliance (37–39), particularly among women with pre-pregnancy overweight (40,41). Similarly, changing post-natal behaviors, such as breastfeeding duration or timing of solid food introduction may be problematic if there is financial pressure on women of lower SES to re-enter workforce sooner (42).
Current findings highlight the pivotal role of child eating behavior in modifying the associations between early life risk and future adiposity. Risk factors during the first 1,000 days could be used to identify children at risk for overweight, to target these families in intervention programs focused on promoting eating behaviors that facilitate healthy growth. Eating behavior interventions have previously shown some short-term effectiveness in pediatric populations. For example, attention modification training has been successfully used to reduce eating in the absence of hunger (43), and eating rates and energy intakes have been reduced by providing within-meal feedback from external prompters (44, 45). Though not tested to date, another strategy to reduce both eating rates and energy intake would be to modify food textures to produce ‘slower foods’ that require more chewing and encourage smaller bite sizes (46) or to promote intake of naturally ‘slow’ foods (47). Future studies should test the feasibility of these methods in modifying children’s eating behaviors long-term. Such interventions could be more challenging among the lower SES groups (48), but have the potential to be the most effective when introduced earlier rather than later (49).
This study had some limitations worth noting. Composite risk scores were created from a small number of a priori selected risk factors, though the list of all potential risk factors relevant to the local population is likely longer. For example, maternal smoking and alcohol intake have been previously identified as risk factors for childhood obesity (7), yet negligible rates of these behaviors in our sample (< 2.6%) precluded adding them as part of the analysis. Still, risk factors considered here have been previously identified as important predictors of overweight in the GUSTO cohort (8). Furthermore, it needs to be highlighted that some of the non-transient risk factors present in the first 1,000 days, most notably parental overweight, continue to impact adiposity outcomes and child’s eating behaviors beyond the first 1,000 days. Although we hypothesized that eating behaviors would moderate the associations between risk factors and adiposity, the alternative interpretation that risk factors moderate the association between eating behaviors and adiposity needs to be considered. This study was focused on candidate eating behaviors measured in laboratory. The meals pre-selected for this study may be different than the usual food choices, and it is not possible to ascertain the extent to which they reflect a child’s usual eating behaviors. Finally, the sample size of the current study was substantially smaller than the initial sample size of the GUSTO cohort, posing a risk for selection bias from those lost to follow up. Residual confounding due to other unmeasured factors, such as for example pre-pregnancy physical activity levels, as well as generalizability to other populations, need to be considered.
To conclude, the results of this study demonstrate that higher composite risk scores based on risk factors in the first 1,000 days were associated with larger portion sizes, faster eating rates and higher energy intakes at a meal among 6 year old Singaporean children. Supporting the previous findings, children with higher risk scores had higher BMI and greater adiposity, although these associations were not homogenous across the sample, but were more pronounced among children who selected larger food portions, ate faster and ate more. These findings highlight specific eating behaviors such as eating rates or meal sizes that could be targeted to lower energy intakes to mitigate the impact of the early life risk factors on future obesity risk.
Supplementary Material
Acknowledgements
The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira., Jonathan Tze Liang Choo, Jonathan Y. Bernard, Joshua J. Gooley, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Kuan Jin Lee, Leher Singh, Lieng Hsi Ling, Lin Lin Su, Ling-Wei Chen, Lourdes Mary Daniel, Marielle V. Fortier, Mark Hanson, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, See Ling Loy, S. Sendhil Velan, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shiao-Yng Chan, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Yin Bun Cheung and Yiong Huak Chan.
Sources of Support
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore - NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (as an NIHR Senior Investigator (NF-SI-0515-10042) and through the NIHR Southampton Biomedical Research Centre and by the European Union's Erasmus+ Capacity-Building ENeASEA Project and Seventh Framework Programme (FP7/2007-2013), project Early Nutrition under grant agreement n°289346.
Abbreviations
- BMIz
Body Mass Index z-score
- EAH
Eating in the absence of hunger
- eGWG
excessive gestational weight gain
- FPG
fasting plasma glucose
- PPO
pre-pregnancy overweight/obesity
- SSF
Sum of skinfolds
Footnotes
Clinical Trial Registry Number: NCT01174875; https://clinicaltrials.gov/
Data described in the manuscript, code book, and analytic code will be made available upon request pending application.
Conflicts of interest: KMG, CGF, YSL and YSC have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. They are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. CGF currently serves on the scientific advisory council for Kerry Taste and Nutrition. The other authors have no financial or personal conflict of interests.
Authors' contributions: This study was conceived and designed by AF, KMC and CGF. KMC and CGF designed the eating behavior measures. ATG, AF and KMC processed the eating behavior data. IMA, YSL, WWP and WLY processed the risk score data. Analyses were performed and interpreted by AF. AF, KMC and CGF prepared the draft manuscript. YSC, KHT, FY, LPS, MJM, BFPB, CS, YSL and KMG were responsible for conception and recruitment for the GUSTO cohort. All authors reviewed and approved the final draft.
References
- 1.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J of Prev Med. 2016;50(6):761–79. doi: 10.1016/j.amepre.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Weng SF, Redsell SA, Nathan D, Swift JA, Yang M, Glazebrook C. Estimating overweight risk in childhood from predictors during infancy. Pediatrics. 2013:2012–3858. doi: 10.1542/peds.2012-3858. peds. [DOI] [PubMed] [Google Scholar]
- 3.Xue F, Willett WC, Rosner BA, Forman MR, Michels KB. Parental characteristics as predictors of birthweight. Hum Reprod. 2007;23(1):168–77. doi: 10.1093/humrep/dem316. [DOI] [PubMed] [Google Scholar]
- 4.Haschke F, Grathwohl D, Haiden N. Metabolic programming: effects of early nutrition on growth, metabolism and body composition. Protein in Neonatal and Infant Nutrition: Recent Updates: Karger Publishers. 2016:87–95. doi: 10.1159/000442728. [DOI] [PubMed] [Google Scholar]
- 5.Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med. 2013;369(23):2173–5. doi: 10.1056/NEJMp1310577. [DOI] [PubMed] [Google Scholar]
- 6.Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity. 2008;16(7):1651–6. doi: 10.1038/oby.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, Cooper C, Inskip HM. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention–. Am J Clin Nutr. 2014;101(2):368–75. doi: 10.3945/ajcn.114.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aris IM, Bernard JY, Chen LW, Tint M, Pang WW, Soh S-E, Saw SM, Shek LP, Godfrey KM, Gluckman PD, et al. Modifiable risk factors in the first 1000 days for subsequent risk of childhood overweight in an Asian cohort: significance of parental overweight status. Int J Obes. 2018;41(1):44. doi: 10.1038/ijo.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kral TV, Moore RH, Chittams J, Jones E, O'Malley L, Fisher JO. Identifying behavioral phenotypes for childhood obesity. Appetite. 2018 doi: 10.1016/j.appet.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage JS, Haisfield L, Fisher JO, Marini M, Birch LL. Do children eat less at meals when allowed to serve themselves? American Journal of Clinical Nutrition. 2012;96(1):36–43. doi: 10.3945/ajcn.112.035261. [DOI] [PubMed] [Google Scholar]
- 11.Fisher JO, Liu Y, Birch LL, Rolls BJ. Effects of portion size and energy density on young children's intake at a meal. Am J Clin Nutr. 2007;86(1):174–9. doi: 10.1093/ajcn/86.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syrad H, Llewellyn CH, Johnson L, Boniface D, Jebb SA, Van Jaarsveld CH, Wardle J. Meal size is a critical driver of weight gain in early childhood. Scien Rep. 2016;6 doi: 10.1038/srep28368. 28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogel A, Goh AT, Fries LR, Sadananthan SA, Velan SS, Michael N, Tint MT, Fortier MV, Chan MJ, Toh JY, et al. Faster eating rates are associated with higher energy intakes during an ad libitum meal, higher BMI and greater adiposity among 4.5-year-old children: results from the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort. Brit J Nutr. 2017;117(7):1042–51. doi: 10.1017/s0007114517000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkowitz RI, Moore RH, Faith MS, Stallings VA, Kral TV, Stunkard AJ. Identification of an obese eating style in 4-year-old children born at high and low risk for obesity. Obesity. 2010;18(3):505–12. doi: 10.1038/oby.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr. 2002;76(1):226–31. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogel A, Mccrickerd K, Fries LR, Goh AT, Quah PL, Chan MJ, Toh JY, Chong Y-S, Tan KH, Yap F. Eating in the absence of hunger: Stability over time and associations with eating behaviours and body composition in children. Phys & Beh. 2018 doi: 10.1016/j.physbeh.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lansigan RK, Emond JA, Gilbert-Diamond D. Understanding eating in the absence of hunger among young children: A systematic review of existing studies. Appetite. 2015;85:36–47. doi: 10.1016/j.appet.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr. 2003;78(2):215–20. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollins BY, Loken E, Savage JS, Birch LL. Maternal controlling feeding practices and girls’ inhibitory control interact to predict changes in BMI and eating in the absence of hunger from 5 to 7 y. Am J Clin Nutr. 2014;99(2):249–57. doi: 10.3945/ajcn.113.063545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stunkel W, Holbrook JD, Kwek K, Chong YS, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidem. 2014;43(5):1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 21.IOM. The National Academies Collection: Reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US)National Academy of Sciences; 2009. [PubMed] [Google Scholar]
- 22.Metzger B, Gabbe S, Persson B, Buchanan T, Catalano P, Damm P. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diab Care. 2010;33(3):676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haidet KK, Tate J, Divirgilio-Thomas D, Kolanowski A, Happ MB. Methods to Improve Reliability of Video Recorded Behavioral Data. Res Nurs Health. 2009;32(4):465–74. doi: 10.1002/nur.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Onis M, Onyango AW, Van den Broeck J, Chumlea CW, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25(1_suppl1):S27–S36. doi: 10.1177/15648265040251S104. [DOI] [PubMed] [Google Scholar]
- 25.Phenxtoolkit. https://www.phenxtoolkit.org.
- 26.WHO. Internet: http://www.who.int/childgrowth/standards/Technical_report.pdf.
- 27.Hayes AF. A versatile computational tool for bserved variable mediation, moderation, and conditional process modeling [White paper] 2012 [Google Scholar]
- 28.Yee AZ, Lwin MO, Ho SS. The influence of parental practices on child promotive and preventive food consumption behaviors: a systematic review and meta-analysis. Nutr Phys Act. 2017;14(1):47. doi: 10.1186/s12966-017-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savage JS, Fisher JO, Birch LL. Parental influence on eating behavior: conception to adolescence. Journal Law, Med Ethics. 2007;35(1):22–34. doi: 10.1111/j.1748-720X.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher J, Birch L, Zhang J, Grusak M, Hughes S. External influences on children’s self-served portions at meals. Int J Obes. 2013;37(7):954–60. doi: 10.1038/ijo.2012.216. [DOI] [PubMed] [Google Scholar]
- 31.DiSantis KI, Birch LL, Davey A, Serrano EL, Zhang J, Bruton Y, Fisher JO. Plate Size and Children’s Appetite: Effects of Larger Dishware on Self-Served Portions and Intake. Pediatrics. 2013;131(5):e1451–e8. doi: 10.1542/peds.2012-2330. [DOI] [PubMed] [Google Scholar]
- 32.Fisher JO, Rolls BJ, Birch LL. Children’s bite size and intake of an entrée are greater with large portions than with age-appropriate or self-selected portions. Am J Clin Nutr. 2003;77(5):1164–70. doi: 10.1093/ajcn/77.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llewellyn C, Wardle J. Genetic Influences on Child Eating Behavior. Encyclopedia on Early Childhood Development. 2013 [Google Scholar]
- 34.Drewnowski A, Moudon AV, Jiao J, Aggarwal A, Charreire H, Chaix B. Food environment and socioeconomic status influence obesity rates in Seattle and in Paris. Int J Obes. 2014;38(2):306. doi: 10.1038/ijo.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes rev. 2008;9(2):140–50. doi: 10.1111/j.1467-789X.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 36.Abrevaya J, Tang H. Body mass index in families: spousal correlation, endogeneity, and intergenerational transmission. Emp Econ. 2011;41(3):841–64. [Google Scholar]
- 37.Inskip HM, Crozier SR, Godfrey KM, Borland SE, Cooper C, Robinson SM. Women’s compliance with nutrition and lifestyle recommendations before pregnancy: general population cohort study. Brit Med J. 2009;338:b481. doi: 10.1136/bmj.b481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke PE, Gross H. Women's Behavior, beliefs and information sources about physical exercise in pregnancy. Midwifery. 2004;20(2):133–41. doi: 10.1016/j.midw.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Gardner B, Wardle J, Poston L, Croker H. Changing diet and physical activity to reduce gestational weight gain: a meta-analysis. Obesity reviews. 2011;12(7):e602–e20. doi: 10.1111/j.1467-789X.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 40.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gyn. 2010;202(2):135–e1-. e8. doi: 10.1016/j.ajog.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, Hayes L, Khazaezadeh N, Nelson SM, Oteng-Ntim E. Effect of a Behavioral intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. The lancet Diabetes & endocrinology. 2015;3(10):767–77. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 42.Chua L, Win AM. Prevalence of breastfeeding in Singapore. Statistics Singapore Newsletter. 2013;10:15. [Google Scholar]
- 43.Boutelle KN, Kuckertz JM, Carlson J, Amir N. A pilot study evaluating a one-session attention modification training to decrease overeating in obese children. Appetite. 2014;76:180–5. doi: 10.1016/j.appet.2014.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ford AL, Bergh C, Södersten P, Sabin MA, Hollinghurst S, Hunt LP, Shield JPH. Treatment of childhood obesity by retraining eating Behavior: Randomised controlled trial. Brit Med J (Online) 2010;340(7740):250. doi: 10.1136/bmj.b5388. [DOI] [PubMed] [Google Scholar]
- 45.Faith MS, Diewald LK, Crabbe S, Burgess B, Berkowitz RI. Reduced Eating Pace (RePace) Behavioral Intervention for Children Prone to or with Obesity: Does the Turtle Win the Race? Obesity (Silver Spring, Md) 2018 doi: 10.1002/oby.22329. [DOI] [PubMed] [Google Scholar]
- 46.McCrickerd K, Lim CMH, Leong C, Chia EM, Forde CG. Texture-Based Differences in Eating Rate Reduce the Impact of Increased Energy Density and Large Portions on Meal Size in Adults. J Nutr. 2017;147(6):1208–17. doi: 10.3945/jn.116.244251. [DOI] [PubMed] [Google Scholar]
- 47.Forde C, Leong C, Chia-Ming E, McCrickerd K. Fast or slow-foods? Describing natural variations in oral processing characteristics across a wide range of Asian foods. Food & Function. 2017;8(2):595–606. doi: 10.1039/c6fo01286h. [DOI] [PubMed] [Google Scholar]
- 48.Barkin SL, Heerman WJ, Sommer EC, Martin NC, Buchowski MS, Schlundt D, Po’e EK, Burgess LE, Escarfuller J, Pratt C. Effect of a behavioral intervention for underserved preschool-age children on change in body mass index: a randomized clinical trial. JAMA. 2018;320(5):450–60. doi: 10.1001/jama.2018.9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psych. 2007;26(4):381–91. doi: 10.1002/oby.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.