Abstract
Neurons have multiple dendrites and single axon. This neuronal polarity is gradually established during early processes of neuronal differentiation: generation of multiple neurites (stages 1-2); differentiation (stage 3) and maturation (stages 4-5) of an axon and dendrites. In this study, we demonstrated that the neuron-specific n-glycosylated protein NELL2 is important for neuronal polarization and axon growth using cultured rat embryonic hippocampal neurons. Endogenous NELL2 expression was gradually increased in parallel with the progression of developmental stages of hippocampal neurons, and overexpression of NELL2 stimulated neuronal polarization and axon growth. In line with these results, knockdown of NELL2 expression resulted in deterioration of neuronal development, including inhibition of neuronal development progression, decreased axon growth and increased axon branching. Inhibitor against extracellular signal-regulated kinase (ERK) dramatically inhibited NELL2-induced progression of neuronal development and axon growth. These results suggest that NELL2 is an important regulator for the morphological development for neuronal polarization and axon growth.
Keywords: axon growth, extracellular signal-regulated kinase, hippocampal neurons, neural epidermal growth factor-like like protein 2, neuronal polarity
INTRODUCTION
The secreted N-glycosylated protein NELL2 is specifically expressed in neural tissues (Matsuhashi et al., 1995; Oyasu et al., 2000). NELL2 contains a signal peptide and multiple functional domains such as a thrombospondin-1-like domain, six epidermal growth factor-like domains and Ca2+ binding domains, suggesting its multifunctional roles in neural cells (Matsuhashi et al., 1995; Rao et al., 1995). One possible NELL2 function in neural cell differentiation has been proposed based on several findings: its spatial expression generally corresponds to regions of neurogenesis and it functions in neural cell differentiation during development (Jeong et al., 2008; Kim et al., 2002; Nelson et al., 2004). Our recent study showed that NELL2 regulates neuronal differentiation of mouse embryonic carcinoma P19 cells via control of N-cadherin-mediated cell aggregation (Kim et al., 2014). In addition to this function, here we report a novel role for NELL2 in neural cell differentiation, specifically in the generation of neuronal polarity and axon growth.
Neurons typically consist of a single axon and multiple dendrites. This structural polarity of neurons creates a cellular basis for neural networking and the unidirectional signal transduction from dendrites to axon. The polarity of neurons is established during early processes of neuronal differentiation, which have been well studied using cultured hippocampal neurons (Dotti et al., 1988). First, the cells extend multiple neurites (stages 1-2); next, one of the neurites is selected to differentiate into the axon (stage 3), whereas others give rise to dendrites, followed by maturation of the axon and dendrites at stages 4 and 5. This neuronal polarization is regulated by numerous factors and mechanisms (Shi et al., 2003; Yan et al., 2006). In this study, we show that NELL2 promoted axon development of neurons using hippocampal primary cultured neurons transfected with NELL2 expression vectors and small interfering RNA (siRNA) targeting NELL2.
MATERIALS AND METHODS
Hippocampal primary neuron culture
Whole brains were isolated from rats at embryonic day 18. Animal care and procedures were conducted according to the protocols and guidelines approved by the University of Ulsan Animal Care and Use Committee (UOUACUC-No. BJL-17-020). The hippocampi were digested with papain (Worthington Biochem, USA) at 37°C for 30 min, followed by trituration with pipettes in the plating media (minimal essential medium [MEM] containing 10% fetal bovine serum, 0.5% glucose, 1 mM sodium pyruvate, 25 μM glutamine, and 1× penicillin/streptomycin) (Shi et al., 2003). Dissociated neurons were plated onto 18 mm glass coverslips coated with poly-D-lysine (50 μg/ml; Sigma-Aldrich, USA) (5 slips/60 mm culture dish) at density of 1.2 × 105 cells/dish. After 4 h, the plating media were replaced with Neurobasal media (Thermo Scientific, USA) containing B27 supplement (Gibco, USA) and 0.5 mM Glutamax (Thermo Scientific). The media were replaced with Neurobasal media 4 h after plating. At 6 h after plating, neurons were transfected with the pDS-GFP-XB control vector (pDS-GFP-XB) or pDS-GFP-XB expression vector that encodes NELL2 (pDS-NELL2-GFP) using jetPRIME (Polyplus, USA) according to the manufacturer’s instructions. Cells were incubated with jetPRIME buffer (Polyplus) for 15 min at room temperature before the mixture was added to the cells. To confirm involvement of NELL2 in the development of neuronal polarity, hippocampal primary neurons were treated with human NELL2 protein (Cat. No. 13132-H08B; Sino Biological, USA). To determine intracellular signaling in NELL2-induced neuronal polarity, hippocampal neuronal cells were incubated with extracellular signal-regulated kinase (ERK) inhibitor, U0126 (10 μM; Calbiochem, USA); phosphoinositide 3-kinase (PI3K) inhibitor, LY294002 (5 μM; Sigma-Aldrich); or mitogen-activated protein kinase (MAPK) inhibitor, SB203580 (5 μM; Calbiochem) during the neuronal development period. All the inhibitors were directly added to Neurobasal media at indicated concentrations 8 h after plating. Neurons were then fixed and analyzed at 2 to 3 days after plating.
Immunocytochemistry
Hippocampal primary neurons were washed with phosphate buffered saline and fixed with pre-warmed 4% paraformaldehyde at room temperature for 15 min. Fixed neurons were then incubated for 30 min at room temperature in a blocking solution containing 1% bovine serum albumin and 0.1% Triton X-100 in 0.1 M PB. The neurons were then washed and incubated with rabbit anti-NELL2 antiserum (1:1,000) (Jeong et al., 2008) and chicken anti-Tau1 antibody (1:1,000, Cat. No. ab75714; Abcam, USA) overnight at 4°C. For immunofluorescence, sections were incubated with a combination of Alexa Fluor 594-labeled goat anti-rabbit (1:1,000, Cat. No. A11012; Invitrogen, USA) and Alexa Fluor 488-labeled goat anti-chicken secondary antibody (1:1,000, Cat. No. 4410; Cell Signaling Technology, USA) for 1 h at room temperature. Images were taken with a FV1200 confocal laser-scanning microscope (Olympus America, USA) and EVOS FL Auto2 Imaging System (Thermo Scientific).
Analysis of images for neurons and axon branch morphology
Axon was defined as a 45 μm long neurite, which is 10 μm longer than other neurites (Goslin and Banker, 1989), with positive Tau1 (axon marker) expression. The length of axon and dendrites were measured using ImageJ software (National Institutes of Health, USA). The primary branch extends from the soma. The secondary, tertiary and fourth branches are branches extending from the primary, secondary and tertiary branches, respectively. The length of the total branches is the sum of the lengths of primary, secondary, tertiary and fourth branches. The axon branches were traced using NeuronJ program (https://imagescience.org/meijering/software/neuronj/).
RNA interference
For siRNA-mediated knockdown of NELL2, receptors roundabout (Robo)2 and Robo3, neurons were transfected with 100 pmole of either the fluorescent dye (FAM)-labeled negative control siRNA (siCTL), FAM-labeled NELL2 siRNA (siNELL2) (Bioneer Primer Synthesis Service, Korea), Robo2 siRNA or Robo3 siRNA using Lipofectamine 3000 for 24 h. The siRNA sequences are as follows: NELL2 sense, 5′-GGA CGA AAG CCU UCC UCU U-3′; NELL2 antisense, 5′-AAG AGG AAG GCU UUC GUC C-3′; Robo2 sense, 5′-GUC AGU CAC UCC AAC CCA U-3′; Robo2 antisense, 5′-AUG GGU UGG AGU GAC UGA C-3′. Robo3 sense, 5′-GUC AGU CAC UCC AAC CCA U-3′; Robo3 antisense, 5′-AUG GGU UGG AGU GAC UGA C-3′; control siRNA sense, 5′-CCU ACG CCA AUU UCG-3′; control siRNA antisense, 5′-ACG AAA UUG GUG GCG UAG G-3′.
Real-time polymerase chain reaction (PCR)
Total RNA was extracted with TRIzol reagent (Invitrogen) according to the protocol supplied by the manufacturer and a previous report (Jin et al., 2016). RNA purity and concentration were determined by NanoDrop ND-1000 Spectrophotometer (Nano Drop Technologies, USA) using absorbance at 260 nm and 280 nm. cDNA was synthetized using the Qiagen Whole Transcriptome Kit (Qiagen, USA) and analyzed by real-time PCR using the following primer sets: NELL2 sense primer, 5′-GGT AGC CGT CTC TGC ACT CA-3′, NELL2 antisense primer, 5′-TGT AGA AAC GGA GCG TG-3′; Robo2 sense primer, 5′-GCT ACG ATC CAA GAC CAA GG -3′, Robo2 antisense primer, 5′-CAG ACT CTG TCA CAT CCA GC-3′; Robo3 sense primer, 5′-GGA GAT CCC CAG CCC AAT CT-3′, Robo3 antisense primer, 5′-TCG GCC CAC ACT GTT TTC T-3′; GAPDH sense primer, 5′-GGG GCC AAA AGG GTC ATC AT-3′, GAPDH antisense primer, 5′-GTG ATG GCA TGG ACT GTG GT-3′. Real-time PCR experiments were performed by BrightGreen 2× qPCR MasterMix-ROX (ABM, Canada) using the StepOnePlus Real-Time PCR System (Applied Biosystems; Thermo Scientific) for ~40 cycles. Data were normalized for gene expression by using GAPDH as an internal control. The 2–ΔΔCT method was used to analyze the relative quantification of gene expression.
Statistics
The results were analyzed with a one way ANOVA followed by the Student Neuman–Keuls multiple comparison test for unequal replications. Student’s t-test was used to compare two groups. Two-way ANOVA was performed to detect significant interaction between groups. The data were analyzed using GraphPad Prism 5 software (GraphPad Software, USA).
RESULTS
Effect of NELL2 on the differentiation process of cultured hippocampal neurons
To determine the effect of NELL2 on neuronal differentiation, we used primary cultured cells from rat fetus at embryonic day 18. The primary culture of dissociated hippocampal neurons is a well-established system to study the development of neuronal polarity (Craig and Banker, 1994). The developing hippocampal neurons in primary cultures were subdivided into five stages as previously reported (Dotti et al., 1988). After embryonic hippocampal cells were plated onto culture plates, neurons were initially surrounded by lamellipodia (stage 1, 0-12 h). Neurons gave rise to multiple immature neurites (stage 2, 12-24 h). Next, one of the neurites rapidly extended and became the axon (stage 3, 24-48 h). After 2 to 3 days in culture, the remaining neurites became dendrites (stage 4, more than 3 days) and then neurons formed synapses (stage 5, more than one week).
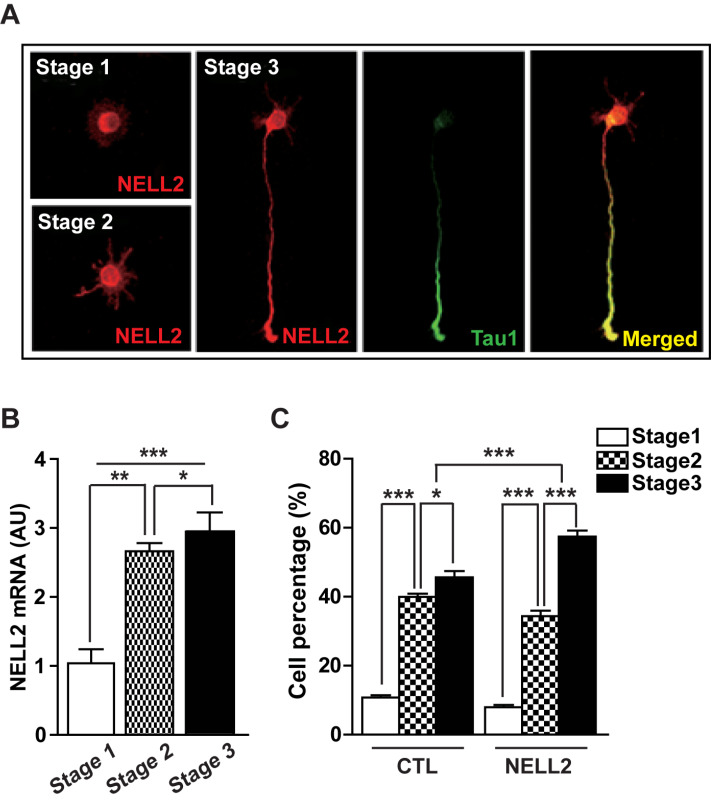
We examined NELL2 expression in the cultured rat hippocampal neurons using NELL2 antibody. NELL2 was expressed and distributed in all parts of the neurons at stages 1 to 3 (Fig. 1A). NELL2 mRNA level was increased in parallel with progression from stages 1 to 3 (Fig. 1B), suggesting that NELL2 is involved in neural differentiation. Thus, we further investigated whether NELL2 promotes the progression of neural differentiation stages. Hippocampal primary cells were transfected with a NELL2 expression vector (pDS-NELL2-GFP, NELL2) or control vector (pDS-GFP-XB, CTL) 6 h after plating. Thereafter, hippocampal primary cells were cultured for 2 days before analysis of differentiation stage. Approximately 94% of cells showed GFP expression (data not shown). Cells transfected with NELL2 expression vectors revealed an increased progression in the stages of neuronal differentiation (Fig. 1C). These results together suggest that NELL2 is involved in the differentiation of hippocampal neurons.
Fig. 1. NELL2 function in the differentiation of cultured hippocampal neurons.
(A) Expression of NELL2 in cultured hippocampal neurons during stages 1 to 3. Hippocampal neurons were stained with anti-NELL2 and anti-Tau1 antibodies. Scale bar = 20 µm. (B) Change of NELL2 mRNA level in cultured hippocampal neurons during developmental stages 1 to 3. (C) Effect of NELL2 on the development of cultured hippocampal neurons. Neurons were transfected with control (pDS-GFP-XB, CTL) or NELL2-overexpresion vector (pDS-NELL2-GFP, NELL2). The progression of developmental stage was observed in the transfected cells that were identified with fluorescence. All data are presented as mean ± SEM. n = 60 (CTL) and 62 (NELL2) cells. *P < 0.05; **P < 0.01; ***P < 0.001. AU, arbitrary units. P values for unpaired comparisons were analyzed by two-tailed Student’s t-test. Two-way repeated-measures ANOVA was performed to detect significant interaction between groups.
Effect of NELL2 on neuronal polarization
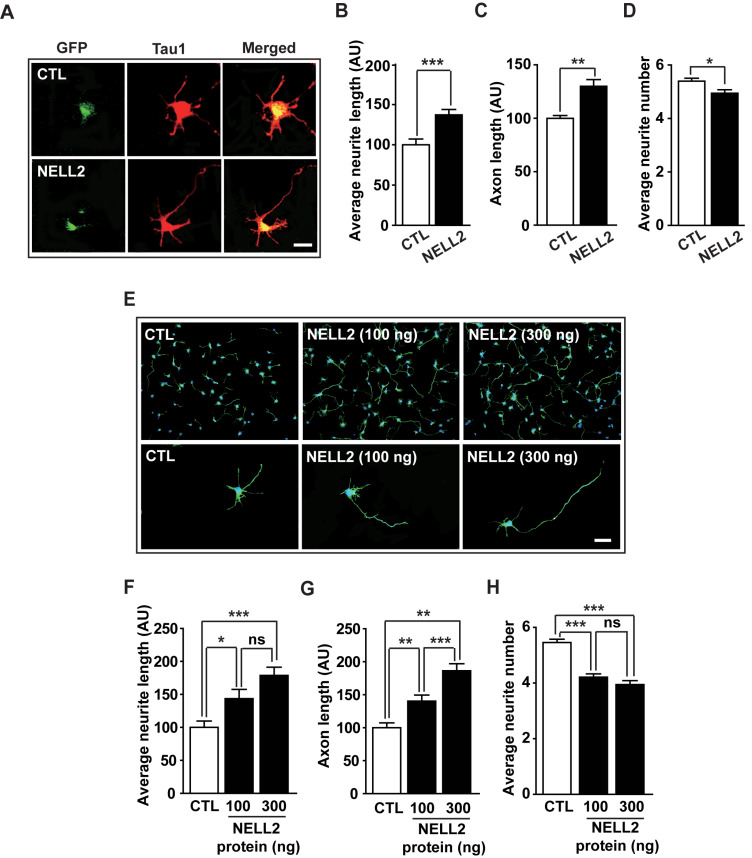
As NELL2 promoted the progression of developmental stages of cultured hippocampal neurons, we next investigated whether NELL2 affects neurite growth and neuronal polarization. Neurons transfected with NELL2 expression vectors were cultured for 2 days and their morphology was analyzed (Fig. 2A). NELL2 overexpression resulted in increased average neurite length (Fig. 2B) and axon length of neurons (Fig. 2C); however, it decreased average neurite numbers per neuron (Fig. 2D). To confirm NELL2 function in neurite outgrowth and neuronal polarization of hippocampal neurons, we cultured hippocampal neurons treated with human NELL2 protein for 2 days (Fig. 2E). NELL2 protein significantly increased average neurite length (Fig. 2F) and axon length (Fig. 2G), whereas average neurite number was decreased by the NELL2 protein (Fig. 2H). These results suggest that NELL2 promotes neuronal polarity and axon growth during the development of hippocampal neurons.
Fig. 2. Effect of NELL2 on neuronal polarization.
(A) Representative microphotographs of immunocytochemistry. Hippocampal neurons were cultured and transfected with pDS-GFP-XB (CTL) or pDS-NELL2-GFP (NELL2) vectors. Neurons were fixed at 2 days after transfection and stained with anti-Tau1 antibody. (B-D) Hippocampal primary cells transfected with CTL or NELL2 vectors were analyzed to determine the average neurite length (B), axon length (C), and average neurite number (D). All data are presented as mean ± SEM. n = 41 (CTL) and 48 (NELL2) cells. (E-H) Hippocampal primary cells were treated with human NELL2 proteins and their neuronal polarization was analyzed after staining with anti-Tau1 antibody: representative microphotographs (E), neurite length (F), axon length (G), and average neurite number number (H). Scale bars = 20 µm (A and E). n = 35 (CTL), 48 (NELL2, 100 ng), and 40 (NELL2, 300 ng) cells. *P < 0.05; **P < 0.01; ***P < 0.001; ns, no significance. AU, arbitrary units. P values for unpaired comparisons were analyzed by two-tailed Student’s t-test.
Effect of NELL2 siRNA on the morphological development of axon
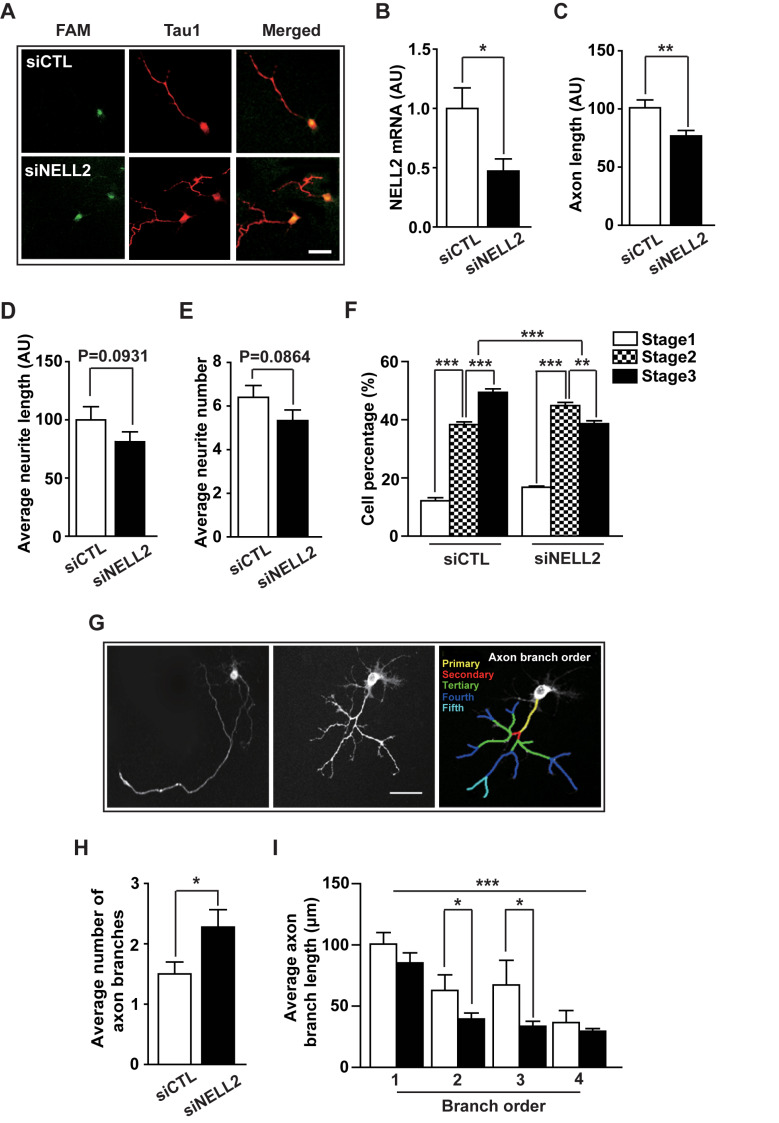
To further confirm the effect of NELL2 on neuronal polarization, cultured hippocampal neurons were transfected with fluorescent FAM-labelled negative control siRNA (siCTL) or FAM-labelled NELL2 siRNA (siNELL2) and cultured for 2 days, and the morphology of FAM-labelled cells was analyzed (Fig. 3A). The NELL2 siRNA resulted in about 50% decrease in NELL2 mRNA level (Fig. 3B), and then caused a significant decrease in the axon length (Fig. 3C), but did not significantly affect average neurite length (Fig. 3D) or average neurite number (Fig. 3E), which further suggests the importance of NELL2 in axon development. Moreover, cells transfected with NELL2 siRNA showed a delayed progression of neuronal differentiation (Fig. 3F), suggesting that NELL2 is important in the normal progression of neuronal differentiation. Our results above indicate that NELL2 is important in the generation of neural polarity and normal axon growth. Surprisingly, siNELL2 increased the formation of axon branches (collaterals); many neurons transfected with siNELL2 showed multiple secondary, tertiary or fourth branches and some showed more than five branches (Fig. 3G). The average number of axon branches was significantly increased by siNELL2 transfection (Fig. 3H). We then measured the average lengths of primary, secondary, tertiary and fourth axon branches. Neurons transfected with siNELL2 showed a significantly shorter average length of secondary and tertiary axon branches compared with the control group (Fig. 3I). Because axons with abnormal morphology, such as ramified and curled axon branches, cannot fulfill normal functions for transmission of information to different neurons (Díaz-Hernandez et al., 2006), these results indicate that NELL2 is a critical regulator for the normal development of axons and thus for the normal function of neurons.
Fig. 3. Effect of NELL2 siRNA on the morphological development of axon.
(A-F) Primary cultured neurons were transfected with FAM-labelled negative control siRNA (siCTL) or FAM-labelled NELL2 siRNA (siNELL2). The cells were fixed at 2 days after transfection and stained with anti-Tau1 antibody, and their axon development was analyzed: representative microphotographs (A), NELL2 mRNA level determined by real-time PCR analysis (B), axon length (C), average neurite length (D), average neurite number (E), and progression of development stages (F). n = 46 (siCTL) and 48 (siNELL2) cells. (G) Representative microphotographs showing neurons transfected with FAM-labelled negative control siRNA (siCTL) or FAM-labelled NELL2 siRNA (siNELL2). Scale bar = 20 µm (A and G). (H and I) The cultured neurons transfected with siRNA were analyzed to determine the average number of axon branches (H) and length of average axon branches at different branch order (I). All data are presented as mean ± SEM. n = 75 (siCTL) and 82 (siNELL2) cells. *P < 0.05; **P < 0.01; ***P < 0.001. AU, arbitrary units. P values for unpaired comparisons were analyzed by two-tailed Student’s t-test. Two-way repeated-measures ANOVA was performed to detect significant interaction between groups.
Effect of NELL2 signaling on the morphological development of axon
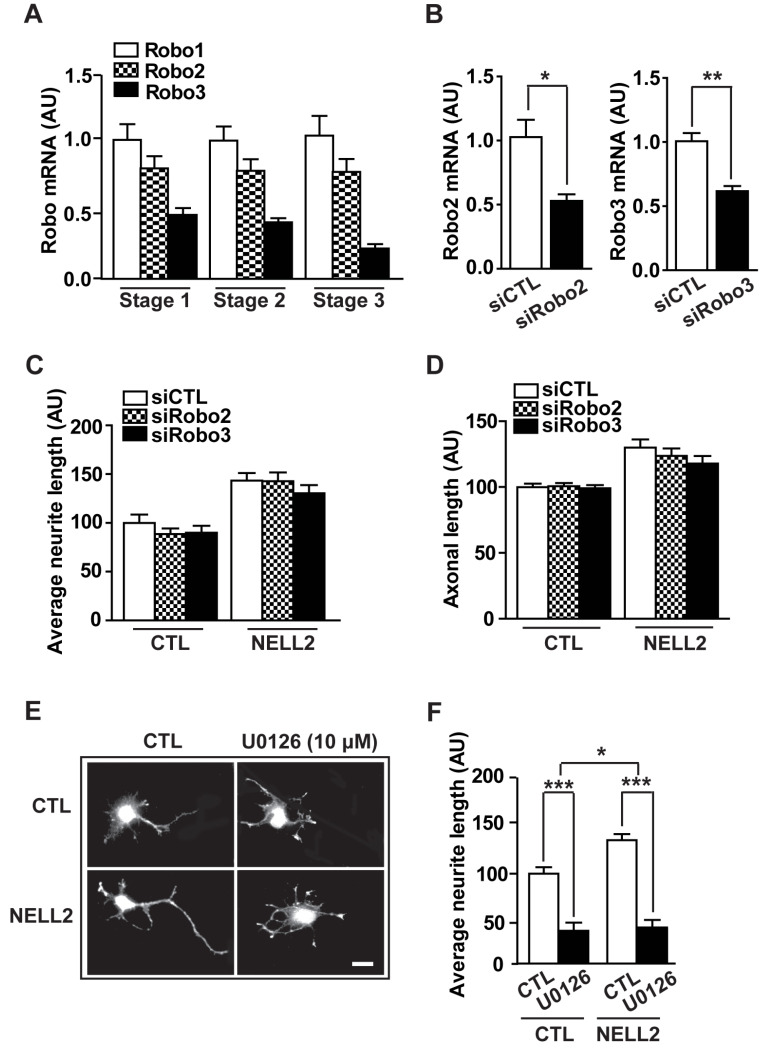
Recent studies have identified NELL2 as a novel ligand for the receptors roundabout (Robo)2 and 3 that are receptors for chemorepellent Slit proteins during neural development (Jaworski et al., 2015; Yamamoto et al., 2019). Thus, we next investigated whether NELL2 action on axon development is carried out via Robo2 and Robo3. First, we analyzed mRNA expression of Robo receptors during developmental stages of hippocampal primary neurons. The mRNA levels of Robo1 to Robo3 were relatively constant throughout the developmental stages (Fig. 4A). In addition, hippocampal primary cells were transfected with siRNA to knockdown Robo2 and Robo3 expression (Fig. 4B) and their neurite and axon length were determined (Figs. 4C and 4D). Neither average neurite length nor average axon length was affected by knocking down Robo2 or Robo3 expression, suggesting that NELL2 acts on axon development through another signaling system.
Fig. 4. Signaling pathway of NELL2 action for axon development.
(A) Expression of Robo1, Robo2, and Robo3 mRNA in the different developmental stage of hippocampal primary cells. (B) Robo2 and Robo3 mRNA levels were determined in the hippocampal primary cells transfected with negative control siRNA (siCTL), siRNA Robo2 (siRobo2), or siRNA Robo3 (siRobo3). (C and D) Quantitative analysis for the average neurite length (C) and axon length (D) by treatment with siRobo2 and siRobo3. n = 53 (siCTL), 56 (siRobo2), and 55 (siRobo3) cells. (E and F) Effect of ERK inhibitor (U0126, 10 µM) on NELL2 action on neurites and axon development: representative microphotographs showing primary cultured neurons (E) transfected with pDS-GFP-XB (CTL) or pDS-NELL2-GFP (NELL2) and calculated average neurite length (F). n = 46 (CTL), 28 (CTL-U0126), 43 (NELL2-CTL), and 22 (NELL2-U0126) cells. Scale bar = 20 µm. All data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. AU, arbitrary units. P values for unpaired comparisons were analyzed by two-tailed Student’s t-test. Two-way repeated-measures ANOVA was performed to detect significant interaction between groups.
Various signaling pathways are involved in neuronal polarity and axon growth, including PI3K, MAPK and ERK pathways (Barnes and Polleux, 2009; Kim and Baek, 2019; Nix et al., 2011; Perron and Bixby, 1999; Shi et al., 2003). To identify whether NELL2 action on axon development is mediated through one of these signaling pathways, hippocampal neurons transfected with NELL2 expression vectors were treated with PI3K inhibitor (LY294002), MAPK inhibitor (SB203580) or ERK inhibitor (U0126). ERK inhibitor markedly decreased the average neurite length compared with control cells (Figs. 4E and 4F) as previously reported (Ma et al., 2017). In NELL2-overexpressing cells, the ERK inhibitor significantly decreased neurite length and, moreover, inhibited axon formation and development. No axon was found in these cells. In contrast, inhibition of PI3K and MAPK signaling did not induce any change in NELL2-transfected neurons (data not shown). Together, these results indicate that NELL2 regulates neuronal polarization not via Robo2 or Robo3, but through the ERK signaling pathway.
DISCUSSION
NELL2 has been reported to be involved in neural development. However, the detailed functions of NELL2 in neural development have been unknown. In this study, we found a novel NELL2 function in neuronal polarity and axon development of hippocampal neurons. Previous studies reported NELL2 as a novel neurotrophin (Choi et al., 2010; Kim et al., 2014; Nelson et al., 2004). Neurotrophins are growth factors that induce the differentiation, survival, and development of neurons (Huang and Reichardt, 2001; Park and Poo, 2013). Several neurotrophins are also involved in the development of axons; axon formation and growth are positively regulated by neurotrophins such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and NT4 (Chao, 2003; Park and Poo, 2013). During axon-dendrite polarization of cultured hippocampal neurons, BDNF promoted differentiation of unpolarized neurites into an axon and dendrites via activation of its receptor (TrkB) signaling (Cheng et al., 2011). BDNF secreted by neurons contributed to axon initiation via autocrine and paracrine actions, whereas short hairpin RNA-mediated downregulation of BDNF synthesis resulted in markedly impaired axon initiation. Similarly, our results suggest that NELL2 released from transfected cells acts on neural polarization of the same cells and neighboring cells via autocrine and/or paracrine routes.
In this study, knockdown of NELL2 expression resulted in abnormal axon morphology that was characterized with changed branch formation. The extension of axon branching allows a neuron to connect to broad regions and combine information from diverse regions in the nervous system (Acebes and Ferrús, 2000; Kalil and Dent, 2014). According to previous reports, NELL2 knockout (KO) mice showed enhanced long-term potentiation in the hippocampal dentate gyrus (Matsuyama et al., 2004), but poor learning in the Morris water maze test (Matsuyama et al., 2005). How these results were caused by NELL2 KO had been unclear. Our present results suggest an importance of NELL2 in normal axon development of hippocampal neurons that are pivotal for the learning and memory system.
In mammals, four members of the Robo family (Robo1 to Robo4) have been identified as receptors for Slit ligands (Slit1 to Slit3), the axon guidance cues (Seiradake et al., 2016; Ypsilanti et al., 2010). Recent studies have identified NELL2 as a novel ligand for Robo2 (Yamamoto et al., 2019) and Robo3 (Jaworski et al., 2015). However, our siRNA-mediated knockdown of Robo2 and Robo3 expressions did not affect NELL2-induced axon development in the hippocampal neurons. These results indicated that another receptor-mediated intracellular signaling system may be responsible for NELL2 action in axon development.
Our previous studies showed that siRNA-mediated knockdown of NELL2 resulted in a decrease of ERK phosphorylation and increase of Bax expression in neuroprogenitor cells (Choi et al., 2010) and revealed that NELL2 stimulated intracellular ERK signaling for the regulation of N-cadherin in neuronal differentiation (Kim et al., 2014). In this study, treatment with an ERK inhibitor resulted in a loss of neuronal polarity and a significant decrease of neurite length in cells transfected with NELL2. These interesting results suggest that the function of NELL2 in axon development is also mediated through the ERK signaling pathway, though the specific receptor and intracellular mediators are unclear.
In summary, our results revealed that NELL2 plays an important role in axon development during neuronal differentiation through the ERK intracellular signaling pathway in cultured hippocampal neurons.
ACKNOWLEDGMENTS
This research was supported by the Priority Research Centers Program (2014R1A6A1030318) and research fund (NRF-2015R1C1A1A01054848) through the National Research Foundation of Korea and supported by KBRI basic research program through Korea Brain Research Institute funded by the Ministry of Science, ICT (20-BR-04-02).
Footnotes
AUTHOR CONTRIBUTIONS
H.R.K., D.H.K., J.Y.A., C.M.H., and B.J.L. made experimental design, interpreted results and wrote the manuscript. H.R.K., D.H.K., and J.Y.A. performed and analyzed most of the experiments. D.H.K. and D.K. performed primary neuron culture and histological analysis. E.J.S. and I.H.J. performed image analysis. J.W.P. and E.M.H. provided intellectual inputs.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Acebes A., Ferrús A. Cellular and molecular features of axon collaterals and dendrites. Trends Neurosci. 2000;23:557–565. doi: 10.1016/S0166-2236(00)01646-5. [DOI] [PubMed] [Google Scholar]
- Barnes A.P., Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M.V. Neurotrophins and their receptors: a convergence point for many signaling pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Cheng P.L., Song A.H., Wong Y.H., Wang S., Zhang X., Poo M.M. Self-amplifying autocrine actions of BDNF in axon development. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.J., Kim D.H., Kim J.G., Kim D.Y., Kim J.D., Seol O.J., Jeong C.S., Park J.W., Choi M.Y., Kang S.G., et al. Estrogen-dependent transcription of the NEL-like 2 (NELL2) gene and its role in protection from cell death. J. Biol. Chem. 2010;285:25074–25084. doi: 10.1074/jbc.M110.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.M., Banker G. Neuronal polarity. Annu. Rev. Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Díaz-Hernandez M., del Puerto A., Díaz-Hernandez J.I., Diez-Zaera M., Lucas J.J., Garrido J.J., Miras-Portugal M.T. Inhibition of the ATP-gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J. Cell Sci. 2006;15:3717–3728. doi: 10.1242/jcs.034082. [DOI] [PubMed] [Google Scholar]
- Dotti C.G., Sullivan C.A., Banker G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K., Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E.M., Kim D.G., Lee B.J., Choi J., Kim E., Park N., Kang D., Han J., Choi W.S., Hong S.G., et al. Alternative splicing generates a novel non-secretable cytosolicisoform of NELL2. Biochem. Biophys. Res. Commun. 2007;353:805–811. doi: 10.1016/j.bbrc.2006.12.115. [DOI] [PubMed] [Google Scholar]
- Jaworski A., Tom I., Tong R.K., Gildea H.K., Koch A.W., Gonzalez L.C., Tessier-Lavigne M. Operational redundancy in axon guidance through the multifunctional receptor Robo3 and its ligand NELL2. Science. 2015;350:961–965. doi: 10.1126/science.aad2615. [DOI] [PubMed] [Google Scholar]
- Jeong J.K., Kim H.R., Hwang S.M., Park J.W., Lee B.J. Region- and neuronal phenotype specific expression of NELL2 in the adult rat brain. Mol. Cells. 2008;26:186–192. [PubMed] [Google Scholar]
- Jin S., Kim J.G., Park J.W., Koch M., Horvath T.L., Lee B.J. Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis. Sci. Rep. 2016;6:29424. doi: 10.1038/srep29424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K., Dent E.W. Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat. Rev. Neurosci. 2014;15:7–18. doi: 10.1038/nrn3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Kim H.R., Choi E.J., Kim D.Y., Kim K.K., Kim B.S., Park J.W., Lee B.J. Neural epidermal growth factor-like like protein 2 (NELL2) promotes aggregation of embryonic carcinoma P19 cells by inducing N-cadherin expression. PLoS One. 2014;9:e85898. doi: 10.1371/journal.pone.0085898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Ha C.M., Choi J., Choi E.J., Jeon J., Kim C., Park S.K., Kang S.S., Kim K., Lee B.J. Ontogeny and the possible function of a novel epidermal growth factor-like repeat domain containing protein, NELL2, in the rat brain. J. Neurochem. 2002;83:1389–1400. doi: 10.1046/j.1471-4159.2002.01245.x. [DOI] [PubMed] [Google Scholar]
- Kim Y.E., Baek S.T. Neurodevelopmental Aspects of RASopathies. Mol. Cells. 2019;42:441–447. doi: 10.14348/molcells.2019.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Zhou Y., Chai Y., Wang X., Huang X. Stat3 controls maturation and terminal differentiation in mouse hippocampal neurons. J. Mol. Neurosci. 2017;61:88–95. doi: 10.1007/s12031-016-0820-x. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S., Noji S., Koyama E., Myokai F., Ohuchi H., Taniguchi S., Hori K. New gene, nel, encoding a Mr 93 K protein with EGF-like repeats is strong expressed in neural tissues of early stage chick embryos. Dev. Dyn. 1995;203:212–222. doi: 10.1002/aja.1002030209. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Aihara K., Nishino N., Takeda S., Tanizawa K., Kuroda S., Horie M. Enhanced long-term potentiation in vivo in dentate gyrus of NELL2-deficient mice. Neuroreport. 2004;15:417–420. doi: 10.1097/00001756-200403010-00007. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Doe N., Kurihara N., Tanizawa K., Kuroda S., Iso H., Horie M. Spatial learning of mice lacking a neuron-specific epidermal growth factor family protein, NELL2. J. Pharmacol. Sci. 2005;98:239–243. doi: 10.1254/jphs.FP0050211. [DOI] [PubMed] [Google Scholar]
- Nelson B.R., Claes K., Todd V., Chaverra M., Lefcort F. NELL2 promotes motor and sensory neuron differentiation and stimulates mitogenesis in DRG in vivo. Dev. Biol. 2004;270:322–335. doi: 10.1016/j.ydbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Nix P., Hisamoto N., Matsumoto K., Bastiani M. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc. Natl. Acad. Sci. U. S. A. 2011;28:10738–10743. doi: 10.1073/pnas.1104830108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyasu M., Kuroda S., Nakashita M., Fujimiya M., Kikkawa U., Saito N. Immunocytochemical localization of a neuron-specific thrombospondin-1-like protein, NELL2: light and electron microscopic studies in the rat brain. Brain Res. Mol. Brain Res. 2000;76:151–160. doi: 10.1016/S0169-328X(99)00342-3. [DOI] [PubMed] [Google Scholar]
- Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Perron J.C., Bixby J.L. Distinct neurite outgrowth signaling pathways converge on ERK activation. Mol. Cell Neurosci. 1999;13:362–378. doi: 10.1006/mcne.1999.0753. [DOI] [PubMed] [Google Scholar]
- Rao Z., Handford P., Mayhew M., Knott V., Brownlee G.G., Stuart D. The structure of a Ca2+-binding epidermal growth factor-like domain: its role in protein-protein interactions. Cell. 1995;82:131–141. doi: 10.1016/0092-8674(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Seiradake E., Jones E.Y., Klein R. Structural perspectives on axon guidance. Annu. Rev. Cell Dev. Biol. 2016;32:577–608. doi: 10.1146/annurev-cellbio-111315-125008. [DOI] [PubMed] [Google Scholar]
- Shi S.H., Jan L.Y., Jan Y.N. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/S0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Kashiwagi M., Ishihara M., Kojima T., Maturana A.D., Kuroda S., Niimi T. Robo2 contains a cryptic binding site for neural EGFL-like (NELL) protein 1/2. J. Biol. Chem. 2019;294:4693–4703. doi: 10.1074/jbc.RA118.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Guo L., Wang Y. Requirement of dendritic Akt degradation by the ubiquitin-proteasome system for neuronal polarity. J. Cell Biol. 2006;174:415–424. doi: 10.1083/jcb.200511028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypsilanti A.R., Zagar Y., Chédotal A. Moving away from the midline: new developments for Slit and Robo. Development. 2010;137:1939–1952. doi: 10.1242/dev.044511. [DOI] [PubMed] [Google Scholar]