Abstract
In eukaryotes, membraneous cellular compartmentation essentially requires vesicle trafficking for communications among distinct organelles. A donor organelle-generated vesicle releases its cargo into a target compartment by fusing two distinct vesicle and target membranes. Vesicle fusion, the final step of vesicle trafficking, is driven intrinsically by complex formation of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs). Although SNAREs are well-conserved across eukaryotes, genomic studies revealed that plants have dramatically increased the number of SNARE genes than other eukaryotes. This increase is attributed to the sessile nature of plants, likely for more sensitive and harmonized responses to environmental stresses. In this review, we therefore try to summarize and discuss the current understanding of plant SNAREs function in responses to biotic and abiotic stresses.
Keywords: abiotic stress, biotic stress, plant, SNARE, trafficking
INTRODUCTION
A plant cell, as a eukaryotic one, contains several membrane-separated compartments such as nucleus, endoplasmic reticulum (ER), Golgi body, mitochondria and plastids. Although each subcellular compartment performs its own tasks, a more complex cellular work requires cooperative activities among distinct organelles, even between neighboring cells. For this, cellular compartments communicate by exchanging their contents which are transported by small membraneous containers called vesicles. A cargo-loaded vesicle from a donor organelle moves to a target site and releases its cargo. However, to transport the cargo into a target place, membranes between a vesicle and a target compartment should be fused, which is energetically unfavorable. To overcome this, eukaryotes have successfully invented a membrane-merging machinery that consists of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) (Jahn and Scheller, 2006).
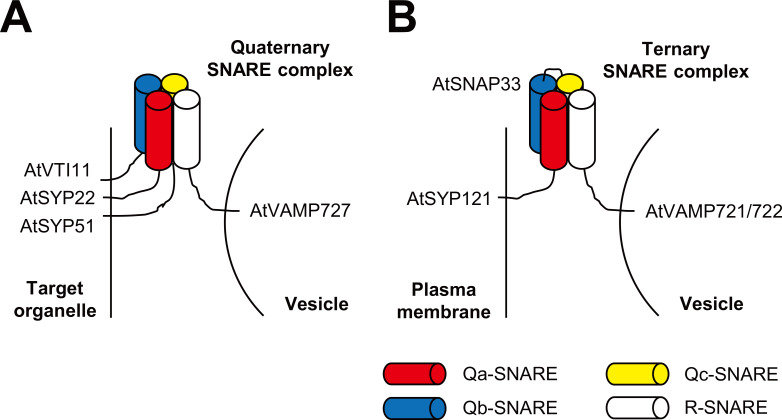
Simply based on their locations, SNAREs can be categorized as t (target)-SNARE that is placed on the target compartment membrane and v (vesicle)-SNARE that is localized on the vesicle membrane (Sollner et al., 1993). Based on the conserved central amino acid in the SNARE motif that is responsible for interactions between SNAREs, they can be additionally grouped into Q (Gln)-SNARE that generally corresponds to t-SNARE and R (Arg)-SNARE that corresponds to v-SNARE (Fasshauer et al., 1998). Q-SNAREs, on the basis of their sequence similarity, can be further classified into Qa-, Qb-, Qc-, and two SNARE motifs-containing Qbc-SNARE (Bock et al., 2001). Although most SNAREs are membrane-inserted by transmembrane motif, some SNAREs, such as SNAP25 (synaptosome-associated protein 25) Qbc-SNAREs and YKT6 R-SNAREs, are peripherally attached to the membrane by posttranslational lipidation (Hong, 2005). To drive the energy-required mergence of two distinct membranes, SNAREs form a SDS-resistant tight complex that contains four α-helical SNARE motifs (Hanson et al., 1997; Lin and Scheller, 2000). In general, four distinct SNAREs (Qa + Qb + Qc + R; e.g., AtSYP22 [syntaxin of plant 22] + AtVTI11 [vesicle transport v-SNARE 11] + AtSYP51 + AtVAMP727 [vesicle-associated membrane protein 727] in Arabidopsis) engage in the fusion between a vesicle and an intracellular endomembrane compartment (Fig. 1A), whereas three different SNAREs (Qa + Qbc + R; e.g., AtSYP121 + AtSNAP33 + AtVAMP721/722 in Arabidopsis) involve in the fusion between the plasma membrane (PM) and a vesicle (Fig. 1B) (Antonin et al., 2002; Ebine et al., 2008; Kwon et al., 2008b; Sutton et al., 1998).
Fig. 1. Two distinct types of SNARE complexes containing four α-helices to drive vesicle fusion events.
While four different SNAREs (Qa + Qb + Qc + R) form a quaternary SNARE complex for fusing a vesicle with an intracellular compartment (A), three different ones (Qa + Qbc + R) do a ternary SNARE complex for exocytosis (vesicle fusion with the PM) (B). Known Arabidopsis SNARE complexes are; (A) AtSYP22-AtVTI11-AtSYP51-AtVAMP727 (Qa-Qb-Qc-R) quaternary SNARE complex (Ebine et al., 2008), and (B) AtSYP121-AtSNAP33-AtVAMP721/722 (Qa-Qbc-R) ternary SNARE complex (Kwon et al., 2008b).
The first functionally identified SNARE in plants is the Arabidopsis AtSYP111 (also called KNOLLE) (Lauber et al., 1997; Lukowitz et al., 1996). Mitosis-specific expression of AtSYP111, division plane localization of AtSYP111, and cytokinesis defect in atsyp111 (Lauber et al., 1997; Lukowitz et al., 1996) indicate that AtSYP111 is specifically involved in cell division, especially cytokinesis, in plants. Later biochemical works revealed that AtSYP111 forms two distinct SNARE complexes to drive cytokinesis with either AtSNAP33 Qbc-SNARE and AtVAMP721/722 R-SNAREs (ternary SNARE complex) (Heese et al., 2001), or AtNPSN11 (novel plant SNARE 11) Qb-SNARE, AtSYP71 Qc-SNARE and AtVAMP721/722 R-SNAREs (quaternary SNARE complex) (El Kasmi et al., 2013). Failure of other Qa-SNAREs except AtSYP132 in rescuing the cytokinesis-defective atsyp111 phenotype indicates that AtSYP111 is specialized for cytokinesis (Muller et al., 2003; Reichardt et al., 2011). Partial rescue of the atsyp111 phenotype by AtSYP132 and the presence of an AtSYP132 but not AtSYP111 counterpart in lower plants suggest that AtSYP111 is an AtSYP132-derived Qa-SNARE evolutionarily specialized for cytokinesis in angiosperms (Park et al., 2018; Reichardt et al., 2011). This may explain why the number of SNARE genes are increased in higher plants, most likely due to complex physiological processes under their sessile nature.
Unlike animals, plants are immobile once rooted in the soil. This indicates that plants are continuously exposed to potential threats. However, they are healthy and well living in nature, suggesting that plants have evolved a sophisticated system to effectively resist to variable environmental stresses. Such a system includes correct detection of extrinsic stresses, proper signaling to synthesize defense molecules, and accurate delivery of those molecules to right places. Since these stress-resistant molecules are likely harmful to plants themselves, they have to be transported in a safely membrane-separated vesicle. SNAREs are the minimal core factors to drive the vesicle fusion with a destination compartment to discharge the cargo. Therefore, we will discuss in this review the current understanding of the importance of SNAREs in stress responses in plants.
SNAREs IN BIOTIC STRESS RESPONSES
Due to the lack of the circulatory system and mobile immune cells, plants solely depend on the cell-autonomous innate immunity. Plants detect a pathogen by recognizing a pathogen-associated molecular pattern (PAMP) by a surface receptor called a pattern-recognition receptor (PRR) (Dodds and Rathjen, 2010; Jones and Dangl, 2006). A cognate PRR-PAMP pairing initiates an immune signal that leads to the transcriptional reprogramming in a pathogen-challenged plant cell via the mitogen-activated protein kinase (MAPK) cascade and WRKY transcription factors (Dodds and Rathjen, 2010; Jones and Dangl, 2006). To expel extracellular pathogens, plant cells finally secrete immune molecules to pathogen-attempting sites (Yun and Kwon, 2017).
So far, two distinct immune secretory pathways have been identified (Kwon et al., 2008a; Yun and Kwon, 2017). One is a transporter-mediated secretion which involves the mitochondrium/peroxisome-localized AtPEN2 (penetration 2) myrosinase and the PM-residing AtPEN3 ABC transporter (Fuchs et al., 2016; Lipka et al., 2005; Stein et al., 2006). The other is a SNARE-assisted exocytosis. The first identified SNARE that is required for plant immunity is the Arabidopsis PM-residing AtSYP121 (also called AtPEN1) Qa-SNARE, whose barley ortholog is HvROR2 (required for mlo-specified disease resistance 2) (Collins et al., 2003). Elevated fungal penetration in syp121 plants suggests that AtSYP121 at the PM facilitates the immune exocytosis to fungal pathogens (Collins et al., 2003). Later biochemical and genetic approaches revealed that AtSYP121 Qa-SNARE forms the SDS-resistant immune SNARE complex with the AtSNAP33 Qbc-SNARE and functionally redundant AtVAMP721/722 R-SNAREs (Kwon et al., 2008b) (Fig. 1), which is the first report to identify the whole component SNAREs to form a SNARE complex in plants. Interestingly, the dicotyledonous Arabidopsis AtSYP121-AtSNAP33-AtVAMP721/722-driven immune exocytosis is conserved in the monocotyledonous barley as the HvROR2-HvSNAP34-HvVAMP721-assisted exocytosis (Kwon et al., 2008b). This indicates that the SNARE-mediated immune exocytosis has been invented in plants before the monocot-dicot divergence. The requirement of SNAREs involved in this immune exocytosis for basal growth and development in plants additionally suggests that plants have co-opted a default exocytosis for immunity (Kwon et al., 2008b).
SYP121 is required for resistance to fungal and oomycete pathogens but not to bacterial ones (Kwon et al., 2008b). The compromised defense against Pseudomonas syringae pathovar (pv.) tabaci bacterium by silencing NbSYP132 Qa-SNARE gene but not NbSYP121 in Nicotiana benthamiana suggests that SYP132 rather than SYP121 is engaged in immune responses to bacterial pathogens (Kalde et al., 2007). Since AtSYP132 specifically interacts with AtVAMP721/722 in plant cells (Yun et al., 2013a), and since AtSNAP33 is the major SNAP25-like genes that is expressed in the leaf tissue (Kwon et al., 2008b), it is likely that the SYP132-SNAP33-VAMP721/722 SNARE complex drives an immune exocytosis to bacterial pathogens in plants (Fig. 1). This additionally suggests that plants employ common SNAP33 and VAMP721/722 but distinct PM Qa-SNAREs depending on pathogen types for immune responses.
Although AtVAMP721/722 are engaged in multiple immune responses to distinct types of pathogens, what are transported and secreted via AtVAMP721/722 vesicles remain poor yet. Recent proteomic approaches for either Arabidopsis seedling-grown liquid media (likely containing plant-secreted proteins) or leaf apoplastic fractions revealed that AtSYP121 and AtVAMP721/722 are important for the secretion of many cell wall-associated proteins (Uemura et al., 2019; Waghmare et al., 2018). A reciprocal proteomic approach to compare intracellular proteins between wild-type (WT) and AtVAMP721/722-depleted plants additionally found that a lignin biosynthetic enzyme, caffeoyl-CoA O-methyltransferase 1 (CCOAOMT1), might be transported out of plant cells by AtVAMP721/722 vesicles (Kwon et al., 2020). AtVAMP722 vesicles were found to directionally move to the fungal entry sites where AtSYP121 is focally accumulated at the PM (Assaad et al., 2004; Kwon et al., 2008b). In addition, AtSYP121 and AtVAMP721/722 are required for the timely formation of secondary cell walls called papillae at fungal challenging sites in plant cells (Assaad et al., 2004; Kwon et al., 2008b). This suggests that AtVAMP721/722 vesicles deliver cell wall-modifying proteins and/or cell wall materials to reinforce the local cell walls at pathogen attempting areas. Beyond cell wall-related molecules, the powdery mildew resistance protein RPW8.2, whose precise function is unknown, is the only identified immune protein transported by AtVAMP721/722 vesicles. Its colocalization with AtVAMP721/722 and delayed trafficking to plant-fungal interface in AtVAMP721/722-depleted plants suggest that AtVAMP721/722 vesicles focally deliver RPW8.2 to fungal entry sites for immunity (Kim et al., 2014). Extracellular release of those immune molecules in plants is likely to be mediated by SYP121-VAMP721/722 interactions for defense against fungal pathogens but by SYP132-VAMP721/722 interactions against bacterial ones.
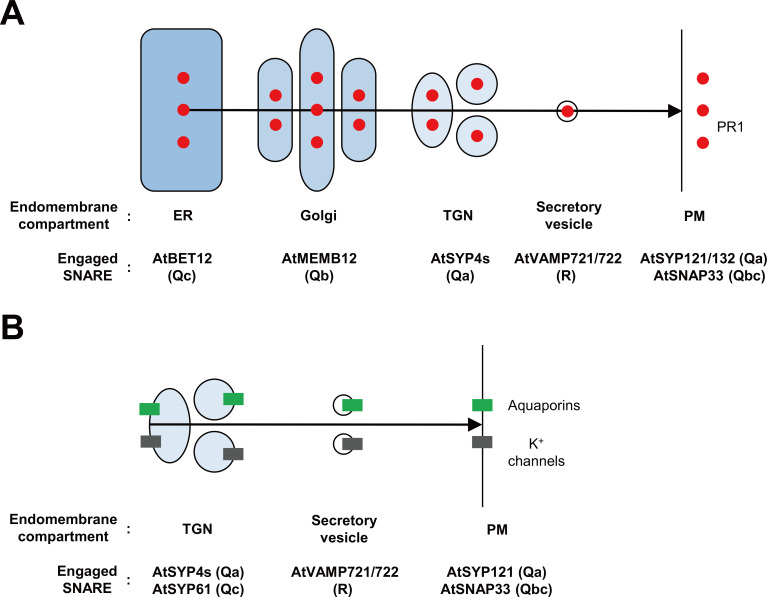
Pathogenesis-related (PR) proteins are well-known plant- secreted immune proteins through the default secretory pathway in response to pathogen attack (van Loon et al., 2006). Indeed, disruption in ER translocation or modification of default-secreted proteins reduces the secretion of PR1, a representative PR, resulting in impaired immune responses to P. syringae pv. maculicola ES4326 bacterium (Wang et al., 2005). AtBET12 Qc-SNARE that exports the ER and localizes to the Golgi and trans-Golgi network (TGN) is regarded to be engaged in the ER-to-Golgi anterograde protein trafficking (Chung et al., 2018). Intriguingly, in plant cells expressing the ER export-defective BET12 mutant, the Golgi-localized AtMEMB12 Qb-SNARE but not other Golgi-localized ones is trapped in the ER (Chung et al., 2018). Increased extracellular PR1 levels accompanied by elevated resistance to bacterial pathogens, but WT-like growth phenotype in atmemb12 plants suggest that AtMEMB12 is specifically involved in the Golgi-to-ER retrograde trafficking of immune proteins (Zhang et al., 2011). In planta interactions between AtBET12 and AtMEMB12, and elevated PR1 retention in the ER by overexpressing either AtBET12 or AtMEMB12 suggest that these SNAREs are specialized for controlling the ER-Golgi trafficking of immune proteins (Chung et al., 2018). The TGN is regarded as a sorting platform of endocytosed and PM- or vacuole-targeted vesicles. The TGN-localized AtSYP41/42/43 Qa-SNAREs are required for immune responses to fungal pathogens in Arabidopsis (Uemura et al., 2012; 2019). No more redirected AtVAMP721 vesicles to fungal entry sites in AtSYP4s-depleted plants (Uemura et al., 2019) suggests that VAMP721/722 vesicles containing immune proteins bud at the TGN, which is aided by SYP4s. Therefore, it is likely that secreted immune proteins including PR proteins are translated and modified in the ER, transported from the ER to the Golgi by BET12-MEMB12 interactions, packed into VAMP721/722 vesicles in the TGN by SYP4s, and finally released out of plant cells by SYP121/132-SNAP33-VAMP721/722 SNARE complex-driven exocytosis (Fig. 2A).
Fig. 2. Cellular routes of stress-related proteins in Arabidopsis.
(A) PR1 is regarded to be secreted via ER, Golgi, TGN and secretory vesicle as shown by an arrow. For the secretion of PR1, AtBET12 and AtMEMB12 control the ER-Golgi trafficking, AtSYP4s (AtSYP41/42/43) does the budding of AtVAMP721/722 vesicles at the TGN, AtSYP121/132-AtSNAP33 at the PM regulate the fusion of AtVAMP721/722 vesicles with the PM. (B) K+ channels and aquaporins are thought to be delivered from the TGN to the PM via AtVAMP721/722 secretory vesicles. Their transport is controlled by AtSYP4s/AtSYP61 at the TGN and by AtSYP121/AtSNAP33 at the PM.
Orthologous ternary SNARE complexes required for immune responses between Arabidopsis (AtSYP121-AtSNAP33- AtVAMP721/722) and barley (HvROR2-HvSNAP34-HvVAMP721) (Collins et al., 2003; Kwon et al., 2008b) indicate that this SNARE complex-driven exocytosis is a conserved ancient secretory pathway for immunity in plants. Recently, OsSYP121 that interacts with OsSNAP32 and OsVAMP714/724 was reported to be required for rice resistance to the Magnaporthe ryzae rice blast fungus (Cao et al., 2019). In addition, overexpression of AtSNAP33 orthologs (CkSNAP33 in Cynanchum komarovii and GhSNAP33 in Gossypium hirsutum) in Arabidopsis results in elevated resistance to the Verticillium dahliae fungus (Wang et al., 2017; 2018). These results support that the SYP121-SNAP33-VAMP721/722 SNARE complex is the common immune exocytosis-driving machinery in plants. However, requirement of GmSYP31 Qa-SNARE in soybean for defense against the Heterodera glycines nematode and TaSYP71 Qc-SNARE in wheat for resistance to the Puccinia striiformis fungus (Liu et al., 2016; Pant et al., 2014) suggests that different plant species may have adopted additional SNAREs to the above-mentioned common SNARE complex for immunity depending on pathogen types.
SNAREs IN ABIOTIC STRESS RESPONSES
Plants are also continuously exposed to a variety of abiotic stresses such as drought, high salinity, heat, cold, freezing, UV-B and osmotic stresses as well as to biotic stresses during their life cycles. The existence of a large number of SNARE genes in plants (Sanderfoot, 2007) implies that at least a fraction of SNAREs could be involved in abiotic stress responses. Indeed, several genetic and biochemical studies isolated many SNAREs participating in abiotic stress signaling, mostly based on phenotypic analysis according to their altered expressions. In spite of their functional involvement in abiotic stress responses, however, their detailed action mechanisms are largely unknown.
Suppression of the AtVAMP7C genes (AtVAMP711, AtVAMP712, AtVAMP713, and AtVAMP714) expression results in enhanced tolerance to salt stress (Leshem et al., 2006). Moreover, functional defect of AtVAMP711 leads to a slower stomatal closure through enhancement of PM H+-ATPase activity, after drought and abscisic acid (ABA, plant abiotic stress hormone) treatments (Leshem et al., 2010; Xue et al., 2018). Collectively, these results show a regulatory role of AtVAMP7C proteins in salt and drought stress responses. AtVAMP721/722-depleted lines display retarded growth pattern compared to WT after ABA application (Yi et al., 2013). Decreased AtVAMP721/722 protein levels by ABA and salinity treatments (Yi et al., 2013; Yun et al., 2013b) imply their involvement in drought and salt stress responses.
In addition to R-SNAREs, Q-SNAREs are also found to be engaged in abiotic stress responses. Before the identification of AtSYP121 in plant immunity in Arabidopsis, its tobacco ortholog, NtSYP121 (also called NtSyr1 [syntaxin-related protein 1]), was isolated as a component to be required for ion channel control at the PM in response to ABA (Leyman et al., 1999). Although functional loss mutant of AtSYP121 (atsyp121) does not affect stomatal closure in response to high Ca2+ levels or ABA, stomatal reopening is retarded in the light and following Ca2+-evoked closure, and K+ uptake process for stomatal opening is suppressed in the atsyp121 mutant (Eisenach et al., 2012), indicating that SYP121 is responsible for stomatal control. Moreover, under conditions of low humidity and high light intensities, atsyp121 exhibits low stomatal conductance and retardation of vegetative growth (Eisenach et al., 2012), implying its involvement in drought stress response. Indeed, it was revealed that the AtSYP121-AtSNAP33-AtVAMP721 SNARE complex is involved in the delivery of K+ channels to the PM (Honsbein et al., 2009; Waghmare et al., 2019; Zhang et al., 2015). The AtSYP4 group consisting of AtSYP41, AtSYP42, and AtSYP43 positively affects tolerance to salinity and osmotic stresses (Uemura et al., 2012). In addition, defect of AtSYP61, a component in the AtSYP41 complex, leads to altered osmotic stress tolerance and stomatal responses (Zhu et al., 2002). A co-immunoprecipitation assay with AtSYP41 identified a large At-SYP41-interacting protein called AtTNO1 (TGN-localized SYP41-interacting protein 1) (Kim and Bassham, 2011). The loss-of-function of AtTNO1 results in increased sensitivity to salt and osmotic stresses, accompanied by partial secretion of vacuolar proteins to the apoplast (Kim and Bassham, 2011). Rescue of the attno1 phenotypes, abnormal salt sensitivity and vacuolar trafficking, by overexpression of AtSYP41 or AtSYP61 suggests AtTNO1 as a tethering factor to regulate AtSYP41-AtSYP61 complex (Yang et al., 2019). These findings reveal the importance of SYP4s/SYP61-mediated process in those stress responses. Moreover, AtSYP61 and AtSYP121 together regulate the water permeability of PM through coordinating trafficking process of aquaporin AtPIP2;7 (PM intrinsic protein 2;7) (Hachez et al., 2014; Piofczyk et al., 2015). Taken together, it is therefore likely that abiotic stress-related proteins such as K+ channels and aquaporins are delivered to the PM via VAMP721/722 vesicles with the aid of SYP4s/SYP61 at the TGN and SYP121/SNAP33 at the PM (Fig. 2B). Furthermore, it was reported that growth inhibition by UV-B is reduced by functional defect of AtNPSN12 (Piofczyk et al., 2015). These additionally imply that other SNAREs than SYP121/SYP4s/SYP61 are also involved in plant responses to UV-B stress, as well as drought and salt stresses.
Abiotic stress response-related SNAREs from other plant species than Arabidopsis have also been recently reported. A SNARE-like superfamily protein-encoding gene, SbSLSP, was identified from an extreme halophyte Salicornia brachiata (Singh et al., 2016). In agreement with its inducibility to salt and drought stresses, SbSLSP-overexpressing tobacco lines show enhanced tolerance to salt and drought stresses by modulating membrane stability, K+/Na+ ratio and ROS levels (Singh et al., 2016). HbSYR1 protein from Tibetan barley (Hordeum vulgare) possesses a SNARE family characteristic motif (Xu et al., 2017). Alleviated drought tolerance in HbSYR1-silenced transgenic lines indicates that HbSYR1 positively affects drought resistance in barley (Xu et al., 2017). Overexpression of TaVAP (vesicle-associated membrane protein-associated protein gene in Triticum aestivum) and GsSNAP33 (a SNAP25-like gene in Glycine soja) in Arabidopsis plants confer tolerance to drought stress, showing their possible contribution to drought stress tolerance (Nisa et al., 2017; Singh et al., 2018). Transcriptomic profiling under salt stress indicates that six differentially expressed genes (c92920_g1_i1, c64313_g1_i1, c45795_g1_i2, c49659_g1_i1, c98985_g1_i1, c33236_g2_i1) by salt stress are categorized into “SNARE interactions in vesicular transport” in asparagus bean, supporting recent reports that SNARE-mediated membrane trafficking is required for proper response of plants to salt stress (Pan et al., 2019).
CONCLUDING REMARKS
In response to environmental stresses, plants utilize SNAREs to transport pathogenesis-terminating and abiotic stress-alleviating molecules. Since SNAREs drive vesicle fusion between donor and target compartments, those otherwise harmful molecules might be safely delivered to the working places via membrane-contained vesicles. The understanding of those transported molecules during stress responses undoubtedly helps to improve crop productivity especially in this rapidly climate-changing era. However, a single molecule of known secreted PR proteins and secondary metabolites in response to pathogen infection has a limited immune activity. This indicates that stress-relieving molecules may work in a more complex way than expected, likely as a cocktail rather than a single compound. Therefore, precise and comparative isolation and identification of trafficked molecules in response to a single pure stress would be critical to correctly understand how those molecules act in a specific combination for plant resistance to an environmental stress.
Additional difficulty in understanding molecules that are SNARE-transported in a specific response is that a same SNARE is engaged in multiple stress responses. For example, AtSYP121 and AtVAMP721/722 are required for both biotic and abiotic stress responses in Arabidopsis (Collins et al., 2003; Eisenach et al., 2012; Kwon et al., 2008b; Leyman et al., 1999; Yi et al., 2013; Yun et al., 2013a). Interestingly, plant SNAREs promiscuously form a SNARE complex in vitro (Kwon et al., 2008b), indicating that their specific in planta interactions should be regulated by an accessory factor. Indeed, KEULE also called SEC11 is found to control the formation of AtSYP121-AtSNAP33-AtVAMP721/722 and AtSYP111-AtSNAP33-AtVAMP721/722 (Karnik et al., 2013; Park et al., 2012). In addition, ARA6, a plant-unique RAB5 small GTPase required for salt stress tolerance, promotes unexpected AtSYP121-AtVAMP727 interaction (Ebine et al., 2011). Therefore, in addition to pure isolation, the knowledge on a biological function of a SNARE complex-regulating protein would be greatly helpful to understand the exact nature of transported molecules for a specific stress response in plants.
ACKNOWLEDGMENTS
This work was supported by grants from the National Research Foundation, Korea (and 2016R1D1A1B02007322 to C.K., 2019R1F1A1041226 to J.H.L., and 2017R1D1A1B03029802 to H.S.Y.), and a grant (PJ01477001 to C.K.) from Rural Development Administration, Korea.
Footnotes
AUTHOR CONTRIBUTIONS
J.H.L. wrote the part of SNAREs in abiotic stress responses, and C.K. and H.S.Y. wrote the other parts of the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Antonin W., Fasshauer D., Becker S., Jahn R., Schneider T.R. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat. Struct. Biol. 2002;9:107–111. doi: 10.1038/nsb746. [DOI] [PubMed] [Google Scholar]
- Assaad F.F., Qiu J.L., Youngs H., Ehrhardt D., Zimmerli L., Kalde M., Wanner G., Peck S.C., Edwards H., Ramonell K., et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol. Biol. Cell. 2004;15:5118–5129. doi: 10.1091/mbc.e04-02-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J.B., Matern H.T., Peden A.A., Scheller R.H. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Cao W.L., Yu Y., Li M.Y., Luo J., Wang R.S., Tang H.J., Huang J., Wang J.F., Zhang H.S., Bao Y.M. OsSYP121 accumulates at fungal penetration sites and mediates host resistance to rice blast. Plant Physiol. 2019;179:1330–1342. doi: 10.1104/pp.18.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K.P., Zeng Y., Li Y., Ji C., Xia Y., Jiang L. Signal motif-dependent ER export of the Qc-SNARE BET12 interacts with MEMB12 and affects PR1 trafficking in Arabidopsis. J. Cell Sci. 2018;131:jcs202838. doi: 10.1242/jcs.202838. [DOI] [PubMed] [Google Scholar]
- Collins N.C., Thordal-Christensen H., Lipka V., Bau S., Kombrink E., Qiu J.L., Huckelhoven R., Stein M., Freialdenhoven A., Somerville S.C., et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature. 2003;425:973–977. doi: 10.1038/nature02076. [DOI] [PubMed] [Google Scholar]
- Dodds P.N., Rathjen J.P. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- Ebine K., Fujimoto M., Okatani Y., Nishiyama T., Goh T., Ito E., Dainobu T., Nishitani A., Uemura T., Sato M.H., et al. A membrane trafficking pathway regulated by the plant-specific RAB GTPase ARA6. Nat. Cell Biol. 2011;13:853–859. doi: 10.1038/ncb2270. [DOI] [PubMed] [Google Scholar]
- Ebine K., Okatani Y., Uemura T., Goh T., Shoda K., Niihama M., Morita M.T., Spitzer C., Otegui M.S., Nakano A., et al. A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. Plant Cell. 2008;20:3006–3021. doi: 10.1105/tpc.107.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C., Chen Z.H., Grefen C., Blatt M.R. The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K(+) channel activity with vegetative growth. Plant J. 2012;69:241–251. doi: 10.1111/j.1365-313X.2011.04786.x. [DOI] [PubMed] [Google Scholar]
- El Kasmi F., Krause C., Hiller U., Stierhof Y.D., Mayer U., Conner L., Kong L., Reichardt I., Sanderfoot A.A., Jurgens G. SNARE complexes of different composition jointly mediate membrane fusion in Arabidopsis cytokinesis. Mol. Biol. Cell. 2013;24:1593–1601. doi: 10.1091/mbc.e13-02-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Sutton R.B., Brunger A.T., Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Kopischke M., Klapprodt C., Hause G., Meyer A.J., Schwarzlander M., Fricker M.D., Lipka V. Immobilized subpopulations of leaf epidermal mitochondria mediate PENETRATION2-dependent pathogen entry control in Arabidopsis. Plant Cell. 2016;28:130–145. doi: 10.1105/tpc.15.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C., Laloux T., Reinhardt H., Cavez D., Degand H., Grefen C., De Rycke R., Inze D., Blatt M.R., Russinova E., et al. Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability. Plant Cell. 2014;26:3132–3147. doi: 10.1105/tpc.114.127159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P.I., Heuser J.E., Jahn R. Neurotransmitter release - four years of SNARE complexes. Curr. Opin. Neurobiol. 1997;7:310–315. doi: 10.1016/S0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- Heese M., Gansel X., Sticher L., Wick P., Grebe M., Granier F., Jurgens G. Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J. Cell Biol. 2001;155:239–249. doi: 10.1083/jcb.200107126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochim. Biophys. Acta. 2005;1744:120–144. doi: 10.1016/j.bbamcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Honsbein A., Sokolovski S., Grefen C., Campanoni P., Pratelli R., Panaque M., Chen Z., Johansson I., Blatt M.R. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell. 2009;21:2859–2877. doi: 10.1105/tpc.109.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Scheller R.H. SNAREs--engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kalde M., Nuhse T.S., Findlay K., Peck S.C. The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11850–11855. doi: 10.1073/pnas.0701083104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik R., Grefen C., Bayne R., Honsbein A., Kohler T., Kioumourtzoglou D., Williams M., Bryant N.J., Blatt M.R. Arabidopsis Sec1/Munc18 protein SEC11 is a competitive and dynamic modulator of SNARE binding and SYP121-dependent vesicle traffic. Plant Cell. 2013;25:1368–1382. doi: 10.1105/tpc.112.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., O'Connell R., Maekawa-Yoshikawa M., Uemura T., Neumann U., Schulze-Lefert P. The powdery mildew resistance protein RPW8.2 is carried on VAMP721/722 vesicles to the extrahaustorial membrane of haustorial complexes. Plant J. 2014;79:835–847. doi: 10.1111/tpj.12591. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Bassham D.C. TNO1 is involved in salt tolerance and vacuolar trafficking in Arabidopsis. Plant Physiol. 2011;156:514–526. doi: 10.1104/pp.110.168963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C., Bednarek P., Schulze-Lefert P. Secretory pathways in plant immune responses. Plant Physiol. 2008a;147:1575–1583. doi: 10.1104/pp.108.121566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C., Neu C., Pajonk S., Yun H.S., Lipka U., Humphry M., Bau S., Straus M., Kwaaitaal M., Rampelt H., et al. Co-option of a default secretory pathway for plant immune responses. Nature. 2008b;451:835–840. doi: 10.1038/nature06545. [DOI] [PubMed] [Google Scholar]
- Kwon H., Cho D.J., Lee H., Nam M.H., Kwon C., Yun H.S. CCOAOMT1, a candidate cargo secreted via VAMP721/722 secretory vesicles in Arabidopsis. Biochem. Biophys. Res. Commun. 2020;524:977–982. doi: 10.1016/j.bbrc.2020.02.029. [DOI] [PubMed] [Google Scholar]
- Lauber M.H., Waizenegger I., Steinmann T., Schwarz H., Mayer U., Hwang I., Lukowitz W., Jurgens G. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol. 1997;139:1485–1493. doi: 10.1083/jcb.139.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y., Golani Y., Kaye Y., Levine A. Reduced expression of the v-SNAREs AtVAMP71/AtVAMP7C gene family in Arabidopsis reduces drought tolerance by suppression of abscisic acid-dependent stomatal closure. J. Exp. Bot. 2010;61:2615–2622. doi: 10.1093/jxb/erq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y., Melamed-Book N., Cagnac O., Ronen G., Nishri Y., Solomon M., Cohen G., Levine A. Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18008–18013. doi: 10.1073/pnas.0604421103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyman B., Geelen D., Quintero F.J., Blatt M.R. A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science. 1999;283:537–540. doi: 10.1126/science.283.5401.537. [DOI] [PubMed] [Google Scholar]
- Lin R.C., Scheller R.H. Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell Dev. Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- Lipka V., Dittgen J., Bednarek P., Bhat R., Wiermer M., Stein M., Landtag J., Brandt W., Rosahl S., Scheel D., et al. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science. 2005;310:1180–1183. doi: 10.1126/science.1119409. [DOI] [PubMed] [Google Scholar]
- Liu M., Peng Y., Li H., Deng L., Wang X., Kang Z. TaSYP71, a Qc-SNARE, contributes to wheat resistance against Puccinia striiformis f. sp. tritici. Front. Plant Sci. 2016;7:544. doi: 10.3389/fpls.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W., Mayer U., Jurgens G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell. 1996;84:61–71. doi: 10.1016/S0092-8674(00)80993-9. [DOI] [PubMed] [Google Scholar]
- Muller I., Wagner W., Volker A., Schellmann S., Nacry P., Kuttner F., Schwarz-Sommer Z., Mayer U., Jurgens G. Syntaxin specificity of cytokinesis in Arabidopsis. Nat. Cell Biol. 2003;5:531–534. doi: 10.1038/ncb991. [DOI] [PubMed] [Google Scholar]
- Nisa Z.U., Mallano A.I., Yu Y., Chen C., Duan X., Amanullah S., Kousar A., Baloch A.W., Sun X., Tabys D., et al. GsSNAP33, a novel Glycine soja SNAP25-type protein gene: improvement of plant salt and drought tolerances in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2017;119:9–20. doi: 10.1016/j.plaphy.2017.07.029. [DOI] [PubMed] [Google Scholar]
- Pan L., Yu X., Shao J., Liu Z., Gao T., Zheng Y., Zeng C., Liang C., Chen C. Transcriptomic profiling and analysis of differentially expressed genes in asparagus bean (Vigna unguiculata ssp sesquipedalis) under salt stress. PLoS One. 2019;14:e0219799. doi: 10.1371/journal.pone.0219799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant S.R., Matsye P.D., McNeece B.T., Sharma K., Krishnavajhala A., Lawrence G.W., Klink V.P. Syntaxin 31 functions in Glycine max resistance to the plant parasitic nematode Heterodera glycines. Plant Mol. Biol. 2014;85:107–121. doi: 10.1007/s11103-014-0172-2. [DOI] [PubMed] [Google Scholar]
- Park M., Krause C., Karnahl M., Reichardt I., El Kasmi F., Mayer U., Stierhof Y.D., Hiller U., Strompen G., Bayer M., et al. Concerted action of evolutionarily ancient and novel SNARE complexes in flowering-plant cytokinesis. Dev. Cell. 2018;44:500–511. doi: 10.1016/j.devcel.2017.12.027. [DOI] [PubMed] [Google Scholar]
- Park M., Touihri S., Muller I., Mayer U., Jurgens G. Sec1/Munc18 protein stabilizes fusion-competent syntaxin for membrane fusion in Arabidopsis cytokinesis. Dev. Cell. 2012;22:989–1000. doi: 10.1016/j.devcel.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Piofczyk T., Jeena G., Pecinka A. Arabidopsis thaliana natural variation reveals connections between UV radiation stress and plant pathogen-like defense responses. Plant Physiol. Biochem. 2015;93:34–43. doi: 10.1016/j.plaphy.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Reichardt I., Slane D., El Kasmi F., Knoll C., Fuchs R., Mayer U., Lipka V., Jurgens G. Mechanisms of functional specificity among plasma-membrane syntaxins in Arabidopsis. Traffic. 2011;12:1269–1280. doi: 10.1111/j.1600-0854.2011.01222.x. [DOI] [PubMed] [Google Scholar]
- Sanderfoot A. Increases in the number of SNARE genes parallels the rise of multicellularity among the green plants. Plant Physiol. 2007;144:6–17. doi: 10.1104/pp.106.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Khurana P., Khurana J.P., Singh P. Gene encoding vesicle-associated membrane protein-associated protein from Triticum aestivum (TaVAP) confers tolerance to drought stress. Cell Stress Chaperones. 2018;23:411–428. doi: 10.1007/s12192-017-0854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Yadav N.S., Tiwari V., Agarwal P.K., Jha B. A SNARE-like superfamily protein SbSLSP from the halophyte Salicornia brachiata confers salt and drought tolerance by maintaining membrane stability, K(+)/Na(+) ratio, and antioxidant cachinery. Front. Plant Sci. 2016;7:737. doi: 10.3389/fpls.2016.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Stein M., Dittgen J., Sanchez-Rodriguez C., Hou B.H., Molina A., Schulze-Lefert P., Lipka V., Somerville S. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell. 2006;18:731–746. doi: 10.1105/tpc.105.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer D., Jahn R., Brunger A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Uemura T., Kim H., Saito C., Ebine K., Ueda T., Schulze-Lefert P., Nakano A. Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1784–1789. doi: 10.1073/pnas.1115146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Nakano R.T., Takagi J., Wang Y., Kramer K., Finkemeier I., Nakagami H., Tsuda K., Ueda T., Schulze-Lefert P., et al. A Golgi-released subpopulation of the trans-Golgi network mediates protein secretion in Arabidopsis. Plant Physiol. 2019;179:519–532. doi: 10.1104/pp.18.01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L.C., Rep M., Pieterse C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- Waghmare S., Lefoulon C., Zhang B., Liliekyte E., Donald N., Blatt M.R. K+ channel-SEC11 binding exchange regulates SNARE assembly for secretory traffic. Plant Physiol. 2019;181:1096–1113. doi: 10.1104/pp.19.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare S., Liliekyte E., Karnik R., Goodman J.K., Blatt M.R., Jones A.M.E. SNAREs SYP121 and SYP122 mediate the secretion of distinct cargo subsets. Plant Physiol. 2018;178:1679–1688. doi: 10.1104/pp.18.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Weaver N.D., Kesarwani M., Dong X. Induction of protein secretory pathway is required for systemic acquired resistance. Science. 2005;308:1036–1040. doi: 10.1126/science.1108791. [DOI] [PubMed] [Google Scholar]
- Wang P., Sun Y., Pei Y., Li X., Zhang X., Li F., Hou Y. GhSNAP33, a t-SNARE protein from Gossypium hirsutum, mediates resistance to Verticillium dahliae infection and tolerance to drought stress. Front. Plant Sci. 2018;9:896. doi: 10.3389/fpls.2018.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Zhang X., Ma X., Sun Y., Liu N., Li F., Hou Y. Identification of CkSNAP33, a gene encoding synaptosomal-associated protein from Cynanchum komarovii, that enhances Arabidopsis resistance to Verticillium dahliae. PLoS One. 2017;12:e0178101. doi: 10.1371/journal.pone.0178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.J., Wang Y.L., Wei Z.X., Yuan H.J., Zeng X.Q., Tashi N. Cloning and functional characterization of the HbSYR1 gene encoding a syntaxin-related protein in Tibetan hulless barley (Hordeum vulgare L. var. nudum HK. f.) Genet. Mol. Res. 2017;16:gmr16038909. doi: 10.4238/gmr16038909. [DOI] [PubMed] [Google Scholar]
- Xue Y., Yang Y., Yang Z., Wang X., Guo Y. VAMP711 is required for abscisic acid-mediated inhibition of plasma membrane H(+)-ATPase activity. Plant Physiol. 2018;178:1332–1343. doi: 10.1104/pp.18.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Liao C.Y., Tang J., Bassham D.C. Overexpression of trans-Golgi network t-SNAREs rescues vacuolar trafficking and TGN morphology defects in a putative tethering factor mutant. Plant J. 2019;99:703–716. doi: 10.1111/tpj.14353. [DOI] [PubMed] [Google Scholar]
- Yi C., Park S., Yun H.S., Kwon C. Vesicle-associated membrane proteins 721 and 722 are required for unimpeded growth of Arabidopsis under ABA application. J. Plant Physiol. 2013;170:529–533. doi: 10.1016/j.jplph.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Yun H.S., Kwaaitaal M., Kato N., Yi C., Park S., Sato M.H., Schulze-Lefert P., Kwon C. Requirement of vesicle-associated membrane protein 721 and 722 for sustained growth during immune responses in Arabidopsis. Mol. Cells. 2013a;35:481–488. doi: 10.1007/s10059-013-2130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H.S., Kwon C. Vesicle trafficking in plant immunity. Curr. Opin. Plant Biol. 2017;40:34–42. doi: 10.1016/j.pbi.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Yun H.S., Yi C., Kwon H., Kwon C. Model for regulation of VAMP721/722-mediated secretion: growth vs. stress responses. Plant Signal. Behav. 2013b;8:e27116. doi: 10.4161/psb.27116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Karnik R., Wang Y., Wallmeroth N., Blatt M.R., Grefen C. The Arabidopsis R-SNARE VAMP721 interacts with KAT1 and KC1 K+ channels to moderate K+ current at the plasma membrane. Plant Cell. 2015;27:1697–1717. doi: 10.1105/tpc.15.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao H., Gao S., Wang W.C., Katiyar-Agarwal S., Huang H.D., Raikhel N., Jin H. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393(*)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol. Cell. 2011;42:356–366. doi: 10.1016/j.molcel.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Gong Z., Zhang C., Song C.P., Damsz B., Inan G., Koiwa H., Zhu J.K., Hasegawa P.M., Bressan R.A. OSM1/SYP61: a syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell. 2002;14:3009–3028. doi: 10.1105/tpc.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]