Abstract
As the anucleate cells responsible for hemostasis and thrombosis, platelets are exposed to a myriad of biophysical and biochemical stimuli within vasculature and heterogeneous blood clots. Highly controlled, reductionist in vitro imaging studies have been instrumental in providing a detailed and quantitative understanding of platelet biology and behavior, and have helped elucidate some surprising functions of platelets. In this review, we highlight the tools and approaches that enable visualization of platelets in conjunction with precise control over the local biofluidic and biochemical microenvironment. We also discuss next generation tools that add further control over microenvironment cell stiffness or enable visualization of the interactions between platelets and endothelial cells. Throughout the review, we include pragmatic knowledge on imaging systems, experimental conditions, and approaches that have proved to be useful to our in vitro imaging studies of platelets under flow.
Introduction
Platelets are blood cell fragments that aggregate at intravascular injury sites to collectively stem hemorrhage and restore hemostasis. Hemostasis is a hypersensitive process that is regulated through feedback mechanisms to achieve a balance between bleeding (too little clotting) and thrombosis (too much clotting). During this process, a highly complex myriad of biophysical and biochemical signaling occurs, including tissue factor-induced thrombin generation and inter-platelet signaling. As platelets are central to hemostatic process, understanding their role in the initiation, development, and retraction of a blood clot is key to understanding both healthy hemostasis and pathological bleeding or clotting. In vivo investigations have been vital to our understanding of hemostasis; however, the native clotting environment is innately heterogeneous, complex, and dynamic, making it difficult to determine the underlying mechanisms that govern platelet behavior. Highly controlled, reductionist in vitro studies have been instrumental in providing a detailed and quantitative understanding of platelet biology and behavior within the context of healthy and pathological clotting. In vitro systems enable exceptional control over the presented ligands, substrate stiffness, biochemical agonist identity and concentrations, and shear stresses experienced by platelets. As such, in vitro systems are often used synergistically for detailed investigations into platelet behavior observed in vivo, which is demonstrated by a recent study that investigated pathologic clot formation in diabetes, which was shown to be mediated by biophysical forces[1]. Using in vitro-based approaches, the authors were able to show that increased compression forces applied to circulating platelets in diabetes were linked to increased in vivo clot formation that was resistant to standard antiplatelet therapies.
The majority of in vitro studies that investigate the nuanced behavior of platelets rely on imaging methodologies to capture the dynamic response of platelets to various experimental inputs. The findings of these studies have been critical in developing a body of literature that has shaped our understanding of fundamental platelet biology. Previous work has indicated that microenvironmental cues, such as the mechanical properties of the underlying matrix substrate[2, 3], matrix geometry[4], biochemical conditions[5], and shear stress[6, 7], mediate platelet physiology at the single cell level. Recent work using in vitro imaging setups has elucidated intriguing aspects regarding factors that influence platelet behavior and platelet function itself, such as the aforementioned sensitivity to compressive forces[1], links between single platelet force and bleeding[8], and the ability of platelets to mechanoscavenge bacteria in circulation[9]. As in vitro-based systems can be optimized for high resolution imaging, they have also enabled insights into the unusual morphologies that platelets may undertake when exposed to physiological or pathological conditions[10].
Technological advances that enable precise control of the local mechanical, shear, and biochemical micro-environment, have led to new experimental systems that are conducive to imaging, portable, inexpensive, and easy to use. The goal of this work is to provide the reader with an overview of the current state of the art in platelet imaging techniques by discussing of the types of experiments and experimental setups that are currently possible in addition to their limitations. We discuss how modern imaging systems have enabled measurements of single platelets, discrete sub-populations, and indiscriminate aggregates. We also seek to complement a number of excellent reviews on platelets and blood-based microfluidic devices [11–13], by including pragmatic information on imaging setups and conditions that we have found to be successful.
Imaging considerations
A critical consideration for in vitro investigations into platelet behavior is their isolation and purification from whole blood via centrifugation without inadvertent activation, as platelets are shear sensitive. Platelet purification is often necessary for reductionist studies on platelet behavior or non-specific cell staining protocols, in which a serum-free environment or wash step is required to minimize background noise. Numerous protocols have been developed to minimize platelet activation by the addition of anticoagulants and platelet inhibitors or by optimizing centrifugation time, acceleration, and spin speed[14, 15]. In our experience, achieving perfectly quiescent platelets after more than one high-speed centrifugation step, even in the presence of prostaglandins, low pH, and high citrate concentrations, remains difficult. Each cycle of high-speed centrifugation increases the likelihood of pre-activation, visualized by shape change, and/or platelet clumping, which makes it more difficult to resuspend the platelet plug into solution. One strategy our group has used to limit centrifugation cycles is to isolate platelets using sepharose gel filtration [8]. This method enables the isolation of quiescent platelets while simultaneously limiting centrifugation cycles, especially when prepared from platelet rich plasma. However, final platelet concentrations are lower than those prepared using centrifugal-based washes.
For both single platelet and multi-platelet in vitro studies, we have found continued success using membrane dyes (e.g., CellMask) as they label the platelets quickly, produce minimal background signal, and work for morphological studies with washed platelets and aggregation studies. Prolonged fluorescent exposure from high intensity light or long-term experiments can lead to phototoxicity, blebbing, and apoptosis, so it can be helpful to optimize imaging parameters in advance by balancing signal and noise with acquisition time and platelet activation. For whole blood, we have found success using platelet specific antibody stains, such as CD41. Acridine orange also works well for whole blood but also labels leukocytes in addition to platelets, so they may not be applicable for all experimental designs. In some instances, it can be advantageous to use platelets stained with different fluorophores to distinguish a unique population of platelets or better understand platelet interactions within a thrombus. Isolating and staining multiple populations of platelets with membrane dyes can be a viable approach, but it is important to note that the platelets will slowly acquire both stains over time, so the lifetime of these studies is limited.
Immunofluorescent staining can also elucidate the dynamics of key platelet signaling events. Visualizing early stage platelet events such as cytosolic[16–19] or mitochondrial calcium dynamics[20] have been instrumental for measuring the differing responses to ligands under flow. Staining of late stage markers of platelet activation, such as phosphatidylserine, a component of the inner cell membrane that is a key marker for apoptosis; P-selectin, a cell adhesion molecule stored in alpha-granules of unactivated platelets and released when activated; or ligand binding to αIIbβ3, a receptor for fibrinogen that aids in platelet activation, have enhanced understanding of platelet activation and how platelets respond to varying microenvironments[3, 8]. Standard platelet dyes, concentrations, and sources are provided in Table 1.
Table 1:
Common live cell stains for imaging platelets in vitro under flow conditions. Vendor list is not complete and not intended as an endorsement, but instead is a suggested starting point to help the reader. Unlabeled primary antibodies may also be pre-labeled with fluorophore conjugation kits.
| Purpose | Dye | Concentrations | Vendors | Incubation time/temperature | Useful Tips |
|---|---|---|---|---|---|
| Platelet-specific staining | CD41 primary antibody (clone M148) + Any color tagged Secondary Antibody (I.e Goat anti-mouse IgG) | CD41: 1:400 Secondary Antibody: 1:400 |
CD 41: Novus Biologicals Secondary Antibody: ThermoFisher |
Pre-incubation not necessary | Mix primary and secondary together immediately before the experiment |
| Membrane stain (non-specific) | CellMask (Orange, Deep Red, or Green) | 1:1000 | ThermoFisher | Pre-incubation not necessary | For prolonged live studies, minimize dye concentration and fluorescent imaging exposure times |
| Cytosolic calcium | Oregon Green BAPTA-1, AM + Fura Red, AM [66] | BAPTA-1: 1uM, Fura: 1.25 uM |
Molecular Probes, ThermoFisher | Incubate for 30 min at 37C | Can be used as ratio for more precision, or used independently |
| Mitochondrial Staining (non-specific) | MitoTracker Red CMXRos MitoTracker Green FM |
100nM [67] | ThermoFisher | Incubate for 15 min at 37C | Keep dye concentration low, high concentrations stain other cellular structures |
| Platelet activation (phosphatidyl serine) | Annexin V 488 [68] | 1:20 | Sigma; ThermoFisher | Pre-incubation not necessary, 5 mins sufficient if not staining in real-time | Conjugate fluorophores ranging from AF 250 to AF 680 available |
| Platelet activation (P-Selectin) | AK4, SC-19996 Ab + Any color tagged Secondary Antibody | 1:300 [3] | BD Biosciences, Santa Cruz Biotechnology | AK4: 1 hr incubation Secondary Antibody: 1 hr incubation |
May block function, but can be used in low conc. to minimally impact agg [5] |
| Platelet activation (αIIbβ3) | PAC-1 Ab (human) [69] JON/A (murine) [70] |
In conjunction with secondary (1μg/mL) [66], Conjugated with fluorophore (PAC1 FITC) (40μg/ml) [71] | Unconjugated: Invitrogen, ThermoFisher Conjugated: BD Biosciences (25ug/mL, conjugated) |
5 min sufficient if not staining in real-time [3] | Competitively binds, can reduce adhesion. |
If use of human platelets is not necessary, platelets from transgenic animal models that express a fluorescent protein can be used. LifeAct murine platelets can be used for single platelet investigations as they that appear to have fully functioning and stained actin cytoskeletons [21]. For multi-platelet studies, confetti platelets from transgenic mice [9] can be useful to distinguish individual platelets within an aggregate as they are randomly colored, which is a useful functionality when studying thrombus growth and dynamics on the single platelet scale.
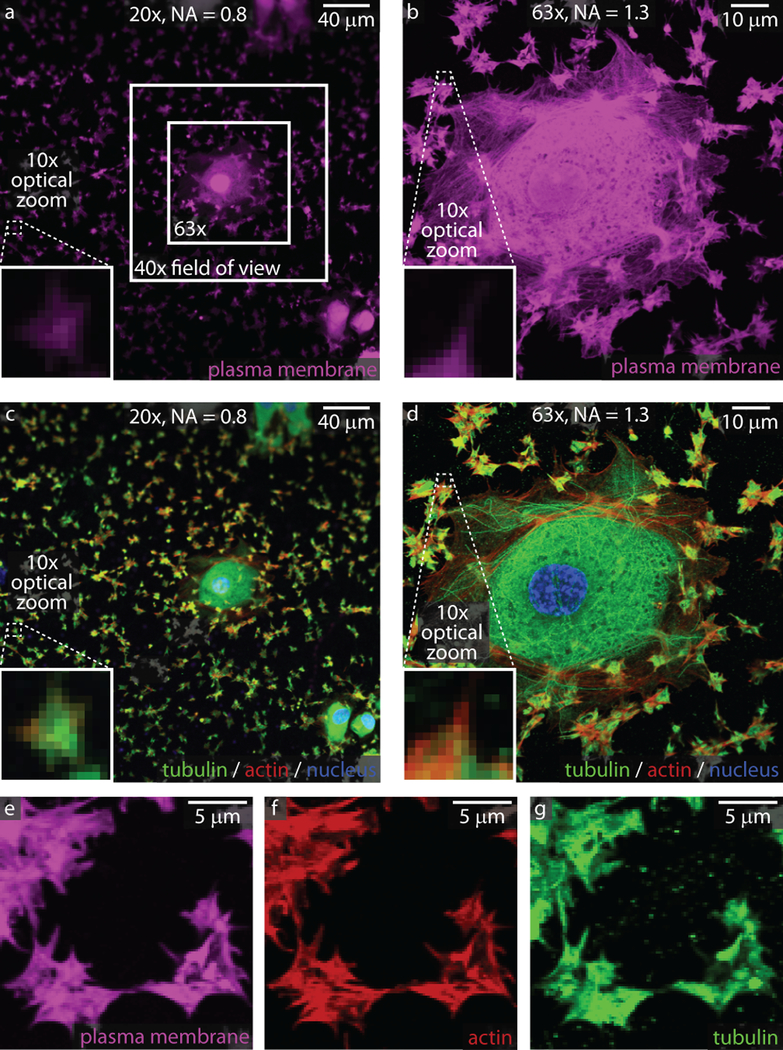
Another significant consideration in developing in vitro imaging methodologies to study platelets is their size. Human platelets have a resting diameter of approximately 2 μm, whereas murine platelets have a resting diameter of closer to 0.5 μm. Dogs, cows, and pigs, which are used for various hemostatic models, have platelet diameters similar to the size of human platelets. Platelets adhered to a fixed protein ligand or glass substrate will spread to approximately 8 μm (human) in diameter. The size discrepancy between platelets and other cell types can lead to challenges when an experiment requires the simultaneous imaging of both cell types in the same field of view, which is exemplified in Figure 1, which shows human umbilical vein endothelial cells (HUVECs) next to platelets. During the initial planning stages of an experiment, it is important to determine which aspects of platelet behavior are to be investigated and imaged. This will dictate the appropriate objective(s), imaging modality, fluorescent stain (if any), and the setup and logistical considerations of the experiment.
Figure 1: Imaging considerations for single platelets and aggregates.
Platelets are much smaller than most cells in the body, as can be seen next to a spread endothelial cell adhered to collagen and stained with a plasma membrane dye (a, b) or with actin/tubulin staining (c, d). At 20x, the general platelet morphology can be discerned. At higher magnifications and resolutions (63x), subcellular structures such as filipodia are visible with plasma dyes (b and e) or actin/tubulin staining (d, f, and g). Nuclei for the endothelial cell are shown in blue in panels c and d.
As image resolution can be estimated by dividing the wavelength of light by twice the numerical aperture, platelet features larger than 250 to 350 nm can be detected, depending on the fluorophore used. For multi-platelet imaging studies that measure platelet aggregation or thrombus size, lower magnifications can be useful as they image larger fields of view [22–27]. For single platelet studies, our group has found success imaging the number and morphology of human platelets on coverslips and hydrogels using a 20x lens with a numerical aperture (NA) of 0.8 [3, 8]. As the resolution increases at shorter wavelengths and larger numerical apertures, an objective lens with 40x or 63x magnification and a NA of 1.3 is typically used in our group to obtain data on the arrangement of proteins and organelles within single platelets [3, 8, 24, 25] (Figure 1). It is important to note that subcellular imaging of platelets works best on platelets that are fully spread, typically on glass substrates. Higher resolution images can be obtained using more advanced techniques such as super-resolution microscopy that requires additional sample preparation, such as platelet fixation and mounting, which is detailed in another excellent review in this series (REF from this series).
Several commonly used imaging modalities such as epifluorescent imaging, confocal imaging, and total internal reflectance imaging (TIRF) are applicable for imaging platelets under flow. Epifluorescent imaging illuminates the entire sample area and is widely accessible due to lower equipment costs. This imaging modality is superb for quick measurements and for visualizing whole platelets and platelet-platelet interactions. However, since light illuminates the entire sample, background noise from the surrounding liquid or out-of-plane sources can be problematic, particularly when image quantification is desired. To combat this and improve the signal to noise ratio, either confocal or TIRF imaging can be used. TIRF imaging illuminates a thin plane adjacent to the glass and has been used to great effect with DNA force reporters that measure platelet forces at the glass interface[28]. Although TIRF has yet to be performed on samples under flow, there is no pragmatic reason that prohibits its adaption to more dynamic experimental setups. Confocal microscopy illuminates a thicker plane than TIRF and can be used to image an entire cell volume to create 3D images of platelets. While 3D images have minimal utility in studying platelets at the single cell level, 3D renderings of platelet aggregates and thrombi can provide useful information on structure and composition. As our groups have access to superb equipment maintained by institutional microscopy cores, our preferred imaging modality for static and flow experiments has been confocal microscopy, as it is facile to operate, produces high-quality images with low signal-to-noise ratio, and offers multiple image acquisition modes (e.g., tile scans and z-stacks). Historically, line-scan confocal microscopes require a tradeoff between speed and resolution; however, this has been eliminated in spinning disk confocal microscopes [14, 15, 21].
Experimental considerations for imaging studies under flow
The first in vitro systems developed to image platelets under flow were focused on imaging aggregates of platelets and platelet thrombus formation. The original systems used collagen-coated glass tubes with a wall thickness of 200 μm[29]. Later systems improved the imaging resolution by creating flow chambers constructed around glass coverslips [30]. Microfluidic devices were the natural evolution of these flow chambers and enabled several key improvements, including reduced blood volumes, improved ease of use, and more complex geometries. Microfluidic devices generally display a ligand or protein to facilitate initial platelet adhesion, such as collagen, fibrinogen, fibronectin, or molecular fragments, such as specified domains of von Willebrand Factor or RGD.
In designing an experiment to image platelet behavior under flow, microfluidic devices are an excellent choice given their accessibility. Several companies sell premade microfluidic devices of standard geometries, such as straight channels, that can be used to perform in vitro imaging experiments under multiple shear conditions [31–33]. As the technology is well-established, it is possible to find contract manufacturers to fabricate a custom mold by either submitting mold designs created with software (e.g., AutoCAD) or providing high-level input for the mold design. Using the molds to fabricate microfluidic devices is fairly straightforward and requires PDMS and access to a plasma cleaner to bond the microfluidic device together[34]. With the addition of tubing and a syringe pump, microfluidic devices become portable, enabling flow-based experiments to be conducted at nearly any inverted microscope. Some microfluidic devices employ a vacuum or non-permanent interaction to attach the microfluidic mold to glass instead of plasma bonding [11, 12]. While these offer the advantage of accessing the sample after the experiment for further testing or different analyses, they delaminate from the surface more easily so great care must be taken to ensure that leaks do not occur during the experiment. This is of particular concern as leakage can skews experimental results and may require cleaning and decontamination of the housing unit or microscopy equipment.
A number of interesting microfluidic geometries have simplified the collection of data or led to key insights into platelet physiology. To introduce the reader to the vast number of possibilities, a few unique geometries highlighting the capabilities of microfluidic devices are mentioned here. Such devices have made key insights into our understanding of platelet biology, such as the 90% stenosis model [6], which demonstrated that a rapid flow acceleration and deceleration leads to stabilized discoid platelet aggregates. Microfluidic devices enable the patterning of multiple substrates, biomolecules, and cells onto a surface[35], enabling studies on the effects of exposing platelets to multiple ligands, which more aptly recapitulates the in vivo environment. Due to their small size, another key advantage of microfluidic devices is the ability to monitor multiple experimental conditions at the same time in the same field of view [36]. The majority of devices require imaging of platelets and clots from the bottom surface; however, microfluidic devices can be designed to enable imaging along the side of a developing platelet aggregate or clot[37]. This side-view device highlights the extensive control that can be achieved over the flow conditions, as in this case, both the shear stress and pressure gradients were controlled independently.
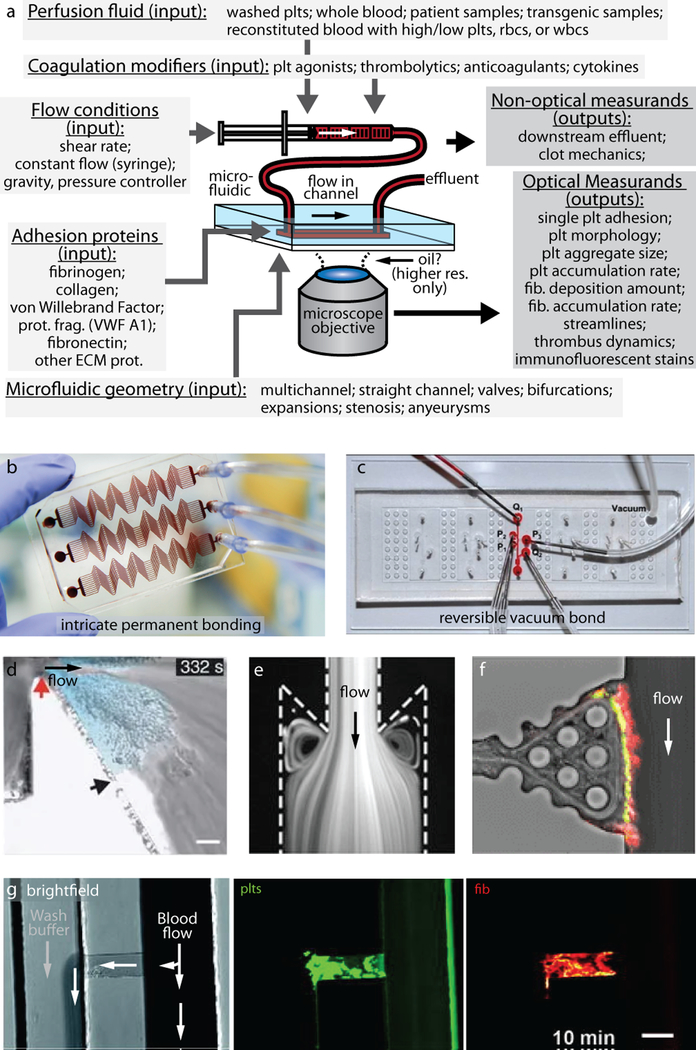
When designing a flow-based experiment using microfluidic devices, it can be helpful to consider the inputs that can be modulated and the experimental outputs that can be easily measured (Figure 2). The microfluidic geometry can be varied to model multiple areas in the body including venous valves[38], injured blood vessels with stenosis[12, 39], arteries[11], and extravascular compartments[40]. Experimental parameters within the microfluidic device can be adjusted to mimic in vivo conditions, such as the microfluidic channel coating (e.g., fibrin[40] and von Willebrand factor[12]), varying blood compositions (e.g., modifying hematocrit levels[41] and genetically modified culture-derived platelets[42]), and modifiers of coagulation (e.g., factor VIII antigen levels[43], nitric oxide concentration[44]). The typical measurements performed in these experiments include determining the thrombus area[39], extent of platelet accumulation and aggregation[11, 12], the rate of thrombus formation[40], extent of fibrin deposition[45], determining the optimal shear rate for platelet deposition[46], determining aggregate and thrombus volume [16], and identifying streamlines formed by blood flow[38]. Many of these systems can be adapted to study single platelet behaviors under flow condition by using a lower platelet number, such that single platelets are spread apart from one another on the surface of interest. In addition, higher magnification objectives are typically employed to enable better resolution as only a few platelets are imaged at a time.
Figure 2: Microfluidic devices represent an extremely versatile tool for studying platelets under flow conditions.
(a) Platelet and fibrin deposition parameters can be measured as a function of perfusion fluid, coagulation modifiers, flow conditions, adhesion proteins, and microfluidic geometry. Permanently bonded microfluidic devices[62] typically achieve more intricate geometries (b) then reversibly bonded microfluidic devices[37] (c), which offer access to the sample for further analysis. A variety of geometries such as stenoses (d) [6], valves (e) [38], or plugs[40] (g) can be created with microfluidic. Clots may be imaged from the side[37] (f) and platelets, fibrin, and coagulation proteins may be visualized utilizing immunofluorescent labels[40] (f, g).
Animal models have long been advantageous in the study of both hemostasis and thrombotic disorders as the highly complex in vivo environment has increased our mechanistic understanding and knowledge of disease outcomes that are relevant to human health. Murine platelets have played a vital role in hemostasis and thrombosis studies because of their genetic and functional similarity to human platelets, despite differences in size and structure. As such, combining in vitro microfluidic studies with genetically modified animal models has played an imperative role in illuminating biochemical and/or biophysical adhesion and aggregation processes that are difficult to discern in vivo. Gene knockout models have provided insight into the importance of specific gene mutations and various mechanistic processes. Various microfluidic systems have leveraged gene knockouts in order to specifically decipher the importance of α2β1 and PAR4[47], the importance of the PI3K signaling pathway[48], and the importance of talin1[49] in adhesion and aggregation of platelets to collagen and fibrinogen. Utilizing a commercial microfluidic system, an ADAMTS13 knockout mouse model has been used to better understand the pathophysiology of thrombotic thrombocytopenia purpura [50]. One study employed both knockout models of mice lacking GPVI and ex vivo inhibitors of αIIbβ3 and Src kinase to reveal the existence of two potential routes of platelet adhesion to collagen[51].
Next generation in vitro systems for studying platelets under flow
Next generation in vitro systems with increased complexity have begun to address some of the key limitations seen in standard microfluidic devices by providing new methods to optically measure platelet function or offering enhanced control over the mechanical or cellular microenvironment. There even appear to be translational potential of in vitro imaging from these While these systems overcome certain limitations, challenges remain regarding imaging resolution and system complexity.
Several approaches have recently been developed that enable the measurement the contraction force of single platelets that incorporate flow or can easily be adapted to introduce flow. Traction force microscopy determines platelet contraction on a hydrogel substrate by monitoring the displacement of a bead that is embedded in the hydrogel[52]. This has recently been adapted to flow conditions and used to demonstrate that platelets orient forces perpendicular to flow in high shear conditions[53]. Traction force microscopy is limited by the need to perform non-trivial computational analysis to calculate traction forces from bead displacements, as well as the need to have continuous images of beads before, during, and after contraction. Building on traction force microscopy, we recently overcame these challenges by developing the first microfluidic devices with patterned hydrogels to create a high-throughput platelet contraction cytometer capable of measuring the contraction force of hundreds to thousands of platelets on the single platelet level[8]. This system has translational potential as platelet contraction force has shown to be correlated with phenotypic bleeding and thus could be utilized as a biophysical biomarker to assess bleeding risk. A key advantage of these systems is that the hydrogel stiffness and ligand density can be independently controlled to test over the entire range of mechanical, shear, and biochemical conditions that are encountered in a clot.
Other work has focused on both aggregate and subcellular force measurements of platelets. Through the clever use of microengineering, other groups have designed systems that are well suited to measure forces associated with a developing aggregate, which may find use in the clinical setting[54]. Another interesting approach has enabled the measurements of elasticity and contraction on a platelet/collagen hybrid[55]. Finally, several DNA-based tension sensors[28, 56, 57] have recently been published and have revealed exquisite details on the generation of subcellular forces on a glass substrate or lipid membrane. While these have been employed under static flow conditions, these systems could be adapted for use in microfluidic devices and tested under flow conditions.
Hemostasis includes a plethora of interactions between platelets, the endothelium, coagulation factors, and red and white blood cells. However, most assays investigate one or two parts of hemostasis in isolation, despite the importance of combined interactions in various diseased states. Using the advances of in vitro technology, researches have been able to investigate blood-endothelial interactions by fabricating an “endothelialized” microfluidic device that closely recapitulates the microvasculature with control over the biochemical and shear environment. Monitoring this system with spinning disc confocal microscopy enables investigations into the interactions between, platelets, white blood cells, and endothelial cells that cause adhesion, aggregation, and cytokine production. These systems have resulted in new insights and enhanced our understanding of hematologic diseases, especially those involving microvascular occlusion and thrombosis, such as sickle cell disease, hemolytic uremic syndrome, and malaria [23, 34, 58, 59]. These systems have even been simplified, such that they can be fabricated in under 2 hrs using commonly available lab supplies [60]. More complex systems have been created to model bleeding in order to study hemostasis after a mechanical vascular injury is sustained with single cell resolution by way of confocal microscopy [61]. By coupling an endothelialized microchannel with a pneumatic valve that induces injury, the in vitro system models in vivo mechanical vascular injury. This enabled the visualization of hemostatic plug formation and quantitative measurements of altered hemostasis in pathological clotting states, such as inhibition of αIIbβ3 and factor VIII deficiency. Moreover, chemical fixation of endothelial cells in these microfluidic devices has been used to improve the longevity of these devices. It was shown that hemostasis and thrombus formation was retained after storage for 36 hrs at 4°C [62]. However, loss of functional response, such as impaired release of bioactive messengers, was seen after storage.
Conclusion
While we have endeavored to highlight the significant opportunities available for in vitro imaging of platelets under flow, we also would like to highlight some of the limitations of these systems. First, re-calcifying the blood or platelet solution while in the syringe will activate the platelets and eventually lead to clots in the syringe that will clog the microfluidic device. However, it is possible to incorporate microfluidic mixers that mix a calcium solution with blood before entering a region of interest within the microfluidic channel [63, 64]. More recent work has also shown that platelets are mechanosensitive [3, 8, 53]. As many microfluidic devices employ stiff materials, platelet mechanosensitivity may influence results. Next generation systems can be fabricated from soft materials [8, 23, 53] but introduce additional imaging and experimental complications. For example, polyacrylamide gels are commonly used in these systems but have varied optical properties and are even opaque in some cases. Low cross-linker concentrations help to maintain transparent gels to enable the collection of clear and precise images [65]. Overall, the significant advantages of flow-based in vitro systems for optical investigations of platelets overshadows the few limitations, which is illustrated by the profound insights and knowledge gained through these investigations.
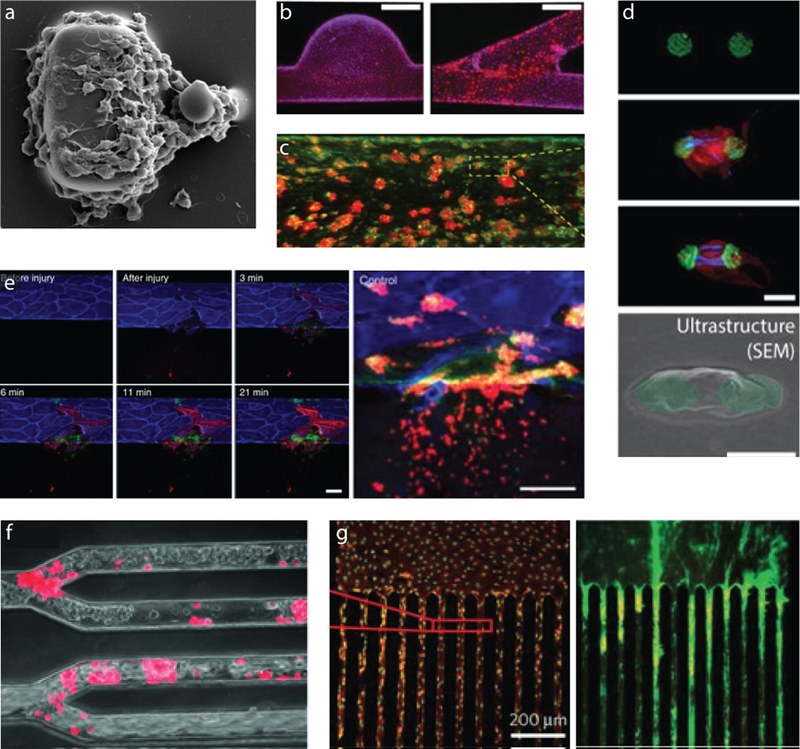
Figure 3: Next generation in vitro platelet imaging systems incorporate flow to measure dynamic functions of platelets in vitro.
(a) The combined aggregation and force potential of flowing platelets is measured by measuring the deformation of the smaller post [54]. (b) Various geometries covered by endothelial cells enable studies of how geometry, flow, and the presence of an endothelium influence platelet aggregation[60]. (c) More portable devices utilizing fixed endothelial cells can also measure platelet function[62]. (d) Single platelet contraction forces can be measured in a high-throughput manner, using large numbers of these arrays under flow conditions in microfluidic devices[8]. (e) Integrating valves into microfluidic channels with endothelial cells can even lead to a system that can be injured and “bleed”, which is useful for modeling hemostasis[61]. (f) Multi-channel microfluidic devices reveal that platelets can interact with endothelial cells in numerous geometries[58] and can be used to (g) image multiple shear conditions simultaneously[36].
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ju L, et al. , Compression force sensing regulates integrin αIIbβ3 adhesive function on diabetic platelets. Nature Communications, 2018. 9(1): p. 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam WA, et al. , Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nature materials, 2011. 10(1): p. 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu Y, et al. , Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proceedings of the National Academy of Sciences of the United States of America, 2014. 111(40): p. 14430–14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kita A, et al. , Microenvironmental geometry guides platelet adhesion and spreading: a quantitative analysis at the single cell level. PloS one, 2011. 6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stalker TJ, et al. , Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood, 2013. 121(10): p. 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nesbitt WS, et al. , A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nature medicine, 2009. 15(6): p. 665–673. [DOI] [PubMed] [Google Scholar]
- 7.Kroll MH, et al. , Platelets and shear stress. Blood, 1996. 88(5): p. 1525–1541. [PubMed] [Google Scholar]
- 8.Myers DR, et al. , Single-platelet nanomechanics measured by high-throughput cytometry. Nature materials, 2017. 16(2): p. 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaertner F, et al. , Migrating Platelets Are Mechano-scavengers that Collect and Bundle Bacteria. Cell, 2017. 171(6): p. 1368–1382.e23. [DOI] [PubMed] [Google Scholar]
- 10.Schwertz H, et al. , Anucleate platelets generate progeny. Blood, 2010. 115(18): p. 3801–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branchford BR, et al. , Microfluidic technology as an emerging clinical tool to evaluate thrombosis and hemostasis. Thrombosis Research, 2015. 136(1): p. 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu S, et al. , In microfluidico: Recreating in vivo hemodynamics using miniaturized devices. Biorheology, 2015. 52(5–6): p. 303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastings SM, Griffin MT, and Ku DN, Hemodynamic studies of platelet thrombosis using microfluidics. Platelets, 2017. 28(5): p. 427–433. [DOI] [PubMed] [Google Scholar]
- 14.Watson SP and Authi KS, Platelets : a practical approach. 1996, Oxford; New York: IRL Press at Oxford University Press. [Google Scholar]
- 15.White MM, Jennings LK, and Condry MP, Platelet protocols : research and clinical laboratory procedures. 2008, San Diego: Academic Press. [Google Scholar]
- 16.Goto S, et al. , Dependence of Platelet Thrombus Stability on Sustained Glycoprotein IIb/IIIa Activation Through Adenosine 5′-Diphosphate Receptor Stimulation and Cyclic Calcium Signaling. Journal of the American College of Cardiology, 2006. 47(1): p. 155–162. [DOI] [PubMed] [Google Scholar]
- 17.Nesbitt WS, et al. , Distinct glycoprotein Ib/V/IX and integrin alpha IIbbeta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem, 2002. 277(4): p. 2965–72. [DOI] [PubMed] [Google Scholar]
- 18.Kuwahara M, et al. , Cytosolic calcium changes in a process of platelet adhesion and cohesion on a von Willebrand factor-coated surface under flow conditions. Blood, 1999. 94(4): p. 1149–1155. [PubMed] [Google Scholar]
- 19.Mazzucato M, et al. , Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibα mechanoreceptor. Blood, 2002. 100(8): p. 2793–2800. [DOI] [PubMed] [Google Scholar]
- 20.Choo H-J, et al. , Mitochondrial calcium and reactive oxygen species regulate agonist-initiated platelet phosphatidylserine exposure. Arteriosclerosis, thrombosis, and vascular biology, 2012. 32(12): p. 2946–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schachtner H, et al. , Tissue inducible Lifeact expression allows visualization of actin dynamics in vivo and ex vivo. European Journal of Cell Biology, 2012. 91(11): p. 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown AC, et al. , Ultrasoft microgels displaying emergent platelet-like behaviours. Nature Materials, 2014. 13: p. 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu Y, et al. , Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nature Biomedical Engineering, 2018. 2(6): p. 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen CE, et al. , Platelet–Microcapsule Hybrids Leverage Contractile Force for Targeted Delivery of Hemostatic Agents. ACS Nano, 2017. 11(6): p. 5579–5589. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai Y, et al. , Platelet geometry sensing spatially regulates α-granule secretion to enable matrix self-deposition. Blood, 2015. 126(4): p. 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciciliano JC, et al. , Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. Blood, 2015. 126(6): p. 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciciliano JC, et al. , Probing blood cell mechanics of hematologic processes at the single micron level. Lab on a Chip, 2017. 17(22): p. 3804–3816. [DOI] [PubMed] [Google Scholar]
- 28.Brockman JM, et al. , Mapping the 3D orientation of piconewton integrin traction forces. Nature Methods, 2017. 15: p. 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams GA, et al. , Kinetics of platelet adhesion and thrombus growth. Blood, 1983. 62(1): p. 69–74. [PubMed] [Google Scholar]
- 30.Hubbell JA and McIntire LV, Technique for visualization and analysis of mural thrombogenesis. Review of Scientific Instruments, 1986. 57(5): p. 892–897. [DOI] [PubMed] [Google Scholar]
- 31.ibidi GmbH. 2019; Available from: www.ibidi.com.
- 32.Cellix Ltd. 2019; Available from: www.wearecellix.com.
- 33.Fluxion Biosciences Inc. 2019; Available from: www.fluxionbio.com.
- 34.Myers DR, et al. , Endothelialized Microfluidics for Studying Microvascular Interactions in Hematologic Diseases. JoVE, 2012(64): p. e3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rana K, Timmer BJ, and Neeves KB, A combined microfluidic-microstencil method for patterning biomolecules and cells. Biomicrofluidics, 2014. 8(5): p. 056502–056502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown AC, et al. , Ultrasoft microgels displaying emergent platelet-like behaviours. Nat Mater, 2014. 13(12): p. 1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthard RW and Diamond SL, Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab on a chip, 2013. 13(10): p. 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehmann M, et al. , Platelets Drive Thrombus Propagation in a Hematocrit and Glycoprotein VI–Dependent Manner in an In Vitro Venous Thrombosis Model. Arteriosclerosis, Thrombosis, and Vascular Biology, 2018. 38(5): p. 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin MT, Kim D, and Ku DN, Shear-induced platelet aggregation: 3D-grayscale microfluidics for repeatable and localized occlusive thrombosis. Biomicrofluidics, 2019. 13(5): p. 054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoeman RM, et al. , A Microfluidic Model of Hemostasis Sensitive to Platelet Function and Coagulation. Cellular and Molecular Bioengineering, 2017. 10(1): p. 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walton BL, et al. , Elevated hematocrit enhances platelet accumulation following vascular injury. Blood, 2017. 129(18): p. 2537–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamat V, et al. , Microfluidic assessment of functional culture-derived platelets in human thrombi under flow. Experimental Hematology, 2015. 43(10): p. 891–900.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoeman RM, Lehmann M, and Neeves KB, Flow chamber and microfluidic approaches for measuring thrombus formation in genetic bleeding disorders. Platelets, 2017. 28(5): p. 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sylman JL, et al. , The Relative Role of Soluble Guanylyl Cylase Dependent and Independent Pathways in Nitric Oxide Inhibition of Platelet Aggregation Under Flow. Cellular and Molecular Bioengineering, 2014. 7(3): p. 421–431. [Google Scholar]
- 45.Colace TV, et al. , Microfluidic assay of hemophilic blood clotting: distinct deficits in platelet and fibrin deposition at low factor levels. Journal of thrombosis and haemostasis : JTH, 2014. 12(2): p. 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neeves KB, et al. , Sources of Variability in Platelet Accumulation on Type 1 Fibrillar Collagen in Microfluidic Flow Assays. PLOS ONE, 2013. 8(1): p. e54680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neeves KB, et al. , Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. Journal of Thrombosis and Haemostasis, 2008. 6(12): p. 2193–2201. [DOI] [PubMed] [Google Scholar]
- 48.Martin V, et al. , Deletion of the p110β isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood, 2010. 115(10): p. 2008–2013. [DOI] [PubMed] [Google Scholar]
- 49.Nieswandt B, et al. , Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. The Journal of Experimental Medicine, 2007. 204(13): p. 3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bao J, et al. , Carboxyl terminus of ADAMTS13 directly inhibits platelet aggregation and ultra large von Willebrand factor string formation under flow in a free-thiol-dependent manner. Arteriosclerosis, thrombosis, and vascular biology, 2014. 34(2): p. 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auger JM, et al. , Adhesion of human and mouse platelets to collagen under shear: a unifying model. The FASEB Journal, 2005. 19(7): p. 825–827. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz Henriques S, et al. , Force field evolution during human blood platelet activation. Journal of Cell Science, 2012. 125(16): p. 3914. [DOI] [PubMed] [Google Scholar]
- 53.Hanke J, et al. , Human blood platelets contract in perpendicular direction to shear flow. Soft Matter, 2019. 15(9): p. 2009–2019. [DOI] [PubMed] [Google Scholar]
- 54.Ting LH, et al. , Contractile forces in platelet aggregates under microfluidic shear gradients reflect platelet inhibition and bleeding risk. Nature Communications, 2019. 10(1): p. 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Z, et al. , Microclot array elastometry for integrated measurement of thrombus formation and clot biomechanics under fluid shear. Nature Communications, 2019. 10(1): p. 2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, et al. , Platelet integrins exhibit anisotropic mechanosensing and harness piconewton forces to mediate platelet aggregation. Proceedings of the National Academy of Sciences, 2018. 115(2): p. 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, et al. , Force-activatable biosensor enables single platelet force mapping directly by fluorescence imaging. Biosensors and Bioelectronics, 2018. 100: p. 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai M, et al. , In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. The Journal of Clinical Investigation, 2012. 122(1): p. 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Y, et al. , In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the National Academy of Sciences, 2012. 109(24): p. 9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mannino RG, et al. , “Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions”. Scientific Reports, 2015. 5: p. 12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakurai Y, et al. , A microengineered vascularized bleeding model that integrates the principal components of hemostasis. Nature Communications, 2018. 9(1): p. 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jain A, et al. , Assessment of whole blood thrombosis in a microfluidic device lined by fixed human endothelium. Biomedical microdevices, 2016. 18(4): p. 73–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehmann M, et al. , On-chip recalcification of citrated whole blood using a microfluidic herringbone mixer. Biomicrofluidics, 2015. 9(6): p. 064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muthard RW and Diamond SL, Rapid on-chip recalcification and drug dosing of citrated whole blood using microfluidic buffer sheath flow. Biorheology, 2014. 51(2–3): p. 227–37. [DOI] [PubMed] [Google Scholar]
- 65.Denisin AK and Pruitt BL, Tuning the Range of Polyacrylamide Gel Stiffness for Mechanobiology Applications. ACS Applied Materials & Interfaces, 2016. 8(34): p. 21893–21902. [DOI] [PubMed] [Google Scholar]
- 66.Yap CL, et al. , Synergistic adhesive interactions and signaling mechanisms operating between platelet glycoprotein Ib/IX and integrin alpha IIbbeta 3. Studies in human platelets ans transfected Chinese hamster ovary cells. J Biol Chem, 2000. 275(52): p. 41377–88. [DOI] [PubMed] [Google Scholar]
- 67.Marcondes NA, et al. , Comparison of JC-1 and MitoTracker probes for mitochondrial viability assessment in stored canine platelet concentrates: A flow cytometry study. Cytometry. Part A : the journal of the International Society for Analytical Cytology, 2019. 95(2): p. 214–218. [DOI] [PubMed] [Google Scholar]
- 68.Schoenwaelder SM, et al. , 14–3-3ζ regulates the mitochondrial respiratory reserve linked to platelet phosphatidylserine exposure and procoagulant function. Nature Communications, 2016. 7(1): p. 12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shattil SJ, et al. , Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. The Journal of biological chemistry, 1985. 260(20): p. 11107–11114. [PubMed] [Google Scholar]
- 70.Bergmeier W, et al. , Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry, 2002. 48(2): p. 80–86. [DOI] [PubMed] [Google Scholar]
- 71.Frelinger AL 3rd, et al. , Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood, 2015. 126(7): p. 873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]