Abstract
Background/Objectives:
The winter holiday season in the US, which spans mid-November to mid-January, contributes to over half of annual body weight gain. Although self-reported data has linked this weight change to both increased energy intake and reduced physical activity, objective techniques have never been used and thus the actual cause of holiday weight gain is controversial. Here, we aimed to determine changes in components of energy balance leading to the holiday weight gain.
Methods:
Body weight change was compared between the pre-holiday (mid-September to mid-November) and the holiday period (mid-November to early January). Total energy expenditure (TEE) was measured using doubly labeled water during holiday time (early to mid-December). Subjective (ratings) and physiological (appetite-regulating hormones) measures of appetite, eating-away-from-home frequency, and incentive salience of food pictures were also evaluated.
Results:
In 23 obese adults (87% female), body weight change during the holidays (0.41 ± 0.42 kg) was significantly higher (P=0.02) than the body weight change during the pre-holiday period (−0.86 kg ± 0.42 kg). The TEE was unchanged during the two periods, suggesting no role of energy expenditure on weight gain. However, participants reported lower satisfaction after a meal pre-load which was significantly correlated with increased body weight during the holiday period. An increase in number of episodes of eating at sit-down restaurants was also reported during that period. Overall, these changing behaviors were supported by a non-significant increase in energy intake (+80 kcal/day, P=0.07) observed during the study holiday period.
Conclusion:
We conclude that a decrease in energy expenditure does not result in the weight increase, but that an increase in food intake is the more likely cause. Our data imply that compromised internal satiety mechanisms in presence of external food cues and diet related behavioral variables during the holidays may influence this weight gain.
INTRODUCTION
As the prevalence of obesity continues to rise in the United States1, considerable efforts are made to understand the environmental factors that impact weight gain. Multiple longitudinal studies indicate that small seasonal fluctuations in body weight, especially between the holiday months of mid-November and mid-January (~8 weeks), contribute to more than half of the weight gained annually2–9. Most importantly, this weight is not subsequently lost4 and can lead to a substantial increase of 15–30 pounds over multiple decades. Obese adults in particular are vulnerable to gaining more weight during this critical time4, 5. Self-reported data imply that this weight increase could be due to excess energy intake and/or lower physical activity4. A secondary analysis of a cross-sectional dataset testing whether high TEE measured prior to the holiday months (late summer or early fall) is predictive of weight gain during the holiday period, did not support the role of energy expenditure on the holiday weight gain6. Similarly, exercise performed regularly during the Christmas period did not protect against weight gain7. Evidently, objective techniques have never been used and thus the actual cause for holiday weight gain is controversial.
Characteristically, the holidays are accompanied with easy access to a large variety of calorie-dense foods. Previous work shows that constant exposure to such food cues10, combined with social facilitation11, and stress due to increase in alcohol intake, changes in sleep and activity patterns, increased contact with family members, financial stress, loneliness, party planning, holiday shopping, meal preparations etc.12 can increase energy intake from highly rewarding foods11, 13, something that is common around the holidays and humorously captured in Ode on Health and Holidays14. These rewarding foods may trigger alterations in neural pathways and cause a conscious increase in eating, leading to the holiday weight gain, particularly in obese adults. Continuous exposure to food may also cause disruption of food reward circuitry by dysregulating appetite-related hormones15. Another trait typical to the holidays, and a possible contributor to related weight increases, is frequent opportunities for prolonged and socially expected overconsumption of food outside the home16. With a myriad of possible contributors, research has yet to determine whether changes in one or multiple factors may drive weight gain during the holidays.
Here we aimed to test our hypothesis that an increase in body weight during the holiday period will be largely due to an increase in energy intake rather than a decrease in energy expenditure. In the US, the period between Thanksgiving and New Year’s Eve involves a series of events such as holiday parties, frequent dinners with friends and family, vacation, watching football games, office holiday bake-offs etc. providing ample opportunities to indulge in overeating and thus may significantly impact these energy balance components. We also examined differences in endocrinological (such as ghrelin, leptin, PYY, and insulin), and behavioral factors (such as, eating-away-from-home (EH) behavior, stress, motivational eating with exposure to food cues) between the pre-holiday and the holiday period in obese adults. We predicted that the increase in energy intake during the holiday period will be influenced by amplifying pro-feeding hormones, increased frequency of EH, higher perceived value of food cues, and high subjective appetite levels. This information is critical for developing targeted behavior modification approaches for weight gain prevention during this weight gain period.
SUBJECTS AND METHODS
Subjects
Independently living adults (BMI 30–39.9 kg/m2; age 21–50 years) were recruited from the Madison metropolitan area by means of flyers and newspaper advertisements. Conditions that restrict eating and/or physical activity were considered for inclusion/exclusion in the study. Specifically, we excluded individuals with ambulatory inability, dietary restraints (restraint score >10, Three-Factor Eating Questionnaire)17, or diabetes (fasting blood glucose >126 mg/dL), history of eating disorders. People taking medication that affects metabolism, actively trying weight loss, and pregnant or post-menopausal women were also excluded. People with travel plans during doubly labeled water (DLW) procedure days were also excluded to avoid measurement errors18. The study was approved by the University of Wisconsin-Madison Institutional Review Board, and all volunteers gave their written consent to participate.
Experimental design overview
Based on the holiday timeline described by Yanovski et al.4, we implemented a 16-week longitudinal study to assess changes in body weight, body composition, energy balance components, and behavioral measures during two consecutive periods: 1) an 8-week pre-holiday period (15th-30thSeptember to 9th-25thNovember), and 2) an 8-week holiday period (9th-25thNovember to 4th-15thJanuary) (Fig. 1; Supplementary Table 1). The visits fell on a range of dates because we were limited in the number of participants that could be handled in the clinic on a given day. Subject recruitment and data collection were conducted at the Clinical Research Unit (CRU) in quiet private rooms at University of Wisconsin Hospital.
Figure 1. Experimental design.
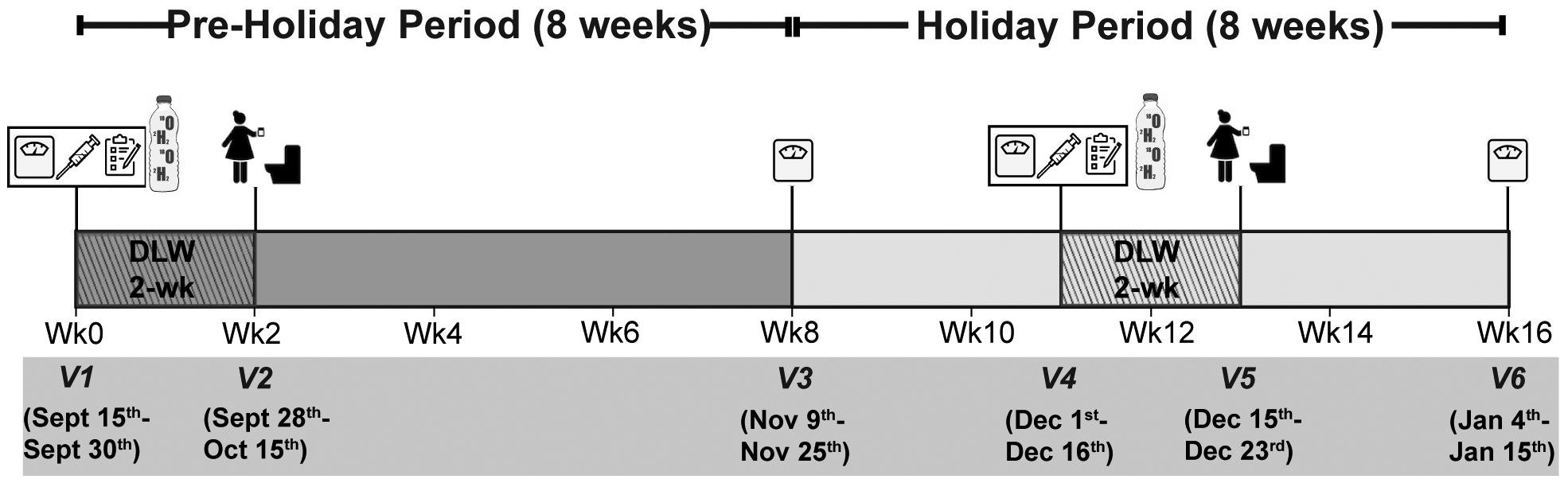
(a) Study protocol for longitudinal study design with 8-week pre-holiday and 8-week holiday period. Anthropometric measures, appetite ratings, blood samples, and questionnaire data, and DLW administered at visit 1 (V1) and visit 4 (V4). On visit 2 (V2) and visit 5 (V5) follow-up urine samples were collected.
Pre-holiday period.
Participants visited the study center three times during this phase: at baseline (visit 1, V1), at week 3 (visit 2, V2), and at the end of the phase (week 8; visit 3, V3). At V1 we collected anthropometric data, administered stable isotope labeled water, and collected urine samples for total body water (TBW) measurement, after an overnight fast. We also collected subjective appetite ratings and blood samples, and implemented a computer-based Leeds Food Preference Questionnaire (LFPQ) task19, all in response to a test meal. Participants also completed the Seasonal Pattern Assessment questionnaire (SPAQ) to identify Affective Disorder20 and EH frequency questionnaire to determine eating out behavior. At V2, we collected follow up urine samples for total energy expenditure (TEE) assessment. At V3 (end of the pre-holiday period), TBW and body weight were measured again for changes in fat mass and fat-free mass.
The Holiday period.
This phase began at the completion of the pre-holiday period. Participants visited the study center at week 11 (visit 4, V4), at week 13 (visit 5, V5), and at week 16 (visit 6, V6). All measures from baseline V1 (pre-holiday period) were repeated during the holiday period (December 1st through December 16th) at V4. At V5 (December 15th through December 23rd), we collected follow up urine samples to calculate TEE. At V6, TBW was measured to determine body composition change from the end of pre-holiday period (V3 vs. V6).
Assessment of body weight and body composition
At each visit, body weight was measured to the nearest 0.5 kg using a calibrated balance beam scale (HealthOMeter, Sunbeam, Boca Raton, FL) and height measured using a wall-mounted stadiometer without shoes and with the subject’s head held in the Frankfort plain. Waist (WC) and hip circumference (HC) were measured with a flexible tape to the nearest 0.1 cm midway between the lower costal margin and super iliac crest during a period of expiration. Blood pressure was measured in triplicate using a digital automatic blood pressure monitor (Omron HEM 705 LP, Kyoto, Japan) after a 10-min rest. Fat-free mass (FFM) was calculated from TBW, assuming 73.2% hydration21. Fat mass was estimated using FM = body mass – FFM22, 23 and caloric densities of 1 kcal/g for FFM and 9.45 kcal/g for fat mass24.
Assessment of TEE
DLW measured TEE over a 2-week period for both the pre-holiday and the holiday periods. DLW was administered orally at a dose of 2g of 10 atom % 18O labeled water and 0.12g of 99.9 atom % deuterium-labeled water per kilogram of estimated total body water, along with a subsequent 50-ml water rinse of the dose bottle. Urine samples were collected immediately before DLW administration and 2, 3, and 4 hours post-dosing. Additional urine samples were collected after two weeks, at the same time of the day as 3- and 4-hour post-dosing samples. TEE was calculated using the equation described by de Weir et al.25 Dilution spaces for 2H and 18O were calculated according to Cole at al.26.
Estimation of energy intake
The DLW technique allowed us to measure the “TEE” and “body stores” components, and “energy intake” for each period was calculated using the following equation:
Energy Intake = TEE + Change in body energy stores
Change in body energy stores was calculated from initial and final TBW.
Collection of blood samples
At V1 (mid-September) and V4 (early- to late-December), participants arrived at the CRU after an overnight fast. To verify the fasting status, all participants were asked to record the time of last meal/liquid consumed. Based on that data, we determined that all participants followed the fasting regimen as instructed. After that a peripheral intravenous line was placed in the antecubital vein by a trained nursing staff. Blood samples were drawn at baseline (before test meal at either 8:00am or 10:00 am) and at 30 min, 60 min, and 120 min post-test meal consumption. The test-meal (Dannon full fat yogurt with Del Monte canned peaches in syrup and a cinnamon roll). was designed based on the principles described by Gibbons et al.27 and to effectively detect changes in “wanting” and liking” components (consultation with the co-author, Dr. Finlayson). Calories of the test meal were determined for each individual based on their height, weight, age, and sex,28 and composed 15% of the daily estimated energy requirement (55% calories carbohydrate, 10% calories protein, 34% calories fat). Blood samples were centrifuged at 2000 g for 15 min at 4˚C and plasma was stored at - 80˚C until assay. We used radioimmunoassay (Millipore, Billerica, MA, USA) to measure plasma active ghrelin, plasma leptin, plasma total PYY, and plasma insulin levels.
Assessment of appetite
Subjects completed a paper version of visual analogue scale (0–10 cm) to measure motivation to eat at baseline (before test meal) and 15 min, 30 min, 60 min, and 120 min after meal initiation (V1 and V4)29. At each time point the following questions were asked: (1) How hungry do you feel? (2) How full do you feel? (3) How satisfied do you feel? (4) How much do you think you can eat? Ratings were made on a 10 cm scale with text at each end indicating the most positive and most negative rating29.
Collection of saliva samples
Subjects performed an in-home saliva collection for three consecutive weekdays during the week following V1 and V4. One mL samples were collected in pre-labeled glass tubes at the following timepoints: 1) Immediately upon waking up, 2) 30 minutes after waking up, 3) between 2pm and 5pm, 4) between 7pm and 10pm. Participants were instructed to avoid eating/drinking, brushing teeth, or engaging in physical activity between the two morning sample collections. Tubes were stored in subject’s standard freezer until brought back to the lab, where they were stored at −80˚C until assay.
Behavioral measures
At each visit during the pre-holiday and the holiday period, questionnaires were administered to collect self-reported information on EH. Specifically, we asked how frequently participants consumed a meal at fast food restaurants, fast-casual restaurants, all-you-can-eat restaurants, sit-down restaurants, takeout meal/frozen boxed meal. Fast-food restaurants were defined as places that serve fast-food cuisine and have minimal table service with limited menu items; fast casual restaurants were defined as somewhat quieter and slower paced and offer complex foods that take longer to prepare; All-you-can-eat restaurants were places where unlimited meals are served at one price; and sit-down restaurants were places where people sit and a staff takes an order. The response scale for eating out at each restaurant type was (1) Never, (2) Rarely, (3) Sometimes (1–3/month), (4) 1–2 times/week (5) 3–4 times/week, and (6) >5 times/week. This questionnaire was developed by the Survey of Health of Wisconsin (SHOW) and is validated16, 30. These categories were combined and scored as follows: (1) Never/Rarely, given a value of 0, (2) Sometimes, given a value of 0.7, (3) 1–2 times/week, given a value of 1.5, and (4) >3 times/week, given a value of 3. An average for number of visits per week was calculated and compared for the pre-holiday and the holiday period. The Seasonal Pattern Assessment Questionnaire (SPAQ)20 was also administered at V1 and V4 to identify Seasonal Affective Disorder31, 32. The questionnaire consisted of six questions, each graded on a response from 0 to 4. Responses were added to generate a total SPAQ score20 between 0 and 24.
Leeds Food Preference Questionnaire (LFPQ)
Participants completed the LFPQ task at V1 and V4, 60 minutes after test meal consumption. The LFPQ is a computerized hedonic analysis platform that measures implicit and explicit measures of food reward.19 Participants rated 20 holiday-related food pictures varying in 2 dimensions: fat (low and high) and taste (sweet and savory). For explicit measures, participants were asked to rate on a visual-analogue scale how much they “liked” (“How pleasant would it be to experience a mouthful of this food now?”) or “wanted” (“How much do you want to eat this food item now?”) food items shown on the screen. In addition, random food image pairs were presented on the screen and participants were instructed to quickly and accurately select the food item they would “most want to eat now.” With this, both frequency and preferred choice (relative food preference), and reaction time were measured, and a standardized implicit wanting score was calculated.33 Because we were most interested in the fat content of foods and differences between the two time periods, values for sweet and savory were averaged for each individual to give us a high-fat and a low-fat food score for each measure.
Statistical Analysis
Statistical analyses were performed using SPSS (version 24; SPSS Inc., Chicago, IL, USA). Sample size was based on the prior studies5, 6 to detect an increase of 0.8±1.4 kg in body weight with power of 80% alpha of 5%. For changes in body weight, body composition, and other anthropometric measures, we calculated the difference between V1 and V3 for the pre-holiday period, and between V3 and V6 for the holiday period. These differences were then compared using a paired t-test. TEE, energy intake, and behavioral measures were compared between V1 and V4 using paired t-tests. A two-way repeated measure ANOVA was used to analyze differences in hormones and subjective appetite levels. We used a mixed design ANOVA to analyze explicit wanting, explicit liking, implicit wanting, and preferred choice from the LFPQ measure. Pearson’s correlations were used to assess relationships between variables. Data are reported as mean ± standard error of mean (SEM) and statistical significance was set at p ≤ 0.05. All data met the assumption for statistical tests. We use one-tailed thresholds to test our directed hypothesis that holiday related weight gain will be largely due to an increase in energy intake rather than a decrease in energy expenditure6, and this increase in energy intake will be driven by changes in eating patterns.
RESULTS
Data from 23 participants (n=27 enrolled) were included in the analyses. One participant dropped out of the study due to scheduling conflict. For three subjects, DLW results did not pass quality control (poor precision between the TEE calculated from successive urine specimens at the start and end of sample collection) and their data were excluded. All participants in the study were obese (BMI 33.1 ±1.0) with an average age of 37 ± 0.01 years.
Changes in body weight and body composition are reported in Table 1. The difference between V3 and V1 (pre-holiday) was compared with the difference between V6 and V3 (holiday) for all these variables. We observed a decrease of 0.86 kg during the pre-holiday period vs an increase of 0.41 kg during the holiday period. Interestingly, the composition of weight change did not differ between the two periods with fat comprising of 25% of weight change during the pre-holiday vs 22% of weight change during the holiday period.
Table 1.
Changes in anthropometric measures within the pre-holiday and holiday period.
| N= 23 | Pre-Holiday (V3-V1) |
Holiday (V6-V3) |
P value |
|---|---|---|---|
| Δ Body Weight (kg) | −0.86 (± 0.25) | 0.41 (± 0.25) | 0.018 |
| Δ Fat Mass as % body weight | 25% (± 30%) | 22% (± 33%) | 0.947 |
| Δ Fat Free Mass as % body weight | 75% (± 30%) | 78% (± 33%) | 0.946 |
| Δ Waist Circumference (cm) | 0.54 (± 1.38) | 0.14 (± 1.38) | 0.887 |
| Δ Hip Circumference (cm) | −1.17 (± 0.84) | 0.14 (± 0.84) | 0.444 |
| Δ Systolic Blood Pressure | −3.83 (± 1.96) | 1.30 (± 1.96) | 0.204 |
| Δ Diastolic Blood Pressure | −3.70 (± 1.22) | 1.74 (± 1.22) | 0.037 |
Values reported as mean ±SEM. Pre-holiday and Holiday period values represent change within the periods (V3-V1 for Preholiday period; V6-V3 for Holiday period). A paired t-test was used to make the comparisons.
In line with our hypotheses, our most important finding was that TEE did not change between the two periods (V1, Pre-holiday 2745 ± 96kcal/day; V4, Holiday 2742 ± 86 kcal/day, P = 0.96), indicating no effect of season on energy expenditure. As such the energy density of weight change, as calculated in a paper by Bhutani et al.34, averaged 3000 kcal/kg. Thus, our data suggests that the weight gain may be influenced by changes in eating behavior during the holiday period rather than decreased energy expenditure.
To assess potential effects of holiday-related depression and stress on holiday weight gain, we compared the scores generated from the SPAQ as well as salivary cortisol, a physiological marker of stress, between V1 and V4. We did not find any differences in the SPAQ scores (P = 0.40) or cortisol levels (P = 0.73) (Fig. 2a and 2b), suggesting no role of stress and depression on holiday related weight gain. We next tested if the body weight was influenced by subjective perception of appetite. Our self-reported data indicated that participants were equally hungry (V1, pre-holiday 6.4 ± 0.25; V4, holiday 7.0 ± 0.25; P = 0.2) when they arrived at the study center at both periods. Interestingly, at V4 (the holiday period), subjective feelings of hunger (P=0.02, one-tailed) and desire to eat more (P=0.01, one-tailed) remained high and satisfaction from meal remained low (P=0.01, one-tailed) immediately after the test meal consumption. Participants continued to feel less satisfied (main effect period, P = 0.02, one-tailed) after 15 min, 60 min, and 120 minutes of test meal consumption (Fig. 3). Furthermore, the change in average satisfaction score was negatively correlated with change in body weight (r = −0.47, P = 0.02) between the two periods.
Figure 2. Comparison of probable confounders to energy balance during the holiday period.
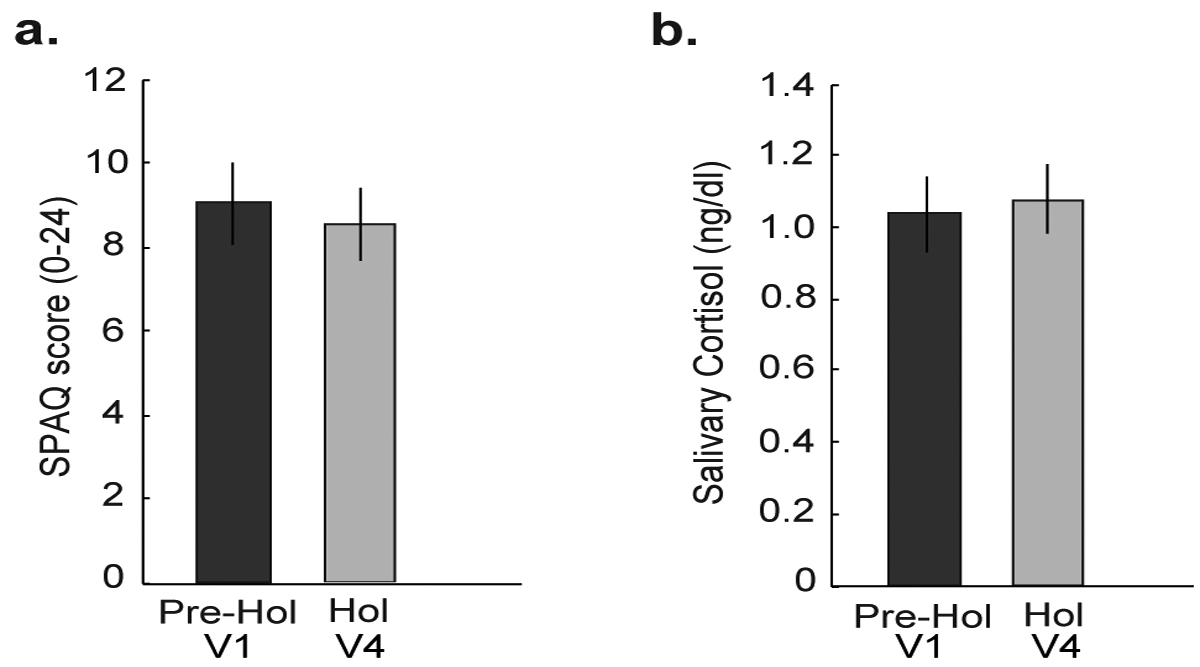
(a) The Seasonal Pattern Assessment Questionnaire (SPAQ) was used to measure markers of Seasonal Affective Disorder (SAD), rated on a total scale of 0 to 24, with higher numbers indicating SAD. There were no differences in SPAQ scores between the pre-holiday (V1) and the holiday period (V4), indicating no SAD. (b) An average of salivary cortisol samples collected on three consecutive weekdays, were not different between the holiday (the week following V1) and pre-holiday period (the week following V4), indicating no differences in stress levels. *p<0.05. Data are represented as mean ± SEM. A paired t-test was used to make the comparisons.
Figure 3: Comparison of subjective measures of appetite prior to and in response to a test meal.
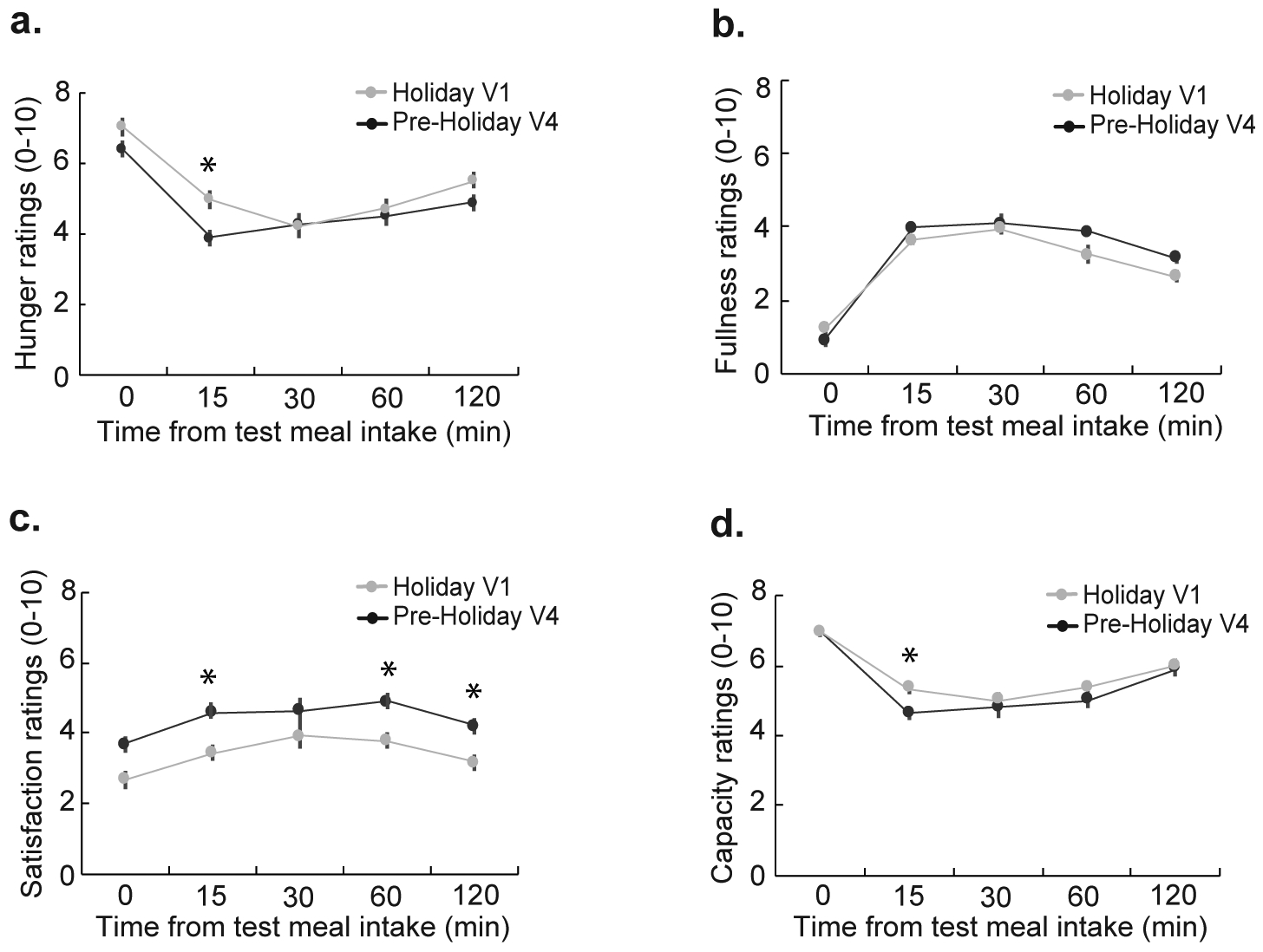
Participants rated hunger, fullness, capacity to eat more, and satisfaction on a 10 cm scale. Participants were significantly less satisfied (p = 0.02) with their test meal during the holiday period (V4 vs V1). There were no differences in hunger, fullness, or capacity between the pre-holiday and holiday period. A two-way repeated measure ANOVA was used to make the comparisons.
Based on the evidence that EH increases body weight35, we next tested whether self-reported eating out behavior changed during the holiday period. Partially replicating these previous findings, subjects reported a significant increase in number of episodes of eating at sit-down restaurants (P = 0.03, one-tailed) during the holiday period (average of V4, V5, V6) compared to the pre-holiday period (average of V1, V2, V3) (Fig. 4a). We observed no changes in consumption of fast-food (P = 0.38), fast-casual (P = 0.72), all-you-can-eat (P = 0.16), or takeout and frozen meals (P = 0.78) between the two periods (Supplementary Table 2.)
Figure 4. Changes in energy balance components and effect of eating-away-from-home frequency on energy intake during the holiday period.
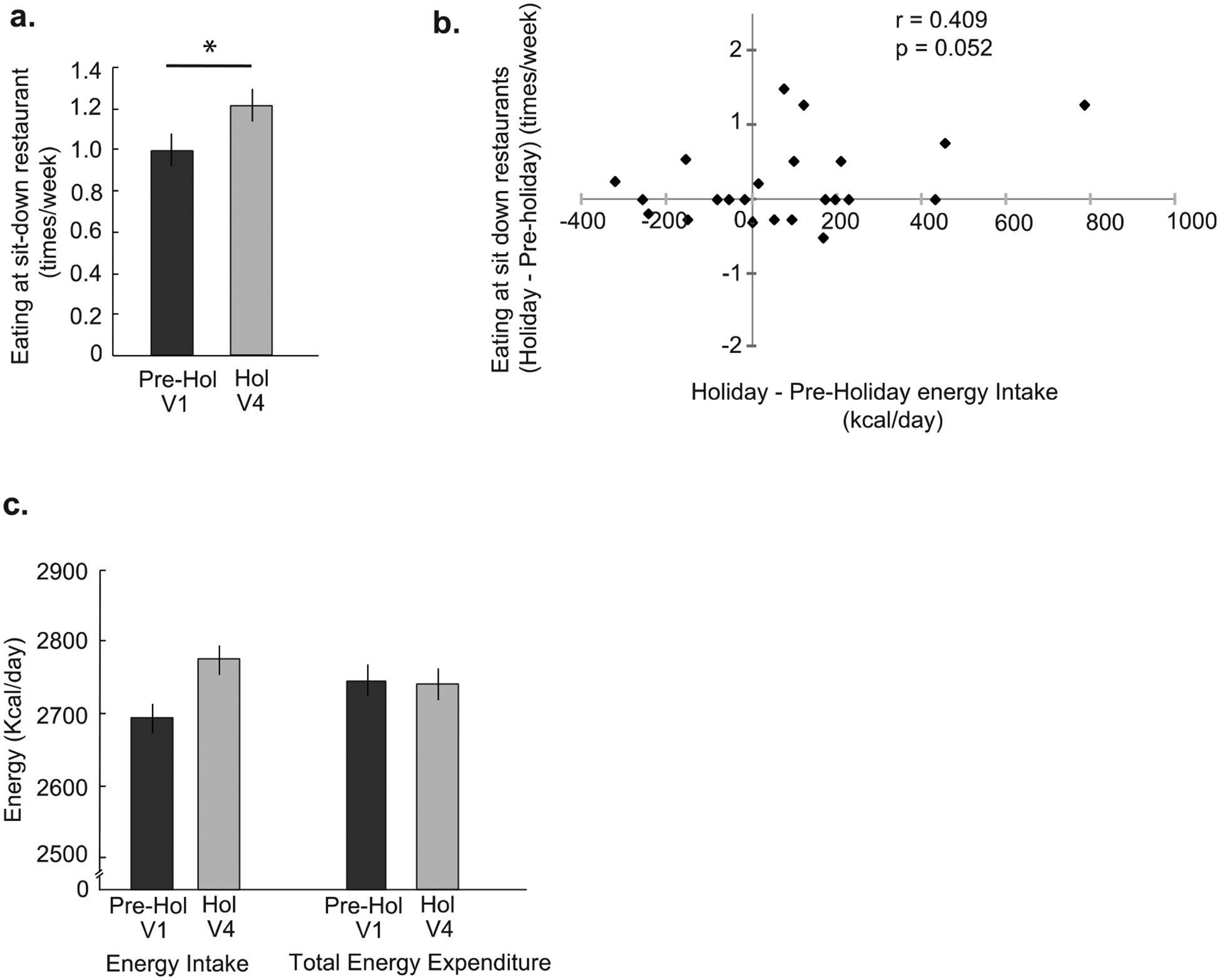
(a) The average number of times per week participants reported eating at sit-down restaurants during the pre-holiday period (average of V1, V2, V3) vs the holiday period (average of V4, V5, V6). Eating at sit-down restaurants increased (one-tailed, p = 0.03) during the holiday period. (b) There was a trending positive correlation (p = 0.05) between eating at sit-down restaurants and energy intake over the pre-holiday and the holiday periods. Pearson’s correlation was used for this analysis. (c) The changes in energy intake and expenditure as measured by collection of doubly labeled water (DLW) during the pre-holiday (V1) and holiday periods (V4). The total energy expenditure did not differ between two periods, while energy intake increased. Data are represented as mean ± SEM. A paired t-test was used to make the comparison.
We next tested the possible contribution of constant exposure to high caloric food cues on holiday related weight gain using LFPQ. Intriguingly, the LFPQ data showed no main effect of period and no interaction between period and fat for explicit wanting (P = 0.46), explicit liking (P = 0.39), or food choice (P = 0.10) measures (Table 2). However, a significant interaction between period and fat for implicit wanting was observed in the opposite direction than expected.
Table 2.
Leeds Food Preference Questionnaire measures of liking and wanting.
| Pre-Holiday (V1) |
Holiday (V4) |
P value Period*Fat |
|||
|---|---|---|---|---|---|
| High Fat | Low Fat | High Fat | Low Fat | ||
| Explicit Liking | 58.0 (± 2.5) | 57.7 (± 1.8) | 51.8 (± 2.5) | 56.2 (± 1.8) | 0.455 |
| Explicit Wanting | 52.7 (± 2.3) | 53.3 (± 2.1) | 48.6 (± 2.3) | 54.5 (± 2.1) | 0.394 |
| Implicit Wanting | 1.2 (± 0.9) | −1.2 (± 0.9) | −2.4 (± 0.9) | 2.4 (± 0.9) | 0.008 |
| Food Choice | 24.0 (± 0.6) | 24.0 (± 0.6) | 22.7 (± 0.6) | 25.4 (± 0.6) | 0.103 |
Values reported as mean (±SEM). There was no effect of the holiday period on measures of explicit liking, explicit wanting, implicit wanting, or food choice. A mixed design ANOVA was used to make the comparisons.
We further tested if alterations in circulating gut hormones may have appetite modulatory effects during the holiday period. Our analysis did not indicate an increase in hunger inducing hormones (active ghrelin, P = 0.46) or satiety causing hormones (leptin, P = 0.64, total PYY, P= 0. 90; insulin, P= 0.52) between the pre-holiday and the holiday-period (Supplementary Table 3.), suggesting no influence of these regulatory hormones on excessive energy intake. However, in line with our hypothesis that it is increase in energy intake that has larger contribution to the holiday weight gain than energy expenditure, we observed a non-significant change with estimated energy intake in the expected direction (+80 kcal/day; one-tailed, P = 0.07) (pre-holiday 2695± 26kcal/day; holiday 2775± 26 kcal/day) (Fig. 4c). Further, our correlational analysis revealed that there is a strongly trending correlation between change in eating out episodes at sit-down restaurants and change in energy intake (r = 0.41, P = 0.05) between the two periods (Fig. 4b).
DISCUSSION
In previous studies, six-weeks of the holiday period (Thanksgiving through New Year’s) is well documented as a critical time for weight gain in adults2–9. Although studies have implemented dietary9, 36, 37 and physical activity7 interventions to prevent weight increases, the actual cause of the holiday weight gain is controversial. Here, we are the first to quantify energy balance components using DLW to test our hypothesis that no change in TEE and a possible increase in energy intake will contribute to weight gain during the holiday period. Following this hypothesis, we observed no decrease in TEE during the holiday period. We also recorded lower post-meal satisfaction and increased EH, as possible contributors to increased body weight. In agreement with the above findings, we also recorded a non-significant increase in energy intake during the holiday period.
As reported previously, weight change over the winter holidays is highly variable, ranging from 0.4 to 0.9 kg3–7, 38, with a modest average gain of 0.5 kg across all studies39. In line with this published literature, we observed a similar weight gain of 0.4 kg in obese adults during the 57 days of the holiday period. An average adult gains 0.4 to 1.0 kg40 in a year, with a daily weight gain of 0.0012 to 0.0027 kg/day. Comparing these with the rate of weight changes in our study, our numbers are significantly higher (0.007 kg/day) and thus sufficient to contribute to excess weight gain. Studies that included a measure of body composition indicate that the weight gain during this period is largely due to fat gain2, with pronounced effects in obese individuals7. Interestingly, in our sample fat comprised of only 25% weight gain, and thus weight change was not attributed to fat gain alone. It is possible that a significant increase in energy intake would have resulted in a significant increase in body fat. Our data also suggests that body water may have contributed to the holiday weight gain. Glycogen, a component of fat free mass, in particular, is hydrated with water41. We suspect that daily variation in carbohydrate intake or utilization during the holiday period may have resulted in glycogen related water retention, hence weight change. Additionally, intestinal contents and fecal water contribute to a large variability in body water42 and may have fluctuated with changing eating patterns during the holiday period.
It is worth noting that despite no detectable decline in TEE, participants still gained weight. These findings are supported by observational studies where TEE did not predict subsequent weight gain6, and regular exercisers were not protected against weight increases during the winter holidays7, 43. If energy expenditure was not a contributor to this rise in body weight, change in eating patterns likely explains our results. Indeed, eating in a social setting16, constant exposure to food cues44, 45 and stress13 influence calorie consumption, all of which are likely to occur during the holidays. Though our study participants did not have high-stress levels, they visited sit-down restaurants more often during the holiday period than the pre-holiday period. We also noted a correlation between this increased frequency of eating outside of the home and calorie intake during the holiday period, suggesting this social factor to be a contributor to holiday weight gain. Participants also felt less satisfied after an energy-dense test meal was consumed at the study center. Moreover, reduced meal satisfaction ratings were correlated with a change in body weight. One plausible explanation is that excessive exposure to food cues during the holidays, overrides and undermines the internal appetite signaling system, making it difficult for people to regulate food intake46, 47. Particularly, in potentially social settings like restaurants, external cues extend the decision to actively terminate consumption beyond internal satiation. We expected the appetite-related hormones to reflect these effects, but none of that data supported our theory.
Environmental cues such as sight and smell of food can affect one’s motivational drive to eat46. The learned association between the real or anticipated reward value of hedonic foods may trigger wanting (drive for food) and liking (pleasantness of food). Consequently, we expected that during the time of year when people are surrounded by these high reward value foods, we will observe a surge in ratings associated with wanting/liking. Nevertheless, we did not observe an increase in wanting and liking for pictures of foods consumed frequently during the holiday period, demonstrating that the hedonic value and motivation for food does not change during this time. Our inability to show any effects for foods that are high in reward value, especially during the holiday period, warrant further investigation with larger sample size. This may also reflect possible individual differences in mechanisms underlying motivational behavior, or effect of external modulators such as a change in temperature, daylight, or social and cultural practices. Future studies investigating such individual differences during the holiday period are warranted.
No decrease in objectively measured TEE suggests that altered eating patterns produced weight gain during the holiday period. Indeed, we observed a non-significant increase in energy intake in our study. Our objective measure of energy intake is the first to potentially support the limited self-reported dietary data collected during the holiday period4, 48. While we did not track body weight or energy intake over the long-term, studies show that people fail to return to baseline body weight after the winter holiday4, 43. Some overfeeding studies with a remarkably high energy surplus have demonstrated this phenomenon49, 50. Whether a modest energy imbalance of 80 kcal/day during the holiday period in our study will be sufficient to trigger compensatory action warrants long-term measurement of energy balance components. We speculate that this non-significant increase in daily energy intake may not be strong enough to trigger biological compensatory response to lose accrued body weight and will contribute to permanent weight gain4, 43.
There were some limitations to our study. First, we were underpowered to detect significant increases in energy intake in this modestly sized cohort. With a larger sample size of participants in future studies, we expect to observe significant increases in energy intake. Second, weight gain within the holiday period was not significant by itself but was only significant when compared to the pre-holiday values. There are two possible contributors to this challenge that we cannot adjust for. First, we could not randomize the order of the periods to control for an order effect, due to the inherent nature of the study. Second, participants were not blinded to the purpose of the experiment because of the timeline and measurement. This may have caused them to be more aware of what they were eating and could have influenced their energy intake and food choice. To avoid this, future studies should measure results over a larger period to blind participants to the study period of interest. We also lack physical activity data to show no change in the physical activity component of TEE. Additionally, we recognize that due to cultural differences, eating patterns in the US during the holiday period may differ from other countries. Thus, our findings may not be generalized. Furthermore, while impractical to implement, the data that also captured variations in energy expenditure and food intake patterns on Christmas holiday would have been ideal. Finally, considering that alcohol intake can significantly add to the calorie intake during vacation43, future studies should consider collecting alcohol intake data during the holiday period.
Altogether, this study is the first to report on the changes in energy balance components during the winter holiday period in obese adults. With no reduction in TEE and a trend in increased calorie intake during the holiday period, the latter is likely the major contributor to this weight gain. Further, a trend in increased energy intake was most likely influenced by disrupted internal satiety signals coupled with external cues in a food cue rich environment. These findings may help inform the development of targeted interventions focused on satiety control and environmental factors. Further, examining the physiological and neural mechanisms involved will give us better insight into holiday weight gain and design effective weight loss or weight gain prevention strategies.
Supplementary Material
ACKNOWLEDGEMENTS
S.B. and D.A.S conceived and designed the experiment; S.B. conducted the experiment and acquired the data; S.B., D.A.S, N.W. and G.F. analyzed and interpreted the data; S.B., D.A.S, N.W. and G.F. wrote the paper. The corresponding author is supported by NIH MANTP training grant (T32 DK 007665). The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
List of Abbreviations
- TBW
Total body water
- TEE
Total energy expenditure
- DLW
Doubly labeled water
- FM
Fat mass
- FFM
Fat free mass
- WC
Waist circumference
- HC
Hip circumference
- EH
Eating-away-from-home
- SPAQ
Seasonal Pattern Assessment Questionnaire
- LFPQ
Leeds Foods Preference Questionnaire
Footnotes
COMEPTING INTERESTS
The authors declare no competing interests.
REFERENCE
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016; 315(21): 2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hull HR, Hester CN, Fields DA. The effect of the holiday season on body weight and composition in college students. Nutr Metab (Lond) 2006; 3: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull HR, Radley D, Dinger MK, Fields DA. The effect of the Thanksgiving holiday on weight gain. Nutr J 2006; 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O’Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med 2000; 342(12): 861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoeller DA. The effect of holiday weight gain on body weight. Physiol Behav 2014; 134: 66–9. [DOI] [PubMed] [Google Scholar]
- 6.Cook CM, Subar AF, Troiano RP, Schoeller DA. Relation between holiday weight gain and total energy expenditure among 40- to 69-y-old men and women (OPEN study). Am J Clin Nutr 2012; 95(3): 726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson JL, Krishnan S, Stoner MA, Goktas Z, Cooper JA. Effects of exercise during the holiday season on changes in body weight, body composition and blood pressure. Eur J Clin Nutr 2013; 67(9): 944–9. [DOI] [PubMed] [Google Scholar]
- 8.Rees SG, Holman RR, Turner RC. The Christmas feast. Br Med J (Clin Res Ed) 1985; 291(6511): 1764–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watras AC, Buchholz AC, Close RN, Zhang Z, Schoeller DA. The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain. Int J Obes (Lond) 2007; 31(3): 481–7. [DOI] [PubMed] [Google Scholar]
- 10.Painter JE, Wansink B, Hieggelke JB. How visibility and convenience influence candy consumption. Appetite 2002; 38(3): 237–8. [DOI] [PubMed] [Google Scholar]
- 11.de Castro JM. Family and friends produce greater social facilitation of food intake than other companions. Physiol Behav 1994; 56(3): 445–5. [DOI] [PubMed] [Google Scholar]
- 12.The Baier M. “holiday blues” as a stress reaction. Perspect Psychiatr Care 1987; 24(2): 64–8. [PubMed] [Google Scholar]
- 13.Sominsky L, Spencer SJ. Eating behavior and stress: a pathway to obesity. Front Psychol 2014; 5: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan C Ode on health and the holidays. CMAJ 1998; 159(12): 1499. [PMC free article] [PubMed] [Google Scholar]
- 15.Reichelt AC, Westbrook RF, Morris MJ. Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses. Br J Pharmacol 2015; 172(22): 5225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhutani S, Schoeller DA, Walsh MC, McWilliams C. Frequency of Eating Out at Both Fast-Food and Sit-Down Restaurants Was Associated With High Body Mass Index in Non-Large Metropolitan Communities in Midwest. Am J Health Promot 2018; 32(1): 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29(1): 71–83. [DOI] [PubMed] [Google Scholar]
- 18.Bhutani S, Racine N, Shriver T, Schoeller DA. Special Considerations for Measuring Energy Expenditure with Doubly Labeled Water under Atypical Conditions. J Obes Weight Loss Ther 2015; 5(Suppl 5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlayson G, King N, Blundell J. The role of implicit wanting in relation to explicit liking and wanting for food: implications for appetite control. Appetite 2008; 50(1): 120–7. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal N BG, Wehr T Seasonal Pattern Assessment Questionnaire (SPAQ) In: Bethesda M, USA: National Institute of Mental Health, (ed), 1984. [Google Scholar]
- 21.Robotham DR, Schoeller DA, Mercado AB, Mirch MC, Theim KR, Reynolds JC et al. Estimates of body fat in children by Hologic QDR-2000 and QDR-4500A dual-energy X-ray absorptiometers compared with deuterium dilution. J Pediatr Gastroenterol Nutr 2006; 42(3): 331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: new physiological modeling approach. Am J Physiol 1999; 276(6): E995–E1003. [DOI] [PubMed] [Google Scholar]
- 23.Hydrometry DAS. Human Body Composition: Champaign, IL, 1996. [Google Scholar]
- 24.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr 2010; 92(6): 1326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109(1–2): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole TJ, Coward WA. Precision and accuracy of doubly labeled water energy expenditure by multipoint and two-point methods. Am J Physiol 1992; 263(5 Pt 1): E965–73. [DOI] [PubMed] [Google Scholar]
- 27.Gibbons C, Finlayson G, Dalton M, Caudwell P, Blundell JE. Metabolic Phenotyping Guidelines: studying eating behaviour in humans. J Endocrinol 2014; 222(2): G1–12. [DOI] [PubMed] [Google Scholar]
- 28.The Health and Medicine Division of the National Academies of Sciences EaM. Dietary Reference Intakes Calculator for Healthcare Professionals. In: United States Department of Agriculture. [Google Scholar]
- 29.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000; 24(1): 38–48. [DOI] [PubMed] [Google Scholar]
- 30.Nieto FJ, Peppard PE, Engelman CD, McElroy JA, Galvao LW, Friedman EM et al. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Public Health 2010; 10: 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson C, Stinson D, Fernandez M, Fine J, Isaacs G. A comparison of normal, bipolar and seasonal affective disorder subjects using the Seasonal Pattern Assessment Questionnaire. J Affect Disord 1988; 14(3): 257–64. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal NE, Genhart M, Jacobsen FM, Skwerer RG, Wehr TA. Disturbances of appetite and weight regulation in seasonal affective disorder. Ann N Y Acad Sci 1987; 499: 216–30. [DOI] [PubMed] [Google Scholar]
- 33.Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. J Pers Soc Psychol 2003; 85(2): 197–216. [DOI] [PubMed] [Google Scholar]
- 34.Bhutani S, Kahn E, Tasali E, Schoeller DA. Composition of two-week change in body weight under unrestricted free-living conditions. Physiol Rep 2017; 5(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachat C, Nago E, Verstraeten R, Roberfroid D, Van Camp J, Kolsteren P. Eating out of home and its association with dietary intake: a systematic review of the evidence. Obes Rev 2012; 13(4): 329–46. [DOI] [PubMed] [Google Scholar]
- 36.Hirsh SP, Pons M, Joyal SV, Swick AG. Avoiding holiday seasonal weight gain with nutrient-supported intermittent energy restriction: a pilot study. J Nutr Sci 2019; 8: e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason F, Farley A, Pallan M, Sitch A, Easter C, Daley AJ. Effectiveness of a brief behavioural intervention to prevent weight gain over the Christmas holiday period: randomised controlled trial. BMJ 2018; 363: k4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts SB, Mayer J. Holiday weight gain: fact or fiction? Nutr Rev 2000; 58(12): 378–9. [DOI] [PubMed] [Google Scholar]
- 39.Diaz-Zavala RG, Castro-Cantu MF, Valencia ME, Alvarez-Hernandez G, Haby MM, Esparza-Romero J. Effect of the Holiday Season on Weight Gain: A Narrative Review. J Obes 2017; 2017: 2085136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief 2015; (219): 1–8. [PubMed] [Google Scholar]
- 41.Heymsfield SB, Thomas D, Nguyen AM, Peng JZ, Martin C, Shen W et al. Voluntary weight loss: systematic review of early phase body composition changes. Obes Rev 2011; 12(5): e348–61. [DOI] [PubMed] [Google Scholar]
- 42.Academies IoMotN. Water. National Academy Press:Washington, DC., 2005. [Google Scholar]
- 43.Cooper JA, Tokar T. A prospective study on vacation weight gain in adults. Physiol Behav 2016; 156: 43–7. [DOI] [PubMed] [Google Scholar]
- 44.Elliston KG, Ferguson SG, Schuz N, Schuz B. Situational cues and momentary food environment predict everyday eating behavior in adults with overweight and obesity. Health Psychol 2017; 36(4): 337–345. [DOI] [PubMed] [Google Scholar]
- 45.Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev 2016; 17(2): 159–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bilman E, van Kleef E, van Trijp H. External cues challenging the internal appetite control system-Overview and practical implications. Crit Rev Food Sci Nutr 2017; 57(13): 2825–2834. [DOI] [PubMed] [Google Scholar]
- 47.Johnson AW. Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends Neurosci 2013; 36(2): 101–9. [DOI] [PubMed] [Google Scholar]
- 48.Jahns L, Johnson LK, Scheett AJ, Stote KS, Raatz SK, Subar AF et al. Measures of Diet Quality across Calendar and Winter Holiday Seasons among Midlife Women: A 1-Year Longitudinal Study Using the Automated Self-Administered 24-Hour Recall. J Acad Nutr Diet 2016; 116(12): 1961–1969. [DOI] [PubMed] [Google Scholar]
- 49.Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite 2004; 43(3): 253–9. [DOI] [PubMed] [Google Scholar]
- 50.Levitsky DA, Obarzanek E, Mrdjenovic G, Strupp BJ. Imprecise control of energy intake: absence of a reduction in food intake following overfeeding in young adults. Physiol Behav 2005; 84(5): 669–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


